- 1College of Acupuncture and Tuina, Changchun University of Chinese Medicine, Changchun, China
- 2College of Integration of Chinese and Western Medicine, Changchun University of Chinese Medicine, Changchun, China
Background: Type 2 diabetes mellitus (T2DM) is a public health challenge that urgently needs to be addressed globally. Short-chain fatty acids (SCFAs), as metabolic products of gut microbiota, have increasingly attracted attention for their role in the pathogenesis of T2DM. This study employs bibliometric and visual analysis methods, aiming to systematically depict the knowledge structure, research hotspots, and future directions in this field.
Method: We screened 965 publications on SCFAs and T2DM from the Scopus and Web of Science Core Collection databases. Visual analysis was conducted by using tools such as VOSviewer and CiteSpace.
Result: Since 2016 publications in this field have increased rapidly. China leads in publication volume, while the United States plays a central role in collaboration and academic influence. Key contributors include Max Nieuwdorp and Fredrik Bäckhed. King's College London shows strong academic impact, and Nature is the most influential journal. Keyword analysis highlights the importance of “gut microbiota,” “metabolomics,” “microbial metabolism,” “insulin resistance,” and “dietary intervention.” These topics suggest future research will focus on signaling pathways, personalized nutrition, and microbial interventions.
Conclusions: The current research focuses on a deep exploration of the role and mechanism of SCFAs in T2DM, and it aims to transition from basic research to clinical research. In terms of application, clinical workers are shifting from macroscopic dietary intervention to more precise dietary formulation strategies. The development of technologies such as metabolomics is expected to provide more powerful support for basic research and promote further progress in this field.
1 Introduction
T2DM is a chronic metabolic disorder that has become a significant global public health issue (Danaei et al., 2011). It is defined by hyperglycemia, insulin resistance, and low-grade inflammation, predominantly impacting the metabolism of glucose, lipids, and proteins. This chronic metabolic imbalance is believed to arise from the interplay of genetic and environmental variables, encompassing diet, lifestyle, and gut flora. According to credible sources, approximately 828 million adults worldwide were estimated to have diabetes in 2022, the vast majority being type 2 diabetes, with prevalence increasing markedly in both men and women compared with 1990 (Zhou et al., 2024). Numerous consequences, such as kidney failure, neuropathy, and cardiovascular disease, are closely linked to T2DM (Stratton, 2000; Lu et al., 2024). Not only does it severely reduce patients' quality of life and increase their economic burden, but it also imposes enormous pressure on the medical system (Cannon et al., 2018).
In the past few years, there has been a surge in the number of studies that have emphasized the importance of the intestinal microbiota and its metabolites in the development of T2DM (Canfora et al., 2019; Hosomi et al., 2022). SCFAs represent one of the principal metabolites of the gut microbiota, consisting primarily of acetate, propionate, and butyrate. They are generated under the anaerobic conditions of the intestine through the microbial fermentation of fermentable carbohydrates such as dietary fiber, resistant starch, and oligosaccharides (Cummings et al., 1987). Current studies suggest that SCFAs regulate host metabolism through multiple pathways and thereby influence the progression of T2DM (Lin et al., 2012; Yamashita et al., 2007). On one hand, SCFAs tend to activate specific G protein-coupled receptors (GPCRs) (Li et al., 2018), trigger intestinal L cells to generate GLP-1 and PYY (Tolhurst et al., 2012), increase the production of insulin, and improve insulin sensitivity (Drucker, 2018). On the other hand, they help to maintain the intestinal barrier's effectiveness (Kelly et al., 2015) and successfully lessen intestinal endotoxin leaks into the circulation, which lowers the systemic inflammatory response (Zhang and Mo, 2023). Moreover, the gut's pH can drop due to SCFAs generation, establishing an acidic milieu that is detrimental to pathogenic bacteria while promoting the proliferation of beneficial bacteria, thus aiding in the preservation of long-term metabolic equilibrium (Den Besten et al., 2013). These mechanisms collectively demonstrate the pivotal role of SCFAs in regulating T2DM (Mukhopadhya and Louis, 2025; Pham et al., 2024).
The number of studies on SCFAs and T2DM continues to increase. However, previous studies have not systematically summarized global trends, core hotspots, or evolving directions in this field. Bibliometric analysis, by evaluating publication output, collaboration networks, and keyword co-occurrence, can reveal the knowledge structure and emerging frontiers of a research domain (Jiang et al., 2023). In medical research, this approach has been extensively employed to identify prevalent research trends and offer recommendations for future paths (Ganti et al., 2025). Therefore, this study combines bibliometric and visualization strategies for performing a system evaluation of publications on SCFAs and T2DM from 1995 to 2024 to provide researchers with a macroscopic perspective and evidence to inform future investigations.
2 Methods
2.1 Sources of information and search methods
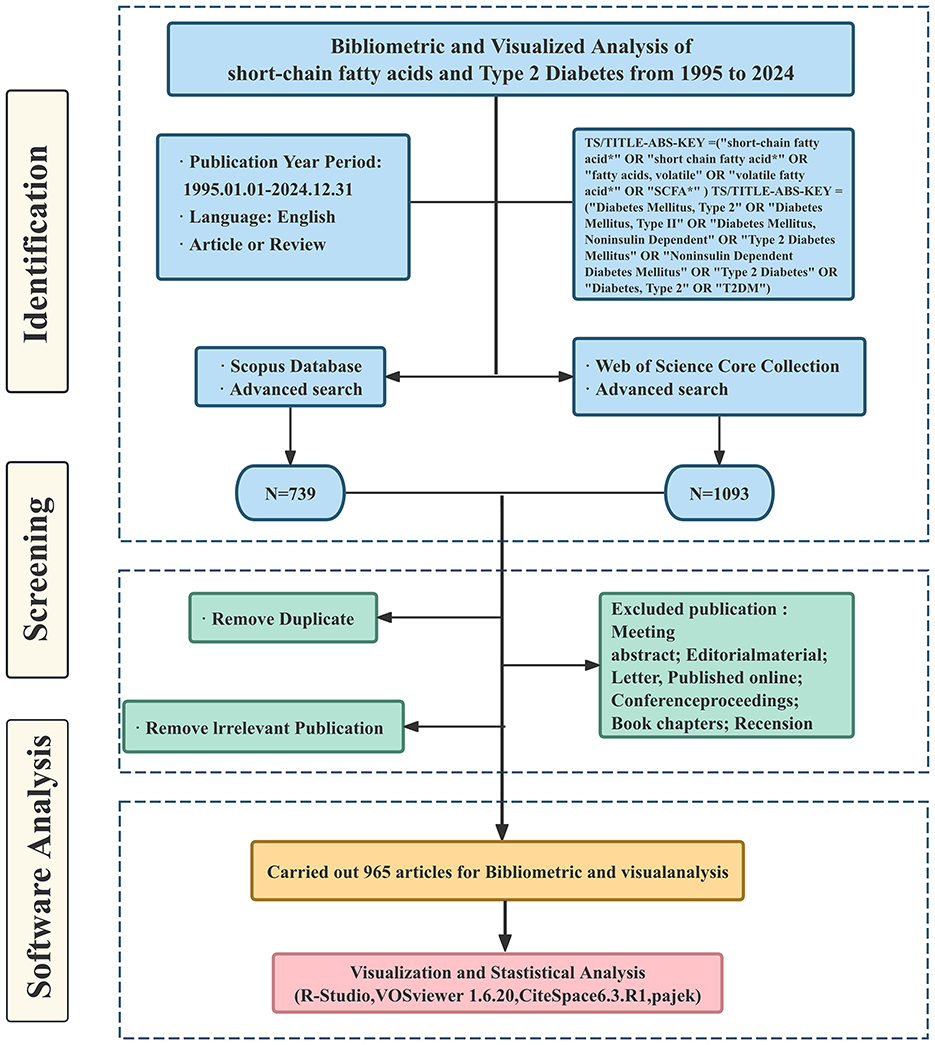
The WoSCC and Scopus databases were the sources of information for these publications. The search was conducted on May 10, 2025. The main search terms included SCFAs and T2DM, along with related free-text terms. Table 1 describes each database's unique search approach. The search encompassed publications published from January 1, 1995, until December 31, 2024. Figure 1 illustrates the comprehensive screening process.
2.2 Literature screening and data extraction
Records from Scopus were exported as complete entries in CSV format, while records exported from WoSCC were obtained as “complete records and cited references” in plain text format. All the data was downloaded in a single day to maintain consistency. Two researchers (ZLK and JRS) independently screened the literature. They reviewed titles, abstracts, and references, then cross-checked all included studies.
Inclusion criteria: (1) Research type: Publicly published original research articles (Article) or systematic reviews/review articles (Review) (2) Research Object/Topic: The research must simultaneously involve the following two core contents: ① Research related to SCFAs, such as their levels, metabolism, or mechanisms of action ② Human studies, animal models, or cellular mechanism studies related to T2DM. (3) Language: English. (4) Publication period: January 1, 1995, to December 31, 2024.
Exclusion criteria for publications: (1) Publication type: Exclude conference abstracts, editorials, letters, reviews, book chapters, and other non-research or non-review literature. (2) Literature not related to the research content, for example: ① The application of SCFAs in non-biomedical fields such as ruminant nutrition, wastewater treatment, and industrial fermentation ② Type 1 diabetes, gestational diabetes, or other types of diabetes, and T2DM have not been clearly distinguished or discussed. ③ SCFAs have not been directly measured or discussed. Studies that only briefly mention “gut microbiota” or “dietary fiber” (3) cannot obtain the full text of the literature.
2.3 Data analysis
Bibliometric analysis employs mathematical and statistical methods. This method is considered a quantitative analysis methodology that reveals the hotspots and present condition of a study topic while forecasting its future development. This approach enables a comprehensive assessment of research trajectories across several aspects, among them research hotspots, networks of collaboration, and temporal trends (Barrington et al., 2023). R-Studio is used to plot the annual growth trend of publications (Aria and Cuccurullo, 2017), and SCImago Graphica (version 1.0.39) is used to analyze the scientific research output and cooperation pattern of countries and regions (Hassan-Montero et al., 2022). VOSviewer (version 1.6.20) is dedicated to building and measuring co-occurrence/collaboration/co-citation networks (country, institution, journal, author, keyword co-occurrence) and outputting overlay views (Van Eck and Waltman, 2010); CiteSpace (version 6.3.R1) is used for keyword burst analysis (Chen, 2004); Pajek only performs layout and beautification on the large-scale network exported by VOSviewer, without changing any weights or metrics, to enhance the readability of the image (Chen et al., 2025).
3 Results
3.1 Annual publication trends
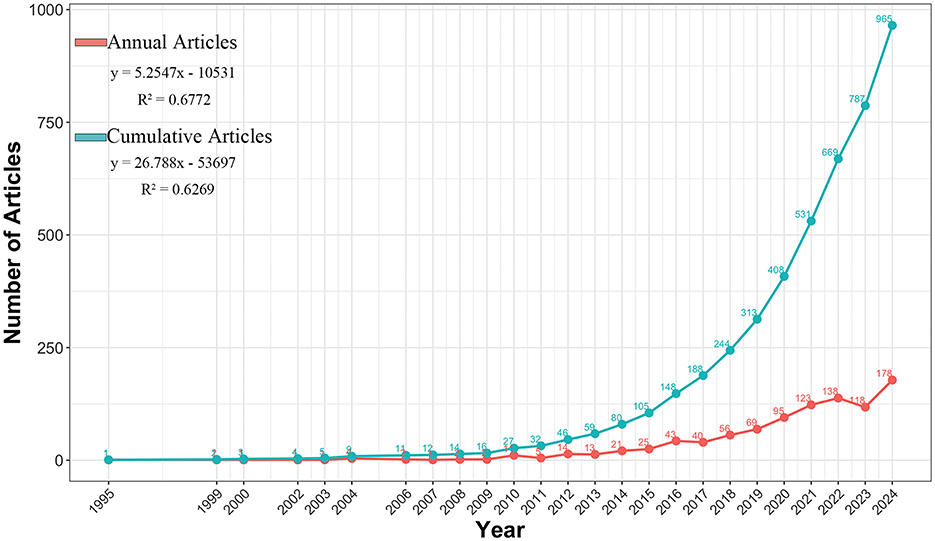
Figure 2 shows the changing trends of the annual and cumulative publication volumes in this field from 1995 to 2024, which can be divided into three stages: (1) The start-up period (1995–2009): The output was relatively small; (2) Initial growth period (2010–2014): Output steadily increased; (3) Rapid expansion period (2015–2024): Witness explosive growth. The two linear regression models in the figure describe the overall average situation of historical data, corresponding to “annual articles” and “cumulative articles,” respectively. Here, y represents the number of articles published annually or the cumulative total of articles up to a certain year, and x represents the year. The linear regression model for the annual publication volume is y = 5.2547x−10531. The coefficient of determination is R2 = 0.6772. The linear regression model of the cumulative publication volume is y = 26.788x−53697, and the coefficient of determination is R2 = 0.6269. This field saw a major rise in research over the last 10 years. The upward trend is expected to continue.
3.2 Country and regional analysis
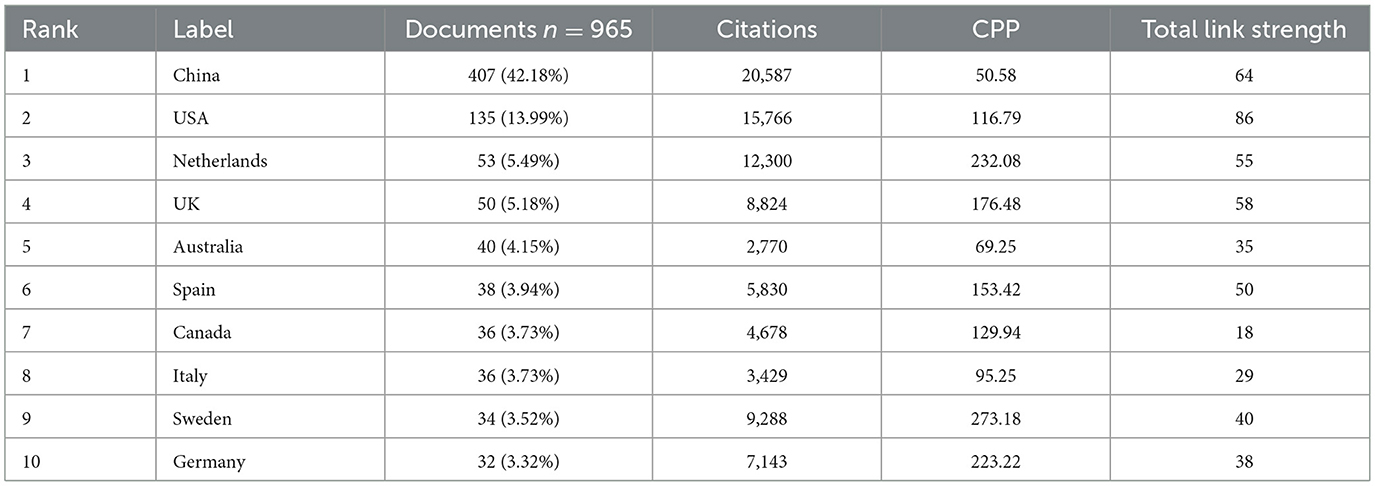
Publications on T2DM and SCFAs come from 75 different countries. Table 2 lists the 10 nations in the world that produce the largest quantities. China ranked first with 407 papers (42.18%), followed by the United States with 135 papers (13.99%) and the Netherlands with 53 papers (5.49%). The United Kingdom and Australia ranked fourth and fifth, with 50 papers (5.18%) and 40 papers (4.15%), respectively. In terms of total citation counts, China leads with 20,587 citations, followed by the United States with 15,766 and the Netherlands with 12,300. Regarding the intensity of international cooperation, the United States ranks first with a total link strength of 86, followed by China with 64 and the United Kingdom with 58. Finally, in terms of citations per publication (CPP), Sweden ranks first, with the Netherlands and Germany in second and third place, respectively.
Figure 3A shows the international collaboration network generated with VOSviewer. Each node represents a country or region, with node size weighted by publication volume. The thickness of the connecting lines indicates the strength of collaboration. The visualization uses publication volume (documents) as the weighting parameter, while the color scale reflects the average citation impact of each country. Lighter colors indicate higher average citation influence. Figure 3B further illustrates the global co-occurrence of countries and regions. The results show that this research topic is distributed worldwide, reflecting broad international attention and participation.
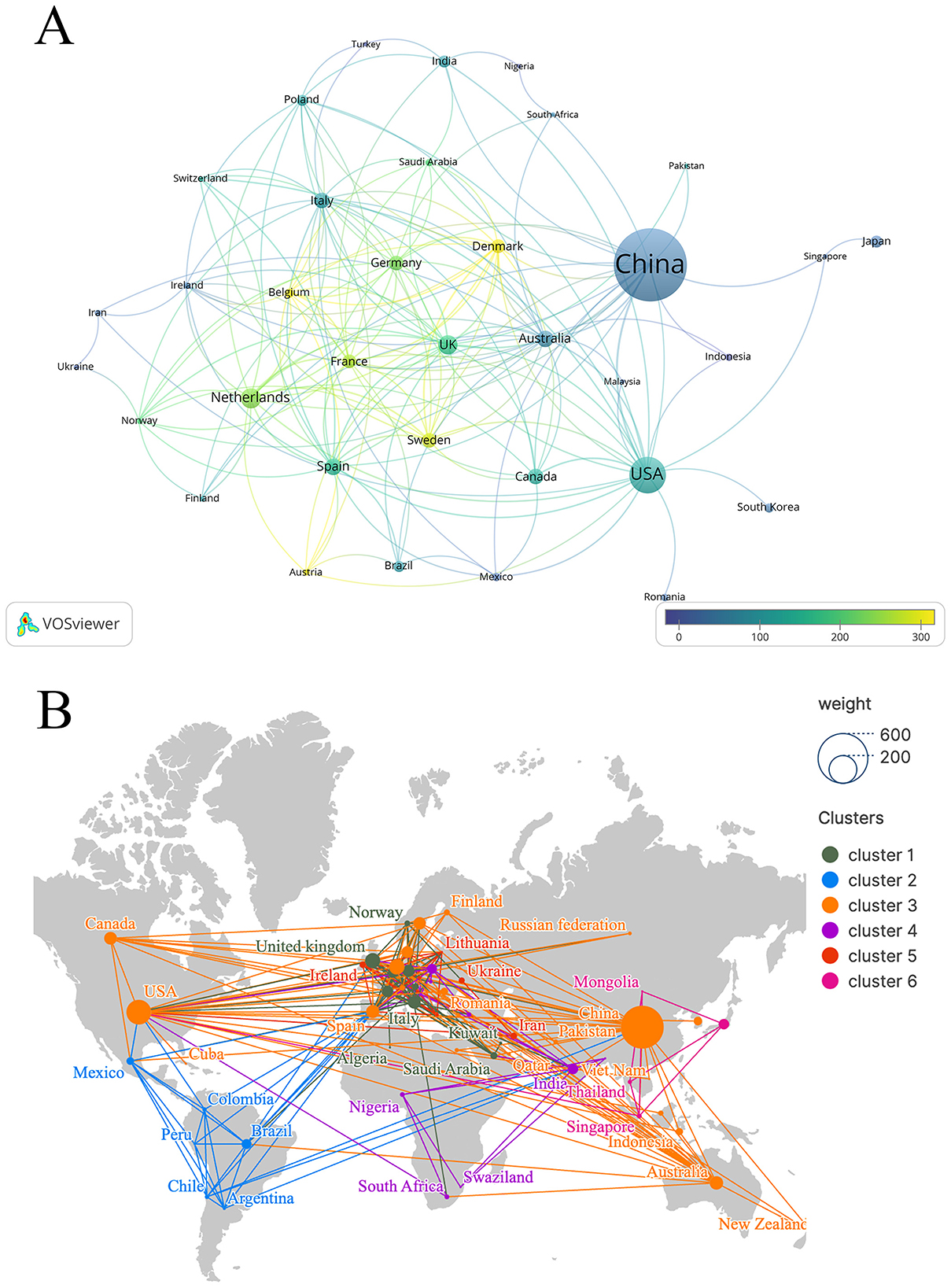
Figure 3. Country/region visual analysis. (A) Country/region co-authorship of publication and citation overlay map. (B) World map of countries/regions' distribution in this field.
3.3 Author analysis
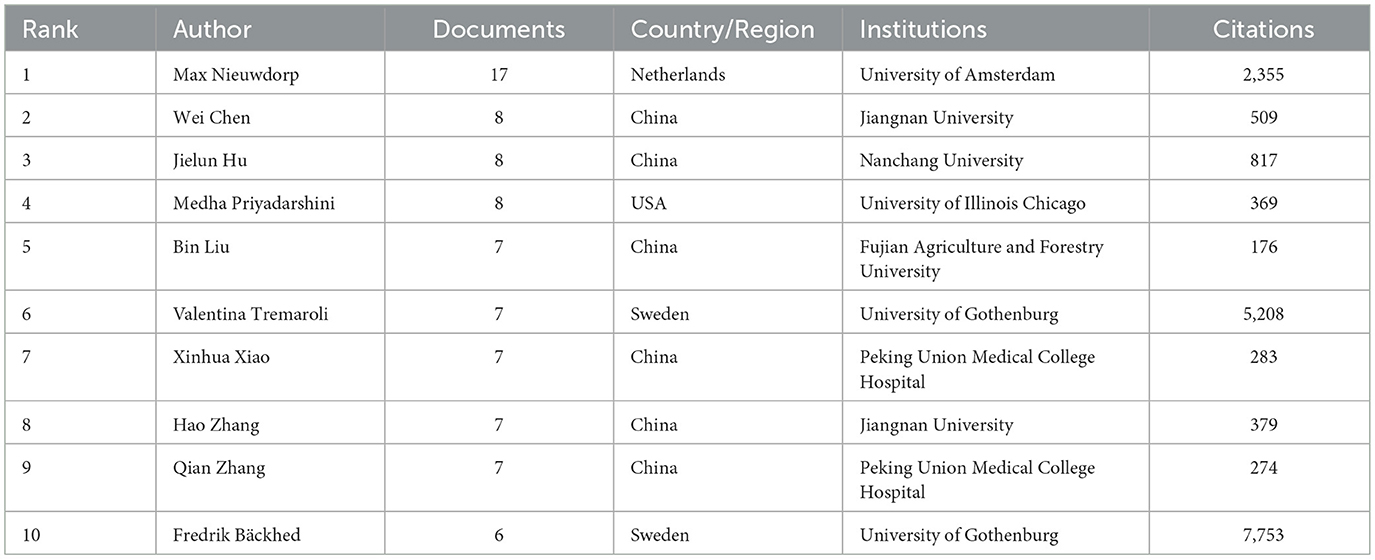
Research on T2DM and SCFAs has been provided by 5,147 authors overall. The top 10 most productive authors' publication and citation statistics were gathered in Table 3, who collectively published 82 papers. As shown in Figure 4A, nodes represent authors, and the size of nodes represents the number of published articles. Dutch scholar Max Nieuwdorp ranked first with 17 publications. Chinese scholars Wei Chen and Jielun Hu, along with U.S. scholar Medha Priyadarshini, each published eight papers, tying for second place. Among the top 10 prolific authors, six were from China, which shows the remarkable activity of Chinese scholars in this field.
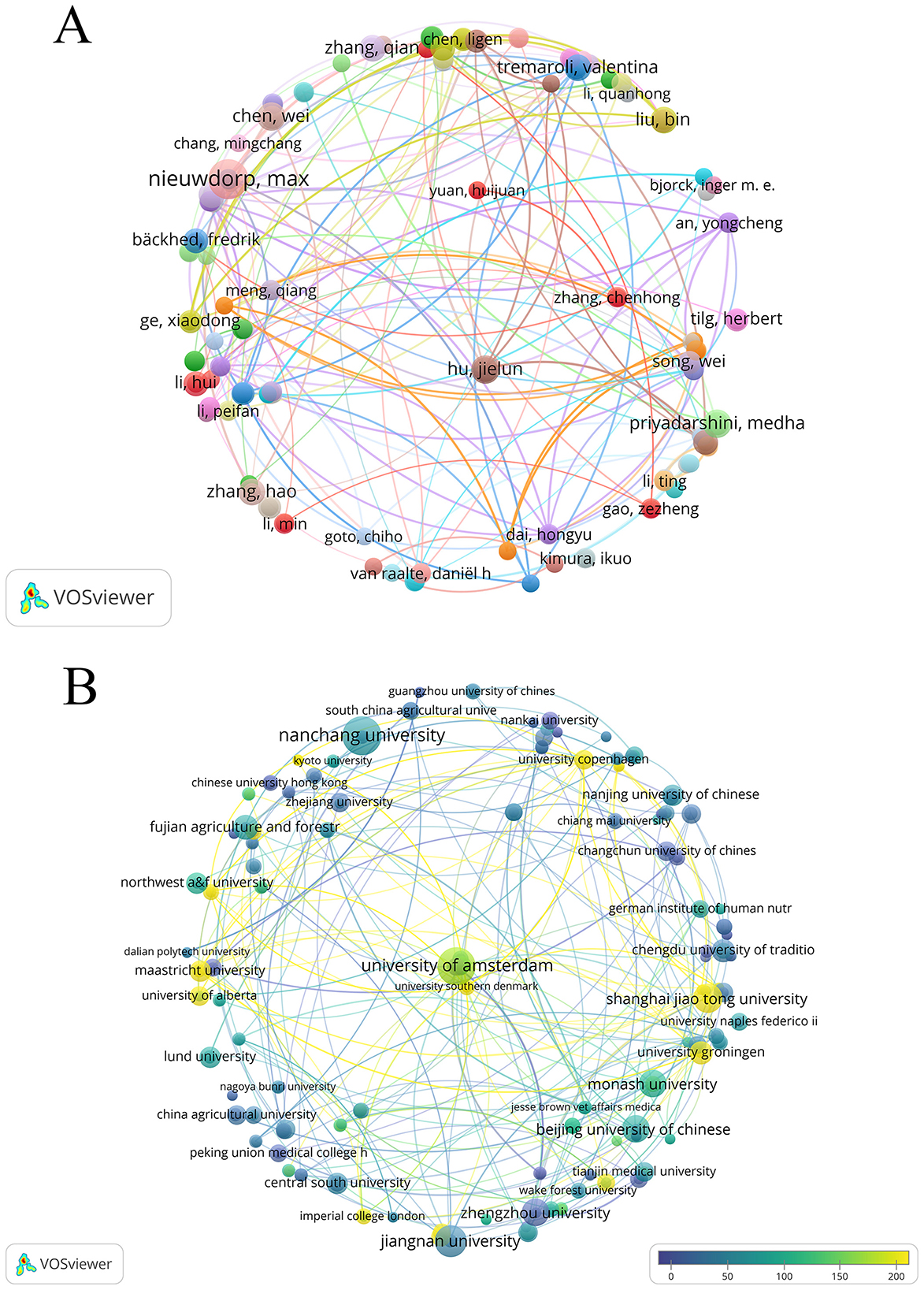
Figure 4. (A) Map visualizing author co-occurrence. (B) Organization of publication and citation overlay map.
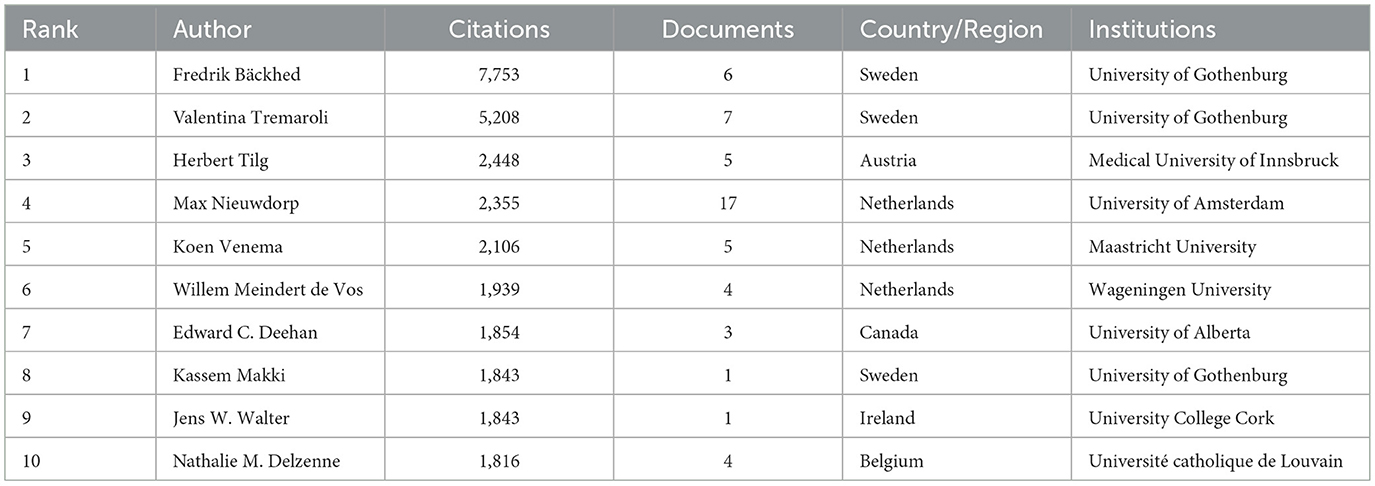
The frequency of citations is a crucial indicator for assessing an author's impact. This survey revealed 75 writers with more than 1,000 citations, with Table 4 displaying the 10 most often cited experts. Swedish scholar Fredrik Bäckhed leads with 7,753 citations, indicating significant academic influence; Valentina Tremaroli of the same institution ranked second with 5,208 citations. Austrian scholar Herbert Tilg and Dutch scholar Max Nieuwdorp ranked third and fourth with 2,448 and 2,355 times respectively. Notably, Max Nieuwdorp and Valentina Tremaroli appear on both the most-productive and most-cited lists, underscoring their significant contributions to this field.
3.4 Institutional analysis
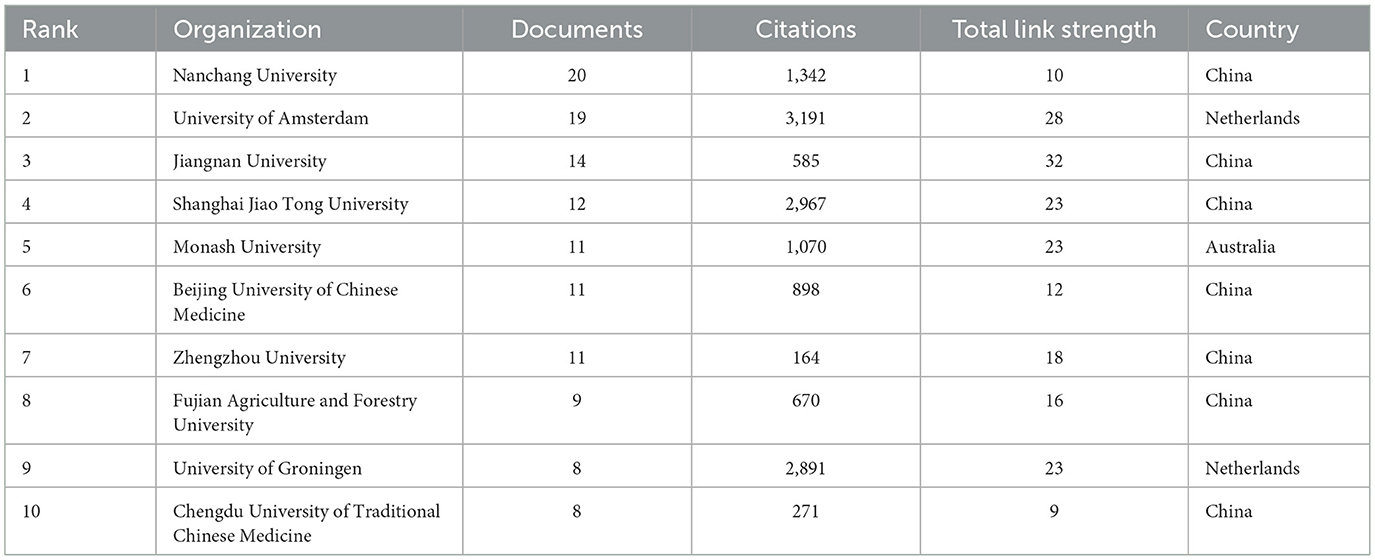
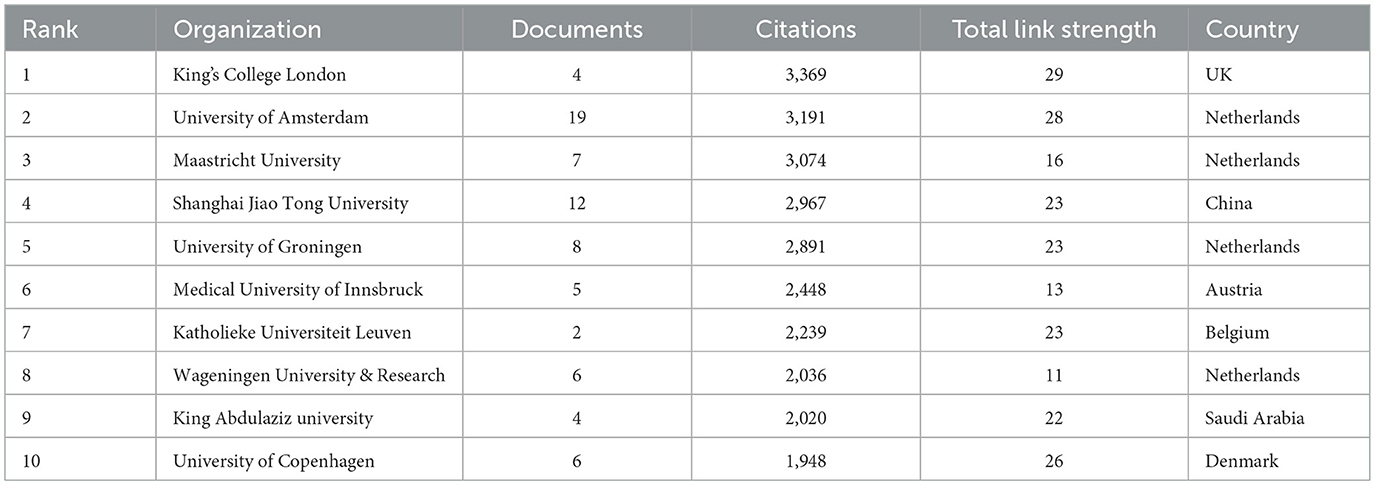
A total of 1,303 institutions' relevant publications were retrieved in this study (Figure 4B). Each node represents an institution, with node size indicating the number of published articles and color scales reflecting the average citations per article. Lighter colors denote higher average citation impact. In terms of the number of published articles (Table 5), Nanchang University of China ranked first with 20 articles, followed closely by University of Amsterdam of the Netherlands with 19 articles. In terms of academic influence, King's College London in the UK has the highest citation count, with a total of 3,369 citations, followed by the University of Amsterdam in the Netherlands with 3,191 citations (Table 6). The University of Amsterdam not only ranks second in the number of published papers, but also performs outstandingly in citation influence, indicating that it has both output and influence in this field.
It is notable that the University of Amsterdam, Shanghai Jiao Tong University, and University of Groningen appear both among the top 10 institutions by publications and the top 10 institutions by citations. This shows that these institutions have outstanding overall strength in the field.
3.5 Journal analysis
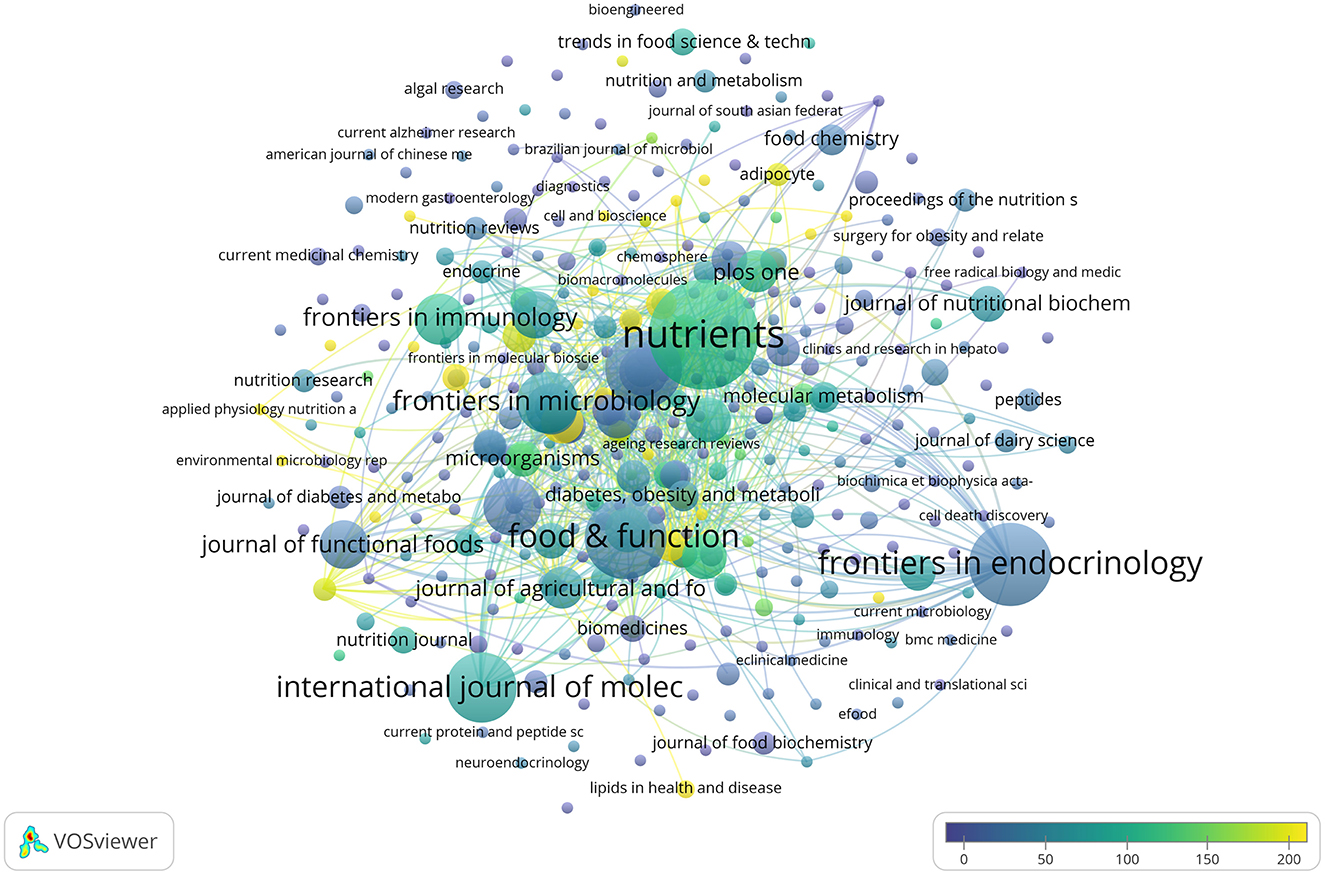
During the past nearly 30 years, research on SCFAs and T2DM has been published across 398 journals. A network representation of these journals can be seen in Figure 5, nodes represent journals, node size indicates the number of published articles, and color scales reflect the average number of citations per article for each journal. The lighter the color, the higher the average citation influence of the research.
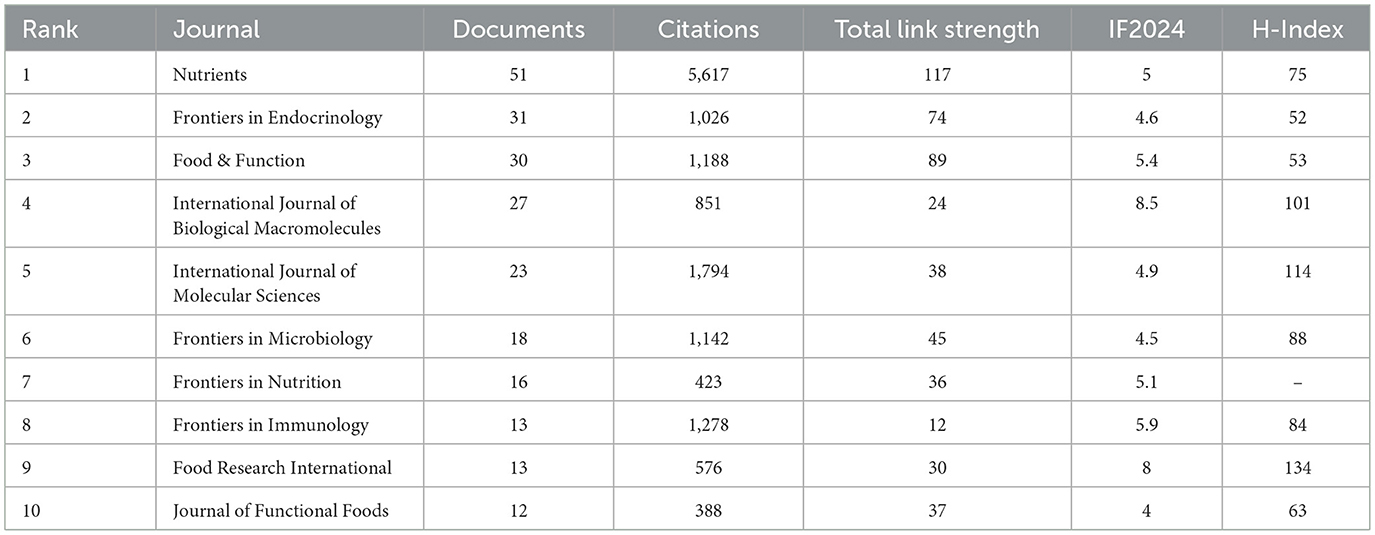
Table 7 enumerates the 10 journals with the highest number of publications. In terms of publication volume, the journal Nutrients ranked first with 51 papers (H-index = 75), followed by Frontiers in Endocrinology with 31 papers (H-index = 52) and Food & Function with 30 papers (H-index = 53). These three journals also ranked among the highest in total link strength, indicating their strong academic cohesion and influence within the field.
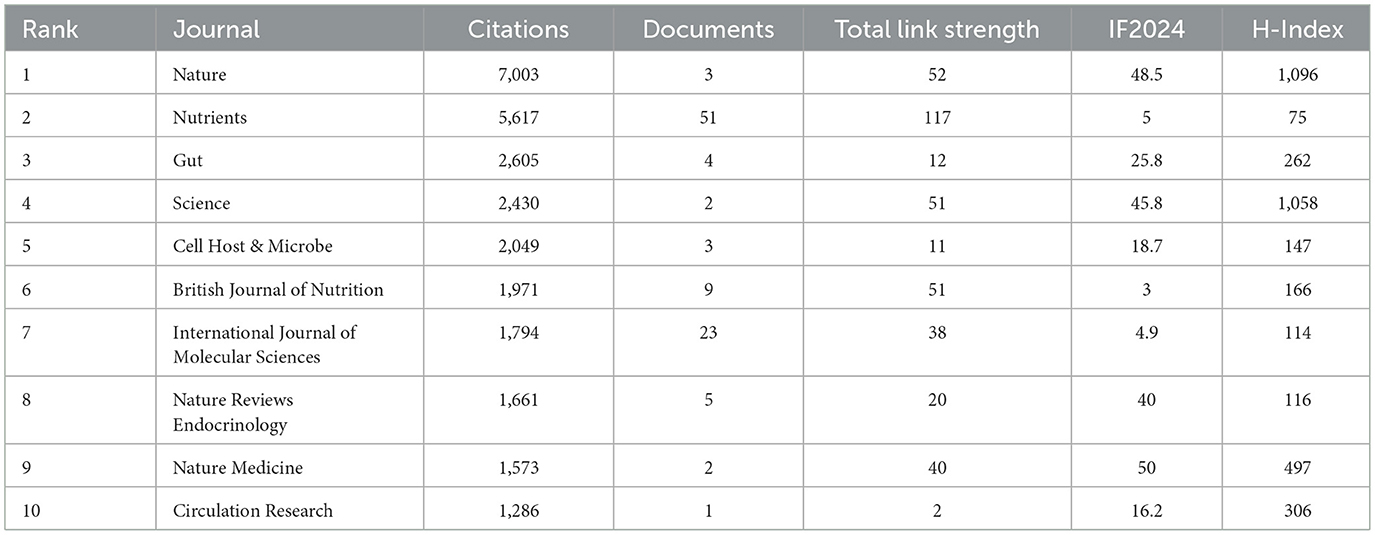
In terms of citation impact (Table 8), Nature published only three papers but received 7,003 citations, with 2,334 references annually for each article, ranking first. Nutrients ranked second, with 51 papers accumulating 5,617 citations (an average of 110 citations per paper). Gut ranked third, with four papers receiving 2,605 citations (an average of 651 citations per paper). Science contributed only two papers but achieved 2,430 citations (an average of 1,215 citations per paper).
3.6 Keyword analysis
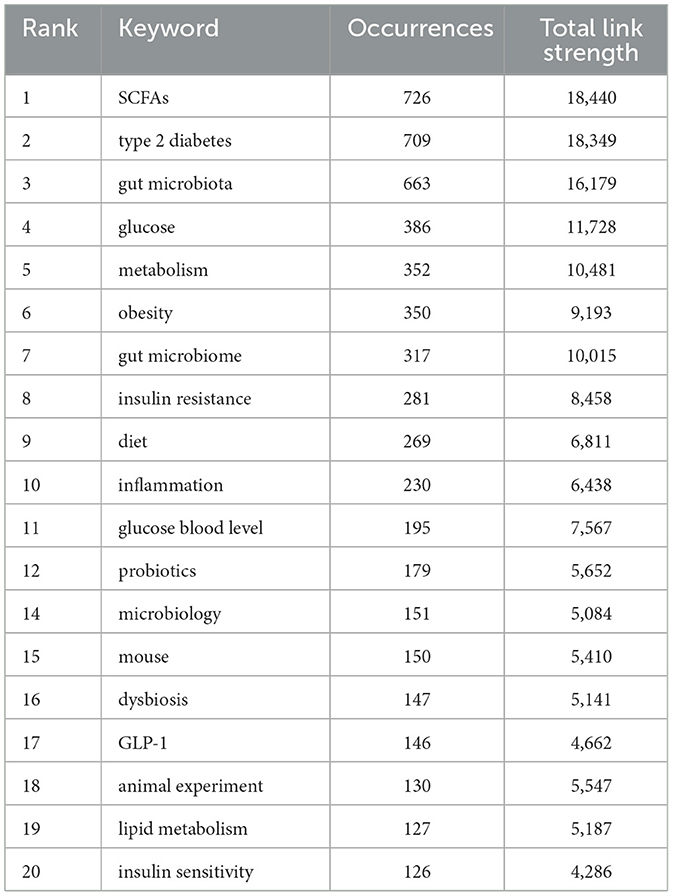
Key words reflect the main idea and core content of publications. Through co-occurrence and cluster analysis of key words, the distribution of research topics, field hotspots, and frontier directions can be revealed (Zhong et al., 2022). Figure 6A shows nodes representing keywords, with node size indicating the frequency of keyword occurrence. Table 9 presents the top 20 keywords of Co-occurrence Frequency, among which the most common keyword is “SCFAs” (n = 726), “type 2 diabetes” (n = 709), and “gut microbiota” (n = 663), which together represent the central themes of this field. Other high-frequency keywords included “glucose” (n = 386), “metabolism” (n = 352), “obesity” (n = 350), “gut microbiome” (n = 317), “insulin resistance” (n = 281), “diet” (n = 269), and “inflammation” (n = 230).
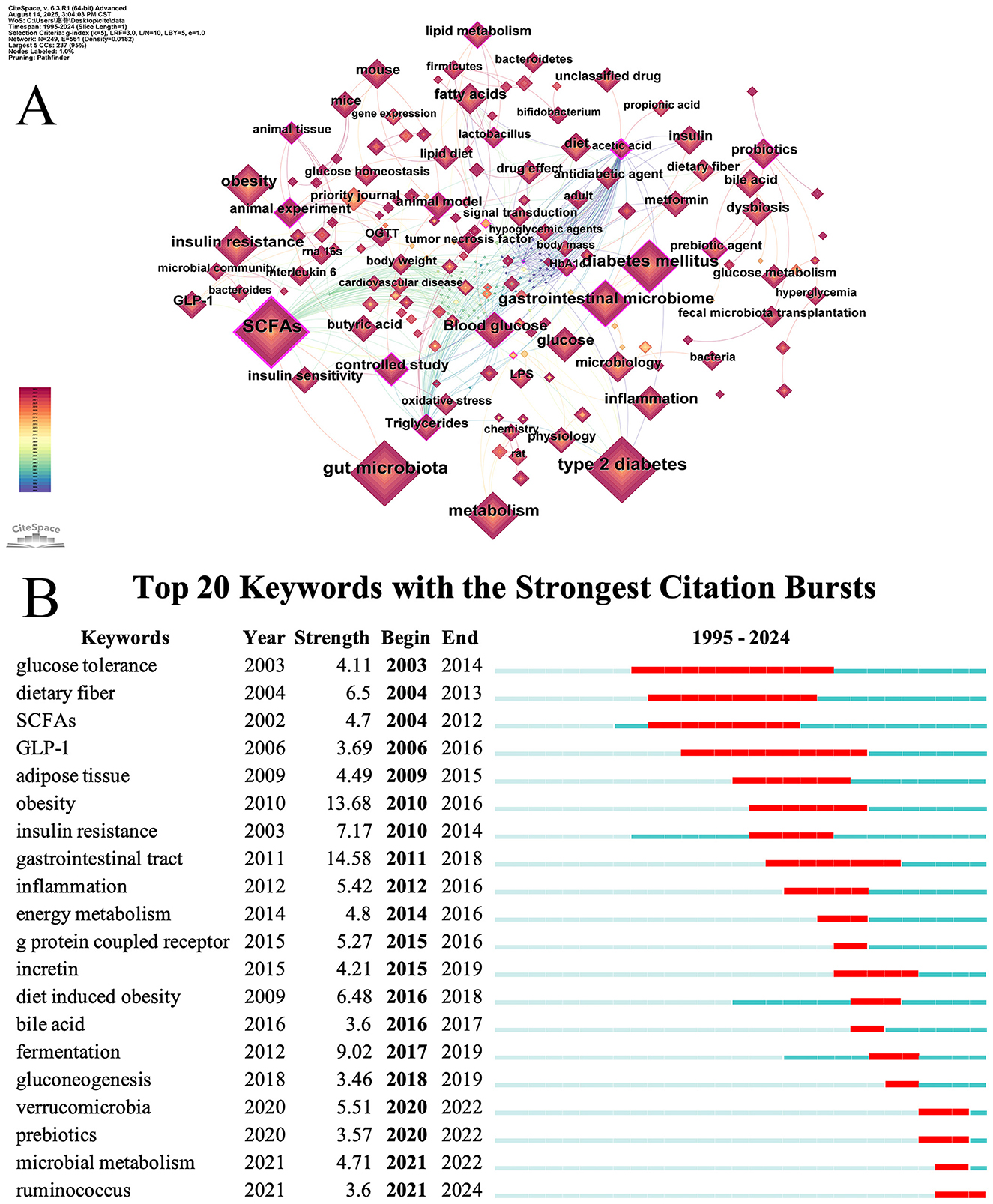
We generated a keyword co-occurrence map using VOSviewer and refined the layout in Pajek to improve visual clarity. This process identified six clusters. As shown in Figure 7A, each node represents a keyword. The node size indicates the frequency of the keyword, and different colors mark different thematic clusters. The themes were identified through an integrated analysis of the most frequent and most central keywords within each cluster. The yellow cluster highlighted the core of the research field, focusing on dietary interventions involving SCFAs in relation to T2DM.Keywords include “diet,” “whole grain,” “low fat diet,” “fruit,” “fermentation,” and “sugar intake.” The green cluster focuses on gut microbiota and microbiology, examining their roles in SCFAs production and T2DM. Keywords include “bacteroides,” “firmicutes,” “lactobacillus,” “ruminococcus,” “probiotics” and “synbiotics.” The Red Cluster focuses on SCFAs regulating T2DM-related signaling pathways. Keywords include “ffar3,” “glp-1,” “insulin,” “leptin,” “interleukin 6,” and “signal transduction.” The blue cluster (middle right) focused on animal experiments investigating the function of SCFAs in glucose metabolism. Keywords include “animal experiment,” “mouse,” “rat,” “streptozotocin,” and “correlation analysis.” The Purple Research Group has examined the impact of acetate, propionate, and butyrate, which are specific SCFAs, on T2DM. Keywords include “acetic acid,” “butyric acid,” “propanoic acid,” “intestinal mucosa,” “permeability,” and “zonulin.” The light-blue cluster (far left) concentrated on metabolomics approaches, underscoring their importance in elucidating the relationship between SCFAs and T2DM. Core keywords include “metabolomics” and common small-molecule analytes in metabolomics, such as “amino acid,” “acylcarnitine,” “leucine,” and “taurine.”
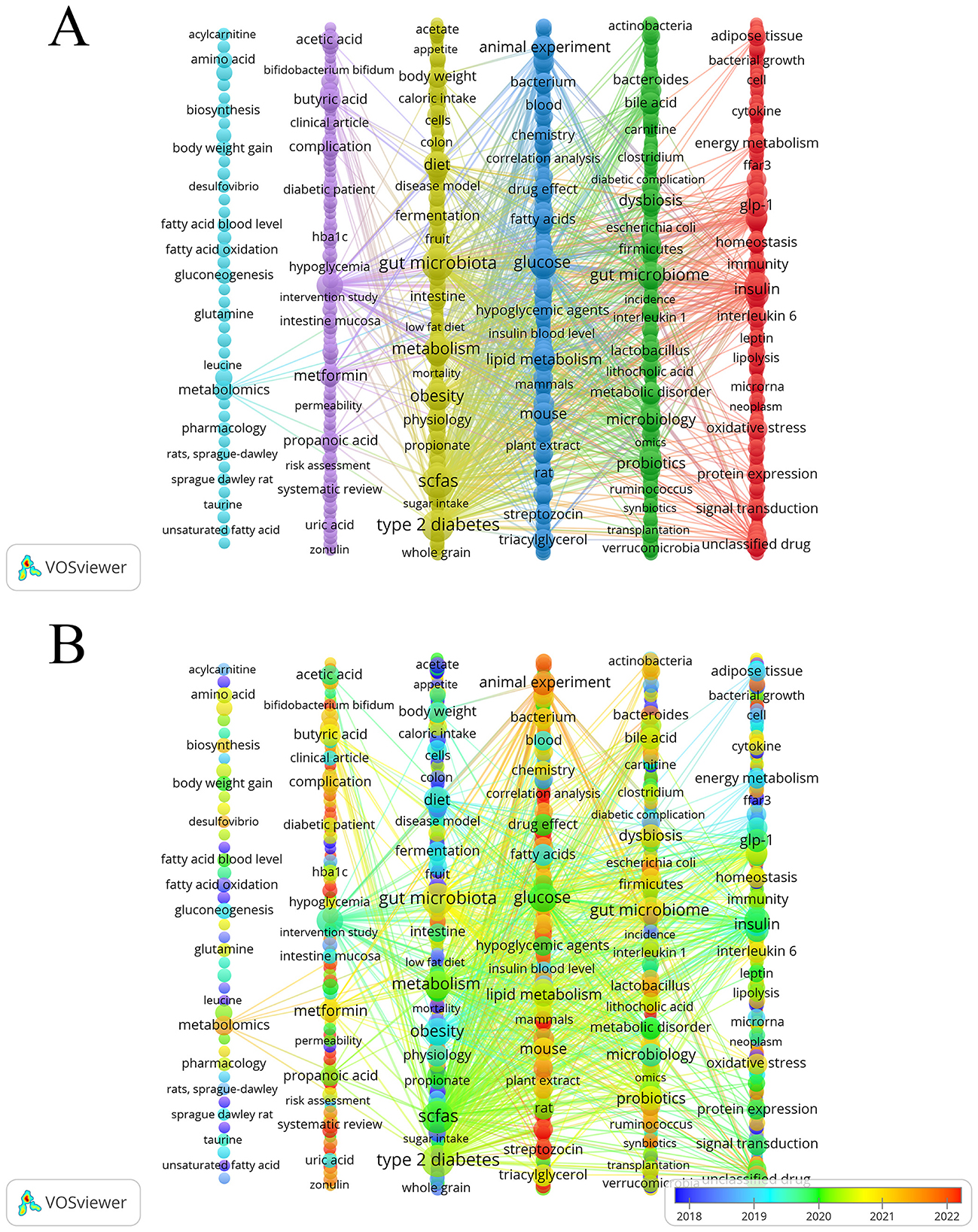
Figure 7. (A) Keywords clustering visualization. (B) Keywords intensity visualization timing overlay.
By overlaying time on the keyword clustering map, we analyzed changes in research directions within the field. Figure 7B shows the average publication year of keywords. The color of each node represents the average year when the keyword became a research hotspot. Blue indicates earlier occurrence, while yellow indicates more recent occurrence. In the early stage (blue nodes), keywords such as “acylcarnitine,” “unsaturated fatty acid,” “acetate,” “ffar3,” “colon,” and “fatty acid oxidation.” During the early-to-middle transitional stage (blue-green nodes), keywords such as “diet,” “obesity,” “cell,” “taurine,” “Sprague Dawley rat,” and “whole grain.” In the middle stage (green nodes), keywords such as “acetic acid,” “glp-1,” “insulin,” “leptin,” “lipolysis,” “metabolic disorder,” “microbiology,” “scfas,” “type 2 diabetes,” “glucose,” and “protein expression.” In the later stage (yellow–red nodes), keywords such as “bifidobacterium bifidum,” “systematic review,” “animal experiment,” “streptozocin,” “synbiotics,” and “correlation analysis.”
Burst keyword analysis can reveal the emergence, development, and decline of research hotspots and help researchers identify emerging fields, frontier directions, and future trends (Li, 2017). As shown in Figure 6B, the red bar chart to the right of each keyword indicates the duration of the outbreak for that keyword. The figure displays 20 burst keywords in studies of SCFAs and T2DM. The keyword “gastrointestinal tract” exhibited the strongest burst intensity (Strength = 14.58), followed by “obesity” (Strength = 13.68) and “fermentation” (Strength = 9.02). “Glucose tolerance” is the keyword with the highest continuous attention.
3.7 Citation analysis
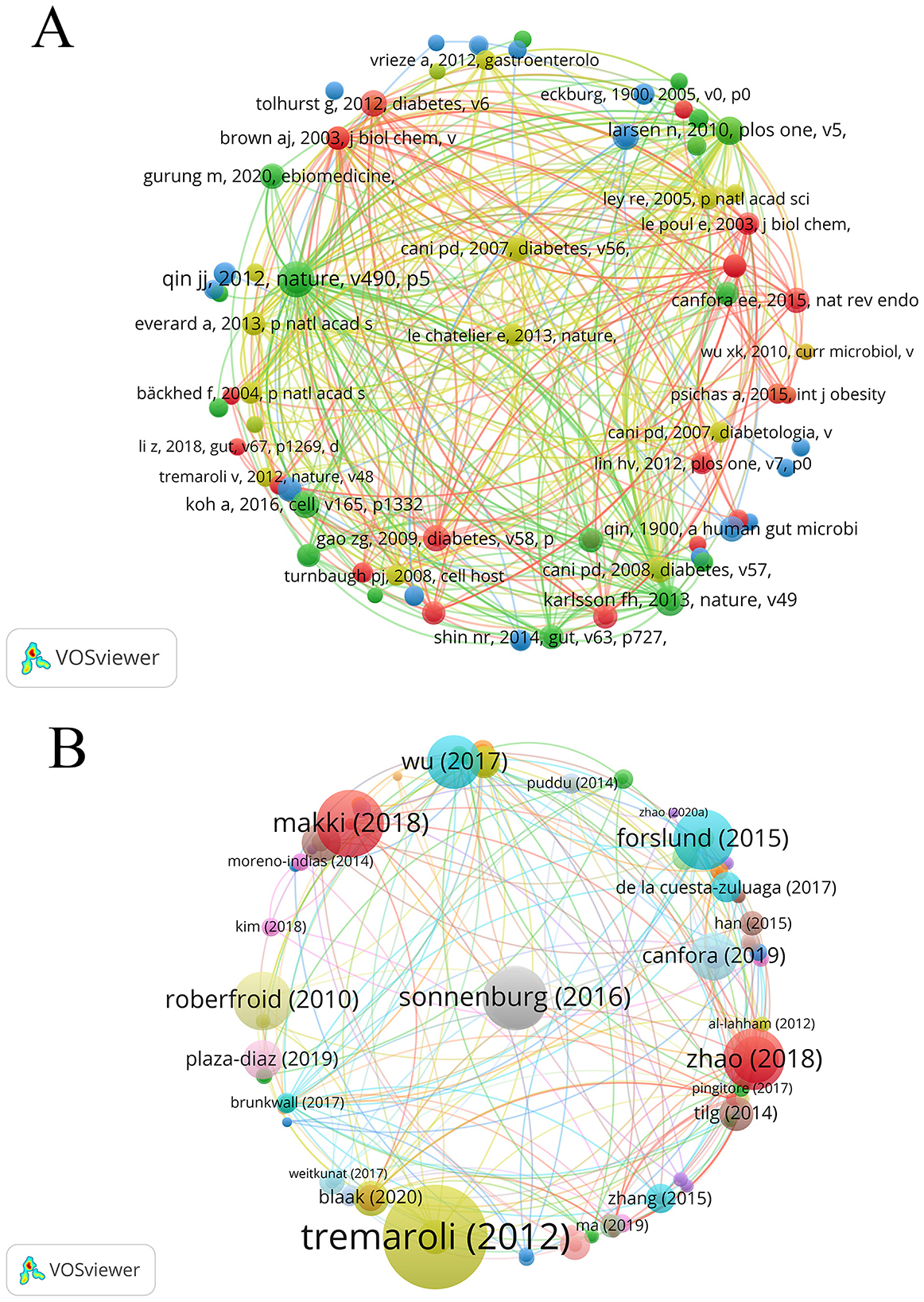
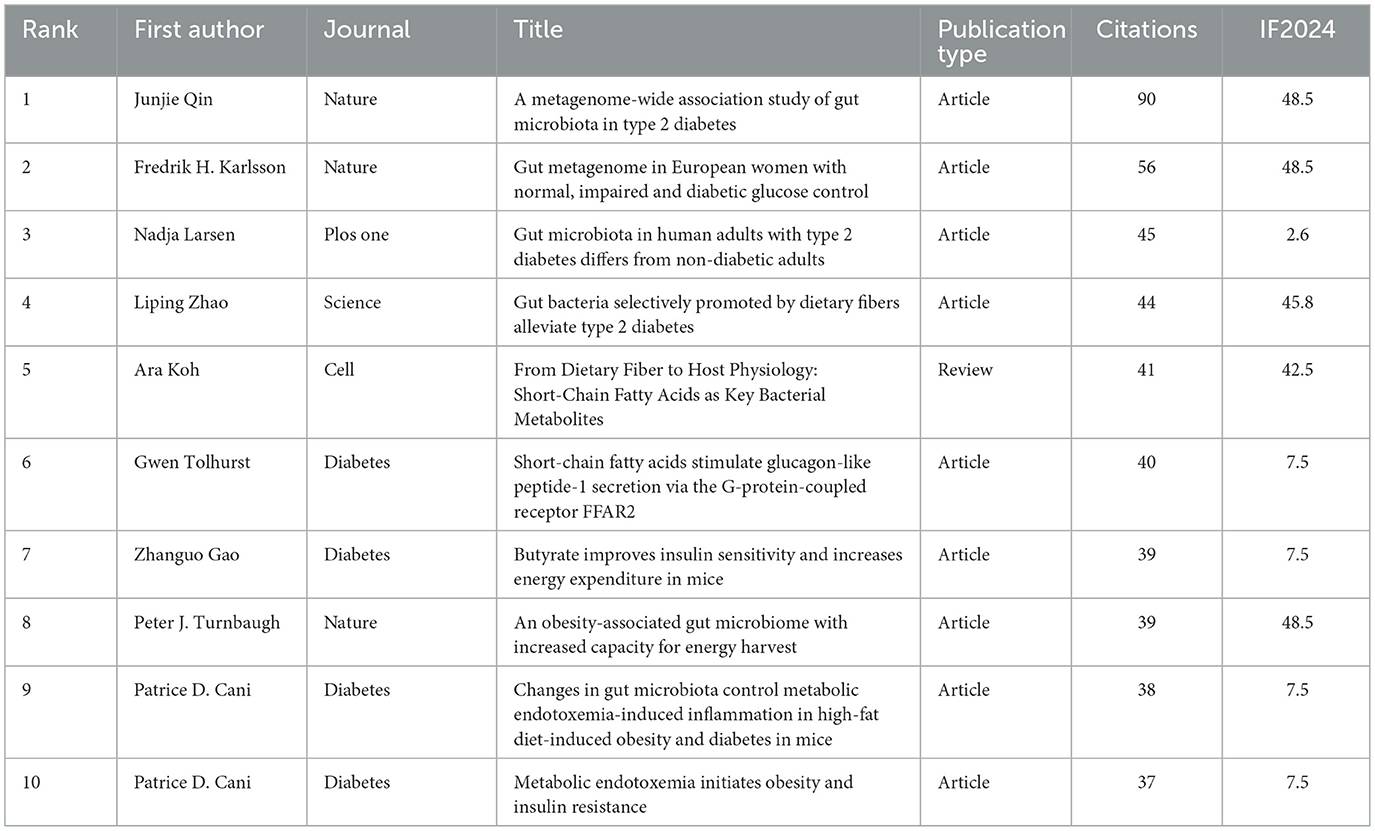
A total of 20,131 references were cited among the 965 included publications, with 44 of those citations occurring more than 20 times. Table 10 enumerates the 10 most co-cited references in the domain of SCFAs and T2DM, comprising nine research articles and one review, with co-citation counts ranging from 37 to 90. As shown in Figure 8A, each node represents a publication. The size of a node reflects the number of citations. The larger the node, the higher the number of citations and the greater the influence. The lines connecting nodes indicate the co-citation relationship. The most frequently co-cited reference was a scholarly paper in Nature by Qin et al. (2012) (impact factor 48.5), which was co-cited 90 times.
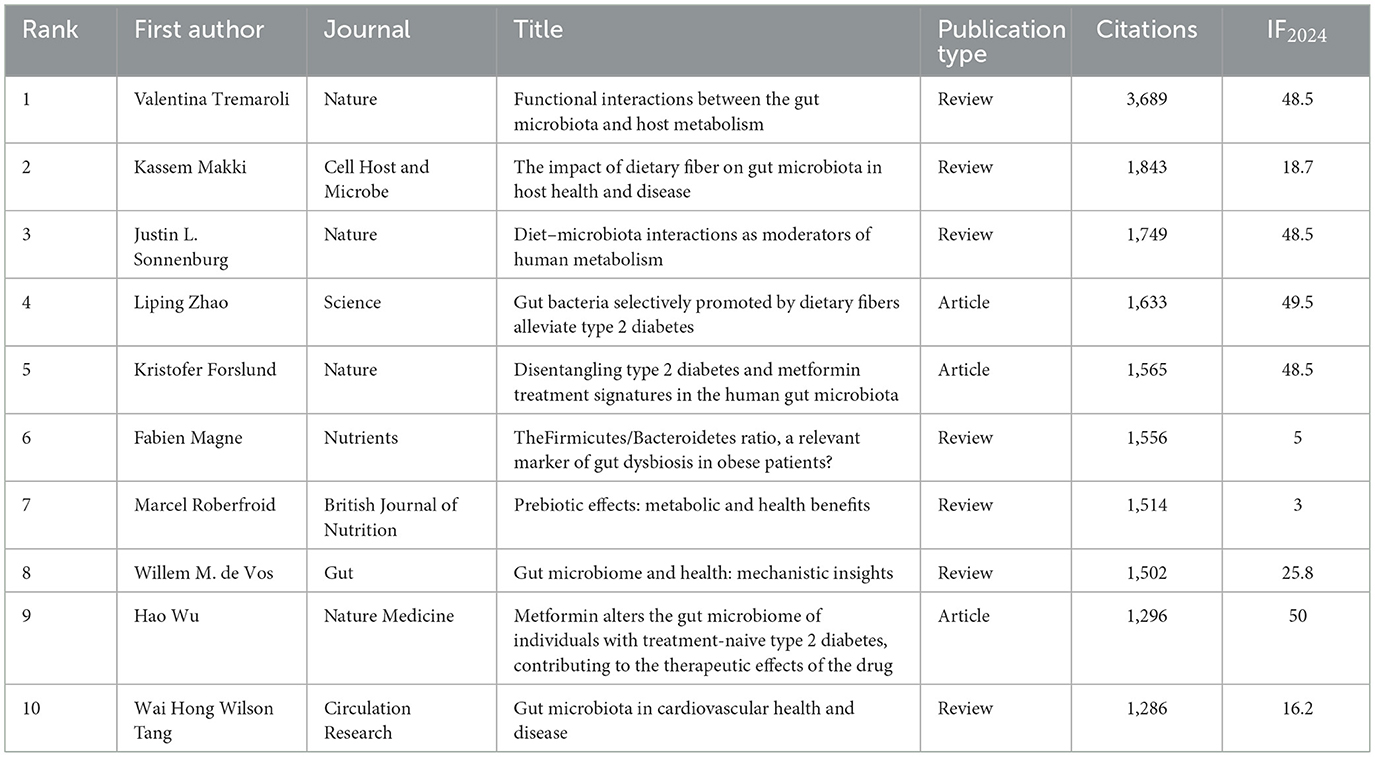
Across all publications, 84 articles received more than 200 citations. Table 11 lists the top ten most cited works, including three articles and seven reviews. Figure 8B shows the node size, which reflects the citation frequency of the publication. The larger the node, the higher the number of citations and the greater the impact, the largest node corresponds to the review by Tremaroli published in Nature (Tremaroli and Bäckhed, 2012), which has been cited 3,689 times, ranking first in Table 10 with an impact factor of 48.5. The second and third positions are occupied by the reviews authored by Makki et al. (2018) and Sonnenburg and Bäckhed (2016), with 1,843 and 1,749 citations, respectively.
4 Discussion
4.1 General information
This study uses bibliometric methods to analyze research related to SCFAs and T2DM, and it can be observed that researchers' attention to this field has increased year by year in recent years. In the initial stage, the research mainly focused on the substrate metabolism related to SCFAs in T2DM, such as resistant starch and high fiber diet (Alles et al., 1999; Wolever et al., 2002). During the early stage, research gradually turned to how dietary interventions affect the production of SCFAs and to exploring their mechanisms in the development of T2DM (Tolhurst et al., 2012; Cornall et al., 2013). After 2015, the development of technologies such as metabolomics accelerated publication growth (Clish, 2015), accompanied by a surge of animal experiments and clinical trials. These studies explored the preventive and therapeutic effects of SCFAs or their precursors, such as dietary fiber and prebiotics, on T2DM, with growing attention to individual variability (Vliex et al., 2024; Zhang et al., 2020).
China has the largest number of publications, while the United States shows the highest total link strength. Both countries have made important contributions to this research field. It is worth noting that, in terms of average citations per paper, which reflect the average impact of research, European countries such as Sweden and the Netherlands perform better. Several factors may contribute to this. On the one hand, these countries have infrastructure such as large cohorts and microbiome sample banks, which supports high quality empirical research. For example, the Dutch Microbiome Project is a large-scale study on the function of gut microbiota (Gacesa et al., 2022). These achievements are the result of long-term accumulation. This also indicates that research in this field started earlier in these countries, and their publications have had more time to accumulate citations. On the other hand, since English is the main language in these countries and their publications are often in open access or top journals, their work is more easily noticed and cited by the international academic community.
Dutch scholar Max Nieuwdorp and Swedish scholar Fredrik Bäckhed were identified as the most productive and the most frequently cited authors, respectively. Nieuwdorp has focused on the relationship between gut microbiota and various diseases, emphasizing the regulatory role of SCFAs in human health (Baars et al., 2024; Kullberg et al., 2025). Medha Priyadarshini and Jielun Hu concentrated on SCFAs receptors (FFAR2/3, GPR109A) and their roles in glucose metabolism, intestinal barrier function, and inflammation (Priyadarshini et al., 2016; Nie et al., 2020). Xinhua Xiao and Qian Zhang investigated the mechanisms of drug-mediated modulation of gut microbiota (Guo et al., 2025; Ding et al., 2022). Wei Chen and Hao Zhang explored probiotics and functional foods (Zhu et al., 2025), while Bin Liu focused on dietary polysaccharides and hepatic pathways in improving insulin resistance and lipid metabolism (Lee et al., 2024).
We found that the highly cited scholars mainly concentrate their research interests in the following areas. Fredrik Bäckhed, Valentina Tremaroli, Herbert Tilg, and Max Nieuwdorp focus on animal experiments, human intervention studies, and review analyses, seeking to elucidate the processes by which gut microbiota influence host metabolism (Tremaroli and Bäckhed, 2012; De Vos et al., 2022; Wu et al., 2017). Edward C. Deehan, Kassem Makki, Jens Walter, Nathalie M. Delzenne, and Koen Venema primarily investigate how dietary fibers and prebiotics enhance SCFAs production, with particular attention to their applications in nutritional interventions and metabolic regulation (Den Besten et al., 2013; Makki et al., 2018; Seethaler et al., 2022; Yang et al., 2013). Willem M. de Vos emphasizes the identification of key functional bacteria and their translational applications, which have advanced the practical implementation of microbiome research findings (Zhang et al., 2024).
From the perspective of institutional distribution, seven of the top 10 institutions by publication volume are from China, highlighting the contribution of Chinese institutions in this field. Among the top 10 institutions by citation count, four are from the Netherlands, reflecting the strong academic influence of Dutch institutions in this area. Among the top 10 journals in terms of publication volume, five focus on food science and nutrition, two on molecular biology, and the remaining three belong to the fields of endocrinology, microbiology, and immunology, respectively. These journals have a strong disciplinary correlation with each other, reflecting the interdisciplinary characteristics of this research topic. And although Nature and Science have published relatively few papers in this field, their high citation rates highlight their leading academic influence.
Co-citation analysis of references facilitates the rapid accumulation of domain knowledge and helps identify research dynamics. In this study, the top 10 most frequently cited 10 references were further analyzed and categorized into four groups based on their research content. (1) Population-based observational studies: Larsen et al. (2010) utilized 16S pyrosequencing and qPCR in Danish adult males, revealing a substantial integration of the relative number of particular bacterial phyla and plasma glucose concentrations. Qin et al. (2012) used shotgun metagenomic sequencing in Chinese adults and found gut microbiota dysbiosis in T2DM patients, distinguished by a significant decrease in bacteria that produce butyrate. Karlsson et al. (2013) investigated European women and, through metagenomic clustering (MGCs), reported heightened prevalence of Lactobacillus and diminished prevalence of Clostridium in people with T2DM. (2) Clinical dietary intervention studies: Zhao et al. intervened in Chinese T2DM patients through a high-fiber diet, combined with fecal microbiota transplantation experiments, revealed an increase in 15 SCFA-producing bacterial strains. These changes were closely associated with reductions in HbA1c, elevations in GLP-1 and PYY, and improvements in glycemic control (Zhao et al., 2018). (3) Mechanistic studies: Tolhurst et al. (2012) conducted experimental studies incorporating wild-type mice and FFAR2/FFAR3 knockout mice. They demonstrated that acetate, propionate, and butyrate promote GLP-1 secretion via FFAR2/3, thereby influencing glucose metabolism. Gao et al. (2009) supplemented butyrate in mice subjected to a high-fat diet and discovered that it enhanced energy expenditure and improved insulin sensitivity. Cani et al. (2007, 2008), through mouse experiments, revealed that bacterial lipopolysaccharide (LPS) levels were closely associated with inflammation, metabolic disturbances, and endotoxemia. Turnbaugh et al. (2006) compared obese and lean mice, demonstrating that concentrations of acetate and butyrate were markedly increased in the cecum of fat mice. (4) Review studies: Koh et al. (2016) offered an in-depth investigation of the origins and targets of SCFAs, emphasizing that butyrate and propionate can suppress histone deacetylases (HDACs) to modulate gene transcription. SCFAs can also directly activate GPCRs, thereby modulating multiple signaling pathways and host physiological functions.
We further analyzed the top 10 most cited publications and found that their research themes were primarily centered on diet mediating the microbiota, generating SCFAs, and regulating the chain of metabolic processes and associated mechanisms. Diet composition and microbial metabolism: Tremaroli and Bäckhed (2012); Makki et al. (2018); Sonnenburg and Bäckhed (2016); Zhao et al. (2018) focused on the influence of dietary structure on gut microbiota and metabolic outcomes, providing theoretical support for the dietary fiber-driven modulation of SCFAs production Mechanistic insights: De Vos et al. (2022) and Tang et al. (2017) systematically described the mechanisms of SCFAs in T2DM, indicating that SCFAs activate GPR41/43 to promote the production of PYY and GLP-1, consequently affecting hunger, energy metabolism, and insulin release. Prebiotics and gut environment: Roberfroid et al. (2010) emphasized the health effects of prebiotics, noting that fermentable fibers can alter the gut environment (e.g., reduce fecal pH) and modulate the SCFA profile. Drugs and microbiota remodeling: Forslund et al. (2015) and Wu et al. (2017) reported that metformin and other medications reshape the gut microbiota, with part of their metabolic effects mediated through alterations in community structure. Microbiota composition and disease associations: Magne et al. (2020) analyzed the correlation between gut microbial makeup and disease progression, emphasizing how varying community patterns may influence the onset of metabolic problems.
The research in the above-mentioned hotspot literature mainly focuses on dietary interventions, such as increasing dietary fiber intake or supplementing with probiotics, to regulate the composition and function of the gut microbiota and promote the production of key metabolites such as SCFAs. Acting as core signaling molecules, SCFAs activate GPCRs and inhibit HDACs, thereby regulating the host's hormone secretion, glucose and lipid metabolism, and inflammatory responses. This series of studies, from population observation, clinical intervention to molecular mechanism, jointly confirmed the key role of SCFAs in regulating T2DM.
4.2 Research hotspots
Keyword analysis can reveal the research hotspots of the discipline and reflect the knowledge structure. We summarize the research hotspots in the field by interpreting these six clusters, which are summarized into three parts, they are, respectively the generation of SCFAs, the research on the mechanism of SCFAs mediating T2DM, and the application of omics technology.
4.2.1 Generation of SCFAs
The core of the yellow cluster, “dietary intervention,” and the core of the green cluster, “gut microbiota,” jointly constitute the regulatory axis of SCFAs generation. Research indicates that high-fiber dietary patterns, exemplified by the Mediterranean diet, can significantly increase SCFA levels, enhance intestinal barrier integrity, and diminish plasma concentrations of lipopolysaccharide-binding protein, thereby contributing to the regulation of T2DM onset and progression (Seethaler et al., 2022; Salamone et al., 2021). These studies point the way to the modulation of T2DM through dietary promotion of SCFAs production. At the same time, the production of SCFAs is closely related to the composition of gut microbiota, and enhancing the beneficial bacteria that produce SCFAs in the gut helps maintain host metabolic homeostasis (Antony et al., 2023). Supplementing with probiotics or prebiotics can increase the abundance of beneficial bacteria such as Lactobacillus and Bifidobacterium in the gut, thereby raising SCFAs levels (Li et al., 2021). In recent studies, “prebiotics” can serve as a key dietary factor. By promoting the growth of probiotics and optimizing the intestinal environment, they can increase the production of SCFAs such as acetic acid and butyric acid, playing a crucial role in improving insulin secretion and regulating appetite (Kume et al., 2018; Baba et al., 2025) The core of this process is “microbial metabolism,” which refers to the gut microbiota producing a variety of bioactive products, including SCFAs, by breaking down substrates such as dietary fiber and prebiotics (Baba et al., 2025; Myhrstad et al., 2020). Emerging research hotspots further focus on specific functional bacteria, such as Akkermansia muciniphila within the Verrucomicrobia phylum, which can produce acetate and propionate to enhance the gut barrier (De Vos, 2017; He et al., 2023). And the genus Ruminococcus, which is positively correlated with dietary fiber intake, has main metabolic products of butyric acid and propionic acid, which are crucial for maintaining energy metabolism (Yang et al., 2013; Van-Wehle and Vital, 2024). The production of SCFAs depends on the supply of dietary substrates and the metabolic activity of the gut microbiota. In terms of diet, particularly the intake of high fiber and prebiotics, it can influence SCFAs production by shaping the gut ecosystem.
4.2.2 Research on the mechanism of SCFAs mediating T2DM
The red cluster focuses on SCFAs that regulate T2DM related signaling pathways. SCFAs can act as compounds to activate various GPCRs, including GPR41/FFAR3 and GPR43/FFAR2 (Brown et al., 2003). These receptors are widely expressed in enteroendocrine cells, pancreatic β-cells, adipose tissue, liver, and immune cells. Upon activation, they stimulate the secretion of gastrointestinal hormones, including GLP-1 and PYY, thereby increasing insulin release, inhibiting glucagon secretion, and improving glucose homeostasis (Kimura et al., 2014; González Hernández et al., 2019). Meanwhile, the purple cluster focuses on mechanistic studies of specific SCFAs, such as acetate, propionate, and butyrate. Acetate has been shown to activate the AMPK pathway, thereby promoting energy metabolism and lipid oxidation, and improving energy balance (Frost et al., 2014). Propionic acid can act as a substrate for gluconeogenesis to regulate glucose homeostasis (Todesco et al., 1991) and participate in lipid metabolism by inhibiting HMG-CoA reductase (Lin et al., 1995). Butyrate is essential for the protection of the intestinal barrier, contributing to the integrity of the intestinal epithelium and the tightness of its connections, which reduces endotoxin translocation into the bloodstream, decreases inflammation and improves insulin sensitivity (Xu et al., 2018). In addition, butyrate promotes peripherally induced Treg cell differentiation, enhances immune tolerance, and attenuates pancreatic inflammation (Hao et al., 2021). These findings emphasize the multiple roles of different types of SCFAs in energy metabolism, glucose and lipid homeostasis, intestinal barrier, and immune regulation, and provide a theoretical basis and research direction for intervening in T2DM by targeting SCFAs mechanisms. Meanwhile, the effectiveness of these mechanisms is mainly verified through animal experiments, as shown by blue clustering. A meta-analysis of SCFAs intervention in a diabetic mouse model showed that butyrate could reduce triglyceride (TG), total cholesterol (TC), and fasting blood glucose (FBG) levels in T2DM mice (Zheng et al., 2024). Overall, the related studies mainly explored the deep mechanism through animal models, and found that SCFAs can effectively regulate the onset and progression of T2DM through the physiological regulatory network of multi-level and multi-targets. At the signal transduction level, SCFAs regulates the release of enteroendocrine hormones (such as GLP-1 and PYY), insulin secretion and inflammatory response by activating GPCRs. At the metabolic level, they are involved in energy homeostasis and lipid metabolism, promote glucose utilization and improve insulin sensitivity. At the same time, SCFAs can maintain the integrity of the intestinal barrier, reduce endotoxin translocation, and inhibit chronic low-grade inflammation; its immunoregulatory effects can also promote the differentiation of anti-inflammatory cells and restore metabolic homeostasis.
4.2.3 Omics technologies and applications of SCFAs in T2DM
The light blue cluster reveals the central role of metabolomics as a cutting-edge technology in advancing research in this field. Analytical techniques represented by gas chromatography–mass spectrometry (GC-MS) and liquid chromatography–mass spectrometry (LC-MS) provide essential technical support for the precise quantification of SCFAs and other metabolites, as well as for elucidating the complex interactions between the gut microbiota and the host (Saha et al., 2021; Llauradó et al., 2025). Untargeted metabolomics is also widely applied to explore the roles of microbial metabolites in metabolic diseases. For example, some researchers have revealed the mechanism by which fecal microbiota transplantation improves T2DM mouse models through an integrated study combining untargeted metabolomics and 16S rRNA analysis (Yang et al., 2023). With technological advancements, research hotspots are gradually shifting from the macroscopic “phylum” level toward analyses at the functional genus and species levels. Integrated analyses combining metabolomics, metagenomics, and transcriptomics have enabled researchers to focus on specific beneficial microbial communities.
4.3 Clinical translation and future trend analysis
In recent years, multiple studies have shown from different levels that interventions surrounding SCFAs production may provide new pathways for the management of T2DM. A clinical trial found that the combination of probiotics and metformin can reshape the fecal microbiome composition of patients with T2DM, especially by increasing the abundance of various SCFAs-producing bacteria. Meanwhile, their combination significantly enhances the hypoglycemic effect and increases insulin secretion, improving pancreatic function (Chen et al., 2023). A randomized crossover trial showed that short-term supplementation with multivitamins and minerals, at doses near recommended levels, also altered the gut microbiota composition and metabolite profile in healthy adults, characterized by increased levels of SCFAs such as propionate and butyrate. It is worth noting that this effect is not determined solely by the supplement but is influenced by the individual's existing dietary structure (Mckirdy et al., 2025). In terms of dietary intervention, the high-fiber diet proposed by the researchers can significantly improve the SCFAs level in feces and simultaneously improve clinical outcomes such as HbA1c, fasting blood sugar and insulin resistance; at the same time, the study also identified a group of core strains that produce acetic acid and butyric acid. Based on this, the researcher proposed the concept of “ecological therapy”: by restoring or promoting the abundance of SCFAs producing bacteria and rebalancing the intestinal microecology, the metabolic health of the host can be improved. The research also pointed out that personalized nutrition strategies can be utilized to specifically cultivate highly active SCFAs producers into “ecosystem service providers (ESP),” with the aim of managing T2DM more effectively (Zhao et al., 2018). In addition to nutritional and microbiota intervention, delivery technology is also expanding the clinical application possibilities of SCFAs. Researchers have developed the oral multifunctional carrier system “nano-in-microparticles,” which uses supercritical emulsion extraction to co-encapsulate butyric acid and propionic acid for targeted release in the colon. The findings of this study represent an innovative approach, providing a new perspective for the clinical use of SCFAs to mediate T2DM (Carvalho et al., 2025). In population studies, heterogeneity should also be considered. A randomized, double-blind, placebo-controlled, crossover clinical trial showed that short-term high-dose inulin increased SCFAs production in both young and older adults, with a more pronounced rise in butyrate. However, the increase in older adults was relatively limited (Kirschner et al., 2025). Consistent with this, the analysis of nine types of SCFAs producing bacteria genera found that their abundance significantly decreased with the aging process of the human body, and there was no significant difference between men and women (Wu et al., 2024). In addition, there are systematic differences in the composition of basic microbiota and dietary habits among different regions and populations (such as the East and the West, tropical/temperate zones, and urban and rural areas), which will significantly affect the generation potential and intervention response of SCFAs. Therefore, an effective SCFAs intervention strategy in one population may not achieve the same effect in another (Sheng et al., 2024).
Taken together, SCFAs related interventions have certain advantages in clinical applications, and there are multiple pathways to enhance the production of SCFAs in vivo, with the sources relying mainly on dietary fiber, prebiotics, or probiotics, with a high degree of safety and acceptability. However, clinical translation still faces many challenges, including marked interindividual differences across populations, issues of tolerance and absorption with direct SCFAs supplementation, and the lack of evidence from large-scale, long-term randomized controlled trials. In the future, with the advancement of multi-omics technologies and personalized medicine, SCFAs-related interventions are expected to find broader application in precision nutrition and combined strategies. This may drive a shift from general dietary guidelines to individualized nutrition management, offering new pathways for T2DM management and public health improvement.
4.4 Limitations
(1) This study was analyzed based on data from two databases, WoSCC and Scopus, so other database-related research may be missed. Future research should be included in databases such as PubMed and Embase to minimize biases caused by database selection and increase coverage. (2) Both WOSCC and Scopus primarily index high-impact, English-language international journals, so non-English research may be excluded. In future studies, we recommend developing bibliometric tools with multilingual analysis capabilities to address these shortcomings. (3) This research analysis mainly focuses on articles and reviews, and excludes other types of publications such as conference papers, book chapters, etc., which may ignore the potential important research results. Future research should integrate these diverse literature types to build a more complete and dynamic view of domain development. (4) The citation rate of articles is usually influenced by multiple factors such as self-citations. Future research should conduct longitudinal tracking of relevant citations to assess their effectiveness. (5) This study has a certain time lag in the literature; the latest research may not have been cited or included. In the future, the most recent literature can be incorporated to update the development status of the field, thereby providing a more comprehensive depiction of its evolutionary panorama. (6) By leveraging the unique advantages of different tools (CiteSpace, VOSviewer), we obtain a more comprehensive picture of the research field. However, a methodological limitation is that we did not systematically cross-validate multiple tools with different core algorithms, so the specific results may exhibit slight variations. Although we observed a high consistency in the major research themes at the macro level across analyses using different tools, future research should more systematically compare the effects of different algorithms on bibliometric results to enhance the robustness of the conclusions. (7) This study is based on published scientific literature, but it is undeniable that we did not consider publication bias. The proportion of positive findings in the literature is too high. This bias should be reduced in future research.
5 Conclusion
This study systematically analyzed the development trends, current hotspots, and future directions of research on SCFAs related to T2DM. The results help researchers understand the knowledge structure in this field and provide a reference for future studies.
In the future, for researchers, combining modern technologies such as metabolomics and metagenomics with multidisciplinary approaches from nutrition, microbiology, and endocrinology can be used to more deeply elucidate the specific mechanisms by which SCFAs improve T2DM. For clinical practice, optimizing SCFAs-based interventions and developing individualized treatment strategies that consider patients' ethnicity, age, and gender differences is expected to bring better clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LZ: Data curation, Writing – original draft, Conceptualization. RJ: Writing – original draft, Formal analysis, Data curation. JF: Conceptualization, Methodology, Writing – original draft. YT: Writing – review & editing, Validation. YW: Data curation, Writing – review & editing. LL: Writing – original draft, Formal analysis. ML: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China, grant number: 82174525; Jilin Provincial Natural Science Foundation, grant number: YDZJ202201ZYTS195; Jilin Province Doctoral Student Support Program, grant number: BST202528.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alles, M. S., De Roos, N. M., Bakx, J. C., Van De Lisdonk, E., Zock, P. L., and Hautvast, J. G. (1999). Consumption of fructooligosaccharides does not favorably affect blood glucose and serum lipid concentrations in patients with type 2 diabetes. Am. J. Clin. Nutr. 69, 64–69. doi: 10.1093/ajcn/69.1.64
Antony, M. A., Chowdhury, A., Edem, D., Raj, R., Nain, P., Joglekar, M., et al. (2023). Gut microbiome supplementation as therapy for metabolic syndrome. World J. Diabetes 14, 1502–1513. doi: 10.4239/wjd.v14.i10.1502
Aria, M., and Cuccurullo, C. (2017). bibliometrix : an R-tool for comprehensive science mapping analysis. J. Informetr. 11, 959–975. doi: 10.1016/j.joi.2017.08.007
Baars, D. P., Fondevila, M. F., Meijnikman, A. S., and Nieuwdorp, M. (2024). The central role of the gut microbiota in the pathophysiology and management of type 2 diabetes. Cell Host Microbe 32, 1280–1300. doi: 10.1016/j.chom.2024.07.017
Baba, Y., Tsuge, D., and Aoki, R. (2025). Enhancement of carbohydrate metabolism by probiotic and prebiotic intake promotes short-chain fatty acid production in the gut microbiome: a randomized, double-blind, placebo-controlled crossover trial. Biosci. Biotechnol. Biochem 89, 1191–1202. doi: 10.1093/bbb/zbaf071
Barrington, N. M., Gupta, N., Musmar, B., Doyle, D., Panico, N., Godbole, N., et al. (2023). bibliometric analysis of the rise of ChatGPT in medical research. Med. Sci. 11:61. doi: 10.3390/medsci11030061
Brown, A. J., Goldsworthy, S. M., Barnes, A. A., Eilert, M. M., Tcheang, L., Daniels, D., et al. (2003). The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278, 11312–11319. doi: 10.1074/jbc.M211609200
Canfora, E. E., Meex, R. C. R., Venema, K., and Blaak, E. E. (2019). Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15, 261–273. doi: 10.1038/s41574-019-0156-z
Cani, P. D., Amar, J., Iglesias, M. A., Poggi, M., Knauf, C., Bastelica, D., et al. (2007). Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772. doi: 10.2337/db06-1491
Cani, P. D., Bibiloni, R., Knauf, C., Waget, A., Neyrinck, A. M., Delzenne, N. M., et al. (2008). Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes 57, 1470–1481. doi: 10.2337/db07-1403
Cannon, A., Handelsman, Y., Heile, M., and Shannon, M. (2018). Burden of illness in type 2 diabetes mellitus. J. Manag. Care Spec. Pharm. 24, S5–S13. doi: 10.18553/jmcp.2018.24.9-a.s5
Carvalho, S. G., Reverchon, E., Scognamiglio, M., Baldino, L., and Chorilli, M. (2025). Continuous supercritical emulsion extraction for the co-encapsulation of butyrate and propionate nanoparticles in PLGA microparticles for colonic release. Colloids Surf. 725:137606. doi: 10.1016/j.colsurfa.2025.137606
Chen, B-. F., Liu, L., Lin, F-. Z., Zeng, H-. M., Huang, H-. Q., Zhang, C-. F., et al. (2025). Comprehensive bibliometric analysis of pharmacotherapy for bipolar disorders: present trends and future directions. World J. Psychiatry 15:100685. doi: 10.5498/wjp.v15.i1.100685
Chen, C. (2004). Searching for intellectual turning points: progressive knowledge domain visualization. Proc. Natl. Acad. Sci. 101, 5303–5310. doi: 10.1073/pnas.0307513100
Chen, Y., Shen, X., Ma, T., Yu, X., Kwok, L-.Y., Li, Y., et al. (2023). Adjunctive probio-X treatment enhances the therapeutic effect of a conventional drug in managing type 2 diabetes mellitus by promoting short-chain fatty acid-producing bacteria and bile acid pathways. Msystems 8:e01300–22. doi: 10.1128/msystems.01300-22
Clish, C. B. (2015). Metabolomics: an emerging but powerful tool for precision medicine. Mol. Case Stud. 1:a000588. doi: 10.1101/mcs.a000588
Cornall, L. M., Mathai, M. L., Hryciw, D. H., and McAinch, A. J. (2013). The therapeutic potential of GPR43: a novel role in modulating metabolic health. Cell. Mol. Life Sci. 70, 4759–4770. doi: 10.1007/s00018-013-1419-9
Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P., and Macfarlane, G. T. (1987). Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227. doi: 10.1136/gut.28.10.1221
Danaei, G., Finucane, M. M., Lu, Y., Singh, G. M., Cowan, M. J., Paciorek, C. J., et al. (2011). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378, 31–40. doi: 10.1016/S0140-6736(11)60679-X
De Vos, WM. (2017). Microbe profile: akkermansia muciniphila: a conserved intestinal symbiont that acts as the gatekeeper of our mucosa: this article is part of the microbe profiles collection. Microbiology 163, 646–648. doi: 10.1099/mic.0.000444
De Vos, W. M., Tilg, H., Van Hul, M., and Cani, P. D. (2022). Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032. doi: 10.1136/gutjnl-2021-326789
Den Besten, G., Van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D-. J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
Ding, L., Liu, J., Zhou, L., Jia, X., Li, S., Zhang, Q., et al. (2022). A high-fat diet disrupts the hepatic and adipose circadian rhythms and modulates the diurnal rhythm of gut microbiota-derived short-chain fatty acids in gestational mice. Front. Nutr. 9:925390. doi: 10.3389/fnut.2022.925390
Drucker, D. J. (2018). Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell. Metab. 27, 740–756. doi: 10.1016/j.cmet.2018.03.001
Forslund, K., Hildebrand, F., Nielsen, T., Falony, G., Le Chatelier, E., et al. (2015). Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528, 262–266. doi: 10.1038/nature15766
Frost, G., Sleeth, M. L., Sahuri-Arisoylu, M., Lizarbe, B., Cerdan, S., Brody, L., et al. (2014). The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5:3611. doi: 10.1038/ncomms4611
Gacesa, R., Kurilshikov, A., Vich Vila, A., Sinha, T., Klaassen, M. A. Y., Bolte, L. A., et al. (2022). Environmental factors shaping the gut microbiome in a dutch population. Nature 604, 732–739. doi: 10.1038/s41586-022-04567-7
Ganti, L., Persaud, N. A., and Stead, T. S. (2025). Bibliometric analysis methods for the medical literature. Acad. Med. Surg. doi: 10.62186/001c.129134
Gao, Z., Yin, J., Zhang, J., Ward, R. E., Martin, R. J., Lefevre, M., et al. (2009). Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517. doi: 10.2337/db08-1637
González Hernández, M. A., Canfora, E. E., Jocken, J. W. E., and Blaak, E. E. (2019). The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 11:1943. doi: 10.3390/nu11081943
Guo, X., Su, Y., Du, Y., Zhang, F., Yu, W., Ren, W., et al. (2025). Vinegar-processed schisandra chinensis polysaccharide ameliorates type 2 diabetes via modulation serum metabolic profiles, gut microbiota, and fecal SCFAs. Int. J. Biol. Macromol 294:139514. doi: 10.1016/j.ijbiomac.2025.139514
Hao, F., Tian, M., Zhang, X., Jin, X., Jiang, Y., Sun, X., et al. (2021). Butyrate enhances CPT1A activity to promote fatty acid oxidation and iTreg differentiation. Proc. Natl. Acad. Sci. 118:e2014681118. doi: 10.1073/pnas.2014681118
Hassan-Montero, Y., De-Moya-Anegón, F., and Guerrero-Bote, V. P. (2022). SCImago graphica: a new tool for exploring and visually communicating data. Prof. Inf. 31:e310502. doi: 10.3145/epi.2022.sep.02
He, K-. Y., Lei, X-. Y., Wu, D-. H., Zhang, L., Li, J-. Q., Li, Q-. T., et al. (2023). Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes 15:2293312. doi: 10.1080/19490976.2023.2293312
Hosomi, K., Saito, M., Park, J., Murakami, H., Shibata, N., Ando, M., et al. (2022). Oral administration of blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 13:4477. doi: 10.1038/s41467-022-32015-7
Jiang, S., Ma, D., Tan, X., Yang, M., Jiao, Q., and Xu, L. (2023). Bibliometric analysis of the current status and trends on medical hyperspectral imaging. Front. Med. 10:1235955. doi: 10.3389/fmed.2023.1235955
Karlsson, F. H., Tremaroli, V., Nookaew, I., Bergström, G., Behre, C. J., Fagerberg, B., et al. (2013). Gut metagenome in european women with normal, impaired and diabetic glucose control. Nature 498, 99–103. doi: 10.1038/nature12198
Kelly, C. J., Zheng, L., Campbell, E. L., Saeedi, B., Scholz, C. C., Bayless, A. J., et al. (2015). Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell. Host Microbe. 17, 662–671. doi: 10.1016/j.chom.2015.03.005
Kimura, I., Inoue, D., Hirano, K., and Tsujimoto, G. (2014). The SCFA receptor GPR43 and energy metabolism. Front. Endocrinol. 5:85. doi: 10.3389/fendo.2014.00085
Kirschner, S. K., Engelen, M. P., Haas, P., Bischoff, S. C., and Deutz, N. E. (2025). Short-chain fatty acid kinetics and concentrations are higher after inulin supplementation in young and older adults: a randomized trial. Am. J. Clin. Nutr. 121, 1224–1235. doi: 10.1016/j.ajcnut.2025.04.018
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Kullberg, R. F. J., Van Linge, C. C. A., De Vos, A. F., Brands, X., Bui, T. P. N., Butler, J. M., et al. (2025). Butyrate-producing gut bacterium faecalibacterium prausnitzii protects against bacterial pneumonia. Eur. Respir. J. 7:2402208. doi: 10.1183/13993003.02208-2024
Kume, H., Okazaki, K., Yamamoto, N., Omae, Y., Ashida, K., Takahashi, T., et al. (2018). An enteral formula containing fermented milk products and prebiotics promotes glucagon-like peptide-1 secretion via short chain fatty acid signaling. J. Nutr. Food Sci. 8:662. doi: 10.4172/2155-9600.1000662
Larsen, N., Vogensen, F. K., Van Den Berg, F. W. J., Nielsen, D. S., Andreasen, A. S., Pedersen, B. K., et al. (2010). Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5:e9085. doi: 10.1371/journal.pone.0009085
Lee, Q., Xue, Z., Luo, Y., Lin, Y., Lai, M., Xu, H., et al. (2024). Low molecular weight polysaccharide of tremella fuciformis exhibits stronger antioxidant and immunomodulatory activities than high molecular weight polysaccharide. Int. J. Biol. Macromol. 281:136097. doi: 10.1016/j.ijbiomac.2024.136097
Li, H.-Y., Zhou, D.-D., Gan, R.-Y., Huang, S.-Y., Zhao, C.-N., Shang, A., et al. (2021). Mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: a narrative review. Nutrients 13:3211. doi: 10.3390/nu13093211
Li, M. (2017). An exploration to visualise the emerging trends of technology foresight based on an improved technique of co-word analysis and relevant literature data of WOS. Technol. Anal. Strateg. Manag. 29, 655–671. doi: 10.1080/09537325.2016.1220518
Li, M., Van Esch, B. C. A. M., Henricks, P. A. J., Folkerts, G., and Garssen, J. (2018). The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front. Pharmacol. 9:533. doi: 10.3389/fphar.2018.00533
Lin, H. V., Frassetto, A., Kowalik Jr, E. J., Nawrocki, A. R., Lu, M. M., Kosinski, J. R., et al. (2012). Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE. 7:e35240. doi: 10.1371/journal.pone.0035240
Lin, Y., Vonk, R. J., Slooff, M. J. H., Kuipers, F., and Smit, M. J. (1995). Differences in propionate-induced inhibition of cholesterol and triacylglycerol synthesis between human and rat hepatocytes in primary culture. Br. J. Nutr. 74, 197–207. doi: 10.1079/BJN19950123
Llauradó, G., Cedó, L., Climent, E., Badia, J., Rojo-Martínez, G., Flores-Le Roux, J., et al. (2025). Circulating short-chain fatty acids and Mediterranean food patterns. A potential role for the prediction of type 2 diabetes risk: the ZGlAYmV0LmVz study. BMC Med. 23:337. doi: 10.1186/s12916-025-04186-9
Lu, X., Xie, Q., Pan, X., Zhang, R., Zhang, X., Peng, G., et al. (2024). Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal Transduct Target. Ther. 9, 262. doi: 10.1038/s41392-024-01951-9
Magne, F., Gotteland, M., Gauthier, L., Zazueta, A., Pesoa, S., Navarrete, P., et al. (2020). The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients 12:1474. doi: 10.3390/nu12051474
Makki, K., Deehan, E. C., Walter, J., and Bäckhed, F. (2018). The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715. doi: 10.1016/j.chom.2018.05.012
Mckirdy, S., Koutsos, A., Nichols, B., Anderson, M., Dhami, S., Chowdhury, C. R., et al. (2025). Micronutrient supplementation influences the composition and diet-originating function of the gut microbiome in healthy adults. Clin. Nutr. 51, 293–303. doi: 10.1016/j.clnu.2025.06.020
Mukhopadhya, I., and Louis, P. (2025). Gut microbiota-derived short-chain fatty acids and their role in human health and disease. Nat. Rev. Microbiol. 23, 635–651. doi: 10.1038/s41579-025-01183-w
Myhrstad, M. C. W., Tunsjø, H., Charnock, C., and Telle-Hansen, V. H. (2020). Dietary fiber, gut microbiota, and metabolic regulation—current status in human randomized trials. Nutrients 12:859. doi: 10.3390/nu12030859
Nie, Q., Chen, H., Hu, J., Tan, H., Nie, S., and Xie, M. (2020). Effects of nondigestible oligosaccharides on obesity. Annu. Rev. Food Sci. Technol. 11, 205–233. doi: 10.1146/annurev-food-032519-051743
Pham, N. H. T., Joglekar, M. V., Wong, W. K. M., Nassif, N. T., Simpson, A. M., and Hardikar, A. A. (2024). Short-chain fatty acids and insulin sensitivity: a systematic review and meta-analysis. Nutr. Rev. 82, 193–209. doi: 10.1093/nutrit/nuad042
Priyadarshini, M., Wicksteed, B., Schiltz, G. E., Gilchrist, A., and Layden, B. T. (2016). SCFA receptors in pancreatic β cells: novel diabetes targets? Trends Endocrinol. Metab. 27, 653–664. doi: 10.1016/j.tem.2016.03.011
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. doi: 10.1038/nature11450
Roberfroid, M., Gibson, G. R., Hoyles, L., McCartney, A. L., Rastall, R., Rowland, I., et al. (2010). Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 104, S1–S63. doi: 10.1017/S0007114510003363
Saha, S., Day-Walsh, P., Shehata, E., and Kroon, P. A. (2021). Development and validation of a LC-MS/MS technique for the analysis of short chain fatty acids in tissues and biological fluids without derivatisation using isotope labelled internal standards. Molecules 26:6444. doi: 10.3390/molecules26216444
Salamone, D., Rivellese, A. A., and Vetrani, C. (2021). The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: the possible role of dietary fibre. Acta Diabetol. 58, 1131–1138. doi: 10.1007/s00592-021-01727-5
Seethaler, B., Nguyen, N. K., Basrai, M., Kiechle, M., Walter, J., Delzenne, N. M., et al. (2022). Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: data from the randomized controlled LIBRE trial. Am. J. Clin. Nutr. 116, 928–942. doi: 10.1093/ajcn/nqac175
Sheng, Y., Wang, J., Gao, Y., Peng, Y., Li, X., Huang, W., et al. (2024). Combined analysis of cross-population healthy adult human microbiome reveals consistent differences in gut microbial characteristics between western and non-western countries. Comput. Struct. Biotechnol. J. 23, 87–95. doi: 10.1016/j.csbj.2023.11.047
Sonnenburg, J. L., and Bäckhed, F. (2016). Diet–microbiota interactions as moderators of human metabolism. Nature 535, 56–64. doi: 10.1038/nature18846
Stratton, I. M. (2000). Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321, 405–412. doi: 10.1136/bmj.321.7258.405
Tang, W. H. W., Kitai, T., and Hazen, S. L. (2017). Gut microbiota in cardiovascular health and disease. Circ. Res. 120, 1183–1196. doi: 10.1161/CIRCRESAHA.117.309715
Todesco, T., Rao, A., Bosello, O., and Jenkins, D. (1991). Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am. J. Clin. Nutr. 54, 860–865. doi: 10.1093/ajcn/54.5.860
Tolhurst, G., Heffron, H., Lam, Y. S., Parker, H. E., Habib, A. M., Diakogiannaki, E., et al. (2012). Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes 61, 364–371. doi: 10.2337/db11-1019
Tremaroli, V., and Bäckhed, F. (2012). Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249. doi: 10.1038/nature11552
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., and Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. doi: 10.1038/nature05414
Van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
Van-Wehle, T., and Vital, M. (2024). Investigating the response of the butyrate production potential to major fibers in dietary intervention studies. npj Biofilms Microb. 10:63. doi: 10.1038/s41522-024-00533-5
Vliex, L. M. M., Penders, J., Nauta, A., Zoetendal, E. G., and Blaak, E. E. (2024). The individual response to antibiotics and diet — insights into gut microbial resilience and host metabolism. Nat. Rev. Endocrinol. 20, 387–398. doi: 10.1038/s41574-024-00966-0
Wolever, T. M., Schrade, K. B., Vogt, J. A., Tsihlias, E. B., and McBurney, M. I. (2002). Do colonic short-chain fatty acids contribute to the long-term adaptation of blood lipids in subjects with type 2 diabetes consuming a high-fiber diet? Am. J. Clin. Nutr. 75, 1023–1030. doi: 10.1093/ajcn/75.6.1023
Wu, H., Esteve, E., Tremaroli, V., Khan, M. T., Caesar, R., Mannerås-Holm, L., et al. (2017). Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858. doi: 10.1038/nm.4345
Wu, J., Shen, H., Lv, Y., He, J., Xie, X., Xu, Z., et al. (2024). Age over sex: evaluating gut microbiota differences in healthy chinese populations. Front. Microbiol. 15:1412991. doi: 10.3389/fmicb.2024.1412991
Xu, Y-. H., Gao, C-. L., Guo, H-. L., Zhang, W-. Q., Huang, W., Tang, S-. S., et al. (2018). Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J. Endocrinol. 238, 231–244. doi: 10.1530/JOE-18-0137
Yamashita, H., Fujisawa, K., Ito, E., Idei, S., Kawaguchi, N., Kimoto, M., et al. (2007). Improvement of obesity and glucose tolerance by acetate in type 2 diabetic otsuka long-evans tokushima fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 71, 1236–1243. doi: 10.1271/bbb.60668
Yang, J., Martínez, I., Walter, J., Keshavarzian, A., and Rose, D. J. (2013). In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 23, 74–81. doi: 10.1016/j.anaerobe.2013.06.012
Yang, W., Xia, Z., Zhu, Y., Tang, H., Xu, H., Hu, X., et al. (2023). Comprehensive study of untargeted metabolomics and 16S rRNA reveals the mechanism of fecal microbiota transplantation in improving a mouse model of T2D. Diabetes Metab. Syndr. Obes. 16, 1367–1381. doi: 10.2147/DMSO.S404352
Zhang, C., Ma, S., Wu, J., Luo, L., Qiao, S., Li, R., et al. (2020). A specific gut microbiota and metabolomic profiles shifts related to antidiabetic action: the similar and complementary antidiabetic properties of type 3 resistant starch from canna edulis and metformin. Pharmacol. Res. 159:104985. doi: 10.1016/j.phrs.2020.104985
Zhang, H., and Mo, Y. (2023). The gut-retina axis: a new perspective in the prevention and treatment of diabetic retinopathy. Front. Endocrinol. 14:1205846. doi: 10.3389/fendo.2023.1205846
Zhang, Z., Zhang, H., Yang, D., Hao, Q., Yang, H., Meng, D., et al. (2024). Lactobacillus rhamnosus GG triggers intestinal epithelium injury in zebrafish revealing host dependent beneficial effects. Imeta 3:e181. doi: 10.1002/imt2.181
Zhao, L., Zhang, F., Ding, X., Wu, G., Lam, Y. Y., Wang, X., et al. (2018). Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359, 1151–1156. doi: 10.1126/science.aao5774
Zheng, J., An, Y., Du, Y., Song, Y., Zhao, Q., and Lu, Y. (2024). Effects of short-chain fatty acids on blood glucose and lipid levels in mouse models of diabetes mellitus: a systematic review and network meta-analysis. Pharmacol. Res. 199:107041. doi: 10.1016/j.phrs.2023.107041
Zhong, D., Li, Y., Huang, Y., Hong, X., Li, J., and Jin, R. (2022). Molecular mechanisms of exercise on cancer: a bibliometrics study and visualization analysis via CiteSpace. Front. Mol. Biosci. 8:797902. doi: 10.3389/fmolb.2021.797902
Zhou, B., Rayner, A. W., Gregg, E. W., Sheffer, K. E., Carrillo-Larco, R. M., Bennett, J. E., et al. (2024). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 404, 2077–2093. doi: 10.1016/S0140-6736(24)02317-1
Keywords: short-chain fatty acids, type 2 diabetes, gut microbiota, metabolomics, microbial metabolism, dietary intervention, bibliometric analysis
Citation: Zheng L, Jiang R, Fang J, Tan Y, Wang Y, Li L and Liu M (2025) Short-chain fatty acids and type 2 diabetes: a bibliometric analysis of knowledge structure, research hotspots, and future directions. Front. Microbiol. 16:1697421. doi: 10.3389/fmicb.2025.1697421
Received: 05 September 2025; Accepted: 24 October 2025;
Published: 12 November 2025.
Edited by:
Yan Liu, Southwest University, ChinaReviewed by:
Zhengrui Li, Shanghai Jiao Tong University, ChinaJinwei Zhang, University of Exeter, United Kingdom
Copyright © 2025 Zheng, Jiang, Fang, Tan, Wang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjun Liu, Y2N6dGxtakAxNjMuY29t
 Likun Zheng
Likun Zheng Rongsheng Jiang
Rongsheng Jiang Jinxu Fang
Jinxu Fang Yi Tan1
Yi Tan1