- 1Institute of Crop Sciences, Fujian Academy of Agricultural Sciences (Fujian Germplasm Resources Center), Fuzhou, China
- 2Fujian Forestry Science and Technology Extension General Station, Fuzhou, China
- 3Sanming Academy of Agricultural Sciences, Sanming, China
Introduction: Prolonged monoculture cultivation of Paris polyphylla has been linked to challenges such as disruption of rhizosphere soil microbial balance and deterioration in crop quality. Intercropping has emerged as a viable strategy to address these issues.
Methods: In the present study, monoculture of P. polyphylla (PP) served as the control, while two intercropping systems were implemented: P. polyphylla with Polygonatum cyrtonema (PPPC) and P. polyphylla with Ganoderma lucidum (PPG). The objective was to evaluate the P. polyphylla quality, soil physicochemical properties, and the structure of the microbial community.
Results: Findings revealed that the PPG intercropping system significantly increased available potassium levels by 50.28% and enhanced the abundance of Trichoderma by 3,022% via the G. lucidum network. These alterations were associated with improvements in P. polyphylla yield (51.30%), polyphyllin VII content (34.16%), and total polyphyllin content (30.59%). Conversely, the PPPC system promoted the enrichment of Cupriavidus and nitrogen-fixing bacteria such as Hyphomicrobiales, leading to a 26.78% rise in available phosphorus and a 20.00% increase in polyphyllin II content in P. polyphylla. Both intercropping approaches markedly elevated the abundance of Basidiomycota, with the PPPC system further enriching functional microbial taxa including Glomeromycota and Saitozyma.
Discussion: Collectively, these results demonstrate that intercropping P. polyphylla with either P. cyrtonema (PPPC) or G. lucidum (PPG) enhances soil physicochemical attributes, optimizes the composition of rhizosphere microbial communities, and positively influences the accumulation and yield of bioactive compounds.
1 Introduction
Paris polyphylla is recognized as a nationally designated second-class rare medicinal resource. Its rhizome contains polyphyllins I, II, and VII, which constitute the principal bioactive compounds in traditional Chinese patent medicines renowned for their heat-clearing and detoxifying effects (Wu et al., 2017; Liu L. L. et al., 2025). In recent years, the scale of artificial cultivation of P. polyphylla has expanded steadily. Nevertheless, prolonged monoculture or continuous cropping of traditional Chinese medicinal plants frequently results in a triad of challenges, including soil microbial imbalance, nutrient depletion, and a consequent decline in crop quality (Li M. L. et al., 2025). Recent studies conducted in extensive cultivation areas have demonstrated that the species' 5–7 years growth cycle, combined with low root exudation, leads to a marked decrease in available potassium within the rhizosphere, which declines to 93 mg·kg−1 after 4 to 5 years of continuous planting—approximately 55% of the levels observed in newly cultivated soils (Xian et al., 2022; Su et al., 2024). Concurrently, the relative abundance of Basidiomycota fungi diminished significantly from 18% to 6%, while potentially pathogenic taxa such as Fusarium proliferated. These alterations were correlated with a reduction exceeding 15% in polyphyllin VII content (Xian et al., 2022; Su et al., 2024).
For crops with a long growth period, intercropping has emerged as an effective strategy to alleviate these issues. This approach has been systematically investigated in model plant and food crop systems. For example, the pineapple-banana rotation promotes the proliferation of Pseudomonas via root-exuded carboxylic acids, resulting in a increase in soil available phosphorus and a significant suppression of Fusarium wilt disease (Yang et al., 2023). Similarly, wheat-fava bean intercropping leverages the nitrogen-fixing capacity and flavonoid secretion of fava bean to restructure bacterial-fungal interaction networks, thereby improving both yield and soil health (Li et al., 2016). Within the context of medicinal plants, continuous cultivation of ginseng over 2 years has been linked to reductions in soil available phosphorus as well as declines in quality and yield (Rao et al., 2025). Intercropping with Asari Radix et Rhizoma, which emits volatile oils capable of inhibiting ginseng pathogens and promoting phosphate-solubilizing bacteria, has been demonstrated to increase saponin content in ginseng (Feng et al., 2008; Wei et al., 2024). Collectively, these examples highlight the effectiveness of disrupting the deleterious microecological cycles induced by monoculture through integrated systems involving companion plants, functional microorganisms, and soil interactions.
Two medicinal plants, Polygonatum cyrtonema and Ganoderma lucidum, share similar ecological characteristics with P. polyphylla. All three species are shade- tolerant plants and exhibit complementary ecological traits. Notably, P. cyrtonema enhances soil phosphorus and potassium availability via root-secreted organic acids (Lu et al., 2025; Chen et al., 2002), whereas G. lucidum, a white rot fungus, decomposes lignocellulose through its mycelial network, thereby releasing bioavailable potassium and secreting polysaccharides that induce plant resistance (Fang et al., 2024). However, systematic investigations remain limited regarding whether the tripartite “plant-fungus-plant” interactions among these species can selectively reconstruct the rhizosphere microecology of P. polyphylla and improve the quality of its medicinal components. This study focuses on P. polyphylla, which is notably affected by continuous cropping obstacles. Three cultivation regimes were established: monoculture of P. polyphylla (PP), intercropping with P. cyrtonema (PPPC), and intercropping with G. lucidum (PPG). The present research examines changes in agronomic traits, active ingredient concentrations, rhizosphere soil physicochemical properties, and microbial community composition of P. polyphylla. Furthermore, it explores the effects of intercropping on the quality of P. polyphylla and elucidates the underlying mechanisms, thereby providing a theoretical foundation and technical framework for the high-efficiency cultivation of this species.
2 Materials and methods
2.1 Study location and experimental conditions
The study was conducted at the Taozhou Base, located in Guangze County, Nanping City, Fujian Province, China (27°18′N, 117°0′E, 298 meters above sea level). The climate is subtropical monsoon, with an annual accumulated temperature of 6,336 °C, total sunshine duration of 1,615 h annual precipitation of 1,864 mm, and a frost-free period of 271 days (Su et al., 2024). The experiment was carried out under the canopy of a 20 year old Chinese fir (Cunninghamia lanceolate) forest, providing approximately 50% shading. The soil type at the site was sandy loam.
2.2 Experimental design and treatments
The experiment was carried out from March 2022 to October 2024. The experimental design comprised a monoculture system of P. polyphylla (PP) and two intercropping treatments: P. polyphylla intercropped with P. cyrtonema (PPPC) and P. polyphylla intercropped with G. lucidum (PPG) (Figure 1). Each treatment included 100 plants, with three replicates per treatment. Seedlings of P. polyphylla and P. cyrtonema were 3 years old at transplantation, planted at a density of 25 cm between individual plants and 25 cm between rows. The G. lucidum mushroom sticks used in the intercropping treatments consisted of a composite substrate containing miscellaneous wood chips, corn cobs, and cottonseed materials. For intercropping, the outer membrane of the G. lucidum sticks was removed, and trenches were excavated 5 to 8 cm from the base of the P. polyphylla plants to embed the mushroom sticks.
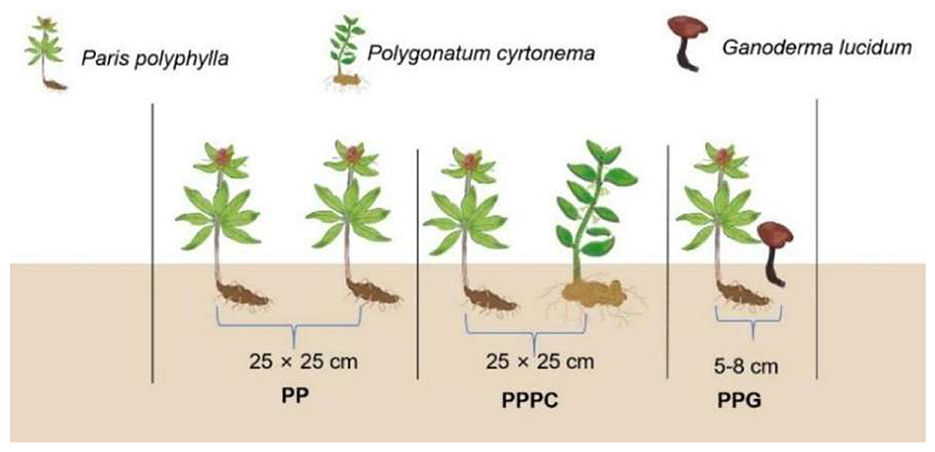
Figure 1. Diagram illustrating the different intercropping treatments and planting configurations used in this study. The monoculture systems of P. polyphylla (PP) was compared with two intercropping treatments: P. polyphylla with P. cyrtonema (PPPC) and P. polyphylla with G. lucidum (PPG). Each treatment features distinct plant spacing and root configurations, with the spacing between plants shown as 25 × 25 cm for PP and PPPC systems, while 5-8 cm distances for PPG systems.
Rhizosphere soil samples were collected in October 2024 employing the root-shaking method. For each treatment, ten plants exhibiting uniform size and growth were selected. Soil adhering to the roots was carefully brushed off using sterile brushes and placed into sampling bags. The collected soil samples were then divided into two parts: one part was immediately frozen in liquid nitrogen for 10 to 15 mins and subsequently stored at −80° C for microbial diversity analyses; the other portion was air-dried for subsequent physicochemical soil properties analysis.
The remaining rhizosphere soil samples were subjected to air-drying. Subsequently, the dried soil was ground and sieved following the soil treatment protocol outlined by Li F. Q. et al. (2025) to facilitate the analysis of soil physicochemical properties. The subterranean plant parts were carefully washed and oven-dried at 60° C. Following drying, these plant samples were ground and prepared for further analytical procedures.
2.3 Agronomic traits
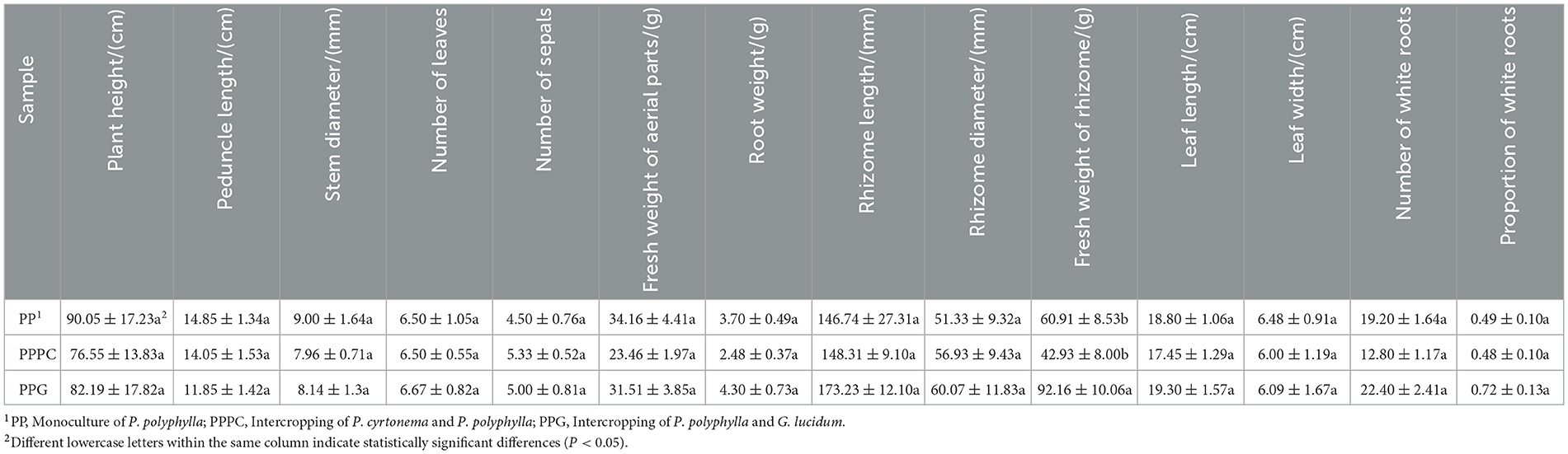
The agronomic characteristics of P. cyrtonema were assessed following a 2 year cultivation period of P. polyphylla. In August 2024, measurements were taken for plant height, stem length, stem diameter, leaf length, leaf width, leaf count, and sepal count. Subsequently, in October 2024, additional parameters were assessed, including the fresh weight of the aboveground parts (comprising leaves, flowers, fruits, and stems), total root biomass, rhizome length and diameter, fresh rhizome weight, as well as the quantity and proportion of white roots relative to the entire root system.
2.4 Quantification of bioactive constituents in P. polyphylla rhizomes
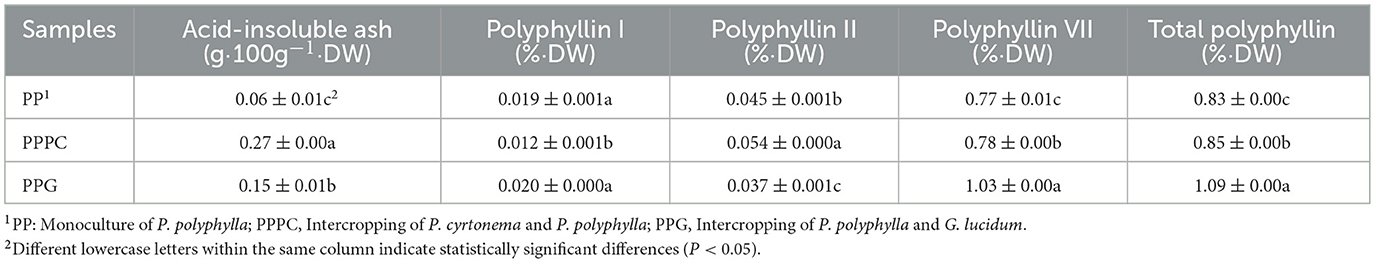
The acid-insoluble ash content of the rhizomes was determined in accordance with the National Food Safety Standard for Ash Content in Foods. Furthermore, concentrations of polyphyllin I, II, and VII within the rhizomes were quantified following the protocols outlined in the 2020 edition of the Pharmacopeia of the People's Republic of China (Su et al., 2024). Specifically, 0.500 g of the test samplewas accurately weighed and placed into a stoppered conical flask, to which 25 mL of ethanolwas added. The flaskwas weighed, then subjected to reflux heating for 30 mins, cooled, and weighed again. The weight loss was compensated with ethanol, the mixture was thoroughly shaken and filtered to obtain test solution. Reference standards ofpolyphyllin I, polyphyllin II, and polyphyllin VII (4 mg each) were separately weighed, dissolved in 1 mL of methanol, and mixed thoroughly. Chromatographic analysis was conducted using a ZORBAX SB-C18 column (Agilent) was used, with acetonitrile (A) and water (B) as the mobile phase, maintained 25° C, a flow rate of 0.8 mL/min, and detection wavelength at 203 nm. A 10 μL aliquot of each reference standard and test solution was injected into the high-performance liquid chromatography (Agilent, 1,290 series, USA) system for quantification.
2.5 Soil analysis
Soil properties were analyzed following the methodologies described in the Soil Agrochemical Analysis by (Bao 2000). Soil pH was measured using a pH meter (Shanghai Yidian Scientific Instruments Co., Ltd. China), with a soil-to-water ratio of 1:25. The soil and water mixture was stirred for 5 mins and then allowed to stand for 1 h before measurement. Soil Organic Matter (SOM) content was assessed using the potassium dichromate-sulfuric acid oxidation method. Alkali-hydrolyzable nitrogen (AHN) was quantified using the Kjeldahl method. Available phosphorus (AP) was extracted using ammonium fluoride and hydrochloric acid and subsequently measured by molybdenum-antimony colorimetry. Available potassium (AK) was measured using flame photometry with an ammonium acetate solution. Total nitrogen (TN) was quantified using an automatic nitrogen analyzer. The contents of total phosphorus (TP) and total potassium (TK) contents were determined by digestion with nitric acid and perchloric acid, followed by hydrofluoric acid decomposition and dissolution in hydrochloric acid, with subsequent analysis via flame photometry.
2.6 Extraction of microbial DNA from soil, PCR amplification and sequencing
Microbial genomic DNA was extracted using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) through a service provided by Shanghai Majorbio Biopharmaceutical Technology Co., Ltd. DNA quality and concentration were assessed via 1.0% agarose gel electrophoresis and quantified using a NanoDrop 2,000 spectrophotometer (Thermo Fisher Scientific, USA). DNA sequencing was performed using the Illumina PE300 platform. Raw sequencing data were submitted to the NCBI Sequence Read Archive (SRA) database (Li F. Q. et al., 2025).
2.7 Data analysis
Statistical analysis of agronomic traits, rhizosphere soil physicochemical properties, and bioactive components in P. polyphylla rhizomes under different intercropping systems was conducted using the Least Significant Difference (LSD) test implemented in the DPS (Data Processing System) software. Microbial diversity analyses were performed using the Majorbio cloud platform. Alpha diversity indices, including Shannon, Simpson, Ace, Chao, and Sobs, were calculated using Mothur (version v.1.30.2). Principal Component Analysis (PCA) was performed to analyze the variation in microbial community composition. Analysis of Similarities (ANOSIM) was used to test the significance of differences in community structure among groups. Redundancy analysis (RDA) was conducted to investigate relationships between soil physicochemical properties and soil micro bial communities. Pearson correlation coefficient was employed to examine the relationships between soil physical and chemical properties, root weight, rhizome fresh weight, effective components of P. polyphylla, and soil microorganisms at the phylum level. Visualization of data, including bar charts and heatmaps, was performed using the R programming language (version 3.3.1).
3 Results
3.1 Influence of intercropping systems on the agronomic characteristics of P. polyphylla
The impact of various intercropping systems on the agronomic characteristics of P. polyphylla exhibited variation (Table 1). Specifically, the fresh weight of rhizomes under the PPG intercropping system was significantly greater than that observed in both the PP and PPPC systems (P < 0.05), demonstrating a 51.30% increase relative to the PP monoculture, thereby indicating a pronounced enhancement in biomass accumulation. No statistically significant differences were detected among the three planting patterns concerning other agronomic parameters, including plant height, flower stem length, and stem thickness. Nonetheless, parameters such as leaf number, root weight, rhizome length, stem thickness, leaf length, number of white roots, and the ratio of white roots were slightly higher in the PPG system compared to the PP and PPPC systems. Notably, the proportion of white roots in the PPG system (0.72) increased by 46.90% relative to the PP system (0.49), suggesting that intercropping with G. lucidum may enhance nutrient uptake by promoting root vitality. In contrast, most agronomic traits measured in the PPPC system were slightly reduced compared to those in the PP system.
3.2 Variation in active ingredient content of P. polyphylla under different intercropping systems
Significant differences were observed in the acid-insoluble ash content and the concentrations of active compounds in P. polyphylla across the all three cultivation treatments (Table 2). The acid-insoluble ash content reached its maximum in the PPPC treatment, measuring 0.27 g/100 g, which was significantly greater than the levels recorded in the PPG and PP treatments (P < 0.05), representing a 350.00% increase relative to the PP monoculture. Additionally, the concentration of polyphyllin I was significantly higher in the PPG treatment compared to PPPC, although no significant difference was detected between PPG and PP (Table 2). The highest concentration of polyphyllin II (0.05%) was observed in the PPPC treatment, corresponding to a 20.00% increase compared to the PP monoculture (P < 0.05). Polyphyllin VII and total polyphyllin contents were greatest in the PPG treatment, at 1.03% and 1.09%, respectively, which were significantly higher than those in PPPC and PP (P < 0.05), reflecting increases of 34.16% and 30.60% relative to the PP monoculture (P < 0.05). Furthermore, the levels of polyphyllin VII and total polyphyllin in PPPC were significantly elevated compared to PP (P < 0.05), with increases of 1.6% and 1.7%, respectively. These results indicate that intercropping P. polyphylla with G. lucidum and P. cyrtonema notably enhances the accumulation of active constituents in P. polyphylla
3.3 Characteristics of rhizosphere soil of P. polyphylla under different intercropping systems
As shown in Table 3, compared to monoculture, the PPPC and PPG intercropping systems significantly enhanced soil nutrient parameters—including Soil Organic Matter (SOM), available phosphorus (AP), available potassium (AK), total nitrogen (TN), and total potassium (TK)—relative to monoculture cultivation. Specifically, the PPPC system increased these parameters by 16.93%, 4.90%, 20.67%, 11.64%, and 2.61%, respectively, whereas the PPG system exhibited increases of 2.82%, 26.78%, 50.28%, 0.87%, and 2.87%, respectively. Under the PPPC intercropping regime, SOM and TN concentrations were significantly higher than those observed in the PPG system; conversely, the contents of alkali-hydrolyzable nitrogen (AHN), AP, and AK were significantly lower in PPPC compared to PPG. No significant differences were detected in total potassium (TK) content between the two intercropping patterns. Additionally, the PPPC system significantly elevated AHN content by 4.63% while markedly reducing total phosphorus (TP) levels by 15.84%. In contrast, the PPG system significantly decreased AHN by 4.82%, with no significant variation in TP content relative to the PP monoculture. Across all three cultivation treatments, rhizosphere soil pH ranged narrowly from 6.49 to 6.52, indicating a slightly acidic environment. These results suggest that intercropping, particularly the PPG system, more effectively promotes the accumulation of key soil nutrients than monoculture.
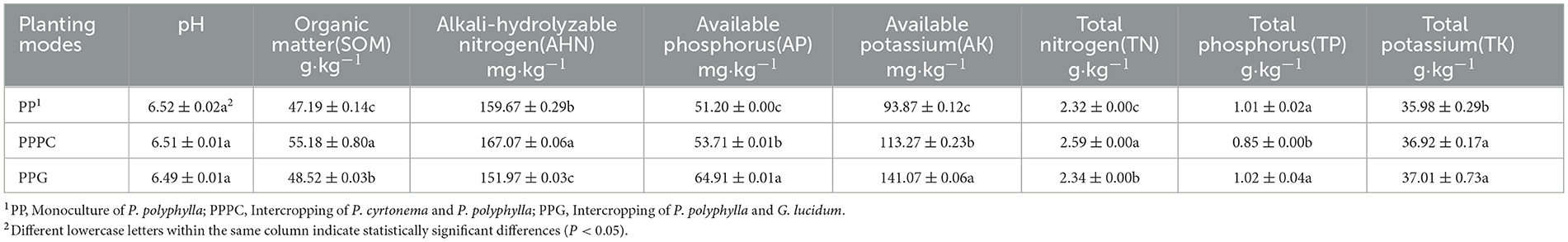
Table 3. Physical and chemical properties of rhizosphere soil of P. polyphylla under different intercropping modes.
3.4 Characteristics of rhizosphere soil microbial communities of P. polyphylla under various intercropping systems
3.4.1 Soil microbial diversity
In this investigation, diversity analyses conducted on nine soil samples yielded a total of 554,751 optimized fungal sequences and 529,512 optimized bacterial sequences. The fungal sequence libraries demonstrated coverage values ranging from 99.95% to 99.98%, while bacterial sequence libraries exhibited coverage between 99.80% and 99.90%, indicating comprehensive representation of microbial diversity within the rhizosphere soil. A total of 3,562 fungal Operational Taxonomic Units (OTUs) and 9,373 bacterial OTUs were identified.
Alpha diversity indices for soil fungi revealed that the Ace, Chao, and Sobs indices under the PPPC and PPG intercropping patterns were significantly higher than those observed in the PP monoculture system (P < 0.05) (Table 4). In contrast, the Shannon and Simpson indices did not exhibit statistically significant differences among these treatments. Although the alpha diversity indices for PPPC were marginally higher than those for PPG, this difference was not statistically significant. Principal component analysis (PCA) of fungal community composition (Figure 2A) further indicated that the fungal community structures in the rhizosphere soils of PP, PPPC, and PPG were broadly similar.
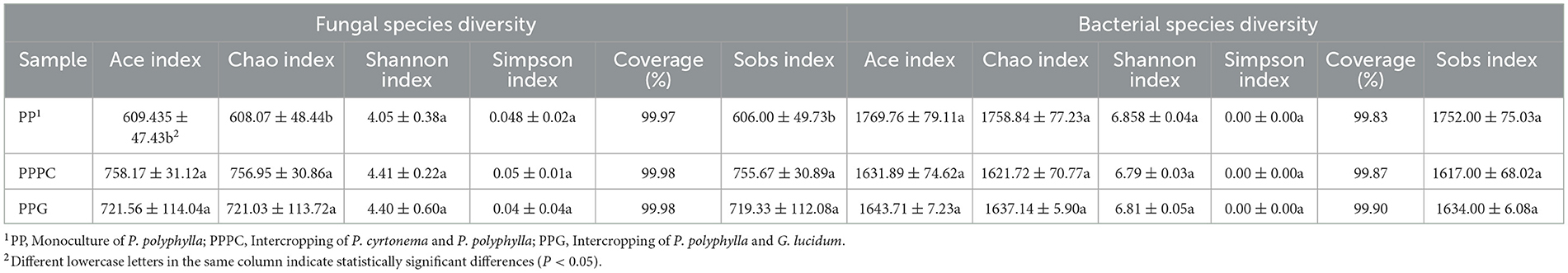
Table 4. Alpha diversity indices of fungal and bacterial communities in rhizosphere soil of P. polyphylla under different intercropping modes.
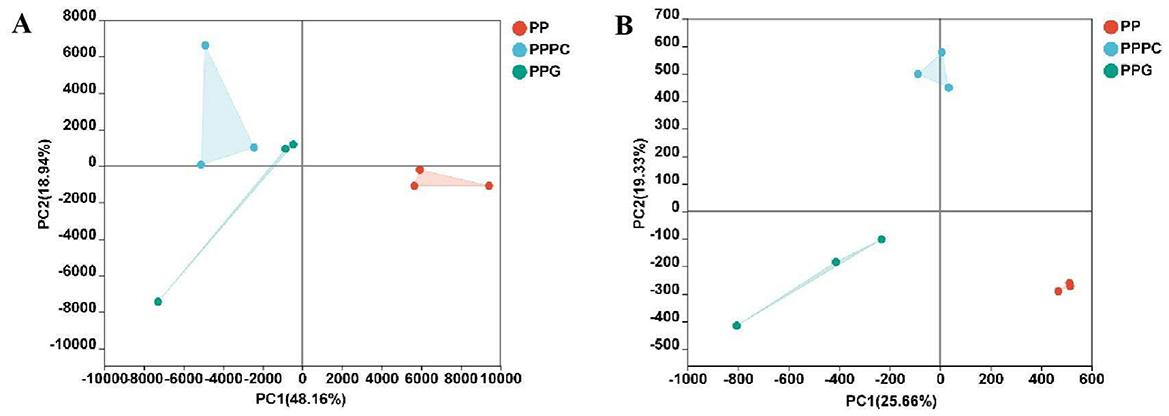
Figure 2. The influence of various intercropping patterns on microbial species diversity. (A) Fungal species diversity; (B) Bacterial species diversity. PP, Monoculture of P. polyphylla; PPPC, Intercropping of P. cyrtonema and P. polyphylla; PPG, Intercropping of P. polyphylla and G. lucidum.
In contrast, bacterial alpha diversity indices revealed no significant differences in the Ace, Chao, and Sobs indices among the PP, PPPC, and PPG treatments (Table 4). Notably, the Ace, Chao, Shannon, and Sobs indices were marginally higher in the PP treatment relative to PPPC and PPG. PCA revealed significant differences in bacterial community structures in the PPPC and PPG treatments differed significantly from those observed in the PP treatment (Figure 2B).
3.4.2 Composition of soil microbial communities
Analysis identified 15 fungal phyla and 592 fungal genera across the nine soil samples. The horizontal distribution of fungal phyla within the rhizosphere soil across different intercropping systems was depicted in Figure 3A. The ten most abundant fungal phyla, ranked by relative abundance, included Ascomycota, Basidiomycota, Fungi_phy_Incertae_sedis, Mortierellomycota, unclassified_k_Fungi, Rozellomycota, Chytridiomycota, Aphelidiomycota, Mucoromycota, and Monoblepharomycota. Ascomycota and Basidiomycota predominated across all treatments, with Ascomycota accounting for 76.89%, 53.41%, and 61.97% in the PP, PPPC, and PPG models, respectively. Basidiomycota showed relative abundances of 6.85%, 15.96%, and 15.52% in the corresponding models.
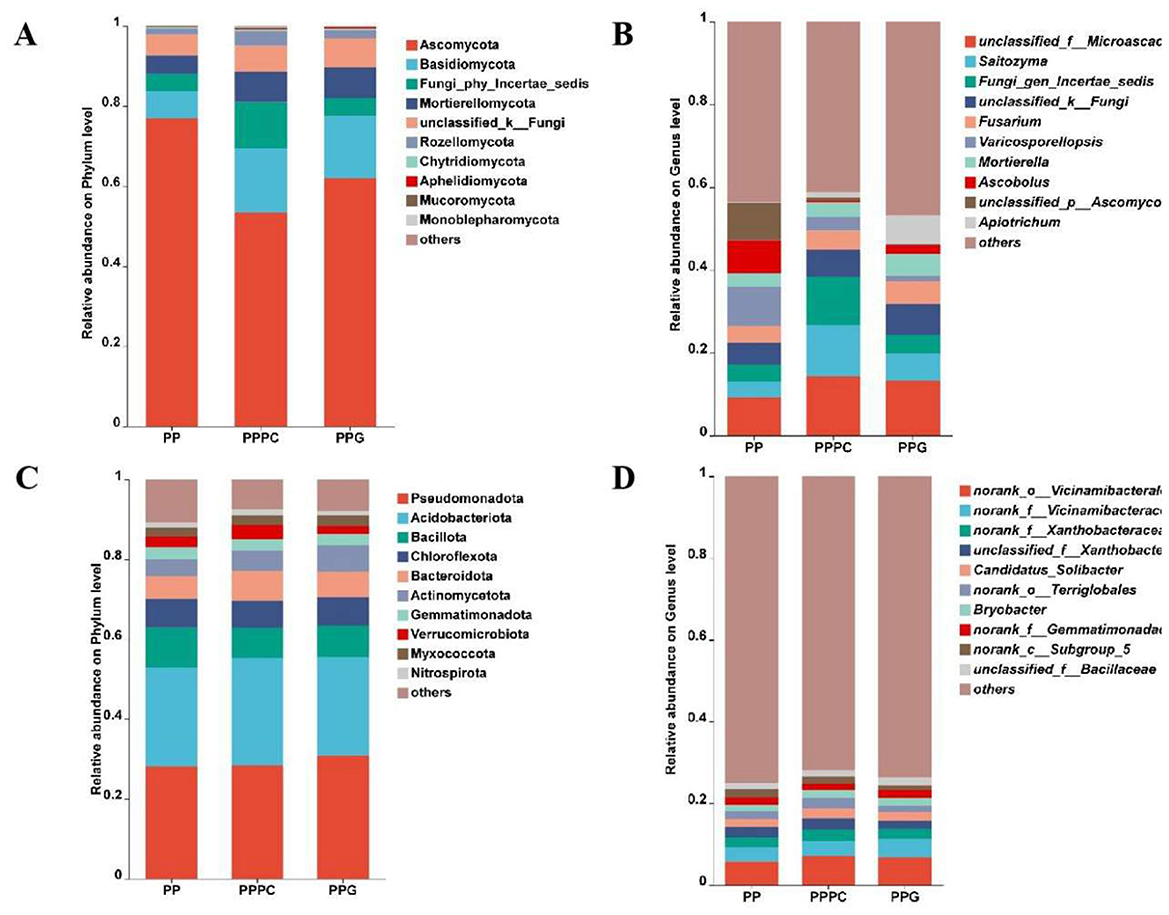
Figure 3. The effects of different intercropping patterns on the composition of fungal species in the rhizosphere soil of P. polyphylla. (A) Fungal phylum level; (B) Fungal genus level; (C) Bacterial phylum level; (D) Bacterial genus level. PP, Monoculture of P. polyphylla; PPPC, Intercropping of P. cyrtonema and P. polyphylla; PPG, Intercropping of P. polyphylla and G. lucidum.
At the genus level (Figure 3B), the ten most abundant fungal genera were unclassified_f_Microascaceae (9.25%−14.35%), Saitozyma (3.78%−12.45%), Fungi_gen_Incertae_sedis (4.22%−11.57%), unclassified_k_Fungi (5.28%−7.40%), Fusarium (3.99%−5.42%), Varicosporellopsis (1.41%−9.41%), Mortierella (3.24%−5.22%), Ascobolus (0.51%−8.10%), unclassified_p_Ascomycota (0.29%−9.00%), and Apiotrichum (0.15%−6.94%).
Bacterial community analysis identified 43 bacterial phyla and 907 bacterial genera within the same soil samples. At the phylum level (Figure 3C), the ten most abundant bacterial phyla were Pseudomonadota, Acidobacteriota, Bacillota, Chloroflexota, Bacteroidota, Actinomycetota, Gemmatimonadota, Verrucomicrobiota, Myxococcota, and Nitrospiraota. Pseudomonadota and Acidobacteriota dominated across all treatments, with relative abundances of 28.05%, 28.50%, and 30.84% for Pseudomonadota and 24.74%, 26.87%, and 24.69% for Acidobacteriota in PP, PPPC, and PPG, respectively.
At the genus level (Figure 3D), the ten most abundant bacterial genera included norank_o_Vicinamibacterales (5.76%−7.12%), norank_f_Vicinamibacteraceae (3.54%−4.46%), norank_f_Xanthobacteraceae (2.31%−2.76%), unclassified_f_Xanthobacteraceae (1.93%−2.70%), Candidatus_Solibacter (2.04%−2.39%), norank_o_Terriglobales (1.51%−2.52%), Bryobacter (1.55%−1.96%), norank_f_Gemmatimonadaceae (1.69%−1.84%), norank_c_Subgroup_5 (1.31%−2.04%), and classified_f_Bacillaceae (1.51%−1.90%).
3.4.3 Variations in soil microbial community composition
At the fungal phylum level (Figure 4A), the relative abundance of Glomeromycota in the rhizosphere soil of P. polyphylla under the PPPC intercropping model was markedly greater than that observed in the PP and PPG models, with an increase of 6,298% relative to the PP model (P < 0.05). At the fungal genus level, 61 genera demonstrated statistically significant differences in relative abundance (P < 0.05) across the three cultivation patterns. The ten most differentially abundant fungal genera were selected for detailed analysis (Figures 4B–K). Compared to the PP model, both the PPPC and PPG models significantly decreased the relative abundance of the genera Ascobolus, Paraboremia, Cyberlindnera, and Coniothyrium. Within the PPPC model, the relative abundances of Saitozyma, Furcasterigium, and Podila in the rhizosphere soil were significantly elevated compared to PP, with increases of 229%, 2,612%, and 565%, respectively. Additionally, the abundances of Saitozyma and Furcasterigium were significantly higher in PPPC than in PPG. Conversely, in the PPG model, the genera Apiotrichum, Trichoderma, and Corallomycetella exhibited significantly greater relative abundances than in PP, increasing by 4,529%, 3,022%, and 697%, respectively; moreover, the abundances of Trichoderma and Corallomycetella were significantly higher than those observed in PPPC. These results indicate that distinctly influence the composition and abundance of specific fungal taxa in the rhizosphere.
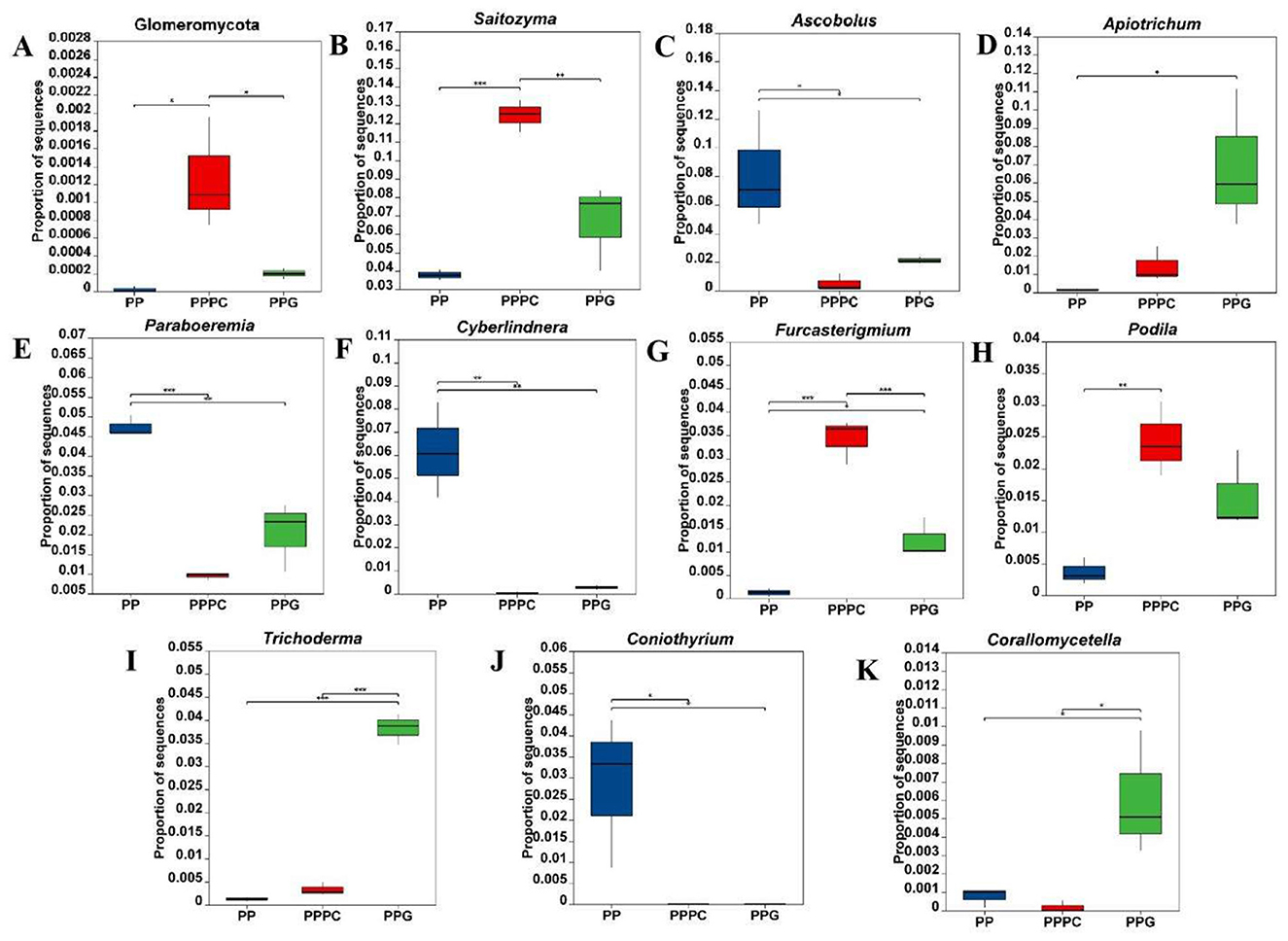
Figure 4. Relative abundance changes of differential fungi in the rhizosphere soil of P. polyphylla under different intercropping patterns. (A) Phylum level; (B-K) Genus level. PP, Monoculture of P. polyphylla; PPPC, Intercropping of P. cyrtonema and P. polyphylla; PPG, Intercropping of P. polyphylla and G. lucidum. * indicates a significant difference between the two groups (*P < 0.05; **P < 0.01; ***P < 0.001).
At the bacterial phylum level (Figures 5A, B), the relative abundances of NB1-j and Zixibacteria in the rhizosphere soil of P. polyphylla were significantly lower in the PPPC intercropping system compared to PP. At the genus level, 58 bacterial genera exhibited significant differences in relative abundance (P < 0.05) across the three planting regimes in the rhizosphere soil of P. polyphylla. The top ten differentially abundant genera were selected for further analysis (Figures 5C–L). Compared to PP, the PPPC intercropping mode significantly increased the relative abundances of Cupriavidus and norank_f__Hypomicrobiales by 101% and 260%, respectively. Concurrently, the relative abundances of Bauldia, norank_c__S0134_terrestrial-group, norank_p__NB1-j, and Arenimonas were significantly decreased. Both PPPC and PPG intercropping modes markedly enhanced the relative abundance of Puia by 393% and 591%, respectively, while reducing norank_f__A21b. Additionally, under the PPG mode, the relative abundances of unclassified_f__Chitinophagaceae and Ureibacillus were significantly lower than those observed in PP and PPPC. These findings demonstrate that distinct intercropping patterns can modulate the composition and abundance of specific bacterial taxa within the rhizosphere soil of P. polyphylla.
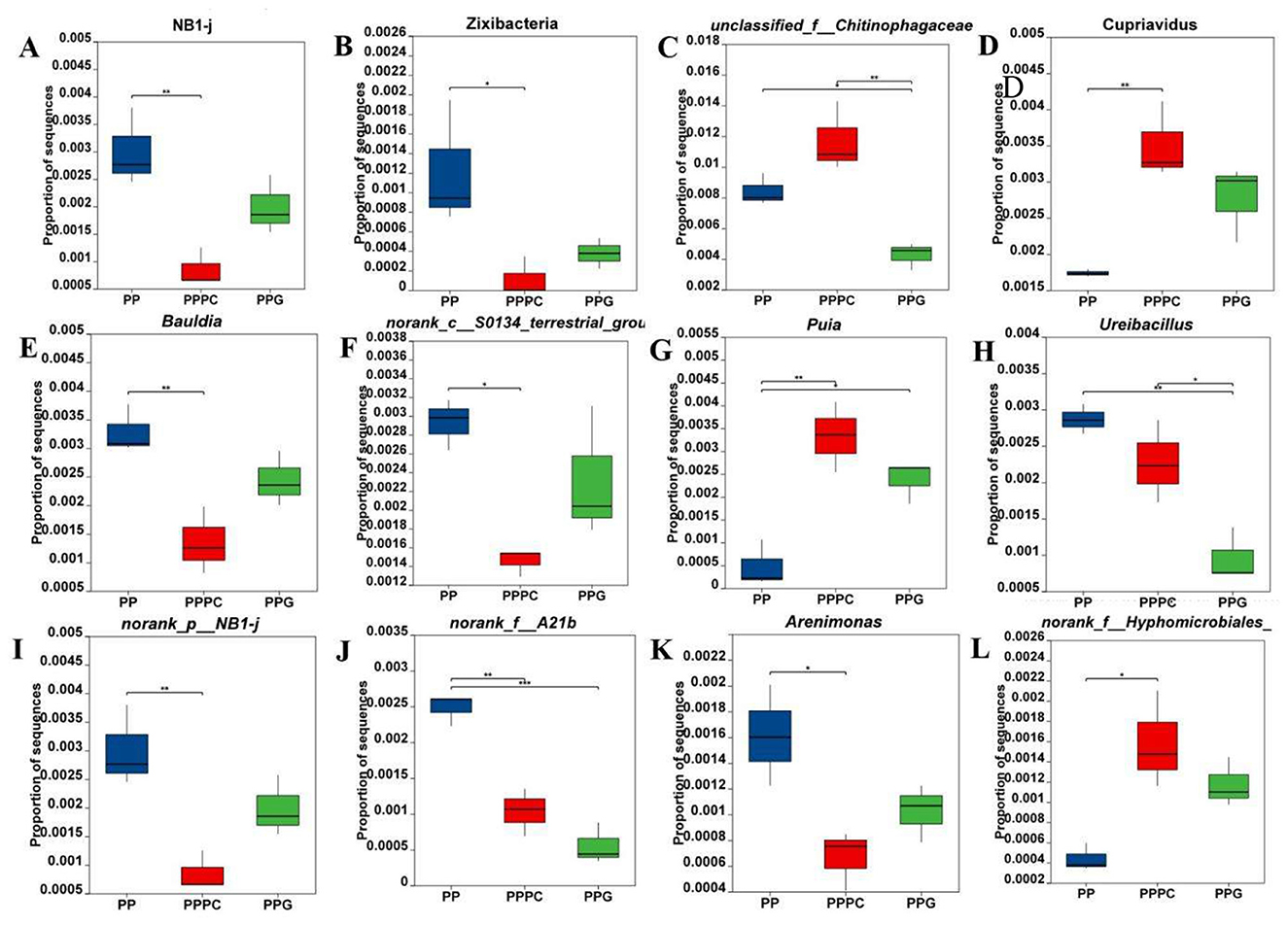
Figure 5. Changes in relative abundance of differential bacteria in rhizosphere soil of P. polyphylla under different intercropping modes (A, B) Phylum level; (C-L) Genus level. PP, Monoculture of P. polyphylla; PPPC, Intercropping of P. cyrtonema and P. polyphylla; PPG, Intercropping of P. polyphylla and G. lucidum. * indicates a significant difference between the two groups (*P < 0.05; **P < 0.01; ***P < 0.001).
3.4.4 Analysis of the relationship between soil microbial diversity and soil physicochemical properties
Redundancy analysis (RDA) of the top ten microorganisms exhibiting the highest relative abundance in rhizosphere soil demonstrated that the combined variance explained by the first two RDA axes accounted for 95.46% at the fungal community level (Figure 6A). Soil physicochemical parameters significantly influenced the relative abundances of fungal taxa such as Ascomycota, Basidiomycota, and Fungi_phy_Incertae_sedis (Figure 6C). Similarly, for bacterial communities, the first two RDA axes explained 91.91% of the total variance, and soil physicochemical factors exerted a notable influence on the relative abundance of bacterial phyla such as Pseudomonadota, Acidobacteriota, and Bacillota (Figure 6B). Both RDA and Pearson correlation analyses indicated that the relative abundances of dominant fungal and bacterial phyla were predominantly affected by soil environmental variables, with available potassium (AK) and available phosphorus (AP) contents identified as particularly influential (Figures 6B, D).
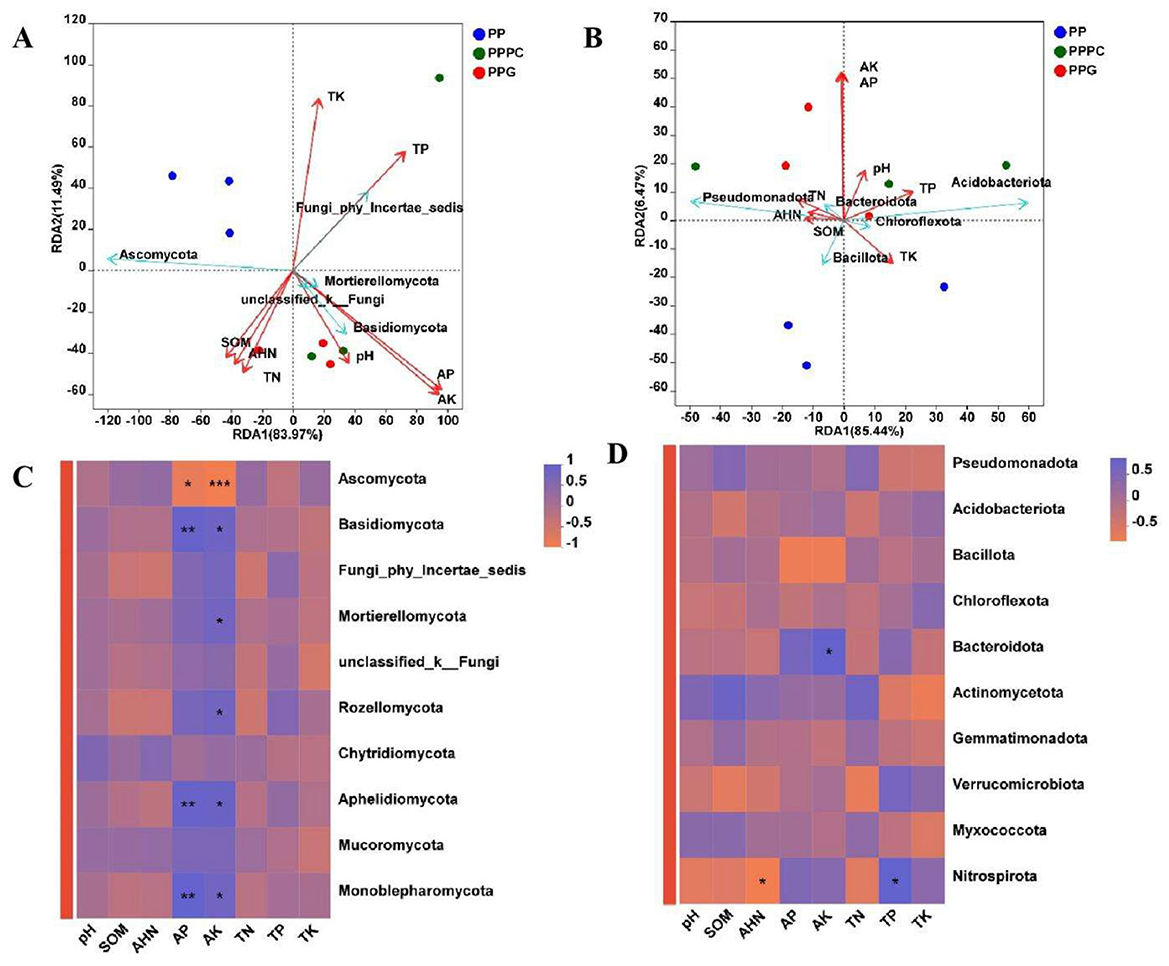
Figure 6. Analysis of the relationship between soil microbial community composition and soil physical and chemical characteristics. (A, C): Fungal phylum level; (B, D): Bacterial phylum level. PP, Monoculture of P. polyphylla; PPPC, Intercropping of P. cyrtonema and P. polyphylla; PPG, Intercropping of P. polyphylla and G. lucidum; SOM, Organic matter; AHN: Alkali-hydrolyzable nitrogen; AP, Available phosphorus; AK, Available potassium; TN, Total nitrogen; TP, Total phosphorus; TK, Total potassium. * indicates a significant difference between the two groups (*P < 0.05; **P < 0.01; ***P < 0.001).
3.5 Analysis of the relationship between soil microbial diversity and the quality of P. polyphylla across various intercropping systems
A correlation analysis was performed to examine the associations between the yield and active ingredient content of P. polyphylla under different intercropping patterns and the relative abundances of the top ten dominant fungal and bacterial phyla present in the soil. The acid-insoluble ash content of P. polyphylla demonstrated a highly significant negative correlation with the abundance of Ascomycota (R = −0.8815, P < 0.01), while showing significant positive correlations with Mortierellomycota (R = 0.7120), Rozellomycota (R = 0.6781), Aphelidiomycota (R = 0.8001), and Monoblepharomycota (R = 0.6809). Furthermore, polyphyllin VII (R = 0.6833) and total polyphyllin content (R = 0.7120) exhibited notable positive correlations with the Mortierellomycota phylum (Figure 7A). A significant positive correlation was also identified between the total polyphyllin content in P. polyphylla and the Nitrospiraota phylum (R = 0.7967). Regarding biomass, the fresh weight of the P. polyphylla rhizome was significantly positively correlated with Gemmatimonadota (R = 0.6667, P < 0.05). Conversely, acid-insoluble ash content (R = −0.7628), polyphyllin VII (R = −0.6667), and total polyphyllin content (R = −0.7120) were significantly negatively correlated with Bacillota. Additionally, polyphyllin I was strongly negatively correlated with Nitrospiraota (R = −0.8530), whereas polyphyllin II showed a strong positive correlation with Nitrospiraota (R = 0.7500) (Figure 7B).
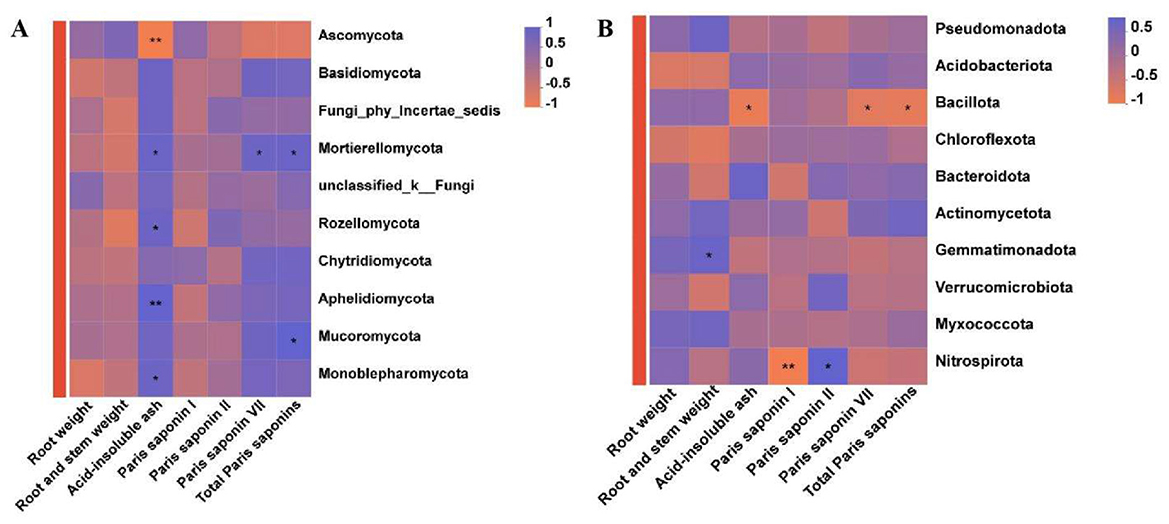
Figure 7. Correlation between the active components of P. polyphylla and the dominant soil microorganism phyla. (A) Components of P. polyphylla and dominant fungal phyla; (B) Components of P. polyphylla and dominant bacterial phyla. * indicates a significant difference between the two groups (*P < 0.05; **P < 0.01.
4 Discussion
4.1 Impact of various intercropping patterns on the quality of P. polyphylla
Different intercropping configurations influence the growth and quality of medicinal plants by modulating interspecific resource allocation and microenvironmental conditions (Machiani et al., 2018; Fallah et al., 2018; Zhang et al., 2024; Ma et al., 2017). In the present study, both the P. polyphylla–P. cyrtonema (PPPC) and P. polyphylla–G. lucidum (PPG) intercropping systems enhanced the quality of P. polyphylla, a phenomenon closely associated with interspecific niche complementarity inherent in these patterns (Wang Q. C. et al., 2024). Both P. polyphylla and P. cyrtonema exhibit shade tolerance. However, P. polyphylla demonstrates a higher potassium requirement, whereas P. cyrtonema preferentially assimilates nitrogen (Su et al., 2024). This differentiation in nutrient assimilation reduces interspecific competition, leading to a 4.6% increase in soil alkali-hydrolyzable nitrogen (AHN) under the PPPC system. This nutrient enrichment underpins the synthesis of acid-insoluble ash (linked to mineral accumulation) and polyphyllin II, whose contents increased by 350.00% and 20.00%, respectively. In the PPG system, G. lucidum, functioning as a beneficial fungus, secretes cellulase and lignin peroxidase (Zhou et al., 2018), facilitating the decomposition of insoluble organic matter into small-molecule carbon sources, which was evidenced by a 2.8% increase in soil organic matter (SOM). This process supplies energy for polyphyllin biosynthesis in P. polyphylla. Concurrently, the available phosphorus (AP) content, elevated by 26.78% relative to monoculture (PP), promotes the accumulation of polyphyllin VII and total polyphyllin by enhancing the activity of key enzymes in the mevalonate pathway, such as 3-hydroxy-3-methylglutaryl-CoA reductase (Cho et al., 2022). Consequently, polyphyllin VII and total polyphyllin contents increased by 34.16% and 30.60%, respectively, compared to PP. These species-specific regulatory mechanisms suggest that intercropping patterns can be strategically selected based on desired active compounds: PPPC is preferable for augmenting acid-insoluble ash, whereas PPG is advantageous for enhancing total polyphyllin content.
4.2 Influence of intercropping patterns on soil physicochemical properties associated with P. polyphylla
Alterations in soil nutrient content constitute a critical determinant of plant growth, and appropriate intercropping practices can modify soil physicochemical characteristics (Lan et al., 2025). This investigation suggested that soil nutrient levels under both intercropping patterns (PPPC and PPG) were significantly increased compared to monoculture (PP). Specifically, PPPC markedly improved SOM and AK, whereas PPG was most effective in enhancing AP and AK. These findings align previous report that intercropping medicinal plants beneath forest canopies improves soil physicochemical properties and fertility by establishing a synergistic “plant-microbe” nutrient transformation network (Zhang et al., 2016). The 2.82% increase in SOM observed in PPG is primarily attributable to the biological characteristics of G. lucidum, including the composition and decomposition of its fungal bag and the release of mycelial metabolites into the soil (Cen et al., 2024). As a wood-decaying fungus with potent lignocellulose-degrading capabilities, G. lucidum mycelia extend into the fungal bag and adjacent soil, secreting extracellular enzymes such as cellulase, ligninase, and hemicellulase (Ematou et al., 2025; Zhou et al., 2013). Moreover, G. lucidum promotes mineralization of native soil organic matter via the “priming effect” (Zhang et al., 2017), which indirectly enhances AP by 26.78% and AK by 50.28%. In addition, the PPPC cultivation pattern demonstrated the highest AHN content, potentially attributable to flavonoids secreted by P. cyrtonema roots that promote the proliferation of nitrogen-fixing bacteria, such as Hyphomicrobiales (Wu et al., 2025). This process increases soil nitrogen availability, thereby providing an additional nitrogen source for P. polyphylla. The enrichment of specific microbial taxa, including Saitozyma, within the rhizosphere of P. polyphylla contributes to the improvement of soil structure and the enhancement of nutrient cycling (Bastida et al., 2016), thereby fostering a more conducive growth environment for P. cyrtonema. This microbially mediated resource complementarity mechanism mitigates interspecific resource competition and facilitates mutualistic interactions between the two species (Han et al., 2025). Further investigation of this “plant–nitrogen-fixing bacteria” interaction through metagenomic sequencing is warranted to substantiate these findings.
4.3 Effects of intercropping patterns on rhizosphere soil microbial communities of P. polyphylla
Soil microbial communities are integral to nutrient cycling and plant–microbe interactions, with community structure predominantly influenced by environmental factors such as intercropping regimes and soil physicochemical properties (Li et al., 2024; Wang R. Y. et al., 2024; Liu Y. et al., 2025). This study demonstrated that intercropping systems induced more pronounced alterations in rhizosphere microbial diversity and composition than monoculture, with fungal communities exhibiting a stronger response than bacterial communities. Arbuscular mycorrhizal fungi within the Glomeromycota phylum, known for forming symbiotic associations that enhance nutrient uptake and stress tolerance (Abarca et al., 2024), showed significantly increased relative abundance under the PPPC system. Additionally, genera such as Saitozyma (Basidiomycota), Furcasterigium (Ascomycota), and Podila (Mortierellomycota), which contribute to soil organic matter decomposition and pathogen suppression (Bastida et al., 2016; Liu H. et al., 2025; Zhao et al., 2020), were enriched under PPPC. These taxa contribute to the accumulation of soil nitrogen, phosphorus, and potassium, thereby promoting healthy plant development. Within the PCG intercropping system, Trichoderma demonstrates capabilities for solubilizing phosphorus and potassium, in addition to exhibiting a synergistic interaction with nitrogen-fixing bacteria (Qin et al., 2024). Consequently, the PPPC and PCG intercropping models enhance soil nutrient availability by optimizing the functional groups of fungi.
At the bacterial genus level, PPPC intercropping significantly increased the relative abundance of Cupriavidus, norank_f_Hyphomicrobiales, and Puia. Cupriavidus, through its metabolic activities, may enhance soil phosphorus availability, indirectly facilitating plant phosphorus uptake (Wang, 2023). Hyphomicrobiales encompasses various rhizobia, including Rhizobiaceae strains capable of symbiotic nitrogen fixation with legumes (Volpiano et al., 2021), indicating that PPPC also augments soil nitrogen fixation capacity. Puia, a Gram-negative, aerobic, rod-shaped, non-motile bacterium (Lv et al., 2017), exhibited increased abundance in both PPPC and PPG systems. Furthermore, research has demonstrated that crops can modulate the rhizosphere microbial communities of adjacent plants through interspecific interactions. For instance, root exudates from potato onion (Allium cepa var. agrogatum Don.), particularly flavonoids such as taxifolin, have been shown to modify the chemical profile of tomato (Solanum lycopersicum) root exudates, thereby facilitating the recruitment of beneficial bacteria, including those belonging to the genus Bacillus (Zhou et al., 2023; Zhu et al., 2025). Similarly, root exudates from ginger, which contain compounds such as sinapyl alcohol and 6-gingerol, can induce chrysanthemum roots to secrete specific metabolites that enhance the proliferation and colonization of Burkholderia species within the chrysanthemum rhizosphere (Zhu et al., 2025). Therefore, variations in microbial community composition among intercropping patterns may be attributable to differences in root exudate profiles of P. cyrtonema, P. polyphylla, and G. lucidum, a hypothesis that could be further examined through exudate compositional analyses.
4.4 Influence of rhizosphere microbial community structure on the quality of P. polyphylla
The modulation of medicinal herb quality via intercropping fundamentally represents a cascade process involving soil nutrients, microorganisms, and secondary metabolites (Gong et al., 2021; Duan et al., 2024). Correlation analyses in this study confirmed that specific microbial taxa are closely associated with the accumulation of active compounds, underscoring the pivotal role of functional microorganisms as mediators. Central to this cascade is the capacity of microorganisms to link soil nutrient dynamics with plant secondary metabolism through their metabolic activities (Bettina et al., 2025; Bikash et al., 2018). For instance, the fungal phylum Mortierellomycota exhibited positive correlations with polyphyllin VII and total polyphyllin content in P. polyphylla, suggesting potential secretion of enzymes that facilitate polyphyllin biosynthesis. Bacterial taxa also contributed to quality regulation. The abundance of Gemmatimonadota was positively correlated with rhizome fresh weight, while the abundance of Bacillota was negatively correlated with acid-insoluble ash, polyphyllin VII, and total polyphyllin. These findings imply that intercropping not only enriches beneficial microbial populations but may also suppress potentially deleterious groups, thereby maintaining a balanced quality formation process. This research extends current understanding of microbial regulation of medicinal plant quality (Wang R. Y. et al., 2024) and provides a foundation for optimizing intercropping strategies through targeted microbial management. Various intercropping configurations have been shown to significantly elevate the abundance of Trichoderma populations. The proliferation of this microbial group has been demonstrated to reduce the incidence of crop disease (Xu et al., 2022) while concurrently enhancing crop yield substantially (Szczech et al., 2024). Moreover, during crop cultivation, the deliberate inoculation of Trichoderma not only achieves these benefits but also markedly increases the accumulation of flavonoids and terpenoids within the crops (Kulbat-Warycha et al., 2024). These findings further corroborate the role of Trichoderma in modulating plant secondary metabolic pathways and promoting the biosynthesis of secondary metabolites (Jurić et al., 2020). In the present investigation, the PPG intercropping pattern was found to enrich Trichoderma populations and augment the accumulation of polyphyllin I and VII in P. polyphylla, suggesting that Trichoderma may facilitate polyphyllin biosynthesis in this species; however, additional experimental validation is warranted in future studies.
5 Conclusions
The results indicate that the PPPC intercropping pattern (P. polyphylla—P. cyrtonema) enriches the phylum Glomeromycota and the genera Saitozyma and Cupriavidus, thereby enhancing soil nitrogen and phosphorus cycling and promoting the accumulation of acid-insoluble ash and polyphyllin II. This pattern is particularly suitable for cultivation scenarios aiming to balance mineral content with specific saponin profiles. Conversely, the PPG intercropping pattern (P. polyphylla—G. lucidum) leverages the mycelial network of G. lucidum to increase available potassium, activate fungal taxa such as Trichoderma, and significantly elevate levels of polyphyllin VII and total saponins through synergistic interactions involving the Chaetomiaceae family and associated metabolic pathways. This configuration is optimal for cultivation objectives prioritizing total saponin content. Collectively, this study elucidates the directional relationships between rhizosphere microbial communities and target phytochemical components under diverse intercropping regimes of medicinal plants, thereby providing a theoretical framework to address continuous cropping challenges in the cultivation of rare medicinal species. Practically, intercropping patterns can be selected based on desired phytochemical targets, or their efficacy can be enhanced through the artificial inoculation of functional microorganisms.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/, accession number PRJNA1347261.
Author contributions
HS: Writing – original draft. PL: Investigation, Writing – review & editing. HC: Writing – review & editing, Resources. FL: Methodology, Writing – review & editing. MZ: Investigation, Writing – review & editing. YZ: Project administration, Writing – review & editing. WY: Resources, Writing – review & editing. FM: Data curation, Writing – review & editing. YuZ: Supervision, Writing – review & editing. YF: Writing – review & editing, Funding acquisition. YaZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Outstanding Scientific and Technological Innovation Talent Program of Fujian Academy of Agricultural Sciences (Grant No.: YCZX202501), Major Science and Technology Program of Fujian Province (Grant No.: 2022NZ029017), and Young Talent Program of Fujian Academy of Agricultural Sciences (Grant No.: YC2021002).
Acknowledgments
We thank Shanghai Majorbio Biopharmaceutical Technology Co., Ltd, for their support during detection, and quantitative analysis of metabolites.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abarca, C., Bidondo, L. F., Bompadre, J., and Velázquez, M. S. (2024). Arbuscularmycorrhizal fungi in tomato tolerance to pathogens and nematodes: a comprehensive review. Sci. Hortic. 329:112969. doi: 10.1016/j.scienta.2024.112969
Bao, S. D. (2000). Soil and Agricultural Chemistry Analysis, 3rd Edn. Beijing:China Agriculture Press.
Bastida, F., Torres, I. F., Moreno, J. L., Baldrian, P., Ondoño, S., Ruiz-Navarro, A., et al. (2016). The active microbial diversity drives ecosystem multifunctionality and is physiologically related to carbon availability in Mediterranean semi-arid soils. Mol. Ecol. 25, 4660–4467. doi: 10.1111/mec.13783
Bettina, B.e, M., Marco, A. N., and Mariangela, H. (2025). Microbial secondary metabolites and their use in achieving sustainable agriculture: present achievements and future challenges. Agronomy. 15, 1350–1350. doi: 10.3390/agronomy15061350
Bikash, B., Amir, A., and Mikko, M. K. (2018). Activation of microbial secondary metabolic pathways: avenues and challenges. Synth. Syst. Biotechnol. 3, 163–178. doi: 10.1016/j.synbio.2018.09.001
Cen, Q., Fan, J., Zhang, R., Chen, H. Y., Hui, F. Y., Li, J. M., et al. (2024). Impact of Ganoderma lucidum fermentation on the nutritional composition, structural characterization, metabolites, and antioxidant activity of Soybean, sweet potato and Zanthoxylum pericarpium residues. Food Chem. X. 21:101078. doi: 10.1016/j.fochx.2023.101078
Chen, Y. L., Guo, Y. Q., Han, S. J., Zou, C. J., Zhou, Y. M., and Cheng, G. L. (2002). Effect of root derived organic acids on the activation of nutrients in the rhizosphere soil. J. For. Res.13, 115–118. doi: 10.1007/BF02857233
Cho, S. H., Tóth, K., Kim, D., Vo, P. H., Lin, C. H., Handakumbura, P. P., et al. (2022). Activation of the plant mevalonate pathway by extracellular ATP. Nat. Commun. 13, 450–450. doi: 10.1038/s41467-022-28150-w
Duan, W. Y., Chen, X. L., Ding, Y., Mao, X. Y., Song, Z., et al. (2024). Intricate microbe-plant-metabolic remodeling mediated by intercropping enhances the quality of Panax quinquefolius L. Physiol. Plant. 176:e14499. doi: 10.1111/ppl.14499
Ematou, N. L. N., Njouonkou, A. L., and Moundipa, F. P. (2025). Assessment of extracellular enzymes in mycelial culture of some fungi from the Western highlands of Cameroon. J. Mater. Environ. Sci. 16, 328–340. doi: 10.1016/j.funbio.2025.08.012
Fallah, S., Rostaei, M., Lorigooini, Z., and Surki, A. A. (2018). Chemical compositions of essential oil and antioxidant activity of dragonhead (Dracocephalum moldavica) in sole crop and dragonhead-soybean (Glycine max) intercropping system under organic manure and chemical fertilizers. Ind. Crops Prod. 115, 158–165. doi: 10.1016/j.indcrop.2018.02.003
Fang, L. X., Li, J. C., Chen, X. X., and Xu, X. Q. (2024). How lignocellulose degradation can promote the quality and function of dietary fiber from bamboo shoot residue by Inonotus obliquus fermentation. Food Chem. 451:139479. doi: 10.1016/j.foodchem.2024.139479
Feng, S. F., Wu, L. J., Guan, Y. M., Wang, Z. Q., and Wang, Y. P. (2008). Inhibition effects of volatile oil of asarum on 4 kinds of pathogens of ginseng using in vitro tests. Special Wild Econ. Anim. Plant Res. 37–39. doi: 10.16720/j.cnki.tcyj.2008.03.003
Gong, X. Y., Shi, J. B., Zhou, X. G., Yuan, T., Gao, D. M., and Wu, F. Z. (2021). Crop rotation with cress increases cucumber yields by regulating the composition of the rhizosphere soil microbial community. Front. Microbiol. 12:631882. doi: 10.3389/fmicb.2021.631882
Han, M., Zhang, Z., Yang, H., Du, J., Wu, X., and Fu, Y. (2025). The effect of intercropping with eucommia ulmoides on the growth and quality of abelmoschus manihot and its rhizosphere microbial community. Agronomy 15:863. doi: 10.3390/agronomy15040863
Jurić, S., Sopko, S. K., Król-Kilińska, Ż., Žutić, I., Uher, S. F., Ðermić, E., et al. (2020). The enhancement of plant secondary metabolites content in Lactuca sativa L. by encapsulated bioactive agents. Sci. Rep. 10:3737. doi: 10.1038/s41598-020-60690-3
Kulbat-Warycha, K., Nawrocka, J., Kozłowska, L., and Żyżelewicz, D. (2024). Effect of light conditions, trichoderma fungi and food polymers on growth and profile of biologically active compounds in Thymus vulgaris and Thymus serpyllum. Int. J. Mol. Sci.. 25:4846. doi: 10.3390/ijms25094846
Lan, X., Liang, Z. H., Yang, H. X., Li, H. R., Ruan, L. X., Wei, W. L., et al. (2025). Effects of sugarcane and Platostoma palustre intercropping on soil physicochemical properties and crop yield. Crops. 1–9. [Epub ahead of print].
Li, B., Li, Y. Y., Wu, H. M., Zhang, F. F., Li, C. J., Li, X. X., et al. (2016). Root exudates drive interspecific facilitation by enhancing nodulation and N2 fixation. Proc. Natl. Acad. Sci. USA. 113, 6496–6501. doi: 10.1073/pnas.1523580113
Li, F. Q., Liu, S. Z., Luo, G. S., Zou, Y. L., Huang, W., and Zeng, M. S. (2025). Analysis of bacterial community structure and diversity in rhizosphere soil of Monochasma savatieri in different habitats. Sci. Silvae Sin. 61, 47–56. doi: 10.11707/j.1001-7488.LYKX20240002
Li, M. L., Chen, X. B., Cui, Y. S., Yue, X., Qi, L. F., Huang, Y. L., et al. (2025). Mechanism of soil microbial community degradation under long-term tomato monoculture in greenhouse. Front. Microbiol. 16:1587397. doi: 10.3389/fmicb.2025.1587397
Li, Y., Ding, Z., Xu, T., Wang, Y. L., Wu, Q. L., Song, T. J., et al. (2024). Synthetic consortia of four strains promote Schisandra chinensis growth by regulating soil microbial community and improving soil fertility. Planta. 259, 135–135. doi: 10.1007/s00425-024-04410-5
Liu, H., Ma, Z. H., Liu, W. F., Wan, M. H., Ma, F. L., Wu, N., et al. (2025). Effects of different tillage practices with organic fertilizers on rhizosphere soil microbial communities of maize in saline - alkali soils. Chin. J. Eco-Agric. 33, 25–39. doi: 10.12357/cjsa.20240308
Liu, L. L., Ding, M. Y., Xie, Q., Luo, W. B., Chen, Q. X., and Su, H. L. (2025). Genetic diversity analysis of Paris polyphylla Sm. and construction of primary core germplasm. Fujian J. Agric. Sci. 40, 234–244. doi: 10.19303/j.issn.1008-0384.2025.03.003
Liu, Y., Fan, Y., Kuzyakov, Y., Dai, J., Zhang, C., and Li, C. (2025). Effects of maize/soybean intercropping on nitrogen mineralization and fungal communities in soil. Plant Soil 1-15. doi: 10.1007/s11104-025-07824-6
Lu, Y. P., Gao, Z., Zhu, Y. L., Mao, J. P., Yao, D. L., and Wang, X. L. (2025). Construction and evaluation of Polygonatum cyrtonema Hua intercropping basedon the growth and physiological adaptability. Plant Sci. J. 43, 253–264. doi: 10.11913/PSJ.2095-0837.24100
Lv, Y. Y., Gao, Z. H., Xia, F., Chen, M. H., and Qiu, L. H. (2017). Puia dinghuensis gen. nov., sp. nov., isolated from monsoon evergreen broad-leaved forest soil. Int. J. Syst. Evol. Microbiol. 67:46. doi: 10.1099/ijsem.0.002346
Ma, Y. H., Fu, S. L., Zhang, X. P., Zhao, K., and Chen, H. Y. H. (2017). Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Agric. Ecosyst. Environ. Appl. Soil Ecol. 119, 171–178. doi: 10.1016/j.apsoil.2017.06.028
Machiani, M. A., Javanmard, A., Morshedloo, M. R., and Filippo, M. (2018). Evaluation of yield, essential oil content and compositions of peppermint (Mentha piperita L.) intercropped with faba bean (Vicia faba L.). J. Clean. Prod.. 171, 529–537. doi: 10.1016/j.jclepro.2017.10.062
Qin, L., Gao, Y., Wang, L., Ran, J., Ou, X., Wang, Y., et al. (2024). Trichoderma consortium compost corncob promotes the growth of Pseudostellaria heterophylla and alleviates its diseases incidence via reshaping the soil microbiome and improving the soil nutrients under continuous monocropping conditions. Ind. Crops Prod. 216:118800. doi: 10.1016/j.indcrop.2024.118800
Rao, Z. W., Sun, Y. Y., Guo, J. J., Jin, H., Yang, Z. P., and Zhang, Q. (2025). A review on the continuous cropping obstacles of rhizomatous medicinal plants. Soil Fertil. Sci. China. 4, 258–272. doi: 10.11838/sfsc.1673-6257.24469
Su, H. L., Zheng, M. X., Chen, H., Zhu, Y. M., and Zhu, Y. J. (2024). Optimum nitrogen, phosphorus and potassium combination increasesthe rhizome yield and saponin content of Paris polyphylla. J. Plant Nutr. Fertil. 30, 128–136. doi: 10.11674/zwyf.2023279
Szczech, M., Kowalska, B., Wurm, F. R., Ptaszek, M., Boncela, A. J., Trzciński, P., et al. (2024). The effects of tomato intercropping with medicinal aromatic plants combined with trichoderma applications in organic cultivation. Agronomy 14, 2572–2572. doi: 10.3390/agronomy14112572
Sant'A F, H., Ambrosini, A., De, S. J. J. F. B., Beneduzi, A., Whitman, W. B., et al. (2021). Genomic metrics applied to rhizobiales (Hyphomicrobiales): species reclassification, identification of unauthentic genomes and false type strains. Front. Microbiol. 12:614957. doi: 10.3389/fmicb.2021.614957
Wang, H. X. (2023). Effect of organic fertilizer on soil, leaf nutrition, fruit quality and microbial community in citrus orchards. (MS Thesis of Southwest University, Chongqing). 1–82. doi: 10.27684/d.cnki.gxndx.2023.003493
Wang, Q. C., Peng, X. J., Yuan, Y. X., Zhou, X. D., Huang, J. Q., and Wang, H. N. (2024). The effect of Torreya grandis inter-cropping with Polygonatum sibiricum on soil microbial community. Front. Microbiol. 15:1487619. doi: 10.3389/fmicb.2024.1487619
Wang, R. Y., Liu, C. Y., Bie, X. S., Dai, Y., Feng, X., Wang, R., et al. (2024). Pecan-medicinal crops intercropping improved soil fertility and promoted interactions between soil microorganisms and metabolites. Chem. Biol. Technol. Agric. 11,162–162. doi: 10.1186/s40538-024-00693-8
Wei, F. H., Zhang, J. X., Zhou, D. P., Xu, Y. H., Gong, J. Z., and Zhao, L. (2024). Effect of ginseng-asarum strip cropping on the soil microhabitat of ginseng rhizosphere. SSRN. 35:ssrn4789910. doi: 10.2139/ssrn.4789910
Wu, J., Liu, S., Zhang, H., Chen, S., Si, J., Liu, L., et al. (2025). Flavones enrich rhizosphere Pseudomonas to enhance nitrogen utilization and secondary root growth in Populus. Nat. Commun. 16:1461. doi: 10.1038/s41467-025-56226-w
Wu, Z., Zhang, J., Xu, F. R., Wang, Y. Z., and Zhang, J. Y. (2017). Rapid and simple determination of polyphyllin I, II, VI, and VII in different harvest times of cultivated Paris polyphylla Smith var. yunnanensis (Franch.) Hand.-Mazz by UPLC-MS/MS and FT-IR. J. Nat. Med. 71, 139–147. doi: 10.1007/s11418-016-1043-8
Xian, K., Su, J., Fu, C., He, W., Liu, B., Xie, D., et al. (2022). Microbial diversity in rhizosphere soil of Paris polyphylla var. chinensis in different growth years. in Chinese. Guihaia 42, 2087–2098. guihaia.gxzw202103004
Xu, H., Yan, L., Zhang, M., Chang, X., Zhu, D., Wei, D., et al. (2022). Changes in the density and composition of rhizosphere pathogenic fusarium and beneficial trichoderma contributing to reduced root rot of intercropped soybean. Pathogens 11, 478–478. doi: 10.3390/pathogens11040478
Yang, J. M., Wu, Q. H., Wang, Y. T., Chen, X. Y., Gao, W., Zhao, Y., et al. (2023). Suppression of banana fusarium wilt disease with soil microbial mechanisms via pineapple rotation and residue amendment. Agronomy 13, 377–377. doi: 10.3390/agronomy13020377
Zhang, C., Liu, G..B., Xue, S., and Wang, G. L. (2016). Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 97, 40–49. doi: 10.1016/j.soilbio.2016.02.013
Zhang, R., Li, X. C., Qu, J. X., Zhang, D. D., Cao, L. X., Qin, X. M., et al. (2024). Intercropping with maize and sorghum-induced saikosaponin accumulation in Bupleurum chinense DC. by liquid chromatography-mass spectrometry-based metabolomics. J. Mass Spectrom. 59, e5035–e5035. doi: 10.1002/jms.5035
Zhang, X. W., Han, X. Z., Yu, W. T., Wang, P., and Cheng, W. X. (2017). Priming effects on labile and stable soil organic carbon decomposition: pulse dynamics over two years. PLoS ONE 12:e0184978. doi: 10.1371/journal.pone.0184978
Zhao, X. H., Chai, S. S., Zhang, M. M., Fan, Y. C., Mao, Y. F., Mao, Z. Q., et al. (2020). Effects of shell powder on microbial diversity in acidified soil and growth of malus hupehensis var. mengshanensis seedlings. Sci. Silvae Sin. 56, 153–163. doi: 10.11707/j.1001-7488.20200917
Zhou, S., Zhang, J. S., Ma, F. Y., Tang, C. H., Tang, Q. J., and Zhang, X. Y. (2018). Investigation of lignocellulolytic enzymes during different growth phases of Ganoderma lucidum strain G0119 using genomic, transcriptomic and secretomic analyses. PLoS ONE 13:e0198404. doi: 10.1371/journal.pone.0198404
Zhou, X., Zhang, J., Khashi, U. R. M., Gao, D., Wei, Z., Wu, F., et al. (2023). Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant. 16, 849–864. doi: 10.1016/j.molp.2023.03.009
Zhou, X. W., Cong, W. R., Su, K. Q., and Zhang, Y. M. (2013). Ligninolytic enzymes from Ganoderma spp: current status and potential applications. Crit. Rev. Microbiol. 39, 416–426. doi: 10.3109/1040841X.2012.722606
Keywords: Paris polyphylla, intercropping, rhizospheric microorganism, soil physicochemical properties, polyphyllin
Citation: Su H, Liao P, Chen H, Lin F, Zheng M, Niu Y, Ye W, Mao F, Zhu Y, Fang Y and Zhu Y (2025) The influence of intercropping Paris polyphylla with Polygonatum cyrtonema or Ganoderma lucidum on rhizosphere soil microbial community structure and quality of Paris polyphylla. Front. Microbiol. 16:1697736. doi: 10.3389/fmicb.2025.1697736
Received: 02 September 2025; Accepted: 15 October 2025;
Published: 06 November 2025.
Edited by:
Mir Muhammad Nizamani, Shantou University, ChinaReviewed by:
Waqar Islam, Xinjiang Institute of Ecology and Geography, ChinaRubab Sarfraz, Gyeongsang National University, Republic of Korea
Copyright © 2025 Su, Liao, Chen, Lin, Zheng, Niu, Ye, Mao, Zhu, Fang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanghui Fang, MTg4MDU5OTA1NzNAMTYzLmNvbQ==; Yanming Zhu, bXVtdXp5bUAxNjMuY29t
 Hailan Su1
Hailan Su1 Wei Ye
Wei Ye