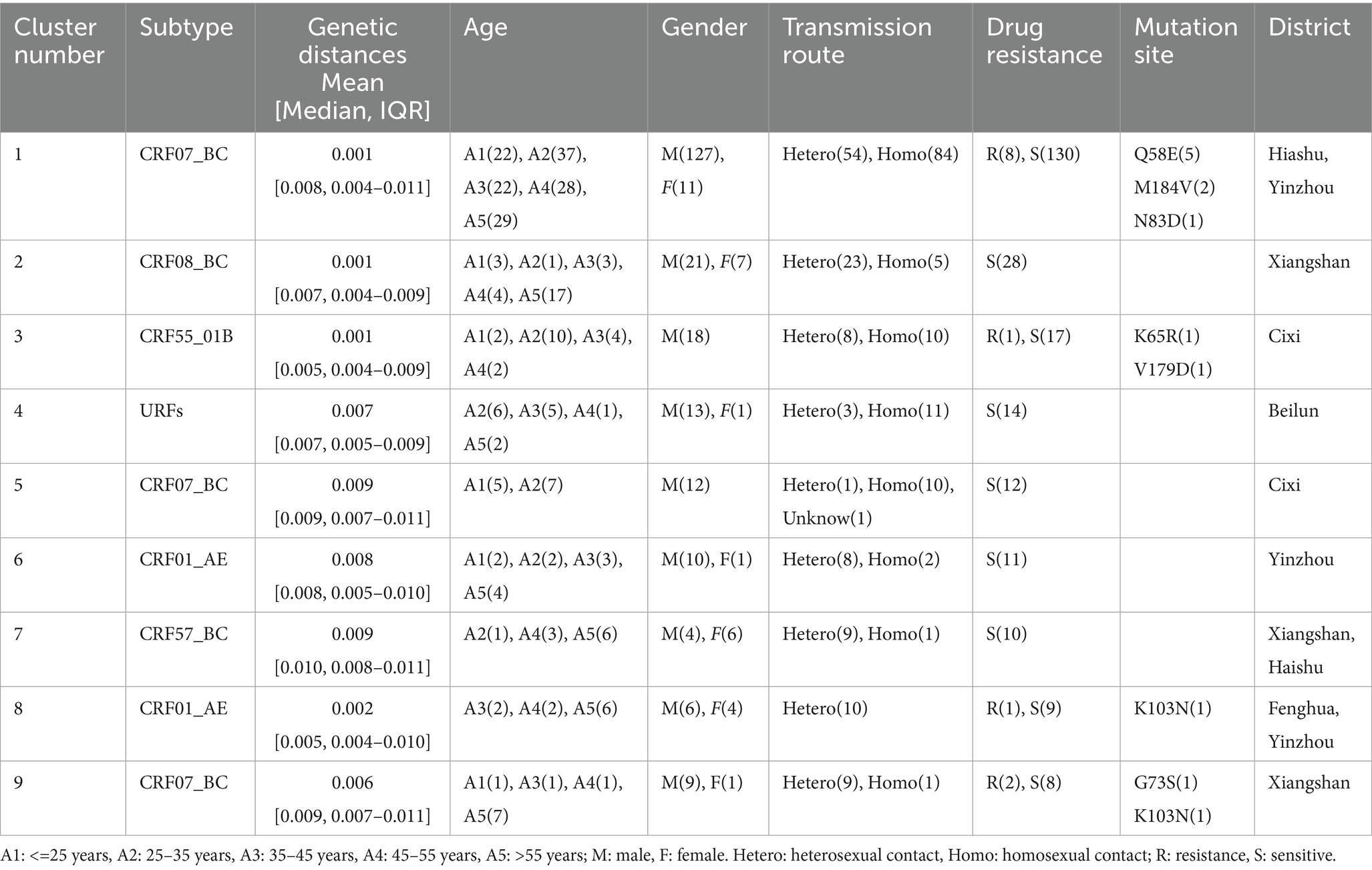
- 1Department of Data Center, Yinzhou District Center for Disease Control and Prevention, Ningbo, Zhejiang, China
- 2Ningbo Center for Disease Control and Prevention, Ningbo, Zhejiang, China
- 3Nanjing Jiangning Hospital, Nanjing, Jiangsu, China
Introduction: Understanding molecular transmission patterns is critical for HIV prevention designed with key populations. This study aimed to characterize the molecular epidemiology, transmission networks, and underlying factors associated with HIV-1 transmission in Ningbo during 2018–2022.
Methods: We analyzed data from 1,409 newly diagnosed people living with HIV who had successful genotyping. A maximum likelihood phylogenetic tree was constructed, and transmission clusters were identified using 1.3% distance and 0.9 bootstrap values. Multivariate logistic regression was applied to identify factors associated with clustered, large clusters (≥10 nodes) and fast-growing clusters.
Results: Molecular analysis revealed 11 distinct HIV-1 subtypes and some unique recombinant forms (URFs), with CRF07_BC (41.6%) and CRF01_AE (33.2%) as the most prevalent. CRF07_BC consistently tended to form larger, more densely connected clusters, whereas CRF01_AE networks primarily exhibited sparse, fragmented distributions. Molecular transmission network analysis identified 9 large clusters and 12 fast-growing clusters. HIV-1 subtypes were associated with the large clusters and fast-growing clusters. CRF07_BC formed larger clusters (aOR = 7.80, 95%CI: 4.70–13.49) and fast-growing clusters (aOR = 6.02, 95%CI: 3.80–9.78) compared to CRF01_AE. Temporally, the molecular transmission networks (MTNs) expanded rapidly in 2020–2021.
Conclusion: This study elucidates the MTNs of HIV-1 in Ningbo, highlighting the role of subtype diversity and demographic traits in shaping transmission networks. Continuous monitoring of HIV-1 molecular subtypes among key populations may serve as feasible and focused prevention strategies to curb HIV transmission.
1 Introduction
Despite significant global efforts to control the transmission of HIV-1, Acquired Immune Deficiency Syndrome (AIDS) remains a major global public health issue. At the end of 2024, an estimated 40.8 million (37.0–45.6 million) people were living with HIV globally, including 1.4 million (1.1–1.8 million) children (0–14 years old) and 39.4 million (35.7–44.0 million) adults (15 + years old) (UNAIDS, Global HIV, 2025). In China, as of Dec. 31, 2024, there were 1,355,017 reported cases of people living with HIV, with 491,437 reported deaths (National Center for STD/AIDS Control, Chinese Center for Disease Control and Prevention, 2025). Over the past decade, the incidence of AIDS has shown a sustained upward trend across most regions of China, characterized by continuous spread from south to north and west to east, coupled with increasing disease intensity and a growing burden of illness (Wang et al., 2019). From 1990 to 2021, the incidence, mortality, and disability-adjusted life years (DALYs) of HIV increased in the general population, with age-standardized incidence, mortality, and DALY rates rising at average annual rates of 0.051, 0.056, and 2.629, respectively (He et al., 2025). These trends underscore the critical need for enhanced HIV prevention and control strategies to curb transmission, reduce incidence, and alleviate the escalating disease burden on affected populations.
On June 8, 2021, The Joint United Nations Program on HIV/AIDS (UNAIDS) put forward the “95–95-95 targets” (95% of all PLWH knowing their status, 95% of those diagnosed accessing treatment, and 95% of those on treatment achieving viral suppression) to be achieved by 2025 (United Nations General Assembly, 2021). The “95–95-95” targets are not only the global action plan for the response to HIV but also the core indicators for measuring progress. Their significance lies in transforming AIDS from an “incurable epidemic” into a “controllable chronic disease” through systematic and multi-level prevention and control measures, and ultimately achieving the vision of ending the AIDS epidemic. Following this, WHO has rolled out a series of action plans and guidelines to advance these goals (World Health Organization, 2022). Globally, as of 2024, 87% (69–98%) of all PLWH knew their HIV status. Among those aware of their status, 89% (71–98%) were accessing treatment, and 94% (75–98%) of those on treatment achieved viral suppression (UNAIDS, Global HIV, 2025). In China, since implementing the “treat-all” strategy (initiating antiretroviral therapy (ART) regardless of CD4 cell count) in 2016, ART coverage has further improved (Bai et al., 2020). By the end of 2022, China’s progress toward the “95-95-95 targets” stood at 84-93-97% (Ye et al., 2024). While significant progress has been made in scaling up treatment coverage and improving treatment outcomes, a substantial gap persists in case detection compared to the global targets. Addressing this gap remains a critical challenge.
Molecular transmission networks (MTNs), constructed from HIV genetic sequence data, have emerged as critical tools for dissecting HIV-1 transmission dynamics. Initially formalized as a prevention strategy by the U. S. CDC in 2018 (Oster et al., 2018), MTNs were firstly adapted into China’s guidelines for monitoring and intervention by the National Center for AIDS/STD Control and Prevention in 2019 (China CDC, 2019). By reconstructing evolutionary and transmission-related associations among viral sequences, MTNs facilitate the identification of HIV transmission clusters and the delineation of ancestral-contemporary infection relationships. Their applications have expanded beyond fundamental research, now encompassing early case detection of HIV infections, long-term surveillance of HIV drug resistance, and the design of precision-focused intervention strategies (Zhao et al., 2022; Han et al., 2020; Liu et al., 2020). Currently, MTNs play a pivotal role in the operational control of HIV epidemics: they assist in determining the timing and geographical locations of new HIV infections, quantifying HIV transmission velocity, and evaluating the efficacy of HIV prevention interventions. By integrating genetic sequence analysis with real-world patterns of HIV spread, MTNs provide evidence-based and actionable insights to inform strategies aimed at curbing the transmission of HIV.
Zhejiang Province, home to a population exceeding 60 million, reported 4,279 newly diagnosed HIV infections in 2022 alone (He et al., 2025). Epidemiological data from 2018 indicate an HIV incidence rate of 1.67 per 10,000 population and an HIV testing positivity rate of 45.1 per 10,000 population in the province (Chen et al., 2021). These figures underscore the persistent challenges in controlling HIV transmission in Zhejiang, particularly given its status as a major economic hub with substantial population mobility. Zhejiang Province has conducted a series of studies leveraging MTNs to investigate multiple dimensions of HIV transmission, including network characteristics across distinct populations and regions, and the evaluation of HIV intervention efficacy. These research efforts have yielded evidence-based contributions to the regional control of HIV transmission (Chen et al., 2021; Dai et al., 2024; Chen et al., 2024). Ningbo, a coastal city in eastern Zhejiang Province, possesses the longest coastline within the province and is home to the Ningbo-Zhoushan Port—one of the world’s largest cargo throughput ports, which has maintained this leading position for 16 consecutive years. With a permanent population of approximately 10 million (accounting for one-sixth of Zhejiang’s total population), Ningbo also grapples with a distinct HIV epidemic: the city recorded an HIV incidence rate of 1.24 per 10,000 population in 2018 (Chen et al., 2021). In our previous work, we performed an analysis of the HIV molecular transmission network among men who have sex with men (MSM) in Ningbo and further explored drug resistance-associated HIV transmission patterns through complementary network-based studies (Hong et al., 2018; Hong et al., 2023; Shi et al., 2024). Building on these foundational investigations, the present study aims to comprehensively characterize the HIV molecular transmission network in Ningbo spanning the period 2018–2022. A specific focus will be placed on analyzing the influencing factors underlying the formation of large-scale transmission networks and the expansion of rapidly growing networks in this region. Findings from this work are expected to provide actionable insights for designing focused HIV intervention strategies.
2 Method
2.1 Study population and laboratory tests
This study enrolled all individuals aged ≥18 years who were newly diagnosed with HIV-1 infection in Ningbo from January 2018 to December 2022 and had not initiated antiretroviral therapy (ART). Written informed consent was obtained from all participants prior to study inclusion. Blood samples were processed following standardized protocols, and the partial pol gene segment (HXB2: 2253–3,283) was sequenced using a previously validated protocol (Hong et al., 2023). Only sequences exceeding 1,000 base pairs in length were included for subsequent phylogenetic and molecular transmission network analyses.
2.2 Sequences analysis
Raw sequence reads were assembled and aligned using ChromasPro 1.6 (Technelysium Pty Ltd.), and the Gene Cutter online tool.1 Reads with <1,000 nucleotides in length or >1.5% ambiguous bases were excluded. Duplicate sequences were identified and removed using the online tool ElimDupes.2 To facilitate subtype classification and phylogenetic analysis, all remaining sequences were submitted to the LANL HIV Database (see text footnote 1, respectively). For each query sequence, the top 10 genetically similar reference sequences were downloaded from the database. These reference sequences, along with the query sequences, were then used to construct maximum likelihood (ML) phylogenetic trees using FastTree v2.1.10 under the GTR + G + I nucleotide substitution model. Local support values for clades were calculated via the Shimodaira–Hasegawa approximate likelihood ratio test (SH-aLRT) to assess phylogenetic robustness. The reference sequences cited in this study are provided in Supplementary Table S1.
Drug-resistance mutations were identified and scored using the Stanford HIVdb Program,3 following the 2009 WHO Surveillance Drug Resistance Mutations (SDRMs) list (Bennett et al., 2009). The database automatically assigns a resistance score to each detected mutation based on its impact on drug susceptibility, with scores stratified as: 0–9 (sensitive), 10–14 (potential low-level resistance), 15–29 (low-level resistance), 30–59 (moderate resistance), and ≥60 (high-level resistance). For each sample, the highest score across all detected mutations was selected as its representative resistance level. Samples with a total score >15 were classified as resistant, whereas those with scores ≤15 were deemed sensitive.
2.3 HIV-1 transmission network analysis
Potential transmission clusters were extracted from the phylogenetic tree using Cluster Picker v1.2.5, with inclusion criteria of bootstrap support values ≥90% and maximum pairwise genetic distance ≤1.3% nucleotide substitutions per site (Chen et al., 2024; Hong et al., 2023), detailed information is provided in Supplementary Figure S1. For all extracted sequences, Tamura-Nei 93 pairwise genetic distances were calculated using HyPhy 2.2.4, and clusters were required to contain at least two sequences. The transmission network was visualized using Cytoscape v3.7.0, where edges represent potential transmission relationships between connected subjects, and network degree (the number of links per sequence node) was used to quantify transmission propensity—higher degree values indicating a greater likelihood of viral transmission. To guide HIV intervention strategies, we further characterized cluster size and growth dynamics: large clusters were defined as those containing ≥10 sequences, small clusters were defined as those containing 2 to 9 sequences, while fast-growing clusters were defined as exhibiting a ≥ 5-sequence increase in node count within a 1-year sequence collection period (Yin et al., 2021; Wertheim et al., 2018; Shi et al., 2024). The baseline transmission network was constructed using samples collected during the first 2 years of the study.
2.4 Statistical analysis
Statistical analyses were conducted using R version 4.4.2. Continuous variables with non-normal distributions were summarized using descriptive statistics, including interquartile ranges (IQR) and medians, while categorical variables were described with frequencies and percentages. To identify factors associated with transmission within molecular clusters, large molecular clusters, and fast-growing clusters, univariate and multivariate logistic regression models were employed. Variables showing significance in univariate analysis (p < 0.2) were included in the multivariate model. All statistical tests were two-tailed, with a significance level set at p < 0.05.
3 Result
3.1 Study population
Between January 2018 and December 2022, we enrolled 1,518 individuals newly diagnosed with HIV-1 infection. Of these, 1,409 (92.8%) underwent successful HIV-1 genotyping and were included in subsequent MTNs analyses. Demographic characteristics revealed male predominance (85.0%), a median age of 40.6 years (interquartile range [IQR]: 28.2–53.5 years; mean ± SD: 41.9 ± 15.2 years), and 95.7% self-identifying as Han Chinese. Marital status distribution showed 42.7% married and 37.4% single. The highest age proportion was in the 25–35 group (25.9%), followed by individuals aged >55 years (22.4%). Educational attainment was low, with only 23.0% holding a college degree or higher. Occupational distribution indicated service-sector employment as the primary occupation (43.8%). Transmission routes included heterosexual contact (53.7%), exceeding homosexual contact (45.9%). Discovery pathways were dominated by general outpatient clinics (22.0%), preoperative examinations (20.9%), and counseling/testing services (20.3%). At diagnosis, 37.8% of individuals had CD4 counts <200 cells/μL. Geographic distribution highlighted local residency (72.3%), with Ningbo’s Yinzhou (20.6%) and Haishu (19.0%) districts accounting for the highest proportions. Subtype distribution exhibited significant regional variation (p < 0.001; Supplementary Table S2).
3.2 Molecular epidemiology and drug resistance characteristics
We employed a 1,030-bp fragment of the partial pol gene to construct a ML phylogenetic tree (Supplementary Figure S2). A total of 12 distinct HIV-1 genotypes were identified, with CRF07_BC (41.6%) and CRF01_AE (33.2%) being the predominant subtypes. The remaining genotypes included CRF08_BC (6.7%), CRF55_01B (5.7%), URF (4.6%), CRF85_BC (2.4%), B (2.3%), CRF57_BC (1.5%), C (0.8%), CRF59_01B (0.4%), CRF67_01B (0.4%), and CRF68_01B (0.2%).
According to the 2009 WHO SDRMs list, 7.9% (n = 111/1,409) of sequences exhibited SDRMs, including 14 NRTI-associated mutations, 15 NNRTI-associated mutations, and 2 PI-associated mutations (Supplementary Figure S3A). Notably, 17 antiretroviral drugs showed varying levels of resistance, with high-grade resistance detected against EFV (efavirenz), NVP (nevirapine), FTC (emtricitabine), and 3TC (lamivudine) (Supplementary Figure S3B). Subtype-specific resistance patterns were also observed (p = 0.015; Supplementary Table S2).
3.3 Characteristics of HIV-1 transmission networks
A total of 168 HIV-1 molecular transmission networks were identified, encompassing 692 individuals (49.1% of the 1,409 successfully genotyped individuals) and 2,063 edges. Network sizes ranged from 2 to 139 nodes, with the majority (59.5%) comprising dyadic clusters (two nodes). Notably, 9 clusters contained ≥10 sequences (Supplementary Figure S4A). Node connectivity exhibited marked heterogeneity: 36.6% of nodes (253/692) formed single linkages, 49.3% (341/692) engaged in 2–10 linkages, and 14.2% (98/692) demonstrated extensive connectivity (>20 linkages) (Supplementary Figure S4B). Under a 1.5% genetic distance threshold, 30.0% of edges (618/2,063) exhibited minimal genetic divergence (<0.005), suggesting recent or ongoing transmission events (Supplementary Figure S4C).
Figure 1A illustrates the MTNs stratified by different HIV-1 subtypes. CRF07_BC dominated cluster composition with 320 nodes forming 63 clusters, including 60 small clusters comprising 160 nodes. Similarly, CRF01_AE contributed 187 nodes across 62 clusters, with 60 small clusters containing 166 nodes. CRF08_BC formed 9 clusters (49 nodes), 8 of which were small (21 nodes). CRF55_01B was distributed across 12 clusters (45 nodes), including 11 small clusters (27 nodes). The URF subtype comprised 5 clusters (31 nodes), 4 of which were small (17 nodes). CRF57_BC formed 4 clusters (16 nodes), 3 of which were small (6 nodes). Remaining subtypes all formed small clusters: CRF85_BC (26 nodes/7 clusters), subtype B (8 nodes/3 clusters), subtype C (7 nodes/2 clusters), and CRF59_01B (3 nodes/1 cluster). The largest molecular cluster was CRF07_BC, which consisted of 126 males and 11 females, with the primary mode of transmission being homosexual transmission (60.9%), details showed in Table 1. Furthermore, the dominant drug resistance mutation in the largest MTNs was Q58E, whereas other resistance-associated mutations were either scattered across the network or confined to smaller clusters. The genotypic and age-specific drug resistance profiles of MTNs are characterized in Supplementary Figure S5. Analysis of individuals with potential transmission links.
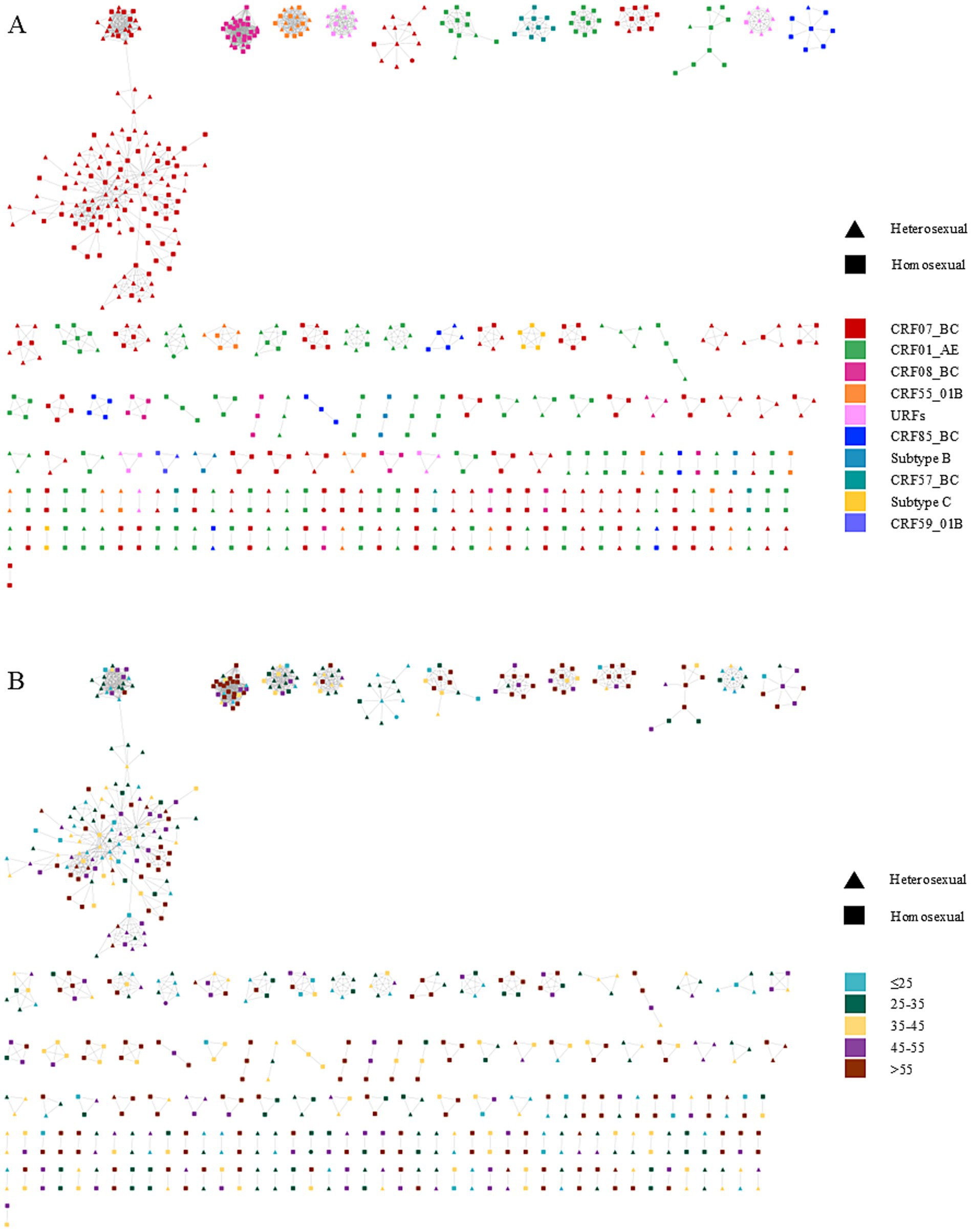
Figure 1. Molecular transmission network of people living with HIV in Ningbo, China (2018–2022). Clusters are ordered by size within each panel. Node shapes denote distinct transmission routes, and node colors represent participant characteristics, including HIV-1 genotypes and age groups. (A) corresponds to the subtype distribution of the network. (B) represents the age distribution of the network.
Univariate and multivariate logistic regression models were used to compare sequences included in the transmission networks versus those not included. Results are presented in Figure 1; Supplementary Table S3. The multivariate analysis revealed that individuals aged >55 years were more likely to cluster compared with those aged ≤25 years [adjusted odds ratio (aOR) = 2.379, 95% confidence interval (CI) = 1.623–3.502, p < 0.001]. Compared with Han ethnic patients, those from other ethnic groups showed reduced odds of clustering (aOR = 0.494, 95% CI = 0.270–0.873, p = 0.018). Patients with CD4 counts >200 cells/μL exhibited higher odds of clustering than those with CD4 ≤ 200 cells/μL (aOR = 1.295, 95% CI = 1.023–1.641, p = 0.032). Temporally, cases diagnosed in 2020 (aOR = 1.571, 95% CI = 1.055–2.349, p = 0.027) and 2021 (aOR = 1.765, 95% CI = 1.208–2.589, p = 0.003) exhibited higher odds of being part of transmission clusters compared to 2018. In contrast, 2022 showed no statistically significant difference in clustering odds relative to 2018 (aOR = 1.242, 95% CI = 0.856–1.809, p = 0.256). Regarding viral genotypes, CRF07_BC (aOR = 1.806, 95% CI = 1.395–2.342, p < 0.001) and others (aOR = 1.577, 95% CI = 1.177–2.115, p = 0.002) showed stronger clustering tendencies than CRF01_AE.
3.4 Characterization of large clusters
Nine large clusters (≥10 nodes) were identified, comprising 251 individuals (220 males and 31 females). Among these clusters, homosexual transmission (50.2%) and heterosexual transmission (49.4%) accounted for nearly equal proportions, with other routes contributing minimally (0.4%). A total of 12 transmitted drug resistance (TDR) cases were distributed across four large clusters. Within the nine large clusters, three were predominantly located in Xiangshan district (Table 1). Residents from Xiangshan exhibited significantly higher odds of clustering compared with Haishu district (aOR = 2.219, 95% CI = 1.086–4.594, p = 0.030). Conversely, Ninghai (aOR = 0.242, 95% CI = 0.064–0.736, p = 0.020), Yuyao (aOR = 0.395, 95% CI = 0.195–0.778, p = 0.008), and Zhenhai (aOR = 0.307, 95% CI = 0.094–0.853, p = 0.033) showed reduced odds of clustering relative to Haishu. Regarding viral genotypes, individuals infected with CRF07_BC (aOR = 7.796, 95% CI = 4.699–13.486, p < 0.001) and others (aOR = 4.619, 95% CI = 2.613–8.417, p < 0.001) exhibited stronger clustering tendencies than those with CRF01_AE (Table 2). A summary of large cluster characteristics (Table 1) further highlighted that 3 out of 9 identified large clusters (encompassing 251 individuals) were dominated by CRF07_BC, including the largest cluster in this study. Additionally, URFs constituted a large transmission network comprising 14 sequences, with infected individuals primarily residing in Beilun District, Ningbo City—an area adjacent to Ningbo-Zhoushan Port, one of China’s largest seaports (Table 3).
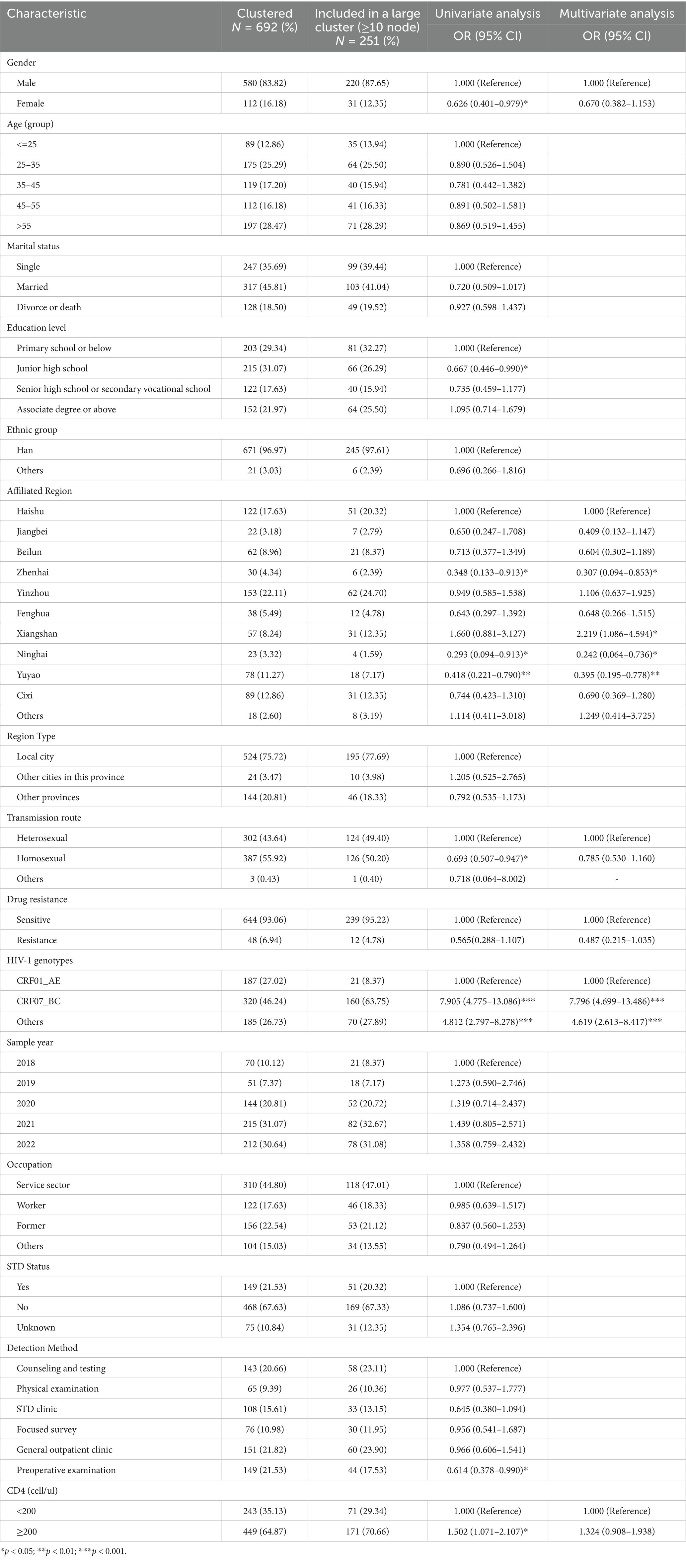
Table 2. Factors influencing the inclusion of individuals into the large molecular transmission clusters.
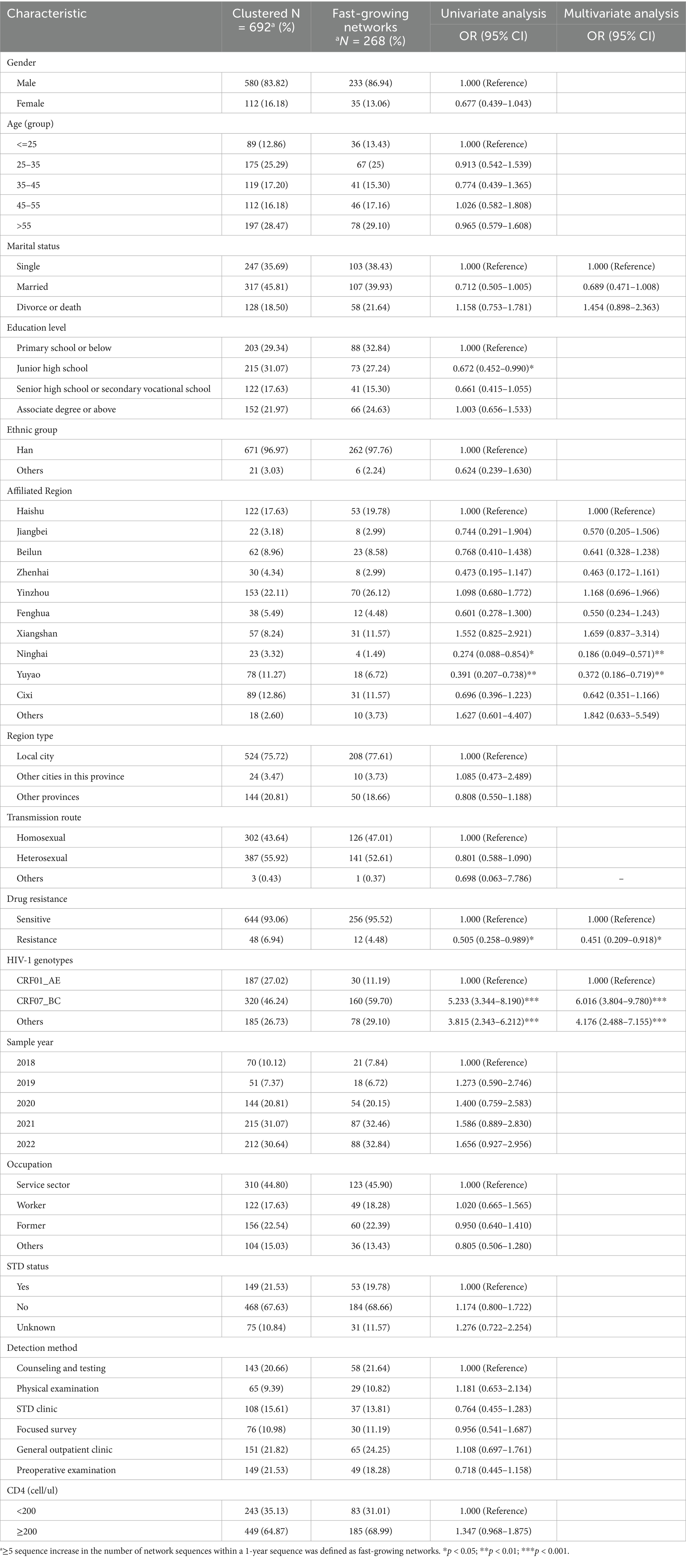
Table 3. Factors influencing the inclusion of individuals into the fast-growing molecular transmission clusters.
3.5 Characterization of fast-growing clusters
To elucidate temporal trends, annual HIV-1 transmission networks were reconstructed for each study year (Figure 2). During 2020, four clusters emerged. By 2021, one cluster originating in 2020 had expanded significantly in size, while two smaller clusters formed independently. In 2022, five new small clusters appeared, and the previously expanded cluster continued to grow. Multivariate analysis identified underlying factors associated with network expansion dynamics. Residents from Ninghai (aOR = 0.186, 95% CI = 0.049–0.571, p = 0.006) and Yuyao (aOR = 0.372, 95% CI = 0.186–0.719, p = 0.004) exhibited reduced odds of clustering compared with Haishu residents. Drug-resistant individuals showed lower odds of involvement in rapid transmission networks (aOR = 0.451, 95% CI = 0.209–0.918, p = 0.034). Furthermore, infections with CRF07_BC (aOR = 6.016, 95% CI = 3.804–9.780, p < 0.001) and other subtypes (aOR = 4.176, 95% CI = 2.4889–7.155, p < 0.001) exhibited significantly stronger clustering propensities compared with CRF01_AE. Detailed information is listed in Supplementary Table 3.
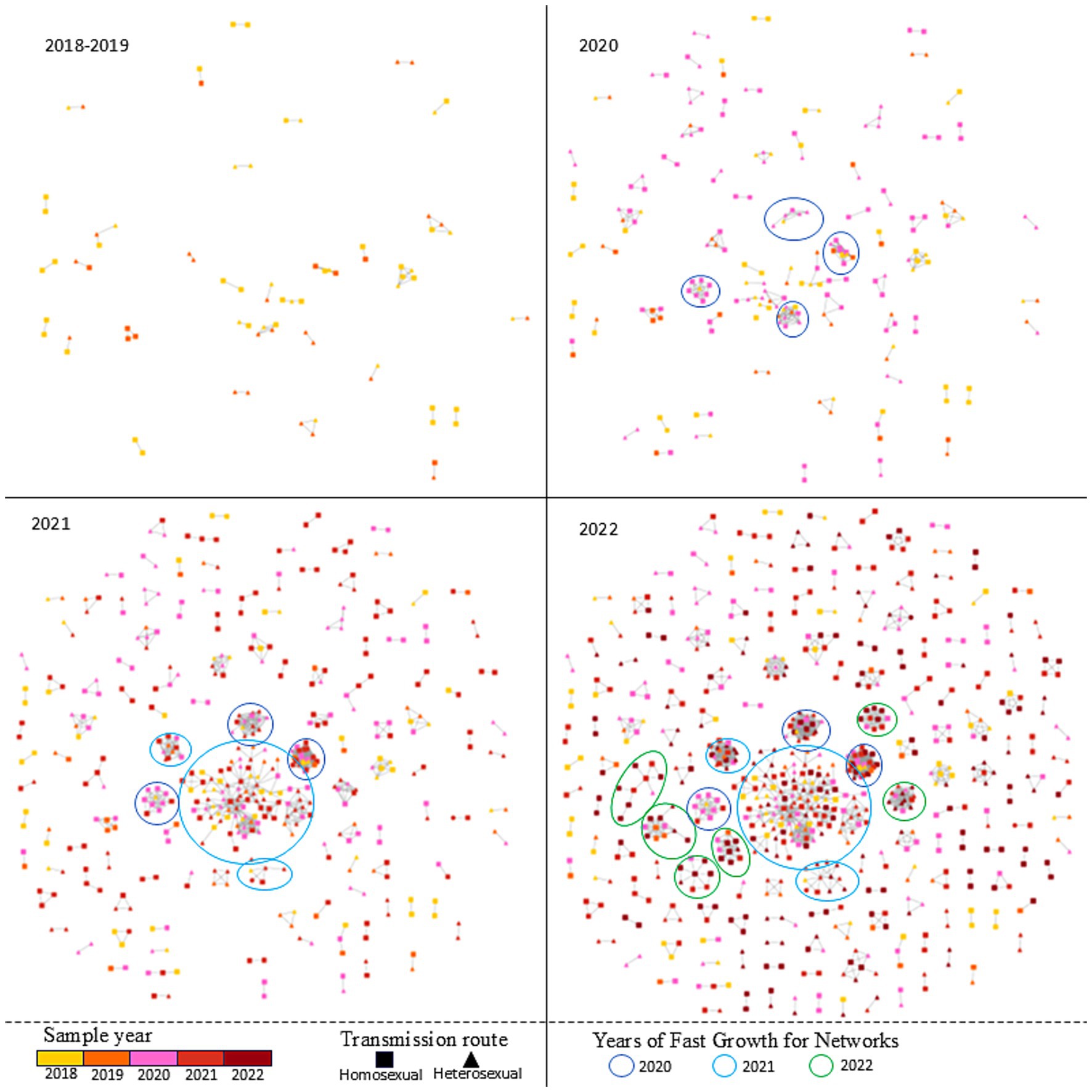
Figure 2. The temporal trends and Fast-growing clusters of HIV-1 transmission network in Ningbo (2018–2022).
4 Discussion
This study systematically investigated the molecular epidemiology, transmission dynamics, and risk factors of HIV-1 among 1,409 newly diagnosed, genotyped individuals in Ningbo City from 2018 to 2022. Molecular transmission network analysis revealed 168 distinct clusters involving 692 individuals, with 59.5% being dyadic (two-node) clusters and 9 clusters containing ≥10 nodes. Molecular transmission network analysis identified 9 large clusters and 12 fast-growing clusters. Age and HIV-1 subtype were associated with in the large clusters and fast-growing clusters, highlighting their critical roles in shaping local HIV transmission patterns. These findings provide critical insights into the structure and underlying factors associated with HIV transmission networks, which are essential for focused prevention and control strategies in Ningbo.
Previously, we analyzed molecular transmission networks in Ningbo from 2018 to 2021, revealing that the overall distribution of these networks remained relatively consistent over time. Specifically, CRF07_BC consistently tended to form larger, more densely connected clusters, whereas CRF01_AE networks primarily exhibited sparse, fragmented distributions. This aligns with nationwide trends in China, where CRF07_BC has shown increasing prevalence and CRF01_AE has declined in recent years (Li et al., 2016; Yin et al., 2019). Factors associated with cluster formation also showed consistency: CRF07_BC demonstrated a higher propensity to drive network formation during that period. Building on this foundation, the current study distinguishes itself by focusing specifically on the formation and influencing factors of large clusters and fast-growing clusters. While prior research emphasized the need for surveillance and comprehensive interventions focused on key clusters to mitigate potential HIV-1 transmission risks, it did not explicitly define or characterize what constituted these “key clusters.” In contrast, our work explicitly identifies large and fast-growing clusters as key areas for focused prevention and confirms that their formation remains consistently driven by HIV subtypes. Notably, CRF07_BC-associated clusters involved the most demographically heterogeneous populations, a pattern consistent with observations from Fujian, Anhui, and Jiangsu provinces (Wang et al., 2023; Zheng et al., 2021; Li et al., 2024). Similarly, analysis of fast-growing clusters reinforced the link between HIV-1 subtype and cluster dynamics: CRF07_BC showed a pronounced association with rapid network expansion. Structural analyses revealed that CRF07_BC harbors specific mutations/deletions in the p6Gag protein (e.g., PTAPPE insertion and/or PIDKELY deletion), which may attenuate virulence while enhancing transmissibility. Moreover, CRF07_BC variants with reduced net charge in the V3 loop exclusively utilize the CCR5 co-receptor and exhibit slower replication kinetics in primary target cells (Hu et al., 2022). These molecular features collectively suggest that CRF07_BC may possess superior fitness for initiating infections in key populations (Ge et al., 2021). In recent years, China has witnessed a bidirectional upward trend in HIV-1 infections, with notable increases among both adolescents and older adults. Our study revealed that older age (>55 years) was associated with a 2.38-fold higher likelihood of being included in MTNs compared to younger individuals—a pattern consistent with the national epidemiological trajectory (Ma et al., 2021; Cai et al., 2020). Similarly, a molecular transmission network analysis of HIV-1 in Huzhou, Zhejiang Province (2017–2022) highlighted that older adults (≥50 years) play a critical role in local network formation. This age-related disparity may be attributed to factors such as prolonged unprotected sexual encounters and a higher cumulative number of sexual partners among older individuals (Luo et al., 2019). However, our analysis revealed no age-related disparities in the formation of large clusters or fast-growing clusters. While prior research has established that distinct molecular transmission networks contribute disproportionately to HIV spread (Novitsky et al., 2023), we posit that the large clusters and fast-growing clusters prioritized for intervention in Ningbo should not be constrained by age. Given limited HIV prevention resources, prioritizing networks with greater future impact is imperative.
Beyond this, CRF57_BC—first identified in Yunnan Province (Li et al., 2012; Wei et al., 2014)—was absent from subtype surveys in Zhejiang during 2012–2016 (Ding et al., 2022) but was first reported in the province’s 2021 surveillance data (Fan et al., 2025). Notably, our study revealed that CRF57_BC had already formed large transmission networks by 2018–2022, a rapid dissemination pattern that warrants attention. Additionally, URFs constituted a large transmission network in Beilun District, one of China’s major seaports. Given the region’s unique geographical location and high population mobility—factors known to facilitate viral recombination between diverse subtypes—additional experimental validation is warranted to clarify these dynamics.
From a temporal perspective, our analysis showed that the number of sequences included in transmission networks in 2020 and 2021 was 1.57-fold and 1.77-fold higher, respectively, than that in 2018. However, no statistically significant difference was observed between 2022 and 2018. Visually, the MTNs expanded rapidly in 2020–2021 but plateaued in 2022—a divergent trend compared to other regions in Zhejiang Province and nationally, where networks have continued to grow unchecked. Ningbo features a well-developed medical information infrastructure and has allocated substantial financial and human resources to HIV prevention and control efforts. In 2020, Ningbo conducted a cost-effectiveness prediction for HIV interventions focused on MSM, projecting that scaling up intervention coverage by 3.0-fold (with a 2.4-fold increase in funding) from the 2020 baseline could reduce cumulative new HIV infections by 7.9% and AIDS-related deaths by 1.7% between 2021 and 2030 (Wang et al., 2022). Guided by this evidence, Ningbo has since intensified its prevention efforts through evidence-based strategies, including enhanced public education, optimized testing networks, improved treatment services, and technological innovation. Notable initiatives include the development of a medical-police collaborative big data system for efficient HIV source tracing, the “4 + 1” intensive management program for key HIV cases, and a risk prediction and early warning system for HIV transmission. These innovations were showcased at the 2024 “Fast-Track Cities” network symposium on ending the AIDS epidemic, where Ningbo shared its progress with domestic and international experts (Ningbo CDC, 2024). Collectively, these measures could have been instrumental in curbing the spread of HIV-1 in Ningbo.
In this study, we found no statistically significant association between drug resistance status and either sequences entered the transmission network or large clusters formed. However, sensitive sequences exhibited a significantly higher propensity to form fast-growing networks compared to their resistant sequences. While some prior studies have emphasized that factors such as HIV-1 subtype, antiretroviral treatment history, and transmission route critically influence drug resistance (Chen et al., 2023; Tan et al., 2023; Lan et al., 2021), evidence linking drug resistance itself to transmission network formation remains scarce. This gap may be attributed to China’s relatively low overall drug resistance rate, which has thus far prevented the emergence of large-scale, drug-resistant strain-dominated transmission networks. A study in Guangxi Province identified viral load (50–1,000 copies/mL) and immunological treatment failure as significant correlates of clustering (Chen et al., 2021). In contrast, U. S.-based research demonstrated that antiretroviral therapy (ART) focused on people living with HIV effectively curbed secondary HIV-1 transmission (Little et al., 2014). Similarly, a nationwide study in China reported that large clusters in Shenyang were controlled through ART interventions (Liu et al., 2020). However, in our study, neither CD4 count nor drug resistance factors yielded statistically significant associations with clustering. This observed discrepancy may be associated with Ningbo’s high ART coverage, which potentially saturates the preventive benefits derived from treatment-mediated viral suppression. Consistent with this hypothesis, a national HIV intervention prioritization analysis revealed that oral PrEP serves as an effective strategic intervention for MSM in regions with high ART coverage (e.g., Ningbo) under idealized scenarios (Zhang et al., 2024). MSM populations in cities like Beijing (Sun et al., 2023) and Nanjing (Chen et al., 2024) have shown high willingness to use PrEP; however, their actual adherence and usage rates remain extremely low (mostly <5%) (Du et al., 2025; Peng et al., 2022). Three key scenarios may contribute to drug resistance during PrEP use: initiating PrEP without prior HIV diagnosis or awareness of infection status, inconsistent adherence to PrEP medications, and resuming high-risk behaviors shortly after discontinuing PrEP while residual prophylactic drugs remain in the body. All of these scenarios can lead to the acquisition of drug-resistant HIV. Research has further shown that drug resistance is particularly prevalent among individuals who start PrEP during acute HIV infection without a confirmed diagnosis; however, widespread PrEP implementation with sustained high adherence does not significantly increase the transmission of resistant strains (Johnson et al., 2021). To date, Ningbo has not implemented focused PrEP policies, and no local studies have investigated PrEP adoption. Only information on post-exposure prophylaxis (PEP) clinics is publicly available on the Ningbo Center for Disease Control and Prevention (CDC) website. We therefore hypothesize that PrEP usage in Ningbo is minimal, and its potential confounding effect on our drug resistance findings is likely negligible.
This study has several limitations that should be acknowledged. First, the analysis was restricted to newly diagnosed people living with HIV with successful genotype data, potentially introducing selection bias by excluding cases with poor-quality or unsequenced samples. This may constrain the generalizability of findings to the broader community of people living with HIV in Ningbo, particularly among individuals with undiagnosed or unsequenced infections. Second, the study focused on a 5-year period (2018–2022), which may not capture long-term trends or the impact of very recent intervention adjustments (e.g., post-2022 policy changes). Third, while molecular transmission networks were constructed using genetic sequence data, the analysis did not integrate detailed behavioral or socioeconomic factors (e.g., sexual partner dynamics, socioeconomic status) that may influence transmission patterns, potentially overlooking factors associated with cluster formation. Fourth, the evaluation of intervention effectiveness relied on observational data, which may be confounded by concurrent public health initiatives or regional disparities in service access, making causal inferences challenging. Finally, the findings are specific to Ningbo’s unique epidemiological and geographic context (e.g., port-related population mobility), limiting direct extrapolation to other cities or regions with distinct demographic, behavioral, or structural features. Future studies should address these gaps by expanding sample diversity, incorporating longitudinal behavioral data, and exploring external validity through multi-region comparisons.
5 Conclusion
This study systematically characterized the molecular transmission dynamics of HIV-1 in Ningbo City from 2018 to 2022. Key findings revealed that the CRF07_BC subtype exhibited a significant propensity to form large transmission clusters and rapidly expanding transmission clusters; adults aged ≥55 years played a critical role in the formation of local transmission networks. Continuous monitoring of HIV-1 molecular subtypes may inform feasible and focused prevention strategies to curb HIV transmission.
Data availability statement
The data presented in the study are deposited in GenBank, accession number PX482927 to PX484313.
Ethics statement
The studies involving humans were approved by School of Medicine, Ningbo University Institutional Review Board (No. NBU-2022-135) in Medical Ethics Committee of School of Medicine, Ningbo University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YY: Writing – original draft, Conceptualization, Data curation, Formal analysis, Funding acquisition, Visualization. YL: Formal analysis, Investigation, Resources, Validation, Writing – review & editing. JZ: Formal analysis, Funding acquisition, Methodology, Resources, Writing – review & editing. PS: Data curation, Project administration, Supervision, Validation, Writing – review & editing. YC: Formal analysis, Software, Validation, Writing – review & editing. ZJ: Project administration, Resources, Supervision, Writing – review & editing. HL: Project administration, Resources, Supervision, Writing – review & editing. HN: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Validation, Writing – review & editing. YS: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Zhejiang Provincial Natural Science Foundation of China under Grant (LQ23H260002), Ningbo Key Laboratory of Virus Research (20221CXJD030031), and Nanjing Medical Science and technology development Foundation (YKK24228). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1701408/full#supplementary-material
Abbreviations
URFs, Unique recombinant forms; MTNs, Molecular transmission networks; WHO, World Health Organization; DALYs, Disability-adjusted life years; ART, Antiretroviral therapy; PI, Protease inhibitors; NRTIs, Nucleoside reverse transcriptase inhibitors; NNRTIs, Non-nucleoside reverse transcriptase inhibitors; aOR, Adjusted odds ratio; CIs, Confidences intervals.
Footnotes
1. ^Los Alamos National Laboratory, LANL; www.hiv.lanl.gov.
2. ^https://www.hiv.lanl.gov/content/sequence/elimdupesv2/elimdupes.html.
References
Bai, R. J., Dai, L. L., and Wu, H. (2020). Advances and challenges in antiretroviral therapy for acquired immunodeficiency syndrome. Chin. Med. J. 133, 2775–2777. doi: 10.1097/CM9.0000000000001226
Bennett, D. E., Camacho, R. J., Otelea, D., Kuritzkes, D. R., Fleury, H., Kiuchi, M., et al. (2009). Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 4:e4724. doi: 10.1371/journal.pone.0004724
Cai, C., Tang, H., Chen, F., Li, D., and Lyu, F. (2020). Characteristics and trends of newly reported HIV infection in young students in China, 2010-2019. Zhonghua Liu Xing Bing Xue Za Zhi 41, 1455–1459. doi: 10.3760/cma.j.cn112338-20200417-00592
Chen, W., Chen, L., Ni, Z., He, L., and Pan, X. (2024). Role of HIV Serostatus communication on frequent HIV testing and self-testing among men who have sex with men who seek sexual partners on the internet in Zhejiang, China: cross-sectional study. JMIR Form Res. 8:e57244. doi: 10.2196/57244
Chen, H., Hao, J., Hu, J., Song, C., Zhou, Y., Li, M., et al. (2023). Pretreatment HIV drug resistance and the molecular transmission network among HIV-positive individuals in China in 2022: multicenter observational study. JMIR Public Health Surveill. 9:e50894. doi: 10.2196/50894
Chen, L., Luo, M., Xu, Y., Xia, Y., Zhou, X., Chen, W., et al. (2021). The first 90: Progress in HIV detection in Zhejiang Province, 2008-2018. PLoS One 16:e0249517. doi: 10.1371/journal.pone.0249517
Chen, X., Qin, C., Chen, R., Huang, Y., Xu, Y., Tang, Q., et al. (2021). Epidemiological profile and molecular genetic characterization of HIV-1 among female sex workers and elderly male clients in Guangxi, China. Emerg. Microbes Infect. 10, 384–395. doi: 10.1080/22221751.2021.1888659
Chen, W., Yang, J., Jiang, J., He, L., Xu, Y., Zheng, J., et al. (2021). A spatial analysis of the epidemiology of HIV-infected students in Zhejiang province, China. BMC Infect. Dis. 21:430. doi: 10.1186/s12879-021-06033-7
China CDC. (2019). Technical guidelines for HIV transmission network surveillance and intervention (trial) have been officially released in China. Available online at: https://ncaids.chinacdc.cn/zxzx/zxzx/201909/t20190929_205904.htm.
Dai, B., Peng, X., Sun, J., Zhu, X., Liu, X., Xiong, Y., et al. (2024). Distinct clusters of HIV-1 CRF01_AE in Zhejiang, China: high-risk transmission cluster 4 requires heightened surveillance. Infect Drug Resist. 17, 4333–4342. doi: 10.2147/IDR.S480192
Ding, X., Chaillon, A., Pan, X., Zhang, J., Zhong, P., He, L., et al. (2022). Characterizing genetic transmission networks among newly diagnosed HIV-1 infected individuals in eastern China: 2012–2016. PLoS One 17:e0269973. doi: 10.1371/journal.pone.0269973
Du, J., Wang, S., Zhang, H., Liu, T., Sun, S., Yang, C., et al. (2025). Pre-exposure prophylaxis (PrEP) awareness and engagement among MSM at high risk of HIV infection in China: a Multi-City cross-sectional survey. AIDS Behav. 29, 1629–1639. doi: 10.1007/s10461-025-04633-x
Fan, Q., Zhang, J., Pan, X., Ding, X., Xing, H., Feng, Y., et al. (2025). Insights into the molecular network characteristics of major HIV-1 subtypes in developed eastern China: a study based on comprehensive molecular surveillance data. Infection 53, 905–920. doi: 10.1007/s15010-024-02389-5
Ge, Z., Feng, Y., Zhang, H., Rashid, A., Zaongo, S. D., Li, K., et al. (2021). HIV-1 CRF07_BC transmission dynamics in China: two decades of national molecular surveillance. Emerg Microbes Infect. 10, 1919–1930. doi: 10.1080/22221751.2021.1978822
Han, X., Zhao, B., An, M., Zhong, P., and Shang, H. (2020). Molecular network-based intervention brings us closer to ending the HIV pandemic. Front. Med. 14, 136–148. doi: 10.1007/s11684-020-0756-y
He, L., Pan, X., Yang, J., Zheng, J., Cheng, W., and Chai, C. (2025). HIV transmission risk among people living with HIV in Zhejiang Province, China: data from a large cross-sectional study, 2022. Front. Public Health 13:1550565. doi: 10.3389/fpubh.2025.1550565
He, X., Zheng, W., Wang, X., Jiang, Y., and Zhu, W. (2025). Trend of HIV/AIDS disease burden in China from 1990 to 2021: An age-period-cohort model analysis. HIV Med. 26, 773–784. doi: 10.1111/hiv.70009
Hong, H., Tang, C., Liu, Y., Jiang, H., Fang, T., and Xu, G. (2023). HIV-1 drug resistance and genetic transmission network among newly diagnosed people living with HIV/AIDS in Ningbo, China between 2018 and 2021. Virol. J. 20:233. doi: 10.1186/s12985-023-02193-x
Hong, H., Xu, J., McGoogan, J., Dong, H., Xu, G., and Wu, Z. (2018). Relationship between the use of gay mobile phone applications and HIV infection among men who have sex with men in Ningbo, China: a cross-sectional study. Int. J. STD AIDS 29, 491–497. doi: 10.1177/0956462417738468
Hu, X., Feng, Y., Li, K., Yu, Y., Rashid, A., Xing, H., et al. (2022). Unique profile of predominant CCR5-tropic in CRF07_BC HIV-1 infections and discovery of an unusual CXCR4-tropic strain. Front. Immunol. 13:911806. doi: 10.3389/fimmu.2022.911806
Johnson, K. A., Chen, M. J., Kohn, R., Sachdev, D., Bacon, O., Lee, S., et al. (2021). Acute HIV at the time of initiation of pre-exposure or post-exposure prophylaxis: impact on drug resistance and clinical outcomes. J. Acquir. Immune Defic. Syndr. 87, 818–825. doi: 10.1097/QAI.0000000000002638
Lan, Y., Deng, X., Li, L., Cai, W., Li, J., Cai, X., et al. (2021). HIV-1 drug resistance and genetic transmission networks among MSM failing antiretroviral therapy in South China 2014-2019. Infect Drug Resist. 14, 2977–2989. doi: 10.2147/IDR.S317187
Li, L., Chen, L., Yang, S., Li, T., Li, J., Liu, Y., et al. (2012). Recombination form and epidemiology of HIV-1 unique recombinant strains identified in Yunnan, China. PLoS One 7:e46777. doi: 10.1371/journal.pone.0046777
Li, X., Li, W., Zhong, P., Fang, K., Zhu, K., Musa, T. H., et al. (2016). Nationwide trends in molecular epidemiology of HIV-1 in China. AIDS Res. Hum. Retrovir. 32, 851–859. doi: 10.1089/aid.2016.0029
Li, X., Shi, H., Shi, H., Xu, Y., Wu, S., Wu, R., et al. (2024). Transmission network and phylogenetic analysis highlight the role of suburban population in HIV-1 transmission among older adults in Nanjing, Jiangsu Province, China. J. Med. Virol. 96:e70035. doi: 10.1002/jmv.70035
Little, S. J., Kosakovsky Pond, S. L., Anderson, C. M., Young, J. A., Wertheim, J. O., Mehta, S. R., et al. (2014). Using HIV networks to inform real time prevention interventions. PLoS One 9:e98443. doi: 10.1371/journal.pone.0098443
Liu, M., Han, X., Zhao, B., An, M., He, W., Wang, Z., et al. (2020). Dynamics of HIV-1 molecular networks reveal effective control of large transmission clusters in an area affected by an epidemic of multiple HIV subtypes. Front. Microbiol. 11:604993. doi: 10.3389/fmicb.2020.604993
Luo, M., Pan, X., Fan, Q., Zhang, J., Ge, R., Jiang, J., et al. (2019). Epidemiological characteristics of molecular transmission cluster among reported HIV/AIDS cases in Jiaxing city, Zhejiang province, 2017. Zhonghua Liu Xing Bing Xue Za Zhi 40, 202–206. doi: 10.3760/cma.j.issn.0254-6450.2019.02.015
Ma, Y., Cui, Y., Hu, Q., Mubarik, S., Yang, D., Jiang, Y., et al. (2021). Long-term changes of HIV/AIDS incidence rate in China and the U.S. population from 1994 to 2019: a join-point and age-period-cohort analysis. Front. Public Health 9:652868. doi: 10.3389/fpubh.2021.652868
National Center for STD/AIDS Control, Chinese Center for Disease Control and Prevention (2025). The national AIDS and sexually transmitted disease epidemic in December 2024. Chin. J. AIDS STD. 31:225.
Ningbo CDC. (2024). The AIDS prevention and control work in Ningbo City shared its experience at the ‘fast track to end the AIDS epidemic’ city webinar. Available online at: http://wjw.ningbo.gov.cn/art/2024/8/9/art_1229793627_58963633.html.
Novitsky, V., Steingrimsson, J., Howison, M., Dunn, C. W., Gillani, F. S., Fulton, J., et al. (2023). Not all clusters are equal: dynamics of molecular HIV-1 clusters in a statewide Rhode Island epidemic. AIDS 37, 389–399. doi: 10.1097/QAD.0000000000003426
Oster, A. M., France, A. M., and Mermin, J. (2018). Molecular epidemiology and the transformation of HIV prevention. JAMA 319:1657. doi: 10.1001/jama.2018.1513
Peng, Q., Liu, X., Tang, X., Zhang, Q., Zhao, J., Zheng, C., et al. (2022). Low rate of pre-exposure prophylaxis and post-exposure prophylaxis uptake and high prevalence of transmitted drug resistance among newly diagnosed primary HIV infections in Shenzhen, China: a real-world retrospective study. Chin. Med. J. 135, 2730–2737. doi: 10.1097/CM9.0000000000002510
Shi, H., Li, X., Wang, S., Dong, X., Qiao, M., Wu, S., et al. (2024). Molecular transmission network analysis of newly diagnosed HIV-1 infections in Nanjing from 2019 to 2021. BMC Infect. Dis. 24:583. doi: 10.1186/s12879-024-09337-6
Shi, S., Tan, S., Ye, Z., Jiang, H., Pan, X., Chu, K., et al. (2024). Cohort study of incidence of high transmission risk of HIV/AIDS cases in Ningbo, Zhejiang, 2017−2022. Dis. Surveill. 39, 1288–1293.
Sun, Y., Lu, H., Ye, J., Li, D., and Li, G. (2023). Awareness and use of HIV pre-exposure prophylaxis and factors associated with awareness among MSM in Beijing, China. Sci. Rep. 13:554. doi: 10.1038/s41598-023-27485-8
Tan, T., Bai, C., Lu, R., Chen, F., Li, L., Zhou, C., et al. (2023). HIV-1 molecular transmission network and drug resistance in Chongqing, China, among men who have sex with men (2018-2021). Virol. J. 20:147. doi: 10.1186/s12985-023-02112-0
UNAIDS, Global HIV. (2025) AIDS statistics — Fact sheet. Available online at: https://www.unaids.org/en/resources/fact-sheet (Accessed October 2, 2025).
United Nations General Assembly (2021). Political declaration on HIV and AIDS: Ending inequalities and getting on track to end AIDS by 2030. Switzerland, United Nations: United Nations General Assembly.
Wang, L., Hong, H., Zhang, Y., Shi, H., Chen, L., Jiang, H., et al. (2022). Cost-effectiveness prediction of AIDS interventions amongmen who have sex withmen in Ningbo. Chin. J. Epidemiol. 43, 2008–2014. doi: 10.3760/cma.j.cn112338-20220410-00275
Wang, Z., Liu, H., and Wang, Y. (2019). Spatial distribution and clustering characteristics of AIDS epidemic in China, 2005 – 2017. Chin. J. Public Health 35, 1593–1597.
Wang, Z., Wang, D., Lin, L., Qiu, Y., Zhang, C., Xie, M., et al. (2023). Epidemiological characteristics of HIV transmission in southeastern China from 2015 to 2020 based on HIV molecular network. Front. Public Health 11:1225883. doi: 10.3389/fpubh.2023.1225883
Wei, H., Liu, Y., Feng, Y., Hsi, J., Xing, H., He, X., et al. (2014). Genome sequence of a novel HIV-1 circulating recombinant form (CRF57_BC) identified from Yunnan, China. AIDS Res. Hum. Retrovir. 30, 384–388. doi: 10.1089/aid.2013.0228
Wertheim, J. O., Murrell, B., Mehta, S. R., Forgione, L. A., Kosakovsky Pond, S. L., Smith, D. M., et al. (2018). Growth of HIV-1 molecular transmission clusters in new York City. J. Infect. Dis. 218, 1943–1953. doi: 10.1093/infdis/jiy431
World Health Organization (2022). Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030. Switzerland: World Health Organization.
Ye, J., Lan, Y., Wang, J., Feng, Y., Lin, Y., Zhou, Y., et al. (2024). Improvement in the 95-95-95 targets is accompanied by a reduction in both the human immunodeficiency virus transmission rate and incidence in China. J. Infect. Dis. 230, 1202–1214. doi: 10.1093/infdis/jiae302
Yin, Y., Liu, Y., Zhu, J., Hong, X., Yuan, R., Fu, G., et al. (2019). The prevalence, temporal trends, and geographical distribution of HIV-1 subtypes among men who have sex with men in China: a systematic review and meta-analysis. Epidemiol. Infect. 147:e83. doi: 10.1017/S0950268818003400
Yin, Y., Zhou, Y., Lu, J., Liu, X., Shi, L., Fu, G., et al. (2021). Molecular epidemiology of HIV-1 in Jiangsu Province, Southeast China: genotypes and HIV-1 transmission networks among newly diagnosed men having sex with men in 2017. AIDS Res. Hum. Retrovir. 37, 62–69. doi: 10.1089/aid.2020.0165
Zhang, Y., Wang, L., Jiang, Z., Yan, H., Liu, X., Gu, J., et al. (2024). Exploration for the priority of HIV intervention: modelling health impact and cost-effectiveness - six cities, eastern China, 2019-2028. China CDC Wkly. 6, 463–469. doi: 10.46234/ccdcw2024.089
Zhao, B., Qiu, Y., Song, W., Kang, M., Dong, X., Li, X., et al. (2022). Undiagnosed HIV infections May drive HIV transmission in the era of “treat all”: a deep-sampling molecular network study in Northeast China during 2016 to 2019. Viruses 14:1895. doi: 10.3390/v14091895
Keywords: HIV-1, molecular transmission networks, transmission clusters, subtype, prevention strategies
Citation: Yin Y-Q, Liu Y-H, Zhu J, Shen P, Chen Y-P, Jiang Z-Q, Lin H-B, Ni H-X and Sun Y-X (2025) Molecular transmission network analysis of newly diagnosed HIV-1 infections in Ningbo from 2018–2022. Front. Microbiol. 16:1701408. doi: 10.3389/fmicb.2025.1701408
Edited by:
Leiliang Zhang, Shandong First Medical University and Shandong Academy of Medical Sciences, ChinaReviewed by:
Machiko Otani, World Health Organization (Denmark), DenmarkQin Fan, Zhejiang Provincial Center for Disease Control and Prevention, China
Copyright © 2025 Yin, Liu, Zhu, Shen, Chen, Jiang, Lin, Ni and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye-Xiang Sun, eWV4aWFuZ19zdW4yMDIyQDE2My5jb20=; Hong-Xia Ni, bmlob25neGlhQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Yue-Qi Yin
Yue-Qi Yin Yu-Hui Liu
Yu-Hui Liu Jing Zhu
Jing Zhu Peng Shen
Peng Shen Yun-Peng Chen
Yun-Peng Chen Zhi-Qin Jiang
Zhi-Qin Jiang Hong-Bo Lin
Hong-Bo Lin Hong-Xia Ni2*
Hong-Xia Ni2* Ye-Xiang Sun
Ye-Xiang Sun