- 1College of Medical Technology, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Chongqing Key Laboratory of Sichuan-Chongqing Co-construction for Diagnosis and Treatment of Infectious Diseases Integrated Traditional Chinese and Western Medicine, Chengdu, China
- 3Key Laboratory of Non-coding RNA and Drug Discovery at Chengdu Medical College, Chengdu, China
- 4Department of Pharmacy, Shaoxing People’s Hospital, Shaoxing, Zhejiang, China
- 5School of Basic Medical Sciences, Chengdu Medical College, Chengdu, China
In the intensifying global crisis of antimicrobial resistance (AMR), the “old” antibiotic fosfomycin has regained prominence because of its unique mechanism of action and potent activity against numerous multidrug-resistant (MDR) pathogens. However, its clinical application is hampered by the rapid emergence of resistance during monotherapy. Rational combination therapy represents a strategic necessity to preserve and enhance the efficacy of fosfomycin. This review systematically analyzes the antibacterial and molecular mechanisms of resistance to fosfomycin, with a focus on the growing threat posed by plasmid-mediated resistance genes. The preclinical and clinical evidence of key combination regimens (including β-lactams, aminoglycosides, fluoroquinolones, polymyxins, and daptomycin) has been comprehensively evaluated, with detailed discussions of the mechanistic foundations for the observed synergistic effects. Although in vitro and animal models show substantial promise, we critically examine the translational gap between positive preclinical results and clinical realities, discussing major barriers to clinical advancement. Finally, we outline a prospective research agenda, encompassing pharmacokinetic/pharmacodynamic (PK/PD)-guided precision dosing, exploring non-antibiotic adjuvants, and developing more predictive preclinical models to unlock the full potential of fosfomycin-based combinations against MDR infections.
1 Introduction
Antimicrobial resistance (AMR) has emerged as a significant global public health threat, as highlighted by organizations such as the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) (Castanheira et al., 2023). The relentless emergence of multidrug-resistant (MDR), extensively drug-resistant (XDR), and even pandrug-resistant (PDR) pathogens severely limits clinical therapeutic options, necessitating urgent innovative strategies (Falagas et al., 2016). According to CDC data, at least 2.8 million antibiotic-resistant infections occur in the United States each year, resulting in more than 35,000 deaths (Morrison and Zembower, 2020). This crisis has prompted scientific and medical communities to reevaluate the existing antimicrobial arsenal.
Faced with the depletion of novel antibiotic development channels, the repurposing and optimized use of previously neglected “old drugs” has become a key strategy (Dijkmans et al., 2017). The use of fosfomycin, a natural antibiotic discovered more than 45 years ago, has experienced a significant revival because of its unique mechanism of action and potent activity against numerous MDR pathogens. Its broad-spectrum bactericidal activity and extremely favorable toxicity profile make it an attractive option for addressing complex infections (Dijkmans et al., 2017; Silver, 2017). However, the application of fosfomycin faces a central paradox: despite being a potent bactericide with good safety, the rapid development of resistance during both in vitro and in vivo monotherapy severely restricts its clinical utility (Silver, 2017). This contradiction highlights the need to transition from monotherapy to rational combination therapies. Indeed, the renewed focus on fosfomycin presents a double-edged sword. Its activity against MDR bacteria also creates selective pressure for the emergence and spread of fosfomycin resistance. With the increasing use of fosfomycin, particularly in the treatment of multidrug-resistant infections, its resistance rate is also rising, a trend confirmed by usage data from regions such as Spain (Gardiner et al., 2019), which establishes a concerning feedback loop: the AMR crisis drives fosfomycin use, which in turn contributes to the emergence of the novel problem of fosfomycin resistance. This dynamic suggests that the only viable approach to breaking this cycle lies in strategies that suppress resistance development, with combination regimens being central to this effort. Research indicates that combination therapy is not only a multi-drug option but also a strategic necessity to enhance bactericidal activity through co-administration, suppressing the emergence and selection of resistance, and potentially restoring susceptibility in strains resistant to antibacterial drugs (Antonello et al., 2020).
This review aims to provide basic microbiology researchers with a detailed report, systematically dissecting the mechanistic basis, preclinical evidence, and clinical results of fosfomycin combination therapy while also exploring future research directions.
2 Pharmacology of fosfomycin
2.1 Unique chemical structures and formulations
Fosfomycin [chemical name: (1R,2S)-epoxypropylphosphonic acid] is an extremely small (138 g/mol), highly polar molecule that was first isolated from Streptomyces cultures in 1969 and is characterized by high tissue penetration and low toxicity (Hendlin et al., 1969; Popovic et al., 2010). Its structural similarity to phosphoenolpyruvate (PEP) underlies its broad-spectrum antibacterial activity against both gram-positive and gram-negative bacteria. Clinically, two major salt formulations of FOS (C3H7O4P) are utilized: oral fosfomycin trometamol (C3H7O4P ⋅ C4H11NO3), with a bioavailability of 34%–58%, and intravenous fosfomycin disodium (C3H5Na2O4P), which is employed to achieve higher systemic concentrations (Kwan and Beahm, 2020; Marino et al., 2022). Trometamol, an alkaline organic compound, is believed to mitigate acid-catalyzed hydrolysis. Compared with the oral calcium salt formulation (bioavailability 12%–37%), fosfomycin trometamol achieves serum concentrations 2- to 4-fold greater, establishing it as the preferred oral formulation for treating uncomplicated urinary tract infections (UTIs) (Bergan, 1990; Dijkmans et al., 2017; Neuner et al., 2012). Fosfomycin disodium is currently approved in many countries for the treatment of soft tissue infections and sepsis (Michalopoulos et al., 2011).
2.2 Direct bactericidal action
Unlike other mainstream antibiotics, fosfomycin (FOS) exerts its core mechanism by interfering with the first step of bacterial cell wall synthesis: the formation of the peptidoglycan precursor UDP-N-acetylmuramic acid (UDP-MurNAc), which acts earlier than β-lactams and glycopeptides (Figure 1A; Borisova et al., 2014; Falagas et al., 2016). FOS acts on the bacterial cytoplasm (Kahan et al., 1974). Once inside the cytoplasm, FOS functions as a structural analog of phosphoenolpyruvate (PEP) and covalently binds to the active site Cys115 of MurA (UDP-GlcNAc enolpyruvyl transferase), thereby inactivating the MurA enzyme (Aghamali et al., 2019). MurA catalyzes the condensation of UDP-N-acetylglucosamine (UDP-GlcNAc) with PEP to form UDP-MurNAc (Silver, 2017). Once the MurA enzyme is inhibited, UDP-MurNAc cannot be produced, ultimately leading to bacterial cell lysis. The interaction between FOS and MurA is highly specific, contributing to the low toxicity of this complex in mammals.
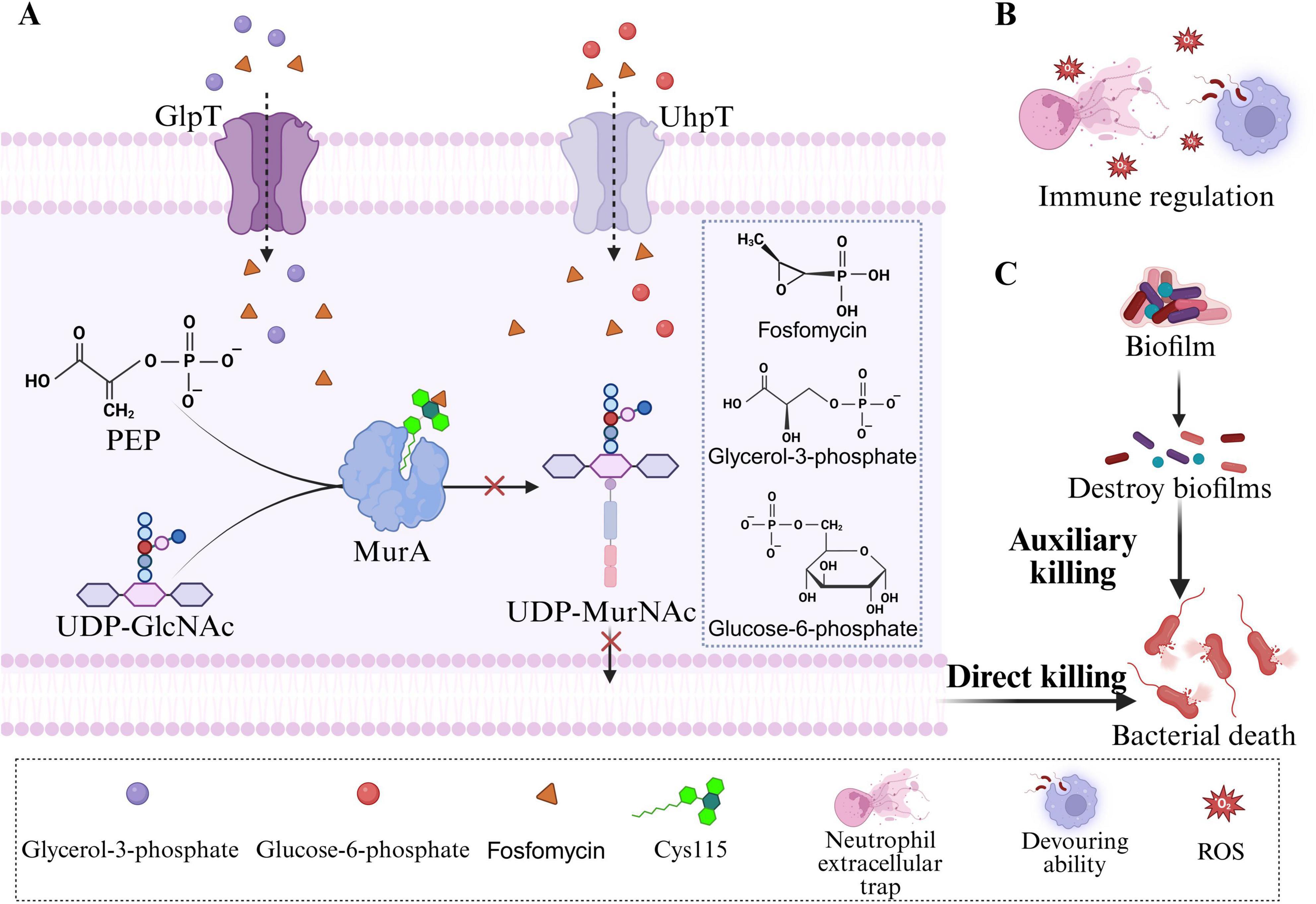
Figure 1. Antibacterial mechanism of fosfomycin. (A) FOS inhibits the formation of UDP-MurNAc, thereby exerting a direct bactericidal effect on bacteria. (B) FOS exerts an indirect bactericidal effect on bacteria through immunomodulation. (C) FOS exerts an indirect bactericidal effect by destroying bacterial biofilms.
To enter cells, FOS relies primarily on two transport systems in Escherichia coli: GlpT (glycerol-3-phosphate transporter) and UhpT (hexose-6-phosphate transporter) (Castañeda-García et al., 2013). The expression of these transporters is induced by their respective substrates, glycerol-3-phosphate (G3P) and glucose-6-phosphate (G6P). Extracellular G3P and G6P enter bacterial cells via GlpT and UhpT, respectively, and induce high expression of GlpT and UhpT in the presence of the cAMP-CRP complex (Castañeda-García et al., 2013; Yang et al., 2016). This characteristic explains why the addition of G6P to the culture medium is essential for reliable fosfomycin susceptibility testing. G6P supplementation enhances the expression of the UhpT system, ensuring adequate drug uptake and generating accurate minimum inhibitory concentration (MIC) values.
2.3 Auxiliary bactericidal effects
2.3.1 Immunomodulation
In addition to its core bactericidal mechanism, FOS has auxiliary properties that are beneficial for clinical therapy. Notably, FOS exerts complex modulatory effects on the host immune system. Studies indicate that FOS enhances the phagocytic and bactericidal capacity of phagocytes (neutrophils and macrophages) against invading pathogens, including promoting phagocytosis, inducing reactive oxygen species (ROS) generation, and stimulating the production of extracellular traps (ETs) (Figure 1B; Shen et al., 2016). FOS inhibits the production of IL-2 in T cells, leukotriene B4 (LTB4) in neutrophils, and the expression of IL-8 mRNA in monocytes (Honda et al., 1998; Morikawa et al., 1993). Furthermore, FOS has been shown to modulate the production of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-1β, and IL-6, in vivo (Matsumoto et al., 1999). However, a study evaluating the effects of FOS on proinflammatory cytokines in healthy volunteers revealed that the protein and mRNA expression levels of TNF-α, IL-1β, and IL-6 exhibited little difference in the presence of FOS (Sauermann et al., 2007). Although the clinical significance of these effects is still being explored, they suggest that fosfomycin may confer dual benefits by modulating host immune and inflammatory responses.
2.3.2 Anti-biofilm activity
Fosfomycin also effectively penetrates and disrupts biofilms formed by various pathogens, including Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Enterococcus spp. (Figure 1C; Descourouez et al., 2013; Hajdu et al., 2009; Mikuniya et al., 2007; Shi et al., 2014). Multiple in vitro studies have shown that FOS, either alone or in combination with other antimicrobial agents, not only reduces or eradicates clinically significant bacteria within biofilms but also induces structural alterations in biofilms (Ai et al., 2025; Anderson et al., 2013; Mihailescu et al., 2014). For example, in a recent in vitro biofilm infection model, the combination of fosfomycin and daptomycin exhibited superior antibiofilm activity, demonstrating synergistic antibacterial effects against methicillin-resistant Staphylococcus aureus (MRSA) (Ai et al., 2025). In a rat urinary tract infection model, scanning electron microscopy revealed that the combination of fosfomycin and prulifloxacin disrupted and eliminated multilayered P. aeruginosa biofilms from polyethylene tube surfaces (Mikuniya et al., 2007). Fosfomycin monotherapy has also been reported to reduce the density of S. epidermidis biofilms (Hajdu et al., 2009). Furthermore, combinations of fosfomycin with traditional Chinese medicines have shown significant potential in combating biofilms (Chrisostomo et al., 2025). The combination of fosfomycin and cryptotanshinone was found to inhibit biofilm formation by fosfomycin-resistant S. aureus, thereby reducing fosfomycin resistance (Ruan et al., 2020). Collectively, the antibiofilm activity of fosfomycin highlights its considerable potential for treating chronic infections and infections associated with medical devices, providing strong support for its use in combination therapeutic regimens.
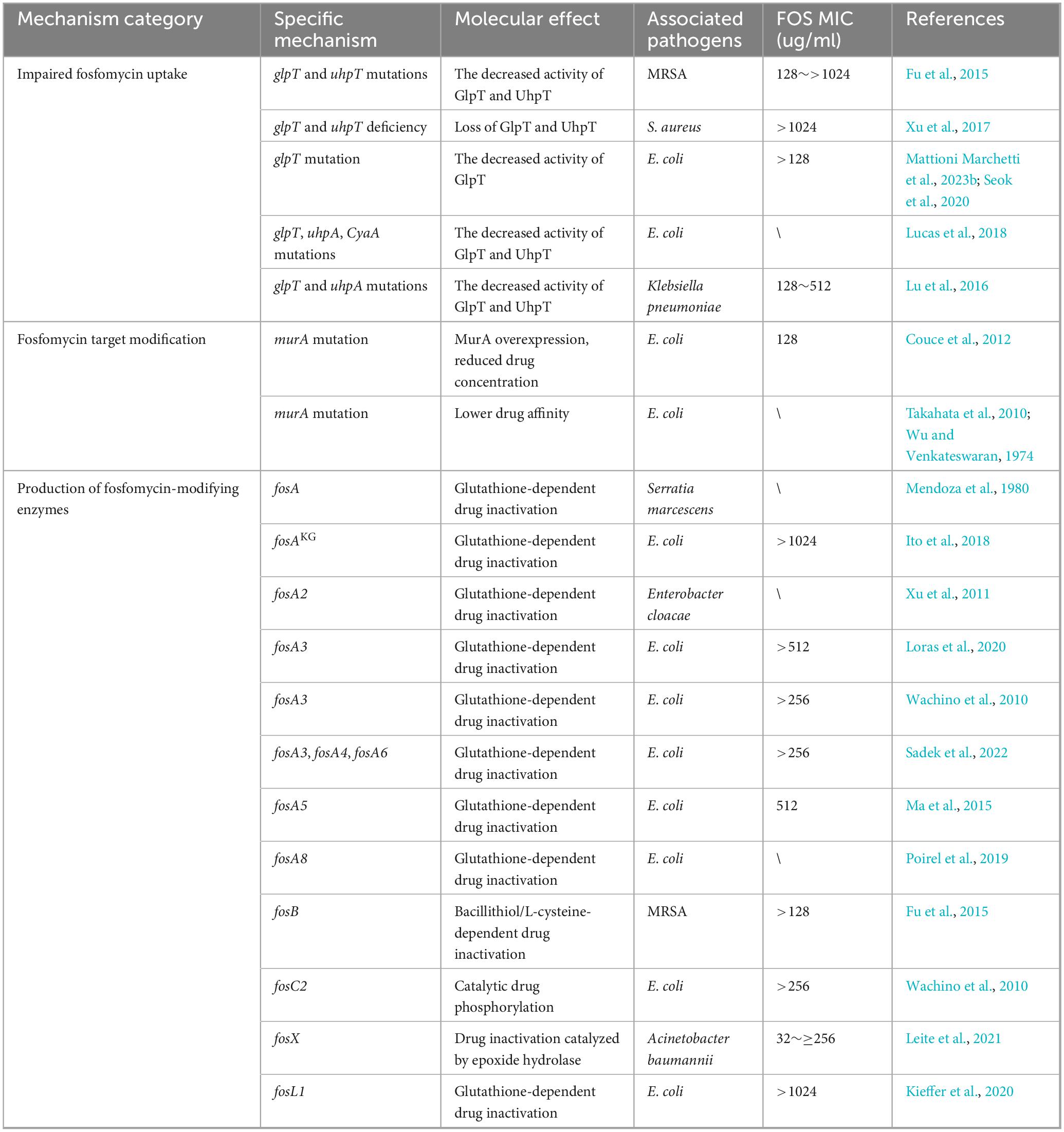
3 Molecular mechanisms of fosfomycin resistance
A thorough understanding of how bacteria evade the action of fosfomycin is fundamental for designing effective combination therapies to overcome antibiotic resistance. The mechanisms of fosfomycin resistance are broadly categorized into two classes: chromosomally mediated resistance and plasmid-mediated resistance (Table 1; Hosoi et al., 2024). Chromosomally mediated resistance typically arises from mutations in a bacterium’s genes and is transmitted vertically to its progeny. In contrast, plasmid-mediated resistance involves bacteria acquiring mobile genetic elements (such as plasmids) carrying the fos gene through horizontal gene transfer. These genes encode enzymes that modify and inactivate fosfomycin (Mattioni Marchetti et al., 2023a).
3.1 Chromosomally mediated resistance
3.1.1 Impaired fosfomycin uptake
Mutations (such as insertions, deletions, or truncations) in the transporter-encoding genes glpT and uhpT result in impaired function or complete loss of the GlpT and UhpT transporters, respectively, thereby blocking or reducing fosfomycin uptake (Castañeda-García et al., 2013). The expression of these genes (glpT, uhpT) requires the presence of cAMP. Mutations can reduce cAMP levels in the ptsI or cyaA genes, which also affect the catabolism of various carbohydrates. Furthermore, high-level expression of the uhpT gene requires the regulatory gene uhpA (Falagas et al., 2019). Mutations in any of these regulatory pathway genes diminish antibiotic uptake, leading to varying degrees of fosfomycin resistance (Nilsson et al., 2003). Notably, such mutations often incur a significant “fitness cost,” such as the inability of bacteria to metabolize carbon sources such as G3P and G6P. This may explain why the clinical resistance rate for treating uncomplicated urinary tract infections is considerably lower than that observed in vitro (Silver, 2017). However, for some pathogens, such as P. aeruginosa, GlpT is the sole functional fosfomycin transporter; consequently, the fitness cost associated with its mutation might be lower, making it a clinically significant resistance mechanism (Pipitone et al., 2023). In Listeria monocytogenes, bacteria exhibit intrinsic resistance to fosfomycin due to the absence of antibiotic transporters. However, the virulence factor Hpt (a glucose-6-phosphate permease) mediates fosfomycin uptake, conferring antibiotic susceptibility during infection (Castañeda-García et al., 2013).
3.1.2 Fosfomycin target modification
Modification of the antibiotic target MurA constitutes another mechanism leading to fosfomycin resistance. Mutations in the murA gene, which encodes the MurA enzyme, can result in the substitution of the active site cysteine (Cys115) with other amino acids, such as Cys115Asp or Cys115Glu, thereby reducing its affinity for fosfomycin (Kim et al., 1996). Additionally, overexpression of the murA gene can dilute the intracellular fosfomycin concentration, effectively lowering the drug level within the cell and enabling bacteria to acquire resistance at a low fitness cost (Couce et al., 2012). Due to the absence of MurA, Pseudomonas species are considered to have inherent resistance to fosfomycin; however, reports indicate variability in fosfomycin activity, with 61% of P. aeruginosa isolates being susceptible to fosfomycin (MIC ≤ 64 μg/ml) (Humphries et al., 2021). However, mutations in the murA gene are rare in clinical isolates. This rarity is attributed to the isolation of murA mutants in mutagenized E. coli by Wu and Venkateswaran (1974) and the identification of two murA mutants in a Japanese study of clinical E. coli isolates (Takahata et al., 2010).
3.2 Plasmid-mediated resistance
Another critical pathway for clinical fosfomycin resistance is the plasmid-mediated production of modifying enzymes. Diverse fosfomycin-modifying enzymes, including FosA and its variants (FosA1–FosA10), FosB, FosC2, FosX, and FosL1–L2, have been identified (Zhang et al., 2025). These enzymes are encoded by the fos gene (located on plasmids) and catalyze the opening of the epoxide ring of fosfomycin, rendering it inactive. FosA (glutathione S-transferase) is the most prevalent modifying enzyme and is widespread in the Enterobacteriaceae. It inactivates fosfomycin by catalyzing the addition of glutathione to its epoxide ring. Among these, the FosA3 variant is the most widely disseminated acquired fosfomycin resistance determinant and is speculated to originate from Kluyvera georgiana (Ito et al., 2018; Yu et al., 2024). According to a study of 350 strains of extended-spectrum β-lactamase (ESBL)-producing E. coli in Mexico, 60.5% of fosfomycin-resistant strains harbored fos genes, with 60% of these carrying fosA3 (Galindo-Méndez et al., 2022). FosB (bacillithiol/L-cysteine transferase), which is primarily found in gram-positive bacteria such as Staphylococci, Bacilli, and Enterococci, inactivates fosfomycin by adding bacillithiol or L-cysteine (Bruce et al., 2022; DiCicco et al., 2014; Haenni et al., 2012). FosC2, an enzyme structurally similar to FosA, catalyzes the phosphorylation of fosfomycin in the presence of ATP to inactivate it (Wachino et al., 2010). FosX (epoxide hydrolase) functions similarly to other Fos enzymes, inactivating fosfomycin by adding water at the C1 position to open the epoxide ring (Castañeda-García et al., 2013). FosL1 and FosL2 are recently described novel glutathione-S-transferases with 63% identity to FosA8, exhibiting high-level resistance to fosfomycin (Kieffer et al., 2020).
The most concerning trend in fosfomycin resistance today is not only the presence of fos genes but also their frequent colocalization on the same plasmids with other critical resistance genes, particularly those encoding ESBLs such as blaCTX–M and blaNDM (Kaewnirat et al., 2022; Zhang et al., 2025). FosA3 is most commonly found on conjugative plasmids that carry CTX-M subtype ESBL genes. It may be cotransferred via shared mobile genetic elements (He et al., 2013; Loayza-Villa et al., 2020; Yao et al., 2016), implying that the selective pressure exerted by one class of antibiotics (such as cephalosporins or carbapenems) can coselect for and promote the dissemination of plasmids that also carry fos genes, enabling pathogens to develop resistance to multiple antibiotics simultaneously, thereby severely compromising our therapeutic arsenal. Consequently, fosfomycin combination therapy is not only a sound therapeutic option but also an imperative strategy for combating pathogens harboring multidrug-resistant plasmids.
4 Combination therapy with fosfomycin to combat bacterial resistance
4.1 Synergy with β-lactams: sequential inhibition of cell wall synthesis
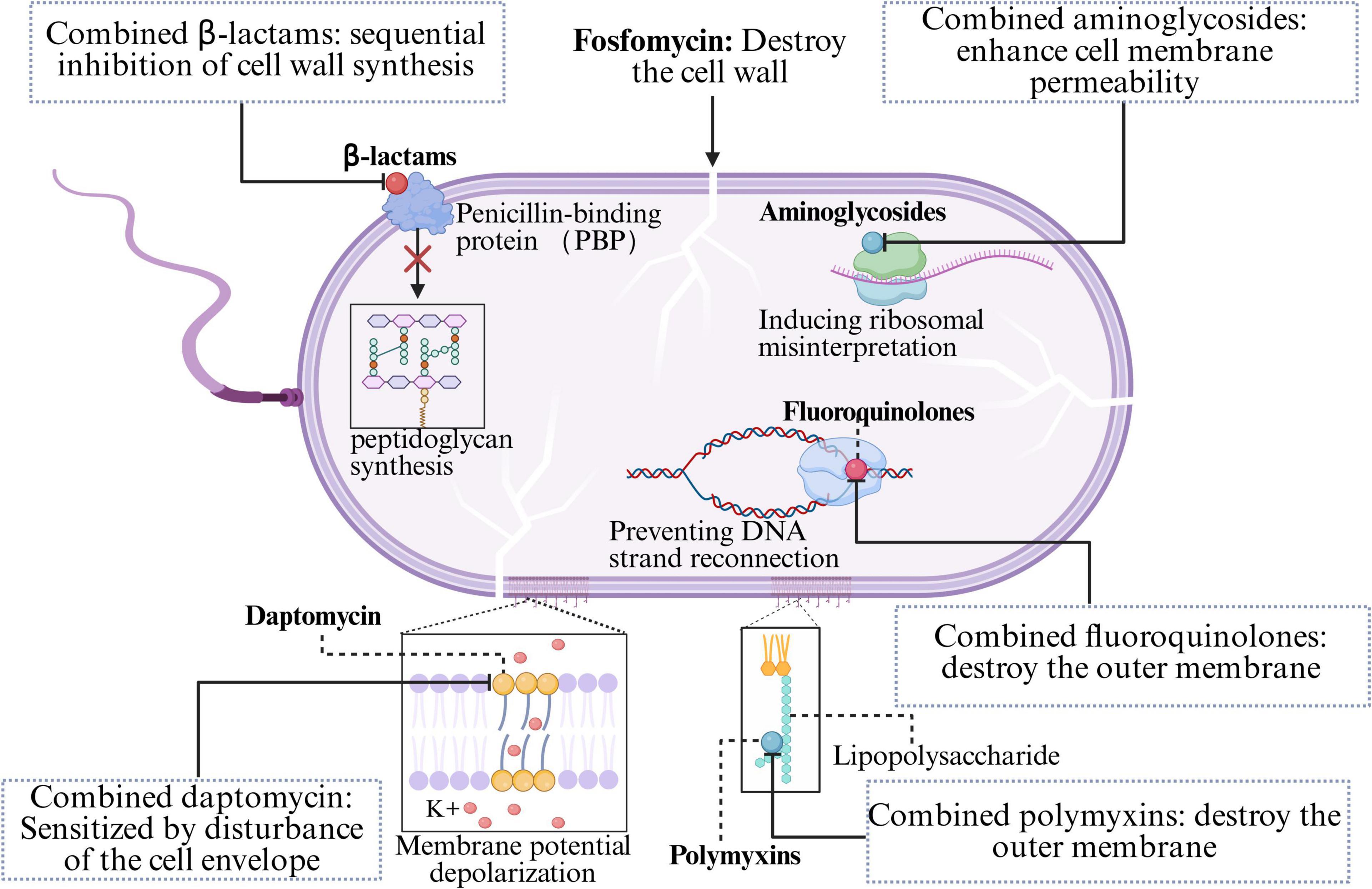
β-Lactam antibiotics (carbapenems, cephalosporins, penicillins, and monobactams) constitute an exceptionally broad class of antibiotics characterized by a β-lactam ring in their chemical structure, which inhibits peptidoglycan synthesis (Flores-Kim et al., 2019). The β-lactam ring binds to the active site of penicillin-binding proteins (PBPs), thereby blocking PBP-catalyzed transpeptidation (cross-linking), which results in weakened bacterial cell walls and subsequent cell lysis (Flores-Kim et al., 2019; Mora-Ochomogo and Lohans, 2021). Due to their safety profile and broad spectrum of activity, β-lactams and newer β-lactamase inhibitor combinations remain among the most reliable and effective antibiotic classes globally for treating both simple and severe infections (Venuti et al., 2023). However, the rapid global spread of resistance challenges the efficacy of single antibiotic classes (Munoz-Price et al., 2013). Since fosfomycin inhibits the initial and critical step of bacterial cell wall synthesis (the MurA enzyme), it renders the cell wall biosynthesis system in a “fragile” state, characterized by enhanced permeability. In contrast, β-lactams exert their effect by irreversibly binding to PBPs, thereby inhibiting the final cross-linking/transpeptidation steps. This “front-and-rear attack” strategy induces severe cell wall stress and increases bacterial susceptibility to lysis, resulting in rapid and synergistic bactericidal activity (Figure 2; Bakthavatchalam et al., 2020; Silver, 2017). Furthermore, fosfomycin-induced cell wall stress may modulate bacterial stress response pathways, altering the expression and distribution of cell membrane and cell wall components, which in turn improves the accessibility of β-lactams to their PBPs. Additionally, studies have shown that the combination of fosfomycin and β-lactams leads to a more pronounced reduction in the functional expression or activity of PBPs, further elucidating the mechanistic basis for their synergistic antibacterial effect (del Río et al., 2016).
4.1.1 Combinations with carbapenems
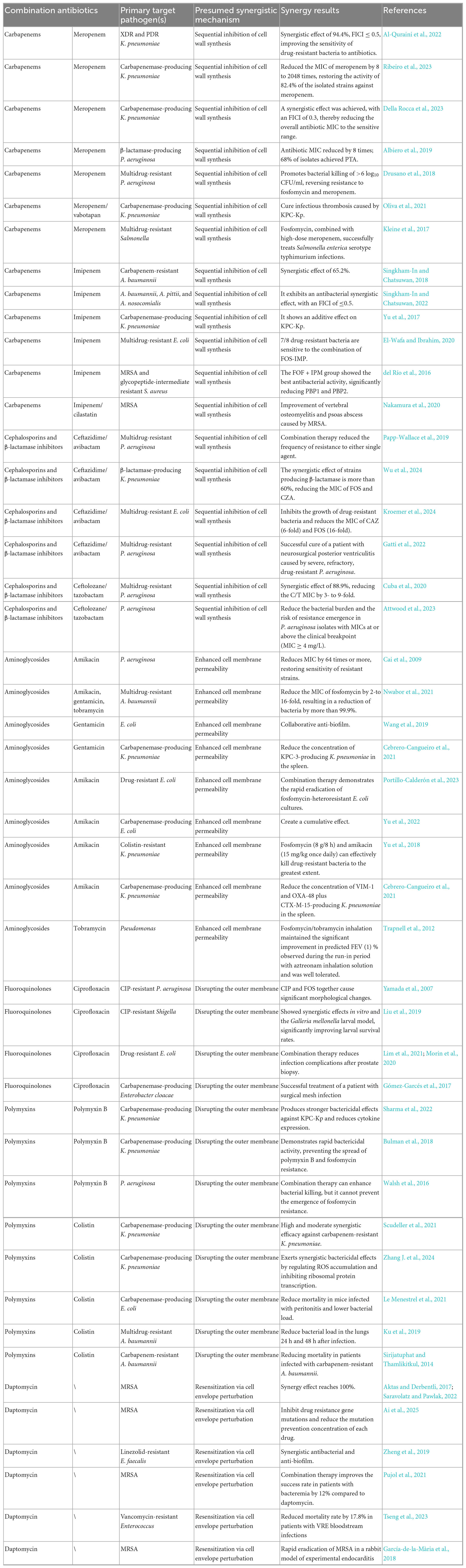
Carbapenems (such as meropenem and imipenem) are considered last-resort antibiotics for treating bacterial infections. They are widely used to manage severe infections, including hospital-acquired pneumonia (HAP), complicated intra-abdominal infections (cIAIs), and bloodstream infections (BSIs) (Zhang S. et al., 2024). Consequently, resistance to carbapenems poses a serious clinical challenge. Substantial evidence has demonstrated that combination therapy with fosfomycin and carbapenem antibiotics has significant synergistic effects and has potential for combating resistant bacteria (Adaleti et al., 2023; Al-Quraini et al., 2022).
Meropenem (MEM), a broad-spectrum carbapenem with potent activity against gram-negative bacteria, has synergistic potential with fosfomycin against carbapenem-resistant Enterobacterales (CRE). For example, Al-Quraini et al. (2022) used a FOS-MEM combination against extensively drug-resistant (XDR) and pandrug-resistant (PDR) Klebsiella pneumoniae. Despite harboring a formidable array of resistance genes, including β-lactamases (blaSHV–11, blaTEM–1b, blaCTX–M–15, blaOXA–232) and fosfomycin resistance determinants (fosA5, fosA6, mutated uhpT), excellent combination therapy (94.4%) with a fractional inhibitory concentration index (FICI) ≤ 0.50 was observed. Another study used the same combination against carbapenemase-producing K. pneumoniae (KPC-Kp), which reduced the meropenem MIC by 8- to 2048-fold, restoring meropenem susceptibility in 82.4% of the isolates (Ribeiro et al., 2023). Furthermore, the FOS-MEM combined regimen achieved 100% survival in a Galleria mellonella larval model. Albiero et al. (2016) also demonstrated a good combination regimen with FOS-MEM against KPC-2-producing K. pneumoniae, reducing both meropenem and fosfomycin MICs to susceptible ranges. Additionally, a recent clinical case reported the successful cure of postneurosurgical ventriculitis caused by KPC-producing K. pneumoniae via a combination of meropenem/vaborbactam (MVB) and intravenous fosfomycin (Volpicelli et al., 2024), further confirming the clinical utility of FOS-MEM combinations. In addition to K. pneumoniae, fosfomycin combined with meropenem also demonstrates effective synergistic bactericidal activity in vitro and in vivo against other Gram-negative pathogens, including β-lactamase-producing P. aeruginosa and carbapenem-resistant E. coli (Albiero et al., 2019; Drusano et al., 2018; Suich et al., 2022).
The imipenem (IPM)-fosfomycin combination has similar synergistic potential against carbapenem-resistant gram-negative bacteria. Studies have reported that FOS-IPM achieves synergistic effects against carbapenem-resistant Acinetobacter baumannii (CRAB) in 65.2% of cases, indicating that FOS-IPM is more effective than imipenem combined with other antibiotics, even in fosfomycin-resistant isolates (MIC ≥ 64 mg/L) (Singkham-In and Chatsuwan, 2018). Another study revealed that the combination of imipenem-relebactam with fosfomycin had a synergistic effect on 60% of 100 carbapenem-resistant gram-negative isolates and was additive on 40%, exhibiting synergistic activity against all the tested K. pneumoniae and A. baumannii isolates (Xu et al., 2022). Among the carbapenem-resistant Acinetobacter calcoaceticus-baumannii (ACB) complex isolates, while fosfomycin monotherapy resulted in insufficient killing of A. baumannii, A. pittii, and A. nosocomialis, the FOS-IPM combination significantly enhanced antibacterial efficacy, with a FICI of ≤0.5 (Singkham-In and Chatsuwan, 2022), demonstrating its utility against carbapenem-resistant ACB infections. Furthermore, a clinical case highlights FOS-IPM as a potential salvage therapy for MRSA infections (Nakamura et al., 2020). Continuous administration of imipenem/cilastatin (1.5 g daily) and fosfomycin (4.0 g daily) for 4 weeks improved vertebral osteomyelitis and a psoas abscess caused by MRSA, with E-test combination therapy tests confirming the optimal co-administration combination of imipenem/cilastatin and fosfomycin. Collectively, these studies demonstrate the potential of fosfomycin as an adjuvant to carbapenems, which jointly inhibit cell wall synthesis and reduce the rates of resistance.
4.1.2 Combinations with cephalosporins and β-lactamase inhibitors
Compared with other types of antibiotics, cephalosporins represent a highly efficacious and indispensable class of β-lactam antibiotics, offering a broader spectrum of antimicrobial activity and fewer side effects, and are often utilized for mild to severe infectious diseases (Lin and Kück, 2022). However, the overuse and misuse of cephalosporins for prophylaxis, therapy, or food production have significantly contributed to the emergence of numerous drug-resistant pathogens. The development of β-lactamase inhibitors has helped preserve the efficacy of β-lactams against β-lactamase-producing pathogens. Novel cephalosporin/β-lactamase inhibitor combinations, such as ceftazidime/avibactam (CZA) and ceftolozane/tazobactam (C/T), are particularly important for treating MDR gram-negative pathogens (Katsarou et al., 2025).
Ceftazidime/avibactam has been employed as a first-line therapy for carbapenem-resistant K. pneumoniae (CRKP) infections. However, it is notable that the rate of resistance to CZA is also increasing (Wang et al., 2020; Zhang et al., 2020). Studies have revealed that the combination of CZA with FOS restores antimicrobial susceptibility in MDR P. aeruginosa by targeting PBP3, Pseudomonas-derived cephalosporinase (PDC), and MurA (Winkler et al., 2015). Subsequently, Papp-Wallace et al. (2019) demonstrated the efficacy of the CZA-FOS combination against the MDR P. aeruginosa clinical isolate CL232, which harbored mutations conferring β-lactam resistance and exhibited upregulated expression of blaPDC, the mexAB-oprM efflux pump, and murA. Combination regimen with CZA-FOS reduced the frequency of resistance to either single agent. Furthermore, Tüzemen et al. (2024) reported that the FOS-CZA combination exhibited enhanced antibacterial activity against CRKP, achieving combination treatment in 63.6% of isolates, with susceptibility rates of 89.1% to CZA and 47.3% to FOS among CRKP isolates. Another study reported similar synergistic results (>60%) when the FOS-CZA combination was used against MBL-producing K. pneumoniae (Wu et al., 2024). Unlike ceftazidime/avibactam, ceftolozane/tazobactam (C/T) has enhanced activity against certain AmpC β-lactamases and P. aeruginosa (Cho et al., 2015). The synergistic effect of C/T and FOS against MDR P. aeruginosa was reported to be 88.9% (24/27), reducing the C/T MIC by 3- to 9-fold, despite the strains being resistant to C/T and FOS individually (Cuba et al., 2020). Attwood et al. (2023) demonstrated that adding fosfomycin or tobramycin to C/T at simulated human clinically observed concentrations reduced the bacterial burden and the risk of resistance emergence in P. aeruginosa isolates with MICs at or above the clinical breakpoint (MIC ≥ 4 mg/L).
Although promising in vitro data exist for the use of fosfomycin combined with cephalosporins against MDR pathogens, clinical cases reporting on this combination appear limited. In one case, clinical cure was achieved in a patient with post-neurosurgical ventriculitis caused by severe, refractory MDR P. aeruginosa (FOS MIC = 64 mg/L) treated with a continuous infusion of CZA and FOS, and reached optimal PK/PD targets (Gatti et al., 2022). However, a retrospective study reported mortality in all six patients with P. aeruginosa infections treated with the combination of FOS and CZA (Anastasia et al., 2023). Therefore, MDR P. aeruginosa, particularly in immunocompromised patients and despite susceptibility at high doses, remains a significant threat. Further studies are warranted to optimize combination therapy with fosfomycin and confirm its efficacy.
4.2 Synergy with aminoglycosides: enhanced cell membrane permeability
Aminoglycoside antibiotics (amikacin, tobramycin, gentamicin) constitute a vital component of the therapeutic arsenal for certain bacterial infections, particularly those caused by aerobic, Gram-negative pathogens, characterized by their broad spectrum, rapid action, and low allergenic potential (Le et al., 2023). The antibacterial mechanism involves the irreversible binding of the compound to the bacterial 30S ribosomal subunit, resulting in misreading during protein translation. This results in the production of aberrant proteins, which further disrupts cell membrane integrity, leading to the leakage of cellular contents and facilitating a massive influx of aminoglycoside molecules into the cytoplasm, ultimately resulting in rapid, concentration-dependent bactericidal killing (Davis, 1988; Taber et al., 1987). When combined with fosfomycin, the inhibition of cell wall synthesis by fosfomycin compromises bacterial cell membrane integrity, thereby increasing bacterial permeability (Figure 2). This facilitates the enhanced entry of aminoglycosides into the cell, allowing greater access to their ribosomal targets.
Reports of in vitro combination therapy between fosfomycin and aminoglycosides are highly consistent. Studies have demonstrated significant synergistic effects of fosfomycin combined with amikacin or isepamicin against resistant P. aeruginosa, markedly reducing aminoglycoside MICs by up to 64-fold or more (Cai et al., 2009). This not only restores susceptibility in highly resistant strains but also allows for the use of lower, less toxic doses. Time-kill kinetics confirmed the synergistic bactericidal activity of fosfomycin combined with aminoglycosides (amikacin, gentamicin, and tobramycin) against fosfomycin-resistant A. baumannii (MIC ≥ 128 μg/mL), resulting in >99.9% bacterial reduction and a 2- to 16-fold decrease in the fosfomycin MIC (Nwabor et al., 2021). Furthermore, the combination of fosfomycin and gentamicin was shown to act synergistically against E. coli biofilm strains (Wang et al., 2019). In a hollow-fiber infection model (HFIM), co-administration with fosfomycin (8 g/8 h) and amikacin (15 mg/kg every 24 h) achieved rapid eradication of fosfomycin-heteroresistant E. coli cultures, whereas neither fosfomycin nor amikacin monotherapy was effective in sterilizing the cultures (Portillo-Calderón et al., 2023). These results further support the notion that combinations of aminoglycosides and fosfomycin can rapidly reduce the bacterial burden and prevent the emergence of resistant subpopulations to antibiotics such as fosfomycin.
In in vivo studies, the combination of fosfomycin and aminoglycosides also demonstrated synergistic antibacterial activity. In a murine peritoneal sepsis model, the combination of fosfomycin and amikacin reduced the spleen concentrations of VIM-1-producing and OXA-48 plus CTX-M-15-producing K. pneumoniae, whereas the combination of fosfomycin and gentamicin reduced the spleen concentrations of KPC-3-producing strains (Cebrero-Cangueiro et al., 2021). Notably, none of these combinations improved survival in mice infected with carbapenemase-producing K. pneumoniae strains. Therefore, the evaluation of combinations of fosfomycin and aminoglycosides for other types of infections warrants further consideration. In patients with cystic fibrosis and Pseudomonas airway infection, fosfomycin/tobramycin inhalation (FTI) significantly improved the predicted FEV1% observed during an aztreonam inhalation solution (AZLI) run-in period and was well tolerated (Trapnell et al., 2012). These findings demonstrate that FTI is a promising therapeutic option for anti-Pseudomonas treatment in cystic fibrosis patients. Additionally, a novel composite susceptibility breakpoint threshold for predicting successful combination therapy has been proposed: when the product of the MICs of two drugs is less than 256, successful co-administration can be predicted (Darlow et al., 2021), representing a significant conceptual advancement beyond simple FICI values, offering a more clinically translatable, PK/PD-based threshold for predicting the efficacy of combination therapies.
4.3 Synergy with fluoroquinolones: disrupting the outer membrane
Fluoroquinolone antibiotics (ciprofloxacin, levofloxacin) are potent, synthetically derived broad-spectrum antibacterial agents that exhibit bactericidal activity against both gram-positive and gram-negative bacteria (Rusu et al., 2023). The mechanism relies on targeting two enzymes essential for DNA replication and repair: DNA gyrase and topoisomerase IV (Kabbani et al., 2018; Werner et al., 2011). Fluoroquinolones block DNA strand rejoining by reversibly and noncovalently binding to the cleavage complex at the cleavage-ligation active site, thereby compromising DNA replication (Correia et al., 2017). These irreparable DNA strand breaks trigger the bacterial SOS response, followed by a protein cascade that ultimately leads to bacterial death (Blondeau, 2004). The synergistic mechanism of combining fluoroquinolones with FOS involves the disruption of the bacterial outer membrane and cell wall integrity by FOS (Figure 2), enhancing the penetration of fluoroquinolones and increasing their bactericidal efficiency.
However, studies have reported that the fluoroquinolone ciprofloxacin (CIP) can disrupt the outer membrane structure itself. Combined CIP and FOS treatment induced significant morphological changes, and adding CIP first rather than FOS first produced a stronger synergistic effect on CIP-resistant P. aeruginosa (Yamada et al., 2007). In a study of CIP-resistant Shigella isolates, the CIP-FOS combination demonstrated synergistic effects both in vitro and in a Galleria mellonella larval model, significantly improving larval survival (Liu et al., 2019). Furthermore, studies indicate that combined FOS-CIP therapy reduces the incidence of postoperative infectious complications. Following transrectal ultrasound-guided prostate biopsy (TRUSPB), co-administration of CIP and FOS was associated with fewer infectious complications (0.3%), suggesting its potential applicability in the Era of high rectal flora resistance (Lim et al., 2021). A retrospective study revealed that the primary infectious outcome after prostate needle biopsy (PNB) was urosepsis. The incidence of urosepsis was 1.1% (12/1090) with CIP alone, which was reduced to 0.2% (2/1197) with CIP-FOS combination therapy (Morin et al., 2020). Additionally, Gómez-Garcés et al. (2017) described a case of infection involving surgical mesh by a carbapenemase-producing Enterobacter cloacae strain that was resistant to various antimicrobial regimens and was successfully treated with a combination of ciprofloxacin and fosfomycin. Collectively, these results demonstrate the prophylactic and synergistic antibacterial potential of ciprofloxacin-fosfomycin combination therapy against resistant strains, particularly CIP-resistant Enterobacterales.
4.4 Synergy with polymyxins: disrupting the outer membrane
Polymyxins are polypeptide antibiotics that target aerobic, Gram-negative pathogens; however, only two are currently available: polymyxin E (colistin) and polymyxin B (El-Sayed Ahmed et al., 2020). The core mechanism involves the binding of the hydrophilic (positively charged) moiety of polymyxin molecules to the anionic lipopolysaccharide (LPS) in the outer membrane of Gram-negative bacteria, which disrupts the outer membrane’s structure and function, facilitating further penetration into the inner membrane and ultimately leading to bacterial cell death (Kaye et al., 2016). This action significantly enhances FOS uptake into bacterial cells (Figure 2).
Mechanism-based models suggest that, compared to monotherapy, the combination of fosfomycin and polymyxin B (PMB) exhibits greater bactericidal efficacy against KPC-2-producing K. pneumoniae (KPC-Kp) (Sharma et al., 2022). Furthermore, the combination therapy achieved greater reductions in cytokine expression. In a hollow-fiber infection model, monotherapy resulted in >3 log10 CFU/mL killing of KPC-Kp within 3 h; however, resistant subpopulations regrew and proliferated by 48 h. In contrast, the PMB-FOS combination achieved rapid bactericidal killing (>6 log10 CFU/mL reduction) while preventing the emergence of resistance to both PMB and FOS (Bulman et al., 2018). In P. aeruginosa, the combination of FOS and PMB increased bacterial killing but did not suppress the emergence of fosfomycin resistance (Walsh et al., 2016). Additionally, Scudeller et al. (2021) described high and moderate synergy rates (high synergy, ES ≥ 0.75; moderate synergy, 0.35 < ES < 0.75) for FOS combined with colistin (COL) against CRKP through a systematic review and meta-analysis. Compared with monotherapy, time-kill assays demonstrated that FOS combined with COL exhibited superior bactericidal activity against KPC-Kp (Zhang J. et al., 2024). The FOS-COL combination also increased the survival of KPC-Kp-infected Thp-1 cells while reducing their cytotoxicity and resistance rates. Moreover, this study revealed that the synergistic bactericidal effect of FOS-COL involves the modulation of ROS accumulation and the suppression of ribosomal protein transcription (Zhang J. et al., 2024). Recently, Khalifa et al. (2025) confirmed the synergistic activity of the FOS-COL combination against E. coli and Salmonella strains via time-kill assays, further supporting its potential against multidrug-resistant E. coli and Salmonella.
In murine models, combinations of fosfomycin and polymyxin have also demonstrated significant synergistic effects. In an NDM-1-producing E. coli mouse peritonitis infection model, compared with monotherapy, the combination of FOS with COL resulted in reduced mortality and lower bacterial counts in the spleen in all strains tested (Le Menestrel et al., 2021). Furthermore, the combination prevented the selection of resistant mutants. In a murine model of multidrug-resistant A. baumannii pneumonia, compared with monotherapy, FOS combined with COL significantly reduced lung bacterial loads at 24 and 48 h (Ku et al., 2019). A clinical study further demonstrated that compared with COL monotherapy, FOS combined with COL yielded more favorable microbiological outcomes in patients infected with carbapenem-resistant A. baumannii (CRAB), along with trends toward improved clinical outcomes and lower mortality (Sirijatuphat and Thamlikitkul, 2014). Although fosfomycin-polymyxin combinations demonstrate objective efficacy, future multicenter studies with larger patient cohorts are warranted to definitively establish their benefit for patients infected with carbapenem-resistant strains.
4.5 Synergy with daptomycin: resensitization via cell envelope perturbation
Daptomycin (DAP) is a critical cyclic lipopeptide antibiotic used to treat infections caused by gram-positive bacteria (Miller et al., 2016). DAP has a distinct mechanism of action: it binds in a calcium-dependent manner to the bacterial membrane lipids phosphatidylglycerol (PG) and cardiolipin (CL), inserts into the lipid bilayer, and forms transmembrane ion channels, resulting in rapid depolarization of the membrane potential and subsequent bactericidal killing (Huang, 2020). In recent years, reports of daptomycin resistance in some gram-positive pathogens (such as S. aureus, Enterococcus faecium, and Enterococcus faecalis) have increased. In daptomycin-resistant MRSA and VRE, resistance is often associated with adaptive changes in the cell envelope, such as increased surface positive charge, altered membrane fluidity, and redistribution of anionic cardiolipin microdomains. Fosfomycin appears to counteract these adaptations by disrupting the bacterial membrane potential (Hall Snyder et al., 2016; Mishra et al., 2022). It perturbs the cell envelope, reducing the net surface positive charge and altering membrane fluidity and cardiolipin localization, thereby resensitizing bacteria to the membrane-depolarizing effects of daptomycin (Figure 2).
Numerous in vitro studies have reported significant synergy between FOS and DAP against MRSA, with synergy rates of up to 100% (Aktas and Derbentli, 2017; Saravolatz and Pawlak, 2022). This combination not only enhances the killing of both daptomycin-susceptible and daptomycin-resistant MRSA but also effectively prevents the evolution of resistance and resensitizes resistant strains (Mishra et al., 2022). Recently, Ai et al. (2025) reported that the FOS-DAP combination therapy against MRSA suppressed the emergence of resistance-conferring mutations and lowered the minimum inhibitory concentration of each drug in mutants. They noted that mutations in the mprF and murA genes were detected in the DAP and FOS monotherapy groups, respectively, whereas no such mutations were found in the combination group. These findings provide crucial insights into preventing the evolution of MRSA resistance.
Furthermore, DAP combined with FOS demonstrated superior antibacterial and antibiofilm effects against both planktonic and adherent linezolid-resistant E. faecalis isolates (Zheng et al., 2019). DAP is approved for treating MRSA bacteremia and endocarditis in the clinic (Shaw et al., 2015). A randomized clinical trial demonstrated a 12% higher treatment success rate for patients with MRSA bacteremia who received DAP-FOS compared to those who received DAP alone, with fewer cases of clinical or microbiological failure observed in the combination group (Pujol et al., 2021). Another clinical trial reported that FOS-DAP co-administration reduced mortality by 17.8% in patients with vancomycin-resistant Enterococcus (VRE) bloodstream infections, with particularly significant effects in patients with lower Pitt bacteremia scores or FOS MICs ≤ 64 mg/L (Tseng et al., 2023). The combination of DAP and FOS was also shown to be synergistic and rapidly bactericidal against MRSA in a rabbit model of experimental endocarditis (García-de-la-Mària et al., 2018).
However, combined regimens are often accompanied by adverse events, which can reduce treatment success and limit clinical utility. Hypernatremia and hypokalemia, which are related to sodium overload, are the most common concerns. Studies indicate that compared with daptomycin monotherapy, fosfomycin combination therapy leads to higher rates of hypernatremia and hypokalemia (Tseng et al., 2023). The high sodium salt content of fosfomycin is likely responsible for the significantly increased incidence of hypernatraemia. Hypokalemia appears to be associated with increased renal excretion of fosfomycin in the distal renal tubules. Consequently, optimizing the pharmacodynamics of fosfomycin combination regimens is imperative for future use. Table 2 summarizes the key studies on fosfomycin combination therapy.
5 Clinical application and translational challenges
Despite promising preclinical data for fosfomycin combination therapy, significant obstacles impede the effective translation of these findings into routine clinical practice. This section critically examines these challenges.
5.1 Clinical evidence from case reports and trials
Although reports on fosfomycin combination therapies are increasingly encountered in clinical practice, much of the current evidence still originates from isolated case reports or small series. Examples include successful treatment of neurosurgical ventriculitis caused by KPC-producing K. pneumoniae, vertebral osteomyelitis due to MRSA, and postoperative infections caused by Enterobacter cloacae (Gómez-Garcés et al., 2017; Nakamura et al., 2020; Volpicelli et al., 2024). While these reports are encouraging, they generally lack direct comparison with non-fosfomycin-containing regimens. This limitation restricts the strength of clinical inferences and makes it difficult to attribute outcomes solely to fosfomycin use. Only a limited number of comparative studies are available. For instance, in the context of prostate biopsy prophylaxis, the combination of ciprofloxacin with fosfomycin significantly reduced the incidence of sepsis compared to ciprofloxacin monotherapy (Lim et al., 2021). Similarly, fosfomycin combined with colistin demonstrated a trend toward improved microbiological clearance and reduced mortality in infections caused by carbapenem-resistant organisms (Sirijatuphat and Thamlikitkul, 2014). Furthermore, a randomized clinical trial demonstrated that combining daptomycin with fosfomycin for MRSA bacteremia resulted in a 12% higher success rate compared to daptomycin alone, and led to a 17.8% reduction in mortality in vancomycin-resistant Enterococcus bloodstream infections (Pujol et al., 2021). These data provide useful preliminary comparative evidence, but their scope remains limited.
In summary, the clinical evidence supporting fosfomycin combination regimens remains incomplete and often lacks robustness. Due to the observational nature of most studies, heterogeneity in patient populations, and the absence of standardized comparators, the majority of available reports do not permit definitive conclusions. Therefore, it is essential to clearly acknowledge these limitations and conduct larger, multicenter randomized controlled trials to validate the efficacy and safety of fosfomycin-based combinations across various infection types and resistant pathogens.
5.2 Efficacy disconnect
A disconnect exists between the in vitro and in vivo efficacy of fosfomycin combinations, as notably exemplified in cases of S. aureus bacteremia. Although in vitro studies have demonstrated robust synergy and a solid mechanistic basis for combining fosfomycin with antistaphylococcal agents (such as β-lactams and daptomycin) (Aktas and Derbentli, 2017; del Río et al., 2016; Saravolatz and Pawlak, 2022), a recent systematic review and meta-analysis of three randomized controlled trials (RCTs) by Mourad et al. (2025) concluded that combination therapy did not significantly improve patient mortality (RR 0.85) or reduce rates of persistent bacteremia. This discrepancy may stem from several factors: (1) Patient and infection complexity: Randomized controlled trial (RCT) populations exhibit high heterogeneity, and the complexity of infections (such as endocarditis and deep-seated abscesses) far exceeds that of standardized laboratory models; (2) Suboptimal dosing regimens: The dosing regimens used in trials may fail to achieve the PK/PD targets required for synergy at the infection site; (3) Adverse events (AEs): The meta-analysis revealed a nonsignificant trend toward greater treatment discontinuation due to AEs in the combination arms, which may have potentially confounded the efficacy assessment. In contrast, fosfomycin combinations have a more positive outlook for MDR gram-negative infections. A 2024 review of clinical studies in severe gram-negative infections reported clinical success rates of approximately 75%–80% for fosfomycin combination therapy regimens, even in patients with MDR pathogens (Butler et al., 2024). These findings suggest that fosfomycin combinations can yield synergistic effects that are comparable to or better than those of other common combination therapies.
5.3 Standardization gap
The lack of standardization presents a pervasive challenge. First, standardized, readily available susceptibility testing methods are lacking. Both the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommend agar dilution supplemented with G6P as the reference method for fosfomycin susceptibility testing (Pipitone et al., 2023). However, this method is laborious and time-consuming and is not routinely performed in most clinical laboratories. Alternative methods, such as broth microdilution, E-test, and disk diffusion, exhibit poor consistency and lack universal endorsement by authorities, including the CLSI and EUCAST (Smith et al., 2020). Second, validated clinical susceptibility breakpoints are lacking. Clinically validated fosfomycin breakpoints have not been established for many significant pathogens, including P. aeruginosa and A. baumannii (Zheng et al., 2022). Clinicians and researchers are often compelled to utilize E. coli breakpoints and extrapolate to other pathogens, which can be risky. Finally, the impossibility of uniformly defining patient inclusion criteria and microbiological outcomes across different study centers complicates the execution of high-quality, large-scale RCTs. This results in a scarcity of robust RCT data, hindering the establishment of optimal, approved dosing regimens for severe systemic infections. In the absence of optimized dosing and clear evidence of clinical benefit, clinicians remain cautious about the clinical use of these combination regimens.
5.4 Dosing dilemma
The dosing of fosfomycin presents a persistent clinical conundrum, largely due to the absence of standardized dosing guidelines. Oral fosfomycin is approved only as a single 3 g dose for uncomplicated urinary tract infections (UTIs), as its bioavailability is insufficient for systemic infections. Systemic infections require high-dose intravenous (IV) fosfomycin (Dijkmans et al., 2017). For severe infections caused by MDR organisms, there is currently no consensus on the optimal IV dosing regimen, with proposals ranging from 8 to 12 g/day for gram-positive pathogens to 16–24 g/day for gram-negative pathogens (Candel et al., 2019). High-dose fosfomycin carries the risk of saline overload (1 g of fosfomycin disodium provides 0.33 g of sodium), potentially causing patient intolerance and treatment discontinuation. Furthermore, in critically ill patients, particularly those with renal impairment, fosfomycin pharmacokinetics exhibit high interindividual variability. This makes standardized dosing unreliable and necessitates PK/PD-guided therapeutic drug monitoring (TDM) to inform individualized dosing (Wu et al., 2024).
6 Prospects
As an “old drug,” fosfomycin has gained renewed importance in the current antimicrobial resistance crisis because of its unique mechanism of inhibiting cell wall synthesis and its activity against diverse multidrug-resistant strains. However, its inherent susceptibility to resistance development during monotherapy indicates that its future is inextricably linked to combination regimens. Extensive preclinical research, encompassing in vitro studies and animal models, provides compelling evidence in support of various fosfomycin combination regimens. Combinations of β-lactams, aminoglycosides, fluoroquinolones, polymyxins, and daptomycin, which are based on well-defined synergistic mechanisms (such as sequential blockade, enhanced penetration, and resensitization), have demonstrated potent synergistic bactericidal activity and an effective capacity to suppress the development of resistance. These findings offer a solid theoretical foundation and promise for clinical application. Nevertheless, the path from the laboratory to the clinic is fraught with challenges, necessitating further planning for future directions.
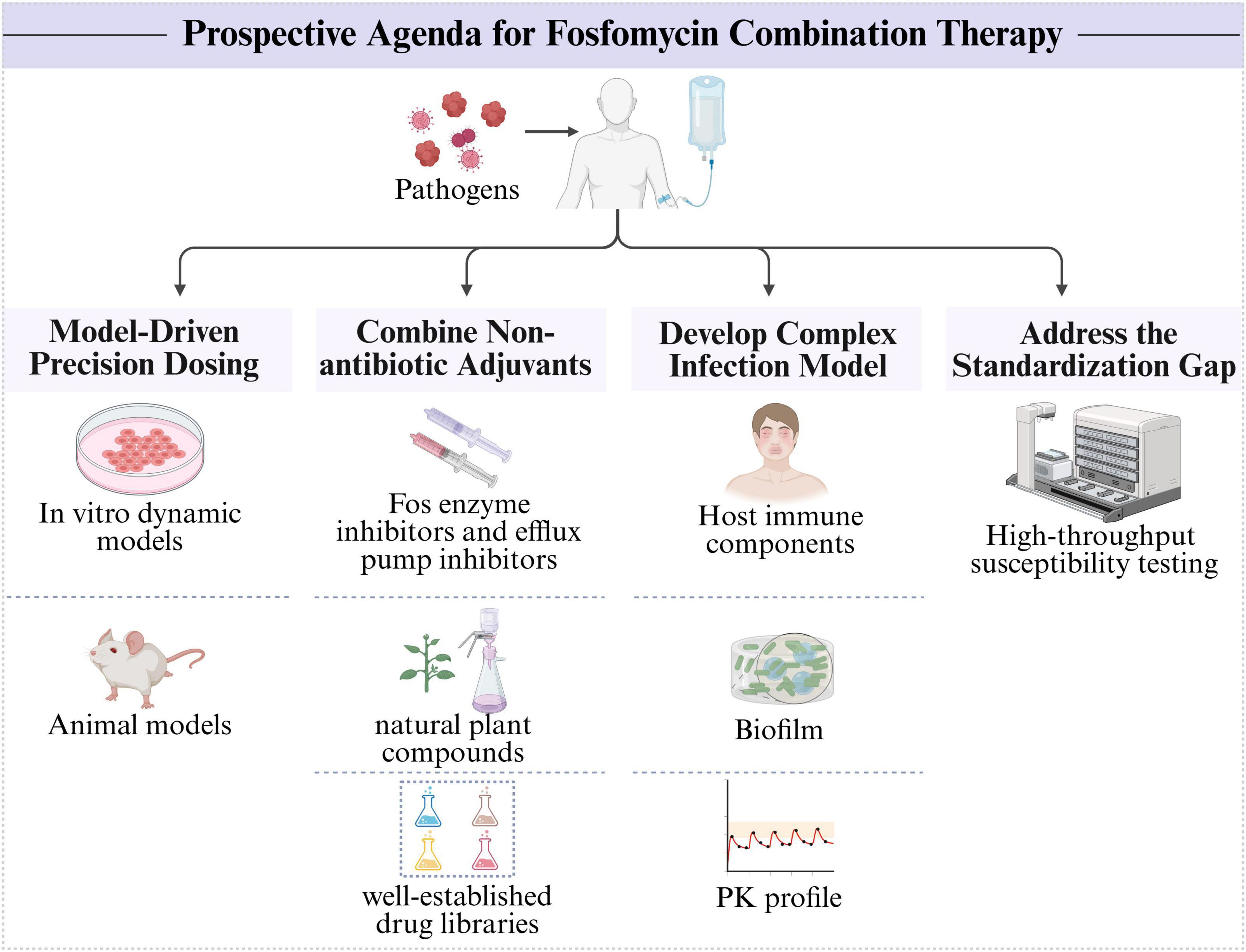
To unlock the full potential of fosfomycin combination therapy against MDR pathogens and advance its clinical application, we propose a prospective research agenda (Figure 3). First, the future of fosfomycin combination therapy hinges on a transition from empirical dosing to model-informed precision dosing. Future efforts should utilize in vitro dynamic models (such as the hollow-fiber infection model) and animal models to comprehensively understand the PK/PD properties of drugs, thereby enabling dosing regimens that provide maximal synergy while minimizing toxicity and resistance development (Wale et al., 2024). Second, the discovery and identification of nonantibiotic adjuvants that potentiate fosfomycin activity (such as FOS enzyme inhibitors and efflux pump inhibitors) to assist in fosfomycin therapy are crucial (Dhanda et al., 2023). High-throughput screening of well-established drug libraries and exploration of natural plant compounds represent important avenues for discovering unexpected synergistic partners (Tiwana et al., 2024). Furthermore, there is a need for improved in vitro and in vivo models that better simulate the complexity of human infections (such as by integrating host immune components, biofilm formation, and realistic PK profiles) to increase the predictive value of preclinical synergy studies (Wale et al., 2024). Finally, the standardization gap needs to be addressed. Basic researchers can contribute by developing and validating new, more reliable, high-throughput susceptibility testing methods that could form the basis for future clinical standards.
Author contributions
YW: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. JL: Writing – review & editing. FQ: Conceptualization, Supervision, Writing – review & editing. JG: Supervision, Writing – review & editing. LZ: Methodology, Writing – review & editing. XJ: Conceptualization, Methodology, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (32170119) and Funded by CMC Excellent-Talent Program (2024kjTzn01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adaleti, R., Nakipoglu, Y., Arici, N., Kansak, N., Calik, S., Senbayrak, S., et al. (2023). [Evaluation of in vitro efficacy of meropenem/colistin and meropenem/fosfomycin combinations on multidrug resistant gram-negative Bacilli]. Mikrobiyol. Bul. 57, 365–377. doi: 10.5578/mb.20239930
Aghamali, M., Sedighi, M., Zahedi Bialvaei, A., Mohammadzadeh, N., Abbasian, S., Ghafouri, Z., et al. (2019). Fosfomycin: Mechanisms and the increasing prevalence of resistance. J. Med. Microbiol. 68, 11–25. doi: 10.1099/jmm.0.000874
Ai, Q., Wang, S., Chen, Z., Zheng, S., Chen, Z., Zhang, N., et al. (2025). Synergistic antibacterial activity and prevention of drug resistance of daptomycin combined with fosfomycin against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 69:e0160924. doi: 10.1128/aac.01609-24
Aktas, G., and Derbentli, S. (2017). In vitro activity of daptomycin combinations with rifampicin, gentamicin, fosfomycin and fusidic acid against MRSA strains. J. Glob. Antimicrob. Resist. 10, 223–227. doi: 10.1016/j.jgar.2017.05.022
Albiero, J., Mazucheli, J., Barros, J. P. D. R., Szczerepa, M. M. D. A., Nishiyama, S. A. B., Carrara-Marroni, F. E., et al. (2019). Pharmacodynamic attainment of the synergism of meropenem and fosfomycin combination against Pseudomonas aeruginosa producing Metallo-β-Lactamase. Antimicrob. Agents Chemother. 63:e00126-19. doi: 10.1128/AAC.00126-19
Albiero, J., Sy, S. K., Mazucheli, J., Caparroz-Assef, S. M., Costa, B. B., Alves, J. L., et al. (2016). Pharmacodynamic evaluation of the potential clinical utility of fosfomycin and meropenem in combination therapy against KPC-2-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 60, 4128–4139. doi: 10.1128/AAC.03099-15
Al-Quraini, M., Rizvi, M., Al-Jabri, Z., Sami, H., Al-Muzahmi, M., Al-Muharrmi, Z., et al. (2022). Assessment of in-vitro synergy of fosfomycin with meropenem, amikacin and tigecycline in whole genome sequenced extended and pan drug resistant Klebsiella Pneumoniae: Exploring a colistin sparing protocol. Antibiotics 11:153. doi: 10.3390/antibiotics11020153
Anastasia, A., Bonura, S., Rubino, R., Giammanco, G. M., Miccichè, I., Di Pace, M. R., et al. (2023). the use of intravenous fosfomycin in clinical practice: A 5-Year retrospective study in a tertiary hospital in Italy. Antibiotics 12:971. doi: 10.3390/antibiotics12060971
Anderson, G. G., Kenney, T. F., Macleod, D. L., Henig, N. R., and O’Toole, G. A. (2013). Eradication of Pseudomonas aeruginosa biofilms on cultured airway cells by a fosfomycin/tobramycin antibiotic combination. Pathog Dis. 67, 39–45. doi: 10.1111/2049-632X.12015
Antonello, R. M., Principe, L., Maraolo, A. E., Viaggi, V., Pol, R., Fabbiani, M., et al. (2020). Fosfomycin as partner drug for systemic infection management. a systematic review of its synergistic properties from in vitro and in vivo studies. Antibiotics 9:500. doi: 10.3390/antibiotics9080500
Attwood, M., Griffin, P., Noel, A. R., Albur, M., and Macgowan, A. P. (2023). Antibacterial effect of seven days exposure to ceftolozane-tazobactam as monotherapy and in combination with fosfomycin or tobramycin against Pseudomonas aeruginosa with ceftolozane-tazobactam MICs at or above 4 mg/l in an in vitro pharmacokinetic model. J. Antimicrob. Chemother. 78, 2254–2262. doi: 10.1093/jac/dkad230
Bakthavatchalam, Y. D., Shankar, A., Muthuirulandi Sethuvel, D. P., Asokan, K., Kanthan, K., and Veeraraghavan, B. (2020). Synergistic activity of fosfomycin-meropenem and fosfomycin-colistin against carbapenem resistant Klebsiella pneumoniae: An in vitro evidence. Future Sci. OA 6:FSO461. doi: 10.2144/fsoa-2019-0074
Bergan, T. (1990). Degree of absorption, pharmacokinetics of fosfomycin trometamol and duration of urinary antibacterial activity. Infection 18(Suppl. 2), S65–S69. doi: 10.1007/BF01643430
Blondeau, J. M. (2004). Fluoroquinolones: Mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 49(Suppl. 2), S73–S78. doi: 10.1016/j.survophthal.2004.01.005
Borisova, M., Gisin, J., and Mayer, C. (2014). Blocking peptidoglycan recycling in Pseudomonas aeruginosa attenuates intrinsic resistance to fosfomycin. Microb. Drug Resist. 20, 231–237. doi: 10.1089/mdr.2014.0036
Bruce, S. A., Smith, J. T., Mydosh, J. L., Ball, J., Needle, D. B., Gibson, R., et al. (2022). Shared antibiotic resistance and virulence genes in Staphylococcus aureus from diverse animal hosts. Sci. Rep. 12:4413. doi: 10.1038/s41598-022-08230-z
Bulman, Z. P., Zhao, M., Satlin, M. J., Chen, L., Kreiswirth, B. N., Walsh, T. J., et al. (2018). Polymyxin B and fosfomycin thwart KPC-producing Klebsiella pneumoniae in the hollow-fibre infection model. Int. J. Antimicrob. Agents 52, 114–118. doi: 10.1016/j.ijantimicag.2018.02.010
Butler, D. A., Patel, N., O’Donnell, J. N., and Lodise, T. P. (2024). Combination therapy with IV fosfomycin for adult patients with serious Gram-negative infections: A review of the literature. J. Antimicrob. Chemother. 79, 2421–2459. doi: 10.1093/jac/dkae253
Cai, Y., Fan, Y., Wang, R., An, M. M., and Liang, B. B. (2009). Synergistic effects of aminoglycosides and fosfomycin on Pseudomonas aeruginosa in vitro and biofilm infections in a rat model. J. Antimicrob. Chemother. 64, 563–566. doi: 10.1093/jac/dkp224
Candel, F. J., Matesanz David, M., and Barberán, J. (2019). New perspectives for reassessing fosfomycin: Applicability in current clinical practice. Rev. Esp. Quimioter. 32(Suppl. 1), 1–7.
Castañeda-García, A., Blázquez, J., and Rodríguez-Rojas, A. (2013). Molecular mechanisms and clinical impact of acquired and intrinsic fosfomycin resistance. Antibiotics 2, 217–236. doi: 10.3390/antibiotics2020217
Castanheira, M., Mendes, R. E., and Gales, A. C. (2023). Global epidemiology and mechanisms of resistance of Acinetobacter baumannii-calcoaceticus complex. Clin. Infect. Dis. 76(Suppl. 2), S166–S178. doi: 10.1093/cid/ciad109
Cebrero-Cangueiro, T., Labrador-Herrera, G., Pascual, Á,Díaz, C., Rodríguez-Baño, J., Pachón, J., et al. (2021). Efficacy of fosfomycin and its combination with aminoglycosides in an experimental sepsis model by carbapenemase-producing Klebsiella pneumoniae clinical strains. Front. Med. 8:615540. doi: 10.3389/fmed.2021.615540
Cho, J. C., Fiorenza, M. A., and Estrada, S. J. (2015). Ceftolozane/Tazobactam: A novel cephalosporin/β-lactamase inhibitor combination. Pharmacotherapy 35, 701–715. doi: 10.1002/phar.1609
Chrisostomo, D. A., Pereira, J. A., Scaffa, P. M. C., Gouveia, Z., Abuna, G. F., Plotnikov, S. V., et al. (2025). Antibiofilm properties, cytotoxicity, and effect on protease activity of antibiotics and EGCG-based medications for endodontic purposes. J. Dent. 156:105660. doi: 10.1016/j.jdent.2025.105660
Correia, S., Poeta, P., Hébraud, M., Capelo, J. L., and Igrejas, G. (2017). Mechanisms of quinolone action and resistance: Where do we stand? J. Med. Microbiol. 66, 551–559. doi: 10.1099/jmm.0.000475
Couce, A., Briales, A., Rodríguez-Rojas, A., Costas, C., Pascual, A., and Blázquez, J. (2012). Genomewide overexpression screen for fosfomycin resistance in Escherichia coli: Mura confers clinical resistance at low fitness cost. Antimicrob. Agents Chemother. 56, 2767–2769. doi: 10.1128/AAC.06122-11
Cuba, G. T., Rocha-Santos, G., Cayô, R., Streling, A. P., Nodari, C. S., Gales, A. C., et al. (2020). In vitro synergy of ceftolozane/tazobactam in combination with fosfomycin or aztreonam against MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 75, 1874–1878. doi: 10.1093/jac/dkaa095
Darlow, C. A., Docobo-Perez, F., Farrington, N., Johnson, A., McEntee, L., Unsworth, J., et al. (2021). Amikacin combined with fosfomycin for treatment of neonatal sepsis in the setting of highly prevalent antimicrobial resistance. Antimicrob. Agents Chemother. 65:e0029321. doi: 10.1128/AAC.00293-21
Davis, B. B. (1988). The lethal action of aminoglycosides. J. Antimicrob. Chemother. 22, 1–3. doi: 10.1093/jac/22.1.1
del Río, A., García-de-la-Mària, C., Entenza, J. M., Gasch, O., Armero, Y., Soy, D., et al. (2016). Fosfomycin plus β-Lactams as synergistic bactericidal combinations for experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 60, 478–486. doi: 10.1128/AAC.02139-15
Della Rocca, M. T., Di Caprio, G., Colucci, F., Merola, F., Panetta, V., Cordua, E., et al. (2023). Fosfomycin-meropenem synergistic combination against NDM carbapenemase-producing Klebsiella pneumoniae strains. New Microbiol. 46, 264–270.
Descourouez, J. L., Jorgenson, M. R., Wergin, J. E., and Rose, W. E. (2013). Fosfomycin synergy in vitro with amoxicillin, daptomycin, and linezolid against vancomycin-resistant Enterococcus faecium from renal transplant patients with infected urinary stents. Antimicrob. Agents Chemother. 57, 1518–1520. doi: 10.1128/AAC.02099-12
Dhanda, G., Acharya, Y., and Haldar, J. (2023). Antibiotic adjuvants: A versatile approach to combat antibiotic resistance. ACS Omega 8, 10757–10783. doi: 10.1021/acsomega.3c00312
DiCicco, M., Weese, S., Neethirajan, S., Rousseau, J., and Singh, A. (2014). Fosfomycin susceptibility of canine methicillin-resistant Staphylococcus pseudintermedius isolates. Res. Vet. Sci. 96, 251–253. doi: 10.1016/j.rvsc.2014.02.004
Dijkmans, A. C., Zacarías, N. V. O., Burggraaf, J., Mouton, J. W., Wilms, E. B., van Nieuwkoop, C., et al. (2017). Fosfomycin: Pharmacological, clinical and future perspectives. Antibiotics 6:24. doi: 10.3390/antibiotics6040024
Drusano, G. L., Neely, M. N., Yamada, W. M., Duncanson, B., Brown, D., Maynard, M., et al. (2018). The combination of fosfomycin plus meropenem is synergistic for Pseudomonas aeruginosa PAO1 in a hollow-fiber infection model. Antimicrob. Agents Chemother. 62:e01682-18. doi: 10.1128/AAC.01682-18
El-Sayed Ahmed, M. A. E., Zhong, L. L., Shen, C., Yang, Y., Doi, Y., and Tian, G. B. (2020). Colistin and its role in the Era of antibiotic resistance: An extended review (2000-2019). Emerg. Microbes Infect. 9, 868–885. doi: 10.1080/22221751.2020.1754133
El-Wafa, W. M. A., and Ibrahim, Y. M. (2020). In vitro activity of fosfomycin in double and triple combinations with imipenem, ciprofloxacin and tobramycin against multidrug-resistant Escherichia coli. Curr. Microbiol. 77, 755–761. doi: 10.1007/s00284-019-01871-w
Falagas, M. E., Athanasaki, F., Voulgaris, G. L., Triarides, N. A., and Vardakas, K. Z. (2019). Resistance to fosfomycin: Mechanisms, frequency and clinical consequences. Int. J. Antimicrob. Agents 53, 22–28. doi: 10.1016/j.ijantimicag.2018.09.013
Falagas, M. E., Vouloumanou, E. K., Samonis, G., and Vardakas, K. Z. (2016). Fosfomycin. Clin. Microbiol. Rev. 29, 321–347. doi: 10.1128/CMR.00068-15
Flores-Kim, J., Dobihal, G. S., Fenton, A., Rudner, D. Z., and Bernhardt, T. G. (2019). A switch in surface polymer biogenesis triggers growth-phase-dependent and antibiotic-induced bacteriolysis. Elife 8:e44912. doi: 10.7554/eLife.44912
Fu, Z., Ma, Y., Chen, C., Guo, Y., Hu, F., Liu, Y., et al. (2015). Prevalence of fosfomycin resistance and mutations in murA, glpT, and uhpT in methicillin-resistant Staphylococcus aureus strains isolated from blood and cerebrospinal fluid samples. Front. Microbiol. 6:1544. doi: 10.3389/fmicb.2015.01544
Galindo-Méndez, M., Navarrete-Salazar, H., Baltazar-Jiménez, F., Muñoz-de la Paz, E., Sánchez-Mawcinitt, M. F., Gómez-Pardo, A., et al. (2022). Emergence of fosfomycin resistance by plasmid-mediated fos genes in uropathogenic ESBL-Producing E. coli isolates in Mexico. Antibiotics 11:1383. doi: 10.3390/antibiotics11101383
García-de-la-Mària, C., Gasch, O., García-Gonzalez, J., Soy, D., Shaw, E., Ambrosioni, J., et al. (2018). The combination of daptomycin and fosfomycin has synergistic, potent, and rapid bactericidal activity against methicillin-resistant Staphylococcus aureus in a rabbit model of experimental endocarditis. Antimicrob. Agents Chemother. 62:e02633-17. doi: 10.1128/AAC.02633-17
Gardiner, B. J., Stewardson, A. J., Abbott, I. J., and Peleg, A. Y. (2019). Nitrofurantoin and fosfomycin for resistant urinary tract infections: Old drugs for emerging problems. Aust. Prescr. 42, 14–19. doi: 10.18773/austprescr.2019.002
Gatti, M., Virgili, G., Cojutti, P. G., Gaibani, P., Conti, M., Sturiale, C., et al. (2022). Real-Time optimization of pharmacodynamic target attainment at infection site during treatment of post-neurosurgical ventriculitis caused by carbapenem-resistant gram negatives with ceftazidime-avibactam-based regimens: A report of two cases. Microorganisms 10:154. doi: 10.3390/microorganisms10010154
Gómez-Garcés, J. L., Gil-Romero, Y., Vazquez, O., and Merino, F. (2017). [Synergistic activity and clinical efficacy of fosfomycin and ciprofloxacin combination treatment for soft tissue infection caused by carbapenemase-producing Enterobacter cloacae]. Enferm. Infecc. Microbiol. Clin. 35, 135–136. doi: 10.1016/j.eimc.2016.05.009
Haenni, M., Saras, E., Châtre, P., Médaille, C., Bes, M., Madec, J. Y., et al. (2012). A USA300 variant and other human-related methicillin-resistant Staphylococcus aureus strains infecting cats and dogs in France. J. Antimicrob. Chemother. 67, 326–329. doi: 10.1093/jac/dkr499
Hajdu, S., Lassnigg, A., Graninger, W., Hirschl, A. M., and Presterl, E. (2009). Effects of vancomycin, daptomycin, fosfomycin, tigecycline, and ceftriaxone on Staphylococcus epidermidis biofilms. J. Orthop. Res. 27, 1361–1365. doi: 10.1002/jor.20902
Hall Snyder, A. D., Werth, B. J., Nonejuie, P., McRoberts, J. P., Pogliano, J., Sakoulas, G., et al. (2016). Fosfomycin enhances the activity of daptomycin against vancomycin-resistant enterococci in an in vitro pharmacokinetic-pharmacodynamic model. Antimicrob. Agents Chemother. 60, 5716–5723. doi: 10.1128/AAC.00687-16
He, L., Partridge, S. R., Yang, X., Hou, J., Deng, Y., Yao, Q., et al. (2013). Complete nucleotide sequence of pHN7A8, an F33:a-:b- type epidemic plasmid carrying blaCTX-M-65, fosA3 and rmtB from China. J. Antimicrob. Chemother. 68, 46–50. doi: 10.1093/jac/dks369
Hendlin, D., Stapley, E. O., Jackson, M., Wallick, H., Miller, A. K., Wolf, F. J., et al. (1969). Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science 166, 122–123. doi: 10.1126/science.166.3901.122
Honda, J., Okubo, Y., Kusaba, M., Kumagai, M., Saruwatari, N., and Oizumi, K. (1998). Fosfomycin (FOM: 1 R-2S-epoxypropylphosphonic acid) suppress the production of IL-8 from monocytes via the suppression of neutrophil function. Immunopharmacology 39, 149–155. doi: 10.1016/s0162-3109(98)00003-4
Hosoi, Y., Kawanishi, M., Harada, S., Kumakawa, M., Matsuda, M., and Sekiguchi, H. (2024). The prevalence and the underlying mechanisms of fosfomycin resistance of Escherichia coli and Salmonella spp. among cattle in Japan. Int. J. Mol. Sci. 25:13723. doi: 10.3390/ijms252413723
Huang, H. W. (2020). DAPTOMYCIN, its membrane-active mechanism vs. that of other antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 1862:183395. doi: 10.1016/j.bbamem.2020.183395
Humphries, R., Bobenchik, A. M., Hindler, J. A., and Schuetz, A. N. (2021). Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st edition. J. Clin. Microbiol. 59:e0021321. doi: 10.1128/JCM.00213-21
Ito, R., Pacey, M. P., Mettus, R. T., Sluis-Cremer, N., and Doi, Y. (2018). Origin of the plasmid-mediated fosfomycin resistance gene fosA3. J. Antimicrob. Chemother. 73, 373–376. doi: 10.1093/jac/dkx389
Kabbani, S., Hersh, A. L., Shapiro, D. J., Fleming-Dutra, K. E., Pavia, A. T., and Hicks, L. A. (2018). Opportunities to improve fluoroquinolone prescribing in the United States for adult ambulatory care visits. Clin. Infect. Dis. 67, 134–136. doi: 10.1093/cid/ciy035
Kaewnirat, K., Chuaychob, S., Chukamnerd, A., Pomwised, R., Surachat, K., Phoo, M. T. P., et al. (2022). In vitro synergistic activities of fosfomycin in combination with other antimicrobial agents against carbapenem-resistant Escherichia coli harboring bla NDM-1 on the IncN2 plasmid and a study of the genomic characteristics of these pathogens. Infect. Drug Resist. 15, 1777–1791. doi: 10.2147/IDR.S357965
Kahan, F. M., Kahan, J. S., Cassidy, P. J., and Kropp, H. (1974). The mechanism of action of fosfomycin (phosphonomycin). Ann. N. Y. Acad. Sci. 235, 364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x
Katsarou, A., Stathopoulos, P., Tzvetanova, I. D., Asimotou, C. M., and Falagas, M. E. (2025). β-Lactam/β-Lactamase inhibitor combination antibiotics under development. Pathogens 14:168. doi: 10.3390/pathogens14020168
Kaye, K. S., Pogue, J. M., Tran, T. B., Nation, R. L., and Li, J. (2016). Agents of last resort: Polymyxin resistance. Infect. Dis. Clin. North Am. 30, 391–414. doi: 10.1016/j.idc.2016.02.005
Khalifa, H. O., Mohammed, T., Mohamed, M. I., Hashem, H., and Habib, I. (2025). In vitro assessment of the synergistic effects of cefotaxime, colistin, and fosfomycin combinations against foodborne resistant Escherichia coli and Salmonella isolates. J. Antibiot. 78, 265–273. doi: 10.1038/s41429-025-00808-9
Kieffer, N., Poirel, L., Descombes, M. C., and Nordmann, P. (2020). Characterization of FosL1, a plasmid-encoded fosfomycin resistance protein identified in Escherichia coli. Antimicrob. Agents Chemother. 64:e02042-19. doi: 10.1128/AAC.02042-19
Kim, D. H., Lees, W. J., Kempsell, K. E., Lane, W. S., Duncan, K., and Walsh, C. T. (1996). Characterization of a Cys115 to Asp substitution in the Escherichia coli cell wall biosynthetic enzyme UDP-GlcNAc enolpyruvyl transferase (MurA) that confers resistance to inactivation by the antibiotic fosfomycin. Biochemistry 35, 4923–4928. doi: 10.1021/bi952937w
Kleine, C. E., Schlabe, S., Hischebeth, G. T. R., Molitor, E., Pfeifer, Y., Wasmuth, J. C., et al. (2017). Successful therapy of a multidrug-resistant extended-spectrum β-Lactamase-Producing and fluoroquinolone-resistant Salmonella enterica subspecies enterica serovar typhi infection using combination therapy of meropenem and fosfomycin. Clin. Infect. Dis. 65, 1754–1756. doi: 10.1093/cid/cix652
Kroemer, N., Amann, L. F., Farooq, A., Pfaffendorf, C., Martens, M., Decousser, J.-W., et al. (2024). Pharmacokinetic/pharmacodynamic analysis of ceftazidime/avibactam and fosfomycin combinations in an in vitro hollow fiber infection model against multidrug-resistant Escherichia coli. Microbiol. Spectr. 12:e0331823. doi: 10.1128/spectrum.03318-23
Ku, N. S., Lee, S. H., Lim, Y. S., Choi, H., Ahn, J. Y., Jeong, S. J., et al. (2019). In vivo efficacy of combination of colistin with fosfomycin or minocycline in a mouse model of multidrug-resistant Acinetobacter baumannii pneumonia. Sci. Rep. 9:17127. doi: 10.1038/s41598-019-53714-0
Kwan, A. C. F., and Beahm, N. P. (2020). Fosfomycin for bacterial prostatitis: A review. Int. J. Antimicrob Agents 56:106106. doi: 10.1016/j.ijantimicag.2020.106106
Le Menestrel, A., Guerin, F., Chau, F., Massias, L., Benchetrit, L., Cattoir, V., et al. (2021). Activity of the combination of colistin and fosfomycin against NDM-1-producing Escherichia coli with variable levels of susceptibility to colistin and fosfomycin in a murine model of peritonitis. J. Antimicrob. Chemother. 77, 155–163. doi: 10.1093/jac/dkab378
Le, T. A., Hiba, T., Chaudhari, D., Preston, A. N., Palowsky, Z. R., Ahmadzadeh, S., et al. (2023). Aminoglycoside-Related nephrotoxicity and ototoxicity in clinical practice: A review of pathophysiological mechanism and treatment options. Adv. Ther. 40, 1357–1365. doi: 10.1007/s12325-023-02436-x
Leite, G. C., Perdigão-Neto, L. V., Ruedas Martins, R. C., Rizek, C., Levin, A. S., and Costa, S. F. (2021). Genetic factors involved in fosfomycin resistance of multidrug-resistant Acinetobacter baumannii. Infect. Genet. Evol. 93:104943. doi: 10.1016/j.meegid.2021.104943
Lim, D. G., Jung, S. I., Kim, M. S., Chung, H. S., Hwang, E. C., and Kwon, D. D. (2021). Comparison of a combined regimen of fosfomycin and ciprofloxacin with ciprofloxacin alone as antimicrobial prophylaxis for transrectal prostate biopsy in the era of high fluoroquinolone-resistant rectal flora. Prostate Int. 9, 163–168. doi: 10.1016/j.prnil.2021.03.001
Lin, X., and Kück, U. (2022). Cephalosporins as key lead generation beta-lactam antibiotics. Appl. Microbiol. Biotechnol. 106, 8007–8020. doi: 10.1007/s00253-022-12272-8
Liu, Y., Li, H., Zhang, Y., Ye, Y., Gao, Y., and Li, J. (2019). In vitro and in vivo activity of ciprofloxacin/fosfomycin combination therapy against ciprofloxacin-resistant Shigella flexneri isolates. Infect. Drug Resist. 12, 1619–1628. doi: 10.2147/IDR.S208071
Loayza-Villa, F., Salinas, L., Tijet, N., Villavicencio, F., Tamayo, R., Salas, S., et al. (2020). Diverse Escherichia coli lineages from domestic animals carrying colistin resistance gene mcr-1 in an Ecuadorian household. J. Glob. Antimicrob. Resist. 22, 63–67. doi: 10.1016/j.jgar.2019.12.002
Loras, C., Mendes, A. C., Peixe, L., Novais, Â, and Alós, J. I. (2020). Escherichia coli resistant to fosfomycin from urinary tract infections: Detection of the fosA3 gene in Spain. J. Glob. Antimicrob. Resist. 21, 414–416. doi: 10.1016/j.jgar.2020.01.023
Lu, P. L., Hsieh, Y. J., Lin, J. E., Huang, J. W., Yang, T. Y., Lin, L., et al. (2016). Characterisation of fosfomycin resistance mechanisms and molecular epidemiology in extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates. Int. J. Antimicrob. Agents 48, 564–568. doi: 10.1016/j.ijantimicag.2016.08.013
Lucas, A. E., Ito, R., Mustapha, M. M., McElheny, C. L., Mettus, R. T., Bowler, S. L., et al. (2018). Frequency and mechanisms of spontaneous fosfomycin nonsusceptibility observed upon disk diffusion testing of Escherichia coli. J. Clin. Microbiol. 56:e01368-17. doi: 10.1128/JCM.01368-17.
Ma, Y., Xu, X., Guo, Q., Wang, P., Wang, W., and Wang, M. (2015). Characterization of fosA5, a new plasmid-mediated fosfomycin resistance gene in Escherichia coli. Lett. Appl. Microbiol. 60, 259–264. doi: 10.1111/lam.12366
Marino, A., Stracquadanio, S., Bellanca, C. M., Augello, E., Ceccarelli, M., Cantarella, G., et al. (2022). Oral fosfomycin formulation in bacterial prostatitis: New role for an old molecule-brief literature review and clinical considerations. Infect. Dis. Rep. 14, 621–634. doi: 10.3390/idr14040067
Matsumoto, T., Tateda, K., Miyazaki, S., Furuya, N., Ohno, A., Ishii, Y., et al. (1999). Fosfomycin alters lipopolysaccharide-induced inflammatory cytokine production in mice. Antimicrob. Agents Chemother. 43, 697–698. doi: 10.1128/AAC.43.3.697
Mattioni Marchetti, V., Hrabak, J., and Bitar, I. (2023a). Fosfomycin resistance mechanisms in enterobacterales: An increasing threat. Front. Cell. Infect. Microbiol. 13:1178547. doi: 10.3389/fcimb.2023.1178547
Mattioni Marchetti, V., Kraftova, L., Finianos, M., Sourenian, T., Hrabak, J., and Bitar, I. (2023b). Polyclonal spread of fosfomycin resistance among carbapenemase-producing members of the enterobacterales in the Czech Republic. Microbiol. Spectr. 11:e0009523. doi: 10.1128/spectrum.00095-23
Mendoza, C., Garcia, J. M., Llaneza, J., Mendez, F. J., Hardisson, C., and Ortiz, J. M. (1980). Plasmid-determined resistance to fosfomycin in Serratia marcescens. Antimicrob. Agents Chemother. 18, 215–219. doi: 10.1128/AAC.18.2.215
Michalopoulos, A. S., Livaditis, I. G., and Gougoutas, V. (2011). The revival of fosfomycin. Int. J. Infect. Dis. 15, e732–e739. doi: 10.1016/j.ijid.2011.07.007
Mihailescu, R., Furustrand Tafin, U., Corvec, S., Oliva, A., Betrisey, B., Borens, O., et al. (2014). High activity of Fosfomycin and Rifampin against methicillin-resistant Staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 58, 2547–2553. doi: 10.1128/AAC.02420-12
Mikuniya, T., Kato, Y., Ida, T., Maebashi, K., Monden, K., Kariyama, R., et al. (2007). Treatment of Pseudomonas aeruginosa biofilms with a combination of fluoroquinolones and fosfomycin in a rat urinary tract infection model. J. Infect. Chemother. 13, 285–290. doi: 10.1007/s10156-007-0534-7
Miller, W. R., Bayer, A. S., and Arias, C. A. (2016). Mechanism of action and resistance to daptomycin in Staphylococcus aureus and enterococci. Cold Spring Harb. Perspect. Med. 6:a026997. doi: 10.1101/cshperspect.a026997
Mishra, N. N., Lew, C., Abdelhady, W., Lapitan, C. K., Proctor, R. A., Rose, W. E., et al. (2022). Synergy mechanisms of daptomycin-fosfomycin combinations in daptomycin-susceptible and -resistant methicillin-resistant staphylococcus aureus: In vitro, ex vivo, and in vivo metrics. Antimicrob. Agents Chemother. 66:e0164921. doi: 10.1128/AAC.01649-21
Mora-Ochomogo, M., and Lohans, C. T. (2021). β-Lactam antibiotic targets and resistance mechanisms: From covalent inhibitors to substrates. RSC Med. Chem. 12, 1623–1639. doi: 10.1039/d1md00200g
Morikawa, K., Oseko, F., Morikawa, S., and Sawada, M. (1993). Immunosuppressive activity of fosfomycin on human T-lymphocyte function in vitro. Antimicrob. Agents Chemother. 37, 2684–2687. doi: 10.1128/AAC.37.12.2684
Morin, A., Bergevin, M., Rivest, N., and Lapointe, S. P. (2020). Antibiotic prophylaxis for transrectal ultrasound-guided prostate needle biopsy: Compared efficacy of ciprofloxacin vs. the ciprofloxacin/fosfomycin tromethamine combination. Can. Urol. Assoc. J. 14, 267–272. doi: 10.5489/cuaj.6248
Morrison, L., and Zembower, T. R. (2020). Antimicrobial resistance. Gastrointest Endosc. Clin. N. Am. 30, 619–635. doi: 10.1016/j.giec.2020.06.004
Mourad, A., Parsons, J. B., Skalla, L. A., Holland, T. L., and Jenkins, T. C. (2025). Combination therapy with fosfomycin for Staphylococcus aureus bacteraemia or endocarditis: A systematic review and meta-analysis of randomized trials. JAC Antimicrob. Resist. 7:dlaf101. doi: 10.1093/jacamr/dlaf101
Munoz-Price, L. S., Poirel, L., Bonomo, R. A., Schwaber, M. J., Daikos, G. L., Cormican, M., et al. (2013). Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13, 785–796. doi: 10.1016/S1473-3099(13)70190-7
Nakamura, I., Yamaguchi, T., Aoki, K., Miura, Y., Sato, S., Fujita, H., et al. (2020). Imipenem plus fosfomycin as salvage therapy for vertebral osteomyelitis. Antimicrob. Agents Chemother. 65:e01746-20. doi: 10.1128/AAC.01746-20
Neuner, E. A., Sekeres, J., Hall, G. S., and van Duin, D. (2012). Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob. Agents Chemother. 56, 5744–5748. doi: 10.1128/AAC.00402-12
Nilsson, A. I., Berg, O. G., Aspevall, O., Kahlmeter, G., and Andersson, D. I. (2003). Biological costs and mechanisms of fosfomycin resistance in Escherichia coli. Antimicrob. Agents Chemother. 47, 2850–2858. doi: 10.1128/AAC.47.9.2850-2858.2003
Nwabor, O. F., Terbtothakun, P., Voravuthikunchai, S. P., and Chusri, S. (2021). Evaluation of the synergistic antibacterial effects of fosfomycin in combination with selected antibiotics against carbapenem-resistant Acinetobacter baumannii. Pharmaceuticals 14:185. doi: 10.3390/ph14030185
Oliva, A., Curtolo, A., Volpicelli, L., Cogliati Dezza, F., De Angelis, M., Cairoli, S., et al. (2021). Synergistic meropenem/vaborbactam plus fosfomycin treatment of KPC Producing K. pneumoniae septic thrombosis unresponsive to ceftazidime/avibactam: From the bench to the bedside. Antibiotics 10:781. doi: 10.3390/antibiotics10070781
Papp-Wallace, K. M., Zeiser, E. T., Becka, S. A., Park, S., Wilson, B. M., Winkler, M. L., et al. (2019). Ceftazidime-Avibactam in combination with fosfomycin: A novel therapeutic strategy against multidrug-resistant Pseudomonas aeruginosa. J. Infect. Dis. 220, 666–676. doi: 10.1093/infdis/jiz149
Pipitone, G., Di Bella, S., Maraolo, A. E., Granata, G., Gatti, M., Principe, L., et al. (2023). Intravenous fosfomycin for systemic multidrug-resistant Pseudomonas aeruginosa infections. Antibiotics 12:1653. doi: 10.3390/antibiotics12121653
Poirel, L., Vuillemin, X., Kieffer, N., Mueller, L., Descombes, M. C., and Nordmann, P. (2019). Identification of FosA8, a plasmid-encoded fosfomycin resistance determinant from Escherichia coli, and its origin in Leclercia adecarboxylata. Antimicrob. Agents Chemother. 63:e01403-19. doi: 10.1128/AAC.01403-19
Popovic, M., Steinort, D., Pillai, S., and Joukhadar, C. (2010). Fosfomycin: An old, new friend? Eur. J. Clin. Microbiol. Infect. Dis. 29, 127–142. doi: 10.1007/s10096-009-0833-2
Portillo-Calderón, I., Ortiz-Padilla, M., de Gregorio-Iaria, B., Merino-Bohorquez, V., Blázquez, J., Rodríguez-Baño, J., et al. (2023). Activity of fosfomycin and amikacin against fosfomycin-heteroresistant Escherichia coli strains in a hollow-fiber infection model. Antimicrob. Agents Chemother. 65:e02213-20. doi: 10.1128/AAC.02213-20
Pujol, M., Miró, J. M., Shaw, E., Aguado, J. M., San-Juan, R., Puig-Asensio, M., et al. (2021). Daptomycin plus fosfomycin versus daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia and endocarditis: A randomized clinical trial. Clin. Infect. Dis. 72, 1517–1525. doi: 10.1093/cid/ciaa1081
Ribeiro, A. C. D. S., Chikhani, Y. C. D. S. A., Valiatti, T. B., Valêncio, A., Kurihara, M. N. L., Santos, F. F., et al. (2023). In vitro and in vivo synergism of fosfomycin in combination with meropenem or polymyxin B against KPC-2-Producing Klebsiella pneumoniae clinical isolates. Antibiotics 12:237. doi: 10.3390/antibiotics12020237
Ruan, Z., Cui, J., He, Z., Guo, Y., Jia, X., and Huang, X. (2020). Synergistic effects from combination of cryptotanshinone and fosfomycin against fosfomycin-susceptible and fosfomycin-resistant Staphylococcus aureus. Infect. Drug Resist. 13, 2837–2844. doi: 10.2147/IDR.S255296
Rusu, A., Munteanu, A. C., Arbănaşi, E. M., and Uivarosi, V. (2023). Overview of side-effects of antibacterial fluoroquinolones: New drugs versus old drugs, a step forward in the safety profile? Pharmaceutics 15:804. doi: 10.3390/pharmaceutics15030804
Sadek, M., Ortiz de la Rosa, J. M., Ramadan, M., Nordmann, P., and Poirel, L. (2022). Molecular characterization of extended-spectrum ß-lactamase producers, carbapenemase producers, polymyxin-resistant, and fosfomycin-resistant Enterobacterales among pigs from Egypt. J. Glob. Antimicrob. Resist. 30, 81–87. doi: 10.1016/j.jgar.2022.05.022
Saravolatz, L. D., and Pawlak, J. (2022). In vitro activity of fosfomycin alone and in combination against Staphylococcus aureus with reduced susceptibility or resistance to methicillin, vancomycin, daptomycin or linezolid. J. Antimicrob. Chemother. 78, 238–241. doi: 10.1093/jac/dkac380
Sauermann, R., Marsik, C., Steiner, I., Seir, K., Cvitko, T., Zeitlinger, M., et al. (2007). Immunomodulatory effects of fosfomycin in experimental human endotoxemia. Antimicrob. Agents Chemother. 51, 1879–1881. doi: 10.1128/AAC.00914-06
Scudeller, L., Righi, E., Chiamenti, M., Bragantini, D., Menchinelli, G., Cattaneo, P., et al. (2021). Systematic review and meta-analysis of in vitro efficacy of antibiotic combination therapy against carbapenem-resistant Gram-negative bacilli. Int. J. Antimicrob. Agents 57:106344. doi: 10.1016/j.ijantimicag.2021.106344
Seok, H., Choi, J. Y., Wi, Y. M., Park, D. W., Peck, K. R., and Ko, K. S. (2020). Fosfomycin resistance in Escherichia coli isolates from South Korea and in vitro activity of fosfomycin alone and in combination with other antibiotics. Antibiotics 9:112. doi: 10.3390/antibiotics9030112
Sharma, R., Garcia, E., Diep, J. K., Lee, V. H., Minhaj, F., Jermain, B., et al. (2022). Pharmacodynamic and immunomodulatory effects of polymyxin B in combination with fosfomycin against KPC-2-producing Klebsiella pneumoniae. Int. J. Antimicrob. Agents 59:106566. doi: 10.1016/j.ijantimicag.2022.106566
Shaw, E., Miró, J. M., Puig-Asensio, M., Pigrau, C., Barcenilla, F., Murillas, J., et al. (2015). Daptomycin plus fosfomycin versus daptomycin monotherapy in treating MRSA: Protocol of a multicentre, randomised, phase III trial. BMJ Open 5:e006723. doi: 10.1136/bmjopen-2014-006723
Shen, F., Tang, X., Cheng, W., Wang, Y., Wang, C., Shi, X., et al. (2016). Fosfomycin enhances phagocyte-mediated killing of Staphylococcus aureus by extracellular traps and reactive oxygen species. Sci. Rep. 6:19262. doi: 10.1038/srep19262
Shi, J., Mao, N. F., Wang, L., Zhang, H. B., Chen, Q., Liu, H., et al. (2014). Efficacy of combined vancomycin and fosfomycin against methicillin-resistant Staphylococcus aureus in biofilms in vivo. PLoS One 9:e113133. doi: 10.1371/journal.pone.0113133
Silver, L. L. (2017). Fosfomycin: Mechanism and resistance. Cold Spring Harb. Perspect. Med. 7:a025262. doi: 10.1101/cshperspect.a025262
Singkham-In, U., and Chatsuwan, T. (2018). In vitro activities of carbapenems in combination with amikacin, colistin, or fosfomycin against carbapenem-resistant Acinetobacter baumannii clinical isolates. Diagn. Microbiol. Infect. Dis. 91, 169–174. doi: 10.1016/j.diagmicrobio.2018.01.008
Singkham-In, U., and Chatsuwan, T. (2022). Synergism of imipenem with fosfomycin associated with the active cell wall recycling and heteroresistance in Acinetobacter calcoaceticus-baumannii complex. Sci. Rep. 12:230. doi: 10.1038/s41598-021-04303-7
Sirijatuphat, R., and Thamlikitkul, V. (2014). Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 58, 5598–5601. doi: 10.1128/AAC.02435-13
Smith, E. C., Brigman, H. V., Anderson, J. C., Emery, C. L., Bias, T. E., Bergen, P. J., et al. (2020). Performance of four fosfomycin susceptibility testing methods against an international collection of clinical Pseudomonas aeruginosa isolates. J. Clin. Microbiol. 58:e01121-20. doi: 10.1128/JCM.01121-20
Suich, J., Mawer, D., van der Woude, M., Wearmouth, D., Burns, P., Smeets, T., et al. (2022). Evaluation of in vitro activity of fosfomycin, and synergy in combination, in Gram-negative bloodstream infection isolates in a UK teaching hospital. J. Med. Microbiol. 71. doi: 10.1099/jmm.0.001524
Taber, H. W., Mueller, J. P., Miller, P. F., and Arrow, A. S. (1987). Bacterial uptake of aminoglycoside antibiotics. Microbiol. Rev. 51, 439–457. doi: 10.1128/mr.51.4.439-457.1987
Takahata, S., Ida, T., Hiraishi, T., Sakakibara, S., Maebashi, K., Terada, S., et al. (2010). Molecular mechanisms of fosfomycin resistance in clinical isolates of Escherichia coli. Int. J. Antimicrob. Agents 35, 333–337. doi: 10.1016/j.ijantimicag.2009.11.011
Tiwana, G., Cock, I. E., and Cheesman, M. J. (2024). Combinations of Terminalia bellirica (Gaertn.) Roxb. and Terminalia chebula Retz. extracts with selected antibiotics against antibiotic-resistant bacteria: Bioactivity and phytochemistry. Antibiotics 13:994. doi: 10.3390/antibiotics13100994
Trapnell, B. C., McColley, S. A., Kissner, D. G., Rolfe, M. W., Rosen, J. M., McKevitt, M., et al. (2012). Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with Pseudomonas airway infection. Am. J. Respir. Crit. Care Med. 185, 171–178. doi: 10.1164/rccm.201105-0924OC
Tseng, T. C., Chuang, Y. C., Yang, J. L., Lin, C. Y., Huang, S. H., Wang, J. T., et al. (2023). The combination of daptomycin with fosfomycin is more effective than daptomycin alone in reducing mortality of vancomycin-resistant enterococcal bloodstream infections: A retrospective, comparative cohort study. Infect. Dis. Ther. 12, 589–606. doi: 10.1007/s40121-022-00754-1
Tüzemen, N. Ü,Önal, U., Merdan, O., Akca, B., Ener, B., Özakın, C., et al. (2024). Synergistic antibacterial activity of ceftazidime-avibactam in combination with colistin, gentamicin, amikacin, and fosfomycin against carbapenem-resistant Klebsiella pneumoniae. Sci. Rep. 14:17567. doi: 10.1038/s41598-024-67347-5
Venuti, F., Trunfio, M., Martson, A. G., Lipani, F., Audagnotto, S., Di Perri, G., et al. (2023). Extended and continuous infusion of novel protected β-lactam antibiotics: A narrative review. Drugs 83, 967–983. doi: 10.1007/s40265-023-01893-6
Volpicelli, L., Cairoli, S., Al Ismail, D., Baisi, F., Sacco, F., Goffredo, B. M., et al. (2024). Simultaneous post-neurosurgical ventriculitis and bacteraemia by two different strains of KPC-producing K. pneumoniae successfully treated with meropenem/vaborbactam and high dose of fosfomycin. J. Glob. Antimicrob. Resist. 37, 86–90. doi: 10.1016/j.jgar.2024.03.003
Wachino, J., Yamane, K., Suzuki, S., Kimura, K., and Arakawa, Y. (2010). Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob. Agents Chemother. 54, 3061–3064. doi: 10.1128/AAC.01834-09
Wale, Y. M., Roberts, J. A., and Sime, F. B. (2024). Dynamic in vitro PK/PD infection models for the development and optimisation of antimicrobial regimens: A narrative review. Antibiotics 13:1201. doi: 10.3390/antibiotics13121201
Walsh, C. C., Landersdorfer, C. B., McIntosh, M. P., Peleg, A. Y., Hirsch, E. B., Kirkpatrick, C. M., et al. (2016). Clinically relevant concentrations of fosfomycin combined with polymyxin B, tobramycin or ciprofloxacin enhance bacterial killing of Pseudomonas aeruginosa, but do not suppress the emergence of fosfomycin resistance. J. Antimicrob. Chemother. 71, 2218–2229. doi: 10.1093/jac/dkw115
Wang, L., Di Luca, M., Tkhilaishvili, T., Trampuz, A., and Gonzalez Moreno, M. (2019). Synergistic activity of fosfomycin, ciprofloxacin, and gentamicin against Escherichia coli and Pseudomonas aeruginosa biofilms. Front. Microbiol. 10:2522. doi: 10.3389/fmicb.2019.02522
Wang, Y., Wang, J., Wang, R., and Cai, Y. (2020). Resistance to ceftazidime-avibactam and underlying mechanisms. J. Glob. Antimicrob. Resist. 22, 18–27. doi: 10.1016/j.jgar.2019.12.009
Werner, N. L., Hecker, M. T., Sethi, A. K., and Donskey, C. J. (2011). Unnecessary use of fluoroquinolone antibiotics in hospitalized patients. BMC Infect. Dis. 11:187. doi: 10.1186/1471-2334-11-187
Winkler, M. L., Papp-Wallace, K. M., Hujer, A. M., Domitrovic, T. N., Hujer, K. M., Hurless, K. N., et al. (2015). Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: Resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 59, 1020–1029. doi: 10.1128/AAC.04238-14
Wu, H. C., and Venkateswaran, P. S. (1974). Fosfomycin-resistant mutant of Escherichia coli. Ann. N. Y. Acad. Sci. 235, 587–592. doi: 10.1111/j.1749-6632.1974.tb43292.x
Wu, Y., Yu, W., Chu, X., Zhang, J., Jia, P., Liu, X., et al. (2024). Effect of ceftazidime-avibactam combined with different antimicrobials against carbapenem-resistant Klebsiella pneumoniae. Microbiol. Spectr. 12:e0010724. doi: 10.1128/spectrum.00107-24
Xu, C., Chen, T., Zhang, S., Zhou, C., Liao, W., Fang, R., et al. (2022). In vitro activity of imipenem-relebactam alone and in combination with fosfomycin against carbapenem-resistant gram-negative pathogens. Diagn. Microbiol. Infect. Dis. 103:115712. doi: 10.1016/j.diagmicrobio.2022.115712
Xu, H., Miao, V., Kwong, W., Xia, R., and Davies, J. (2011). Identification of a novel fosfomycin resistance gene (fosA2) in Enterobacter cloacae from the Salmon River. Canada. Lett. Appl. Microbiol. 52, 427–429. doi: 10.1111/j.1472-765X.2011.03016.x
Xu, S., Fu, Z., Zhou, Y., Liu, Y., Xu, X., and Wang, M. (2017). Mutations of the transporter proteins GlpT and UhpT confer fosfomycin resistance in Staphylococcus aureus. Front. Microbiol. 8:914. doi: 10.3389/fmicb.2017.00914
Yamada, S., Hyo, Y., Ohmori, S., and Ohuchi, M. (2007). Role of ciprofloxacin in its synergistic effect with fosfomycin on drug-resistant strains of Pseudomonas aeruginosa. Chemotherapy 53, 202–209. doi: 10.1159/000100811
Yang, Y., Sun, H., Liu, X., Wang, M., Xue, T., and Sun, B. (2016). Regulatory mechanism of the three-component system HptRSA in glucose-6-phosphate uptake in Staphylococcus aureus. Med. Microbiol. Immunol. 205, 241–253. doi: 10.1007/s00430-015-0446-6
Yao, H., Wu, D., Lei, L., Shen, Z., Wang, Y., and Liao, K. (2016). The detection of fosfomycin resistance genes in Enterobacteriaceae from pets and their owners. Vet. Microbiol. 193, 67–71. doi: 10.1016/j.vetmic.2016.07.019
Yu, H., Ma, D., Liu, B., Yang, S., Lin, Q., Yu, R., et al. (2022). Differences in the distribution of species, carbapenemases, sequence types, antimicrobial heteroresistance and mortality rates between pediatric and adult carbapenemase-producing enterobacterales in bloodstream infections. Front. Med. 9:827474. doi: 10.3389/fmed.2022.827474
Yu, W., Luo, Q., Shi, Q., Huang, C., Yu, X., Niu, T., et al. (2018). In vitro antibacterial effect of fosfomycin combination therapy against colistin-resistant Klebsiella pneumoniae. Infect. Drug Resist. 11, 577–585. doi: 10.2147/IDR.S160474
Yu, W., Shen, P., Bao, Z., Zhou, K., Zheng, B., Ji, J., et al. (2017). In vitro antibacterial activity of fosfomycin combined with other antimicrobials against KPC-producing Klebsiella pneumoniae. Int. J. Antimicrob. Agents 50, 237–241. doi: 10.1016/j.ijantimicag.2017.03.011
Yu, Y., Li, X., Wu, Y., Lou, N., Jia, H., Liu, N., et al. (2024). Global prevalence of fosfomycin resistance genes fosA and fosB in multidrug-resistant bacteria. Int. J. Antimicrob. Agents 64:107272. doi: 10.1016/j.ijantimicag.2024.107272
Zhang, J., Xu, L., Zhang, K., Yue, J., Dong, K., Luo, Q., et al. (2024). Synergistic effect of fosfomycin and colistin against KPC-producing Klebsiella pneumoniae: Pharmacokinetics-pharmacodynamics combined with transcriptomic approach. BMC Microbiol. 24:430. doi: 10.1186/s12866-024-03581-1
Zhang, P., Shi, Q., Hu, H., Hong, B., Wu, X., Du, X., et al. (2020). Emergence of ceftazidime/avibactam resistance in carbapenem-resistant Klebsiella pneumoniae in China. Clin. Microbiol. Infect. 26, 124.e1–124.e4. doi: 10.1016/j.cmi.2019.08.020
Zhang, S., Di, L., Qi, Y., Qian, X., and Wang, S. (2024). Treatment of infections caused by carbapenem-resistant Acinetobacter baumannii. Front. Cell Infect. Microbiol. 14:1395260. doi: 10.3389/fcimb.2024.1395260
Zhang, S., Yang, J., Yang, Q., Li, Q., Zhong, Z., Wang, M., et al. (2025). High prevalence of plasmid-mediated Fosfomycin resistance in waterfowl-derived Escherichia coli strains: Insights into genetic context and transmission dynamics in China. Front. Vet. Sci. 12:1481822. doi: 10.3389/fvets.2025.1481822
Zheng, D., Bergen, P. J., Landersdorfer, C. B., and Hirsch, E. B. (2022). Differences in fosfomycin resistance mechanisms between Pseudomonas aeruginosa and enterobacterales. Antimicrob. Agents Chemother. 66:e0144621. doi: 10.1128/AAC.01446-21
Keywords: fosfomycin, combination therapy, antimicrobial resistance, multidrug resistance, antimicrobial co-administration
Citation: Wu Y, Li J, Qiao F, Guo J, Zhang L and Jia X (2025) Overcoming the drug resistance barrier: progress in fosfomycin combination therapy against multidrug-resistant pathogens. Front. Microbiol. 16:1702881. doi: 10.3389/fmicb.2025.1702881
Received: 11 September 2025; Accepted: 30 October 2025;
Published: 18 November 2025.
Edited by:
João Pedro Rueda Furlan, Federal University of Paraíba, BrazilReviewed by:
Vittoria Mattioni Marchetti, University of Pavia, ItalyMohamed Kettani Halabi, Mohammed VI University of Health Sciences, Morocco
Copyright © 2025 Wu, Li, Qiao, Guo, Zhang and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengling Qiao, cWlhb3poYW95aUBjZHV0Y20uZWR1LmNu; Lin Zhang, emhhbmdsQHVzeC5lZHUuY24=; Xu Jia, amlheHVAY21jLmVkdS5jbg==
 Yan Wu1,2,3
Yan Wu1,2,3 Jinlin Guo
Jinlin Guo Lin Zhang
Lin Zhang Xu Jia
Xu Jia