- Sleep Research Laboratory, School of Life Sciences, Jawaharlal Nehru University, New Delhi, India
Sleep is characterized as rapid eye movement (REM) and non-rapid eye movement (NREM) sleep. Studies suggest that wake-related neurons in the basal forebrain, posterior hypothalamus and brainstem, and NREM sleep-related neurons in the anterior-hypothalamic area inhibit each other, thus alternating sleep–wakefulness. Similarly, pontine REM-ON and REM-OFF neurons reciprocally inhibit each other for REM sleep modulation. It has been proposed that inhibition of locus coeruleus (LC) REM-OFF neurons is pre-requisite for REM sleep genesis, but it remains ambiguous how REM-OFF neurons are hyperpolarized at REM sleep onset. The frequency of breathing pattern remains high during wake, slows down during NREM sleep but further escalates during REM sleep. As a result, brain CO2 level increases during NREM sleep, which may alter REM sleep manifestation. It has been reported that hypocapnia decreases REM sleep while hypercapnia increases REM sleep periods. The groups of brainstem chemosensory neurons, including those present in LC, sense the alteration in CO2 level and respond accordingly. For example, one group of LC neurons depolarize while other hyperpolarize during hypercapnia. In another group, hypercapnia initially depolarizes but later hyperpolarizes LC neurons. Besides chemosensory functions, LC REM-OFF neurons are an integral part of REM sleep executive machinery. We reason that increased CO2 level during a stable NREM sleep period may hyperpolarize LC neurons including REM-OFF, which may help initiate REM sleep. We propose that REM sleep might act as a sentinel to help maintain normal CO2 level for unperturbed sleep.
Introduction
Coordinated reciprocal interactions between specific hypothalamic and brainstem neuronal groups are involved in the generation of electrographic and behavioral aspects of sleep and wakefulness (S–W). Non-rapid eye movement (NREM) sleep-related neuronal groups in the medial and ventro-lateral preoptic areas of anterior hypothalamus, and wakefulness related neuronal groups in the basal forebrain, posterior hypothalamus, and brainstem impart mutual inhibitory signals (McGinty and Szymusiak, 2000; Saper et al., 2001; Saper, 2006; Jha and Mallick, 2011). As a result, one of the circuitries remains up-regulated when the other is down-regulated (McGinty and Szymusiak, 2000; Saper et al., 2001; Saper, 2006; Jha and Mallick, 2011). Several reports suggest that the activation of the arousal system induces alertness by down-regulating the executive circuitries of NREM sleep (McGinty and Szymusiak, 2000; Saper et al., 2001; Saper, 2006). Similarly, NREM sleep appears after deactivation of arousal system by NREM sleep-related neurons (McGinty and Szymusiak, 2000; Saper et al., 2001; Saper, 2006; Jha and Mallick, 2011). Additionally, there are neuronal groups in the brainstem [the tegmental cholinergic neurons, locus coeruleus (LC) norepinephrinergic (NE-ergic), and dorsal raphe nucleus (DRN) serotonergic (5HT-nergic) neurons] that regulate rapid eye movement (REM) sleep (Aston-Jones and Bloom, 1981; Jha and Mallick, 2011). Two distinct neuronal populations unique to REM sleep have been identified in the brainstem: REM-ON neurons in the tegmental area (exclusively fire during REM sleep) and REM-OFF neurons in LC and DRN (remain silent during REM sleep; Hobson et al., 1974; Trulson and Jacobs, 1979; Aston-Jones and Bloom, 1981; Sakai and Koyama, 1996). REM-ON and REM-OFF neurons mutually inhibit each other for REM sleep regulation (Hobson et al., 1975). It is evident that progression to sleep from wakefulness requires the simultaneous inhibition of multiple arousal systems while the deactivation of the sleep center begets alertness. However, it is still ambiguous how NREM sleep sporadically leads to REM sleep.
Although several theories have been proposed regarding REM sleep generation, the neural processes participating in the transition from NREM to REM sleep are still obscure. Hobson et al. (1975) originally reasoned a reciprocal interaction between the REM-ON and REM-OFF neurons for REM sleep generation. Subsequently, several modified and elaborated propositions have been made (Sakai, 1988; Mallick et al., 2001; Lu et al., 2006). Now a well-acknowledged fact is that the cholinergic REM-ON neurons in the latero-dorsal (LDT) and pedunculopontine (PPT) tegmental nuclei inhibit the REM-OFF neurons of LC and DRN to initiate REM sleep (Figure 1). Similarly, the activated aminergic REM-OFF group of cells inhibit REM-ON neurons (Figure 1), which either terminates REM sleep (if the subject is already in REM sleep) or does not allow REM sleep to occur (Singh and Mallick, 1996). Such phenomenon helps us to understand the mechanism for REM sleep maintenance; yet it does not explain the precise neural events participating in state transition from NREM sleep to REM sleep.
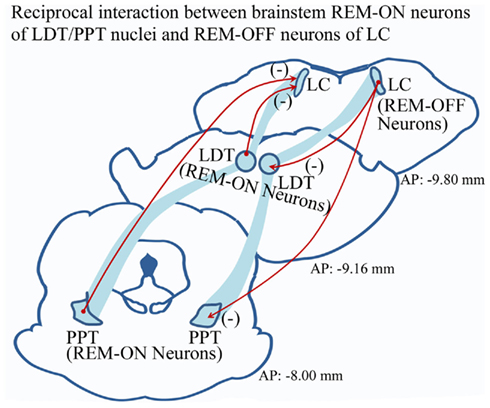
Figure 1. Widely accepted model of reciprocal interaction between aminergic REM-OFF neurons of LC and cholinergic REM-ON cells of LDT and PPT. It has been proposed that activated LC REM-OFF cells inhibit REM-ON neurons in LDT and PPT, which does not allow REM sleep to occur. Hyperpolarization of REM-OFF cells in turn causes disinhibition of REM-ON neurons, which may initiate REM sleep. (Abbreviation – LC, locus coeruleus; LDT, latero-dorsal tegmentum; PPT, pedunculopontine tegmentum; (−), inhibitory circuitry).
In addition to their role in REM sleep regulation, LC neurons modulate several physiological functions, for example, pulmonary ventilation, thermoregulation, autonomic regulation, etc (Morilak et al., 1987a,b; Dean et al., 2001). The LC contains high proportions of chemosensitive neurons (Pineda and Aghajanian, 1997) and focal acidification of LC stimulates pulmonary ventilation and blood pressure (Coates et al., 1993). The brainstem respiratory rhythm generator and CO2, both modulate the excitability of LC neurons (Ballantyne and Scheid, 2000; Dean et al., 2001). For example, progressive increase in CO2 concentration excites LC neurons but interestingly, if the increased level persists, it turns-off the neurons (Dean et al., 2001). The brain CO2 level gradually increases as NREM sleep progresses (McKay et al., 2011) and increased CO2 excites the chemosensory neurons including those in LC (Dean et al., 2001) to help prevent hypercapnia. In view of the fact that high concentration of CO2 for a sustained period switches-off LC neurons, it is likely that during prolonged and stable NREM sleep period, the elevated CO2 level may stay for a while (Trinder et al., 1997) and may instead turn-off LC neurons including the population of REM-OFF neurons. It has been proposed that cessation of LC REM-OFF neurons disinhibit brainstem REM-ON neurons, which further helps initiate REM sleep over NREM sleep (Jha and Mallick, 2011). In this review, we assess the viable role of physiological CO2 level in state transition from NREM sleep to REM sleep. We reason that the critically elevated CO2 level for sustained period during NREM sleep may facilitate the cessation of LC REM-OFF cells, an obligatory condition for REM sleep to occur. Further, we propose that one of the functions of REM sleep is to maintain normal brain CO2 level during sleep for a sustained and unperturbed slumber.
Why Do We Need Uninterrupted but Composite Bimodal Sleep?
Prolonged wakefulness and/or frequent arousal from sleep, equally and independently, affect several physiological and behavioral functions. Repetitive arousal from sleep produces cardiovascular dysfunctions in normal healthy individuals (Carrington and Trinder, 2008). Also, there is a remarkable decrease in the daytime performance of patients suffering from periodic leg movements disorder and central sleep apnea (which manifests fragmented nocturnal sleep) (Bonnet, 1996). Several reports suggest that disturbed sleep or no sleep after successful learning of a novel task causes reduction of performance because it impairs memory consolidation in rats (Smith and Rose, 1996; Chowdhury et al., 2011) as well as in humans (Stickgold et al., 2000, 2001). Brain activity occurring during NREM sleep and REM sleep seems to be particularly important during the post-natal period for the development of neural circuitries (Marks et al., 1995; Frank et al., 2001; Jha et al., 2005a; Aton et al., 2009). REM sleep loss during development decreases total brain mass later in life (Mirmiran et al., 1983a,b) and also abnormally alters neuronal excitability (Madan and Mallick, 2011), which may cause neuronal loss (Morrissey et al., 2004). Further, it has been noted that REM sleep and/or its associative components such as pontine P-wave density (PGO wave) are augmented following successful learning in the rat (Datta, 2000). Therefore, it seems that NREM and REM sleep have discrete functions and their enduring or partial loss may induce several deficits.
Sleep also plays an important role in the modulation of several physiological functions. Body physiology manifests dipping effects during NREM sleep and a mounting trend during REM sleep. For example, heart rate and blood pressure decrease during NREM sleep but increase during REM sleep (Verrier et al., 2005). Similarly body temperature also decreases during NREM sleep and goes up during REM sleep (McGinty and Szymusiak, 1990). Since metabolic activities descend during NREM sleep, one of the functions of sleep could be to conserve energy (Berger and Phillips, 1995). Ironically, NREM sleep dampens pulmonary ventilation, as a result decreasing blood pH significantly (Robin et al., 1958). The respiratory groups of neurons in the brainstem perceive the elevated hydrogen ion concentration through chemosensors and in turn accelerate pulmonary ventilation to help protect the neural damage from this altered pH. Therefore, it seems obligatory that the subject should go periodically into either wakefulness or REM sleep to help maintain physiological range of blood CO2. Since frequent arousal from sleep may not be physiologically and cognitively sustainable, intermittent REM sleep between NREM sleep would thus be favored to help maintain optimum CO2 level and long-lasting sleep periods.
Does Blood CO2 Level Variation Alter REM Sleep?
If one of the functions of REM sleep is to help maintain normal blood CO2 level then one would assume that the expression of REM sleep amount and/or episode numbers in a subject would be in proportion to the concentration of blood CO2. In few investigations, the responses to hypercapnia (elevated level of blood CO2) and hypocapnia (reduced level of blood CO2) have been studied during different vigilant states. It has been observed that REM sleep helps regulate breathing response during altered blood CO2 level (Netick et al., 1984). During hypocapnia, REM sleep amount is decreased and the effect was entirely due to change in CO2 level but not due to any other non-specific reasons (Ryan et al., 1983; Lovering et al., 2003, 2004). Further, it has also been observed that decrease in REM sleep amount correlates proportionally with a relative decrease in CO2 level (Lovering et al., 2003). On the other hand, with mild hypercapnia (2% increase in CO2 level in the inspired air), sleep amount significantly increased with concurrent increase in the number of REM sleep episodes (Fraigne et al., 2008). In contrast, acute hypercapnia (6% increase in CO2 level in the inspired air), significantly decreased sleep and induced awaking (Fraigne et al., 2008). The induced wakefulness during acute hypercapnia can help readjust blood CO2 rapidly to the normal level by inducing fast ventilatory responses. Therefore, it seems that moderately raised CO2 level (probably within a range of set points) enhances REM sleep while alarmingly increased CO2 level induces wakefulness.
Interestingly, some animals are reared with high CO2 content in their breathing air and they manifest proportionally very high REM sleep amount. For example, altricial babies of marsupials such as Didelphis virginiana receive high CO2 gas in their breathing air because the pouch environment is relatively high in CO2 and low in O2 concentration (Farber and Tenney, 1971). The CO2 concentration in the pouch increases as the young increases in age (Reynolds, 1952) and it is broadly known that marsupials (van Twyver and Allison, 1970) and their infants (Astic and Saucier, 1978) spend much of their time in REM sleep. Although the manifestation of high REM sleep amount in these animals could also be attributed to their phylogenetic status as it has been hypothesized that evolutionarily older mammals have REM sleep in high proportion (Jha et al., 2006). It is not yet clear why phylogenetically older mammals exhibit so much REM sleep, but one of the plausible reasons could be that these animals are reared in an environment where they are exposed to a very high CO2 concentration. These evidences indicate that change in blood CO2 concentration proportionally alters REM sleep and hence REM sleep might help in maintaining normocapnia for persistent and stable sleep.
How Does REM Sleep Contribute to These Effects?
There are several chemosensory areas in the brain, which seem to play a specific role in ventilatory response during different vigilant states. For example, focal acidification of the nucleus tractus solitarius increased ventilation significantly both during sleep and wakefulness (Nattie and Li, 2002). On the other hand, acidosis in the retrotrapezoid nucleus increased breathing only during the waking period and not during sleep (Li et al., 1999). However, focal acidification of LC and medial raphe significantly induced ventilatory responses even in an anesthetized condition (Coates et al., 1993; Bernard et al., 1996). Additionally, these two areas play an important role in the modulation of REM sleep (Fuller et al., 2007; Jha and Mallick, 2011). These studies suggest that there are different brain areas, which play an important role in state specific modulation of breathing. In a recent study, it was found that the firing property of the medullary respiratory group of neurons increased significantly during REM sleep apnea (Orem et al., 2000). These neurons fired non-rhythmically at low CO2 but exhibited rhythmic firing pattern at high CO2 level during REM sleep (Orem et al., 2000). Brainstem LC and DRN aminergic neurons modulate the hypoglossal motoneurons, a key regulator of pharyngeal dilator muscle activity. It is believed that a decrement in the firing frequency of raphe and LC neurons during sleep could lead to disfacilitation of hypoglossal motoneurons, thereby contributing to pharyngeal collapse (White, 2006). It has been observed that CO2 concentration increased during sleep apnea (Klingelhofer et al., 1992), which may cause sleep fragmentation by stimulating LC neurons (Dean et al., 2001). Further, the breathing pattern during phasic-REM sleep (with abundant REM sleep associated bursts) remains irregular but it becomes regular during tonic-REM sleep (when REM sleep associated bursts are absent) (Krieger, 2005). Since the medullary respiratory group of neurons become extremely sensitive to CO2 level during REM sleep (Orem et al., 2000), the differential breathing pattern during phasic and tonic-REM sleep could be attributed to the discrete firing pattern of these neurons at different CO2 concentrations.
Some of the chemosensory neurons are electrically coupled and as a result fire together in a synchronous fashion (Andrzejewski et al., 2001). In one fascinating study, Dean et al. (2001) simultaneously recorded two chemosensory neurons in LC in brain slice and observed that these neurons fire together in synchronous fashion (Figure 2). Further, they noticed that these neurons increased their firing rate with incrementing CO2 concentration and synchronous firing of both the neurons remains maintained (Dean et al., 2001). However, continued exposure of high CO2 concentration induced two discrete responses: (a) initially neurons lost their synchronous firing pattern, and later (b) the firing rate was completely abolished (Figure 2) (Dean et al., 2001). These neurons nevertheless regained their spontaneous activity, once the normal CO2 level was restored. These finding thus suggest that if higher CO2 concentration persists, it initially uncouples the LC neural network and subsequently turns them off. Although it is not known if some of the chemosensory neurons are also part of the REM sleep executive machinery, given the total number of 1500 densely packed cells in each side of LC (Aston-Jones et al., 1995), it would be reasonable to presume that some chemosensory cells could be REM-OFF type and can be turned silent if hypercapnia continues. It is also possible that chemosensory cells may directly modulate REM-OFF cells through gap junctions. Anions may spill over to REM-OFF cells from the hyperpolarized chemosensory cells and turn them off during sustained hypercapnia.
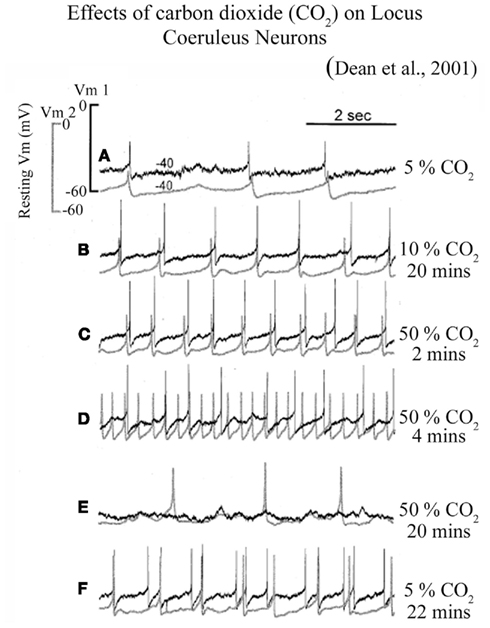
Figure 2. Firing patterns of LC neurons at different CO2 levels in slice preparation. Increased CO2 level from (A) 5% control level to (B) 10% level for 20 min and (C) 50% for 2 min induced a graded stimulation of firing rate which remained synchronized between two neurons; (D) Prolonged exposure to 50% CO2 for 4 min disrupted synchronized firing; while (E) a continued exposure to 50% CO2 for 20 min completely hyperpolarized one of the cells; (F) The same neurons resumed their firing rate after exposing them to 5% CO2 for 22 min, although the lost synchrony was not regained. (Figure taken from Dean et al., 2001; after obtaining copyright permission from the Copyright clearance center, Elsevier Limited, UK through license number: 2820791193001).
Is Silencing of LC REM-OFF Neurons Obligatory for REM Sleep Generation?
The widely accepted reciprocal inhibitory model of REM sleep regulation suggests that REM-OFF neurons of LC are the key regulators of REM sleep (Hobson et al., 1975; Mallick et al., 2001; Fuller et al., 2007). The LC contains REM-OFF neurons, which do not fire during REM sleep (Aston-Jones and Bloom, 1981) and remain highly active during REM sleep deprivation (Mallick et al., 1990). It was proposed that if these neurons are kept persistently active, it should prevent REM sleep generation. Based on this preposition, the obtained results demonstrate that when the LC was stimulated bilaterally with mild, low intensity, and low frequency electrical pulses (an average frequency at which LC neurons normally fire), REM sleep was significantly reduced (Singh and Mallick, 1996). REM sleep remained suppressed throughout the period of stimulation (8 h) and when the stimulation was terminated, it attained normal level with a rebound increase (Singh and Mallick, 1996). Further, it was observed that the effect of electrical stimulation was annulled in the presence of NE-ergic antagonist, which suggests the activity dependent role of LC NE-ergic neurons in the generation of REM sleep (Mallick et al., 2005). It was also found that when these neurons were stimulated pharmacologically using microinjection of picrotoxin (a GABA-A receptor antagonist) in LC, REM sleep was reduced (Kaur et al., 1997). These effects were so pronounced that six such microinjections of picrotoxin into the LC at an interval of every 6 h for 36 h, kept REM sleep inhibited (Kaur et al., 2004). In addition to LC, REM-OFF neurons have been characterized in DRN (Trulson and Jacobs, 1979). Similar to LC neurons, activation of DRN neurons also inhibits REM-ON neurons and REM sleep (Monti and Monti, 2000; Jha et al., 2005b) suggesting that if the REM-OFF neurons are kept active, it would not allow REM sleep to occur normally. These findings thus strongly support the view that REM-OFF neurons must be silent for REM sleep to occur.
How Do LC REM-OFF Neurons Hyperpolarize during REM Sleep?
An elevated CO2 level sustained for a while during the stable and lengthy episodes of NREM sleep may switch off LC REM-OFF neurons for REM sleep genesis. Two populations of LC neurons which exhibit different types of activity patterns or modes (phasic and tonic mode), may differentially be associated with the regulation of attention and vigilance states (Aston-Jones and Cohen, 2005). In addition, LC neurons also show differential responses to hypoxia; some get hyperpolarized and some others get depolarized during a brief period of hypoxia (Yang et al., 1997). It is not known, however, if these two neuronal populations belong to morphologically two distinct groups that release completely different or perhaps similar neurotransmitters. Further, Dean et al. (2001) have found that sustained hypercapnia induced two discrete responses in LC neurons: initially, it dissociated the synchronous firing of the chemosensory neurons, and shortly after, it hyperpolarized the chemosensory neurons while hypercapnia lasted. Similar to hypercapnic conditions, some LC neurons also become completely hyperpolarized during REM sleep (Hobson et al., 1974, 1975; Sakai and Koyama, 1996). These findings clearly demonstrate that LC neurons are completely hyperpolarized under two conditions; one during hypercapnia and another during REM sleep. Therefore, it is possible that the elevated CO2 level within a set range during stable NREM sleep episodes may hyperpolarize REM-OFF neurons. As mentioned earlier, the cessation of LC REM-OFF neurons is pre-requisite for REM sleep to occur, which can possibly be attributed to the altered level of CO2 in LC during a stable NREM sleep period. Since LC neurons modulate the brainstem inspiratory neurons via an excitatory drive (Hakuno et al., 2004), we reason that the group of neurons depolarized during hypoxia and/or hypercapnia would be associated with an intrinsic excitatory drive to help maintain the increased breathing pattern during REM sleep. These findings suggest that an obligatory condition for REM sleep genesis, i.e., the complete cessation of REM-OFF neurons is possibly accomplished through hypercapnic conditions during NREM sleep. This raises a further question, however, “does NREM sleep determine the timing and duration of REM sleep”?
Although the timing and distribution of REM sleep occurrence could be regulated by several homeostatic and circadian factors (Franken, 2002), it has been argued that the accumulation of REM sleep propensity during NREM sleep period also determines the occurrence of REM sleep timing (Benington and Heller, 1994b). It has been observed that the duration of NREM sleep episodes is positively correlated with prior REM sleep episode duration (Benington and Heller, 1994a,b). These studies suggest that the long NREM sleep episodes may progress toward REM sleep and sufficiently long REM sleep episodes may restore a stable NREM sleep episode, otherwise it may terminate into wakefulness. It can thus reasonably be said that the CO2 level during sleep could be one of the determinants that plays a permissive role for the state transformation from NREM sleep to REM sleep to wakefulness or NREM sleep to wakefulness. If CO2 level during NREM sleep increases moderately then it may progress to REM sleep otherwise sharply increased CO2 level would induce wakefulness. Further, if CO2 level is brought back to normal at the end of REM sleep, it would induce another stable NREM sleep episode or else REM sleep would be terminated into wakefulness.
Conclusion
Several evidences clearly suggest that REM sleep begins when LC REM-OFF neurons are hyperpolarized, a condition that seems to be notably permissible for REM sleep. LC neurons also play an important role in breathing pattern during REM sleep. Permanent lesioning of LC neurons increases breathing variability only during REM sleep and not in any other vigilant state. This suggests that LC neurons help modulate REM sleep-dependent breathing patterns (Li and Nattie, 2006). Also, LC neurons are highly responsive to changes in CO2, hence it is considered to be one of the many putative central chemoreceptor sites. A different group of LC neurons is hyperpolarized with increased CO2, while another group (REM-OFF neurons) gets hyperpolarized during REM sleep. We propose that some of the chemosensory neurons could also be REM-OFF type and a moderately elevated CO2 level can hyperpolarize them. However, it needs to be investigated in depth (i) if the majority of REM-OFF cells in LC are also chemosensory neurons; (ii) the alteration of CO2 level in and around LC during NREM and REM sleep; (iii) if blocking of hydrogen pump/exchanger in LC alters REM sleep. But given the facts that (i) change in CO2 level alters REM sleep (Ryan et al., 1983; Lovering et al., 2003, 2004), (ii) LC plays an important role in the regulation of REM sleep (Hobson et al., 1974; Jha and Mallick, 2011) and breathing patterns (Orem et al., 2000; Dean et al., 2001; Viemari, 2008), (iii) majority of LC neurons are highly responsive to the changes in CO2 level (Orem et al., 2000; Dean et al., 2001), and (iv) CO2 significantly increases during NREM sleep (Robin et al., 1958); we hypothesize that LC REM-OFF cells are hyperpolarized by elevated CO2 level during stable NREM sleep, a condition that permits REM sleep to occur.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding to Sushil K. Jha from CSIR, DBT, DST, Capacity Build-up Funds, and UGC resource networking funds is highly acknowledged.
References
Andrzejewski, M., Muckenhoff, K., Scheid, P., and Ballantyne, D. (2001). Synchronized rhythms in chemosensitive neurons of the locus coeruleus in the absence of chemical synaptic transmission. Respir. Physiol. 129, 123–140.
Astic, L., and Saucier, D. (1978). Sleep in Marsupio, the kangaroo rat (Potorous apicalis). Physiol. Behav. 20, 363–368.
Aston-Jones, G., and Bloom, F. E. (1981). Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1, 876–886.
Aston-Jones, G., and Cohen, J. D. (2005). Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 493, 99–110.
Aston-Jones, G., Shipley, M. T., and Grzanna, R. (1995). “The locus coeruleus, A5 and A7 noradrenergic cell groups,” in The Rat Nervous System, ed. G. Paxinos (San Diego: Academic Press), 182–213.
Aton, S. J., Seibt, J., Dumoulin, M., Jha, S. K., Steinmetz, N., Coleman, T., Naidoo, N., and Frank, M. G. (2009). Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron 61, 454–466.
Ballantyne, D., and Scheid, P. (2000). Mammalian brainstem chemosensitive neurones: linking them to respiration in vitro. J. Physiol. (Lond.) 525(Pt 3), 567–577.
Benington, J. H., and Heller, H. C. (1994a). Does the function of REM sleep concern non-REM sleep or waking? Prog. Neurobiol. 44, 433–449.
Benington, J. H., and Heller, H. C. (1994b). REM-sleep timing is controlled homeostatically by accumulation of REM-sleep propensity in non-REM sleep. Am. J. Physiol. 266, R1992–R2000.
Berger, R. J., and Phillips, N. H. (1995). Energy conservation and sleep. Behav. Brain Res. 69, 65–73.
Bernard, D. G., Li, A., and Nattie, E. E. (1996). Evidence for central chemoreception in the midline raphe. J. Appl. Physiol. 80, 108–115.
Bonnet, M. H. (1996). Sleep fragmentation as the cause of daytime sleepiness and reduced performance. Wien. Med. Wochenschr. 146, 332–334.
Carrington, M. J., and Trinder, J. (2008). Blood pressure and heart rate during continuous experimental sleep fragmentation in healthy adults. Sleep 31, 1701–1712.
Chowdhury, A., Chandra, R., and Jha, S. K. (2011). Total sleep deprivation impairs the encoding of trace-conditioned memory in the rat. Neurobiol Learn Mem 95, 355–360.
Coates, L. E., Li, A., and Nattie, E. E. (1993). Widespread sites of brain stem ventilatory chemoreceptors. J. Appl. Physiol. 94, 5–14.
Datta, S. (2000). Avoidance task training potentiates phasic pontine-wave density in the rat: a mechanism for sleep-dependent plasticity. J. Neurosci. 20, 8607–8613.
Dean, J. B., Kinkade, E. A., and Putnam, R. W. (2001). Cell-cell coupling in CO(2)/H(+)-excited neurons in brainstem slices. Respir. Physiol. 129, 83–100.
Farber, J. P., and Tenney, S. M. (1971). The pouch gas of the Virginia opossum (Didelphis virginiana). Respir. Physiol. 11, 335–345.
Fraigne, J. J., Dunin-Barkowski, W. L., and Orem, J. M. (2008). Effect of hypercapnia on sleep and breathing in unanesthetized cats. Sleep 31, 1025–1033.
Frank, M. G., Issa, N. P., and Stryker, M. P. (2001). Sleep enhances plasticity in the developing visual cortex. Neuron 30, 275–287.
Franken, P. (2002). Long-term vs. short-term processes regulating REM sleep. J. Sleep Res. 11, 17–28.
Fuller, P. M., Saper, C. B., and Lu, J. (2007). The pontine REM switch: past and present. J. Physiol. (Lond.) 584, 735–741.
Hakuno, H., Oyamada, Y., Murai, M., Ito, Y., and Yamaguchi, K. (2004). Effects of inactivation and stimulation of locus coeruleus on respiratory activity of neonatal rat. Respir. Physiol. Neurobiol. 140, 9–18.
Hobson, J. A., McCarley, R. W., Pivik, R. T., and Freedman, R. (1974). Selective firing by cat pontine brain stem neurons in desynchronized sleep. J. Neurophysiol. 37, 497–511.
Hobson, J. A., McCarley, R. W., and Wyzinski, P. W. (1975). Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 189, 55–58.
Jha, S. K., Coleman, T., and Frank, M. G. (2006). Sleep and sleep regulation in the ferret (Mustela putorius furo). Behav. Brain Res. 172, 106–113.
Jha, S. K., Jones, B. E., Coleman, T., Steinmetz, N., Law, C. T., Griffin, G., Hawk, J., Dabbish, N., Kalatsky, V. A., and Frank, M. G. (2005a). Sleep-dependent plasticity requires cortical activity. J. Neurosci. 25, 9266–9274.
Jha, S. K., Ross, R. J., and Morrison, A. R. (2005b). Sleep-related neurons in the central nucleus of the amygdala of rats and their modulation by the dorsal raphe nucleus. Physiol. Behav. 86, 415–426.
Jha, S. K., and Mallick, B. N. (2011). “Modulation of REM sleep by non-REM sleep and waking areas in the brain,” in Rapid Eye Movement Sleep, eds B. N. Mallick, S. R. Pandi-Perumal, R. W. McCarley, and A. R. Morrison (New York: Cambridge University Press), 173–182.
Kaur, S., Panchal, M., Faisal, M., Madan, V., Nangia, P., and Mallick, B. N. (2004). Long term blocking of GABA-A receptor in locus coeruleus by bilateral microinfusion of picrotoxin reduced rapid eye movement sleep and increased brain Na-K ATPase activity in freely moving normally behaving rats. Behav. Brain Res. 151, 185–190.
Kaur, S., Saxena, R. N., and Mallick, B. N. (1997). GABA in locus coeruleus regulates spontaneous rapid eye movement sleep by acting on GABAA receptors in freely moving rats. Neurosci. Lett. 223, 105–108.
Klingelhofer, J., Hajak, G., Sander, D., Schulz-Varszegi, M., Ruther, E., and Conrad, B. (1992). Assessment of intracranial hemodynamics in sleep apnea syndrome. Stroke 23, 1427–1433.
Krieger, J. (2005). Respiratory Physiology: Breathing in Normal Subjects. Philadelphia: Elsevier Saunders.
Li, A., and Nattie, E. (2006). Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J. Physiol. (Lond.) 570, 385–396.
Li, A., Randall, M., and Nattie, E. E. (1999). CO(2) microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J. Appl. Physiol. 87, 910–919.
Lovering, A. T., Fraigne, J. J., Dunin-Barkowski, W. L., Vidruk, E. H., and Orem, J. M. (2003). Hypocapnia decreases the amount of rapid eye movement sleep in cats. Sleep 26, 961–967.
Lovering, A. T., Fraigne, J. J., Dunin-Barkowski, W. L., Vidruk, E. H., and Orem, J. M. (2004). Hypocapnia decreases the amount of rapid eye movement sleep in cats. High Alt. Med. Biol. 5, 467; author reply 468.
Lu, J., Sherman, D., Devor, M., and Saper, C. B. (2006). A putative flip-flop switch for control of REM sleep. Nature 441, 589–594.
Madan, V., and Mallick, B. N. (2011). “The role of REM sleep in maintaining neuronal excitibility and its possible mechanism of action,” in Rapid Eye Movement Sleep, eds B. N. Mallick, S. R. Pandi-Perumal, R. W. McCarley, and A. R. Morrison (New York: Cambridge University Press), 359–367.
Mallick, B. N., Kaur, S., and Saxena, R. N. (2001). Interactions between cholinergic and GABAergic neurotransmitters in and around the locus coeruleus for the induction and maintenance of rapid eye movement sleep in rats. Neuroscience 104, 467–485.
Mallick, B. N., Siegel, J. M., and Fahringer, H. (1990). Changes in pontine unit activity with REM sleep deprivation. Brain Res. 515, 94–98.
Mallick, B. N., Singh, S., and Pal, D. (2005). Role of alpha and beta adrenoceptors in locus coeruleus stimulation-induced reduction in rapid eye movement sleep in freely moving rats. Behav. Brain Res. 158, 9–21.
Marks, G. A., Shaffery, J. P., Oksenberg, A., Speciale, S. G., and Roffwarg, H. P. (1995). A functional role for REM sleep in brain maturation. Behav. Brain Res. 69, 1–11.
McGinty, D., and Szymusiak, R. (1990). Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 13, 480–487.
McGinty, D., and Szymusiak, R. (2000). The sleep-wake switch: a neuronal alarm clock. Nat. Med. 6, 510–511.
McKay, L. C., Atalla, A., and Morrell, M. J. (2011). “Physiology and neural control of breathing during sleep,” in Sleep Apnoea, eds W. T. McNicholas and M. R. Bonsignore (Sheffield: European Respiratory Society), 1–16.
Mirmiran, M., Scholtens, J., van de Poll, N. E., Uylings, H. B., van der Gugten, J., and Boer, G. J. (1983a). Effects of experimental suppression of active (REM) sleep during early development upon adult brain and behavior in the rat. Brain Res. 283, 277–286.
Mirmiran, M., Uylings, H. B., and Corner, M. A. (1983b). Pharmacological suppression of REM sleep prior to weaning counteracts the effectiveness of subsequent environmental enrichment on cortical growth in rats. Brain Res. 283, 102–105.
Monti, J. M., and Monti, D. (2000). Role of dorsal raphe nucleus serotonin 5-HT1A receptor in the regulation of REM sleep. Life Sci. 66, 1999–2012.
Morilak, D. A., Fornal, C. A., and Jacobs, B. L. (1987a). Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. I. Thermoregulatory challenge. Brain Res. 422, 17–23.
Morilak, D. A., Fornal, C. A., and Jacobs, B. L. (1987b). Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. II. Cardiovascular challenge. Brain Res. 422, 24–31.
Morrissey, M. J., Duntley, S. P., Anch, A. M., and Nonneman, R. (2004). Active sleep and its role in the prevention of apoptosis in the developing brain. Med. Hypotheses 62, 876–879.
Nattie, E. E., and Li, A. (2002). CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J. Appl. Physiol. 92, 2119–2130.
Netick, A., Dugger, W. J., and Symmons, R. A. (1984). Ventilatory response to hypercapnia during sleep and wakefulness in cats. J. Appl. Physiol. 56, 1347–1354.
Orem, J., Lovering, A. T., Dunin-Barkowski, W., and Vidruk, E. H. (2000). Endogenous excitatory drive to the respiratory system in rapid eye movement sleep in cats. J. Physiol. (Lond.) 527(Pt 2), 365–376.
Pineda, J., and Aghajanian, G. K. (1997). Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77, 723–743.
Reynolds, H. C. (1952). Studies on Reproduction in the Opossum, Didelphis virginiana. Berkeley: University of California Press.
Robin, E. D., Whaley, R. D., Crump, C. H., and Travis, D. M. (1958). Alveolar gas tensions, pulmonary ventilation and blood pH during physiologic sleep in normal subjects. J. Clin. Invest. 37, 981–989.
Ryan, A. T., Hale, B., Megirian, D., and Sherrey, J. H. (1983). The effects of hypoxia and CO2 on the sleep-waking pattern of the potoroo (Potorous tridactylus apicalis). Physiol. Behav. 30, 237–242.
Sakai, K., and Koyama, Y. (1996). Are there cholinergic and non-cholinergic paradoxical sleep-on neurones in the pons? Neuroreport 7, 2449–2453.
Saper, C. B. (2006). Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog. Brain Res. 153, 243–252.
Saper, C. B., Chou, T. C., and Scammell, T. E. (2001). The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 24, 726–731.
Singh, S., and Mallick, B. N. (1996). Mild electrical stimulation of pontine tegmentum around locus coeruleus reduces rapid eye movement sleep in rats. Neurosci. Res. 24, 227–235.
Smith, C., and Rose, G. M. (1996). Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol. Behav. 59, 93–97.
Stickgold, R., Hobson, J. A., Fosse, R., and Fosse, M. (2001). Sleep, learning, and dreams: off-line memory reprocessing. Science 294, 1052–1057.
Stickgold, R., Whidbee, D., Schirmer, B., Patel, V., and Hobson, J. A. (2000). Visual discrimination task improvement: a multi-step process occurring during sleep. J. Cogn. Neurosci. 12, 246–254.
Trinder, J., Van Beveren, J. A., Smith, P., Kleiman, J., and Kay, A. (1997). Correlation between ventilation and EEG-defined arousal during sleep onset in young subjects. J. Appl. Physiol. 83, 2005–2011.
Trulson, M. E., and Jacobs, B. L. (1979). Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 163, 135–150.
van Twyver, H., and Allison, T. (1970). Sleep in the opossum Didelphis marsupialis. Electroencephalogr. Clin. Neurophysiol. 29, 181–189.
Verrier, R. L., Harper, R. M., and Hobson, J. A. (2005). “Cardiovascular physiology: central and autonomic regulation,” in Principles and Practce of Sleep Medicine, eds M. H. Kryger, T. Roth, and W. C. Dement (Philadelphia: Elsevier Saunders), 192–202.
Viemari, J. C. (2008). Noradrenergic modulation of the respiratory neural network. Respir. Physiol. Neurobiol. 164, 123–130.
White, D. P. (2006). The pathogenesis of obstructive sleep apnea: advances in the past 100 years. Am. J. Respir. Cell Mol. Biol. 34, 1–6.
Keywords: sleep, brainstem, breathing, paradoxical sleep, REM-OFF and REM-ON neurons
Citation: Madan V and Jha SK (2012) A moderate increase of physiological CO2 in a critical range during stable NREM sleep episode: a potential gateway to REM sleep. Front. Neur. 3:19. doi: 10.3389/fneur.2012.00019
Received: 01 November 2011;
Accepted: 01 February 2012;
Published online: 20 February 2012.
Edited by:
Larry Sanford, Eastern Virginia Medical School, USAReviewed by:
Angel Nunez, Universidad Autonoma de Madrid, SpainJames Shaffery, University of Mississippi Medical Center, USA
Copyright: © 2012 Madan and Jha. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Sushil K. Jha, Sleep Research Laboratory, School of Life Sciences, Jawaharlal Nehru University, New Delhi 110067, India. e-mail:c3VzaGlsa2poYUBtYWlsLmpudS5hYy5pbg==;c3VzaGlsXzEwMDBAeWFob28uY29t
 Vibha Madan
Vibha Madan