Abstract
Background: The data on the relationship between statin use and clinical outcomes after intravenous thrombolysis (IVT) for acute ischemic stroke (AIS) are in controversy.
Objective: This systematic review and meta-analysis aimed to evaluate the safety and efficacy of statins administered prior to onset and during hospitalization in patients with AIS treated with IVT.
Methods: We searched PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials from inception until June 8, 2021. Comparative studies investigating statin effect on intracranial hemorrhage (ICH), functional outcomes, and mortality in adults with AIS treated with IVT were screened. Random-effect meta-analyses of odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were performed. The protocol was registered in PROSPERO (CRD42021254919).
Results: Twenty-two observational studies were included, which involved 17,554 patients. The pooled estimates showed that pre-stroke statin use was associated with a higher likelihood of symptomatic ICH (OR 1.31; 95% CI 1.07–1.59; p = 0.008) and any ICH (OR 1.21; 95% CI 1.03–1.43; p = 0.02). However, the pre-stroke statin use was not significantly associated with the 3-month mortality, 3-month favorable functional outcome (FFO, modified Rankin Scale [mRS] score 0–1), and 3-month functional independence (FI; mRS score 0–2). However, in-hospital statin use was associated with a reduced risk of symptomatic ICH (OR 0.46; 95% CI 0.21–1.00; p = 0.045), any ICH (OR 0.51; 95% CI 0.27–0.98; p = 0.04), and 3-month mortality (OR 0.42; 95% CI 0.29–0.62; p < 0.001) and an increased probability of 3-month FFO (OR 1.33; 95% CI 1.02–1.744; p = 0.04) and 3-month FI (OR 1.41; 95% C, 1.11–1.80; p = 0.005).
Conclusions: The present systematic review and meta-analysis suggests that in-hospital statin use after IVT may be safe and may have a favorable impact on clinical outcomes, a finding not observed in studies restricted to patients with pre-stroke statin use.
Highlights
-
- Twenty-two observational articles with more than 15,000 patients were enrolled.
-
- Pre-stroke statin use probably increase the risk of intracranial hemorrhage, but has no effect on functional outcome or mortality.
-
- In-hospital statin use probably decrease the risk of intracranial hemorrhage and mortality and increase the odds of a good functional outcome.
Introduction
Stroke is a common devastating neurological condition and one of the top causes of disability and mortality worldwide (1, 2). There are two major types: ischemic stroke and hemorrhagic stroke. Of note, acute ischemic stroke (AIS) accounts for ~80% of total strokes (3). In terms of treatment strategy of AIS, timely reperfusion of ischemic tissue to save the ischemic penumbra is the key to avoid severe disability and premature death (4). Intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator, which is the only thrombolytic drug approved by the US Food and Drug Administration for AIS (5, 6), is considered to be most effective when administered within the first few hours of stroke onset (7).
For many years, researchers and medical doctors have been looking for a combination therapy to reduce the risk of mortality and improve functional outcomes for AIS patients treated with IVT. Statins, one of the most commonly prescribed medications for treatment of dyslipidemia, have gained attention recently as promising therapeutic agents for neurological conditions (8). Studies in animal models have shown that statins have pleiotropic effects on neuronal survival, angiogenesis, neurogenesis, and brain remodeling in ischemic stroke brain injury (9–12). Thereby, statins have potential neuroprotective and neurorestorative effects for AIS. Previous meta-analyses driven mostly by observational studies showed that statin use in AIS patients may be associated with improved functional outcome and short-term survival (13, 14). Accordingly, a recent guideline from the American Heart Association/American Stroke Association (15) recommends that AIS patients qualified for statin treatment should receive statin therapy as soon as possible. However, this recommendation is mainly based on observational studies of AIS patients with heterogeneous treatments. The existing observational studies on whether the use of statin is associated with any clinical benefit in AIS patients after IVT have reported fragmentary and conflicting results. Thus, a relatively homogeneous set of participants (AIS patients receiving IVT) was enrolled in this meta-analysis.
We hypothesize that statin use is likely to be associated with improved mortality and functional outcomes in AIS patients treated with IVT. Given that there is no randomized clinical trial (RCT) to date evaluating the safety and efficacy of statin therapy in patients with AIS treated with IVT, we performed a comprehensive systematic review and meta-analysis of post-hoc analyses of RCTs and observational studies to investigate its comparative safety and efficacy.
Methods
This meta-analysis was conducted strictly in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (16). It was prospectively registered in the PROSPERO (International Prospective Register of Systematic Reviews) registry, with registration number of CRD42021254919. The PRISMA checklist is available in Supplementary Table 1.
Search Strategy
One investigator (QB) performed a comprehensive literature search in multiple electronic databases (PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials) until June 8, 2021, without any restrictions. MeSH (in PubMed) and Emtree (in EMBASE) terms were used, as well as text words. Search terms included those related to stroke, thrombolysis, statins, and their variants. The detailed search strategy is available in Supplementary Table 2. Two investigators (YG and JY) manually searched all the references from relevant reviews and meta-analyses for additional studies.
Inclusion and Exclusion Criteria
Inclusion criteria included the following: (1) types of studies: post-hoc analyses of RCT, prospective or retrospective cohort study; (2) characteristics of participants: adult patients (≥18 years) with AIS treated with IVT (with recombinant tissue plasminogen activator); (3) types of interventions: statin therapy regardless of type and dose; and (4) types of outcome measures: at least one outcome of interest, including symptomatic intracranial hemorrhage (ICH), any ICH, 3-month mortality, 3-month favorable functional outcome (FFO), and 3-month functional independence (FI), with odds ratio (OR) or clinical data to calculate OR.
Exclusion criteria included the following: (1) abstract with insufficient data; (2) studies that included fewer than 50 patients; (3) statin use only as a covariate in the statistical model; (4) studies providing only overlapping data with previous publication.
Study Selection
The following study selection processes were performed. Step 1: the records obtained from initial search were imported into the Zotero citation management software (www.zotero.org) and duplicates were removed. Step 2: two investigators (YG and JY) screened the titles and abstracts of remaining articles and excluded the non-relevant articles. Step 3: the full texts of the relevant articles were retrieved for further assessment of eligibility. Disagreements were resolved through group discussion with another investigator.
Data Extraction
Two investigators (YG and XG) independently extracted data from each included study using a standardized form. The following information was extracted: (1) study characteristics: name of first author, year of publication, country of origin, type of design, and total number of patients; (2) patient characteristics: age, sex, and baseline National Institutes of Health Stroke Scale (NIHSS) score; (3) intervention characteristics: use of statins; and (4) data on outcomes of interest, etc. Disagreements were resolved through group discussion with another investigator.
Risk of Bias Assessment
The Newcastle–Ottawa scale (NOS) (17) was used to evaluate the methodological quality of post-hoc analyses of RCTs and cohort studies included in this meta-analysis. The quality control and bias assessment were performed independently by two investigators (YG and XG). NOS score >7, 7 ≥ NOS score > 5, and NOS score ≤5 indicated good quality, fair quality, and poor quality, respectively. Disagreements were resolved through group discussion with another investigator.
Statistical Analysis
We investigated the association between statin use and clinical outcomes using pooled ORs and their corresponding 95% confidence intervals (CIs). To stabilize the variance and normalize the distribution, ORs with corresponding 95% CIs were extracted from each study and transformed into log OR and standard error (18). For studies that did not report risk estimates for the comparison of user vs. non-user of statins, we calculated ORs based on the available published data (19). Meta-analyses were performed using a random-effect model accounting for clinical heterogeneity (20). The effects of pre-stroke and in-hospital statin use were considered separately. p < 0.05 was considered statistically significant.
Statistical heterogeneity across studies was assessed by the Cochran Q test and quantified by the I2 statistic. For the qualitative interpretation of heterogeneity, I2 > 50% was considered significant (21). Potential publication bias across studies was graphically evaluated using a funnel plot and estimated through Egger's test (with p < 0.1 indicating significance) (22).
Meta-analyses were performed using RevMan 5.3 software (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark). Egger's test was conducted with Stata 15.0 software (Stata Corporation, College Station, TX, USA).
Results
Literature Search and Study Selection
Our literature searches in the PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials databases yielded 652, 1,324, and 39 records, respectively. After review of titles and abstracts, and exclusion of duplicate records, 33 potentially eligible studies were retrieved. After careful evaluation of full texts, 11 studies were excluded (data available from Supplemental Table 3), and 22 studies (23–44) were included. The study selection process is illustrated in Figure 1.
Figure 1

PRISMA flow diagram.
Study Characteristics
Among the included 22 studies (23–44), there were 2 post-hoc analyses of RCTs (35, 40), 13 prospective cohort studies (24, 27–29, 31, 33, 34, 37–39, 42–44), and 7 retrospective cohort studies (23, 25, 26, 30, 32, 36, 40). The 22 included studies were published from 2007 to 2021, with sample sizes ranging from 55 to 4,012 participants and a total of 17,554 participants. The mean age of participants ranged from 50 to 71 years, and most of them were male. The baseline NIHSS score varied from 7 to 17. The main outcomes were ICH, functional outcomes, and mortality after at least 3 months of follow-up. Statin therapy was classified into two major types: pre-stroke statin use and in-hospital statin use. Characteristics of included studies are summarized in Table 1.
Table 1
| References | Country | Study design | Total-n | Age-y | Male-% | Baseline NIHSS | Exposure | Statin-% | Follow up-m | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Alvarez-Sabín et al. (23) | Spain | RC | 145 | 72 | 52 | 17 | Statin① | 17.9 | 3 | Ⓔ |
| Bruning et al. (24) | Germany | PC | 542 | 72 | 51 | 11 | Statin①② | 26.4①, 35.7② | 3 | ⒶⒸ |
| Cappellari et al. (25) | Italy | RC | 178 | NR | 58 | NR | Statin④ | 35.4 | 3 | ⒶⒺ |
| Cappellari et al. (26) | Italy | RC | 2,072 | 67 | 58 | 13 | Statin⑥ | 40.5 | 3 | ⒶⒸⒹⒺ |
| Cui et al. (27) | China | PC | 215 | 71 | 53 | 9 | Statin② | 83.7 | 3 | ⒷⒺ |
| Engelter et al. (28) | Europe | PC | 4,012 | 68 | 56 | 12 | Statin① | 22.9 | 3 | ⒶⒷⒸⒹⒺ |
| Faivre et al. (29) | France | PC | 101 | 63 | 59 | 15 | Statin① | 25.0 | 3 | ⒶⒺ |
| Geng et al. (30) | China | RC | 119 | 62 | 71 | NR | Statin③ | 59.7 | 3 | ⒶⒷⒸⒹ |
| Kang et al. (31) | Korea | PC | 86 | NR | NR | NR | Statin⑤ | 17.4 | 3 | ⒶⒹⒺ |
| Makihara et al. (32) | Japan | RC | 489 | 71 | 65 | 12 | Statin① | 31.7 | 3 | ⒷⒹ |
| Martinez-Ramirez et al. (33) | Spain | PC | 182 | 68 | 54 | 14 | Statin① | 16.3 | 3 | ⒶⒷⒸⒺ |
| Miedema et al. (34) | Netherlands | PC | 476 | 69 | 54 | 13 | Statin① | 20.6 | 3 | ⒶⒺ |
| Montaner et al. (35) | Spain | Post-hoc RCT | 55 | NR | NR | 7 | Simvastatin③ | 49.1 | 3 | ⒶⒷⒸⒺ |
| Mowla et al. (36) | USA | RC | 834 | 71 | 51 | 12 | Statin① | 33.8 | 3 | ⒶⒺ |
| Rocco et al. (37) | Germay | PC | 1,066 | 73 | 53 | 12 | Statin① | 20.5 | 3 | ⒶⒷⒸⒹ |
| Scheitz et al. (38) | Germany | PC | 481 | 74 | 50 | 11 | Statin② | 17.2 | 3 | ⒸⒺ |
| Scheitz et al. (39) | Germany, Switzerland | PC | 1,446 | 75 | 54 | 11 | Statin① | 21.9 | 3 | ⒶⒺ |
| Scheitz et al. (40) | International | Post-hoc RCT | 2,583 | 68 | 57 | 14 | Statin① | 15.3 | 3 | Ⓐ |
| Tong et al. (41) | China | RC | 367 | 69 | 55 | 9 | Statin⑥ | 51.2 | 3 | ⒶⒺ |
| Tsivgoulis et al. (42) | International | PC | 1,660 | 67 | 59 | 11 | Statin① | 22.5 | 3 | ⒶⒸⒹⒺ |
| Uyttenboogaart et al. (43) | Netherlands | PC | 252 | 68 | 54 | 12 | Statin① | 12.3 | 3 | ⒶⒸⒹⒺ |
| Zhao et al. (44) | China | PC | 193 | 65 | 64 | 9 | Statin① | 24.4 | 3 | ⒶⒸⒹⒺ |
Baseline characteristics of included studies.
NR, not report; PC, prospective cohort; RC, retrospective cohort; RCT, randomized clinical trial.
① pre-stroke statin use; ② post-stroke statin use; ③ started statin within 12 h of stroke onset; ④ started statin within 24 h of stroke onset; ⑤ started statin within 48 h of stroke onset; ⑥ started statin within 72 h of stroke onset; Ⓐ symptomatic intracranial hemorrhage; Ⓑ any intracranial hemorrhage; Ⓒ 3 month-mortality; Ⓓ 3 month-favorable functional outcome; Ⓔ 3 month-functional independence.
Study Quality
Risk of bias among the post-hoc analyses of RCTs and cohort studies was assessed with NOS. The results showed that 15 studies were graded as good quality (25–28, 30, 32, 34–39, 41–43) and the remaining 7 studies were graded as fair quality. The overall score of the NOS was 173 of 198 (87%), which is considered to represent an overall high quality. Details of the quality assessment are shown in Table 2.
Table 2
| References | Selection | Comparability | Outcome | Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis * | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Alvarez-Sabín et al. (23) | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | 7 | ||
| Bruning et al. (24) | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | 7 | |
| Cappellari et al. (25) | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 8 | |
| Cappellari et al. (26) | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 8 | |
| Cui et al. (27) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | 8 | |
| Engelter et al. (28) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 9 |
| Faivre et al. (29) | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | 7 | |
| Geng et al. (30) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 9 |
| Kang et al. (31) | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | 6 | ||
| Makihara et al. (32) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 9 |
| Martinez-Ramirez et al. (33) | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | 6 | ||
| Miedema et al. (34) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 9 |
| Montaner et al. (35) | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 8 | |
| Mowla et al. (36) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 9 |
| Rocco et al. (37) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 9 |
| Scheitz et al. (38) | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 8 | |
| Scheitz et al. (39) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 9 |
| Scheitz et al. (40) | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | 6 | ||
| Tong et al. (41) | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 8 | |
| Tsivgoulis et al. (42) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 9 |
| Uyttenboogaart et al. (43) | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | 8 | |
| Zhao et al. (44) | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | 6 | ||
| Total | 13/22 | 22/22 | 22/22 | 22/22 | 32/44 | 22/22 | 22/22 | 18/22 | 173/198 |
Risk of bias assessment.
A maximum of 2 stars can be allotted in this category; one for age, and the other for other controlled factors.
Association Between Statin Use and Outcomes
Table 3 provides a comprehensive overview of the association between pre-stroke or in-hospital statin use and various clinical outcomes.
Table 3
| Outcome | Pre-stroke statin use | In-hospital statin use | ||||||
|---|---|---|---|---|---|---|---|---|
| Studies, n | OR (95% CI) | p-value |
Heterogeneity (I2, p for Cochran Q) |
Studies, n | OR (95% CI) | p-value |
Heterogeneity (I2, p for Cochran Q) |
|
| sICH | 12 | 1.31 (1.07–1.59) | 0.008 | I 2 = 20%, p = 0.25 | 5 | 0.46 (0.21–1.00) | 0.05* | I 2 = 0%, p = 0.88 |
| Any ICH | 4 | 1.21 (1.03–1.43) | 0.02 | I 2 = 0%, p = 0.91 | 3 | 0.51 (0.27–0.98) | 0.04 | I 2 = 0%, p = 0.53 |
| Mortality (3 mo) | 7 | 1.06 (0.74–1.51) | 0.76 | I 2 = 64%, p = 0.01 | 5 | 0.42 (0.29–0.62) | <0.001 | I 2 = 0%, p = 0.44 |
| FFO (3 mo) | 6 | 0.93 (0.81–1.07) | 0.33 | I 2 = 0%, p = 0.67 | 3 | 1.33 (1.02–1.74) | 0.04 | I 2 = 0%, p = 0.72 |
| FI (3 mo) | 10 | 1.14 (0.86–1.52) | 0.37 | I 2 = 66%, p = 0.002 | 7 | 1.41 (1.11–1.80) | 0.005 | I 2 = 6%, p = 0.38 |
Overview of the safety and efficacy analyses on different endpoints.
CI, confidence interval; FFO, favorable functional outcome; FI, functional independence; ICH, intracranial hemorrhage; OR, odds ratio; sICH, symptomatic intracranial hemorrhage.
The p-value was 0.045, approximately equal to 0.05.
Pre-stroke Statin Use and Outcomes
We identified 14 studies (23, 24, 28, 29, 32–34, 36, 37, 39, 40, 42–44) involving 13,990 participants that explored the effect of pre-stroke statin use on ICH, mortality, and functional outcome in patients with AIS treated with IVT. The pooled estimates showed that pre-stroke statin use was associated with an increased odds of symptomatic ICH (12 studies, OR 1.31; 95% CI 1.07–1.59; p = 0.008; p for Cochran Q statistic = 0.25, I2 = 20%; Figure 2A; Table 3) and any ICH (four studies, OR 1.21; 95% CI 1.03–1.43; p = 0.02; p for Cochran Q statistic = 0.91, I2 = 0%; Figure 2B; Table 3). However, pre-stroke statin use was not significantly related to 3-month mortality (seven studies, OR 1.06; 95% CI 0.74–1.51; p = 0.76; p for Cochran Q statistic = 0.01, I2 = 64%; Figure 2C; Table 3), 3-month FFO (six studies, OR 0.93; 95% CI 0.81–1.07; p = 0.33; p for Cochran Q statistic = 0.67, I2 = 0%; Figure 2D; Table 3), and 3-month FI (10 studies, OR 1.14; 95% CI 0.86–1.52; p = 0.37; p for Cochran Q statistic = 0.002, I2 = 66%; Figure 2E; Table 3).
Figure 2
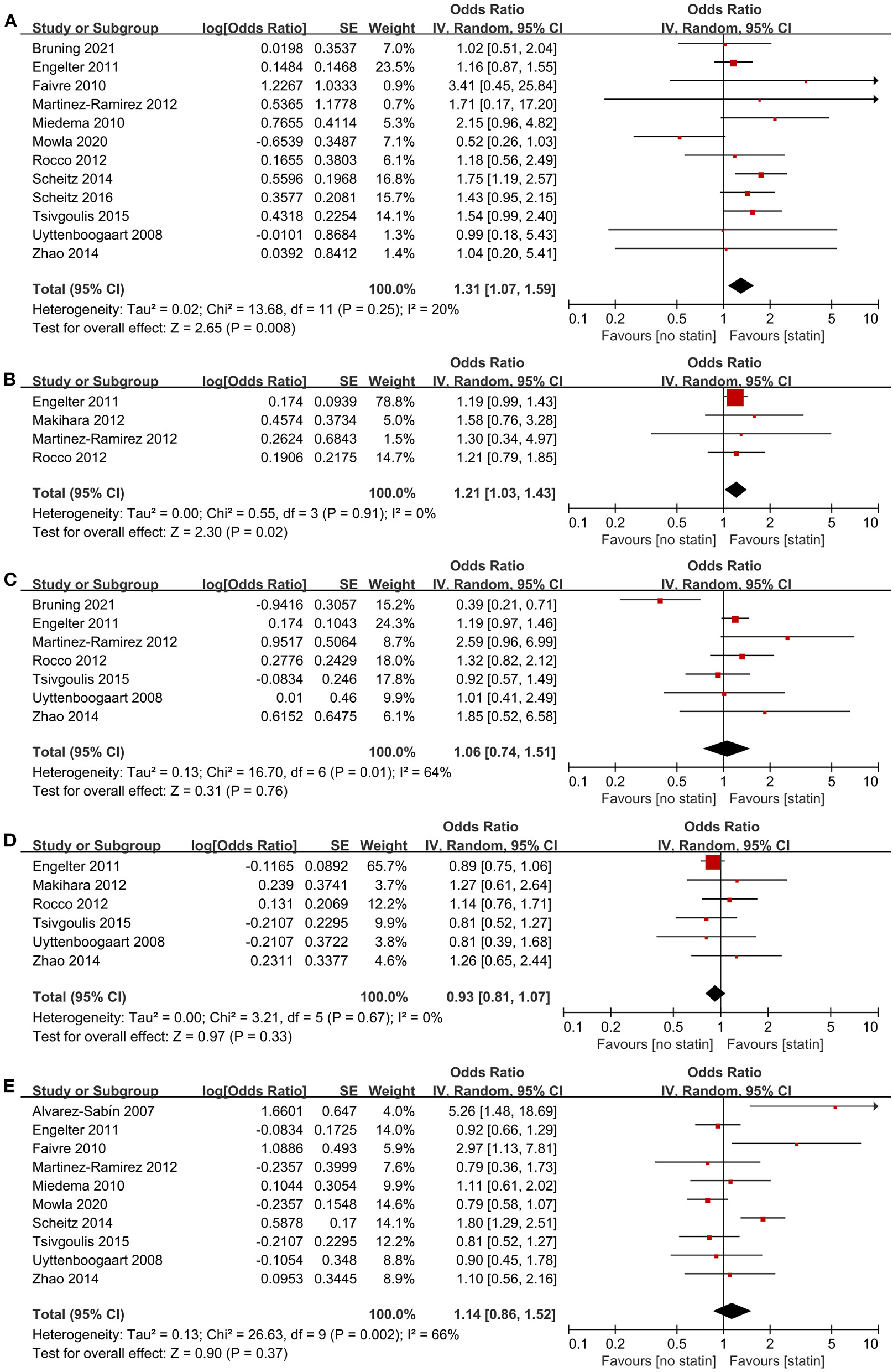
Association of pre-stroke statin use with (A) symptomatic intracranial hemorrhage, (B) any intracranial hemorrhage, (C) 3-month mortality, (D) 3-month favorable functional outcome, and (E) 3-month functional independence.
In-hospital Statin Use and Outcomes
Nine studies (24–27, 30, 31, 35, 38, 41) involving 4,115 patients reported outcomes according to in-hospital statin use. The pooled estimates showed that in-hospital statin use was associated with a lower likelihood of symptomatic ICH (five studies, OR 0.46; 95% CI 0.21–1.00; p = 0.045; p for Cochran Q statistic = 0.88, I2 = 0%; Figure 3A; Table 3), any ICH (three studies, OR 0.51; 95% CI 0.27–0.98; p = 0.04; p for Cochran Q statistic = 0.53, I2 = 0%; Figure 3B; Table 3), and 3-month mortality (five studies, OR 0.42; 95% CI 0.29–0.62; p < 0.001; p for Cochran Q statistic = 0.44, I2 = 0%; Figure 3C; Table 3). The pooled estimates also showed that in-hospital statin use was associated with 3-month FFO (three studies, OR 1.33; 95% CI 1.02–1.74; p = 0.04; p for Cochran Q statistic = 0.72, I2 = 0%; Figure 3D; Table 3) and 3-month FI (seven studies, OR 1.41; 95% CI 1.11–1.80; p = 0.005; p for Cochran Q statistic = 0.38, I2 = 6%; Figure 3E; Table 3).
Figure 3

Association of in-hospital statin use with (A) symptomatic intracranial hemorrhage, (B) any intracranial hemorrhage, (C) 3-month mortality, (D) 3-month favorable functional outcome, and (E) 3-month functional independence.
Publication Bias
For the safety and efficacy analyses on different endpoints, visual inspection of the funnel plot and the Egger statistical test revealed no evidence of asymmetry, indicating no potential publication bias (data available from Supplemental Figures 1, 2).
Discussion
There were two major findings in this comprehensive systematic review and meta-analysis with 22 studies involving more than 15,000 participants. The primary finding was that pre-stroke statin use was associated with a potentially higher risk of systematic ICH in AIS patients treated with IVT whereas in-hospital statin use was related with a lower likelihood of symptomatic ICH. The secondary finding was that in-hospital statin use was associated with improved outcome in AIS patients treated with IVT, a finding not observed in patients using statin prior to hospital admission.
According to the American Heart Association/American Stroke Association guidelines updated in 2019 (15), it is reasonable to initiate statin therapy in eligible AIS patients. This is supported by previously published meta-analyses (13, 14), which have shown that the use of statins was associated with improved outcome. However, conflicting data were observed in a subgroup restricted to thrombolysis-treated patients (13, 14, 45). The heterogeneity in the previous studies may be due to several reasons. Firstly, a heterogeneous population undergoing different treatment modalities, including mechanical recanalization, IVT, and intra-arterial thrombolysis, was included. Secondly, the starting time of statin administration, including pre-stroke statin use and in-hospital statin use, was not considered separately. A large multicenter RCT should be the best way to address the question whether the use of statin is associated with any clinical benefit in AIS patients after IVT. Such a trial may be challenging in determining the duration and frequency of statin. However, to date, there is only one small RCT with 310 patients that has investigated the safety and efficacy of intensive statin in the acute phase of ischemic stroke after IVT therapy (46). In this trial, because of the recommendation from the American Heart Association/American Stroke Association guidelines (15), the ethics committee did not approve the no-statin group based on the principles of non-maleficence and beneficence. Therefore, we performed a comprehensive systematic review and meta-analysis of observational studies and post-hoc RCT analyses. Our findings may provide a good basis for determining the use of statin in combination with IVT for patients with AIS.
Our findings have important implications for both policymakers and clinicians. Firstly, previously published systematic reviews have raised concerns that statin therapy could increase the risk of ICH (47, 48). We found that in-hospital statin use probably decreased the risk of systematic ICH. Our findings provide evidence against the theoretical concerns of increased ICH risk with statin treatment. Additionally, previously published systematic reviews found that statin therapy at stroke onset was associated with improved outcome; however, inconclusive results were observed in studies restricted to thrombolysis-treated patients (13, 14, 33, 45). Our meta-analysis found that, in AIS patients receiving IVT, statin use during hospitalization was associated with improved outcome. We consider that our findings further support current international recommendations that AIS patients qualified for statin treatment should receive statin therapy as soon as possible (class of recommendation = II, level of evidence = C) (15). In addition, we believe that pretreatment with statins is not recommended as it does not improve outcomes of AIS patient treated with IVT but increases the risk of ICH.
Our findings might be attributed to the cholesterol-independent (pleiotropic) protective effects of statins. Among these, the pleiotropic effects can inhibit the differentiation of microglia to M1 cells and the release of inflammatory factors after tissue plasminogen activator treatment, thereby protecting neurovascular function. Reducing blood–brain barrier destruction may explain the positive effect of in-hospital statin treatment on the incidence of hemorrhagic transformation and clinical outcomes (49, 50). In a rat model of embolic stroke, combination treatment with atorvastatin and tissue plasminogen activator at 4 h after stroke significantly reduced the infarct volume, improved the neurologic function, and decreased the incidence of hemorrhagic transformation by decreasing neutrophil infiltration and metalloproteinase-9 expression (49). In addition, Lu et al. also found that rosuvastatin combined with tissue plasminogen activator after stroke onset prevented the activation of astrocytes and microglia and reduced the release of inflammatory factors, thereby alleviating blood–brain barrier disruption and hemorrhagic transformation severity (50). However, in stroke patients receiving IVT, the beneficial effects have not been observed consistently in prior statin users, because the beneficial effects of statins may diminish after withdrawal (51, 52), which is in agreement with one previous study (44). In addition, compared with statin treatment after thrombolysis, statin use before stroke significantly increased the fibrinolytic effect and disrupted homeostasis between coagulation and fibrinolysis (25). Hence, it might be possible that pre-stroke statin use associates with a potential higher risk of systematic ICH in AIS patients treated with IVT.
Certain limitations of the present study warrant further consideration. Firstly, this is a meta-analysis of observational studies. Our findings were exclusively based on data of observational studies that predispose to inherent biases, especially selection bias. Secondly, despite the use of adjusted ORs whenever applicable, unmeasured confounders cannot be eliminated due to a lack of individual study patient data. It is possible that differences in cardiovascular risk factors might account for observed associations, while the confounding role of pharmacologic differences in statins cannot be excluded. Thirdly, specific data for statin, including dosage, duration, compliance, pharmacokinetics, and statin type, were not assessed. These parameters could have introduced unmeasured biases in our analysis.
Our study also has several strengths. Firstly, to our knowledge, this is the first systematic review and meta-analysis to explore the effects of starting time of statin administration (pre-stroke or in-hospital) in patients with AIS treated with IVT. Secondly, the majority of the included studies were prospective cohort studies or post-hoc analysis of RCTs with high quality and had adequately adjusted for confounders. This might reduce the influences of other cardiovascular risk factors on the association of pre-stroke statin use with clinical outcomes. Thirdly, the number of available studies and the sample size were large, which allowed us to explore the association of pre-stroke and in-hospital statin administration with clinical outcomes.
Conclusion
In AIS patients treated with IVT, pre-stroke statin use was probably associated with increased risk of ICH, but had no effect on good functional outcome or mortality at 3 months. On the contrary, in-hospital statin use probably decreased the risk of ICH and 3-month mortality and was associated with good functional outcome at 3 months.
Funding
This research was funded by the Science and Technology Department of Qinghai Province (Grant No. 2019-ZJ-7040) and the National Key R&D Program of China (Grant Nos. 2018YFC1312600 and 2018YFC1312601).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YG: study concept and design, acquisition of data, analysis and interpretation, and critical revision of the manuscript for important intellectual content. XG: acquisition of data, analysis and interpretation, and critical revision of the manuscript for important intellectual content. KZ: critical revision of the manuscript for important intellectual content. QB and JY: acquisition of data. MY: study supervision and critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.734927/full#supplementary-material
- AIS
acute ischemic stroke
- CI
confidence interval
- FFO
favorable functional outcome
- FI
functional independence
- ICH
intracranial hemorrhage
- IVT
intravenous thrombolysis
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- NOS
Newcastle-Ottawa scale
- OR
odds ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- RCT
randomized clinical trial.
Abbreviations
References
1.
Katan M Luft A . Global burden of stroke. Semin Neurol. (2018) 38:208–11. 10.1055/s-0038-1649503
2.
Benjamin EJ Virani SS Callaway CW Chamberlain AM Chang AR Cheng S et al . Heart Disease and Stroke Statistics-2018 update: a report from the American Heart Association. Circulation. (2018) 137:e67–492. 10.1161/CIR.0000000000000573
3.
Feigin VL Forouzanfar MH Krishnamurthi R Mensah GA Connor M Bennett DA et al . Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. (2014) 383:245–54. 10.1016/S0140-6736(13)61953-4
4.
Furie KL Jayaraman MV . 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. (2018) 49:509–10. 10.1161/STROKEAHA.118.020176
5.
Jauch EC Saver JL Adams HP Jr Bruno A Connors JJB Demaerschalk BM et al . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:870–947. 10.1161/STR.0b013e318284056a
6.
Xu AD Wang YJ Wang DZ Chinese Stroke Therapy Expert Panel for Intravenous Recombinant Tissue Plasminogen Activator . Consensus statement on the use of intravenous recombinant tissue plasminogen activator to treat acute ischemic stroke by the Chinese Stroke Therapy Expert Panel. CNS Neurosci Ther. (2013) 19:543–8. 10.1111/cns.12126
7.
Gumbinger C Reuter B Stock C Sauer T Wiethölter H Bruder I et al . Time to treatment with recombinant tissueplasminogen activator and outcome of stroke in clinical practice: retrospective analysis of hospital quality assurance data withcomparison with results from randomised clinical trials. BMJ. (2014) 348:g3429. 10.1136/bmj.g3429
8.
Fracassi A Marangoni M Rosso P Pallottini V Fioramonti M Siteni S et al . Statins and the brain: more than lipid lowering agents?Curr Neuropharmacol. (2019) 17:59–83. 10.2174/1570159X15666170703101816
9.
Chen J Zhang ZG Li Y Wang Y Wang L Jiang H et al . Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. (2003) 53:743–51. 10.1002/ana.10555
10.
Kilic E Reitmeir R Kilic Ü Caglayan AB Beker MC Kelestemur T et al . HMG-CoA reductase inhibition promotes neurological recovery, peri-lesional tissue remodeling, and contralesional pyramidal tract plasticity after focal cerebral ischemia. Front Cell Neurosci. (2014) 8:422. 10.3389/fncel.2014.00422
11.
Yan L Zhu T . Effects of rosuvastatin on neuronal apoptosis in cerebral ischemic stroke rats via Sirt1/NF-kappa B signaling pathway. Eur Rev Med Pharmacol Sci. (2019) 23:5449–55. 10.26355/eurrev_201906_18214
12.
Carloni S Balduini W . Simvastatin preconditioning confers neuroprotection against hypoxia-ischemia induced brain damage in neonatal rats via autophagy and silent information regulator 1 (SIRT1) activation. Exp Neurol. (2020) 324:113117. 10.1016/j.expneurol.2019.113117
13.
Ni Chroinin D Asplund K Asberg S Callaly E Cuadrado-Godia E DiezTejedor E et al . Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke. (2013) 44:448–56. 10.1161/STROKEAHA.112.668277
14.
Hong KS Lee JS . Statins in acute ischemic stroke: a systematic review. J Stroke. (2015) 17:282–301. 10.5853/jos.2015.17.3.282
15.
Powers WJ Rabinstein AA Ackerson T Adeoye OM Bambakidis NC Becker K et al . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. 10.1161/STR.0000000000000211
16.
Moher D Liberati A Tetzlaff J Altman DG PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. 10.1136/bmj.b2535
17.
Stang A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z
18.
Higgins JPT Green S . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration (2011). Available online at: www.handbook.cochrane.org (accessed August 10, 2021).
19.
Bland JM Altman DG . Statistics notes. The odds ratio. BMJ. (2000) 320:1468. 10.1136/bmj.320.7247.1468
20.
DerSimonian R Laird N . Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. 10.1016/0197-2456(86)90046-2
21.
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. 10.1002/sim.1186
22.
Sterne JAC Sutton AJ Ioannidis JPA Terrin N Jones DR Lau J et al . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. 10.1136/bmj.d4002
23.
Alvarez-Sabín J Huertas R Quintana M Rubiera M Delgado P Ribó M et al . Prior statin use may be associated with improved stroke outcome after tissue plasminogen activator. Stroke. (2007) 38:1076–8. 10.1161/01.STR.0000258075.58283.8f
24.
Bruning T Al-Khaled M . Do statins reduce the mortality rate in stroke patients treated with systemic thrombolysis in a 5-year. Neural Regen Res. (2021) 16:1807–12. 10.4103/1673-5374.306088
25.
Cappellari M Deluca C Tinazzi M Tomelleri G Carletti M Fiaschi A et al . Does statin in the acute phase of ischemic stroke improve outcome after intravenous thrombolysis? A retrospective study. J Neurol Sci. (2011) 308:128–34. 10.1016/j.jns.2011.05.026
26.
Cappellari M Bovi P Moretto G Zini A Nencini P Sessa M et al . The THRombolysis and STatins (THRaST) study. Neurology. (2013) 80:655–61. 10.1212/WNL.0b013e318281cc83
27.
Cui C Li Y Bao J Dong S Gao L He L . Low dose statins improve prognosis of ischemic stroke patients with intravenous thrombolysis. BMC Neurol. (2021) 21:220. 10.1186/s12883-021-02259-9
28.
Engelter ST Soinne L Ringleb P Sarikaya H Bordet R Berrouschot J et al . IV thrombolysis and statins. Neurology. (2011) 77:888–95. 10.1212/WNL.0b013e31822c9135
29.
Faivre A Sagui E Canini F Wybrecht D Bounolleau P Grapperon J et al . [Intravenous thrombolysis with rt-PA in stroke: experience of the French military hospital of Toulon from September 2003 to June 2009]. Rev Neurol (Paris). (2010) 166:909–20. 10.1016/j.neurol.2010.03.020
30.
Geng J Song Y Mu Z Xu Q Shi G Sun Y et al . Early use of statin in patients treated with alteplase for acute ischemic stroke. Acta Neurochir Suppl. (2016) 121:269–75. 10.1007/978-3-319-18497-5_47
31.
Kang J Kim N Park TH Bang OY Lee JS Lee J et al . Early statin use in ischemic stroke patients treated with recanalization therapy: retrospective observational study. BMC Neurol. (2015) 15:122. 10.1186/s12883-015-0367-4
32.
Makihara N Okada Y Koga M Shiokawa Y Nakagawara J Furui E et al . Effect of serum lipid levels on stroke outcome after rt-PA therapy: SAMURAI rt-PA registry. Cerebrovasc Dis. (2012) 33:240–7. 10.1159/000334664
33.
Martinez-Ramirez S Delgado-Mederos R Marin R Suárez-Calvet M Sáinz MP Alejaldre A et al . Premorbid use of statin may increase the risk of symptomatic intracranial haemorrhage in thrombolysis for ischemic stroke: results from a case-control study and a meta-analysis. J Neurol. (2012) 259:111–8. 10.1007/s00415-011-6137-3
34.
Miedema I Uyttenboogaart M Koopman K Keyser JD Luijckx GJ . Statin use and functional outcome after tissue plasminogen activator treatment in acute ischaemic stroke. Cerebrovasc Dis. (2010) 29:263–7. 10.1159/000275500
35.
Montaner J Bustamante A Garcia-Matas S Martínez-Zabaleta M Jiménez C de la Torre J et al . Combination of thrombolysis and statins in acute stroke is safe: results of the STARS randomized trial (stroke treatment with acute reperfusion and simvastatin). Stroke. (2016) 47:2870–3. 10.1161/STROKEAHA.116.014600
36.
Mowla A Shah H Lail NS Vaughn CB Shirani P Sawyer RN . Statins use and outcome of acute ischemic stroke patients after systemic thrombolysis. Cerebrovasc Dis. (2020) 49:503–8. 10.1159/000510095
37.
Rocco A Sykora M Ringleb P Diedler J . Impact of statin use and lipid profile on symptomatic intracerebral haemorrhage, outcome and mortality after intravenous thrombolysis in acute stroke. Cerebrovasc Dis. (2012) 33:362–8. 10.1159/000335840
38.
Scheitz JF Endres M Heuschmann PU Audebert HJ Nolte CH . Reduced risk of poststroke pneumonia in thrombolyzed stroke patients with continued statin treatment. Int J Stroke. (2015) 10:61–6. 10.1111/j.1747-4949.2012.00864.x
39.
Scheitz JF Seiffge DJ Tütüncü S Gensicke H Audebert HJ Bonati LH et al . Dose-related effects of statins on symptomatic intracerebral hemorrhage and outcome after thrombolysis for ischemic stroke. Stroke. (2014) 45:509–14. 10.1161/STROKEAHA.113.002751
40.
Scheitz JF MacIsaac RL Abdul-Rahim AH Siegerink B Bath PM Endres M et al . Statins and risk of poststroke hemorrhagic complications. Neurology. (2016) 86:1590–6. 10.1212/WNL.0000000000002606
41.
Tong LS Hu HT Zhang S Yan SQ Lou M . Statin withdrawal beyond acute phase affected outcome of thrombolytic stroke patients: an observational retrospective study. Medicine (Baltimore). (2015) 94:e779. 10.1097/MD.0000000000000779
42.
Tsivgoulis G Kadlecova P Kobayashi A Czlonkowska A Brozman M Švigelj V et al . Safety of premorbid use of statin in intravenous thrombolysis for acute ischemic stroke. Stroke. (2015) 46:2681–4. 10.1161/STROKEAHA.115.010244
43.
Uyttenboogaart M Koch MW Koopman K Vroomen PC Luijckx GJ Keyser JD . Lipid profile, statin use, and outcome after intravenous thrombolysis for acute ischaemic stroke. J Neurol. (2008) 255:875–80. 10.1007/s00415-008-0797-7
44.
Zhao HD Zhang YD . The effects of previous statin treatment on plasma matrix metalloproteinase-9 level in Chinese stroke patients undergoing thrombolysis. J Stroke Cerebrovasc Dis. (2014) 23:2788–93. 10.1016/j.jstrokecerebrovasdis.2014.07.001
45.
Liu J Wang Q Ye C Li G Zhang B Ji Z et al . Premorbid use of statin and outcome of acute ischemic stroke after intravenous thrombolysis: a meta-analysis. Front Neurol. (2020) 11:585592. 10.3389/fneur.2020.585592
46.
Wan-Yong Y Yu-Feng L Zi-Ran W Tian-Xia Y Dong-Juan X Nan Y et al . Combined therapy of intensive statin plus intravenous rt-PA in acute ischemic stroke: the INSPIRE randomized clinical trial. J Neurol. (2021) 268:2560–9. 10.1007/s00415-020-10388-3
47.
Tan C Liu X Mo L Wei X Peng W Wang H et al . Statin, cholesterol, and sICH after acute ischemic stroke: systematic review and meta-analysis. Neurol Sci. (2019) 40:2267–75. 10.1007/s10072-019-03995-0
48.
Ziff OJ Banerjee G Ambler G Werring DJ . Statins and the risk of intracerebral haemorrhage in patients with stroke: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. (2019) 90:75–83. 10.1136/jnnp-2018-318483
49.
Zhang L Chopp M Jia L Cui Y Lu M Zhang ZG . Atorvastatin extends the therapeutic window for tPA to 6 h after the onset of embolic stroke in rats. J Cereb Blood Flow Metab. (2009) 29:1816–24. 10.1038/jcbfm.2009.105
50.
Lu D Liu Y Mai H Zang J Shen L Zhang Y et al . Rosuvastatin reduces neuroinflammation in the hemorrhagic transformation after rt-PA treatment in a mouse model of experimental stroke. Front Cell Neurosci. (2018) 12:225. 10.3389/fncel.2018.00225
51.
Endres M Laufs U . Discontinuation of statin treatment in stroke patients. Stroke. (2006) 37:2640–3. 10.1161/01.STR.0000240690.69406.28
52.
Vitturi BK Gagliardi RJ . The influence of statin withdrawal and adherence on stroke outcomes. Neurol Sci. (2021) 42:2317–23. 10.1007/s10072-020-04790-y
Summary
Keywords
stroke, thrombolysis, statin, intracranial hemorrhage, functional outcomes, mortality, meta-analysis
Citation
Guo Y, Guo X, Zhao K, Bao Q, Yang J and Yang M (2021) Statin Use and Outcomes of Patients With Acute Ischemic Stroke Treated With Intravenous Thrombolysis: A Systematic Review and Meta-Analysis. Front. Neurol. 12:734927. doi: 10.3389/fneur.2021.734927
Received
01 July 2021
Accepted
19 August 2021
Published
22 September 2021
Volume
12 - 2021
Edited by
Peter Sporns, University Hospital of Basel, Switzerland
Reviewed by
Yujie Wang, The People's Hospital of Liaoning Province, China; Michele Romoli, University of Perugia, Italy
Updates
Copyright
© 2021 Guo, Guo, Zhao, Bao, Yang and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingfei Yang iloveyoucmu@163.com
†These authors have contributed equally to this work and share first authorship
This article was submitted to Stroke, a section of the journal Frontiers in Neurology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.