Abstract
Objectives:
The aim of the study was to assess the effect of the stroke health management model on the prognosis and recurrence of mild to moderate ischemic stroke, guided by the stroke health manager based on the patients' needs. In addition, up-to-date evidence of healthcare resource allocation, planning, and optimization is provided.
Methods:
The current research was a retrospective, observational, single-center, history-controlled study with patients divided into two groups, namely, the intervention group and the control group, following the guidance of the stroke health manager. The control group patients received standard medical care during hospitalization, which consisted of advice on healthy lifestyle choices carried out by the bed nurse, but no structured education, WeChat group, or clinical consultation was included. The intervention group patients, in addition to the standard medical care, received health management and health education from the stroke health manager, and after hospital discharge, the patients were followed up over the telephone by the health manager to see if there was any recurrence or readmission.
Results:
From 1 January 2018 to 31 December 2020, 382 patients with acute ischemic stroke were enrolled in this study. Through the univariate regression analysis, we found that SHM intervention was associated with a significantly lower risk of recurrence (HR = 0.459). We constructed a nomogram based on the significant variables from the regression analysis and also analyzed the association between the control group and the SHM intervention group among all subgroups using the Cox proportional hazards model to assess the effect of the stroke health management model. Most patients in this study had a total risk point between 170 and 270. The C-index value was 0.76, and the time-dependent AUC for predicting recurrence was >0.7.
Conclusion:
The stroke health manager-guided management model based on patients' needs can better control the risk factors of stroke and significantly reduce the recurrence rate of mild to moderate ischemic stroke within 1 year.
Introduction
In China, the burden of stroke has increased over the past 30 years and has become the leading cause of mortality and morbidity, accounting for 1.57 million deaths in 2018 (1). Notably, ischemic stroke accounts for up to 80% of strokes in China, with a high recurrence rate (1). The Chinese stroke survey showed that the rate of stroke recurrence at 1 year was around 8.2–16% and that the rate of stroke recurrence at 5 years was as high as 41% (2, 3), which is significantly higher than the international report (10–15%) (4).
A study on the risk factors for ischemic stroke in 22 countries (INTERSTROKE) found 10 risk factors that can explain 91.5% of the population-attributable risk of ischemic stroke (4–7). The prevalence of major risk factors for stroke is high in the general population and among stroke survivors, and most of the risk factors have increased over time (1, 5, 8). For preventing recurrent ischemic stroke, the development of measures and standardized strategies targeting etiology and risk factors is important (7, 9, 10). In addition, the intervention of behavioral risk factors for ischemic stroke by improving diet and physical activity, controlling smoking, and limiting alcohol consumption is also of great significance for preventing stroke recurrence (1, 5, 11). Increasing evidence shows that tracking risk factors, clinical characteristics, management patterns, and outcomes of patients with stroke facilitate resource allocation and priority setting in the healthcare system (1, 2, 12–15).
Driven by this principle, the Stroke Screening and Prevention Project Committee of the National Health Commission sponsored the “Stroke Health Manager (SHM)” training program in 2017 for training the stroke health manager nationwide, combined with professional stroke managers and continuous health management (1). The combination of models provides comprehensive, one-stop, and professional stroke health management for patients with stroke (5, 13). A stroke management model should be oriented to the needs of patients with stroke, led by the stroke health manager, and inclusive of multiple disciplines to conduct comprehensive and targeted assessments for patients, through regular and standardized healthcare, medication consultation, rehabilitation guidance, and other stroke management work (9, 15–17).
In this report, a retrospective analysis was conducted to assess the effect of the stroke health management model on the prognosis and recurrence of mild to moderate ischemic stroke, as guided by the stroke health manager based on patients' needs (18, 19).
Methods
The study design was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Approve ID: 2021-SR-382).
Study design and participants
This work was a retrospective, observational, single-center, history-controlled study comprising two groups. Based on the electronic medical records, patients who presented to the Stroke Emergency Green Channel of the First Affiliated Hospital of Nanjing Medical University were consecutively recruited from 1 January 2018 to 31 December 2020 by a senior nurse, if they met the following criteria: (1) patients aged ≥ 18 years; (2) patients diagnosed with ischemic stroke as per the Chinese Guidelines for Diagnosis and Treatment of Acute Ischemic Stroke 2018; (3) patients with the first episode of ischemic stroke; (4) patients with mild to moderate stroke with an NIHSS score at admission between 1 and 15; (5) patients with a 12-month follow-up after hospital discharge; and/or (6) patients who gave, or authorized a representative to give, informed consent. Patients with incomplete follow-up information were excluded from the study. The included patients were divided into a control group (1 January 2018 to 30 April 2019) and an SHM intervention group (1 May 2018 to 31 December 2020), following the guidance of the stroke health manager.
In the Hospital Quality Monitoring System (HQMS), recurrent stroke diagnosis and comorbidities were identified by the main diagnosis using National Clinical V.2.0 of the International Classification of Diseases, 10th Revision, disease codes. In the Chinese Stroke Center Alliance (CSCA), stroke diagnosis was determined at discharge. Procedures or interventions were identified by the International Classification of Diseases, Ninth Revision, Clinical Modification, Volume 3.
Health management
Stroke health manager
Stroke health manager is a new profession promoted by the Stroke Prevention and Treatment Engineering Committee of China for which senior nurses are recommended. The stroke health manager of our hospital conducted full-time management of patients with ischemic stroke during their hospitalization and followed them up after their discharge from the hospital, based on evidence-based medicine, and as a bridge and coordination between medical research and health management.
Health education
The stroke health manager used multimedia devices to conduct half-hour courses for all hospitalized patients with stroke and their family members in the stroke center. The course content was in line with the American Heart Association/American Stroke Association Guidelines (11), which included management of risk factors for stroke, prevention of deep vein thrombosis in lower extremities, healthy dietary guidance, early exercise rehabilitation therapy, and health education on constipation (13). It was among the stroke health manager's responsibilities to ensure the quality and continuity of the educational content so that patients could learn more about stroke prevention and care during hospitalization as well as make correlative video and promotional films (e.g., ankle pump exercises), which were to be played on the display screen of the outpatient hall and treatment area.
Clinical consultation
The control group patients received standard medical care during hospitalization, which consisted of advice on healthy lifestyle choices carried out by the bed nurse, but no structured education, WeChat group, or clinical consultation was included. The intervention group patients, in addition to standard medical care, received health management and health education from the stroke health manager, which include the following processes: (1) evaluating patients and establishing health records; (2) adding patients to the group of stroke health management; (3) establishing follow-up relationship and formulating the health education plan; (4) guiding patients to cooperate with doctors for diagnosis and treatment; (5) providing health education through information technology, such as nursing information system, WeChat official account, and health management WeChat group; and (6) conducting full-time “face-to-face” follow-up with patients, carrying out weekly patient education lectures, or hosting irregular in-hospital training meetings and other forms.
Post-discharge advice
The stroke health manager issues a follow-up notice to patients who plan to discharge from the hospital, informing them of relevant matters, and a follow-up manual to the patients. The management of patients after hospital discharge is carried out through WeChat public groups, telephone follow-ups, and face-to-face follow-ups. A telephone follow-up is conducted 3, 6, and 12 months after hospital discharge for patients who have not been followed up in the follow-up clinic, including (1) checking the patient's rehabilitation status; (2) guiding healthy lifestyle and chronic disease management; and (3) evaluating the existing or potential risk factors and scale (Figure 1).
Figure 1
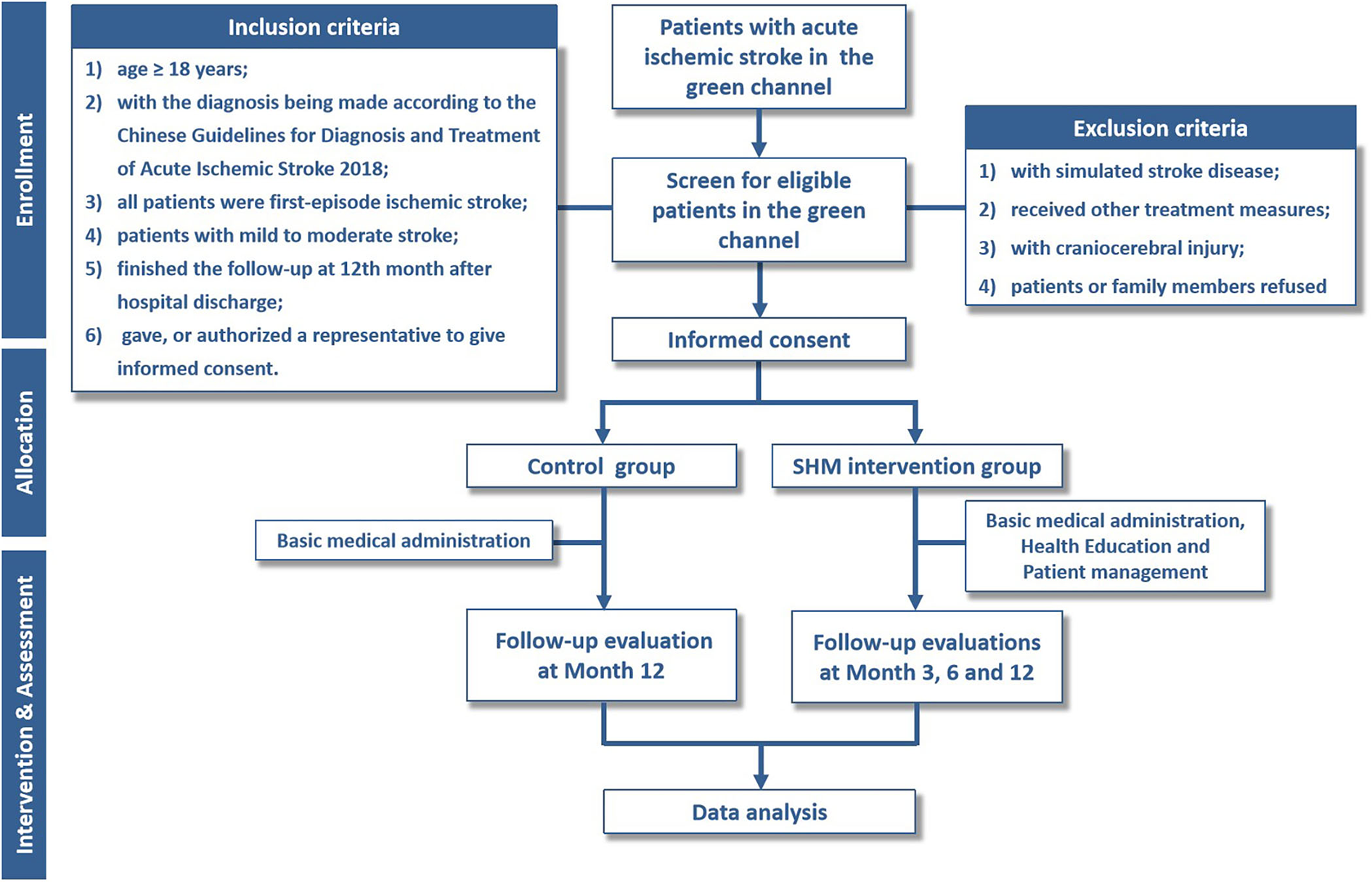
Flowchart of the trial. SHM, stroke health manager.
Data collection and outcome assessment
The stroke health manager used a paper-based registry for collecting study data. We used the results of patients' examination the day before their hospital discharge as baseline data, which were retrieved from the medical record. The results of the tests conducted in the follow-up clinic 3 months after discharge were used as outcome indicators, which included sitting systolic blood pressure (SBP), diastolic blood pressure (DBP), serum lipids, modified Rankin Scale (mRS), and recurrence rate. Blood pressure was measured in the seated position after 5 min of rest. An mRS score ≤ 2 at 3 months post-discharge was defined as a good functional outcome. A Barthel index score ≥ 60 was defined as basic self-care in daily life. Fasting blood glucose was not a routine examination for all patients, and only patients with a history of diabetes as listed in the follow-up manual were given a blood glucose examination during the follow-up. Blood pressure and blood glucose levels were compared only between patients with hypertension and diabetes. At 6 months after discharge, the patients were followed up over the telephone by the health manager to see if there was any recurrence or readmission. All the data were then entered into a specialized database.
Statistical analysis
Descriptive statistics were used to summarize sociodemographic and health-related characteristics. The chi-square test and the t-test were conducted to compare the baseline characteristics between the control group and the SHM intervention group. The distribution of variables is given as mean and SD for continuous variables and as number and percentage for categorical variables. For comparison between the two groups, the Mann–Whitney U test was used for continuous variables. All significance tests were two-sided and conducted at a 5% significance level. The recurrence probabilities were estimated using the nomogram. The concordance index (C-index) and the area under the time-dependent receiver operating characteristic curve (time-dependent AUC) calculated by bootstrapping were used to evaluate discriminative ability. Calibration plots were used to evaluate calibrating ability. C-index and AUC values varied from 0.5 to 1.0, where 0.5 represents random chance and 1.0 indicates a perfect fit. However, C-index and AUC values >0.7 suggest a reasonable estimation.
Results
Demographic and clinical characteristics
From 1 January 2018 to 31 December 2020, 382 patients with acute ischemic stroke were enrolled in this study (from 1 January 2018 to 30 April 2019, 169 patients in the control group; from 1 May 2019 to 31 December 2020, 213 patients in the SHM intervention group). The characteristics of the study participants are shown in Table 1. The baseline characteristics of the participants included in the analysis were well balanced. In total, 193 (50.66%) participants received intravenous thrombolysis therapy, 99 (25.98%) participants received mechanical thrombectomy, and 90 (23.62%) participants received combination therapy. In the CT scan, 51.57% of the patients showed a unilateral implicative range, with 50.52% of the involved vasculum being basal ganglia. From CTA results, the mainly involved vasculum in 54.71% of the patients was the middle cerebral artery. The most common comorbidities in this population were hypertension (57.59%), diabetes mellitus (23.82%), and atrial fibrillation (20.68%).
Table 1
| Patient characteristics | Total ( n = 382) | Control ( n = 169) | SHM intervention ( n = 213) | P | |||
|---|---|---|---|---|---|---|---|
| Gender, n(%) | 0.350 | ||||||
| Men | 241 | (63.10) | 111 | (65.68) | 130 | (76.92) | |
| Women | 141 | (36.91) | 58 | (34.32) | 83 | (49.11) | |
| Age in year, mean (SD) | 67.46 | (11.52) | 67.23 | (11.53) | 67.95 | (11.55) | 0.544 |
| BMI, mean (SD) | 23.91 | (3.00) | 23.63 | (3.34) | 23.93 | (2.60) | 0.332 |
| Time to treatment, mean (SD) | 3.1 | (2.52) | 2.92 | (2.68) | 3.21 | (2.09) | 0.241 |
| NIHSS at admission, mean (SD) | 11.37 | (6.27) | 11.51 | (6.48) | 11.37 | (6.03) | 0.820 |
| Therapy Interventions, n(%) | 0.052 | ||||||
| Intravenous thrombolysis | 193 | (50.52) | 96 | (56.80) | 97 | (57.40) | |
| Mechanical thrombectomy | 99 | (25.92) | 37 | (21.89) | 62 | (36.69) | |
| Both | 90 | (23.56) | 36 | (21.30) | 54 | (31.95) | |
| Implicative range in CT, n(%) | 0.127 | ||||||
| Unilateral | 197 | (51.57) | 83 | (49.11) | 114 | (67.46) | |
| Bilateral /Multiple | 166 | (43.46) | 70 | (41.42) | 96 | (56.80) | |
| No abnormality | 19 | (4.97) | 16 | (9.47) | 3 | (1.78) | |
| Involved territory in CT, n(%) | |||||||
| Basal ganglia | 193 | (50.52) | 81 | (47.93) | 120 | (71.01) | 0.102 |
| Lateral ventricle | 166 | (43.46) | 68 | (40.24) | 98 | (57.99) | 0.258 |
| Cerebral cortex | 126 | (32.98) | 54 | (31.95) | 72 | (42.6) | 0.702 |
| Semioval center | 24 | (6.28) | 12 | (7.10) | 12 | (7.10) | 0.557 |
| Brainstem | 19 | (4.97) | 6 | (3.55) | 13 | (7.69) | 0.254 |
| Cerebellum | 6 | (1.57) | 4 | (2.37) | 2 | (1.18) | 0.412 |
| Implicative range in CTA, n(%) | 0.626 | ||||||
| Unilateral | 239 | (62.57) | 104 | (61.54) | 135 | (79.88) | |
| Bilateral/Multiple | 62 | (16.23) | 31 | (18.34) | 31 | (18.34) | |
| No abnormality | 74 | (19.37) | 32 | (18.93) | 42 | (24.85) | |
| Mainly involved vasculum in CTA, n(%) | |||||||
| Carotid artery | 84 | (21.99) | 35 | (20.71) | 49 | (28.99) | 0.591 |
| Vertebral artery/Basilar artery | 41 | (10.73) | 19 | (11.24) | 22 | (13.02) | 0.774 |
| Middle cerebral artery | 209 | (54.71) | 84 | (49.70) | 125 | (73.96) | 0.080 |
| Anterior cerebral artery | 23 | (6.02) | 9 | (5.33) | 14 | (8.28) | 0.611 |
| Posterior cerebral artery | 26 | (6.81) | 11 | (6.51) | 15 | (8.88) | 0.837 |
| Comorbidities, n(%) | |||||||
| None | 44 | (11.52) | 23 | (13.61) | 21 | (12.43) | 0.254 |
| Hypertension | 220 | (57.59) | 105 | (62.13) | 113 | (66.86) | 0.075 |
| Diabetes mellitus | 91 | (23.82) | 45 | (26.63) | 41 | (24.26) | 0.086 |
| Atrial fibrillation | 79 | (20.68) | 28 | (16.57) | 51 | (30.18) | 0.077 |
| Surgical operation | 69 | (18.06) | 30 | (17.75) | 39 | (23.08) | 0.888 |
| Ischemic stroke | 62 | (16.23) | 28 | (16.57) | 34 | (20.12) | 0.873 |
| Cardiovascular disorders | 36 | (9.42) | 19 | (11.24) | 14 | (8.28) | 0.107 |
| Malignant tumor | 19 | (4.97) | 9 | (5.33) | 10 | (5.92) | 0.778 |
| Hyperlipidemia | 12 | (3.14) | 25 | (14.79) | 40 | (23.67) | 0.303 |
| Thyropathy | 8 | (2.09) | 3 | (1.78) | 5 | (2.96) | 0.698 |
| Valvular heart disease | 7 | (1.83) | 2 | (1.18) | 5 | (2.96) | 0.471 |
| Neurological/Psychiatric disorder | 7 | (1.83) | 2 | (1.18) | 5 | (2.96) | 0.471 |
| Hemorrhagic stroke | 6 | (1.57) | 3 | (1.78) | 3 | (1.78) | 0.775 |
| NIHSS at discharge, mean (SD) | 5.94 | (7.92) | 6.55 | (9.38) | 5.46 | (6.53) | 0.182 |
| mRS at discharge, n (%) | 0.617 | ||||||
| 0 | 71 | (18.59) | 28 | (16.57) | 43 | (25.44) | |
| 1 | 83 | (21.73) | 38 | (22.49) | 45 | (26.63) | |
| 2 | 71 | (18.59) | 33 | (19.53) | 38 | (22.49) | |
| 3 | 55 | (14.40) | 26 | (15.38) | 29 | (17.16) | |
| 4 | 39 | (10.21) | 19 | (11.24) | 20 | (11.83) | |
| 5 | 49 | (12.83) | 11 | (6.51) | 38 | (22.49) | |
| 6 | 14 | (3.66) | 14 | (8.28) | 0 | (0) | |
| mRS at 3 months, n(%) | / | ||||||
| 0 | 45 | (11.78) | 0 | (0) | 45 | (26.63) | |
| 1 | 47 | (12.30) | 0 | (0) | 47 | (27.81) | |
| 2 | 43 | (11.26) | 0 | (0) | 43 | (25.44) | |
| 3 | 42 | (10.99) | 0 | (0) | 42 | (24.85) | |
| 4 | 18 | (4.71) | 0 | (0) | 18 | (10.65) | |
| 5 | 18 | (4.71) | 0 | (0) | 18 | (10.65) | |
| 6 | 0 | (0) | 0 | (0) | 0 | (0) | |
| mRS at 6 months, n(%) | / | ||||||
| 0 | 49 | (12.83) | 0 | (0) | 49 | (28.99) | |
| 1 | 48 | (12.57) | 0 | (0) | 48 | (28.40) | |
| 2 | 38 | (9.95) | 0 | (0) | 38 | (22.49) | |
| 3 | 43 | (11.26) | 0 | (0) | 43 | (25.44) | |
| 4 | 19 | (4.97) | 0 | (0) | 19 | (11.24) | |
| 5 | 15 | (3.93) | 0 | (0) | 15 | (8.88) | |
| 6 | 0 | (0) | 0 | (0) | 0 | (0) | |
| mRS at 12 months, n(%) | 0.122 | ||||||
| 0 | 122 | (31.94) | 62 | (36.69) | 60 | (35.50) | |
| 1 | 90 | (23.56) | 44 | (26.04) | 46 | (27.22) | |
| 2 | 53 | (13.87) | 18 | (10.65) | 35 | (20.71) | |
| 3 | 54 | (14.14) | 14 | (8.28) | 40 | (23.67) | |
| 4 | 20 | (5.24) | 5 | (2.96) | 15 | (8.88) | |
| 5 | 17 | (4.45) | 4 | (2.37) | 13 | (7.69) | |
| 6 | 26 | (6.81) | 22 | (13.02) | 4 | (2.37) | |
Demographics of the study population.
SHM, stroke health manager; BMI, body mass index; NIHSS, National Institutes of Health Stroke Scale; CT, computed tomography; CTA, computed tomography angiography; mRS, modified Rankin Scale.
SHM intervention and the risk of recurrent ischemic stroke
We analyzed the association between the SHM intervention and the risk of ischemic stroke recurrence using the Cox proportional hazard models (Figure 2). The univariate regression analysis revealed that SHM intervention was associated with a lower risk of recurrence (HR = 0.459), showing a 54.1% risk reduction in the SHM intervention group compared with the control group.
Figure 2
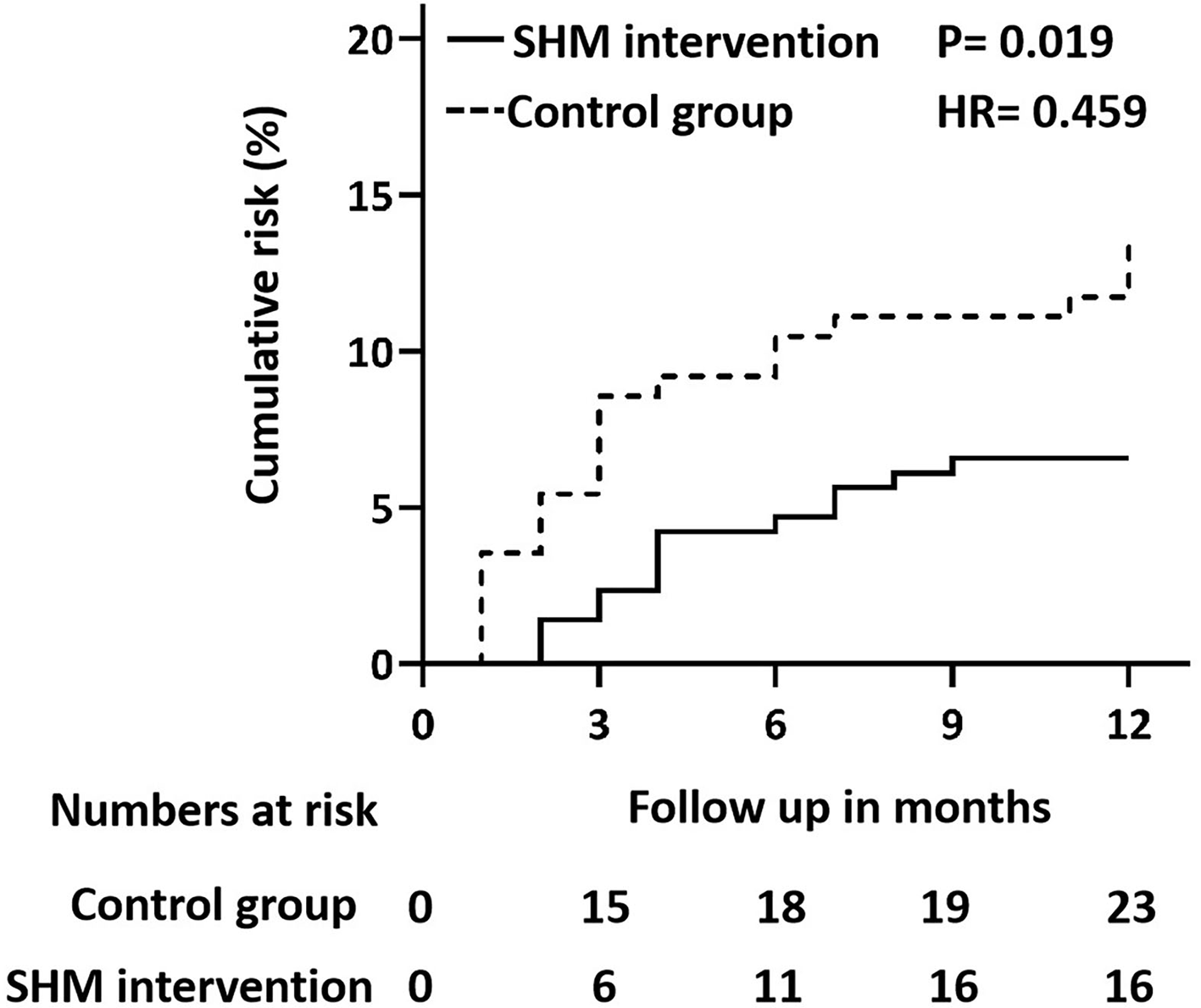
Cox regression model (proportional hazards model) plot for recurrent ischemic stroke in the control group and SHM intervention group. SHM, stroke health manager.
Nomogram variable screening and nomogram construction and validation
The results of the univariate regression analysis are given in Table 2. We found that two variables (SHM intervention and atrial fibrillation) were significantly associated with recurrence. In a multivariate regression analysis of SHM intervention, age, NIHSS on admission, atrial fibrillation, history of ischemic stroke, and treatment modality interventions, three variables (NIHSS on admission, atrial fibrillation, and history of ischemic stroke) were identified as independent prognostic factors for recurrence.
Table 2
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| SHM interventions | 0.470 | 0.233–0.950 | 0.035 | |||
| Gender | 1.097 | 0.542–2.221 | 0.796 | |||
| Age in year | 1.032 | 1.000–1.064 | 0.052 | |||
| BMI | 0.915 | 0.815–1.027 | 0.130 | |||
| Time to treatment | 0.926 | 0.773–1.108 | 0.400 | |||
| NIHSS at admission | 1.053 | 0.999–1.110 | 0.056 | 1.068 | 1.013–1.127 | 0.015 |
| Therapy interventions | ||||||
| Intravenous thrombolysis | 1.000 | |||||
| Mechanical thrombectomy | 0.714 | 0.270–1.885 | 0.496 | |||
| Both | 2.038 | 0.947–4.384 | 0.069 | |||
| Implicative range in CT | ||||||
| No abnormality | 1.000 | |||||
| Unilateral | 0.536 | 0.143–2.018 | 0.536 | |||
| Bilateral/Multiple | 0.530 | 0.138–2.028 | 0.354 | |||
| Involved territory in CT | ||||||
| Basal ganglia | 0.614 | 0.307–1.232 | 0.170 | |||
| Lateral ventricle | 0.812 | 0.402–1.640 | 0.562 | |||
| Cerebral cortex | 1.512 | 0.751–3.045 | 0.247 | |||
| Semioval center | 0.401 | 0.053–3.062 | 0.378 | |||
| Brainstem | 1.875 | 0.519–6.771 | 0.337 | |||
| Cerebellum | 1.949 | 0.221–17.152 | 0.548 | |||
| Implicative range in CTA | ||||||
| No abnormality | 1.000 | |||||
| Unilateral | 1.035 | 0.399–2.682 | 0.944 | |||
| Bilateral/Multiple | 2.179 | 0.744–6.383 | 0.155 | |||
| Mainly involved vasculum in CTA | ||||||
| Carotid artery | 0.830 | 0.374–1.841 | 0.647 | |||
| Vertebral artery/Basilar artery | 0.958 | 0.321–2.860 | 0.939 | |||
| Middle cerebral artery | 0.850 | 0.424–1.704 | 0.647 | |||
| Anterior cerebral artery | 1.482 | 0.418–5.251 | 0.542 | |||
| Posterior cerebral artery | 0.543 | 0.176–1.674 | 0.288 | |||
| Comorbidities | ||||||
| None | 1.621 | 0.634–4.146 | 0.313 | |||
| Hypertension | 1.549 | 0.778–3.084 | 0.213 | |||
| Diabetes mellitus | 1.838 | 0.878–3.848 | 0.107 | |||
| Atrial fibrillation | 0.206 | 0.048–0.875 | 0.032 | 0.168 | 0.039–0.726 | 0.017 |
| Surgical operation | 1.106 | 0.463–2.639 | 0.821 | |||
| Ischemic stroke | 2.175 | 0.990–4.775 | 0.053 | 2.267 | 1.013–5.072 | 0.046 |
| Cardiovascular disorders | 0.958 | 0.277–3.308 | 0.945 | |||
| Malignant tumor | 1.138 | 0.252–5.138 | 0.866 | |||
| Hyperlipidemia | 0.584 | 0.199–1.712 | 0.327 | |||
| Thyropathy | 1.384 | 0.165–11.574 | 0.764 | |||
| Valvular heart disease | 0.000 | 0.000–∞ | 0.999 | |||
| Neurological/Psychiatric disorders | 1.619 | 0.189–13.836 | 0.660 | |||
| Hemorrhagic stroke | 0.000 | 0.000–∞ | 0.999 | |||
| NIHSS at discharge | 1.021 | 0.984–1.061 | 0.272 | |||
| mRS at discharge | ||||||
| 0 | 1.000 | |||||
| 1 | 4.196 | 0.876–20.106 | 0.073 | |||
| 2 | 5.656 | 1.192–26.828 | 0.029 | |||
| 3 | 1.990 | 0.321–12.346 | 0.460 | |||
| 4 | 7.547 | 1.484–38.380 | 0.015 | |||
| 5 | 1.468 | 0.200–10.791 | 0.706 | |||
| 6 | 9.409 | 1.409–62.844 | 0.021 | |||
Univariate and multivariate logistic analyses of variables for the prediction of recurrence rate of ischemic stroke.
SHM, stroke health manager; BMI, body mass index; NIHSS, National Institute of Health Stroke Scale; CT, computed tomography; CTA, computed tomography angiography; mRS, modified Rankin Scale.
We constructed a nomogram based on these identified variables. Figure 3 shows the total score based on the individual scores calculated using the nomogram; most patients in this study had a total risk point between 170 and 270. The C-index value was 0.76, and the time-dependent AUC for predicting recurrence was >0.7, which indicated that the nomogram was beneficial for discrimination.
Figure 3
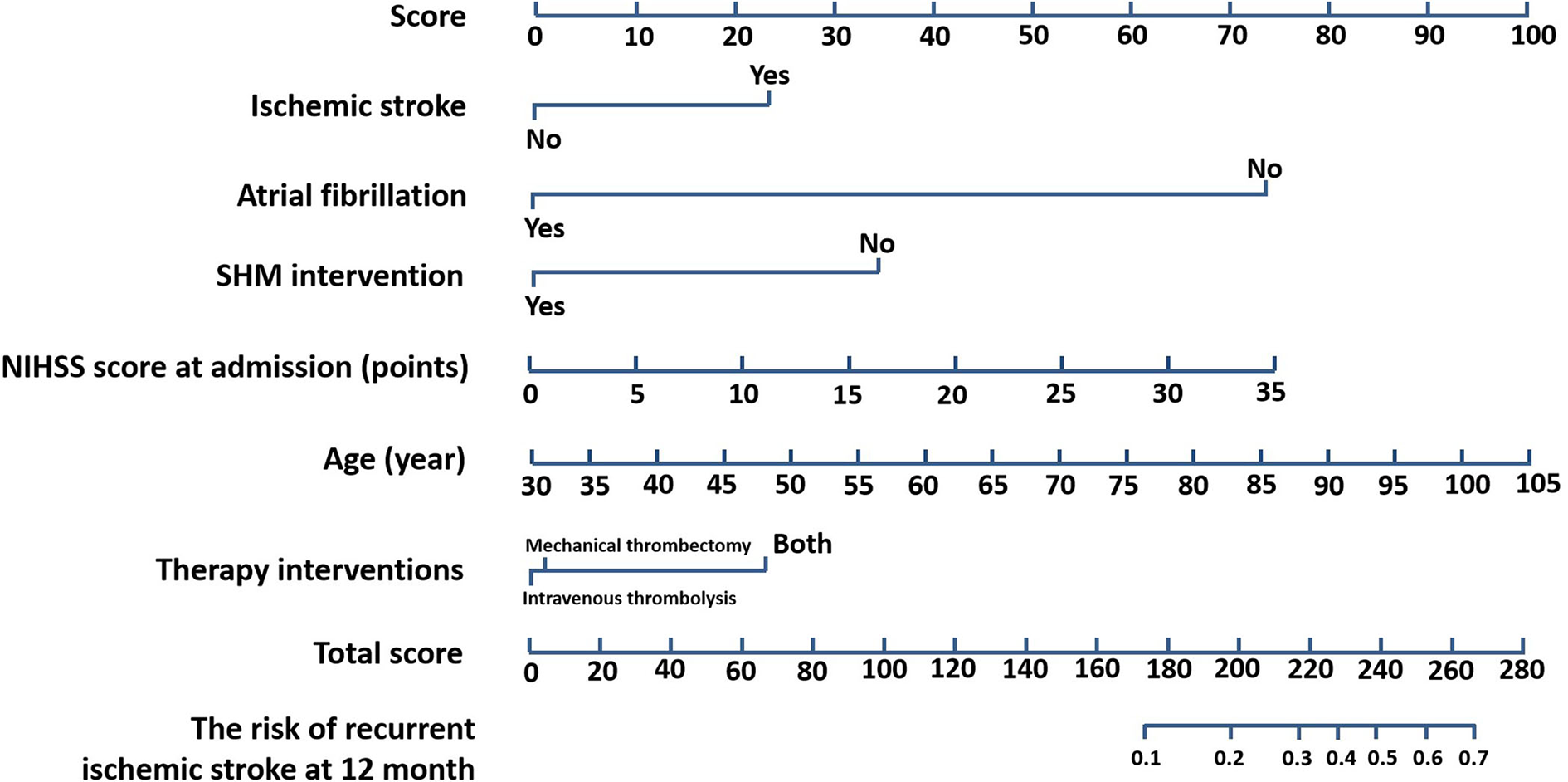
Nomogram for prognostic prediction. Nomogram (SHM intervention, age, NIHSS score at admission, atrial fibrillation, ischemic stroke, therapy interventions) for predicting the risk of recurrent ischemic stroke at 12 months. The importance of each variable was ranked according to the standard deviation along nomogram scales. SHM, stroke health manager; NIHSS, National Institutes of Health Stroke Scale.
Subgroup analyses between the control and the SHM intervention group
We analyzed the association between the control group and the SHM intervention group among all subgroups using the Cox proportional hazards model. Figure 4 shows that the SHM intervention group was associated with a lower risk of recurrence in all four subgroups. In the BMI ≥ 24 kg/m2 subgroup, the SHM intervention group had a 90.0% lower risk of recurrence than the control group (P = 0.028; HR = 0.459). In the admission at NIHSS ≤ 16 subgroup, the SHM intervention group had a 57.4% lower risk of recurrence than the control group (P = 0.048; HR = 0.426). In the intravenous thrombolysis subgroup, the SHM intervention group had a 78.4% lower risk of recurrence than the control group (P = 0.017; HR = 0.216). In the discharge at NIHSS ≤ 16 subgroup, the SHM intervention group had a 46.8% lower risk than the control group (P = 0.065; HR = 0.532).
Figure 4
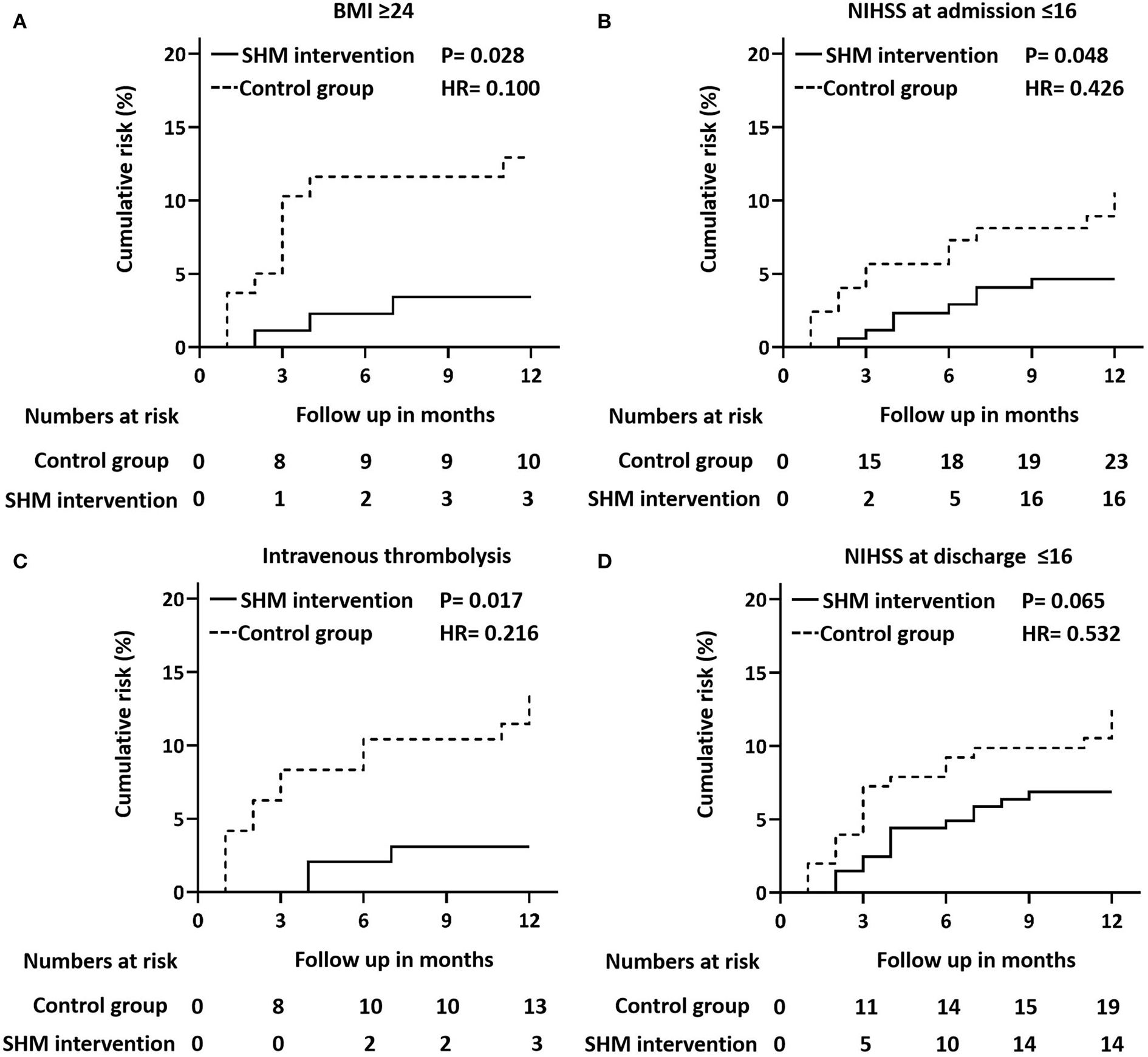
Cox proportional hazard model plots. Cox proportional hazard models plots of outcomes stratified by BMI ≥ 24 kg/m2(A), NIHSS at discharge ≤ 16 (B), intravenous thrombolysis (C), and NIHSS at admission ≤ 16 (D). P-values for the overall comparison of the difference between the control group and the SHM intervention group. BMI, body mass index; SHM, stroke health manager; NIHSS, National Institutes of Health Stroke Scale.
Discussion
Controlling risk factors and continued investment in public health projects have helped reduce the stroke burden in the United States over the past 100 years (16, 20). Conversely, in China, risk factors are highly prevalent among patients with stroke, and the prevalence of major risk factors for stroke in the general population increased from 2002 to 2012 (5). Furthermore, according to the data from NESS-China 2013, the most prevalent risk factors in stroke survivors were hypertension (84.2%), smoking (47.6%), and alcohol use (43.9%) (1, 2). The Chinese government has implemented several public education and primary prevention initiatives for stroke, with some success (2). From 2002 to 2012, the awareness rate, the treatment rate, and the control rate of hypertension improved by 16.3, 16.4, and 7.7%, respectively, although the stroke incidence is still considered at a high level (1, 16). About one-half of patients who survive an ischemic stroke or TIA are at an increased risk of recurrent stroke within a few days or weeks of the initial event, with the greatest risk during the first week (1, 5). Recurrent events lead to prolonged hospitalization, worsened functional outcomes, and increased mortality (12, 16).
In this study, in the intervention group, the stroke health management model oriented to the needs of patients with stroke, and guidance and positive stroke management work was used. During the 1-year follow-up, blood pressure, fasting blood glucose, and LDL-C levels of the patients significantly decreased compared with those before the SHM intervention, that is, the control group. This indicates that the knowledge of stroke prevention and treatment provided for patients and their families should be improved by the stroke health manager, as well as the enthusiasm and initiative of patients for self-management. We also observed the effect of age, NIHSS score, atrial fibrillation, history of previous ischemic stroke, and treatment modalities on recurrence. In this study, the rate of stroke recurrence in patients with a history of atrial fibrillation was reduced in the SHM group (consistent with the China Stroke Statistics 2019) (1). It may be due to the strong awareness of risk factors created by the stroke health manager and high compliance of intervention group patients with atrial fibrillation. Furthermore, it can be concluded from the multivariate logistics regression analysis that ischemic stroke patients with diabetes mellitus should pay more attention to early prevention and early rehabilitation. It may be because the lifestyle change for patients with diabetes is difficult. The full-time follow-up plays a poor role in supervising and controlling underlying diseases.
China is still in its infancy in the whole process of ischemic stroke diagnosis and follow-up process dominated by the stroke health manager, and the work of relevant foreign health consultants has been carried out earlier. The follow-up method is better than the one-way method of sending health education information. In addition, several information methods (e.g., information sent by an AI system and auto-telephone calls) can be used to reduce the manual workload and expand the follow-up population. For example, in 2016, an app called ICTUS3R was developed in Italy specifically to educate patients with stroke by providing information regarding symptoms and coping measures of early stroke, and to encourage people to actively change risky lifestyles; the suitable for all ages part (21) serves as a good inspiration for people to pursue the recommended course.
In future, we should establish health records and track patients' health status for long-range lifetime, improve patient compliance and health awareness, and change lifestyle to promote the control of other chronic diseases in addition to stroke (3, 19). The standardization and humanization of the whole process of stroke health management could improve the knowledge and compliance of patients, which is beneficial to the prognosis of the disease (6, 19, 22). Selection bias is inevitable for a single-center, history-controlled study; therefore, the results should be further verified and validated with larger sample studies.
Conclusion
The stroke health manager-guided management model based on patients' needs better controlled risk factors for stroke and significantly reduced the recurrence rate of mild to moderate ischemic stroke within 1 year (1, 3–5, 11, 13, 23). This study corroborates the role of the stroke health manager and recommends their use in the secondary prevention of ischemic stroke.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81701872), the Clinical Capability Improvement Project of Jiangsu Province Hospital (Grant JSPH-MC-2021-5), and the Key Clinical Projects of Jiangsu Provincial Finance (Grant (2020)155).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HSu, LeJ, and XZ were responsible for conceptualization, methodology, statistical analysis, and original draft—review and editing. XJ, LiJ, and LZ were responsible for designing the study. LW, HSh, QL, BH, and YW enrolled the participants and collected data. HSu and XJ revised the original manuscript and reanalyzed the data. All authors contributed to the interpretation of the data, critical revision, and approval of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We are grateful to the respondents of the studies for their time and all members of the First Affiliated Hospital of Nanjing Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Wang YJ Li ZX Gu HQ Zhai Y Jiang Y Zhao XQ et al . China stroke statistics 2019: a report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. (2020) 5:211–39. 10.1136/svn-2020-000457
2.
Wang Y Li Z Zhao X Wang C Wang X Wang D et al . Effect of a multifaceted quality improvement intervention on hospital personnel adherence to performance measures in patients with acute ischemic stroke in China: a randomized clinical trial. JAMA. (2018) 320:245–54. 10.1001/jama.2018.8802
3.
Sicras-Mainar A Planas-Comes A Frias-Garrido X Navarro-Artieda R de Salas-Cansado M Rejas-Gutierrez J . Statins after recent stroke reduces recurrence and improves survival in an aging Mediterranean population without known coronary heart disease. J Clin Pharm Ther. (2012) 37:441–7. 10.1111/j.1365-2710.2011.01318.x
4.
Flach C Muruet W Wolfe CDA Bhalla A Douiri A . Risk and secondary prevention of stroke recurrence: a population-base cohort study. Stroke. (2020) 51:2435–44. 10.1161/STROKEAHA.120.028992
5.
Wang W Jiang B Sun H Ru X Sun D Wang L et al . Prevalence, incidence, and mortality of stroke in china: results from a nationwide population-based survey of 480 687 adults. Circulation. (2017) 21:135:759–71. 10.1161/CIRCULATIONAHA.116.025250
6.
Wu J Zhang H Li L Hu M Chen L Xu B et al . A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun (Lond). (2020) 40:301–12. 10.1002/cac2.12067
7.
Pennlert J Eriksson M Carlberg B Wiklund PG . Long-term risk and predictors of recurrent stroke beyond the acute phase. Stroke. (2014) 45:1839–41. 10.1161/STROKEAHA.114.005060
8.
Mohan KM Wolfe CD Rudd AG Heuschmann PU Kolominsky-Rabas PL Grieve AP . Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. (2011) 42:1489–94. 10.1161/STROKEAHA.110.602615
9.
Karlinski M Kobayashi A Czlonkowska A Mikulik R Vaclavik D Brozman M et al . Intravenous thrombolysis for stroke recurring within 3 months from the previous event. Stroke. (2015) 46:3184–9. 10.1161/STROKEAHA.115.010420
10.
Yuan K Chen J Xu P Zhang X Gong X Wu M et al . A nomogram for predicting stroke recurrence among young adults. Stroke. (2020) 51:1865–7. 10.1161/STROKEAHA.120.029740
11.
Virani SS Alonso A Aparicio HJ Benjamin EJ Bittencourt MS Callaway CW et al . Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. (2021) 143:e254–743. 10.1161/CIR.0000000000000950
12.
Haesebaert J Termoz A Polazzi S Mouchoux C Mechtouff L Derex L et al . Can hospital discharge databases be used to follow ischemic stroke incidence?Stroke. (2013) 44:1770–4. 10.1161/STROKEAHA.113.001300
13.
Jiang B Wang WZ Wu SP Du XL Bao QJ . Effects of urban community intervention on 3-year survival and recurrence after first-ever stroke. Stroke. (2004) 35:1242–7. 10.1161/01.STR.0000128417.88694.9f
14.
Craig LE Bernhardt J Langhorne P Wu O . Early mobilization after stroke: an example of an individual patient data meta-analysis of a complex intervention. Stroke. (2010) 41:2632–6. 10.1161/STROKEAHA.110.588244
15.
Persson J Holmegaard L Karlberg I Redfors P Jood K Jern C et al . Spouses of stroke survivors report reduced health-related quality of life even in long-term follow-up: results from Sahlgrenska Academy Study on ischemic stroke. Stroke. (2015) 46:2584–90. 10.1161/STROKEAHA.115.009791
16.
Ovbiagele B Saver JL Fredieu A Suzuki S Selco S Rajajee V et al . In-hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow-up. Stroke. (2004) 35:2879–83. 10.1161/01.STR.0000147967.49567.d6
17.
Fjaertoft H Indredavik B Lydersen S . Stroke unit care combined with early supported discharge: long-term follow-up of a randomized controlled trial. Stroke. (2003) 34:2687–91. 10.1161/01.STR.0000095189.21659.4F
18.
Dutta D Bowen E Foy C . Four-year follow-up of transient ischemic attacks, strokes, and mimics: a retrospective transient ischemic attack clinic cohort study. Stroke. (2015) 46:1227–32. 10.1161/STROKEAHA.114.008632
19.
Gil-Salcedo A Dugravot A Fayosse A Jacob L Bloomberg M Sabia S et al . Long-term evolution of functional limitations in stroke survivors compared with stroke-free controls: findings from 15 years of follow-up across 3 international surveys of aging. Stroke. (2022) 53:228–37. 10.1161/STROKEAHA.121.034534
20.
Hillen T Coshall C Tilling K Rudd AG McGovern R Wolfe CD . Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke. (2003) 34:1457–63. 10.1161/01.STR.0000072985.24967.7F
21.
Baldereschi M Di Carlo A Piccardi B Inzitari D . The Italian stroke-app: ICTUS3R. Neurol Sci. (2016) 37:991–4. 10.1007/s10072-016-2506-0
22.
Yang XM Rao ZZ Gu HQ Zhao XQ Wang CJ Liu LP et al . Atrial fibrillation known before or detected after stroke share similar risk of ischemic stroke recurrence and death. Stroke. (2019) 50:1124–9. 10.1161/STROKEAHA.118.024176
23.
Yaghi S Trivedi T Henninger N Giles J Liu A Nagy M et al . Anticoagulation timing in cardioembolic stroke and recurrent event risk. Ann Neurol. (2020) 88:807–16. 10.1002/ana.25844
Summary
Keywords
stroke health manager, ischemic stroke, recurrence, health management, follow-up
Citation
Jiang L, Zhou Y, Zhang L, Wu L, Shi H, He B, Wang Y, Liu Q, Ji X, Zhang X, Jiang L and Sun H (2022) Stroke health management: Novel strategies for the prevention of recurrent ischemic stroke. Front. Neurol. 13:1018794. doi: 10.3389/fneur.2022.1018794
Received
13 August 2022
Accepted
31 August 2022
Published
26 October 2022
Volume
13 - 2022
Edited by
Yue Lan, Guangzhou First People's Hospital, China
Reviewed by
Lu Wang, Qingdao Municipal Hospital, China; Haiming Wang, First Affiliated Hospital of Zhengzhou University, China
Updates
Copyright
© 2022 Jiang, Zhou, Zhang, Wu, Shi, He, Wang, Liu, Ji, Zhang, Jiang and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueli Ji 365785102@qq.comXintong Zhang zhangxintong1002@163.comLei Jiang racheljl@126.comHao Sun haosun@njmu.edu.cn
†These authors have contributed equally to this work
This article was submitted to Neurorehabilitation, a section of the journal Frontiers in Neurology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.