Abstract
Introduction:
X-linked adrenoleukodystrophy (X-ALD) is the most common inherited peroxisomal disorder caused by variants in the ABCD1 gene. The main phenotypes observed in men with X-ALD are primary adrenal insufficiency, adrenomyeloneuropathy, and cerebral ALD (cALD). Cerebral ALD consists of a demyelinating progressive cerebral white matter (WM) disease associated with rapid clinical decline and is fatal if left untreated. Hematopoietic stem cell transplantation is the standard treatment for cALD as it stabilizes WM degeneration when performed early in the disease. For this reason, early diagnosis is crucial, and several countries have already implemented their newborn screening programs (NBS) with the assessment of C26:0-lysophosphatidylcholine (C26:0-LPC) values as screening for X-ALD.
Methods:
In June 2021, an Italian group in Lombardy launched a pilot study for the implementation of X-ALD in the Italian NBS program. A three-tiered approach was adopted, and it involved quantifying the values of C26:0-LPC and other metabolites in dried blood spots with FIA-MS/MS first, followed by the more specific ultra-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) technique and, finally, the genetic confirmation via focused NGS.
Discussion:
Genetically confirmed patients are set to undergo a follow-up protocol and are periodically evaluated to promptly start a specific treatment if and when the first signs of brain damage appear, as suggested by international guidelines. A specific disease monitoring protocol has been created based on literature data and personal direct experience.
Conclusion:
The primary aim of this study was to develop a model able to improve the early diagnosis and subsequent follow-up and timely treatment of X-ALD.
Ethics:
The study was approved by the local ethics committee. The research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Introduction
X-linked adrenoleukodystrophy (X-ALD) is the most common inherited peroxisomal disorder, with an estimated prevalence of 1/15.000–17.000 (1). It consists of an inborn error of very long-chain fatty acids (VLCFA) beta-oxidation caused by variants in the X chromosome ATP binding cassette subfamily D member 1 (ABCD1) gene. This gene encodes a peroxisomal transmembrane protein necessary for the entrance of VLCFA into the peroxisome where they are digested and eliminated (2, 3). A defect in ABCD1 protein leads to increased concentrations of the VLCFA in tissues, including the brain, the spinal cord, and the adrenal cortex, and in the plasma (4, 5).
The main phenotypes observed in men with X-ALD are primary adrenal insufficiency (Addison disease), adrenomyeloneuropathy (AMN), and cerebral ALD (cALD), alone or in any combination. Addison's disease occurs in the first decade of life with a lifetime prevalence of 80–90% (6). Demyelinating progressive cerebral white matter (WM) disease with rapid clinical declining (cALD) occurs in about 35% of boys with ABCD1 mutation, most often during the first decade of life (3–10 years) and turns fatal if untreated (7). Most men who do not develop cALD during childhood develop AMN in adult life. In this latter case, AMN is clinically characterized by spastic paraparesis, sensory-motor peripheral neuropathy, sphincter disturbances, and sexual dysfunction as the main symptoms (5).
On the other hand, female carriers of pathogenetic variants of ABCD1 (in up to 88% of cases) develop a milder and later phenotype of AMN, with peripheral neuropathy or myelopathy as the main manifestation (8). Central involvement or adrenal insufficiency is not commonly observed in women (<1%) (3).
Early diagnosis, therefore, is crucially important. Hematopoietic stem cell transplantation (HSCT) is the standard treatment for cALD. It is not able to recover WM degeneration but it leads to its stabilization, and as a consequence, the earlier it is performed, the better the final outcome (9). Given the significant associated morbidity and mortality related to HSCT, and considering that only some men with X-ALD develop cALD, this is only recommended for patients with an active cerebral disease diagnosed at an early stage (Loes score <10 and no neurological signs) (10, 11). As WM lesions on magnetic resonance imaging (MRI) precede clinical neurological manifestations, their early recognition offers the opportunity to intervene in a pre-symptomatic stage of the disease.
Gene therapy is also being studied as an alternative if allogeneic donor options are poor, but it is not yet available for routine care (12).
Most X-ALD men also develop Addison's disease during their lifetime, which is associated with high morbidity and mortality. The early identification of mutated ABCD1 among men with X-ALD is therefore essential to monitor adrenal hormone levels and start hormone replacement therapy when needed (13).
Given the fundamental importance of early diagnosis of X-ALD, over the past ten years, several countries have implemented their newborn screening (NBS) program with the assessment of C26:0-lysophosphatidylcholine (C26:0-LPC) values in dried blood spot (DBS) for screening X-ALD. The first state to introduce this screening was the state of New York in 2013 (14), and since February 2016, X-ALD has been added to the United States Recommended Uniform Screening Panel (RUSP). Since then, NBS for X-ALD has been implemented in other states of the USA (15–21). Preliminary studies have been conducted in China and India to promote its implementation (22, 23). In the Netherlands, a pilot study (the SCAN study) has been set up recently to develop a gender-specific screening for X-ALD (24). Indeed, the Dutch Health Council recommended ALD screening only for men because “symptoms in women usually develop later and are untreatable” (Health Council of Netherlands 2015) (24). Inspired by the New York state's three-tier screening algorithm, the Dutch ALD screening is based on four tiers: (1) the quantification of C26:0-LPC by FIA-MS/MS in both men and women, (2) the “X-Counter” to distinguish boys from girls genetically, (3) the quantification of C26:0-LPC by the more specific HPLC-MS/MS technique (only in male newborns), and (4) the ABCD1gene sequencing (24).
The Italian NBS program currently includes 49 conditions and is conducted between 48 and 72 h after birth. A review of the diseases included in the Italian NBS is carried out every 2 years according to the evolution of scientific evidence on rare genetic diseases. In June 2021, we launched a pilot study for the implementation of X-ALD in the Italian NBS program. To our knowledge, this is the first project on newborn screening for X-ALD that was officially started in Italy.
C26:0-LPC can be positive in other peroxisomal disorders (PD), including Zellweger Spectrum Disorders, ACOX1, HSD1B4, ACBD5 deficiency, and CADDS (ContiguousABCD1 DXS1357/BCAP31 Deletion Syndrome). These conditions can therefore be considered the cause of “false positivity” in X-ALD screening in NBS. Peroxisomal disorders constitute a group of severe neurodegenerative conditions that can present at all ages, usually with progressive severe neurological abnormalities due to the central and peripheral nervous system involvement associated with ophthalmological abnormalities, sensorineural hearing loss, hepato-digestive problems, kidney involvement, and adrenocortical insufficiency. MRI images exceedingly show the coexistence of both developmental abnormalities and progressive WM degeneration. Treatment is essentially symptomatic, and hematopoietic stem cell transplantations have been proposed anecdotally as a possible therapeutic option (25). Recently, it was demonstrated that another leukodystrophy, namely Aicardi-Goutières Syndrome (AGS), can be detected early through C26:0-LPC (14, 26). AGS is the prototype of leukodystrophy with cerebral calcification. The most common clinical presentations are neonatal and infantile AGS. Symptoms of congenital AGS are microcephaly severe jitteriness, irritability, muscular tone and movement abnormalities, poor feeding, and possible seizures. The onset of the infantile form at around 4 months of age occurs with less specific signs: irritability, unexplained fevers, sleep-wake disorders, feeding difficulties, followed by a loss of motor skills, the appearance of spastic-dystonic tetraplegia, and intellectual disability. To date, JAK 1/2 Inhibitors and Reverse-Transcriptase Inhibitors seem to be promising therapeutic options. Preliminary results in a small subset of patients have shown encouraging results (27–29).
The primary aim of our study was to develop a model for a regional NBS program to be able to offer to all newborns X-ALD screening as a part of the general NBS program and therefore improve the early diagnosis and subsequent follow-up and timely treatment of X-ALD. In this pilot phase, we decided to evaluate the possibility that some of the false positive subjects could be affected by PD or AGS considering the important therapeutic implication, namely early diagnosis and early symptomatic care in PD and early access to new therapeutic options in AGS. Therefore, secondary targets of the project are the early identification, follow-up, and treatment of those with PD and AGS that tested “false positive” by X-ALD-NBS.
Algorithm for X-ALD newborn screening
The pilot study presented in this report is ongoing (started in June 2021 and will end in June 2024).
So far, the Neonatal Care Units from 33 hospitals in the Italian region of Lombardy have joined the program, led by a multidisciplinary team from V. Buzzi Children's Hospital in Milano. The clinical component of this group consists of three child neurologists, two geneticists, two pediatric endocrinologists, one pediatrician expert in child nutrition, one dietician, three pediatric neuroradiologists, and two physical therapists.
The program integrates the expertise of two labs: the laboratory of Newborns Screening at V. Buzzi Children's Hospital, which is the reference lab for NBS in Lombardy since 1977, and the Medical Genetics lab of Papa Giovanni XXIII Hospital in Bergamo, which is one of the main reference centers for the genetic diagnosis of rare diseases in Italy.
Ethics approval and informed consent
The study was centrally approved by the Ethical Committee of Milano area 1 (2020/ST/395).
X-ALD neonatal screening program was proposed and explained to the parents of all newborns with gestational age ≥ 37 + 0 born in Lombardy. Both male and female newborns were included in the study. Adherence to screening for X-ALD was voluntary and participation in this study was based on informed consent signed by both parents.
A separate written consent specific for genetic analysis was proposed in case of positive tests for the first and the second tier screening.
Sample collection and biochemical analysis
A three-tiered approach is adopted (Figure 1). DBS specimens for X-ALD screening are collected from the newborns using the standard heel prick method. DBS cards are then sent to the Newborn Screening and Metabolic Diseases Unit at V. Buzzi Children's Hospital for analysis.
Figure 1

Diagnostic process of X-ALD newborn screening.
The screening for X-ALD is based on the quantification of not only C20:0-LPC, C22:0-LPC, C24:0-LPC, C26:0-LPC but also C20:0-, C22:0-, C24:0-, and C26:0-acylcarnitine.
The first tier involves the measurement of these metabolites with FIA-MS/MS and the selection of a “non-negative” sample is done using a web-based post-analytical tool in Collaborative Laboratory Integrated Reports (CLIR) and the cut-offs determined from our reference population (samples of newborn ≥ 37 W.G.E. obtained between 48 and 72 h of life). In the first month of the study, reference intervals and cut-off values were analyzed in 1,000 anonymous DBS samples already available in the lab.
The analysis of C20:0-LPC, C22:0-LPC, C24:0-LPC, C26:0-LPC, and C20:0-, C22:0-, C24:0-, and C26:0-acylcarnitine are performed using the Neobase 2 newborn screening kit (PerkinElmer) following the manufacturer's instructions. Single 3.2 mm disks are punched with DBS and transferred into 96-well plates. A total of 125 mL of the PerkinElmer Neobase 2 extraction working solution (EWS) is added to each well. The microplate is covered with an adhesive microplate cover and shaken for 30 min at 650–750 rpm at 45°C. The microplate cover is removed and 100 mL was transferred to a new microplate and covered with an adhesive microplate cover before being analyzed with a PerkinElmer QSight 210. According to our reference population, the first tier is considered “non-negative” if C:26:0-LPC values, measured along with FIA-MS/MS, are greater than or equal to 0.5 uM.
Ultra-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) is performed as a second-tier test on the same well as the first tier. Analysis is done using a QSight MD UHPLC Pump system (Perkin Elmer) consisting of a binary solvent manager, a vacuum degasser, a column heater, and a sample manager. About 5 μL of the extract is injected into a Gemini 3 μm C6-Phenyl 110 Å 100 mm x 2.0 mm column from Phenomenex (Torrance, CA, USA). Metabolites are separated by a linear gradient between solution A (0.1% formic acid in H2O) and solution B (0.1% formic acid in acetonitrile). The UHPLC run is 15 min at a flow rate of 0.4 mL/min. All gradient steps are linear and as follows: at T = 0 min: 70% B, toward T= 10 min: 100% B; T=10–13 min 100% B isocratic, and T = 13–15 min back to 70% B. For the mass spectrometric detection, a QSight 225 MD (Perkin Elmer) is used in the positive electrospray ionization mode with the following parameters: ESI voltage: 5200 V, HSID 320°C, Nebulizer gas: 120 L/H, Drying gas: 90L/H, source: 325°C. For the acylcarnitines, the following multiple reaction monitoring (MRMs) is used: C26:0-carnitine (540.50 > 85.00) and D4-C26:0-carnitine (544.50 > 85.00), both using a dwell time of 115 ms, CCL2-96 V and a collision energy of 44 eV. For C26:0-LPC, the following MRMs were used: C26:0-lysoPC (636.60 > 104.10) and D4- C26:0-LPC (640.60 > 104.10), both using a dwell time of 100 ms, CCL2−112 V and collision energy of 46 eV. C26:0-carnitine and C26:0-LPC levels were calculated using Simplicity software. According to our reference population, the second tier is considered positive if C26:0-LPC values, measured along with UHPLC-MS/MS, are >0.1 uM.
Genetic counseling and gene sequencing
The newborns who test positive also at the second tier screening are referred to C.O.A.L.A. (Center for the diagnosis and treatment of leukodystrophies and genetic leukoencephalopathies) at V. Buzzi Children's Hospital in Milan, where multidisciplinary counseling with neurologists and geneticists took place first. A second written informed consent for specific genetic analyses is collected after providing families with specific information regarding X-ALD, its possible manifestations over time, and its dissimilar impact on different sexes. More specifically related to female newborns, the importance of the investigation is emphasized for the possible identification of other affected male babies and not for an effective possibility of therapeutic intervention on the newborn. If the family agrees to these investigations, focused NGS is performed first on the patient sample and then, if positive, on the parental samples for segregation analysis.
Venous blood samples are collected and sent for analysis to the Laboratory of Medical Genetics at Papa Giovanni XXIII Hospital in Bergamo. Introns and exons of the ABCD1 gene are sequenced from genomic DNA extracted from blood in both male and female infants. If this is negative, the analysis is extended to the Zellweger Spectrum Disorders and Aicardi-Goutières syndrome genes (see Table 1).
Table 1
| X-linked adrenoleukodystrophy |
| ABCD1 |
| Aicardi-Goutières syndrome |
| TREX1 |
| RNASEH2B |
| RNASEH2A |
| RNASEH2C |
| SAMHD1 |
| ADAR1 |
| IFIH1 |
| LSM11 |
| RNU7-1 |
| RNASET2 |
| Zellweger Spectrum Disorders |
| HSD17B4 |
| SCP2 |
| ACOX1 |
| ACBD5 |
| DNM1L |
| PEX1 |
| PEX2 |
| PEX3 |
| PEX5 |
| PEX6 |
| PEX10 |
| PEX11B |
| PEX12 |
| PEX13 |
| PEX14 |
| PEX16 |
| PEX19 |
| PEX26 |
Peroxisomal and Aicardi-Goutières syndrome genes.
Briefly, whole-genome sequencing (WGS, Illumina DNA PCR-Free kit) is performed on a Novaseq 6,000 by 150 base pair paired-end reads. To create the sequencing files targeted only to the genomic regions of interest, the WGS bam file (generated by Dragen v 3.0 Illumina) is cut by Samtols to develop a smaller bam file encompassing only the target region. The bam file is then reconverted in fastq while all original fastQ are deleted. Ultimately, the raw file (fastq) containing only the target region is obtained. The small and specific bam file is re-run with Dragen to get the vcf file, which was annotated and filtered. A bioinformatics pipeline in bash language is created to minimize errors and speed up the process.
Variants in the ABCD1 gene are evaluated using the Adrenoleukodystrophy Variant Database (https://adrenoleukodystrophy.info), the Human Gene Mutation Database (www.hgmd.cf.ac.uk), and published literature. Variants are also assessed for their frequency in the general population as reported in public databases (e.g., gnomAD.broadinstitute.org) and for their predicted effect. The American College of Medical Genomics guidelines are used to classify the identified variants (29).
A diagnosis of X-ALD is considered confirmed if a known pathogenic variant, likely pathogenic variant, or VUS are detected in the ABCD1 gene.
Genetically confirmed patients will undergo a second neuro-genetic counseling to discuss the results of the analyses; an extended family screening to identify other family members with the same variant is also proposed.
X-ALD follow-up
The results of the genetic investigations are obtained within one week of the collection of the samples.
Then, genetically confirmed patients are set to undergo a follow-up protocol and are periodically evaluated to promptly start a specific treatment if and when the first signs of brain damage appear, as suggested by international guidelines.
A specific disease monitoring protocol has been created based on literature data and personal direct experience (Figure 2).
Figure 2
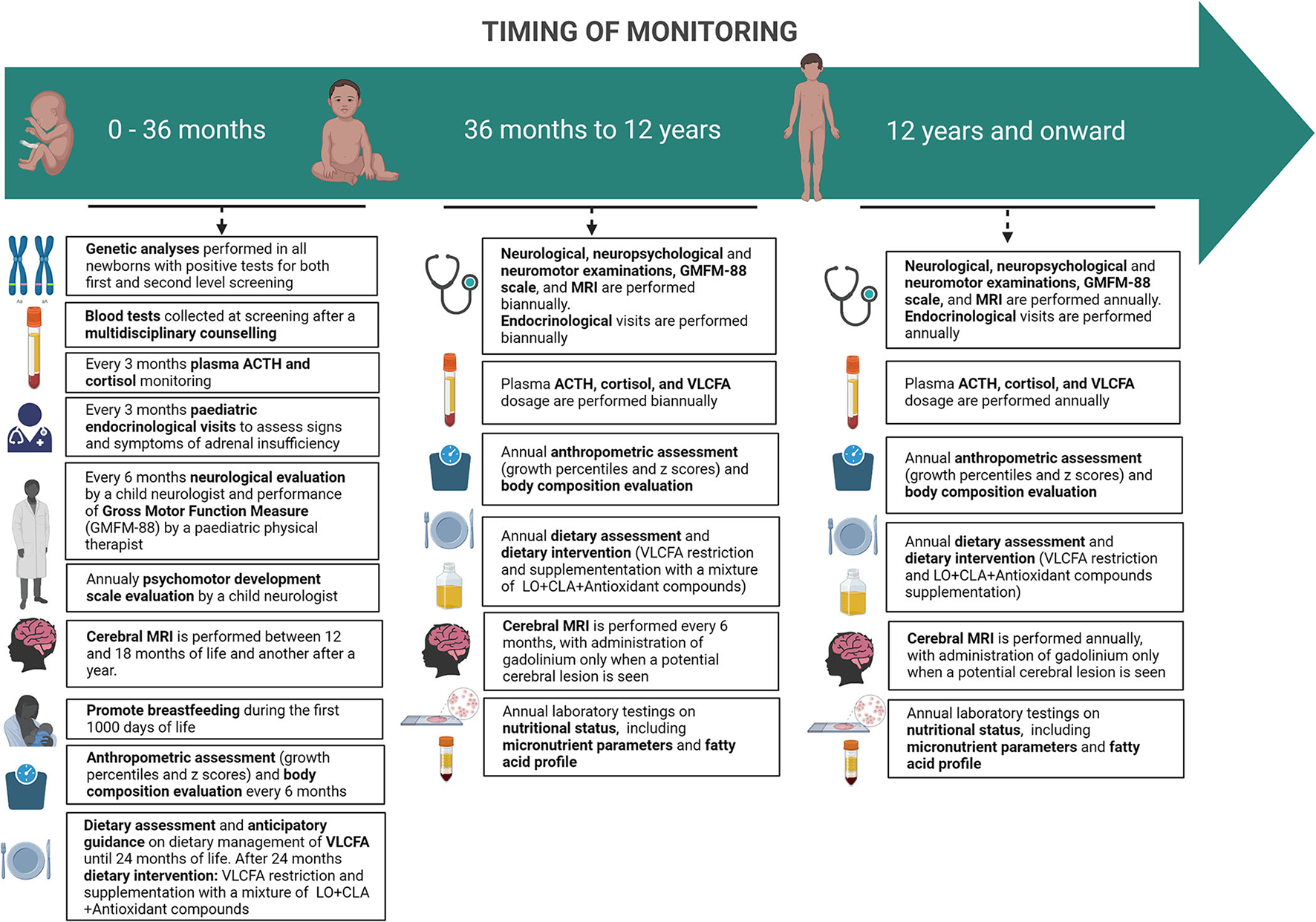
Timing of monitoring of pre-symptomatic ABCD1-mutated males subjects.
Neuromotor assessment
From 0 to 12 years, a neurological evaluation by a child neurologist and the Gross Motor Function Measure (GMFM-88) by a pediatric physical therapist is set to be performed every 6 months. In addition, in the first 42 months of life, patients undergo a psychomotor development scale evaluation (Bayley Scales of Infant and Toddler Development—Third Edition) by a child neurologist and then substituted by an age-appropriate full intellectual evaluation through the age-appropriate Wechsler Intelligence Scales.
The same assessments are set to be carried out annually from the age of 12 onwards.
Endocrinological assessment
In the first 24 months of life, pediatric endocrinological visits are scheduled to take place every 3 months to assess signs and symptoms of adrenal insufficiency; plasma ACTH and cortisol are also monitored quarterly. When the diagnosis is confirmed, basal serum cortisol and ACTH are performed as soon as possible. Within 6 months from birth, samples of ACTH and cortisol may be taken randomly, considering that diurnal secretion has not been established yet. After 6 months from birth hormonal sample should be taken at 8 am. If a normal adrenal function is detected (ACTH <100 pg/ml and cortisol ≥5 mcg/dl), hormonal exams should be repeated in 3 months. When ACTH is between 100 and 299 pg/ml, with a cortisol <10 mcg/dl, adrenal insufficiency cannot be ruled out and a high-dose ACTH test must be performed. An ACTH ≥300 pg/ml and cortisol <18 mcg/dl are indicative of adrenal insufficiency and glucocorticoid replacement therapy must begin (30).
From 2 to 12 years of age, children should follow the same schedule every four to 6 months (30) and after 12 years of age annually (12).
Nutritional assessment and treatment
A pediatric nutritional visit is scheduled within 3 months from birth after neonatal screening by a specialized nutritional team composed of a nutritional pediatric doctor, dietitians, and biologists that is part of the multidisciplinary group involved in the follow-up of patients with X-ALD. Subsequently starting with a complementary feeding period, patients' anthropometric parameters are to be recorded every 6 months, along with dietary assessment until 36 months of life.
From 36 months to 12 years, nutritional and anthropometric assessments are to be performed annually. Thereafter, during adolescence, the same assessments are to be carried out annually.
Dietary intervention
The dietary management of X-ALD has three main focuses: the restriction of Very Long Chain Fatty Acids (VLCFA) with emphasis on avoidance of C26:0 (hexacosanoic acid), the inhibition of fatty acids elongation, and the enhancement of peroxisomal beta-oxidation by the administration of a mixture of Lorenzo's oil (LO) [a 4:1 mixture of glyceryl trioleate (GTO) (C18:1 n-9) and glyceryl trierucate (GTE) (C22:1 n-9)], conjugated linoleic acid (CLA), and antioxidants compounds.
Considering the crucial role of dietary lipids for growth and neurodevelopment in the early stages of life, no fat intake restrictions are suggested for patients until 2 years of life, but foods with naturally low content VLFCA are suggested (see Table 2). During this period of life, a balanced diet according to Dietary Reference Values (DRV) is provided to the family (32). Dietary fats restriction starts after two years of life, and the diet aims to reduce C26:0 intake to <2–3 mg/day (33). Thus, fat intake through food is <15% of the total calories. Families are instructed on the main foods to be avoided or selected according to their C26:0 content (31). Particularly, attention has to be paid concerning fatty food and to the constituent of the cut in the outer covering of plants, fruits, vegetables, and nuts (31, 33) (see Table 2).
Table 2
| Fish, meat, egg, dairy, and legumes | Pasta, bread, and cereals | Fruits and vegetables | Fats and oils | ||||
|---|---|---|---|---|---|---|---|
| Low content (<0.5 mg/100 g) | Low content (<0.5 mg/100 g) | Low content (<0.5 mg/100 g) | Low content (<0.5 mg/100 g) | ||||
| Egg whites | 0.007 | Potato, peeled, boiled | 0.096 | Green apple, peeled | 0.045 | Medium-chain triglyceride oil | 0.450 |
| Milk, skim | 0.050 | Commercial breakfast cereal | 0.220 | Mushrooms, fresh, boiled | 0.055 | ||
| Chicken, breast, skinned, broiled | 0.060 | Rice, long grain, cooked | 0.270 | Carrots, peeled | 0.061 | ||
| Flounder, fresh, broiled | 0.068 | Flour, white | 0.320 | Strawberries, fresh, deseeded | 0.064 | ||
| Ham, lean, fat trimmed, boiled | 0.072 | Cantaloupe, fresh | 0.066 | ||||
| Steak, fat trimmed, broiled | 0.083 | Tomato, peeled, deseeded | 0.071 | ||||
| Sausage, lean, grilled | 0.084 | Cherries, fresh, peeled | 0.077 | ||||
| Beef, ground round, broiled | 0.120 | Eggplant, fresh, peeled, deseeded, cooked | 0.088 | ||||
| Cottage cheese, 1% | 0.120 | Turnips, fresh, peeled, cooked | 0.088 | ||||
| Pork chops, extra lean, fat trimmed | 0.130 | Peach, fresh, with peel | 0.091 | ||||
| Green pees, frozen, cooked | 0.180 | Green pepper, fresh | 0.092 | ||||
| Turkey, breast, skinned, broiled | 0.220 | Strawberries, fresh, with seeds | 0.094 | ||||
| Yogurt, strawberry | 0.470 | Purple plum, fresh, with peel | 0.160 | ||||
| Cod, fresh, broiled | 0.480 | Watermelon | 0.160 | ||||
| Butternut squash, cooked | 0.190 | ||||||
| Tomato, fresh, with skin and seeds | 0.290 | ||||||
| Green grapes, fresh, peeled | 0.310 | ||||||
| Lettuce hearts | 0.370 | ||||||
| Broccoli, frozen, boiled | 0.450 | ||||||
| Medium content (0.5–1 mg/100 g) | Medium content (0.5–1 mg/100 g) | Medium content (0.5–1 mg/100 g) | Medium content (0.5–1 mg/100 g) | ||||
| Pinto beans, fresh, soaked and cooked | 0.590 | Bread, homemade | 0.700 | Green apple with peel | 0.500 | ||
| Shrimp, fresh, broiled | 0.760 | Spinach, frozen, cooked | 0.510 | ||||
| Tofu | 0.850 | Apricots, dried, stewed | 0.710 | ||||
| Banana | 0.780 | ||||||
| High content (>1 mg/100 g) | High content (>1 mg/100 g) | High content (>1 mg/100 g) | High content (>1 mg/100 g) | ||||
| Beef frank | 1.23 | Rye bread | 1.02 | Green beans, string, canned | 1.48 | Milk, chocolate, low fat | 3.33 |
| White bread, commercial brand, nocrust | 1.39 | Cabbage, fresh, uncooked | 2.19 | Safflower oil | 5.57 | ||
| White bread, commercial brand, with crust | 1.48 | Zucchini, fresh, skinned, cooked | 2.87 | Coconut oil | 5.99 | ||
| Commercial French fries, frozen, baked | 2.29 | Sunflower oil | 8.81 | ||||
| All grain commercial breakfast cereal | 5.63 | Olive oil | 19.9 | ||||
| Potato chips | 7.46 | Corn oil | 31.3 | ||||
| Peanut oil | 208.4 | ||||||
Content of C26:0 (mg) in 100 g of different edible foods.
Modified from Van Duyn et al. (31).
Simultaneously, a mixture of LO, CLA, and antioxidant compounds is to be administered orally in a dosage that provides ~20% of total caloric intake. Patients can either take trioleate glycerol (GTO) separately or incorporate it into food during meals to replace margarine, butter, mayonnaise, and cooking oils (34, 35). Considering pre-clinical studies (36–38) and clinical outcomes reported in ALD female carriers (39), the mixture containing LO, CLA, and antioxidants is a promising therapeutic approach targeted to contrast VLCFA accumulation and to reduce inflammatory markers, although more extensive studies are required.
Multivitamin and mineral supplements are prescribed according to individual needs, with particular attention to dietetic intakes of Vitamin D and B12. To avoid essential fatty acid deficiency, eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) intakes are evaluated (35, 40).
Laboratory parameters
Since children with neurological impairment are at major risk of having poor micronutrient status, the following laboratory tests are to be performed annually as part of the nutritional assessment: vitamin A, B12, D (25-OH), E, folic acid, iron, ferritin, calcium, zinc, and phosphorus (41). Other blood laboratory testing includes serum albumin, prealbumin, urea, creatinine, and glucose (see Table 3). Considering the possible side effects of Lorenzo's oil on platelet levels (mild platelet anisocytosis), it is important to evaluate the mean corpuscular volume (42).
Table 3
| Standard blood tests | Guthrie test |
|---|---|
| Hemoglobin, mean corpuscular volume, ferritin, iron | Analysis of fatty acids (FA) profile, particularly A |
| Calcium, magnesium, phosphate and zinc | |
| Vitamins A, B12, D, E, folic acid | |
| VLCFA blood levels | |
| Glucose | |
| Creatinine | |
| Total protein | |
| Albumin, prealbumin | |
| Liver enzymes | |
| Urea and electrolytes |
Laboratory tests for nutritional assessment.
Guthrie test paper is used to collect whole blood for the analysis of fatty acids (FA) profile, particularly docosahexaenoic acid (DHA). FA profile is evaluated in a drop of blood collected on a Guthrie paper embedded with butylated hydroxytoluene (BHT) as an antioxidant and stored in a refrigerator until analysis. Later, FA methyl esters are analyzed by gas chromatography using a GC-2100 (Shimadzu Italia S.r.l., Milano, Italy) equipped with a 15 m capillary column (DBB Agilent), PTV injector, and FID detection.
Neuroradiological assessment
A cerebral MRI is performed every 6 months between 2 and 12 years and annually from the age of 12 onwards. MRI studies are performed on a 3 Tesla scanner (MAGNETOM VIDA, SIEMENS), with a protocol including T1, T2, and FLAIR sequences (see Table 4), in accordance with the consensus guidelines for the imaging surveillance of asymptomatic X-ALD patients (12, 43). For the studies performed before 3 years of age, the use of Gadolinium is not recommended because the likelihood of developing an active, inflammatory brain lesion remains low (44). Advanced Imaging sequences like Diffusion Tensor Tractography (DTI) or spectroscopy are not recommended in the routine clinical surveillance protocol; we decided to include them as a part of a research endeavor.
Table 4
| Sequences | |
|---|---|
| Standard | 3D-T1-GRE |
| TSE-T2_AX | |
| TSE-T2_COR | |
| STIR-T2_SAG | |
| FLAIR_AX | |
| FLAIR_SAG | |
| DWI_AX | |
| SWI_AX | |
| (DTI) | |
| (Spectroscopy) | |
| STIR-T2 SAG SPINE | |
| TSE-T1 SAG SPINE | |
| Lesion | TSE-T1 SAG SPINE post-Gad |
| 3D-T1-GRE post-Gad | |
| AX T1-SE post-Gad |
MRI protocol.
From 3 to 12 years, imaging guidelines recommend the use of contrast-enhanced brain MRI every 6 months, as the likelihood of developing an active brain lesion is highest in this age group. To limit the exposure of patients to repeated contrast, we perform a real-time MRI reading with the administration of gadolinium only when a potential cerebral lesion is seen in agreement with the literature (12, 45). If a lesion or questionable lesion is seen, then GAD is applied. The same assessments are carried out annually from the age of 12 onwards. After this age, the use of gadolinium is limited to those cases with evidence of a cerebral lesion on a previous MRI or when there is a clinical concern.
In the case of a first lesion in an asymptomatic patient, before proceeding to HSCT, MRI is repeated after 3 months to exclude an arrested cALD (12).
MRI is evaluated through a standardized severity score (Loes score) which measures the location and extent of WM lesions and the presence of local or global atrophy (46). Each area is scored as 0 if normal, 0.5 in case of unilateral involvement, and 1 if the lesion or atrophy is bilateral. According to the literature, values ≤9 are considered early Loes scores within which HSCT should be performed (9). More specifically, in the early stages, cALD lesions are characterized by small T2 hyperintensities most often involving the genu or the splenium of the corpus callosum and may not exhibit clear gadolinium enhancement (47); alternatively, some authors reported a potential self-arrest of a subset of early lesions (48). In these cases, an MRI is repeated in 3 months to reassess the signal alteration or for gadolinium enhancement whose presence indicates active cALD and represents the need for HSCT. If a more prominent, clearly gadolinium-enhancing early cALD lesion is detected, the patient is triaged for HSCT.
Female carriers of ABCD1
Female carriers of ABCD1 pathogenetic variants typically develop a milder and later phenotype and symptoms are usually untreatable. For these reasons, no regular follow-up at a pediatric age is proposed. Considering the absence of a specific treatment, an early diagnosis in these cases could be a major source of parental stress. However, genetic data are still important for identifying and eventually treating other family members with the same variant and for counseling in case of further pregnancies.
To investigate the level of parental stress related to X-ALD newborn screening and to find the best way to assist and support parents in the process of diagnosis, in addition to the pilot study presented here, another study based on parent interviews is currently underway. Moreover, families are offered the possibility of consulting a psychologist who collaborates with C.O.A.L.A.
Expected outcome
About 70,000 neonates are born in Lombardy every year. Excluding premature infants (around 10% of newborns) and newborns from families and Neonatal Units that refuse to participate in the study, we expect to include at least 40,000 newborns each year. Given the prevalence of X-ALD (1:17.000), other peroxisomal diseases (1:50.000), direct experience with AGS (no epidemiological data available), and other published pilot studies (15–21), we expect to select 10 “non-negative patients” per year. We expect that genetic analyses will confirm the results of NBS in 80% of patients, who will be immediately recruited in the surveillance protocol.
We aim to validate the NBS program linked to the clinical monitoring program and eventually investigate the optimal therapeutic strategies.
Conclusion
Early diagnosis of X-ALD allows for monitoring and timely therapeutic intervention in children who would otherwise die from progressive and irreparable cerebral damage.
Considering the evidence of the efficacy of HSCT in arresting disease progression of cerebral phenotype, when performed in the early stage of the disease, the introduction of X-ALD into NBS would significantly change the natural history of the disease in these patients. Furthermore, early diagnosis would allow for endocrinological monitoring and early intervention, as Addison's disease is frequent and potentially lethal in X-ALD.
The results from our pilot study and those conducted internationally will constitute the basis on which the X-ALD screening can be introduced into the current Italian NBS program to offer all newborns early diagnosis, follow-up, and timely treatment to affected patients.
XALD-NBS study group
Gianluca Lista and Paola Fontana: Department of Neonatology and Neonatal Intensive Care Unit, V. Buzzi Children's Hospital, ASST Fatebenefratelli-Sacco, Milan, Italy. Tiziana Varisco and Olivia Casati: Department of Paediatrics, Desio Hospital, ASST Brianza, Monza, Italy. Alberto Fabio Podestà and Maddalena Gibelli: Department of Paediatrics and Neonatology, San Carlo Borromeo Hospital, ASST Santi Paolo e Carlo, Milan, Italy. Stefano Martinelli and Roberta Restelli: Neonatology and Neonatal Intensive Care Unit, Niguarda Hospital, Milan, Italy. Laura Maria Pogliani and Roberta Agistri: Department of Paediatrics, Neonatology and Neonatal Pathology, Legnano Hospital, ASST Ovest MI, Milan, Italy. Marco Giuseppe Nedbal and Paolo Vaglia: Department of Paediatrics and Neonatology, Gallarate Hospital, ASST Valleolona, Milan, Italy. Chryssoula Tzialla and Luisa Magnani: Department of Paediatrics and Neonatology, Oltrepò Pavese Hospital, ASST Pavia, Pavia, Italy. Elena Sala and Laura Lorioli: Department of Neonatal Pathology, ASST Papa Giovanni XXIII, Bergamo, Italy. Giuseppe Banderali and Diana Ghisleni, Clinical Department of Neonatology, San Paolo Hospital, ASST Santi Paolo e Carlo, Milan, Italy. Bruno Drera and Marta Frittoli: Department of Neonatal Pathology, ASST Cremona, Cremona, Italy. Francesca Lizzoli and Marta Bellini: Department of Paediatrics, Neonatology and Neonatal Pathology, Magenta Hospital, ASST Ovest Milanese, Milan, Italy. Paola Bruni and Ilaria Giulini, Department of Paediatrics, ASST Melegnano-Martesana, Milan, Italy. Valentina Benedetti and Valentina Polimeni: Department Neonatology and Neonatal Intensive Care Unit, IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy. Nadia Salvoni and Masotina Raffaele: Department Neonatology and Neonatal Pathology, Sacco Hospital, ASST Fatebenefratelli-Sacco, Milan, Italy. Cristina Bellan and Roberto Bottino: Department Neonatology and Neonatal Intensive Care Unit, Bolognini Hospital, ASST Bergamo Est, Bergamo, Italy. Graziano Barera and Antonella Poloniato: Neonatal Unit, San Raffaele Scientific Institute, Milan, Italy. Marta Odoni and Ilaria Dalla Verde: Department of Paediatrics and Neonatology, Policlinico San Pietro, Gruppo Ospedaliero San Donato, Bergamo, Italy. Massimo Agosti and Angela Bossi: Department of Paediatrics, Neonatology and Neonatal Intensive Care Unit, ASST Settelaghi, Varese, Italy. Anna Tosi and Anna Elisabetta Bussolini: Department of Paediatrics, Tradate Hospital, ASST Settelaghi, Varese, Italy. Francesco Maria Risso and Vania Spinoni: Department of Neonatology and Neonatal Intensive Care Unit, Children Hospital, ASST Spedali Civili, Brescia, Italy. Nicola Altamura and Patrizia Ballista: Department of Paediatrics and Neonatology, Sesto San Giovanni Hospital, ASST Nord Milano, Milan, Italy. Silvia Di Chio and Luciana Pagani: Department of Neonatal Pathology and Neonatal Intensive Care Unit, Macedonio Melloni Hospital, ASST Fatebenefratelli-Sacco, Milan, Milano. Lidia Decembrino and Michela Grignani: Department of Paediatrics and Neonatology, Vigevano Hospital, ASST Pavia, Pavia, Italy. Grazia Morandi and Valeria Angela Fasolato: Department of Neonatology and Neonatal Intensive Care Unit, Carlo Poma Hospital, ASST Mantova, Mantova, Italy. Lorella Rossi and Emilio Palumbo: Department of Paediatrics and Neonatology, Eugenio Morelli Hospital, ATS Montagna, Sondrio, Italy. Alessandro Lepore and Maria Forestieri: Department of Paediatrics and Neonatology, Busto Arsizio Hospital, ASST Valleolona, Varese, Italy. Stefano Ghirardello and Elisa Civardi: Department of Neonatal Pathology and Neonatal Intensive Care Unit, San Matteo Research Hospital, Pavia, Italy. Paolo Adamoli: Department of Paediatrics, Moriggia Pelascini Hospital, Gravedona, Italy. Roberta Giacchero: Department of Paediatrics and Neonatology, ASST Lodi, Lodi, Italy. Giovanni Traina: Department of Paediatrics, ASST-Melegnano Martesana, Melzo, Italy. Salvatore Barberi: Department of Paediatrics, Rho Hospital, ASST-Rhodense, RHO, Milan, Italy. Patrizia Calzi and Fenesia Pedace: Department of Neonatology, Carate Brianza Hospital, ASST Brianza, Carate, Italy. Marco Sala: Department of Paediatrics, Vimercate Hospital, Vimercate, Italy.
Statements
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Milano area 1 (2020/ST/395). Written informed consent to participate in this study was provided by the patients/participants' legal guardian/next of kin.
Author contributions
Study conceived by: EB, LA, MF, SM, LS, MI, EV, CC, and DT. Drafting of the manuscript: EB, LA, GI, CP, ED, GF, MF, AS, LS, MI, EV, CC, and DT. All authors critical revision of the manuscript for important intellectual content, read, and approved the final manuscript.
Funding
The study was supported by the Italian Ministry of Health, GR-2019-12368701.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Moser AB Jones RO Hubbard WC Tortorelli S Orsini JJ Caggana M et al . Newborn screening for X-linked adrenoleukodystrophy. Int J Neonatal Screen. (2016) 2:15. 10.3390/ijns2040015
2.
Kemp S Wanders RJA . X-linked adrenoleukodystrophy: Very long-chain fatty acid metabolism, ABC half-transporters and the complicated route to treatment. Mol Genet Metab. (2007) 90:268–76. 10.1016/j.ymgme.2006.10.001
3.
Bezman L Moser AB Raymond GV Rinaldo P Watkins PA Smith KD et al . Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol. (2001) 49:512–7. 10.1002/ana.101
4.
Moser HW Mahmood A Raymond GV . X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. (2007) 3:140–51. 10.1038/ncpneuro0421
5.
Turk BR Theda C Fatemi A Moser AB . X-linked adrenoleukodystrophy: Pathology, pathophysiology, diagnostic testing, newborn screening and therapies. Int J Dev Neurosci. (2020) 80:52–72. 10.1002/jdn.10003
6.
Huffnagel IC Laheji FK Aziz-Bose R Tritos NA Marino R Linthorst GE et al . The natural history of adrenal insufficiency in X-linked adrenoleukodystrophy: an international collaboration. J Clin Endocrinol Metab. (2019) 104:118–26. 10.1210/jc.2018-01307
7.
Mahmood A Dubey P Moser HW Moser A . X-linked adrenoleukodystrophy: therapeutic approaches to distinct phenotypes. Pediatr Transplant. (2005) 9:55–62. 10.1111/j.1399-3046.2005.00447.x
8.
Engelen M Barbier M Dijkstra IME Schür R De Bie RMA Verhamme C et al . X-linked adrenoleukodystrophy in women: a cross-sectional cohort study. Brain. (2014) 137:693–706. 10.1093/brain/awt361
9.
Raymond GV Aubourg P Paker A Escolar M Fischer A Blanche S et al . Survival and functional outcomes in boys with cerebral adrenoleukodystrophy with and without hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2019) 25:538–48. 10.1016/j.bbmt.2018.09.036
10.
Miller WP Rothman SM Nascene D Kivisto T Defor TE Ziegler RS et al . Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. (2011) 118:1971–8. 10.1182/blood-2011-01-329235
11.
Page KM Stenger EO Connelly JA Shyr D West T Wood S et al . Hematopoietic stem cell transplantation to treat leukodystrophies: clinical practice guidelines from the hunter's hope leukodystrophy care network. Biol Blood Marrow Transplant. (2019) 25:363–74. 10.1016/j.bbmt.2019.09.003
12.
Engelen M van Ballegoij WJC Mallack EJ Van Haren KP Köhler W Salsano E et al . International recommendations for the diagnosis and management of patients with adrenoleukodystrophy: a consensus-based approach. Neurology. (2022) 99:940–51. 10.1212/WNL.0000000000201374
13.
Dubey P Raymond GV Moser AB Kharkar S Bezman LMH . Adrenal insufficiency in asymptomatic adrenoleukodystrophy patients identified by very long-chain fatty acid screening. J Pediatr. (2005) 146:528–32. 10.1016/j.jpeds.2004.10.067
14.
Vogel BH Bradley SE Adams DJ D'Aco K Erbe RW Fong C et al . Newborn screening for X-linked adrenoleukodystrophy in New York State: diagnostic protocol, surveillance protocol and treatment guidelines. Mol Genet Metab. (2015) 114:599–603. 10.1016/j.ymgme.2015.02.002
15.
Wiens K Berry SA Choi H Gaviglio A Gupta A Hietala A et al . A report on state-wide implementation of newborn screening for X-linked Adrenoleukodystrophy. Am J Med Genet. (2019) 179:1205–13. 10.1002/ajmg.a.61171
16.
Tang H Matteson J Rinaldo P Tortorelli S Currier RSS . The clinical impact of CLIR tools toward rapid resolution of post-newborn screening confirmatory testing for X-linked adrenoleukodystrophy in California. Int J Neonatal Screen. (2020) 6:62. 10.3390/ijns6030062
17.
Lee S Clinard K Young SP Rehder CW Fan Z Calikoglu AS et al . Evaluation of X-linked adrenoleukodystrophy newborn screening in North Carolina. JAMA Netw Open. (2020) 3:1–12. 10.1001/jamanetworkopen.2019.20356
18.
Hall PL Li H Hagar AF Caleb Jerris S Wittenauer A Wilcox W . Newborn screening for X-linked Adrenoleukodystrophy in Georgia: experiences from a pilot study screening of 51,081 newborns. Int J Neonatal Screen. (2020) 6:81. 10.3390/ijns6040081
19.
Matteson J Sciortino S Feuchtbaum L Bishop T Olney RS Tang H . Neonatal screening adrenoleukodystrophy newborn screening in California since 2016: programmatic outcomes and follow-Up. Int J Neonatal Screen. (2021) 7:22. 10.3390/ijns7020022
20.
Moser AB Raymond GV Burton BK Hickey R Hitchins L Shively V et al . Neonatal screening newborn screening for X-linked adrenoleukodystrophy: the initial illinois experience. Int J Neonatal Screen. (2022) 8:6. 10.3390/ijns8010006
21.
Priestley JRC Adang LA Drewes Williams S Lichter-Konecki U Menello C Engelhardt NM et al . Newborn screening for X-linked adrenoleukodystrophy: review of data and outcomes in Pennsylvania. Int J Neonatal Screen. (2022) 8:24. 10.3390/ijns8020024
22.
Tian GL Xu F Jiang K Wang YM Ji W Zhuang YP . Evaluation of a panel of very long-chain lysophosphatidylcholines and acylcarnitines for screening of X-linked adrenoleukodystrophy in China. Clin Chim Acta. (2020) 503:157–62. 10.1016/j.cca.2020.01.016
23.
Natarajan A Christopher R Palakuzhiyil SV Chandra SR . Utility of measuring very long-chain fatty-acyl carnitines in dried blood spots for newborn screening of X-linked Adrenoleukodystrophy. Mol Genet Metab Rep. (2021) 26:100720. 10.1016/j.ymgmr.2021.100720
24.
Barendsen RW Dijkstra IME Visser WF Alders M Bliek J Boelen A et al . Adrenoleukodystrophy newborn screening in the Netherlands (SCAN Study): the X-factor. Front. Cell Develop. Biol. (2020) 8:499. 10.3389/fcell.2020.00499
25.
Wang RY Monuki ES Powers J Schwartz PH Watkins PA Shi Y et al . Effects of hematopoietic stem cell transplantation on acyl-CoA oxidase deficiency: a sibling comparison study. J Inherit Metab Dis. (2014) 37:791–9. 10.1007/s10545-014-9698-3
26.
Armangue T Orsini JJ Takanohashi A Gavazzi F Conant A Ulrick N et al . Neonatal detection of Aicardi Goutières Syndrome by increased C26:0 lysophosphatidylcholine and interferon signature on newborn screening blood spots. Mol Genet Metab. (2017) 122:134–9. 10.1016/j.ymgme.2017.07.006
27.
Rodero MP Frémond ML Rice GI Neven B Crow YJ . JAK inhibition in STING-associated interferonopathy. Ann Rheum Dis. (2016) 75:e76. 10.1136/annrheumdis-2016-210565
28.
Kothur K Bandodkar S Chu S Wienholt L Johnson A Barclay P et al . An open-label trial of JAK 1/2 blockade in progressive IFIH1-associated neuroinflammation. Neurology. (2018) 90:289–91. 10.1212/WNL.0000000000004921
29.
AlbaRice GI Meyzer C Bouazza N Hully M Boddaert N Semeraro M et al . Reverse-transcriptase inhibitors in the Aicardi–Goutières syndrome. N Engl J Med. (2018) 379:2275–7. 10.1056/NEJMc1810983
30.
Regelmann MO Kamboj MK Miller BS Nakamoto JM Sarafoglou K Shah S et al . Adrenoleukodystrophy: guidance for adrenal surveillance in males identified by newborn screen. J Clin Endocrinol Metab. (2018) 103:4324–31. 10.1210/jc.2018-00920
31.
Van Duyn MA Moser AE Brown FR Sacktor N Liu AMH . The design of a diet restricted in saturated very long-chain fatty acids: therapeutic application in adrenoleukodystrophy. Am J Clin Nutr. (1984) 40:277–84. 10.1093/ajcn/40.2.277
32.
European Food Safety Authority (EFSA) . Dietary Reference Values for Nutrients Summary Report. Parma: EFSA Support Publication (2017). p. 14. 10.2903/sp.efsa.2017.e15121
33.
Moser AB Borel J Odone A Naidu S Cornblath D Sanders DB et al . A new dietary therapy for adrenoleukodystrophy: biochemical and preliminary clinical results in 36 patients. Ann Neurol. (1987) 21:240–9. 10.1002/ana.410210305
34.
Tenreiro AM Lázaro MÁ Celda RA Fernández MF Mateo MC Hernández AD . Tratamiento dietético de la adrenoleucodistrofia ligada a X: ¿es útil el aceite de Lorenzo?Endocrinol y Nutr. (2013) 60:37–9. 10.1016/j.endonu.2012.01.011
35.
Moser HW Raymond GV Lu SE Muenz LR Moser AB Xu J et al . Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo's oil. Arch Neurol. (2005) 62:1073–80. 10.1001/archneur.62.7.1073
36.
López-Erauskin J Fourcade S Galino J Ruiz M Schlüter A Naudi A et al . Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann Neurol. (2011) 70:84–92. 10.1002/ana.22363
37.
Fa M Diana A Carta G Cordeddu L Melis MP Murru E et al . Incorporation and metabolism of c9, t11 and t10, c12 conjugated linoleic acid (CLA) isomers in rat brain. Biochim Biophys Acta Mol Cell Biol Lipids. (2005) 1736:61–6. 10.1016/j.bbalip.2005.06.010
38.
Golovko MY Murphy EJ . Uptake and metabolism of plasma-derived erucic acid by rat brain. J Lipid Res. (2006) 47:1289–97. 10.1194/jlr.M600029-JLR200
39.
Cappa M Bizzarri C Petroni A Carta G Cordeddu L Valeriani M et al . A mixture of oleic, erucic and conjugated linoleic acids modulates cerebrospinal fluid inflammatory markers and improve somatosensorial evoked potential in X-linked adrenoleukodystrophy female carriers. J Inherit Metab Dis. (2012) 35:899–907. 10.1007/s10545-011-9432-3
40.
Aubourg P Adamsbaum C Lavallard-Rousseau M-C Rocchiccioli F Cartier N Jambaque I et al . A two-year trial of Oleic and Erucic Acids (“Lorenzo's Oil”) as treatment for Adrenomyeloneuropathy. N Engl J Med. (1993) 329:745–52. 10.1056/NEJM199309093291101
41.
Romano C Van Wynckel M Hulst J Broekaert I Bronsky J Dall'Oglio L et al . European society for paediatric gastroenterology, hepatology and nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr. (2017) 65:242–64. 10.1097/MPG.0000000000001646
42.
Revell P Green A Green S . Platelets in treated Adrenoleukodystrophy: a brief report. J Inherit Metab Dis. (1995) 18:635–7. 10.1007/BF02436010
43.
Mallack EJ Turk BR Yan H Price C Demetres M Moser AB et al . MRI surveillance of boys with X-linked adrenoleukodystrophy identified by newborn screening: meta-analysis and consensus guidelines. J Inherit Metab Dis. (2021) 44:728–39. 10.1002/jimd.12356
44.
Moser HW Loes DJ Melhem ER Raymond GV Bezman L Cox CS et al . X-linked adrenoleukodystrophy: Overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics. (2000) 31:227–39. 10.1055/s-2000-9236
45.
Turk B Mallack E Adang L Becker C Eichler F Van Haren K et al . Consensus guidelines: MRI surveillance of children with presymptomatic Adrenoleukodystrophy (P4.6-047). Neurology. (2019) 92:15.
46.
Loes DJ Fatemi A Melhem ER Gupte N Bezman L Moser HW et al . Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology. (2003) 61:369–74. 10.1212/01.WNL.0000079050.91337.83
47.
Liberato AP Mallack EJ Aziz-Bose R Hayden D Lauer A Caruso PA et al . MRI brain lesions in asymptomatic boys with X-linked adrenoleukodystrophy. Neurology. (2019) 92:e1698–708. 10.1212/WNL.0000000000007294
48.
Mallack EJ van de Stadt S Caruso PA Musolino PL Sadjadi R Engelen M et al . Clinical and radiographic course of arrested cerebral adrenoleukodystrophy. Neurology. (2020) 94:e2499–507. 10.1212/WNL.0000000000009626
Summary
Keywords
X-ALD, X-linked adrenoleukodystrophy (X-ALD), Zellweger Spectrum Disorders, Aicardi-Goutières syndrome (AGS), hematopoietic stem cell transplantation (HCST), newborn screening (NBS), DBS, C26:0-lysophosphatidylcholine
Citation
Bonaventura E, Alberti L, Lucchi S, Cappelletti L, Fazzone S, Cattaneo E, Bellini M, Izzo G, Parazzini C, Bosetti A, Di Profio E, Fiore G, Ferrario M, Mameli C, Sangiorgio A, Masnada S, Zuccotti GV, Veggiotti P, Spaccini L, Iascone M, Verduci E, Cereda C, Tonduti D and XALD-NBS Study Group (2023) Newborn screening for X-linked adrenoleukodystrophy in Italy: Diagnostic algorithm and disease monitoring. Front. Neurol. 13:1072256. doi: 10.3389/fneur.2022.1072256
Received
17 October 2022
Accepted
07 December 2022
Published
09 January 2023
Volume
13 - 2022
Edited by
Kette D. Valente, University of São Paulo, Brazil
Reviewed by
Dalia Goldhaber, Leiden University, Netherlands; Ann B. Moser, Kennedy Krieger Institute, United States
Updates
Copyright
© 2023 Bonaventura, Alberti, Lucchi, Cappelletti, Fazzone, Cattaneo, Bellini, Izzo, Parazzini, Bosetti, Di Profio, Fiore, Ferrario, Mameli, Sangiorgio, Masnada, Zuccotti, Veggiotti, Spaccini, Iascone, Verduci, Cereda, Tonduti and XALD-NBS Study Group.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Davide Tonduti ✉ davide.tonduti@asst-fbf-sacco.itEleonora Bonaventura ✉ eleonora.bonaventura@asst-fbf-sacco.it
This article was submitted to Pediatric Neurology, a section of the journal Frontiers in Neurology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.