Abstract
Background:
The progress of Moyamoya disease (MMD) is often accompanied by the occurrence of new ischemia or hemorrhagic events, which was difficult to predict. This systematic review and meta-analysis aimed to identify predictors for progression in MMD patients.
Methods:
We searched PubMed, Web of Science, Cochrane Library, and Embase databases up to December 10th, 2022 for randomized controlled trials, case-control studies, or cohort studies reporting predictors of disease progression in MMD patients. The results of each predictor were pooled by meta-analysis and further analyzed by subgroup analysis for predictors of unilateral to bilateral progression of MMD.
Results:
A total of 842 patients from 12 studies were included. The estimated pooled means indicated lower age (standard mean difference [SMD]: −0.29, 95% confidence interval [CI]: −0.55 to −0.03; P = 0.03), family history (odds ratio [OR] 3.97, 95% CI: 1.96 to 8.03; P < 0.001) and contralateral abnormality (OR 3.95, 95% CI: 1.10 to 14.20; P = 0.04) were associated with progression in MMD patients. Subgroup analyses indicated that the same three factors were associated with the progression of unilateral to bilateral MMD.
Conclusions:
This meta-analysis revealed that lower age, family history and contralateral abnormality were associated with progression in MMD patients. The same three factors are associated with the progression of unilateral to bilateral MMD. Further studies are needed to validate our results.
Introduction
Moyamoya disease (MMD) is a chronic cerebrovascular disease characterized by progressive narrowing of the distal internal carotid arteries (ICA) and their proximal branches, finally developing abnormal collateral vessels, including basal moyamoya vessels, leptomeningeal anastomosis, and transdural anastomosis(1, 2). MMD is more common in people living in East Asian countries such as Japan (3–6) and Korea (7, 8) than in the Western Hemisphere (9, 10). In Japan, the incidence rate is 0.94–1.13 per 100,000 people, and the prevalence rate is 5.22–10.50 per 100,000 people (4, 6). Cognitive function and physical dysfunction caused by MMD seriously affect the quality of life of patients (1, 11). With the development and application of radiological techniques, various neuroimaging methods with different advantages have furthered the understanding of MMD in terms of its structural, functional, spatial, and temporal aspects. Such angiographic and morphological changes of this chronically progressive disease have been gradually understood, but the mechanisms of the disease progression have not been fully elucidated.
Disease progression may be related to the development of new ischemic or hemorrhagic events (12). For patients with MMD, the mortality rate of the first hemorrhage is 6.8%. Once the hemorrhage occurs again, the mortality rate will rise sharply to 28.6% (13). Predicting disease progression may reverse the current passive situation of waiting for hemorrhage before treatment. At the same time, it can assist clinical decision support and help patients clearly understand their own disease status, thereby indirectly improving their quality of life during treatment.
Some studies have explored the factors that can predict the progression of MMD, but there are also some controversies between them. Contralateral abnormality, age, family history and so on were found to be different between progression and non-progression groups in previous studies (2, 14, 15). However, Tian et al. (16) didn't find any predictors to distinguish these two groups. As most related studies are observational ones with a low level of evidence, a systematic review and meta-analysis to determine predictors of progression in MMD patients was necessary.
Methods
Eligibility criteria
The inclusion criteria were as following:
-
Patients: Patients were diagnosed with MMD which Refer to the diagnostic criteria revised in 2021 (17).
-
Potential predictors: Include at least one of the following potential predictors: age, sex, initial lesion side, Suzuki grade, hypertension, diabetes, family history, smoking and contralateral abnormality.
-
Outcome: After at least 6 months of follow-up, the disease was found to have progressed, which meant progression of the occlusive lesion in the major intracranial arteries or from unilateral to bilateral lesions.
-
Study type: Randomized controlled trials, case-control studies or cohort studies.
-
Publication date and language: Limited to articles published on or before December 10th 2022, English.
Studies were excluded based on the following criteria:
-
Patients: Moyamoya syndrome, atherosclerotic lesion and inflammatory disease.
-
Potential predictors: None of the above potential predictors were reported.
-
Study type: Case reports, expert consensus, abstracts, conferences, animal studies, guidelines, and comments.
-
Language: Non-English.
Search strategy
RCTs, case-control and cohort studies reporting progression of MMD patients were included after searching PubMed, Web of science, Cochrane library and Embase databases. There were no restrictions on the type of study used, and the publication date and language filters corresponding to the eligible criteria were used. The following search terms were used in different combinations: moyamoya disease; moyamoya syndrome; disease progression; disease exacerbation; progression.
Study selection and data extraction
Two authors (JC and ZX) independently screened and selected the eligible studies according to the inclusion and exclusion criteria. Data were independently extracted by 2 authors (JC and ZX) from all the included studies and subsequently cross-checked to ensure their accuracy. Any discrepancies in study extraction and data extraction were resolved by the senior author during cross-checking process. Data extracts included the first author of the study, year of publication, definition of progression, quality assessment, recruitment period, follow-up duration and characteristics of the study population in total, including the number of participants with potential predictors like age, sex, Suzuki grade, comorbidities et al. which were available in the literature. Authors were contacted for missing information when necessary. Studies were excluded when there was no response upon exposure.
Assessment of risk of bias
Two authors (JC and ZX) independently assessed the risk of bias for included studies according to the principle of the Newcastle-Ottawa Scale (NOS) (18). Potential predictors that were included in <10 studies were not suitable for performing funnel plots because of the potential difficulty in obtaining sufficient power to distinguish random from genuine occurrence (19). Any disagreements were resolved by the 2 authors through discussion, with the help of a third author (LD).
Assessment of heterogeneity
The I-square test was used to test the heterogeneity. We defined heterogeneity as follows: I2 = 25–49%, low heterogeneity; I2 = 50–74%, moderate heterogeneity; and I2 > 75%, severe heterogeneity.
Data synthesis and statistical analysis
Continuous outcomes were presented as standardized mean difference (SMD) with 95% confidence interval (CI), and dichotomous outcomes were described as odds ratio (OR) with 95% CI. A meta-analysis was conducted using the software Review Manager if the effect sizes were available or calculable in 3 or more studies for specific potential predictors. A random-effects model was used to analyze the outcomes for included studies, but a fixed-effect model was used when there was little evidence of heterogeneity (I2 < 50%). Moreover, Subgroup analysis would be performed according to the content of included studies.
Results
Study selection and study characteristics
There were 1,750 potentially relevant studies being systematically identified through an electronic database search, 589 of which were excluded due to duplication. Further, 1,086 studies were excluded after a screening of the titles and abstracts. Another 63 studies were excluded after reviewing the full text. These studies were excluded because they were not original research, didn't mention potential predictors or unable to extract data. Finally, 12 studies enrolling a total of 842 patients (158 patients with progression and 684 patients with non-progression) were included in the meta-analysis (2, 12, 14–16, 20–26). The flowchart for studies screening was presented in Figure 1.
Figure 1

Flow chart of the study selection.
The included studies had been published between 1994 and 2022. There were no randomized controlled trials. The mean incidence of MMD progression of the included studies was 18.8% (range 8.2–58.8%). Most of studies focused on the progression from unilateral to bilateral lesions. The detailed characteristics of the 11 included studies were listed in Table 1.
Table 1
| Studies | Country | Period | Definition of progression | Follow-up duration | Patients | Total patients | Rate of progression | Factors included in analysis | Quality (NOS) |
|---|---|---|---|---|---|---|---|---|---|
| Kawano et al. (26) | Japan | NA | From unilateral to bilateral lesions | Progression: 0.7–7 (mean 2.6) years Non-progression: 0.2–13 (mean 3.1) years |
Children and adults | 32 | 53.1% | Age, sex, family history | 5 |
| Kuroda et al. (12) | Japan | 1990–2004 | progression of the occlusive lesion in the major intracranial arteries | 73.6 ± 49.0 months | Adults only | 63 | 23.8% | Age, sex | 6 |
| Kelly et al. (20) | USA | January 1, 1991–December 31, 2005 | From unilateral to bilateral lesions | Progression: 17.1 ± 3.5 months Non-progression: 19.0 ± 3.3 months |
Children and adults | 18 | 38.9% | Sex, hypertension, diabetes, contralateral abnormality | 6 |
| Smith and Scott (21) | USA | January 1, 1985–June 30, 2006 | From unilateral to bilateral lesions | 5.3 years (first operation) and 4.3 years (second operation) | Children and adults | 33 | 30.3% | Family history | 5 |
| Park et al. (14) | South Korea | January 2000–June 2008 | From unilateral to bilateral lesions | 18.1–100 (mean 35.3) months | Children only | 34 | 58.8% | Age, sex, family history | 7 |
| Yeon et al. (22) | South Korea | March 1995–February 2009 | From unilateral to bilateral lesions | 13–157 (mean 53.4) months | Children only | 45 | 17.8% | Sex, initial lesion side, Suzuki grade | 7 |
| Lee et al. (15) | South Korea | 2001–2011 | From unilateral to bilateral lesions | 50.1 ± 28.0 months | Adults only | 41 | 14.6% | Age, sex, hypertension, family history, smoking, contralateral abnormality | 7 |
| Zhang et al. (23) | China | January 2002–May 2014 | From unilateral to bilateral lesions | 43.8 ± 21.3 months | Children and adults | 109 | 16.5% | Sex, initial lesion side, Suzuki grade, hypertension, diabetes, smoking, contralateral abnormality | 7 |
| Church et al. (25) | USA | 1991–2017 | From unilateral to bilateral lesions | 1–22 (average 5.8) years | Adults | 217 | 8.3% | Sex, hypertension, family history, smoking | 7 |
| Mineharu et al. (2) | Japan | 1987–2017 | From unilateral to bilateral lesions | 0–355 (mean 72.2) months | Children and adults | 93 | 24.7% | Age, sex, hypertension, diabetes, family history, smoking, contralateral abnormality | 7 |
| Oomori et al. (24) | Japan | May 2008–September 2015 | Scores in one or more main cerebral arteries were increased | 5 years | Adults only | 68 | 11.8% | Age, sex, hypertension, diabetes | 8 |
| Tian et al. (16) | China | January 2015–January 2017 | From unilateral to bilateral lesions | 48–78 (median 43) months | Adults only | 89 | 8.2% | Sex, initial lesion side, Suzuki grade, hypertension, diabetes, family history | 7 |
Characteristics of included studies for progression of MMD.
MMD, moyamoya disease; NOS, Newcastle-Ottawa Scale; NA, not available.
Risk of bias of included studies
The Newcastle–Ottawa scale was used to assess the bias risk of observational studies (Table 1). All the included studies were assessed with 5 to 9 points by NOS. Funnel plot was unsuitable for assessment of publication bias because the number of included studies for analyzing potential predictors were small.
Meta-analysis
We found lower age (SMD: −0.29, 95% CI: −0.55-−0.03; P = 0.03), family history (OR: 3.97, 95% CI: 1.96–8.03; P < 0.001) and contralateral abnormality (OR: 3.95, 95% CI: 1.10–14.20; P = 0.04) were associated with progression in MMD patients (Figure 2), while sex, initial lesion side, Suzuki grade, hypertension, diabetes and smoking were not (Figure 3).
Figure 2

Positive predictor for progression in MMD patients. MMD, moyamoya disease; SD, standard deviation; IV, Inverse variance; M-H, Mantel-Haenszel; df, degrees of freedom; CI, Confidence Interval; Std. Mean Difference, standardized mean difference.
Figure 3

Potential predictors which were not significantly associated with the progression in this meta-analysis. MMD, moyamoya disease; M-H, Mantel-Haenszel; df, degrees of freedom; CI, Confidence Interval.
Subgroup analysis
Considering the inclusion of an “age” factor in two studies (12, 24) and the definition of progression in these two studies differently from the other studies (12, 24), we further performed a subgroup analysis of the remaining studies which focused on unilateral MMD patients. Same results as above, lower age (SMD: −0.44, 95% CI: −0.76–0.12; P = 0.007) was the predictor for progression of this subgroup (Figure 4). The results of family history (OR: 3.97, 95% CI: 1.96–8.03; P < 0.001) and contralateral abnormality (OR: 3.95, 95% CI: 1.10–14.20; P = 0.04) were consistent with those of the previous analysis due to consistent definitions about progression in unilateral MMD patients (Figures 2B, C).
Figure 4
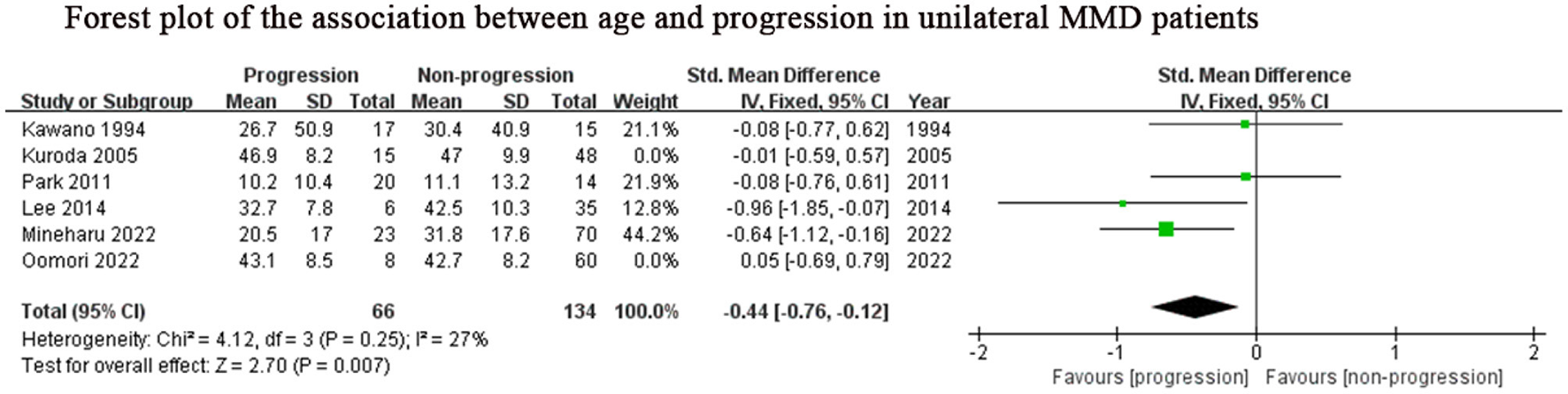
Positive predictor for progression in unilateral MMD patients. MMD, moyamoya disease; SD, standard deviation; IV, Inverse variance; M-H, Mantel-Haenszel; df, degrees of freedom; CI, Confidence Interval; Std. Mean Difference, standardized mean difference.
Discussion
Predicting the progression of the disease can achieve timely warning, early intervention, and reduce the disability and mortality of the disease, but the factors that predict the progression of MMD were still controversial. In this systematic review and meta-analysis of clinical studies reporting predictors of disease progression in MMD patients, a total of 842 patients were included, and we found three important findings: (1) the progression rate for MMD patients was around 18.8%; (2) lower age, family history and contralateral abnormality were associated with progression in MMD patients, while sex, initial lesion side, Suzuki grade, hypertension, diabetes and smoking were not predictors for progression; (3) lower age, family history and contralateral abnormality were associated with progression in unilateral MMD patients.
With the development and application of radiological techniques, more and more MMD patients are being identified (27). The incidence of progression in MMD patients was reported differently in these included literatures, varying from 8.2 to 58.8%. However, as for included literature, it seems that the newer the literature, the lower the incidence of progression, which may be due to the early prediction of disease recurrence, death, disability, and complications. The progression of the disease is often accompanied by the onset of symptoms, with more serious consequences for the patient (23). Identifying the predictors can inform the future progress of individual diseases and the probability of a certain outcome, so as to guide doctors and patients to jointly decide on future prevention, treatment, and rehabilitation programs.
The age group with the highest incidence of moyamoya disease is 0–10 years old, followed by 30–50 years old (6, 28–30). In addition, it is reported that children with unilateral often exhibit progression to typical bilateral MMD (31). In 2011, Yeon et al. (22) reported that contralateral progression was mostly observed in patients under the age of 9 years old and they developed contralateral lesions within 3 years after the initial diagnosis. Smith et al. (21) reported that a younger age at diagnosis was associated with a rapid contralateral progression. Park et al. (14) also found younger age was associated with more rapid rate of progression (age <8 years, 14.18 months and age >8, 22.38 months). Consistent with their data, our results also showed lower age was associated with progression in MMD patients. One thing worth noting, moyamoya disease is mainly divided into hemorrhagic type and ischemic type, in which children mainly present with ischemic symptoms and adult patients mainly present with hemorrhagic symptoms with high mortality (11, 32, 33). Therefore, a bias in younger age groups at greater susceptibility to progression may exist due to rapid death of patients without observed disease progression. But in any case, closer follow-up using MRI would be necessary for child patients or younger adults. Especially for children, active surgical intervention has a significant effect on restoring normal brain function before irreversible brain damage occurs.
The incidence of people with a family history of MMD are 30–40 times higher than the incidence of the general population, and patients with MMD have shown the genetic characteristics of autosomal dominant (34). Mineharu et al. found that 50% of the children of MMD patients could progress to MMD (35). Accordingly, it can be considered that patients with family history of MMD and angiographic features of MMD can be diagnosed as MMD. Our results suggested that MMD patients with family history were more likely to progress. Therefore, patients with moyamoya disease with a family history should take precautions to reduce the probability of adverse events.
Contralateral abnormality indicated equivocal or mild M1, A1, and intracranial segment of ICA stenosis on the contralateral side. We found that contralateral abnormality was a robust predictor for progression in MMD patients. Zhang et al. (23) also showed that contralateral progression survival was significantly higher in patients with contralateral abnormality than those without it. As MMD is a chronic cerebrovascular disease, some signs, especially the appearance of stenosis, are often indicative of later development. It is important to carefully assess the contralateral vessels for even very minor involvement, and if present, follow this subgroup closely.
There are some limitations. First, lack of randomized controlled studies influenced the quality of the article. Second, fewer factors that could be included in the study affected a comprehensive study of the predictors. Third, although there were two literatures with different definitions of progression, most of the articles have the same definition.
In conclusion, this meta-analysis revealed that lower age group, family history and contralateral abnormality were associated with progression in MMD patients. The same three factors are associated with the progression of unilateral MMD to bilateral. Further studies are needed to validate our results.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JC and ZX completed data extraction and manuscript writing. LD completed data checking. TW completed manuscript revision. YZ and YF completed charts production. YC provided funding and guidance. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer SH declared a shared affiliation with the authors JC, TW, YF, and YC to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Scott RM Smith ER . Moyamoya disease and moyamoya syndrome. N Engl J Med. (2009) 360:1226–37. 10.1056/NEJMra0804622
2.
Mineharu Y Takagi Y Koizumi A Morimoto T Funaki T Hishikawa T et al . Genetic and nongenetic factors for contralateral progression of unilateral moyamoya disease: the first report from the supra Japan study group. J Neurosurg. (2022) 136:1005–14. 10.3171/2021.3.JNS203913
3.
Sato Y Kazumata K Nakatani E Houkin K Kanatani Y . Characteristics of moyamoya disease based on national registry data in Japan. Stroke. (2019) 50:1973–80. 10.1161/STROKEAHA.119.024689
4.
Hayashi K Horie N Suyama K Nagata I . An epidemiological survey of moyamoya disease, unilateral moyamoya disease and quasi-moyamoya disease in Japan. Clin Neurol Neurosurg. (2013) 115:930–3. 10.1016/j.clineuro.2012.09.020
5.
Kuriyama S Kusaka Y Fujimura M Wakai K Tamakoshi A Hashimoto S et al . Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. (2008) 39:42–7. 10.1161/STROKEAHA.107.490714
6.
Baba T Houkin K Kuroda S . Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. (2008) 79, 900–4. 10.1136/jnnp.2007.130666
7.
Kim T Lee H Bang JS Kwon OK Hwang G Oh CW . Epidemiology of moyamoya disease in Korea: based on national health insurance service data. J Korean Neurosurg Soc. (2015) 57:390–5. 10.3340/jkns.2015.57.6.390
8.
Ahn IM Park DH Hann HJ Kim KH Kim HJ Ahn HS . Incidence, prevalence, and survival of moyamoya disease in Korea: a nationwide, population-based study. Stroke. (2014) 45:1090–5. 10.1161/STROKEAHA.113.004273
9.
Uchino K Johnston SC Becker KJ Tirschwell DL . Moyamoya disease in washington state and California. Neurology. (2005) 65:956–8. 10.1212/01.wnl.0000176066.33797.82
10.
Ghaffari-Rafi A Ghaffari-Rafi S Leon-Rojas J . Socioeconomic and demographic disparities of moyamoya disease in the United States. Clin Neurol Neurosurg. (2020) 192:105719. 10.1016/j.clineuro.2020.105719
11.
Shang S Zhou D Ya J Li S Yang Q Ding Y et al . Progress in moyamoya disease. Neurosurg Rev. (2020) 43:371–82. 10.1007/s10143-018-0994-5
12.
Kuroda S Ishikawa T Houkin K Nanba R Hokari M Iwasaki Y . Incidence and clinical features of disease progression in adult moyamoya disease. Stroke. (2005) 36:2148–53. 10.1161/01.STR.0000182256.32489.99
13.
Kobayashi E Saeki N Oishi H Hirai S Yamaura A . Long-term natural history of hemorrhagic moyamoya disease in 42 patients. J Neurosurg. (2000) 93:976–80. 10.3171/jns.2000.93.6.0976
14.
Park EK Lee YH Shim KW Choi JU Kim DS . Natural history and progression factors of unilateral moyamoya disease in pediatric patients. Childs Nerv Syst. (2011) 27, 1281–7. 10.1007/s00381-011-1469-y
15.
Lee SC Jeon JS Kim JE Chung YS Ahn JH Cho WS et al . Contralateral progression and its risk factor in surgically treated unilateral adult moyamoya disease with a review of pertinent literature. Acta Neurochir. (2014) 156:103–11. 10.1007/s00701-013-1921-8
16.
Tian X Hu M Zhang J . The contralateral progression in a cohort of chinese adult patients with unilateral moyamoya disease after revascularization: a single-center long-term retrospective study. Acta Neurochir. (2022) 164:1837–44. 10.1007/s00701-022-05153-6
17.
Kuroda S Fujimura M Takahashi J Kataoka H Ogasawara K Iwama T et al . Diagnostic criteria for moyamoya disease-2021 revised version. Neurol Med Chir. (2022) 62:307–12. 10.2176/jns-nmc.2022-0072
18.
Feng Y Bai X Zhang X Wang T Lu X Yang K et al . Risk factors for new ischemic cerebral lesions after carotid artery stenting: a systematic review and meta-analysis. Ann Vasc Surg. (2021) 77:296–305. 10.1016/j.avsg.2021.05.031
19.
Hartemink N Boshuizen HC Nagelkerke NJ Jacobs MA Van Houwelingen HC . Combining risk estimates from observational studies with different exposure cutpoints: a meta-analysis on body mass index and diabetes type 2. Am J Epidemiol. (2006) 163:1042–52. 10.1093/aje/kwj141
20.
Kelly ME Bell-Stephens TE Marks MP Do HM Steinberg GK . Progression of unilateral moyamoya disease: a clinical series. Cerebrovasc Dis. (2006) 22:109–15. 10.1159/000093238
21.
Smith ER Scott RM . Progression of disease in unilateral moyamoya syndrome. Neurosurg Focus. (2008) 24:E17. 10.3171/FOC/2008/24/2/E17
22.
Yeon JY Shin HJ Kong DS Seol HJ Kim JS Hong SC et al . The prediction of contralateral progression in children and adolescents with unilateral moyamoya disease. Stroke. (2011) 42:2973–6. 10.1161/STROKEAHA.111.622522
23.
Zhang Q Wang R Liu Y Zhang Y Wang S Cao Y et al . Clinical features and long-term outcomes of unilateral moyamoya disease. World Neurosurg. (2016) 96:474–82. 10.1016/j.wneu.2016.09.018
24.
Oomori D Kubo Y Yabuki M Kitakami K Fujiwara S Yoshida K et al . Angiographic disease progression in medically treated adult patients with ischemic moyamoya disease without cerebral misery perfusion: supplementary analysis of a 5-year prospective cohort. Neurosurg Rev. (2022) 45:1553–61. 10.1007/s10143-021-01677-0
25.
Church EW Bell-Stephens TE Bigder MG Gummidipundi S Han SS Steinberg GK . Clinical course of unilateral moyamoya disease. Neurosurgery. (2020) 87:1262–8. 10.1093/neuros/nyaa284
26.
Kawano T Fukui M Hashimoto N Yonekawa Y . Follow-up study of patients with “unilateral” moyamoya disease. Neurol Med Chir. (1994) 34:744–7. 10.2176/nmc.34.744
27.
Zhang X Xiao W Zhang Q Xia D Gao P Su J et al . Progression in moyamoya disease: clinical features, neuroimaging evaluation, and treatment. Curr Neuropharmacol. (2022) 20:292–308. 10.2174/1570159X19666210716114016
28.
Lee DJ Liebeskind DS . Characterization of inpatient moyamoya in the United States: 1988–2004. Front Neurol. (2011) 2:43. 10.3389/fneur.2011.00043
29.
Wakai K Tamakoshi A Ikezaki K Fukui M Kawamura T Aoki R et al . Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg. (1997) 99:S1–5. 10.1016/S0303-8467(97)00031-0
30.
Chen PC Yang SH Chien KL Tsai IJ Kuo MF . Epidemiology of moyamoya disease in taiwan: a nationwide population-based study. Stroke. (2014) 45:1258–63. 10.1161/STROKEAHA.113.004160
31.
Kim TW Seo BR Kim JH Kim YO . Rapid progression of unilateral moyamoya disease. J Korean Neurosurg Soc. (2011) 49, 65–67. 10.3340/jkns.2011.49.1.65
32.
Graham JF Matoba A . A survey of moyamoya disease in Hawaii. Clin Neurol Neurosurg. (1997) 99, S31–5. 10.1016/S0303-8467(97)00037-1
33.
Fujimura M Bang OY Kim JS . Moyamoya disease. Front Neurol Neurosci. (2016) 40:204–20. 10.1159/000448314
34.
Kim JS . Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke. (2016) 18:2–11. 10.5853/jos.2015.01627
35.
Mineharu Y Takenaka K Yamakawa H Inoue K Ikeda H Kikuta KI et al . Inheritance pattern of familial moyamoya disease: autosomal dominant mode and genomic imprinting. J Neurol Neurosurg Psychiatry. (2006) 77:1025–9. 10.1136/jnnp.2006.096040
Summary
Keywords
moyamoya disease, potential predictors, progression, systematic review, meta-analysis
Citation
Cao J, Xing Z, Dai L, Wang T, Zhang Y, Feng Y and Chen Y (2023) Potential predictors for progression of moyamoya disease: A systematic review and meta-analysis. Front. Neurol. 14:1128338. doi: 10.3389/fneur.2023.1128338
Received
20 December 2022
Accepted
13 February 2023
Published
02 March 2023
Volume
14 - 2023
Edited by
Teodor Svedung Wettervik, Uppsala University, Sweden
Reviewed by
Hiroki Hongo, The University of Tokyo, Japan; Shihao He, Beijing Tiantan Hospital, Capital Medical University, China; Satoshi Koizumi, The University of Tokyo Hospital, Japan
Updates
Copyright
© 2023 Cao, Xing, Dai, Wang, Zhang, Feng and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfei Chen chenyanflying@126.com
†These authors have contributed equally to this work
This article was submitted to Endovascular and Interventional Neurology, a section of the journal Frontiers in Neurology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.