Abstract
Hemiplegic migraine (HM) can cause significant functional impairment and negatively affect the quality of life of affected individuals. Emerging evidence suggests an association between migraines and congenital patent foramen ovale (PFO), which is a small opening between the atria of the heart that normally closes shortly after birth. This report describes a 34 years-old woman with sporadic hemiplegic migraine (SHM) who was diagnosed with PFO. Following percutaneous PFO closure, her hemiplegic symptoms disappeared, but her headache exacerbated. After 3 years of follow-up, her headache severity gradually reduced, and the frequency remained consistent at 2–3 times per year with no aura symptoms. This case highlights the dissociation between the resolution of hemiplegic symptoms and the persistence of headaches after PFO closure in sporadic HM. Patients with HM may experience changes in aura symptoms and headache severity after PFO closure. Before performing PFO closure in patients with hemiplegic migraine, the indications should be thoroughly understood.
1. Introduction
Hemiplegic migraine (HM) is a rare subtype of migraine with aura (MA) characterised by recurrent attacks of reversible motor weakness or paralysis (hemiplegia) on one side of the body (1). These attacks typically last from minutes to hours and are often accompanied by other migraine symptoms such as headaches, visual disturbances, and sensory changes (2). Sporadic HM (SHM) is a subtype of HM, and its exact prevalence is unknown; it is estimated to affect <0.01% of the population (3). The disease burden of SHM can be significant because of the frequency and severity of attacks. Hemiplegic episodes can cause significant functional impairment and negatively affect the quality of life of affected individuals (4).
Emerging evidence suggests an association between migraines and congenital patent foramen ovale (PFO) (5), which is a small opening between the atria of the heart that normally close shortly after birth (6). PFO allows the passage of small blood clots or other substances from the venous to the arterial circulation, potentially triggering migraines or stroke-like symptoms. A few case reports suggest that patients with HM may have co-existing PFO and that the patient’s clinical symptoms are alleviated after percutaneous PFO closure (7–10). Here, we report a case of SHM in a patient with a PFO. After percutaneous PFO closure, the hemiplegic symptoms disappeared, but the headache persisted.
2. Case presentation
A 34 years-old female patient began experiencing headaches at around age 6, which occurred 2–3 times per year. No apparent triggers were identified. Prior to each attack, the patient noticed neck stiffness. After noting Z-shaped flashing lights in her vision for about 10 min, she would develop a moderate throbbing headache (visual analogue scale [VAS] score of 5) on the right temporal and frontal areas, usually lasting for about 8 h and accompanied by nausea, vomiting, phonophobia, and dizziness. Resting in a quiet environment or consuming ibuprofen relieved these symptoms. After age 30, she started experiencing right-sided hemiparesis half an hour before the onset of headaches, which lasted for 2 weeks and occurred 2–3 times per year. Her migraine disability assessment questionnaire and headache impact test scores were 2 and 41, respectively. She was engaged in physical labour, denied smoking or alcohol consumption, and had no history of chronic or infectious diseases. Her mother died suddenly at age 66, and her father had bronchitis. She had six sisters and four brothers, and none had a history of similar episodes.
When she was 34 years old, she was readmitted to the hospital because of right-sided limb weakness and a headache. A brain magnetic resonance imaging (MRI) scan during headache attacks with hemiparesis (Figure 1A) revealed no significant abnormalities. Computed tomography angiography revealed left vertebral artery hypoplasia (Figure 1C). During hospitalisation, a PFO with right-to-left shunting was observed. Transoesophageal echocardiography showed that her PFO was “long tunnel”-like (Figure 2A), which carried a higher risk of thromboembolic events. Blood tests, liver and kidney function tests, thyroid function tests, coagulation studies, and immunological examinations revealed no significant abnormalities. After evaluation by the cardiovascular department, she underwent transcatheter PFO closure according to the guidelines (11) and was discharged 2 weeks later (Figures 2B–D).
Figure 1

Magnetic resonance imaging (MRI). From left to right: sagittal, diffusion-weighted, fluid-attenuated inversion recovery, T1, and T2 sequences. (A) MRI showed no significant abnormalities during headache attacks with hemiparesis. (B) MRI showed no significant abnormalities at 6 months after patent foramen ovale closure. (C) Computed tomography angiography revealed left vertebral artery hypoplasia.
Figure 2
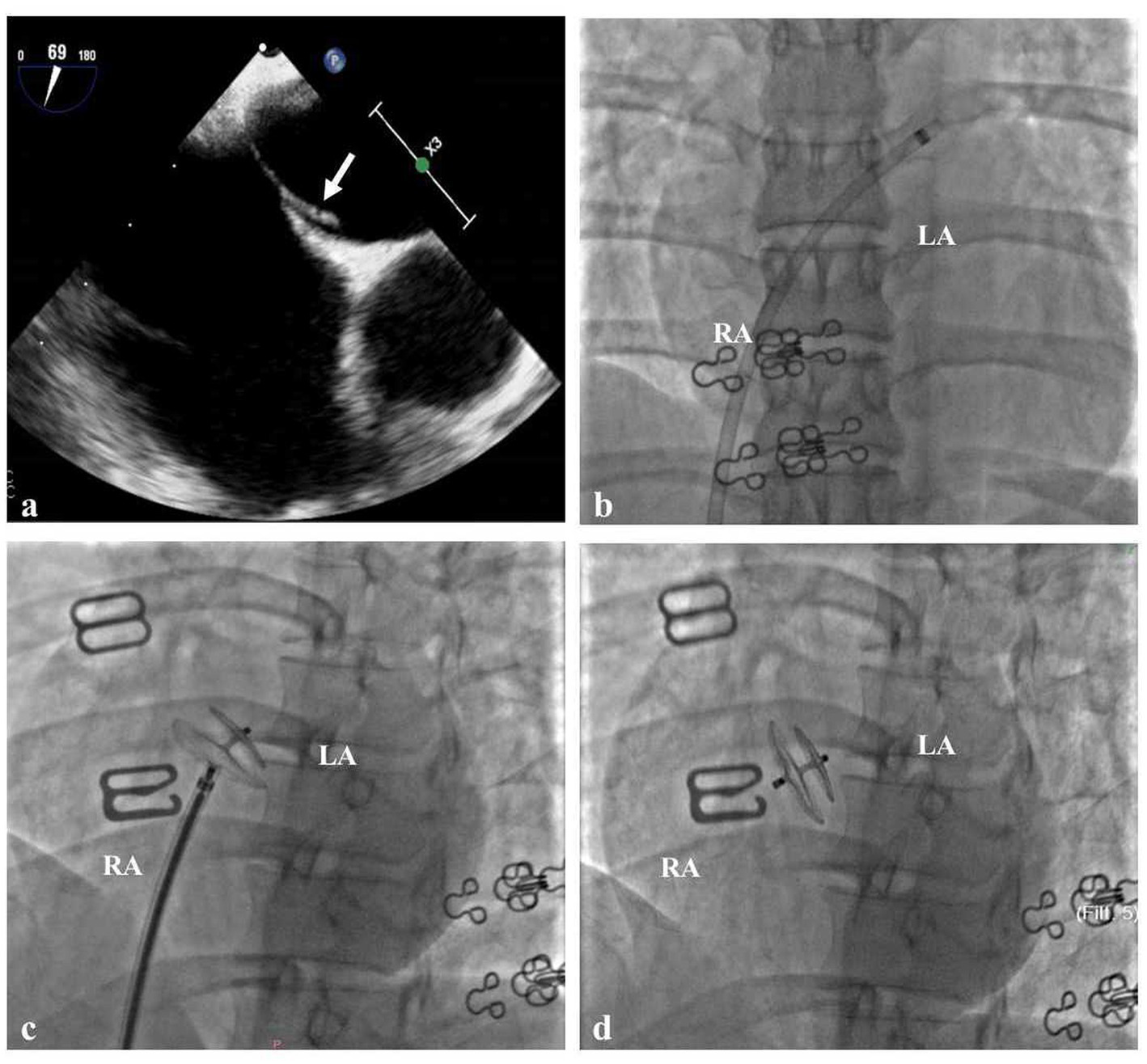
Cardiac Imaging in the Patient (A) Transoesophageal echocardiography showed that the patent foramen ovale was long tunnel-like (white arrow). (B) The delivery sheath passes through the PFO from the right to the left atrium. (C) The traction test of PFO closure was successful. (D) Made occluder successfully released.
After she was discharged from the hospital, we followed her for 3 years. Six months after PFO closure, her headache severity worsened (VAS score 9) and she experienced a migraine attack lasting for 8 h, but without visual aura or hemiplegia. Her brain MRI was also normal (Figure 1B). Three years after PFO closure, she experiences a throbbing headache (VAS score 4) at the right temporal and frontal areas 2–3 times per year, with each attack lasting about 8 h. She had not experienced any more right-sided limb weakness; the Z-shaped flashing lights in her vision no longer occurred before headaches; and the accompanying dizziness during headaches had disappeared. Figure 3 shows the changes in prodromal symptoms, aura, headache severity, hemiparesis, and accompanying symptoms from the age of 6 years to the 3 years follow-up after PFO closure. With her informed consent, whole-exome sequencing was performed, and no genetic abnormalities were found.
Figure 3
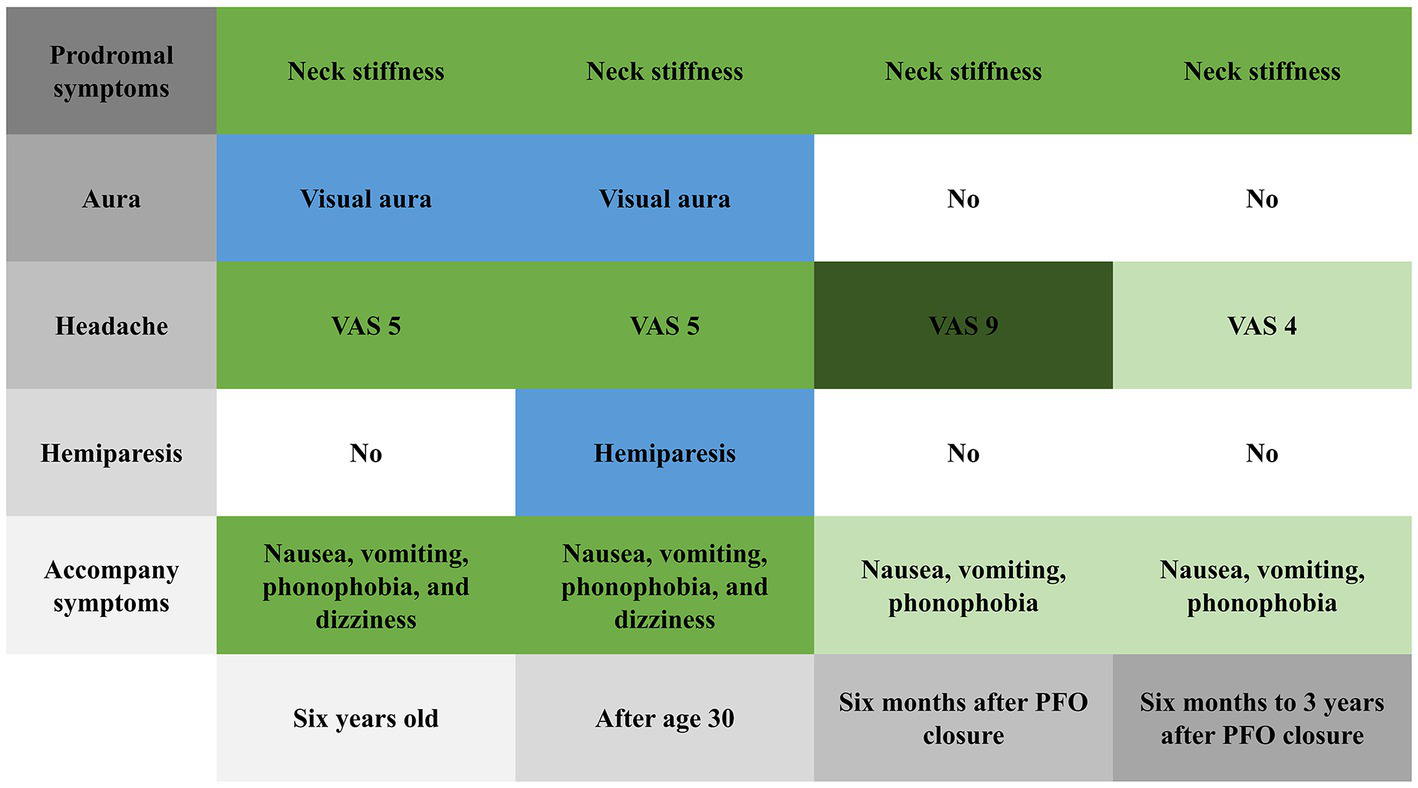
Changes in prodromal symptoms, aura, headache severity, hemiparesis, and accompanying symptoms from 6 years old to the 3 years follow-up after patent foramen ovale closure.
3. Discussion
We report a case of migraine in a patient with PFO who experienced relief from aura symptoms after treatment. In this case, we considered the clinical diagnosis of HM valid. According to the International Classification of Headache Disorders 3rd Edition (ICHD-3) criteria (12), HM is a specific subtype of MA. The patient had a typical visual aura preceding each headache episode from age 6–30 years, which lasted for approximately 10 min. During this period, we diagnosed her with MA. Between the ages of 30 and 34, the patient also experienced right-sided limb weakness, occurring 30 min before each headache and lasting for approximately 2 weeks, which resolved completely after the attack. A brain MRI performed during headache episodes accompanied by hemiplegia did not reveal any significant abnormalities. Therefore, we revised the diagnosis to HM. According to the ICHD-3 description, hemiplegic symptoms can persist for several weeks (12), as in the present case. Finally, the patient underwent whole-exome sequencing, which did not reveal any pathogenic gene mutations associated with familial hemiplegic migraines. Therefore, we confirmed the diagnosis of SHM.
PFO is a residual opening between the septa primum and secundum that allows blood to flow from the right to the left atrium (13). Approximately 40–60% of individuals with MA have PFO, compared to 20–30% of the general adult population (14, 15). Although previous studies have demonstrated the impact of PFO on migraines, a definitive conclusion regarding the association between PFO and migraines has not been reached (16). HM has been sporadically reported in case studies on the relationship between HM and PFO closure (12). Table 1 summarises the cases of HM treated with PFO closure, showing partial or complete relief of symptoms in patients after closure. Interestingly, all previous case reports involved male patients. However, as this is a rare condition, sporadic case reports cannot be used to make inferences about the general population.
Table 1
| Case report | Hemiplegic age | Gender | Aura symptoms | Diagnosis | Genetic tests | Radiological examinations | Heart exam findings | Prognosis after PFO closure |
|---|---|---|---|---|---|---|---|---|
| Brighina et al. (8) | 30 | Male | Basilar aura and left hemiplegia for 3 weeks | Atypical SHM | NA | Brain MR imaging was normal and MRA demonstrated hypoplasia of the basilar artery | PFO with a large right-to-left shunt. | Four year persisting remission of migraine |
| Perrotta et al. (9) | 9 | Male | Visual aura, paresis and paresthesia | FHM | ATP1A2 | Brain MR imaging was normal | Large PFO and atrial septal aneurysm | Sudden improvement of the frequency of HM attacks |
| Lemka et al. (7) | 14 | Male | Visual aura, dysarthria, left hemiplegia, facial asymmetry | SHM | Normal | MRI + MRA imaging was normal | PFO with left-to-right shunt | Been free of SHM attacks for 3 years |
| Hu et al. (10) | Since childhood | Male | Visual aura, hemiparesis and hemianesthesia | HM | Normal | Brain MRI showed cortical necrosis and MRA demonstrated vasodilation of the branches of the left MCA and PCA | PFO | Substantially reduced frequency of episodes |
Cases of hemiplegic migraine treated with patent foramen ovale closure.
HM, hemiplegic migraine; PFO, patent foramen ovale; NA, not available; MRA, magnetic resonance angiography; FHM, familial hemiplegic migraine; SHM, sporadic hemiplegic migraine; MCA, middle cerebral artery; PCA, posterior cerebral artery.
The exact pathogenesis of MA is still unclear. One proposed mechanism is cortical spreading depression (CSD), which is a transient wave of depolarisation originating from neurons and glia in the occipital cortex that slowly self-propagates in the cerebral cortex, followed by long-term inhibition of brain activity (17). However, the mechanisms by which CSD initiates, propagates, and triggers MA symptoms are undetermined. PFO may trigger migraine attacks by facilitating a microembolism, which activates the trigeminovascular system and leads to CSD (16). Genetic factors are also believed to play a role in the development of MA (18). Mutations in genes involved in ion-channel function, neurotransmitter release, and cortical excitability have been identified in some individuals with this condition (19). Our patient did not have any genetic mutations identified by whole-exome sequencing. Interestingly, as no further transient hemiparesis or other auras were observed after PFO closure, this clinical manifestation suggests that PFO may activate the trigeminovascular system and can lead to CSD by facilitating a microembolism.
Kato et al. (20) conducted a retrospective cohort study evaluating the effect of atrial septal defect (ASD) closure on migraine attacks and found that 11 of the 29 patients who had HM prior to ASD closure reported exacerbations of migraine attacks, but most of them improved after a median of 2.5 months post-surgery. Yankovsky and Kuritzky reported a 48 years-old male with migraines with sensory and visual aura who experienced daily attacks for 6 months the day after undergoing percutaneous ASD closure (21). Riederer et al. reported a 27 years-old female with MA who experienced recurrent migraine attacks almost daily after percutaneous ASD closure (22). Six months after PFO closure, headache severity worsened in our patient. Platelet activation on the surface of the implanted device and subsequent secretion of serotonin by activated platelets have been proposed as one potential mechanism for headache exacerbation (20). Implanted devices or tensile deformation of the interatrial septum may induce the release of atrial natriuretic peptide, which is associated with migraine attacks (21, 22).
In our case, the patient presented with hypoplasia of the left vertebral artery. Autopsy and angiography studies have reported that the frequency of this congenital variation ranges from 2 to 6% (23). Brighina et al. described a patient with atypical SHM associated with a PFO and basilar artery hypoplasia (8). The relationship between anatomical variations in blood vessels and hemiplegic migraines remains under investigation. MA was found to be associated with a perfusion deficit not limited to a specific vascular territory by Förster et al. (24). In previous studies, up to 23% of patients with HM were found to have white-matter signal abnormalities on T2 sequences outside migraine attacks (25, 26). Additionally, cortical oedema has been documented during HM attacks, both in FHM (26, 27) and SHM (28, 29). However, no brain MRI abnormalities were found in our case.
MIST (Migraine Intervention with STARFlex Technology) (30), PRIMA (Percutaneous Closure of PFO in MA) (31), and PREMIUM (Prospective, Randomized Investigation to Evaluate Incidence of Headache Reduction in Subjects With Migraine and PFO Using the Amplatzer PFO Occluder to Medical Management) (32) are three randomized controlled trials that investigated the efficacy of PFO closure in patients with migraine. Although a meta-analysis of these studies indicated that PFO closure may offer benefits in reducing migraine attacks and migraine days (5, 33–36), there is still no good-quality evidence to support PFO closure reducing the intensity and severity of migraine headaches (37). From our perspective, based on the safety of PFO closure, relieving migraines should be regarded as an additional benefit rather than a primary goal. These interventions reduce the risk of stroke or myocardial infarction resulting from paradoxical embolism, and preventing systemic embolic events is a lifelong endeavour (38).
4. Conclusion
This case highlights the dissociation between the resolution of MA and the persistence of headaches after percutaneous PFO closure in a patient with SHM. There is still a lack of effective evidence that PFO closure is beneficial for the prevention of HM attacks. Patients with HM may experience changes in aura symptoms and headache severity after PFO closure. Before performing PFO closure on patients with HM, the indications should be thoroughly understood.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PW: Supervision, Writing – original draft. FY: Writing – review & editing. HZ: Investigation, Writing – original draft. QY: Performed the PFO closure procedure and advised on the manuscript. YW: Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the Sichuan Provincial Health Commission Popularization Project (21PJ150); Hypertension (Tai Ge) Special Research Project of Sichuan Medical Association (No. 2019TG37) and the Chengdu High-Level Clinical Key Specialty Construction project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Di Stefano V Rispoli MG Pellegrino N Graziosi A Rotondo E Napoli C et al . Diagnostic and therapeutic aspects of hemiplegic migraine. J Neurol Neurosurg Psychiatry. (2020) 91:764–71. doi: 10.1136/jnnp-2020-322850
2.
Hiekkala ME Vuola P Artto V Häppölä P Häppölä E Vepsäläinen S et al . The contribution of CACNA1A, ATP1A2 and SCN1A mutations in hemiplegic migraine: a clinical and genetic study in Finnish migraine families. Cephalalgia. (2018) 38:1849–63. doi: 10.1177/0333102418761041
3.
Nandyala A Shah T Ailani J . Hemiplegic migraine. Curr Neurol Neurosci Rep. (2023) 23:381–7. doi: 10.1007/s11910-023-01277-z
4.
Yamanaka G Go S Morichi S Takeshita M Morishita N Suzuki S et al . Clinical features and burden scores in Japanese pediatric migraines with brainstem aura, hemiplegic migraine, and retinal migraine. J Child Neurol. (2020) 35:667–73. doi: 10.1177/0883073820927840
5.
Kheiri B Abdalla A Osman M Ahmed S Hassan M Bachuwa G et al . Percutaneous closure of patent foramen ovale in migraine: a meta-analysis of randomized clinical trials. JACC Cardiovasc Interv. (2018) 11:816–8. doi: 10.1016/j.jcin.2018.01.232
6.
Romano V Gallinoro CM Mottola R Serio A Di Meglio F Castaldo C et al . Patent foramen ovale-a not so innocuous septal atrial defect in adults. J Cardiovasc Dev Dis. (2021) 8:60. doi: 10.3390/jcdd8060060
7.
Lemka M Pienczk-Reclawowicz K Pilarska E Szmuda M . Cessation of sporadic hemiplegic migraine attacks after patent foramen ovale closure. Dev Med Child Neurol. (2009) 51:923–4. doi: 10.1111/j.1469-8749.2009.03466.x
8.
Brighina F Gurgone G Gaglio RM Palermo A Cosentino G Fierro B . A case of atypical sporadic hemiplegic migraine associated with PFO and hypoplasia of vertebro-basilar system. J Headache Pain. (2009) 10:303–6. doi: 10.1007/s10194-009-0125-3
9.
Perrotta A Gambardella S Ambrosini A Anastasio MG Albano V Fornai F et al . A novel ATP1A2 gene variant associated with pure sporadic hemiplegic migraine improved after patent foramen ovale closure: a case report. Front Neurol. (2018) 9:332. doi: 10.3389/fneur.2018.00332
10.
Hu Y Wang Z Zhou L Sun Q . Prolonged hemiplegic migraine led to persistent hyperperfusion and cortical necrosis: case report and literature review. Front Neurol. (2021) 12:748034. doi: 10.3389/fneur.2021.748034
11.
Kavinsky CJ Szerlip M Goldsweig AM Amin Z Boudoulas KD Carroll JD et al . SCAI guidelines for the management of patent foramen ovale. J Soc Cardiovasc Angiogr Interv. (2022) 1:100039. doi: 10.1016/j.jscai.2022.100039
12.
Olesen J. (2018). Headache classification Committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 3rd ed.38:1–211. doi: 10.1177/0333102417738202
13.
Fisher DC Fisher EA Budd JH Rosen SE Goldman ME . The incidence of patent foramen ovale in 1,000 consecutive patients. A contrast transesophageal echocardiography study. Chest. (1995) 107:1504–9. doi: 10.1378/chest.107.6.1504
14.
Kumar P Kijima Y West BH Tobis JM . The connection between patent foramen ovale and migraine. Neuroimaging Clin N Am. (2019) 29:261–70. doi: 10.1016/j.nic.2019.01.006
15.
Takagi H Umemoto T . ALICE (all-literature investigation of cardiovascular evidence) group. A meta-analysis of case-control studies of the association of migraine and patent foramen ovale. J Cardiol. (2016) 67:493–503. doi: 10.1016/j.jjcc.2015.09.016
16.
Cao W Shen Y Zhong J Chen Z Wang N Yang J . The patent foramen ovale and migraine: associated mechanisms and perspectives from MRI evidence. Brain Sci. (2022):12. doi: 10.3390/brainsci12070941
17.
Charles AC Baca SM . Cortical spreading depression and migraine. Nat Rev Neurol. (2013) 9:637–44. doi: 10.1038/nrneurol.2013.192
18.
de Boer I Terwindt GM van den Maagdenberg AMJM . Genetics of migraine aura: an update. J Headache Pain. (2020) 21:64. doi: 10.1186/s10194-020-01125-2
19.
Sutherland HG Albury CL Griffiths LR . Advances in genetics of migraine. J Headache Pain. (2019) 20:72. doi: 10.1186/s10194-019-1017-9
20.
Kato Y Kobayashi T Ishido H Hayashi T Furuya D Tanahashi N . Migraine attacks after transcatheter closure of atrial septal defect. Cephalalgia. (2013) 33:1229–37. doi: 10.1177/0333102413490350
21.
Yankovsky AE Kuritzky A . Transformation into daily migraine with aura following transcutaneous atrial septal defect closure. Headache. (2003) 43:496–8. doi: 10.1046/j.1526-4610.2003.03096.x
22.
Riederer F Kaya M Christina P Harald G Peter W . Migraine with aura related to closure of atrial septal defects. Headache. (2005) 45:953–6. doi: 10.1111/j.1526-4610.2005.05166_2.x
23.
Chuang YM Chan L Wu HM Lee SP Chu YT . The clinical relevance of vertebral artery hypoplasia. Acta Neurol Taiwanica. (2012) 21:1–7. PMID:
24.
Förster A Wenz H Kerl HU Brockmann MA Groden C . Perfusion patterns in migraine with aura. Cephalalgia. (2014) 34:870–6. doi: 10.1177/0333102414523339
25.
Marconi R De Fusco M Aridon P Plewnia K Rossi M Carapelli S et al . Familial hemiplegic migraine type 2 is linked to 0.9-Mb region on chromosome 1q23. Ann Neurol. (2003) 53:376–81. doi: 10.1002/ana.10464
26.
Jurkat-Rott K Freilinger T Dreier JP Herzog J Göbel H Petzold GC et al . Variability of familial hemiplegic migraine with novel A1A2 Na+/K+-ATPase variants. Neurology. (2004) 62:1857–61. doi: 10.1212/01.WNL.0000127310.11526.FD
27.
Toldo I Brunello F Morao V Perissinotto E Valeriani M Pruna D et al . First attack and clinical presentation of hemiplegic migraine in pediatric age: a multicenter retrospective study and literature review. Front Neurol. (2019) 10:1079. doi: 10.3389/fneur.2019.01079
28.
Pelzer N Haan J Stam AH Vijfhuizen LS Koelewijn SC Smagge A et al . Clinical spectrum of hemiplegic migraine and chances of finding a pathogenic mutation. Neurology. (2018) 90:e575–82. doi: 10.1212/WNL.0000000000004966
29.
Toldo I Cecchin D Sartori S Calderone M Mardari R Cattelan F et al . Multimodal neuroimaging in a child with sporadic hemiplegic migraine: a contribution to understanding pathogenesis. Cephalalgia. (2011) 31:751–6. doi: 10.1177/0333102410392068
30.
Dowson A Mullen MJ Peatfield R Muir K Khan AA Wells C et al . Migraine intervention with STARFlex technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation. (2008) 117:1397–404. doi: 10.1161/CIRCULATIONAHA.107.727271
31.
Mattle HP Evers S Hildick-Smith D Becker WJ Baumgartner H Chataway J et al . Percutaneous closure of patent foramen ovale in migraine with aura, a randomized controlled trial. Eur Heart J. (2016) 37:2029–36. doi: 10.1093/eurheartj/ehw027
32.
Stone GW Adams DH Abraham WT Kappetein AP Généreux P Vranckx P et al . Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the mitral valve academic research consortium. J Am Coll Cardiol. (2015) 66:308–21. doi: 10.1016/j.jacc.2015.05.049
33.
Elbadawi A Barssoum K Abuzaid AS Rezq A Biniwale N Alotaki E et al . Meta-analysis of randomized trials on percutaneous patent foramen ovale closure for prevention of migraine. Acta Cardiol. (2019) 74:124–9. doi: 10.1080/00015385.2018.1475027
34.
Wang YL Wang FZ Zhang Y Jiang J Jia Z Liu X et al . Association of migraine with patent foramen ovale closure: a systematic review and meta-analysis. Int J Cardiol Heart Vasc. (2022) 39:100992. doi: 10.1016/j.ijcha.2022.100992
35.
Zhang Y Wang H Liu L . Patent foramen ovale closure for treating migraine: a meta-analysis. J Interv Cardiol. (2022) 2022:6456272. doi: 10.1155/2022/6456272
36.
Mojadidi MK Kumar P Mahmoud AN Elgendy IY Shapiro H West B et al . Pooled analysis of PFO occluder device trials in patients with PFO and migraine. J Am Coll Cardiol. (2021) 77:667–76. doi: 10.1016/j.jacc.2020.11.068
37.
Tariq N Tepper SJ Kriegler JS . Patent foramen ovale and migraine: closing the debate--a review. Headache. (2016) 56:462–78. doi: 10.1111/head.12779
38.
Meier B Meier B . Stroke and migraine: a cardiologist’s headache. Heart. (2009) 95:595–602. doi: 10.1136/hrt.2008.154963
Summary
Keywords
sporadic hemiplegic migraine, whole-exome sequencing, aura symptoms, patent foramen ovale, headache
Citation
Wang P, Yao F, Zhang H, Yu Q and Wang Y (2023) Disappearance of aura symptoms in patients with hemiplegic migraine after patent foramen ovale closure: a case report and literature review. Front. Neurol. 14:1267100. doi: 10.3389/fneur.2023.1267100
Received
26 July 2023
Accepted
15 September 2023
Published
12 October 2023
Volume
14 - 2023
Edited by
Simona Sacco, University of L'Aquila, Italy
Reviewed by
Marco Russo, IRCCS Local Health Authority of Reggio Emilia, Italy; Tao Qiu, Zigong First People's Hospital, China; Simiao Wu, Sichuan University, China
Updates
Copyright
© 2023 Wang, Yao, Zhang, Yu and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Wang, 17340085006@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.