Abstract
Background and purpose:
The efficacy and safety of endovascular treatment (EVT) in acute basilar artery occlusion (ABAO) has been confirmed by four randomized clinical trials. Nevertheless, the predictors of a 90-day favorable outcome after EVT have not been elucidated. We attempted to establish a nomogram for the prediction of a 90-day favorable outcome in ABAO patients with EVT.
Methods:
Clinical data of ABAO patients with EVT were obtained from two nationwide clinical trial registries in China. Factors associated with a 90-day favorable outcome were screened by multivariable step-wise regression on the basis of univariable analysis. A nomogram was established to predict 90-day favorable outcome after EVT.
Results:
The proportion of ABAO patients with a favorable outcome was 41.53% (157/378). Seven variables, including baseline National Institutes of Health Stroke Scale (NIHSS) <20 [odds ratio (OR): 8.330; P-value < 0.0001], posterior circulation Alberta Stroke Program Early CT (pc-ASPECT) score ≥7 (OR: 1.948; P-value = 0.0296), Pons-Midbrain Index (PMI) score < 2 (OR: 2.108; P-value = 0.0128), Posterior Circulation Collateral Score (PC-CS) ≥5 (OR: 3.288; P-value < 0.0001), local anesthesia (OR: 0.389; P-value = 0.0017), time from onset to recanalization (OTR) <330 min (OR: 2.594; P-value = 0.0013), and no occurrence of early neurological deterioration (END; OR: 0.039; P-value < 0.0001) were included into the nomogram, with C-index values of 0.8730 and 0.8857 in the training and the internal validation set, respectively.
Conclusions:
The proposed nomogram provided a reliable prognostic scale, which can be employed in clinical settings for the selection and clinical management of ABAO patients.
Registration:
https://www.clinicaltrials.gov, identifier: NCT03370939.
1. Introduction
The clinical benefits of endovascular treatment (EVT) for acute ischemic stroke (AIS) patients with intracranial large artery occlusions (LVOs) in the anterior circulation, whether within or beyond the time window, have been confirmed in seven randomized clinical trials (RCTs) (1–7). The previous Basilar Artery Occlusion Endovascular Intervention Versus Standard Medical Treatment (BEST) (8) and Basilar Artery International Cooperation Study (BASICS) (9) clinical trials failed to demonstrate the superior effectiveness of EVT over standard medical therapies in acute basilar artery occlusion (ABAO). Nevertheless, these two randomized trials confirmed the safety of EVT in ABAO. The subsequent Basilar Artery Occlusion Chinese Endovascular Trial (BAOCHE) (10) and the Endovascular Treatment for Acute Basilar Artery Occlusion (ATTENTION) (11) randomized clinical trials decisively demonstrated the beneficial effects of EVT in ABAO patients within 24 h of symptom onset, of which, the rate of favorable outcome is consistent with previous observations in patients with anterior circulation large vessel occlusion (AC-LVO) ischemic stroke. Compared to the insufficient imaging-related parameters in patient selection for EVT in anterior circulation strokes, a more rigorous imaging selection of patients was employed in EVT on ABAO patients, which might contribute to the added benefit of EVT in posterior circulation strokes. Besides the imaging parameters such as the posterior circulation Acute Stroke Prognosis Early CT Scores (pc-ASPECTS) and collateral status, the occlusion site, the time period between the onset and the treatment, and the severity of the initial infarction were also identified to be the predictors of the outcomes of EVT for ABAO patients (12–17). However, most of the studies that included these parameters to predict the functional outcome of EVT were obtained from small samples or retrospective studies (18–20). Being a simple statistical visual tool, the nomogram has been used to predict the incidence of complication, prognostic recovery and mortality after EVT of AC-LVO (21–23). Nevertheless, nomograms for predicting clinically favorable outcomes of EVT in patients with posterior circulation large vessel occlusion (PC-LVO) are scarce.
In this study, we established a nomogram to identify the preprocedural and peri-procedural factors for EVT outcome in ABAO patients that enable the prediction of functional outcome and assist clinicians in patient selection and clinical management.
2. Materials and methods
2.1. Data availability
For purposes of replicating the procedure or reproducing the results, data are available to researchers upon request, by directly contacting the corresponding author.
2.2. Study design and population
This study is a retrospective analysis of the prospective data from the Acute Ischemic Stroke Cooperation Group of Endovascular Treatment (ANGEL) (24) and the Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke (ANGEL-ACT) (25) clinical trial registries. Details regarding the design of these clinical trial studies, the criteria for patient inclusion/exclusion, and standards for data collection, have been discussed in previous publications. The ethics committees of all participating centers approved these study protocols, and written informed consent was provided by the study participants or their legal representatives. This study was performed in compliance with the 1964 Declaration of Helsinki and its later amendments. Of the 2,710 patients included in these registries, 2,299 were excluded from the present analysis, due to anterior circulation occlusion (n = 2,071), vertebral artery occlusion (n = 174), or posterior cerebral artery occlusion (n = 54). The remaining 411 ABAO patients were further excluded if they have not undergone angiographic collateral assessment (n = 25), or a 90-day follow-up stage (n = 8). In total, 378 patients were retained in the cohort for subsequent analyses.
2.3. Data collection
The prospectively collected variables included age, sex, baseline modified Rankin Scale (mRS) score, baseline National Institutes of Health Stroke Scale (NIHSS) score, intravenous thrombolysis, mechanical thrombectomy (MT) procedure details, and follow-up outcomes. Neuroimaging variables included the posterior circulation Alberta Stroke Program Early CT Score [pc-ASPECTS] (26), the posterior circulation collateral score (PC-CS) (27), and the Basilar Artery on Computed Tomography Angiography (BATMAN) score (28).
2.4. Outcome definitions
Functional outcomes in this study included a favorable outcome [modified Rankin Scale (mRS) of 0–2] and patient mortality within 90 days. Early neurological deterioration (END) was defined as an increase of the NIHSS score of ≥4 points compared to the baseline NIHSS, or if the patient died within 24 h after EVT. Successful recanalization was defined as a modified thrombolysis in a cerebral infarction grade (mTICI) score of 2b or 3, while futile recanalization was defined as 90-day mRS score of 3–6, despite a technically successful recanalization (mTICI score of 2b or 3). Symptomatic intracranial hemorrhage (sICH) was defined as a hemorrhage detected by CT/MRI, with an increase of ≥4 points or an increase of ≥2 points of the NIHSS score in any NIHSS domain compared with the immediate prehemorrhage neurological status, or the need for surgical treatment according to Heidelberg Bleeding Classification criteria (29). Trained investigators, who were blinded to patient baseline conditions, conducted 90-day follow-up outcome assessments via telephone interviews, using a standard interview protocol.
2.5. Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 4.2.1 software (http://www.R-project.org, foundation for statistical computing, Vienna, Austria). For continuous variables, data are presented as the median and interquartile ranges (IQRs). For categorical variables, data are presented as frequency and percentage. The optimal cutoff value for each continuous variable in predicting the probability of favorable outcome after EVT in ABAO patients was calculated by a receiver operating characteristics (ROC) curve, in which all continuous variables were converted into categorical variables. Univariable logistic regression analysis was performed to compare variables in the framework of demographic, medical history, etiology, imaging parameters, procedural parameters, times and incidence of complication, between the favorable outcome and the unfavorable outcome groups. To test for collinearity between predictors of the multivariable logistic model, the variance inflation factor (VIF) of each predictor was calculated. No predictors with a VIF >5 were included in the final regression model (30). All statistical tests were two-tailed and a P-value < 0.05 indicated a statistically significant difference, and variables with a P-value < 0.05 in the univariable analysis were included into a step-wise regression model. Furthermore, a nomogram was constructed for the prediction of a 90-day favorable outcome in ABAO patients undergoing EVT, by assigning a preliminary score to each predictor, ranging from 0 to 100 points. The total scores were obtained by summing the individual scores of each predictor. Then an internal validation step was performed by bootstrap resampling with 1,000 replications, according to an 8:2 ratio from the whole training set (302 cases). The discriminative performance of the nomogram model was assessed by a concordance index value, with values ranging between 0.5 (for a noninformative model) and 1 (for a perfectly discriminating model). The calibration plot was used to evaluate the degree of fit between the actual and the nomogram-predicted favorable outcomes. Decision curve analysis was used to discriminate the net benefit and the probability of a favorable outcome at 90 days, for ABAO patients undergoing EVT.
3. Results
3.1. General characteristics of the participants
In the training set, a total of 115 patients in the ANGEL and 296 patients in the ANGEL-ACT registries were included. Thirty-three patients were excluded for missing collateral assessment data (n = 25), or due to the absence of follow-up information (n = 8). Finally, 378 basilar artery occlusion patients who underwent EVT were included in the final analyses (Figure 1). The mean age of the ABAO patients who underwent EVT was 62 years (IQR: 55–68), and 81.75% (309/378) of the included patients were male. The median baseline NIHSS score was 20 (IQR: 10–33), the median pc-ASPECT score was 7 (IQR: 6–8), the median PMI was 2 (IQR: 0–3), the median PC-CS score was 4 (IQR: 3–6), and the median BATMAN score was 4 (IQR: 4–6). The median time from symptom onset to puncture was 360 min (IQR: 240–540). 22.22% (84/378) patients received intravenous recombinant tissue plasminogen activator (IV-rtPA). The rate of successful recanalization was 85.71% (324/378), of which 56.17% (182/324) of the patients still suffered from futile recanalization. Concerning complications within 24 h, END was observed in 12.96% (49/378) ABAO patients post-EVT, ICH occurred in 14.78% (55/378) ABAO patients, and 4.05% (15/378) patients developed sICH after EVT. The 90-day mortality rate was 20.90% (79/378). A favorable outcome was found in 41.53% (157/378) of ABAO patients during the 90 days follow-up. The general characteristics and procedure parameters are listed in Supplementary Table 1. The modified Rankin Scale (mRS) scores distribution of patients with successful recanalization vs. un-successful recanalization, the END vs. no-END, and the ICH vs. no-ICH were compared and are shown in Figure 2.
Figure 1

Flow chart of eligible ABAO patients with EVT, obtained from the ANGEL and ANGEL-ACT clinical trial registries. ABAO, acute basilar artery occlusion; EVT, endovascular treatment; ANGEL, Acute Ischemic Stroke Cooperation Group of Endovascular Treatment; ANGEL-ACT, Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke.
Figure 2

The distribution of the modified Rankin Scale (mRS) of patients with SR vs. Un-SR; END vs. no-END; and ICH vs. no-ICH. The mRS scores ranged from 0 to 6, with the higher scores indicating a more severe patient disability. SR, successful recanalization; Un-SR, un-successful recanalization; END, early neurological deterioration; ICH, intracerebral hemorrhage.
3.2. Predictors of a 90-day favorable outcome in ABAO patients undergoing endovascular treatment
The baseline characteristics of the ABAO patients in the training and in the internal validation set, grouped by 90-day favorable outcome (mRS 0–2 vs. 3–6), are listed in Table 1. The continuous variables were converted into dichotomized variables by the optimal cutoff identified by receiver operating curve analysis in differentiating the favorable outcome. The detailed information is listed in Supplementary Table 2. By using a univariable analysis, the baseline NIHSS score, four neuroimaging variables including pc-ASPECT, PMI, PC-CS, and BATMAN, the anesthesia method, the final mTICI, OTP, END, and 24 h ICH were identified as potential predictors (P-values < 0.05). After screening by step-wise logistic regression method, a baseline NIHSS < 20 [OR: 8.330 (95% CI: 4.661–14.888); P-value < 0.0001], PC-ASPECT score ≥7 [OR: 1.948 (95% CI: 1.068–3.554); P-value = 0.0296], PMI score < 2 [OR: 2.108 (95% CI: 1.172–3.792); P-value = 0.0128], PC-CS score ≥5 [OR: 3.288 (95% CI: 1.876–5.760); P-value < 0.0001], local anesthesia method [OR: 0.389 (95% CI: 0.215–0.701); P-value = 0.0017], time from symptom onset to puncture < 330 min [OR: 2.594 (95% CI: 1.453–4.629); P-value = 0.0013], and no occurrence of END [OR: 0.039 (95% CI: 0.011–0.142); P-value < 0.0001] were associated with the 90-day favorable outcome in ABAO patients with EVT (Table 2).
Table 1
| Variables | Statistics | Training set ( n = 378) | Internal validation set ( n = 302) | ||
|---|---|---|---|---|---|
| mRS ≤ 2 (n = 157) | mRS ≥3 (n = 221) | mRS ≤ 2 (n = 131) | mRS ≥3 (n = 171) | ||
| Demographics | |||||
| Age | Median, IQR | 62 IQR (54~68) | 62 IQR (55~69) | 61 IQR (52~67) | 63 IQR (56~69) |
| ≥68 | 26.11% (41/157) | 29.41% (65/221) | 24.43% (32/131) | 30.41% (52/171) | |
| < 68 | 73.89% (116/157) | 70.59% (156/221) | 75.57% (99/131) | 69.59% (119/171) | |
| P-value | 0.5614 | 0.3001 | |||
| Gender | Male | 82.80% (130/157) | 81.00% (179/221) | 84.73% (111/131) | 78.36% (134/171) |
| Female | 17.20% (27/157) | 19.00% (42/221) | 15.27% (20/131) | 21.64% (37/171) | |
| P-value | 0.6871 | 0.1831 | |||
| Medical history | |||||
| Smoking habits | Yes | 38.22% (60/157) | 43.44% (96/221) | 42.75% (56/131) | 38.01% (65/171) |
| No | 42.68% (67/157) | 37.10% (82/221) | 38.93% (51/131) | 42.69% (73/171) | |
| Quit | 19.11% (30/157) | 19.46% (43/221) | 18.32% (24/131) | 19.30% (33/171) | |
| P-value | 0.513 | 0.7021 | |||
| Hypertension | Yes | 71.34% (112/157) | 71.49% (158/221) | 73.28% (96/131) | 72.51% (124/171) |
| No | 28.66% (45/157) | 28.51% (63/221) | 26.72% (35/131) | 27.49% (47/171) | |
| P-value | 1.0000 | 0.8969 | |||
| Diabetes mellitus | Yes | 23.57% (37/157) | 26.70% (59/221) | 24.43% (32/131) | 23.98% (41/171) |
| No | 76.43% (120/157) | 73.30% (162/221) | 75.57% (99/131) | 76.02% (130/171) | |
| P-value | 0.5493 | 1.0000 | |||
| Hyperlipemia | Yes | 17.83% (28/157) | 14.03% (31/221) | 17.56% (23/131) | 14.04% (24/171) |
| No | 82.17% (129/157) | 85.97% (190/221) | 82.44% (108/131) | 85.96% (147/171) | |
| P-value | 0.3184 | 0.4264 | |||
| Coronary heart disease | Yes | 9.55% (15/157) | 13.12% (29/221) | 10.69% (14/131) | 13.45% (23/171) |
| No | 90.45% (142/157) | 86.88% (192/221) | 89.31% (117/131) | 86.55% (148/171) | |
| P-value | 0.3307 | 0.4858 | |||
| Atrial fibrillation | Yes | 9.55% (15/157) | 9.05% (20/221) | 9.16% (12/131) | 9.94% (17/171) |
| No | 90.45% (142/157) | 90.95% (201/221) | 90.84% (119/131) | 90.06% (154/171) | |
| P-value | 0.8593 | 0.8468 | |||
| Previous stroke | Yes | 23.57% (37/157) | 24.43% (54/221) | 23.66% (31/131) | 22.81% (39/171) |
| No | 76.43% (120/157) | 75.57% (167/221) | 76.34% (100/131) | 77.19% (132/171) | |
| P-value | 0.9031 | 0.8911 | |||
| Pre mRS score | =0 | 90.45% (142/157) | 90.05% (199/221) | 9.92% (13/131) | 8.19% (14/171) |
| ≥1 | 9.55% (15/157) | 9.95% (22/221) | 90.08% (118/131) | 91.81% (157/171) | |
| P-value | 1.0000 | 0.6853 | |||
| Pre-IVT | Yes | 24.84% (39/157) | 20.36% (45/221) | 23.66% (31/131) | 21.05% (36/171) |
| No | 75.16% (118/157) | 79.64% (176/221) | 76.34% (100/131) | 78.95% (135/171) | |
| P-value | 0.3172 | 0.6753 | |||
| Etiology (TOAST) | |||||
| LAA | 75.80% (119/157) | 73.30% (162/221) | 77.10% (101/131) | 73.10% (125/171) | |
| CE | 19.11% (30/157) | 16.29% (36/221) | 18.32% (24/131) | 16.37% (28/171) | |
| SOE | 2.55% (4/157) | 3.62% (8/221) | 2.29% (3/131) | 3.51% (6/171) | |
| SUE | 2.55% (4/157) | 6.79% (15/221) | 2.29% (3/131) | 7.02% (12/171) | |
| P-value | 0.2208 | 0.2261 | |||
| SBP | Median, IQR | 157 IQR (140~175) | 152 IQR (140~170) | 158 IQR (142~175) | 152 IQR (40~170) |
| ≥155 | 56.05% (88/157) | 46.61% (103/221) | 58.78% (77/131) | 45.61% (78/171) | |
| < 155 | 43.95% (69/157) | 53.39% (118/221) | 41.22% (54/131) | 54.39% (93/171) | |
| P-value | 0.0765 | 0.0274 | |||
| DBP | Median, IQR | 89 IQR (80~100) | 90 IQR (80~97) | 89 IQR (80~100) | 89 IQR (80~95) |
| ≥84 | 68.15% (107/157) | 60.73% (133/219) | 69.47% (91/131) | 59.17% (100/169) | |
| < 84 | 31.85% (50/157) | 39.27% (86/219) | 30.53% (40/131) | 40.83% (69/169) | |
| P-value | 0.1575 | 0.0706 | |||
| Baseline NIHSS | Median, IQR | 12 IQR (6~20) | 28 IQR (16~35) | 12 IQR (6~21) | 29 IQR (16~35) |
| ≥20 | 29.30% (46/157) | 68.78% (152/221) | 29.01% (38/131) | 69.59% (119/171) | |
| < 20 | 70.70% (111/157) | 31.22% (69/221) | 70.99% (93/131) | 30.41% (52/171) | |
| P-value | < 0.0001 | < 0.0001 | |||
| Neuroimaging variables | |||||
| PC-ASPECT | Median, IQR | 8 IQR (7~9) | 6 IQR (5~8) | 8 IQR (7~9) | 6 IQR (5~8) |
| ≥7 | 75.80% (119/157) | 49.32% (109/221) | 75.57% (99/131) | 49.71% (85/171) | |
| < 7 | 24.20% (38/157) | 50.68% (112/221) | 24.43% (32/131) | 50.29% (86/171) | |
| P-value | < 0.0001 | < 0.0001 | |||
| PMI | Median, IQR | 1 IQR (0~2) | 2 IQR (1~4) | 1 IQR (0~2) | 2 IQR (0~4) |
| ≥2 | 46.50% (73/157) | 70.59% (156/221) | 45.04% (59/131) | 67.84% (116/171) | |
| < 2 | 53.50% (84/157) | 29.41% (65/221) | 54.96% (72/131) | 32.16% (55/171) | |
| P-value | < 0.0001 | < 0.0001 | |||
| PC-CS | Median, IQR | 5 IQR (4~7) | 4 IQR (3~5) | 6 IQR (4~7) | 4 IQR (3~5) |
| ≥5 | 64.97% (102/157) | 33.94% (75/221) | 66.41% (87/131) | 34.50% (59/171) | |
| < 5 | 35.03% (55/157) | 66.06% (146/221) | 33.59% (44/131) | 65.50% (112/171) | |
| P-value | < 0.0001 | < 0.0001 | |||
| BATMAN | Median, IQR | 5 IQR (3~7) | 4 IQR (2~5) | 5 IQR (3~7) | 4 IQR (2~5) |
| ≥6 | 40.76% (64/157) | 19.91% (44/221) | 43.51% (57/131) | 21.05% (36/171) | |
| < 6 | 59.24% (93/157) | 80.09% (177/221) | 56.49% (74/131) | 78.95% (135/171) | |
| P-value | < 0.0001 | < 0.0001 | |||
| Procedures | |||||
| Anesthesia | GA | 56.69% (89/157) | 76.02% (168/221) | 55.73% (73/131) | 74.85% (128/171) |
| Local | 43.31% (68/157) | 23.98% (53/221) | 44.27% (58/131) | 25.15% (43/171) | |
| P-value | < 0.0001 | 0.0006 | |||
| Heparin | Yes | 43.31% (68/157) | 46.61% (103/221) | 45.04% (59/131) | 44.44% (76/171) |
| No | 56.69% (89/157) | 53.39% (118/221) | 54.96% (72/131) | 55.56% (95/171) | |
| P-value | 0.5314 | 1.0000 | |||
| Antagonist (GP2b3a) | Yes | 70.06% (110/157) | 70.59% (156/221) | 71.76% (94/131) | 72.51% (124/171) |
| No | 29.94% (47/157) | 29.41% (65/221) | 28.24% (37/131) | 27.49% (47/171) | |
| P-value | 0.9096 | 0.8975 | |||
| Tandem | Yes | 10.19% (16/157) | 14.03% (31/221) | 8.40% (11/131) | 14.04% (24/171) |
| No | 89.81% (141/157) | 85.97% (190/221) | 91.6% (120/131) | 85.96% (147/171) | |
| P-value | 0.3427 | 0.1489 | |||
| Residual severe stenosis | Yes | 57.32% (90/157) | 59.73% (132/221) | 59.54% (78/131) | 60.82% (104/171) |
| No | 38.22% (60/157) | 37.10% (82/221) | 35.88% (47/131) | 35.67% (61/171) | |
| Unknown | 4.46% (7/157) | 3.17% (7/221) | 4.58% (6/131) | 3.51% (6/171) | |
| P-value | 0.7680 | 0.8899 | |||
| Numbers of thrombectomy | ≤ 3 | 94.90% (149/157) | 91.86% (203/221) | 95.42% (125/131) | 91.23% (156/171) |
| >3 | 5.10% (8/157) | 8.14% (18/221) | 4.58% (6/131) | 8.77% (15/171) | |
| P-value | 0.3049 | 0.1772 | |||
| Number of aspirations | 0 | 88.54% (139/157) | 92.76% (205/221) | 90.08% (118/131) | 92.98% (159/171) |
| 1 | 8.28% (13/157) | 4.98% (11/221) | 7.63% (10/131) | 4.09% (7/171) | |
| 2 | 1.91% (3/157) | 0.45% (1/221) | 2.29% (3/131) | 0.58% (1/171) | |
| 3 | 1.27% (2/157) | 0.45% (1/221) | 0.00% (0/131) | 0.58% (1/171) | |
| 4 | 0.00% (0/157) | 1.36% (3/221) | 0.00% (0/131) | 1.75% (3/171) | |
| P-value | 0.1089 | 0.0953 | |||
| Number of balloon dilatation | 0 | 63.69% (100/157) | 51.13% (113/221) | 64.12% (84/131) | 48.54% (83/171) |
| 1 | 31.21% (49/157) | 44.34% (98/221) | 30.53% (40/131) | 46.78% (80/171) | |
| ≥2 | 5.10% (8/157) | 4.52% (10/221) | 5.34% (7/131) | 4.68% (8/171) | |
| P-value | 0.0337 | 0.0154 | |||
| Number of stent implantation | 0 | 61.15% (96/157) | 55.66% (123/221) | 58.78% (77/131) | 54.39% (93/171) |
| 1 | 36.31% (57/157) | 42.53% (94/221) | 38.17% (50/131) | 45.03% (77/171) | |
| 2 | 2.55% (4/157) | 0.90% (2/221) | 3.05% (4/131) | 0.00% (0/171) | |
| 3 | 0.00% (0/157) | 0.90% (2/221) | 0.00% (0/131) | 0.58% (1/171) | |
| P-value | 0.1674 | 0.0305 | |||
| Number of IA thrombolysis | 0 | 83.44% (131/157) | 82.81% (183/221) | 86.26% (113/131) | 81.87% (140/171) |
| 1 | 15.92% (25/157) | 16.74% (37/221) | 12.98% (17/131) | 18.13% (31/171) | |
| 2 | 0.64% (1/157) | 0.45% (1/221) | 0.76% (1/131) | 0.00% (0/171) | |
| P-value | 0.9511 | 0.2119 | |||
| Final mTICI | 0 (0-2a) | 9.55% (15/157) | 17.65% (39/221) | 7.63% (10/131) | 16.96% (29/171) |
| 1 (2b-c) | 90.45% (142/157) | 82.35% (182/221) | 92.37% (121/131) | 83.04% (142/171) | |
| P-value | 0.0360 | 0.0234 | |||
| Remote embolization | Yes | 3.18% (5/157) | 7.69% (17/221) | 3.05% (4/131) | 8.19% (14/171) |
| No | 96.82% (152/157) | 92.31% (204/221) | 96.95% (127/131) | 91.81% (157/171) | |
| P-value | 0.0760 | 0.0849 | |||
| Dissection | Yes | 1.91% (3/157) | 3.62% (8/221) | 2.29% (3/131) | 3.51% (6/171) |
| No | 98.09% (154/157) | 96.38% (213/221) | 97.71% (128/131) | 96.49% (165/171) | |
| P-value | 0.3737 | 0.7364 | |||
| Times | |||||
| OTA | Median, IQR | 181 IQR (62~344) | 261 IQR (120~427) | 181 IQR (80~357) | 270 IQR (130~437) |
| ≥314 | 26.11% (41/157) | 40.27% (89/221) | 25.95% (34/131) | 39.77% (68/171) | |
| < 314 | 73.89% (116/157) | 59.73% (132/221) | 74.05% (97/131) | 60.23% (103/171) | |
| P-value | 0.0044 | 0.0140 | |||
| OTP | Median, IQR | 310 IQR (208~495) | 405 IQR (270~560) | 330 IQR (194~495) | 390 IQR (258~540) |
| ≥330 | 49.68% (78/157) | 68.78% (152/221) | 50.38% (66/131) | 68.42% (117/171) | |
| < 330 | 50.32% (79/157) | 31.22% (69/221) | 49.62% (65/131) | 31.58% (54/171) | |
| P-value | 0.0003 | 0.0020 | |||
| PTR | Median, IQR | 91 IQR (60~120) | 111 IQR (60~156) | 91 IQR (60~120) | 111 IQR (60~154) |
| ≥139 | 17.20% (27/157) | 31.67% (70/221) | 16.03% (21/131) | 30.41% (52/171) | |
| < 139 | 82.80% (130/157) | 68.33% (151/221) | 83.97% (110/131) | 69.59% (119/171) | |
| P-value | 0.0018 | 0.0043 | |||
| Complications | |||||
| END | Yes | 1.91% (3/157) | 20.81% (46/221) | 0.76% (1/131) | 19.88% (34/171) |
| No | 98.09% (154/157) | 79.19% (175/221) | 99.24% (130/131) | 80.12% (137/171) | |
| P-value | < 0.0001 | < 0.0001 | |||
| 24ICH | Yes | 10.19% (16/157) | 18.14% (39/215) | 11.45% (15/131) | 21.08% (35/166) |
| No | 89.81% (141/157) | 81.86% (176/215) | 88.55% (116/131) | 78.92% (131/166) | |
| P-value | 0.0383 | 0.0295 | |||
| 24h sICH | Yes | 2.56% (4/156) | 5.14% (11/214) | 2.31% (3/130) | 6.02% (10/166) |
| No | 97.44% (152/156) | 94.86% (203/214) | 97.69% (127/130) | 93.98% (156/166) | |
| P-value | 0.2887 | 0.1571 | |||
Univariable analysis of the factor associated with favorable outcome (90D mRS ≤ 2) in acute basilar artery occlusion (ABAO) patients undergoing endovascular treatment (EVT).
LAA, large-artery atherosclerosis; CE, cardioembolic; SOE, stroke of other determined etiology; SUE, stroke of underdetermined etiology; SBP, systolic blood pressure; DBP, diastolic blood pressure; NIHSS, National Institute of Health Stroke Scale; mRS, modified Rankin scale; PC-ASPECT, posterior circulation Alberta Stroke Program Early CT Score; PMI, Pons-Midbrain Index; PC-CS, the posterior circulation collateral score; BATMAN, the Basilar Artery on Computed Tomography Angiography; mTICI, modified thrombolysis in cerebral infarction grade; OTA, onset to admission time; OTP, onset to puncture time; END, early neurological deterioration; PTR, puncture to reperfusion time; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage.
Table 2
| Variables | Training set (90D mRS of 0–2, n = 157) | Validation set (90D mRS of 0–2, n = 131) | ||
|---|---|---|---|---|
| OR (95% CI) | P -value | OR (95% CI) | P -value | |
| Baseline NIHSS (< 20) | 8.330 (4.661–14.888) | < 0.0001 | 10.736 (5.568–20.701) | < 0.0001 |
| PC-ASPECT (≥7) | 1.948 (1.068–3.554) | 0.0296 | ||
| PMI (< 2) | 2.108 (1.172–3.792) | 0.0128 | 2.656 (1.410–5.004) | 0.0025 |
| PC-CS (≥5) | 3.288 (1.876–5.760) | < 0.0001 | 3.879 (2.044–7.364) | < 0.0001 |
| Anesthesia method | 0.389 (0.215–0.701) | 0.0017 | 0.368 (0.190–0.712) | 0.0030 |
| OTP (< 330 min) | 2.594 (1.453–4.629) | 0.0013 | 2.828 (1.472–5.435) | 0.0018 |
| END | 0.039 (0.011–0.142) | < 0.0001 | 0.013 (0.002–0.110) | < 0.0001 |
Multivariate analysis of the predictors of 90D favorable outcome in acute basilar artery occlusion (ABAO) patients undergoing endovascular treatment (EVT).
NIHSS, National Institute of Health Stroke Scale; PC-ASPECT, posterior circulation Alberta Stroke Program Early CT Score; PMI, Pons-Midbrain Index; PC-CS, the posterior circulation collateral score; OTP, onset to puncture time; END, early neurological deterioration.
3.3. Construction of a nomogram for the prediction of a 90-day favorable outcome in ABAO patients undergoing EVT and validation of its performance
Based on the multivariable logistic regression model, a nomogram was developed to predict the probability of a 90-day favorable outcome, by assigning each independent predictor with a score ranging from 0 to 100 points. The cumulative sum of the assigned points for each factor in the nomogram represented the probability of a 90-day favorable outcome and ranged from 0 to 350 points. The probability of a 90-day favorable outcome ranged between 0.1 and 0.9 (Figure 3A). In order to evaluate the performance of the prognostic nomogram model, we carried out an internal validation for the nomogram (n = 302). The results of the corresponding univariable and step-wise multivariable logistic regression analysis of the internal validation set are listed in Table 2. The performance of the nomogram in the training and in the internal validation sets was evaluated by C-index, with results of 0.873 (95% CI: 0.838–0.908) and 0.886 (95% CI: 0.849–0.923), respectively. A calibration plot was further adjusted to assess the comparison between key summary features of the predictive factor scores and the agreement of a 90-day favorable outcome, between nomogram predictions and actual observations. These scores revealed a good predictive accuracy, both in the training and in the internal validation sets (Figures 3B, C). Finally, decision curve analysis was performed to evaluate the net benefit of the nomogram model in predicting a 90-day favorable outcome in ABAO patients after EVT. Figures 4A, B showed that when the risk threshold ranged from 1 to 96% (in the training set), and from 5 to 97%, (in internal validation sets), respectively, using the nomogram model to predict a 90-day favorable outcome resulted in a greater benefit than either all or none of the ABAO patients undergoing EVT. For example, if the personal threshold probability of a patient was 50%, then the net benefit in this nomogram model was 53.5% (95% CI: 40.7%−62.7%) in the training set and 55.7% (95% CI: 44.5%−66.2%) in the internal validation sets, respectively.
Figure 3

Nomogram model for the prediction of a 90-day favorable outcome (mRS of 0–2) in ABAO patients after EVT. (A) The nomogram developed in the present study; (B) Calibration curve of the training set. (C) Calibration curve of the internal validation set. ABAO, acute basilar artery occlusion; EVT, endovascular treatment; NIHSS indicates national Institute of Health Stroke Scale; PC-ASPECT, posterior circulation Alberta Stroke Program Early CT Score; PMI, Pons-Midbrain Index; PC-CS, the posterior circulation collateral score; OTP, onset to puncture time; END, early neurological deterioration.
Figure 4
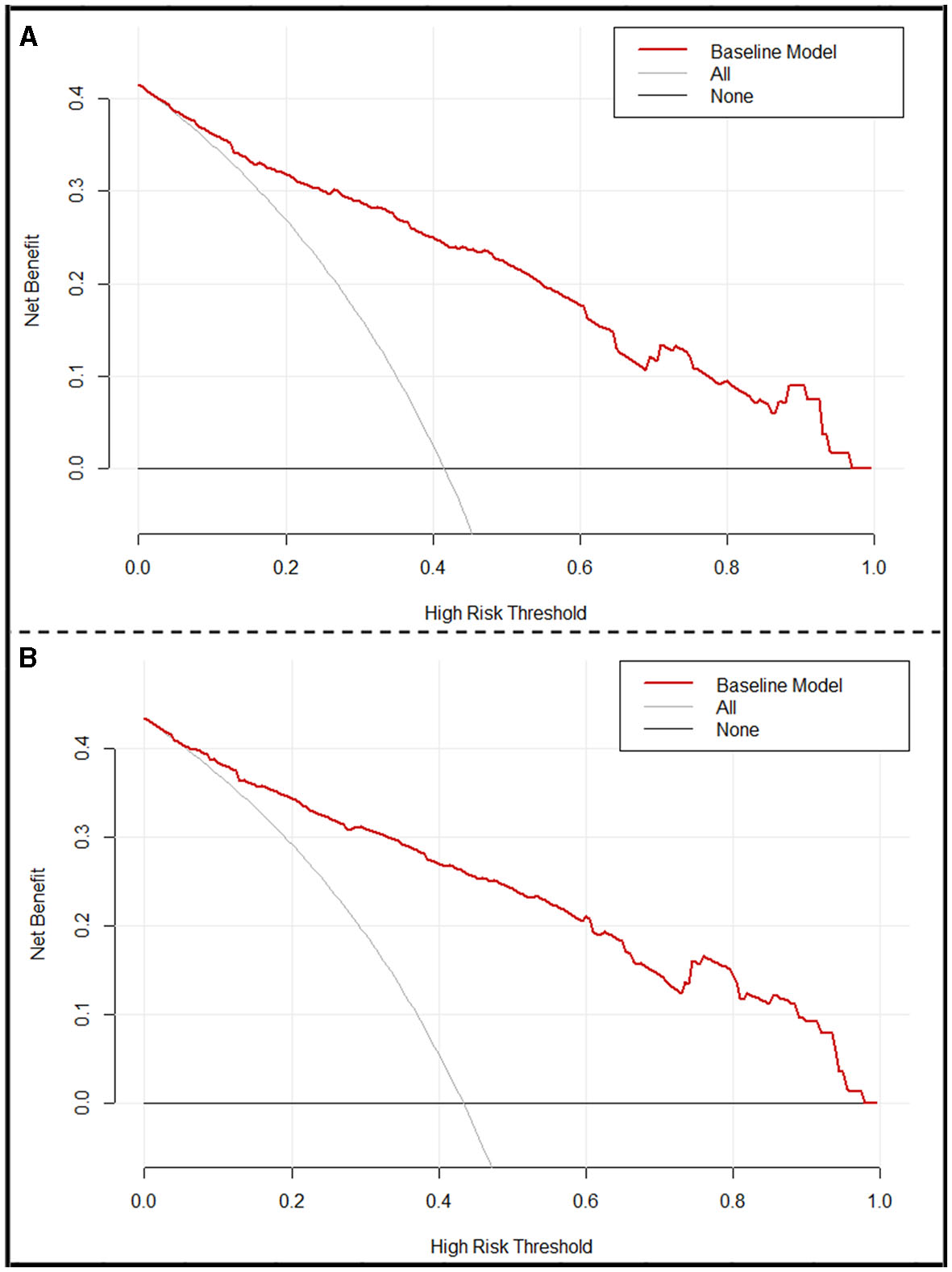
Decision curve analysis (DCA) of the nomogram. (A) DCA of the training set. (B) DCA of the internal validation set. x-axis, the threshold probability; y-axis, the net benefit. The gray line indicates that all ABAO patients undergoing EVT will achieve a favorable functional outcome 90 days after EVT. The black line indicates that no acute ischemic stroke patients undergoing EVT will obtain a favorable functional outcome 90 days after EVT. The red line corresponds to the nomogram to predict the 90-day favorable functional outcome in ABAO patients undergoing EVT. ABAO, acute basilar artery occlusion; EVT, endovascular treatment.
3.4. Translation of the nomogram model into clinical practice
Finally, we translated the predictive model into practice, by assigning the corresponding scores to each indicator, according to their contributions to the nomogram model, with 62 points for Baseline NIHSS scores < 20, 20 points for PC-ASPECT scores ≥7, 22.68 points for PMI scores < 2, 34.28 points for PC-CS scores ≥5, 52.57 points for the local anesthesia method and 25.97 points for OTP < 330 min, and 100 points for no-END. And the total scores of each patient were calculated as follows: total scores = (0 for NIHSS ≥20 or 1 for NIHSS < 20) × 62 + (0 for PC-ASPECT scores < 7 or 1 for PC-ASPECT scores ≥7) × 20 + (0 for PMI scores ≥2 or 1 for PMI scores < 2) × 22.68 + (0 for PC-CS scores < 5 or 1 for PC-CS scores ≥5) × 34.28 + (0 for general anesthesia method or 1 for local anesthesia) × 52.57 + (0 for OTP ≥330 min or 1 for OTP < 330 min) × 25.97 + (0 for END or 1 for no-END) × 100. The thresholds were 188.05 (in the training set) and 186.5 (in the internal validation set). Their sensitivities were 0.78344 (training set) and 0.82456 (internal validation set), and specificities were 0.82805 (training set) and 0.79389 (internal validation set). The probability of achieving functional independence was about 48%. The receiver operating curve and the scatter plot of the total scores of each patient in the training and in the internal validation sets are shown in Figures 5A, B.
Figure 5

Translation of the nomogram model into clinical practice. (A) Receiver operating characteristics (ROC) for identifying the optimal threshold (left) and the scatter plot of the total scores (right) in ABAO patients undergoing EVT, in the training set. (B) ROC for identifying the optimal threshold (left) and scatter plot of the total scores (right) in ABAO patients undergoing EVT, in the internal validation set. ABAO, acute basilar artery occlusion; EVT, endovascular treatment.
4. Discussion
The present study introduces a nomogram model, which incorporated with both pre- and peri-procedural parameters, including baseline NIHSS, PC-ASPECT, PMI, PC-CS, the anesthesia method, OTP, and END, and with a good performance of C-index of 0.873, to predict a favorable outcome at 90 days after EVT in patients with ABAO.
In this study, the successful reperfusion rate was 87.04% (329/378), which was slightly higher than the 71% rate in the HERMES (highly effective reperfusion evaluated in multiple endovascular stroke) (31) clinical trial, the 81% rate in the pooled 15 studies in a meta-analysis (32), and the 85% pooled rate in a meta-analysis (33) of four randomized clinical trials (RCTs), as well as the pooled 83% (95% CI: 81%−86%) rates of 50 studies that facilitated EVT on PC-LVO (Figure 6A) summarized in our study. The favorable outcome of this study was 41.5%, which was comparable to the 46% rate in the HEMERS analysis (31) and 42% reported by Gory et al. (32), and slightly higher than the pooled 35% rate in the meta-analysis (33) and the pooled 38% (95% CI: 36%−40%) rates of 52 studies (Figure 6B) summarized in our study. Given the relatively lower sICH rates of 4.05% and mortality rate of 20.9%, compared to those in the HERMES study (31), the meta-analysis of 15 studies (32) and the meta-analysis of four RCTs (33), our study also confirmed that EVT was safe for treating ABAO patients within 24 h of symptom onset.
Figure 6

Forest plot of pooled incidence of successful recanalization (A) and 90-day favorable outcome (mRS of 0–2) (B) with random-effect methods in PC-LVO patients underwent EVT. PC-LVO, posterior circulation large vessel occlusion; EVT, endovascular treatment.
The scoring system (34, 35) for predicting the prognosis and complications following EVT of AC-LVO have been extensively elucidated. Despite the benefit for MT reported by BOACHE (10) and ATTENTION (11) trials, the risk factors for functional outcome in ABAO patients after MT have not been elucidated. The POST-VB score (20) utilized post-procedure scans as the independent predictors of a 90-day favorable outcome, which did not specifically address patient selection. Besides, the generation of ASIAN KR Posterior Calculator (19) was substantially limited by its small sample size and the retrospective nature. To our knowledge, our model is the first predictive nomogram model incorporated with both pre- and peri-procedural factors for patient selection and clinical management of peri-operation in ABAO. Currently, the factors independently associated with a good outcome after EVT in PC-LVO (summarized in Supplementary Table 3) have considerably varied. Among which, baseline NIHSS was a well-recognized predictor associated with 90-day functional independence following the EVT of PC-LVO. Our study also found that a baseline NIHSS score < 20 was an independent predictor for a 90-day favorable outcome in ABAO patients, accounting for 17.7% (62/350) of total scores for the nomogram models. Rigorous imaging parameters have been confirmed to be an important selection criterion, which improved the substantial benefit of thrombectomy. Consistent with previous studies (13–15), we also identified that a PC-ASPECT score ≥7 was significantly correlated with a 90-day functional independence of ABAO patients who underwent EVT. The most recently published ATTENTION random trial (33) in the Chinese population has achieved an even higher successful recanalization rate of 93% in ABAO patients after EVT, which was largely attributed to the rigorous imaging selection of patients in the absence of a large baseline infarct. In addition to PC-ASPECT, our study found that patients with a PC-CS score ≥5 might achieve a more likely favorable outcome than those with a PC-CS score < 5 and a PMI score < 2 was an independent predictor of a favorable outcome in ABAO patients. Taken together, these findings strengthened the importance of imaging selection in predicting the prognosis of EVT in ABAO patients. The peri-procedural factors have been addressed in the studies of AC-LVO (21, 22), but few factors have been reported in PC-LVO studies. The higher incidence of END is often accompanied with a poor outcome, as well as by the increased disability and mortality following EVT in AC-LVO patients (36–40). Our study showed that no occurrences of END was associated with a favorable outcome in ABAO patients. Reducing END incidence is expected to ameliorate the prognosis. In agreement with the results from previous studies (19), we also found an association between the OTP and the clinical outcome in ABAO patients.
4.1. Limitation
The limitations of this study are as follows. Although an internal validation of the nomogram has been conducted in this study, a future external verification would be required to determine the generalizability of the model. In addition, the high proportion of intracranial atherosclerotic disease (ICAD) due to the different ethnicity of the population in this study and the low proportion of intravenous thrombolysis might affect the extrapolation of the study's findings. Nevertheless, there might be additional unknown factors that were not included in the model, which would require further extension studies.
5. Conclusion
Taken together, the proposed nomogram demonstrated a good discriminative performance in evaluating the probability of a 90-day favorable outcome for ABAO patients after EVT. This nomogram can be easily translated into practice, providing a reliable prognostic scale that can be employed in clinical settings, for the selection and clinical management of acute BAO patients.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Institutional Review Boards at Beijing Tiantan Hospital and at each trial site (KY2014-51-01 and 2017-048-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LL: Writing—original draft, Data curation, Investigation. JL: Writing—original draft, Conceptualization, Methodology, Writing—review and editing. J-jH: Conceptualization, Investigation, Writing—original draft. YG: Methodology, Writing—review and editing. Z-xY: Methodology, Writing—review and editing. QW: Investigation, Writing—review and editing. X-lZ: Data curation, Formal analysis, Methodology, Software, Writing—review and editing. FG: Conceptualization, Funding acquisition, Project administration, Resources, Writing—original draft, Writing—review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Beijing Natural Science Foundation (grant No. Z220016), and the National Natural Science Foundation of China (grant No. 62272325).
Acknowledgments
We thank all the participating hospitals, relevant clinicians, statisticians, and imaging and laboratory technicians of ANGEL and ANGEL-ACT Collaborative Group for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1277189/full#supplementary-material
Abbreviations
ABAO, acute basilar artery occlusion; EVT, endovascular therapy; AIS, acute ischemic stroke; LVOs, large vessel occlusions; RCTs, randomized clinical trials; ANGEL, acute ischemic stroke cooperation group of endovascular treatment; ANGELACT, endovascular treatment key technique and emergency work flow improvement of acute ischemic stroke; BEST, basilar artery occlusion endovascular intervention versus standard medical treatment; BASICS, basilar artery international cooperation study; BAOCHE, basilar artery occlusion chinese endovascular trial; ATTENTION, endovascular treatment for acute basilar artery occlusion; AC-LVO, anterior circulation large vessel occlusion; PC-LVO, posterior circulation large vessel occlusion; pc-ASPECT, posterior circulation Alberta Stroke Program Early CT; PMI, Pons-Midbrain Index; PC-CS, posterior circulation collateral score; BATMAN, basilar artery on computed tomography angiography; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; END, early neurological deterioration; OTR, time from onset to recanalization; MT, mechanical thrombectomy.
References
1.
Albers GW Marks MP Kemp S Christensen S Tsai JP Ortega-Gutierrez S et al . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. 10.1056/NEJMoa1713973
2.
Berkhemer OA Fransen PS Beumer D van den Berg LA Lingsma HF Yoo AJ et al . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. 10.1056/NEJMoa1411587
3.
Campbell BC Mitchell PJ Kleinig TJ Dewey HM Churilov L Yassi N et al . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. 10.1056/NEJMoa1414792
4.
Goyal M Demchuk AM Menon BK Eesa M Rempel JL Thornton J et al . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. 10.1056/NEJMoa1414905
5.
Jovin TG Chamorro A Cobo E de Miquel MA Molina CA Rovira A et al . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. (2015) 372:2296–306. 10.1056/NEJMoa1503780
6.
Saver JL Goyal M Bonafe A Diener HC Levy EI Pereira VM et al . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. (2015) 372:2285–95. 10.1056/NEJMoa1415061
7.
Nogueira RG Jadhav AP Haussen DC Bonafe A Budzik RF Bhuva P et al . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. 10.1056/NEJMoa1706442
8.
Liu X Dai Q Ye R Zi W Liu Y Wang H et al . Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (best): an open-label, randomised controlled trial. Lancet Neurol. (2020) 19:115–22. 10.1016/S1474-4422(19)30395-3
9.
Langezaal LCM van der Hoeven E Mont'Alverne FJA de Carvalho JJF Lima FO Dippel DWJ et al . Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. (2021) 384:1910–20. 10.1056/NEJMoa2030297
10.
Jovin TG Li C Wu L Wu C Chen J Jiang C et al . Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. (2022) 387:1373–84. 10.1056/NEJMoa2207576
11.
Tao C Nogueira RG Zhu Y Sun J Han H Yuan G et al . Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. (2022) 387:1361–72. 10.1056/NEJMoa2206317
12.
Bouslama M Haussen DC Aghaebrahim A Grossberg JA Walker G Rangaraju S et al . Predictors of good outcome after endovascular therapy for vertebrobasilar occlusion stroke. Stroke. (2017) 48:3252–7. 10.1161/STROKEAHA.117.018270
13.
Luo G Mo D Tong X Liebeskind DS Song L Ma N et al . Factors associated with 90-day outcomes of patients with acute posterior circulation stroke treated by mechanical thrombectomy. World Neurosurg. (2018) 109:e318–28. 10.1016/j.wneu.2017.09.171
14.
Pazuello GB de Castro-Afonso LH Fornazari VR Nakiri GS Abud TG Monsignore LM et al . Thrombectomy for posterior circulation stroke: predictors of outcomes in a brazilian registry. World Neurosurg. (2021) 147:e363–72. 10.1016/j.wneu.2020.12.060
15.
Yoon W Kim SK Heo TW Baek BH Lee YY Kang HK . Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke. (2015) 46:2972–5. 10.1161/STROKEAHA.115.010840
16.
Zhang X Luo G Mo D Ma N Gao F Zhang J et al . Predictors of good outcome after endovascular treatment for patients with vertebrobasilar artery occlusion due to intracranial atherosclerotic stenosis. Clin Neuroradiol. (2019) 29:693–700. 10.1007/s00062-018-0731-z
17.
Baik SH Jung C Kim BM Kim DJ . Mechanical thrombectomy for acute posterior cerebral artery stroke; feasibility and predictors of outcome. Neuroradiology. (2022) 64:1419–27. 10.1007/s00234-022-02910-3
18.
Gao F Tong X Sun X Miao Z . A new angiographic collateral grading system for acute basilar artery occlusion treated with endovascular therapy. Transl Stroke Res. (2021) 12:559–68. 10.1007/s12975-020-00856-3
19.
Lee SJ Hong JM Choi JW Park JH Park B Kang DH et al . Predicting endovascular treatment outcomes in acute vertebrobasilar artery occlusion: a model to aid patient selection from the asian kr registry. Radiology. (2020) 294:628–37. 10.1148/radiol.2020191227
20.
Jadhav AP Desai SM Panczykowski DM Rangaraju S Campbell D Ritvonen JK et al . Predicting outcomes after acute reperfusion therapy for basilar artery occlusion. Eur J Neurol. (2020) 27:2176–84. 10.1111/ene.14406
21.
Cappellari M Mangiafico S Saia V Pracucci G Nappini S Nencini P et al . Ier-sich nomogram to predict symptomatic intracerebral hemorrhage after thrombectomy for stroke. Stroke. (2019) 50:909–16. 10.1161/STR.0000000000000209
22.
Cappellari M Mangiafico S Saia V Pracucci G Nappini S Nencini P et al . Ier-start nomogram for prediction of three-month unfavorable outcome after thrombectomy for stroke. Int J Stroke. (2020) 15:412–20. 10.1177/1747493019837756
23.
Li X Zou Y Hu J Li XM Huang CP Shan YJ et al . A NAC nomogram to predict the probability of three-month unfavorable outcome in Chinese acute ischemic stroke patients treated with mechanical thrombectomy. Int J Neurosci. (2021) 131:163–9. 10.1080/00207454.2020.1733565
24.
Huo X Ma N Mo D Gao F Yang M Wang Y et al . Acute ischaemic stroke cooperation group of endovascular treatment (angel) registry: study protocol for a prospective, multicentre registry in china. Stroke Vasc Neurol. (2019) 4:57–60. 10.1136/svn-2018-000188
25.
Jia B Ren Z Mokin M Burgin WS Bauer CT Fiehler J et al . Current status of endovascular treatment for acute large vessel occlusion in china: a real-world nationwide registry. Stroke. (2021) 52:1203–12. 10.1161/STROKEAHA.120.031869
26.
Puetz V Sylaja PN Coutts SB Hill MD Dzialowski I Mueller P et al . Extent of hypoattenuation on ct angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. (2008) 39:2485–90. 10.1161/STROKEAHA.107.511162
27.
van der Hoeven EJ McVerry F Vos JA Algra A Puetz V Kappelle LJ et al . Collateral flow predicts outcome after basilar artery occlusion: the posterior circulation collateral score. Int J Stroke. (2016) 11:768–75. 10.1177/1747493016641951
28.
Alemseged F Shah DG Diomedi M Sallustio F Bivard A Sharma G et al . The basilar artery on computed tomography angiography prognostic score for basilar artery occlusion. Stroke. (2017) 48:631–7. 10.1161/STROKEAHA.116.015492
29.
von Kummer R Broderick JP Campbell BC Demchuk A Goyal M Hill MD et al . The heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. 10.1161/STROKEAHA.115.010049
30.
Swets JA . Measuring the accuracy of diagnostic systems. Science. (1988) 240:1285–93. 10.1126/science.3287615
31.
Goyal M Menon BK van Zwam WH Dippel DW Mitchell PJ Demchuk AM et al . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. 10.1016/S0140-6736(16)00163-X
32.
Gory B Eldesouky I Sivan-Hoffmann R Rabilloud M Ong E Riva R et al . Outcomes of stent retriever thrombectomy in basilar artery occlusion: an observational study and systematic review. J Neurol Neurosurg Psychiatry. (2016) 87:520–5. 10.1136/jnnp-2014-310250
33.
Adusumilli G Kobeissi H Ghozy S Hardy N Kallmes KM Hutchison K et al . Endovascular thrombectomy after acute ischemic stroke of the basilar artery: a meta-analysis of four randomized controlled trials. J Neurointerv Surg. (2022). 10.1136/jnis-2022-019776
34.
Rangaraju S Aghaebrahim A Streib C Sun CH Ribo M Muchada M et al . Pittsburgh response to endovascular therapy (pre) score: optimizing patient selection for endovascular therapy for large vessel occlusion strokes. J Neurointerv Surg. (2015) 7:783–8. 10.1136/neurintsurg-2014-011351
35.
Sarraj A Albright K Barreto AD Boehme AK Sitton CW Choi J et al . Optimizing prediction scores for poor outcome after intra-arterial therapy in anterior circulation acute ischemic stroke. Stroke. (2013) 44:3324–30. 10.1161/STROKEAHA.113.001050
36.
Shi HX Li C Zhang YQ Li X Liu AF Liu YE et al . Predictors of early neurological deterioration occurring within 24 h in acute ischemic stroke following reperfusion therapy: a systematic review and meta-analysis. J Integr Neurosci. (2023) 22:52. 10.31083/j.jin2202052
37.
Zhang YB Su YY He YB Liu YF Liu G Fan LL . Early neurological deterioration after recanalization treatment in patients with acute ischemic stroke: a retrospective study. Chin Med J. (2018) 131:137–43. 10.4103/0366-6999.222343
38.
Seners P Turc G Oppenheim C Baron JC . Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. (2015) 86:87–94. 10.1136/jnnp-2014-308327
39.
Helleberg BH Ellekjaer H Indredavik B . Outcomes after early neurological deterioration and transitory deterioration in acute ischemic stroke patients. Cerebrovasc Dis. (2016) 42:378–86. 10.1159/000447130
40.
Li Z Zhang H Han J Chu Z Zhao S Yang Q et al . Time course and clinical relevance of neurological deterioration after endovascular recanalization therapy for anterior circulation large vessel occlusion stroke. Front Aging Neurosci. (2021) 13:651614. 10.3389/fnagi.2021.651614
Summary
Keywords
acute basilar artery occlusion, nomogram, endovascular treatment, favorable outcome, large vessel occlusion
Citation
Li L, Lv J, Han J-j, Gao Y, Yan Z-x, Wu Q, Zhang X-l and Gao F (2023) Nomogram model of functional outcome for endovascular treatment in patients with acute basilar artery occlusion. Front. Neurol. 14:1277189. doi: 10.3389/fneur.2023.1277189
Received
14 August 2023
Accepted
29 September 2023
Published
19 October 2023
Volume
14 - 2023
Edited by
Tianxiao Li, Henan Provincial People's Hospital, China
Reviewed by
Yingkun He, Henan Provincial People's Hospital, China; Ali Reza Malek, St. Mary's Medical Center, United States
Updates
Copyright
© 2023 Li, Lv, Han, Gao, Yan, Wu, Zhang and Gao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Gao gaofengletter@sina.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.