Abstract
Background:
Growing evidence suggests that headache disorders and atopic dermatitis share similar pathological mechanisms and risk factors. The aim of this study was to assess the risk for headache disorders in patients with atopic dermatitis.
Methods:
We systematically searched the PubMed and Embase databases from inception to December 1, 2023, for observational studies that examined risk of migraine in subjects with atopic dermatitis. Risk estimates from individual studies were pooled using random-effects models.
Results:
Ten studies with 12,717,747 subjects were included in the meta-analysis. Our results showed that patients with atopic dermatitis were associated with a higher risk of headache disorder (OR, 1.46, 95% CI = 1.36–1.56; P < 0.001; I2 = 98%) or migraine (OR, 1.32, 95% CI = 1.18–1.47; P < 0.001; I2 = 98.9%). Most of the results of the subgroup analyses were consistent with the overall results.
Conclusion:
The findings of this meta-analysis suggest that atopic dermatitis is a potential risk indicator for headache disorder or migraine. Further studies are still needed to verify our findings due to the substantial heterogeneity in our analyses.
Introduction
Headaches, including migraine, tension-type headaches, and cluster headaches, are among the most prevalent neurological disorders associated with pain sensation, and significantly diminish patients' quality of life (1). Approximately 52% of the global population experience a headache disorder annually (2). Factors such as family history, smoking, alcohol consumption, male predominance, and head trauma are known to predict the occurrence of headaches (3). Early identification and treatment of these risk factors are crucial for high-risk patients susceptible to early-onset headaches. Additionally, several chronic inflammatory conditions such as systemic lupus erythematosus (4), inflammatory bowel disease (5), and chronic periodontitis (6) are recognized as significant risk factors for headaches.
In recent years, atopic diseases have emerged as a growing global public health concern, affecting ~20–40% of the population worldwide, with incidence rising sharply in developing countries (7). Atopic dermatitis (AD) is the most common chronic condition in early childhood and often marks the start of the atopic march: the progression from AD in infancy to allergic rhinitis and asthma in later childhood (8, 9). As an inflammatory response mediated by diverse immunological pathways (10), AD warrants closer examination in patients with headaches. While several prior systematic reviews have established a positive link between asthma and migraines (11–13), studies focusing specifically on AD have yielded inconclusive results. Subsequent to these reviews, new research has begun to investigate the potential relationship between AD and headaches (14–23). The connection between AD and headache disorders is not yet fully understood, but the increasing number of reports on AD in patients with headaches has raised concerns among healthcare providers. This study aimed to review systematically the available evidence on the association between AD and the onset of headaches, and to estimate the strength of this association through a meta-analysis.
Methods
Search strategy
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A thorough search of the PubMed and Embase databases was conducted, covering all publications from their inception up to December 1, 2023. The search terms used were “(dermatitis OR atopic dermatitis OR eczema) AND (migraine OR hemicrania OR headache OR cephalgia OR cephalalgia)” with no restrictions on language. Additionally, the reference lists of the selected articles and related reviews were scrutinized to identify pertinent articles not captured in the electronic search.
Inclusion criteria
The criteria for inclusion in this review were as follows: (1) the study design was either cross-sectional, case-control, or cohort; (2) the study investigated the association between AD and the risk of headaches; (3) the study provided risk estimates with confidence intervals (CIs) or sufficient data to calculate these estimates; (4) the data were published in a complete text format; and (5) the study had a sample size > 1,000. Exclusions were made for case reports, animal studies, editorials, correspondences, and reviews.
Data extraction and quality assessment
Data extraction was independently performed by two double-blinded authors. The extracted data included the first author's name, publication year, study design, location, subjects, methods of AD and headache ascertainment, adjustments for confounders, results, and quality. The quality of the included studies was evaluated using the Newcastle-Ottawa Scale (24), as recommended by the Cochrane Collaboration. This scale comprises eight items and assigns scores ranging from 0 (indicating a high risk of bias) to 9 (indicating a low risk of bias). Studies scoring ≥ 7 were deemed high quality. One author conducted the initial details and assessment of the included studies, which was then reviewed by another author for accuracy. Any discrepancies were resolved through discussion.
Statistical analysis
Statistical analysis was conducted using STATA 10.0 software (StataCorp LP, College Station, TX, United States). To account for clinical heterogeneity among studies, due to variations in study designs and measurement criteria, the random-effects model was applied in this meta-analysis (25). Heterogeneity among the included studies was quantified using the I2 statistic. Heterogeneity levels were interpreted as follows: I2 < 25% indicated an absence of heterogeneity; 25% ≤ I2 < 50% suggested low heterogeneity; 50% ≤ I2 < 75% represented moderate heterogeneity; and I2 ≥ 75% indicated substantial heterogeneity (26). We either extracted the most fully adjusted effect estimates (ORs, RRs, or HRs) or calculated the unadjusted OR using raw data. The association between AD and headache risk was estimated using odds ratios (ORs) and their corresponding 95% CIs, derived from comparisons between cases and controls (27). To assess publication bias, Egger’s regression test was used for quantitative evaluation, and funnel plots of the logarithm of OR versus the standard error were examined for qualitative assessment (28, 29). P < 0.05 was considered statistically significant (Table 1).
Table 1
| Author, year | Study population | Study design/period | Target group | Atopic disease measurement | Headache assessment | Number of subjects | Adjustment | Nos. quality |
|---|---|---|---|---|---|---|---|---|
| Silverberg (14) | National Health Interview Survey in USA | Cross-sectional/1997-2013 | Children and adolescents | Self-reported diagnosis by questionnaire | Self-reported diagnosis by questionnaire | 401,002 | Age, sex, race/ethnicity, household income, highest level of education in the family, insurance coverage, number of persons in the household, birthplace in the United States, ever history of asthma, hay fever, and food allergy | 6 |
| Shreberk-Hassidim et al. (15) | Israeli civilians undergo a routine medical evaluation and fitness-for-service | Cross-sectional/1998-2003 | Adolescents (16-21 years) | Medical record review | Medical record review | 1,187,757 | Age, region of birth, predicted socioeconomic status (SES), and comorbidities | 6 |
| Wei et al. (16) | National Health Insurance in China | Case-control | Children (7-18 years) | ICD-9 | ICD-9 | 16,130/64,520 | Comorbidity | 7 |
| Smirnova et al. (17) | Life and Health (Liv och Hälsa) in Sweden | Cross-sectional/2007 | Adults (>20 years) | Self-reported diagnosis by questionnaire | Self-reported diagnosis by questionnaire | 34313 | Sex, age group, smoking, and education level | 6 |
| Manjunath and Silverberg (18) | The Fragile Families and Child Wellbeing Study in USA | Case-control/1998-2015 | Adolescents (15 years) | Parent questionnaire | Parent questionnaire | 4898 | Sex and race/ethnicity | 5 |
| Roh et al. (19) | IBM MarketScan Commercial Claims and Encounters database in USA | Case-control/1998-2015 | Adults (>18 years) | ICD-10-CM | ICD-10-CM | 39,779/353,743 | Age, sex, and number of outpatient encounters | 7 |
| Fan et al. (20) | National Institutes of Health database in USA | Cross-sectional | Adults (≥18 years) | ICD-9-CM or ICD-10-CM | ICD-9-CM or ICD-10-CM | 12,008/202,118 | Age, sex, race/ethnicity, smoking status, body mass index, hypertension, hyperlipidemia, cardiovascular disease, Type 2 diabetes mellitus, sleep apnea, and hypothyroidism | 8 |
| Fuxench et al. (21) | The Health Improvement Network in UK | Cohort/NA | Child and adult | Diagnostic codes and 2 AD-treatment codes | Diagnostic codes | 1,034,514/2,219,898 | Age, sex, Townsend index, history of allergic rhinitis, asthma, depression, anxiety, and hormonal medications | 8 |
| Han et al. (22) | Korean National Health Insurance Service database | Cohort/2009 | Adults (>20 years) | ICD-10-CM | ICD-10-CM | 3,607,599 | Age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, myocardial infarction, stroke, anxiety disorder, bipolar disorder, and depression | 8 |
| Lee et al. (23) | Korean National Health Insurance Service (K-NHIS) claims database | Cohort/2009 | Adults (≥18 years) | Least one diagnosis with prescription of the topical agent or three or more occasions of diagnosis with dedicated ICD-10 codes | ICD-10 | 48,983/3,490,485 | Age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, myocardial infarction, stroke, anxiety disorder, bipolar disorder, and depression | 8 |
Characteristics of the included studies.
Results
Search results
Through keyword searches, a total of 3,088 citations were retrieved from two databases, following the removal of duplicates. After reviewing titles and abstracts, 733 articles were deemed irrelevant and excluded. A detailed full-text review was conducted for 65 articles. Ultimately, 10 studies met the criteria and were included in our analysis. Figure 1 illustrates the excluded studies along with the reasons for their exclusion after assessment of the full text.
Figure 1

Flow-chart of the studies considered and finally selected for review.
Characteristics of the included studies
The principal characteristics of the 10 studies included in this review are presented in Table 2. These studies were published between 2016 and 2023. The sample sizes varied, ranging from 4,898 to 3,607,599, totaling 12,717,747 subjects. Geographically, three cohort studies were conducted in Korea and the United States, three case-control studies in Europe, China, and the United States, and four cross-sectional studies in the United States, Israel, and Sweden. Participant age was > 6 years in all studies: four studies focused on children and adolescents, one included both children and adults, while the remaining five involved only adults. For diagnosing AD, two studies relied on questionnaires or medical record reviews, and four used the International Classification of Diseases (ICD) criteria. In a study by Fuxench et al., AD diagnosis was based on at least one of five specific AD-diagnostic codes and two AD-treatment codes. Another study identified AD cases through prescription records of topical agents or at least three diagnoses using specific ICD-10 codes. The criteria for headache disorder diagnosis varied considerably: four studies used questionnaires or medical records, and five used ICD criteria. One study diagnosed headache disorders through diagnostic codes.
Table 2
| Subgroup analysis | Number of studies | Number of estimates | Pooled OR (95% CI), I2 statistics (%), P-value for the heterogeneity Q test | Model used |
|---|---|---|---|---|
| Headache disorder | 10 | 16 | 1.46 (1.36–1.56); I2 = 98.1%, P < 0.001 | Random effects |
| Study design | ||||
| Cross-section | 4 | 8 | 1.67 (1.5–1.86); I2 = 89.5%, P < 0.001 | Random effects |
| Case-control | 4 | 4 | 1.5 (1.07–2.08); I2 = 97.1%, P < 0.001 | Random effects |
| Cohort | 3 | 4 | 1.2 (1.13–1.27); I2 = 97.6%, P < 0.001 | Random effects |
| Study location | ||||
| Asia | 4 | 5 | 1.39 (1.27–1.53); I2 = 92.2%, P < 0.001 | Random effects |
| Europe | 3 | 7 | 1.4 (1.28–1.52); I2 = 97.1%, P < 0.001 | Random effects |
| North Amerca | 4 | 4 | 1.53 (1.18–1.99); I2 = 99%, P < 0.001 | Random effects |
| Age range | ||||
| Children and adolescents | 6 | 7 | 1.46 (1.2–1.78); I2 = 98.2%, P < 0.001 | Random effects |
| Adults | 6 | 9 | 1.47 (1.34–1.61); I2 = 98%, P < 0.001 | Random effects |
| Study quality | ||||
| High quality | 6 | 7 | 1.33 (1.22–1.45); I2 = 98.9%, P < 0.001 | Random effects |
| Low quality | 4 | 8 | 1.61 (1.47–1.75); I2 = 69%, P = 0.002 | Random effects |
| Headache measurement | ||||
| Questionnaires or medical review | 4 | 8 | 1.61 (1.47–1.75); I2 = 69%, P = 0.002 | Random effects |
| Diagnostic code | 6 | 7 | 1.33 (1.22–1.45); I2 = 98.9%, P < 0.001 | Random effects |
| Adjusting for at least 10 variables | 5 | 5 | 1.34 (1.22–1.48); I2 = 99.1%, P < 0.001 | Random effects |
| Severity of AD | ||||
| Mild | 4 | 6 | 1.26 (1.18–1.34); I2 = 95.4%, P < 0.001 | Random effects |
| Moderate or severe | 4 | 9 | 1.23 (1.15–1.32); I2 = 94.7%, P < 0.001 | Random effects |
| Migraine | 6 | 7 | 1.32 (1.18–1.47); I2 = 98.9%, P < 0.001 | Random effects |
| Study design | ||||
| Cross-section | 1 | 1 | 1.89 (1.8–1.99) | Random effects |
| Case-control | 2 | 2 | 1.41 (0.94–2.12); I2 = 98.3%, P < 0.001 | Random effects |
| Cohort | 3 | 4 | 1.17 (1.08–1.27); I2 = 97.6%, P < 0.001 | Random effects |
| Study location | ||||
| Asia | 3 | 3 | 1.38 (1.23–1.55); I2 = 95.5%, P < 0.001 | Random effects |
| Europe | 1 | 2 | 1.09 (1.01–1.19); I2 = 96%, P < 0.001 | Random effects |
| North Amerca | 2 | 2 | 1.47 (0.91–2.4); I2 = 99.6%, P < 0.001 | Random effects |
| Age range | ||||
| Children and adolescents | 2 | 2 | 1.37 (1.14–1.65); I2 = 97.9%, P < 0.001 | Random effects |
| Adults | 4 | 4 | 1.47 (1.34–1.61); I2 = 98%, P < 0.001 | Random effects |
| Sex | ||||
| Male | 3 | 3 | 1.46 (1.18–1.81); I2 = 92.7%, P < 0.001 | Random effects |
| Female | 3 | 3 | 1.52 (1.35–1.72); I2 = 76.5%, P < 0.001 | Random effects |
| Adjusting for at least 10 variables | 4 | 4 | 1.29 (1.12–1.47); I2 = 99.1%, P < 0.001 | Random effects |
Meta-analysis for studies included in the analysis.
Regarding methodological quality, assessed using Supplementary Tables S1, S2, six studies were classified as high quality, and four studies were of low quality. The average quality score for the 10 included studies was 6.9.
Meta-analysis
Headache disorder
Ten studies with a total of 12,717,747 subjects, provided data for analyzing the risk of headache disorder in patients with AD. Figure 2 demonstrates significant heterogeneity among these studies (I2 = 98.1%). The combined OR was 1.46 (95% CI, 1.36–1.56), suggesting a markedly increased risk of headache disorder in patients with AD. As shown in Supplementary Figure S1, there was no evidence of publication bias (Egger’s test, p = 0.06).
Figure 2
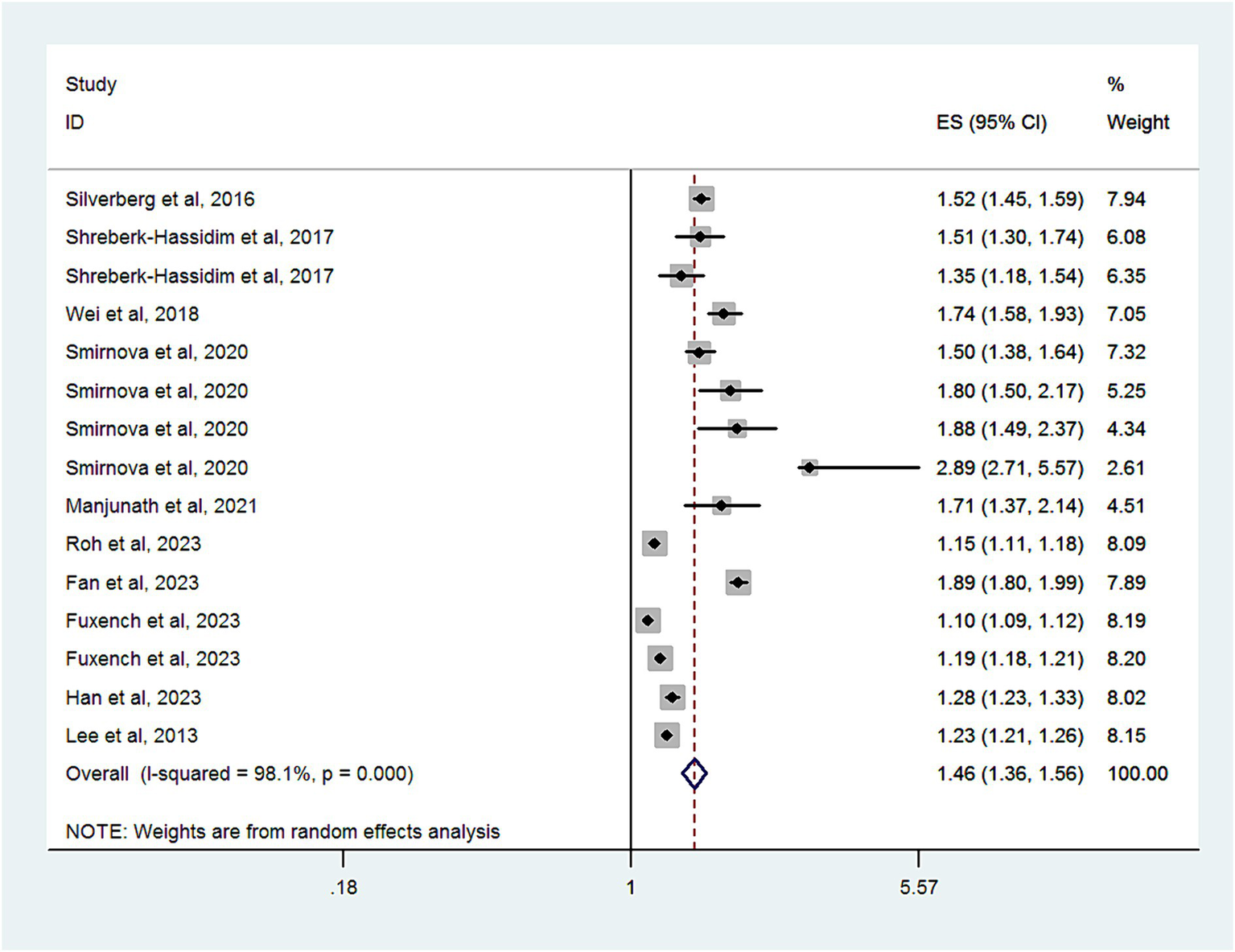
Forest plot of the overall risk of headache disorders in relation to AD.
Subgroup analyses are summarized in Table 2. When categorized by study design, significant associations were found in cross-sectional studies (OR, 1.67; 95% CI = 1.5–1.86; P < 0.001; I2 = 89.5%), cohort studies (OR, 1.2; 95% CI = 1.13–1.27; P < 0.001; I2 = 97.6%), and case-control studies (OR, 1.5; 95% CI = 1.07–2.08; P < 0.001; I2 = 97.1%).
When grouped by study location, significant associations were observed in studies from Asia (OR, 1.39; 95% CI = 1.27–1.53; P < 0.001; I2 = 92.2%), Europe (OR, 1.4; 95% CI = 1.28–1.52; P < 0.001; I2 = 97.1%), and North America (OR, 1.53; 95% CI = 1.18–1.99; P = 0.001; I2 = 99%).
In a subgroup analysis of study quality, both high-quality studies (OR, 1.61; 95% CI = 1.47–1.75; P < 0.001; I2 = 69%) and low-quality studies (OR, 1.33; 95% CI = 1.22–1.45; P < 0.001; I2 = 98.9%) showed significant associations.
Age-based subgroup analysis indicated significant associations in both children and adolescents (OR, 1.46; 95% CI = 1.2–1.78; P = 0.001; I2 = 98.2%) and adults (OR, 1.47; 95% CI = 1.34–1.61; P < 0.001; I2 = 98%).
In a subgroup analysis by headache measurement, a significant association was observed in those studies using questionnaires or medical review (OR, 1.61; 95% CI = 1.47–1.75; P < 0.001; I2 = 69%) and using diagnostic code (OR, 1.33; 95% CI = 1.22–1.45; P < 0.001; I2 = 98.9%).
Four studies that assessed the impact of AD severity on headache disorder risk found a higher risk among patients with mild (OR, 1.26; 95% CI = 1.18–1.34; P < 0.001; I2 = 95.4%) or moderate to severe AD (OR, 1.23; 95% CI = 1.15–1.32; P < 0.001; I2 = 94.7%).
When we limited our analysis to studies adjusting for at least 10 variables, a significant positive association between headache disorder and AD risk was observed (OR, 1.34; 95% CI = 1.22–1.45; P < 0.001; I2 = 99.1%).
Migraine
Six studies with a total of 11,090,412 subjects, contributed data for the analysis of migraine risk in patients with AD. Substantial heterogeneity was noted (I2 = 98.9%) (Figure 3). The overall OR was 1.32 (95% CI, 1.18–1.47), indicating a significantly increased risk of migraine in patients with AD. As shown in Supplementary Figure S2, there was no evidence of publication bias (Egger’s test, p = 0.6).
Figure 3

Forest plot of the overall risk of Migraine in relation to AD.
In a subgroup analysis by study design, significant associations were found in cross-sectional studies (OR, 1.89; 95% CI = 1.8–1.99) and cohort studies (OR, 1.17; 95% CI = 1.08–1.27; P < 0.001; I2 = 97.6%). However, case-control studies did not show an increased risk of migraine (OR, 1.41; 95% CI = 0.94–2.12; P = 0.097; I2 = 98.3%).
Grouping by study location revealed significant associations in studies from Asia (OR, 1.38; 95% CI = 1.23–1.55; P < 0.001; I2 = 95.5%) and Europe (OR, 1.09; 95% CI = 1.01–1.19; P < 0.001; I2 = 96%). Studies from North America, however, did not show an increased risk (OR, 1.47; 95% CI = 0.91–2.4; P = 0.12; I2 = 99.6%).
In an age-based subgroup analysis, a significant association was observed in adults (OR, 1.31; 95% CI = 1.16–1.48; P < 0.001; I2 = 98.9%), but not in children and adolescents (OR, 1.35; 95% CI = 0.82–2.21; P = 0.237; I2 = 98.9%).
Regarding sex, significant associations were observed in both males (OR, 1.52; 95% CI = 1.35–1.72; P = 0.001; I2 = 92.7%) and females (OR, 1.46; 95% CI = 1.18–1.81; P < 0.001; I2 = 92.7%).
When we limited our analysis to studies adjusting for at least 10 variables, a significant positive association between migraine and AD risk was observed (OR, 1.29; 95% CI = 1.12–1.47; P < 0.001; I2 = 99.2%).
Discussion
The present study revealed that AD is associated with a moderately increased risk of headache disorder or migraine. This finding was consistent across various subgroup meta-analyses.
To our knowledge, this is the first meta-analysis to assess the association between AD and headache disorder. The fairly frequent coexistence of migraine and allergic diseases was well described in previous epidemiologic studies (30, 31). While recent meta-analyses (11–13) have indicated a bidirectional association between asthma and headache disorder, our study extends this understanding. The concept of the atopic march describes the progression of atopic disorders from AD in infancy to allergic rhinitis and asthma in childhood (32). Therefore, it is plausible that AD might also elevate the risk of headache disorder. The mechanisms linking AD and migraine could involve inflammatory responses and psychological stress triggers. Prior research has identified elevated levels of histamine, leukotrienes, and prostaglandins in migraine patients (33–35), which are crucial immune modulators in atopic disorders. Additionally, one study reported fluctuating levels of IL-10, IL-4, and IL-5 during headache attacks, suggesting a role for TH2 inflammation in both AD and headache disorders (36, 37). Moreover, a significant portion of AD patients suffer from psychiatric disorders such as depression, anxiety, and insomnia (38), which are known to have a bidirectional relationship with headache disorders. A recent extensive genome-wide study also indicated a genetic correlation between headache disorders and psychiatric conditions (39), hinting at potential common genetic factors or shared pathways.
However, the general conclusion of our study is not definitive due to considerable heterogeneity. This clinical heterogeneity is likely to have contributed to statistical heterogeneity, potentially arising from differences in study design, definitions of headache disorders or AD, age of subjects, and severity of AD. We conducted subgroup analyses based on these factors to investigate potential sources of heterogeneity. Notably, the pooled estimate from cross-sectional studies was the highest, possibly owing to inherent limitations of this study design. Cross-sectional studies allow categorization of subjects by previous exposure at a specific time point, but they can also introduce recall bias. This bias may occur because the presence of a disease or its assessment could influence participants’ responses, potentially leading to an overestimation of associations.
Furthermore, the varied definitions of headache disorder or AD may contribute to the heterogeneity observed in our findings. Several studies included in our analysis relied on questionnaires to confirm cases of headache disorder or AD, potentially impacting the accuracy and completeness of diagnoses. Consequently, misclassification of headache disorder or AD might weaken the robustness of our results. However, the results from subgroup analyses based on diagnostic criteria were still consistent with the overall analysis. However, the pooled OR of studies using diagnostic code was lower, indicating that the unreliability of the diagnoses may overestimate this association. Prior study has shown a positive association between inflammatory cytokines and the severity of AD (40), leading to the hypothesis that the risk of headache disorders could vary with the severity of AD. Interestingly, our study did not find a severity-dependent association between AD and headache disorder, but this finding is limited by the sample size and necessitates further investigation.
The strength of our study lies in its large sample size and extensive search methodology, which enabled a detailed examination of the association. Additionally, we conducted comprehensive subgroup analyses based on various factors such as study region and design, type of headache disorder, measurement methods for headache disorder or AD, study quality, severity of AD, and gender. However, there are several significant limitations to consider. The primary limitation is the potential influence of unknown confounders. As we known, the pathogenesis of the headache disorder is complex and related to many factors. Therefore, only adjusted risk estimates were included in our meta-analysis. Furthermore, we conducted analyses limited to studies adjusting at least 10 variables and the pooled ORs were reduced but still significant. More well-designed studies that consider additional important covariates are needed. Second, the limited number of eligible high-quality studies may affect the precision of our findings. Third, there is a lack of data regarding the impact of medications for AD on the risk of headache disorder. A previous study (41) suggested that anti-asthmatic or anti-allergic treatments might reduce migraine risk in children and adolescents. Future research should include consideration of anti-allergic drug use to clarify further the link between AD and headache disorders.
In conclusion, our results indicate that AD may be a potential risk factor for headache disorder. Clinicians should be mindful of the increased incidence of headache disorder in patients with AD. To examine these findings further and gain a more comprehensive understanding, future prospective cohort studies with more comprehensive data collection and consideration of potential confounders are necessary to provide additional evidence on the detailed association.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WY: Writing – review & editing, Data curation, Methodology, Software, Visualization. HD: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Writing – review & editing. X-fX: Writing – review & editing, Formal analysis, Project administration, Resources, Supervision. H-yJ: Supervision, Validation, Writing – original draft, Writing – review & editing. J-yD: Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the project of training talent for Zhejiang province young and middle-aged clinical TCM experts (Zhejiang TCM [2022] No. 7) and Hangzhou Municipal Health and Family Planning Commission Science and Technology Project (project no. ZD20200004). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1383832/full#supplementary-material
References
1.
Magis D Schoenen J . Advances and challenges in neurostimulation for headaches. Lancet Neurol. (2012) 11:708–19. doi: 10.1016/s1474-4422(12)70139-4
2.
Cheema S Mehta D Ray JC Hutton EJ Matharu MS . New daily persistent headache: a systematic review and meta-analysis. Cephalalgia. (2023) 43:3331024231168089. doi: 10.1177/03331024231168089
3.
Tana C Raffaelli B Souza MNP de la Torre ER Massi DG Kisani N et al . Health equity, care access and quality in headache - part 1. J Headache Pain. (2024) 25:12. doi: 10.1186/s10194-024-01712-7
4.
Mitsikostas DD Sfikakis PP Goadsby PJ . A meta-analysis for headache in systemic lupus erythematosus: the evidence and the myth. Brain. (2004) 127:1200–9. doi: 10.1093/brain/awh146
5.
Dimitrova AK Ungaro RC Lebwohl B Lewis SK Tennyson CA Green MW et al . Prevalence of migraine in patients with celiac disease and inflammatory bowel disease. Headache. (2013) 53:344–55. doi: 10.1111/j.1526-4610.2012.02260.x
6.
Dholakia SB Rao P Talluri S Khan J . The association between migraines and periodontal disease: a systematic review of clinical studies. J Oral Biosci. (2023) 65:137–45. doi: 10.1016/j.job.2023.04.001
7.
Gutowska-Ślesik J Samoliński B Krzych-Fałta E . The increase in allergic conditions based on a review of literature. Postepy Dermatol Alergol. (2023) 40:1–7. doi: 10.5114/ada.2022.119009
8.
Langan SM Irvine AD Weidinger S . Atopic dermatitis. Lancet. (2020) 396:345–60. doi: 10.1016/s0140-6736(20)31286-1
9.
Grangeon L Lange KS Waliszewska-Prosół M Onan D Marschollek K Wiels W et al . Genetics of migraine: where are we now?J Headache Pain. (2023) 24:12. doi: 10.1186/s10194-023-01547-8
10.
Villar-Martínez MD Moreno-Ajona D Chan C Goadsby PJ . Indomethacin-responsive headaches: a narrative review. Headache. (2021) 61:700–14. doi: 10.1111/head.14111
11.
Kang LL Chen PE Tung TH Chien CW . Association between asthma and migraine: a systematic review and meta-analysis of observational studies. Front Allergy. (2021) 2:741135. doi: 10.3389/falgy.2021.741135
12.
Wang L Deng ZR Zu MD Zhang J Wang Y . The comorbid relationship between migraine and asthma: a systematic review and meta-analysis of population-based studies. Front Med. (2020) 7:609528. doi: 10.3389/fmed.2020.609528
13.
Sayyah M Saki-Malehi A Javanmardi F Forouzan A Shirbandi K Rahim F . Which came first, the risk of migraine or the risk of asthma? A systematic review. Neurol Neurochir Pol. (2018) 52:562–9. doi: 10.1016/j.pjnns.2018.07.004
14.
Silverberg JI . Association between childhood eczema and headaches: an analysis of 19 US population-based studies. J Allergy Clin Immunol. (2016) 137:492–499.e5. doi: 10.1016/j.jaci.2015.07.020
15.
Shreberk-Hassidim R Hassidim A Gronovich Y Dalal A Molho-Pessach V Zlotogorski A . Atopic dermatitis in Israeli adolescents from 1998 to 2013: trends in time and association with migraine. Pediatr Dermatol. (2017) 34:247–52. doi: 10.1111/pde.13084
16.
Wei CC Lin CL Shen TC Chen AC . Children with allergic diseases have an increased subsequent risk of migraine upon reaching school age. J Investig Med. (2018) 66:1064–8. doi: 10.1136/jim-2018-000715
17.
Smirnova J Montgomery S Lindberg M Svensson Å von Kobyletzki L . Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. (2020) 20:23. doi: 10.1186/s12895-020-00117-8
18.
Manjunath J Silverberg JI . Association between atopic dermatitis and headaches throughout childhood and adolescence-a longitudinal birth cohort study. Pediatr Dermatol. (2021) 38:780–6. doi: 10.1111/pde.14607
19.
Roh YS Huang AH Sutaria N Choi U Wongvibulsin S Choi J et al . Real-world comorbidities of atopic dermatitis in the US adult ambulatory population. J Am Acad Dermatol. (2022) 86:835–45. doi: 10.1016/j.jaad.2021.11.014
20.
Fan R Leasure AC Damsky W Cohen JM . Migraine among adults with atopic dermatitis: a cross-sectional study in the All of Us research programme. Clin Exp Dermatol. (2023) 48:24–6. doi: 10.1093/ced/llac004
21.
Fuxench ZCC Wan J Wang S Syed MN Shin DB Abuabara K et al . Atopic dermatitis and risk for headache disorders and migraines: a population-based cohort study in children and adults from the UK. Br J Dermatol. (2023) 190:120–3. doi: 10.1093/bjd/ljad325
22.
Han JH Lee HJ Yook HJ Han K Lee JH Park YM . Atopic disorders and their risks of migraine: a nationwide population-based cohort study. Allergy Asthma Immunol Res. (2023) 15:55–66. doi: 10.4168/aair.2023.15.1.55
23.
Lee JH Kim SH Lee GN Han K Lee JH . Association of atopic dermatitis with new-onset migraine: a nationwide population-based cohort study. J Eur Acad Dermatol Venereol. (2023) 37:e236–8. doi: 10.1111/jdv.18680
24.
Stang A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
25.
Higgins JP Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26.
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
27.
Zhang J Yu KF . What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
28.
Egger M Davey Smith G Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
29.
Lau J Ioannidis JP Terrin N Schmid CH Olkin I . The case of the misleading funnel plot. BMJ. (2006) 333:597–600. doi: 10.1136/bmj.333.7568.597
30.
Karlsson WK Ashina H Cullum CK Christensen RH Al-Khazali HM Amin FM et al . The Registry for Migraine (REFORM) study: methodology, demographics, and baseline clinical characteristics. J Headache Pain. (2023) 24:70. doi: 10.1186/s10194-023-01604-2
31.
Waliszewska-Prosół M Straburzyński M Czapińska-Ciepiela EK Nowaczewska M Gryglas-Dworak A Budrewicz S . Migraine symptoms, healthcare resources utilization and disease burden in a large Polish migraine cohort: results from 'Migraine in Poland'-a nationwide cross-sectional survey. J Headache Pain. (2023) 24:40. doi: 10.1186/s10194-023-01575-4
32.
Mrkić Kobal I Plavec D Vlašić Lončarić Ž Jerković I Turkalj M . Atopic March or atopic multimorbidity-overview of current research. Medicina. (2023) 60:21. doi: 10.3390/medicina60010021
33.
Ferretti A Gatto M Velardi M Di Nardo G Foiadelli T Terrin G et al . Migraine, allergy, and histamine: is there a link?J Clin Med. (2023) 12:3566. doi: 10.3390/jcm12103566
34.
Blanco MJ Benesh DR Knobelsdorf JA Khilevich A Cortez GS Mokube F et al . Discovery of dual positive allosteric modulators (PAMs) of the metabotropic glutamate 2 receptor and CysLT1 antagonists for treating migraine headache. Bioorg Med Chem Lett. (2017) 27:323–8. doi: 10.1016/j.bmcl.2016.11.049
35.
Harder AVE Onderwater GLJ van Dongen RM Heijink M van Zwet EW Giera M et al . Prostaglandin-E(2) levels over the course of glyceryl trinitrate provoked migraine attacks. Neurobiol Pain. (2023) 13:100112. doi: 10.1016/j.ynpai.2022.100112
36.
Munno I Centonze V Marinaro M Bassi A Lacedra G Causarano V et al . Cytokines and migraine: increase of IL-5 and IL-4 plasma levels. Headache. (1998) 38:465–7. doi: 10.1046/j.1526-4610.1998.3806465.x
37.
Munno I Marinaro M Bassi A Cassiano MA Causarano V Centonze V . Immunological aspects in migraine: increase of IL-10 plasma levels during attack. Headache. (2001) 41:764–7. doi: 10.1046/j.1526-4610.2001.01140.x
38.
McCracken HT Thaxter LY Smitherman TA . Psychiatric comorbidities of migraine. Handb Clin Neurol. (2024) 199:505–16. doi: 10.1016/b978-0-12-823357-3.00013-6
39.
Winsvold BS Harder AVE Ran C Chalmer MA Dalmasso MC Ferkingstad E et al . Cluster headache genomewide association study and meta-analysis identifies eight loci and implicates smoking as causal risk factor. Ann Neurol. (2023) 94:713–26. doi: 10.1002/ana.26743
40.
Maintz L Welchowski T Herrmann N Brauer J Traidl-Hoffmann C Havenith R et al . IL-13, periostin and dipeptidyl-peptidase-4 reveal endotype-phenotype associations in atopic dermatitis. Allergy. (2023) 78:1554–69. doi: 10.1111/all.15647
41.
Aupiais C Wanin S Romanello S Spiri D Moretti R Boizeau P et al . Association between migraine and atopic diseases in childhood: a potential protective role of anti-allergic drugs. Headache. (2017) 57:612–24. doi: 10.1111/head.13032
Summary
Keywords
allergic, atopic, eczema, cephalgia, cephalalgia
Citation
Yang W, Dai H, Xu X-f, Jiang H-y and Ding J-y (2024) Association of atopic dermatitis and headache disorder: a systematic review and meta-analyses. Front. Neurol. 15:1383832. doi: 10.3389/fneur.2024.1383832
Received
08 February 2024
Accepted
12 March 2024
Published
21 March 2024
Volume
15 - 2024
Edited by
Nina Riggins, Brain Performance Center and Research Institute, United States
Reviewed by
Aynur Özge, Board Member of International Headache Society, United Kingdom
Marta Waliszewska-Prosół, Wroclaw Medical University, Poland
Updates
Copyright
© 2024 Yang, Dai, Xu, Jiang and Ding.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-yuan Ding, dingjiyuan1888@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.