- 1State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing, China
- 2School of System Science, Beijing Normal University, Beijing, China
- 3BABRI Centre, Beijing Normal University, Beijing, China
Background: Evidence regarding the relationship between the use of statins and cognitive outcomes presents varying findings. This study aims to analyze the relationship between sustained statin use and cognitive performance in dyslipidemia patients.
Methods: This study presents findings from the Beijing Ageing Brain Rejuvenation Initiative (BABRI) study, in which a cohort of community-dwelling dyslipidemia patients (Entire sample, N = 1,062, aged 50–86) was recruited. Participants were divided into two groups based on their sustained use statins (Statins group, N = 677) or not use any lipid-lowering agents (Untreated group, N = 385). Furthermore, the entire sample was stratified by age into the middle-aged sample (N = 451) and the older people sample (N = 611), following a similar categorization based on statin application. ANCOVA was used to evaluate the relationship between sustained statin use and cognitive function.
Results: Overall, in the total sample, the statins group demonstrated better cognition in overall cognition, memory, visuospatial ability, attention, executive function, and language domains compared to the untreated group. Moreover, the statins group only showed better performance in attention among the middle-aged sample. In the older people sample, statins group exhibited superior cognitive performance across various cognitive domains compared to untreated group.
Conclusion: Among dyslipidemia patients in Beijing community, sustained statin users exhibited superior cognitive function across all domains compared to untreated individuals, with particularly noticeable improvements among those aged 65 and above. These findings underscore the protective effect of statins on cognitive function in dyslipidemia patients, highlighting significant benefits for the older people population.
1 Introduction
Statins, commonly prescribed for cardiovascular conditions such as dyslipidemia and etc., primarily act by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, thereby reducing cholesterol synthesis (1). Given the established link between elevated cholesterol and cardiovascular diseases, statins play a pivotal role in lowering peripheral blood cholesterol, particularly in reducing low-density lipoprotein cholesterol (LDL-C), thereby aiding in the reduction of atherosclerotic cardiovascular disease and other cardiovascular risks (2, 3). Beyond their established role in managing lipid metabolism and serving as primary prevention or secondary prevention for coronary heart disease and cerebrovascular diseases, research suggests that statins may offer potential benefits in reduce the risk of cognitive impairment-associated conditions such as Alzheimer’s disease (AD) and dementia (4, 5). The observed effect could be attributed to the ability of statins to penetrate the blood–brain barrier (BBB) (6, 7), along with their anti-inflammatory, antioxidant, and synaptic plasticity-regulating properties (8, 9). Moreover, they regulate cerebral cholesterol metabolism, thereby promoting neuroprotection (10, 11). At the same time, other studies have found that statins can regulate hippocampal neurogenesis by upregulating Wnt signaling through dependent inhibition of the Mevalonate (MVA) Pathway (12). Additionally, there is evidence that statins are effective inducers of axon and dendrite growth (13). In summary, the regulation of cognitive function by statins may involve two key mechanisms: first, by modulating cholesterol metabolism and other pathways in the central nervous system, and second, by lowering peripheral blood cholesterol to reduce cardiovascular and cerebrovascular risks (14). These multifaceted effects provide potential biological explanations for the effects of statins on cognitive function. Despite these benefits, controversy remains surrounding the relationship between statin use and cognition in the general population. On February 28, 2012, the U.S. Food and Drug Administration (FDA) mandated the inclusion of a warning label on statins, due to insights provided from post-marketing surveillance, observational studies (15–19), and randomized controlled trials (RCTs) (20, 21) hinting at potential adverse effects on cognitive function. Critiques of RCTs and observational data suggest a potential link between this adverse effect and the use of high-dose statins (22). A review summarizing a series of randomized controlled trials found that statins did not exhibit clear adverse effects on patients’ cognitive function in short-term studies. However, long-term follow-up research indicated a significant reduction in the incidence of dementia among patients treated with statins (23). However, there is still no conclusive evidence that statins cause clinically significant cognitive impairment (22, 24, 25) or that statins reduce the risk of dementia or cognitive impairment (26).
Based on the above background, we decided to use data from the Beijing Aged Brain Rejuvenation Initiative (BABRI) cohort, which is a community-based cohort study that mainly focuses on population aging, especially cognitive aging and its determinants. A screening of 1,062 dyslipidemia patients was conducted using the baseline database of the BABRI cohort. The primary objective was to assess sustained use of statin drugs affect various cognitive functions in dyslipidemia patients compared to those not using any lipid-lowering medication. The aim was to precisely pinpoint the impact of statins on various cognitive functions in individuals dealing with dyslipidemia. Subsequently, dyslipidemia patients were categorized into two sample—older people and middle-aged—in order to investigate more comprehensively the effects of statins on cognitive function in hyperlipidemic patients across different age groups.
2 Materials and methods
2.1 Study cohort and measures
Participants in the cross-sectional study were sourced from the Beijing community, a prospective, community-based cohort (27, 28). This study selected a group of dyslipidemia patients from the BABRI baseline database as follows. Among the 7,625 participants included in the BABRI database and meeting the specified inclusion and exclusion criteria, a group consisting of 1,477 individuals with dyslipidemia was identified. The inclusion criteria as follows: (1) individuals aged 50 or above; (2) attainment of 6 or more years of formal education; (3) all diagnoses of dyslipidemia were made by physicians in Beijing area’s tertiary hospitals, according to the 2018 AHA Guideline on the Management of Blood Cholesterol (29), validated by medical records from community healthcare centers; (4) willingness to engage in face-to-face interviews. Exclusion criteria are as follows: (1) individuals diagnosed with dementia, Parkinson’s disease, other degenerative neurological disorders, psychiatric conditions, or brain tumors; (2) incapacity to undergo cognitive assessments due to physical or mental disabilities.
Furthermore, the medication status of dyslipidemia patients was determined through their medical records or self-reports, with exclusive attention given to the utilization of any medication falling within the class of HMG-CoA reductase inhibitors (Including any hydrophilic or lipophilic statins, with no dose restrictions, such as simvastatin, atorvastatin, rosuvastatin, etc.), with a minimum duration of continuous usage exceeding 6 months. Following the exclusion of patients with unclear or irregular medication records and those taking alternative lipidlowering agents, a final sample of 1,062 dyslipidemia patients with well-documented medication records were categorized into a statins-user group (n = 677) and an untreated group (n = 385).
Other covariates encompassed age, gender, education, diabetes, hypertension, smoking and obesity. Face-to-face interviews were used to assess age, education, gender and smoking and obesity. Smoking status was determined by self-reported smoking habits. Participants were classified as obese if their body mass index (BMI) exceeded ≥30, based on criteria from the World Health Organization Global Health Observatory data. Additionally, in accordance with guidelines from the ADA and AHA, diagnoses of type 2 diabetes and hypertension were performed by physicians at tertiary hospitals in the Beijing area, with patient medical records reviewed at community health care centers.
2.2 Neuropsychological tests
The current study employed a comprehensive neuropsychological test battery to evaluate general cognition and five cognitive domains, consistent with previous research (27). The Mini-Mental State Examination (MMSE) (30) and Montreal Cognitive Assessment (MoCA) (31) acted as comprehensive tools to measure general cognitive function. Memory assessment involved the Auditory Verbal Learning Test (AVLT) (32) and recall in the Rey-Osterrieth Complex Figure Test (CFT) (33). Evaluating visuospatial ability involved administering the CFT copy and the Clock-Drawing Test (CDT) (34). Language proficiency was gauged using the Category Verbal Fluency Test (CVFT) (35) and the Boston Naming Test (BNT) (36). Attention was scrutinized via the Trail-Making Test (TMT) (37) part A and the Symbol Digit Modalities Tests (SDMT) (38), while executive function was measured by the TMT part B and the Stroop Color-Word Test (SCWT) (39).
2.3 Statistical analysis
Demographic characteristics, cognitive performance, and disease status were reported separately for the total sample (n = 1,062), the middle-aged sample (n = 451), and the older people sample (n = 611). One-way ANOVA or the χ2 test was used to test for significant differences between the groups. Given the known impact of age on cognitive function, participants were further categorized into middle-aged and older people sub sample. In these sub sample, the effects of regular statins use on each cognitive test was assessed using one-way ANCOVA, with age, gender, education, hypertension, and diabetes as concomitant variables. All analyses were performed in SPSS 27.0 (IBM Corp, Armonk, NY).
3 Results
Among the 7,625 participants screened in the BABRI database, participants from non-Beijing communities (n = 2,549) were first excluded, as well as those with incomplete basic medical records (n = 3,420) and incomplete cognitive assessments (n = 209). Based on this criterion, a total of 1,477 patients with dyslipidemia were identified. Of these, 385 patients were excluded due to unclear or non-standardized treatment records. Among the remaining 1,062 patients, 677 regularly used statins for more than 6 months (Statins group), while 385 patients did not receive any intervention (Untreated group). To investigate the potential benefits of statin usage across various age groups, the 1,062 patients were further divided into a middle-aged sample (n = 611) and an older people sample (n = 451). Within these two samples, the middle-aged statin group comprised 266 individuals, with the middle-aged untreated group consisting of 185 individuals. The older people statin group encompassed 411 individuals, whereas the older people untreated group comprised 200 individuals (see Figure 1).
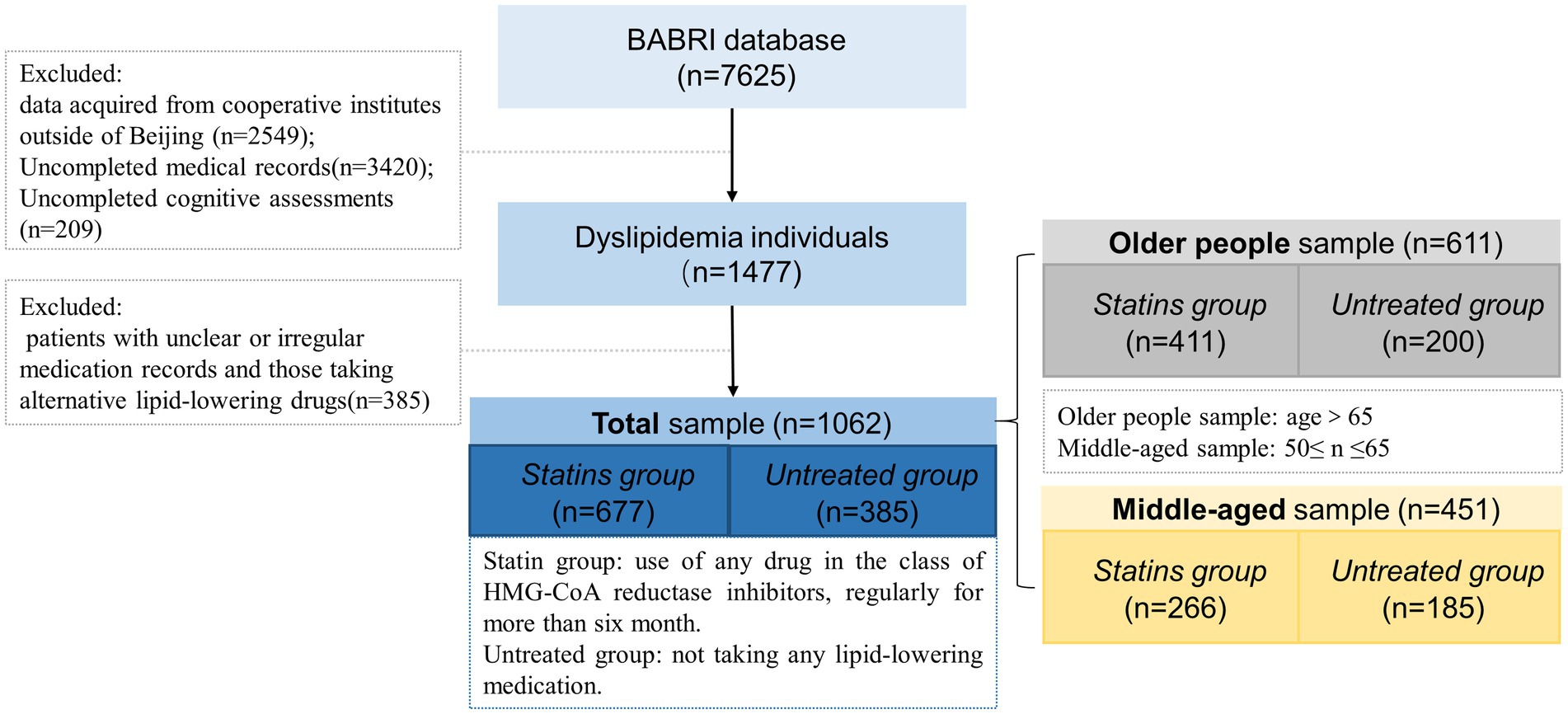
Figure 1. Flow chart of the Dyslipidemia patients inclusion process. The total data volume from the BABRI database (n = 7,625) presented in this flowchart is accurate up to January 2018. Since that time, the database has continually expanded, and BABRI has now enrolled over 10,000 participants.
Among the total sample of 1,062 participants with dyslipidemia, the average age was 65.91 ± 7.37 years. The statins group, with an average age of 66.46 ± 7.33 years, was older than the untreated group (64.95 ± 7.38 years, F = 10.4, p = 0.001). Education (F = 2.05, p = 0.15) and gender (χ2 = 0.02, p = 0.889) showed no significant differences between the two groups (Table 1). In the middle-aged sample, the statins group (59.21 ± 3.52 years) and untreated group (58.81 ± 3.73 years) exhibited no significant differences in age (F = 1.34, p = 0.25), education (F = 1.17, p = 0.28), and gender (χ2 = 1, p = 0.49) (Table 2). Within the older people sample (average age 76.4 ± 6.7 years), the statins group (71.16 ± 4.95 years) and untreated group (70.63 ± 4.97 years) showed no significant differences in age (F = 1.53, p = 0.21), education (F = 1.22, p = 0.27), or gender (χ2 = 0.52, p = 0.29) (Table 3). Simultaneously, both in the total sample and within the middle-aged and older people sub sample, the prevalence of hypertension and diabetes was significantly higher in the statins group in comparison to the untreated group.
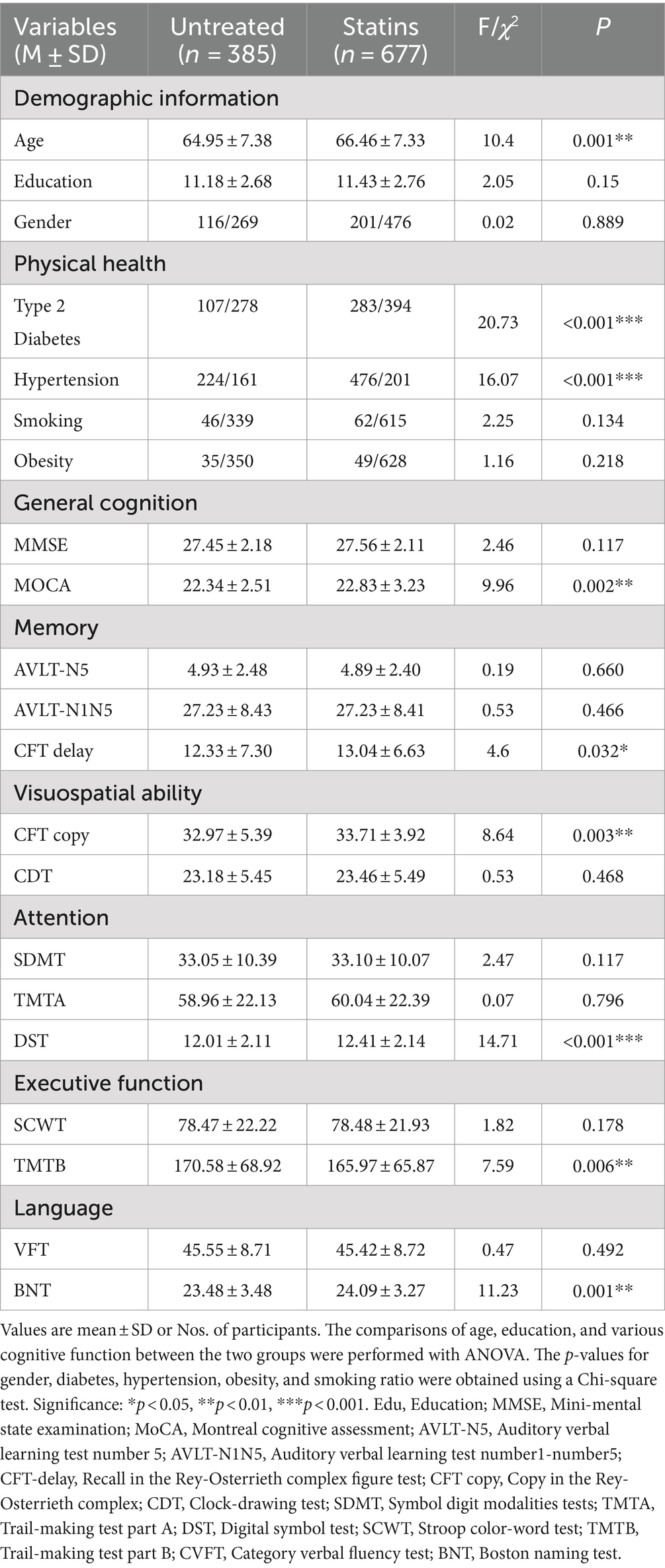
Table 1. Significant intergroup differences in demographic data and multidomain cognitive performance of the two groups in total patients.
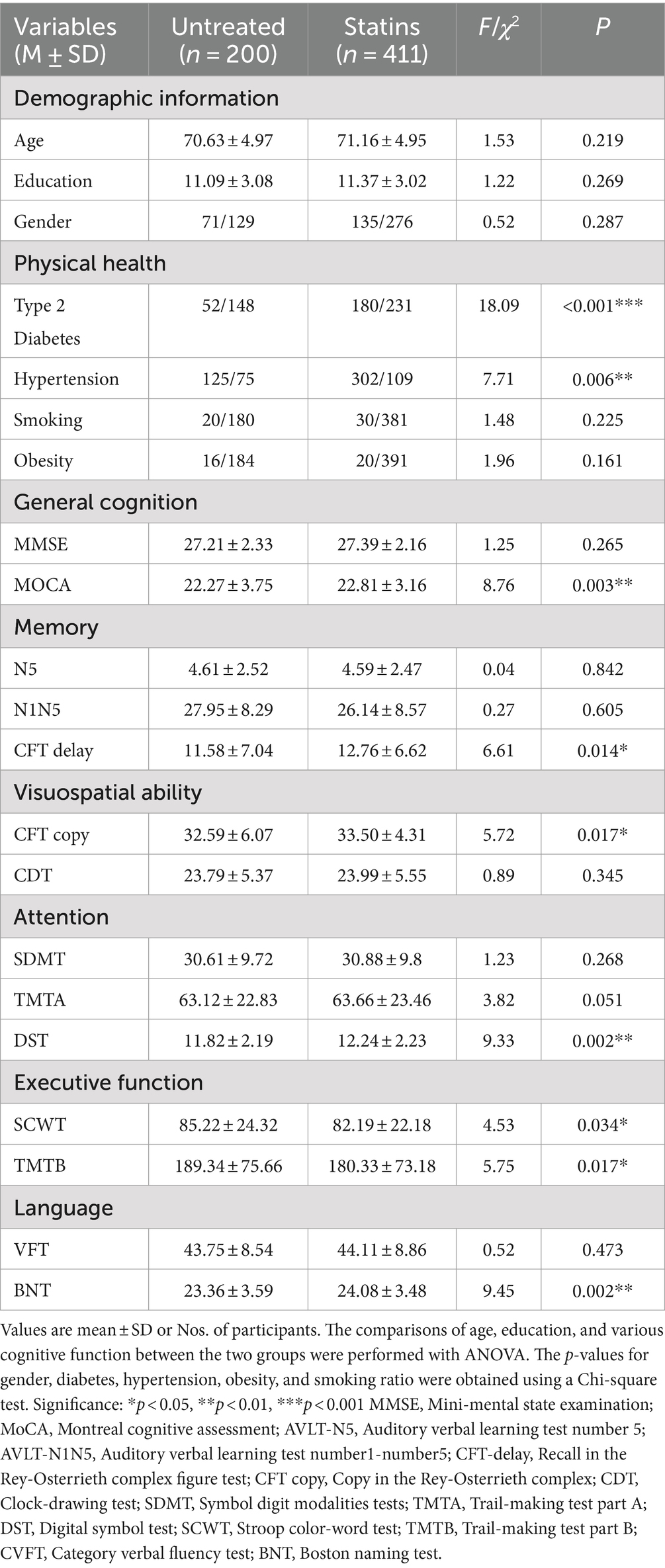
Table 2. Significant intergroup differences in demographic data and multi domain cognitive performance of the two groups in older people sample.
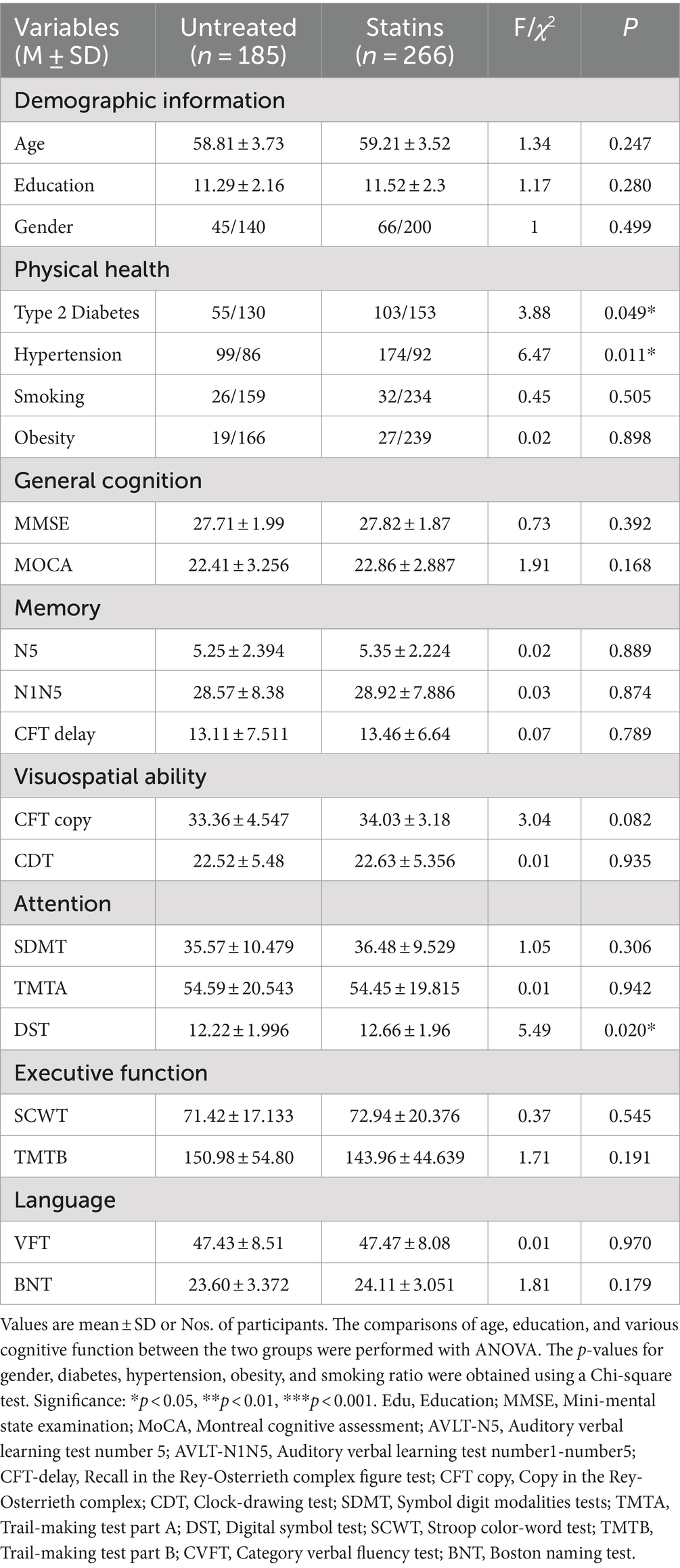
Table 3. Significant intergroup differences in demographic data and multidomain cognitive performance of the two groups in middle age patients.
In the comprehensive sample of 1,062 patients, significant differences were observed between the statin group and the untreated group across multiple cognitive domains, including general cognitive function (MoCA, F = 9.96, p = 0.002), memory (CFT delay, F = 4.6, p = 0.032), visual–spatial function (CFT copy, F = 8.64, p = 0.003), attention (DST, F = 14.71, p < 0.001), executive function (TMTB, F = 7.588, p = 0.006), and language (BNT, F = 11.23, p = 0.001) (see Table 1). In the older people sample, the statin group exhibited differences compared to the untreated group across general cognitive function (MoCA, F = 8.76, p = 0.003), memory (CFT delay, F = 6.61, p = 0.014), visual–spatial function (CFT copy, F = 5.72, p = 0.017), attention (DST, F = 9.33, p = 0.002), executive function (SCWT, F = 4.5, p = 0.034; TMTB, F = 5.75, p = 0.017), and language (BNT, F = 9.445, p = 0.002) (see Table 2). Finally, in the middle-aged sample, the statin group exhibited differences only in the attention domain (DST, F = 5.488, p = 0.02) (see Table 3).
4 Discussion
Our population-based study unveiled a significant correlation between sustained statin use and enhanced cognitive performance among dyslipidemia patients in Beijing communities. Even after adjusting for demographic variables and potential confounders, such as other cardiovascular risks, dyslipidemia patients who regularly took statins demonstrated notably improved cognitive performance, particularly in executive function, memory, and language. Furthermore, this trend persisted across both middle-aged and older people samples, albeit with a slight decrease observed in the middle-aged group and a more pronounced effect in individuals aged 65 and above.
The lipid peroxidation theory of dementia suggests that damage to the BBB in dementia patients leads to the entry of external lipids into the brain, resulting in the accumulation of “adipose inclusion” and abnormalities in brain lipid metabolism, brain cholesterol alters the degradation of amyloid precursor protein, triggering the onset of dementia and accelerating the progression of dementia (40, 41). Consequently, dyslipidemia is thought to be an important risk factor for cognitive dysfunction and dementia (42, 43). Cognitive impairment is frequently observed to be accompanied by elevated serum cholesterol and low-density lipoprotein (LDL) levels (44). Even after adjusting for factors such as age and the APOEε4 allele, an increase in serum cholesterol remains associated with a threefold increase in dementia risk (45). It is commonly believed that lipid-lowering therapy is thought to be beneficial in reducing the incidence of AD and delaying cognitive decline (46). Our study emphasizes that regular use of statin medications has a positive impact on the cognitive function of patients with dyslipidemia. In addition, the beneficial effect is more pronounced in individuals aged 65 and above, especially among those who are already susceptible to cognitive impairment. These results are in line with previous observational cohort studies (16, 47), whether employed for cholesterol regulation in dyslipidemia patients or as a preventive measure against various cardiovascular and cerebrovascular conditions (48), statins have demonstrated a favorable influence on cognitive function. The explanation for differences across older people and middle-aged samples can be attributed to the fact that older people dyslipidemia participants, who are more likely to use statin medications over an extended period and often have a higher prevalence of chronic conditions such as hypertension and diabetes, may benefit from the potential accumulation of long-term protective effects and the mitigation of cognitive decline by controlling underlying risk factors, as well as from potential neuroprotective effects due to their aging nervous system. The differences across different age groups have also been confirmed in previous studies, indicating that statin use can reduce the incidence of dementia in healthy older people populations (49). Certainly, dietary therapy is also an important treatment method for patients with dyslipidemia (50). In this study, all patients received dietary therapy guidance and advice from hospitals, community health service centers, and our team. However, since the majority of the dyslipidemia patients included in this study are from northern China, where dietary habits tend to be rich in oil, salt, sugar, and fat, it is challenging for them to adhere to a healthy dietary regimen. Therefore, for most patients, taking medication regularly is easier than maintaining a healthy diet.
Additionally, it is worth noting that this study primarily examines the benefits of sustained statin use in community-dwelling patients with dyslipidemia, with a focus on a population that is not comprised of cognitive impairment or dementia patients. Therefore, it does not address potential time-dependent confounding factors related to worsened medication adherence due to cognitive impairment (51). Of course, there are conflicting views in epidemiological studies regarding the impact of statin drugs on dementia (51, 52), particularly in randomized controlled trials (RCTs) where the use of statin drugs has been shown not to reduce the risk of dementia (53, 54). Currently, there is no consensus on the potential efficacy of statins in preventing dementia or AD (22, 51). Padala et al. (55, 56) found in their studies on populations with dementia and cognitive impairment that statin use can lead to cognitive decline, while discontinuing statins may result in the reversal of cognitive impairment. In contrast, the Rotterdam Study found that statin use, whether lipophilic or hydrophilic, was associated with a reduced risk of Alzheimer’s disease in the general population (57). An absence of a consensus may stem from differences in experimental design across studies, such as the selection of study populations. Patients who already have cognitive impairments may respond differently to medication compared to those with normal cognition. This is particularly relevant for lipophilic statins, which may regulate cholesterol production in the brain, affecting neuronal structure and function and leading to transient cognitive decline (69). Additionally, factors such as study duration, medication dosage and concurrent drug use, the cognitive assessment tools used, and potential biases introduced by different study populations may also contribute to the variability in results. However, patients who accept and continue statin therapy are significantly associated with their education level, socioeconomic status, and cholesterol levels, all of which are closely related to the risk of dementia. Since the participants in this study were all older individuals from urban areas, this group tends to have better health awareness and medication adherence compared to older individuals from rural areas, this “healthy user effect” is also one of the issues that this study needs to address (58).
Our study has several strengths. Firstly, it explores the relationship between statin medication use and cognitive function in a relatively large community population, reflecting real-world conditions. Additionally, the study provides a comprehensive assessment of multidimensional cognitive function in all dyslipidemia patients. The findings indicate that the statins group outperformed the untreated group in various cognitive domains. Previous research has often been limited by focusing solely on employing one or two cognitive tests (47, 53), while our study comprehensively assessed the impact of statins on cognitive function in various domains among dyslipidemia patients. Furthermore, this study also investigated the relationship between statin medications and cognitive function in patients with dyslipidemia across different age groups. The findings revealed that the benefits of regular statin use are more significant in the older people sample, while in the middle-aged group, cognitive function gains from regular statin use are only evident as a trend.
While this study provides valuable insights, it is not without limitations. First, as a cross-sectional study, it cannot determine the long-term effects of statins on individuals with dyslipidemia. Longitudinal follow-up studies or well-designed randomized controlled trials are needed to optimize experimental design and analysis methods. These studies should precisely account for various confounding variables, including lipid fluctuations (59), different genetic variations (such as APOE, LDLR, CETP, etc.) (60, 61), guidelines for treatment of dyslipidemia, statin dosage (62, 63), and sex differences (64), to minimize bias and accurately quantify the specific cognitive benefits of statins for individuals with dyslipidemia. Additionally, this study solely explores the impact of statin therapy on the cognitive function of dyslipidemia patients and does not include individuals at cardiovascular risk who use statins for preventive purposes. Due to constraints in acquiring biological specimens, this study was not able to account for the APOE ε4 carriage status among subjects. Recent research suggests that the advantageous cognitive effects of statin therapy might be more pronounced among carriers of the APOE ε4 allele (65). Additionally, it is important to note that due to limitations in the available data, our study did not differentiate between the hydrophilic and lipophilic properties of statin medications among participants. While prior research has explored this issue, consensus on whether different types of statins exhibit divergent effects on cognition remains inconclusive (23, 66). Despite lipophilic statins being more likely to enter the central nervous system compared to hydrophilic statins, hydrophilic statins can also cross the blood–brain barrier with the assistance of certain active transport proteins, such as the OATP family transporters (67, 68). The impact of these confounding factors may constrain inferences drawn from observational studies, leading to conclusions that could vary to some extent based on the specific cohorts examined and the potential confounding variables controlled for in multivariate analyses.
5 Conclusion
Our population-based study has unveiled a notable correlation between consistent statin usage and improved cognitive performance among dyslipidemia patients residing in Beijing communities. Even after adjusting for demographic variables and potential confounders, such as other cardiovascular risks, dyslipidemia patients who maintained regular statin intake exhibited enhanced cognitive performance, notably in executive function, memory, and language. Moreover, this effect remained consistent across both middle-aged and older people samples, although a slight decrease was observed in the middle-aged group compared to a more pronounced impact in individuals aged 65 and above. Looking ahead, there is a pressing need for long-term follow-up studies or meticulously designed randomized controlled trials to comprehensively understand and quantify the specific cognitive benefits conferred by statin medications in dyslipidemia patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Imaging Center for Brain Research at Beijing Normal University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WW: Writing – original draft. XL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of China (grant no. 32171085).
Acknowledgments
We thank all the volunteers for their participation in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vaughan, CJ, and Gotto, AM Jr. Update on statins: 2003. Circulation. (2004) 110:886–92. doi: 10.1161/01.CIR.0000139312.10076.BA
2. Goldstein, JL, and Brown, MS. Regulation of the mevalonate pathway. Nature. (1990) 343:425–30. doi: 10.1038/343425a0
3. Mortensen, MB, and Falk, E. Primary prevention with statins in the elderly. J Am Coll Cardiol. (2018) 71:85–94. doi: 10.1016/j.jacc.2017.10.080
4. Caniglia, EC, Rojas-Saunero, LP, Hilal, S, Licher, S, Logan, R, Stricker, B, et al. Emulating a target trial of statin use and risk of dementia using cohort data. Neurology. (2020) 95:e1322–32. doi: 10.1212/WNL.0000000000010433
5. Zingel, R, Bohlken, J, Riedel-Heller, S, Barth, S, and Kostev, K. Association between low-density lipoprotein cholesterol levels, statin use, and dementia in patients followed in German general practices. J Alzheimers Dis. (2021) 79:37–46. doi: 10.3233/JAD-201176
6. Fong, CW . Statins in therapy: understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur J Med Chem. (2014) 85:661–74. doi: 10.1016/j.ejmech.2014.08.037
7. Sierra, S, Ramos, MC, Molina, P, Esteo, C, Vázquez, JA, and Burgos, JS. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis. (2011) 23:307–18. doi: 10.3233/JAD-2010-101179
8. Reis, PA, Alexandre, PC, D’Avila, JC, Siqueira, LD, Antunes, B, Estato, V, et al. Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav Immun. (2017) 60:293–303. doi: 10.1016/j.bbi.2016.11.006
9. Roy, A, Jana, M, Kundu, M, Corbett, GT, Rangaswamy, SB, Mishra, RK, et al. HMG-CoA reductase inhibitors bind to PPARα to upregulate neurotrophin expression in the brain and improve memory in mice. Cell Metab. (2015) 22:253–65. doi: 10.1016/j.cmet.2015.05.022
10. Lu, D, Qu, C, Goussev, A, Jiang, H, Lu, C, Schallert, T, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. (2007) 24:1132–46. doi: 10.1089/neu.2007.0288
11. Segatto, M, Manduca, A, Lecis, C, Rosso, P, Jozwiak, A, Swiezewska, E, et al. Simvastatin treatment highlights a new role for the isoprenoid/cholesterol biosynthetic pathway in the modulation of emotional reactivity and cognitive performance in rats. Neuropsychopharmacology. (2014) 39:841–54. doi: 10.1038/npp.2013.284
12. Robin, NC, Agoston, Z, Biechele, TL, James, RG, Berndt, JD, and Moon, RT. Simvastatin promotes adult hippocampal neurogenesis by enhancing Wnt/β-catenin signaling. Stem Cell Rep. (2014) 2:9–17. doi: 10.1016/j.stemcr.2013.11.002
13. Wu, H, Lu, D, Jiang, H, Xiong, Y, Qu, C, Li, B, et al. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. (2008) 25:130–9. doi: 10.1089/neu.2007.0369
14. Fracassi, A, Marangoni, M, Rosso, P, Pallottini, V, Fioramonti, M, Siteni, S, et al. Statins and the brain: more than lipid lowering agents? Curr Neuropharmacol. (2019) 17:59–83. doi: 10.2174/1570159X15666170703101816
15. Benito-León, J, Louis, ED, Vega, S, and Bermejo-Pareja, F. Statins and cognitive functioning in the elderly: a population-based study. J Alzheimers Dis. (2010) 21:95–102. doi: 10.3233/JAD-2010-100180
16. Bettermann, K, Arnold, AM, Williamson, J, Rapp, S, Sink, K, Toole, JF, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis. (2012) 21:436–44. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.002
17. Beydoun, MA, Beason-Held, LL, Kitner-Triolo, MH, Beydoun, HA, Ferrucci, L, Resnick, SM, et al. Statins and serum cholesterol's associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health. (2011) 65:949–57. doi: 10.1136/jech.2009.100826
18. Parker, BA, Polk, DM, Rabdiya, V, Meda, SA, Anderson, K, Hawkins, KA, et al. Changes in memory function and neuronal activation associated with atorvastatin therapy. Pharmacotherapy: the journal of human pharmacology and drug. Therapy. (2010) 30:625–5. doi: 10.1592/phco.30.6.625
19. Poly, TN, Islam, MM, Walther, BA, Yang, H-C, Wu, C-C, Lin, M-C, et al. Association between use of statin and risk of dementia: a meta-analysis of observational studies. Neuroepidemiology. (2020) 54:214–26. doi: 10.1159/000503105
20. Muldoon, MF, Barger, SD, Ryan, CM, Flory, JD, Lehoczky, JP, Matthews, KA, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. (2000) 108:538–46. doi: 10.1016/S0002-9343(00)00353-3
21. Muldoon, MF, Ryan, CM, Sereika, SM, Flory, JD, and Manuck, SB. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med. (2004) 117:823–9. doi: 10.1016/j.amjmed.2004.07.041
22. Schultz, BG, Patten, DK, and Berlau, DJ. The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Transl Neurodegen. (2018) 7:1–11. doi: 10.1186/s40035-018-0110-3
23. Swiger, KJ, Manalac, RJ, Blumenthal, RS, Blaha, MJ, and Martin, SS. Statins and cognition: a systematic review and meta-analysis of short-and long-term cognitive effects. Mayo Clin Proc. (2013) 88:1213–21. doi: 10.1016/j.mayocp.2013.07.013
24. Ott, BR, Daiello, LA, Dahabreh, IJ, Springate, BA, Bixby, K, Murali, M, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. (2015) 30:348–58. doi: 10.1007/s11606-014-3115-3
25. Richardson, K, Schoen, M, French, B, Umscheid, CA, Mitchell, MD, Arnold, SE, et al. Statins and cognitive function: a systematic review. Ann Intern Med. (2013) 159:688–97. doi: 10.7326/0003-4819-159-10-201311190-00007
26. Jick, H, Zornberg, G, Jick, S, Seshadri, S, and Drachman, D. Statins and the risk of dementia. Lancet. (2000) 356:1627–31. doi: 10.1016/S0140-6736(00)03155-X
27. Li, X, Ma, C, Zhang, J, Liang, Y, Chen, Y, Chen, K, et al. Prevalence of and otential risk factors for mild cognitive impairment in community-dwelling residents of Beijing. J Am Geriatr Soc. (2013) 61:2111–9. doi: 10.1111/jgs.12552
28. Yang, C, Li, X, Zhang, J, Chen, Y, Li, H, Wei, D, et al. Early prevention of cognitive impairment in the community population: the Beijing aging brain Rejuvenation Initiative. Alzheimers Dement. (2021) 17:1610–8. doi: 10.1002/alz.12326
29. Grundy, SM, Stone, NJ, Bailey, AL, Beam, C, Birtcher, KK, Blumenthal, RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2019) 73:e285–350. doi: 10.1016/j.jacc.2018.11.003
30. Katzman, R, Zhang, MY, Ouang Ya, Q, Wang, ZY, Liu, WT, Yu, E, et al. A Chinese version of the Mini-mental state examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. (1988) 41:971–8. doi: 10.1016/0895-4356(88)90034-0
31. Nasreddine, ZS, Phillips, NA, Bédirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
32. Ivnik, RJ, Malec, JF, Tangalos, EG, Petersen, RC, Kokmen, E, and Kurland, LT. The auditory-verbal learning test (AVLT): norms for ages 55 years and older. Psychol Assessment J Consulting Clin Psychol. (1990) 2:304–12. doi: 10.1037/1040-3590.2.3.304
33. Osterrieth, PA . Le test de copie d'une figure complexe; contribution a l'etude de la perception et de la memoire. Arch Psychol. (1944) 30:206–356.
34. Rouleau, I, Salmon, DP, Butters, N, Kennedy, C, and McGuire, K. Quantitative and qualitative analyses of clock drawings in Alzheimer's and Huntington's disease. Brain Cogn. (1992) 18:70–87. doi: 10.1016/0278-2626(92)90112-Y
35. Eysenck, H . Primary mental abilities (psychometric monographs no. 1). Br J Educ Psychol. (1939) 9:270–5. doi: 10.1111/j.2044-8279.1939.tb03214.x
36. Williams, BW, Mack, W, and Henderson, VW. Boston naming test in Alzheimer's disease. Neuropsychologia. (1989) 27:1073–9. doi: 10.1016/0028-3932(89)90186-3
37. Partington, J. E., and Leiter, R. G. (1949). Partington's pathways test. Psychol Serv Center J. 1:11–20.
39. Stroop, JR . Studies of interference in serial verbal reactions. J Exp Psychol. (1935) 18:643–62. doi: 10.1037/h0054651
40. Martins, IJ, Berger, T, Sharman, MJ, Verdile, G, Fuller, SJ, and Martins, RN. Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J Neurochem. (2009) 111:1275–308. doi: 10.1111/j.1471-4159.2009.06408.x
41. Vance, JE . Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech. (2012) 5:746–55. doi: 10.1242/dmm.010124
42. Li, G, Shofer, J, Kukull, W, Peskind, E, Tsuang, D, Breitner, J, et al. Serum cholesterol and risk of Alzheimer disease: a community-based cohort study. Neurology. (2005) 65:1045–50. doi: 10.1212/01.wnl.0000178989.87072.11
43. Vemuri, P, Lesnick, TG, Przybelski, SA, Knopman, DS, Lowe, VJ, Graff-Radford, J, et al. Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol. (2017) 82:706–18. doi: 10.1002/ana.25071
44. Liu, Y, Zhong, X, Shen, J, Jiao, L, Tong, J, Zhao, W, et al. Elevated serum TC and LDL-C levels in Alzheimer’s disease and mild cognitive impairment: a meta-analysis study. Brain Res. (2020) 1727:146554. doi: 10.1016/j.brainres.2019.146554
45. Notkola, I-L, Sulkava, R, Pekkanen, J, Erkinjuntti, T, Ehnholm, C, Kivinen, P, et al. Serum Total cholesterol, apolipoprotein E {FC12}e4 allele, and Alzheimer’s disease. Neuroepidemiology. (1998) 17:14–20. doi: 10.1159/000026149
46. Smith, KB, Kang, P, Sabbagh, MN, and Initiative, A. The effect of statins on rate of cognitive decline in mild cognitive impairment. Alzheimer's Dementia Transl Res Clin Interv. (2017) 3:149–56. doi: 10.1016/j.trci.2017.01.001
47. Bernick, C, Katz, R, Smith, N, Rapp, S, Bhadelia, R, Carlson, M, et al. Statins and cognitive function in the elderly: the cardiovascular health study. Neurology. (2005) 65:1388–94. doi: 10.1212/01.wnl.0000182897.18229.ec
48. Sun, M, Chen, W-M, Wu, S-Y, and Zhang, J. Protective effects against dementia undergo different statin type, intensity, and cumulative dose in older adult type 2 diabetes mellitus patients. J Am Med Dir Assoc. (2024) 25:470–479.e1. e471. doi: 10.1016/j.jamda.2023.11.010
49. Steenland, K, Zhao, L, Goldstein, FC, and Levey, AI. Statins and cognitive decline in older adults with normal cognition or mild cognitive impairment. J Am Geriatr Soc. (2013) 61:1449–55. doi: 10.1111/jgs.12414
50. Ahmed, SM, Clasen, ME, and Donnelly, JF. Management of dyslipidemia in adults. Am Fam Physician. (1998) 57:2192–204.
51. Power, MC, Weuve, J, Sharrett, AR, Blacker, D, and Gottesman, RF. Statins, cognition, and dementia—systematic review and methodological commentary. Nat Rev Neurol. (2015) 11:220–9. doi: 10.1038/nrneurol.2015.35
52. Smeeth, L, Douglas, I, Hall, AJ, Hubbard, R, and Evans, S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. (2009) 67:99–109. doi: 10.1111/j.1365-2125.2008.03308.x
53. Sano, M, Bell, K, Galasko, D, Galvin, J, Thomas, R, Van Dyck, C, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. (2011) 77:556–63. doi: 10.1212/WNL.0b013e318228bf11
54. Trompet, S, van Vliet, P, de Craen, AJ, Jolles, J, Buckley, BM, Murphy, MB, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. (2010) 257:85–90. doi: 10.1007/s00415-009-5271-7
55. Padala, KP, Padala, PR, McNeilly, DP, Geske, JA, Sullivan, DH, and Potter, JF. The effect of HMG-CoA reductase inhibitors on cognition in patients with Alzheimer's dementia: a prospective withdrawal and rechallenge pilot study. Am J Geriatr Pharmacother. (2012) 10:296–302. doi: 10.1016/j.amjopharm.2012.08.002
56. Padala, KP, Padala, PR, and Potter, JF. Statins: a case for drug withdrawal in patients with dementia. J Am Geriatr Soc. (2010) 58:1214–6. doi: 10.1111/j.1532-5415.2010.02889.x
57. Haag, MD, Hofman, A, Koudstaal, PJ, Stricker, BH, and Breteler, MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam study. J Neurol Neurosurg Psychiatry. (2009) 80:13–7. doi: 10.1136/jnnp.2008.150433
58. Brookhart, MA, Patrick, AR, Dormuth, C, Avorn, J, Shrank, W, Cadarette, SM, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. (2007) 166:348–54. doi: 10.1093/aje/kwm070
59. Moser, ED, Manemann, SM, Larson, NB, St Sauver, JL, Takahashi, PY, Mielke, MM, et al. Association between fluctuations in blood lipid levels over time with incident Alzheimer disease and Alzheimer disease–related dementias. Neurology. (2023) 101:e1127–36. doi: 10.1212/WNL.0000000000207595
60. de Oliveira, FF, Bertolucci, PHF, Chen, ES, and Smith, MC. Pharmacogenetic analyses of therapeutic effects of lipophilic statins on cognitive and functional changes in Alzheimer’s disease. J Alzheimers Dis. (2022) 87:359–72. doi: 10.3233/JAD-215735
61. de Oliveira, FF, Chen, ES, Smith, MC, and Bertolucci, PHF. Selected LDLR and APOE polymorphisms affect cognitive and functional response to lipophilic statins in Alzheimer’s disease. J Mol Neurosci. (2020) 70:1574–88. doi: 10.1007/s12031-020-01588-7
62. Li, G, Higdon, R, Kukull, W, Peskind, E, Van Valen Moore, K, Tsuang, D, et al. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. (2004) 63:1624–8. doi: 10.1212/01.WNL.0000142963.90204.58
63. Zhang, X, Wen, J, and Zhang, Z. Statins use and risk of dementia: a dose–response meta analysis. Medicine. (2018) 97:e11304. doi: 10.1097/MD.0000000000011304
64. Sun, L, Hu, C, Zheng, C, Huang, Z, Lv, Z, Huang, J, et al. Gene–gene interaction between CETP and APOE polymorphisms confers higher risk for hypertriglyceridemia in oldest-old Chinese women. Exp Gerontol. (2014) 55:129–33. doi: 10.1016/j.exger.2014.04.003
65. Geifman, N, Brinton, RD, Kennedy, RE, Schneider, LS, and Butte, AJ. Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer’s disease. Alzheimers Res Ther. (2017) 9:1–10. doi: 10.1186/s13195-017-0237-y
66. Wong, WB, Lin, VW, Boudreau, D, and Devine, EB. Statins in the prevention of dementia and Alzheimer's disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf. (2013) 22:345–58. doi: 10.1002/pds.3381
67. Niemi, M . Role of OATP transporters in the disposition of drugs. Pharmacogenomics. (2007) 8:787–802. doi: 10.2217/14622416.8.7.787
68. Ronaldson, PT, Brzica, H, Abdullahi, W, Reilly, BG, and Davis, TP. Transport properties of statins by organic anion transporting polypeptide 1A2 and regulation by transforming growth factor-β signaling in human endothelial cells. J Pharmacol Exp Ther. (2021) 376:148–60. doi: 10.1124/jpet.120.000267
Keywords: cognitive function, statins, dyslipidemia, aging cohorts, middle-aged, elderly
Citation: Wang W and Li X (2024) Cognitive function in dyslipidemia patients: exploring the impact of statins. Front. Neurol. 15:1436010. doi: 10.3389/fneur.2024.1436010
Edited by:
Takao Yamasaki, Minkodo Minohara Hospital, JapanReviewed by:
Fabricio Ferreira de Oliveira, Elysian Clinic, BrazilManjula Ramu, Yale University, United States
David M. Diamond, University of South Florida, United States
Copyright © 2024 Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, bGl4aW45OUBibnUuZWR1LmNu
 Wenxiao Wang
Wenxiao Wang Xin Li
Xin Li