Abstract
Introduction:
Patients with Parkinson's disease (pwPD) and atypical parkinsonism usually complain of impaired balance. Instrumental posturography is widely used to quantitatively assess static balance in pwPD but many posturographic parameters and protocols have been suggested. We aimed to appraise the use of static posturography in pwPD and atypical parkinsonism, and identify gaps hindering its translation into clinical routine.
Methods:
A systematic review on four databases. Study methodology, clinical aspects, assessment protocol, technical aspects, and transferability to clinical practice were critically appraised by a set of quality questions, scored on three levels (0, 0.5, 1). Total scores were used to assess overall studies' quality.
Results:
132 studies were included. The majority (105/132) was rated medium-quality. The domains “transferability to clinical practice” and “assessment protocol” received the lowest scores. The main flaw hindering portability to clinical settings was the lack of a stated rationale behind the choice of a specific protocol and the selection of the posturographic parameters. Missing reporting about the technical aspects employed to manage posturographic data and comprehensive instructions given to the patients further contributed to lower quality.
Discussion:
We provided recommendations for enhancing the clinical transferability of studies on static posturography to assess pwPD, including (1) discussing the rationale for choosing the assessment protocols and posturographic parameters, (2) detailing the inclusion criteria and select appropriate samples, and (3) reporting all the technical information to replicate the procedures and computations.
Systematic review registration:
International Prospective Register of Systematic Reviews (PROSPERO) on 6th February 2024 (ID CRD42024500777), https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024500777.
1 Introduction
Patients with Parkinson's disease (pwPD) or atypical parkinsonism [e.g., multiple system atrophy (MSA), progressive supranuclear palsy (PSP)] (1, 2) usually complain of impaired balance, reduced postural reflexes, freezing of gait, festination, and difficulty in reacting to an external perturbance (3). It has been estimated that pwPD experience two to three times more falls than healthy older adults (4–7). Such increased risk of falls and associated injuries progressively reduce patients' autonomy from caregivers and affect their quality of life (8).
The assessment of balance in pwPD is usually based on clinical scales (9–13), providing a qualitative evaluation of postural instability. Other dynamic tests have been developed to obtain quantitative information about patients' ability to maintain postural control (13–15). These assessments have been suggested to be more sensitive to advanced stages of PD, while they fail to detect differences in the early stages of the disease (16).
Instrumental posturography represents a valuable technique for assessing and monitoring pwPD, providing centimeter-accurate measurements, and therefore capable of early discriminating different patterns among patients (16). It has been suggested to monitor disease progression and understand the effectiveness of physiotherapeutic, pharmacological therapy, and invasive treatments such as Deep Brain Stimulation (DBS) (17–19). The wide availability of instrumentation for stabilometric assessment, with relatively low costs, has encouraged its dissemination in healthcare institutions, leading to increased scientific publications on the topic. A search on Pubmed, including the keywords “Parkinson's disease” and “posturography,” yielded nearly 500 scientific articles published in the past 24 years, with about 40 papers/year in recent years. More than 80% of these papers were published in Journals categorized as clinical, according to Scimago Journal and Country Rank (20). The remaining 20% have been published in engineering or multidisciplinary journals and focused on developing new indicators to be extracted from posturography data to describe patients' conditions. On the one hand, this helps to refine the research for the best outcome measures able to discriminate different patients; on the other hand, newly generated parameters must have a relevant clinical meaning for clinicians to interpret them accurately and integrate them effectively into their daily practice. Among the main limiting factors of instrumental posturography highlighted in the literature there are: the absence of a defined “normal pattern”, the lack of standardization of the protocols, and the large number of parameters that can be computed (21). This heterogeneous array of options in the literature, particularly in the absence of engineering expertise, can lead to uncertainty among physicians who need to select which parameter to use in their daily practice. Transferability to clinical practice should be the ultimate goal of biomedical research, aimed at improving clinicians' knowledge and, consequently, patient management from diagnosis to treatment.
We designed a critical appraisal to evaluate whether the reporting of studies on static posturography in pwPD is sufficiently comprehensive or requires targeted guidance to enhance quality. This approach aligns with similar reviews of other innovative methods, such as gait analysis combined with artificial intelligence (22). While previous works have clearly highlighted the heterogeneity of outcomes used in posturography research (16, 23, 24), none have offered practical guidance to establish a reference framework for improving the reporting of future studies.
This study aims to systematically review the literature on the use of static posturography to assess pwPD and atypical parkinsonism, appraise its clinical and technical aspects, and identify gaps hindering its translation into the clinical routine. The findings will be used to offer valuable recommendations aimed at enhancing external validity and replicability of future studies.
2 Methods
The current systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) 2020 guideline (25).
To increase this research's clarity, transparency, and reproducibility, the protocol was a-priori registered on the International Prospective Register of Systematic Reviews (PROSPERO) on 6th February 2024 (ID CRD42024500777).
2.1 Research question and strategies
The leading question for this investigation was: “How is posturography used in the assessment of static balance in pwPD and atypical parkinsonism?”.
A scientific librarian developed comprehensive and systematic searches. We did not use the PICO framework because neither the Intervention nor the Comparison components could be defined for most studies included in this review, which does not focus on evaluating the effectiveness of a specific intervention on a particular outcome. Therefore, the search strings were developed through an iterative process. The research was conducted in January 2024 within the following databases: Medline, Cinahl, Embase, and Scopus. Furthermore, a manual cross-reference search was performed on the reference lists of included articles. No time limits were set for publications to be included.
The complete search strategies can be consulted in Table 1.
Table 1
| Database | Strategy |
|---|---|
| Medline (Pubmed) | (Parkinson* OR canvas OR “multisystem* atrophy” OR “progressive supranuclear palsy” OR corticobasal OR “GBA Parkinson” OR ”Parkinson Disease“[Mesh] OR ”Parkinsonian Disorders“[Mesh]) AND (balance OR stability OR equilibrium OR ”Postural Balance“[Mesh]) AND (posturograph* OR ”force plate“ OR stabilo* OR ”center of pressure“ OR ”centre of pressure“ OR COP) |
| Cinhal | ('parkinson disease'/mj OR 'parkinsonism'/mj OR parkinson*:ab,ti OR canvas:ab,ti OR 'multisystem* atrophy':ab,ti OR 'progressive supranuclear palsy':ab,ti OR corticobasal:ab,ti OR 'gba parkinson':ab,ti) AND (balance:ab,ti OR stability:ab,ti OR equilibrium:ab,ti OR 'body equilibrium'/exp) AND (posturograph*:ab,ti OR 'force plate':ab,ti OR stabilo*:ab,ti OR 'center of pressure':ab,ti OR 'centre of pressure':ab,ti OR cop:ab,ti) |
| Embase | [(MH ”Parkinson Disease“) OR (MH ”Parkinsonian Disorders“)] OR TI (Parkinson* OR canvas OR “multisystem* atrophy” OR “progressive supranuclear palsy” OR corticobasal OR “GBA Parkinson”) OR AB (Parkinson* OR canvas OR “multisystem* atrophy” OR “progressive supranuclear palsy” OR corticobasal OR “GBA Parkinson”) AND (MH ”Balance, Postural“) OR TI (balance OR stability OR equilibrium) OR AB (balance OR stability OR equilibrium) AND TI (posturograph* OR ”force plate“ OR stabilo* OR ”center of pressure“ OR ”centre of pressure“ OR COP) OR AB (posturograph* OR ”force plate“ OR stabilo* OR ”center of pressure“ OR ”centre of pressure“ OR COP) |
| Scopus | [TITLE-ABS-KEY (parkinson* OR canvas OR ”multisystem* atrophy“ OR ”progressive supranuclear palsy“ OR corticobasal OR ”GBA Parkinson“) AND TITLE-ABS-KEY (balance OR stability OR equilibrium) AND TITLE-ABS-KEY (posturograph* OR ”force plate“ OR stabilo* OR ”center of pressure“ OR ”centre of pressure“ OR cop)] |
Search strategies, according to each database.
2.2 Eligibility criteria
The inclusion criteria were: (a) studies involving adult pwPD or other types of atypical parkinsonism; (b) studies investigating pwPD using static posturography, with any protocol that included barefoot bipedal upright stance acquired with eyes open on a firm surface, which will be from now on considered the “baseline condition” (along with the baseline condition, protocols could then involve other conditions, e.g., open/closed eyes, feet apart/together, firm/foam surface, cognitive tasks); (c) primary peer-reviewed studies (e.g., RCT, clinical study, observational study); (d) full text available in English.
The exclusion criteria were: (a) studies including only healthy individuals or patients with other pathologies; (b) studies not involving humans (i.e., modeling studies); (c) acquisition protocol based on dynamic posturography only (e.g., Sensory Organization Test, Functional Reach Test); (d) sessions acquired while wearing goggles or similar headsets; (e) studies in which patients were provided onscreen feedback of the position of their center of pressure to stabilize during the assessment; (f) patients holding their arms away from the body so that the center of pressure is shifted (e.g., Romberg position).
We were interested in all outcome measures derived from static posturography acquired in the baseline condition.
2.3 Study selection
After all databases were searched, reports were exported to EndNote20 (Clarivate Analytics, PA, USA), and duplicates were removed. The remaining studies were imported to Rayyan (26) online software. Two independent reviewers (LC and MCB) blindly screened their title, abstract, and full text. Discrepancies were discussed in a consensus meeting. If agreement could not be reached, a third researcher was called upon to solve any discrepancy (AM).
2.4 Data extraction and report
Two reviewers (MCB and LC) independently extracted the data. When the computation methods of the parameters were unclear, a third author (AM), a bioengineer with 20 years of experience in instrumental motion and posture analysis, was consulted. When necessary, the authors were contacted by email to obtain missing data from the reports, and information was added if they replied in 1 month.
The following information was summarized in a custom table: first author, year of publication, journal, aims of the study, sample size, patients' demographic and clinical characteristics, any treatment undergone by patients, protocol adopted for the posturographic assessment (including different conditions tested, number and duration of repetitions, and dosage of any drugs or brain stimulation), task conditions (including foot positioning, gaze fixation, and arm positions), technical details of the instrumentations and data processing (e.g., filters applied), any posturographic parameter derived from baseline posture. Parameters (means and standard deviations, median, or ranges) were retrieved from text, tables, or obtained from figures when necessary. Findings were also presented in a narrative synthesis, grouped by the five domains illustrated in the following paragraph.
2.5 Quality assessment
The three reviewers involved in the previous steps critically appraised the included studies. In case of doubts about the clinical characteristics of the sample, a neurologist with extensive expertise in pwPD was consulted.
To standardize the quality assessment procedure, a tailored set of quality questions was designed, tested on a random sample of 30 studies, and refined progressively until its final version, reported in Table 2.
Table 2
| Q | Domain | Question |
|---|---|---|
| Q1 | Study methodology | Are the objectives of the study clearly stated? 0 = completely not stated 0.5 = authors described/introduced what they did 1 = the aim is clearly stated at the end of the introduction |
| Q2 | Transferability to clinical practice | Are the objectives linked to daily clinical applications? 0 = no clinical application introduced 0.5 = clinical applications are not stated but can be derived from the introduction 1 = clinical applications are explicitly stated |
| Q3 | Study methodology | Is the study design stated and the manuscript written accordingly? 0 = study design is not stated and the paper is not structured according to any appropriated checklist 0.5 = study design is not explicitly stated but the paper is structured according to the specific checklist 1 = study design is made clear in the manuscript and the paper is written accordingly |
| Q4 | Clinical aspects | Are the characteristics of the sample sufficiently described (inclusion and diagnostic criteria, age, severity of the disease, etc.)? 0 = no disease-related information is provided to characterize the sample 0.5 = general inclusion criteria or sample characteristics are provided (diagnostic criteria only, or clinical scales only) 1 = sample is described according to diagnostic criteria, precise inclusion criteria, and clinical scales specific for the pathology (only for DBS study: stimulation parameters reported) |
| Q5 | Study methodology | Are the sample characteristics adequate to address the aim of the study in terms of disease severity (e.g., homogeneity or stratification in descriptive and intervention studies, values distributed between minimum and maximum possible values in correlation studies)? NA = not applicable (for case studies or case series) 0 = no disease-related information is provided 0.5 = descriptive/intervention study includes a heterogeneous sample without stratification; correlation study includes a homogeneous sample but this represents only a subgroup of the population 1 = descriptive/intervention study includes a homogeneous sample or stratifies it; correlation study includes a heterogeneous sample representing the entire population |
| Q6 | Study methodology | Is the included sample size justified by power computations or other methods, if applicable, or was an a-posteriori power analysis included? NA = not applicable (for case studies or case series) 0 = sample size was based on data availability and no a-posteriori power analysis was included 0.5 = sample size was based on data availability but a-posteriori power analysis was included 1 = sample size was a-priori designed based on power analysis computations |
| Q7 | Clinical aspects | Are the characteristics of the rehabilitation treatment sufficiently described, when delivered (type of intervention, duration of each session, session weekly frequency, timeline and follow-ups….)? NA = not applicable (for observational studies) 0 = intervention is only cited 0.5 = intervention is described with few details (e.g., weekly frequency and duration) 1 = characteristics of the intervention are completely described to allow reproducibility |
| Q8 | Technical aspects | Are the data acquisition and preprocessing parameters that may affect the posturographic parameters clearly reported (e.g., sampling frequency, low pass cut-off frequency, mean value removal)? 0 = no information is provided 0.5 = partial information is provided or can be retrieved from cited references 1 = parameters are entirely reported |
| Q9 | Assessment protocol | Is the acquisition protocol sufficiently detailed to permit replication (duration of repetitions, number of repetitions, compliant/soft surface material)? 0 = no information is provided 0.5 = partial information is provided or can be retrieved from cited references 1 = parameters are entirely reported |
| Q10 | Assessment protocol | Are the task conditions sufficiently detailed to permit replication (foot positioning, upper limb position, gaze fixation, instructions to the patient)? 0 = no information is provided 0.5 = partial information is provided or can be retrieved from cited references 1 = parameters are entirely reported |
| Q11 | Assessment protocol | Are the testing conditions clear (ON/OFF drug condition, ON/OFF brain stimulation)? NA = not applicable for studies including patients diagnosed with parkinsonisms 0 = no testing information is reported 0.5 = testing conditions are cited 1 = information is reported in terms of dosage (LEDD or usual dosage), time of the day the drug was administered (washout clearly stated in case of OFF condition), hours from DBS starting |
| Q12 | Transferability to clinical practice | Is the rationale for the selection of the assessment protocol (e.g., eyes open/closed, firm/foam surface, dual cognitive task, other foot positions) clearly stated? 0 = the rationale behind the choice of the protocol is not explained or cannot be derived across the manuscript 0.5 = the rationale behind the choice of the protocol can be derived across the manuscript 1 = the rationale behind the choice of the protocol is clearly stated |
| Q13 | Transferability to clinical practice | Did the authors provide a rationale or discuss a-posteriori the choice of the posturographic parameters computed? 0 = the rationale behind the choice of the parameters is not explained or cannot be derived across the manuscript 0.5 = the rationale behind the choice of the parameters can be derived across the manuscript 1 = the rationale behind the choice of the parameters is clearly stated |
| Q14 | Technical aspects | Was the computation of the posturographic parameters clearly described (definition, formulas, or reference to a paper with formulas)? 0 = definitions, formulas, or references for parameter computation are not reported 0.5 = some definitions, formulas, or references for parameter computation are reported 1 = definitions, formulas or references are provided for all parameters |
| Q15 | Technical aspects | Are the units of measurement for each parameter clearly stated? 0 = units of measurements are never stated or are wrong 0.5 = units of measurements are stated only for few parameters or are not consistent across the manuscript 1 = units of measurements are clearly stated for each parameter |
| Q16 | Technical aspects | Are the numerical data consistent with current literature? NA = not applicable when the parameter was used by fewer than five research groupsa 0 = data exceeding by 3 median absolute deviation (MAD) the overall median value 0.5 = data exceeding by 2 MAD the overall median value 1 = data are consistent with the median and MAD derived from the total number of the studies |
| Q17 | Study methodology | Are the limitations of the study discussed (control arm, sample size, power analysis, external validity, follow-up timeline, protocol feasibility)? 0 = limitations are not discussed 0.5 = only few limitations are reported with limited discussion 1 = limitations are properly addressed |
| Q18 | Study methodology | Are the conclusions consistent with the aim of the study? 0 = conclusions do not align with the aim 0.5 = conclusions partially recall the aim of the study 1 = conclusions are in line with the declared aim |
| Q19 | Transferability to clinical practice | Are the clinical utility and transferability of the posturographic assessment addressed and discussed in the study? 0 = no clinical utility or transferability are discussed 0.5 = clinical utility or transferability are no explicitly addressed but can be derived from the discussion 1 = authors made clear the clinical implications and transferability of their study |
Tailored critical appraisal for the assessment of the included studies, addressing five domains that should always be described.
avariables used by at least five research groups: 95% ellipse area (mm2); 90% ellipse area (mm2); area as sum of the triangles (mm2); RMS/SD_AP (mm); RMS/SD_ML (mm); RMS/SD (mm); velocity_AP (mm/s); velocity_ML (mm/s); velocity (mm/s); COP length_AP (mm); COP length_ML (mm); COP length (mm); COP position_AP (mm); COP position_ML (mm); sway range_AP (mm); sway range_ML (mm); COP sway_AP (mm) - mean displacement with respect to the center of the force plate; COP sway_ML (mm) - mean displacement with respect to the center of the force plate; maximum displacement_AP (mm); maximum displacement_ML (mm); mean displacement_AP (mm); radius (mm).
The questions, listed according to the traditional structure of the paper, explore five domains:
-
1) Study methodology (Q1, Q3, Q5, Q6, Q17, Q18), including study design, research question, homogeneity or stratification according to disease severity, sample size or power analysis computation, and statistical analysis;
-
2) Clinical aspects (Q4, Q7), including the description of the sample, and description of the intervention—if any;
-
3) Assessment protocol (Q9, Q10, Q11), including information about balance assessment procedures and their replicability;
-
4) Technical aspects (Q8, Q14, Q15, Q16), including computational operations and engineering data management;
-
5) Transferability to clinical practice (Q2, Q12, Q13, Q19), including the paper's added value to the literature to improve clinical management of pwPD.
Each question was scored on a three-level basis: 1 for yes, 0.5 for limited details, and 0 for no. For some items, the score Not Applicable (NA) was added. The total score was calculated for each study to evaluate its overall quality. The total score of each question—and, consequently, of each category—was then expressed as a percentage of the maximum achievable score (i.e., considering only the assessable studies), not to penalize studies that received an NA score for some items.
Finally, studies were categorized into three groups: high-quality (total score exceeding 80%), medium-quality (total score ranging between 51 and 79%), and low-quality (total score below 50%) as in Samadi Kohnehshahri et al. (22). We also separately evaluated the quality of each category, determined by the ratio of question scores at each level (low, medium, high) and the total number of questions linked to that category.
2.6 Differences in critical appraisal scores due to the journal subject area
We also investigated whether studies published in strictly clinical or mixed (i.e., clinical and bioengineering, bioengineering only, neurophysiology) journals received different scores in the categories of critical appraisal. We labeled the journal as clinical or mixed based on the main “subject area and category” on the Scimago Journal and Country Rank portal (20). When journals were not indexed on Scimago or classified as “multidisciplinary” (e.g., PlosOne), classification was carried out by examining the journal's aims, the study itself, and the authors' affiliations. Critical appraisal analysis was then performed for these two subgroups.
3 Results
The original search identified 1,893 articles, which were turned into 859 after removing duplicates. These were screened for title and abstract according to the eligibility criteria. In this phase, the main reasons for exclusion were wrong outcomes or protocols employed (e.g., not involving the baseline condition, having patients performing dynamic balance tests) and wrong publication type (e.g., conference abstracts). Twenty-two studies required a third reviewer's intervention, and nine were included after consultation, leading to 130 eligible papers. In addition, two out of ten studies identified by hand searching were included, for a total of 132 studies included in the review (see the PRISMA Flowchart in Figure 1) (17–19, 27–155).
Figure 1

Prisma flowchart of the literature search on instrumental static balance assessment for patients with Parkinson's disease and atypical parkinsonism.
3.1 Data extraction
The comprehensive table containing all information extracted from the included studies can be found in Supplementary material 1.
Of the 132 studies, 115 focused on pwPD and 17 on atypical parkinsonism (MSA, PSP). Ninety-five were observational studies, and 37 were interventional studies. The former were generally cross-sectional studies aimed at describing the features of balance management of the included sample. The latter mainly evaluated a drug's or treatment's effectiveness on patients' postural ability.
Overall, 4,262 patients, aged 43–91, were assessed with static posturography in baseline condition.
3.2 Quality assessment
The results obtained from the scores of each quality question are reported in Supplementary material 2. Of the 132 studies, 17 (13%) were rated high-quality, 105 (79%) medium-quality, and 10 (8%) low-quality (Figure 2A). The category “clinical aspects” received high-quality scores in half of the studies (see Figure 2B). The “technical aspects” and “study methodology” categories received mostly medium-quality ratings. Finally, the two categories with the lowest scores were “transferability to clinical practice” and “assessment protocol”.
Figure 2
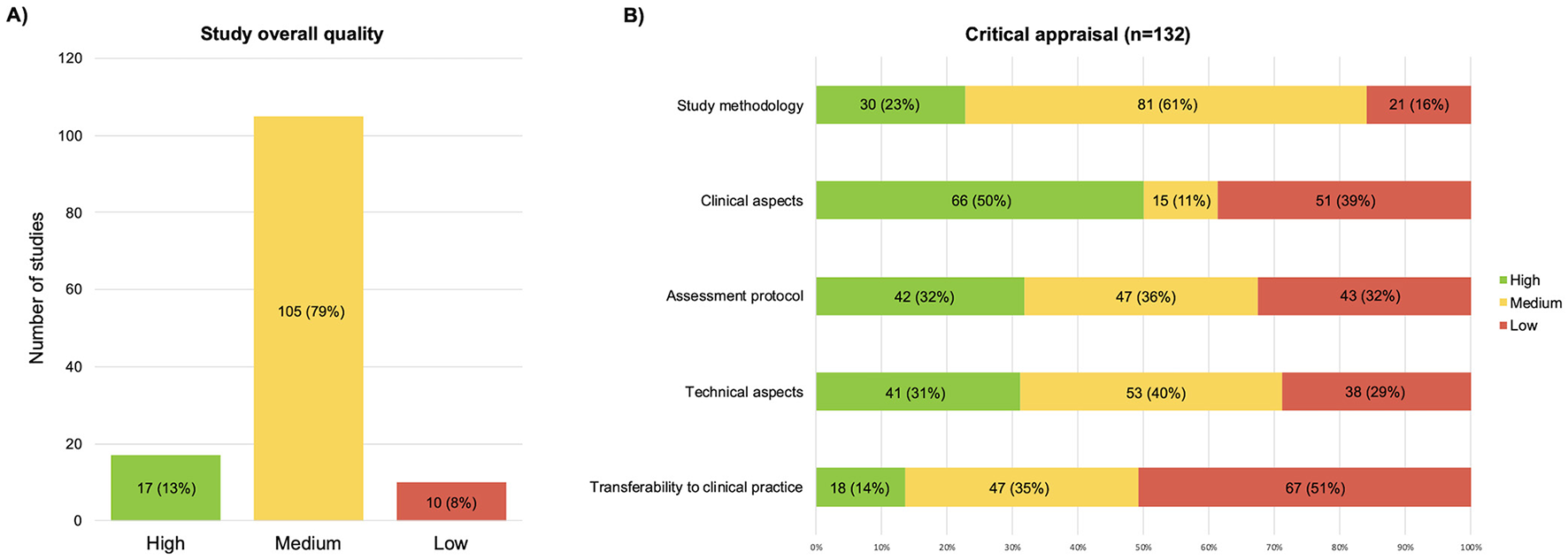
Graphic representation of the results of the critical appraisal, (A) overall scores; (B) scores split by the five domains of the critical appraisal.
Table 3 shows the results of each item from the five domains, detailing the average rating scores (see Supplementary material 2 for details of individual studies). The total score of each question was calculated as a percentage, excluding studies assessed as “NA” to avoid penalizing those questions. Consequently, some ratios have denominators lower than the total number of included studies (e.g., 74/128).
Table 3
| Assessed domain | Results |
|---|---|
| Study methodology | Mean rating: 69% (17% SD) |
| Q1—Study aims | 115/132 (87%) authors specified the aims of their studies. |
| Q3—Study design | 43/132 (33%) authors explicitly specified the study design. |
| Q5—Sample characteristics | - The included sample was appropriate in 74/128 studies (57.8%). - It was partially adequate in 45/128 studies (35.2%). |
| Q6—Sample size | Only 26 out of 127 (21%) studies computed a-priori the sample size or performed a-posteriori power analysis; specifically, 15/32 (47%) interventional and 11/95 (12%) observational studies did it. |
| Q17—Limitations | The limitation section was appropriately discussed in only 62/132 (47%) cases; barely mentioned in 40/132 (30%); and not even mentioned in as many as 30/132 (23%) cases. |
| Q18—Conclusions | 119/132 (90%) authors wrote their conclusions consistently with the aims. |
| Clinical aspects | Mean rating: 75% (29% SD) |
| Q4—Sample description | 68/132 (52%) of the studies accurately listed the eligibility criteria. |
| Q7—Intervention description | 32/37 (87%) of the interventional studies described the intervention protocol in detail, allowing reproducibility. |
| Assessment protocol | Mean rating: 65% (23% SD) |
| Q9—Protocol description | 89/132 (67%) and 32/132 (24%) studies accurately or sufficiently, respectively, described the acquisition protocol. The assessment protocol consisted of: - three balance trials in 49 (37%) studies; - one single trial in 47 (36%) studies, - two trials in 20 (15%) studies; - more trials (range 4–6) in 6 (4%) studies; - no information about the number of trials was reported in ten studies (8%) The duration of recordings ranged from 3 to 180 s, with 60/132 (45%) studies choosing 30 s as the duration of each session; 8/132 (6%) studies never stated the acquisition duration. |
| Q10—Task conditions | 39/132 (30%) studies appropriately detailed the task conditions, while 70/132 (53%) authors provided only limited information: - 85/132 (64%) specified the instructions given to patients; - 67/132 (51%) specified the arm position; - 28/132 (21%) specified the position and type of visual target; - 60/132 (46%) explicitly stated they used the barefoot condition; - 50/132 (38%) provided numerical references for foot placement (e.g., intermalleolar distance, angle between feet); - 22/132 (17%) ensured standardized positioning by means of foot shapes or similar; - 23/132 (17%) gave general instructions to keep the feet at shoulder or hip width; - 6/132 (5%) let patients place their feet in undefined comfortable positions; - 29/132 (22%) specified nothing about foot positioning on platforms; - 7/132 (5%) reported that a quiet setting was ensured while assessing the patients. Beyond baseline conditions, the following conditions were also assessed among studies: - eyes closed on a firm surface (n = 83 studies), - eyes open on a soft surface (n = 14), - eyes closed on a soft surface (n = 14). - dual-task condition (n = 17 studies), usually asking patients to repeat sequences of numbers, count forward or backward, or say as many words as possible beginning with a given letter or belonging to a given category. - further protocols also included tandem and semi-tandem positions, unipedal standing, standing with feet together, or trunk or head inclined. |
| Q11—Drug and stimulation conditions | −52/126 (41%) studies adequately specified the drug or stimulation conditions while performing the balance assessment (i.e., the authors also specified the mean time since medication intake or the duration of the wash-out period). - Likewise, some of these studies also involved DBS and explained in the methods section the stimulation frequency used, the stimulus voltage, and the elapsed time between DBS onset and balance assessment. |
| Technical aspects | Mean rating: 68% (22% SD) |
| Q8—Data acquisition | −45/132 (34%) studies correctly described both the sampling frequency and the filtering process. - 53/132 (40%) papers reported only the sampling frequency, with no specification about the filters applied. Devices from about 20 different producers were used, including: - 3-axial for plates (n = 62), - monoaxial force plates - i.e., with vertical force sensors only (n = 46), - pressure mats (n = 18). - Nintendo Wii (n = 6) - Eleven studies did not report information on the device. |
| Q14—Parameter computation | −51/132 (39%) studies exhaustively described the computation process, with formulas, references, or clear definitions of the posturographic parameters. - 45/132 (34%) studies never provided any reference for parameter calculation. - The remaining 36 studies (27%) only partially described the formulas and computation methods. |
| Q15—Units of measurement | The units of measurements were uniformly made explicit in 122/132 (92%) studies, while five studies (4%) never reported any. |
| Q16—Parameter reliability | −82/109 (75%) studies reported values consistent with current literature. - 26/109 (24%) studies presented values even greater than three median absolute deviations away from the median for at least one parameter. |
| Transferability to clinical practice | Mean rating: 59% (19% SD) |
| Q2—Aims and clinical applications | The objectives of the studies were almost always explicitly related to clinical practice (n = 113/132, 86%). |
| Q12—Rationale behind the protocol | The rationale behind the protocol selection was never made explicit in 80/132 studies (61%); it could at most be derived from the text despite not being made explicit in 19/132 studies (14%). |
| Q13—Rationale behind the parameters | The rationale behind the choice of the parameter was explicitly stated in only 32/132 (24%) studies, while in the majority of the papers it was completely omitted (n = 68/132, 52%). |
| Q19—Discussion of clinical utility | Clinical utility was either made explicit (n = 76/132, 58%) or could be inferred from the discussions (n = 50/132, 38%). |
Results of the critical appraisal for each item.
SD, standard deviation; DBS, deep brain stimulation.
3.3 Differences in critical appraisal scores due to the journal subject area
Of the included studies, 102/132 were strictly clinical, and 30/132 were mixed studies.
Of the 102 clinical studies, 13 (13%), were rated high-quality, 81 (79%) medium-quality, and 8 (8%) low-quality. Of the 30 mixed studies, 4 (13%) were rated high-quality, 24 (80%) medium-quality, and 2 (7%) low-quality.
Regarding clinical studies, the domains with the highest scores were “clinical aspects” and “assessment protocol” (see Figure 3A). The categories with the lowest scores were “transferability to clinical practice” and “technical aspects”. Thirteen clinical studies were cumulatively classified as high-quality. Only one study received high-quality ratings for all five domains (35).
Figure 3
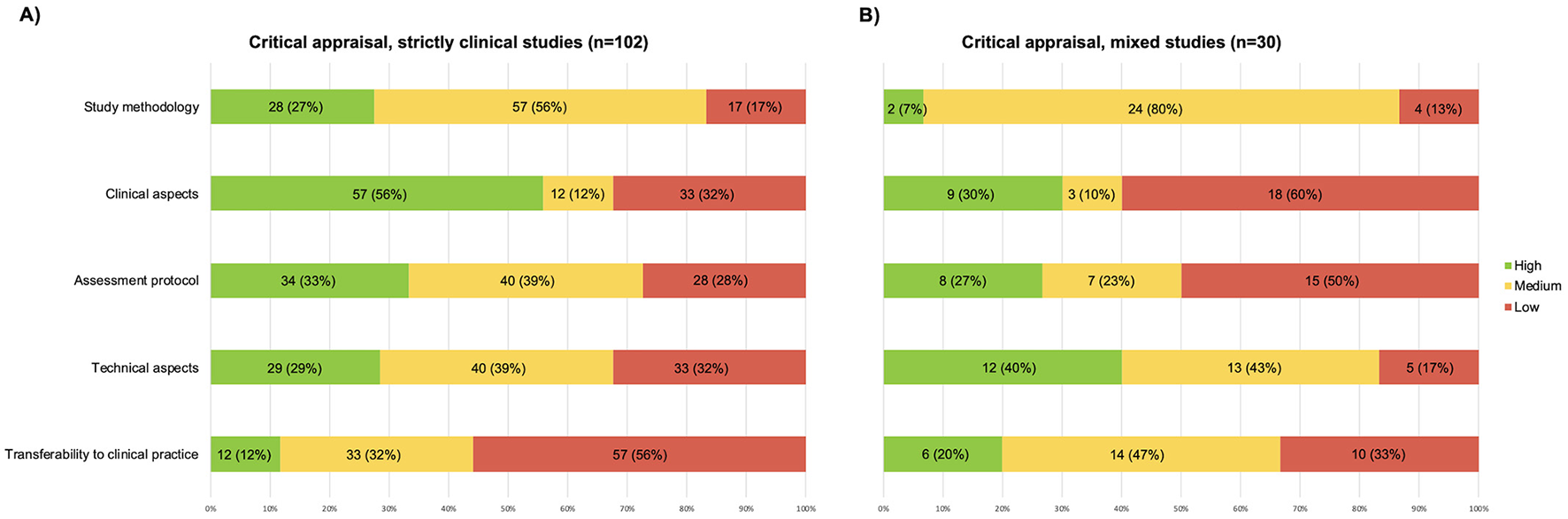
Graphic representation of the results of the critical appraisal, (A) papers published in clinical journals; (B) papers published in mixed journals.
Regarding mixed studies, the domain with the highest score was “technical aspects” (see Figure 3B). The categories with the lowest scores were “clinical aspects” and “assessment protocol”. Four mixed studies received high-quality global evaluation but no study received high-quality ratings for all five domains.
It is also worth mentioning that more than half of the studies received medium-quality scores for the domain “study methodology”.
4 Discussion
We conducted this systematic review to assess the use of static posturography in pwPD, analyzing which types of information are more detailed and how they may impact clinical transferability.
Based on the appraisal conducted, most of the included studies were rated as medium-quality, and only 17 studies were considered of high-quality (17, 32, 34–36, 47, 57, 63, 70, 112, 115, 117, 125, 126, 147, 148, 150).
The “”transferability to clinical practice” was the lowest-scoring domain at the critical appraisal, with only 14% of high-quality studies. From the results reported in Figure 2B and Table 3, this finding is attributable to three main factors. These are (1) The lack of discussion on the rationale adopted for choosing the posturographic protocol and computing the parameters used; (2) The conduction of the studies on limited, very heterogeneous, or insufficiently characterized samples from a clinical point of view; and (3) The lack of transparent reporting on the procedures for conducting the assessment and computing the parameters analyzed.
The interplay of these factors, identified through the critical appraisal items, prevented us from taking the next step in this review: summarizing the results as mean baseline values for pwPD at different stages of the pathology (e.g., H&Y score) or assessing the average variation in posturographic parameters following an intervention. A selection of studies with the highest appraisal scores was indicated above. The following paragraphs analyze these three points in the included studies, their impact on clinical transferability, and suggest possible solutions.
4.1 The rationale supporting the posturographic assessment of pwPD in the literature
Despite being rarely mentioned as a key point among the studies, three main clinical uses of the posturographic assessment in pwPD emerge from this review. These are: (1) to evaluate the effectiveness of rehabilitation treatments (37 clinical trials), (2) to monitor the course of the disease, and (3) to distinguish subgroups of patients (95 observational studies).
Only 16 studies have explicitly discussed how the assessment protocol and investigated posturographic parameters can be used in clinical routine (34–36, 38, 39, 42, 43, 50, 63, 72, 110, 115, 138, 147, 148, 150). Surprisingly, the lack of explanation of a clear rationale is particularly notable in studies published in purely clinical journals (see Figure 3A), which, on the contrary, are expected to be more accurate about this. This makes it difficult for readers to translate the literature into their daily clinical practice.
The reasons for this flaw may be manifold. It is possible that the authors took the rationale behind their choices for granted, as they are used to using these tools and dealing with these parameters every day. Also, the authors might have focused on the research-related aspects of their studies, so they did not discuss the clinical rationale and relevance of both their protocol and results. Next, several journals did not require to address this point explicitly. Concerning the choice of the posturographic parameters used in the studies included in this review, their selection may be due to—and limited by—the software of the commercial device used in the study. These, in fact, typically provide only the most used and standard outcomes, such as COP velocity and area, which may not be the most informative ones when assessing pwPD.
It is known that it takes about two decades to implement evidence into clinical practice (156). To bridge this time gap, the literature must focus on clinical relevance and transferability of findings. Many scientific journals require a short paragraph on clinical implications when submitting a manuscript. In line with this, future studies should discuss which additional information the posturographic test provides other than the clinical assessment, why it is essential to perform it in pwPD, and the possible contribution of the assessing protocol (task and parameters) to differential diagnosis or early recognition of symptoms or treatment effect assessment or patient status monitoring.
4.2 Characteristics of pwPD assessed by posturography in the literature
This systematic review included both pwPD and patients with atypical parkinsonism. To our knowledge, this is new in the literature, as this is the only review that included both populations. Despite being rarer, diseases such as PSP and MSA manifest clinical symptoms at onset that can be confused with PD and, therefore, deserve to be investigated as well and differentiated.
4.2.1 The choice and description of the sample
A limited number of studies reported all the details about the sample, which is necessary to ensure the external validity of the results. Accurately describing sample characteristics allows readers to decide whether or not to compare with their reference patients. Clinical aspects were better described by the studies published in clinical journals (56% of high-quality studies), while the rate dropped to 30% for papers published in mixed journals. This is likely due to a greater sensitivity of clinical teams in reporting aspects that characterize the sample, compared to engineering groups.
Only 68/132 (52%) studies fully and accurately described the sample (see Q4). For pwPD, many studies reported diagnostic criteria and characterized patients through the Unified Parkinson's Disease Rating Scale (UPDRS) (157) and the Hoehn and Yahr staging (H&Y) (158) scales, as well as additional functional tests. For patients with atypical parkinsonism, a variety of specific scales can be used, depending on the disease, including the Unified Multiple System Atrophy Rating Scale, Progressive Supranuclear Palsy Rating Scale, and Scale for the Assessment and Rating of Ataxia (159–161). In progressive diseases such as PD, it might be relevant to report the time from the onset of the first symptoms or diagnosis, as well as the age and gender of the sample. In our review, the sample was considered “adequate” when it included patients with homogeneous characteristics in the case of intervention/descriptive studies and patients with heterogeneous characteristics in the case of correlation studies (see Q5). Among the included studies, 58% set the inclusion criteria consistently with the aim of the study. The adequacy of the sample allows the authors to identify specific findings for a homogeneous subgroup in terms of disease severity, supporting the translation of the results into clinical practice. Finally, the authors should specify whether the study was conducted solely for research purposes or if posturography acquisition was integrated as standard clinical practice.
When conducting a clinical study, the intervention should also be described. In our review, 86.5% of the studies detailed their protocols, including the type of intervention, frequency, and dosage, thus allowing reproducibility (see Q7). The TIDieR checklist was specifically developed in 2014 to improve the reporting quality for healthcare interventions and may further support authors during the writing of their Section 2 (162).
4.2.2 The methodology used
The methodology domain has contributed to lowering the overall quality of the studies. The number of high-quality studies, already quite low when considering all studies (23%), drops to 7% when considering only studies published in mixed journals.
The main methodological limitation found in the included studies is the rare a-priori sample size calculation (see Q6). Only 24/127 (21%) of the studies calculated the sample size a-priori, along with two other studies (2%) that calculated statistical power a-posteriori. Focusing on interventional studies only, surprisingly, only half (47%) of them were conducted on a sample with an adequately calculated sample size. This is a relevant flaw because it can result in the absence of a statistically significant difference in the posturographic outcomes between the experimental and the control group in the presence of an actual difference (type-II error). The lack of this calculation limits the generalizability of their results and, again, their clinical transferability. Furthermore, it hinders the observation of potential differences between groups (in both observational and clinical studies), thereby impacting clinical decision-making (163).
When considering methodology, it is also appropriate to clarify the weaknesses of a study, which helps naïve readers not to overestimate the results and be aware of the limitations they might encounter if they intend to replicate the work in their daily activities. Only 62/132 studies comprehensively reported the limitations of their work (see Q17), and authors rarely referred to guidelines used during writing. Several reporting guidelines have been published since the late 1990s (e.g., CONSORT for experimental studies–1996) (164) and STROBE for observational studies in 2007 (165). More and more scientific journals require them to be completed during submission, proving that they are a handy tool for ensuring that the required methodological rigor has been met and that all the necessary information has been reported.
4.3 Issues in the reporting of protocol details and technical information in the literature
Two domains with similar percentages of high-quality studies are “assessment protocol” (32%) and “technical aspects” (31%). Many studies obtained fair scores at the critical appraisal as they correctly provided the necessary information. However, several technical and assessment-related aspects still deserve consideration.
4.3.1 Assessment protocol
Studies that include posturographic examination should thoroughly describe how it is performed, and which protocol is adopted so as to allow replicability both in subsequent studies and in clinical practice. Key information to be reported is the number of sessions, their duration, the positioning of the feet and arms, the visual target provided, and the instructions given to the patient before the test.
4.3.1.1 Number and duration of repetitions
In the current review, almost the same number of studies performed one or three repetitions, with durations ranging from 3 s to 3 min. Such short durations must never be included, as they are dramatically shorter than even the 5–10 s of recording recommended in some studies before collecting data for analysis (166, 167) and, therefore, provide no helpful information on COP characteristics. In the specific case (46) the authors reported the static balance assessment preliminary to gait analysis acquisitions.
The importance of averaging the parameters obtained in at least three successive trials to obtain values close to the subject's actual value is highlighted in the literature (168, 169). As the number of trials increases, the average value of the most commonly used posturographic parameters, such as COP area and velocity, stabilizes. At least 3–4 trials are necessary to stabilize posturographic parameters (167–170). In addition, the availability of multiple trials makes it possible to observe the variability of posturographic parameters between trials and provide further insight into the patient's condition.
The duration of the single test also affects the value of posturographic parameters. The literature has long investigated the best methods of standardizing the examination (166, 171–175), indicating durations between 30 and 90 s. Most (98/132, 74%) of the studies included in this review involved acquisitions with durations between 30 s (60/132, 45%) and 60 s (26/132, 20%).
The duration of 51.2 s—or multiples—observed in several studies (42, 53, 75, 101, 113, 129, 130, 146, 176) may seem strange to the reader and deserves a brief comment. This duration, adequate if not strenuous for the patient, does not derive from a physiological rationale. It depends on the technical characteristics of specific devices available in the 1980s and used for the definition of the first normative data through the work of the group of Gagey and Bizzo (172, 177). The availability of normative values encouraged, at first, the adoption of this duration in subsequent literature and by device manufacturers.
Furthermore, it should be kept in mind that for individuals such as pwPD, excessive duration could induce high levels of fatigue (178). Performing very long trials should be a conscious choice by researchers, possibly justified as done by Workman and colleagues (148). Therefore, evaluations for clinical uses must be based on a trade-off between several factors: the assessment duration, patient fatigue, and the need to obtain multiple trials to compare and mediate. It seems advisable to record 3–4 trials of 30–60s each.
4.3.1.2 Task instructions
Task instructions are another critical factor for the clinical transferability of posturographic assessment. Of the included studies, only 39/132 (30%) specified the conditions under which the patients performed the balance tests, such as the directions given to the patients, the position of the arms and feet, and the visual target.
Instructions given to patients (e.g., “stand as still as possible” and “maintain a comfortable posture”) affect the reactions and the focus they will maintain during the test and, therefore, should be made explicit (179, 180). Similarly, the position of the arms (e.g., along the body, crossed) and the visual aim provided (e.g., “look straight ahead”, “look at a specific point at eye level”) could result in changes in the positioning and sway of the center of pressure (175). If possible, it would also be appropriate to specify that a quiet setting has been ensured throughout the performance of the test, mainly to avoid acoustic spatial orientation (166, 175), as done by some authors (65, 111–113, 116, 120, 148).
Unambiguous clarification of foot positioning is also essential (181). It is not sufficient to ask the patient to position themselves with their feet shoulder-width apart comfortably, nor to ask them to keep them open at shoulder or hip width (28/132 studies). This clearly does not allow for reproducibility. Other 18/132 studies let the patients position themselves at will but ensured position repeatability between trials by marking the initial position acquired. This is certainly a strength for internal validity, but it does not allow comparison of them with external literature and, again, limits the transferability of findings. Therefore, proper reporting of foot placement should always include numerical values for the distance between the feet and the angular opening between them. The literature suggests a distance of 3–5 cm at an angle of 30° (182) for patients with pathologies such that testing with feet together is not feasible and safe. Although it may seem obvious, researchers should always clarify whether the patient wears footwear or is barefoot during testing (only 60/132, 46%, studies among those included explicitly stated this), as the increased surface area—and sometimes, a slight ankle restraint provided by the footwear—, could affect the subject's stability (166).
The current review focused on the fair reporting of posturographic assessment under “baseline” conditions (i.e., eyes open, on a stable surface). Still, the same rigor must be maintained for any further testing. Specific tasks may reveal the patient's hidden postural issues, depending on individual strategies, as the dual-task condition (183). In this case, it is necessary to describe the required task (e.g., “count backward by three units starting from 100”, “list all the names you can think of referable to the animal category”) (18, 54, 67, 101, 103, 121, 140, 150, 153), without merely mentioning a generic cognitive task. Tasks of increasing difficulty require additional attention and may alter simultaneous balance control (184). Again, in the case of tests on unstable surfaces, the material (e.g., foam, gel), thickness (50, 82, 119, 121), and gel viscosity should be described (36, 118), as they change the multiple biomechanical variables in the foot, resulting in an alteration to the distribution of plantar pressures (185).
Finally, since these pwPD are almost always under a drug regimen, it is relevant to clarify the phase (ON/OFF) in which the examination is performed: it is not sufficient to state whether the patient was in the ON or OFF phase. If in OFF, the patient must have performed a wash-out of at least 12 h; if in ON, the peak of maximum action occurs about 1–2 h after the last intake (186). Only 52/126 (41%) studies included in the current review described it correctly. As with the rehabilitative intervention, it is equally important to specify the frequency, voltage of stimulus administration, and timing of stimulation.
4.3.2 Technical aspects
In addition to information about the protocol, details on data analysis should always be reported in the manuscripts because of their impact on the results and to allow study reproducibility.
In particular, mean value removal, data filtering procedures, calculation of the parameters, and subsequent synthesis methods (e.g., calculation of the mean or median among the many trials, exclusion of the first and last seconds of each trial) should be reported. Using filters and techniques that deviate from traditional practice should be justified, as did Schmit et al. (132), who chose not to apply filters because they intended to characterize dynamic COP patterns in PD.
Studies published in mixed journals, i.e., engineering-contributing journals, better addressed technical aspects (40% high-quality studies vs. 28% high-quality studies published in clinical journals), probably reflecting a greater focus of bioengineering authors on this information. Overall, we observed a lack of standardization in data acquisition, filtering methods, and variable computation, when reported. Low pass filtering frequency, for example, was rarely reported despite its relevant effect on the computed parameters (see Supplementary material 1). This prevents meaningful comparisons between studies and hinders the possibility of synthesizing results.
4.3.2.1 Instrumental set-up
1D- and 3D force plates were the most used devices in the studies included in this review. From a technical point of view, 1D force plates with three vertical load cells placed in the shape of an equilateral triangle or four vertical load cells placed in the shape of a rectangle are suited to obtain COP. 3D force plates, which are more expensive, were reasonably available in the centers and used for additional applications, such as gait analysis. Furthermore, despite the adequate technical characteristics of the Nintendo Wii (1D force plate), this device is not certified as a medical device. It cannot be used with patients for clinical assessments. Recent literature is investigating wearable sensors as low-cost tools capable of providing data on patients' balance that is easier to obtain (187). Under quasi-static conditions, the center-of-mass acceleration (measured by inertial sensors) is related to that of CoP (measured by force platforms) (188) and may serve as a cost-effective alternative to force platforms in specific contexts after validation specific to each pathology and impairment level.
Only forty-five out of 132 (34%) authors accurately reported the sampling frequencies and filters applied to the raw data, demonstrating knowledge of their impact on the data. PwPDs usually exhibit a tremor oscillating between 4 and 6 Hz (189). Hence, the sampling frequency must be high enough to observe information regarding COP oscillations.
4.3.2.2 Computation of the posturographic parameters
Of the included studies, 51/132 (39%) correctly described the computed parameters using mathematical formulas, precise definitions, or citing other articles. Some terms are often used arbitrarily in the literature, which can lead to confusion and misinterpretation, with the high risk for the inexperienced reader of comparing parameters with the same name that hide different underlying calculations. The most striking example is that of the term “sway area/path area/total area,” used in as many as 20 studies without referring to any formula and therefore with potentially very different meanings (30, 40, 41, 45, 76, 78, 93, 97, 99, 103, 107, 111, 112, 130, 137, 144, 146, 151, 154, 176).
The urgency to create an unambiguous taxonomy emerges from this heterogeneous scenario. The historical reference to which most of the literature refers is Prieto et al. (190). Quijoux et al. have recently suggested a new classification of parameters into four categories, including positional (e.g., COP mean position), dynamic (e.g., COP velocity), frequency (e.g., spectral total power), and stochastic (e.g., sample entropy) features (23). Researchers can also rely on open libraries for complete analysis of posturographic data (23). A multidisciplinary approach, including bioengineers in the team, may be adopted to develop specific codes for calculating parameters that are useful to the clinician or to use the available open-source libraries. Moreover, a consensus conference with clinical and engineering experts may establish the best pwPD-specific parameters, providing helpful guidance for software development companies. Alongside the development of new parameters and the informed choice of them, research should be directed toward identifying their psychometric properties and threshold values, capable of distinguishing clinically significant changes, as has been done for spatiotemporal gait parameters for pwPD (191) or some posturographic parameters in healthy subjects (168, 170).
In the current review, five studies (19, 39, 60, 64, 176) never reported the units of measurement of posturographic parameters, and other five studies (18, 43, 55, 57, 155) only partially reported them. As many as 26/109 (24%) studies reported values much higher (>3 MAD) than those found in the literature. In some cases, this could be due to typos in the reported unit of measurement, but this confirms the need to promote clear standards and guidelines in the performance, analysis, and reporting of posturographic data.
To compare the posturographic data obtained during daily clinical practice with those published in the literature, it is mandatory that the clinical characteristics of the samples are comparable and that the acquisition protocol and technical handling of the data are identical. Studies that have accurately reported the necessary information and presented reliable data for comparison are: (17, 35, 115, 130) for pwPD with H&Y ≤ 2, (17, 101, 112, 115, 125, 130, 131) for pwPD with H&Y > 2, and (112) for atypical parkinsonism.
4.4 Limitations
This review has some limitations that need to be considered.
First, we included studies that reported balance assessment under the “baseline” condition. So, other protocols (e.g., dynamic posturography), which could provide additional relevant information and valuable hints for clinical practice, were not considered.
The search was conducted on four databases and by hand searching the references, but some relevant papers not indexed through the keywords entered in the search strings may have been missed.
Finally, the appraisal we used to assess the included studies on posturography in pwPD is new to the literature and is not a validated tool, as it was constructed ad hoc by the authors. Although we relied on similar examples to conceive the items (16, 23, 24) and develop the scoring system (22), followed an iterative process, and consulted with experts for its design, it is possible that some important aspects were not considered.
5 Conclusion
This systematic review assessed the use of static posturography for quantifying static balance in pwPD and atypical parkinsonism, focusing on the aspects that may hinder the transferability of research results to clinical practice. The main issues identified include a lack of rationale behind posturographic protocols and parameters and unclear sample inclusions. We highlighted several opportunities for enhancing the quality of studies on static posturography in assessing pwPD and atypical parkinsonism. In future studies, authors should: (1) discuss the rationale behind the choice of a specific assessment protocol and a posturographic parameter, (2) detail the inclusion criteria and select appropriate samples according to the aim of the study, and (3) report all the technical information necessary to replicate the procedures and computations. Addressing these areas can significantly improve scientific literature's external validity and clinical transferability to daily practice. This review provided valuable references for each of the five domains considered, supporting the rapid portability of findings to clinical settings.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. LC: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. MBò: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. FC: Resources, Writing – review & editing. MBa: Investigation, Writing – review & editing. BD: Writing – review & editing. SS: Writing – review & editing. VF: Writing – review & editing. GD: Writing – review & editing. GP: Writing – review & editing. FV: Writing – review & editing. ML: Writing – review & editing. IC: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was entirely funded by the Azienda USL-IRCCS of Reggio Emilia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1528191/full#supplementary-material
Supplementary 1Characteristics of the included studies, instructions given to the patients for the static balance test, and posturographic parameters computed in each study.
Supplementary 2Results of the critical appraisal for each item and each of the included studies.
References
1.
Dickson DW . Parkinson's disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med (2012) 2:a009258. 10.1101/cshperspect.a009258
2.
Lo R . Epidemiology of atypical parkinsonian syndromes. Tzu Chi Med J. (2022) 34:169–81. 10.4103/tcmj.tcmj_218_20
3.
Palakurthi B Burugupally SP . Postural instability in Parkinson's disease: a review. Brain Sci. (2019) 9:239. 10.3390/brainsci9090239
4.
Bloem BR Hausdorff JM Visser JE Giladi N . Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. (2004) 19:871–84. 10.1002/mds.20115
5.
Paul SS Sherrington C Canning CG Fung VSC Close JCT Lord SR . The relative contribution of physical and cognitive fall risk factors in people with Parkinson's disease: a large prospective cohort study. Neurorehabil Neural Repair. (2014) 28:282–90. 10.1177/1545968313508470
6.
Allen NE Schwarzel AK Canning CG . Recurrent falls in Parkinson's disease: a systematic review. Parkinsons Dis. (2013) 2013:1–16. 10.1155/2013/906274
7.
Pickering RM Grimbergen YAM Rigney U Ashburn A Mazibrada G Wood B et al . Meta-analysis of six prospective studies of falling in Parkinson's disease. Mov Disord. (2007) 22:1892–900. 10.1002/mds.21598
8.
Michałowska M Fiszer U Krygowska-Wajs A Owczarek K . Falls in Parkinson's disease. causes and impact on patients' quality of life. Funct Neurol. (2005) 20:163–8.
9.
Goetz CG Tilley BC Shaftman SR Stebbins GT Fahn S Martinez-Martin P et al . Movement disorder society-sponsored revision of the unified Parkinson's disease rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. 10.1002/mds.22340
10.
Ramaker C Marinus J Stiggelbout AM van Hilten BJ . Systematic evaluation of rating scales for impairment and disability in Parkinson's disease. Mov Disord. (2002) 17:867–76. 10.1002/mds.10248
11.
Qutubuddin AA Pegg PO Cifu DX Brown R McNamee S Carne W . Validating the Berg Balance Scale for patients with Parkinson's disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil. (2005) 86:789–92. 10.1016/j.apmr.2004.11.005
12.
Kegelmeyer DA Kloos AD Thomas KM Kostyk SK . Reliability and validity of the Tinetti mobility test for individuals with Parkinson disease. Phys Ther. (2007) 87:1369–78. 10.2522/ptj.20070007
13.
Winser SJ Kannan P Bello UM Whitney SL . Measures of balance and falls risk prediction in people with Parkinson's disease: a systematic review of psychometric properties. Clin Rehabil. (2019) 33:1949–62. 10.1177/0269215519877498
14.
Balash Y Peretz C Leibovich G Herman T Hausdorff JM Giladi N . Falls in outpatients with Parkinson's disease: frequency, impact and identifying factors. J Neurol. (2005) 252:1310–5. 10.1007/s00415-005-0855-3
15.
Schenkman M Ellis T Christiansen C Barón AE Tickle-Degnen L Hall DA et al . Profile of functional limitations and task performance among people with early- and middle-stage Parkinson disease. Phys Ther. (2011) 91:1339–54. 10.2522/ptj.20100236
16.
Kamieniarz A Michalska J Brachman A Pawłowski M Słomka KJ Juras G et al . Posturographic procedure assessing balance disorders in parkinson's disease: a systematic review. Clin Interv Aging. (2018) 13:2301–16. 10.2147/CIA.S180894
17.
Sebastia-Amat S Tortosa-Martínez J Pueo B . The use of the static posturography to assess balance performance in a Parkinson's disease population. Int J Environ Res Public Health. (2023) 20:981. 10.3390/ijerph20020981
18.
De la Casa-Fages B Alonso-Frech F Grandas F . Effect of subthalamic nucleus deep brain stimulation on balance in Parkinson's disease: a static posturographic analysis. Gait Post. (2017) 52:374–80. 10.1016/j.gaitpost.2016.12.025
19.
Raethjen J Raethjen P Schmalbach B Wasner G . Dynamic posturography and posturographic training for Parkinson's disease in a routine clinical setting. Gait Post. (2020) 82:281–6. 10.1016/j.gaitpost.2020.09.013
20.
SCImago . Scimago Journal & Country Rank. Available at: https://www.scimagojr.com/ (accessed February 14, 2024). Available at: https://www.scimagojr.com/aboutus.php
21.
Chiari L . Stabilometry. In:BinderMDHirokawaNWindhorstU, editors. Encyclopedia of Neuroscience. Berlin, Heidelberg: Springer Berlin Heidelberg (2009). p. 3830–3.
22.
Samadi Kohnehshahri F Merlo A Mazzoli D Bò MC Stagni R . Machine learning applied to gait analysis data in cerebral palsy and stroke: a systematic review. Gait Posture. (2024) 111:105–21. 10.1016/j.gaitpost.2024.04.007
23.
Quijoux F Nicolaï A Chairi I Bargiotas I Ricard D Yelnik A et al . A review of center of pressure (COP) variables to quantify standing balance in elderly people: algorithms and open-access code*. Physiol Rep. (2021) 9:15067. 10.14814/phy2.15067
24.
Quijoux F Vienne-Jumeau A Bertin-Hugault F Zawieja P Lefèvre M Vidal PP et al . Center of pressure displacement characteristics differentiate fall risk in older people: a systematic review with meta-analysis. Ageing Res Rev. (2020) 62:101117. 10.1016/j.arr.2020.101117
25.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71
26.
Ouzzani M Hammady H Fedorowicz Z Elmagarmid A . Rayyan-a web and mobile app for systematic reviews. Syst Rev (2016) 5:210. 10.1186/s13643-016-0384-4
27.
Ali F Loushin SR Botha H Josephs KA Whitwell JL Kaufman K . Laboratory based assessment of gait and balance impairment in patients with progressive supranuclear palsy. J Neurol Sci. (2021) 429:118054. 10.1016/j.jns.2021.118054
28.
Apthorp D Smith A Ilschner S Vlieger R Das C Lueck CJ et al . Postural sway correlates with cognition and quality of life in Parkinson's disease. BMJ Neurol Open. (2020) 2:86. 10.1136/bmjno-2020-000086
29.
Armand S Landis T Sztajzel R Burkhard PR . Dyskinesia-induced postural instability in Parkinson's disease. Parkinsonism Relat Disord. (2009) 15:359–64. 10.1016/j.parkreldis.2008.08.007
30.
Ayán C Bidaurrazaga-Letona I Martin A Lejonagoitia-Garmendia M Torres-Unda J Esain I . Effects of stretching vs. in Hatha yoga people with mild to moderate Parkinson's disease: a randomized controlled trial. Sci Sports. (2023) 38:631–5. 10.1016/j.scispo.2022.09.010
31.
Bacha JMR da Cunha MCC de Freitas TB Nuvolini RA Doná F da Silva KG et al . Effects of virtual rehabilitation on postural control of individuals with Parkinson disease. Motricidade. (2021) 17:220–7. 10.6063/motricidade.20207
32.
Bao W Tan Y Yang Y Chen K Liu J . Correlation of balance posturographic parameters during quiet standing with the Berg Balance Scale in patients with Parkinson's disease. BMC Neurol. (2023) 23:362. 10.1186/s12883-023-03386-1
33.
Baratto L Morasso PG Re C Spada G . A new look at posturographic analysis in the clinical context: sway-density versus other parameterization techniques. Motor Control. (2002) 6:246–70. 10.1123/mcj.6.3.246
34.
Barbieri FA Polastri PF Baptista AM Lirani-Silva E Simieli L Orcioli-Silva D et al . Effects of disease severity and medication state on postural control asymmetry during challenging postural tasks in individuals with Parkinson's disease. Hum Mov Sci. (2016) 46:96–103. 10.1016/j.humov.2015.12.009
35.
Barbieri FA Carpenter M Beretta VS Orcioli-Silva D Simieli L Vitório R et al . Postural control, falls and Parkinson's disease: are fallers more asymmetric than non-fallers?Hum Mov Sci. (2019) 63:129–37. 10.1016/j.humov.2018.10.008
36.
Bekkers EMJ Dockx K Heremans E Vercruysse S Verschueren SMP Mirelman A et al . The contribution of proprioceptive information to postural control in elderly and patients with Parkinson's disease with a history of falls. Front Hum Neurosci. (2014) 8:939. 10.3389/fnhum.2014.00939
37.
Bekkers EMJ Dockx K Devan S Van Rossom S Verschueren SMP Bloem BR et al . The impact of dual-tasking on postural stability in people with Parkinson's disease with and without freezing of gait. Neurorehabil Neural Repair. (2018) 32:166–74. 10.1177/1545968318761121
38.
Bello O Sanchez JA Lopez-Alonso V Márquez G Morenilla L Castro X et al . The effects of treadmill or overground walking training program on gait in Parkinson's disease. Gait Post. (2013) 38:590–5. 10.1016/j.gaitpost.2013.02.005
39.
Beretta VS Gobbi LTB Lirani-Silva E Simieli L Orcioli-Silva D Barbieri FA . Challenging postural tasks increase asymmetry in patients with Parkinson's disease. PLoS ONE. (2015) 10:e0137722. 10.1371/journal.pone.0137722
40.
Błaszczyk JW Orawiec R Duda-Kłodowska D Opala G . Assessment of postural instability in patients with Parkinson's disease. Exp Brain Res. (2007) 183:107–14. 10.1007/s00221-007-1024-y
41.
Błaszczyk JW Orawiec R . Assessment of postural control in patients with Parkinson's disease: sway ratio analysis. Hum Mov Sci. (2011) 30:396–404. 10.1016/j.humov.2010.07.017
42.
Błaszczyk JW . The use of force-plate posturography in the assessment of postural instability. Gait Posture. (2016) 44:1–6. 10.1016/j.gaitpost.2015.10.014
43.
Bonnet CT Delval A Singh T Kechabia YR Defebvre L . New insight into Parkinson's disease-related impairment of the automatic control of upright stance. Eur J Neurosci. (2020) 52:4851–62. 10.1111/ejn.14870
44.
Brachman A Marszałek W Kamieniarz A Michalska J Pawłowski M Juras G . Biomechanical measures of balance after balance-based exergaming training dedicated for patients with Parkinson's disease. Gait Posture. (2021) 87:170–6. 10.1016/j.gaitpost.2021.04.036
45.
Buated W Lolekha P Hidaka S Fujinami T . Impact of cognitive loading on postural control in Parkinson's disease with freezing of gait. Gerontol Geriatr Med. (2016) 2:233372141667375. 10.1177/2333721416673751
46.
Burleigh A Horak F Nutt J Frank J . Levodopa reduces muscle tone and lower extremity tremor in Parkinson's disease. Can J Neurol Sci. (1995) 22:280–5. 10.1017/S0317167100039470
47.
Cabeleira MEP Pagnussat AS do Pinho AS Asquidamini ACD Freire AB Pereira BT et al . Impairments in gait kinematics and postural control may not correlate with dopamine transporter depletion in individuals with mild to moderate Parkinson's disease. Eur J Neurosci. (2019) 49:1640–8. 10.1111/ejn.14328
48.
Cabrera-Martos I Jiménez-Martín AT López-López L Rodríguez-Torres J Ortiz-Rubio A Valenza MC . Effects of a core stabilization training program on balance ability in persons with Parkinson's disease: a randomized controlled trial. Clin Rehabil. (2020) 34:764–72. 10.1177/0269215520918631
49.
Cancela JM Rodriguez G Machado I Mollinedo I . Pilates as physiotherapy in patients with Parkinson disease: a pilot study. Asian J Gerontol Geriatr. (2021) 16:73–81. 10.12809/ajgg-2020-449-oa
50.
Silva TC Da Felippe LA Carregaro RL Christofoletti G . Postural instability in subjects with Parkinson's disease undergoing different sensory pitfalls. Hum Mov. (2017) 18:55–60. 10.1515/humo-2017-0031
51.
Ahn JH Lee D Kim M Cho JW Chang WH Youn J . M1 and cerebellar tDCS for MSA-C: a double-blind, randomized, sham-controlled, crossover study. Cerebellum. (2023) 22:386–93. 10.1007/s12311-022-01416-1
52.
Carpinella I Cattaneo D Bonora G Bowman T Martina L Montesano A Ferrarin M . Wearable sensor-based biofeedback training for balance and gait in Parkinson disease: a pilot randomized controlled trial. Arch Phys Med Rehabil. (2017) 98:622–30.e3. 10.1016/j.apmr.2016.11.003
53.
Chastan N Debono B Maltête D Weber J . Discordance between measured postural instability and absence of clinical symptoms in Parkinson's disease patients in the early stages of the disease. Mov Disord. (2008) 23:366–72. 10.1002/mds.21840
54.
Chen J Chien HF Francato DCV Barbosa AF Souza C de O Voos MC et al . Effects of resistance training on postural control in Parkinson's disease: a randomized controlled trial. Arq Neuropsiquiatr. (2021) 79:511–20. 10.1590/0004-282x-anp-2020-0285
55.
Colnat-Coulbois S Gauchard GC Maillard L Barroche G Vespignani H Auque J et al . Bilateral subthalamic nucleus stimulation improves balance control in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2005) 76:780–7. 10.1136/jnnp.2004.047829
56.
Colnat-Coulbois S Gauchard GC Maillard L Barroche G Vespignani H Auque J et al . Management of postural sensory conflict and dynamic balance control in late-stage Parkinson's disease. Neuroscience. (2011) 193:363–9. 10.1016/j.neuroscience.2011.04.043
57.
Corrêa PS Pagnussat AS Cabeleira MEP Schifino GP Rieder CR de M da Silva Junior N et al . Is the dopaminergic loss associated with gait and postural impairments in subjects with Parkinson's disease at different motor stages?Eur J Neurosci. (2019) 50:3889–95. 10.1111/ejn.14522
58.
D'Andréa Greve JM Luna NMS de Siqueira JP Prota C Alonso AC . Assessment of postural balance among individuals with Parkinson disease with and without effects from dopaminergic medications. Am J Phys Med Rehabil. (2014) 93:365–71. 10.1097/PHM.0b013e3182a92aa9
59.
Dallaire M Houde-Thibeault A Bouchard-Tremblay J Wotto EA Côté S Santos Oliveira C et al . Impact of frailty and sex-related differences on postural control and gait in older adults with Parkinson's disease. Exp Gerontol. (2024) 186:112360. 10.1016/j.exger.2024.112360
60.
Dana A Shams A Allafan N Bahrami A . The relationship between attention and static balance disturbance in patients with Parkinson's disease. Neurol Sci. (2021) 42:5107–15. 10.1007/s10072-021-05184-4
61.
Costa E de C Santinelli FB Moretto GF Figueiredo C von Ah Morano AE Barela JA et al . Multiple domain postural control assessment in people with Parkinson's disease: traditional, non-linear, and rambling and trembling trajectories analysis. Gait Posture. (2022) 97:130–6. 10.1016/j.gaitpost.2022.07.250
62.
Degani AM Cardoso VS Magalhães AT Assunção ALS Soares E de C Danna-dos-Santos A . Postural behavior in medicated parkinson disease patients: a preliminary study searching for indicators to track progress. J Cent Nerv Syst Dis. (2020) 12:1179573520922645. 10.1177/1179573520922645
63.
Doná F Aquino CC Gazzola JM Borges V Silva SMCA Ganança FF et al . Changes in postural control in patients with Parkinson's disease: a posturographic study. Physiotherapy. (2016) 102:272–9. 10.1016/j.physio.2015.08.009
64.
Km E . Dopaminergic medication amplifies sensory integration deficits during static balance in Parkinson's patients with freezing of gait. BAOJ Neurol. (2016) 2:122. 10.24947/baojn/2/3/122
65.
Espinoza-Valdés Y Córdova-Arellano R Espinoza-Espinoza M Méndez-Alfaro D Bustamante-Aguirre JP Maureira-Pareja HA et al . Association between cardiac autonomic control and postural control in patients with Parkinson's disease. Int J Environ Res Public Health. (2020) 18:249. 10.3390/ijerph18010249
66.
Fadil R Huether AXA Sadeghian F Verma AK Blaber AP Lou JS et al . The effect of skeletal muscle-pump on blood pressure and postural control in Parkinson's disease. Cardiovasc Eng Technol. (2023) 14:755–73. 10.1007/s13239-023-00685-z
67.
Fernandes  Coelho T Vitória A Ferreira A Santos R Rocha N et al . Standing balance in individuals with Parkinson's disease during single and dual-task conditions. Gait Post. (2015) 42:323–8. 10.1016/j.gaitpost.2015.06.188
68.
Barbosa AF Souza C de O Chen J Francato DV Caromano FA Chien HF et al . The competition with a concurrent cognitive task affects posturographic measures in patients with Parkinson disease. Arq Neuropsiquiatr. (2015) 73:906–12. 10.1590/0004-282X20150153
69.
Silvia Aparecida FP Carlos Henrique Ferreira C Marise Bueno Z Pessoa Renata R Renato Puppi M Hélio Afonso Ghizoni T . Static posturography analysis for postural instability in patients with Parkinson's disease. Int J Neurosci. (2023) 134:1551–63. 10.1080/00207454.2023.2273765
70.
Franzoni L Monteiro E Oliveira H da Rosa R Costa R Rieder C et al . 9-week nordic and free walking improve postural balance in Parkinson's disease. Sports Med Int Open. (2018) 02:E28–34. 10.1055/s-0043-124757
71.
Geroin C Smania N Schena F Dimitrova E Verzini E Bombieri F et al . Does the Pisa syndrome affect postural control, balance, and gait in patients with Parkinson's disease? An observational cross-sectional study. Parkinsonism Relat Disord. (2015) 21:736–41. 10.1016/j.parkreldis.2015.04.020
72.
Geroin C Gandolfi M Maddalena I Smania N Tinazzi M . Do upper and lower camptocormias affect gait and postural control in patients with Parkinson's disease? An observational cross-sectional study. Parkinsons Dis. (2019) 2019:9026890. 10.1155/2019/9026890
73.
Gervasoni E Cattaneo D Messina P Casati E Montesano A Bianchi E et al . Clinical and stabilometric measures predicting falls in Parkinson disease/parkinsonisms. Acta Neurol Scand. (2015) 132:235–41. 10.1111/ane.12388
74.
Geurts ACH Boonstra TA Voermans NC Diender MG Weerdesteyn V Bloem BR . Assessment of postural asymmetry in mild to moderate Parkinson's disease. Gait Post. (2011) 33:143–5. 10.1016/j.gaitpost.2010.09.018
75.
Guehl D Dehail P De Sèze MP Cuny E Faux P Tison F et al . Evolution of postural stability after subthalamic nucleus stimulation in Parkinson's disease: a combined clinical and posturometric study. Exp Brain Res. (2006) 170:206–15. 10.1007/s00221-005-0202-z
76.
Gulcan K Guclu-Gunduz A Yasar E Ar U Sucullu Karadag Y Saygili F . The effects of augmented and virtual reality gait training on balance and gait in patients with Parkinson's disease. Acta Neurol Belg. (2023) 123:1917–25. 10.1007/s13760-022-02147-0
77.
Halmi Z Dinya E Málly J . Destroyed non-dopaminergic pathways in the early stage of Parkinson's disease assessed by posturography. Brain Res Bull. (2019) 152:45–51. 10.1016/j.brainresbull.2019.07.001
78.
Han J Jung J Lee J Kim E Lee M Lee K . Effect of muscle vibration on postural balance of Parkinson's diseases patients in bipedal quiet standing. J Phys Ther Sci. (2013) 25:1433–5. 10.1589/jpts.25.1433
79.
Hasegawa N Ishikawa K Sato Y Nakayama Y Asaka T . Short-term effects of postural control by standing on a tilting board in patients with Parkinson's disease. Physiother Theory Pract. (2021) 37:1306–12. 10.1080/09593985.2019.1695302
80.
High CM McHugh HF Mills SC Amano S Freund JE Vallabhajosula S . Vibrotactile feedback alters dynamics of static postural control in persons with Parkinson's disease but not older adults at high fall risk. Gait Posture. (2018) 63:202–7. 10.1016/j.gaitpost.2018.05.010
81.
Iwai M Koyama S Takeda K Hirakawa Y Motoya I Kumazawa N et al . Effect of LSVT® BIG on standing balance in a Parkinson's patient: a case report. Physiotherapy Res Int. (2021) 26:1921. 10.1002/pri.1921
82.
Jazaeri SZ Azad A Mehdizadeh H Habibi SA Najafabadi MM Saberi ZS et al . The effects of anxiety and external attentional focus on postural control in patients with Parkinson's disease. PLoS ONE. (2018) 13:e0192168. 10.1371/journal.pone.0192168
83.
Jehu D Nantel J . Fallers with Parkinson's disease exhibit restrictive trunk control during walking. Gait Post. (2018) 65:246–50. 10.1016/j.gaitpost.2018.07.181
84.
Johnson L James I Rodrigues J Stell R Thickbroom G Mastaglia F . Clinical and posturographic correlates of falling in Parkinson's disease. Mov Disord. (2013) 28:1250–6. 10.1002/mds.25449
85.
Johnson L Rodrigues J Teo WP Walters S Stell R Thickbroom G et al . Interactive effects of GPI stimulation and levodopa on postural control in Parkinson's disease. Gait Post. (2015) 41:929–34. 10.1016/j.gaitpost.2015.03.346
86.
Kamieniarz A Michalska J Marszałek W Stania M Słomka KJ Gorzkowska A et al . Detection of postural control in early Parkinson's disease: Clinical testing vs. modulation of center of pressure. PLoS ONE. (2021) 16:e0245353. 10.1371/journal.pone.0245353
87.
Karimi M Sadeghisani M Omar AHH Kouchaki E Mirahmadi M Fatoye F . Stability analysis in patients with neurological and musculoskeletal disorders using linear and non-linear approaches. J Mech Med Biol. (2015) 15:45. 10.1142/S0219519415300045
88.
Kim JY Son MJ Kim YK Lee MG Kim JH Youm CH . Effects of freezing of gait and visual information on the static postural control ability in patients with Parkinson's disease. Kor J Sport Biomech. (2016) 26:293–301. 10.5103/KJSB.2016.26.3.293
89.
Kim A Darakjian N Finley JM . Walking in fully immersive virtual environments: an evaluation of potential adverse effects in older adults and individuals with Parkinson's disease. J Neuroeng Rehabil. (2017) 14:16. 10.1186/s12984-017-0225-2
90.
Korkusuz S Seçkinogullari B Özcan A Demircan EN Çakmakli GY Armutlu K et al . Effects of freezing of gait on balance in patients with Parkinson's disease. Neurol Res. (2023) 45:407–14. 10.1080/01616412.2022.2149510
91.
Kudrevatykh A Senkevich K Miliukhina I . Postural instability and neuropsychiatric disturbance in the overlapping phenotype of essential tremor and Parkinson's disease. Neurophysiol Clin. (2020) 50:489–94. 10.1016/j.neucli.2020.07.001
92.
Kwon D-Y Choi Y-H Kwon Y-R Eom G-M Kim J-W . Comparison of static postural balance in patients with SWEDDS and Parkinson's disease. J Mech Med Biol. (2020) 20:2040013. 10.1142/S0219519420400138
93.
Kwon DY Kwon Y Choi JA Ko J Kim JW . Quantitative Analysis of postural balance in faller and nonfaller patients with Parkinson's disease. Parkinsons Dis. (2023) 2023:9688025. 10.1155/2023/9688025
94.
Kwon YR Kim JW . Reliability of static balance test in faller and nonfaller Parkinson disease patients. J Mech Med Biol. (2023) 2023:9688025. 10.1142/S0219519423401036
95.
Lahr J Pereira MP Pelicioni PHS de Morais LC Gobbi LTB . Parkinson's disease patients with dominant hemibody affected by the disease rely more on vision to maintain upright postural control. Percept Mot Skills. (2015) 121:923–34. 10.2466/15.PMS.121c26x0
96.
Lauretani F Galuppo L Costantino C Ticinesi A Ceda G Ruffini L et al . Parkinson's disease (PD) with dementia and falls is improved by AChEI? A preliminary study report. Aging Clin Exp Res. (2016) 28:551–5. 10.1007/s40520-015-0437-x
97.
Lazarotto L Bobbo GZG Siega J da Silva AZ Iucksch DD Israel VL et al . Static and dynamic postural control: comparison between community old adults and people with Parkinson's disease. Physiotherapy Res Int. (2020) 25:1844. 10.1002/pri.1844
98.
Lee HK Altmann LJP McFarland N Hass CJ . The relationship between balance confidence and control in individuals with Parkinson's disease. Parkinsonism Relat Disord. (2016) 26:24–8. 10.1016/j.parkreldis.2016.02.015
99.
Li Y Zhang S Odeh C . Automated classification of postural control for individuals with Parkinson's disease using a machine learning approach: a preliminary study. J Appl Biomech. (2020) 36:334–9. 10.1123/jab.2019-0400
100.
Mancini M Horak FB Zampieri C Carlson-Kuhta P Nutt JG Chiari L . Trunk accelerometry reveals postural instability in untreated Parkinson's disease. Parkinsonism Relat Disord. (2011) 17:557–62. 10.1016/j.parkreldis.2011.05.010
101.
Marchese R Bove M Abbruzzese G . Effect of cognitive and motor tasks on postural stability in Parkinson's disease: a posturographic study. Mov Disord. (2003) 18:652–8. 10.1002/mds.10418
102.
Mirahmadi M Karimi MT Esrafilian A . An evaluation of the effect of vision on standing stability in the early stage of Parkinson's disease. Eur Neurol. (2019) 80:261–7. 10.1159/000497041
103.
Morenilla L Márquez G Sánchez JA Bello O López-Alonso V Fernández-Lago H et al . Postural stability and cognitive performance of subjects with Parkinson's disease during a dual-task in an upright stance. Front Psychol. (2020) 11:1256. 10.3389/fpsyg.2020.01256
104.
Nantel J McDonald JC Bronte-Stewart H . Effect of medication and STN-DBS on postural control in subjects with Parkinson's disease. Parkinsonism Relat Disord. (2012) 18:285–9. 10.1016/j.parkreldis.2011.11.005
105.
Nantel J Bronte-Stewart H . The effect of medication and the role of postural instability in different components of freezing of gait (FOG). Parkinsonism Relat Disord. (2014) 20:447–51. 10.1016/j.parkreldis.2014.01.017
106.
Nardone A Schieppati M . The role of instrumental assessment of balance in clinical decision making. Eur J Phys Rehabil Med. (2010) 46:221–37.
107.
Nikaido Y Akisue T Kajimoto Y Tucker A Kawami Y Urakami H et al . Postural instability differences between idiopathic normal pressure hydrocephalus and Parkinson's disease. Clin Neurol Neurosurg. (2018) 165:103–7. 10.1016/j.clineuro.2018.01.012
108.
Nocera JR Price C Fernandez HH Amano S Vallabhajosula S Okun MS et al . Tests of dorsolateral frontal function correlate with objective tests of postural stability in early to moderate stage Parkinson's disease. Parkinsonism Relat Disord. (2010) 16:590–4. 10.1016/j.parkreldis.2010.08.008
109.
Oz F Yucekeya B Huzmeli I Yilmaz A . Does subthalamic nucleus deep brain stimulation affect the static balance at different frequencies?Neurocirugia. (2023) 34:60–6. 10.1016/j.neucir.2022.01.001
110.
Padovan L Becker-Bense S Flanagin VL Strobl R Limburg K Lahmann C et al . Anxiety and physical impairment in patients with central vestibular disorders. J Neurol. (2023) 270:5589–99. 10.1007/s00415-023-11871-3
111.
Panyakaew P Anan C Bhidayasiri R . Visual deprivation elicits subclinical postural inflexibilities in early Parkinson's disease. J Neurol Sci. (2015) 349:214–9. 10.1016/j.jns.2015.01.022
112.
Panyakaew P Anan C Bhidayasiri R . Posturographic abnormalities in ambulatory atypical parkinsonian disorders: differentiating characteristics. Parkinsonism Relat Disord. (2019) 66:94–9. 10.1016/j.parkreldis.2019.07.016
113.
Paolucci T Iosa M Morone G Fratte MD Paolucci S Saraceni VM et al . Romberg ratio coefficient in quiet stance and postural control in Parkinson's disease. Neurol Sci. (2018) 39:1355–60. 10.1007/s10072-018-3423-1
114.
Park JH Youm S Jeon Y Park SH . Development of a balance analysis system for early diagnosis of Parkinson's disease. Int J Ind Ergon. (2015) 48:139–48. 10.1016/j.ergon.2015.05.005
115.
Pelykh O Klein AM Bötzel K Kosutzka Z Ilmberger J . Dynamics of postural control in Parkinson patients with and without symptoms of freezing of gait. Gait Post. (2015) 42:246–50. 10.1016/j.gaitpost.2014.09.021
116.
Perera T Tan JL Cole MH Yohanandan SAC Silberstein P Cook R et al . Balance control systems in Parkinson's disease and the impact of pedunculopontine area stimulation. Brain. (2018) 141:3009–22. 10.1093/brain/awy216
117.
Piras A Trofè A Meoni A Raffi M . Influence of radial optic flow stimulation on static postural balance in Parkinson's disease: a preliminary study. Hum Mov Sci. (2022) 81:102905. 10.1016/j.humov.2021.102905
118.
Qiu F Cole MH Davids KW Hennig EM Silburn PA Netscher H et al . Effects of textured insoles on balance in people with Parkinson's disease. PLoS ONE. (2013) 8:e0083309. 10.1371/journal.pone.0083309
119.
Rahmati Z Behzadipour S Schouten AC Taghizadeh G Firoozbakhsh K . Postural control learning dynamics in Parkinson's disease: early improvement with plateau in stability, and continuous progression in flexibility and mobility. Biomed Eng Online. (2020) 19:29. 10.1186/s12938-020-00776-1
120.
Pereira CR Criado MB Machado J Pereira CT Santos MJ . Acute effects of acupuncture in balance and gait of Parkinson disease patients – a preliminary study. Complement Ther Clin Pract. (2021) 45:101479. 10.1016/j.ctcp.2021.101479
121.
Raymakers JA Samson MM Verhaar HJJ . The assessment of body sway and the choice of the stability parameter(s). Gait Posture. (2005) 21:48–58. 10.1016/j.gaitpost.2003.11.006
122.
Rezvanian S Lockhart T Frames C Soangra R Lieberman A . Motor subtypes of Parkinson's disease can be identified by frequency component of postural stability. Sensors. (2018) 18:1102. 10.3390/s18041102
123.
Rocchi L Chiari L Cappello A Gross A Horak FB . Comparison between subthalamic nucleus and globus pallidus internus stimulation for postural performance in Parkinson's disease. Gait Post. (2004) 19:172–83. 10.1016/S0966-6362(03)00059-6
124.
Rocchi L Chiari L Cappello A Horak FB . Identification of distinct characteristics of postural sway in Parkinson's disease: a feature selection procedure based on principal component analysis. Neurosci Lett. (2006) 394:140–5. 10.1016/j.neulet.2005.10.020
125.
Santos SM Da Silva RA Terra MB Almeida IA De Melo LB Ferraz HB . Balance versus resistance training on postural control in patients with Parkinson's disease: a randomized controlled trial. Eur J Phys Rehabil Med. (2017) 53:173–83. 10.23736/S1973-9087.16.04313-6
126.
Santos L Fernandez-Rio J Winge K Barragán-Pérez B González-Gómez L Rodríguez-Pérez V et al . Effects of progressive resistance exercise in akinetic-rigid Parkinson's disease patients: a randomized controlled trial. Eur J Phys Rehabil Med. (2017) 53:651–63. 10.23736/S1973-9087.17.04572-5
127.
Santos L Fernandez-Rio J Winge K Barragán-Pérez B Rodríguez-Pérez V González-Díez V et al . Effects of supervised slackline training on postural instability, freezing of gait, and falls efficacy in people with Parkinson's disease. Disabil Rehabil. (2017) 39:1573–80. 10.1080/09638288.2016.1207104
128.
Sato K Hokari Y Kitahara E Izawa N Hatori K Honaga K et al . Short-term motor outcomes in Parkinson's disease after subthalamic nucleus deep brain stimulation combined with post-operative rehabilitation: a pre-post comparison study. Parkinsons Dis. (2022) 2022:8448638. 10.1155/2022/8448638
129.
Schieppati M Hugon M Grasso M Nardone A Galante M . The limits of equilibrium in young elderly normal subjects and in parkinsonians. Electroencephalogr Clin Neurophysiol. (1994) 93:286–98. 10.1016/0013-4694(94)00036-K
130.
Schieppati M Tacchini E Nardone A Tarantola J Corna S . Subjective perception of body sway. Neurol Neurosurg Psychiatry. (1999) 66:313–22. 10.1136/jnnp.66.3.313
131.
Schlenstedt C Muthuraman M Witt K Weisser B Fasano A Deuschl G . Postural control and freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. (2016) 24:107–12. 10.1016/j.parkreldis.2015.12.011
132.
Schmit JM Riley MA Dalvi A Sahay A Shear PK Shockley KD et al . Deterministic center of pressure patterns characterize postural instability in Parkinson's disease. Exp Brain Res. (2006) 168:357–67. 10.1007/s00221-005-0094-y
133.
Sebastiá-Amat S Tortosa-Martínez J García-Jaén M Pueo B . Within-subject variation in the Cognitive Timed Up and Go test as an explanatory variable in fall risk in patients with Parkinson's disease. J Rehabil Med. (2021) 53:0. 10.2340/16501977-2874
134.
Severo AR Silveira MC Mota CB Rhoden EC Filippin NT . Immediate effects of an approach in high cervical and occipitomastoidon postural control and mobility of individuals with Parkinson's disease: case series. Manual Ther Posturol Rehabil J. (2016) 14:322. 10.17784/mtprehabjournal.2016.14.322
135.
Smart RR Toumi A Harris OD Cremoux S Dalton BH Wile DJ et al . Intermuscular coherence of plantar and dorsiflexor muscles in older adults with Parkinson's disease and age-matched controls during bipedal and unipedal stance. Front Aging Neurosci. (2023) 15:1093295. 10.3389/fnagi.2023.1093295
136.
Sowalsky KL Sonke J Altmann LJP Almeida L Hass CJ . Biomechanical analysis of dance for Parkinson's disease: a paradoxical case study of balance and gait effects?Explore. (2017) 13:409–13. 10.1016/j.explore.2017.03.009
137.
Spolaor F Romanato M Annamaria G Peppe A Bakdounes L To D-K et al . Relationship between muscular activity and postural control changes after proprioceptive focal stimulation (Equistasi®) in middle-moderate parkinson's disease patients: an explorative study. Sensors. (2021) 21:560. 10.3390/s21020560
138.
Suarez H Geisinger D Ferreira ED Nogueira S Arocena S Roman CS et al . Balance in Parkinson's disease patients changing the visual input. Braz J Otorhinolaryngol. (2011) 77:651–5. 10.1590/S1808-86942011000500019
139.
Terra MB Da Silva RA Bueno MEB Ferraz HB Smaili SM . Center of pressure-based balance evaluation in individuals with Parkinson's disease: a reliability study. Physiother Theory Pract. (2020) 36:826–33. 10.1080/09593985.2018.1508261
140.
Terra MB Caramaschi IKF de Oliveira Araújo HAG de Souza RJ da Silva TCO Nascimento TS et al . Is fatigue associated with balance in Parkinson's disease?Motriz Revista de Educacao Fisica. (2022) 28:13921. 10.1590/s1980-657420220013921
141.
Tollár J Nagy F Kovács N Hortobágyi T . A high-intensity multicomponent agility intervention improves Parkinson patients' clinical and motor symptoms. Arch Phys Med Rehabil. (2018) 99:2478–84.e1. 10.1016/j.apmr.2018.05.007
142.
Tollar J Nagy F Hortobágyi T . Vastly different exercise programs similarly improve parkinsonian symptoms: a randomized clinical trial. Gerontology. (2019) 65:120–7. 10.1159/000493127
143.
Tollár J Nagy F Kovács N Hortobágyi T . Two-year agility maintenance training slows the progression of parkinsonian symptoms. Med Sci Sports Exerc. (2019) 51:237–45. 10.1249/MSS.0000000000001793
144.
Tsai CL Lai YR Lien CY Huang CC Chiu WC Chen YS et al . Feasibility of combining disease-specific and balance-related measures as risk predictors of future falls in patients with Parkinson's disease. J Clin Med. (2023) 12:10127. 10.3390/jcm12010127
145.
Vasconcellos LS de Silva RS Pachêco TBF Nagem DAP Sousa C de O Ribeiro TS . Telerehabilitation-based trunk exercise training for motor symptoms of individuals with Parkinson's disease: a randomized controlled clinical trial. J Telemed Telecare. (2023) 29:698–706. 10.1177/1357633X211021740
146.
Volpe D Giantin MG Fasano A . A wearable proprioceptive stabilizer (Equistasi®) for rehabilitation of postural instability in Parkinson's disease: a phase II randomized double-blind, double-dummy, controlled study. PLoS ONE. (2014) 9:e0112065. 10.1371/journal.pone.0112065
147.
Wodarski P Jurkojć J Michalska J Kamieniarz A Juras G Gzik M . Balance assessment in selected stages of Parkinson's disease using trend change analysis. J Neuroeng Rehabil. (2023) 20:99. 10.1186/s12984-023-01229-1
148.
Workman CD Thrasher TA . The influence of dopaminergic medication on balance automaticity in Parkinson's disease. Gait Posture. (2019) 70:98–103. 10.1016/j.gaitpost.2019.02.015
149.
Yoon JP Shin SP Park J Yoo WP . The effects of stooped posture on gait and postural sway in Korean patients with Parkinson's disease. Neurol Asia. (2019) 24:243–7.
150.
Yozu A Kaminishi K Ishii D Omura Y Matsushita A Kohno Y et al . Effects of medication and dual tasking on postural sway in Parkinson's disease: a pilot case study. Adv Robot. (2021) 35:889–97. 10.1080/01691864.2021.1948353
151.
Zarucchi A Vismara L Frazzitta G Mauro A Priano L Maestri R et al . Efficacy of osteopathic manipulative treatment on postural control in Parkinsonian patients with Pisa syndrome: a pilot randomized placebo-controlled trial. NeuroRehabilitation. (2020) 46:529–37. 10.3233/NRE-203068
152.
Zawadka-Kunikowska M Zalewski P Klawe JJ Pawlak J Tafil-Klawe M Kedziora-Kornatowska K et al . Age-related changes in cognitive function and postural control in Parkinson's disease. Aging Clin Exp Res. (2014) 26:505–10. 10.1007/s40520-014-0209-z
153.
Zawadka-Kunikowska M Klawe JJ Tafil-Klawe M Bejtka M Rzepiński Ł Cieślicka M . Cognitive function and postural control strategies in relation to disease progression in patients with Parkinson's disease. Int J Environ Res Public Health. (2022) 19:1912694. 10.3390/ijerph191912694
154.
Zulai LC Albuquerque AM Papcke C Louzada FM Scheeren EM . Postural impairments in Parkinson's disease are not associated with changes in circadian rhythms changes. Chronobiol Int. (2020) 37:135–41. 10.1080/07420528.2019.1692350
155.
Zwergal A La Fougère C Lorenzl S Rominger A Xiong G Deutschenbaur L et al . Postural imbalance and falls in PSP correlate with functional pathology of the thalamus. Neurology. (2011) 101–9. 10.1212/WNL.0b013e318223c79d
156.
Rubin R . It takes an average of 17 years for evidence to change practice—the burgeoning field of implementation science seeks to speed things up. JAMA. (2023) 329:1333. 10.1001/jama.2023.4387
157.
Fahn S Elton RL . Unified Parkinson's disease rating scale. In:StanleyFDavid MarsdenCGoldsteinMCalneDB, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information (1987). p. 153–163.
158.
Goetz CG Poewe W Rascol O Sampaio C Stebbins GT Counsell C et al . Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. (2004) 19:1020–8. 10.1002/mds.20213
159.
Wenning GK Tison F Seppi K Sampiao C Diem A Yekhlef F et al . Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord. (2004) 19:1391–402. 10.1002/mds.20255
160.
Golbe LI Ohman-Strickland PA . A clinical rating scale for progressive supranuclear palsy. Brain. (2007) 130:1552–65. 10.1093/brain/awm032
161.
Schmitz-Hübsch T du Montcel ST Baliko L Berciano J Boesch S Depondt C et al . Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. (2006) 66:1717–20. 10.1212/01.wnl.0000219042.60538.92
162.
Hoffmann TC Glasziou PP Boutron I Milne R Perera R Moher D et al . Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. (2014) 348:g1687–g1687. 10.1136/bmj.g1687
163.
Faber J Fonseca LM . How sample size influences research outcomes. Dental Press J Orthod. (2014) 19:27–9. 10.1590/2176-9451.19.4.027-029.ebo
164.
Schulz KF Altman DG Moher D . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. (2010) 340:698–702. 10.1016/j.ijsu.2010.09.006
165.
Von Elm E Altman DG Egger M Pocock SJ Gøtzsche PC Vandenbroucke JP et al . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. (2007) 370:14537. 10.1016/S0140-6736(07)61602-X
166.
Kapteyn TS Bles W Njiokiktjien CJ Kodde L Massen CH Mol JM . Standardization in platform stabilometry being a part of posturography. Agressologie. (1983) 24:321–6.
167.
Scoppa F Capra R Gallamini M Shiffer R . Clinical stabilometry standardization. Basic definitions - acquisition interval - sampling frequency. Gait Post. (2013) 37:290–2. 10.1016/j.gaitpost.2012.07.009
168.
Lafond D Corriveau H Hébert R Prince F . Intrasession reliability of center of pressure measures of postural steadiness in healthy elderly people. Arch Phys Med Rehabil. (2004) 85:896–901. 10.1016/j.apmr.2003.08.089
169.
Corriveau H Heébert R Prince F Rache M . Postural control in the elderly: an analysis of test-retest and interrater reliability of the COP-COM variable. Arch Phys Med Rehabil. (2001) 82:80–5. 10.1053/apmr.2001.18678
170.
Merlo A Prati P Mazzoli D . How many trials should be acquired to investigate the open-eyes posture of a subject?Gait Post. (2009) 29:e5. 10.1016/j.gaitpost.2008.10.009
171.
Floirat N Bares F Ferrey G Gaudet E Kemoun G Carette P et al . Aporie des normes stabilométriques. Available at: ada-posturologie.fr
172.
Gagey PM Weber B . Study of intra-subject random variations of stabilometric parameters. Med Biol Eng Comput. (2010) 48:833–5. 10.1007/s11517-010-0656-4
173.
Pinsault N Vuillerme N . Test-retest reliability of centre of foot pressure measures to assess postural control during unperturbed stance. Med Eng Phys. (2009) 31:276–86. 10.1016/j.medengphy.2008.08.003
174.
Chiari L . Standardization in Clinical Stabilometry: Towards a Consensus (2014). Available at: ada-posturologie.fr
175.
Yamamoto M Ishikawa K Aoki M Mizuta K Ito Y Asai M et al . Japanese standard for clinical stabilometry assessment: current status and future directions. Auris Nasus Larynx. (2018) 45:201–6. 10.1016/j.anl.2017.06.006
176.
Nardone A Schieppati M . Balance in Parkinson's disease under static and dynamic conditions. Mov Disord. (2006) 21:1515–20. 10.1002/mds.21015
177.
Bizzo G Guillet N Patat A Gagey PM . Specifications for building a vertical force platform designed for clinical stabilometry. Med Biol Eng Comput. (1985) 23:474–6. 10.1007/BF02448937
178.
Paillard T Noé F . Techniques and methods for testing the postural function in healthy and pathological subjects. Biomed Res Int. (2015) 2015:891390. 10.1155/2015/891390
179.
Zok M Mazzà C Cappozzo A . Should the instructions issued to the subject in traditional static posturography be standardised?Med Eng Phys. (2008) 30:913–6. 10.1016/j.medengphy.2007.12.002
180.
Nishiwaki Y Takebayashi T Imai A Yamamoto M Omae K . Difference by instructional set in stabilometry. J Vest Res. (2000) 10:157–61. 10.3233/VES-2000-10305
181.
Chiari L Rocchi L Cappello A . Stabilometric parameters are affected by anthropometry and foot placement. Clin Biomech. (2002) 17:666–77. 10.1016/S0268-0033(02)00107-9
182.
Scoppa F Messina G Gallamini M Belloni G . Clinical stabilometry standardization: Feet position in the static stabilometric assessment of postural stability. Acta Med Mediterranea. (2017) 33:707–13. 10.19193/0393-6384_2017_4_105k
183.
Bloem BR Grimbergen YAM van Dijk JG Munneke M . The “posture second” strategy: a review of wrong priorities in Parkinson's disease. J Neurol Sci. (2006) 248:196–204. 10.1016/j.jns.2006.05.010
184.
de Barros GM Melo F Domingos J Oliveira R Silva L Fernandes JB Godinho C . The effects of different types of dual tasking on balance in healthy older adults. J Pers Med (2021) 11:933. 10.3390/jpm11090933
185.
Chiang J-H Wu G . The influence of foam surfaces on biomechanid variables contributing to postural control. Gait Post. (1997) 5:239–45. 10.1016/S0966-6362(96)01091-0
186.
Defer GL Widner H Marié RM Rémy P Levivier M . Core assessment program for surgical interventional therapies in Parkinson's disease (CAPSIT-PD). Mov Disord. (1999) 14:572–84. 10.1002/1531-8257(199907)14:4<572::AID-MDS1005>3.0.CO;2-C
187.
Hubble RP Naughton GA Silburn PA Cole MH . Wearable sensor use for assessing standing balance and walking stability in people with Parkinson's disease: a systematic review. PLoS ONE. (2015) 10:e0123705. 10.1371/journal.pone.0123705
188.
Morasso PG Spada G Capra R . Computing the COM from the COP in postural sway movements. Hum Mov Sci. (1999) 18:759–67. 10.1016/S0167-9457(99)00039-1
189.
Beuter A Barbo E Rigal R Blanchet PJ . Characterization of subclinical tremor in Parkinson's disease. Mov Disord. (2005) 20:945–50. 10.1002/mds.20467
190.
Prieto TE Myklebust JB Hoffmann RG Lovett EG Myklebust BM . Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng. (1996) 43:956–66. 10.1109/10.532130
191.
Baudendistel ST Haussler AM Rawson KS Earhart GM . Minimal clinically important differences of spatiotemporal gait variables in Parkinson disease. Gait Post. (2024) 108:257–63. 10.1016/j.gaitpost.2023.11.016
Summary
Keywords
Parkinson's disease, atypical parkinsonism, posturography, static balance, stabilometry
Citation
Merlo A, Cavazzuti L, Bò MC, Cavallieri F, Bassi MC, Damiano B, Scaltriti S, Fioravanti V, Di Rauso G, Portaro G, Valzania F, Lusuardi M and Campanini I (2025) Instrumental balance assessment in Parkinson's disease and parkinsonism. A systematic review with critical appraisal of clinical applications and quality of reporting. Front. Neurol. 16:1528191. doi: 10.3389/fneur.2025.1528191
Received
14 November 2024
Accepted
02 January 2025
Published
29 January 2025
Volume
16 - 2025
Edited by
Marina Picillo, University of Salerno, Italy
Reviewed by
Carlo Ricciardi, University of Naples Federico II, Italy
Michela Russo, University of Naples Federico II, Italy
Updates
Copyright
© 2025 Merlo, Cavazzuti, Bò, Cavallieri, Bassi, Damiano, Scaltriti, Fioravanti, Di Rauso, Portaro, Valzania, Lusuardi and Campanini.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Chiara Bò chiarabo88@gmail.com
†These authors have contributed equally to this work and share first authorship
‡ORCID: Andrea Merlo orcid.org/0000-0002-5587-5686
Lorenzo Cavazzuti orcid.org/0000-0003-0299-3598
Maria Chiara Bò orcid.org/0000-0001-5704-503X
Francesco Cavallieri orcid.org/0000-0001-5836-1982
Maria Chiara Bassi orcid.org/0000-0002-5418-2837
Benedetta Damiano orcid.org/0000-0003-2726-0008
Sara Scaltriti orcid.org/0009-0007-0965-3197
Valentina Fioravanti orcid.org/0009-0003-4631-8591
Giulia Di Rauso orcid.org/0000-0001-7159-9311
Giacomo Portaro orcid.org/0009-0005-7594-7826
Franco Valzania orcid.org/0000-0003-4887-1692
Mirco Lusuardi orcid.org/0000-0002-5348-4147
Isabella Campanini orcid.org/0000-0002-9286-6711
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.