Abstract
Background:
Epilepsy is a major public health issue worldwide, often leading to physical and cognitive impairments that limit employment, independence, and social interaction. Health-related quality of life (HRQoL) is a crucial outcome in the treatment of chronic epilepsy as it is linked to reduced independence, treatment challenges, and lower life expectancy. HRQoL serves as an important health indicator for assessing the impact of the disease on daily living activities.
Objective:
This study aimed to estimate the mean score of health-related quality of life (HRQoL) and factors associated with lower HRQoL in people living with epilepsy (PLWE) in sub-Saharan African (SSA) countries.
Methods:
A comprehensive literature search was conducted using PubMed, Cochrane Library, Scopus, and Google Scholar databases. This review has been registered with PROSPERO (CRD42024620363). The eligibility criteria were established, and this review included cross-sectional and observational studies assessing HRQOL in PLWE in SSA countries, published in English from the inception of databases through November 2024. The pooled HRQoL was reported as the mean score with accompanying 95% confidence intervals. Finally, publication bias was evaluated using a funnel plot and Egger’s regression test.
Results:
The pooled mean score of HRQoL among PLWE in SSA was 63.79 (95% CI: 59.75–67.84%). Owing to significant heterogeneity across the studies, a random-effects model was utilized for the meta-analysis (I2 = 98.96%, p < 0.001). This meta-analysis indicated that anxiety (β = −4.762, p = 0.0029), depression (β = −4.591, p < 0.0001), uncontrolled seizures (β = −4.321, p < 0.0001), and a family history of epilepsy (β = −5.093, p = 0.0013) had statistically significant negative impacts on HRQoL in PLWE. Despite some asymmetry in the funnel plot, Egger’s test showed no significant publication bias, with a p-value of 0.321.
Conclusion:
This review found a moderate pooled mean score of HRQoL among PLWE in SSA countries. Factors that negatively affect HRQoL in these regions include anxiety, depression, uncontrolled seizures, comorbidities, and a family history of epilepsy.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/search, identifier CRD42024620363.
Background
Epilepsy is a prevalent chronic neurological disorder characterized by recurrent unprovoked seizures that vary greatly in clinical presentations (1). It is a significant public health concern worldwide and is often linked to physical and cognitive impairments that can restrict employment opportunities, independence, and social engagement (2). Globally, 70 million people suffer from epilepsy, with a high prevalence in developing countries, particularly sub-Saharan African (SSA) countries. Approximately 80% of people living with epilepsy (PLWE) reside in developing countries with limited resources, and as a result, the majority of epilepsy patients remain untreated (3, 4). Epilepsy is a major burden in developing countries, and it affects not only individuals with epilepsy but also their families and society as a whole (5). It is not only a medical problem, but also a social problem that has a negative impact on patients, both socially and culturally (6–8).
Epilepsy has a significant impact on health-related quality of life (HRQoL) (9). The HRQoL of PLWE may be impaired by various factors, including seizure complications, emotional changes (depression and anxiety), stigma, and low social support or social isolation (10). Ineffective Anti-seizure drugs (ASDs) and prolonged disease duration affect the HRQoL of PLWE (11). In SSA, up to 90% of PLWEs have been reported to receive inadequate treatment or no treatment, which significantly affects HRQoL (5). The administration of ASDs to treat seizures and prevent further complications; however, the side effects of these drugs can potentially affect patients’ HRQoL (12).
Epilepsy is a condition that has been culturally devalued worldwide throughout history, and there is often a lack of awareness and understanding of epilepsy, which leads to stigmatization, discrimination, and exclusion of PLWE from society (13). In addition to that epilepsy affects life in many ways and should be studied taking into account the cultural conditions and psychosocial composition of each community (14, 15). PLWE is at risk of physical harm due to the potential dangers of a seizure, which can result in injury or even death (16).
HRQoL is recognized as an important outcome of epilepsy treatment and analysis of HRQoL in patients with chronic epilepsy because it is associated with lower personal independence, treatment-related problems, and lower life expectancy (17). To measure the burden of the disease on daily living activities, HRQOL has been used as a measure of health indicators, apart from morbidity and mortality (18–20). Several instruments have been developed to assess HRQoL in epilepsy patients and frequently used instruments in SSA countries were the World Health Organization’s Quality of Life Questionnaire (WHOQOLBREF), Quality Of Life in Epilepsy (QOLIE)-31, Hospital Anxiety and Depression Scale (HADS) (21–24).
Evaluating the impact of epilepsy on the HRQoL of PLWE in SSA would shed light on the various factors that affect HRQoL (23–27). There is a growing consensus that successful treatment should not only target the severity of symptoms but also impairment of functioning and HRQoL, leading to the restoration of health (22). In SSA countries, there is a lack of reliable evidence for quantifying the impact of epilepsy on HRQoL. The issue of HRQoL assessment and contributing factors is often overlooked in PLWE in SSA countries.
Few studies have been conducted in SSA countries on the HRQoL of PLWE. The reported mean score results showed significant discrepancies, ranging from 49.9% in Uganda (28) to 94.03 ± 10.61% in Nigeria (29). Therefore, it is crucial to estimate the magnitude of this problem and determine the factors that affect the HRQoL of PLWE to develop effective interventions and policy responses that can help improve HRQoL. This study is a comprehensive systematic review and meta-analysis of findings from various SSA countries. The findings of this study can help various organizations working to support PLWE to strengthen their actions in line with the magnitude of the problem.
Materials and methods
Protocol and registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to prepare this systematic review and meta-analysis [SF 1 (PRISMA_2020).docx]. The review protocol is registered with the International Registration of Systematic Reviews (PROSPERO) (30) with, registration number CRD42024620363.
Study eligibility criteria
This systematic review included all published research on the mean scores of health-related quality of life (HRQoL) and the factors associated with HRQoL in PLWE in Sub-Saharan Africa (SSA). Studies were eligible if they reported HRQoL data using validated instruments and presented the mean HRQoL scores. This review includes cross-sectional, observational, and studies published in English from inception to November 2024.
Conference abstracts, qualitative studies review articles, case–control studies, unpublished works (thesis), commentaries, gray literature, and those not fully accessible were excluded.
Data source and search strategy
To identify relevant publications for the systematic review and meta-analysis, we conducted both manual and electronic searches. The databases searched included PubMed, MEDLINE, EMBASE, the Cochrane Library, Scopus, and Google Scholar. We employed the following free-text keywords: (“Epilepsy” OR “Seizure Disorder”) AND (“Quality of Life” OR “Health-Related Quality of Life” OR “HRQoL” OR “QoL”) OR (“Associated Factors” OR “Determinants” OR “Predictors”) AND (“Sub-Saharan Africa” OR “Africa South of the Sahara” OR the specific names of Sub-Saharan African countries such as Angola, Benin, Botswana, Burkina Faso, Burundi, Cabo Verde, Cameroon, Central African Republic, Chad, Comoros, Congo, Democratic Republic of Congo, Djibouti, Equatorial Guinea, Eritrea, Eswatini, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, Somalia, South Africa, South Sudan, Sudan, Togo, Uganda, Tanzania, Zambia, Zimbabwe).
Data extraction
Three authors (DAB, ZDA, and DGD) independently extracted the required data from the articles using a format prepared in Microsoft Excel. This form was designed to gather relevant information, including authors’ names, publication years, study country, study designs, settings, participants, sampling methods, sample sizes, study duration in months, mean HRQoL scores with standard deviations, quality of life assessment tools used to assess HRQoL, and factors associated with HRQoL.
Quality and risk of bias assessment
The Joanna Briggs Institute (JBI) tool for cross-sectional study quality assessment was used to evaluate the methodological quality of the studies included in this review (31). Two authors (ZDA and DGD) independently assessed the quality of the original research using JBI criteria and discrepancies between the two reviewers were resolved through discussion. Studies that scored seven or higher on a nine-point scale were included in the analysis. The JBI tool assesses nine key areas: whether the sample frame is appropriate for addressing the target population if study participants were sampled appropriately, whether the sample size was adequate if study subjects and settings were described in detail, whether data analysis sufficiently covered the identified sample if valid methods were used to identify the condition, whether the condition was measured reliably for all participants, if the statistical analysis was appropriate, and whether an adequate response rate was achieved, or if a low response rate was properly managed.
Outcome measurement
Primary outcome
Health-Related Quality of Life (HRQoL): The main outcome of this study was the pooled estimate of the mean HRQoL scores among PLWE in SSA, which were assessed using validated instruments.
Secondary outcome
Associated Factors of HRQoL among PLWE in Sub-Saharan Africa (SSA).
Data analysis
To estimate the pooled mean HRQoL score, both mean and 95% confidence intervals (CIs) were reported. The mean standard error was calculated by dividing the standard deviation by the square root of the sample size. For the associated factors, odds ratios, logarithms, and standard errors of these logarithms were computed. Data were initially extracted into Microsoft Excel and subsequently imported into STATA 17.0 for further analysis. A random-effects model was employed to summarize the data, while heterogeneity was assessed using the Q test and I2 statistic. The thresholds for I2, indicating low, moderate, substantial, and high heterogeneity, were defined as ≤25%, 25–50%, 50–75%, and ≥ 75%, respectively. Meta-regression analyses were conducted to assess the impact of continuous and categorical moderator variables (e.g., study year, sample size, and study quality) on HRQoL scores. Additionally, subgroup analyses were performed based on the study region in Africa and the type of HRQoL instrument used to identify potential sources of heterogeneity. Meta-analysis and narrative analyses were used to present the findings. The pooled HRQoL was reported as the mean score with accompanying 95% confidence intervals. A sensitivity analysis was performed to verify that the results were robust against potentially influential decisions. Finally, publication bias was evaluated using a funnel plot and Egger’s regression test. If Egger’s test indicated statistical significance (p < 0.05) or if the funnel plot exhibited asymmetry, publication bias was noted.
Results
Search results
A total of 5,138 publications were identified through database searches of PubMed (451), Embase (2,587), and Scopus (2,100). After removing 3,274 duplicates, 1,421 papers were excluded during the title and abstract screening. We attempted to retrieve 443 reports; however, 294 could not be obtained because they were conference abstracts. Of the 149 full-text articles assessed for eligibility, 94 were excluded because HRQoL was not calculated, 29 due to HRQoL data being described only qualitatively, and six due to a JBI score of less than seven. Ultimately, 20 studies were included in this systematic review and meta-analysis (Figure 1).
Figure 1

PRISMA Flow diagram for study selection for systematic review and meta-analysis.
Quality and risk of bias assessment
In this meta-analysis, 26 articles were assessed and six articles were excluded because their JBI scores were < seven. A total of 20 studies were included, all of which had a JBI score higher than seven with scores ranging from 77.7 to 100% (Table 1). Only studies that scored seven or higher on a nine-point scale were included in the analysis [SF 2 (JBI).docx].
Table 1
| No | Author ID | Study area (Country) | Regions in Africa | Sample size | Study design | Tool | Minimum and maximum score | Mean score HRQoL | JBI-Quality score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mesafint et al. (22) | Ethiopia | East Africa | 439 | Cross-sectional | WHOQOL-BREF | 0 to 100 | 61.1 ± 11.6 | 100 |
| 2 | Tegegne et al. (27) | Ethiopia | East Africa | 415 | Cross-sectional | WHOQOL-BREF | 0 to 100 | 56.36 ± 13.37 | 88.9 |
| 3 | Tefera et al. (21) | Ethiopia | East Africa | 121 | Cross-sectional | WHOQOL-BREF | 0 to 100 | 56.42 ± 10.96 | 77.7 |
| 4 | Kassie et al. (32) | Ethiopia | East Africa | 395 | Cross-sectional | QOLIE-31 | 0 to 100 | 63.43 ± 26.56 | 100 |
| 5 | Abadiga et al. (40) | Ethiopia | East Africa | 392 | Cross-sectional | WHOQOL-BREF | 0 to 100 | 60.47 ± 23.07 | 88.9 |
| 6 | Shiferaw et al. (33) | Ethiopia | East Africa | 304 | Cross-sectional | QOLIE-31 | 0 to 100 | 58.8 ± 10.6 | 77.7 |
| 7 | Gebre et al. (26) | Ethiopia | East Africa | 175 | Cross-sectional | QOLIE-31 | 0 to 100 | 77.97 ± 20.78 | 88.9 |
| 8 | Addis et al. (24) | Ethiopia | East Africa | 376 | Cross-sectional | QOLIE-31 | 0 to 100 | 55.81 ± 14 | 77.8 |
| 9 | Adewuya et al. (43) | Nigeria | West Africa | 86 | Cross-sectional | QOLIE-AD-48 | 0 to 100 | 66.8 ± 5.04 | 77.7 |
| 10 | Ogundare et al. (34) | Nigeria | West Africa | 270 | Cross-sectional | QOLIE-31 | 0 to 100 | 77.98 ± 13.32 | 88.9 |
| 11 | Nabukenya et al. (28) | Uganda | East Africa | 175 | Cross-sectional | QOLIE-31 | 0 to 100 | 58 ± 13 | 77.7 |
| 12 | Kaddumukasa et al. (35) | Uganda | East Africa | 48 | Cross-sectional | QOLIE-31 | 0 to 100 | 62.5 ± 14.5 | 88.9 |
| 13 | Iwuozo et al. (41) | Nigeria | West Africa | 103 | Cross-sectional | WHOQOL-BREF | 0 to 100 | 65.2 ± 16.4 | 77.7 |
| 14 | Fawale et al. (36) | Nigeria | West Africa | 93 | Cross-sectional | QOLIE-31 | 0 to 100 | 64.2 ± 13.6 | 88.9 |
| 15 | Amaral et al. (37) | Tanzania | East Africa | 96 | cross-sectional | QOLIE-31 | 0 to 100 | 66.9 ± 13 | 88.9 |
| 16 | Onwuekwe et al. (29) | Nigeria | West Africa | 66 | cross-sectional | WHOQOL-BREF | 0 to 100 | 94.03 ± 10.61 | 77.8 |
| 17 | Luqman et al. (38) | Nigeria | West Africa | 100 | Cross-sectional | QOLIE-31 | 0 to 100 | 49.69 ± 13.45 | 88.9 |
| 18 | Mohamed et al. (42) | Sudan | East Africa | 50 | Cross-sectional | QOLIE-AD-48 | 0 to 100 | 78.95 ± 12.9 | 88.9 |
| 19 | Nubukpo et al. (39) | Togo | West Africa | 281 | Cross-sectional | QOLIE-31 | 0 to 100 | 49.5 ± 14.4 | 100 |
| 20 | Nubukpo et al. (39) | Benin | West Africa | 215 | Cross-sectional | QOLIE-31 | 0 to 100 | 52.1 ± 33.4 | 66.7 |
Characteristics and quality assessment of studies on the quality of life of people with epilepsy in SSA.
Studies characteristics
This systematic review and meta-analysis included 20 studies from SSA, comprising a total of 4,200 participants with sample sizes ranging from 50 to 439. All included studies utilized a cross-sectional study design. Of the 20 studies, eight were conducted in Ethiopia, six in Nigeria, two in Uganda, and one each in Tanzania, Sudan, Togo, and Benin. Geographically, 12 studies were based in East Africa, while eight were from West Africa. In terms of assessment tools, 12 studies used the QOLIE-31 questionnaire (24, 26, 28, 32–39), six used the WHOQOL-BREF scale (21, 27, 29, 40, 41), and two used the HRQoL in Epilepsy for Adolescents (QOLIE-AD)-48 (42, 43). All instruments evaluated HRQoL on a scale of 0 to 100, with higher scores representing better HRQoL (Table 1).
The pooled mean score of health-related quality of life of patients with epilepsy in Sub-Saharan Africa.
The pooled mean score of HRQoL among PLWE in SSA countries was 63.79% (95% CI: 59.75–67.84%). Owing to significant heterogeneity across the studies, a random-effects model was applied for the meta-analysis (I2 = 98.96%, p < 0.001). As shown in Figure 2, 20 studies were included in this meta-analysis to estimate the overall mean score of HRQoL among PLWE in the region.
Figure 2
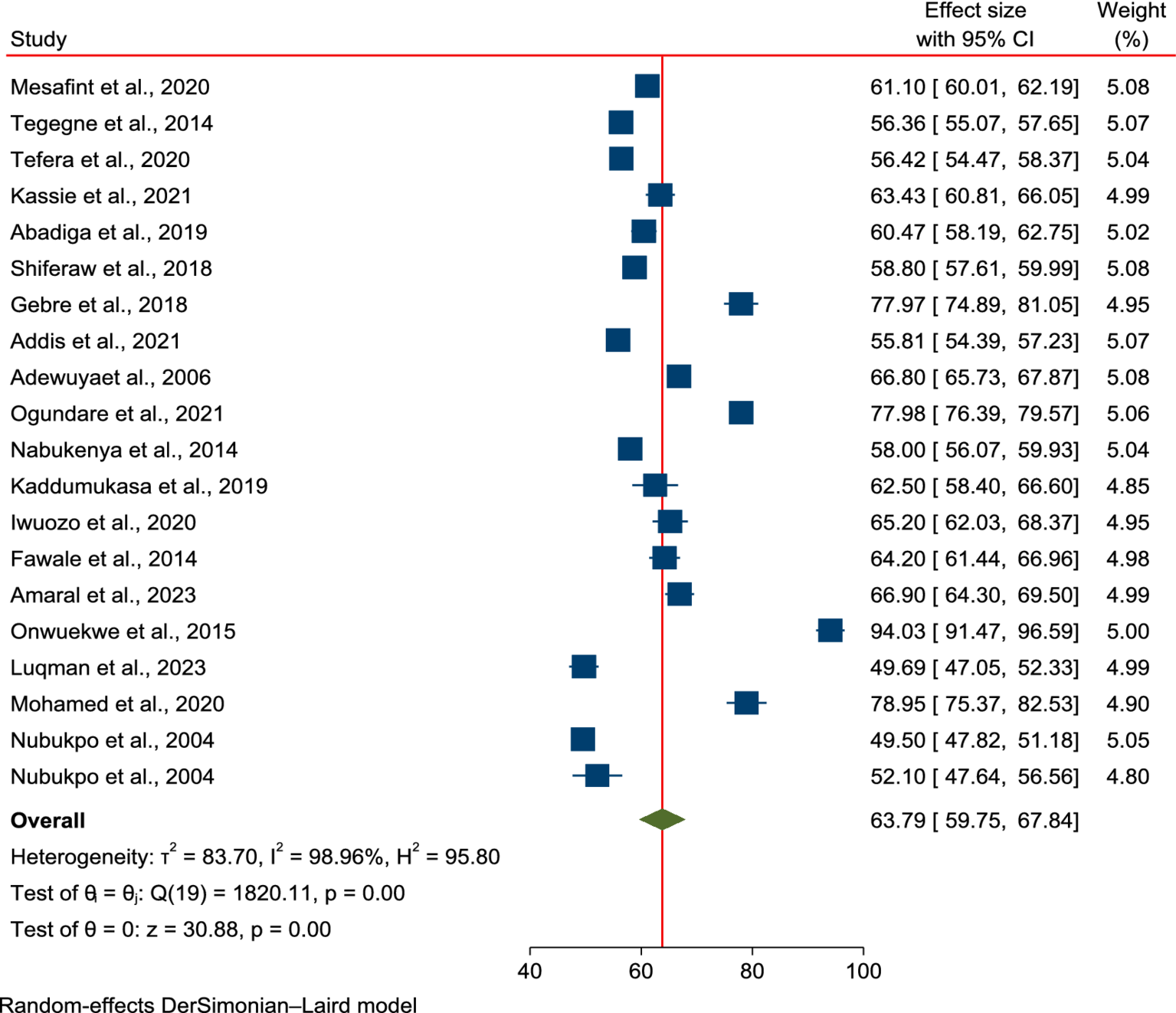
Forest plot of the pooled mean score of HRQoL among patients with epilepsy in SSA.
Subgroup and meta-regression analysis
The selected studies demonstrated significant heterogeneity (I2 = 98.96%, p < 0.001), indicating that the variability among the studies exceeded what would be expected. This led to an inconsistent overall estimate of the pooled mean score for HRQoL among PLWE in Sub-Saharan Africa. To address this, a random-effects model was used for the pooled estimates. Meta-regression and subgroup analyses were performed to explore the sources of heterogeneity. Factors such as sample size, study region in Africa, and tools used to assess HRQoL were included in the meta-regression. The sample size was found to be a significant contributor to heterogeneity [SF 3 (heterogeneity).docx].
The subgroup analysis based on study regions in Africa and the tools used to assess HRQoL in PLWE revealed no significant differences in the mean scores between East and West African countries. The mean score of HRQoL for patients in East Africa was 62.86 (95% CI: 59.83–65.88, p = 0.00), while in West Africa it was 64.97 (95% CI: 59.4–74.55, p = 0.00; Figure 3), with no reduction in heterogeneity (I2 = 98.96%, p < 0.001). Similarly, stratification by the tools used to assess HRQoL showed no changes in mean score or heterogeneity: QULIE-31 yielded 61.41 (95% CI: 55.96–66.87, p = 0.00), QOLE-AD-48 gave 72.75 (95% CI: 60.85–84.65, p = 0.00), and WHOQOL-BREF produced 65.56 (95% CI: 56.9–74.22, p = 0.00), with high heterogeneity (I2 = 98.86%, p < 0.001; Figure 4).
Figure 3
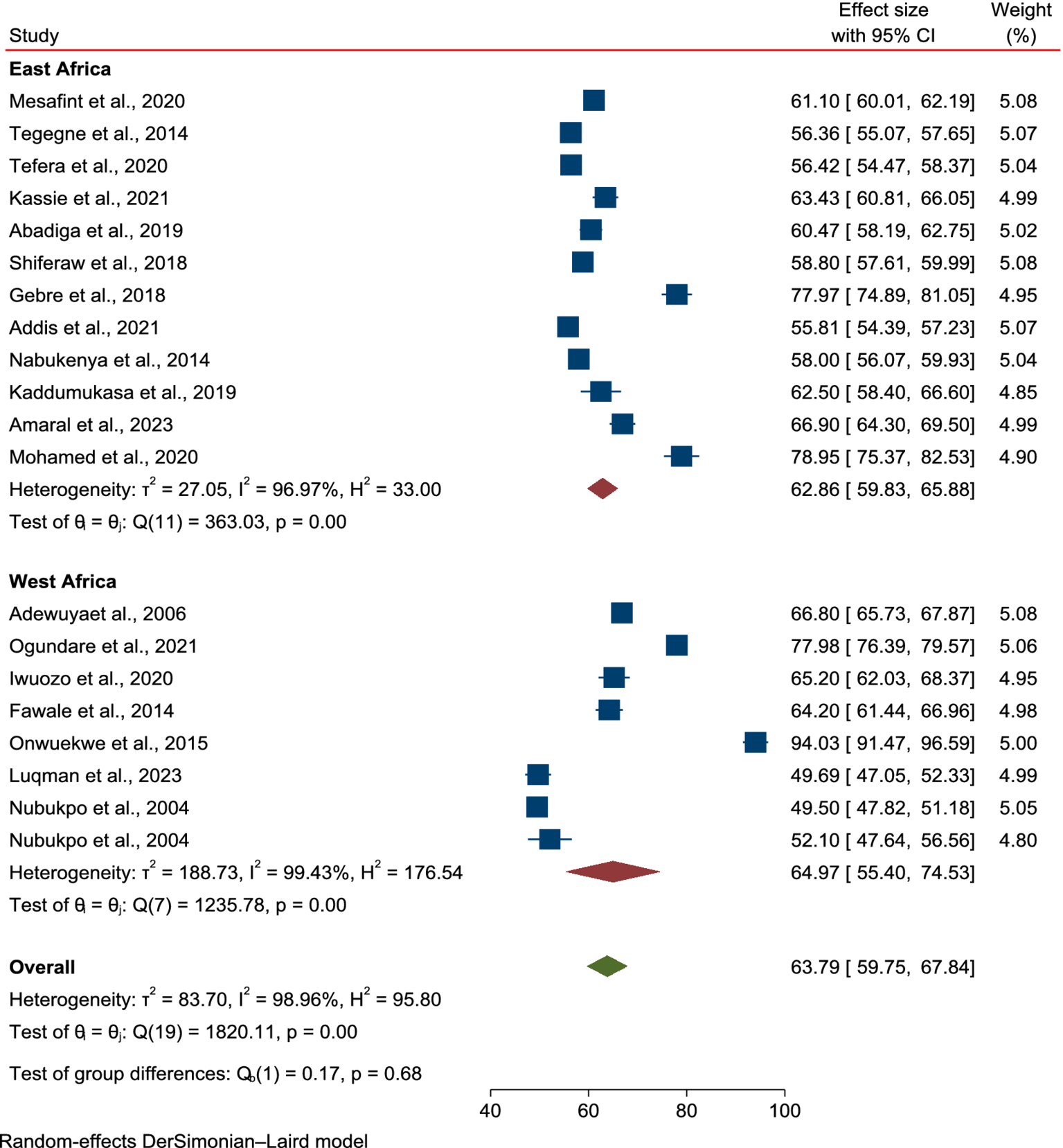
Subgroup analysis based on regions in Africa for the mean score of HRQoL among epilepsy patients in SSA.
Figure 4
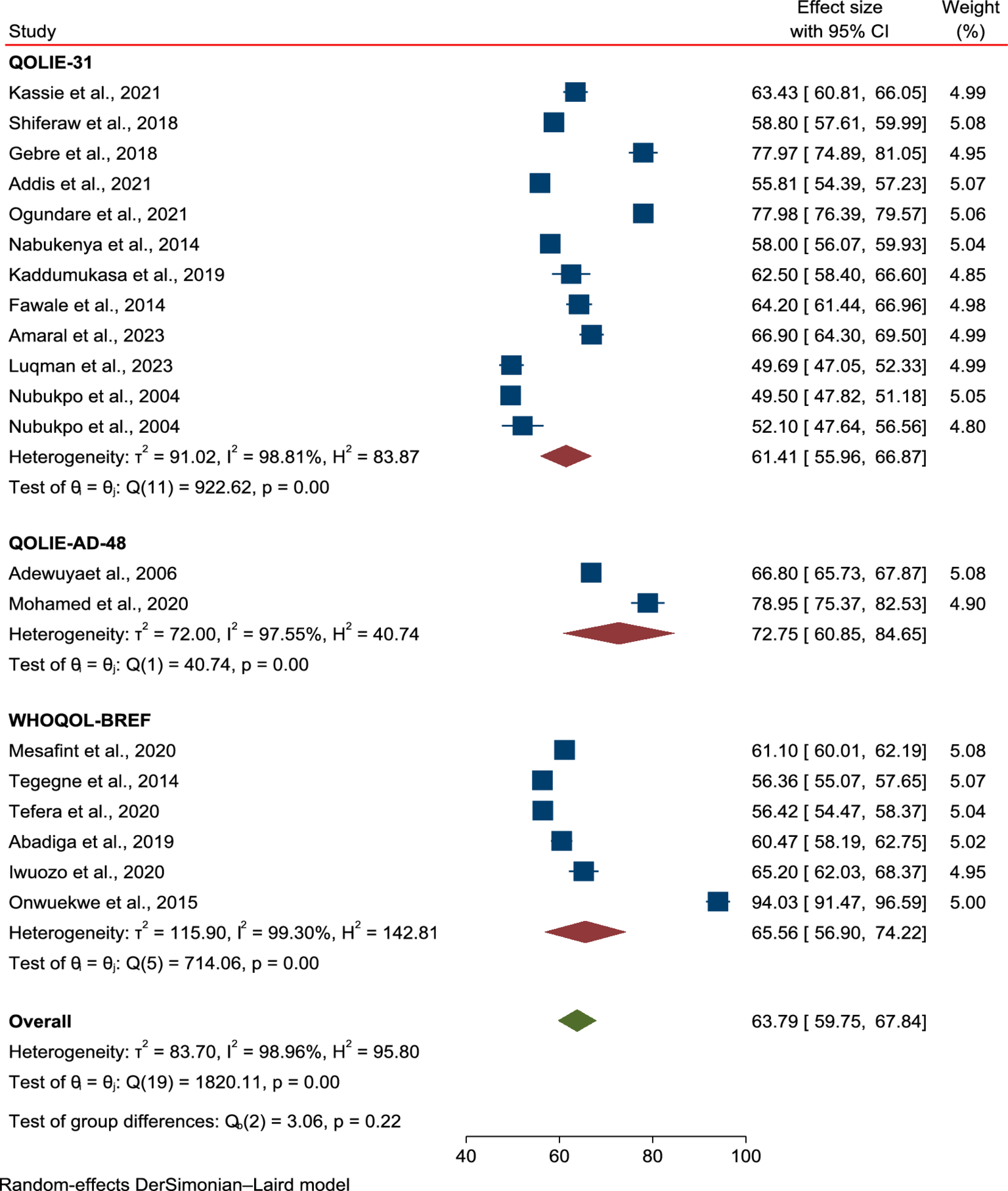
Subgroup analysis based on the tools used to assess HRQoL among epilepsy patients in SSA.
A Galbraith plot was generated to identify any studies that deviated significantly from the others, which could contribute to heterogeneity. As shown in Figure 5, 20 studies were analyzed, with one study noticeably displaced in the plot. However, after removing this outlier, the heterogeneity across the studies remained high (I2 = 98.51%, p = 0.00), indicating no reduction in variability [SF 4 (Galbraith plot).docx]. Sensitivity analysis.
Figure 5
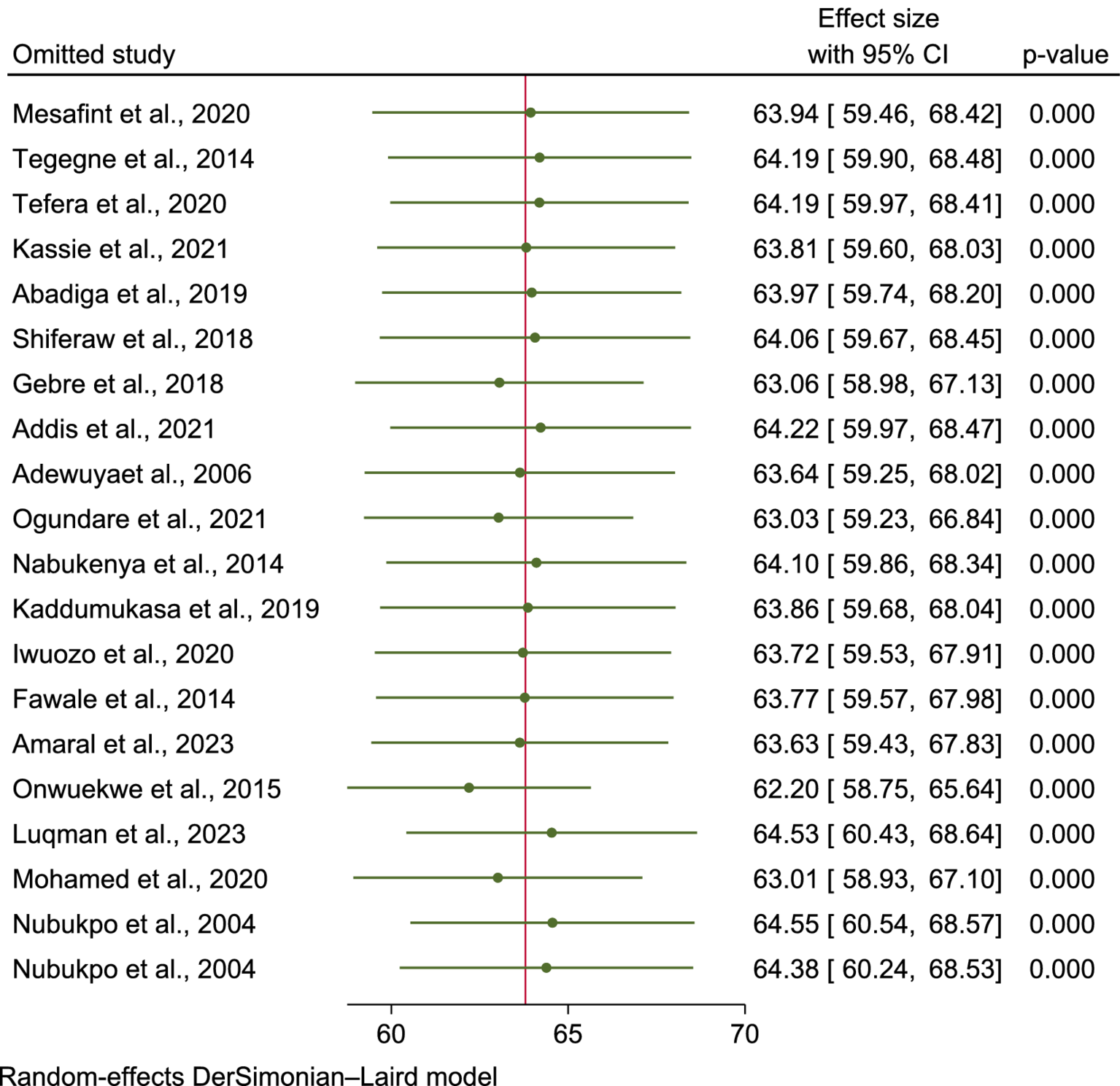
Leave-one-out sensitivity analysis.
To assess the influence of each study on the pooled mean score of HRQoL among PLWE, a leave-one-out meta-analysis was performed. The analysis showed that when each study was excluded, the pooled mean score remained within the confidence interval of the overall estimate, suggesting that no single study significantly affected the pooled result or the statistical significance (Figure 5).
Publication bias
Despite the asymmetry of the funnel plot, a formal evaluation using Egger’s test did not indicate significant publication bias in reporting the mean HRQoL score in PLWE. Publication bias was not statistically significant, with a p-value of 0.321 (SF 5 (Publication bias).docx).
Factors associated with poor quality of life patients in people living with epilepsy in SSA
Key findings from this meta-analysis indicated that anxiety (β = −4.762, p = 0.0029), depression (β = −4.591, p < 0.0001), and uncontrolled seizures (β = −4.321, p < 0.0001) significantly contributed to reduced HRQoL (Table 2). These factors demonstrated not only strong statistical significance but also high levels of heterogeneity, with I2 values of 95.91, 95.16, and 75.78%, respectively, indicating variability in the study results. Additionally, perceived stigma negatively impacted HRQoL (β = −3.047, p = 0.0017), while comorbidity showed a profound effect (β = −9.176, p < 0.0001), making it the most important factor among those analyzed.
Table 2
| Variables | Model | Beta Coefficient (95% CI) | p-value | Heterogeneity (I2%) | Number of studies | Egger Test (Publication bias) |
|---|---|---|---|---|---|---|
| No formal education | Random | −2.082 (−9.675, 5.510) | 0.5909 | 88.35 | 3 | 0.6228 |
| Anxiety | Random | −4.762 (−7.893, −1.632) | 0.0029 | 95.91 | 4 | 0.0000 |
| Depression | Random | −4.591 (−6.948, −2.233) | 0.0000 | 95.16 | 6 | 0.0000 |
| Lack of family/social support | Random | −0.344 (−1.088, 0.400) | 0.3644 | 87.87 | 4 | 0.0000 |
| Antiepileptic drug side effect | Random | −0.678 (−1.437, 0.081) | 0.0802 | 88.03 | 5 | 0.0005 |
| uncontrolled seizure | Random | −4.321 (−5.916, −2.726) | 0.0000 | 75.78 | 9 | 0.0038 |
| Perceived stigma | Random | −3.047 (−4.947, −1.147) | 0.0017 | 92.51 | 5 | 0.0000 |
| Polypharmacy | Random | −0.915 (−2.752, 0.923) | 0.3293 | 76.86 | 5 | 0.1935 |
| Comorbidity | Random | −9.176 (−13.863, −4.489) | 0.0000 | 76.59 | 3 | 0.5259 |
| Family history of epilepsy | Random | −5.093 (−8.196, −1.989) | 0.0013 | 0.00 | 2 | 0.8548 |
Factors associated with poor quality of life in patients with epilepsy in Sub-Saharan Africa.
Conversely, other variables, such as no formal education (β = −2.082, p = 0.5909), lack of family/social support (β = −0.344, p = 0.3644), and polypharmacy (β = −0.915, p = 0.3293), were not statistically significant, suggesting that they may not play a critical role in determining HRQoL for these patients in SSA. The side effects of antiepileptic drugs (β = −0.678, p = 0.0802) also did not reach significance, although the p-value was close to the threshold, indicating potential relevance. Lastly, a family history of epilepsy showed a statistically significant negative impact on HRQoL (β = −5.093, p = 0.0013).
Assessment of publication bias using Egger’s test revealed a significant bias for anxiety (p < 0.0001), depression (p < 0.0001), perceived stigma (p < 0.0001), and uncontrolled seizures (p = 0.0038) suggesting that these factors may be underreported in the literature. Overall, anxiety, depression, uncontrolled seizures, comorbidities, and perceived stigma emerged as critical factors significantly affecting HRQoL for PLWE in SSA, highlighting the need for comprehensive management strategies that address these psychological and social dimensions of care.
Discussion
This systematic review and meta-analysis aimed to determine the pooled mean score of HRQoL and the factors associated with it in PLWE in SSA. A total of 20 cross-sectional studies involving 4,200 participants with sample sizes ranging from 50 to 439 were retrieved and analyzed from various SSA countries. The findings from the studies examined in this review highlight several key factors that contribute to the decreased HRQoL in PLWE in SSA countries. Uncontrolled seizures were identified in nine studies, depression in six studies, perceived stigma in five studies, anxiety in four studies, and comorbidity in three studies.
The pooled mean score of HRQoL among PLWE in SSA countries was 63.79% (95% CI: 59.75–67.84%). Regarding specific countries, Togo recorded the lowest HRQoL score, measured at 49.5 ± 14.4 (39), suggesting significant challenges faced by individuals in that area regarding their health and well-being. In contrast, Nigeria achieved the highest HRQoL score at 94.03 ± 10.61 (29), indicating that individuals in this country experience a considerably better HRQoL compared to their counterparts in Togo. These variations reflect the diverse circumstances and support systems available to the PLWE in different countries within SSA.
Regarding the subgroup analysis based on study regions in Africa, there were no significant differences in the mean scores between East and West African countries. The mean HRQoL score for patients in East Africa was 62.86 (95% CI: 59.83–65.88, p = 0.001), whereas, in West Africa, it was 64.97 (95% CI: 59.4–74.55, p = 0.001), with no reduction in heterogeneity (I2 = 98.96%, p < 0.001). The findings indicate that the mean HRQoL value among PLWE remained unaffected by regional variations in the study conducted across countries in SSA. Moreover, subgroup analysis based on the tools used to assess HRQoL in PLWE showed no changes in the mean score or heterogeneity. QULIE-31 yielded 61.41 (95% CI: 55.96–66.87, p = 0.00), QOLE-AD-48 gave 72.75 (95% CI: 60.85–84.65, p = 0.001), and WHOQOL-BREF produced 65.56 (95% CI: 56.9–74.22, p = 0.001), with heterogeneity remaining high (I2 = 98.86%, p < 0.001). The review findings indicated that there was no statistically significant difference in HRQoL based on the tool used to assess HRQoL in PLWE. Therefore, we can use one of the validated tools mentioned above to assess HRQoL in SSA countries.
Psychiatric disorders are more prevalent in PLWE than in the general population and among individuals with other neurological diseases (44). In contrast, epilepsy significantly impairs physical health and causes psychological disturbances such as depression and anxiety, which adversely affect patients’ HRQoL (41). This meta-analysis indicated that PLWE who had comorbid anxiety (β = −4.762, p = 0.0029) experienced poor HRQoL in four studies (22, 24, 40, 45). Symptoms of anxiety disorders have the most significant impact on HRQoL compared with comorbid conditions. Higher levels of anxiety traits are associated with poorer HRQoL in various areas, including emotional and mental well-being, pain, energy and vitality, cognitive function, and social functioning in PLWE (46, 47). In addition, subjective anxiety has a greater effect than short-term seizure control on HRQoL scores of PLWE (48). These factors should be simultaneously considered when evaluating the effects of treatment on HRQoL.
Depression frequently occurs alongside epilepsy and affects the HRQoL of many patients (49). In this meta-analysis, depression (β = −4.591, p < 0.0001) was associated with poor HRQoL in PLWE in six studies (22, 24, 34, 43, 45, 50). Various depressive disorders can occur in PLWE and are often related to hyperactivity of the hypothalamic–pituitary–adrenal axis, neuroinflammation, and disruptions in serotonin transmission, among other factors (51, 52). Additionally, certain ASDs medications may have adverse psychotropic effects, potentially causing or worsening depression, which can ultimately compromise HRQoL in PLWE (53).
One of the reasons for the poor HRQoL in PLWE is the unpredictable nature of seizures, particularly when they are uncontrolled. Research has shown that uncontrolled seizures are associated with a decline in HRQoL (54). In this meta-analysis, uncontrolled seizures (β = −4.321, p < 0.0001) significantly contributed to reduced HRQoL in nine studies (22, 24, 28, 32, 34, 36, 40, 45). Fears and worries about experiencing seizures in public create a constant source of anxiety for PLWE (55, 56). They often dread the possibility of being mocked, scarring others, or witnessing others’ helplessness during a seizure. The sudden and unpredictable nature of seizures, along with concerns about potential injuries during an episode, heightens anxiety (57). This fear contributes to a feeling of lack of control over their seizures, which negatively affects their HRQoL (56, 58).
Stigma has debilitating effects on HRQoL in PLWE and the pooled mean score of perceived stigma and self-stigma among PLWE in SSA is 43.9%, it is linked to various challenges in their daily lives, such as internalizing negative attitudes, exhibiting decreased adherence to treatment, and experiencing higher rates of unemployment, leading to lower self-esteem and levels of hope (59). In many societies in SSA, there is a belief that epilepsy is contagious. This misconception can lead patients to feel the need to isolate themselves due to the stigma associated with their condition, resulting in a loss of confidence and feelings of embarrassment (60). Such stigma creates significant challenges for individuals in accessing education, finding stable employment, and forming intimate relationships (61). In this meta-analysis, perceived stigma negatively impacted HRQoL (β = −3.047, p = 0.0017) in five studies (22, 24, 36, 40, 45). Distress from stigma often surpasses that of the disease itself, leading to feelings of guilt and increased depression. In PLWE, stigma arises from seizure unpredictability and social exclusion resulting from negative societal attitudes (62). This can result in challenges related to education, starting a family, and finding employment, even when these opportunities are feasible (63). Epilepsy significantly affects HRQoL and overall well-being due to the nature of seizures, which can be unpredictable and may affect awareness. Additionally, PLWE often experiences psychiatric and cognitive challenges. Changes in personal, social, and community aspects of life can further affect a person’s HRQoL, leading to limitations in autonomy and an increased perception of stigma (64).
In this meta-analysis, comorbidity has a significant negative impact on HRQoL in PLWE (β = −9.176, p < 0.0001) in three studies (21, 32, 40), and demonstrated the most substantial effect among the factors analyzed. This detrimental effect was observed in three different studies. Additionally, a family history of epilepsy also showed a statistically significant negative effect on HRQoL (β = −5.093, p = 0.0013) in two studies (32, 34).
The selected studies exhibited significant heterogeneity (I2 = 98.96%, p < 0.001), indicating that the variability among the studies was greater than what would typically be expected. To identify the source of this heterogeneity, the author analyzed factors such as sample size, country, study region in Africa, and the tools used to assess HRQoL using a random effects model. In this meta-analysis, only sample size was found to be a significant source of heterogeneity (p = 0.048). A leave-one-out meta-analysis was conducted to evaluate the impact of each study on the pooled mean HRQoL score in PLWE. This analysis indicated that no single study significantly affected the overall results. Furthermore, an assessment of publication bias showed no statistically significant bias (p = 0.321). Although there was some asymmetry in the funnel plot, a formal evaluation using Egger’s test did not indicate significant publication bias in the reporting of mean HRQoL scores for PLWE.
Challenges in improving the HRQoL of PLWE in SSA stem from a widespread belief that epilepsy is caused by demonic possession (65). This belief contributes to a significant social stigma surrounding the condition. In many societies in SSA, there is a belief that epilepsy is contagious (60). Such perceptions can fuel fear and misunderstanding within communities, often resulting in the isolation of those with epilepsy. Additionally, traditional healing practices may be sought instead of, or alongside, medical treatment, highlighting the cultural nuances that affect how epilepsy is perceived and managed in this region. One of the main challenges in SSA countries is that a significant portion of the population resides in rural areas. In these regions, the impact of inadequate healthcare on the HRQoL for PLWE is notably more pronounced. Additionally, resources are limited, and access to healthcare services is insufficient, which greatly affects the HRQoL of PLWE (5).
The potential interventions to enhance HRQoL among PLWE involve targeting psychological approaches, including cognitive-behavioral and behaviorally based interventions and mindfulness-based interventions (such as acceptance and commitment therapy) (65, 66). The other potential intervention strategy will be (psycho-) educational interventions that will increase knowledge about epilepsy, its comorbidities, and its treatments, as well as the functions and activities of the brain (67–69). The HRQoL for PLWE in low and middle-income countries is significantly correlated with levels of resilience and internalized stigma. Enhancing resilience may effectively mitigate the adverse effects of perceived stigma on HRQoL, thereby contributing to improved overall well-being for PLWE in these regions (70, 71).
Limitations of the study
This systematic review has certain limitations. It only included papers published in English, which restricted the scope owing to the relevance of the topic and most studies utilized a variety of instruments to measure HRQoL, which compromises the conclusions. Additionally, the number of studies from Ethiopia is relatively high, and the review focused solely on cross-sectional studies, which may have affected the generalizability of the findings.
Conclusion
This review found a moderate pooled mean score of HRQoL among PLWE in SSA countries. Factors that negatively affect HRQoL in these regions include anxiety, depression, uncontrolled seizures, comorbidities, and a family history of epilepsy. This study may provide valuable insights to relevant organizations focused on the early screening and management of HRQoL in PLWE. Additionally, it is important to include screening and treatment of anxiety and depression as part of regular epilepsy care management. Our study highlights the need for further studies, particularly those using longitudinal designs.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DD: Methodology, Writing – original draft, Writing – review & editing. CT: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. TY: Investigation, Software, Supervision, Writing – original draft, Writing – review & editing. DE: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. TT: Validation, Visualization, Writing – original draft, Writing – review & editing. ZA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1546911/full#supplementary-material
Abbreviations
ASDs, Anti-seizure drugs; CIs, confidence intervals; HADS, Hospital Anxiety and Depression Scale; HRQoL, Health-related quality of life; JBI, Joanna Briggs Institute; PLWE, People living with epilepsy; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QOLIE, Quality Of Life in Epilepsy-31; SSA, Sub-Saharan African; WHOQOLBREF, World Health Organization’s Quality of Life Questionnaire.
References
1.
Fisher RS Cross JH French JA Higurashi N Hirsch E Jansen FE et al . Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Paediatr Child Health. (2017) 58:522–30. doi: 10.1111/epi.13670
2.
Shanmukhi S Jayalakshmi S Anand B . Factors associated with quality of life in adult epilepsy patients: a hospital based study from South India. Res Neurol Int. (2015) 2015:1–9. doi: 10.5171/2015.766328
3.
Epilepsy W . World Health Organization, Epilepsy: A public health imperative World health organization (2019). [Epubh ahead of print].
4.
Ba-Diop A Marin B Druet-Cabanac M Ngoungou EB Newton CR Preux PM . Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. (2014) 13:1029–44. doi: 10.1016/S1474-4422(14)70114-0
5.
Boling W Means M Fletcher A . Quality of life and stigma in epilepsy, perspectives from selected regions of Asia and sub-Saharan Africa. Brain Sci. (2018) 8:59. doi: 10.3390/brainsci8040059
6.
Mushi D Hunter E Mtuya C Mshana G Aris E Walker R . Social–cultural aspects of epilepsy in Kilimanjaro region, Tanzania: knowledge and experience among patients and carers. Epilepsy Behav. (2011) 20:338–43. doi: 10.1016/j.yebeh.2010.11.016
7.
Szaflarski M . Social determinants of health in epilepsy. Epilepsy Behav. (2014) 41:283–9. doi: 10.1016/j.yebeh.2014.06.013
8.
Gotlieb EG Blank L Willis AW Agarwal P Jette N . Health equity integrated epilepsy care and research: a narrative review. Epilepsia. (2023) 64:2878–90. doi: 10.1111/epi.17728
9.
Jadhav P . Assessment and comparison of health-related quality-of-life (HRQOL) in patients with epilepsy in India. Epilepsy Behav. (2013) 27:165–8. doi: 10.1016/j.yebeh.2012.12.027
10.
Norsa’adah B Zainab J Knight A . The quality of life of people with epilepsy at a tertiary referral Centre in Malaysia. Health Qual Life Outcomes. (2013) 11:143–6. doi: 10.1186/1477-7525-11-143
11.
Szaflarski M Meckler JM Privitera MD Szaflarski JP . Quality of life in medication-resistant epilepsy: the effects of patient’s age, age at seizure onset, and disease duration. Epilepsy Behav. (2006) 8:547–51. doi: 10.1016/j.yebeh.2006.01.001
12.
Perucca P Gilliam FG Schmitz B . Epilepsy treatment as a predeterminant of psychosocial ill health. Epilepsy Behav. (2009) 15:S46–50. doi: 10.1016/j.yebeh.2009.03.016
13.
Fanta T Azale T Assefa D Getachew M . Prevalence and factors associated with perceived stigma among patients with epilepsy in Ethiopia. Psychiatry J. (2015) 2015:1–7. doi: 10.1155/2015/627345
14.
Bishop M Allen CA . The impact of epilepsy on quality of life: a qualitative analysis. Epilepsy Behav. (2003) 4:226–33. doi: 10.1016/S1525-5050(03)00111-2
15.
Jacoby A Snape D Baker GA . Determinants of quality of life in people with epilepsy. Neurol Clin. (2009) 27:843–63. doi: 10.1016/j.ncl.2009.06.003
16.
Mayor R Gunn S Reuber M Simpson J . Experiences of stigma in people with epilepsy: a meta-synthesis of qualitative evidence. Seizure. (2022) 94:142–60. doi: 10.1016/j.seizure.2021.11.021
17.
Siebenbrodt K Willems LM von Podewils F Mross PM Strüber M Langenbruch L et al . Determinants of quality of life in adults with epilepsy: a multicenter, cross-sectional study from Germany. Neurolog Res Prac. (2023) 5:41. doi: 10.1186/s42466-023-00265-5
18.
Gao L Xia L Pan SQ Xiong T Li SC . Burden of epilepsy: a prevalence-based cost of illness study of direct, indirect and intangible costs for epilepsy. Epilepsy Res. (2015) 110:146–56. doi: 10.1016/j.eplepsyres.2014.12.001
19.
van Hezik-Wester V de Groot S Kanters T Versteegh M Wagner L Ardesch J et al . Burden of illness in people with medically refractory epilepsy who suffer from daily to weekly seizures: 12-month follow-up of participants in the EPISODE study. Front Neurol. (2022) 13:1012486. doi: 10.3389/fneur.2022.1012486
20.
Beghi E . Addressing the burden of epilepsy: many unmet needs. Pharmacol Res. (2016) 107:79–84. doi: 10.1016/j.phrs.2016.03.003
21.
Tefera GM Megersa WA Gadisa DA . Health-related quality of life and its determinants among ambulatory patients with epilepsy at ambo general hospital, Ethiopia: using WHOQOL-BREF. PLoS One. (2020) 15:e0227858. doi: 10.1371/journal.pone.0227858
22.
Mesafint G Fanta T Habtamu Y Molla G Shumet S . Quality of life and associated factors among patients with epilepsy attending outpatient department of Saint Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia. J Multidiscip Healthc. (2019) 13:2021–30. doi: 10.2147/JMDH.S284958
23.
Stotaw AS Kumar P Beyene DA Tadesse TA Abiye AA . Health-related quality of life and its predictors among people living with epilepsy at Dessie referral hospital, Amhara, Ethiopia: a cross-sectional study. SAGE Open Med. (2022) 10:20503121221129146. doi: 10.1177/20503121221129146
24.
Addis B Minyihun A Aschalew AY . Health-related quality of life and associated factors among patients with epilepsy at the University of Gondar comprehensive specialized hospital, Northwest Ethiopia. Qual Life Res. (2021) 30:729–36. doi: 10.1007/s11136-020-02666-4
25.
Teshome Y Solomon Y Talargia F Worku N Shitaw A Leminie AA . Level of acceptance of illness and its association with quality of life among patients with Epilepsy in north Shewa, Ethiopia. Behav Neurol. (2022) 2022:1–6. doi: 10.1155/2022/1142215
26.
Gebre AK Haylay A . Sociodemographic, clinical variables, and quality of life in patients with epilepsy in Mekelle City, northern Ethiopia. Behav Neurol. (2018) 2018:1–6. doi: 10.1155/2018/7593573
27.
Tegegne MT . Assessment of quality of life and associated factors among people with epilepsy attending at Amanuel mental specialized hospital, Addis Ababa. Ethiopia Sci. (2014) 2:378–273. doi: 10.11648/j.sjph.20140205.12
28.
Nabukenya AM Matovu JKB Wabwire-Mangen F Wanyenze RK Makumbi F . Health-related quality of life in epilepsy patients receiving anti-epileptic drugs at National Referral Hospitals in Uganda: a cross-sectional study. Health Qual Life Outcomes. (2014) 12:49–8. doi: 10.1186/1477-7525-12-49
29.
Onwuekwe I . Health-related quality of life and its determinants in adult Nigerians with epileptic seizures. Epilepsia. (2015) 2:1013.
30.
Sideri S Papageorgiou SN Eliades T . Registration in the international prospective register of systematic reviews (PROSPERO) of systematic review protocols was associated with increased review quality. J Clin Epidemiol. (2018) 100:103–10. doi: 10.1016/j.jclinepi.2018.01.003
31.
Munn Z Moola S Lisy K Riitano D Tufanaru C . Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. JBI Evidence Implementation. (2015) 13:147–53. doi: 10.1097/XEB.0000000000000054
32.
Kassie AM Abate BB Kassaw MW Getie A Wondmieneh A Tegegne KM et al . Quality of life and its associated factors among epileptic patients attending public hospitals in north Wollo zone. Northeast Ethiopia: A cross-sectional study. (2021) 16:e0247336. doi: 10.1371/journal.pone.0247336
33.
Shiferaw D Hailu EJ . Quality of life assessment among adult epileptic patients taking follow up care at Jimma University medical center, Jimma, south West Ethiopia: Using quality of life in epilepsy Inventory31instrument. Global Journal of medical research (2018):18.
34.
Ogundare T Adebowale TO Okonkwo LR . Quality of life among patients with epilepsy in Nigeria: predictors and barriers to routine clinical use of QOLIE-31. Qual Life Res. (2021) 30:487–96. doi: 10.1007/s11136-020-02643-x
35.
Kaddumukasa M Mugenyi L Lhatoo S Sewankambo N Blixen C Sajatovic M et al . Seizure severity is associated with poor quality of life in people living with epilepsy (PLWE) in Uganda: a cross-sectional study. Epilepsy Behav. (2019) 96:104–8. doi: 10.1016/j.yebeh.2019.04.033
36.
Fawale MB Owolabi MO Ogunniyi A . Effects of seizure severity and seizure freedom on the health-related quality of life of an African population of people with epilepsy. Epilepsy Behav. (2014) 32:9–14. doi: 10.1016/j.yebeh.2013.12.026
37.
Amaral L-J Bhwana D Fomo MF Mmbando BP Chigoho CN Colebunders R . Quality of life of persons with epilepsy in Mahenge, an onchocerciasis-endemic area in Tanzania: A cross-sectional study. Epilepsy Behav. (2023) 145:109302. doi: 10.1016/j.yebeh.2023.109302
38.
Luqman O Joseph Y Akintomiwa M Akinyinka A Aderonke A Bamidele O et al . Determinants of quality of life in Nigerian female patients with epilepsy on carbamazepine and levetiracetam monotherapy. Egyptian J Neurol Psychiatry Neurosurg. (2023) 59:32. doi: 10.1186/s41983-023-00631-9
39.
Nubukpo P Clément JP Houinato D Radji A Grunitzky EK Avodé G et al . Psychosocial issues in people with epilepsy in Togo and Benin (West Africa) II: quality of life measured using the QOLIE-31 scale. Epilepsy Behav. (2004) 5:728–34. doi: 10.1016/j.yebeh.2004.07.002
40.
Abadiga M Mosisa G Amente T Oluma A . Health-related quality of life and associated factors among epileptic patients on treatment follow up at public hospitals of Wollega zones, Ethiopia. BMC Res Notes. (2018) 12:1–7. doi: 10.1186/s13104-019-4720-3
41.
Iwuozo EU Obiako RO Ogunniyi A Abubakar SA . A comparative assessment of health-related quality of life in people with epilepsy and healthy controls in a tertiary hospital in Northwest Nigeria. Sahel Med J. (2020) 23:215–20. doi: 10.4103/smj.smj_61_19
42.
Mohamed I Mohamed SJKMJ . Quality of life among adolescents with epilepsy: An outpatient based study, Khartoum, Sudan. Khartoum Med J. (2020) 13:1714–21. doi: 10.1002/brb3.2487
43.
Adewuya AOJDM Neurology C . Parental psychopathology and self-rated quality of life in adolescents with epilepsy in Nigeria. Dev Med Child Neurol. (2006) 48:600–3. doi: 10.1111/j.1469-8749.2006.tb01322.x
44.
Lin JJ Mula M Hermann BPJTL . Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. (2012) 380:1180–92.
45.
Yesuf W . Health related quality of life and associated factors among adult patients with epilepsy attending at Mizan Tepi university teaching hospital, south West Ethiopia. Ethiopia: Jimma University (2019).
46.
Kwan P Yu E Leung H Leon T Mychaskiw MA . Association of subjective anxiety, depression, and sleep disturbance with quality-of-life ratings in adults with epilepsy. Epilepsia. (2009) 50:1059–66. doi: 10.1111/j.1528-1167.2008.01938.x
47.
Niu C Li P du X Zhao M Wang H Yang D et al . Risk factors for anxiety in patients with epilepsy: A meta-analysis. Epilepsia. (2024) 153:109665. doi: 10.1016/j.yebeh.2024.109665
48.
Van Patten R . Reduced subjective cognitive concerns with neurobehavioral therapy in functional seizures and traumatic brain injury. Appi Neuropsych. (2024) 36, 197–205. doi: 10.1176/appi.neuropsych.20230138
49.
Kwon C-S Rafati A Gandy M Scott A Newton CR Jette N . Multipsychiatric comorbidity in people with Epilepsy compared with people without Epilepsy: a systematic review and meta-analysis. Neurology. (2024) 103:e209622. doi: 10.1212/WNL.0000000000209622
50.
Shiferaw D Hailu E . Quality of life assessment among adult epileptic patients taking follow up care at Jimma University medical center, Jimma, south West Ethiopia: using quality of life in epilepsy Inventory31instrument. Global J Medical Res. (2018) 18:7–15.
51.
Kwon O-Y . Depression in Epilepsy In: Neuropsychiatric manifestations in neurological diseases. eds. Jong S. Kim (Berlin: Springer) (2024):107–24.
52.
Anand LK . Molecular mechanisms implicated with depression and therapeutic intervention, Precision Medicine and Human Health (2024):205.
53.
Kwon O-Y . Aggression in Epilepsy In: Neuropsychiatric manifestations in neurological diseases. eds. Jong S. Kim (Berlin: Springer) (2024):125–40.
54.
Thomas SV Koshy S Sudhakaran Nair CR Sarma SP . Frequent seizures and polytherapy can impair quality of life in persons with epilepsy. Neurol India. (2005) 53:46–50. doi: 10.4103/0028-3886.15054
55.
Deegbe DA Aziato L Attiogbe A . Experience of epilepsy: Coping strategies and health outcomes among Ghanaians living with epilepsy. Epilepsy Behav. (2020) 104:106900. doi: 10.1016/j.yebeh.2020.106900
56.
Fazekas B Megaw B Eade D Kronfeld N . Insights into the real-life experiences of people living with epilepsy: A qualitative netnographic study. Epilepsy Behav. (2021) 116:107729. doi: 10.1016/j.yebeh.2020.107729
57.
Kılınç S van Wersch A Campbell C Guy A . The experience of living with adult-onset epilepsy. Epilepsy Behav. (2017) 73:189–96. doi: 10.1016/j.yebeh.2017.05.038
58.
Deegbe DA Aziato L Attiogbe AJS . Beliefs of people living with epilepsy in the Accra Metropolis, Ghana. Seizure. (2019) 73:21–5. doi: 10.1016/j.seizure.2019.10.016
59.
Tinsae T Shumet S Takelle GM Rtbey G Fentahun S Getinet W . Perceived and self-stigma in people with epilepsy in East Africa: Systematic review and meta-analysis. Seizure: European J Epilepsy. (2024) 117:261–70. doi: 10.1016/j.seizure.2024.03.003
60.
Baskind R Birbeck GL . Epilepsy-associated stigma in sub-Saharan Africa: the social landscape of a disease. Epilepsy Behav. (2005) 7:68–73. doi: 10.1016/j.yebeh.2005.04.009
61.
Lalatović S Milovanović M Krstić N . Stigma and its association with health-related quality of life in adults with epilepsy. Epilepsy Behav. (2022) 135:108874. doi: 10.1016/j.yebeh.2022.108874
62.
Viteva EJS . Impact of stigma on the quality of life of patients with refractory epilepsy. Seizure. (2013) 22:64–9. doi: 10.1016/j.seizure.2012.10.010
63.
De Boer HM . The global burden and stigma of epilepsy. Epilepsy Behav. (2008) 12:540–6. doi: 10.1016/j.yebeh.2007.12.019
64.
Deleo F Quintas R Pastori C Pappalardo I Didato G di Giacomo R et al . Quality of life, psychiatric symptoms, and stigma perception in three groups of persons with epilepsy. Epilepsy Behav. (2020) 110:107170. doi: 10.1016/j.yebeh.2020.107170
65.
Michaelis R Tang V Wagner JL Modi AC LaFrance WC Jr Goldstein LH et al . Cochrane systematic review and meta-analysis of the impact of psychological treatments for people with epilepsy on health-related quality of life. Epilepsy Behav. (2018) 59:315–32. doi: 10.1111/epi.13989
66.
Caller TA Ferguson RJ Roth RM Secore KL Alexandre FP Zhao W et al . A cognitive behavioral intervention (HOBSCOTCH) improves quality of life and attention in epilepsy. Epilepsy Behav. (2016) 57:111–7. doi: 10.1016/j.yebeh.2016.01.024
67.
Jantzen S Müller-Godeffroy E Hallfahrt-Krisl T Aksu F Püst B Kohl B et al . FLIP&FLAP—A training programme for children and adolescents with epilepsy, and their parents. Seizure. (2009) 18:478–86. doi: 10.1016/j.seizure.2009.04.007
68.
Lua PL Neni WSJJOt Telecare . A randomised controlled trial of an SMS-based mobile epilepsy education system. J Telemed Telecare. (2013) 19:23–8. doi: 10.1177/1357633X12473920
69.
Beretta S Beghi E Messina P Gerardi F Pescini F la Licata A et al . Comprehensive educational plan for patients with epilepsy and comorbidity (EDU-COM): a pragmatic randomised trial. J Neurol Neurosurg Psychiatry. (2014) 85:889–94. doi: 10.1136/jnnp-2013-306553
70.
Du Rausas FP . Quality of life in people with epilepsy: Associations with resilience, internalized stigma, and clinical factors in a low-income population. Epilepsy Behav. (2024) 155:109801. doi: 10.1016/j.yebeh.2024.109801
71.
Tombini M Narducci F Ricci L Sancetta B Boscarino M Quintiliani L et al . Resilience and psychosocial factors in adult with epilepsy: A longitudinal study. Epilepsy Behav. (2024) 154:109746. doi: 10.1016/j.yebeh.2024.109746
Summary
Keywords
health-related quality of life, people living with epilepsy, mean score, sub-Saharan Africa, associated factors
Citation
Beyene DA, Demsie DG, Tafere C, Yazie TS, Endeshaw D, Tadesse TA and Addisu ZD (2025) Health-related quality of life and associated factors among epilepsy patients in sub-Saharan Africa: a systematic review and meta-analysis. Front. Neurol. 16:1546911. doi: 10.3389/fneur.2025.1546911
Received
17 December 2024
Accepted
14 February 2025
Published
05 March 2025
Volume
16 - 2025
Edited by
Jacopo Lanzone, Istituti Clinici Scientifici Maugeri IRCCS, Neurorehabilitation Unit, Italy
Reviewed by
Giovanni Assenza, Campus Bio-Medico University, Italy
Gianni Cutillo, Vita-Salute San Raffaele University, Italy
Updates
Copyright
© 2025 Beyene, Demsie, Tafere, Yazie, Endeshaw, Tadesse and Addisu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dessale Abate Beyene, dessale2010@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.