Abstract
Background:
This study employs network meta-analysis to assess the efficacy of transcranial direct current stimulation (tDCS) combined with different rehabilitation approaches in enhancing motor function in people suffering from stroke-related symptoms (PSSS). The objective is to determine the most effective tDCS-based rehabilitation approach and offer valuable evidence to guide clinical decision-making.
Methods:
This study included randomized controlled trials (RCTs) published before September 23, 2024. We conducted a systematic search across eight databases: PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), China Biology Medicine (SinoMed), Wanfang, and VIP. Network meta-analysis (NMA) was conducted utilizing R Studio and Stata 15.0 for data analysis.
Results:
A total of 74 RCTs were included in this study, encompassing 4,335 PSSS and 11 intervention strategies. The NMA revealed that brain-computer interface therapy (BCIT) in combination with tDCS [surface under the cumulative ranking curve (SUCRA) = 88.34%] was the most effective tDCS-based intervention for improving the Fugl-Meyer Assessment for Upper Extremity score in PSSS. Mirror therapy (MT) in combination with tDCS (SUCRA = 85.96%) was identified as the optimal intervention for enhancing the Action Research Arm Test score in PSSS. MT + tDCS (SUCRA = 84.29%) was the best approach for improving the Fugl-Meyer Assessment for Lower Extremity score. Additionally, acupuncture and moxibustion (AM) in combination with tDCS (SUCRA = 77.16%) was the most effective intervention for increasing the Berg Balance Scale score in PSSS. The two-dimensional clustering analysis showed that MT + tDCS (SUCRA = 75.83%/85.96%) was the optimal tDCS-based rehabilitation strategy for treating upper limb motor dysfunction in PSSS, while AM+tDCS (SUCRA = 76.94%/77.16%) was the best tDCS-based rehabilitation strategy for improving lower limb motor dysfunction in PSSS.
Conclusion:
BCIT+tDCS was identified as the optimal tDCS-based rehabilitation strategy for improving upper limb motor ability in PSSS, MT + tDCS was the most effective intervention for enhancing arm mobility, MT + tDCS was the best protocol for improving lower limb motor ability, while AM+tDCS was the best strategy for improving balance ability. Furthermore, MT + tDCS was the optimal tDCS-based rehabilitation approach for treating upper limb motor dysfunction, whereas AM+tDCS was the most effective strategy for addressing lower limb motor dysfunction in PSSS. Future studies may focus on investigating the therapeutic effects of MT combined with tDCS on Berg Balance Scale score in PSSS, as well as the effects of AM combined with tDCS on Action Research Arm Test score, in order to further explore the therapeutic potential of these two intervention strategies.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/view/CRD42024621998, Identifier PROSPERO CRD42024621998.
1 Introduction
With population aging and lifestyle changes, the number of stroke cases has been increasing annually (1). It has become the world’s third leading cause of disability and the second most common cause of death among adults (2). The high disability rate associated with stroke significantly impacts patients’ quality of life, as many stroke survivors experience varying degrees of motor dysfunction, including marked weakness in the affected limbs, restricted joint range of motion, impaired balance, and coordination deficits (3, 4). Currently, the rehabilitation of motor dysfunction in people suffering from stroke-related symptoms (PSSS) remains a major challenge in clinical practice.
Current research suggests that a range of rehabilitation interventions, including acupuncture and moxibustion, functional electrical stimulation, core stability training, proprioceptive training, and transcranial direct current stimulation (tDCS), are widely employed to manage motor dysfunction in individuals with stroke-related symptoms (PSSS), aiming to enhance motor recovery and functional mobility (5–9). Among these, tDCS has demonstrated particularly promising therapeutic effects (10). tDCS is a non-invasive neuromodulation technique (11) that enhances excitability in the affected cortical region, reduces inhibitory effects from the healthy hemisphere, and improves local cerebral blood flow to protect neurons in ischemic areas (12). Based on these mechanisms, numerous clinical studies have confirmed the effectiveness of tDCS in treating motor dysfunction in PSSS. For example, Lindenberg et al. (13) reported that PSSS receiving tDCS exhibited greater improvements in upper limb motor function compared to those receiving sham tDCS. Similarly, Cha et al. (14) found that tDCS treatment resulted in significantly greater improvements in both upper and lower limb function in PSSS than in those who did not receive tDCS. Over the past two decades, the clinical application of tDCS in PSSS motor rehabilitation has matured, with most studies reporting favorable outcomes. To further enhance the effectiveness of motor rehabilitation in PSSS, researchers have explored the combination of tDCS with other rehabilitation techniques. Tedla et al. (15) found that PSSS receiving tDCS combined with proprioceptive training exhibited significantly greater improvements in upper limb motor ability compared to those receiving sham tDCS. Additionally, a study by Cui et al. (16) demonstrated that tDCS combined with acupuncture and moxibustion led to significantly greater improvements in balance ability than conventional rehabilitation. Compared to standalone tDCS or conventional rehabilitation, combined tDCS-based therapies have consistently shown superior efficacy in motor function rehabilitation for PSSS.
Network meta-analysis (NMA) is a specialized form of meta-analysis that integrates direct, indirect, and mixed comparisons of different interventions. This approach enables the evaluation of the relative efficacy of various treatments and ultimately facilitates the identification of the most effective intervention (17). In this study, we applied NMA to compare the therapeutic effects of tDCS combined with other rehabilitation interventions in the rehabilitation of motor dysfunction in PSSS. The objective is to determine the most effective tDCS-based rehabilitation approach and offer valuable evidence to guide clinical decision-making.
2 Methods
This network meta-analysis (NMA) was performed following the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the methodological framework of the Cochrane Handbook (18). Additionally, this study has been registered in PROSPERO under the registration number CRD42024621998.
2.1 Search strategy
An extensive electronic literature search was conducted across eight databases: PubMed, Embase, Cochrane Library, Web of Science, CNKI, SinoMed, WanFang, and VIP, covering studies published until September 23, 2024. Tailored search strategies were applied to meet the specific criteria of each database, with detailed methodologies available in Supplementary Table 1.
2.2 Inclusion criteria
(1) Participants: studies included adult PSSS (people suffering from stroke-related symptoms) aged ≥18 years who met the diagnostic criteria for stroke and were confirmed to have motor function impairment. (2) Intervention in the Experimental Group: Participants received tDCS or tDCS combined with other rehabilitation therapies, with conventional rehabilitation as the baseline treatment. (3) Intervention in the control group: participants received sham stimulation or conventional rehabilitation. (4) Outcome measures: Fugl-Meyer Assessment-Upper Extremity (FMA-UE): Evaluates upper limb motor ability, with higher scores indicating better motor function. Action Research Arm Test (ARAT): Assesses arm movement ability, with higher scores reflecting better arm mobility. Fugl-Meyer Assessment-Lower Extremity (FMA-LE): Measures lower limb motor ability, where higher scores represent better lower limb function. Berg Balance Scale (BBS): Evaluates balance ability, with higher scores indicating better balance control. This study primarily focuses on NMA, which only requires included studies to report sufficient data on primary outcomes (BBS, FMA-LE, FMA-UE, ARAT). (5) Study design: randomized controlled trials (RCTs).
Due to the presence of certain heterogeneity in the original studies, there were no fully consistent standards regarding electrode placement, stimulation intensity, duration, and rehabilitation training protocols. During data extraction, we made every effort to record these key details for subsequent discussion of potential sources of heterogeneity. However, in the overall analysis, tDCS was treated as a unified intervention approach. Detailed tDCS stimulation parameters for all included studies are summarized in Supplementary Table 2.
2.3 Exclusion criteria
(1) Guidelines, conference abstracts, systematic review, meta-analyses, etc. (2) non-RCTs. (3) Duplicate publications. (4) Studies with interventions or outcome measures that do not meet the inclusion criteria, studies with significant data errors, or studies where data could not be obtained. (5) tDCS combination interventions with fewer than two eligible studies.
2.4 Study selection and data extraction
Three researchers jointly established the inclusion criteria for the studies. After removing duplicate records using EndNote X9 software, two researchers independently conducted the study selection process. Initially, screening was performed based on titles, keywords, and abstracts, followed by a full-text review for data extraction and verification. In cases of disagreement, a third researcher was consulted to resolve discrepancies until a consensus was reached. The extracted baseline data included the following: basic study information (authors, publication year), patient characteristics (age, sample size, disease duration), intervention details (treatment and control group interventions), and outcome measures.
2.5 Quality assessment
Two researchers independently evaluated the methodological quality of the included studies using the Risk of Bias 2.0 (ROB 2.0) tool, as detailed in the Cochrane Handbook. The evaluation results were cross-checked, and any disagreements were resolved through discussion with a third researcher until a consensus was reached. The assessment covered several domains, including the randomization process, deviations from intended interventions, completeness of outcome data, outcome measurement, and selective reporting bias. Each domain comprised 1–7 specific items. The overall risk of bias for each study was classified as “high risk,” “low risk,” or “some concerns (moderate risk).”
2.6 Data analysis
This study utilized R 4.2.1 with the “gemtc” and “coda” packages and Stata 15 with the “gemtc” package to perform NMA. Continuous outcome variables were analyzed using mean difference (MD), with 95% credible interval (CrI) for interval estimation. A consistency model was established with 20,000 burn-ins and 50,000 iterations. The deviance information criterion (DIC) was used to compare the consistency model with the inconsistency model, and if the DIC difference was <5, the direct and indirect comparisons were considered consistent. For each outcome measure, the surface under the cumulative ranking curve (SUCRA) was used to rank the efficacy of different interventions. A two-dimensional clustering analysis was conducted to explore the optimal intervention. A comparison-adjusted funnel plot was generated to assess potential publication bias. A regression analysis between publication year and intervention effect was conducted using R Studio to evaluate whether the publication year influences the intervention effect. This NMA employed a burn-in of 20,000 iterations and 50,000 subsequent iterations. Convergence diagnostics, including the Gelman-Rubin statistic and visual inspection of trace plots, confirmed that the model met the stability requirements.
3 Results
3.1 Literature search results
A total of 4,195 records were initially identified through the search strategy. After removing 1,327 duplicate records and excluding 422 records consisting of reviews, animal studies, non-randomized controlled trials (RCTs), and conference abstracts, 2,446 records remained. Title and abstract screening led to the exclusion of another 2,257 records. Of the 189 remaining studies, 8 lacked full-text access, 78 had inappropriate interventions, 23 had unsuitable outcome measures, and 6 were excluded due to other reasons (e.g., apparent data errors or unavailability of original data). Ultimately, 74 RCTs (13–16, 19–88) were included in this study. The process of study selection is depicted in Figure 1.
Figure 1

Flow chart of literature screening.
3.2 Basic characteristics of the included studies
A total of 74 RCTs were included in this study, involving 4,335 PSSS (people suffering from stroke-related symptoms) and 11 intervention strategies. Both the treatment and control groups received conventional rehabilitation (C) as the baseline therapy. In addition to transcranial direct current stimulation (tDCS), the treatment group included nine combined tDCS interventions: tDCS+MIT (motor imagery therapy), tDCS+MT (mirror therapy), tDCS+AM (acupuncture and moxibustion), tDCS+CIMT (constraint-induced movement therapy), tDCS+BCIT (brain-computer interface therapy), tDCS+VRT (virtual reality technology), tDCS+RRT (robotic rehabilitation therapy), tDCS+PT (proprioceptive training), and tDCS+FES (functional electrical stimulation). Table 1 provides an overview of the basic characteristics of the included studies.
Table 1
| No. | Author | Year | Age (TG) | Age (CG) | N (TG) | N (CG) | Treatment (TG) | Treatment (CG) | Outcome | Duration (TG) | Duration (CG) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Che XW (19) | 2017 | 61.49 ± 10.51 | 58.77 ± 14.3 | 41 | 39 | MIT + tDCS | tDCS | FMA-UE | NR | NR |
| 2 | Chen HB (20) | 2021 | 48.4 ± 13.2 | 50.1 ± 11.5 | 19 | 18 | FES + tDCS | tDCS | FMA-LE BBS | 0-6 m | 0-6 m |
| 3 | Chen H (21) | 2020 | 59.32 ± 8.59 | 61.31 ± 9.13 | 26 | 26 | MT + tDCS | tDCS | FMA-UE | 58.92 ± 17.30d | 62.81 ± 18.14d |
| 4 | Chen TT (22) | 2023 | 56.45 ± 9.57 | 55.10 ± 10.18 | 20 | 20 | FES + tDCS | tDCS | FMA-UE | 28.85 ± 4.65d | 28.15 ± 5.13d |
| 5 | Chen Y (23) | 2022 | 62.03 ± 8.45 | 62.84 ± 8.53 | 55 | 55 | MT + tDCS | tDCS | FMA-UE FMA-LE | 3.46 ± 0.81 m | 3.42 ± 0.76 m |
| 6 | Cheng P (24) | 2015 | 61.08 ± 7.34 | 57.27 ± 6.87 | 19 | 19 | tDCS | C | FMA-UE | 51.12 ± 19.4d | 48 ± 16.45d |
| 7 | Cheng XX (25) | 2024 | 61.59 ± 10.97 | 62.57 ± 12.70 | 13 | 11 | tDCS | C | FMA-UE | 33.23 ± 5.86w | 32.45 ± 5.39w |
| 8 | Cui C (16) | 2021 | 64.87 ± 5.59 | 65.27 ± 5.14 | 28 | 27 | AM+tDCS | C | BBS | 6.47 ± 1.20w | 6.57 ± 1.20w |
| 9 | Deng R (26) | 2024 | 67.82 ± 6.80 | 61.24 ± 7.43 | 43 | 43 | tDCS | C | FMA-UE | NR | NR |
| 10 | Dong K (27) | 2021 | 54.10 ± 7.31 | 52.30 ± 5.03 | 10 | 10 | tDCS | C | BBS | 31.80 ± 5.55d | 32.20 ± 14.02d |
| 11 | Feng CW (28) | 2023 | 58.39 ± 9.12 | 56.29 ± 6.23 | 28 | 28 | CIMT+tDCS | C | FMA-UE | 57.79 ± 32.61d | 52.93 ± 26.02d |
| 12 | Gao L (29) | 2024 | 55.73 ± 8.60 | 54.60 ± 8.23 | 15 | 15 | BCIT+tDCS | tDCS | FMA-UE ARAT | 58.47 ± 30.98d | 70.27 ± 46.49d |
| 13 | Gao Z (30) | 2021 | 55.8 ± 10.9 | 56.1 ± 10.7 | 45 | 45 | MIT + tDCS | tDCS | FMA-UE | 33.6 ± 12.9d | 33.9 ± 13.3d |
| 14 | Gao Z (31) | 2023 | 71.18 ± 5.13 | 71.17 ± 5.15 | 71 | 71 | MT + tDCS | tDCS | FMA-UE | <3 m | <3 m |
| 15 | Han X (32) | 2023 | 67.51 ± 10.72 | 67.04 ± 10.73 | 47 | 49 | tDCS | C | FMA-UE | 39.57 ± 22.25d | 34.27 ± 19.78d |
| 16 | Hu HL (33) | 2023 | 61.45 ± 1.63 | 60.23 ± 1.74 | 40 | 40 | tDCS | C | FMA-UE | 36.41 ± 1.72d | 35.23 ± 1.41d |
| 17 | Huang Y (34) | 2023 | 42.67 ± 15.62 | 43.43 ± 14.57 | 50 | 50 | tDCS | C | FMA-UE | NR | NR |
| 18 | Jiang Y (35) | 2020 | 53.19 ± 5.95 | 52.35 ± 6.03 | 15 | 15 | tDCS | C | FMA-UE | 21.84 ± 13.81d | 22.09 ± 12.05d |
| 19 | Jin J (36) | 2019 | 53.1 ± 5.3 | 52.2 ± 5.2 | 45 | 45 | tDCS | C | FMA-UE ARAT | 36.1 ± 3.8d | 35.5 ± 5.7d |
| 20 | Jin MY (37) | 2020 | 62 ± 5.23 | 62 ± 5.34 | 30 | 30 | tDCS | C | FMA-LE BBS | 1.5 ± 1.02 | 1.5 ± 1.04 |
| 21 | Li XL (38) | 2021 | 50.37 ± 14.03 | 52.43 ± 15.12 | 162 | 162 | MT + tDCS | tDCS | FMA-UE ARAT | 76.06 ± 20.95d | 72.13 ± 21.18d |
| 22 | Li YB (39) | 2019 | 52.43 ± 12.15 | 50.12 ± 11.35 | 14 | 15 | MT + tDCS | tDCS | FMA-UE ARAT | 72.11 ± 22.19d | 75.02 ± 18.17d |
| 23 | Liu LS (40) | 2019 | 48.39 ± 11.2 | 48.39 ± 11.2 | 33 | 32 | tDCS | C | FMA-UE ARAT | 1-6 m | 1-6 m |
| 24 | Liu Y (41) | 2023 | 58.83 ± 5.89 | 58.76 ± 5.62 | 47 | 46 | MT + tDCS | tDCS | FMA-UE | 52.71 ± 11.23d | 53.35 ± 10.85d |
| 25 | Liu YW (42) | 2020 | 62.86 ± 9.05 | 63.53 ± 7.26 | 15 | 15 | VRT + tDCS | tDCS | FMA-UE | 9.33 ± 2.15 m | 9.67 ± 2.26 m |
| 26 | Long SY (43) | 2024 | 54.35 ± 6.02 | 55.47 ± 5.53 | 55 | 55 | tDCS | C | FMA-UE FMA-LE | NR | NR |
| 27 | Pan AH (44) | 2023 | 68.27 ± 7.83 | 67.95 ± 7.96 | 50 | 50 | CIMT+tDCS | tDCS | FMA-UE ARAT | 51.67 ± 13.89d | 49.29 ± 14.12d |
| 28 | Qi YS (45) | 2023 | 63.25 ± 2.21 | 63.18 ± 2.13 | 35 | 35 | RRT + tDCS | C | FMA-UE FMA-LE | 3.21 ± 0.24 m | 3.29 ± 0.25 m |
| 29 | Qu F (46) | 2024 | 59 | 63 | 30 | 30 | AM+tDCS | tDCS | FMA-UE | 59d | 47d |
| 30 | Ren SS (47) | 2023 | 66.18 ± 3.98 | 66.21 ± 4.06 | 28 | 28 | MIT + tDCS | tDCS | FMA-UE | 62.86 ± 12.67d | 56.43 ± 10.18d |
| 31 | Song DW (48) | 2024 | 62.01 ± 1.87 | 61.49 ± 1.83 | 41 | 41 | tDCS | C | ARAT | NR | NR |
| 32 | Sun FB (49) | 2023 | 62.15 ± 9.40 | 61.35 ± 9.82 | 20 | 20 | tDCS | C | FMA-UE | 37.95 ± 5.07d | 38.10 ± 4.75d |
| 33 | Tu M (50) | 2021 | 64.2 ± 6.9 | 63.5 ± 8.0 | 75 | 75 | tDCS | C | FMA-UE | NR | NR |
| 34 | Wang C (51) | 2021 | 61.39 ± 6.52 | 61.20 ± 6.31 | 54 | 53 | tDCS | C | FMA-UE | 5.77 ± 0.27 m | 5.61 ± 0.48 m |
| 35 | Wang CY (52) | 2023 | 62.80 ± 11.16 | 62.67 ± 10.70 | 15 | 15 | BCIT+tDCS | tDCS | ARAT | 13.60 ± 2.16d | 12.53 ± 2.59 |
| 59.13 ± 11.93 | 15 | C | 13.93 ± 2.84 | ||||||||
| 36 | Wang HB (53) | 2023 | 57.24 ± 8.23 | 57.81 ± 9.22 | 20 | 20 | CIMT+tDCS | tDCS | FMA-LE BBS | 35.62 ± 32.14d | 37.22 ± 31.16d |
| 37 | Wang HY (54) | 2023 | 60.25 ± 5.84 | 54.07 ± 6.15 | 40 | 40 | AM+tDCS | tDCS | FMA-UE | 1.15 ± 0.78 m | 1.30 ± 0.71 m |
| 38 | Wang Y (55) | 2021 | 60.95 ± 9.31 | 58.95 ± 7.2 | 19 | 20 | AM+tDCS | tDCS | FMA-UE | 1.2 ± 0.66 m | 1.22 ± 0.74 m |
| 39 | Yin Y (56) | 2015 | 55.70 ± 12.32 | 57.68 ± 13.54 | 40 | 40 | tDCS | C | FMA-UE ARAT | 31.55 ± 20.13d | 35.90 ± 19.60d |
| 40 | Zhang SS (57) | 2022 | 61.83 ± 6.29 | 61.26 ± 5.29 | 46 | 46 | AM+tDCS | C | FMA-UE FMA-LE | 1.32 ± 0.53 m | 1.28 ± 0.58 m |
| 41 | Zhang Y (58) | 2019 | 47.9 ± 6.7 | 48.5 ± 6.4 | 36 | 36 | RRT + tDCS | C | FMA-UE | 4.6 ± 1.5 m | 4.3 ± 1.7 m |
| 42 | Wang W (59) | 2021 | 60 ± 14 | 56 ± 11 | 15 | 15 | PT + Tdcs | C | FMA-UE | 20 ± 14d | 20 ± 12d |
| 43 | Zhao F (60) | 2021 | 60.4 ± 9.25 | 58.70 ± 9.64 | 39 | 39 | VRT + tDCS | C | FMA-UE | 38.3 ± 8.94d | 34.7 ± 12.35d |
| 44 | Zhao JY (61) | 2023 | 54.37 ± 4.54 | 54.98 ± 4.32 | 20 | 20 | tDCS | C | FMA-UE FMA-LE | 13.87 ± 2.42d | 13.65 ± 2.31d |
| 45 | Zheng CJ (62) | 2019 | 59.8 ± 8.3 | 61,1 ± 7.4 | 49 | 47 | tDCS | C | FMA-UE | 31.4 ± 11.4d | 35.3 ± 12.2d |
| 46 | Zheng S (63) | 2020 | 48.9 ± 2.0 | 48.5 ± 2.4 | 40 | 40 | AM+tDCS | tDCS | FMA-UE | 4.2 ± 2.9 m | 4.6 ± 2.8 m |
| 47 | Zhou YP (64) | 2018 | 54.25 ± 8.23 | 53.87 ± 8.91 | 32 | 31 | MIT + Tdcs | C | FMA-UE | 36.16 ± 19.9d | 34.83 ± 22.91d |
| 48 | Alisar (65) | 2020 | 63.56 ± 10.19 | 63.5 ± 12.6 | 16 | 16 | tDCS | C | FMA-UE | 352.62 ± 390.32d | 442.75 ± 687.43d |
| 49 | Cha (14) | 2014 | 59.8 ± 10.4 | 57.8 ± 9.9 | 10 | 10 | tDCS | C | FMA-UE FMA-LE | 13.8 ± 4.6 m | 14.5 ± 3.6 m |
| 50 | Cho (66) | 2015 | 58.29 ± 10.67 | 60.38 ± 10.19 | 14 | 13 | MT + tDCS | C | FMA-UE | 13.2 ± 5.1 m | 15.5 ± 7.8 m |
| 51 | Zeng (67) | 2024 | 55.14 ± 14.76 | 57.24 ± 13.75 | 21 | 21 | tDCS | C | FMA-UE ARAT | 1.25w | 2w |
| 52 | Gong (68) | 2023 | 56.3 ± 2.1 | 56.8 ± 2.8 | 37 | 35 | tDCS | C | FMA-UE | 49.6 ± 6.4d | 48.3 ± 6.5d |
| 53 | Lee (69) | 2014 | 63.1 ± 10.3 | 60.3 ± 11.3 | 20 | 19 | VRT + tDCS | tDCS | FMA-UE | 17.8 ± 7.3d | 17.4 ± 9.4d |
| 54 | Tedla (15) | 2022 | 58.5 ± 6.42 | 58.78 ± 5.46 | 18 | 18 | PT + tDCS | C | FMA-UE | 62.56 ± 17.1d | 62.78 ± 20.98d |
| 55 | Hsu (70) | 2023 | 59.1 ± 11.4 | 59.2 ± 11.8 | 13 | 14 | tDCS | C | FMA-UE FMA-LE ARAT | 20.7 ± 3.5d | 21.1 ± 5.3d |
| 56 | Rabadi (71) | 2020 | 62 ± 11 | 63 ± 6 | 8 | 8 | tDCS | C | ARAT | 6.9 ± 3.7 m | 5.9 ± 2.8 m |
| 57 | Qurat (72) | 2023 | 59 ± 4.61 | 57.95 ± 5.45 | 22 | 22 | tDCS | C | BBS | 16.5 ± 11.8 m | 16.41 ± 10.26 m |
| 58 | Li (73) | 2024 | 58.65 ± 12.677 | 56.54 ± 10.428 | 26 | 26 | tDCS | C | FMA-UE ARAT | 100.12d | 87.58d |
| 59 | Lindenberg (13) | 2010 | 61.7 ± 14.7 | 55.8 ± 12.9 | 10 | 10 | tDCS | C | FMA-UE | 30.5 ± 21.4 m | 40.3 ± 23.4 m |
| 60 | Llorens (74) | 2021 | 57.6 ± 6.9 | 52.3 ± 10.9 | 14 | 15 | VRT + tDCS | C | FMA-UE | 8.7 ± 2.3 m | 9.3 ± 2.4 m |
| 61 | Chang (75) | 2015 | 59.9 ± 10.2 | 65.8 ± 10.6 | 12 | 12 | tDCS | C | FMA-LE BBS | 16 ± 6.2d | 16.6 ± 5.2d |
| 62 | Duan (76) | 2023 | 65.83 ± 8.8 | 66.58 ± 10.3 | 46 | 45 | tDCS | C | FMA-LE | 5 ± 2.9w | 4 ± 2.9w |
| 63 | Youssef (77) | 2023 | 65 | 66 | 11 | 11 | tDCS | C | FMA-UE FMA-LE BBS | NR | NR |
| 64 | Toktas (78) | 2024 | 58.79 ± 10.19 | 62.57 ± 8.53 | 14 | 14 | tDCS | C | FMA-LE BBS | 8.07 ± 5.37 m | 6.86 ± 3.08 m |
| 65 | Allman (79) | 2016 | 59.5 ± 12.1 | 66.8 ± 10.4 | 11 | 13 | tDCS | C | FMA-UE ARAT | 51.2 ± 33.4 m | 56.6 ± 39.8 m |
| 66 | Dinesh (80) | 2011 | 61 ± 12 | 56 ± 15 | 7 | 7 | tDCS | C | FMA-UE | 33 ± 20 m | 28 ± 28 m |
| 67 | Fusco (81) | 2014 | 56.4 | 60 | 5 | 6 | tDCS | C | FMA-UE | NR | NR |
| 68 | Kim (82) | 2010 | 55.3 ± 16.4 | 62.9 ± 9.2 | 6 | 7 | tDCS | C | FMA-UE | 34 ± 27.1d | 22.9 ± 7.5d |
| 69 | Oveisgharan (83) | 2017 | 52.1 ± 12.8 | 65.3 ± 16.5 | 10 | 10 | tDCS | C | FMA-UE ARAT | 2.1 ± 3d | 3.8 ± 5.8d |
| 70 | Pinto (84) | 2021 | 45.6 ± 12.1 | 48.1 ± 9.4 | 31 | 29 | tDCS | C | FMA-UE FMA-LE | 1-411d | 1-411d |
| 71 | Prathum (85) | 2022 | 58.67 ± 3.7 | 56.83 ± 3.58 | 12 | 12 | tDCS | C | FMA-UE FMA-LE | 15.5 ± 2.6 m | 16.33 ± 3.3 m |
| 72 | Rossi (86) | 2013 | 66.1 ± 14.3 | 70.3 ± 13.5 | 25 | 25 | tDCS | C | FMA-UE | 9.8 ± 2.4d | 9.5 ± 2.8d |
| 73 | Lazzaro (87) | 2014 | 71.71 ± 5.25 | 66.43 ± 5.96 | 7 | 7 | tDCS | C | ARAT | 2.71 ± 0.42d | 2.57 ± 0.81d |
| 74 | Kim (88) | 2024 | 65.78 ± 12.6 | 57.13 ± 9.49 | 9 | 8 | tDCS | C | BBS | NR | NR |
The basic characteristics of the included studies.
TG, treatment group; CG, control group; FMA-UE, Fugl-Meyer Assessment for Upper Extremity; ARAT, Action Research Arm Test; FMA-LE, Fugl-Meyer Assessment for Lower Extremity; BBS, Berg Balance Scale. tDCS, transcranial direct current stimulation; C, conventional rehabilitation; MIT, motor imagery therapy; FES, functional electrical stimulation; MT, mirror therapy; AM, acupuncture and moxibustion; CIMT, constraint-induced movement therapy; BCIT, brain-computer interface therapy; VRT, virtual reality technology; RRT, robotic rehabilitation therapy; PT, proprioceptive training. NR, no report.
3.3 Quality assessment of the included studies
Regarding the overall risk of bias, 69 studies (13–15, 19–49, 51–61, 63–75, 77–81, 83–88) were assessed with moderate risk of bias, while 5 studies (16, 50, 62, 76, 82) were found to have high risk of bias. In terms of randomization, 64 studies had low risk of bias, and 10 studies had moderate risk. All studies showed moderate risk of bias concerning whether the intervention deviated from the intended plan. Regarding missing outcome data, 69 studies had low risk of bias, and 5 had high risk. For outcome measurement, 33 studies had low risk of bias, and 41 had moderate risk. Regarding selective outcome reporting, all studies exhibited low risk of bias. In conclusion, the main sources of bias in the included studies were moderate bias due to deviations from the intended interventions and high bias resulting from missing outcome data. A detailed description of the risk of bias for the included studies is provided in Figure 2.
Figure 2

Quality assessment of included studies.
3.4 Network meta-analysis
Figure 3 presents the network evidence diagrams. In the network evidence diagram, each node represents a different intervention, with the size of the node indicating the sample size of that intervention. The connections between nodes reflect the number of studies comparing the corresponding interventions, with line thickness increasing as the study count rises.
Figure 3

Network evidence diagrams (A) FMA-UE; (B) ARAT; (C) FMA-LE; (D) BBS. FMA-UE, Fugl-Meyer Assessment for Upper Extremity; ARAT, Action Research Arm Test; FMA-LE, Fugl-Meyer Assessment for Lower Extremity; BBS, Berg Balance Scale. tDCS, transcranial direct current stimulation; C, conventional rehabilitation; MIT, motor imagery therapy; FES, functional electrical stimulation; MT, mirror therapy; AM, acupuncture and moxibustion; CIMT, constraint-induced movement therapy; BCIT, brain-computer interface therapy; VRT, virtual reality technology; RRT, robotic rehabilitation therapy; PT, proprioceptive training.
3.4.1 Fugl-Meyer assessment-upper extremity
A total of 60 studies (13–15, 19, 21–26, 28–36, 38–47, 49–51, 54–70, 73, 74, 77, 79–86) reported Fugl-Meyer Assessment-Upper Extremity (FMA-UE) scores, covering 11 rehabilitation strategies, including RRT + tDCS, PT + tDCS, MIT + tDCS, AM+tDCS, BCIT+tDCS, tDCS, FES + tDCS, CIMT+tDCS, VRT + tDCS, MT + tDCS, and C. The network evidence diagram for FMA-UE is illustrated in Figure 3A, while the corresponding league table is provided in Table 2. Regarding the enhancement of FMA-UE scores, the following interventions showed better therapeutic effects compared to C: AM+tDCS (MD: 10.72, 95% CrI: 6.96–14.75), BCIT+tDCS (MD: 17.76, 95% CrI: 6.63–28.87), CIMT+tDCS (MD: 9.04, 95% CrI: 3.66–14.45), FES + tDCS (MD: 14.7, 95% CrI: 7.08–22.45), MIT + tDCS (MD: 13.2, 95% CrI: 8.86–17.63), MT + tDCS (MD: 13.08, 95% CrI: 9.79–16.36), PT + tDCS (MD: 8.21, 95% CrI: 0.84–15.55), RRT + tDCS (MD: 7.31, 95% CrI: 1.79–12.94), tDCS (MD: 5.67, 95% CrI: 4.22–7.19) and VRT + tDCS (MD: 8.49, 95% CrI: 4.57–12.4). Moreover, compared to tDCS alone, the following interventions demonstrated better effects: AM+tDCS (MD: 5.05, 95% CrI: 1.42–8.86), BCIT+tDCS (MD: 12.09, 95% CrI: 1.05–23.08), FES + tDCS (MD: 9.04, 95% CrI: 1.49–16.6), MIT + tDCS (MD: 7.53, 95% CrI: 3.32–11.74) and MT + tDCS (MD: 7.42, 95% CrI: 4.34–10.35). All differences were statistically significant.
Table 2
| MD 95% CrI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ARAT | AM+tDCS | 7 (−4.72, 18.57) | −10.72 (−14.75, −6.96) | −1.68 (−8.26, 4.71) | 4 (−4.54, 12.29) | 2.47 (−3.19, 8.02) | 2.35 (−2.55, 7.01) | −2.51 (−10.98, 5.67) | −3.41 (−10.26, 3.32) | −5.05 (−8.86, −1.42) | −2.23 (−7.72, 3.01) | FMA-UE |
| BCIT+tDCS | −17.76 (−28.87, −6.63) | −8.7 (−20.95, 3.54) | −3.04 (−16.37, 10.35) | −4.54 (−16.29, 7.24) | −4.68 (−16.13, 6.74) | −9.53 (−22.88, 3.77) | −10.45 (−22.81, 2.09) | −12.09 (−23.08, −1.05) | −9.27 (−20.99, 2.4) | |||
| 2.64 (−6.58, 12.37) | C | 9.04 (3.66, 14.45) | 14.7 (7.08, 22.45) | 13.2 (8.86, 17.63) | 13.08 (9.79, 16.36) | 8.21 (0.84, 15.55) | 7.31 (1.79, 12.94) | 5.67 (4.22, 7.19) | 8.49 (4.57, 12.4) | |||
| −4.78 (−19.83, 10.97) | −7.38 (−20.49, 5.66) | CIMT+tDCS | 5.69 (−3.58, 14.94) | 4.15 (−2.64, 10.99) | 4.04 (−2.17, 10.14) | −0.83 (−9.93, 8.25) | −1.75 (−9.43, 6.05) | −3.38 (−8.75, 2.03) | −0.56 (−7.16, 6.02) | |||
| FES + tDCS | −1.53 (−10.19, 7.14) | −1.64 (−9.82, 6.41) | −6.5 (−17.18, 4.06) | −7.41 (−16.88, 2.09) | −9.04 (−16.6, −1.49) | −6.23 (−14.77, 2.25) | ||||||
| MIT + tDCS | −0.12 (−5.35, 4.99) | −4.97 (−13.62, 3.53) | −5.89 (−12.97, 1.23) | −7.53 (−11.74, −3.32) | −4.71 (−10.53, 0.96) | |||||||
| −7.81 (−20.37, 5.48) | −10.44 (−20.41, −0.25) | −3.04 (−18.24, 12.54) | MT + tDCS | −4.88 (−12.86, 3.17) | −5.78 (−12.14, 0.82) | −7.42 (−10.35, −4.34) | −4.59 (−9.49, 0.33) | |||||
| PT + tDCS | −0.9 (−10.07, 8.41) | −2.54 (−9.98, 5) | 0.28 (−8.05, 8.58) | |||||||||
| RRT + tDCS | −1.64 (−7.43, 4.11) | 1.19 (−5.72, 7.91) | ||||||||||
| −1.95 (−10.8, 7.36) | −4.6 (−8.82, −0.42) | 2.81 (−9.67, 15.16) | 5.84 (−3.48, 14.86) | tDCS | 2.82 (−1.14, 6.68) | |||||||
| VRT + tDCS | ||||||||||||
League table of FMA-UE and ARAT.
FMA-UE, Fugl-Meyer Assessment for Upper Extremity; ARAT, Action Research Arm Test; tDCS, transcranial direct current stimulation; C, conventional rehabilitation; MIT, motor imagery therapy; FES, functional electrical stimulation; MT, mirror therapy; AM, acupuncture and moxibustion; CIMT, constraint-induced movement therapy; BCIT, brain-computer interface therapy; VRT, virtual reality technology; RRT, robotic rehabilitation therapy; PT, proprioceptive training.
3.4.2 Action research arm test
A total of 16 studies (29, 36, 38–40, 44, 48, 52, 56, 67, 70, 71, 73, 79, 83, 87) reported Action Research Arm Test (ARAT) scores, involving five rehabilitation strategies: C, BCTT+tDCS, tDCS, MT + tDCS, and CIMT+tDCS. The network evidence diagram for ARAT is illustrated in Figure 3B, while the corresponding league table is provided in Table 2. Regarding the enhancement of ARAT scores, the following interventions showed better therapeutic effects compared to C: MT + tDCS (MD: 10.44, 95% CrI: 0.25–20.41) and tDCS (MD: 4.6, 95% CrI: 0.42–8.82). All differences were statistically significant.
3.4.3 Fugl-Meyer assessment-lower extremity
A total of 16 studies (14, 20, 23, 37, 43, 45, 53, 57, 61, 70, 75–78, 84, 85) reported Fugl-Meyer Assessment-Lower Extremity (FMA-LE) scores, involving seven rehabilitation strategies: MT + tDCS, FES + tDCS, CIMT+tDCS, RRT + tDCS, C, AM+tDCS, and tDCS. The network evidence diagram for FMA-LE is illustrated in Figure 3C, while the corresponding league table is provided in Table 3. Regarding the enhancement of FMA-LE scores, the following interventions showed better therapeutic effects compared to C: AM+tDCS (MD: 6.7, 95% CrI: 0.63–12.8), MT + tDCS (MD: 7.82, 95% CrI: 1.19–14.57) and tDCS (MD: 2.52, 95% CrI: 0.64–4.49). All differences were statistically significant.
Table 3
| MD 95% CrI | ||||||||
|---|---|---|---|---|---|---|---|---|
| BBS | AM + tDCS | −6.7 (−12.8, −0.63) | −2.5 (−11.12, 6.28) | −2.66 (−12.34, 7.1) | 1.11 (−7.87, 10.17) | −4.19 (−12.75, 4.31) | −4.18 (−10.51, 2.23) | FMA-LE |
| 8.22 (−2.2, 18.63) | C | 4.21 (−1.96, 10.44) | 4.05 (−3.45, 11.7) | 7.82 (1.19, 14.57) | 2.51 (−3.45, 8.47) | 2.52 (0.64, 4.49) | ||
| 2.13 (−13.47, 17.58) | −6.07 (−17.86, 5.44) | CIMT + tDCS | −0.15 (−9.54, 9.25) | 3.61 (−5.11, 12.29) | −1.69 (−10.37, 6.87) | −1.68 (−7.58, 4.2) | ||
| 2.97 (−13.18, 18.88) | −5.26 (−17.6, 6.96) | 0.83 (−15.1, 16.71) | FES + tDCS | 3.75 (−5.98, 13.49) | −1.55 (−11.23, 8.04) | −1.53 (−8.87, 5.79) | ||
| MT + tDCS | −5.3 (−14.31, 3.63) | −5.29 (−11.72, 1.09) | ||||||
| RRT + tDCS | 0.01 (−6.21, 6.31) | |||||||
| 4.58 (−6.72, 15.69) | −3.64 (−7.88, 0.39) | 2.42 (−8.4, 13.29) | 1.6 (−10.02, 13.18) | tDCS | ||||
League table of FMA-LE and BBS.
FMA-LE, Fugl-Meyer Assessment for Lower Extremity; BBS, Berg Balance Scale; tDCS, transcranial direct current stimulation; C, conventional rehabilitation; AM, acupuncture and moxibustion; CIMT, constraint-induced movement therapy; FES, functional electrical stimulation; MT, mirror therapy; RRT, robotic rehabilitation therapy.
3.4.4 Berg balance scale
A total of 10 studies (16, 20, 27, 37, 53, 72, 75, 77, 78, 88) reported Berg Balance Scale (BBS) scores, involving four rehabilitation strategies: CIMT+tDCS, FES + tDCS, C, tDCS, and AM+tDCS. The network evidence diagram for BBS is illustrated in Figure 3D, while the corresponding league table is provided in Table 3. Regarding the enhancement of BBS scores, none of the rehabilitation strategies demonstrated statistically significant differences in effectiveness.
3.5 SUCRA ranking
The cumulative probability rankings for the four outcome measures are presented in Figure 4 and Table 4. The results indicate that BCIT+tDCS (SUCRA = 88.34%) is the most effective tDCS-based combined intervention for improving FMA-UE scores, followed by FES + tDCS (SUCRA = 80.99%), MT + tDCS (SUCRA = 75.83%), MIT + tDCS (SUCRA = 75.80%), AM+tDCS (SUCRA = 57.18%), CIMT+tDCS (SUCRA = 44.11%), VRT + tDCS (SUCRA = 39.31%), PT + tDCS (SUCRA = 39.07%), RRT + tDCS (SUCRA = 31.75%), tDCS (SUCRA = 17.39%), and C (SUCRA = 0.23%). Regarding ARAT scores, MT + tDCS (SUCRA = 85.96%) demonstrated the best efficacy, ranking above CIMT+tDCS (SUCRA = 66.38%), tDCS (SUCRA = 51.51%), BCIT+tDCS (SUCRA = 35.38%), and C (SUCRA = 10.76%). For FMA-LE scores, MT + tDCS (SUCRA = 84.29%) was identified as the most effective intervention, followed by AM+tDCS (SUCRA = 76.94%), CIMT+tDCS (SUCRA = 55.02%), FES + tDCS (SUCRA = 52.45%), RRT + tDCS (SUCRA = 37.57%), tDCS (SUCRA = 36.79%), and C (SUCRA = 6.94%). In terms of BBS scores, AM+tDCS (SUCRA = 77.16%) ranked highest, followed by CIMT+tDCS (SUCRA = 62.47%), FES + tDCS (SUCRA = 55.79%), tDCS (SUCRA = 45.19%), and C (SUCRA = 9.39%).
Figure 4

Cumulative probability ranking charts (A) FMA-UE; (B) ARAT; (C) FMA-LE; (D) BBS. FMA-UE, Fugl-Meyer Assessment for Upper Extremity; ARAT, Action Research Arm Test; FMA-LE, Fugl-Meyer Assessment for Lower Extremity; BBS, Berg Balance Scale. tDCS, transcranial direct current stimulation; C, conventional rehabilitation; MIT, motor imagery therapy; FES, functional electrical stimulation; MT, mirror therapy; AM, acupuncture and moxibustion; CIMT, constraint-induced movement therapy; BCIT, brain-computer interface therapy; VRT, virtual reality technology; RRT, robotic rehabilitation therapy; PT, proprioceptive training.
Table 4
| Intervention | FMA-UE | ARAT | FMA-LE | BBS |
|---|---|---|---|---|
| AM + tDCS | 57.18 | 0 | 76.94 | 77.16 |
| BCIT + tDCS | 88.34 | 35.38 | 0 | 0 |
| C | 0.23 | 10.76 | 6.94 | 9.39 |
| CIMT + tDCS | 44.11 | 66.38 | 55.02 | 62.47 |
| FES + tDCS | 80.99 | 0 | 52.45 | 55.79 |
| MIT + tDCS | 75.80 | 0 | 0 | 0 |
| MT + tDCS | 75.83 | 85.96 | 84.29 | 0 |
| PT + tDCS | 39.07 | 0 | 0 | 0 |
| RRT + tDCS | 31.75 | 0 | 37.57 | 0 |
| tDCS | 17.39 | 51.51 | 36.79 | 45.19 |
| VRT + tDCS | 39.31 | 0 | 0 | 0 |
Cumulative probability ranking table.
FMA-UE, Fugl-Meyer Assessment for Upper Extremity; ARAT, Action Research Arm Test; FMA-LE, Fugl-Meyer Assessment for Lower Extremity; BBS, Berg Balance Scale. tDCS, transcranial direct current stimulation; C, conventional rehabilitation; MIT, motor imagery therapy; FES, functional electrical stimulation; MT, mirror therapy; AM, acupuncture and moxibustion; CIMT, constraint-induced movement therapy; BCIT, brain-computer interface therapy; VRT, virtual reality technology; RRT, robotic rehabilitation therapy; PT, proprioceptive training.
3.6 Two-dimensional clustering analysis
The two-dimensional clustering analysis for the four outcome measures is shown in Figure 5. As depicted in Figure 5A, MT + tDCS (SUCRA = 75.83%/85.96%) demonstrated significant efficacy in improving both FMA-UE and ARAT scores. FMA-UE evaluates upper limb motor ability in PSSS, while ARAT assesses arm mobility. Together, these two scales comprehensively reflect upper limb function in PSSS. Figure 5B indicates that AM+tDCS (SUCRA = 76.94%/77.16%), FES + tDCS (SUCRA = 52.45%/55.79%), and CIMT+tDCS (SUCRA = 55.02%/62.47%) exhibited notable effectiveness in enhancing FMA-LE and BBS scores. FMA-LE evaluates lower limb motor ability in PSSS, while BBS assesses lower limb balance. Together, these two scales provide a comprehensive reflection of lower limb function in PSSS. In summary, MT + tDCS (SUCRA = 75.83%/85.96%) may represent the optimal intervention for upper limb dysfunction in PSSS, while AM+tDCS (SUCRA = 76.94%/77.16%) may be the most effective strategy for improving lower limb function in PSSS.
Figure 5
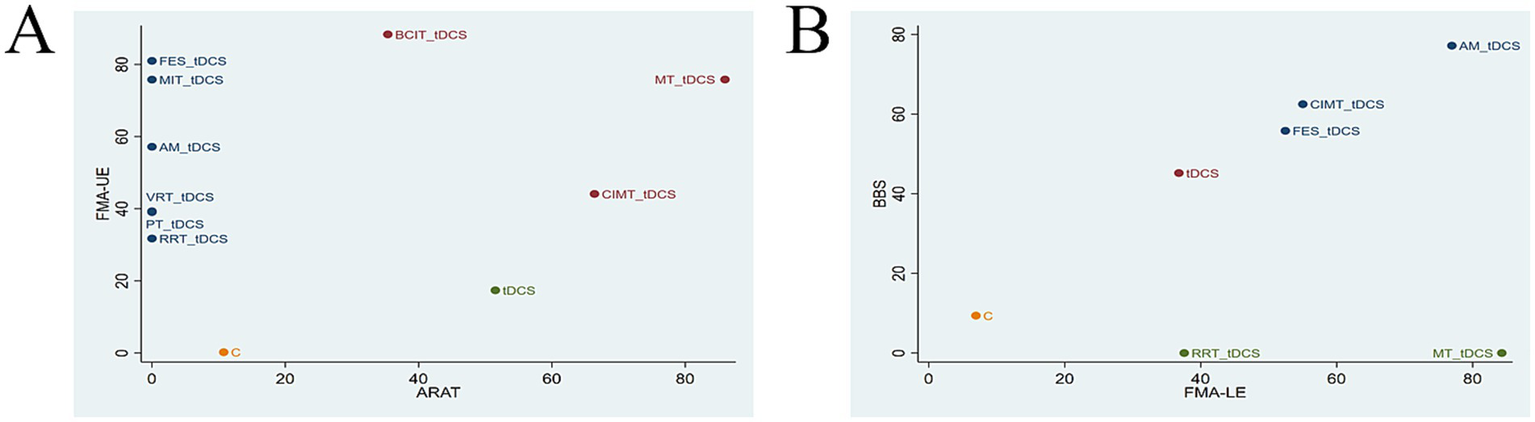
Clustering analysis charts (A) FMA-UE and ARAT; (B) FMA-LE and BBS. FMA-UE, Fugl-Meyer Assessment for Upper Extremity; ARAT, Action Research Arm Test; FMA-LE, Fugl-Meyer Assessment for Lower Extremity; BBS, Berg Balance Scale. tDCS, transcranial direct current stimulation; C, conventional rehabilitation; MIT, motor imagery therapy; FES, functional electrical stimulation; MT, mirror therapy; AM, acupuncture and moxibustion; CIMT, constraint-induced movement therapy; BCIT, brain-computer interface therapy; VRT, virtual reality technology; RRT, robotic rehabilitation therapy; PT, proprioceptive training.
3.7 Publication bias
The funnel plots for the four outcome measures are shown in Figure 6. In Figures 6A,C,D, the plots appear symmetrical overall, with only a few data points falling outside the funnel. It suggests a low likelihood of publication bias for the FMA-UE, FMA-LE, and BBS outcomes. However, in Figure 6B, a lot of data points fall outside the funnel, indicating a higher probability of publication bias for the ARAT outcome. To further assess whether the publication bias in ARAT outcomes was significant, we conducted Begg’s test. The result of Begg’s test showed that p = 0.910 (p > 0.05), indicating a low likelihood of publication bias for the ARAT outcome.
Figure 6
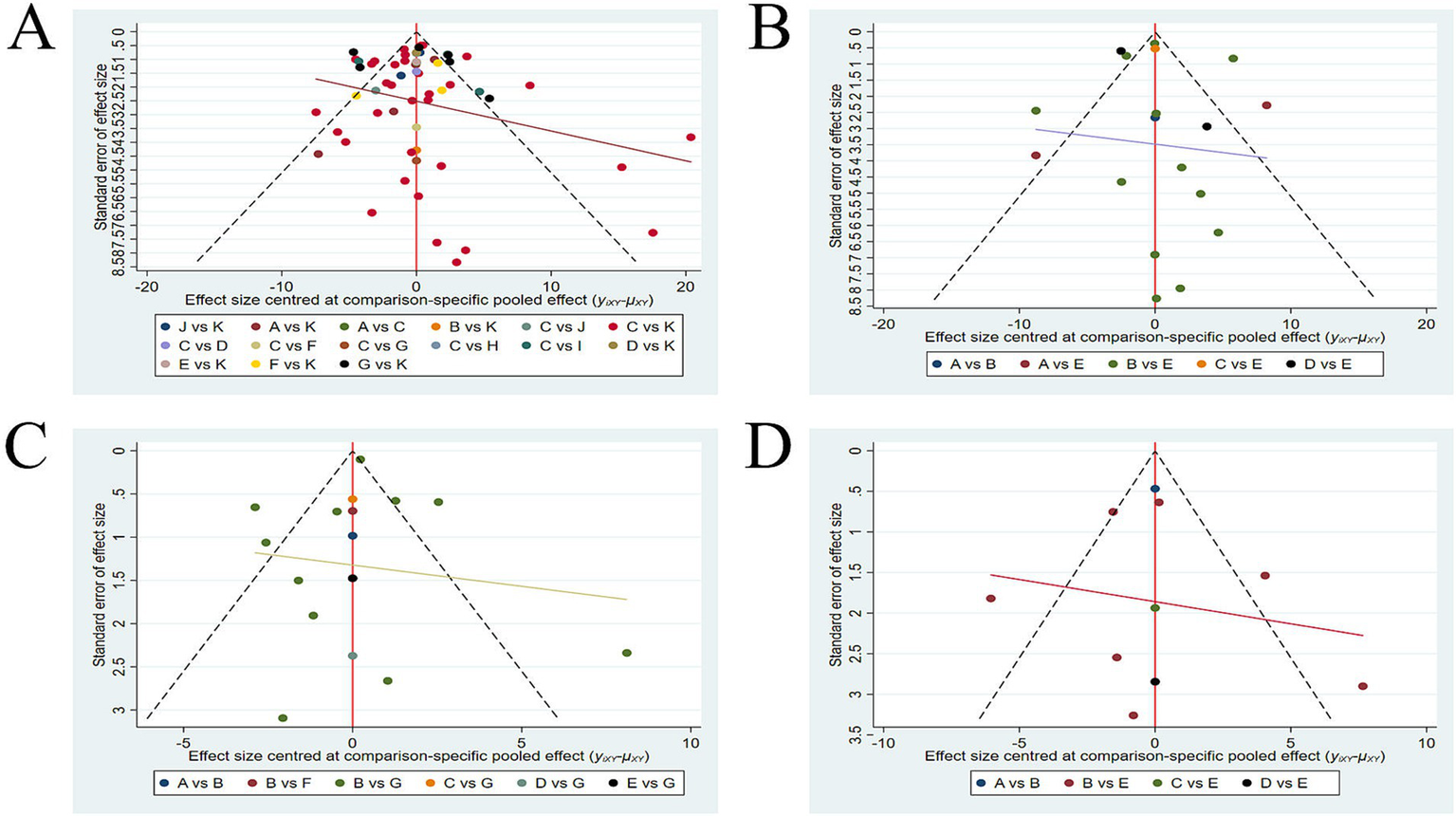
Funnel plots (A) FMA-UE; (B) ARAT; (C) FMA-LE; (D) BBS. FMA-UE, Fugl-Meyer Assessment for Upper Extremity; ARAT, Action Research Arm Test; FMA-LE, Fugl-Meyer Assessment for Lower Extremity; BBS, Berg Balance Scale.
3.8 Regression analysis
As shown in Table 5, the results of the regression analysis between intervention effects and publication year indicate that the credible interval for all four outcome measures do not cross zero. This suggests that publication year is not a moderating factor influencing the treatment effects of the intervention strategies.
Table 5
| Outcomes | 95% CrI |
|---|---|
| FMA-UE | (−2.5145, 3.213) |
| ARAT | (−9.498, 6.0205) |
| FMA-LE | (−1.0525, 5.551) |
| BBS | (−2.6679, 9.772) |
Regression analysis between publication year and intervention effect.
FMA-UE, Fugl-Meyer Assessment for Upper Extremity; ARAT, Action Research Arm Test; FMA-LE, Fugl-Meyer Assessment for Lower Extremity; BBS, Berg Balance Scale.
3.9 Convergence diagnostics
The convergence diagnostics and the trace and density plots are presented in Figures 7–10. We evaluated the convergence and stability of the model by drawing convergence diagnostic maps and trajectory density maps. Before 70,000 iterations, the convergence diagnostic plots and trace plots had converged, and the potential scale reduced factor tended to approach 1. The density plots was a smooth curve with a normal distribution, and the Bandwidth value approached 0, indicating satisfactory convergence and good stability of the model.
Figure 7
Convergence diagnostic plots, trace plots, and density plots of FMA-UE (A) convergence diagnostic plots; (B) trace plots and density plots. FMA-UE, Fugl-Meyer Assessment for Upper Extremity; C, conventional rehabilitation; tDCS, transcranial direct current stimulation; MIT, motor imagery therapy; FES, functional electrical stimulation; MT, mirror therapy; AM, acupuncture and moxibustion; CIMT, constraint-induced movement therapy; BCIT, brain-computer interface therapy; VRT, virtual reality technology; RRT, robotic rehabilitation therapy; PT, proprioceptive training.
Figure 8

Convergence diagnostic plots, trace plots, and density plots of ARAT (A) convergence diagnostic plots; (B) trace plots and density plots. ARAT, Action Research Arm Test; C, conventional rehabilitation; tDCS, transcranial direct current stimulation; CIMT, constraint-induced movement therapy; BCIT, brain-computer interface therapy; MT, mirror therapy.
Figure 9
Convergence diagnostic plots, trace plots, and density plots of FMA-LE (A) convergence diagnostic plots; (B) trace plots and density plots. FMA-LE, Fugl-Meyer Assessment for Lower Extremity; C, conventional rehabilitation; tDCS, transcranial direct current stimulation; AM, acupuncture and moxibustion; RRT, robotic rehabilitation therapy; CIMT, constraint-induced movement therapy; FES, functional electrical stimulation; MT, mirror therapy.
Figure 10
Convergence diagnostic plots, trace plots, and density plots of BBS (A) convergence diagnostic plots; (B) trace plots and density plots. BBS, Berg Balance Scale; C, conventional rehabilitation; tDCS, transcranial direct current stimulation; AM, acupuncture and moxibustion; CIMT, constraint-induced movement therapy; FES, functional electrical stimulation.
4 Discussion
This network meta-analysis (NMA) incorporated 74 randomized controlled trials (RCTs) with a total of 4,335 people suffering from stroke-related symptoms (PSSS) and nine tDCS-based combined intervention strategies. The network meta-analysis (NMA) results indicated that brain-computer interface therapy (BCIT) + tDCS (SUCRA = 88.34%) was the best intervention for enhancing upper limb motor ability in PSSS. For arm mobility and lower limb motor ability, mirror therapy (MT) + tDCS (SUCRA = 85.96%/84.29%) demonstrated the best efficacy, while acupuncture and moxibustion (AM) + tDCS (SUCRA = 77.16%) was the most effective for enhancing balance ability. The two-dimensional clustering analysis showed that MT + tDCS (SUCRA = 75.83%/85.96%) was the optimal tDCS-based rehabilitation strategy for treating upper limb motor dysfunction in PSSS, while AM+tDCS (SUCRA = 76.94%/77.16%) was the best tDCS-based rehabilitation strategy for improving lower limb motor dysfunction in PSSS.
BCIT+tDCS was identified as the most effective intervention for improving upper limb motor ability in PSSS. BCIT is an advanced communication system that integrates hardware and software, enabling the extraction, decoding, and translation of brain motor intention signals into commands that control external devices, thus facilitating direct brain-environment interaction (89, 90). BCIT has been widely used in the rehabilitation of upper limb motor ability in PSSS. Wang et al. (91) reported that BCIT could further enhance upper limb motor function in PSSS when combined with conventional rehabilitation therapy, compared to conventional rehabilitation alone. Additionally, meta-analyses conducted by Nojima et al. (92) and Li et al. (93) confirmed the superior efficacy of BCIT-based training over traditional rehabilitation in improving upper limb function in PSSS patients. In recent years, significant progress has been made in BCIT-based rehabilitation for motor function recovery in PSSS, and integrating BCIT with other rehabilitation strategies appears to be a promising and innovative approach. For instance, BCIT combined with robotic rehabilitation therapy (94) and BCIT combined with functional electrical stimulation therapy (95) have shown enhanced therapeutic effects. The development of these BCIT-based combination therapies supports the findings of this study, which identified BCIT+tDCS as the most effective strategy for improving upper limb motor ability in PSSS. Anodal tDCS applied before BCIT may pre-activate the ipsilesional hemisphere and excite the ipsilesional motor cortex, thereby facilitating faster BCIT activation and enhancing characteristic brain signals (96). Furthermore, tDCS creates a favorable neural environment, which may promote motor learning and enhance BCIT-induced motor learning capacity (97). BCIT, incorporating visual, auditory, and motor feedback, continuously reinforces motor imagery within the brain, providing peripheral visual, auditory, and proprioceptive feedback to the central nervous system. This process strengthens positive feedback loops, reinforces correct motor patterns, and promotes the reconstruction of damaged neural pathways, leading to enhanced therapeutic outcomes beyond those achieved with tDCS alone (98). Moreover, both BCIT and tDCS directly stimulate the central nervous system, enhancing neuroplasticity. Their combined application may produce a synergistic effect, maximizing cortical excitability and neuroplasticity, which could further improve upper limb motor ability in PSSS.
MT + tDCS is considered one of the effective combined treatment approaches for improving arm mobility and lower limb motor ability in PSSS patients. Mirror Therapy (MT), as a neurorehabilitation technique, uses visual illusions created by mirror reflection, imitation, and motor imagery, allowing the brain to perceive the movement of the affected limb as normal (99, 100). Although this process theoretically may involve the activation of the mirror neuron system, thereby facilitating functional recovery of the affected side, it should be noted that the RCTs included in this study did not provide direct neuroimaging or electrophysiological data to support this mechanism. Therefore, we describe this mechanism as a potential explanation. In fact, the multisensory stimulation provided by MT not only potentially activates the mirror neuron system (101), but also enhances the excitability of the primary motor cortex (M1) (102), promotes cortical reorganization, and improves motor cortex plasticity through repeated high-intensity visual feedback, thereby mitigating the phenomenon of “learned non-use” of the affected limb. Furthermore, MT emphasizes bilateral symmetrical movements, which helps to more widely activate the motor cortex, facilitating the activation of residual motor pathways on the affected side (103, 104), thereby promoting motor recovery. In PSSS, an interhemispheric imbalance is often observed, wherein the affected hemisphere exhibits reduced excitability, while the unaffected hemisphere exerts excessive inhibition via the transcallosal pathway (105). Anodal tDCS enhances the excitability of the affected M1 and corrects the pathological interhemispheric inhibition, which may contribute to the restoration of lower limb motor ability and arm mobility. Considering that patients with PSSS often exhibit reduced excitability in the affected hemisphere and excessive inhibition from the healthy hemisphere, anodal tDCS may help correct this interhemispheric imbalance by enhancing the excitability of the affected M1, thus playing a positive role in improving both arm mobility and lower limb motor function (21). Applying anodal tDCS prior to MT may pre-activate the relevant brain regions, creating a more favorable neural environment for subsequent mirror neuron activation, thereby enhancing the efficiency of neural network reorganization (21).
AM combined with tDCS has been recognized as an effective combined therapeutic strategy for improving balance function in PSSS. Acupuncture and moxibustion (AM) is a therapeutic approach based on traditional Chinese medicine principles, involving the insertion of needles at specific angles into the body and applying techniques such as twisting and lifting to stimulate targeted areas for therapeutic effects. AM can enhance excitability in brain regions affected by pathological changes, promote the establishment of collateral cerebral circulation, rapidly alleviate vascular spasms, induce vasodilation, reduce vascular resistance, and increase cerebral blood flow. These effects contribute to alleviating ischemia in brain tissues surrounding the lesion, rescuing ischemic but functionally impaired neurons, and accelerating central nervous system repair and reconstruction (106). However, the proposed mechanisms underlying AM are primarily based on traditional theories and preliminary literature, lacking direct evidence from modern neuroimaging studies. Therefore, these mechanisms remain hypothetical and warrant further investigation through contemporary mechanistic research. The combination of tDCS and AM may further enhance neuroplasticity and functional recovery through synergistic effects. tDCS modulates cortical excitability, increasing activity in the damaged motor cortex while inhibiting excessive excitability in the unaffected hemisphere, thereby restoring interhemispheric balance. Meanwhile, AM stimulates specific acupoints, activating intracerebral neural networks, improving local blood circulation, and facilitating the release of neurotrophic factors, collectively promoting central nervous system repair (57). Additionally, AM reduces pain, alleviates muscle spasticity, and promotes muscle strength recovery in the affected limbs, which further contributes to improved balance ability in PSSS (54). It is noteworthy that all studies involving AM+tDCS were conducted in China, and there may be considerable variation in the specific acupuncture procedures employed—such as acupoint selection, stimulation intensity, needle angle, and frequency of manipulation. These differences could influence the therapeutic outcomes and limit the generalizability of this combined intervention across diverse cultural or clinical settings. Future cross-cultural and multicenter studies are warranted, not only to investigate the relationship between acupuncture parameters and brain functional remodeling using modern neuroimaging techniques, but also to explore the acceptability and efficacy of different acupuncture modalities in varied cultural contexts. Such efforts would provide a more comprehensive foundation for the broader clinical application of AM+tDCS.
In this study, BBS was utilized as a primary outcome measure for evaluating balance ability; however, the results did not indicate statistically significant improvements. Several factors may contribute to this outcome. First, the small sample sizes in studies reporting BBS outcomes may have limited the statistical power, thereby reducing the likelihood of detecting significant effects even in the presence of genuine balance improvements. Second, the intervention itself may have had a relatively modest impact on balance function compared to its effects on upper limb motor ability or other functional domains. Finally, the BBS relies on assessor judgment, which may be influenced by variability in assessment conditions, patient status, and inter-rater differences, potentially contributing to inconsistent results.
Although this study has demonstrated the relative advantages of tDCS combined with various rehabilitation interventions in improving limb dysfunction among PSSS, the practical implementation of these interventions still faces several challenges. Firstly, most existing studies have been conducted in well-equipped specialized hospitals or rehabilitation centers, whereas primary healthcare institutions may lack adequate infrastructure, equipment investment, and trained personnel for implementation and maintenance. Secondly, data on cost-effectiveness remain limited, making it difficult to determine the economic feasibility of these approaches in resource-constrained settings. Lastly, regional disparities in the distribution of rehabilitation resources and technological capabilities may further impede the widespread adoption of these strategies. Therefore, how to balance clinical efficacy and economic costs in real-world practice, and how to tailor these interventions to suit primary care settings, remain critical issues to be addressed. Future efforts should focus on conducting multicenter studies, health economic evaluations, and implementation research to assess the feasibility of these interventions under primary care conditions and to address potential barriers such as equipment procurement and workforce training.
This study has several limitations. Due to the lack of detailed information on stroke stages and the degree of motor dysfunction in most of the original studies, although our network meta-analysis provides relative efficacy for each intervention based on the existing evidence, these factors may lead to significant clinical heterogeneity. This study primarily relied on various clinical assessment scales (e.g., FMA, ARAT, BBS) to evaluate intervention outcomes. While these tools are widely used in clinical practice and offer considerable ease of use and acceptable reliability, they are inherently subjective, as they depend on observer ratings and patients’ self-performance. In contrast, objective indicators such as neuroimaging (e.g., MRI, fMRI) and electroencephalography (EEG) can more directly reflect changes in brain function and structure. Future research should consider integrating neuroimaging, EEG, or other electrophysiological data into study designs to provide more robust scientific evidence for the clinical application of these interventions. Due to insufficient reporting of treatment dosage parameters in combined intervention protocols across some included studies, we were unable to perform more comprehensive dose–response or subgroup analyses. This limitation may constrain the interpretation of intensity-efficacy relationships in our findings. Some studies have defects such as failure to strictly implement blinding or inconsistent procedures during measurements, which may introduce bias in the outcome measures and potentially lead to an overestimation of the efficacy of tDCS or combined tDCS treatment protocols. Therefore, readers should exercise caution when selecting intervention protocols. After excluding studies with high risk of bias and reanalyzing the data, no significant changes in the results were observed, which suggests that the findings of this study are stable.
5 Conclusion
BCIT+tDCS was identified as the optimal tDCS-based rehabilitation strategy for improving upper limb motor ability in PSSS, MT + tDCS was the most effective intervention for enhancing arm mobility, MT + tDCS was the best protocol for improving lower limb motor ability, while AM+tDCS was the best strategy for improving balance ability in PSSS. Furthermore, MT + tDCS was the optimal tDCS-based rehabilitation approach for treating upper limb motor dysfunction, whereas AM+tDCS was the most effective strategy for addressing lower limb motor dysfunction in PSSS. Future studies may focus on investigating the therapeutic effects of MT combined with tDCS on Berg Balance Scale score in PSSS, as well as the effects of AM combined with tDCS on Action Research Arm Test score, in order to further explore the therapeutic potential of these two intervention strategies.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the datasets analysed during the current study are not publicly available but are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to LG, liangguo@m.scnu.edu.cn.
Author contributions
KZ: Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. LG: Funding acquisition, Methodology, Supervision, Writing – review & editing, Writing – original draft. WL: Formal analysis, Methodology, Writing – original draft. PL: Formal analysis, Methodology, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was funded by Guangzhou Science and Technology Bureau (2025B04J0034) and Guangdong Provincial Sports Bureau (GDSS2024N029).
Acknowledgments
I would like to express my heartfelt gratitude to Zhongli Zheng for her meticulous care and unwavering support in my daily life. Her kindness and encouragement have been invaluable throughout my research journey.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. Since the initial draft of this manuscript was written in Chinese, we used ChatGPT and Baidu Translate to assist in translating the Introduction and Discussion sections. However, all final revisions and refinements were made by the authors to ensure accuracy and clarity.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1586685/full#supplementary-material
References
1.
Hankey GJ . Stroke. Lancet. (2017) 389:641–54. doi: 10.1016/S0140-6736(16)30962-X
2.
Feigin VL Brainin M Norrving B Martins S Sacco RL Hacke W et al . World stroke organization (WSO): global stroke fact sheet 2022. Int J Stroke Off Stroke Soc. (2022) 17:18–29. doi: 10.1177/17474930211065917
3.
Liu CH Hsieh YT Tseng HP Lin HC Lin CL Wu TY et al . Acupuncture for a first episode of acute ischaemic stroke: an observer-blinded randomised controlled pilot study. Acupuncture Med J Br Med Acupuncture Soc. (2016) 34:349–55. doi: 10.1136/acupmed-2015-010825
4.
Zheng K Li L Zhou Y Gong X Zheng G Guo L . Optimal proprioceptive training combined with rehabilitation regimen for lower limb dysfunction in stroke patients: a systematic review and network meta-analysis. Front Neurol. (2024) 15:1503585. doi: 10.3389/fneur.2024.1503585
5.
Sánchez-Mila Z Salom-Moreno J Fernández-de-Las-Peñas C . Effects of dry needling on post-stroke spasticity, motor function and stability limits: a randomised clinical trial. Acupuncture Med J Br Med Acupuncture Soc. (2018) 36:358–66. doi: 10.1136/acupmed-2017-011568
6.
Pereira S Mehta S McIntyre A Lobo L Teasell RW . Functional electrical stimulation for improving gait in persons with chronic stroke. Top Stroke Rehabil. (2012) 19:491–8. doi: 10.1310/tsr1906-491
7.
Haruyama K Kawakami M Otsuka T . Effect of Core stability training on trunk function, standing balance, and mobility in stroke patients. Neurorehabil Neural Repair. (2017) 31:240–9. doi: 10.1177/1545968316675431
8.
Chae SH Kim YL Lee SM . Effects of phase proprioceptive training on balance in patients with chronic stroke. J Phys Ther Sci. (2017) 29:839–44. doi: 10.1589/jpts.29.839
9.
Xia W Wang J Zheng C . Effectiveness of anodal transcranial direct current stimulation in stroke patients with upper motor dysfunction: a Meta-analysis. Chin J Rehab. (2015) 30:257–61. doi: 10.3870/zgkf.2015.04.005
10.
Li LL Wu JJ Li KP Jin J Xiang YT Hua XY et al . Comparative efficacy of different noninvasive brain stimulation protocols on upper-extremity motor function and activities of daily living after stroke: a systematic review and network meta-analysis. Neurol Sci Off J Italian Neurol Soc. (2024) 45:3641–81. doi: 10.1007/s10072-024-07437-4
11.
Lefaucheur JP . Methods of therapeutic cortical stimulation. Clin Neurophysiol. (2009) 39:1–14. doi: 10.1016/j.neucli.2008.11.001
12.
Bornheim S Croisier JL Maquet P Kaux JF . Transcranial direct current stimulation associated with physical-therapy in acute stroke patients - a randomized, triple blind, sham-controlled study. Brain Stimul. (2020) 13:329–36. doi: 10.1016/j.brs.2019.10.019
13.
Lindenberg R Renga V Zhu LL Nair D Schlaug G . Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. (2010) 75:2176–84. doi: 10.1212/WNL.0b013e318202013a
14.
Cha HK Ji SG Kim MK Chang JS . Effect of transcranial direct current stimulation of function in patients with stroke. J Phys Ther Sci. (2014) 26:363–5. doi: 10.1589/jpts.26.363
15.
Tedla JS Rodrigues E Ferreira AS Vicente J Reddy RS Gular K et al . Transcranial direct current stimulation combined with trunk-targeted, proprioceptive neuromuscular facilitation in subacute stroke: a randomized controlled trial. PeerJ. (2022) 10:e13329. doi: 10.7717/peerj.13329
16.
Cui C Wang P Shao Y Liu J . Effects of transcranial direct current stimulation combined with eye acupuncture exercise therapy on recovery of limb motor dysfunction after stroke. J Liaoning Univ TCM. (2021) 23:123–6. doi: 10.13194/j.issn.1673-842x.2021.05.028
17.
Cipriani A Higgins JP Geddes JR Salanti G . Conceptual and technical challenges in network meta-analysis. Ann Intern Med. (2013) 159:130–7. doi: 10.7326/0003-4819-159-2-201307160-00008
18.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
19.
Che X Cheng J Jiang D Li H Yuan H Ma H et al . Effects of mental practice combined with transcranial direct current stimulation on the function of the upper limbs in hemiplegic patients with stroke. J Navy Med. (2017) 38:303–6. doi: 10.3969/j.issn.1009-0754.2017.04.008
20.
Chen H Zheng X Lv X Ding L He L Liu C et al . Effects of tDCS synchronized FES on lower limb motor function of stroke patients with hemiplegia:a randomized controlled study. Chin J Rehab Med. (2021) 36:1227–32. doi: 10.3969/j.issn.1001-1242.2021.10.006
21.
Chen H Cai Q Xu L Yang X Song P Liu J et al . Effects of transcranial direct current stimulation and Mirror therapy on upper limb motor function for patients after stroke. Chin J Rehabil Theory Pract. (2020) 26:301–5. doi: 10.3969/j.issn.1006-9771.2020.03.007
22.
Chen T Xu D Sun F Lai J Zeng D . Effect of transcranial direct current stimulation combined with contralateral control functional electrical stimulation on upper limb function of stroke patients. Chin J Rehabil Theory Pract. (2023) 29:527–32. doi: 10.3969/j.issn.1006-9771.2023.05.006
23.
Chen Y Sun Y Zhang Q . Analysis of the therapeutic effect of transcranial direct current stimulation combined with mirror neuron rehabilitation training on post-stroke hemiplegia patients. Modern Med Health Res. (2022) 6:80–3.
24.
Cheng P Jin H Zheng J Liu Z . Clinical research on transcranial direct current stimulation and electrical acupuncture therapy for upper limb spasticity after stroke. Chin Arch Tradit Chin Med. (2015) 33:1994–7. doi: 10.13193/j.issn.1673-7717.2015.08.061
25.
Cheng X Zhang L Liu W Liu L Yang Y Gao R et al . Effect of transcranial direct current stimulation on upper limb motor function in chronic-phase stroke patients with hemiplegia. Chin Med Equip J. (2024) 45:67–73. doi: 10.19745/j.1003-8868.2024032
26.
Deng R . Application effect of transcranial direct current stimulation combined with personalized exercise rehabilitation prescription in post-stroke SHS patients. Chronic Pathematol J. (2021) 22:79–83. doi: 10.16440/j.cnki.1674-8166.2021.01.028
27.
Dong K . The effects of transcranial direct current stimulation on the recovery of balance function after stroke [master’s thesis]. Shanxi: Shanxi Medical University (2021).
28.
Feng C Pu Z Ke Z . Rehabilition effects of transcranial direct current stimulation combined with modified induced movement therapy in stroke patients. Chin J Rehab. (2023) 38:470–4. doi: 10.3870/zgkf.2023.08.005
29.
Gao L Chu F Jia F Zhang J Zhang M . Effect of brain-computer interface based on visual, auditory and motor feedback combined with transcranial direct current stimulation on upper limb function in stroke patients. Chin J Rehabil Theory Pract. (2024) 30:202–9. doi: 10.3969/j.issn.1006-9771.2024.02.010
30.
Gao Z Yang T Wang X Xu L Li X . Combining motor imagery training with transcranial direct current stimulation improves the upper limb functioning of hemiplegic stroke surviors. Chin J Phys Med Rehabil. (2021) 43:611–4. doi: 10.3760/cma.j.issn.0254-1424.2021.07.007
31.
Gao Z Zhang H Yang T Wang H Fan J . Effect of transcranial direct current stimulation combined with mirror therapy for the treatment of patients with upper limb motor dysfunction after cerebral stroke. Guangxi Med J. (2023) 45:2191–5. doi: 10.11675/j.issn.0253-4304.2023.18.05
32.
Han X Li X Song G . Effects of transcranial direct current stimulation on upper limb motor function and somatosensory evoked potential in stroke patients. Chin J Rehab. (2023) 38:272–6. doi: 10.3870/zgkf.2023.05.004
33.
Hu H Yao F Chen J Liu M Guo H Chen Y et al . Clinical study of transcranial direct current combined with rehabilitation training on post-stroke dysfunction. Med Innov China. (2023) 20:49–53. doi: 10.3969/j.issn.1674-4985.2023.03.012
34.
Huang Y Tan Q Shi Z . The effects of limb rehabilitation therapy combined with transcranial direct current stimulation on muscle activity in patients with upper limb dysfunction after cerebral infarction. Med Equipment. (2023) 36:59–73. doi: 10.3969/j.issn.1002-2376.2023.19.022
35.
Jiang Y . Observation of the therapeutic effect of transcranial direct current stimulation on hand dysfunction after stroke [master’s thesis]. Hohhot: Inner Mongolia Medical University (2020).
36.
Jin J Jiang S Pan X Jiang X Shi J . The effect of transcranial direct current stimulation combined with rehabilitation training on cognitive function and limb motor function in stroke patients with hemiplegia. Chin J Phys Med Rehabil. (2019) 41:653–60. doi: 10.3760/cma.j.issn.0254-1424.2019.06.004
37.
Jin M Wang J Liu F Shao Q . Effects of transcranial direct current stimulation combined with Walker view walking training system on walking function in early stroke patients. J Hubei Univ Med. (2020) 39:244–8. doi: 10.13819/j.issn.2096-708X.2020.03.009
38.
Li X . Effects of mirror neuron motor imitation training combined with transcranial direct current stimulation on upper limb function and activities of daily living in hemiplegic patients after stroke. J Xinjiang Med Univ. (2021) 44:713–7. doi: 10.3639/j.issn.1009-5551.2021.06.013
39.
Li Y Feng H Wang H Li J Chen N Yang J et al . Effect of transcranial direct current stimulation combined with mirror neurous rehabilitation training system on upper extremity motor function and somatosensory evoked potentials after stroke. Chin J Rehab. (2019) 34:187–90. doi: 10.3870/zgkf.2019.04.005
40.
Liu L Du W Liu A . Effect of transcranial direct current stimulation on stroke patients. Chin J Prac Nurs. (2019) 35:1537–40. doi: 10.3760/cma.j.issn.1672_7088.2019.20.004
41.
Liu Y Qian S Sun W Liu Y . Effects of transcranial direct current stimulation combined with mirror therapy on functional recovery of upper limbs and hands after stroke. J Harbin Med Univ. (2023) 57:575–9. doi: 10.20010/j.issn.1000-1905.2023.05.0575
42.
Liu Y Huang L Zhang S Ai Y Li L Hu X . Effects of transcranial direct current stimulation combined with virtual reality training on upper limb function of stroke patient: a pilot randomized controlled single-blind trial. West China Med J. (2020) 35:544–9. doi: 10.7507/1002-0179.202003112
43.
Long S Ye J Wang G . Effects of left dorsolateral prefrontal anode transcranial direct current stimulation combined with rehabilitation training on mental state and motor function in patients with limb movement disorders after stroke journal of international. Psychiatry. (2024) 51:1230–6. doi: 10.13479/j.cnki.jip.2024.04.041
44.
Pan A Chen Y Chen J Zhou Y Liu Y Chen R . The effect of CIMT and tDCS combined therapy on upper limb motor function and spasticity in elderly stroke patients. Chin J Gerontol. (2023) 43:5513–6. doi: 10.3969/j.issn.1005-9202.2023.22.038
45.
Qi Y Xie S . Effect of Neurorehabilitation manipulator combined with transcranial direct current stimulation on the recovery of nerve function and limb function in hemiplegic patients after stroke. Reflexol Rehab Med. (2023) 4:66–9. doi: 10.3969/j.issn.2096-7950.2023.5.fshlfykfyx202305018
46.
Qu F He M Peng Q Gong Y Zhang W Luo Y et al . Observation of the therapeutic effect of Tongdu Tiaoshen acupuncture combined with transcranial direct current stimulation on upper limb dysfunction after ischemic stroke. J Shanxi Health Voc College. (2024) 34:73–5.
47.
Ren S Cheng K Xu L Zhou M Gao M . Effects of transcranial direct current stimulation combined with motor imagery training on upper limb motor function and cognitive function in patients with hemiplegia after stroke. Pract Geriatr. (2023) 37:449–53. doi: 10.3969/j.issn.1003-9198.2023.05.005
48.
Song D Yu H . Effect of Cathodic transcranial direct current stimulation on acute phase stroke and its influence on neurological function. Med Innov China. (2024) 21:47–51. doi: 10.3969/j.issn.1674-4985.2024.10.011
49.
Sun F Zhang X Jin Z Chen T . Effect of anodal transcranial direct current stimulation on premotor cortex on upper limb motor function in patients with severe stroke. Chin J Rehabil Theory Pract. (2023) 29:1333–8. doi: 10.3969/j.issn.1006-9771.2023.11.011
50.
Tu M Xiang S Wang H . The effect of rehabilitation training combined with anodic transcranial direct current stimulation on the daily life of patients in the recovery period after cerebral infarction. J Chin Phys. (2021) 23:582–5. doi: 10.3760/cma.j.cn431274-20200303-00229
51.
Wang C Liu D Wu W . Analysis of the effect of tDCS therapy on post-stroke hemiplegic patients and its impact on sensory function. J Med Theor Prac. (2021) 34:2533–4. doi: 10.19381/j.issn.1001-7585.2021.14.079
52.
Wang C . Effect of brain computer interface combined with transcranial direct current stimulation on hand motor dysfunction in cerebral ischemic stroke [master’s thesis]. Qinghai: Qinghai University (2023).
53.
Wang H Tao Y Li J Sun J Xie T Chou H et al . Effect of transcranial direct current stimulation combined with constraint-induced weight training of the affected lower limb on pusher syndrome after stroke. Chin J Rehabil Theory Pract. (2023) 29:269–74. doi: 10.3969/j.issn.1006-9771.2023.03.004
54.
Wang H Wang Y Zhou X He A . Effect of transcranial direct current stimulation combined with acupuncture on central and upper limb function in stroke patients based on central-peripheral-central theory. Chin J Rehabil Theory Pract. (2023) 29:919–25. doi: 10.3969/j.issn.1006-9771.2023.08.008
55.
Wang Y Zhang Y Wang C Sun C Ma L Xue X et al . Clinical effect of transcranial direct current stimulation combined with acupuncture for upper limb dysfunction after stroke. Chin J Rehab. (2021) 36:131–4. doi: 10.3870/zgkf.2021.03.001
56.
Yin Y Zuo X Lv Y Jia Z Zhao Z Huai Y et al . Effects of transcranial direct current stimulation on motor function of upper limbs in stroke patients. Chin J Rehabil Theory Pract. (2015) 21:830–3. doi: 10.3969/j.issn.1006-9771.2015.07.020
57.
Zhang S Shen J . Clinical randomized controlled study of acupuncture combined with transcranial direct current stimulation on rehabilitation of cognitive impairment after stroke. Lab Med Clin. (2022) 19:884–9. doi: 10.3969/j.issn.1672-9455.2022.07.006
58.
Zhang Y Wang J . Effects of transcranial direct current electrical stimulation combined with upper limb robots on upper limb motor function and surface electromyogram signal in patients with cerebral infarction during convalescence. J Hebei Med Univ. (2019) 40:561–4. doi: 10.3969/j.issn.1007-3205.2019.05.016
59.
Wang W Song W Zhang Y Qu S Yan L Zhang D . Effect of transcranial direct current stimulation combined with sensory training on somatosensory recovery and hand function after stroke. Chin J Cerebrovasc Dis. (2021) 18:289–95. doi: 10.3969/j.issn.1672-5921.2021.05.001
60.
Zhao F Tan J Bao X Liu H . Effect of transcranial direct-current stimulation plus virtual reality on the recovery of upper limb function in patients with cerebral infarction. J Navy Med. (2021) 42:466–70. doi: 10.3969/j.issn.1009-0754.2021.04.023
61.
Zhao J Shi M Fan W Chen B Jin L . Rehabilitation effect of transcranial direct current stimulation on increased muscle tone after stroke and brain network mechanism. Clinical education of. Gen Pract. (2023) 21:380–407. doi: 10.13558/j.cnki.issn1672-3686.2023.005.007
62.
Zheng C Xia W Duan C Li Z Zhang X Wang J et al . Effect of transcranial direct current stimulation on upper limb and hand function in stroke: a double-blind randomized controlled trail. Chin J Rehab. (2019) 34:623–6. doi: 10.3870/zgkf.2019.12.002
63.
Zheng S Peng L Mu J . Effect of transcranial direct current stimulation combined with staging acupuncture on upper limb motor function in stroke patients with hemiplegia. China Medical Herald. (2020) 17:86–9. doi: 10.20047/j.issn1673-7210.2020.10.022
64.
Zhou Y Zhang Y Wang G Liu Y Hu S . Effects of transcranial direct current stimulation combined with motor imagery therapy on upper limbs function of stroke surviors. Chin J Phys Med Rehabil. (2018) 40:657–61. doi: 10.3760/cma.j.issn.0254-1424.2018.09.005
65.
Alisar DC Ozen S Sozay S . Effects of Bihemispheric transcranial direct current stimulation on upper extremity function in stroke patients: a randomized double-blind sham-controlled study. J Stroke Cerebrovasc Dis. (2020) 29:104454. doi: 10.1016/j.jstrokecerebrovasdis.2019.104454
66.
Cho HS Cha HG . Effect of mirror therapy with tDCS on functional recovery of the upper extremity of stroke patients. J Phys Ther Sci. (2015) 27:1045–7. doi: 10.1589/jpts.27.1045
67.
Zeng Y Cheng R Zhang L Fang S Zhang S Wang M et al . Clinical comparison between HD-tDCS and tDCS for improving upper limb motor function: a randomized, double-blinded. Sham-Controlled Trial Neural Plasticity. (2024) 2024:1–10. doi: 10.1155/2024/2512796
68.
Gong Q Yan R Chen H Duan X Wu X Zhang X et al . Effects of cerebellar transcranial direct current stimulation on rehabilitation of upper limb motor function after stroke. Front Neurol. (2023) 14:1044333. doi: 10.3389/fneur.2023.1044333
69.
Lee SJ Chun MH . Combination transcranial direct current stimulation and virtual reality therapy for upper extremity training in patients with subacute stroke. Arch Phys Med Rehabil. (2014) 95:431–8. doi: 10.1016/j.apmr.2013.10.027
70.
Hsu SP Lu CF Lin BF Tang CW Kuo IJ Tsai YA et al . Effects of bihemispheric transcranial direct current stimulation on motor recovery in subacute stroke patients: a double-blind, randomized sham-controlled trial. J Neuroeng Rehabil. (2023) 20:27. doi: 10.1186/s12984-023-01153-4
71.
Rabadi MH Aston CE . Effect of transcranial direct current stimulation on severely affected arm-hand motor function in patients after an acute ischemic stroke: a pilot randomized control trial. Am J Phys Med Rehabil. (2017) 96:S178–84. doi: 10.1097/PHM.0000000000000823
72.
Qurat Ul A Ahmad Z Ilyas S Ishtiaq S Tariq I Nawaz Malik A et al . Comparison of a single session of tDCS on cerebellum vs. motor cortex in stroke patients: a randomized sham-controlled trial. Ann Med. (2023) 55:2252439. doi: 10.1080/07853890.2023.2252439
73.
Li C Chen Y Tu S Lin J Lin Y Xu S et al . Dual-tDCS combined with sensorimotor training promotes upper limb function in subacute stroke patients: a randomized, double-blinded, sham-controlled study. CNS Neurosci Ther. (2024) 30:e14530. doi: 10.1111/cns.14530
74.
Llorens R Fuentes MA Borrego A Latorre J Alcañiz M Colomer C et al . Effectiveness of a combined transcranial direct current stimulation and virtual reality-based intervention on upper limb function in chronic individuals post-stroke with persistent severe hemiparesis: a randomized controlled trial. J Neuroeng Rehabil. (2021) 18:108. doi: 10.1186/s12984-021-00896-2
75.
Chang MC Kim DY Park DH . Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimul. (2015) 8:561–6. doi: 10.1016/j.brs.2015.01.411
76.
Duan Q Liu W Yang J Huang B Shen J . Effect of Cathodal transcranial direct current stimulation for lower limb subacute stroke rehabilitation. Neural Plast. (2023) 2023:1–10. doi: 10.1155/2023/1863686
77.
Youssef H Mohamed NAE Hamdy M . Comparison of bihemispheric and unihemispheric M1 transcranial direct current stimulations during physical therapy in subacute stroke patients: a randomized controlled trial. Clin Neurophysiol. (2023) 53:102895. doi: 10.1016/j.neucli.2023.102895
78.
Toktas N Duruturk N Güzel Ş Yürük Ö Özen S . The effect of transcranial direct current stimulation on balance, gait function and quality of life in patients with stroke. Neurol Res. (2024) 46:868–75. doi: 10.1080/01616412.2024.2362583
79.
Allman C Amadi U Winkler AM Wilkins L Filippini N Kischka U et al . Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med. (2016) 8:330re1. doi: 10.1126/scitranslmed.aad5651
80.
Nair DG Renga V Lindenberg R Zhu L Schlaug G . Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. (2011) 29:411–20. doi: 10.3233/RNN-2011-0612
81.
Fusco A Assenza F Iosa M Izzo S Altavilla R Paolucci S et al . The ineffective role of cathodal tDCS in enhancing the functional motor outcomes in early phase of stroke rehabilitation: an experimental trial. Biomed Res Int. (2014) 2014:547290:1–9. doi: 10.1155/2014/547290
82.
Kim DY Lim JY Kang EK You DS Oh MK Oh BM et al . Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. (2010) 89:879–86. doi: 10.1097/PHM.0b013e3181f70aa7
83.
Oveisgharan S Organji H Ghorbani A . Enhancement of motor recovery through left dorsolateral prefrontal cortex stimulation after acute ischemic stroke. J Stroke Cerebrovasc Dis. (2018) 27:185–91. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.026
84.
Pinto EF Gupta A Kulkarni GB Andrade C . A randomized, double-blind, sham-controlled study of transcranial direct current stimulation as an augmentation intervention for the attenuation of motor deficits in patients with stroke. J ECT. (2021) 37:281–90. doi: 10.1097/YCT.0000000000000769
85.
Prathum T Piriyaprasarth P Aneksan B Hiengkaew V Pankhaew T Vachalathiti R et al . Effects of home-based dual-hemispheric transcranial direct current stimulation combined with exercise on upper and lower limb motor performance in patients with chronic stroke. Disabil Rehabil. (2022) 44:3868–79. doi: 10.1080/09638288.2021.1891464
86.
Rossi C Sallustio F Di Legge S Stanzione P Koch G . Transcranial direct current stimulation of the affected hemisphere does not accelerate recovery of acute stroke patients. Eur J Neurol. (2013) 20:202–4. doi: 10.1111/j.1468-1331.2012.03703.x
87.
Di Lazzaro V Dileone M Capone F Pellegrino G Ranieri F Musumeci G et al . Immediate and late modulation of interhemipheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimul. (2014) 7:841–8. doi: 10.1016/j.brs.2014.10.001
88.
Kim HM Na JM Jo HS Kim KH Song MK Park HK et al . Feasibility of simultaneous anodal transcranial direct current stimulation during gait training in chronic stroke patients: a randomized double-blind pilot clinical trial. J Integr Neurosci. (2024) 23:154. doi: 10.31083/j.jin2308154
89.
Hu YQ Gao TH Li J Tao JC Bai YL Lu RR . Motor imagery-based brain-computer Interface combined with multimodal feedback to promote upper limb motor function after stroke: a preliminary study. eCAM. (2021) 2021:1116126
90.
Sinha AM Nair VA Prabhakaran V . Brain-computer Interface training with functional electrical stimulation: facilitating changes in interhemispheric functional connectivity and motor outcomes post-stroke. Front Neurosci. (2021) 15:670953. doi: 10.3389/fnins.2021.670953
91.
Wang A Tian X Jiang D Yang C Xu Q Zhang Y et al . Rehabilitation with brain-computer interface and upper limb motor function in ischemic stroke: a randomized controlled trial. Med. (2024) 5:559–69. doi: 10.1016/j.medj.2024.02.014
92.
Nojima I Sugata H Takeuchi H Mima T . Brain-computer Interface training based on brain activity can induce motor recovery in patients with stroke: a Meta-analysis. Neurorehabil Neural Repair. (2022) 36:83–96. doi: 10.1177/15459683211062895
93.
Li L Yu Y Jia Y Huang H . Effect of brain-computer Interface on upper-limb motor function after stroke: a Meta-analysis. Chin J Rehabil Theory Pract. (2021) 27:765–73. doi: 10.3969/j.issn.1006-9771.2021.07.005
94.
Colucci A Vermehren M Cavallo A Angerhöfer C Peekhaus N Zollo L et al . Brain-computer Interface-controlled exoskeletons in clinical Neurorehabilitation: ready or not?Neurorehabil Neural Repair. (2022) 36:747–56. doi: 10.1177/15459683221138751
95.
Bouton CE . Merging brain-computer interface and functional electrical stimulation technologies for movement restoration. Handb Clin Neurol. (2020) 168:303–9. doi: 10.1016/B978-0-444-63934-9.00022-6
96.
Chew E Teo WP Tang N Ang KK Ng YS Zhou JH et al . Using transcranial direct current stimulation to augment the effect of motor imagery-assisted brain-computer Interface training in chronic stroke patients-cortical reorganization considerations. Front Neurol. (2020) 11:948. doi: 10.3389/fneur.2020.00948
97.
Pires R Baltar A Sanchez MP Antonino GB Brito R Berenguer-Rocha M et al . Do higher transcranial direct current stimulation doses Lead to greater gains in upper limb motor function in post-stroke patients?Int J Environ Res Public Health. (2023) 20:1279. doi: 10.3390/ijerph20021279
98.
Liu X Zhang W Li W Zhang S Lv P Yin Y . Effects of motor imagery based brain-computer interface on upper limb function and attention in stroke patients with hemiplegia: a randomized controlled trial. BMC Neurol. (2023) 23:136. doi: 10.1186/s12883-023-03150-5
99.
Ramachandran VS Rogers-Ramachandran D . Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. (1996) 263:377–86. doi: 10.1098/rspb.1996.0058
100.
Wen X Li L Li X Zha H Liu Z Peng Y et al . Therapeutic role of additional Mirror therapy on the recovery of upper extremity motor function after stroke: a single-blind, randomized controlled trial. Neural Plast. (2022) 2022:1–9. doi: 10.1155/2022/8966920
101.
Garrison KA Winstein CJ Aziz-Zadeh L . The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabil Neural Repair. (2010) 24:404–12. doi: 10.1177/1545968309354536
102.
Michielsen ME Smits M Ribbers GM Stam HJ van der Geest JN Bussmann JB et al . The neuronal correlates of mirror therapy: an fMRI study on mirror induced visual illusions in patients with stroke. J Neurol Neurosurg Psychiatry. (2011) 82:393–8. doi: 10.1136/jnnp.2009.194134
103.
McCombe Waller S Whitall J Jenkins T Magder LS Hanley DF Goldberg A et al . Sequencing bilateral and unilateral task-oriented training versus task oriented training alone to improve arm function in individuals with chronic stroke. BMC Neurol. (2014) 14:236. doi: 10.1186/s12883-014-0236-6
104.
Neva JL Vesia M Singh AM Staines WR . Modulation of left primary motor cortex excitability after bimanual training and intermittent theta burst stimulation to left dorsal premotor cortex. Behav Brain Res. (2014) 261:289–96. doi: 10.1016/j.bbr.2013.12.029
105.
Romero Lauro LJ Rosanova M Mattavelli G Convento S Pisoni A Opitz A et al . TDCS increases cortical excitability: direct evidence from TMS-EEG. Cortex J Devoted Stu Nerv Syst Behav. (2014) 58:99–111. doi: 10.1016/j.cortex.2014.05.003
106.
Chen L Guo X Tao J Zhang B . Effects of acupuncture and muscle strength training on walking function in patients for hemiplegia post stroke. Chin J Rehab Med. (2006) 2:136–9. doi: 10.3969/j.issn.1001-1242.2006.02.012
Summary
Keywords
stroke, network meta-analysis, motor function, upper limb, transcranial direct current stimulation
Citation
Zheng K, Guo L, Liang W and Liu P (2025) Comparison of the effects of transcranial direct current stimulation combined with different rehabilitation interventions on motor function in people suffering from stroke-related symptoms: a systematic review and network meta-analysis. Front. Neurol. 16:1586685. doi: 10.3389/fneur.2025.1586685
Received
04 March 2025
Accepted
05 May 2025
Published
04 June 2025
Volume
16 - 2025
Edited by
Qi Wan, Shenzhen University of Advanced Technology, China
Reviewed by
Juan Chen, Huazhong University of Science and Technology, China
Ling Guan, University of British Columbia, Canada
Updates
Copyright
© 2025 Zheng, Guo, Liang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Guo, liangguo@m.scnu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.