Abstract
Background:
Inflammation plays a crucial role in the onset and progression of epilepsy. However, there is limited information regarding the relationship between diet-related inflammation and epilepsy. This study aimed to investigate the association between dietary inflammatory index (DII) and epilepsy.
Methods:
We conducted a cross-sectional analysis using data from the National Health and Nutrition Examination Survey (NHANES) 2013–2020. The DII scores were calculated and categorized into quartiles. Logistic regression was applied to assess the association between DII and epilepsy. Additionally, restricted cubic spline (RCS) analysis and subgroup analyses were performed.
Results:
The study included a total of 10,761 participants. After adjusting for age, gender, race, body mass index (BMI), smoking status, alcohol consumption, stroke, diabetes, and hypertension, a significant positive association was observed between DII and epilepsy in multivariable logistic regression (quartile 4 vs. 1, OR = 2.66, 95% CI 1.66–4.28, p < 0.001). The RCS analysis further confirmed a positive linear relationship between increasing DII scores and epilepsy risk (p for overall = 0.0007, p for nonlinear = 0.5128). Subgroup analyses showed a consistent association between DII and epilepsy across different subgroups.
Conclusion:
Elevated DII scores are associated with the risk of epilepsy. To improve epilepsy prevention and management, attention to dietary inflammation regulation is essential.
1 Introduction
Epilepsy is one of the most prevalent chronic neurological disorders and a significant cause of disability and mortality. With a global prevalence of 0.5–1% and a lifetime incidence of 1–3%, epilepsy affects nearly 70 million individuals worldwide (1–3). Existing therapies are predominantly based on pharmacological interventions; however, the majority of antiepileptic medications are insufficient in preventing seizures and protecting the brain (4), highlighting the pressing requirement for enhanced preventative and curative strategies.
More and more clinical and experimental evidence reveals that inflammation may play a critical role in the pathophysiology of seizure and epilepsy (5–7). Elevated levels of systemic inflammatory biomarkers, including interleukins (ILs), tumor necrosis factor (TNF), interferon (IFN), and procalcitonin (PCT), have been identified in patients with epilepsy (8–14). Systemic inflammation can lead to the disruption of the blood–brain barrier (BBB), thereby allowing peripheral toxic molecules and cytokine-producing immune cells to infiltrate, which promotes the occurrence of epilepsy (5, 15). Multiple researches have supported that antagonizing peripheral inflammation can reduce the severity of epilepsy, providing new strategies for the prevention and treatment of the condition (15–17).
Diet serves as an essential factor in modulating systemic inflammation within the body. Numerous studies have demonstrated that various nutrients, foods, and non-nutrient food components can regulate inflammation both acutely and chronically (18–20). Highly processed foods, refined grains, foods rich in saturated fatty acids and sodium, simple carbohydrates, and red processed meats are known to be pro-inflammatory. In contrast, vegetables, fruits, whole grains, legumes, low-fat dairy, fish, and foods rich in antioxidants (omega-3 fatty acids, flavonoids) exhibit anti-inflammatory properties (21–23). We propose that dietary interventions capable of modulating systemic inflammation may have a preventative effect on epilepsy.
Clearly, people’s diets are often diverse, rather than consisting of isolated intake of individual foods or food constituents. The inflammatory properties of individual foods are insufficient to assess the inflammation levels across various dietary patterns. Thus, it is essential to assess the dietary inflammatory potential in a comprehensive way. The Dietary Inflammation Index (DII) is a well-validated, reliable, and widely applied nutritional tool that assesses the inflammatory potential of an individual’s diet based on the effects of various dietary components on key inflammatory biomarkers, such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). It has been shown to be associated with systemic inflammation (24). The DII was originally developed by Cavicchia et al. (25) and improved by Shivappa et al. (24). In recent decades, the DII has been evaluated in cancer, diabetes, cardiovascular disease, asthma, neurodevelopment, and mental health disorders (26). However, to our knowledge, the association between DII and epilepsy has not yet been researched. Therefore, in the present study, we utilized the cross-sectional data of the National Health and Nutrition Examination Surveys (NHANES) to explore the relationship between dietary inflammation and epilepsy, with the aim of providing more precise guidance for the prevention strategies of epilepsy.
2 Methods
2.1 Study population and ethics
NHANES, launched by National Center for Health Statistics (NCHS), is an ongoing, nationwide cross-sectional survey that collects health and nutrition information from the U. S. civilian noninstitutional population. All protocols received approval from the NCHS Ethics Review Board (ERB) and were performed in accordance with the Declaration of Helsinki, with all NHANES participants providing signed informed consent (publicly available on the web)1 (27). The cross-sectional data utilized in this study were sourced from the NHANES database, spanning the period from 2013 to March 2020. The data collection for this database was conducted by a team of trained professionals affiliated with the NHANES research initiative. After obtaining these data, we conducted the subsequent statistical analyses independently. Participants missing dietary and prescription medication data were excluded.
2.2 Calculation of the DII
The DII is calculated based on individual dietary components, requiring 45 dietary components in total (24). However, most studies analyze only a subset of these components. Shivappa et al. reported that the DII calculation retains its predictive ability even with fewer than 30 food parameters (28). The DII calculation formula is as follows:
For each dietary component, calculate the Z-score of individual intake:
Convert the Z-score to a percentile score, which is then standardized to a range between −1 and 1:
Multiply the standardized percentile score by the inflammatory effect score for each component, and then sum the scores for all components to obtain the individual’s overall DII score:
A lower DII score indicates a more anti-inflammatory diet, while a higher DII score indicates a more pro-inflammatory diet (24).
Due to the limitations of NHANES data collection, this study, following the approach of other literature, used 28 dietary components for DII calculation (29–31). These components are protein, energy, carbohydrates, dietary fiber, total fat, saturated fat, monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), n-3 fatty acids, n-6 fatty acids, cholesterol, β-carotene, folate, vitamin A, vitamin B1, vitamin B2, niacin, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, magnesium, iron, zinc, selenium, caffeine, and alcohol. Dietary data were collected through 24-h dietary recall interviews by the NHANES Working Group on Nutrition Methods. Two separate dietary recalls were conducted in all participants: the first was a face-to-face interview at the Mobile Examination Center (MEC), and the second was completed via telephone 3 to 10 days later. This approach helps to provide a more comprehensive assessment of each participant’s dietary habits (32). To minimize the potential for recall bias, the dietary data from the two 24-h recalls were averaged.
2.3 Assessment of epilepsy
Epilepsy was defined by NHANES questionnaire data labeled “prescription medications.” In the study, participants who self-reported that their main reason for taking prescription medication in the past 30 days was “epilepsy and recurrent seizures” (International Classification of Disease, Tenth Revision, Clinical Modification [ICD-10-CM] Code: G40) were classified as having epilepsy (33, 34).
2.4 Covariates
Covariates were selected based on prior literature and biological plausibility. The demographic and questionnaire data were obtained through standardized questionnaires and face-to-face interviews, including gender (male, female), age (≤18, >18 years), race (Mexican American, non-Hispanic White, non-Hispanic Black, and other races), alcohol consumption, smoking status, and histories of stroke, diabetes, and hypertension. Based on total weekly alcohol consumption, Alcohol consumption was categorized based on total weekly intake as none, normal (1–14 drinks/week for males and 1–7 drinks/week for females), and heavy (≥15 drinks/week for males and ≥8 drinks/week for females). Smoking status was categorized as never, former, and current, with participants who had smoked at least 100 cigarettes in their lifetime defined as smokers. Physical examination was conducted by experienced medical staff in the MEC. Body mass index (BMI) data, calculated as weight (kg) divided by the square of height (m2), were used to estimate overweight/obesity status. Histories of stroke, diabetes, and hypertension can be defined based on self-reported previous diagnoses by a physician.
2.5 Handling of missing variables
To maximize the sample size and minimize bias from missing covariate data, we employed the multiple-imputation method for participants with incomplete covariate information. Missing values were imputed using chained equations with a 20-fold multiple imputation method. Supplementary Figure S1 presents the distribution of variables with missing data in our study.
2.6 Statistical analysis
We divided the study participants into four groups based on quartiles of the DII scores (Q1 to Q4) and compared differences in baseline characteristics across these quartiles. Categorical variables were presented as frequencies and percentages, whereas continuous variables were expressed as mean ± standard deviation (SD). Comparison of categorical and continuous variables were performed using the Pearson chi-squared test and Student’s t-test, respectively.
The association between DII and epilepsy was examined using logistic regression models, with odds ratios (OR) and 95% confidence intervals (95% CI) reported. In Model 1, no covariate was adjusted for; Model 2 was adjusted for age, gender, and race; Model 3 further included BMI, smoking status, alcohol consumption, stroke, diabetes, and hypertension. The first quartile (Q1) was designated as the reference group. Additionally, multivariate-adjusted (fully adjusted) restricted cubic spline (RCS) logistic regression analyses (choosing 4 knots, 5th, 35th, 65th, and 95th percentiles, respectively) were also conducted to examine the linear and dose–response associations between DII and epilepsy.
Furthermore, we selected covariates including age, gender, race, BMI, smoking status, alcohol consumption, stroke, diabetes, and hypertension for subgroup analyses to evaluate whether these covariates significantly interacted with the association between DII and epilepsy.
Finally, a sensitivity analysis was conducted, excluding participants with missing values for any variable, to verify the robustness of the results.
R software version 4.3.32 was used for all statistical analyses. A p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of the study participants
In this study, cross-sectional data of 35,706 participants from NHANES (2013-March 2020) were initially included. After manual data filtration, a total of 10,761 eligible participants were finally included in our analysis (Figure 1).
Figure 1

Flow diagram of study participants.
The baseline characteristics of all participants, classified into four groups by DII quartiles, are shown in Table 1. Compared with participants in the lowest quartile group (Q1), those with higher DII scores were more likely to be female, younger (≤18 years old), non-Hispanic black, current smokers, non-alcohol consumers, and have higher BMI, as well as a history of stroke and hypertension.
Table 1
| Characteristics | Quartiles of DII | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-value | |
| n | 2,691 | 2,689 | 2,691 | 2,690 | |
| Gender, n (%) | <0.001 | ||||
| Female | 1,182 (43.9) | 1,337 (49.7) | 1,563 (58.1) | 1841 (68.4) | |
| Male | 1,509 (56.1) | 1,352 (50.3) | 1,128 (41.9) | 849 (31.6) | |
| Age, n (%) | <0.001 | ||||
| ≤18 years | 203 (7.5) | 385 (14.3) | 492 (18.3) | 525 (19.5) | |
| >18 years | 2,488 (92.5) | 2,304 (85.7) | 2,199 (81.7) | 2,165 (80.5) | |
| Race, n (%) | <0.001 | ||||
| Mexican American | 332 (12.3) | 299 (11.1) | 300 (11.1) | 285 (10.6) | |
| Non−Hispanic Black | 505 (18.8) | 590 (21.9) | 710 (26.4) | 753 (28.0) | |
| Non−Hispanic White | 1,227 (45.6) | 1,203 (44.7) | 1,147 (42.6) | 1,120 (41.6) | |
| Others | 627 (23.3) | 597 (22.2) | 534 (19.8) | 532 (19.8) | |
| BMI, mean (SD) | 29.09 (7.44) | 29.05 (7.94) | 29.25 (8.37) | 29.67 (8.82) | 0.022 |
| Smoking status, n (%) | <0.001 | ||||
| Never | 1,441 (57.6) | 1,262 (54.0) | 1,213 (54.5) | 1,129 (51.3) | |
| Former | 802 (32.1) | 728 (31.1) | 601 (27.0) | 564 (25.6) | |
| Current | 257 (10.3) | 349 (14.9) | 413 (18.5) | 506 (23.0) | |
| Alcohol consumption, n (%) | <0.001 | ||||
| None | 724 (31.0) | 760 (34.5) | 784 (37.1) | 912 (45.1) | |
| Normal | 1,372 (58.7) | 1,258 (57.1) | 1,141 (54.0) | 1,001 (49.5) | |
| Heavy | 241 (10.3) | 186 (8.4) | 188 (8.9) | 109 (5.4) | |
| DII, mean (SD) | −0.94 (0.92) | 0.91 (0.36) | 2.06 (0.30) | 3.31 (0.49) | <0.001 |
| Stroke, n (%) | <0.001 | ||||
| Yes | 113 (4.6) | 139 (6.1) | 133 (6.1) | 206 (9.7) | |
| No | 2,352 (95.4) | 2,151 (93.9) | 2044 (93.9) | 1921 (90.3) | |
| Diabetes, n (%) | 0.522 | ||||
| Yes | 509 (18.9) | 549 (20.4) | 536 (19.9) | 543 (20.2) | |
| No | 2,182 (81.1) | 2,138 (79.6) | 2,152 (80.1) | 2,147 (79.8) | |
| Hypertension, n (%) | <0.001 | ||||
| Yes | 1,310 (51.8) | 1,255 (52.6) | 1,316 (57.3) | 1,278 (56.2) | |
| No | 1,221 (48.2) | 1,131 (47.4) | 981 (42.7) | 994 (43.8) | |
Baseline characteristics of the study participants grouped by DII quartiles.
Results are shown as n (%) for binary variables, and as mean (standard deviation, SD) for continuous variables. BMI, body mass index.
Additionally, we also summarized the baseline characteristics of all individuals based on the presence of epilepsy (Supplementary Table S1).
3.2 Relationship between DII and epilepsy
We constructed three logistic regression models to examine the association between DII and epilepsy, as presented in Table 2. In the non-adjusted model (Model 1), DII quartiles Q2 and Q4 were statistically significantly associated with epilepsy compared to Q1 (Q2: OR = 1.91, 95% CI 1.16–3.14, p = 0.011; Q4: OR = 2.42, 95% CI 1.47–3.96, p < 0.001). These associations remained significant in both Model 2 (Q2: OR = 1.87, 95% CI 1.14–3.07, p = 0.013; Q4: OR = 2.21, 95% CI 1.37–3.58, p = 0.001) and Model 3 (Q2: OR = 1.98, 95% CI 1.21–3.24, p = 0.007; Q4: OR = 2.66, 95% CI 1.66–4.28, p < 0.001). When DII was treated as a continuous variable, a 1 SD increase in DII was significantly associated with epilepsy across all models (Model 1: OR = 1.34, 95% CI 1.13–1.59, p < 0.001; Model 2: OR = 1.29, 95% CI 1.09–1.52, p = 0.003; Model 3: OR = 1.39, 95% CI 1.18–1.64, p < 0.001). In the fully adjusted RCS regression model, we observed a positive linear association between DII and epilepsy (p for overall = 0.0007, p for nonlinear = 0.5128) (Figure 2).
Table 2
| DII | Cases, n (%) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Quartiles | |||||||
| Q1 | 24 (0.89%) | Reference | - | Reference | - | Reference | - |
| Q2 | 47 (1.75%) | 1.91 (1.16, 3.14) | 0.011 | 1.87 (1.14, 3.07) | 0.013 | 1.98 (1.21, 3.24) | 0.007 |
| Q3 | 37 (1.38%) | 1.50 (0.88, 2.54) | 0.13 | 1.43 (0.85, 2.40) | 0.02 | 1.55 (0.92, 2.60) | 0.10 |
| Q4 | 63 (2.34%) | 2.42 (1.47, 3.96) | <0.001 | 2.21 (1.37, 3.58) | 0.001 | 2.66 (1.66, 4.28) | <0.001 |
| p for trend | - | 0.002 | 0.006 | <0.001 | |||
| Continuous | |||||||
| Per 1 SD increase | - | 1.34 (1.13, 1.59) | <0.001 | 1.29 (1.09, 1.52) | 0.003 | 1.39 (1.18, 1.64) | <0.001 |
Logistic regression analysis on the association between DII and epilepsy.
Model 1 was adjusted for none. Model 2 was adjusted for age, gender, and race. Model 3 was adjusted for age, gender, race, BMI, smoking status, alcohol consumption, stroke, diabetes, and hypertension. OR, odds ratio; CI, confidence interval; DII, dietary inflammation index; Q1, 1st quartile; Q2, 2nd quartile; Q3, 3rd quartile; Q4, 4th quartile; SD, standard deviation.
Figure 2
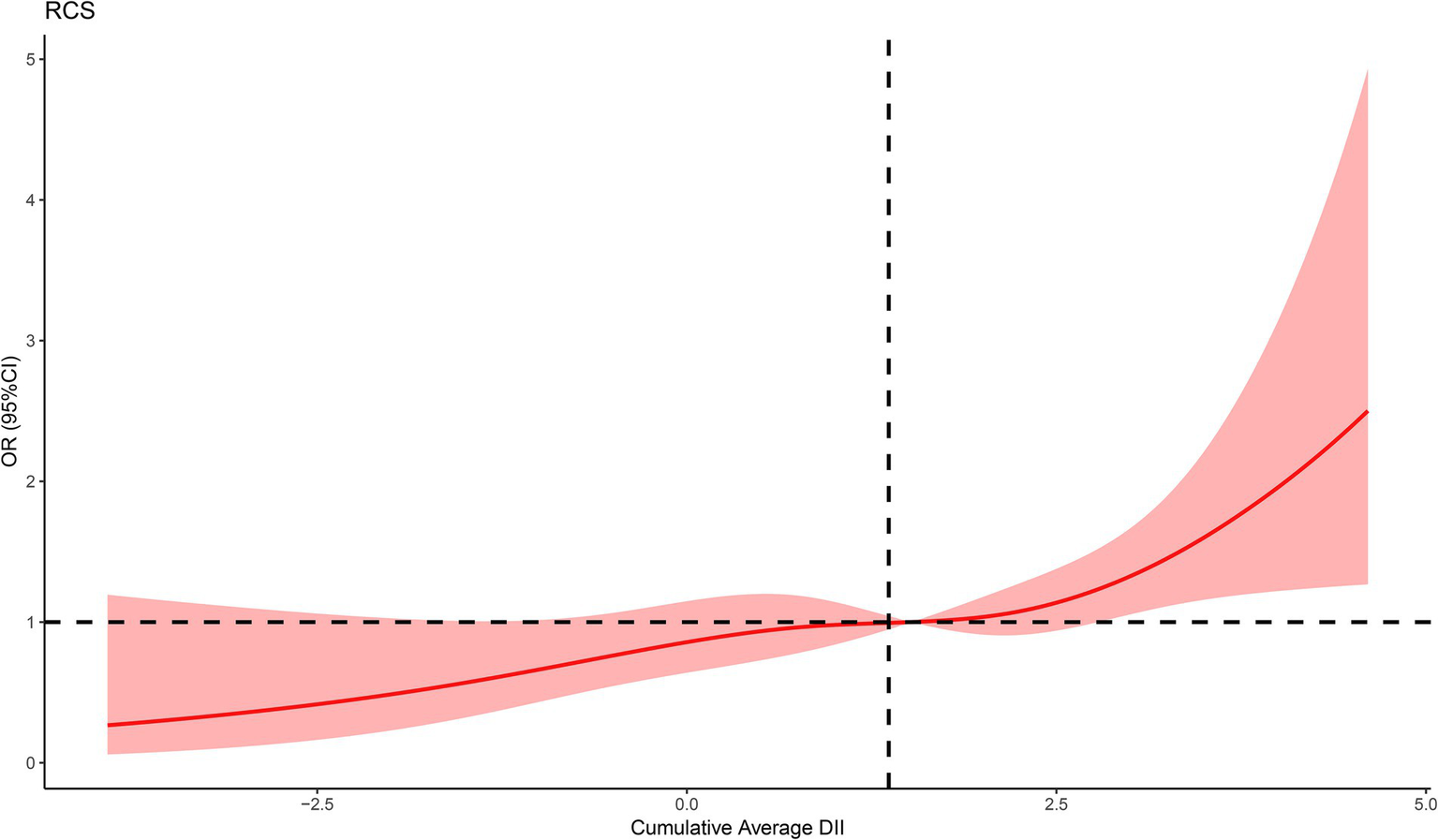
The RCS curve of the association between DII and epilepsy. RCS regression was adjusted for age, gender, race, BMI, smoking status, alcohol consumption, stroke, diabetes, and hypertension.
3.3 Subgroup analysis
We carried out subgroup analysis stratified by age (≤ 18 years, and > 18 years), gender (female and male), race (Mexican American, non-Hispanic White, non-Hispanic Black, and other races), BMI (<25, 25–<30, ≥30), smoking status (never, former, and current), alcohol consumption (none, normal, and heavy), stroke (yes and no), diabetes (yes and no), and hypertension (yes and no) to investigate whether the relationship between DII and epilepsy remained consistent across different subgroups (Figure 3). The results indicated a significant association between DII and epilepsy in most subgroups, while no significant interactions were observed that affected this relationship (all P for interaction > 0.05).
Figure 3

Subgroup analyses of the association between DII and epilepsy. Analyses were stratified for age (≤18 years, and >18 years), gender (female and male), race (Mexican American, non-Hispanic White, non-Hispanic Black, and other races), smoking status (never, former, and current), alcohol consumption (none, normal, and heavy), stroke (yes and no), diabetes (yes and no), and hypertension (yes and no).
3.4 Sensitivity analysis
A sensitivity analysis was performed after excluding participants with missing values for any covariates (Supplementary Table S2). The results from both non-adjusted and adjusted models were consistent with the primary analysis, thereby confirming the stability and reliability of the findings.
4 Discussion
Research on the relationship between the DII and epilepsy remains limited. A study by Ding et al. demonstrated that adult epilepsy patients had higher DII scores compared to non-epileptic subjects (35). However, it did not include data from children under the age of 20, thereby limiting its generalizability. In contrast, our study collected a larger number of cases and included data from pediatric epilepsy patients. In our findings, logistic regression analysis revealed a positive association between high DII scores and epilepsy. Even after adjusting for other covariates, this relationship remained robust. Additionally, dose–response analysis showed a linear positive relationship. Stratified analysis indicated that DII was positively associated with epilepsy in most subgroups.
Although the research on the relationship between the DII and the occurrence and development of epilepsy is still scarce at present, the association between diet and epilepsy has long been a prominent research topic. A recent study by Zhang et al. investigated the association between the comprehensive dietary antioxidant index (CDAI) and epilepsy in the US population and found that a higher CDAI level corresponds to a lower risk of epilepsy, which suggests that a diet rich in antioxidants may help prevent epilepsy (34). He et al. reported that reduced antioxidant intake is associated with an increased risk of psychiatric comorbidities in epilepsy patients (36). Another study demonstrated that diet-derived circulating β-carotene significantly reduces epilepsy risk (37). Park et al. showed that naringin, a flavonoid found in grapefruit and citrus fruits, can reduce spontaneous recurrent seizures in a kainic acid-induced mouse model (38). Thus, these findings indicate that diet-related inflammation can influence epilepsy risk, providing a theoretical basis for our further research on the link between DII and epilepsy.
Several studies have demonstrated that pro-inflammatory diets can elevate systemic inflammation levels. D’Esposito et al. found that red meat consumption is associated with significant rises in IL-6, IL-8, and CRP (39). A study from the United States showed that after consuming an energy-dense, high-fat, fast-food–style meal, participants experienced a significant increase in IL-1β levels (40). An expanding body of evidence now indicates a close relationship between systemic inflammation and epilepsy onset. It has been reported that circulating inflammatory mediators, such as IL-6, TNF-α, and IL-1β, may impair tight junction regulation in brain endothelial cells, leading to heightened BBB permeability, enabling inflammatory mediators to infiltrate the central nervous system and trigger neuroinflammation (41). Additionally, another study revealed that blood monocytes can migrate to the brain through a compromised BBB and mediate neuroinflammation by differentiating into macrophages or microglia-like cells (42). Huang et al. also discovered that inducing systemic inflammation in mice led to TNFα-mediated brain vascular endothelial damage and astrocyte dysfunction, thereby raising the mice’s susceptibility to seizures (6). Therefore, the mechanism by which a high-DII diet increases epilepsy risk appears to be closely related to systemic inflammation.
Diet is well-recognized as a critical factor in shaping and influencing the structure and function of gut microbiota (43, 44). The Western diet, characterized by high levels of fat and cholesterol, is a primary driver of gut microbiota dysbiosis (45). Extensive evidence links changes in gut microbiota to epilepsy (46, 47). Medel-Matus et al. showed that disturbances in the intestinal microbiota of rats, particularly those associated with long-term stress, can increase vulnerability to epilepsy (48). Peng et al. identified a potential relationship between gut microbiota dysbiosis and the pathogenesis of drug-resistant epilepsy (49). Gómez-Eguílaz et al. demonstrated that probiotic therapy aimed at restoring gut microbiota balance can reduce seizure frequency and enhance quality of life in patients with drug-resistant epilepsy (50). Hence, we propose that DII influences epilepsy not only through systemic inflammation but also by modulating gut microbiota.
We further conducted the RCS regression analysis and found a significant positive linear association between DII and the incidence of epilepsy. In subgroup analyses stratified by age, race, gender, and other factors, no between-group differences in the association between DII and epilepsy were observed, which underscores the generalizability of our findings.
This study encompasses several advantages worth considering. Given the substantial sample size included, the study provides a dependable conclusion and ensures accurate statistical power. Additionally, our study utilized RCS analysis to further illustrate the positive linear association between the DII and epilepsy, which could provide novel insights for health policy decision-makers.
We acknowledge several limitations inherent in the present study. First, as a cross-sectional study, it cannot establish causality or temporal relationships between the DII and epilepsy. Second, recall bias may arise when obtaining dietary intake information through self-reporting. Third, the NHANES database does not explicitly differentiate between epilepsy subtypes or the severity of epilepsy. Detecting the connection between dietary-induced inflammatory status and various classifications of epilepsy remains a significant challenge. Fourth, although we have thoroughly screened numerous covariates to mitigate confounding bias, unidentified confounders may still exist and may not be explicitly recorded in the NHANES database.
5 Conclusion
In summary, our research indicates that the DII is closely associated with the risk of epilepsy. The association between the DII and epilepsy is linear and positive. Our findings offer preliminary evidence that may assist public health officials in developing practical strategies. However, more prospective studies are needed, and further investigation is required to explore the potential mechanism through which diet contributes to inflammation in epilepsy.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
YZ: Conceptualization, Methodology, Software, Writing – original draft, Data curation. CL: Visualization, Investigation, Supervision, Writing – review & editing, Data curation, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project was supported by Hainan Province Clinical Medical Center.
Acknowledgments
We acknowledge NHANES database for providing their platforms and contributors for uploading their meaningful datasets.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1599286/full#supplementary-material
Abbreviations
DII, Dietary inflammatory index; NHANES, National Health and Nutrition Examination Survey; RCS, Restricted cubic spline; BMI, Body mass index; ILs, Interleukins; TNF, Tumor necrosis factor; MEC, Mobile examination center; BBB, Blood–brain barrier.
References
1.
Ngugi AK Kariuki SM Bottomley C Kleinschmidt I Sander JW Newton CR . Incidence of epilepsy: a systematic review and meta-analysis. Neurology. (2011) 77:1005–12. doi: 10.1212/WNL.0b013e31822cfc90
2.
Verrotti A Iapadre G Di Francesco L Zagaroli L Farello G . Diet in the treatment of epilepsy: what we know so far. Nutrients. (2020) 12:645. doi: 10.3390/nu12092645
3.
Thijs RD Surges R O'Brien TJ Sander JW . Epilepsy in adults. Lancet. (2019) 393:689–701. doi: 10.1016/S0140-6736(18)32596-0
4.
Radzik I Miziak B Dudka J Chroscinska-Krawczyk M Czuczwar SJ . Prospects of epileptogenesis prevention. Pharmacol Rep. (2015) 67:663–8. doi: 10.1016/j.pharep.2015.01.016
5.
Rana A Musto AE . The role of inflammation in the development of epilepsy. J Neuroinflammation. (2018) 15:144. doi: 10.1186/s12974-018-1192-7
6.
Huang WY Lai YL Liu KH Lin S Chen HY Liang CH et al . TNFalpha-mediated necroptosis in brain endothelial cells as a potential mechanism of increased seizure susceptibility in mice following systemic inflammation. J Neuroinflammation. (2022) 19:29. doi: 10.1186/s12974-022-02406-0
7.
Sun H Ma D Hou S Zhang W Li J Zhao W et al . Exploring causal correlations between systemic inflammatory cytokines and epilepsy: a bidirectional Mendelian randomization study. Seizure. (2024) 114:44–9. doi: 10.1016/j.seizure.2023.11.006
8.
Pacifici R Paris L Di Carlo S Bacosi A Pichini S Zuccaro P . Cytokine production in blood mononuclear cells from epileptic patients. Epilepsia. (1995) 36:384–7. doi: 10.1111/j.1528-1157.1995.tb01013.x
9.
Sutter R Valenca M Tschudin-Sutter S Ruegg S Marsch S . Procalcitonin and mortality in status epilepticus: an observational cohort study. Crit Care. (2015) 19:361. doi: 10.1186/s13054-015-1072-9
10.
Dahlin M Singleton SS David JA Basuchoudhary A Wickstrom R Mazumder R et al . Higher levels of Bifidobacteria and tumor necrosis factor in children with drug-resistant epilepsy are associated with anti-seizure response to the ketogenic diet. EBioMedicine. (2022) 80:104061. doi: 10.1016/j.ebiom.2022.104061
11.
Kamasak T Dilber B Yaman SO Durgut BD Kurt T Coban E et al . HMGB-1, TLR4, IL-1R1, TNF-alpha, and IL-1beta: novel epilepsy markers?Epileptic Disord. (2020) 22:183–93. doi: 10.1684/epd.2020.1155
12.
Omrani S Taheri M Omrani MD Arsang-Jang S Ghafouri-Fard S . The effect of omega-3 fatty acids on clinical and paraclinical features of intractable epileptic patients: a triple blind randomized clinical trial. Clin Transl Med. (2019) 8:3. doi: 10.1186/s40169-019-0220-2
13.
Hara A Chihara N Akatani R Nishigori R Tsuji A Yoshimura H et al . Circulating plasmablasts and follicular helper T-cell subsets are associated with antibody-positive autoimmune epilepsy. Front Immunol. (2022) 13:1048428. doi: 10.3389/fimmu.2022.1048428
14.
Kwon A Kwak BO Kim K Ha J Kim SJ Bae SH et al . Cytokine levels in febrile seizure patients: a systematic review and meta-analysis. Seizure. (2018) 59:5–10. doi: 10.1016/j.seizure.2018.04.023
15.
Wang A Si Z Li X Lu L Pan Y Liu J . FK506 attenuated pilocarpine-induced epilepsy by reducing inflammation in rats. Front Neurol. (2019) 10:971. doi: 10.3389/fneur.2019.00971
16.
Marchi N Fan Q Ghosh C Fazio V Bertolini F Betto G et al . Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. (2009) 33:171–81. doi: 10.1016/j.nbd.2008.10.002
17.
Stredny C Rotenberg A Leviton A Loddenkemper T . Systemic inflammation as a biomarker of seizure propensity and a target for treatment to reduce seizure propensity. Epilepsia Open. (2023) 8:221–34. doi: 10.1002/epi4.12684
18.
Ma TC Zhou J Wang CX Fang M Gao F . Association between dietary inflammatory index and S-klotho plasma levels in middle-aged and elderly people. Front Nutr. (2022) 9:853332. doi: 10.3389/fnut.2022.853332
19.
Malesza IJ Malesza M Walkowiak J Mussin N Walkowiak D Aringazina R et al . High-fat, Western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. (2021) 10:3164. doi: 10.3390/cells10113164
20.
Hart MJ Torres SJ McNaughton SA Milte CM . Dietary patterns and associations with biomarkers of inflammation in adults: a systematic review of observational studies. Nutr J. (2021) 20:24. doi: 10.1186/s12937-021-00674-9
21.
Szypowska A Regulska-Ilow B Zatonska K Szuba A . Comparison of intake of food groups based on dietary inflammatory index (DII) and cardiovascular risk factors in the middle-age population of lower Silesia: results of the PURE Poland study. Antioxidants. (2023) 12:285. doi: 10.3390/antiox12020285
22.
Christ A Lauterbach M Latz E . Western diet and the immune system: an inflammatory connection. Immunity. (2019) 51:794–811. doi: 10.1016/j.immuni.2019.09.020
23.
Hosseini B Berthon BS Saedisomeolia A Starkey MR Collison A Wark PAB et al . Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: a systematic literature review and meta-analysis. Am J Clin Nutr. (2018) 108:136–55. doi: 10.1093/ajcn/nqy082
24.
Shivappa N Steck SE Hurley TG Hussey JR Hebert JR . Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
25.
Cavicchia PP Steck SE Hurley TG Hussey JR Ma Y Ockene IS et al . A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. (2009) 139:2365–72. doi: 10.3945/jn.109.114025
26.
Phillips CM Chen LW Heude B Bernard JY Harvey NC Duijts L et al . Dietary inflammatory index and non-communicable disease risk: a narrative review. Nutrients. (2019) 11:1873. doi: 10.3390/nu11081873
27.
Chuang K Rammon J Shin HC Chen TC . Assessing laboratory method validations for informing inference across survey cycles in the National Health and nutrition examination survey. Vital Health Stat. (2024) 1:1–41. doi: 10.15620/cdc:145591
28.
Shivappa N Steck SE Hurley TG Hussey JR Ma Y Ockene IS et al . A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. (2014) 17:1825–33. doi: 10.1017/S1368980013002565
29.
Xu H Xie P Liu H Tian Z Zhang R Cui M . The relationship between dietary inflammatory index in adults and coronary heart disease: from NHANES 1999-2018. Front Nutr. (2025) 12:1564580. doi: 10.3389/fnut.2025.1564580
30.
Mao Y Weng J Xie Q Wu L Xuan Y Zhang J et al . Association between dietary inflammatory index and stroke in the US population: evidence from NHANES 1999-2018. BMC Public Health. (2024) 24:50. doi: 10.1186/s12889-023-17556-w
31.
Zheng Y Liu W Zhu X Xu M Lin B Bai Y . Associations of dietary inflammation index and composite dietary antioxidant index with preserved ratio impaired spirometry in US adults and the mediating roles of triglyceride-glucose index: NHANES 2007-2012. Redox Biol. (2024) 76:103334. doi: 10.1016/j.redox.2024.103334
32.
Ahluwalia N Dwyer J Terry A Moshfegh A Johnson C . Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. (2016) 7:121–34. doi: 10.3945/an.115.009258
33.
Ran L Xu M Zhang Z Zeng X . The association of nutrient intake with epilepsy: a cross-sectional study from NHANES, 2013-2014. Epilepsy Res. (2024) 200:107297. doi: 10.1016/j.eplepsyres.2024.107297
34.
Zhang Y Shen J Su H Lin C . Association between composite dietary antioxidant index and epilepsy in American population: a cross-sectional study from NHANES. BMC Public Health. (2024) 24:2240. doi: 10.1186/s12889-024-19794-y
35.
Ding R Han Z Gui J Xie L Yang J Yang X et al . Inflammatory properties of diet mediate the effect of epilepsy on moderate to severe depression: results from NHANES 2013-2018. J Affect Disord. (2023) 331:175–83. doi: 10.1016/j.jad.2023.03.054
36.
He X Li Z Wu H Wang L Zhang Y . Composite dietary antioxidant index mediates the effect of epilepsy on psychiatric disorders: results from NHANES 2013-2018. Front Neurol. (2024) 15:1434179. doi: 10.3389/fneur.2024.1434179
37.
Liang Z Lou Y Zheng Z Guo Q Liu S . Diet-derived circulating antioxidants and risk of epilepsy: a study combining metabolomics and mendelian randomization. Heliyon. (2024) 10:e26813. doi: 10.1016/j.heliyon.2024.e26813
38.
Park J Jeong KH Shin WH Bae YS Jung UJ Kim SR . Naringenin ameliorates kainic acid-induced morphological alterations in the dentate gyrus in a mouse model of temporal lobe epilepsy. Neuroreport. (2016) 27:1182–9. doi: 10.1097/WNR.0000000000000678
39.
D'Esposito V Di Tolla MF Lecce M Cavalli F Libutti M Misso S et al . Lifestyle and dietary habits affect plasma levels of specific cytokines in healthy subjects. Front Nutr. (2022) 9:913176. doi: 10.3389/fnut.2022.913176
40.
Devaraj S Wang-Polagruto J Polagruto J Keen CL Jialal I . High-fat, energy-dense, fast-food-style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism. (2008) 57:867–70. doi: 10.1016/j.metabol.2008.02.016
41.
Sun Y Koyama Y Shimada S . Inflammation from peripheral organs to the brain: how does systemic inflammation cause Neuroinflammation?Front Aging Neurosci. (2022) 14:903455. doi: 10.3389/fnagi.2022.903455
42.
Li W Wu J Zeng Y Zheng W . Neuroinflammation in epileptogenesis: from pathophysiology to therapeutic strategies. Front Immunol. (2023) 14:1269241. doi: 10.3389/fimmu.2023.1269241
43.
Makki K Deehan EC Walter J Backhed F . The impact of dietary Fiber on gut microbiota in host health and disease. Cell Host Microbe. (2018) 23:705–15. doi: 10.1016/j.chom.2018.05.012
44.
Cui S Guo W Chen C Tang X Zhao J Mao B et al . Metagenomic analysis of the effects of Lactiplantibacillus plantarum and Fructooligosaccharides (FOS) on the Fecal microbiota structure in mice. Food Secur. (2022) 11:1187. doi: 10.3390/foods11091187
45.
Serino M . Molecular paths linking metabolic diseases, gut microbiota Dysbiosis and Enterobacteria infections. J Mol Biol. (2018) 430:581–90. doi: 10.1016/j.jmb.2018.01.010
46.
Arulsamy A Tan QY Balasubramaniam V O'Brien TJ Shaikh MF . Gut microbiota and epilepsy: a systematic review on their relationship and possible therapeutics. ACS Chem Neurosci. (2020) 11:3488–98. doi: 10.1021/acschemneuro.0c00431
47.
Amlerova J Sroubek J Angelucci F Hort J . Evidences for a role of gut microbiota in pathogenesis and Management of Epilepsy. Int J Mol Sci. (2021) 22:5576. doi: 10.3390/ijms22115576
48.
Medel-Matus JS Shin D Dorfman E Sankar R Mazarati A . Facilitation of kindling epileptogenesis by chronic stress may be mediated by intestinal microbiome. Epilepsia Open. (2018) 3:290–4. doi: 10.1002/epi4.12114
49.
Peng A Qiu X Lai W Li W Zhang L Zhu X et al . Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res. (2018) 147:102–7. doi: 10.1016/j.eplepsyres.2018.09.013
50.
Gomez-Eguilaz M Ramon-Trapero JL Perez-Martinez L Blanco JR . The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Benef Microbes. (2018) 9:875–81. doi: 10.3920/BM2018.0018
Summary
Keywords
NHANES, DII, epilepsy, cross-sectional study, diet
Citation
Zhu Y and Lu C (2025) Association between dietary inflammatory index and epilepsy: findings from NHANES. Front. Neurol. 16:1599286. doi: 10.3389/fneur.2025.1599286
Received
26 March 2025
Accepted
16 May 2025
Published
30 May 2025
Volume
16 - 2025
Edited by
Francesca Felicia Operto, University of Salerno, Italy
Reviewed by
Charalampia Amerikanou, Harokopio University, Greece
Shicun Huang, The First Affiliated Hospital of Soochow University, China
Updates
Copyright
© 2025 Zhu and Lu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuansen Lu, luchuansen@outlook.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.