- 1Department of Neuroscience, Reproductive Science and Dentistry, Section of Audiology, University of Naples “Federico II”, Naples, Italy
- 2Department of Neuroscience, Psychology, Drug’s Area and Child’s Health, University of Florence, Florence, Tuscany, Italy
- 3Department of Neurology, Ospedale San Luca di Vallo della Lucania, ASL Salerno, Salerno, Italy
- 4Department of ENT, University of Perugia, Perugia, Italy
- 5Faculty of Medicine and Surgery, University of Salerno, Salerno, Italy
- 6Department of Medicine and Surgery, University of Perugia, Perugia, Italy
Finding a vertical nystagmus, especially when looking straight ahead, should alert the neurologist/neuro-otologist for other signs of cerebellar or brainstem dysfunction. Upbeat nystagmus (UBN) is a relatively uncommon neuro-otological finding that clinicians may encounter in patients presenting with vertigo. This phenomenon is closely linked to central vestibular dysfunction, making it essential for healthcare providers to recognize and interpret it promptly. Accurate identification of UBN can significantly aid in directing patients toward the appropriate diagnostic and therapeutic pathways. As our understanding of UBN’s pathophysiology has advanced, the clinical significance of this sign has become increasingly evident. It is now recognized that UBN can occur as an isolated finding or more frequently as part of a broader spectrum within defined clinical syndromes. This expanded knowledge has also opened the door to various therapeutic approaches tailored to the underlying cause. In our study, we want to provide as accurate a picture as possible about the origins and clinical presentations of UBN.
1 Introduction
First described by Stengel in 1935 (1), upbeat nystagmus (UBN) significantly impairs patients’ quality of life by producing disabling visual disturbances such as oscillopsia and gaze instability. These symptoms underscore the clinical importance of a thorough understanding of UBN and its management. However, UBN remains a relatively rare finding, limiting opportunities for clinicians and researchers to gain sufficient diagnostic and therapeutic experience. Moreover, UBN is frequently linked to lesions in key regions of the brainstem (particularly the medulla), as well as the flocculus and the brachium conjunctivum (BC). It often presents alongside with other neurological signs, such as saccadic contrapulsion or impaired smooth pursuit, further complicating its recognition and interpretation. Despite its clinical relevance, the neuroanatomical pathways responsible for upward vertical gaze remain incompletely understood. Much of the current knowledge about UBN derives from lesion-based and clinical observational studies (2), highlighting the need for an integrated synthesis of available evidence. Moreover, while individual publications address specific aspects of UBN, a comprehensive, unified resource is lacking, forcing clinicians to consult disparate sources and potentially leading to fragmented understanding. A consolidated review of UBN is therefore warranted to integrate current knowledge, elucidate underlying mechanisms, correlate clinical findings with neuroimaging data, and propose evidence-based approaches to diagnosis and treatment. Such a resource would be invaluable for clinicians, researchers, and patients alike, ultimately supporting improved care and advancing future investigations.
1.1 First descriptions of vertical nystagmus
Vertical nystagmus received limited attention in the early medical literature (3, 4) until 1921, when it was experimentally induced in animals by severing the anterior semicircular canal (SCC) and observed in clinical contexts such as profound bilateral visual loss, drug toxicity (e.g., barbiturates, quinine), multiple sclerosis, spasmus nutans, brainstem lesions (e.g., pontine and medullary tuberculomas and meningiomas), and encephalitis lethargica (5). While some authors emphasized the importance of distinguishing central from peripheral etiologies in cases of vertical or mixed nystagmus (6), Walsh (7) asserted that purely vertical nystagmus was a hallmark of central or cerebellar pathology. He described cases associated with Arnold-Chiari malformation, cerebellar ataxia, bilateral pyramidal tract signs, and hydrocephalus, reinforcing the central origin hypothesis.
2 Characteristics of UBN
Upbeat nystagmus is often a sign of the acute phase of a disease tending to resolve spontaneously before other ocular motor abnormalities. It is typically present in the primary gaze position, both in darkness and during fixation, and must be distinguished from nystagmus evoked exclusively by upward gaze secondary to a vertical gaze holding neural integrator lesions. The slow phases of UBN may have different waveforms, which may be linear, decaying due to a “leaky” neural integrator, or more commonly, increasing due to an unstable neural integrator (2). Unlike downbeat nystagmus (DBN), UBN is minimally affected by lateral gaze (8). In some cases, the upward fast phase may have a horizontal component alternately directed to the right or to the left, in which case it is configured as a “bow-tie nystagmus” (9) or have a torsional component. In this last case, it may be due to the unilateral BC lesion, which in health conveys excitatory upward-torsional eye movement signals from the anterior semicircular canals (10) or to an associated lesion of pathways controlling the VOR in roll (11).
2.1 Alexander law and UBN
Upbeat nystagmus (UBN) typically follows Alexander’s law, exhibiting maximal fast-phase amplitude in upward gaze. However, in some cases, it may paradoxically increase with downward gaze (12, 13) or even convert into downbeat nystagmus (DBN) during upward gaze (13). These atypical patterns have been associated with lesions of the interstitial nucleus of Cajal (INC), which makes unstable the vertical neural integrator responsible for gaze holding - an association supported by corresponding MRI findings (2).
2.2 Effect of convergence and vestibular stimulation on UBN
Convergence has a variable effect on UBN, being able to increase or decrease its intensity up to its suppression or in some cases transforming it into a DBN (14–17). At same time, vestibular stimulation, such as head-shaking and vibration, may reverse nystagmus direction. The changes in nystagmus patterns due to convergence and vestibular stimulation are likely due to disruptions in circuits that process linear acceleration signals from the otoliths, which are essential for generating the correct eye movements during head translation (18). A fundamental role in this is played by the translational vestibulo-ocular reflex (t-VOR), which during movement adjusts compensatory eye movements based on orbital position and vergence angle depending on the target position. In this way, during translation forward or backward the t-VOR generates horizontal movements for targets to the sides, vertical movements for targets above or below, and convergence/divergence for targets straight ahead. Key brain areas involved in these computations include the medial and inferior vestibular nuclei in the spinal cord, which mediate the velocity storage mechanism (19, 20), and their projections to the cerebellar nodulus, which computes the translational components of head movements (21). Disruption of these pathways or velocity storage mechanisms may explain the change in direction of nystagmus in response to convergence and vestibular stimuli, respectively (22).
2.3 Gravitational dependence of UBN
Changes in head position relative to space, and hence in macular input, can abolish (8, 23, 24) UBN, increase its slow-phase velocity (25) and change its direction, often transforming it into a DBN (26, 27). UBN often tends to disappear in the supine or prone position, where the effect of the gravitational vector on the maculae is different (8). In a patient with a focal hemorrhagic lesion of the left BC, anterior vermis and left anterior superior cerebellar hemisphere, UBN was also suppressed by a contralateral head tilt due to otolith-ocular reflex activation (23). In a patient affected with a lesion at the cervicomedullary junction due to multiple sclerosis, the spontaneous upward and counterclockwise nystagmus was suppressed by the prone position, tilting the head to either side while sitting, and turning the head to the right while in the supine position. Moreover, nystagmus direction was reversed by hanging the head straight and turning the head to the left while the patient was in the supine position (24). Change from UBN to DBN were observed in the prone position in a patient with a lesion of the BC and moving from upright to a head-hanging position a patient with cerebellar vermis atrophy (28).
2.4 Spontaneous transformation of UBN in other nystagmus type
Another very peculiar feature of UBN is its possible spontaneous transformation, in the course of the disease, into a hemi-seesaw (29, 30), horizontal, torsional and above all DBN (17, 22, 24, 28, 31, 32). A very interesting case of the above phenomenon is Wernicke’s encephalopathy. The initial thiamine deficiency damages the perihypoglossal nuclei, particularly the nucleus intercalatus of Staderini (SIN) and the nucleus of Roller (RN) more than the PMT nuclei, resulting in a downward slow-phase bias and UBN. Over time, recovery is more complete in the perihypoglossal than in the PMT nuclei, which fails to provide excitatory input to the cerebellar flocculus. The consequence is that the cerebellar flocculus no longer exerts inhibitory control over upward slow-phase pathways resulting in an upward slow-phase bias and hence in DBN (30). Obviously, also the contiguity of the areas responsible for the vestibular syndrome in the sagittal plane may also justify the change in nystagmus direction (33, 34). Overall, changes in nystagmus direction with convergence, head tilt, vestibular stimuli or simply over the course of the disease reflect the tightly interconnected network that maintains balance in the pitch plane.
2.5 Neuronal circuits responsible for upward and downward postural and ocular reflexes
The neuronal circuits responsible for upward and downward postural and ocular reflexes are distinct within the central nervous system. These specialized pathways underlie the generation of different types of vertical nystagmus. Specifically, damage to the circuit facilitating upward compensatory and downward anticompensatory eye movements can result in UBN, while lesions affecting the circuit that facilitates downward compensatory and upward anticompensatory responses can lead to downbeat nystagmus (DBN) (35).
The effectiveness of the circuits controlling upward and downward vestibulo-ocular reflexes is not equal. Actually, upward eye movement reflexes are stronger than downward eye movement. Support for this assertion is the observation that optokinetic nystagmus and optokinetic after nystagmus as well as nystagmus induced by vertical rotation (36, 37) predominate for upward direction. Further confirmation is provided by the fact that the time constant for DBN is about 15 s, which is like that of horizontal nystagmus, whereas for UBN nystagmus it is about 8 s.
One reason for this vertical reflex asymmetry may be due to the anatomy of the eye. In fact, at least in the cat, the center of mass of the eyeballs is located anterior to the center of rotation (38, 39) and therefore, with subject in an upright position and the head in line with the trunk, the force of gravity limits the vertical VOR upwards and favors the vertical VOR downwards, creating a clear and maximum imbalance between the two vertical eye movement systems (Figure 1). On the other hand, the load and viscosity of the eye alone may not necessitate such a pronounced upward reflex preponderance.
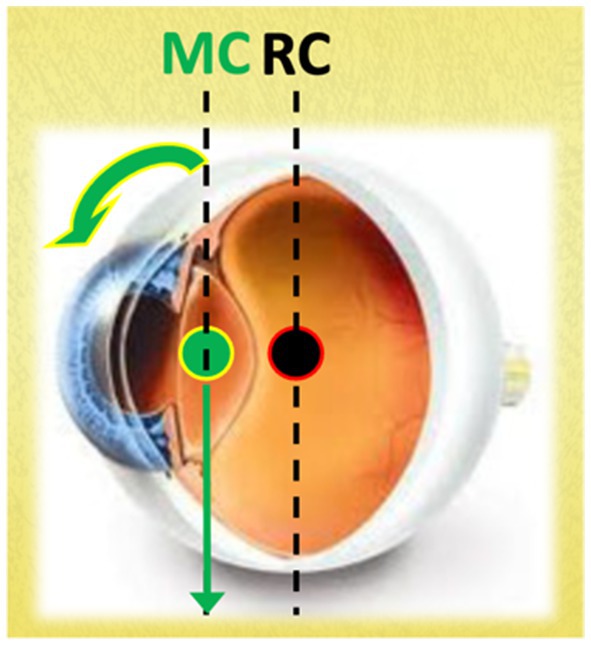
Figure 1. The figure illustrates that the vertical line passing through the center of mass (MC) is positioned anterior to the vertical line passing through the center of rotation (RC). In this configuration, with the subject in an upright position and the head aligned with the trunk, gravity counteracts the upward vertical VOR while facilitating the downward vertical VOR. This asymmetry must be counterbalanced by specific neural pathways.
An alternative explanation considers that gaze stability is maintained not only by the vestibulo-ocular reflex (VOR) but also by the vestibulocollic and cervicocollic reflexes (40). In the case of head reflexes, the influence of gravity is significantly greater than that acting on the eyes, requiring a robust upward enhancement. Consequently, the observed upward bias in the ocular reflex may also serve to compensate for potential gain and phase deficits in the head reflex, thereby providing effective counteraction to gravitational forces.
In addition, the upward preponderance should be modulated depending on the position of the head re-gravity, so that an influence from the otolithic signals, sensing the orientation of the gravity vector, is required for inhibiting or enhancing the upward responses depending either on the direction of the gravity vector and on the load and viscosity in the motor system involved.
2.6 Peripheral source of asymmetry in the vertical eye movement reflexes
At the peripheral level, the upward preponderance in vertical eye movement reflexes is supported by the anatomical and physiological properties of the vertical semicircular canals (VSCCs) (41). VSCC activation occurs via an ampullofugal stimulus, with the receptors of the anterior SCCs being more effectively stimulated by downward rotations and the receptors of the posterior SCC (PSCCs) responding more strongly to upward rotations. Because the anterior SCCs (ASCCs) are more closely aligned with the sagittal plane than the posterior canals, the signals from vertical head rotations are inherently asymmetric, producing a stronger response during downward rotations.
2.7 Central source of asymmetry in the vertical eye movement reflexes
The most important central pathway facilitating upward eye movements is the crossing ventral tegmental tract (CVTT). Additional areas potentially involved in such an asymmetry include a circuit consisting of Superior Vestibular Nucleus (SVN), the RN and the SIN, and a circuit in which the PMT are involved.
3 Pathophysiology for UBN
A damage to one of these circuits would reveal the underlying downward imbalance leading to UBN.
3.1 The CVTT
This complex crossing pathway, responsible for upward eye movement facilitation, originates in the upper pole of the SVN, courses predominantly in the pons, then in lower midbrain tegmentum and finally reaches the III (oculomotor) nucleus (42, 43).
Afferences to SVN come from the anterior semicircular canals, both directly both indirectly through the flocculus, via the interstitial nucleus of the vestibular nerve (2); the maculae, both directly and through the flocculus; the caudal medulla, in which RN and SIN ‘s receives a collateral pathway from the CVTT and projects to the flocculus, for plausible feedback control; the flocculus, which receives inputs from the caudal medulla, ASCCs, maculae and visual apparatus and projects to the SVN, ensuring control of the circuit (44).
The CVTT can be regarded as a true anti-gravitational pathway controlled by macular input (Figure 2) which constantly modulate the tonic activity of the oculomotor nucleus based on the orientation of the head and of the gravitational vector, counterbalancing gravitational forces in relation to the head and eye’s static position and correcting the eyeball’s vertical inertial asymmetry. When the head is in upright position, the gravitational vector facilitates the CVTT so that the upward eye responses are potentiated. In prone or supine positions, the macular input changes and diminishes its facilitatory effect in the CVTT, so that the eye positions balance is therefore guaranteed only by the activity of the pathways that run in the MLF (see below). Finally, in the vertical head-down position, the upward facilitatory effect of CVTT will be minimal or absent and the gravitational vector will tend to favor upward eye movement relative to the head. In absence of CVTT-like circuitry compensating for such an eye movement due to gravity, an upward slow phase and chin-beating nystagmus may occur (45–47). Moreover, by providing the eye elevator muscle motoneurons with precise tonic activity, the CVTT integrates the function of the excitatory MVN-MLF pathway which, in the absence of gravity, would theoretically be sufficient by itself to manage the vertical movements of the eye. To put it more clearly: the excitatory MVN-MLF pathway controls upward eye movements, calculating the eye velocity relative to the orbit in response to rotational and/or translational head movements. The CVTT adjusts these parameters based on the instantaneous gravitational vector, i.e., the spatial position of the head.
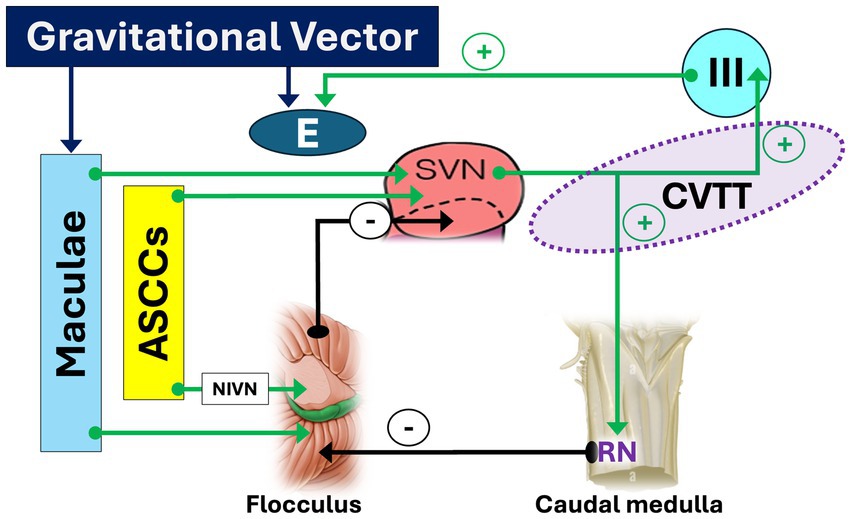
Figure 2. Pathways and circuits facilitating upward slow phase. Afferent inputs to the superior vestibular nucleus (SVN) originate from the anterior semicircular canals - both directly and indirectly via the flocculus through the interstitial nucleus of the vestibular nerve (NIVN)–as well as from the maculae, following similar direct and indirect pathways via the flocculus. From the upper pole of the SVN, the crossing ventral tegmental tract (CVTT) emerges and projects to the oculomotor (III) nucleus. Additionally, a collateral branch of the CVTT reaches the nucleus of Roller (RN) in the caudal medulla, which, by inhibiting the flocculus (−), modulates its inhibitory influence on the SVN. The “+” sign indicates facilitatory effect of CVTT for upward slow phase. LVN: lateral vestibular nucleus.
The existence of a neuronal circuit facilitating upwards eye movements has been confirmed by numerous experiments performed in hypogravity and hypergravity (48–50) and above all from the clinical findings.
3.2 The SVN-RN/SIN-flocculus circuit
The SVN is also controlled by a loop located within the dorsal caudal medulla, consisting mainly of the RN and the SIN, both belonging to the perihypoglossal nuclei (PHN), and the flocculus. For completeness, it is worth mentioning the nucleus prepositus hypoglossi (NPH), which is also part of the PHN but does not participate in this specific circuit.
a. Sublingual nucleus of Roller (RN). This very small nucleus is particularly well developed in higher primates such as chimpanzees and humans (51). It is located just ventral to the cranial tip of the hypoglossal nucleus and appears to be the better candidate to play a role in upward eye movements (10). In health subject, it receives strong excitatory projections from the SVN (52) and send inhibitory projections (51) to the flocculus (53) which in turn finally sends back inhibitory projections to SVN (53–55). A medullary lesion affecting the RN disrupts this inhibitory control over the flocculus, leading to its disinhibition. Consequently, this determines a strong inhibition of the SVN, impaired processing of input from the anterior semicircular canals (ASCCs), loss of tonic activity in the superior rectus and inferior oblique muscles, a downward drift of the eyes, and the emergence UBN (53, 55).
b. Nucleus intercalatus of Staderini (SIN). This nucleus is located between the hypoglossal nucleus and dorsal nucleus of the vagus nerve (56) and creates a circuit that overlaps with the one described for the RN (57). The strong connections existing among perihypoglossal nuclei and flocculus, paraflocculus and nodulus (58, 59) suggest their role as the vertical cerebello-vestibular integrator involved in vertical gaze holding (53, 60–62). Upbeat nystagmus due to SIN involvement has been demonstrated in unilateral medial medullary infarction (60, 61), chronic lateral medullary infarction (63) multiple sclerosis (57), and cavernoma in the medulla oblongata (64). Despite the data presented, we believe it is right to remember that some authors do not fully agree with the gaze holding function; in fact, a patient with a dorsal paramedian lesion involving the SIN showed a constant-velocity slow phase rather than exponential decay, suggesting that this was not a nystagmus due to vertical gaze holding deficit (65).
3.3 The “cell groups of the paramedian tracts” (PMT)
PMT is a collective term used to refer to clusters of neurons scattered along the midline fibers tracts in the pons and medulla (66, 67) which receives inputs from all known premotor cell groups of the oculomotor system (52, 68–73) and project to the flocculus, paraflocculus, and vermis of the cerebellum (50, 66–68). The PMT contributes to neural integrator function by relaying eye movement signals to the vestibulocerebellum (67, 74). Particularly, the mid-medullary PMT-cell group in the monkey, the nucleus pararaphales (75, 76) receives vertical eye position signals from the interstitial nucleus of Cajal and therefore relays vertical eye position signals to the floccular complex. Thus, medullary lesions that affect the nucleus pararaphales may result in UBN (33, 77–81) and vertical gaze-evoked nystagmus (GEN). In addition, the PMT provide the cerebellum with a form of efference copy (or corollary discharge) of eye movement commands for the optimization of gaze control, including the fidelity of the neural integrator for eye movements or more long-term adaptive control of eye movements (74, 82).
3.4 The brachium conjunctivum
For the sake of completeness, we also report the crossing path of the BC, which has long been considered responsible for UBN. This pathway arises from the SVN, runs rostrally in the caudal tegmentum, and decussate in the caudal midbrain before projecting projects to the III (oculomotor) nucleus, which excites the motoneurons of the superior rectus and inferior oblique muscles, bilaterally. For a long time, this pathway was thought to be involved in the excitatory control of upward eye movements in the rabbit (83), until it was shown that this pathway was most likely confused with the CVTT, since these tracts are very close to each other in the lower pontine tegmentum (38, 39), and this is valid also in humans (23, 84, 85). Clinical data would confirm that a lesion of the BC does not determine an up-beat nystagmus consequent to the imbalance due to the predominance of the tonic activity of the system controlling downward movements as one would expect (86) but rather a down beat GEN (87) or a positional downbeat nystagmus (88). Moreover, some cases of spontaneous UBN due to involvement of the region through which the BC passes are characterized by large median tumoral or hemorrhagic lesions, always associated with damage to the cerebellar vermis, which in itself may lead to UBN (2, 78).
3.5 What is the role of the pathways that run through the medial longitudinal fasciculus?
To better understand the role of the pathway running through the medial longitudinal fasciculus (MLF) in the genesis of UBN, valuable insights can be gained from patients with lesions of this pathway, in whom high-acceleration head rotations reveal an asymmetrical vertical vestibulo-ocular reflex (VOR) gain deficit. Specifically, upward VOR gain is less severely affected than downward VOR gain (89, 90). This finding supports the notion that upward VOR signals are transmitted not only via the MLF but also through extra-MLF pathways (91).
Additional noteworthy observations in such patients include the absence of spontaneous vertical nystagmus and the presence of predominantly upbeat GEN (53, 89–91). The lack of spontaneous vertical nystagmus suggests a relatively symmetrical role of the MLF in vertical VOR control. Meanwhile, the presence of upbeat GEN confirms MLF damage, indicating that the lesion deprives the interstitial nucleus of Cajal—a key component of the vertical neural integrator—of vestibular input integrator (2).
Finally, the induction of UBN following an MLF lesion requires selective or predominant damage to the bilateral pathways responsible for the upward VOR, while sparing those involved in the downward VOR. This selective damage may explain why upbeat nystagmus is infrequently observed in cases of internuclear ophthalmoplegia (24).
4 Symptoms of upbeat nystagmus syndrome
The symptomatology of Upbeat Nystagmus Syndrome (UBNS) is essentially characterized by oscillopsia, motion illusion and sagittal imbalance. Oscillopsia, the most disabling symptom, is caused by the displacement of images on the retina due to the involuntary slow phase of nystagmus. In the absence of an efferent copy, which is generated only in the presence of a voluntary movement, the pathological displacement of the eyes creates a motion illusion of the visual scene in the vertical plane. The illusion is less intense compared to the magnitude of the slow-phase eye movements (92) presumably due to a reduction in the sensitivity of visual motion perception which is useful to mitigate the annoyance of illusory oscillations. Consequence of motion illusion due to oscillopsia is a compensatory vestibulo-spinal reflex (VSR) that contributes to the sagittal imbalance. By analogy with other vestibular vertigo syndromes, one would expect a tendency to fall forward due to motor compensation of an apparent backward tilt. In fact, under physiological conditions, extension of the head and/or trunk activates the vertical VOR, which is responsible for a slow downward phase and then the vestibulo-collic and vestibulo-spinal reflexes to compensate for the extension. Under pathological conditions, the tonic asymmetry generated not by the subject’s movement but by the central vestibular pathways dysfunction causes a downward eye movement and generates a retropulsion misperception characterized by an illusory head and/or body extension. The resulting postural compensation aims to compensate for the illusory extension, which is characterized by retropulsion and postural oscillations in the posterior–anterior direction. Contrary to these physiopathological premises some patients behaved differently, showing a tendency to fall backwards, in the same direction as patients with downbeat nystagmus (11).
5 Additional oto-neurological signs
Due to the presence of spontaneous upbeat nystagmus, smooth pursuit in the vertical plane is altered, constituting a “false” (extrinsic) alteration. On the other hand, the alteration of smooth pursuit is greater than would be expected from spontaneous nystagmus alone and the specific impairment of downward vertical pursuit suggests a direct and more extensive lesion affecting the pathways involved (93). Based on the possible associated lesions, it is possible to highlight other possible signs. For example, in patients with Wernicke encephalopathy a bilateral and symmetric loss of the horizontal VOR and a horizontal GEN can be highlighted due to the involvement of the NPH/MVN complex, which is located medial to the medulla and just below the area postrema (30, 94). Another possible finding associated with upbeat nystagmus is saccadic contrapulsion, characterized by hypermetria (overshooting) away from the side of the lesion and hypometria (undershooting) toward it. This phenomenon may result from the disruption of either: (1) the climbing fiber tracts that originate in the inferior olivary nucleus and project to the cerebellar vermis before crossing the midline (77); (2) the outputs from the fastigial nucleus that pass through the BC (85, 95).
6 Sites of lesions
The sites of lesions responsible of upbeat nystagmus must be sought in:
1. Lesion of CVTT, which carries signals from the ASCCs to the elevator muscles of the eyes (Figure 3). Several possible sites of CVTT lesion have been demonstrated in humans:
a. Lesions affecting the upper pole of the SVN and the initial portion of the pathway, in the posterolateral region of the inferior pontine tegmentum (96)
b. Lesions affecting the intermediate segment of the CVTT, near the decussation, in the central part of the pons (44)
c. Lesions affecting the final portion of the CVTT, in the anterior midbrain tegmentum (97)
d. Bilateral lesions affecting the anterior pontine tegmentum and the adjacent posterior basis pontis in the middle and upper pons (8, 98, 99).
2. Lesions affecting caudal medulla (77):
a. Lesion affecting the RN, which play a fundamental role in upward eye movements (33) (Figure 4)
b. Disruption of the mid-medullary nucleus pararaphales of the PMT, involved in vertical gaze holding (67, 74)
c. Lesions affecting the SIN, also involved in vertical gaze holding (53, 60–62) whose role is however questioned (65).
3. Rarer sites of lesions are the anterior vermis of the cerebellum (2, 24, 28, 78) and the thalamus (24), moreover without a precise explanation of the mechanisms.
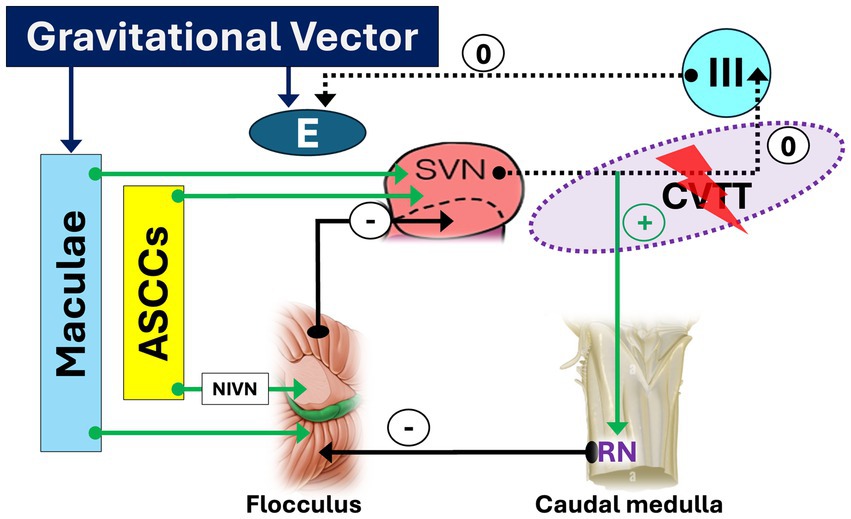
Figure 3. Crossing ventral tegmental tract (CVTT) lesion facilitating down slow phase and upbeat nystagmus. A lesion affecting the segment of the CVTT directed to the oculomotor nucleus deprives the elevator muscles of inputs from the anterior semicircular canals (ASSCs). As a result, a downward-directed slow phase and an upbeat nystagmus (UBN) will occur. The sign “0” indicates the disappearance of the facilitatory effect of CVTT for upward slow phase.
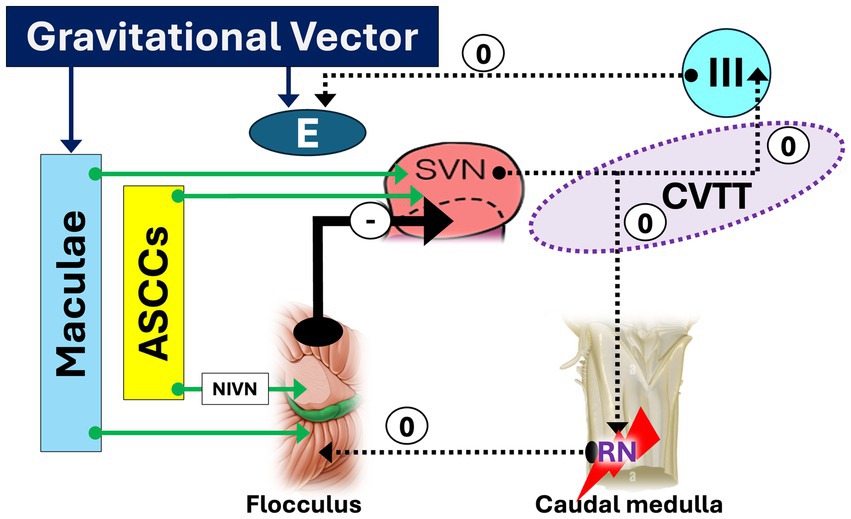
Figure 4. Caudal medulla lesion facilitating down slow phase and upbeat nystagmus. A medullary lesion affecting the nucleus of Roller (RN) disrupts its inhibitory control over the flocculus, resulting in disinhibition. Consequently, this leads to strong inhibition (heavy arrow) of the superior vestibular nucleus (SVN), impaired processing of input from the anterior semicircular canals (ASCCs), loss of tonic activity in the superior rectus and inferior oblique muscles, a downward drift of the eyes, and the emergence of upbeat nystagmus (UBN). The sign “0” indicates the disappearance of the facilitatory effect of CVTT for upward slow phase.
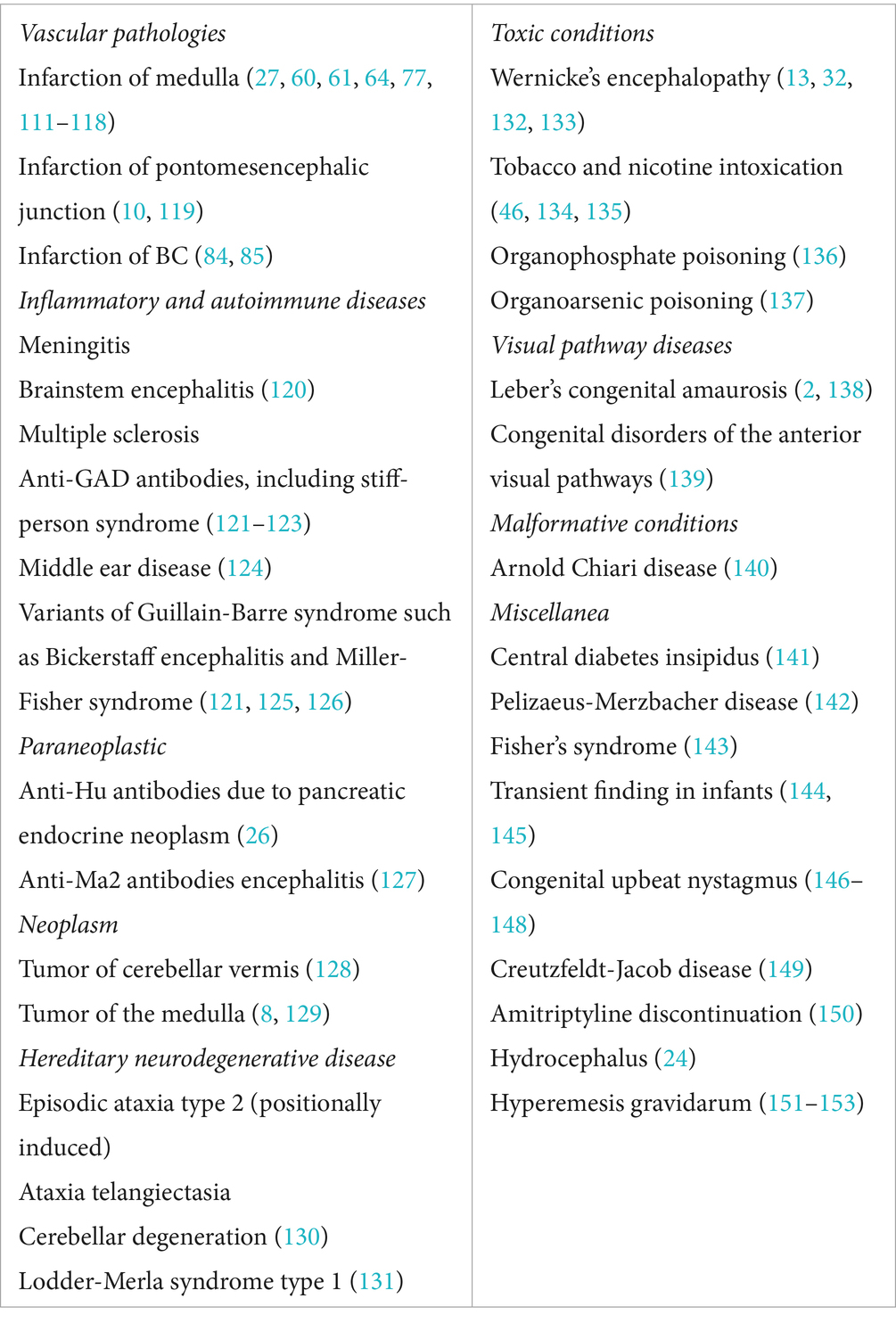
7 Etiology
The most frequent central causes of UBNS are reported in Table 1.
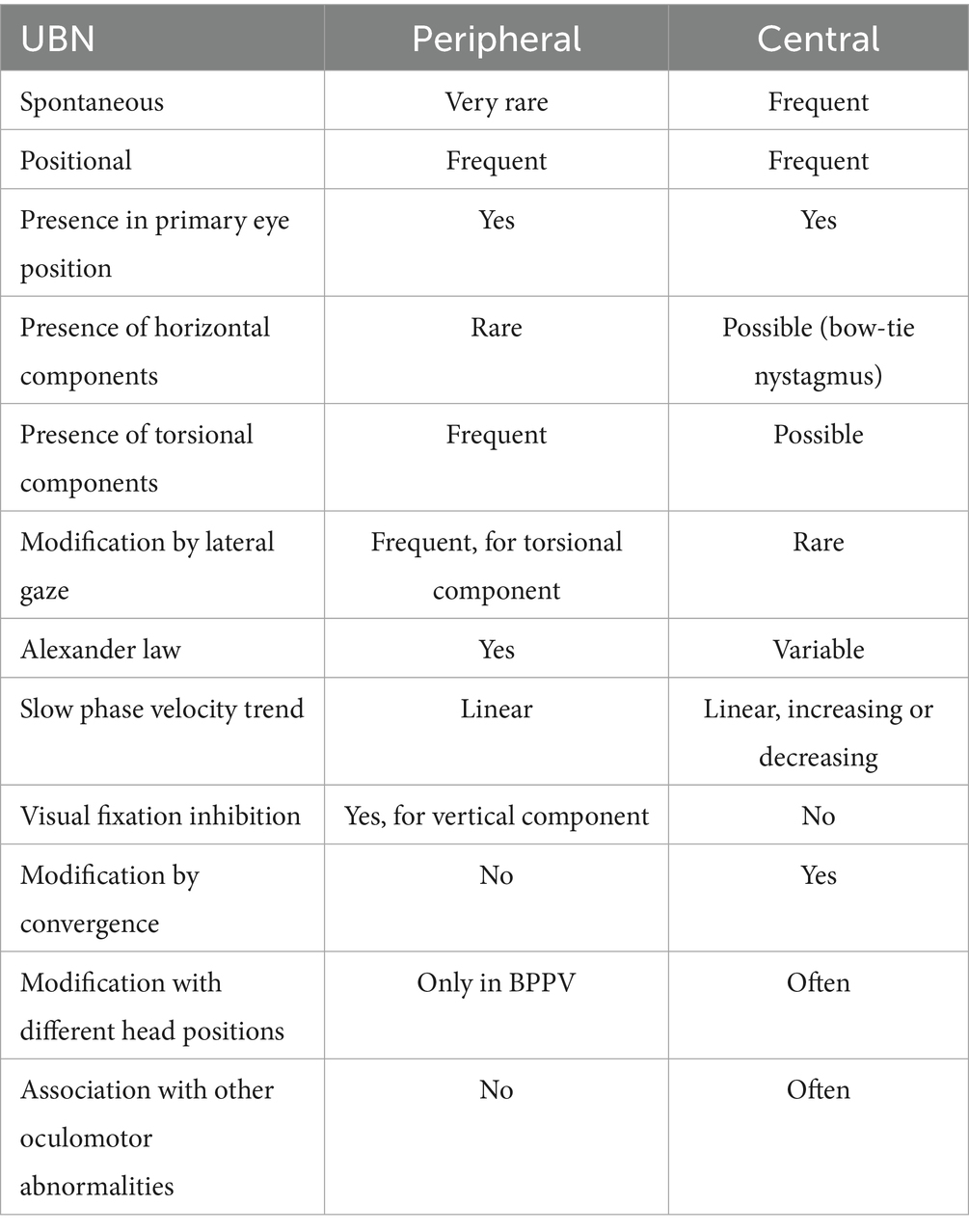
7.1 UBN in peripheral vestibular diseases
Although UBN is typically associated with central vestibular or brainstem lesions, rare cases of peripheral origin have been documented. Ichimura and Itani (100) reported a case of persistent positional UBN in a patient with bilateral posterior canal benign paroxysmal positional vertigo (BPPV) due to canalolithiasis. The nystagmus was elicited during the transition from an upright seated to a straight supine position, characterized by a latency of approximately 2 s and a maximum duration of 110 s. The absence of neurological signs, normal brain imaging, and spontaneous resolution supported a peripheral etiology. Similar cases have been described in earlier literature (101, 102). More frequently, a mixed spontaneous nystagmus with an upbeat component is seen in peripheral vestibular dysfunction. For instance, Fetter and Dichgans documented such a case in superior vestibular neuritis (VN) (103–105). In a retrospective study by Ling et al. (106), 43 patients with UBN were reviewed, and peripheral vestibular disorders were identified in 14 (32.6%) of them. These included 10 cases of superior acute unilateral vestibulopathy (AUVP), 1 complete AUVP, 1 probable labyrinthine infarction, 1 isolated acute unilateral utricular vestibulopathy, and 1 probable Ménière’s disease. Therefore, while rare, peripheral causes of UBN must be considered. A comprehensive diagnostic approach - integrating clinical history, symptomatology, neurological and neuroimaging evaluation, and especially what we call “semeiological features with different diagnostic weight” (e.g., nystagmus direction and characteristics, smooth pursuit, saccadic eye movements, skew deviation) are essential for accurate localization and differentiation.
Table 2 reports key points for distinguish between central or peripheral origins of the UBN.
8 Treatment of UBN and related symptoms
Management UBN may be either causal or symptomatic, depending on the underlying etiology. In cases of toxic, metabolic, deficiency-related, or autoimmune conditions, identification and elimination of the triggering factor may allow for resolution or reduction of the nystagmus. However, when causal treatment is not feasible and spontaneous remission does not occur, symptomatic therapy becomes necessary.
The primary aim of symptomatic treatment is to improve visual stability and reduce oscillopsia, while preserving normal ocular motor function, and enhancing postural control.
Although carbamazepine, an antiepileptic agent, has occasionally been reported to reduce UBN (107), the most commonly employed pharmacological agents include baclofen, 4-aminopyridine (4-AP), and memantine, which may be used individually or, in cases of insufficient efficacy, in various combinations.
Baclofen, a GABA B receptor agonist, reduces the slow-phase velocity of nystagmus and alleviates oscillopsia (108) by potentiating the inhibitory influence of the vestibulocerebellum on vestibular nuclei.
4-aminopyridine (4-AP), a potassium channel blocker, has demonstrated efficacy in attenuating UBN and associated visual disturbances, restore impaired upward smooth pursuit (93, 109), and modulate macular-driven vertical gaze control (27). The lack of efficacy in darkness suggests its action may involve facilitation of visually dependent parallel pathways that suppress UBN in lighted conditions. Moreover, 4-AP may increase cerebellar Purkinje cell excitability, thereby enhancing parallel compensatory circuits.
Memantine, a non-competitive NMDA receptor antagonist, may be beneficial in selected patients (110).
In summary, pharmacological management of UBN should be tailored to the etiology and symptom burden, with consideration given to both targets and patient-specific responses.
9 Conclusion
A highly significant finding, often indicative of central vestibular dysfunction, is UBN. Only recently has its diagnostic importance been widely recognized, thanks to advancements in research on its origin and pathophysiology. We now have a detailed understanding of the neural structures responsible for the precise control of eye movements in the vertical plane, allowing us to focus on these structures when encountering UBN.
Today, UBN is considered a highly localizing pathological sign, and its identification should prompt thorough neuroimaging studies to detect the most common acute or chronic structural causes.
As a result, therapy for UBN and related symptoms can be causal. When this is not feasible, treatment can be symptomatic.
The goal of our study is to provide a comprehensive and detailed overview of UBN and to offer possible explanations for the different functional aspects observed in the presence of this finding. This paper would serve as a valuable resource not only for specialists evaluating these patients in a clinical setting but also for general practitioners who may encounter this sign and pathology in non-specialized contexts, such as in emergency care.
Author contributions
VM: Writing – review & editing, Data curation, Resources, Methodology, Writing – original draft, Conceptualization. BG: Data curation, Conceptualization, Writing – review & editing, Writing – original draft, Methodology. GV: Writing – original draft, Conceptualization, Writing – review & editing. MF: Conceptualization, Methodology, Writing – original draft. EM: Methodology, Writing – original draft, Conceptualization, Resources. MC: Conceptualization, Writing – review & editing. AF: Data curation, Validation, Resources, Conceptualization, Writing – review & editing, Investigation, Writing – original draft. VP: Conceptualization, Resources, Formal Analysis, Data curation, Methodology, Writing – review & editing, Writing – original draft, Investigation, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stengel, E. Zur Frage der Herdlokalisation bei spontanem Vertikalnystagmus. Zeitschr Ges Neurol Psychiat. (1935) 153:417–24. doi: 10.1007/BF02865757
2. Leigh, RJ, and David, SZ. The neurology of eye movements, 5 edn, contemporary neurology series. Online ed. New York: Oxford Academic (2015).
4. Fuchs, E In: A Duane, editor. Fuchs’ text-book of ophthalmology. 3rd ed. Philadelphia: JB Lippincott (1908)
5. Wilbrand, H, and Saenger, A. Die Pathologie der Bahnen und Centren der Augenmuskeln In: JF Bergmann, editor. Die Neurologie des Auges, vol. VIII. München und Wiesbaden (1922)
6. Berens, C, and McAlpine, PT. Motor anomalies of the eye In: C Berens, editor. The eye and its diseases. 2nd ed. Philadelphia: WB Saunders (1950). 765–6.
8. Fisher, A, Gresty, M, Chambers, B, and Rudge, P. Primary position upbeating nystagmus. A variety of central positional nystagmus. Brain. (1983) 106:949–64. doi: 10.1093/brain/106.4.949
9. Weissman, JD, Seidman, SH, Dell’Osso, LF, Naheedy, MH, and Leigh, RJ. Torsional, see-saw, “bow-tie” nystagmus in association with brain stem anomalies. Neuro-Ophthalmology. (1990) 10:315–8. doi: 10.3109/01658109009009629
10. Thurtell, MJ, Tomsak, RL, and Leigh, RJ. Upbeat-torsional nystagmus and contralateral fourth-nerve palsy due to unilateral dorsal ponto mesencephalic lesion. Ann N Y Acad Sci. (2009) 1164:476–8. doi: 10.1111/j.1749-6632.2008.03713.x
12. Kim, HA, Yi, HA, and Lee, H. Can upbeat nystagmus increase in downward, but not upward, gaze? J Clin Neurosci. (2012) 19:600–1. doi: 10.1016/j.jocn.2011.07.033
13. Shin, BS, Oh, SY, Kim, JS, Lee, H, Kim, EJ, and Hwang, SB. Upbeat nystagmus changes to downbeat nystagmus with upward gaze in a patient with Wernicke’s encephalopathy. J Neurol Sci. (2010) 298:145–7. doi: 10.1016/j.jns.2010.08.012
14. Nabors, G, and Lavin, PJM. Convergence induced upbeat nystagmus. Neuro-Ophthalmology. (1990) 10:177–80. doi: 10.3109/01658109008997279
15. Cox, TA, Corbett, JJ, Thompson, HS, and Lennarson, L. Upbeat nystagmus changing to downbeat nystagmus with convergence. Neurology. (1981) 31:891–2. doi: 10.1212/wnl.31.7.891
16. Carl, JR, Yee, RD, and Baloh, RW. Convergence and gaze effects on vertical nystagmus. Invest Ophthalmol Vis Sci (ARVO Suppl). (1982) 22:265.
17. Rousseaux, M, Dupard, T, Lesoin, F, Barbaste, P, and Hache, JC. Upbeat and downbeat nystagmus occurring successively in a patient with posterior medullary haemorrhage. J Neurol Neurosurg Psychiatry. (1991) 54:367–9. doi: 10.1136/jnnp.54.4.367
18. Patel, VR, and Zee, DS. The cerebellum in eye movement control: nystagmus, coordinate frames and disconjugacy. Eye (Lond). (2015) 29:191–5. doi: 10.1038/eye.2014.271
19. Stein, B, and Carpente, MB. Central projections of portions of the vestibular ganglia innervating specific parts of the labyrinth in the rhesus monkey. Am J Anat. (1967) 120:281–317. doi: 10.1002/aja.1001200205
20. Büttner-Ennever, JA. A review of otolith pathways to brainstem and cerebellum. Ann N Y Acad Sci. (1999) 871:51–64. doi: 10.1111/j.1749-6632.1999.tb09175.x
21. Laurens, J, Meng, H, and Angelaki, DE. Computation of linear acceleration through an internal model in the macaque cerebellum. Nat Neurosci. (2013) 16:1701–8. doi: 10.1038/nn.3530
22. Kattah, JC, Tehrani, AS, du Lac, S, Newman-Toker, DE, and Zee, DS. Conversion of upbeat to downbeat nystagmus in Wernicke encephalopathy. Neurology. (2018) 91:790–6. doi: 10.1212/WNL.0000000000006385
23. Kattah, JC, and Dagi, TF. Compensatory head tilt in upbeating nystagmus. J Clin Neuroophthalmol. (1990) 10:27–31. doi: 10.3109/01658109008997258
24. Kim, JS, Yoon, B, Choi, KD, Oh, SY, Park, SH, and Kim, BK. Upbeat nystagmus: clinicoanatomical correlations in 15 patients. J Clin Neurol. (2006) 2:58–65. doi: 10.3988/jcn.2006.2.1.58
25. Habek, M, Gabelić, T, Pavliša, G, and Brinar, VV. Central positioning upbeat nystagmus and vertigo due to pontine stroke. J Clin Neurosci. (2011) 18:977–8. doi: 10.1016/j.jocn.2010.11.021
26. Wray, SH, Martinez-Hernandez, E, Dalmau, J, Maheshwari, A, Chen, A, King, S, et al. Paraneoplastic upbeat nystagmus. Neurology. (2011) 77:691–3. doi: 10.1212/WNL.0b013e318229e6a5
27. Helmchen, C, Sprenger, A, Rambold, H, Sander, T, Kömpf, D, and Straumann, D. Effect of 3, 4-diaminopyridine on the gravity dependence of ocular drift in downbeat nystagmus. Neurology. (2004) 63:752–3. doi: 10.1212/01.WNL.0000136226.52639.35
28. Mizuno, M, Kudo, Y, and Yamane, M. Upbeat nystagmus influenced by posture: report of two cases. Auris Nasus Larynx. (1990) 16:215–21. doi: 10.1016/s0385-8146(12)80129-5
29. Choi, KD, Jung, DS, Park, KP, Jo, JW, and Kim, JS. Bowtie and upbeat nystagmus evolving into hemi-seesaw nystagmus in medial medullary infarction: possible anatomic mechanisms. Neurology. (2004) 62:663–5. doi: 10.1212/01.WNL.0000110186.05217.9B
30. Kattah, JC, McClelland, C, and Zee, DS. Vertical nystagmus in Wernicke’s encephalopathy: pathogenesis and role of central processing of information from the otoliths. J Neurol. (2019) 266:139–45. doi: 10.1007/s00415-019-09326-9
31. Sakuma, A, Kato, I, Ogino, S, Okada, T, and Takeyama, I. Primary position upbeat nystagmus with special reference to alteration to downbeat nystagmus. Acta Otolaryngol Suppl. (1996) 522:43–6.
32. Suzuki, Y, Matsuda, T, Washio, N, and Ohtsuka, K. Transition from upbeat to downbeat nystagmus observed in a patient with Wernicke’s encephalopathy. Jpn J Ophthalmol. (2005) 49:220–2. doi: 10.1007/s10384-004-0182-8
33. Keane, JR, and Itabashi, HH. Upbeat nystagmus: clinicopathologic study of two patients. Neurology. (1987) 37:491–4. doi: 10.1212/wnl.37.3.491
34. Brandt, T, and Dieterich, M. Vestibular syndromes in the roll plane: topographic diagnosis from brainstem to cortex. Ann Neurol. (1994) 36:337–47. doi: 10.1002/ana.410360304
35. Marcelli, V, Giannoni, B, Volpe, G, Faralli, M, Fetoni, AR, and Pettorossi, VE. Downbeat nystagmus: a clinical and pathophysiological review. Front Neurol. (2024) 15:1394859. doi: 10.3389/fneur.2024.1394859
36. Matsuo, V, and Cohen, B. Vertical optokinetic nystagmus and vestibular nystagmus in the monkey: up-down asymmetry and effects of gravity. Exp Brain Res. (1984) 53:197–216. doi: 10.1007/BF00238150
37. Takahashi, M, Sakurai, S, and Kanzaki, J. Horizontal and vertical optokinetic nystagmus in man. ORL J Otorhinolaryngol Relat Spec. (1978) 40:43–52. doi: 10.1159/000275385
38. Harris, LR, Goltz, HC, and Steinbach, MJ. The effect of gravity on the resting position of the cat’s eye. Exp Brain Res. (1993) 96:107–16. doi: 10.1007/BF00230444.PMID: 8243573
39. Pierrot-Deseilligny, C, and Tilikete, C. New insights into the upward vestibulo-oculomotor pathways in the human brainstem. Prog Brain Res. (2008) 171:509–18. doi: 10.1016/S0079-6123(08)00673-0
40. Peterson, BW, Choi, H, Hain, T, Keshner, E, and Peng, GC. Dynamic and kinematic strategies for head movement control. Ann N Y Acad Sci. (2001) 942:381–93. doi: 10.1111/j.1749-6632.2001.tb03761.x
41. Böhmer, A, and Straumann, D. Pathomechanism of mammalian downbeat nystagmus due to cerebellar lesion: a simple hypothesis. Neurosci Lett. (1998) 250:127–30. doi: 10.1016/s0304-3940(98)00450-9
42. Sato, Y, and Kawasaki, T. Target neurons of floccular caudal zone inhibition in y-group nucleus of vestibular nuclear complex. J Neurophysiol. (1987) 57:460–80. doi: 10.1152/jn.1987.57.2.460
43. Carpenter, MB, and Cowie, RG. Connections and oculomotor projections of the superior vestibular nucleus and cell group ‘y’. Brain Res. (1985) 336:265–87. doi: 10.1016/0006-8993(85)90653-5
44. Pierrot-Deseilligny, C, Milea, D, Sirmai, J, Papeix, C, and Rivaud-Péchoux, S. Upbeat nystagmus due to a small pontine lesion: evidence for the existence of a crossing ventral tegmental tract. Eur Neurol. (2005) 54:186–90. doi: 10.1159/000090295
45. Bisdorff, AR, Sancovic, S, Debatisse, D, Bentley, C, Gresty, MA, and Bronstein, AM. Positional nystagmus in the dark in normal subjects. Neuro-Ophthalmology. (2000) 24:283–90. doi: 10.1076/0165-8107(200008)2411-VFT283
46. Kim, JI, Somers, JT, Stahl, JS, Bhidayasiri, R, and Leigh, RJ. Vertical nystagmus in normal subjects: effects of head position, nicotine and scopolamine. J Vestib Res. (2000) 10:291–300. doi: 10.3233/VES-2000-10606
47. Leigh, RJ, Das, VE, and Seidman, SH. A neurobiological approach to acquired nystagmus. Ann N Y Acad Sci. (2002) 956:380–90. doi: 10.1111/j.1749-6632.2002.tb02835.x
48. von Baumgarten, RJ, Baldrighi, G, Vogel, H, and Thümler, R. Physiological response to hyper-and hypogravity during rollercoaster flight. Aviat Space Environ Med. (1980) 51:145–54.
49. Clément, G, Andre-Deshays, C, and Lathan, CE. Effects of gravitoinertial force variations on vertical gaze direction during oculomotor reflexes and visual fixation. Aviat Space Environ Med. (1989) 60:1194–8.
50. Cheung, BS, Money, KE, and Howard, IP. Human gaze instability during brief exposure to reduced gravity. J Vestib Res. (1994) 4:17–27.
51. Büttner-Ennever, JA, and Büttner, U. The reticular formation In: JA Büttner-Ennever, editor. Neuroanatomy of the oculomotor system. Amsterdam: Elsevier Science Publishers BV (1988). 119–76.
52. McCrea, RA, Strassman, A, and Highstein, SM. Anatomical and physiological characteristics of vestibular neurons mediating the vertical vestibulo-ocular reflexes of the squirrel monkey. J Comp Neurol. (1987) 264:571–94. doi: 10.1002/cne.902640409
53. Pierrot-Deseilligny, C, and Milea, D. Vertical nystagmus: clinical facts and hypotheses. Brain. (2005) 128:1237–46. doi: 10.1093/brain/awh532
54. Torvik, A, and Brodal, A. The cerebellar projection of the perihypoglossal nuclei (nucleus intercalatus, nucleus praepositus hypoglossi and nucleus of roller) in the cat. J Neuropathol Exp Neurol. (1954) 13:515–27. doi: 10.1097/00005072-195410000-00002
55. Meling, TR, Nouri, A, May, A, Guinand, N, Vargas, MI, and Destrieux, C. Upbeat vertical nystagmus after brain stem cavernoma resection: a rare case of nucleus intercalatus/nucleus of roller injury. J Neurol. (2020) 267:2865–70. doi: 10.1007/s00415-020-09891-4
56. Cascella, M. The intercalatus nucleus of Staderini. J Hist Neurosci. (2016) 25:408–19. doi: 10.1080/0964704X.2015.1081515
57. Saito, T, Aizawa, H, Sawada, J, Katayama, T, and Hasebe, N. Lesion of the nucleus intercalatus in primary position upbeat nystagmus. Arch Neurol. (2010) 67:1403–4. doi: 10.1001/archneurol.2010.285
58. Brodal, A. Experimental demonstration of cerebellar connexions from the perihypoglossal nuclei (nucleus intercalatus, nucleus praepositus hypoglossal and nucleus of roller) in the cat. J Anat. (1952) 86:110–29.
59. Brodal, A. The perihypoglossal nuclei in the macaque monkey and the chimpanzee. J Comp Neurol. (1983) 218:257–69. doi: 10.1002/cne.902180303
60. Hirose, G, Ogasawara, T, Shirakawa, T, Kawada, J, Kataoka, S, Yoshioka, A, et al. Primary position upbeat nystagmus due to unilateral medial medullary infarction. Ann Neurol. (1998) 43:403–6. doi: 10.1002/ana.410430323
61. Munro, NA, Gaymard, B, Rivaud, S, Majdalani, A, and Pierrot-Deseilligny, C. Upbeat nystagmus in a patient with a small medullary infarct. J Neurol Neurosurg Psychiatry. (1993) 56:1126–8. doi: 10.1136/jnnp.56.10.1126
62. Munro, NA. The role of the nucleus intercalatus in vertical gaze holding. J Neurol Neurosurg Psychiatry. (1999) 66:552–3. doi: 10.1136/jnnp.66.4.552
63. Lee, H, Park, JY, and Kim, HA. Spontaneous upbeat contratorsional nystagmus with downbeat ipsitorsional nystagmus during horizontal gaze in chronic lateral medullary infarction. Neurol Sci. (2021) 42:2565–7. doi: 10.1007/s10072-020-04966-6
64. Choi, H, Kim, CH, Lee, KY, Lee, YJ, and Koh, SH. A probable cavernoma in the medulla oblongata presenting only as upbeat nystagmus. J Clin Neurosci. (2011) 18:1567–9. doi: 10.1016/j.jocn.2011.02.043
65. Larner, AJ, Bronstein, AM, and Farmer, SF. Role of the nucleus intercalatus in upbeat nystagmus. Ann Neurol. (1998) 44:840. doi: 10.1002/ana.410440523
66. Büttner-Ennever, JA, Horn, AK, Schmidtke, K, Büttner-Ennever, JA, Horn, AK, and Schmidtke, K. Cell groups of the medial longitudinal fasciculus and paramedian tracts. Rev Neurol (Paris). (1989) 145:533–9.
67. Büttner-Ennever, JA, and Horn, AK. Pathways from cell groups of the paramedian tracts to the floccular region. Ann N Y Acad Sci. (1996) 781:532–40. doi: 10.1111/j.1749-6632.1996.tb15726.x
68. Büttner-Ennever, JA. Paramedian tract cell groups: a review of connectivity and oculomotor function In: H Shimazu and Y Shinoda, editors. Vestibular and brain stem control of eye-head and body movements. Tokyo: Japan Scientific Societies Press (1992). 323–30.
69. Belknap, DB, and McCrea, RA. Anatomical connections of the prepositus and abducens nuclei in the squirrel monkey. J Comp Neurol. (1988) 268:13–28. doi: 10.1002/cne.902680103
70. McCrea, RA, Strassman, A, and Highstein, SM. Morphology and physiology of abducens motoneurons and internuclear neurons intracellularly injected with horseradish peroxidase in alert squirrel monkeys. J Comp Neurol. (1986) 243:291–308. doi: 10.1002/cne.902430302
71. McCrea, RA, Strassman, A, May, E, and Highstein, SM. Anatomical and physiological characteristics of vestibular neurons mediating the horizontal vestibulo-ocular reflex of the squirrel monkey. J Comp Neurol. (1987) 264:547–70. doi: 10.1002/cne.902640408
72. Strassman, A, Highstein, SM, and McCrea, RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol. (1986) 249:337–57. doi: 10.1002/cne.902490303
73. Strassman, A, Highstein, SM, and McCrea, RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol. (1986) 249:358–80. doi: 10.1002/cne.902490304
74. Nakamagoe, K, Iwamoto, Y, and Yoshida, K. Evidence for brainstem structures participating in oculomotor integration. Science. (2000) 288:857–9. doi: 10.1126/science.288.5467.857
75. Langer, T, Fuchs, AF, Scudder, CA, and Chubb, MC. Afferents to the flocculus of the cerebellum in the rhesus macaque as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. (1985) 235:1–25. doi: 10.1002/cne.902350102
76. Büttner-Ennever, JA, and Horn, AKE. Olszewski and Baxter’s Cytoarchitecture of the human brainstem. 3rd, revised and extended edition. S. Karger AG: Basel (2014).
77. Tilikete, C, Hermier, M, Pelisson, D, and Vighetto, A. Saccadic lateropulsion and upbeat nystagmus: disorders of caudal medulla. Ann Neurol. (2002) 52:658–62. doi: 10.1002/ana.10342
79. Brandt, T, and Dieterich, M. Central vestibular syndromes in roll, pitch, and yaw planes: topographic diagnosis of brainstem disorders. Neuro-Ophthalmology. (1995) 15:291–303. doi: 10.3109/01658109509044618
80. Büttner, U, Helmchen, C, and Büttner-Ennever, J. The localizing value of nystagmus in brainstem disorders. Neuro-Ophthalmology. (1995) 15:283–90. doi: 10.3109/01658109509044617
81. Adamec, I, Gabelić, T, Krbot, M, Ozretić, D, Milivojević, I, and Habek, M. Primary position upbeat nystagmus. J Clin Neurosci. (2012) 19:161–2. doi: 10.1016/j.jocn.2011.05.028
82. Cheron, G, Saussez, S, Gerrits, N, and Godaux, E. Existence in the nucleus incertus of the cat of horizontal-eye-movement-related neurons projecting to the cerebellar flocculus. J Neurophysiol. (1995) 74:1367–72. doi: 10.1152/jn.1995.74.3.1367
83. Yamamoto, M, Shimoyama, I, and Highstein, SM. Vestibular nucleus neurons relaying excitation from the anterior canal to the oculomotor nucleus. Brain Res. (1978) 148:31–42. doi: 10.1016/0006-8993(78)90376-1
84. Nakada, T, and Remler, MP. Primary position upbeat nystagmus. Another central vestibular nystagmus? J Clin Neuroophthalmol. (1981) 1:185–9. doi: 10.3109/01658108109004917
85. Benjamin, EE, Zimmerman, CF, and Troost, BT. Lateropulsion and upbeat nystagmus are manifestations of central vestibular dysfunction. Arch Neurol. (1986) 43:962–4. doi: 10.1001/archneur.1986.00520090086025
86. Mossuto-Agatiello, L. Caudal paramedian midbrain syndrome. Neurology. (2006) 66:1668–71. doi: 10.1212/01.wnl.0000218180.03127.11
87. Frohman, EM, Frohman, TC, Fleckenstein, J, Racke, MK, Hawker, K, and Kramer, PD. Ocular contrapulsion in multiple sclerosis: clinical features and pathophysiological mechanisms. J Neurol Neurosurg Psychiatry. (2001) 70:688–92. doi: 10.1136/jnnp.70.5.688
88. Anagnostou, E, Mandllos, D, Limbitaki, G, Papadimitriou, A, and Anastasopoulos, D. Positional nystagmus and vertigo due to a solitary brachium conjunctivum plaque. J Neurol Neurosurg Psychiatry. (2006) 77:790–2. doi: 10.1136/jnnp.2005.084624
89. Ranalli, PJ, and Sharpe, JA. Vertical vestibulo-ocular reflex, smooth pursuit and eye-head tracking dysfunction in internuclear ophthalmoplegia. Brain. (1988) 111:1299–317. doi: 10.1093/brain/111.6.1299
90. Aw, ST, Chen, L, Todd, MJ, Barnett, MH, and Halmagyi, GM. Vestibulo-ocular reflex deficits with medial longitudinal fasciculus lesions. J Neurol. (2017) 264:2119–29. doi: 10.1007/s00415-017-8607-8
91. Cremer, PD, Migliaccio, AA, Halmagyi, GM, and Curthoys, IS. Vestibulo-ocular reflex pathways in internuclear ophthalmoplegia. Ann Neurol. (1999) 45:529–33. doi: 10.1002/1531-8249(199904)45:4<529::AID-ANA18>3.0.CO;2-H
92. Dieterich, M, and Brandt, TH. Impaired motion perception in congenital nystagmus and acquired ocular motor palsy. Clin Vis Sci. (1987) 1:337–45.
93. Glasauer, S, Strupp, M, Kalla, R, Büttner, U, and Brandt, T. Effect of 4-aminopyridine on upbeat and downbeat nystagmus elucidates the mechanism of downbeat nystagmus. Ann N Y Acad Sci. (2005) 1039:528–31. doi: 10.1196/annals.1325.060
94. Kattah, JC, Dhanani, SS, Pula, JH, Mantokoudis, G, Tehrani, ASS, and Toker, DEN. Vestibular signs of thiamine deficiency during the early phase of suspected Wernicke encephalopathy. Neurol Clin Pract. (2013) 3:460–8. doi: 10.1212/01.CPJ.0000435749.32868.91
95. Ranalli, PJ, and Sharpe, JA. Contrapulsion of saccades and ipsilateral ataxia: a unilateral disorder of the rostral cerebellum. Ann Neurol. (1986) 20:311–6. doi: 10.1002/ana.410200307
96. Tilikete, C, Milea, D, and Pierrot-Deseilligny, C. Upbeat nystagmus from a demyelinating lesion in the caudal pons. J Neuroophthalmol. (2008) 28:202–6. doi: 10.1097/WNO.0b013e318183bd73
97. Yura, S, Sako, K, and Yonemasu, Y. Primary midbrain hemorrhage with upbeat nystagmus. Act Neuro. (1988) 10:239–45.
98. Hankey, GJ, Silbert, PL, and Edis, RH. Localising value of primary position upbeating nystagmus. Aust NZ J Med. (1987) 17:333–5. doi: 10.1111/j.1445-5994.1987.tb01239.x
99. Hirose, G, Kawada, J, Tsukada, K, Yoshioka, A, and Sharpe, JA. Upbeat nystagmus: clinicopathological and pathophysiological considerations. J Neurol Sci. (1991) 105:159–67. doi: 10.1016/0022-510x(91)90140-3
100. Ichimura, A, and Itani, S. Persistent upbeat positional nystagmus in a patient with bilateral Posterior Canal benign paroxysmal positional vertigo. Case Rep Otolaryngol. (2019) 2019:4281641–3. doi: 10.1155/2019/4281641
101. Beyea, JA, and Parnes, LS. Purely vertical upbeat nystagmus in bilateral posterior canal benign paroxysmal positional vertigo: a case report. Laryngoscope. (2010) 120:208–9. doi: 10.1002/lary.20730
102. Yetiser, S, and Ince, D. Vertical nystagmus during the seated-supine positional (straight head-hanging) test in patients with benign paroxysmal positional vertigo. J Laryngol Otol. (2014) 128:674–8. doi: 10.1017/S0022215114001480
103. Fetter, M, and Dichgans, J. Vestibular neuritis spares the inferior division of the vestibular nerve. Brain. (1996) 119:755–63. doi: 10.1093/brain/119.3.755
104. Yagi, T, Koizumi, Y, and Sugizaki, K. 3D analysis of spontaneous nystagmus in early stage of vestibular neuritis. Auris Nasus Larynx. (2010) 37:167–72. doi: 10.1016/j.anl.2009.05.008
105. Kim, JS. When the room is spinning: experience of vestibular neuritis by a neurotologist. Front Neurol. (2020) 11:157. doi: 10.3389/fneur.2020.00157
106. Ling, X, Wu, YX, Feng, YF, Zhao, TT, Zhao, GP, Kim, JS, et al. Spontaneous nystagmus with an upbeat component: central or peripheral vestibular disorders? Front Neurol. (2023) 14:1106084. doi: 10.3389/fneur.2023.1106084
107. Iwata, A, Takao, F, Kunimoto, M, and Inoue, K. Primary position upbeat nystagmus reversed with carbamazepine. Eur J Neurol. (1996) 3:260–3. doi: 10.1111/j.1468-1331.1996.tb00432.x
108. Dieterich, M, Straube, A, Brandt, T, Paulus, W, and Büttner, U. The effects of baclofen and cholinergic drugs on upbeat and downbeat nystagmus. J Neurol Neurosurg Psychiatry. (1991) 54:627–32. doi: 10.1136/jnnp.54.7.627
109. Glasauer, S, Kalla, R, Büttner, U, Strupp, M, and Brandt, T. 4-aminopyridine restores visual ocular motor function in upbeat nystagmus. J Neurol Neurosurg Psychiatry. (2005) 76:451–3. doi: 10.1136/jnnp.2004.045716
110. Thurtell, MJ, Joshi, AC, Leone, AC, Tomsak, RL, Kosmorsky, GS, Stahl, JS, et al. Crossover trial of gabapentin and memantine as treatment for acquired nystagmus. Ann Neurol. (2010) 67:676–80. doi: 10.1002/ana.21991
111. Hirose, G, Kawada, J, Tsukada, K, Komatsuzaki, A, and Sharpe, JA. Primary position upbeat nystagmus. Clinicopathologic study of four patients. Acta Otolaryngol Suppl. (1991) 481:357–60.
112. Kim, JS, and Han, YS. Medial medullary infarction: clinical, imaging, and outcome study in 86 consecutive patients. Stroke. (2009) 40:3221–5. doi: 10.1161/STROKEAHA.109.559864
113. Pongmoragot, J, Parthasarathy, S, Selchen, D, and Saposnik, G. Bilateral medial medullary infarction: a systematic review. J Stroke Cerebrovasc Dis. (2013) 22:775–80. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.010
114. Kim, GS, and Park, C. Upbeat nystagmus in a patient with a medial rostral medullary infarction. J Neurol Sci. (2017) 381:748. doi: 10.1016/j.jns.2017.08.2111
115. Lee, ES, Sung, KB, and Lee, TK. Teaching video neuro images: upbeat and horizontal gaze-evoked nystagmus in bilateral medial medullary infarction. Neurology. (2017) 89:e238. doi: 10.1212/WNL.0000000000004634
116. Md Isa, IA, Halim, SA, and Chuan, CY. Upbeat nystagmus and quadriplegia in a young girl with bilateral medial medullary syndrome. Neurology. (2021) 96:e1921–4. doi: 10.1212/WNL.0000000000011493
117. Kumar, EAA, and Chavalla, K. A rare case of medial medullary syndrome with upbeat nystagmus due to protein “S” deficiency and hypothyroidism (Hashimato’s thyroiditis) - a case report. IAIM. (2021) 8:34–44.
118. Vandana, KV, Jyothi, PJ, Lakshmanan, S, Jeyaraj, KM, Velayutham, SS, Sowmini, PR, et al. Inter-nuclear Opthalmoplegia with upbeat nystagmus and central facial palsy in medial medullary syndrome. Neurol India. (2024) 72:441–2. doi: 10.4103/NI.Neurol-India-D-23-00369
119. Shaikh, AG, Ghasia, FF, Rasouli, G, DeGeorgia, M, and Sundararajan, S. Acute onset of upbeat nystagmus, exotropia, and internuclear ophthalmoplegia—a tell-tale of ponto-mesencephalic infarct. J Neurol Sci. (2013) 332:56–8. doi: 10.1016/j.jns.2013.06.012
120. Furman, JM, Brownstone, PK, and Baloh, RW. Atypical brainstem encephalitis: magnetic resonance imaging and oculographic features. Neurology. (1985) 35:438–40. doi: 10.1212/WNL.35.3.438
121. Mahale, RR, Dutta, D, Kovoor, J, Mailankody, P, Padmanabha, H, and Mathuranath, PS. Upbeat nystagmus in late onset cerebellar ataxia: think of anti-glutamate decarboxylase 65 antibody-associated cerebellar ataxia. Ann Indian Acad Neurol. (2021) 24:441–3. doi: 10.4103/aian.AIAN_470_20
122. Khachikyan, N, Manasyan, A, and Hashemi, N. Nystagmus and imbalance as the presenting symptoms of anti gad antibody syndrome in a 67-year-old female. J Ophthalmol Res Rev Rep. (2024):1–2. doi: 10.47363/JORRR/2024(5)158
123. Feldman, D, Otero-Millan, J, and Shaikh, AG. Gravity-independent upbeat nystagmus in syndrome of anti-GAD antibodies. Cerebellum. (2019) 18:287–90. doi: 10.1007/s12311-018-0972-z
124. Gresty, MA, Bronstein, AM, Brookes, GB, and Rudge, P. Primary position upbeating nystagmus associated with middle ear disease. Neuro-Ophthalmology. (1988) 8:321–8. doi: 10.3109/01658108808996061
125. Odaka, M, Yuki, N, Yamazaki, K, and Hirata, K. Bickerstaff’s brainstem encephalitis associated with nystagmus. Rinsho Shinkeigaku. (1998); 38: 319–322
126. Alroughani, R, Thussu, A, and Guindi, RT. Yet another atypical presentation of anti-GQ1b antibody syndrome. Neurol Int. (2015) 7:5770. doi: 10.4081/ni.2015.5770
127. Garcia-Reitboeck, P, Thompson, G, Johns, P, Al Wahab, Y, Omer, S, and Griffin, C. Upbeat nystagmus in anti-Ma2 encephalitis. Pract Neurol. (2014) 14:36–8. doi: 10.1136/practneurol-2013-000524
128. Higashi-Shingai, K, Imai, T, Takeda, N, Uno, A, Nishiike, S, Horii, A, et al. 3D analysis of spontaneous upbeat nystagmus in a patient with astrocytoma in cerebellum. Auris Nasus Larynx. (2011) 39:216–9. doi: 10.1016/j.anl.2011.03.005
129. Gilman, N, Baloh, RW, and Tomiyasu, U. Primary position upbeat nystagmus. Clinicopathol Study Neurol. (1977) 27:294–8. doi: 10.1212/WNL.27.3.294
130. Furman, JM, Baloh, RW, Chugani, H, Waluch, V, and Bradley, WG. Infantile cerebellar atrophy. Ann Neurol. (1985) 17:399–402. doi: 10.1002/ana.410170417
131. Vernon, H, Cohen, J, De Nittis, P, Fatemi, A, McClellan, R, Goldstein, A, et al. Intellectual developmental disorder with cardiac arrhythmia syndrome in a child with compound heterozygous GNB5 variants. Clin Genet. (2018) 93:1254–6. doi: 10.1111/cge.13194
132. Zumstein, H, and Meienberg, O. Upbeat nystagmus and visual system disorder in Wernicke’s encephalopathy due to starvation. Neuro-Ophthalmology. (1982) 2:157–62. doi: 10.3109/01658108209009697
133. Abouaf, L, Vighetto, A, Magnin, E, Nove-Josserand, A, Mouton, S, and Tilikete, C. Primary position upbeat nystagmus in Wernicke’s encephalopathy. Eur Neurol. (2011) 65:160–3. doi: 10.1159/000324329
134. Neveling, R, and Kruse, KE. Über Nicotinnystagmus. Archiv fur Ohren-Nasen- und Kehlkopfheilkunde. (1961) 177:427–31. doi: 10.1007/BF02103301
135. Sibony, PA, Evinger, C, and Manning, KA. Tobacco-induced primary-position upbeat nystagmus. Ann Neurol. (1987) 21:53–8. doi: 10.1002/ana.410210110
136. Jay, WM, Marcus, RW, and Jay, MS. Primary position upbeat nystagmus with organophosphate poisoning. J Pediatr Ophthalmol Strabismus. (1982) 19:318–9. doi: 10.3928/0191-3913-19821101-09
137. Nakamagoe, K, Ishii, K, Tamaoka, A, and Shoji, S. Upward gaze-evoked nystagmus with organoarsenic poisoning. Neurology. (2006) 66:131–2. doi: 10.1212/01.wnl.0000191396.40700.eb
138. Good, WV, Brodsky, MC, Hoyt, CS, and Ahn, JC. Upbeating nystagmus in infants: a sign of anterior visual pathway disease. Binocular Vision Strabismus Q. (1990) 5:13–8.
139. Simonsz, HJ, Florijn, RJ, van Minderhout, HM, Bergen, AA, and Kamermans, M. Nightblindness-associated transient tonic downgaze (NATTD) in infant boys with chin-up head posture. Strabismus. (2009) 17:158–64. doi: 10.3109/09273970903396893
140. Kumar, A, Patni, AH, and Charbel, F. The Chiari I malformation and the neurotologist. Otol Neurotol. (2002) 23:727–35. doi: 10.1097/00129492-200209000-00021
141. Fujikane, M, Katayama, S, Hirata, K, and Sunami, S. Central diabetes insipidus complicated with upbeat nystagmus and cerebellar ataxia. Rinsho Shinkeigaku. (1992) 32:68–72.
142. Trobe, JD, Sharpe, JA, Hirsh, DK, and Gebarski, SS. Nystagmus of Pelizaeus-Merzbacher disease. Magnetic Search Coil Study Arch Neurol. (1991) 48:87–91. doi: 10.1001/archneur.1991.00530130099026
143. Yamazaki, K, Katayama, S, Ishihara, T, and Hirata, K. A case of Fisher’s syndrome with upbeat nystagmus. Rinsho Shinkeigaku. (1994) 34:489–92.
144. Hoyt, CS. Nystagmus and other abnormal ocular movements in children. Pediatr Clin N Am. (1987) 34:1415–23. doi: 10.1016/S0031-3955(16)36364-7
145. Robert, MP, Michel, S, Amjad, E, Boddaert, N, Desguerre, I, and Vidal, PP. Benign intermittent upbeat nystagmus in infancy: a new clinical entity. Eur J Paediatr Neurol. (2015) 19:262–5. doi: 10.1016/j.ejpn.2014.12.013
146. Forsythe, WI. Congenital hereditary vertical nystagmus. J Neurol Neurosurg Psychiatry. (1955) 18:196–8. doi: 10.1136/jnnp.18.3.196
147. Sogg, RL, and Hoyt, WF. Intermittent vertical nystagmus in a father and son. Arch Ophthalmol. (1962) 68:515–7. doi: 10.1001/archopht.1962.00960030519015
148. Hoyt, CS, and Gelbart, SS. Vertical nystagmus in infants with congenital ocular abnormalities. Ophthalmic Paediatr Genet. (1984) 4:155–61. doi: 10.3109/13816818409006115
149. Zingler, VC, Strupp, M, Jahn, K, Glaser, M, Herberger, S, Kretzschmar, HA, et al. Upbeat nystagmus as the initial clinical sign of Creutzfeldt-Jakob disease. Ann Neurol. (2005) 57:607–8. doi: 10.1002/ana.20435
150. Osborne, SF, and Vivian, AJ. Primary position upbeat nystagmus associated with amitriptyline use. Eye (Lond). (2004) 18:106. doi: 10.1038/sj.eye.6700529
151. Kotha, VK, and De Souza, A. Wernicke’s encephalopathy following hyperemesis gravidarum. A report of three cases. Neuroradiol J. (2013) 26:35–40. doi: 10.1177/197140091302600106
152. Hokazono, K, Geminiani, F, and Bertholdo, D. Acute up-beating nystagmus in a pregnant woman with hyperemesis gravidarum. Am J Ophthalmol Case Rep. (2017) 6:81–3. doi: 10.1016/j.ajoc.2017.01.008
Keywords: UBN, oscillopsia, unsteadiness, vertigo, treatment
Citation: Marcelli V, Giannoni B, Volpe G, Faralli M, Marcelli E, Cavaliere M, Fetoni AR and Pettorossi VE (2025) Upbeat nystagmus: a clinical and pathophysiological review. Front. Neurol. 16:1601434. doi: 10.3389/fneur.2025.1601434
Edited by:
Hubertus Axer, Jena University Hospital, GermanyReviewed by:
Yazhi Xing, Shanghai Jiao Tong University, ChinaMaria Dolores Villar Martinez, King’s College London, United Kingdom
Copyright © 2025 Marcelli, Giannoni, Volpe, Faralli, Marcelli, Cavaliere, Fetoni and Pettorossi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beatrice Giannoni, YmVhdHJpY2UuZ2lhbm5vbmlAdW5pZmkuaXQ=
 Vincenzo Marcelli
Vincenzo Marcelli Beatrice Giannoni
Beatrice Giannoni Giampiero Volpe
Giampiero Volpe Mario Faralli
Mario Faralli Edoardo Marcelli
Edoardo Marcelli Michele Cavaliere
Michele Cavaliere Anna Rita Fetoni
Anna Rita Fetoni Vito Enrico Pettorossi
Vito Enrico Pettorossi