Abstract
Introduction:
There are challenges associated with the timely diagnosis of people with mild cognitive impairment (MCI) and Alzheimer's disease (AD), especially with the future introduction of amyloid-targeting therapies. This study evaluates the current diagnostic journey for MCI and dementia due to AD in Spain.
Methods:
This study used data from the Adelphi Real World Dementia Disease Specific Programme (DSPTM), a cross-sectional survey with retrospective data collection. The survey involved primary care physicians (PCPs) and hospital specialists with experience in managing and treating AD, along with their consulting patients, between January and July 2023 in Spain. Analyses were descriptive.
Results:
Physicians (N = 94; 44.7% PCPs, 38.3% neurologists) reported data for 723 patients. Most patients (78.9%) first consulted a PCP. The median (inter-quartile range) time since symptom onset to first consultation was 21.6 (6.1–48.2) weeks and, in those patients who were not diagnosed immediately, the time from first consultation to diagnosis was 13.0 (6.0–21.9) weeks if diagnosed by a PCP, or 28.9 (17.1–52.1) weeks if diagnosed by a specialist. The diagnosing physician was a specialist or a PCP for 83.2% and 16.8% of patients, respectively. In addition, the most used advanced diagnostic techniques were computed tomography scans and magnetic resonance imaging (57.2% and 43.3% of patients, respectively). CSF determination of AD biomarkers was conducted in 12.4% of patients and AD-specific blood biomarkers in 2.9%. Treatment was mostly initiated by neurologists.
Conclusion:
The diagnostic process for people with MCI and AD could be accelerated by increased awareness of the disease, shorter referral times, and better access to specialized diagnostic services. This study shows limited use of AD-specific biomarker testing in Spain.
1 Introduction
Alzheimer's disease (AD) is a chronic and progressive neurodegenerative condition that ranges from a preclinical stage to mild cognitive impairment (MCI) and, finally, a phase of dementia (1, 2). Epidemiological studies have shown that most people with early-stage AD (MCI and dementia due to mild AD) remain undiagnosed, and misdiagnoses are common (3, 4). The reason is, in part, due to the diagnosis of AD being based exclusively on a clinical evaluation (5). The development of blood-based biomarkers and in vivo biomarkers, such as those based on cerebrospinal fluid (CSF) and positron emission tomography (PET), offer the potential for a more accurate and timely diagnosis of AD (6). Better management of risk factors can help take preventive lifestyle measures and care planning, and anticipate related pathologies (1, 7). In addition, the identification of suitable patients who may benefit from amyloid-targeting therapies as soon as possible after the first symptoms of MCI and dementia due to mild AD is essential (7–10).
In Spain, more than 900,000 people (2.2% of the total population in 2022) are estimated to be affected by AD, with approximately 40,000 new cases diagnosed annually (11, 12). However, accurate data on the epidemiology of the various stages of AD remain unavailable. A recent study showed that the prevalence of treated people with AD was estimated at 760.5 per 100,000 inhabitants (13). In addition to the large numbers of people with AD, the economic impact of dementia in the healthcare system and in society at large is huge and it is estimated to greatly increase with the progressive aging of the population (12). Since primary care is the entry point to the health system in Spain for people with MCI and AD, it is relevant to understand the factors that determine the identification, referral, and management of these patients (14). It is also unclear what specific symptoms drive the search for medical attention in people with AD or their caregivers at its early stages (15, 16), and the impact of diagnosis (17). AD diagnosis can be a multi-step and lengthy process involving several distinct healthcare providers and settings (18–20). Despite the availability of biomarkers and their associated technologies in Spain, an overloaded healthcare system has been identified as a barrier to speedier diagnosis of suspected patients (15). Understanding the current diagnostic process of people with MCI and AD in the Spanish healthcare system is of vital importance to identify areas for improvement.
A recent study evaluated the diagnostic journey and the challenges associated with the timely diagnosis of people with MCI and AD in real-world clinical practice in multiple countries, including Spain (21). This study was based on survey data of physicians relating to patients under their care with MCI and AD. Here, we present a more detailed analysis of the characteristics of the Spanish cohort, focusing on the clinical characteristics of people with MCI and AD and their clinical presentation, from primary care to hospital care, including the main diagnostic tests used and their associated waiting times. The study also analyzed the key factors that influence the search for medical help by people with MCI and AD in Spain, including symptoms, the healthcare professional who is initially consulted, and the causes of the delay in seeking medical help.
2 Materials and methods
Data were drawn from the Adelphi Real World Dementia Disease Specific Programme (DSPTM), a cross-sectional survey with retrospective data collection of primary care physicians and specialists with experience in managing and treating AD, and their consulting patients, between January and July 2023 in Spain. The DSP methodology has been previously published, validated (22–24), and found to be consistent over time (25). Data were collected anonymously and aggregated, ensuring that neither patients nor physicians could be identified. Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines, so ethics committee approval was not required.
2.1 Participants
The physicians included in this study were those who made treatment decisions for ≥5 patients per week [if a primary care physician (PCP)], or ≥10 patients per week (if a specialist, including neurologists, geriatricians, and psychogeriatricians) with a diagnosis of MCI or dementia/AD. PCPs and specialists were invited to take part in the survey through specialized recruitment agencies. Patients were eligible to be included if they were 50 years or older and had a physician-confirmed diagnosis of MCI or a specified form of dementia, including AD. Exclusion criteria included having solely vascular dementia, dementia resulting from environmental factors such as traumatic brain injury or alcoholism, or current participation in a clinical trial at the time of data collection.
2.2 Study procedures
Detailed methodology has been described previously (21). Physicians completed an attitudinal physician survey and then patient record forms for approximately nine consecutively consulting patients with a diagnosis of MCI or dementia/AD. The physician survey assessed perceived reasons for diagnostic delays, key barriers to timely diagnosis, and the role of biomarker testing in MCI/AD dementia. Patient record forms captured demographics, clinical characteristics, symptoms at first consultation, consulting and diagnosing physicians, time to diagnosis, diagnostic tests, disease severity, and current treatment and management. For each patient record form completed by physicians, the corresponding patient was invited to provide a self-completion form reporting reasons for delaying their first consultation about their memory problems. Completion of these forms was voluntary, so data were not obtained from all patients.
This analysis focused only on patients whom physicians classified as having either “MCI” or “AD dementia.” For those with dementia due to AD, physicians assessed current disease severity as mild, moderate, or severe based on medical records, consultation findings, patient/family feedback, and clinical judgment. The Mini-Mental State Examination (MMSE) scores were used to assess severity at the first consultation when available.
For data harmonization, patient self-reported data (e.g., reasons for delaying their first consultation with a healthcare practitioner) were included only when a corresponding patient record form completed by the physician was available for analysis, ensuring that patient-reported information was analyzed only for patients for whom we also have physician-provided data. Additionally, all physicians completed the attitudinal survey before providing information on patients seen in clinical practice, allowing the comparison of physicians' self-reported attitudes toward management and testing with their actual clinical practice at an individual patient level.
2.3 Statistical methods
Descriptive statistics included means and standard deviations for continuous variables and frequencies with percentages for categorical variables. Medians and interquartile ranges were also reported. Missing data were not imputed, leading to variable base sizes. Analyses were conducted using STATA® Version 17 (StataCorp LLC, College Station, Texas, USA).
3 Results
3.1 Physician and patient characteristics
A total of 94 physicians in Spain participated in the DSP, reporting data for 723 patients, and 102 patients' self-reported data. Of the physicians, 44.7% were PCPs, 38.3% neurologists, 7.4% geriatricians, and 9.6% other specialties; 77.2% of patients were seen in public and 22.8% in private settings (Supplementary Table S1). Physicians reported data for 723 patients with MCI or AD, whose sociodemographic and clinical characteristics are shown in Table 1. Patients had a mean (SD) age of 77.5 (7.7) years, and 54.1% were female. The diagnosis at the time of the survey was MCI for 37.3% of patients, and AD for 62.7%. In patients with an available MMSE score at the time of initial diagnosis (N = 501), MCI (MMSE score 27–28) was present in 10.4%, mild dementia (MMSE score 20–26) was found in 67.5%, and moderate or severe dementia (MMSE ≤ 19) in 22.2%. Most patients had a comorbidity (85.9%), with arterial hypertension (44.3%), anxiety (31.0%) and depression (28.1%), dyslipidemia (21.4%), and diabetes without chronic complications (14.2%) frequently reported. A total of 209 patients (33.7%) were taking anti-depressants as a comorbid-related treatment.
Table 1
| Variable | N = 723 |
|---|---|
| Age, mean (SD) | 77.5 (7.7) |
| Sex (women), n (%) | 391 (54.1) |
| Weight (kg), mean (SD) | 71.6 (11.3) |
| Current diagnosis, N(%), N = 711 | |
| MCI - suspected AD | 244 (34.3) |
| MCI - unknown AD | 21 (3.0) |
| AD with mild dementia | 89 (12.5) |
| AD with moderate dementia | 229 (32.2) |
| AD with severe dementia | 128 (18.0) |
| MMSE score at initial diagnosis, N(%), N = 501 | |
| Mild cognitive disease (MMSE = 27–28) | 52 (10.4) |
| Mild dementia (MMSE = 20–26) | 338 (67.5) |
| Moderate dementia (MMSE = 10–19) | 100 (20.0) |
| Severe dementia (MMSE = 0–9) | 11 (2.2) |
| Comorbidities a, N(%), N = 621 (85.9%) | |
| Arterial hypertension | 320 (44.3) |
| Anxiety | 224 (31.0) |
| Depression | 203 (28.1) |
| Dyslipidemia | 155 (21.4) |
| Diabetes without chronic complications | 103 (14.2) |
| Hyperlipidemia | 91 (12.6) |
| Back pain | 82 (11.3) |
| Insomnia/sleep disorders | 79 (10.9) |
| Osteoporosis | 77 (10.7) |
| Treatments for comorbidities, N(%), N = 621 | |
| Anti-depressantsb | 209 (33.7) |
| Anti-platelet | 54 (8.7) |
| Anti-coagulants | 49 (7.9) |
Physician-reported demographics and clinical characteristics of patients with a current MCI or AD dementia diagnosis.
aComorbidities diagnosed and present in ≥10% of patients. Multiple responses were possible.
bIncludes SSRI/SNRI antidepressants, MAOI inhibitors, other anti-depressants.
AD, Alzheimer's disease; MCI, mild cognitive impairment; MMSE, mini mental state examination; SD, standard deviation.
3.2 Pre-diagnosis
The most frequent symptoms that prompted patients to first consult with a physician (N = 697) were the loss of short-term memory (85.2% of patients), difficulties in concentration (37.4%), difficulties in recalling names and words (34.9%), difficulties managing finances and paying bills (29.7%), and other activities of daily life (Figure 1A). The individual who first noticed the person's cognitive decline and prompted initial consultation was generally the patient's partner or spouse (43.8%), followed by a daughter (21.5%) (Figure 1B). Only 13.8% of patients consulted for the first time on their own initiative.
Figure 1
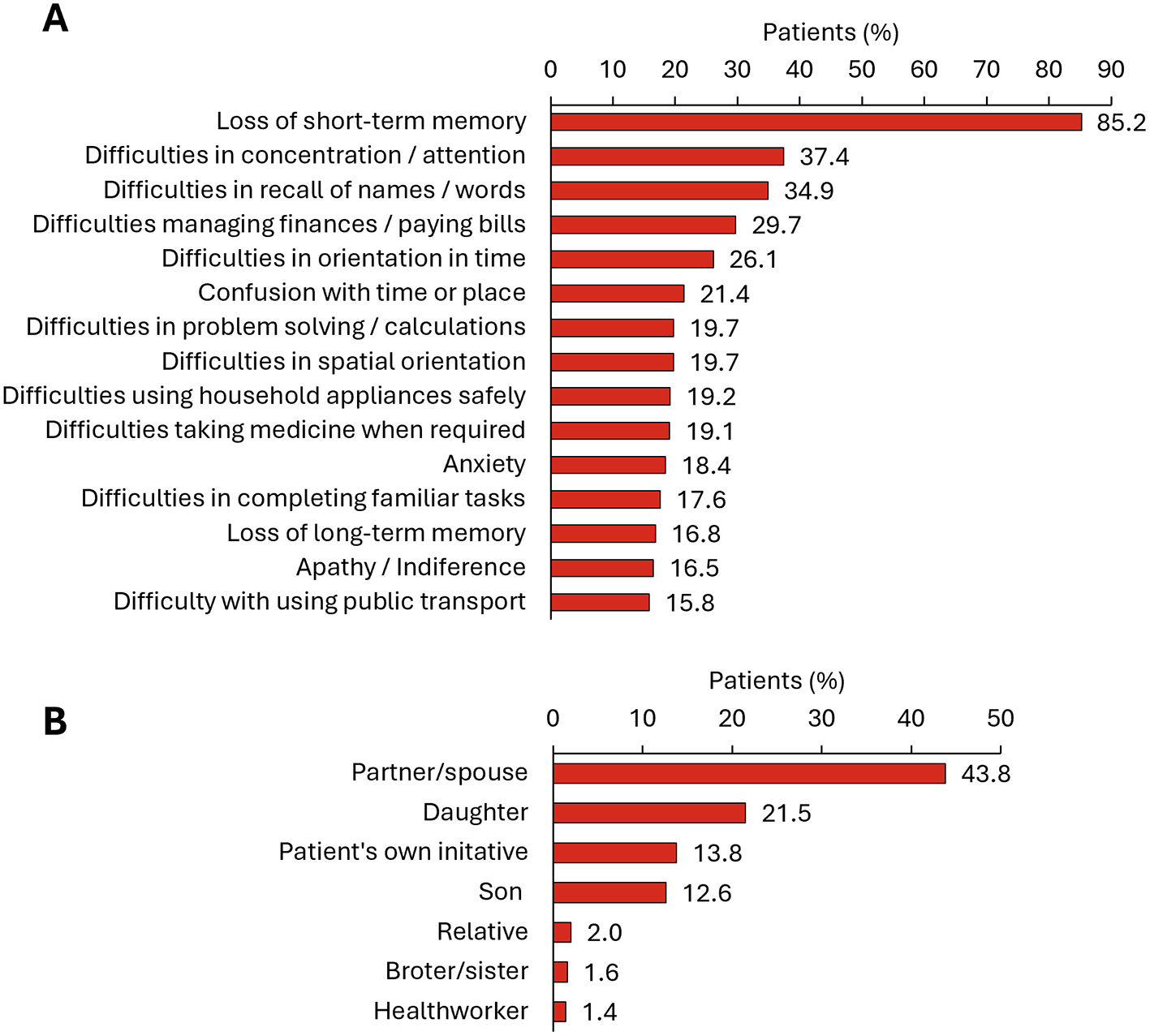
First MCI or dementia related consultation. (A) Symptoms prompting first consultation (list includes those symptoms reported by ≥15% of people with MCI or dementia) (N = 697); (B) Individual who first noticed the person's cognitive decline and prompted initial consultation (N = 708).
The median (IQR) time since the symptoms started to the first consultation was 21.6 (6.1–48.2) weeks (Figure 2A). In this study, 78.9% of patients consulted first with a PCP, and 21.1% to a hospital specialist (Figure 2B). The main reasons given by patients (N = 102) for delaying the first consultation were thinking that their memory problems were a normal part of aging (42.2%), being afraid of the diagnosis (18.6%), being worried about what other people would think (16.7%), or being worried about losing independence (15.7%) (Figure 3A). According to specialists, the main diagnostic barriers for early identification of patients with MCI and dementia due to mild AD was also the patients' delay due to lack of awareness of symptoms or stigma (MCI: 63.5%; AD with mild dementia: 50.0%) and lack of understanding of normal aging process (44.2%; 26.9%) (Figure 3B).
Figure 2
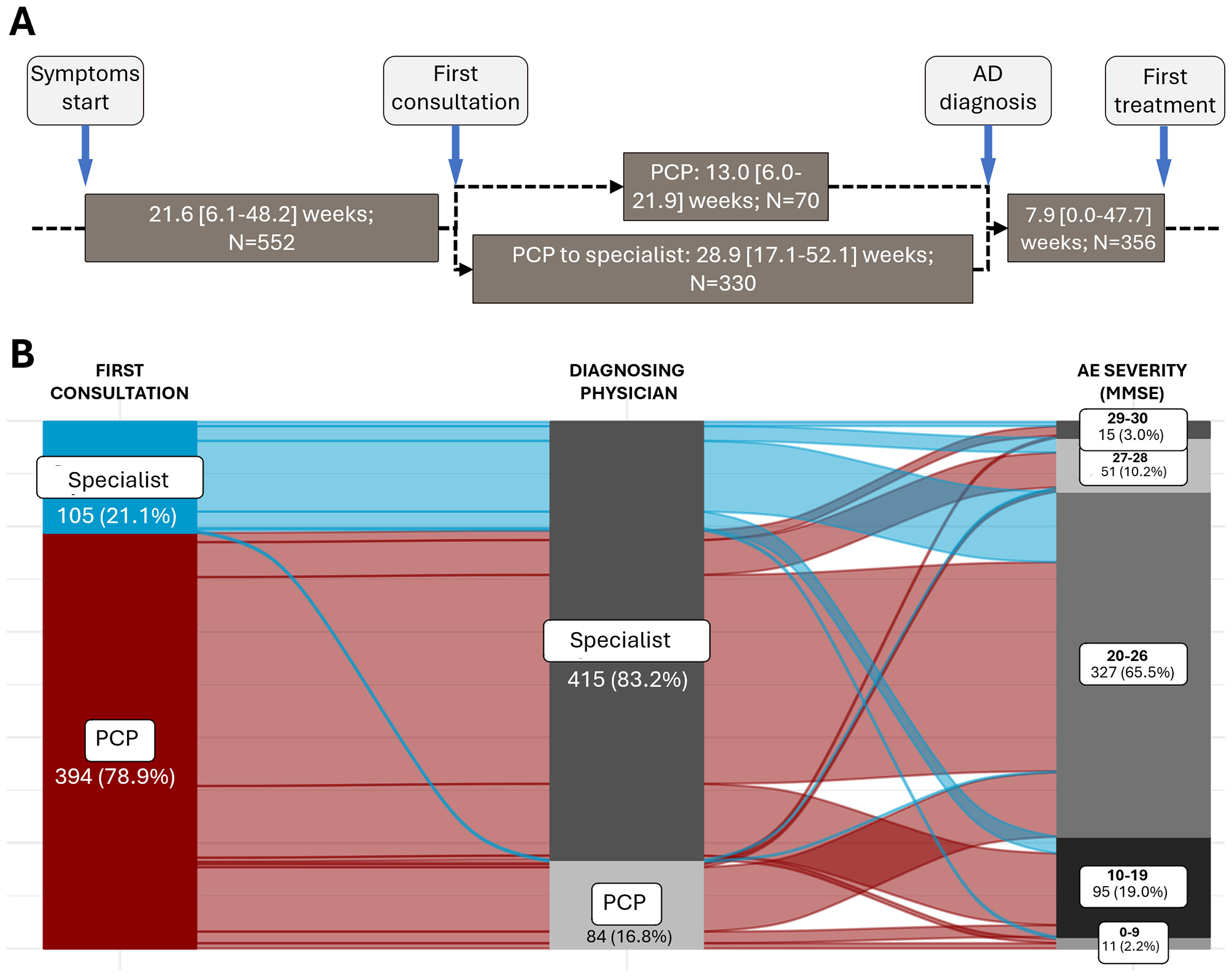
Timeline of AD diagnosis. (A) Timeline of the diagnostic process of people with MCI/AD. The “PCP to specialist” pathway indicates people who first consulted a PCP and are then referred to a hospital specialist for diagnosis. Values are median [IQR]. (B) Sankey graph representing the diagnostic journey of patients at different stages of AD severity according to the MMSE questionnaire. Only patients for whom all the information available in their history regarding the collected variables are shown (N = 499 patients).
Figure 3
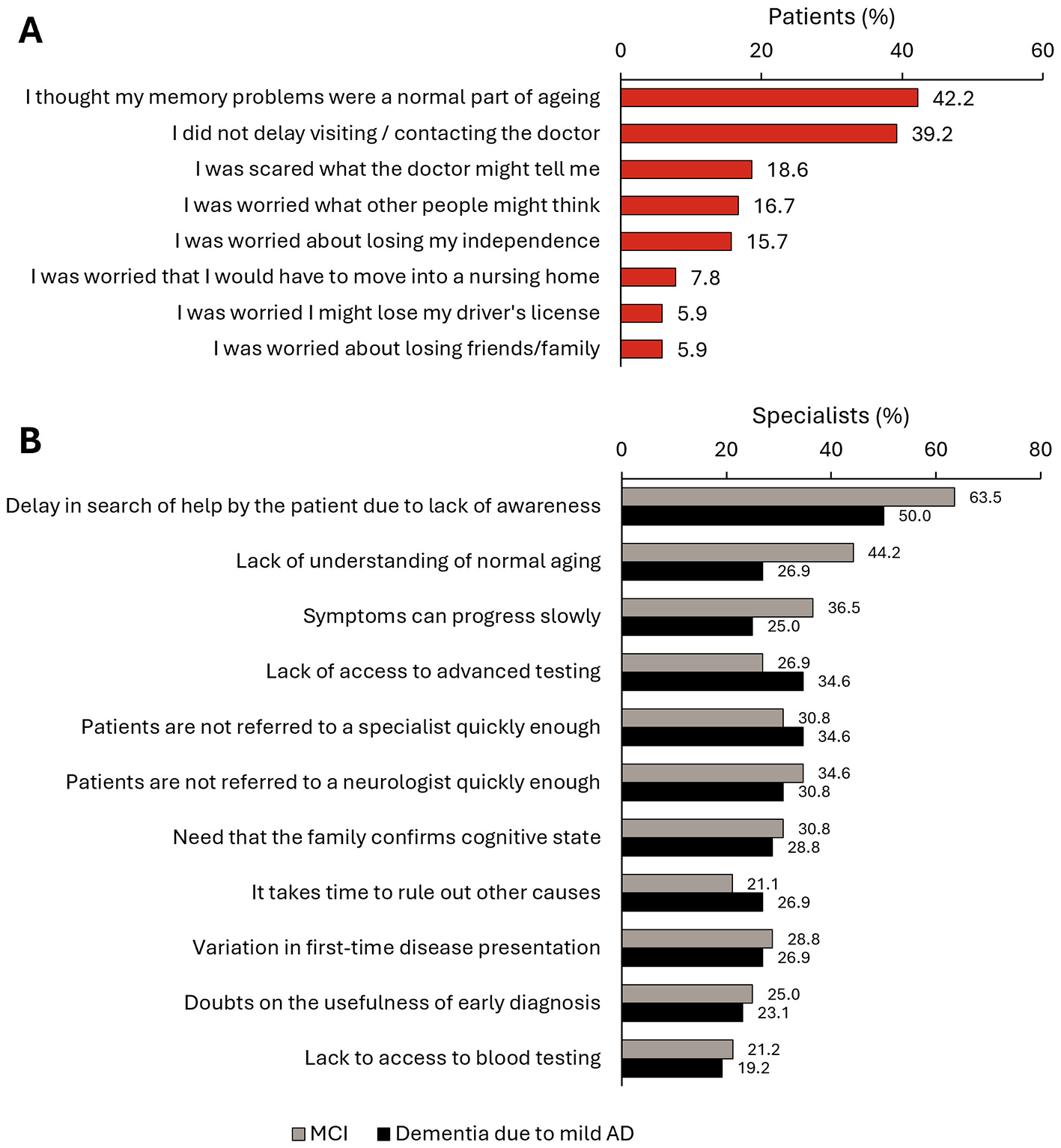
Delay in early diagnosis of dementia. (A) Reasons given by patients (N = 102) for delaying the first MCI-related consultation. (B) Main diagnostic barriers for early identification of patients with MCI and dementia due to mild AD according to specialists (N = 52).
3.3 Diagnosis
Although PCPs primarily conducted the first consultation, most patients were referred to neurologists or other hospital specialists. The diagnosing physician was a specialist for 83.2% of patients, and only 16.8% were diagnosed by a PCP (Figure 2B). Of patients diagnosed by a PCP, 86.9% had an MMSE score of 10–26 at diagnosis; for patients diagnosed by specialists, 84.1% had an MMSE score of 10–26, and 14.5% with MMSE 29–30 at diagnosis (data not shown). In patients who first consulted a PCP and were not diagnosed in the first consultation, the median time from the initial consultation to diagnosis differed if it was the PCP who diagnosed the patient [13.0 (6.0–21.9] weeks), or if the PCP referred the patient to a specialist, in which case the time to diagnosis extended to 28.9 [17.1–52.1] weeks (Figure 2A).
During the diagnosis process, physicians assessed the medical history of the patient and feedback from the patient's family and/or friends, and performed cognitive tests, a complete blood count, thyroid tests, vitamin B12 measurements, and a comprehensive metabolic panel (Figure 4). Among the cognitive tests, the MMSE and the clock draw test were performed on 86.4% and 42.1% of patients, respectively. At diagnosis, most patients (>65%) had MCI or mild dementia due to AD (MMSE score 20–26) (Figure 2B).
Figure 4
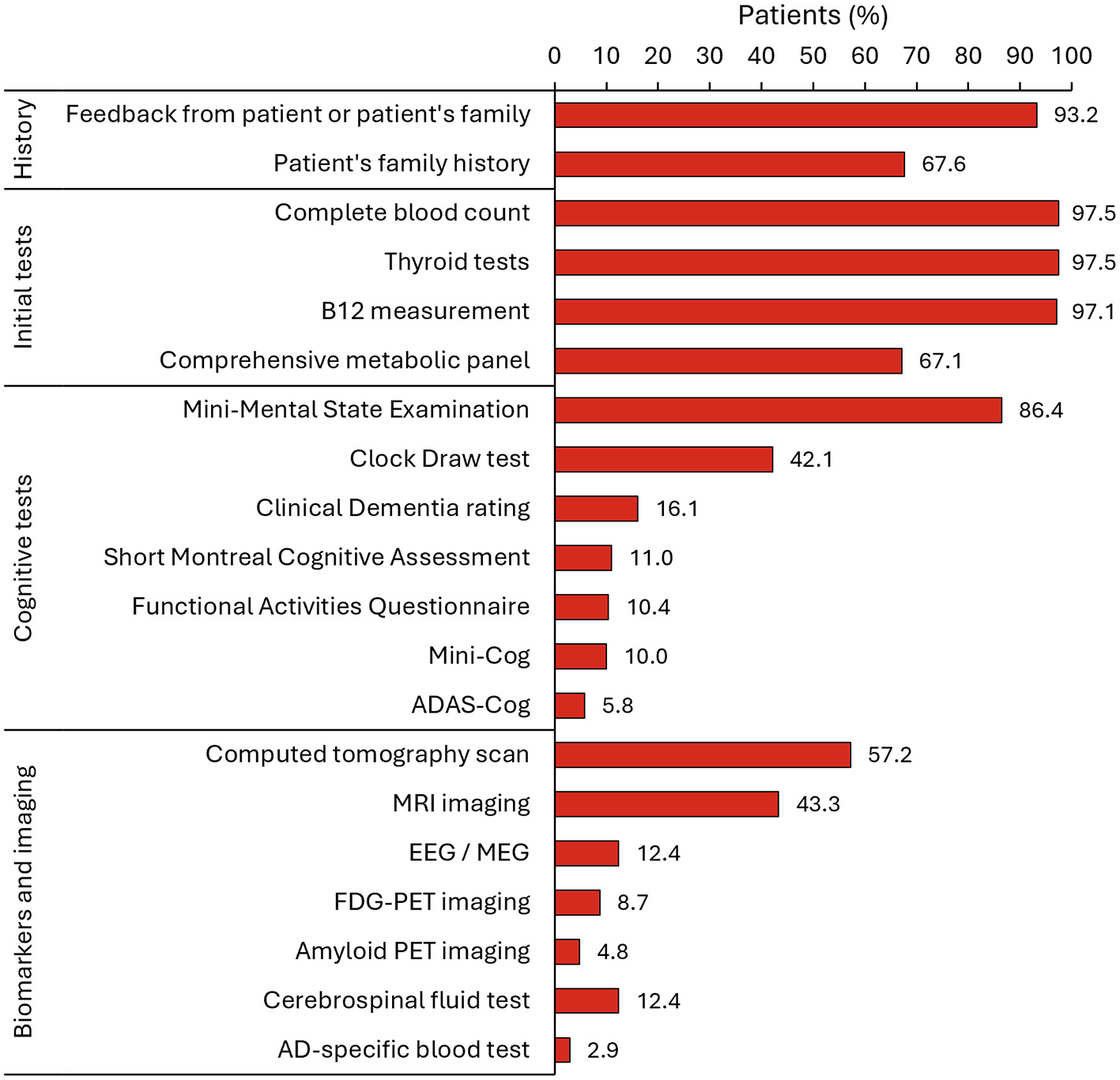
Specified tools that were used to aid in the diagnosis of AD (N = 691). AD, Alzheimer's disease; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; EEG, electroencephalogram; FDG-PET, fluorodeoxyglucose-positron emission tomography; MEG, magneto-encegalogram; MRI, magnetic resonance imaging; PET, positron emission tomography.
Further testing during the diagnosis process included imaging and the use of biomarkers. Computed tomography (CT) scans were performed on 57.2% of patients, magnetic resonance imaging (MRI) in 43.3%, EEG/MEG in 12.4%, FDG-PET in 8.7%, amyloid PET imaging in 4.8%, CSF determination of AD biomarkers in 12.4%, and AD-specific blood biomarkers in 2.9% (Figure 4). Specialists reported that the mean (SD) time elapsed between their referral to patients undergoing the test were 73.1 (48.5) days for amyloid PET, 61.0 (40.0) days for volumetric MRI, 32.1 (29.9) days for a blood test of AD biomarkers, and 30.8 (28.3) days for a CSF test (Supplementary Table S2).
The challenges faced by specialists when adopting AD biomarker testing during diagnosis in routine clinical practice are shown in Figure 5A. For AD specific blood tests, 51.9% of specialists believed that the biggest challenge was their costs to the healthcare system, and 23.1% thought that it has limited capabilities and precision in the prediction of AD. For CSF testing, the major challenges were the patient's reluctance to undergo the testing (53.8%), that it is not suitable for all types of patients (51.9%), and the high costs to the healthcare system (44.2%). Concerning PET imaging, the costs were also the main challenge (78.8%), followed by the requirement for resources and capabilities (46.2%) and the high cost to the patient (42.3%). Specialists considered that the most specific biomarkers for the identification of AD diagnosis were amyloid plaque (71.2%), CSF (total tau) (59.6%), and CSF (Aβ42) (55.8%) (Figure 5B). When asked about the future relevance of validated biomarkers in the identification of patients with AD with a clinical diagnosis of MCI, most specialists responded “important” (38.5%) or “extremely important” (48.1%) (Supplementary Figure S1A). On their plans to use various tests in the future, specialists indicated that they plan to use AD specific blood tests (85.4% of participants), CSF testing (34.1%), or PET imaging (40.4%) (Supplementary Figure S1B).
Figure 5

Use of biomarkers in routine clinical practice. (A) Views of specialists (N = 52) on various challenges in adopting precision biomarker testing in routine clinical practice. (B) Percentage of specialists (n = 52) who consider each specific biomarker during the AD diagnosis. AD, Alzheimer's disease; CSF, cerebrospinal fluid; NTFs, neurofibrilar tangles; PET, positron emission tomography.
3.4 Treatment
Physicians who initiated treatment were mostly neurologists (72.9%). PCPs initiated treatment in only 8.7% of patients, followed by geriatricians (7.4%) and geriatric psychiatrists (6.5%) (Figure 6A). The most commonly administered treatments were donepezil (46.9% of patients), followed by rivastigmine (patches) (31.3%), and memantine (22.5%) (Figure 6B).
Figure 6

Treatment initiation in all patients and patients with MCI or AD with mild dementia. (A) Physician who initiated the patient's first treatment after a diagnosis of AD (N = 565 patients). (B) Treatment received in the first line (N = 565 patients). Only treatments administered to >5% of patients are shown.
4 Discussion
The results of the large survey of Spanish physicians show that most people with MCI and dementia due to AD first see a PCP and are then referred and diagnosed by a hospital specialist (mainly a neurologist), who also initiated the treatment. Most patients were initially diagnosed in the mild dementia phase and a very small proportion of patients received a diagnosis utilizing biomarker tools during the assessment. However, most specialists consider the future use of precision biomarkers to be important for the diagnosis of early-stage AD and hope to incorporate blood-based biomarker testing into their clinical practice. Specialists perceived several challenges associated with the use of CSF-, PET-, or blood-based biomarkers in routine clinical practice, mostly related to the high costs to the healthcare system and the use of resources. According to specialists, the diagnostic process for people with MCI and AD could be accelerated by increased awareness of the disease, shorter referral times, and better access to specialized diagnostic services. Improving diagnosis is essential to optimize treatment as new amyloid-targeting therapies are expected to be used in the near future in clinical settings in Spain.
The characteristics of the patients reported by the participants in the study were consistent with previous studies of the Spanish population of people with MCI and AD, and highlight the high prevalence of comorbidities such as hypertension, depression and anxiety, diabetes, and hyperlipidemia (26–30). About one-third of the people attending the first consultation were already taking anti-depressants (30–32). In this regard, a recent study in Spain showed that people who seek help for cognitive problems reported higher levels of anxiety, depression, and concern about their perceived cognitive decline, compared to those who did not seek help (16).
In Spain, as in other countries, complaints related to memory or cognitive impairment are a common reason for consultation in primary care (33). The survey showed that short-term memory loss is the predominant symptom that drives people with MCI or AD to seek medical attention, usually prompted by their partner/spouse or daughter. Most patients initially consulted a PCP, who referred them to a hospital specialist, usually a neurologist, for diagnosis. As the first point of contact for patients experiencing cognitive decline, PCPs are in a unique position to recognize the early warning signs of AD, as their ongoing relationship with patients allows them to track cognitive changes over time, which can be essential for early intervention (6, 14). Also, PCPs can perform initial cognitive screenings and assessments, ruling out other possible causes of memory loss, such as vitamin deficiencies, depression, or medication side effects. PCPs provide continuous care by managing comorbid conditions, coordinating treatment plans, and offering support to caregivers (34). By advising caregivers on available resources and coping strategies, PCPs can be essential in improving the patient's quality of life (35).
Consistent with prior research, survey participants often estimated a substantial delay between the appearance of the first symptoms of cognitive impairment and the etiologic diagnosis of AD, especially when the diagnosis was conducted by hospital specialists (15, 20). This delay is in part due to the patient and/or the caregivers, who often attribute memory problems to the natural aging process, delaying the search for medical help and a diagnosis (36). Patients and families are often unaware that early diagnosis of AD is vital because it enables them to plan for the future, access appropriate medical treatments, and adopt lifestyle changes that may slow disease progression. However, the determinants of the delay in diagnosis are varied and complex, as shown in research in other countries, and include healthcare system barriers such as long waiting times for neurology appointments or insufficient training of PCPs in dementia diagnosis (15, 37–40). A prior survey in Spain showed that most PCPs considered diagnosis at the early stage of value, but that they did not have sufficient time to manage the patient (14).
Clinical assessment, based on patient and family medical history, conventional blood tests, and the MMSE brief cognitive assessment test, was the most common practice for AD diagnosis, as shown in prior studies (14, 20). Imaging with CT and MRI was also widely used (20, 41), but the use of AD-specific blood tests was very low. Blood tests for Alzheimer's disease are not yet widely available for clinical use in Spain. According to the specialists' perceptions, the main challenges for future routine adoption of AD specific blood tests would be the high costs to the healthcare system and a perceived lack of diagnostic precision for AD. Blood-based biomarker tests offer a less invasive, more accessible alternative, detecting key indicators like amyloid-beta and phosphorylated tau proteins (42, 43). In a recent position paper, the Spanish Society for Neurology advised that more data, training, and appropriate infrastructure are required for the use of blood-based biomarkers in general neurology clinics and in primary care (44). To generate confidence in the use of plasma biomarkers among clinicians, it is essential to understand the factors influencing plasma biomarker measurement and interpretation before incorporating them into routine clinical practice (45). In this sense, the vast majority of specialists in the survey considered the use of precision biomarkers important for the diagnosis of early AD, and plan on incorporating blood biomarker testing into their clinical practice in the future.
This study revealed that CSF testing was used in only 12.4% of patients and amyloid PET was used in 4.8%. In the case of CSF testing, the main challenges for routine adoption were the patient's reluctance to undergo testing, the fact that it is not suitable for all patients, and the costs to the healthcare system. The invasiveness of CSF testing and the associated patient concerns have been observed in other surveys in Spain and elsewhere (18, 20). In contrast, the challenges for the routine adoption of amyloid PET were mostly the costs for the healthcare system and its limited capacity (18). Nevertheless, the underuse of biomarkers of AD such as PET or CSF, which are mostly accessible to hospital specialists, is noteworthy, as these biomarkers could allow for more timely interventions in patients with AD. The recent development and commercialization of amyloid-targeting therapies will accelerate the need for an accurate, fast, and cost-effective diagnosis of patients with early-stage AD (MCI and mild dementia) (46).
Although for most patients the PCP is mainly responsible for identifying the patients, performing the initial screening and referring them to the specialist for diagnostic confirmation, the results of this study show that generally the physician initiating a treatment for dementia was the neurologist. The most common treatments were the acetylcholinesterase inhibitors donepezil and rivastigmine, followed by the NMDA receptor antagonist memantine, consistent with clinical guidelines (47), and previous studies of prescription and of antidementia drugs in Spain and elsewhere (48, 49). Other medications (antidepressants, antipsychotics, and anxiolytics) were also used to manage common neuropsychiatric comorbidities associated to AD like anxiety, depression, and sleep disturbances.
Survey-based studies, like the one presented here, have inherent limitations that should be considered when interpreting the results. First, the sample does not necessarily represent the proportion of specialists diagnosing or treating patients with AD. For example, the participating physicians worked in centers that managed a sufficient number of patients to provide the required patient record forms, and the dataset may not be representative of under-resourced centers. Also, the cross-sectional design of the DSP prevented any conclusions about causal relationships. Recall bias, a common limitation of surveys, might also have affected the responses of physicians. However, physicians had the ability to refer to the patients' records while completing the survey, thus minimizing the possibility of recall bias. The DSP is based on a pseudo-random, convenience sample of physicians; while minimal inclusion criteria governed the selection of physicians, participation was influenced by their willingness to complete the survey. Participating patients may not reflect the general AD dementia population since the DSP only includes patients who are actively consulting with their physician.
This study examined the real-world patient journey and barriers to timely diagnosis of MCI and AD dementia in Spain. The data from the study revealed that the delays in initial consultations were mainly due to lack of patient awareness about the normal course of aging, which led them to believe that their cognitive impairment was part of this process. Conversely, diagnostic delays stemmed from the time required for specialist referrals and scheduling of tests. AD-specific and validated biomarkers are used in a very low percentage of patients and waiting times for them are long. Enhancing patient awareness and utilizing accurate biomarker testing are essential to improving diagnosis and enabling the future initiation of treatments that may slow disease progression.
Statements
Data availability statement
The data that support the findings of this survey are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Sarah Cotton at sarah.cotton@adelphigroup.com. Sarah Cotton is an employee of Adelphi Real World.
Ethics statement
Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines (50) and as such did not require ethics committee approval. Each survey was performed in full accordance with relevant legislation at the time of data collection. No patients or physicians can be identified directly in the data. Data were aggregated before being shared with the subscriber and/or for publication. This study was granted ethical exemption by the Pearl Institutional Review Board, approval number (IRB number: #22-ADRW-172/3).
Author contributions
PS-J: Writing – review & editing, Investigation. PB: Writing – review & editing, Investigation. EA: Writing – review & editing, Investigation. DN: Writing – review & editing, Investigation. SD-C: Writing – review & editing, Investigation. SC: Writing – review & editing, Data curation, Investigation. CW: Writing – review & editing, Data curation, Investigation. ÁT: Writing – review & editing, Investigation. MN: Writing – original draft, Investigation.
Funding
The authors declared that financial support was received for this work and/or its publication. The publication of this article was funded by Eli Lilly and Company.
Acknowledgments
The sponsors acknowledge and thank the physicians and patients involved in the original research. Medical writing support was provided by Francisco López de Saro PhD, funded by Eli Lilly and Company. Andrés Sanz and Mikel Etxebeste assisted with graphics design.
Conflict of interest
The authors declared that this work received funding from Eli Lilly and Company. The funder had the following involvement in the study: interpretation of data, the writing of this article, and the decision to submit it for publication.
DN, SD-C, AT, and MN were employed by Eli Lilly & Company. SC and CW were employed by Adelphi Real World.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1702805/full#supplementary-material
References
1.
Atri A . The Alzheimer's disease clinical spectrum: diagnosis and management. Med Clin North Am. (2019) 103:263–93. doi: 10.1016/j.mcna.2018.10.009
2.
Guzman-Martinez L Calfío C Farias GA Vilches C Prieto R Maccioni RB . New frontiers in the prevention, diagnosis, and treatment of Alzheimer's disease. JAD. (2021) 82:S51–63. doi: 10.3233/JAD-201059
3.
Amjad H Roth DL Sheehan OC Lyketsos CG Wolff JL Samus QM . Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. (2018) 33:1131–8. doi: 10.1007/s11606-018-4377-y
4.
Giebel C Silva-Ribeiro W Watson J Volkmer A Chirico I Diaz A et al . Systematic review on the evidence of misdiagnosis in dementia and its impact on accessing dementia care. Int J Geriatr Psychiatry. (2024) 39:e6158. doi: 10.1002/gps.6158
5.
Dubois B Feldman HH Jacova C Hampel H Molinuevo JL Blennow K et al . Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. (2014) 13:614–29. doi: 10.1016/S1474-4422(14)70090-0
6.
Liss JL Seleri Assunção S Cummings J Atri A Geldmacher DS Candela SF et al . Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer's disease (MCI and dementia) in primary care: a review and synthesis. J Intern Med. (2021) 290:310–34. doi: 10.1111/joim.13244
7.
Rabinovici GD La Joie R . Amyloid-targeting monoclonal antibodies for Alzheimer disease. JAMA. (2023) 330:507–9. doi: 10.1001/jama.2023.11703
8.
Mintun MA Lo AC Duggan Evans C Wessels AM Ardayfio PA Andersen SW et al . Donanemab in early Alzheimer's disease. N Engl J Med. (2021) 384:1691–704. doi: 10.1056/NEJMoa2100708
9.
Sims JR Zimmer JA Evans CD Lu M Ardayfio P Sparks J et al . Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. (2023) 330:512–27. doi: 10.1001/jama.2023.21109
10.
van Dyck CH Swanson CJ Aisen P Bateman RJ Chen C Gee M et al . Lecanemab in early Alzheimer's disease. N Engl J Med. (2023) 388:9–21. doi: 10.1056/NEJMoa2212948
11.
Cantón-Habas V Rich-Ruiz M Romero-Saldaña M Carrera-González MDP . Depression as a risk factor for dementia and Alzheimer's disease. Biomedicines. (2020) 8:457. doi: 10.3390/biomedicines8110457
12.
Zapata Arriaza E López Fernández JC . “Impacto sociosanitario en España de la enfermedad de Alzheimer y otras demencias,” Impacto sociosanitario de las enfermedades neurológicas en España. Sociedad Española de Neurología (2024). Available online at: https://www.sen.es/pdf/2024/Informe_sociosanitario_2024.pdf (Accessed August 10, 2025).
13.
Olazarán J Carnero-Pardo C Fortea J Sánchez-Juan P García-Ribas G Viñuela F et al . Prevalence of treated patients with Alzheimer's disease: current trends and COVID-19 impact. Alzheimers Res Ther. (2023) 15:130. doi: 10.1186/s13195-023-01271-0
14.
Sannemann L Müller T Waterink L Zwan M Wimo A Stomrud E et al . General practitioners' attitude toward early and pre-dementia diagnosis of AD in five European countries—A MOPEAD project survey. Alzheimers Dement. (2021) 13:e12130. doi: 10.1002/dad2.12130
15.
Kerwin D Abdelnour C Caramelli P Ogunniyi A Shi J Zetterberg H et al . Alzheimer's disease diagnosis and management: perspectives from around the world. Alzheimers Dement. (2022) 14:e12334. doi: 10.1002/dad2.12334
16.
Villarejo-Galende A García-Arcelay E Piñol-Ripoll G Del Olmo-Rodríguez A Viñuela F Boada M et al . Medical help-seeking intentions among patients with early Alzheimer's disease. Front Psychiatry. (2023) 14:1290002. doi: 10.3389/fpsyt.2023.1290002
17.
Villarejo-Galende A García-Arcelay E Piñol-Ripoll G Del Olmo-Rodríguez A Viñuela F Boada M et al . Awareness of diagnosis in persons with early-stage Alzheimer's disease: an observational study in Spain. Neurol Ther. (2022) 11:1183–92. doi: 10.1007/s40120-022-00367-3
18.
Judge D Roberts J Khandker RK Ambegaonkar B Black CM . Physician practice patterns associated with diagnostic evaluation of patients with suspected mild cognitive impairment and Alzheimer's disease. Int J Alzheimers Dis. (2019) 2019:4942562. doi: 10.1155/2019/4942562
19.
Volpe U Amin H Ayinde OO Burns A Chan WC David R et al . Pathways to care for people with dementia: an international multicentre study. Int J Geriatr Psychiatry. (2020) 35:163–73. doi: 10.1002/gps.5223
20.
Roth S Burnie N Suridjan I Yan JT Carboni M . Current diagnostic pathways for Alzheimer's disease: a cross-sectional real-world study across six countries. J Alzheimers Dis Rep. (2023) 7:659–74. doi: 10.3233/ADR230007
21.
Vasileva-Metodiev SZ Spargo D Klein EG Quevenco FC Cotton S Sánchez-Juan P et al . Diagnostic journey and current management of patients with mild cognitive impairment and Alzheimer's disease, and challenges associated with timely diagnosis: a multinational, real-world survey. J Alzheimer's Dis. (2025) 104:1212–1234. doi: 10.1177/13872877251322978
22.
Anderson P Benford M Harris N Karavali M Piercy J . Real-world physician and patient behaviour across countries: disease-Specific Programmes - a means to understand. Curr Med Res Opin. (2008) 24:3063–72. doi: 10.1185/03007990802457040
23.
Babineaux SM Curtis B Holbrook T Milligan G Piercy J . Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. (2016) 6:e010352. doi: 10.1136/bmjopen-2015-010352
24.
Anderson P Higgins V Courcy JD Doslikova K Davis VA Karavali M et al . Real-world evidence generation from patients, their caregivers and physicians supporting clinical, regulatory and guideline decisions: an update on Disease Specific Programmes. Curr Med Res Opin. (2023) 39:1707–15. doi: 10.1080/03007995.2023.2279679
25.
Higgins V Piercy J Roughley A Milligan G Leith A Siddall J et al . Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. (2016) 9:371–80. doi: 10.2147/DMSO.S120101
26.
Andreu-Reinón ME Huerta JM Gavrila D Amiano P Mar J Tainta M et al . Incidence of dementia and associated factors in the EPIC-Spain dementia cohort. J Alzheimers Dis. (2020) 78:543–55. doi: 10.3233/JAD-200774
27.
Khandker RK Ritchie CW Black CM Wood R Jones E Hu X et al . Multi-national, cross-sectional survey of healthcare resource utilization in patients with all stages of cognitive impairment, analyzed by disease severity, country, and geographical region. J Alzheimers Dis. (2020) 75:1141–52. doi: 10.3233/JAD-190760
28.
Tortajada-Soler M Sánchez-Valdeón L Blanco-Nistal M Benítez-Andrades JA Liébana-Presa C Bayón-Darkistade E . Prevalence of comorbidities in individuals diagnosed and undiagnosed with Alzheimer's disease in León, Spain and a proposal for contingency procedures to follow in the case of emergencies involving people with Alzheimer's disease. Int J Environ Res Public Health. (2020) 17:3398. doi: 10.3390/ijerph17103398
29.
Villarejo-Galende A García-Arcelay E Piñol-Ripoll G Del Olmo-Rodríguez A Viñuela F Boada M et al . Quality of life and the experience of living with early-stage Alzheimer's disease. J Alzheimers Dis. (2022) 90:719–26. doi: 10.3233/JAD-220696
30.
Gracia-García P Bueno-Notivol J Lipnicki DM De La Cámara C Lobo A Santabárbara J . Clinically significant anxiety as a risk factor for Alzheimer's disease: results from a 10-year follow-up community study. Int J Methods Psych Res. (2023) 32:e1934. doi: 10.1002/mpr.1934
31.
Botto R Callai N Cermelli A Causarano L Rainero I . Anxiety and depression in Alzheimer's disease: a systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol Sci. (2022) 43:4107–24. doi: 10.1007/s10072-022-06068-x
32.
Sevil-Pérez A López-Antón R Gracia-García P De La Cámara C Gascón-Catalán A Santabárbara J . The association between major depression and Alzheimer's disease risk: evidence from a 12-year longitudinal study. JCM. (2024) 13:7039. doi: 10.3390/jcm13237039
33.
Vega Alonso T Miralles Espí M Mangas Reina JM Castrillejo Pérez D Rivas Pérez AI Gil Costa M et al . Prevalence of cognitive impairment in Spain: the Gómez de Caso study in health sentinel networks. Neurologia. (2018) 33:491–8. doi: 10.1016/j.nrleng.2016.10.002
34.
Brunton S Pruzin JJ Alford S Hamersky C Sabharwal A Gopalakrishna G . Perspectives of patients, care partners, and primary care physicians on management of mild cognitive impairment and mild Alzheimer's disease dementia. Postgrad Med. (2023) 135:530–8. doi: 10.1080/00325481.2023.22170s25
35.
Curto Romeu C Mora López G Gavaldà Espelta E Brunet Reverté N Gonçalves AQ Jacques-Aviñó C et al . Designing a multicomponent intervention to support caregivers of persons with dementia in primary care in Spain: a qualitative study of family and professional carers. BMJ Open. (2024) 14:e091599. doi: 10.1136/bmjopen-2024-091599
36.
de Miranda LFJR Matoso R de O Rodrigues MV de Lima TOL Nascimento AF Carvalho FC et al . Factors influencing possible delay in the diagnosis of Alzheimer's disease: findings from a tertiary Public University Hospital. Dement Neuropsychol. (2011) 5:328–31. doi: 10.1590/S1980-57642011DN05040011
37.
Bernstein A Rogers KM Possin KL Steele NZR Ritchie CS Kramer JH et al . Dementia assessment and management in primary care settings: a survey of current provider practices in the United States. BMC Health Serv Res. (2019) 19:919. doi: 10.1186/s12913-019-4603-2
38.
Dumas A Destrebecq F Esposito G Suchonova D Steen Frederiksen K . Rethinking the detection and diagnosis of Alzheimer's disease: outcomes of a European Brain Council project. Aging Brain. (2023) 4:100093. doi: 10.1016/j.nbas.2023.100093
39.
Glass J Boulay L Vasileva-Metiodiev S Walker C Cotton S Tarride J et al . Diagnostic journey and barriers to diagnosis for patients with mild cognitive impairment or dementia due to Alzheimer's disease in Canada: results from a real-world survey. Alzheimers Dement. (2024) 20:e095446. doi: 10.1002/alz.095446
40.
Cox CG Brush BL Kobayashi LC . Roberts JS. Determinants of dementia diagnosis in US primary care in the past decade: a scoping review. J Prev Alzheimers Dis. (2025) 12:100035. doi: 10.1016/j.tjpad.2024.100035
41.
Turró-Garriga O Viñas-Díez V Conde-Sala JL Calvó-Perxas L Cullell-Juncà M Mas-Vall-Llosera G et al . Caregivers' sense of coherence: implications on direct and indirect costs of dementia care. J Alzheimers Dis. (2020) 78:117–26. doi: 10.3233/JAD-200350
42.
Altuna-Azkargorta M Mendioroz-Iriarte M . Blood biomarkers in Alzheimer's disease. Neurologia. (2021) 36:704–10. doi: 10.1016/j.nrleng.2018.03.006
43.
Barthélemy NR Salvadó G Schindler SE He Y Janelidze S Collij LE et al . Highly accurate blood test for Alzheimer's disease is similar or superior to clinical cerebrospinal fluid tests. Nat Med. (2024) 30:1085–95. doi: 10.1038/s41591-024-02869-z
44.
Suárez-Calvet M Abdelnour C Alcolea D Mendióroz-Iriarte M Balasa M Morenas-Rodríguez E et al . Biomarcadores en sangre para la enfermedad de Alzheimer: posicionamiento y recomendaciones de uso del Grupo de Estudio de Conducta y Demencias de la Sociedad Española de Neurología. Neurología. (2025) 40:700–12. doi: 10.1016/j.nrl.2024.08.002
45.
Schöll M Vrillon A Ikeuchi T Quevenco FC Iaccarino L Vasileva-Metodiev SZ et al . Cutting through the noise: a narrative review of Alzheimer's disease plasma biomarkers for routine clinical use. J Prev Alzheimer's Dis. (2025) 12:100056. doi: 10.1016/j.tjpad.2024.100056
46.
Frisoni GB Festari C Massa F Cotta Ramusino M Orini S Aarsland D et al . European intersocietal recommendations for the biomarker-based diagnosis of neurocognitive disorders. Lancet Neurol. (2024) 23:302–12. doi: 10.1016/S1474-4422(23)00447-7
47.
Manzano MS Fortea J Villarejo A Sánchez del Valle R . Guía oficial de práctica clínica en demencia. Soc. Esp. Neurol. (2018). Available online at: https://www.sen.es/pdf/guias/Guia_Demencias_2018.pdf (Accessed August 10, 2025).
48.
Calvó-Perxas L Turró-Garriga O Vilalta-Franch J Lozano-Gallego M de Eugenio R Márquez F et al . Trends in the prescription and long-term utilization of antidementia drugs among patients with Alzheimer's disease in Spain: A cohort study using the registry of dementias of Girona. Drugs Aging. (2017) 34:303–10. doi: 10.1007/s40266-017-0446-x
49.
Garcia MJ Leadley R Lang S Ross J Vinand E Ballard C et al . Real-world use of symptomatic treatments in early Alzheimer's disease. J Alzheimers Dis. (2023) 91:151–67. doi: 10.3233/JAD-220471
50.
EPHMRA. Code of Conduct. (2022). Available online at: https://www.ephmra.org/sites/default/files/2022-08/EPHMRA%202022%20Code%20of%20Conduct.pdf (Accessed May 27, 2025).
Summary
Keywords
Alzheimer's disease, biomarkers, early diagnosis, mild cognitive impairment, neurocognitive disorders
Citation
Sánchez-Juan P, Baz P, Arrieta E, Novick D, Díaz-Cerezo S, Cotton S, Walker C, Trueba Saiz Á and Núñez M (2026) The diagnostic pathway of Alzheimer's disease in real-world clinical practice in Spain: results from the Adelphi Dementia Disease Specific Programme™. Front. Neurol. 16:1702805. doi: 10.3389/fneur.2025.1702805
Received
10 September 2025
Revised
26 November 2025
Accepted
05 December 2025
Published
20 January 2026
Volume
16 - 2025
Edited by
Cristian Sandoval, University of La Frontera, Chile
Reviewed by
Durjoy Lahiri, Queen's University, Canada
Najara Iacovino, University Hospital of Modena, Italy
Updates
Copyright
© 2026 Sánchez-Juan, Baz, Arrieta, Novick, Díaz-Cerezo, Cotton, Walker, Trueba Saiz and Núñez.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mercedes Núñez, nunez_mercedes@lilly.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.