Abstract
Study design:
Prospective cohort study.
Objectives:
To characterize patients with a new traumatic spinal cord injury and their pre-injury profiles.
Setting:
Tampere University Hospital, Finland.
Methods:
Newly injured patients (n = 46, male = 89%, mean age = 66y) with an acute cervical or thoracic traumatic spinal cord injury (TSCI) were recruited. They were evaluated and interviewed within 72 h postinjury. Health and medication history was gathered by interview and from electronic medical records. The International Standards for Neurological Classification of Spinal Cord Injury were used to classify the neurological consequences of TSCI. Epidemiological characteristics were recorded according to the International SCI Core Data Sets.
Results:
The leading causes of injury were low-level falls (48%), high-level falls (26%), and transport accidents (15%). Among patients >60 years, 63% were injured by low-level falls. Tetraplegia occurred in 87% of patients >60, compared to 63% ≤ 60 years. AIS D was the most common injury grade (44%). Complete injuries were seen in 38% of younger patients and 17% of older patients. Most patients had prior medication (72%) and at least one diagnosed disease (87%), both increasing in the older group. Overweight and low physical activity were common pre-injury characteristics. Alcohol preceded injury in 37% of cases. Low-level falls mostly caused cervical injuries (96%) and the patients seemed to have more diseases, fall-risk-increasing drugs and reduced physical activity levels compared to other etiologies.
Conclusion:
Low-level falls, particularly in older patients, were the leading cause of TSCI, often resulting in incomplete tetraplegia. Age-specific prevention strategies, especially fall prevention for older adults, are essential.
Introduction
Traumatic spinal cord injury (TSCI) leads to motor, sensory, and autonomic impairments, affecting multiple body systems and causing a lifelong risk of various secondary complications. It significantly affects the lives of those injured as well as their social environment. Each year, Finland records around 200 new cases of traumatic spinal cord injury (TSCI) (1), and the mortality rate is higher among affected individuals than in the general population (2).
As there is currently no cure for spinal cord injuries, preventive strategies are critical to avoid the burden on individuals and society. Traumatic spinal cord injuries impose significant economic costs on society; therefore, investing in preventive measures would be justified. Recent studies indicate a rising trend in the average age at the time of TSCI, along with an increase in the proportion of cervical injuries and those caused by falls (1, 3). Also, globally, the overall risk of injury from falls increases with age (4). Understanding the epidemiological characteristics of TSCI in even more detail is crucial not only for targeting preventive measures but also for the planning of clinical care and support services for affected individuals. To our knowledge, no previous studies have systematically assessed detailed pre-injury health information. Although the proportion of TSCI resulting from low-level falls is increasing, detailed data on the pre-injury health status of patients who sustain TSCI due to low-level falls is largely lacking. The aim of this study was to provide a more detailed understanding of who is affected by TSCI and to identify pre-injury individual characteristics in different age groups giving special consideration to the pre-injury profiles of patients whose TSCIs are caused by low-level falls.
Methods
Setting
During the time of this study, Finland was divided into 21 hospital districts, each responsible for organizing specialized health care within its designated area. Among these, there are five university hospital districts that support the surrounding districts and provide government-mandated, highly specialized medical care to their primary referral areas. Additionally, as of May 1st, 2011, Finnish government centralized the acute care, subacute rehabilitation and life-long follow-up of SCI patients to three university hospitals: Helsinki, Oulu, and Tampere. In Tampere University Hospital’s primary referral area, Tampere University Hospital serves as the sole tertiary referral center, offering 24/7 neurosurgery and spine surgery services to a population of approximately 490,000 inhabitants.
Study design
SUPERSTAR (Spinal Trauma Prognostication – A Tampere Research Initiative Aiming to Individualize Treatment) study is a prospective cohort study with a broad objective of improving the prognostication of individual long-term functional outcome following cervical and thoracic TSCI. The objective was approached by comprehensive data collection, consisting of pre-injury health information, injury-related characteristics, surgical and neurointensive care parameters, neuroimaging findings, blood-based neurotrauma biomarkers, genetic factors and artificial intelligence. The neurological consequences and the medical information on SCI-related issues were assessed at three time points: within the first 72 h, at 3 months, and at 1 year postinjury. In this initial study, we report the epidemiological findings of the SUPERSTAR cohort and focus on the pre- and peri-injury data. All the data provided in this substudy are based on the initial assessment. The 3-month and 1-year clinical follow-up data are not reported.
The study subjects consist of newly injured patients with acute cervical or thoracic TSCI. Patients were enrolled from the Tampere University Hospital, Tampere, Finland. The patient enrollment period lasted 32 months (Sep 2020–Apr 2023) with a 1-year follow-up period (until Apr 2024). All consecutive acute TSCI patients were applicable to participate in the study pertaining to the following inclusion criteria: (i) cervical or thoracic TSCI, (ii) admission to Tampere University Hospital within 24 h of injury, (iii) age 18 years or older, (iv) Finnish citizenship, (v) written informed consent, and (vi) no evidence of concurrent moderate or severe traumatic brain injury (Glasgow Coma Scale Score < 13 at admission or any time after). New patients were identified through reports by on-call surgeons or by daily review (study personnel) of the patient admission lists from the emergency department, neurosurgical ward, orthopedic ward and intensive care unit. Medical records of all potential patients were evaluated and eligibility was assessed. All enrolled patients gave informed consent to participate in the study, following the Declaration of Helsinki. If the patient was unable to provide a signature, the consent was taken verbally by the study physician accompanied by an additional external witness. In this case, the consent was signed by the study physician and the witness. The Ethics Committee of Pirkanmaa Hospital District, Tampere, approved the study protocol (R18182). Potential TSCI patients were screened daily and information obtained during patient screening was used to present the characteristics of the excluded patients.
Study variables and data collection
Pre-injury health: Health history of the patients was gathered from the electronic medical records utilizing the hospital’s records and national patient data repository (Kanta archive). The medication history was obtained using the national electronic prescription archive. Pre-injury health aspects were verified by patient interview conducted by the study physicians (E.L. and E.K.). Pre-injury diseases were categorized based on the ICD-10 coding. The use of five or more drugs (≥5) was defined as polypharmacy. Fall-risk increasing drugs (FRID) were assessed by the Screening Tool of Older Persons Prescriptions in older adults with high fall risk (STOPPFall) which included 14 medication categories (5). Frailty was assessed by the Clinical Frailty Scale (CFS); scores 1–3 are considered non-frail, a score 4 is considered pre-frail, and 5–9 are considered frail (6). Alcohol Use Disorders Identification Test (AUDIT) was used to assess alcohol consumption habits. Data of locomotion and living residence were gathered. Body Mass Index (BMI) was calculated as body mass divided by height squared, weight and height were measured during the acute hospitalization. Level of leisure-time physical activity (PA) was assessed by a questionnaire with a seven-point scale ranging from activities of daily living to competitive sports (7). The response categories were (1) no activity exceeding basic activities of daily living, (2) light walking or outdoor activity one to two times per week, (3) light walking or outdoor activity several times per week, (4) brisk physical activity causing some decree of sweating and breathlessness one to two times per week, (5) brisk physical activity causing some degree of sweating and breathlessness several times per week, (6) physical activity causing sweating and rather strong shortness of breath several times per week, and (7) competitive sports and related training. For the current analyses, the categories were combined as follow: 1 and 2 = sedentary behavior; 3 and 4 = a low level PA; 5 = a medium level of PA; and 6–7 = a high level of PA.
Injury-related characteristics: A detailed injury background was outlined by combining patient interview and pre-hospital emergency personnel reports using a structured closed-ended case report form. The International Standards for Neurological Classification of Spinal Cord Injury (8) were used to evaluate and classify the neurological consequence of TSCI within 72 h after injury. Tetraplegia and paraplegia terms refer to any impairment or loss of motor and/or sensory function in the cervical or lower segments of the spinal cord. The terms tetra/paraparesis are not used. Instead, the ASIA Impairment Scale (AIS) provides a more precise approach to description of severity (i.e., completeness) of the SCI (8). Epidemiological characteristics were collected and categorized using the International SCI Core Data Set (9). In addition, falls were divided into high-level (≥1 m; ICD-10: W00-09) and low-level (<1 m; ICD-10: W10-W17) falls.
Neuroimaging Findings: As part of routine emergency management and treatment triage, a cervical or trauma computed tomography (CT) was performed at the emergency department. When clinically feasible, spinal magnetic resonance imaging (MRI) was performed after CT scanning to characterize the extent and level of injury and also to aid in the assessment of the need for acute decompressive and/or stabilizing surgery. For this study, the spinal MRI images were interpreted by a neurosurgeon (T.L.) regarding acute traumatic medullopathy. A follow-up spine MRI was performed on enrolled patients two to 3 months after the injury as part of the study protocol. The extent of decompression was evaluated using the follow-up MRI scans.
Statistical analysis
A descriptive overview of the entire cohort was first provided followed by stratified analysis comparing groups based on age (≤60 vs. >60 years) and injury etiology (low-level falls vs. other causes). The age distribution is based on the International SCI Core Data Set and is additionally comparable to previous Finnish epidemiological studies on TSCI. Continuous variables were presented with descriptive statistics (mean = M, standard deviation = SD, median = Md, and range). The Mann–Whitney-U-test or T-test test were used to calculate the differences between patient groups. For categorial variables, the frequencies and percentages were calculated. The differences between groups were examined by the Fisher’s exact test or chi-squared test. A p-value < 0.05 was considered statistically significant. SPSS version 28.0 (IBM, Armonk, NY) was used to perform all the statistical analyses.
Results
A total of 81 cervical and thoracic TSCI patients were identified during the study period. The final enrolled cohort which met the study inclusion criteria consisted of 46 patients. A detailed overview of the inclusion process, as well as the characteristics of the inclusion and exclusion groups, is presented in Figure 1.
Figure 1
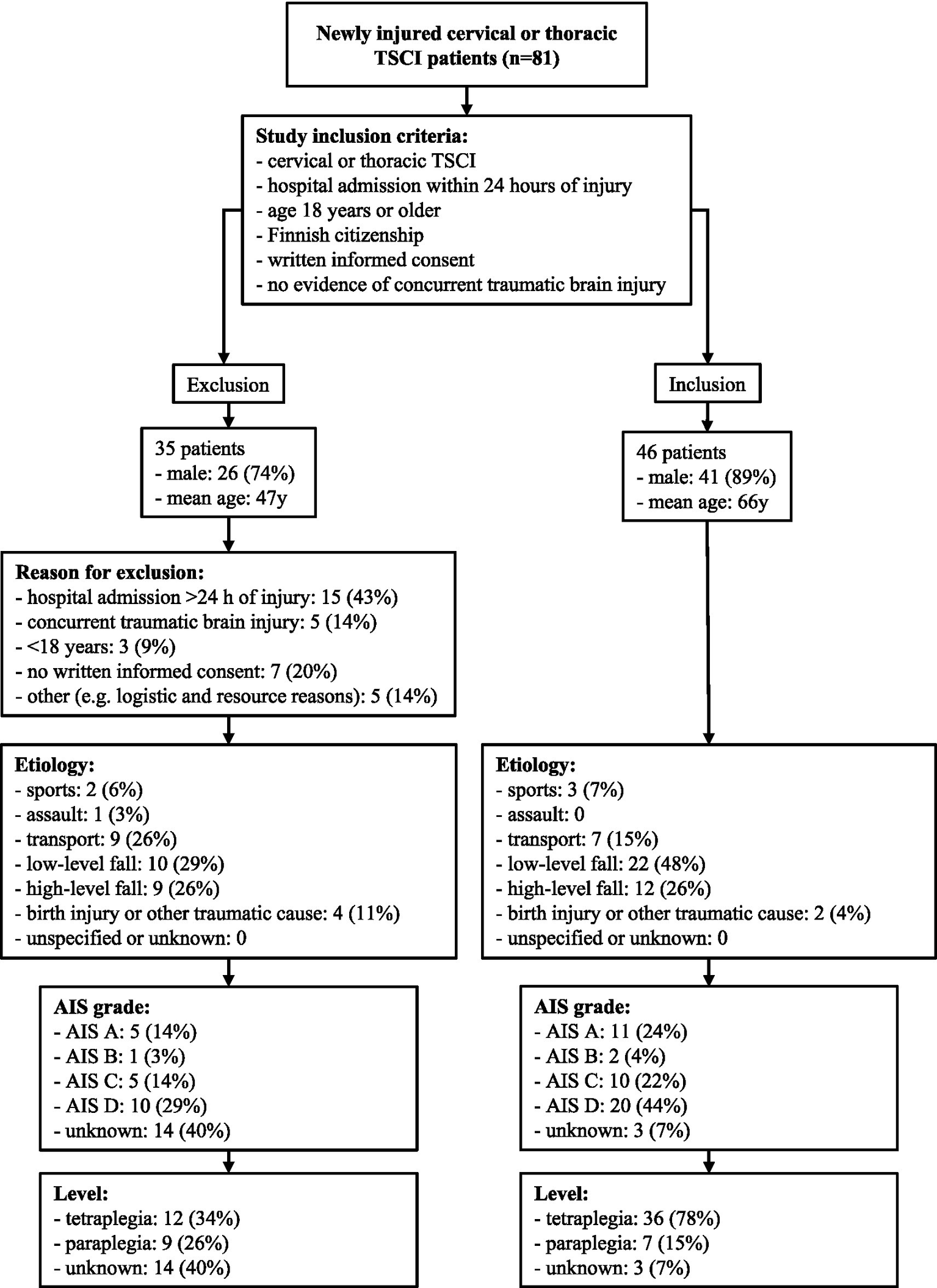
Patient enrollment process of the SUPERSTAR study.
Injury-related characteristics are presented in Table 1. The mean age of the patients was 66 years and 89% were men. The leading cause of injury was low-level fall (48%), followed by high-level fall (26%), transport accidents (15%), sports (7%), and other traumatic causes (4%). Of the patients 78% were tetraplegic and 24% had a complete injury.
Table 1
| Variable | All (n = 46) | Age ≤ 60 years (n = 16) | Age > 60 years (n = 30) | p-value |
|---|---|---|---|---|
| Age at injury, Mean ± SD, Md (range) | 65.9 ± 13.8 68.5(32–93) | |||
| Gender (Male/Female) | 8.2/1 (41/5) | 16/0 | 6.0/1(30/5) | 0.147 |
| Injury etiology (%) | ||||
| Sports | 3 (6.5%) | 3 (18.8%) | 0 (0.0%) | 0.008 |
| Assault | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Transport | 7 (15.2%) | 3 (18.8%) | 4 (13.3%) | |
| Low-level fall | 22 (47.8%) | 3 (18.8%) | 19 (63.3%) | |
| High-level fall | 12 (26.1) | 6 (37.5%) | 6 (20.0%) | |
| Birth injury or other traumatic cause | 2 (4.3%) | 1 (6.3%) | 1 (3.3%) | |
| Unspecified or unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Alcohol intoxication at the time of injury (%) | ||||
| Yes | 17 (37.0%) | 7 (43.8%) | 10 (33.3%) | 0.340 |
| No | 21 (45.7%) | 8 (50.0%) | 13 (43.3%) | |
| Unknown | 8 (17.4%) | 1 (6.3%) | 7 (23.3%) | |
| Vertebral injury (%) | 37 (80.4%) | 13 (81.3%) | 24 (80.0%) | 1.000 |
| Associated injury (%) | 6 (13.0%) | 4 (25.0%) | 2 (6.7%) | 0.163 |
| Spinal surgery (%) | 38 (82.6%) | 15 (93.8%) | 23 (76.7%) | 0.230 |
| Hours from injury to surgery Mean ± SD, Md (range) | 21.8 ± 23.2 12.0 (122) (n = 38) | 24.0 ± 31.0 12.3 (122) (n = 15) | 20.4 ± 17.0 12.0 (66) (n = 23) | 0.436 |
| Neurological level of injury (%) | ||||
| Tetraplegia | 36 (78.3%) | 10 (62.5%) | 26 (86.7%) | 0.003 |
| Paraplegia | 7 (15.2%) | 6 (37.5%) | 1 (3.3%) | |
| Unknown or not applicable | 3 (6.5%) | 0 (0.0%) | 3 (10.0%) | |
| ASIA Impairment Scale (%) | ||||
| A | 11 (23.9%) | 6 (37.5%) | 5 (16.7%) | 0.419 |
| B | 2 (4.3%) | 0 (0.0%) | 2 (6.7%) | |
| C | 10 (21.7%) | 3 (18.8%) | 7 (23.3%) | |
| D | 20 (43.5%) | 7 (43.8%) | 13 (43.3%) | |
| E and unknown or not applicable | 3 (6.5%) | 0 (0.0%) | 3 (10.0%) | |
| Neurological category (%) | ||||
| Ventilator dependent | Not applicable | Not applicable | Not applicable | 0.196 |
| C1-C4 AIS A, B, C | 15 (32.6%) | 4 (25.0%) | 11 (36.7%) | |
| C5-C8 AIS A, B, C | 3 (6.5%) | 1 (6.3%) | 2 (6.7%) | |
| T1-T12 AIS A, B, C | 5 (10.9%) | 4 (25.0%) | 1 (3.3%) | |
| All AIS D | 20 (43.5%) | 7 (43.8%) | 13 (43.3%) | |
| All AIS E | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Unknown or not applicable | 3 (6.5%) | 0 (0.0%) | 3 (10.0%) | |
| Days from injury to ISNCSCI Mean ± SD, Md (range) | 3.6 ± 2.3 3.0 (1–11) (n = 43) | 3.9 ± 2.8 3.0 (1–11) (n = 16) | 3.4 ± 2.0 3.0 (1–9) (n = 27) | 0.837 |
| LOS of acute hospitalization Mean ± SD, Md (range) | 15.5 ± 16.8 11.0 (1–79) (n = 43) | 16.7 ± 16.7 12.5 (4–76) (n = 16) | 14.8 ± 17.1 10.0 (1–79) (n = 27*) | 0.186 |
| ICU LOS Mean ± SD, Md (range) | 7.1 ± 3.1 8.0 (1–16) (n = 42) | 7.9 ± 3.4 8.0 (4–16) (n = 14) | 6.7 ± 2.9 7.5 (1–12) (n = 28) | 0.484 |
Demographic and peri-injury characteristics of TSCI patients.
ISNCSCI, The International Standards for Neurological Classification of Spinal Cord Injury; LOS, length of stay; ICU, intensive care unit; * 3 patients died during the acute hospitalization. p-values < 0.05 are bolded.
Acute MRI was done on 91% (n = 42) of the cases and acute traumatic medullopathy was seen in 79% (n = 33) of those cases. Four of the cases were not MR-imaged because of practical reasons (e.g., instability of vitals and/or spine, and hospital logistics). In 12% (n = 5) of the cases, there were no neuroimaging abnormalities and in 9% (n = 4) of the cases it was impossible to evaluate the images (e.g., movement and metal-induced artifacts). Spinal surgery was performed in 83% (n = 38) of the cases. Of these, in 37 procedures a spinal cord decompression was performed, and sufficient decompression according to the follow-up MRI (2–3 months postinjury) was achieved in 29 cases (78%). Of the operated patients, surgery was performed within the first 12 h after the injury in 50% (n = 19) and within 24 h in 68% (n = 26).
Tables 2, 3 present the patients’ pre-injury background characteristics stratified by age. All but one patient lived independently in a private residence before the injury. Three patients used a stick or crutches for walking, and one used a rollator. The remaining patients were able to walk without any assistive devices. In total, 87% (n = 40) of patients had been diagnosed with one or more diseases prior to the injury. Multimorbidity (≥2 diseases) was more common in the older age group compared to the younger group, 87% (n = 26) and 63% (n = 10), respectively (p = 0.071). Diseases of the circulatory system were the most common, affecting 52% (n = 24) of patients, with hypertension being the most prevalent (50%). Only one patient had dementia (2%). Musculoskeletal system and connective tissue diseases were present in 46% (n = 21), with dorsopathies affecting 22% (n = 10). Prior spine surgery had been performed on 9% (n = 4) of the patients, and only one patient (2%) had sequelae of a previous TSCI. Of all patients, 72% (n = 33) were using regular medication prior to their injury. This proportion was higher in the older age group (>60 years), with 87% (n = 26) using medication, compared to only 44% (n = 7) in the younger age group (≤60 years) (p = 0.005). Polypharmacy was observed in 46% (n = 21) and at least one FRID was used by 39% (n = 18) of the patients. The most used medications were cardiovascular medication, particularly anti-hypertensive medication, which was used by 46% of patients.
Table 2
| Variable | All (n = 46) | Age ≤60 years (n = 16) | Age >60 years (n = 30) | p-value |
|---|---|---|---|---|
| BMI, Mean ± sd, Md (range) | 26.9 ± 3.5 26.1 (21.7–38.9) | 26.7 ± 3.4 26.0 (22.4–33.9) | 27.0 ± 3.5 26.6 (21.7–38.9) | 0.745 |
| Underweight, BMI < 18.5 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.825 |
| Normal weight, BMI 18.5–24.9 | 14 (30.4%) | 6 (37.5%) | 8 (26.7%) | |
| Overweight, BMI 25–29.9 | 26 (56.5%) | 8 (50.0%) | 18 (60.0%) | |
| Obese, BMI ≥ 30 | 6 (13.0%) | 2 (12.5%) | 4 (13.3%) | |
| AUDIT (%) | ||||
| 0–7 points | 32 (69.6%) | 11 (68.8%) | 21 (70.0%) | 0.005 |
| 8–13 points | 3 (6.5%) | 2 (12.5%) | 1 (3.3%) | |
| ≥14 points | 3 (6.5%) | 3 (18.8%) | 0 (0.0%) | |
| Unknown | 8 (17.4%) | 0 (0.0%) | 8 (26.7%) | |
| Clinical Frailty Scale | ||||
| Non-frail | 42 (91.3%) | 16 (100.0%) | 26 (86.7%) | 0.801 |
| Pre-frail | 1 (2.2%) | 0 (0.0%) | 1 (3.3%) | |
| Frail | 1 (2.2%) | 0 (0.0%) | 1 (3.3%) | |
| Unknown | 2 (4.3%) | 0 (0.0%) | 2 (6.7%) | |
| Physical activity | ||||
| 1–2 Sedentary behavior | 7 (15.2%) | 1 (6.3%) | 6 (20.0%) | 0.160 |
| 3–4 Low level physical activity | 14 (30.4%) | 8 (50.0%) | 6 (20.0%) | |
| 5 Medium level physical activity | 13 (28.3%) | 3 (18.8%) | 10 (33.3%) | |
| 6–7 High level physical activity | 9 (19.6%) | 4 (25.0%) | 5 (16.7%) | |
| Unknown | 3 (6.5%) | 0 (0.0%) | 3 (10.0%) | |
| Number of diseases pre-injury | ||||
| 0–1 diseases | 10 (21.7%) | 6 (37.5%) | 4 (13.3%) | 0.071 |
| 2–4 diseases | 26 (56.5%) | 9 (56.3%) | 17 (56.7%) | |
| ≥5 diseases | 10 (21.7%) | 1 (6.3%) | 9 (30.0%) | |
| Polypharmacy (≥5 drugs) | 21 (45.7%) | 2 (12.5%) | 19 (63.3%) | <0.001 |
| Number of fall risk increasing drugs | ||||
| None | 28 (60.9%) | 13 (81.3%) | 15 (50.0%) | 0.039 |
| Any | 18 (39.1%) | 3 (18.8%) | 15 (50.0%) | |
Pre-injury characteristics of TSCI patients.
BMI, body mass index, AUDIT, Alcohol Use Disorders Identification Test. p-values < 0.05 are bolded.
Table 3
| Variable | All (n = 46) | Age ≤60 (n = 16) | Age >60 (n = 30) | p-value |
|---|---|---|---|---|
| Medical History (%) | 40 (87.0%) | 12 (75%) | 28 (93.3%) | 0.163 |
| Neoplasms (C00-D48) | 7 (15.2%) | 0 (0%) | 7 (23.3%) | 0.078 |
| Nervous system | 2 (4.3%) | 0 (0%) | 2 (6.7%) | 0.536 |
| Diseases of the blood and bloodforming organs and certain disorders involving the immune mechanisms (D50-89) | 3 (6.5%) | 0 (0%) | 3 (10%) | 0.542 |
| Anaemia (D50-64) | 3 (6.5%) | 0 (0%) | 3 (10%) | 0.542 |
| Endocrine, nutritional and metabolic diseases (E00-90) | 21 (45.7%) | 5 (31.3%) | 16 (53.3%) | 0.152 |
| Diabetes mellitus (E10-14) | 10 (21.7%) | 2 (12.5%) | 8 (26.7%) | 0.455 |
| Mental and behavioral disorders (F00-99) | 9 (19.6%) | 4 (25%) | 5 (16.7%) | 0.698 |
| Diseases of the nervous system (G00-99) | 8 (17.4%) | 2 (12.5%) | 6 (20.0%) | 0.694 |
| Myelopathy in diseases classified elsewhere (G99.2) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Clinically diagnosed dementia | 1 (2.2%) | 0 (0.0%) | 1 (3.3%) | 1.000 |
| Myelopathy with symptoms | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Disorders of vestibular function (H81) | 0 (0.0%) | 0 (0%) | 0(0%) | |
| Diseases of circulatory system (I00-99) | 24 (52.2%) | 6 (37.5%) | 18 (60.0%) | 0.146 |
| Hypertensive disease (I10-15) | 23 (50.0%) | 5 (31.3%) | 18 (60.0%) | 0.063 |
| Ischemic heart diseases (I20-25) | 4 (8.7%) | 1 (6.3%) | 3 (10%) | 1.000 |
| Cerebrovascular diseases (I60-69) | 2 (4.3%) | 0 (0%) | 2 (6.7%) | 0.536 |
| Diseases of respiratory system (J00-99) | 3 (6.5%) | 1 (6.3%) | 2 (6.7%) | 1.000 |
| Sleep apnea (G47.3) | 4 (8.7%) | 1 (6.3%) | 3 (10%) | 1.000 |
| Diseases of digestive system (K00-93) | 8 (17.4%) | 1 (6.3%) | 7 (23.3%) | 0.230 |
| Sphincter dysfunction unrelated the spinal cord lesion | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Diseases of musculoskeletal system and connective tissue (M00-99) | 21 (45.7%) | 7 (43.8%) | 14 (46.7%) | 0.850 |
| Dorsopathies | 10 (21.7%) | 4 (25.0%) | 6 (20.0%) | 0.720 |
| Spondylopathies | 7 (15.2%) | 3 (18.8%) | 4 (13.3%) | 0.681 |
| Ankylosing spondylitis (M45) | 3 (6.5%) | 1 (6.3%) | 2 (6.7%) | 1.000 |
| Spinal stenosis (M48) | 1 (2.2%) | 1 (6.3%) | 0 (0.0%) | 0.348 |
| Diseases of genitourinary system (N00-99) | 8 (17.4%) | 1 (6.3%) | 7 (23.3%) | 0.230 |
| Urinary tract impairment unrelated to spinal cord lesion | 5 (10.9%) | 0 (0.0%) | 5 (16.7%) | 0.147 |
| Certain conditions originating in the perinatal period (P00-96) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Congenital malformations, deformations and chromosomal abnormalities (Q00-99) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Injury, poisoning and certain other consequences of external causes (S00-T98) | 1 (2.2%) | 0 (0.0%) | 1 (3.3%) | 1.000 |
| Sequelae of intracranial injury (T90.5) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Sequelae of injury of spinal cord (T91.3) | 1 (2.2%) | 0 (0.0%) | 1 (3.3%) | 1.000 |
| Prior surgical treatment of the brain or spine | 5 (10.9%) | 2 (12.5%) | 3 (10.0%) | 1.000 |
| Intracranial surgery | 1 (2.2%) | 0 (0.0%) | 1 (3.3%) | 1.000 |
| Cervical spine surgery | 2 (4.3%) | 1 (6.3%) | 1 (3.3%) | 1.000 |
| Thoracic spine surgery | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Lumbar spine surgery | 2 (4.3%) | 1 (6.3%) | 1 (3.3%) | 1.000 |
| Prior Medication (%) | 33 (71.7%) | 7 (43.8%) | 26 (86.7%) | 0.005 |
| Cardiovascular medication | 23 (50.0%) | 4 (25.0%) | 19 (63.3%) | 0.013 |
| Antihypertensive medication | 21 (45.7%) | 4 (25.0%) | 17 (56.7%) | 0.040 |
| Medication affecting blood clotting and anaemia | 8 (17.4%) | 0 (0.0%) | 8 (26.7) | 0.037 |
| Anticoagulation | 4 (8.7%) | 0 (0.0%) | 4 (13.3%) | 0.282 |
| Antiplatelet medication | 4 (8.7%) | 0 (0.0%) | 4 (13.3%) | 0.282 |
| Central nervous system medication | 8 (17.4%) | 1 (6.3%) | 7 (23.3%) | 0.230 |
| Analgesic medication | 11 (23.9%) | 2 (12.5%) | 9 (30.0%) | 0.282 |
| Opioids | 2 (4.3%) | 0 (0.0%) | 2 (6.7%) | 0.536 |
| Pulmonary medication | 2 (4.3%) | 0 (0.0%) | 2 (6.7%) | 0.536 |
| Gastrointestinal medication | 11 (23.9%) | 1 (6.3%) | 10 (33.3%) | 0.068 |
| Hormones, contraceptives and gynecologic medication | 4 (8.7%) | 1 (6.3%) | 3 (10.0%) | 1.000 |
| Sexual and urinary organ medication | 6 (13.0%) | 1 (6.3%) | 5 (16.7%) | 0.649 |
| Diabetes medication | 8 (17.4%) | 1 (6.3%) | 7 (23.3%) | 0.230 |
| Cancer medication; immunosystem modulators | 2 (4.3%) | 1 (6.3%) | 1 (3.3%) | 1.000 |
| Bone tissue medication | 4 (8.7%) | 0 (0.0%) | 4 (13.3%) | 0.282 |
Pre-injury diseases and medication of TSCI patients.
p-values < 0.05 are bolded.
Low-level falls were the leading cause of TSCI. Detailed characteristics of patients stratified by etiology of low-level falls versus other etiology is presented in Table 4. Of these patients, 96% (n = 21) experienced tetraplegia, compared to 63% (n = 15) in the group with injuries caused by other etiologies (p = 0.009). Neurological category C1-4 AIS A, B, and C seemed to be more common in patients with low-level falls, seen in 46% (n = 10) of cases, versus 21% (n = 5) in those with injuries from other causes (p = 0.094). Among patients injured due to low-level falls, 18% (n = 4) had an AIS grade A injury, compared to 29% (n = 7) those with injuries from other causes (p = 0.856). None of the patients in the fall group had associated injuries, whereas 25% (n = 6) of those injured by other causes did (p = 0.022). Frailty was not prominent in either patient group. Physical activity was more commonly classified as sedentary behavior among patients injured by low-level falls (27%, n = 6) compared to those with injuries from other causes (4%, n = 1) (p = 0.160). Patients in the fall-related group also had slightly more pre-existing medical conditions and use of two or more FRIDs. There were no notable differences between the groups in terms of alcohol use or BMI.
Table 4
| Variable | Low-level fall (n = 22) | Other etiology (n = 24) | p-value |
|---|---|---|---|
| Number of diseases | |||
| 0–1 disease | 4 (18.2%) | 6 (25.0%) | 0.347 |
| 2–4 diseases | 11 (50.0%) | 15 (62.5%) | |
| ≥5 diseases | 7 (31.8%) | 3 (12.5%) | |
| Polypharmacy (≥5 drugs) | 10 (45.5%) | 11 (45.8%) | 1.000 |
| Number of fall risk increasing drugs | |||
| None | 12 (54.5%) | 16 (66.7%) | 0.531 |
| 1 drug | 6 (27.3%) | 6 (25.0%) | |
| 2 or more | 4 (18.2%) | 2 (8.3%) | |
| Clinical frailty scale | |||
| Non-frail | 19 (86.4%) | 23 (95.8%) | 0.603 |
| Pre-frail | 1 (4.5%) | 0 (0%) | |
| Frail | 1 (4.5%) | 0 (0%) | |
| Unknown | 1 (4.5%) | 1 (4.2%) | |
| AUDIT | |||
| 0–7 | 17 (77.3%) | 15 (62.5%) | 0.469 |
| 8–13 | 0 (0%) | 3 (12.5%) | |
| 14–40 | 1 (4.5%) | 2 (8.3%) | |
| Unknown | 4 (18.2%) | 4 (16.7%) | |
| BMI | |||
| Normal weight | 7 (31.8%) | 7 (29.2%) | 1.000 |
| Overweight | 12 (54.5%) | 14 (58.3%) | |
| Obese | 3 (13.6%) | 3 (12.5%) | |
| Physical activity | |||
| Sedentary behavior | 6 (27.3%) | 1 (4.2%) | 0.160 |
| Low level physical activity | 4 (18.2%) | 10 (41.7%) | |
| Medium level physical activity | 6 (27.3%) | 7 (29.2%) | |
| High level physical activity | 4 (18.2%) | 5 (20.8%) | |
| Unknown | 2 (9.1%) | 1 (4.2%) | |
| Alcohol at the time of injury | |||
| No | 10 (45.5%) | 11 (45.8%) | 0.308 |
| Yes | 10 (45.5%) | 7 (29.2%) | |
| Unknown | 2 (9.1%) | 6 (25.0%) | |
| Spinal surgery | |||
| No | 3 (13.6%) | 5 (20.8%) | 0.702 |
| Yes | 19 (86.4%) | 19 (79.2%) | |
| Associated injury | |||
| No | 22 (100.0%) | 18 (75.0%) | 0.022 |
| Yes | 0 (0%) | 6 (25.0%) | |
| Injury level | |||
| Tetraplegia | 21 (95.5%) | 15 (62.5%) | 0.009 |
| Paraplegia | 0 (0%) | 7 (29.2%) | |
| Unknown | 1 (4.5%) | 2 (8.3%) | |
| ASIA impairment scale (%) | |||
| A | 4 (18.2%) | 7 (29.2%) | 0.856 |
| B | 1 (4.5%) | 1 (4.2%) | |
| C | 6 (27.3%) | 4 (16.7%) | |
| D | 10 (45.5%) | 10 (41.7%) | |
| E and unknown or not applicable | 1 (4.5%) | 2 (8.3%) | |
| Neurological category (%) | |||
| Ventilator dependent | Not applicable | Not applicable | 0.094 |
| C1-C4 AIS A, B, C | 10 (45.5%) | 5 (20.8%) | |
| C5-C8 AIS A, B, C | 1 (4.5%) | 2 (8.3%) | |
| T1-T12 AIS A, B, C | 0 (0%) | 5 (20.8%) | |
| All AIS D | 10 (45.5%) | 10 (41.7%) | |
| All AIS E | 0 (0%) | 0 (0%) | |
| Unknown or not applicable | 1 (4.5%) | 2 (8.3%) | |
Characteristics of TSCI patients stratified by trauma etiology.
BMI, body mass index, AUDIT, Alcohol Use Disorders Identification Test. p-values < 0.05 are bolded.
Discussion
Low-level falls, especially in older adults, were the primary cause of TSCI, frequently leading to incomplete tetraplegia. Alcohol use before the injury was frequent. Overweight and physical inactivity were common among TSCI patients. Low-level falls primarily resulted in cervical injuries among older patients. These patients also had more chronic diseases at the time of TSCI, FRIDs, and reduced physical activity levels that most likely contributed to the injury risk and explain the injury mechanism.
Epidemiological characteristics
Overall, low-level falls were the most common etiology of TSCI, particularly in the older age group, where these falls account for most injuries. This aligns with prior studies showing that older adults are more prone to both low-level falls and following fractures and TSCI (1, 3, 4, 10, 11). By contrast, the younger age group experiences a wider variety of TSCI mechanisms, with high-level falls being most common, followed by low-level falls, sports and transport accidents. Previous Nordic studies support these findings (1, 12). The diverse injury mechanisms in younger adults may reflect the higher likelihood of risky behavior.
The distribution of TSCI levels leans toward tetraplegia, particularly in the older age group. In terms of injury severity, AIS D was the most common classification across the age groups. However, younger patients were more likely to experience complete injuries (AIS A) compared to older patients. These findings are consistent with those of a previous Finnish study. This may be due to the more severe injury mechanisms commonly seen in younger adults, which are more likely to result in complete spinal cord transection. Incomplete injuries in older adults may be partially attributed to the biomechanics of low-level falls, which typically exert less force than high-impact injuries. The lack of associated injuries in patients injured by falls further reflects the difference in injury energy compared to other etiologies.
An acute spine MRI was performed in 91% of cases revealing medullopathy signal in 77% of these cases. The percentage of TSCI patients with a MRI-positive spinal cord lesions varies across studies, but a commonly cited figure is around 70–80% (13). Surgical intervention was performed on 83% of TSCI patients, with a slightly smaller proportion in the older age group. Surgical decompression within 24 h of acute TSCI is associated with improved sensorimotor recovery (14) and in our study 68% of patients were operated on within this timeframe. In neighboring countries Sweden and Norway, almost all the patients are surgically treated (96 and 89%, respectively) (12, 15). In Sweden, 61% of acute TSCI patients undergo surgery within 24 h after injury (15).
The total days required in the ICU have decreased compared to a previous Finnish study conducted between 2012 and 2016, with the mean value dropping from 9.8 days to 7.1 days. The reduction is even more pronounced in the age group ≤60 years, where the mean ICU stay decreased from 12.6 days to 7.9 days (1). The median length of stay for acute hospitalization before rehabilitation admission or direct discharge was 11.0 days, consistent with a previous Finnish study conducted between 2011 and 2015, which reported a median of 12 days (16).
Patient characteristics
The majority of TSCI patients across age groups had at least one chronic disease documented in their medical history. This is even more pronounced in the older age group. In the older age group, a significant majority were on medication before their injury, with polypharmacy being substantially more common among older than younger patients. In contrast, medication use was rare in younger TSCI patients, likely to reflect both lower disease prevalence and reduced need for medical interventions. In line with our findings, a Japanese study, reported that most of the cervical spine injury patients had some underlying health condition, and the mean number of medications was 3.9 (17).
It is commonly accepted that alcohol increases the risk of injuries which may subsequently lead to TSCI. It is also documented that both consuming alcohol shortly before an injury and having a history of alcohol use disorder are positively correlated with incidence of TSCI (18). In our study, younger TSCI patients tended to report higher alcohol consumption habits compared to their older counterparts. This aligns with prior studies indicating a link between alcohol use and TSCI, as patients in this age group are often injured while intoxicated (1). However, we found a discrepancy between the low AUDIT scores in the older age group and the high rate of alcohol intoxication at the time of injury. This can be due to interviewer bias as patients can be unwilling to truthfully report their alcohol habits just after an intoxication-related injury. It is also important to note that even small amounts of alcohol can be harmful to older adults with multiple comorbidities and may increase the risk of falls.
The global prevalence of overweight and obesity has been increasing at an alarming rate (19). In our study, most patients with TSCI were classified as being above normal weight, with none categorized as underweight. This skew towards overweight and obesity among TSCI patients is noteworthy, aligning with previous research that shows an elevated risk of injury, particularly from falls, among overweight individuals (20). Furthermore, in the context of TSCI, obesity has been linked to less favorable rehabilitation outcomes (21).
Globally physical inactivity is rising and after 60 years of age it rises even faster (22). In terms of physical activity in our study, while sedentary behavior was more prevalent among older TSCI patients, half of the younger patients were found to engage only in low levels of physical activity. There are no previous studies on physical activity before TSCI. Results in the same direction are seen in earlier studies dealing with injuries in general (1). Although physically more active individuals have greater frequency of exposure to precipitants to injuries, less-active individuals have an increased propensity to activity-related injuries. Also, low fitness levels and physical inactivity increase the risk for walking-related falls (23). Regular physical activity reduces the incidence of non-sport and non-leisure time injuries (24).
Frailty is a multidimensional syndrome characterized by reduced physiological and cognitive reserves leading to increased vulnerability to adverse health outcomes including falls (25). To the best of our knowledge, there are only two studies that have evaluated the effect of frailty on outcomes in TSCI patients. In a recent Canadian study (26), 31% of patients over 61 years were frail. This study reported that frailty predicted adverse events, acute length of stay, and in-hospital mortality in TSCI patients. In another study with data extracted from the US Nationwide Inpatient Sample, 23% of acute TSCI patients were categorized as frail, and of these patients 77% were over the age of 65 years (27). In this inpatient study, frailty was associated with increased risk for adverse outcomes. Previously, chronological age was reported to be a factor influencing treatment decisions after TSCI and younger patients were reported to undergo surgical decompression earlier than older patients (28). Early surgical decompression is associated with greater sensorimotor recovery (14) and in this context, it would be noteworthy to consider the importance of frailty and not only age in treatment decisions. In our study, it was surprising that nearly all patients in both age groups were classified as non-frail according to the CFS. This is even in contrast with the population level prevalence of frailty. Given that frailty is a known risk factor for falls, a higher prevalence of frailty would have been anticipated specifically among patients injured by low-level falls. This clear underrepresentation of frail patients in our sample can be due to methodological limitations of the CFS. The assessment of the CFS contains a rather large degree of rater subjectivity and patients are inclined to rate their preinjury functioning too optimistically (“good-old-days” bias) (29). As a straightforward screening tool, CFS oversimplifies the complex frailty syndrome that involves multiple factors. Thus, CFS only gives a superficial insight instead of a holistic overview.
Low-level fall
TSCI patients with low-level falls as the primary etiology exhibit distinct pre-injury characteristics that highlight the complex interplay between frailty, comorbidities, medication use, and physical activity. In Japan, patients over 65 years injured from ground-level falls tended to have poorer pre-injury health conditions, such as medical comorbidities and frailty, compared with those who fell from higher heights (17). Our research did not provide evidence to support that patients injured by low-level falls were frail prior to the injury. TSCI patients with low-level fall etiology seem to have more pre-existing diseases. Additionally, these patients have more frequent use of FRIDs. Chronic conditions and comorbidities have shown an effect on the risk of injurious falls either directly or indirectly (30). Persistent polypharmacy, particularly combined with FRIDs use, is associated with increased risk for fall injuries (30). A further compounding factor is the lower level of physical activity observed among these patients before their injury. An inactive lifestyle reduces but does not eliminate fall-prone situations. Conversely, it has been shown that physical inactivity increases the risk for walking-related falls (23).
Strength and limitations
The strength of our study is the comprehensive and detailed data collection, prospective design and small amount of missing data among included patients. Extensive pre-injury health data gives new insight into the current characteristics of TSCI patients. The small sample size limited the ability to reliably assess some associations. The quite high percentage of excluded patients is a limitation that hinders the wider generalizability of the results.
Conclusion
Our study of TSCI patients reveals behavioral and physical health patterns across different age groups. Overall, these findings underscore the need for age-specific prevention strategies for TSCI. For younger adults, promoting responsible alcohol use could reduce the likelihood of injury. For older adults, efforts should focus on fall prevention strategies. Efforts to optimize medication regimens, carefully managing chronic conditions and promoting physical activity could be critical in fall and TSCI prevention. Larger prospective studies focusing on low-level falls and the pre-injury vulnerability factors leading to these injuries are urgently needed. These studies would help to design effective prevention interventions to lower the burden of these injuries.
Statements
Data availability statement
The raw data supporting the conclusions of this article is available on request. All inquiries related to the data can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Pirkanmaa Hospital District, Tampere (study code: R18182). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EL: Formal analysis, Writing – review & editing, Writing – original draft, Data curation, Methodology, Visualization, Project administration, Investigation, Validation, Conceptualization. EK: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Supervision, Methodology. TT: Writing – review & editing, Investigation, Conceptualization, Methodology. HM: Writing – review & editing. JL: Conceptualization, Methodology, Writing – review & editing. EJ: Writing – review & editing, Methodology, Conceptualization. TL: Investigation, Writing – original draft, Resources, Funding acquisition, Visualization, Formal analysis, Project administration, Validation, Conceptualization, Data curation, Methodology, Writing – review & editing, Supervision.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the Finnish State Research Funding. Dr Luoto has a received research grant from the Academy of Finland (Terveyden tutkimuksen toimikunta #349758). Open access funding was provided by the Academy of Finland as part of Dr. Luoto’s research grant.
Acknowledgments
We thank research nurse Laura Nevaharju-Sarantis and the rehabilitation staff of Tampere University Hospital, especially Jaana Leivo, for their assistance.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Johansson E Luoto TM Vainionpää A Kauppila AM Kallinen M Väärälä E et al . Epidemiology of traumatic spinal cord injury in Finland. Spinal Cord. (2021) 59:761–8. doi: 10.1038/s41393-020-00575-4,
2.
Johansson E Koskinen E Helminen M Vainionpää A Luoto TM . Mortality and causes of death of traumatic spinal cord injury in Finland. Spinal Cord. (2025) 63:24–30. doi: 10.1038/s41393-024-01047-9
3.
Lee BB Cripps RA Fitzharris M Wing PC . The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. (2014) 52:110–6. doi: 10.1038/sc.2012.158,
4.
Sattin RW A public health perspective ! [internet]. 1992. Available online at: www.annualreviews.org (accessed September 18, 2025).
5.
Seppala LJ Petrovic M Ryg J Bahat G Topinkova E Szczerbinska K et al Stoppfall (screening tool of older persons prescriptions in older adults with high fall risk): a delphi study by the EuGMS task and finish group on fall-risk-increasing drugs. Age Ageing202150(4):1189–1199, doi: DOI: 10.1093/ageing/afaa249, 33349863.
6.
De Biasio JC Mittel AM Mueller AL Ferrante LE Kim DH Shaefi S . Frailty in critical care medicine: a review. 130, Anesth Analg Lippincott Williams and Wilkins; 2020. p. 1462–1473.
7.
Grimby G Frändin K . On the use of a six-level scale for physical activity. Scand J Med Sci Sports. (2018) 28:819–25. doi: 10.1111/sms.12991
8.
Rupp R Biering-Sørensen F Burns SP Graves DE Guest J Jones L et al . International standards for neurological classification of spinal cord injury In: Topics in spinal cord injury rehabilitation, Thomas Land Publishers Inc. (2021). 27:1–22. doi: 10.46292/sci2702-1
9.
Biering-Sørensen F Charlifue S Chen Y New PW Noonan V Post MWM et al . International spinal cord injury Core data set (version 3.0)—including standardization of reporting. Spinal Cord. (2023) 61:65–8. doi: 10.1038/s41393-022-00862-2,
10.
Kannus P Palvanen M Niemi S Parkkari J . Alarming rise in the number and incidence of fall-induced cervical spine injuries among older adults. J Gerontol A Biol Sci Med Sci. (2007), 62:180–3. doi: 10.1093/gerona/62.2.180
11.
Moschovou M Antepohl W Halvorsen A Pettersen AL Divanoglou A . Temporal changes in demographic and injury characteristics of traumatic spinal cord injuries in Nordic countries - a systematic review with meta-analysis. Spinal Cord. (2022) 60:765–73. doi: 10.1038/s41393-022-00772-3,
12.
Halvorsen A Pettersen AL Nilsen SM Halle KK Schaanning EE Rekand T . Epidemiology of traumatic spinal cord injury in Norway in 2012–2016: a registry-based cross-sectional study. Spinal Cord. (2019) 57:331–8. doi: 10.1038/s41393-018-0225-5,
13.
Jentzsch T Cadotte DW Wilson JR Jiang F Badhiwala JH Akbar MA et al . Spinal cord signal change on magnetic resonance imaging may predict worse clinical in-and outpatient outcomes in patients with spinal cord injury: a prospective multicenter study in 459 patients. J Clin Med. (2021) 10:4778. doi: 10.3390/jcm10204778
14.
Badhiwala JH Wilson JR Witiw CD Harrop JS Vaccaro AR Aarabi B et al . The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. (2021) 20:117–26. doi: 10.1016/S1474-4422(20)30406-3,
15.
Joseph C Andersson N Bjelak S Giesecke K Hultling C Nilsson Wikmar L et al . Incidence, aetiology and injury characteristics of traumatic spinal cord injury in Stockholm, Sweden: a prospective, population-based update. J Rehabil Med. (2017) 49:431–6. doi: 10.2340/16501977-2224
16.
Väärälä E Alanen S Öhman J Koskinen E . Hoidon keskittäminen nopeuttaa selkäydinvammapotilaan kotiutumista. Suom Laakarilehti. (2017):72.
17.
Yokogawa N Kato S Sasagawa T Hayashi H Tsuchiya H Ando K et al . Differences in clinical characteristics of cervical spine injuries in older adults by external causes: a multicenter study of 1512 cases. Sci Rep. (2022) 12:15867. doi: 10.1038/s41598-022-19789-y,
18.
Stroud MW Bombardier CH Dyer JR Rimmele CT Esselman PC . Preinjury alcohol and drug use among persons with spinal cord injury: implications for rehabilitation. J Spinal Cord Med. (2011) 34:461–72. doi: 10.1179/2045772311Y.0000000033
19.
Phelps NH Singleton RK Zhou B Heap RA Mishra A Bennett JE et al . Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. (2024) 403:1027–50. doi: 10.1016/S0140-6736(23)02750-2,
20.
Fjeldstad C Fjeldstad AS Acree LS Nickel KJ Gardner AW . The influence of obesity on falls and quality of life. Dyn Med. (2008) 7:18. doi: 10.1186/1476-5918-7-4,
21.
Tian W Hsieh CH Dejong G Backus D Groah S Ballard PH . Role of body weight in therapy participation and rehabilitation outcomes among individuals with traumatic spinal cord injury. Arch Phys Med Rehabil. (2013) 94:S125–36. doi: 10.1016/j.apmr.2012.10.039
22.
Guthold R Stevens GA Riley LM Bull FC . Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. (2018) 6:e1077–86. doi: 10.1016/S2214-109X(18)30357-7,
23.
Mertz KJ Lee D C Sui X Powell KE Blair SN . Falls among adults. The association of cardiorespiratory fitness and physical activity with walking-related falls. Am J Prev Med. (2010) 39:15–24. doi: 10.1016/j.amepre.2010.03.013
24.
Carlson SA Hootman JM Powell KE Macera CA Heath GW Gilchrist J et al . Self-reported injury and physical activity levels: United States 2000 to 2002. Ann Epidemiol. (2006) 16:712–9. doi: 10.1016/j.annepidem.2006.01.002,
25.
Fried LP Tangen CM Walston J Newman AB Hirsch C Gottdiener J et al . Frailty in older adults: evidence for a phenotype [internet]. J Gerontol. (2001) 56:M146–56. doi: 10.1093/gerona/56.3.m146
26.
Banaszek D Inglis T Marion TE Charest-Morin R Moskven E Rivers CS et al . Effect of frailty on outcome after traumatic spinal cord injury. J Neurotrauma. (2020) 37:839–45. doi: 10.1089/neu.2019.6581,
27.
Chu H Chen L Li J Li J Yang D Yang M et al . Impact of frailty on inpatient outcomes of acute traumatic spinal cord injury evidence from US National Inpatient Sample. Neurologist. (2024) 29:82–90. doi: 10.1097/NRL.0000000000000532,
28.
Ahn H Bailey CS Rivers CS Noonan VK Tsai EC Fourney DR et al . Effect of older age on treatment decisions and outcomes among patients with traumatic spinal cord injury. CMAJ. (2015) 187:873–80. doi: 10.1503/cmaj.150085,
29.
Iverson GL Lange RT Brooks BL Rennison VLA . “Good old days” bias following mild traumatic brain injury. Clin Neuropsychol. (2010) 24:17–37. doi: 10.1080/13854040903190797,
30.
FJX P Shorey S . A literature review of factors influencing injurious falls. Clin Nurs Res. (2020) 29:141–8. doi: 10.1177/1054773818802187
Summary
Keywords
elderly, epidemiology, fall accident, prevention, spinal cord injury, spine
Citation
Luoto E, Koskinen E, Thesleff T, Mäntymäki H, Långsjö J, Jämsen E and Luoto TM (2026) Who gets traumatic spinal cord injury? A Finnish tertiary trauma centre study. Front. Neurol. 16:1709012. doi: 10.3389/fneur.2025.1709012
Received
19 September 2025
Revised
04 December 2025
Accepted
08 December 2025
Published
12 January 2026
Volume
16 - 2025
Edited by
Krisztián Pajer, University of Szeged, Hungary
Reviewed by
Alypio Nyandwi, Ministry of Health, Rwanda
Karma Phuentsho, Jigme Dorji Wangchuck National Referral Hospital (JDWNRH), Bhutan
Updates
Copyright
© 2026 Luoto, Koskinen, Thesleff, Mäntymäki, Långsjö, Jämsen and Luoto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elina Luoto, elina.johansson@tuni.fi
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.