Abstract
Background:
Children with autism spectrum disorder (ASD) are at increased risk of epilepsy (EP), but distinguishing epileptic seizures from ASD-associated behavior remains a clinical challenge. Although previous studies have reported changes in peripheral immune markers in adults with EP, it remains unclear whether similar immune signatures are present in pediatric patients with both ASD and EP, and more pronounced than in children with ASD alone.
Methods:
We conducted an exploratory, prospective, cross-sectional study of children aged 9–14 years with mild ASD, with or without EP, recruited from outpatient settings. Peripheral blood samples were analyzed by enzyme-linked immunosorbent assay for 23 immune proteins and by flow cytometry for leukocyte population counts. Analyses included t-tests / Mann–Whitney U-tests, post hoc tests for multiple comparisons, and effect size / power analyses.
Results:
A total of 30 children were included, n = 21 with primarily mild ASD and n = 9 with mild ASD and EP. The epilepsy cases consisted of children with generalized seizures or self-limited epilepsy with centrotemporal spikes. Three immune proteins, Interleukin (IL)-12p70, IL-13 and IL-1β, were significantly increased in the ASD + EP group compared to the ASD-only group. However, the statistical power was low, and group differences did not remain significant after correction for multiple comparisons, even though effect sizes were moderate to large. No differences in the counts of activated leukocyte populations were observed.
Conclusion:
These findings raise the possibility that immune system alterations may be associated with EP in children with ASD and could potentially aid diagnosis, although larger studies are needed to confirm these findings.
1 Introduction
Epilepsy (EP) affects approximately 0.8% of the general population and 0.6% of children (1, 2). Among children with autism spectrum disorder (ASD), prevalence is markedly higher, with an estimated range of 5–46%, and the rate rises further in adults with ASD (3–6). ASD is a neurodevelopmental condition characterized by mild to severe difficulties in social interaction, repetitive behaviors and restricted interests (7).
Growing evidence implicates immune dysregulation both in disease pathogenesis and as a source of biomarkers for neurological disorders, including those of the central nervous system (8, 9). Circulating immune proteins in peripheral blood and cerebrospinal fluid, including cytokines and chemokines, are of particular interest. Members of the cytokine families, such as interleukin (IL)-6 /IL-12, guide T- and B-cell differentiation, whereas chemokines can recruit leukocytes across the blood–brain barrier to sites of neuroinflammation (8, 10, 11).
Previous studies have reported elevated blood levels of cytokines such as IL-6, IL-1β, IL12p70, IL-8 and IL-17 in children with ASD compared to typically developing controls (12–14). The role of inflammation in ASD is complex and may be influenced by comorbid conditions in which immune mechanisms are also implicated. Approximately 50% of children with ASD have comorbid attention deficit hyperactivity disorder (ADHD), and immune protein alterations have been observed both in ASD with ADHD, and in ADHD alone (15–17). Children with ASD also face an increased risk of psychiatric disorders such as depression and anxiety, which have likewise been linked to neuroinflammatory processes (18–20).
EP has similarly been investigated from a neuroimmunological perspective, with immune factors proposed as biomarkers (21–23). Both pro- and anti-inflammatory proteins are thought to play a role in epileptogenesis by modulating the excitatory/inhibitory balance in the central nervous system, potentially facilitating abnormal neuronal activity (24). In adults with EP, interictal peripheral blood studies have reported elevated levels of IL-6, IFN-γ, IL-17A, and TNF-α compared to healthy controls (25–28). IL-1β, among other cytokines, has been suggested as both an interictal marker and a potential therapeutic target in EP (29–31). However, findings remain inconsistent, with some studies reporting no significant changes or even decreased levels of cytokines such as IL-6, TNF-α, and IL-1β (27, 29, 32). Although pediatric studies are limited, altered cytokine levels, including TNF-α, IL-1β, and IL-1R1, have been reported in children with EP (32, 33).
The search for biomarkers to support the early diagnosis of epilepsy has gained momentum, particularly in light of diagnostic delays (34). This challenge is especially pronounced in children with ASD, where hospital/unfamiliar environments, clinical assessments and multiple electroencephalographies (EEGs) can be distressing. Moreover, distinguishing epilepsy symptoms from ASD-related behaviors further complicates diagnosis, underscoring the need for improved clinical tools.
It remains unclear, whether children with both ASD and EP exhibit a distinct immune profile in the blood compared to those with ASD alone. In this exploratory study, we examined immune protein levels and leukocyte activation in peripheral blood from children aged 9–14 years with mild ASD, with and without EP, to investigate potential biomarkers for EP in this population.
2 Method and materials
2.1 Patient inclusion
Patients were recruited between September 2020 and June 2023 through the Department of Pediatric Neurology at Skåne University Hospital (SUS) and Child and Adolescent Psychiatry in Skåne, Sweden. Inclusion criteria were: children aged 9–14 years with a confirmed diagnosis of ASD grade 1–2, with our without EP. Exclusion criteria were: severe developmental delay, traumatic brain injury within the last 6 months, active systemic autoimmune disease, or neurodegenerative disorders. Children with known monogenetic syndromes, other genetic disorders associated with ASD and/or EP, or treatment-resistant EP were not eligible for participation. Demographic data and medical history, including diagnoses, radiological findings, and perinatal data, were obtained from medical records. Child-adjusted study information was provided prior to enrolment. Written informed consent was obtained, and the study was approved by the Swedish Ethical Review Authority (approval no. 2020–03207) in accordance with the Helsinki declaration.
2.2 Blood sampling and biochemical analyses
Two blood samples were collected on one occasion per participant at the Department of Pediatric Neurology, SUS. Sample number 1 was collected in 5 mL BD Vacutainer serum tubes and were left to coagulate for 1 h at room temperature (RT) before centrifugation at 20 °C at 1500 rcf for 10 min. Serum was aliquoted and stored at −80 °C until analysis. Serum concentrations of cytokines and chemokines were determined by multiplex ELISA proinflammatory panel 1 (K15049D; INF-γ, IL1-β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13 and TNF-α), chemokine panel 1 (K15047D; Eotaxin, Eotaxin-3, MIP-1α, MIP-1β, TARC, IP-10, MCP-1, MCP-4 and MDC) and vascular injury panel 2 (K15198D; SAA, CRP, VCAM-1 and ICAM-1) human kits according to the manufacturer’s instructions (MSD, US). Serum samples were diluted 1:2 (proinflammatory panel 1), 1:4 (chemokine panel 1) or 1:1000 (vascular injury panel 2) and run in duplicates along with calibrator standards. The samples were incubated at 4 °C overnight (proinflammatory panel 1, chemokine panel 1), or for 2 h in room temperature (vascular injury panel 2). After washing, detection antibody was added for 2 h at RT. The wash was then repeated and read buffer was added. Plates were read on the MESO QuickPlex SQ 120 and analyzed using discovery workbench 4.0 (MSD, US). Blood sample number 2 was collected in 5 mL whole blood EDTA (ethylenediaminetetraacetic acid tubes and stored at room temperature at a maximum of 24 h prior to flow cytometry analysis of circulating leukocytes). Gating was based on CD45 expression and side scatter (SSC) signature. Cell population counts included: B cells, CD4/CD8 ratio, CD4 + T cells, CD8 + T cells, HLA-DR + cells, HLA-DR + T cells, HLA-DR+/CD4 + T cells, HLA-DR+/CD8 + T cells, lymphocytes, and total T cells. The ELISAs were conducted in house at Lund Biomedical Center, while flow cytometry procedures were performed at the Department of Clinical Immunology and Transfusion Medicine, SUS.
2.3 Statistical analyses
Statistical analyses were conducted using statistical software R and SPSS Statistics version 27. Quantile-quantile plots and the Shapiro–Wilk test for normality were used to assess data distribution. For parametric distribution, data were analyzed with Student’s t-tests and presented as mean (SD), number of samples (n). For nonparametric/skewed distribution, including single outliers, data were analyzed with Mann–Whitney U-tests and presented as median (IQR), n. Undetectable values reported by the software were excluded from analysis. Cohen’s D was used for measuring effect sizes and Benjamini-Hochberg post hoc analysis for correction of multiple comparisons. Subgroup analyses were all performed with Mann–Whitney U-test due to few samples. p-values ≤0.05 were considered statistically significant.
3 Results
3.1 Patient inclusion and clinical characteristics
A total of 33 children were enrolled in the study. Following the exclusion of three participants due to diagnostic uncertainties or somatic comorbidities, the final cohort consisted of n = 30. Participants were divided into an ASD group (n = 21) and an ASD + EP group (n = 9). Clinical characteristics and medical history of the participants are presented in Table 1. A majority of participants were boys, namely 67% of ASD and 89% in ASD + EP groups, respectively. The majority of children in the ASD group and all in the ASD + EP group received additional support in school or attended adapted schooling. All participants were diagnosed with mild (grade 1) ASD, except for two in the ASD-only group who had moderate (grade 2) ASD. One child with ASD + EP had mild to moderate intellectual disability. Approximately two-thirds of participants in both groups had a comorbid diagnosis of ADHD or attention deficit disorder (ADD), and nearly half had received a psychiatric diagnosis such as anxiety disorder, obsessive-compulsive disorder and depressive disorder. Among the ASD + EP group, almost half had focal EP and the remainder had generalized EP. None of the included participants met the criteria for drug-resistant epilepsy (35). N = 6 reported seizure freedom of varying duration; none had seizures weekly at the time of enrollment. Other somatic diagnoses were few, and neuroimaging had been performed in only isolated cases.
Table 1
| Diagnosis | ||
|---|---|---|
| ASD (n = 21) | ASD + EP (n = 9) | |
| Clinical characteristics | ||
| Age: median years (range) | 12 (9–14) | 12 (9–14) |
| Gender: male % (n) | 67 (14) | 89 (8) |
| Schooling: % (n) | ||
| Regular with no support | 24 (5) | 0 (0) |
| Regular with support | 48 (10) | 44 (4) |
| Adapted | 14 (3) | 56 (5) |
| Missing data | 14 (3) | 0 (0) |
| Medical history | ||
| ASD level: % (n) | ||
| Mild | 90 (19) | 100 (9) |
| Moderate | 10 (2) | 0 (0) |
| ADHD/ADDa: % (n) | 71 (15) | 67 (6) |
| Intellectual disabilityb: % (n) | 0 (0) | 11 (1) |
| Other psychiatric diagnosisc: % (n) | 38 (8) | 56 (5) |
| Prematurityd: % (n) | 14 (3) | 11 (1) |
| Missing data | 14 (3) | 22 (2) |
| Epilepsy classification: % (n) | ||
| Focal | 44 (4) | |
| Generalized | 56 (5) | |
| MRT findinge: % (n) | 0 (0) | 33 (3) |
| Missing data | 100 (21) | 33 (3) |
| Somatic diagnosisf: % (n) | 14 (3) | 33 (3) |
| Medicationsg: median number (range) | 2 (0–5) | 3 (0–4) |
Clinical characteristics and medical history.
Demography and medical history of included patients in the ASD and ASD+EP group. Data presented as median and range for age and medications, and as percentage (%) and number of participants (n) for all other parameters.
n = 3 with attention deficit disorder (ADD) and n = 18 with attention deficit hyperactivity disorder (ADHD).
n = 1 with moderate intellectual disability.
n = 13, including n = 1 anxiety disorder, obsessive compulsive disorder and tics, n = 1 impressive and expressive language disorder, specific reading disorder and non-organic enuresis, n = 2 depressive disorder, n = 1 obsessive compulsive disorder, n = 1 depressive disorder and eating disorder, n = 1 pragmatic language disorder, n = 1 non-organic enuresis, n = 1 anxiety disorder, n = 1 non-organic sleeping disorder, n = 1 anxiety disorder and developmental coordination disorder, n = 1 mixed anxiety and depression conditions, and n = 1 generalized language disorder.
n = 1 twin pregnancy born GW 35 + 6, n = 2 twin pregnancy born GW 36 + 4, and n = 1 born GW 33.
n = 1 grey substance heterotopia, n = 1 cortical dysplasia, and n = 1 enlarged ventricles.
n = 6 including n = 1 psoriasis, n = 1 unilateral hearing loss, n = 1 migraine, n = 1 lumbosacral dermal sinus, psoriasis and neuromuscular bladder disorder, n = 1 allergic rhinitis, and n = 1 bilateral sensorineural hearing loss.
Medications in the ASD group included sertraline (n = 3), fluoxetine (n = 1), melatonin/circadin (n = 15), propiomazine (n = 1), metylphenidate (n = 6), guanfacine (n = 5), dexamphetamine (n = 4), atomoxetine (n = 3), hydroxyzine (n = 1), risperidone (n = 1), Grazax (n = 1), and over-the-counter allergy medications (n = 2). In the ASD+EP group, the following medications were reported: fluoxetine (n = 1), sertraline (n = 1), melatonin/circadin (n = 5), metylphenidate (n = 2), dexamphetamine (n = 3), guanfacine (n = 2), and risperidone (n = 1), and anti-seizure medication (ASM) (n = 5); lamotrigine (n = 2), levetiracetam (n = 1), oxcarbazepine (n = 2), valproic acid (n = 1). One ASD+EP participant was on ASM polytherapy with valproic acid and lamotrigine; the others were on monotherapy.
3.2 Indication of altered cytokine profile in children with ASD and EP compared to those with ASD only
Analysis of 23 immune proteins in serum revealed significantly increased levels of three cytokines in the ASD + EP compared to the ASD-only group before correcting for multiple comparisons: IL-12p70 (p = 0.038, large effect size = 0.96, power = 0.6), IL-13 (p = 0.022, large effect size = 0.8, power = 0.33) and IL-1β (p = 0.005, medium-large effect size = 0.6, power = 0.22) (Figure 1). However, the differences did not remain statistically significant after correction for multiple comparisons (adjusted p = 0.29, 0.25, and 0.12, respectively). No other group differences were found, including IFN-γ, IL-6, IL-8, TNF-α, and MCP-1 (Table 2). The detected levels of immune proteins were broadly consistent with previous reports for some of these proteins in pediatric cohorts (36). No participants were excluded due to high C-reactive protein (CRP), which could have indicated ongoing systemic infection.
Figure 1

Immune proteins in serum. (A–C). Scatter and box plots of IL-12p70, IL-13, and IL-1β serum concentrations in children with ASD and ASD + EP. Data presented with mean and SD for IL-12p70 due to normal distrubution and with median and IQR due to non-normal distribution for IL-13 and IL-1β. *p < 0.05, **p < 0.01. Figure was created in BioRender. Taylor, M. (2025) https://BioRender.com/cgu4kk8.
Table 2
| Protein (mg/dl, pg/ml) (min–max) | ASD | ASD+EP | p-value |
|---|---|---|---|
| CRP* (mg/dl) (0.3E-6–1.2E6) | 0.02 (1.64), 21 | 0.0008 (0.04), 9 | 0.33 |
| Eotaxin (pg/ml) (119.25–510.72) | 230.34 (66.79), 21 | 241.91 (120.89), 9 | 0.79 |
| Eotaxin-3 (pg/ml) (6.97–33.83) | 18.75 (6.3), 21 | 19.72 (7.37), 9 | 0.74 |
| ICAM-1* (pg/ml) (0.66–427.94) | 25.72 (52.72), 21 | 55.42 (180.14), 9 | 0.16 |
| IFN-g (pg/ml) (3.26–65.77) | 9.91 (13.95), 21 | 5.23 (1.93), 9 | 0.15 |
| IL-10* (pg/ml) (0.07–0.90) | 0.29 (0.16), 21 | 0.27 (0.18), 9 | 0.76 |
| IL-12p70 (pg/ml) (0.03–0.28) | 0.13 (0.05), 20 | 0.18 (0.06), 9 | **0.04 |
| IL-13* (pg/ml) (0.32–1.74) | 0.55 (0.19), 12 | 0.87 (0.59), 7 | **0.02 |
| IL-1β* (pg/ml) (0.01–14.56) | 0.05 (0.05), 10 | 0.16 (0.63), 7 | **0.01 |
| IL-2* (pg/ml) (0.06–1.33) | 0.19 (0.09), 17 | 0.29 (0.18), 8 | 0.12 |
| IL-4* (pg/ml) (0.0045–0.13) | 0.03 (0.02), 17 | 0.02 (0.01), 9 | 0.22 |
| IL-6* (pg/ml) (0.19–3.82) | 0.63 (0.36), 21 | 0.48 (0.76), 9 | 0.79 |
| IL-8 (pg/ml) (5.64–18.53) | 9.53 (3.27), 21 | 10.12 (3.73), 9 | 0.69 |
| IP-10 (pg/ml)* (57.90–757.00) | 125.63 (59.90), 21 | 100.90 (43.54), 9 | 0.33 |
| MCP-1* (pg/ml) (134.72–294.16) | 208.67 (61.28), 21 | 217.90 (27.64), 9 | 0.37 |
| MCP-4* (pg/ml) (24.12–182.45) | 61.48 (28.92), 21 | 62.35 (20.08), 9 | 0.26 |
| MDC (pg/ml) (811.23–2204.67) | 1403.59 (323.60), 21 | 1209.11 (407.43), 9 | 0.23 |
| MIP-1a* (pg/ml) (8.02–98.50) | 18.45 (7.31), 20 | 19.47 (5.32), 9 | 0.44 |
| MIP-1b (pg/ml) (71.17–256.18) | 118.95 (41.39), 21 | 135.89 (43.61), 9 | 0.34 |
| SAA* (mg/dl) (0.0014–2.67E8) | 0.19 (8.05), 21 | 0.59 (2.91), 9 | 0.76 |
| TARC (pg/ml) (81.16–1144.02) | 409.87 (260.52), 21 | 331.26 (162.35), 9 | 0.33 |
| TNF-a (pg/ml) (0.86–3.44) | 2.26 (0.60), 21 | 2.51 (0.62), 9 | 0.34 |
| VCAM-1* (pg/ml) (5.69–1856.26) | 119.01 (147.39), 21 | 209.62 (1114.73), 9 | 0.11 |
Protein levels in serum.
Protein levels analyzed by ELISA in children with ASD and ASD+EP. Data presented as mean (SD), n. *Data presented as median (IQR), n. **p-value before adjusted for multiple comparisons.
Subgroup analyses were performed separately within the ASD + EP and ASD-only group, based on gender, comorbid of ADHD/ADD, psychiatric or somatic disorders, and focal versus generalized EP. The following proteins were selected based on previous findings identifying them as potential biomarkers: IFN-γ, IL-6, IL-12p70, IL-8, TNF-α, and MCP-1 (16, 25, 28, 29). However, apart from increased TNF-α levels in participants with ASD and comorbid ADHD compared to those without ADHD (2.26 ± 0.60, p = 0.036), no significant differences were found (data not shown). Subgroup analyses for IL-1β and IL-13 were not conducted due to low overall serum detection rates in both patient groups (n = 7–12).
3.3 No differences in the counts of activated leukocyte populations between children with ASD with or without EP
The gating strategies used to isolate cell populations is shown in Figure 2. Leukocytes were gated based on their CD45 expression and side scatter (SSC) signature. The following subpopulations were analyzed: HLA-DR+ cells (CD45+HLA-DR+), B-cells (CD45+CD19+), T-cells (CD45+CD3+), CD4+ T-cells (CD3+CD4+), CD8+ T-cells (CD3+CD8+), and HLA-DR+ T-cell subsets (HLA-DR+CD3+, HLA-DR+CD4+, and HLA-DR+CD8+).
Figure 2
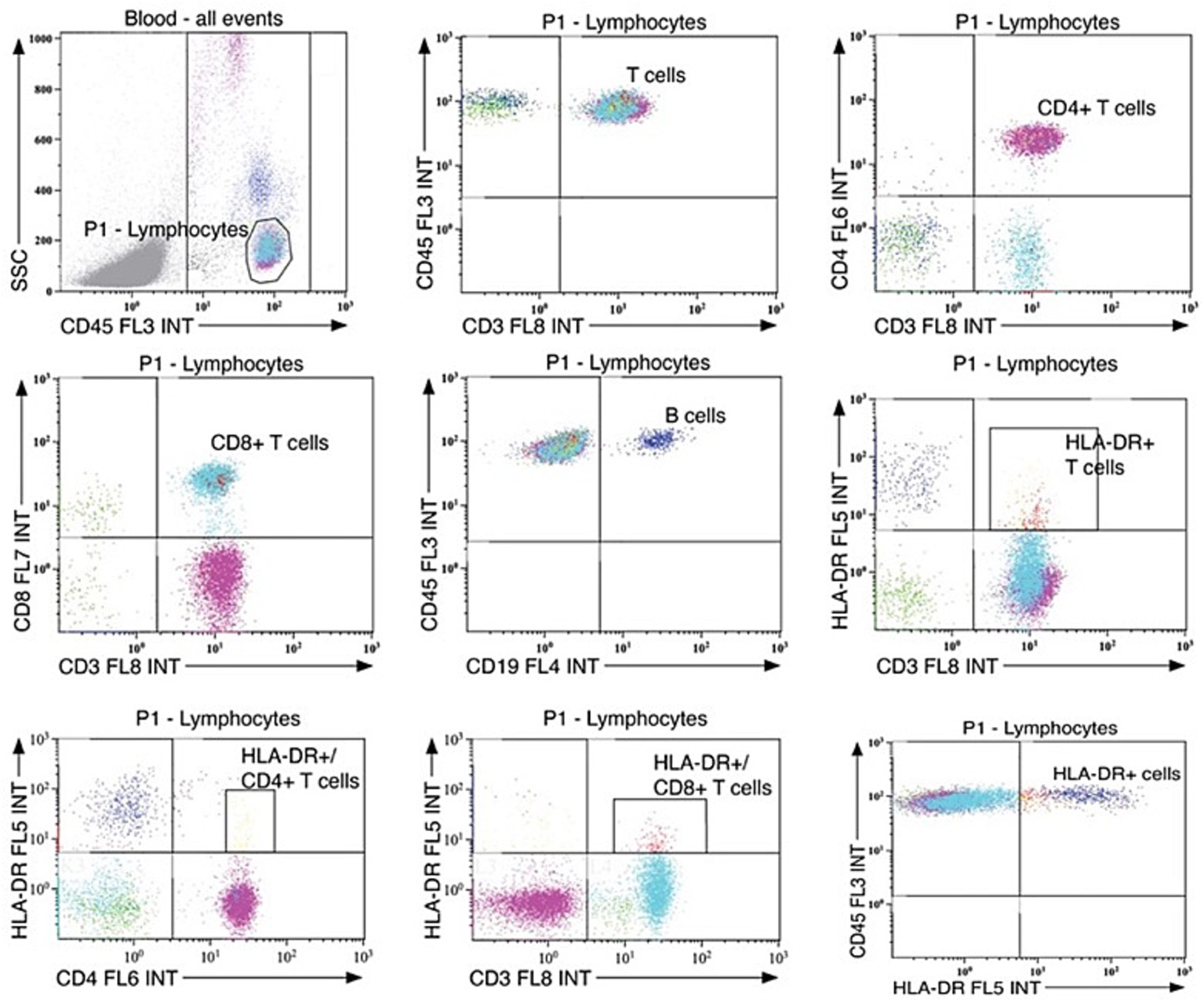
Gating strategies for leukocyte population counts. Standardized gating strategies for flow cytometry of leukocyte populations in serum.
Flow cytometry analysis revealed no significant differences in circulating leukocyte levels between the ASD + EP and ASD-only group (Table 3). Total counts of HLA-DR+cells, HLA-DR+ T-cells, and subsets of HLA-DR+/CD4+ and HLA-DR+/CD8+ T-cells, showed substantial variability within both groups. The leukocyte counts in our cohort were in line with previously established pediatric reference values (37).
Table 3
| Cell population (x 109/L) | ASD | ASD+EP | p-value |
|---|---|---|---|
| B cells* | 0.32 (0.27), 21 | 0.26 (0.21), 9 | 0.15 |
| CD4/CD8 ratio | 1.91 (0.43), 21 | 1.89 (0.50), 9 | 0.94 |
| CD4 + T cells | 1.00 (0.30), 21 | 0.86 (0.22), 9 | 0.18 |
| CD8 + T cells | 0.54 (0.17), 21 | 0.48 (0.18), 9 | 0.41 |
| HLA-DR + cells* | 0.39 (0.28), 21 | 0.31 (0.26), 9 | 0.15 |
| HLA-DR + T cells* | 0.04 (0.04), 21 | 0.05 (0.04), 9 | 0.66 |
| HLA-DR+/CD4 + T cells* | 0.02 (0.02), 21 | 0.02 (0.01), 9 | 0.76 |
| HLA-DR+/CD8 + T cells* | 0.01 (0.01), 18 | 0.02 (0.02), 9 | 0.32 |
| Lymphocytes* | 2.14 (1.34), 21 | 1.88 (0.61), 9 | 0.24 |
| T cells | 1.72 (0.50), 21 | 1.44 (0.36), 9 | 0.11 |
Leukocyte population counts in serum.
Cell populations analyzed by flow cytometry in children with ASD and ASD+EP. Counts are presented as cells × 109/L unless otherwise stated. Data presented as mean (SD), n. *Data presented as median (IQR), n. CD, cluster of differentiation; HLA, human leukocyte antigen.
4 Discussion
In this study, we explored 23 immune parameters significant for neuro-inflammation in a cohort of 30 children aged 9–14 years with ASD with or without EP. All participants had mild ASD, except for two in the ASD-only group with moderate ASD. The ASD + EP group included cases of both focal and generalized EP. Of the 23 immune proteins analyzed, three cytokines IL-12p70, IL-1β and IL-13, were significantly elevated in the ASD + EP group compared to the ASD group, although differences did not remain significant after correction for multiple comparisons. No group differences were observed in leukocyte population counts. Thus, these findings indicate three candidate markers for EP in ASD which warrant further study in larger, confirmatory cohorts.
Previous studies have shown altered immune reactions and functioning in both ASD and EP. In addition, neuroinflammatory processes are of special importance in the context of pediatric EP (38). Given that participants with both ASD and EP were included in our study, a certain degree of overlap in immune activity could be expected. The observed increase in IL-12p70, IL-1β and IL-13 in ASD + EP children, before adjustment for multiple comparisons, suggest enhanced pro- and anti-inflammatory activity associated with EP. The results align with a recent study on adults with temporal lobe EP (39). No group differences were observed for the other evaluated cytokines and chemokines. IL-6, a cytokine frequently studied in the context of neuroinflammation, has previously been reported to be elevated in both ASD and EP (13, 40). In our study, the effect size for IL-6 was moderate (=0.54), suggesting that a chronic increase may have been present in both groups, potentially explaining the absence of significant group differences and thus limiting the relevance of IL-6 in this particular patient cohort. However, larger studies are needed to confirm this.
In subgroup analysis, the only significant association was between a co-diagnosis of ADHD and TNF-α levels within the ASD group. In a previous study of 13 children with ASD and 9 with ASD + ADHD, those with comorbid ADHD had increased levels of proinflammatory cytokine MIF and decreased levels of chemokine IL-8, while children with ASD only showed increased levels of MCP-1 compared to typically developing controls (16). Studies focusing on ADHD have also linked inflammatory proteins to certain biotypes, although how such patterns may interact with comorbid EP remains unknown (15).
Previous studies on the immune reactions in adults undergoing clinical evaluation and video-EEG recordings for frequent seizures have shown that immune profiles differ between interictal and postictal blood sampling (28, 29). Interictal cytokine levels have also be associated with seizure recurrence risk and severity, including IL-1β in children with EP (29, 41, 42). According to medical records and parental reports, all children with EP in the present study had adequate seizure control at the time of enrollment. However, no continuous video-EEG confirmed seizure activity or freedom on the day of blood sampling, which may have influenced immune marker levels. Transient increases in immune factors have been observed postictally after focal seizures (28), and adults with focal EP have shown transient postictal increase in immune cell counts (43). A retrospective study of adults also indicated higher leukocyte count in generalized versus focal EP (44). Due to small sample size in our study, comparisons between focal and generalized EP in the ASD + EP group had low statistical power and need further exploration. The neutrophil-to-lymphocyte ratio (NLR) has also been suggested as a potential biomarker for EP. While some studies have reported increases in NLR, results remain mixed, and the literature is heterogenous (45). In the present study, NLR could not be evaluated due to lack of neutrophil counts. Although technical factors cannot be entirely excluded as an explanation, the substantial variability in leukocyte population counts, is still in line with previously described normal data and variability (37) and may be explained by biological heterogeneity.
Most studies of immune responses in pediatric EP have focused on severe cases, such as epileptic seizures associated with autoimmune encephalitis or febrile infection-related epilepsy syndrome (46). By contrast, the children in our study had relatively mild forms of EP, such as self-limited epilepsy with centrotemporal spikes and generalized absence epilepsy. It is possible that the immune responses observed in this group were also influenced by their underlying ASD. Previous studies have reported elevated immune factors and changes in immune cell populations in children with ASD (12, 13), but these findings are primarily based on young children with more severe ASD diagnosis, potentially reflecting a different immunological profile. Altered immune profiles have also been reported in post-mortem brain tissue from individuals with moderate to severe ASD (47). In contrast, the present study focused on children with mild ASD, which may involve different immune mechanisms. Additionally, many previous studies did not provide information on comorbid EP, limiting direct comparisons.
This exploratory study has some limitations: First, the relatively small sample size limits statistical power, especially given the heterogeneity in prior research. Technical concerns such as low detection rates in certain samples including IL-1β and IL-13 warrants careful interpretation of the results. Second, the study had a skewed gender distribution, with few female participants, which may reduce the generalizability of the findings. Third, the absence of neutrophil counts prevented evaluation of the NLR. Fourth, a potential confounder in this study is the high prevalence of psychiatric medications and ASMs, both of which have previously been reported to affect levels of immune proteins (48, 49).
5 Conclusion
Although systemic immune proteins levels may have potential as diagnostic markers for EP, even in milder cases with infrequent seizures, the presence of neuropsychiatric disorders, such as ASD, complicates the interpretation. Given the exploratory nature of this study, further research is warranted to better understand the immune profile associated with EP in the context of ASD.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Swedish Ethical Review Authority, Uppsala, Sweden. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
MT: Investigation, Writing – review & editing, Writing – original draft, Funding acquisition, Visualization, Formal analysis. FF: Writing – review & editing, Formal analysis, Visualization. MR: Investigation, Writing – review & editing, Visualization. JW: Investigation, Writing – review & editing. OR: Supervision, Writing – review & editing, Resources. CE: Methodology, Funding acquisition, Conceptualization, Writing – original draft, Writing – review & editing, Investigation, Supervision, Resources.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by Swedish Epilepsy Foundation (Epilepsifonden), Sundbyberg, Sweden, Swedish ALF Grant (grant no RSID 130567), and Tore Nilson Foundation for Medical Research, Stockholm, Sweden.
Acknowledgments
We thank staff at Division of Child Neurology, Skåne University Hospital, Lund for valuable technical support. We thank Associate Professor Erik Eklund (Division of Child Neurology, Skåne University Hospital, Lund) for valuable input and discussions. Figure 1 was created in BioRender. Taylor (2025) (https://BioRender.com/cgu4kk8).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Bolin K Berggren F Landtblom AM . Prevalence and cost of epilepsy in Sweden--a register-based approach. Acta Neurol Scand2015;131:37–44, Prevalence and cost of epilepsy in Sweden—a register-based approach, doi: 10.1111/ane.12297, .
2.
Aaberg KM Gunnes N Bakken IJ Lund Soraas C Berntsen A Magnus P et al . Incidence and prevalence of childhood epilepsy: a nationwide cohort study. Pediatrics. (2017) 139:e20163908. doi: 10.1542/peds.2016-3908
3.
Keller R Basta R Salerno L Elia M . Autism, epilepsy, and synaptopathies: a not rare association. Neurol Sci. (2017) 38:1353–61. doi: 10.1007/s10072-017-2974-x,
4.
Danielsson S Gillberg IC Billstedt E Gillberg C Olsson I . Epilepsy in young adults with autism: a prospective population-based follow-up study of 120 individuals diagnosed in childhood. Epilepsia. (2005) 46:918–23. doi: 10.1111/j.1528-1167.2005.57504.x,
5.
Pan PY Bolte S Kaur P Jamil S Jonsson U . Neurological disorders in autism: a systematic review and meta-analysis. Autism. (2021) 25:812–30. doi: 10.1177/1362361320951370,
6.
Liu X Sun X Sun C Zou M Chen Y Huang J et al . Prevalence of epilepsy in autism spectrum disorders: a systematic review and meta-analysis. Autism. (2022) 26:33–50. doi: 10.1177/13623613211045029
7.
American Psychiatric Association . MINI-D 5 Diagnostiska kriterier enligt DSM-5. 2nd ed. Stockholm: Pilgrim Press AB (2015).
8.
Yadav MK Singh SP Egwuagu CE . IL-6/IL-12 superfamily of cytokines and regulatory lymphocytes play critical roles in the etiology and suppression of CNS autoimmune diseases. Front Immunol. (2025) 16:1514080. doi: 10.3389/fimmu.2025.1514080,
9.
Marchi N Granata T Janigro D . Inflammatory pathways of seizure disorders. Trends Neurosci. (2014) 37:55–65. doi: 10.1016/j.tins.2013.11.002,
10.
Hughes CE Nibbs RJB . A guide to chemokines and their receptors. FEBS J. (2018) 285:2944–71. doi: 10.1111/febs.14466,
11.
Song J Wu C Korpos E Zhang X Agrawal SM Wang Y et al . Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. (2015) 10:1040–54. doi: 10.1016/j.celrep.2015.01.037,
12.
Ashwood P Krakowiak P Hertz-Picciotto I Hansen R Pessah I Van de Water J . Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. (2011) 25:40–5. doi: 10.1016/j.bbi.2010.08.003,
13.
Masi A Quintana DS Glozier N Lloyd AR Hickie IB Guastella AJ . Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. (2015) 20:440–6. doi: 10.1038/mp.2014.59,
14.
Suzuki K Matsuzaki H Iwata K Kameno Y Shimmura C Kawai S et al . Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One. (2011) 6:e20470. doi: 10.1371/journal.pone.0020470,
15.
Schnorr I Siegl A Luckhardt S Wenz S Friedrichsen H El Jomaa H et al . Inflammatory biotype of ADHD is linked to chronic stress: a data-driven analysis of the inflammatory proteome. Transl Psychiatry. (2024) 14:37. doi: 10.1038/s41398-023-02729-3,
16.
Han YM Cheung WK Wong CK Sze SL Cheng TW Yeung MK et al . Distinct cytokine and chemokine profiles in autism spectrum disorders. Front Immunol. (2017) 8:11. doi: 10.3389/fimmu.2017.00011,
17.
Ghirardi L Brikell I Kuja-Halkola R Freitag CM Franke B Asherson P et al . The familial co-aggregation of ASD and ADHD: a register-based cohort study. Mol Psychiatry. (2018) 23:257–62. doi: 10.1038/mp.2017.17,
18.
Muller N . The role of intercellular adhesion Molecule-1 in the pathogenesis of psychiatric disorders. Front Pharmacol. (2019) 10:1251. doi: 10.3389/fphar.2019.01251,
19.
Mutluer T Aslan Genc H Ozcan Morey A Yapici Eser H Ertinmaz B Can M et al . Population-based psychiatric comorbidity in children and adolescents with autism spectrum disorder: a meta-analysis. Front Psych. (2022) 13:856208. doi: 10.3389/fpsyt.2022.856208,
20.
Hudson CC Hall L Harkness KL . Prevalence of depressive disorders in individuals with autism spectrum disorder: a meta-analysis. J Abnorm Child Psychol. (2019) 47:165–75. doi: 10.1007/s10802-018-0402-1
21.
Alapirtti T Lehtimaki K Nieminen R Makinen R Raitanen J Moilanen E et al . The production of IL-6 in acute epileptic seizure: a video-EEG study. J Neuroimmunol. (2018) 316:50–5. doi: 10.1016/j.jneuroim.2017.12.008,
22.
Alapirtti T Rinta S Hulkkonen J Makinen R Keranen T Peltola J . Interleukin-6, interleukin-1 receptor antagonist and interleukin-1beta production in patients with focal epilepsy: a video-EEG study. J Neurol Sci. (2009) 280:94–7. doi: 10.1016/j.jns.2009.02.355,
23.
Avdic U Ahl M Oberg M Ekdahl CT . Immune profile in blood following non-convulsive epileptic seizures in rats. Front Neurol. (2019) 10:701. doi: 10.3389/fneur.2019.00701,
24.
Vezzani A Viviani B . Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. (2015) 96:70–82. doi: 10.1016/j.neuropharm.2014.10.027,
25.
Gao F Gao Y Zhang SJ Zhe X Meng FL Qian H et al . Alteration of plasma cytokines in patients with active epilepsy. Acta Neurol Scand. (2017) 135:663–9. doi: 10.1111/ane.12665,
26.
Wang B Li Q Wang H Du X Lai Q Li X et al . TNF-α: a serological marker for evaluating the severity of hippocampal sclerosis in medial temporal lobe epilepsy?J Clin Neurosci. (2024) 123:123–9. doi: 10.1016/j.jocn.2024.03.030,
27.
Nowak M Bauer S Haag A Cepok S Todorova-Rudolph A Tackenberg B et al . Interictal alterations of cytokines and leukocytes in patients with active epilepsy. Brain Behav Immun. (2011) 25:423–8. doi: 10.1016/j.bbi.2010.10.022
28.
Ahl M Taylor MK Avdic U Lundin A Andersson M Amandusson A et al . Immune response in blood before and after epileptic and psychogenic non-epileptic seizures. Heliyon. (2023) 9:e13938. doi: 10.1016/j.heliyon.2023.e13938,
29.
Wang Y Wang D Guo D . Interictal cytokine levels were correlated to seizure severity of epileptic patients: a retrospective study on 1218 epileptic patients. J Transl Med. (2015) 13:378. doi: 10.1186/s12967-015-0742-3,
30.
Lai YC Muscal E Wells E Shukla N Eschbach K Hyeong Lee K et al . Anakinra usage in febrile infection related epilepsy syndrome: an international cohort. Ann Clin Transl Neurol. (2020) 7:2467–74. doi: 10.1002/acn3.51229,
31.
Vezzani A Balosso S Ravizza T . Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. (2019) 15:459–72. doi: 10.1038/s41582-019-0217-x
32.
Saengow VE Chiangjong W Khongkhatithum C Changtong C Chokchaichamnankit D Weeraphan C et al . Proteomic analysis reveals plasma haptoglobin, interferon-gamma, and interleukin-1beta as potential biomarkers of pediatric refractory epilepsy. Brain Dev. (2021) 43:431–9. doi: 10.1016/j.braindev.2020.11.001
33.
Kamasak T Dilber B Yaman SO Durgut BD Kurt T Coban E et al . HMGB-1, TLR4, IL-1R1, TNF-alpha, and IL-1beta: novel epilepsy markers?Epileptic Disord. (2020) 22:183–93. doi: 10.1684/epd.2020.1155,
34.
Pellinen J French J Knupp KG . Diagnostic delay in epilepsy: the scope of the problem. Curr Neurol Neurosci Rep. (2021) 21:71. doi: 10.1007/s11910-021-01161-8,
35.
Kwan P Arzimanoglou A Berg AT Brodie MJ Allen Hauser W Mathern G et al . Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. (2010) 51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x,
36.
Buytaert M El Kaddouri R Hoste L Meertens B Tavernier SJ Claes K et al . Age-dependent signature of serum inflammatory cytokines in healthy children and young adults. J Interf Cytokine Res. (2024) 44:372–8. doi: 10.1089/jir.2024.0053,
37.
Garcia-Prat M Alvarez-Sierra D Aguilo-Cucurull A Salgado-Perandres S Briongos-Sebastian S Franco-Jarava C et al . Extended immunophenotyping reference values in a healthy pediatric population. Cytometry B Clin Cytom. (2019) 96:223–33. doi: 10.1002/cyto.b.21728
38.
Vega Garcia A Lopez-Meraz ML Gonzalez MI Rocha L Peixoto-Santos JE Cavalheiro EA . Immunity and neuroinflammation in early stages of life and epilepsy. Epilepsia. (2025) 66:2157–69. doi: 10.1111/epi.18361,
39.
Mueller C Hong H Sharma AA Qin H Benveniste EN Szaflarski JP . Brain temperature, brain metabolites, and immune system phenotypes in temporal lobe epilepsy. Epilepsia Open. (2024) 9:2454–66. doi: 10.1002/epi4.13082,
40.
Soltani Khaboushan A Yazdanpanah N Rezaei N . Neuroinflammation and proinflammatory cytokines in Epileptogenesis. Mol Neurobiol. (2022) 59:1724–43. doi: 10.1007/s12035-022-02725-6,
41.
Fang W Chen S Xia X Huang W Du Y Liu Z et al . Interictal interleukin-6 and tumor necrosis factor α levels are associated with seizure recurrence in adults with epilepsy. Epilepsy Behav. (2024) 155:109786. doi: 10.1016/j.yebeh.2024.109786
42.
Choi J Kim SY Kim H Lim BC Hwang H Chae JH et al . Serum alpha-synuclein and IL-1beta are increased and correlated with measures of disease severity in children with epilepsy: potential prognostic biomarkers?BMC Neurol. (2020) 20:85. doi: 10.1186/s12883-020-01662-y
43.
Bauer S Koller M Cepok S Todorova-Rudolph A Nowak M Nockher WA et al . NK and CD4+ T cell changes in blood after seizures in temporal lobe epilepsy. Exp Neurol. (2008) 211:370–7. doi: 10.1016/j.expneurol.2008.01.017,
44.
Sarkis RA Jehi L Silveira D Janigro D Najm I . Patients with generalised epilepsy have a higher white blood cell count than patients with focal epilepsy. Epileptic Disord. (2012) 14:57–63. doi: 10.1684/epd.2012.0493,
45.
Hosseini S Mofrad AME Mokarian P Nourigheimasi S Azarhomayoun A Khanzadeh S et al . Neutrophil to lymphocyte ratio in epilepsy: a systematic review. Mediat Inflamm. (2022) 2022:1–7. doi: 10.1155/2022/4973996,
46.
Granata T Fusco L Matricardi S Tozzo A Janigro D Nabbout R . Inflammation in pediatric epilepsies: update on clinical features and treatment options. Epilepsy Behav. (2022) 131:107959. doi: 10.1016/j.yebeh.2021.107959,
47.
Li X Chauhan A Sheikh AM Patil S Chauhan V Li XM et al . Elevated immune response in the brain of autistic patients. J Neuroimmunol. (2009) 207:111–6. doi: 10.1016/j.jneuroim.2008.12.002,
48.
Ormerod M Sheikh MA Ueland T Hjell G Rodevand L Saether LS et al . Minor immunomodulatory effects of psychotropics suggested in severe mental disorders: associations of antipsychotics with beta defensin 2, antidepressants with C-reactive protein, and mood stabilizers with soluble interleukin 2 receptor. Eur Psychiatry. (2025) 68:e140. doi: 10.1192/j.eurpsy.2025.10104
49.
Gulcebi MI Kendirli T Turgan ZA Patsalos PN Onat YF . The effect of serum levetiracetam concentrations on therapeutic response and IL1-beta concentration in patients with epilepsy. Epilepsy Res. (2018) 148:17–22. doi: 10.1016/j.eplepsyres.2018.09.015,
Summary
Keywords
autism spectrum disorder, biomarker, epilepsy, immune reaction, interleukin-13, interleukin-1, interleukin-12
Citation
Taylor MK, Fredlund F, Richter M, Wickham J, Rask O and Ekdahl CT (2026) Immune biomarkers for epilepsy in autism: indications of cytokine alterations in an exploratory cross-sectional pediatric study. Front. Neurol. 16:1720712. doi: 10.3389/fneur.2025.1720712
Received
08 October 2025
Revised
05 December 2025
Accepted
10 December 2025
Published
05 January 2026
Volume
16 - 2025
Edited by
Francesca Felicia Operto, University of Salerno, Italy
Reviewed by
Monica Neagu, Victor Babes National Institute of Pathology (INCDVB), Romania
Israel Jose Pereira Garcia, Universidade Federal de São João del-Rei, Brazil
Updates
Copyright
© 2026 Taylor, Fredlund, Richter, Wickham, Rask and Ekdahl.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine T. Ekdahl, christine.ekdahl_clementson@med.lu.se
† Present addresses: Filip Fredlund, Redoxis AB, Lund, Sweden Jenny Wickham, Electrophysiology Core Facility, Stem Cell Center, Lund University, Lund, Sweden
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.