Abstract
Objectives:
To characterise the pattern of video head impulse test (vHIT) impairments in patients with sudden sensorineural hearing loss with vertigo (SSNHL-V), and to assess its correlation with audiological prognosis.
Design:
We systematically searched four databases (PubMed, Embase, Scopus, Cochrane Library) from inception to February 24, 2025, performed meta-analysis using Stata 18, and had the protocol prospectively registered with PROSPERO (CRD420251025900).
Results:
Among patients with SSNHL-V, impairment of the posterior semicircular canal (PSCC) was found to be more common than that of the horizontal semicircular canal (HSCC) or anterior semicircular canal (ASCC). The pooled prevalence of PSCC abnormality was 50% (95% CI: 0.40–0.60; I2 = 87.65%, z = 14, p < 0.05). Regarding auditory prognosis, patients with reduced PSCC gain on vHIT had a 4.14-fold increased risk of poor hearing recovery (95% CI: 2.64–6.51; I2 = 7%, p = 0.36; z = 6.17, p < 0.05). Similarly, reduced HSCC gain was associated with a 3.06-fold increased risk (95% CI, 1.72–5.44; I2 = 0%, p = 0.96; z = 3.82, p < 0.05).
Conclusion:
In patients with SSNHL-V, vHIT assessment revealed a higher rate of PSCC dysfunction compared to other semicircular canals, and impaired PSCC function serves as a significant predictor of auditory prognosis.
1 Introduction
In the late 19th and early 20th centuries, research into the relationship between head and ocular movements revealed that any rotation of the head induces a unique activation pattern across the six semicircular canals of the vestibular system (1), laying the groundwork for the study of the vestibulo-ocular reflex (VOR). Upon activation of the receptor of the semicircular canals by head rotation, neurons drive ocular movements via rapid neural pathways to correct for head motion. This ensures ocular movements match head movements in angular velocity while opposing them in direction, thereby stabilizing retinal imaging and maintaining visual clarity. In clinical audiological testing, calibrated audiometers/headphones are used to deliver precisely controlled acoustic stimuli and to measure and record patient responses. Similarly, in clinical VOR assessment, tests such as the head-shaking nystagmus test (HSNT) and the head impulse test (HIT) employ natural head acceleration as the stimulus. However, unlike the calibrated audiometer, this mode of stimulation is difficult to control with precision in a clinical setting. In contrast, the vHIT enables the precise measurement of the ocular movement response corresponding to each head impulse. According to MacDougall et al., the scleral search coil method, which is regarded as the gold standard for vestibular-ocular testing in laboratories, and vHIT have similar diagnostic accuracy (2–4).
Sudden sensorineural hearing loss (SSNHL) is clinically characterized by a loss of over 30 dB HL in at least three contiguous frequencies occurring within 72 h (5). Common complications of SSNHL include tinnitus, ear fullness, vertigo, and unsteadiness. The phenomenon of vestibular involvement in this condition was first reported in 1949 (6). Given the embryological and anatomical interrelationship between the cochlear and vestibular systems, approximately 30–60% (7–9) of patients with SSNHL experience vestibular dysfunction concurrently with their hearing impairment. While vestibular dysfunction has been identified in multiple studies as a key indicator of hearing prognosis, there is no consensus on which specific test holds superior predictive value. All six semicircular canals’ high-frequency VOR function may be individually evaluated using the vHIT, which allows for both qualitative and quantitative analysis of the semicircular canals and their neural pathways. Gain and the pathological catch-up saccades are two of the main criteria of vHIT that indicate the state of vestibular compensation and the functional status of the vestibular system. Compared to other vestibular function tests, vHIT offers better patient tolerance and can be conveniently administered as a bedside examination, leading to its widespread application and in-depth study across various vestibular disorders. This systematic review examines the performance of the vHIT in assessing patients with SSNHL-V and explores the correlation between vHIT findings and audiological prognosis.
2 Materials and methods
2.1 Data sources and search strategy
This systematic review was conducted in accordance with the PRISMA guidelines (10). Two authors independently performed a comprehensive literature search of the PubMed, Cochrane Library, Embase, and Scopus databases for studies published up to February 24, 2025. No restrictions were placed on the publication date. Using all available free-text synonyms and the pertinent Medical Subject Headings (MeSH), such as “Hearing Loss, Sensorineural” and “video head impulse test”, the literature search was conducted. The complete search strategy for all databases is available in Supplementary Table S1. The following were the requirements for inclusion in the study: (1) the study population had to be SSNHL-V patients; (2) vHIT results included in the vestibular assessment; and (3) availability of pure-tone audiometry results or data on hearing recovery. Exclusion criteria included: (1) non-English publications; (2) animal studies, reviews, conference reports, and case reports; and (3) studies with incomplete data necessary for analysis.
2.2 Data abstraction and quality assessment
In accordance with the guidelines, the retrieved potential studies were imported into the reference management software EndNote 21. Two staff members independently conducted a preliminary assessment and exclusion based on titles and abstracts. The information and data used to determine the inclusion of studies were independently extracted from each selected study. The hearing recovery rate was defined as the proportion of patients with incomplete hearing recovery in each group relative to the total number of patients in that group. The retrieved characteristics included: the surname of the primary author, year of publication, country, research design, study duration, number of SSNHL subjects, abnormal rate of vHIT results, audiologic test outcomes, and hearing recovery.
The quality of the included studies was evaluated separately by two authors using the Newcastle-Ottawa Scale (NOS). The quality was examined across three dimensions: study population, outcome assessment, and comparability of groups, comprising a total of 8 items. Each item was scored using a star system, with the overall score ranging from 0 to 9 stars. A superior score signifies enhanced study quality. Studies having a score of 6 or above were deemed high-quality (11). The quality assessment results are presented in Supplementary Table S2.
2.3 Statistical analysis
STATA 18 software was used to conduct the meta-analysis, and forest plots were used to display the findings. The pooled abnormal rate of the semicircular canal in vHIT results was determined through a single-arm meta-analysis. The hearing recovery rate was coded as dichotomous data, and the relationship between auditory prognosis and vHIT was analyzed by combining odds ratios (ORs) using the Mantel–Haenszel method. The Q-test and I2 test were utilized to quantify heterogeneity across the included studies. A p-value < 0.05 and/or I2 > 50% indicated substantial heterogeneity (12). A fixed-effects model was employed when I2 < 50% and p > 0.1; otherwise, a random-effects model was utilized. The assessment of publication bias in the included studies was conducted using funnel plots and symmetry tests.
3 Results
3.1 Literature search results
The PRISMA flowchart in Figure 1 illustrates the process of searching, deleting, and filtering for relevant studies. As of February 24, 2025, a total of 363 records were identified through searches using subject headings and free-text terms for “Hearing Loss, Sensorineural” and “video head impulse test”. Before abstract screening, 188 duplicate records were removed. Among the remaining records, 12 case reports, 3 review articles, and 1 editorial were excluded. After screening titles and abstracts, 139 records were further excluded. Two studies could not be retrieved in full text (13, 14), and five studies did not provide detailed vHIT parameter data (15–18). Ultimately, 13 studies were deemed eligible for inclusion (19–31).
Figure 1
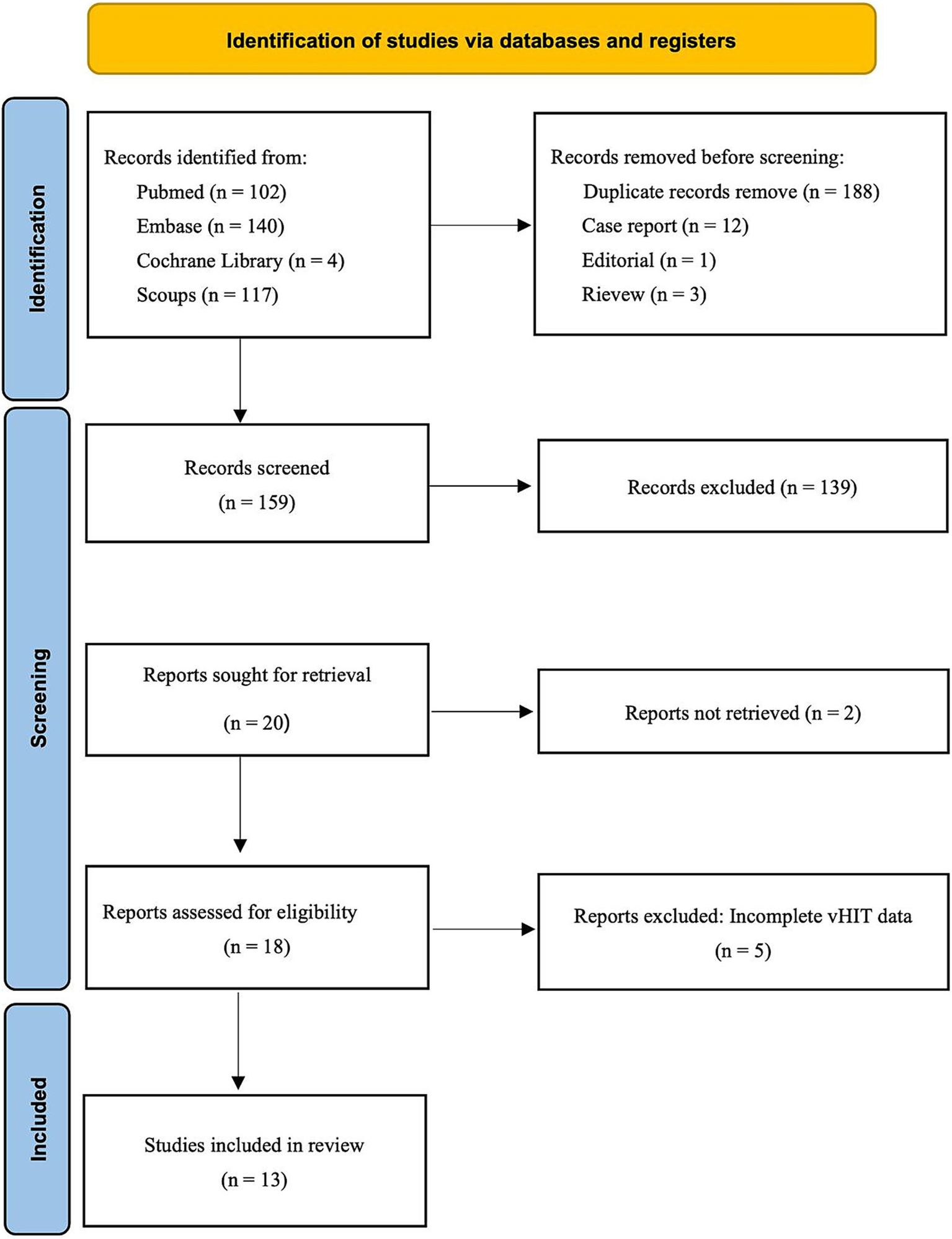
The PRISMA flowchart of selecting the relevant studies for this review is depicted.
3.2 Study characteristics
Table 1 presents the essential characteristics of the 13 selected studies, conducted between 2016 and 2025 and covering China, Australia, North Korea, South Korea, and Japan. These studies included 1,141 participants, with sample sizes ranging from 23 to 148, and a male-to-female ratio of 501:640. Four studies included other patients with acute vestibular syndrome, such as vestibular neuritis (VN) and Ramsay Hunt syndrome (RHSD), to compare vHIT results with those of patients with SSNHL-V (19, 21, 22, 24). The criteria for hearing recovery varied across the included studies. Two studies employed Siegel’s criteria (25, 29), two defined recovery as a pure-tone average (PTA) within 10 dB HL of the unaffected ear (23, 26), one applied a threshold of PTA < 20 dB HL (20), and another used PTA < 25 dB HL as the criterion (31). The median Newcastle-Ottawa score for the 13 included studies was 4 (range: 4–6; See Supplementary Table S2). All studies were observational studies with moderately low study quality.
Table 1
| Study | Research type | Patients with SSNHL-V | Patients with SSNHL without vertigo | vHIT results | Hearing recovery criteria | ||
|---|---|---|---|---|---|---|---|
| HSCC | ASCC | PSCC | |||||
| Liu Y (2025), (19) | R | 70 | 0 | 16/70 | 3/70 | 39/70 | |
| Qian Y (2024), (20) | R | 112 | 114 | 24/112 | 20/112 | 60/112 | PTA < 20dBHL |
| Nakamichi N (2024), (21) | R | 15 | 0 | 8/15 | 2/15 | 11/15 | |
| Liu Y (2023) (22) | R | 57 | 0 | 12/57 | 3/57 | 30/57 | |
| Hong JP (2023), (23) | R | 73 | 79 | 15/73 | 4/73 | 41/73 | Within 10 dB HL of unaffected ear |
| Hong JP (2023), (24) | R | 81 | 0 | 33/81 | 9/81 | 42/81 | |
| Hao W (2023), (25) | P | 86 | 0 | 46/86 | 10/86 | 48/86 | Siegel |
| Cho JW (2023), (31) | R | 23 | 0 | 16/23 | 11/23 | 19/23 | PTA < 25 dB HL |
| Seo HW (2022), (26) | R | 54 | 81 | 8/54 | 7/54 | 20/54 | Within 10 dB HL of unaffected ear |
| Jiang Z (2021), (27) | R | 29 | 21 | 2/29 | 10/29 | 17/29 | |
| Lee JY (2020), (28) | R | 71 | 0 | 10/71 | 5/71 | 21/71 | |
| Byun H (2020), (29) | R | 148 | 0 | 25/148 | 19/148 | 28/148 | Siegel |
| Pogson JM (2016), (30) | R | 27 | 0 | 11/27 | 8/27 | 20/27 | |
Literature reports of selected studies.
R, retrospective; P, prospective.
A total of 7 out of the 13 included studies demonstrated a higher prevalence of abnormal vHIT results for the PSCC relative to the other canals in patients with SSNHL-V (19–24, 28). One study (Jiang et al., 2021) was excluded from this subgroup owing to insufficient data on canal-specific abnormality rates (27). Heterogeneity testing across the remaining 12 studies revealed significant heterogeneity, with I2 > 50% and p < 0.1 of the Q-test. A sensitivity analysis was conducted on 12 studies (Figure 2), revealing that no single study significantly disrupted the meta-analysis results, indicating robust stability of the research. Consequently, a random-effects model was utilized to pool the abnormal rates of the three semicircular canals according to vHIT results. The analysis showed an abnormal PSCC rate of 50% (95% CI: 0.40–0.60; I2 = 87.65%, p = 0; z = 14, p < 0.05), an abnormal HSCC rate of 25% (95% CI: 0.18–0.34; I2 = 84.35%, p = 0; z = 10.15, p < 0.05), and an abnormal ASCC rate of 12% (95% CI: 0.08–0.16; I2 = 64.51%, p = 0; z = 9.5, p < 0.05) (Figure 3). The assessment of publication bias was conducted through a funnel plot (Figure 4), with symmetry tests revealing no significant evidence of publication bias.
Figure 2
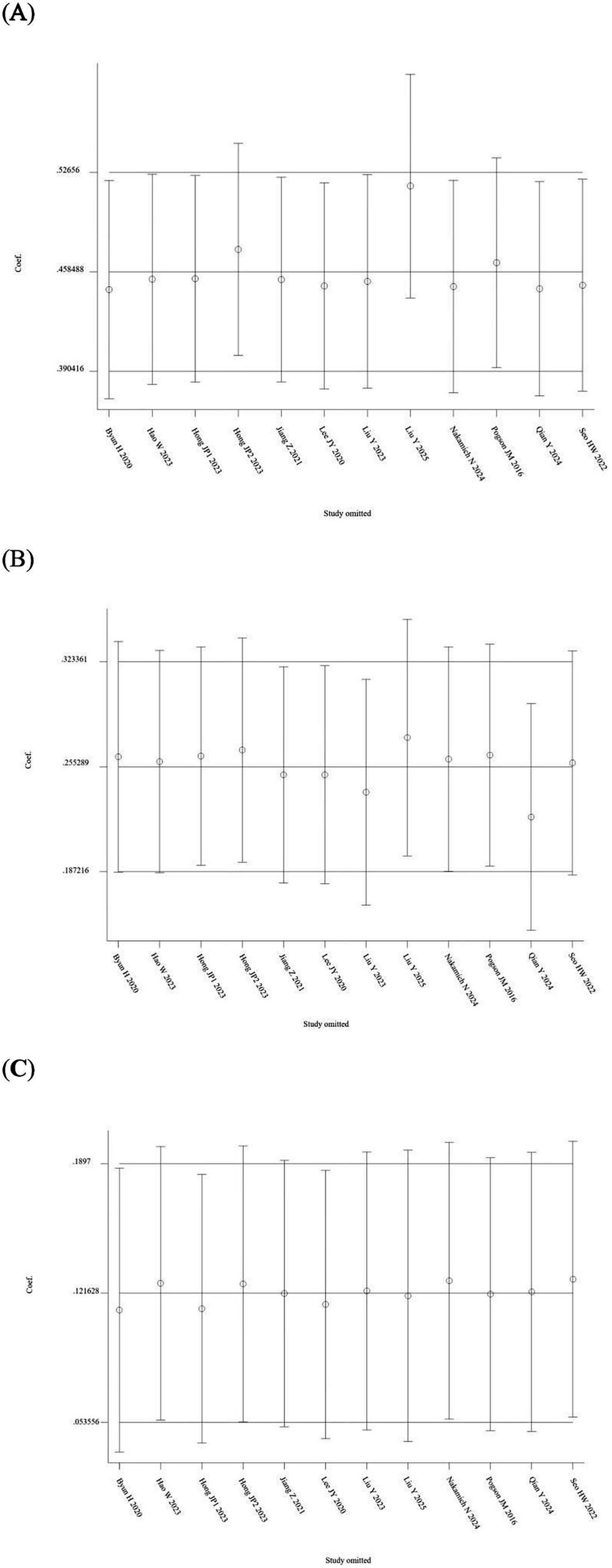
Sensitivity analysis of the random-effects model of the three semicircular canal abnormality rates. (A) Sensitivity analysis of PSCC. (B) Sensitivity analysis of HSCC. (C) Sensitivity analysis of ASCC.
Figure 3
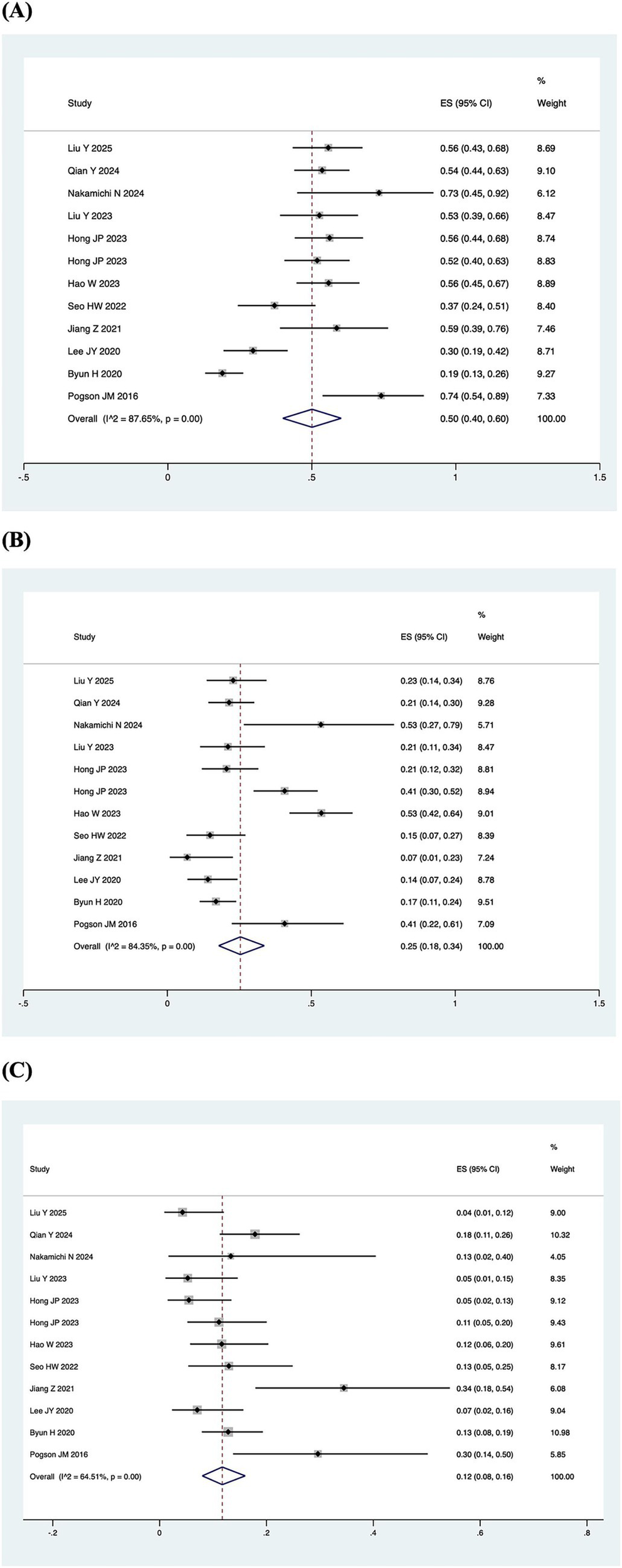
Pooled abnormal rate of the semicircular canal in the vHIT result. (A) Forest plot diagram of the abnormality rate in PSCC. (B) Forest plot diagram of the abnormality rate in HSCC. (C) Forest plot diagram of the abnormality rate in ASCC.
Figure 4
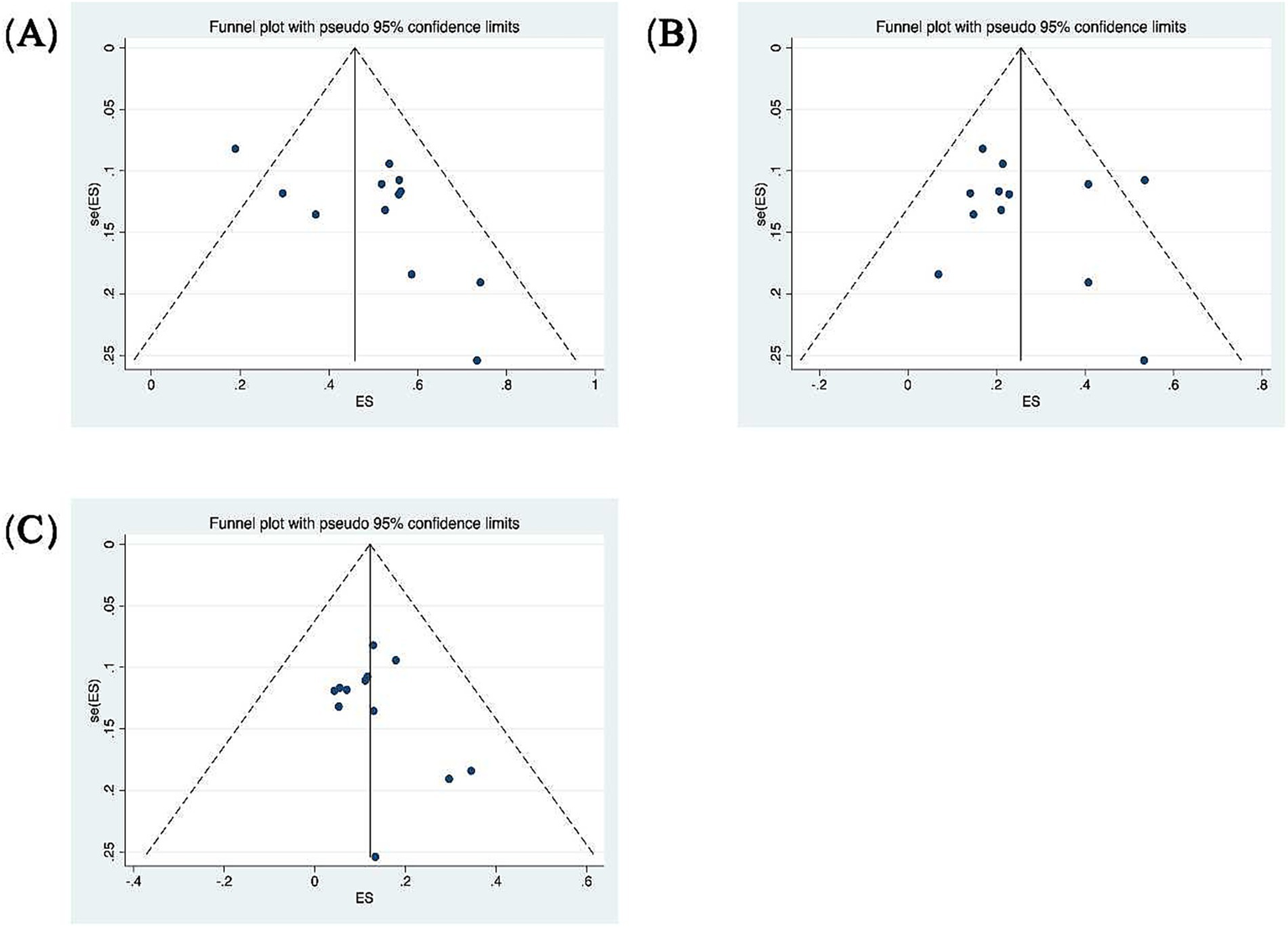
Funnel plots for the evaluation of publication bias. (A) Funnel plot of the included studies in the meta-analysis of the abnormality rate in PSCC. p = 0.373 > 0.05, funnel plot symmetry, no publication bias. (B) Funnel plot of the included studies in the meta-analysis of the abnormality rate in HSCC. p = 0.732 > 0.05, funnel plot symmetry, no publication bias. (C) Funnel plot of the included studies in the meta-analysis of the abnormality rate in ASCC. p = 0.945 > 0.05, funnel plot symmetry, no publication bias.
3.3 Hearing recovery based on vHIT results
Patients were categorized according to whether the gain values for each semicircular canal (abnormal gain: <0.8 for the HSCC, <0.7 for the vertical semicircular canals) in the vHIT results were normal or not, and the association with inadequate hearing recovery was examined using a dichotomous method. Heterogeneity testing across the five included studies revealed I2 < 50% and a Q-test p-value > 0.1, indicating no significant heterogeneity among the studies (20, 23, 26, 29, 31). A fixed-effects model was utilized to assess the subgroup outcomes. The weighted mean odds ratio was 3.06 for the HSCC (95% CI: 1.72–5.44; I2 = 0%, p = 0.96; z = 3.82, p < 0.05), 1.38 for the ASCC (95% CI: 0.77–2.48; I2 = 0%, p = 0.77; z = 1.07, p = 0.29), and 4.14 for the PSCC (95% CI: 2.64–6.51; I2 = 7%, p = 0.36; z = 6.17, p < 0.05). These results indicate a statistically significant association between reduced PSCC gain and poor auditory prognosis (Figure 5). Publication bias was assessed using a funnel plot (Figure 6), and symmetry testing confirmed the funnel plot was symmetric, suggesting no significant publication bias.
Figure 5
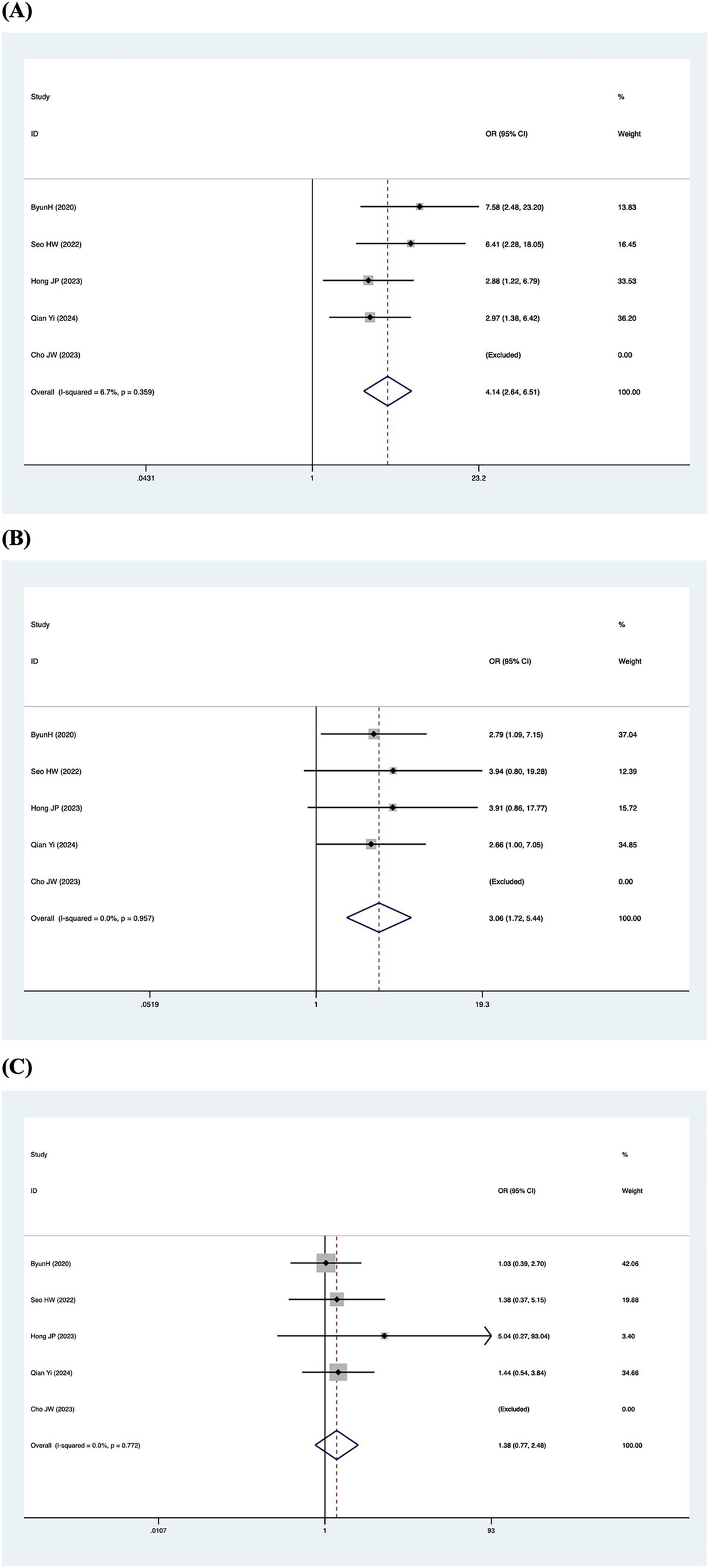
Forest plots of the synthesized data from the selected studies. (A) Forest plot diagram of hearing recovery and PSCC results. (B) Forest plot diagram of hearing recovery and HSCC results. (C) Forest plot diagram of hearing recovery and ASCC results.
Figure 6
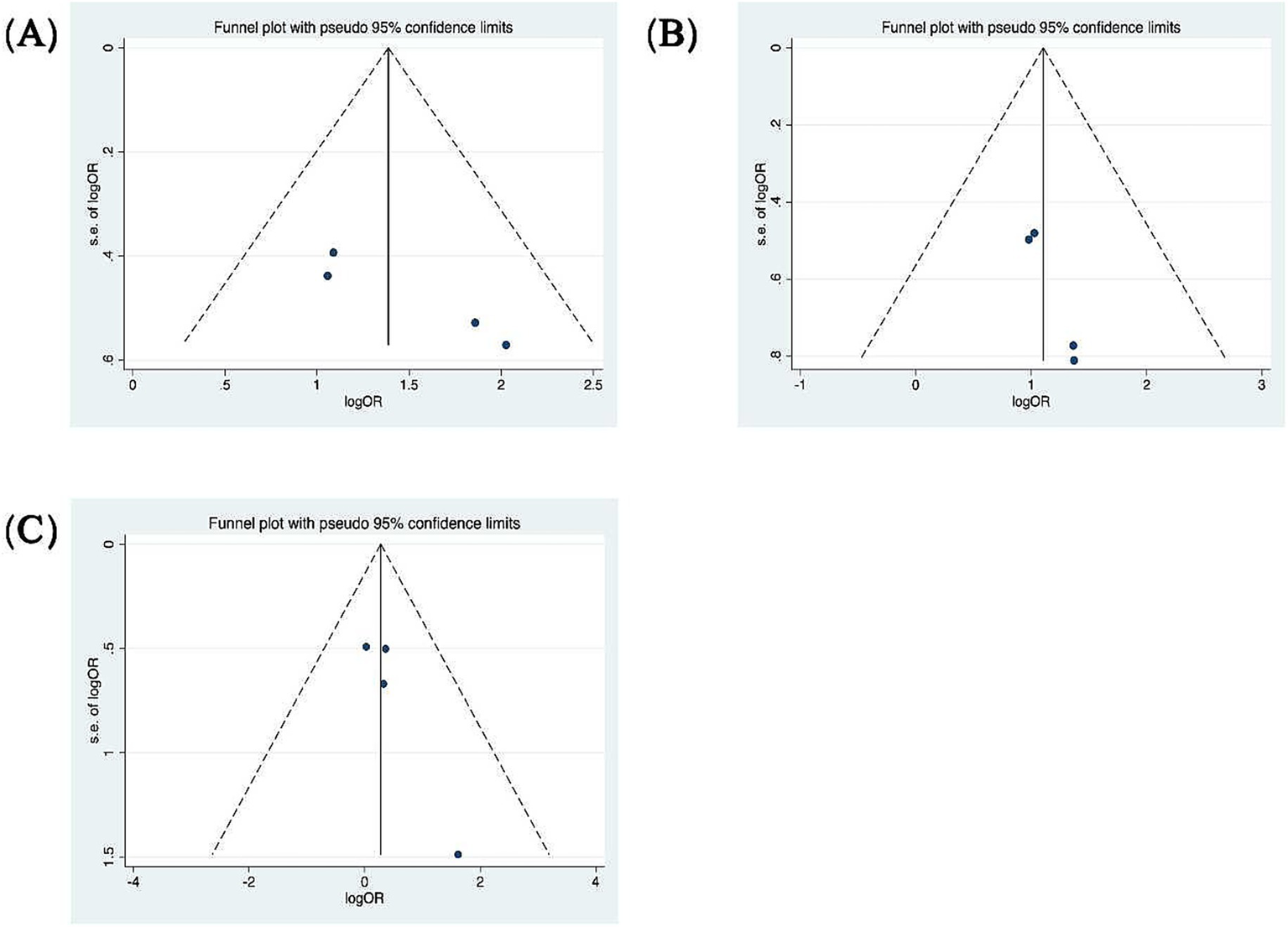
Funnel plots for the evaluation of publication bias. (A) Funnel plot of the included studies in the meta-analysis of hearing retrieval and PSCC. p = 0.045 > 0.05. (B) Funnel plot of the included studies in the meta-analysis of hearing retrieval and HSCC. p = 0.017 < 0.05. (C) Funnel plot of the included studies in the meta-analysis of hearing retrieval and ASCC. p = 0.111 > 0.05.
4 Discussion
Numerous studies have established that vertigo serves as a negative prognostic factor for hearing recovery. However, there is no consensus regarding which specific vestibular function test most effectively predicts hearing outcomes. vHIT is a rapid, easily executable, and well-tolerated examination that can quantitatively assess high-frequency VOR function (4). The main aim of this systematic review and meta-analysis is to examine the impairment patterns of vHIT and the relationship between vHIT outcomes and hearing prognosis in patients with SSNHL-V.
Among the 13 studies ultimately included, four compared the vHIT impairment patterns between patients with other acute vestibular syndromes (VN and RHSD) and those with SSNHL-V (19, 21, 22, 24). The comparative analysis indicated that patients with RHSD and VN exhibit significantly reduced VOR gain and an increased occurrence of pathological saccades in the HSCC and ASCC relative to the SSNHL-V group (19). In cases of RHSD, the impairment in gain is more severe, with a broader involvement of impaired semicircular canals, often manifesting as combined damage to all three semicircular canals (24). Due to the neural clamping mechanism involved in vestibular compensation, not only is the affected side compromised, but the healthy side is also impacted, exhibiting a reduction in gain over a certain period (32). In contrast, among VN patients, the HSCC was the most frequently affected, followed by the ASCC and PSCC (19, 21, 22). This pattern of higher involvement rates for the HSCC and ASCC compared to the PSCC was also corroborated in studies of VN patients by Magliulo and Taylor, respectively (33, 34).
vHIT exhibits distinct impairment patterns that may be associated with viral infection characteristics. RHSD is a polycranial neuropathy triggered by the reactivation of the varicella-zoster virus (VZV) latent in cranial nerve ganglia (35). Due to the neurotropic and polyneuropathic nature of VZV, the lesion scope in RHSD often extends beyond a single nerve. Combined involvement of the 7th (facial) and 8th (vestibulo-cochlear) cranial nerves is most common, and it may also affect the 5th, 9th, and 10th cranial nerves (36). In rare cases, inflammation may retrogradely extend to the brainstem, resulting in vestibular pathology exhibiting both peripheral and central features (37). Consequently, vHIT reveals a more extensive (involving all semicircular canals) and severe (more significant gain reduction) impairment pattern. Although the exact etiology of vestibular neuritis (VN) remains unclear, it is generally attributed to viral infections (particularly HSV-1) (38). The lesion in VN is primarily confined to the vestibular nerve itself, aligning more closely with its anatomical distribution and demonstrating greater selectivity. The predominant vHIT findings in VN are isolated impairment of the HSCC or a combination of impairments in both the HSCC and ASCC.
Compared to the two aforementioned acute vestibular syndromes, patients with SSNHL-V exhibit a higher impairment rate of the PSCC. vHIT testing in SSNHL patients reveals that the PSCC has the highest impairment rate, followed by the HSCC, while the ASCC shows the lowest rate. Common impairment patterns manifest as either isolated or combined PSCC dysfunction. Furthermore, studies have found that PSCC dysfunction has a greater impact on poor hearing prognosis, with reduced PSCC gain increasing the likelihood of poor hearing recovery by 4.14-fold. The explanation for this phenomenon is primarily supported by the inference of vascular compromise. The HSCC, ASCC, the utricular macula, and the superior part of the saccular macula receive blood supply from the anterior vestibular artery (39). As a branch of the common cochlear artery, the posterior vestibular artery supplies the PSCC and the part of the saccular macula. The lack of collateral circulation makes these structures more vulnerable to vascular impairment. Research involving animals has demonstrated that ischemia persisting for 30 min or more can result in irreversible damage (40). In another meta-analysis investigating cVEMP (cervical Vestibular Evoked Myogenic Potential) in SSNHL patients, abnormal cVEMP findings were associated with a 3.22-fold increased risk of poor hearing prognosis (41). This finding is consistent with our conclusion, indicating that involvement of the inferior vestibular pathway has a strong influence on poor hearing prognosis.
In a case of left-sided SSNHL-V, inner ear MRI 3D-FIESTA sequences revealed a filling defect in the PSCC, indicative of fibrosis due to ischemia, which further supports the vascular theory (42). Furthermore, a multivariate analysis by Byun et al. indicated that PSCC involvement exhibited the highest odds ratio among the considered clinical factors (e.g., age, initial hearing level) (29). While many unaccounted variables may influence auditory recovery in SSNHL, the relationship between partial/absent recovery and vascular pathophysiology should not be regarded as coincidental.
5 Limitations
This study presents several limitations. First, the included studies are limited in number and do not consist of multicenter studies or controlled trials. Second, all incorporated studies were retrospective in design, which may introduce significant confounding factors. Third, nearly all the studies employed different assessment methods or combinations of tests to evaluate vestibular function, making it difficult to compare the results. Currently, a standardised, multi-center-validated prognostic scoring system for hearing outcomes based on vestibular function test parameters has not yet been established.
6 Conclusion
This meta-analysis indicates that in patients with SSNHL-V, the rate of impairment is higher in the PSCC than in the other semicircular canals. The findings suggest that reduced vHIT gain of either the HSCC or PSCC is associated with an increased risk of poor hearing prognosis. Notably, PSCC involvement carries a substantially greater risk, with 4.14-fold higher odds of poor hearing recovery, establishing it as a key predictor. Furthermore, standardised vHIT assessment shows potential clinical utility for informing treatment decisions, predicting audiological outcomes, and guiding vestibular rehabilitation in all SSNHL patients with pathological vHIT, regardless of the presence of subjective vertigo.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
QG: Data curation, Methodology, Software, Writing – original draft. YL: Conceptualization, Supervision, Writing – review & editing. ZW: Data curation, Writing – original draft, Investigation. PH: Data curation, Investigation, Writing – original draft. DZ: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by Innovative Research Team on the Fundamentals and Clinical Applications of Sensorineural Hearing Loss (grant number: 2023-CX-TD-70), Shaanxi Provincial Health Science Research and Innovation Platform for Otorhinolaryngology (grant number: 2024PT-07), and Research on Key Technologies for the Prevention and Control of Noise-Induced Hearing Loss (2024SF-ZDCYL-01-16) to QG.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1756795/full#supplementary-material
References
1.
Curthoys IS Blanks RH Markham CH . Semicircular canal functional anatomy in cat, Guinea pig and man. Acta Otolaryngol. (1977) 83:258–65. doi: 10.3109/00016487709128843,
2.
MacDougall HG McGarvie LA Halmagyi GM Curthoys IS Weber KP . Application of the video head impulse test to detect vertical semicircular canal dysfunction. Otol Neurotol. (2013) 34:974–9. doi: 10.1097/MAO.0b013e31828d676d,
3.
MacDougall HG McGarvie LA Halmagyi GM Curthoys IS Weber KP . The video head impulse test (vHIT) detects vertical semicircular canal dysfunction. PLoS One. (2013) 8:e61488. doi: 10.1371/journal.pone.0061488,
4.
MacDougall HG Weber KP McGarvie LA Halmagyi GM Curthoys IS . The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. (2009) 73:1134–41. doi: 10.1212/WNL.0b013e3181bacf85,
5.
Chandrasekhar SS Tsai Do BS Schwartz SR Bontempo LJ Faucett EA Finestone SA et al . Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. (2019) 161. doi: 10.1177/0194599819859885
6.
Yu H Li H . Vestibular dysfunctions in sudden sensorineural hearing loss: a systematic review and meta-analysis. Front Neurol. (2018) 9:45. doi: 10.3389/fneur.2018.00045,
7.
Fetterman BL Saunders JE Luxford WM . Prognosis and treatment of sudden sensorineural hearing loss. Am J Otol. (1996) 17:529–36.
8.
Shaia FT Sheehy JL . Sudden sensori-neural hearing impairment: a report of 1,220 cases. Laryngoscope. (1976) 86:389–98. doi: 10.1288/00005537-197603000-00008,
9.
Niu X Zhang Y Zhang Q Xu X Han P Cheng Y et al . The relationship between hearing loss and vestibular dysfunction in patients with sudden sensorineural hearing loss. Acta Otolaryngol. (2016) 136:225–31. doi: 10.3109/00016489.2015.1110750,
10.
Moher D Liberati A Tetzlaff J Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097,
11.
Wells G Shea B O'Connell J . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Health Research Institute Web site (2014).
12.
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186,
13.
Yoo BJ Park CW Byun HY Lee SH Yoon HS Chung JH . PScc hypofunction by v-HIT as a poor prognostic factor for idiopathic SSNHL. Otolaryngol Head Neck Surg. (2019) 161:P259
14.
Murofushi T Tsubota M Daiji S . Idiopathic sudden sensorineural hearing loss: labyrinthitis or ischemia? Consideration based on VHIT and CVEMP. J Neurol Sci. (2019) 405:90–1. doi: 10.1016/j.jns.2019.10.386
15.
Hepkarsi S Kaya I Kirazli T . Vestibular function assessment in idiopathic sudden sensorineural hearing loss: a prospective study. Eur Arch Otorrinolaringol. (2024) 281:2365–72. doi: 10.1007/s00405-023-08361-7,
16.
Guan R Zhao Z Guo X Sun J . The semicircular canal function tests contribute to identifying unilateral idiopathic sudden sensorineural hearing loss with vertigo. Am J Otolaryngol. (2020) 41:102461. doi: 10.1016/j.amjoto.2020.102461,
17.
Lin SC Lin MY Kang BH Lin YS Liu YH Yin CY et al . Video head impulse test coherence predicts vertigo recovery in sudden sensorineural hearing loss with vertigo. Clin Exp Otorhinolaryngol. (2024) 17:282–91. doi: 10.21053/ceo.2024.00068,
18.
Yao Q Xu C Wang H Shi H Yu D . Video head impulse test results suggest that different pathomechanisms underlie sudden sensorineural hearing loss with vertigo and vestibular neuritis: our experience in fifty-two patients. Clin Otolaryngol. (2018) 43:1621–4. doi: 10.1111/coa.13196,
19.
Liu Y Chen X Shen X Xia K Liu Q Zhou R et al . Comparison of vHIT deficits with Ramsay hunt syndrome with dizziness, vestibular neuritis, and idiopathic sudden sensorineural hearing loss with vertigo. J Vestib Res. (2025) 35:9574271251313801. doi: 10.1177/09574271251313801
20.
Qian Y Kang H Zhong S Tao C Zuo W Lei Y et al . The role of asymmetry values, gain, and pathological saccades of the video head impulse test (vHIT) in sudden sensorineural hearing loss. Otol Neurotol. (2024) 45:e509–16. doi: 10.1097/MAO.0000000000004247,
21.
Nakamichi N Shiozaki T Sakagami M Kitahara T . Differences in semicircular canal function in the video head impulse test in patients in the chronic stage of sudden sensorineural hearing loss with vertigo and vestibular neuritis. Acta Otolaryngol. (2024) 144:123–9. doi: 10.1080/00016489.2024.2330680,
22.
Liu Y Leng Y Zhou R Liu J Wang H Xia K et al . Discrepancies of video head impulse test results in patients with idiopathic sudden sensorineural hearing loss with vertigo and vestibular neuritis. Front Neurosci. (2023) 17:1102512. doi: 10.3389/fnins.2023.1102512,
23.
Hong JP Lee JY Kim MB . A comparative study using vestibular mapping in sudden sensorineural hearing loss with and without vertigo. Otolaryngol Head Neck Surg. (2023) 169:1573–81. doi: 10.1002/ohn.422,
24.
Hong JP Lee JY Kim MB . Vestibular mapping in Ramsay-hunt syndrome and idiopathic sudden sensorineural hearing loss. Eur Arch Otorrinolaringol. (2023) 280:5251–8. doi: 10.1007/s00405-023-08029-2,
25.
Hao W Ye L Yu H Li H . Prognosis of vestibular dysfunction in idiopathic sudden sensorineural hearing loss with vertigo: a prospective cohort study. J Neurol. (2023) 270:5516–26. doi: 10.1007/s00415-023-11894-w,
26.
Seo HW Chung JH Byun H Lee SH . Vestibular mapping assessment in idiopathic sudden sensorineural hearing loss. Ear Hear. (2022) 43:242–9. doi: 10.1097/AUD.0000000000001129,
27.
Jiang Z Zhang J Wang Y Huang X Yao Q Feng Y et al . Contribution of audiogram classification in evaluating vestibular dysfunction in sudden sensorineural hearing loss with vertigo. Front Neurol. (2021) 12:667804. doi: 10.3389/fneur.2021.667804,
28.
Lee JY Lee YW Chang SO Kim MB . Vestibular function analysis of sudden sensorineural hearing loss with dizziness. J Vestib Res. (2020) 30:203–12. doi: 10.3233/VES-200703,
29.
Byun H Chung JH Lee SH . Clinical implications of posterior semicircular canal function in idiopathic sudden sensorineural hearing loss. Sci Rep. (2020) 10:8313. doi: 10.1038/s41598-020-65294-5,
30.
Pogson JM Taylor RL Young AS McGarvie LA Flanagan S Halmagyi GM et al . Vertigo with sudden hearing loss: audio-vestibular characteristics. J Neurol. (2016) 263:2086–96. doi: 10.1007/s00415-016-8214-0,
31.
Cho JW Cho SI Baek W Kim MS Nam GS . Significance of baseline inferior vestibular function on the prognosis of patients with Labyrinthitis. Otol Neurotol. (2023) 44:e26–32. doi: 10.1097/MAO.0000000000003746,
32.
Curthoys IS Halmagyi GM . Vestibular compensation In: HerdmanSJ, editor. 3rd ed. Philadelphia: F. A. Davis (2007). 76–97.
33.
Taylor RL McGarvie LA Reid N Young AS Halmagyi GM Welgampola MS . Vestibular neuritis affects both superior and inferior vestibular nerves. Neurology. (2016) 87:1704–12. doi: 10.1212/WNL.0000000000003223,
34.
Magliulo G Gagliardi S Ciniglio Appiani M Iannella G Re M . Vestibular neurolabyrinthitis: a follow-up study with cervical and ocular vestibular evoked myogenic potentials and the video head impulse test. Ann Otol Rhinol Laryngol. (2014) 123:162–73. doi: 10.1177/0003489414522974,
35.
Sweeney CJ Gilden DH . Ramsay hunt syndrome. J Neurol Neurosurg Psychiatry. (2001) 71:149–54. doi: 10.1136/jnnp.71.2.149,
36.
Pavlidis P Camara RJA Kekes G Gouveris H . Bilateral taste disorders in patients with Ramsay hunt syndrome and bell palsy. Ann Neurol. (2018) 83:807–15. doi: 10.1002/ana.25210,
37.
Sun H You H Wu H . Brainstem infarction and vertebral artery vasculopathy after Ramsay hunt syndrome. Neurology. (2022) 98:890–1. doi: 10.1212/WNL.0000000000200522,
38.
Strupp M Bisdorff A Furman J Hornibrook J Jahn K Maire R et al . Acute unilateral vestibulopathy/vestibular neuritis: diagnostic criteria. J Vestib Res. (2022) 32:389–406. doi: 10.3233/VES-220201,
39.
Kim JS Lee H . Inner ear dysfunction due to vertebrobasilar ischemic stroke. Semin Neurol. (2009) 29:534–40. doi: 10.1055/s-0029-1241037,
40.
Tsuji S Tabuchi K Hara A Kusakari J . Long-term observations on the reversibility of cochlear dysfunction after transient ischemia. Hear Res. (2002) 166:72–81. doi: 10.1016/S0378-5955(02)00299-X,
41.
Gonzalez-Garcia M Prieto-Sanchez-de-Puerta L Dominguez-Duran E Sanchez-Gomez S . Auditory prognosis of patients with sudden sensorineural hearing loss in relation to the presence of acute vestibular syndrome: a systematic literature review and meta-analysis. Ear Hear. (2025) 46:8–15. doi: 10.1097/AUD.0000000000001576
42.
Castellucci A Pepponi E Bertellini A Senesi C Bettini M Botti C et al . Case report: filling defect in posterior semicircular canal on MRI with balanced steady-state gradient-echo sequences after labyrinthine ischemia in the common cochlear artery territory as an early sign of fibrosis. Front Neurol. (2020) 11:608838
Summary
Keywords
audiological prognosis, hearing recovery, sudden sensorineural hearing loss, vestibular test, video head impulse test
Citation
Guo Q, Lin Y, Wang Z, Hang P and Zha D (2026) Prognostic value of the video head impulse test in sudden sensorineural hearing loss with vertigo: a systematic review and meta-analysis. Front. Neurol. 16:1756795. doi: 10.3389/fneur.2025.1756795
Received
29 November 2025
Revised
19 December 2025
Accepted
22 December 2025
Published
12 January 2026
Volume
16 - 2025
Edited by
Jose Antonio Lopez-Escamez, University of Sydney, Australia
Reviewed by
Jorge Rey-Martinez, Donostia University Hospital, Spain
Georgios Korres, National and Kapodistrian University of Athens, Greece
Updates
Copyright
© 2026 Guo, Lin, Wang, Hang and Zha.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dingjun Zha, zhadjun@fmmu.edu.cn; Ying Lin, lytemple@fmmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.