Abstract
Objective:
Age-related cognitive decline (ARCD) is highly prevalent in aging populations and is characterized by progressive declines in cognitive function—particularly executive function (EF), which merits focused investigation. This study aimed to compare prefrontal cortex (PFC) activation patterns between ARCD patients with executive dysfunction (ED) and those with non-executive dysfunction (non-ED) using functional near-infrared spectroscopy (fNIRS) during a verbal fluency task (VFT). It further explored correlations between alterations in PFC activation, the severity of EF impairment, and global cognitive function.
Methods:
A total of 36 elderly individuals diagnosed with ARCD were recruited for this study. Participants were stratified into the ED group or the non-ED group based on neuropsychological test performance, with 18 individuals in each group. fNIRS was employed during the VFT to assess cortical activity. Additionally, a comprehensive neuropsychological assessment was conducted to evaluate global cognitive function, as well as specific domains related to memory and EF. Correlations between PFC activation, as reflected by changes in oxyhemoglobin (oxy-Hb) concentration and cognitive outcomes were analyzed.
Results:
The participants of the ED group were older than those in the non-ED group, and had a higher incidence of type 2 diabetes mellitus than those in the non-ED group. The fNIRS-VFT analysis revealed no activation of the PFC in the ED group (p > 0.05), whereas only minimal activation of the left frontal pole was observed in the non-ED group of ARCD (FDR-corrected p < 0.05). Specifically, activation at Channel 13 (located at the left frontal pole) was statistically higher in the non-ED group than in the ED group at the uncorrected level (p < 0.05); however, this difference became non-significant after FDR correction (p > 0.05). Additionally, the Montreal Cognitive Assessment (MoCA) score was significantly correlated with oxy-Hb concentration changes at Channel 21 (located at the left frontal pole, rs = 0.515, FDR-corrected p < 0.05).
Conclusion:
ARCD showed attenuated PFC activation during VFT, with no significant difference between the ED and non-ED groups. The decline in PFC activation was correlated with lower MoCA scores, suggesting that fNIRS-derived PFC activation metrics might serve as a potential biomarker for global cognitive decline in ARCD.
Introduction
With the accelerating aging process in China, the incidence of ARCD has been on the rise, becoming an increasingly prominent challenge (1). Specifically, the incidence has reached 20% (2). ARCD refers to a syndrome characterized by a measurable decline in cognitive performance closely associated with physiological brain aging—defined operationally as a 1 – standard deviation (SD) reduction below the normative mean of cognitive assessment domains—yet crucially, it does not impair an individual’s activities of daily living. Distinct from mild cognitive impairment (MCI) (typically defined as a ≥1.5 SD decline below normative means), ARCD is demarcated from MCI by one core criterion: the absence of documented evidence of underlying cerebral or systemic diseases/conditions known to induce cerebral dysfunction (3, 4). Normal aging is associated with neuroanatomical and physiological changes that can impair specific cognitive domains, particularly working memory, processing speed, and EF (5, 6).
Executive function, known as cognitive control, includes flexibility, planning, working memory, task-switching, and inhibitory control (7, 8). Unlike fundamental cognitive domains—such as working memory and processing speed, which function as discrete, bottom-up information-processing modules—EF serves as a central integrative regulator that orchestrates and deploys these basic cognitive abilities to execute sophisticated, goal-directed behaviors. Neuroanatomically anchored in the PFC and its distributed cortical-subcortical networks (7), EF is not merely a constituent of the broader cognitive system but rather the linchpin that translates raw cognitive capacity into real-world functional competence. Critically, across all cognitive domains, EF has been identified as the sole and strongest predictor of functional independence, as operationalized by Instrumental Activities of Daily Living (IADL)—a key proxy for independent living encompassing complex instrumental tasks (9). In elderly individuals with ARCD, this unique, domain-specific association underscores that even minimal EF impairments can compromise IADL performance, even in the presence of preserved fundamental cognitive processes. Therefore, EF warrants attention in individuals with ARCD. Current EF assessment relies on neuropsychological tasks: the Stroop task evaluates inhibitory control (10), the Go/No-Go task assesses response inhibition (11), the N-back task measures working memory (12), and the Shape Trails Test (STT) evaluates cognitive flexibility (13).
The verbal fluency task (VFT) is a neuropsychological paradigm designed to assess speech fluency, which is divided into two subtypes: the semantic fluency test and the phonemic fluency test. The semantic fluency test is less cognitively demanding than the phonemic fluency test; the latter places greater reliance on EF, while the former requires the engagement of different semantic categories. The number of valid words generated during the VFT is a behavioral indicator of MCI (14).
Functional near-infrared spectroscopy (fNIRS) is a non-invasive neuroimaging technique with excellent temporal resolution that can capture changes in oxy-Hb and deoxyhemoglobin (deoxy-Hb)—changes that reflect cortical activation during task performance. The combination of fNIRS and VFT (fNIRS-VFT) is widely used to assess brain activation patterns in mental illnesses such as depression (15–18); however, few studies have examined the correlation between task-related prefrontal activation and cognitive function (19, 20).
Overall, the phonemic fluency test—a neuropsychological experimental paradigm— serves as a valid tool for evaluating EF, a cognitive process closely associated with the prefrontal lobe. In the present study, we employed fNIRS-VFT to examine prefrontal activation differences between the ED and non-ED groups, providing preliminary insights into the potential mechanisms underlying ED in ARCD.
Materials and methods
Subjects
All participants were recruited from our clinical trial entitled “Effects of cognitive-motor dual-task training on ARCD” (Registration No.: ChiCTR2200064684). Participants were included if they met all the following criteria: (1) aged 65 years or older; (2) MoCA score <26 (21); (3) evidence of gradual cognitive decline persisting for at least 6 months; (4) ability to perform Activities of Daily Living (ADLs) independently; (5) absence of previously diagnosed conditions known to cause cognitive decline. Participants were excluded if they met any of the following criteria: (1) A diagnosis of depression, anxiety, or other significant psychiatric disorders; (2) Dementia secondary to Parkinson’s disease, Alzheimer’s disease (AD), stroke, or other etiologies; (3) Failure to complete the fNIRS assessment.
We collected demographic information from all participants, including age, sex, ethnicity, and education level. Education levels were categorized as: Illiteracy, Primary School, Junior High School, Senior High School, Bachelor’s Degree, Master’s Degree, Doctor’s Degree. All participants were evaluated for global cognitive function using the MoCA. Memory function was assessed using the Auditory Verbal Learning Test Huashan Version (AVLT-H) (14) and the Digit Span Test (DST). Cognitive flexibility and cognitive processing speed were evaluated with the Shape Trails Test Part B (STT-B) and the Symbol Digit Modalities Test (SDMT) (15). The Modified Barthel Index (MBI) and the IADL scale were used to assess ADLs and IADL, respectively. All assessments were performed by a single professional rehabilitation physician. A total of 36 participants with ARCD were recruited for our study between October 2022 and October 2024 from nursing homes and communities in Beijing.
Higher scores on the MoCA, AVLT-H, DST, and SDMT indicate better cognitive function; a shorter completion time on the STT-B test indicates better EF in that domain. There are 5 points for visual-spatial/EF tasks in the MoCA scale, including trail making test, cube copying test, and clock drawing test, as well as 5 points for memory.
Assignment to subgroups
Shape Trails Test Part B and the Montreal Cognitive Assessment Executive Function Subscale (MoCA-EF) were employed to evaluate EF. Participants were categorized into the ED group if their score on either of the two scales fell below the cutoff value; otherwise, they were categorized into the non-ED group. This approach was adopted to minimize the risk of missing older adults with relative EF decline. The cut-off value for STT-B was defined as a score indicating relative EF impairment (specifically, >1 SD below the age-corrected normative mean) (22). The MoCA includes five items assessing EF, for which no standardized cut-off score has been established; thus, we established the cut-off score by adopting the method described in previous studies (3, 23). First, we calculated the mean score on the EF subscales for all participants. Subsequently, the criterion for defining substantial relative EF impairment was set as a score at least 1.0 SD below this mean (3). This method yielded two subgroups: participants with relative EF impairment and those without. The study was approved by the Institutional Review Board of Beijing Rehabilitation Hospital, Capital Medical University. All participants provided written informed consent.
fNIRS data acquisition
A 22-channel near-infrared optical imaging system with 8 sources and 8 detectors (ETG-4000, HITACHI, Japan), which covers the PFC, was utilized. The sampling frequency was set at 16.67 Hz, and the wavelengths were 730 nm and 850 nm. The light sources and detectors were designed according to an internationally used 10/20 electrode distribution system. The bottom probes were located along the PF1-PF2 line. The inter-probe distance was set to 3 cm. The distribution of channels and their corresponding brain regions is presented in Figure 1. This channel distribution has been employed in previous study (24).
Figure 1

Probe distribution of frontal lobe (anterior view) and corresponding brain regions. Red denotes light sources, and blue denotes detector. DLPFC, dorsolateral prefrontal cortex, FP, frontopolar region, IFG, inferior frontal gyrus, L, left, R, right.
The fNIRS recordings were performed concurrently with a VFT in a controlled environment with minimal acoustic noise and stable ambient lighting. The experimental paradigm comprised a 30 s pre-task resting baseline period, a 60 s verbal generation period, and a 30 s post-task recovery phase. Stimuli were presented on a display monitor positioned at a distance of 1 m away from the participants.
During the active task, participants were instructed to generate as many semantically meaningful phrases as possible, rapidly and accurately, using a series of specified Chinese characters [e.g., “白” (white), “大” (big), “天” (sky)]. Prior to the formal task, a practice trial using the character “门” (door) was administered to ensure task comprehension and standardization of the task procedure. The presentation order of the characters was fixed across all participants.
Each character was displayed for 2 s and subsequently followed by a central fixation cross (“+”) presented for 18 s. During both pre- and post-task rest periods, participants were instructed to engage in silent repetitive counting from 1 to 5 to minimize cognitive load and establish a consistent baseline (25).
Data processing and analysis
NIRS_KIT (HuiChuang, Zhenjiang, China) (26) and BrainNet Viewer (27) were employed for data analysis and visualization. The raw light intensity data were first converted to optical density. Subsequently, physiological noise, such as signals arising from cardiac and respiratory activities, was eliminated using a bandpass filter (0.01–0.2 Hz). The filtered optical density signals were then transformed into concentrations of oxy-Hb and deoxy-Hb based on the modified Beer-Lambert law. For all subsequent analyses, oxy-Hb was selected over deoxy-Hb due to its superior signal-to-noise ratio, as documented in previous research (18). A baseline was derived from the 30 s period preceding the task, which was used to calculate the mean changes in oxy-Hb concentration across all 22 prefrontal channels during the VFT.
The clinical data were analyzed using IBM SPSS Statistics version 26.0. Data normality was tested via the Shapiro–Wilk test. Continuous variables including age and scores on cognitive and ADL-related scales (MoCA, AVLT-H, DST, SDMT, STT-B, MBI and IADL) were analyzed using an unpaired t-test. Categorical variables such as sex, education, and clinical variables (hypertension, diabetes, alcohol consumption, smoking status, sedentary lifestyle) were analyzed by Fisher’s exact test. An unpaired t-test was used to compare the difference in oxy-Hb changes within the PFC during the VFT task between the ED and non-ED groups of ARCD participants. Paired t-test was used to compare oxy-Hb changes between non-task and VFT phases to identify task-related brain region activation. Spearman correlation analysis was performed between oxy-Hb changes and the MoCA/MoCA-EF/SDMT/STT-B scores in all the participants. If the data did not follow a normal distribution, the Mann–Whitney U test was used instead. p-values of all the within- and between-group comparisons were corrected by the false discovery rate (FDR) method. We also calculated the effect size (ES) for the group differences of oxy-Hb changes in each channel in order to evaluate whether the differences are independent of sample size using Cliff’s Delta (δ) – a non-parametric ES measure designed to quantify the magnitude of difference between two independent groups, particularly for data that violates the normality assumption. According to the ES classification criteria proposed by Romano et al. (28), an ES of <0.147 is classified as negligible; an ES ranging from 0.147 to 0.33 is defined as a small effect size; an ES between 0.33 and 0.474 is categorized as a medium effect size; and an ES of >0.474 is regarded as a large effect size. A p-value less than 0.05 was considered statistically significant, and all p-values were two-tailed.
Results
Demographic and clinical characteristics
No statistically significant differences were observed between the ED and non-ED groups in terms of sex, education level, AVLT-H scores, DST scores, IADL scores, frequency of smoking and drinking, prevalence of hypertension, or sedentary behavior. All participants achieved an MBI score of 100. Compared with the non-ED group, the ED group had a significantly older age (p < 0.05) and significantly lower MoCA scores (p < 0.05). Additionally, the prevalence of type 2 diabetes mellitus was significantly higher in the ED group than in the non-ED group (p < 0.05). Detailed data are presented in Table 1.
Table 1
| Variable | ED (n = 18) | Non-ED (n = 18) | t/z | p |
|---|---|---|---|---|
| Age (M ± SD) | 78.44 ± 7.52 | 73.33 ± 5.58 | 2.316 | 0.027* |
| Sex (male/female) | 7/11 | 9/9 | 0.738 | |
| MoCA [M (P25, P75)] | 22 (20, 23) | 24 (21, 25) | −2.396 | 0.017* |
| Education | ||||
| Illiteracy | 0 | 0 | 0.742 | |
| Primary school | 0 | 2 | ||
| Junior high school | 4 | 3 | ||
| Senior high school | 3 | 3 | ||
| Bachelor’s degree | 11 | 10 | ||
| Master’s degree | 0 | 0 | ||
| AVLT-H | ||||
| AVLT-D (M ± SD) | 4.3 ± 2.7 | 5.1 ± 2.2 | −0.913 | 0.368 |
| AVLT-R [M (P25, P75)] | 21.0 (19, 22.5) | 21.5 (20.0, 22.8) | −0.272 | 0.785 |
| DST | ||||
| Forward [M (P25, P75)] | 7 (6, 8) | 7 (6, 8) | −0.267 | 0.789 |
| Backward [M (P25, P75)] | 4 (3, 5) | 5 (3, 5) | −0.588 | 0.556 |
| SDMT (M ± SD) | 20.9 ± 10.8 | 33.8 ± 8.7 | −3.860 | 0.000*** |
| STT-B (M ± SD) | 292.5 ± 80.3 | 166.3 ± 50.0 | 5.615 | 0.000*** |
| Smoking status | ||||
| Yes | 1 | 0 | 0.658 | |
| No | 14 | 16 | ||
| Quit | 3 | 2 | ||
| Alcohol consumption | ||||
| Yes | 3 | 3 | 1.000 | |
| No | 14 | 14 | ||
| Quit | 1 | 1 | ||
| Hypertension | ||||
| Yes | 12 | 7 | 0.091 | |
| No | 6 | 11 | ||
| Type 2 diabetes | ||||
| Yes | 8 | 2 | 0.03* | |
| No | 10 | 16 | ||
| Sedentary lifestyle | ||||
| >5 h/day | 3 | 5 | 0.345 | |
| ≤5 h/day | 15 | 13 | ||
| IADL, M (P25, P75) | 22 (21, 24) | 23 (23, 24) | 0.833 | 0.491 |
Demographic characteristics of participants in each group.
ED, executive dysfunction; non-ED, non-executive dysfunction; MoCA, Montreal cognitive assessment; AVLT-H, Auditory Verbal Learning Test Huashan Version; DST, Digit Span Test; D, delayed free recall; R, recognition; SDMT, Symbol Digit Modalities Test; STT-B, Shape Trails Test B; M, mean or median; SD, Standard deviation; P25, 25th percentile; P75, 75th percentile; IADL, Instrumental Activities of Daily Living. *Indicates p < 0.05; ***Indicates p < 0.001.
Behavioral results: verbal fluency task outcomes
The number of correct words generated by the two participant groups during the VFT was analyzed using the Mann–Whitney U test. Results revealed no significant difference between the two groups in the total number of correct words (z = −0.192, p = 0.847), as shown in Figure 2.
Figure 2

The number of correct words during a verbal fluency task. There was no significant difference between the ED group and the non-ED group.
Brain activation in the ED and non-ED group
Oxy-Hb concentration changes in the non-ED group
Non-ED group: Since not all oxy-Hb concentration changes followed a normal distribution, a non-parametric test for two related samples was employed. Statistically significant differences were observed in Channel 5 (Ch5), Channel 13 (Ch13), Channel 18 (Ch18), and Channel 19 (Ch19), which are located in the left dorsolateral prefrontal cortex and bilateral frontal pole (FP). After FDR correction, a statistically significant difference persisted only in Ch13, which is located in the lFP. No statistically significant differences were detected in the remaining channels, as shown in Table 2 and Figure 3. Additionally, to illustrate the typical response pattern, the oxy-Hb time-response curve derived from one channel of a single participant is shown in Figure 4A. It appeared that the oxy-Hb concentration changes exhibited mild magnitude of variation, pronounced fluctuations, along with the absence of distinct peaks and the lack of an initial dip throughout the entire duration of the task paradigm.
Table 2
| Oxy-Hb | M (P25, P75) (μmol/L * mm) | z | p | p (FDR) |
|---|---|---|---|---|
| Baseline 1 | −0.2 (−1.5, 1.1) | −0.370 | 0.711 | 0.823 |
| Task 1 | −0.1 (−0.8, 0.5) | |||
| Baseline 2 | 0.1 (−0.6, 0.6) | −0.196 | 0.845 | 0.885 |
| Task 2 | 0.2 (−0.4, 0.5) | |||
| Baseline 3 | −0.3 (−0.6, 0.3) | −1.372 | 0.17 | 0.468 |
| Task 3 | 0.1 (−0.1, 0.3) | |||
| Baseline 4 | −0.3 (−1.1, 0.2) | −1.633 | 0.102 | 0.449 |
| Task 4 | 0.2 (0, 0.5) | |||
| Baseline 5 | −0.3 (−1.0, 0) | −2.722 | 0.006** | 0.066 |
| Task 5 | 0.2 (−0.1, 0.5) | |||
| Baseline 6 | −0.2 (−0.9, 0.1) | −1.328 | 0.184 | 0.405 |
| Task 6 | 0.1 (−0.2, 0.3) | |||
| Baseline 7 | −0.2 (−1.5, 0.4) | −1.590 | 0.112 | 0.411 |
| Task 7 | 0.1 (−0.3, 0.5) | |||
| Baseline 8 | 0.2 (−1.1, 1.5) | −0.544 | 0.586 | 0.758 |
| Task 8 | −0.2 (−1.4, 0.6) | |||
| Baseline 9 | 0 (−1.3, 1.3) | −0.152 | 0.879 | 0.879 |
| Task 9 | −0.3 (−0.7, 0.6) | |||
| Baseline 10 | 0 (−0.4, 0.3) | −0.893 | 0.372 | 0.585 |
| Task 10 | 0.1 (−0.2, 0.4) | |||
| Baseline 11 | −0.3 (−0.7, 0.4) | −1.024 | 0.306 | 0.561 |
| Task 11 | 0.2 (−0.3, 0.4) | |||
| Baseline 12 | −0.1 (−0.7, 0.3) | −0.632 | 0.528 | 0.726 |
| Task 12 | 0 (0, 0.1) | |||
| Baseline 13 | −0.4 (−0.9, -0.1) | −3.245 | 0.001** | 0.022* |
| Task 13 | 0.4 (0.1, 10) | |||
| Baseline 14 | 0 (−0.6, 0.8) | −0.936 | 0.349 | 0.591 |
| Task 14 | 0.4 (−0.2, 0.6) | |||
| Baseline 15 | 0.1 (−1.5, 0.8) | −0.719 | 0.472 | 0.692 |
| Task 15 | 0.2 (−0.1, 0.7) | |||
| Baseline 16 | 0 (−1.7, 0.9) | −0.327 | 0.744 | 0.818 |
| Task 16 | 0 (−0.9, 0.5) | |||
| Baseline 17 | 0 (−0.5, 0.4) | −0.501 | 0.616 | 0.753 |
| Task 17 | 0 (−0.4, 0.3) | |||
| Baseline 18 | 0 (−1.1, 0.4) | −2.461 | 0.014* | 0.103 |
| Task 18 | 0.3 (0.2, 0.6) | |||
| Baseline 19 | −0.2 (−0.7, 0) | −2.330 | 0.02* | 0.11 |
| Task 19 | 0.2 (0, 0.4) | |||
| Baseline 20 | −0.1 (−1.0, 0.1) | −1.372 | 0.17 | 0.416 |
| Task 20 | 0.3 (−0.1, 0.6) | |||
| Baseline 21 | 0 (−1.7, 0.4) | −1.459 | 0.145 | 0.456 |
| Task 21 | 0.3 (−0.3, 0.7) | |||
| Baseline 22 | −0.1 (−1.1, 0.6) | −1.198 | 0.231 | 0.462 |
| Task 22 | 0.2 (−0.2, 0.7) |
Brain activation of the non-ED group at each channel (n = 18).
*Indicates p < 0.05; **Indicates p < 0.01; oxy-Hb, oxyhemoglobin; FDR, false discovery rate; Baseline, Oxy-Hb concentration changes during silent counting from 1 to 5; Task, Oxy-Hb concentration changes during the VFT; M, median; P25, 25th percentile; P75, 75th percentile; μmol/L * mm, micromole per liter per millimeter.
Figure 3

Brain activation of the ED group and non-ED group: z-value maps with FDR-corrected p-values <0.05 circled in red.
Figure 4

Time course of relative oxy-Hb changes in a non-ED group (A) and an ED group (B). HbO, Oxyhemoglobin.
Oxy-Hb concentration changes of the ED group
The relative concentration changes of oxy-Hb at baseline and during the VFT task across 22 channels did not all show a normal distribution. A statistically significant difference was observed in Ch1, but no statistically significant differences remained after FDR correction. For the other channels, no statistically significant differences were found, as presented in Table 3 and Figure 3. Additionally, the oxy-Hb time-response curve from the ED group is presented in Figure 4B. In comparison to the curve in Figure 4A, this curve also exhibited mild variations in magnitude, pronounced fluctuations, and a complete absence of notable amplitude.
Table 3
| Oxy-Hb | M (P25, P75) (μmol/L * mm) | z | p | p (FDR) |
|---|---|---|---|---|
| Baseline 1 | 0.3 (−0.1, 0.6) | −2.504 | 0.012* | 0.264 |
| Task 1 | −0.1 (−0.5, 0.1) | |||
| Baseline 2 | 0.1 (−0.4, 0.6) | −0.240 | 0.811 | 1 |
| Task 2 | 0.1 (−0.2, 0.3) | |||
| Baseline 3 | −0.1 (−0.9, 0.4) | −0.588 | 0.557 | 1 |
| Task 3 | 0 (−0.5, 0.4) | |||
| Baseline 4 | −0.4 (−1.1, 0.2) | −1.677 | 0.094 | 1 |
| Task 4 | 0.2 (−0.2, 0.5) | |||
| Baseline 5 | −0.3 (−1, 0.5) | −0.588 | 0.557 | 1 |
| Task 5 | 0 (−0.3, 0.2) | |||
| Baseline 6 | 0 (−0.5, 0.4) | −0.936 | 0.349 | 1 |
| Task 6 | 0.2 (−0.2, 0.5) | |||
| Baseline 7 | 0.1 (−0.9, 0.5) | −0.849 | 0.396 | 1 |
| Task 7 | 0 (−0.2, 0.7) | |||
| Baseline 8 | 0.1 (−0.7, 0.8) | −0.065 | 0.948 | 0.993 |
| Task 8 | 0 (−0.4, 0.7) | |||
| Baseline 9 | 0.2 (−0.3, 0.7) | −1.415 | 0.157 | 1 |
| Task 9 | −0.2 (−0.7, 0.3) | |||
| Baseline 10 | 0 (−0.5, 0.5) | −0.414 | 0.679 | 1 |
| Task 10 | 0 (−0.3, 0.5) | |||
| Baseline 11 | 0 (−0.3, 0.1) | −0.501 | 0.616 | 1 |
| Task 11 | 0 (−0.3, 0.2) | |||
| Baseline 12 | −0.1 (−0.8, 0.5) | −0.762 | 0.446 | 1 |
| Task 12 | 0.1 (−0.1, 0.2) | |||
| Baseline 13 | 0.1 (−0.5, 0.2) | −0.240 | 0.811 | 0.991 |
| Task 13 | 0.1 (−0.5, 0.4) | |||
| Baseline 14 | 0.2 (−0.5, 0.6) | −0.327 | 0.744 | 1 |
| Task 14 | −0.1 (−0.5, 0.4) | |||
| Baseline 15 | −0.4 (−0.9, 0.3) | −0.980 | 0.327 | 1 |
| Task 15 | 0.1 (−0.4, 0.5) | |||
| Baseline 16 | 0 (−0.8, 0.4) | −0.022 | 0.983 | 0.983 |
| Task 16 | −0.1 (−0.3, 0.6) | |||
| Baseline 17 | 0.1 (−0.5, 0.4) | −0.501 | 0.616 | 1 |
| Task 17 | −0.1 (−0.3, 0.2) | |||
| Baseline 18 | 0.3 (−0.6, 0.6) | −0.240 | 0.811 | 0.939 |
| Task 18 | 0.1 (−0.4, 0.4) | |||
| Baseline 19 | −0.1 (−1.0, 0.1) | −0.893 | 0.372 | 1 |
| Task 19 | 0 (−0.3, 0.7) | |||
| Baseline 20 | 0.2 (−0.9, 0.6) | −0.283 | 0.777 | 1 |
| Task 20 | 0 (−0.4, 0.6) | |||
| Baseline 21 | −0.2 (−0.6, 0.3) | −0.240 | 0.811 | 0.892 |
| Task 21 | 0 (−0.6, 0.3) | |||
| Baseline 22 | 0 (−0.5, 0.4) | −0.283 | 0.777 | 1 |
| Task 22 | −0.1 (−0.8, 0.6) |
Brain activation of the ED group at each channel (n = 18).
*Indicates p < 0.05; oxy-Hb, oxyhemoglobin; FDR, false discovery rate; Baseline, Oxy-Hb concentration changes during silent counting from 1 to 5; Task, Oxy-Hb concentration changes during the VFT; M, median; P25, 25th percentile; P75, 75th percentile; μmol/L * mm, micromole per liter per millimeter.
The group comparison for relative changes of brain activation between the ED group and non-ED group
Not all relative concentration changes of oxy-Hb across the 22 channels followed a normal distribution. Therefore, we employed an independent-samples Mann–Whitney U test for subsequent analyses, which revealed a statistically significant difference in oxy-Hb concentration changes for Ch13 (z = −2.088, p = 0.035). However, this significance was lost following FDR correction. Additionally, no statistically significant differences in oxy-Hb concentration changes were observed in the remaining channels (all p > 0.05). Detailed results for all 22 channels are provided in Table 4.
Table 4
| Ch | Group | M (P25, P75) (μmol/L * mm) | z | p | p (FDR) | δ |
|---|---|---|---|---|---|---|
| 1 | ED | −0.4 (−0.8, −0.4) | −0.791 | 0.429 | 1 | −0.15 |
| Non-ED | 0.2 (−2.0, 0.2) | |||||
| 2 | ED | 0 (−0.6, 0) | −0.411 | 0.681 | 1 | −0.08 |
| Non-ED | 0.4 (−1.1, 0.4) | |||||
| 3 | ED | 0 (−0.0006, 0) | −0.348 | 0.728 | 0.942 | −0.07 |
| Non-ED | 0.4 (−0.3, 0.4) | |||||
| 4 | EF | 0.6 (−0.3, 0.6) | −0.127 | 0.899 | 0.989 | −0.02 |
| Non-ED | 0.7 (−0.3, 0.7) | |||||
| 5 | ED | 0.3 (−0.8, 0.3) | −0.981 | 0.327 | 1 | −0.19 |
| Non-ED | 0.5 (0.1, 0.5) | |||||
| 6 | ED | 0.2 (−0.4, 0.2) | −0.538 | 0.591 | 1 | −0.1 |
| Non-ED | 0.3 (−0.3, 0.3) | |||||
| 7 | ED | −0.1 (−0.7, −0.1) | −0.696 | 0.486 | 1 | −0.14 |
| Non-ED | 0.2 (−0.6, 0.2) | |||||
| 8 | ED | −0.2 (−1.1, −0.2) | −0.38 | 0.704 | 0.968 | −0.07 |
| Non-ED | −0.1 (−2.4, −0.1) | |||||
| 9 | ED | −0.3 (−0.9, −0.3) | −0.411 | 0.681 | 1 | −0.08 |
| Non-ED | 0 (−2.0, 0) | |||||
| 10 | ED | 0 (−0.6, 0) | −0.285 | 0.776 | 0.948 | −0.06 |
| Non-ED | 0.2 (−0.4, 0.2) | |||||
| 11 | ED | 0 (−0.1, 0) | −1.297 | 0.195 | 1 | −0.25 |
| Non-ED | 0.6 (−0.6, 0.6) | |||||
| 12 | ED | 0.1 (−0.5, 0.1) | −0.127 | 0.899 | 0.942 | −0.02 |
| Non-ED | 0.1 (−0.3, 0.1) | |||||
| 13 | ED | 0.2 (−0.8, 0.2) | −2.088 | 0.037* | 0.814 | −0.41 |
| Non-EF | 0.8 (0.1, 0.8) | |||||
| 14 | ED | −0.2 (−1.3, −0.2) | −0.918 | 0.359 | 1 | −0.18 |
| Non-ED | 0.3 (−0.6, 0.3) | |||||
| 15 | ED | 0.4 (−0.7, 0.4) | −0.158 | 0.874 | 1 | −0.03 |
| Non-ED | 0.3 (−0.7, 0.3) | |||||
| 16 | ED | −0.2 (−0.9, −0.2) | −0.063 | 0.95 | 0.95 | −0.01 |
| Non-ED | 0.1 (−1.4, 0.1) | |||||
| 17 | ED | −0.1 (−0.8, −0.1) | −0.601 | 0.548 | 1 | −0.12 |
| Non-ED | 0.2 (−0.5, 0.2) | |||||
| 18 | ED | −0.5 (−0.8, −0.5) | −1.614 | 0.107 | 1 | −0.31 |
| Non-ED | 0.4 (0, 0.4) | |||||
| 19 | ED | 0.1 (−0.6, 0.1) | −0.601 | 0.548 | 1 | −0.12 |
| Non-ED | 0.4 (0, 0.4) | |||||
| 20 | ED | −0.3 (−1.0, −0.3) | −0.854 | 0.393 | 1 | −0.17 |
| Non-ED | 0.5 (−0.4, 0.5) | |||||
| 21 | ED | 0 (−0.9, 0) | −0.949 | 0.343 | 1 | −0.19 |
| Non-ED | 0.3 (−0.5, 0.3) | |||||
| 22 | ED | −0.1 (−1.1, 1.6) | −0.411 | 0.696 | 1 | −0.08 |
| Non-ED | 0.3 (−0.7, 1.4) |
Comparison of oxy-Hb concentration changes between the two groups.
*Indicates p < 0.05; Ch, channel; ED, executive dysfunction; Non-ED, non-executive dysfunction; FDR, false discovery rate; M, median; P25, 25th percentile; P75, 75th percentile.
Correlational analysis between cognitive function and oxy-Hb concentration changes
Spearman’s rank correlation analysis was performed between relative oxy-Hb concentration changes in various channels and scores on the MoCA, MoCA-EF, SDMT, and STT-B. Statistically significant positive correlations were observed between MoCA scores and average oxy-Hb concentration changes in Ch13 (rs = 0.390, p = 0.019), Ch20 (rs = 0.380, p = 0.022), and Ch21 (rs = 0.515, p = 0.001) – all of which are located in the lFP. After FDR correction, only the correlation between MoCA scores and average oxy-Hb concentration changes in Ch21 remained statistically significant (rs = 0.515, p = 0.022). Additionally, statistically significant positive correlations were found between MoCA-EF scores and average oxy-Hb concentration changes in Ch3 (rs = 0.414, p = 0.012), Ch5 (rs = 0.348, p = 0.037), and Ch18 (rs = 0.468, p = 0.004). Ch3 is located in the rDLPFC, Ch5 in the lDLPFC, and Ch18 in the lFP; however, none of these correlations remained statistical significance after FDR correction. For the STT-B, a statistically significant negative correlation was observed between its scores and average oxy-Hb concentration changes in Ch13 (rs = −0.343, p = 0.041; located in the lFP), but this correlation no longer reached statistical significance following FDR correction. These results are shown in Figure 5. No statistically significant correlations were detected between average oxy-Hb concentration changes in all channels and either SDMT scores or age.
Figure 5
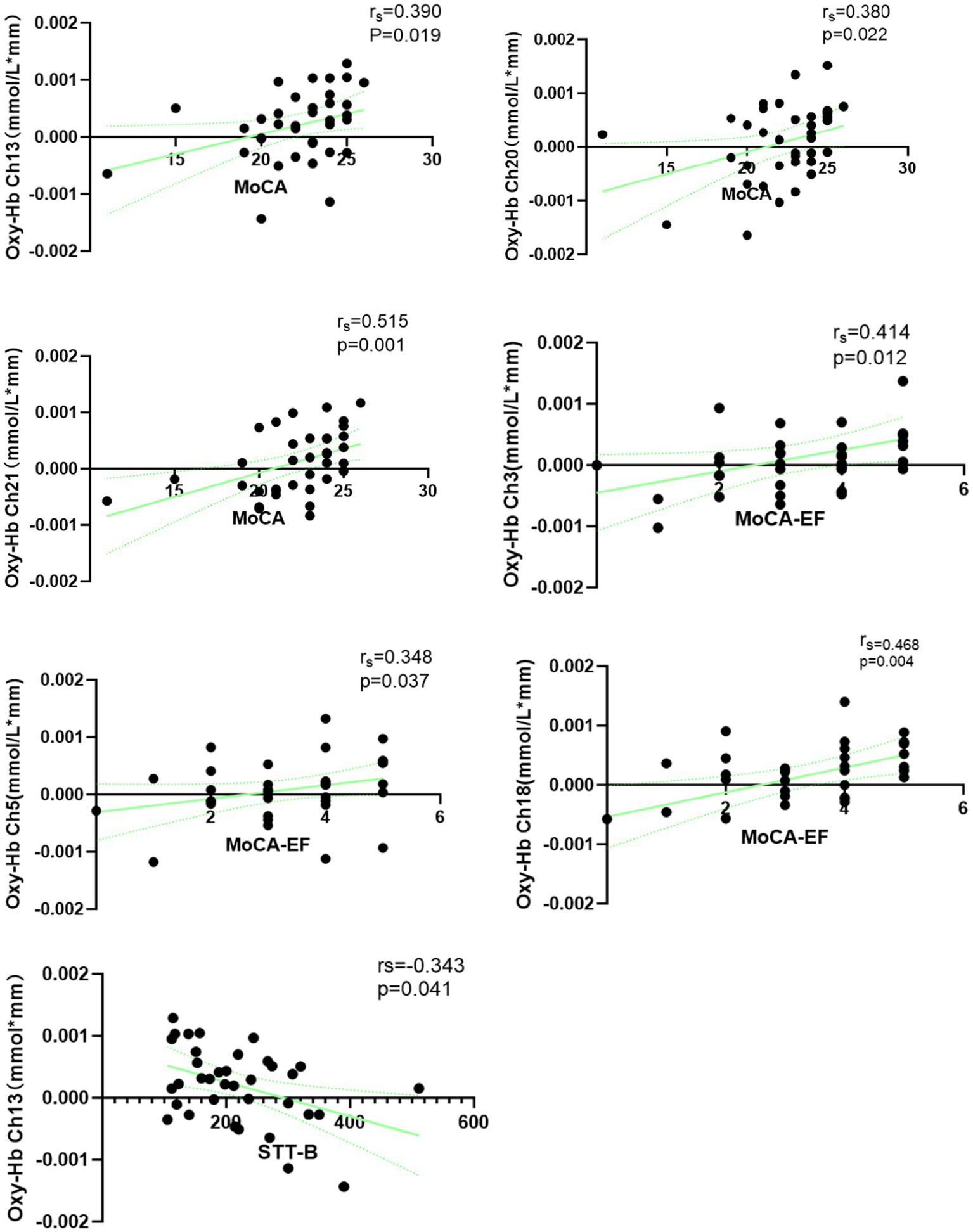
Spearman correlation between oxy-Hb concentration changes and cognition in age-related cognitive decline.
Discussion
The ARCD is associated with advancing age. In addition to age, it may also be related to other aging-related comorbidities, such as hypertension and diabetes. In this study, individuals with ARCD were categorized into the ED group and non-ED group based on performance on the MoCA EF subscale and STT-B. Specifically, the STT-B can be used to evaluate cognitive flexibility, while the clock drawing test within the MoCA can be used to assess planning and working memory. Participants were considered to have ED if their score on either of these two measures fell below the predefined cutoff. No statistically significant differences were observed between the two groups in terms of sex, education level, prevalence of hypertension, frequency of smoking and alcohol intake, or sedentary behavior; nor were there significant differences in memory assessment scores, specifically those from the DST and AVLT-H. However, the ED group was significantly older than the non-ED group, indicating that age may be a risk factor for impaired EF in individuals with ARCD. These findings are consistent with a previous study indicating that EF performance decreases after the age of 65 years (29). This also highlights that EF in ARCD warrants further investigation. We also observed that the incidence of type 2 diabetes in the ED group was higher than that in the non-ED group, suggesting that type 2 diabetes may also be a risk factor for ARCD. Previous studies have demonstrated that diabetes can induce cognitive decline, including impairments in attention, EF, and processing speed (30). Although scores on EF measures, total MoCA scores, and age differed between the two groups, our analyses revealed no significant between-group difference in the total number of words generated during the VFT. This unexpected null finding is most plausibly explained by the multifactorial nature of the VFT performance. Word generation in this task is influenced not only by core cognitive capacities, but also by contextual and individual non-cognitive factors, such as cultural background, emotional state, and task motivation. This finding suggests that the VFT lacked sufficient sensitivity to distinguish between ED and non-ED individuals with ARCD, despite the fact that the VFT has been shown to be a sensitive indicator for distinguishing normal cognition from MCI (14).
Intra-group comparison revealed that no PFC channels were activated in the ED group, whereas only one channel located at the left frontal pole was activated in the non-ED group of ARCD participants. Notably, previous studies have demonstrated that the phonemic fluency test is associated with the PFC, especially the left PFC (31), and it not only activates multiple channels of the PFC in normal elderly individuals and elderly individuals with Parkinson’s disease without cognitive impairment (14, 32), but also activates small patches of the right orbitofrontal cortex in some healthy female adults (31). Consistent with these established findings, our study found that the left PFC was activated in the non ED group. However, our results further highlighted a marked reduction in frontal lobe activation among older adults with ARCD: in stark contrast to the single-channel activation observed in the non-ED group, no prefrontal channels exhibited significant activation in the ED group at all. Beyond cortical activation profiles, our analysis of oxy-Hb concentration curves during the VFT also identified distinct response features, characterized by multiple fluctuations and negligible amplitude variation—a pattern that diverges from the response dynamics reported in prior research (16). Plausibly, this atypical hemodynamic response may be linked to two interrelated factors: aging itself, which is known to induce reduced cerebral hemodynamic responses (33), and the cognitive decline inherent to ARCD (19). Collectively, these observations align with prior evidence indicating that patients with MCI also exhibit diminished PFC activity during cognitive tasks (9, 19, 34).
Inter-group comparisons revealed that the relative change in oxy-Hb concentration at Ch13, located in the left frontal pole, was marginally higher in the non-ED group than the ED group, yet, this between-group discrepancy failed to attain statistical significance following FDR correction. Collectively, these findings tentatively imply that cortical activity within the left frontal hemisphere is attenuated in ED older adults compared with their non-ED counterparts, albeit such a trend did not reach statistical significance. This null result might plausibly stem from a confluence of interrelated factors: specifically, the blunted cerebral hemodynamic responsiveness characteristic of older adults with cognitive decline (19), may have induced a ceiling effect, narrowing the gap in prefrontal activation levels between the two subgroups and thereby obscuring statistically detectable differences. Furthermore, as a preliminary exploratory investigation, the modest sample size of our ARCD cohort likely limits statistical power to detect subtle yet potentially biologically meaningful between-group variations in prefrontal hemodynamic responses. In the future, these results may offer valuable insights to inform subsequent investigations, and the robustness of this trend awaits further verification in cohorts with larger sample sizes.
Previous studies have shown that NIRS-derived regional cerebral activation exhibits a positive correlation with the number of words generated in the VFT (25). Our study found that although there were certain differences in NIRS-derived regional cerebral activation between the two groups, the VFT performance of the two groups was comparable. This may suggest that the observed differences in NIRS activation may be attributed to cognitive impairment itself, and this effect is independent of the VFT performance. Similar findings have been reported in other studies—for instance, those focusing on depression (35, 36)—where no differences were observed in VFT performance, yet statistically significant differences were noted in NIRS-derived activation.
Our study demonstrated that MoCA scores exhibited a positive correlation with oxy-Hb concentration changes across most channels located at lFP. Specifically, EF scores, which are a subcomponent of the MoCA, showed a positive correlation with oxy-Hb changes in a small portion of the lFP cortex and the bilateral DLPFC, while STT-B scores were negatively correlated with those in a small portion of the lFP. However, after FDR correction, only the correlation between MoCA scores and oxy-Hb changes of a small portion of lFP remained significantly significant, indicating that the fNIRS-VFT might be used as an auxiliary tool for cognition assessment. Similar to previous studies, prefrontal hemodynamic response by fNIRS was related to cognitive decline (32, 34, 37), and this technique has also been used to distinguish between individuals with MCI and dementia (34). As an exploratory study, the present work highlights that the correlation between EF and prefrontal cortical activity warrants further investigation.
There are several limitations to our study. Firstly, the ED group and non-ED group were grouped by STT-B and MoCA-EF. However, inhibitory control—one dimension of EF—wasn’t assessed. Consequently, there may have been some elderly who actually had ED but were misclassified into the non-ED group, which could have contributed to null results. Secondly, there were significant differences in age and diabetes incidence between the two groups. According to previous studies, diabetes exerts little impact on oxy-Hb changes in the prefrontal lobe (38); however, age may influence prefrontal oxy-Hb changes during the VFT (39). Thus, it remains difficult to rule out the potential confounding effect of age. To address this limitation, elderly individuals with no cognitive decline should be enrolled as a control group in future studies. Thirdly, while ARCD was the target population of this study, relying solely on MoCA scores and medical history posed challenges for excluding cognitive decline with identified etiologies. This limitation arises because such etiologically defined cognitive changes may overlap with ARCD in clinical manifestations, yet cannot be differentiated by MoCA or basic medical history alone. Finally, we did not perform an a priori sample size calculation, Nevertheless, the concentration change of oxy-Hb at Ch13 was higher in the non-ED group than in the ED group, suggesting our sample did capture the effect in this instance; however, we cannot rule out that a larger sample might yield more stable, generalizable results.
In conclusion, the present study demonstrated that older adults with ARCD exhibited attenuated prefrontal cortical activity during the VFT. Notably, no statistically significant between-group difference in prefrontal activation was detected between the ED and non-ED subgroups of ARCD participants, indicating that EF-related subtyping may not correspond to overt alterations in prefrontal hemodynamic responses during VFT in this ARCD cohort. Furthermore, MoCA scores were positively associated with changes in oxy-Hb concentration in the left frontal pole, suggesting that fNIRS-derived PFC activation may serve as a potential biomarker for global cognitive decline in ARCD. However, if quantitative analysis of fNIRS-VFT data is to be applied in clinical cognitive assessment, a longitudinal study would be required to validate its utility and stability.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Beijing Rehabilitation Hospital, Capital Medical University (Approval number: 2022bkky-059). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QZ: Formal analysis, Writing – original draft, Visualization, Data curation, Methodology, Conceptualization, Investigation. RS: Conceptualization, Data curation, Methodology, Writing – original draft, Supervision, Investigation. CH: Data curation, Investigation, Writing – review & editing. JW: Data curation, Investigation, Writing – review & editing. LS: Writing – review & editing, Investigation, Data curation. WG: Conceptualization, Funding acquisition, Writing – review & editing, Supervision.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by Capital’s Funds for Health Improvement and Research (2022-1-2251).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Yang Y Wang D Hou W Li H . Cognitive decline associated with aging. Adv Exp Med Biol. (2023) 1419:25–46. doi: 10.1007/978-981-99-1627-6_3,
2.
Carson Smith J Callow DD Pena GS Kommula Y Arnold-Nedimala N Won J et al . Exercise and protection from age-related cognitive decline. Curr Top Behav Neurosci. (2024) 67:263–80. doi: 10.1007/7854_2024_501,
3.
Levy R . Aging-associated cognitive decline. Working party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr. (1994) 6:63–8.
4.
Schönknecht P Pantel J Kruse A Schröder J . Prevalence and natural course of aging-associated cognitive decline in a population-based sample of young-old subjects. Am J Psychiatry. (2005) 162:2071–7. doi: 10.1176/appi.ajp.162.11.2071,
5.
Joubert C Chainay H . Aging brain: the effect of combined cognitive and physical training on cognition as compared to cognitive and physical training alone – a systematic review. Clin Interv Aging. (2018) 13:1267–301. doi: 10.2147/CIA.S165399,
6.
Harada CN Love MCN Triebel KL . Normal cognitive aging. Clin Geriatr Med. (2013) 29:737–52. doi: 10.1016/j.cger.2013.07.002,
7.
Diamond A . Executive functions. Annu Rev Psychol. (2013) 64:135–68. doi: 10.1146/annurev-psych-113011-143750,
8.
Ferguson HJ Brunsdon VEA Bradford EEF . The developmental trajectories of executive function from adolescence to old age. Sci Rep. (2021) 11:1382. doi: 10.1038/s41598-020-80866-1,
9.
Raimo S Maggi G Ilardi CR Cavallo ND Torchia V Pilgrom MA et al . The relation between cognitive functioning and activities of daily living in normal aging, mild cognitive impairment, and dementia: a meta-analysis. Neurol Sci. (2024) 45:2427–43. doi: 10.1007/s10072-024-07366-2,
10.
Sjoberg EA Wilner RG D’Souza A Cole GG . The Stroop task sex difference: evolved inhibition or color naming?Arch Sex Behav. (2023) 52:315–23. doi: 10.1007/s10508-022-02439-9,
11.
Watanabe N Kamijo M Nishino T Ashida K Sasamori F Okuhara M et al . The feasibility of using the go/no-go task as a dementia screening test assessed with a cross-sectional design. Sci Rep. (2024) 14:29834. doi: 10.1038/s41598-024-81301-5,
12.
Kimura T Matsuura R . The content-dependent effect of the N-back task on dual-task performance. Behav Brain Res. (2023) 452:114511. doi: 10.1016/j.bbr.2023.114511,
13.
Zhao Q Guo Q Li F Zhou Y Wang B Hong Z . The shape trail test: application of a new variant of the trail making test. PLoS One. (2013) 8:e57333. doi: 10.1371/journal.pone.0057333,
14.
Galtier I Nieto A Lorenzo JN Barroso J . Mild cognitive impairment in Parkinson’s disease: clustering and switching analyses in verbal fluency test. J Int Neuropsychol Soc. (2017) 23:511–20. doi: 10.1017/s1355617717000297,
15.
Zhang H Shan AD Wan CH Cao XY Yuan YS Ye SY et al . Transcutaneous auricular vagus nerve stimulation improves anxiety symptoms and cortical activity during verbal fluency task in Parkinson’s disease with anxiety. J Affect Disord. (2024) 361:556–63. doi: 10.1016/j.jad.2024.06.083,
16.
Lang X Wen D Li Q Yin Q Wang M Xu Y . fNIRS evaluation of frontal and temporal cortex activation by verbal fluency task and high-level cognition task for detecting anxiety and depression. Front Psychiatry. (2021) 12:690121. doi: 10.3389/fpsyt.2021.690121,
17.
Liu J Hu Y Zong B Wang S Zheng Y Guo D et al . Functional characteristics of the frontal cortex during the verbal fluency task in subthreshold depression: a fNIRS study. J Affect Disord. (2025) 384:144–50. doi: 10.1016/j.jad.2025.04.166,
18.
Zhang JM Liu XB Li YX Li HJ Fan J Xue C et al . Characteristic activation pattern and network connectivity of prefrontal cortex in patients with type 2 diabetes mellitus and major depressive disorder during a verbal fluency task: a functional near-infrared spectroscopy study based on network-based statistic prediction. Neuroendocrinology. (2024) 114:1112–23. doi: 10.1159/000542235,
19.
Duan C Chong Y Gong J Wu Q Sun J Zheng C et al . An fNIRS-based investigation of cerebral hemodynamic responses during verbal fluency task and n-back task in individuals with mild cognitive impairment. Front Neurol. (2025) 16:1571964. doi: 10.3389/fneur.2025.1571964,
20.
Wang HY Ren L Yan Z Zhou T Liang Z . Neural basis of dysexecutive and visuospatial impairments in Parkinson’s disease with MCI: a task-based fNIRS study. NPJ Parkinsons Dis. (2025) 11:163. doi: 10.1038/s41531-025-01013-z,
21.
Nasreddine ZS Phillips NA Bédirian V Charbonneau S Whitehead V Collin I et al . The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x,
22.
Huang L Chen K Liu Z Guo Q . A conceptual framework for research on cognitive impairment with no dementia in memory clinic. Curr Alzheimer Res. (2020) 17:517–25. doi: 10.2174/1567205017666200807193253,
23.
Crane PK Groot C Ossenkoppele R Mukherjee S Choi SE Lee M et al . Cognitively defined Alzheimer’s dementia subgroups have distinct atrophy patterns. Alzheimers Dement. (2024) 20:1739–52. doi: 10.1002/alz.13567,
24.
Deng HLLM . Study on the cerebral cortex mechanism of intravesical electrical stimulation therapy for bladder hypomotility using resting state near-infrared spectroscopy brain functional imaging. Zhonghua Nan Ke Xue. (2024) 45:664–70. doi: 10.3760/cma.j.cn112330-20240813-00364
25.
Quan W Wu T Li Z Wang Y Dong W Lv B . Reduced prefrontal activation during a verbal fluency task in Chinese-speaking patients with schizophrenia as measured by near-infrared spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. (2015) 58:51–8. doi: 10.1016/j.pnpbp.2014.12.005,
26.
Hou X Zhang Z Zhao C Duan L Gong Y Li Z et al . NIRS-KIT: a MATLAB toolbox for both resting-state and task fNIRS data analysis. Neurophotonics. (2021) 8:010802. doi: 10.1117/1.NPh.8.1.010802,
27.
Xia M Wang J He Y . BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. (2013) 8:e68910. doi: 10.1371/journal.pone.0068910,
28.
Romano J Kromrey JD Coraggio J Skowronek J Devine L , Exploring methods for evaluating group differences on the NSSE and other surveys: are the t-test and Cohen’s d indices the most appropriate choices?, Proceedings of the 2006 Annual Meeting of the Southern Association for Institutional Research (2006), State College (PA): Penn State College of Information Sciences and Technology
29.
Rattanavichit Y Chaikeeree N Boonsinsukh R Kitiyanant K . The age differences and effect of mild cognitive impairment on perceptual-motor and executive functions. Front Psychol. (2022) 13:906898. doi: 10.3389/fpsyg.2022.906898,
30.
Koekkoek PS Kappelle LJ van den Berg E Rutten GEHM Biessels GJ . Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. (2015) 14:329–40. doi: 10.1016/S1474-4422(14)70249-2,
31.
Herrmann MJ Ehlis AC Fallgatter AJ . Frontal activation during a verbal-fluency task as measured by near-infrared spectroscopy. Brain Res Bull. (2003) 61:51–6. doi: 10.1016/s0361-9230(03)00066-2,
32.
Da C Jj L Metting Z Se R Jm S Jwj E et al . The feasibility of fNIRS as a diagnostic tool for pediatric TBI: a pilot study. Eur J Paediatr Neurol. (2021) 30:22–4. doi: 10.1016/j.ejpn.2020.12.008
33.
Karunakaran KD Ji K Chen DY Chiaravalloti ND Niu H Alvarez TL et al . Relationship between age and cerebral hemodynamic response to breath holding: a functional near-infrared spectroscopy study. Brain Topogr. (2021) 34:154–66. doi: 10.1007/s10548-021-00818-4,
34.
Mei X Liang M Zhao Z Xu T Wu X Zhou D et al . Functional connectivity and cerebral cortex activation during the resting state and verbal fluency tasks for patients with mild cognitive impairment, Lewy body dementia, and Alzheimer’s disease: a multi-channel fNIRS study. J Psychiatr Res. (2024) 179:379–87. doi: 10.1016/j.jpsychires.2024.09.049,
35.
Zhang X Zhang N Yang Y Wang S Yu P Wang CX . Cortical activation during the verbal fluency task for obstructive sleep apnea patients with depressive symptoms: a multi-channel fNIRS study. Brain Behav. (2024) 14:e70038. doi: 10.1002/brb3.70038,
36.
Li Y Li X Zhaung W Yu C Wei S Li Y et al . Relationship between cognitive function and brain activation in major depressive disorder patients with and without insomnia: a functional near-infrared spectroscopy (fNIRS) study. J Psychiatr Res. (2024) 169:134–41. doi: 10.1016/j.jpsychires.2023.11.002,
37.
Katzorke A Zeller JBM Müller LD Lauer M Polak T Deckert J et al . Decreased hemodynamic response in inferior frontotemporal regions in elderly with mild cognitive impairment. Psychiatry Res Neuroimaging. (2018) 274:11–8. doi: 10.1016/j.pscychresns.2018.02.003,
38.
Kwan H Scarapicchia V Halliday D MacDonald S Gawryluk JR . Functional near infrared spectroscopy activation during an executive function task differs between healthy older and younger adults. Aging Brain. (2022) 2:100029. doi: 10.1016/j.nbas.2022.100029,
39.
Hamasaki A Akazawa N Yoshikawa T Myoenzono K Tagawa K Maeda S . Age-related declines in executive function and cerebral oxygenation hemodynamics. Tohoku J Exp Med. (2018) 245:245–50. doi: 10.1620/tjem.245.245,
Summary
Keywords
age-related cognitive decline, executive function, functional near-infrared spectroscopy, prefrontal cortex, verbal fluency task
Citation
Zhen Q, Sun R, Han C, Wu J, Song L and Gong W (2026) Prefrontal cortex activation patterns in age-related cognitive decline: insights from verbal fluency task. Front. Neurol. 17:1691856. doi: 10.3389/fneur.2026.1691856
Received
24 August 2025
Revised
05 January 2026
Accepted
08 January 2026
Published
23 January 2026
Volume
17 - 2026
Edited by
Jin Hyuck Park, Soonchunhyang University, Republic of Korea
Reviewed by
Vassiliy Tsytsarev, University of Maryland, United States
Chiara Abbatantuono, University of Bari Aldo Moro, Italy
Pei-Hsin Ku, Ignite Physio Clinic, Taiwan
Updates
Copyright
© 2026 Zhen, Sun, Han, Wu, Song and Gong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijun Gong, gwj197104@ccmu.edu.cn
‡These authors have contributed equally to this work and share first authorship
†PRESENT ADDRESS: Conglin Han, Department of Rehabilitation Medicine, The Affiliated Hospital of Qingdao University, Qingdao, Shandong Province, China
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.