Abstract
Background:
Zoster-associated pain (ZAP) encompasses acute, subacute, and postherpetic neuralgia stages. It often results in persistent sensory abnormalities and substantial impairment of quality of life. Although oral pharmacotherapy remains the first-line and foundational approach, its effectiveness may be limited in some patients; accordingly, non-oral interventions are investigated as complementary or escalated strategies. However, high-quality evidence investigating the relative efficacy and safety of these interventions remains scarce.
Objective:
This study intended to systematically evaluate the efficacy and safety of a variety of non-oral therapeutic interventions for ZAP, thereby providing evidence to inform clinical decision-making.
Methods:
Web of Science, Cochrane Library, Embase, and PubMed were searched to identify eligible randomized controlled trials (RCTs). Data from the included studies were extracted, and the risk of bias was examined via the Cochrane Risk of Bias Tool 2.0. A Bayesian network meta-analysis (NMA) was carried out to compare different interventions, and surface under the cumulative ranking curve (SUCRA) probabilities were utilized to rank relative treatment effects. STATA 18 and R version 4.4.2 were employed to conduct statistical analyses.
Results:
Fifty-three RCTs involving 4,973 patients were included. The NMA showed that chemical selective neurolysis provided the greatest pain relief compared with other treatments (standardized mean difference [SMD]: 4.34; 95% credible interval [CrI]: 2.18 to 6.49). The analysis showed no statistically significant increase in the incidence of adverse events (AEs) (risk ratio: 32.05; 95% CrI: 0.57 to 3,326.64), though the extremely wide CrI indicated substantial uncertainty in this risk estimate. There were no serious complications. Minimally invasive central nervous system neuromodulation combined with topical and peripheral chemical interventions demonstrated the most favorable overall benefits in both pain relief (SMD = 3.41, 95% CrI: 1.08 to 5.73) and sleep improvement (SMD = 3.71, 95% CrI: 1.86 to 5.77). This was followed by minimally invasive peripheral nerve modulation combined with systemic pharmacological analgesia. Regarding safety, no statistically significant differences in AE incidence were found among interventions. However, SUCRA rankings suggested that oral medication and minimally invasive central nervous system neuromodulation had the most favorable safety profiles.
Conclusion:
Combination therapies utilizing minimally invasive neuromodulation show favorable potential in managing ZAP. While chemical selective neurolysis may benefit refractory cases, its use necessitates careful ethical and safety evaluation. Due to the low overall certainty of the evidence, these findings should be interpreted with caution, underscoring the critical need for rigorous future research to confirm long-term outcomes.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO, identifier CRD420251059913.
1 Introduction
Herpes zoster is caused by the reactivation of the latent varicella-zoster virus. The condition is characterized by inflammation of the dorsal root ganglion (DRG), which results in a unilateral, dermatomal rash accompanied by zoster-associated pain (ZAP) (1). Based on disease progression, ZAP is classified into three clinical stages: (i) acute ZAP, defined as pain persisting for ≤ 30 days after onset, (ii) subacute ZAP, persisting after vesicle healing but resolving within 3 months, and (iii) postherpetic neuralgia (PHN) (2). PHN is the most prevalent complication of Herpes zoster. PHN is marked by mechanical allodynia, thermal hyperalgesia, and spontaneous electric shock–like pain. Approximately 22% of patients experience pain lasting more than one month, which subsequently progresses to PHN. PHN can result in depression, weight loss, sleep disturbances, chronic fatigue, elevated risks of cardiovascular disease and suicide, and substantial healthcare and economic burdens (3).
Current therapeutic strategies for ZAP include conventional pharmacological treatments, various interventional therapies, and complementary and alternative therapies, such as mindfulness meditation (4). Oral pharmacotherapy is the cornerstone of the first-line management for ZAP. Frequently prescribed medications include tricyclic antidepressants, anticonvulsants (e.g., pregabalin, gabapentin), and opioids (e.g., tramadol) (4, 5). However, the use of tricyclic antidepressants is often limited due to adverse effects such as somnolence, constipation, and dry mouth. These drugs have gradually been replaced for older patients because their pronounced sedative and anticholinergic effects increase the risk of falls (6). Lidocaine patches are generally safe and well-tolerated; they can be combined with systemic agents to achieve additive effects (7). Consequently, non-oral interventions are recommended when conventional oral therapies are inadequate, when pain impairs physical function or quality of life, or when adverse effects limit tolerance. These interventions include nerve block, minimally invasive central/peripheral nervous system neuromodulation, targeted peripheral/superficial electrical neuromodulation, physical therapy and energy medicine, medical oxidant therapy, biological therapy, and chemical selective neurolysis (e.g., doxorubicin-mediated DRG destruction) (8). Among these interventions, pulsed radiofrequency—a type of minimally invasive peripheral nerve modulation—is a safe and effective treatment for cervical, lumbar, and sacral ZAP. It reduces pain intensity and improves quality of life in patients who do not respond to standard treatment. Compared with nerve blocks, pulsed radiofrequency provides longer-lasting modulation of pain sensitization. Unlike radiofrequency thermocoagulation, pulsed radiofrequency avoids tissue destruction and lowers the risk of dysesthesia. However, its long-term efficacy in refractory cases is inferior to that of minimally invasive central nervous system neuromodulation (9). A 2025 meta-analysis by Liu et al. confirmed that minimally invasive central nervous system neuromodulation (particularly short-term spinal cord stimulation [SCS]) yields significant efficacy and favorable safety in PHN treatment (10). Other non-pharmacological and non-interventional therapies, such as superficial electrical neuromodulation and cognitive behavioral therapy, have not been sufficiently studied for their efficacy in treating neuropathic pain. Thus, their role in patient management remains uncertain (11).
Recent systematic reviews and meta-analyses have examined the therapeutic effects of various interventions for ZAP. However, these interventions have been limited in scope, and comparisons across different neuromodulation techniques, energy-based physical therapies, drug infusion treatments, immunotherapies, combination therapies, as well as complementary and alternative medicine remain lacking (12–16). Network meta-analysis (NMA) is an extension of traditional meta-analysis that can integrate direct and indirect evidence across multiple interventions. This provides a systematic framework for assessing complex treatment effects and offers comprehensive guidance for clinical decision-making or policy development (17). Therefore, this study conducted a systematic review of articles examining various interventions for ZAP and employed NMA to quantify the relative efficacy and safety of different strategies. The aim was to address the clinical challenge of suboptimal responses to conventional oral therapies in individuals with ZAP by providing an evidence-based framework for individualized treatment decisions.
2 Methods
This study was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, including the extension for NMA (PRISMA checklist) (18). The study protocol was prospectively registered in the International Prospective Register of Systematic Reviews (CRD420251059913).
2.1 Search strategy
Embase, Web of Science, the Cochrane Library, and PubMed were comprehensively searched from inception to May 23, 2025. Studies were restricted to those written in English. The search strategy was designed by combining medical subject headings and free-text terms. The keywords used included herpes zoster, neuralgia, and pain. In addition, reference lists of relevant studies and gray literature were manually reviewed to identify eligible articles. Supplementary file 1 presents the full search strategy.
2.2 Inclusion and exclusion criteria
Inclusion criteria encompassed: (i) participants: adults (≥ 18 years) diagnosed with ZAP; (ii) interventions and comparators: the intervention of interest was any procedure or therapy aimed at pain relief, categorized according to its primary mechanism of action into one of the following: nerve blocks, neuromodulation therapies (including non-invasive and invasive techniques), energy-based and physical therapies, biological therapies, drug and chemical therapies (encompassing both localized and systemic administration), conventional and alternative medicine, or combination therapies. Permissible comparators included standard pharmacotherapy (e.g., first-line oral agents such as pregabalin or gabapentin), placebo, sham procedures, or any intervention differing from the investigated treatment; (iii) outcomes: the primary outcome was pain intensity measured by the Numerical Rating Scale (NRS), Visual Analog Scale (VAS), Zoster Brief Pain Inventory (BPI), or Short-Form McGill Pain Questionnaire version 2. Secondary outcomes included sleep quality assessed by the Insomnia Severity Index (ISI), NRS-Sleep, Sleep Quality Scale, Sleep Disturbance Scores, Quality of Sleep, or Sleep Impairment Scale, as well as adverse events (AEs). If outcome definitions differed, original results were classified according to prespecified standards.
Exclusion criteria included: (i) animal or cell studies, case reports, letters, editorials, study protocols, reviews, and conference abstracts; (ii) studies with missing or erroneous data; (iii) duplicate publications; (iv) studies without accessible full texts; (v) studies with overlapping participant populations.
2.3 Data extraction
All selected articles were imported into EndNote 21. Two reviewers (Hao Yuchen and Liu Xiange) independently screened titles and abstracts according to the eligibility criteria, followed by full-text screening. Disagreements were addressed through discussion or by consulting a third reviewer (Sun Tao). Data were independently extracted by two reviewers via a predefined electronic form. Collected information encompassed: country, year of publication, study design, the first author, intervention and comparator details, sample size, follow-up duration, baseline characteristics of participants, and outcome measures.
2.4 Quality assessment
Two reviewers independently appraised the quality of eligible studies via the Cochrane Risk of Bias tool for randomized trials (RoB 2) (19). The assessment covered five domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result. Each domain was evaluated according to the RoB 2 algorithm and categorized as “low risk,” “some concerns,” or “high risk.” The overall risk of bias for each study was determined by the highest risk level across all domains: “high risk” if any domain was rated as high risk, “some concerns” if no domain was rated as high risk but at least one domain raised some concerns, and “low risk” if all domains were rated as low risk. Disagreements were resolved through consensus or adjudication by a third reviewer.
2.5 Statistical analysis
For continuous outcomes (e.g., pain relief, sleep quality), the effect size was expressed as the standardized mean difference (SMD), corrected for small sample sizes using Hedges’ g. To optimize clinical homogeneity and reflect intermediate- to long-term efficacy, the analysis preferentially utilized the mean change from baseline to the primary follow-up endpoint (predominantly at 6 months) and its corresponding standard deviation. This approach synthesized data reported using various scales, including the VAS, NRS, BPI, and ISI. For dichotomous outcomes (e.g., AEs), the risk ratio (RR) served as the effect measure. All effect estimates were presented with their 95% credible intervals (CrI). A Bayesian random-effects NMA model was fitted for each outcome using the gemtc package in R to account for between-study heterogeneity. Vague prior distributions were specified: normal (mean 0, variance 10,000) for treatment effects and uniform (0, 5) for the between-study standard deviation (τ). For multi-arm trials, we split them into independent two-arm comparisons against a common control group and included them in the pairwise network. Models were estimated using a Markov chain Monte Carlo (MCMC) simulation (20). Four chains were run, each with 10,000 burn-in iterations, followed by 50,000 sampling iterations. The thinning interval was set to 10, and the dispersal factor was set to 2.5. Convergence was assessed by confirming that the potential scale reduction factor (PSRF) was below 1.05 for all key parameters and by visually inspecting trace plots. The core NMA assumptions were evaluated. Transitivity was assessed by comparing the distribution of potential effect modifiers (e.g., disease stage, mean patient age, proportion of female patients, baseline pain score, follow-up duration, lesion site, sample size, and year of publication) across treatment comparisons (17). Heterogeneity within pairwise comparisons was quantified using the I2 statistic. Inconsistency was evaluated locally via the node-splitting method and globally via the design-by-treatment interaction model (21). To explore heterogeneity sources, a Bayesian meta-regression was conducted with the aforementioned covariates. A covariate’s impact was considered statistically significant if the 95% CrI of its regression coefficient excluded 0. The relative ranking of interventions was summarized using the surface under the cumulative ranking curve (SUCRA). Publication bias was evaluated through a composite approach that employed a contoured-enhanced funnel plot to assess visual symmetry and an Egger’s test to measure quantitative asymmetry (a p-value < 0.05 suggested potential publication bias or heterogeneity). The Confidence in Network Meta-Analysis (CINeMA) framework was utilized to grade the certainty of evidence across six domains: risk of bias, reporting bias, heterogeneity, imprecision, indirectness, and incoherence. Each domain was rated as having high, moderate, low, or very low certainty. STATA (version 18) and R (version 4.4.2) were used for all statistical analyses.
3 Results
3.1 Literature search and selection process
A total of 27,441 records were obtained from the databases, of which 14,644 were duplicate publications and were removed. After screening the titles and abstracts, an additional 12,707 studies were removed. The remaining studies were assessed in full text based on the inclusion and exclusion criteria, and more studies were removed. Ultimately, 53 studies were included in the NMA (22–74). Figure 1 illustrates the detailed screening process.
Figure 1

Study selection flowchart.
3.2 Characteristics of included studies and quality assessment
The 53 included studies were conducted across eight countries (China, Greece, the Netherlands, Egypt, South Korea, the USA, India, and Denmark) and enrolled 4,973 participants, with 52.09% being female. Participants’ ages ranged from 35 to 89 years old. The eligible studies evaluated multiple interventions targeting ZAP. To mitigate potential bias arising from heterogeneous mechanisms of action and multicomponent combination therapies, the interventions were systematically classified according to their primary mechanism of action before the NMA. The classification scheme is detailed below. 1. Neuromodulation therapies: These were subdivided based on the target site and technical invasiveness: (i) Minimally invasive peripheral nerve modulation: Low-invasiveness techniques targeting peripheral nerves (e.g., pulsed radiofrequency therapy). (ii) Minimally invasive central nervous system neuromodulation: Moderately invasive techniques targeting the spinal cord (e.g., short-term spinal cord stimulation). (iii) Targeted peripheral nerve electrical stimulation: Precise stimulation of specific peripheral nerves (e.g., short-term supraorbital nerve stimulation). (iv) Superficial electrical neuromodulation: Non-invasive transcutaneous electrical stimulation techniques (e.g., transcutaneous electrical nerve stimulation). (v) Non-invasive central nervous system neuromodulation: Non-invasive techniques targeting the brain (e.g., repetitive transcranial magnetic stimulation). 2. Pharmacological and chemical therapies: This category was differentiated by the mechanism of action and route of administration: (i) Topical and peripheral chemical interventions: Locally administered agents (e.g., Botulinum Toxin A subcutaneous injection, lidocaine intradermal injection, steroid/local anesthetic compound preparation). (ii) Systemic pharmacological analgesia: Systemically administered drugs (e.g., Hydromorphone intravenous patient-controlled analgesia). (iii) Chemical selective neurolysis: Neurodestructive procedures (e.g., dorsal root ganglion destruction by Adriamycin). 3. Energy and physical therapies: These interventions were classified according to the mechanism of action of exogenous physical energy: (i) Physical therapy and energy medicine: Modalities utilizing physical energy (e.g., extracorporeal shock wave therapy, pulsed electromagnetic field therapy, light-emitting diode therapy). (ii) Medical oxidant therapy: Approaches based on redox reactions (e.g., ozone autohemotherapy). 4. Biological therapy: This modality aimed to achieve therapeutic effects by modulating intrinsic biological processes, such as immune responses or tissue repair (e.g., BCG polysaccharide and nucleic acid injection, platelet-rich plasma injection, autologous fat grafting). 5. Nerve block: Regional nerve blockade techniques (e.g., stellate ganglion block, erector spinae plane block, paravertebral block). 6. Conventional and alternative medicine: (i) Standard pharmacotherapy: First-line oral medications (e.g., pregabalin, gabapentin). (ii) Complementary and alternative medicine: Mind–body interventions (e.g., mindfulness meditation). 7. Sham control: Placebo interventions simulating authentic treatment procedures. 8. Combination therapy: Original combination formats were preserved (e.g., pulsed radiofrequency + nerve block, spinal cord stimulation + lidocaine patch). Table 1 summarizes the baseline characteristics of the included studies.
Table 1
| Authors | Year | Study design | Area | Intervention groups | Description of interventions | Sample size | Female, n (%) | Ages | Follow-up duration | Disease stage | Lesion site | Baseline pain scores | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y. Li et al. (25) | 2025 | RCT | China | Minimally invasive central nervous system neuromodulation + Topical and peripheral chemical interventions | Short-term spinal cord stimulation + Lidocaine patch | 49 | 25 (51%) | 63.84 ± 10.20 | 3 M | Postherpetic neuralgia | Truncal | 7.20 ± 1.40 | VAS, PSQI |
| Minimally invasive central nervous system neuromodulation | Short-term spinal cord stimulation + Placebo patch | 48 | 25 (52%) | 63.70 ± 3.10 | 24 M | 7.10 ± 1.30 | |||||||
| Y. Li et al. (26) | 2025 | RCT | China | Minimally invasive central nervous system neuromodulation | Short-term Spinal Cord Stimulation | 70 | 46 (66%) | 63.00 ± 4.30 | 6 M | Postherpetic neuralgia | Cervical, Truncal | 7.35 ± 0.90 | VAS, PSQI, SAS, SDS |
| Minimally invasive peripheral nerve modulation | Bipolar pulsed radiofrequency | 70 | 44 (63%) | 61.33 ± 7.96 | 2 M | 7.40 ± 0.90 | |||||||
| C. Wu et al. (24) | 2025 | RCT | China | Targeted peripheral nerve electrical stimulation | Short-term supraorbital nerve stimulation | 31 | 15 (48%) | 59.00 ± 7.60 | 0.5 M | Acute herpes zoster pain | Cranial | 7.26 ± 1.03 | VAS, Adverse events |
| Minimally invasive peripheral nerve modulation | Supraorbital pulsed radiofrequency | 34 | 14 (41%) | / | 6 M | 7.00 ± 1.07 | |||||||
| Y. Wu et al. (23) | 2025 | RCT | China | Biological therapy | BCG polysaccharide and nucleic acid injection | 49 | 25 (51%) | / | 6 M | Postherpetic neuralgia | / | 6.89 ± 1.82 | VAS, Adverse events |
| Standard treatment | Standard oral therapy | 49 | 26 (53%) | 56.80 ± 3.10 | 2 M | 7.03 ± 1.68 | |||||||
| C. Zheng et al. (22) | 2025 | RCT | China | Standard treatment | Standard oral therapy | 40 | 22 (55%) | 56.20 ± 3.70 | 1 M | Postherpetic neuralgia | Cranial | 4.36 ± 2.32 | VAS, Adverse events |
| Nerve block | Stellate ganglion block | 52 | 26 (50%) | 53.14 ± 4.64 | 6 M | 4.00 ± 1.52 | |||||||
| Medical oxidant therapy | Ozone autohemotherapy | 53 | 28 (53%) | 54.50 ± 4.85 | 1 M | 4.00 ± 1.52 | |||||||
| Medical oxidant therapy + Nerve block | Ozone autohemotherapy + Stellate ganglion block | 45 | 21 (47%) | 73.14 ± 6.64 | 6 M | 4.35 ± 2.30 | |||||||
| Z. Zhou et al. (27) | 2024 | RCT | China | Biological therapy | Platelet-rich plasma injection | 40 | 17 (43%) | 71.18 ± 7.30 | 6 M | Acute herpes zoster pain | Cervical, Truncal | 5.80 ± 0.60 | NRS |
| Standard treatment | Standard oral therapy | 40 | 19 (48%) | 73.20 ± 10.50 | 3 M | 5.90 ± 0.40 | |||||||
| R. Wang et al. (28) | 2024 | RCT | China | Minimally invasive peripheral nerve modulation + Medical oxidant therapy | Pulsed radiofrequency + Dorsal root ganglion ozone injection | 81 | 47 (58%) | 77.50 ± 8.20 | 2 M | Acute herpes zoster pain | / | 6.06 ± 0.64 | NRS |
| Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 83 | 47 (57%) | 66.82 ± 7.78 | 1.5 M | 6.10 ± 0.63 | |||||||
| A. Patil et al. (29) | 2024 | RCT | India | Nerve block | Erector spinae plane block | 20 | 13 (65%) | 66.75 ± 10.98 | 12 M | Acute herpes zoster pain | Truncal | 7.70 ± 1.12 | NRS, Adverse events |
| Nerve block | Paravertebral block | 20 | 9 (45%) | 67.21 ± 9.37 | 6 M | 8.00 ± 1.17 | |||||||
| Y. Liu et al. (30) | 2024 | RCT | China | Standard treatment | Standard oral therapy | 25 | 11 (44%) | 60.60 ± 2.20 | 6 M | 7.50 ± 1.19 | VAS, PSQI, Adverse events | ||
| Targeted peripheral nerve electrical stimulation | Short-term supraorbital nerve stimulation | 25 | 12 (48%) | 59.60 ± 3.20 | 2 M | Acute herpes zoster pain, Subacute herpes zoster pain | Cranial | 7.69 ± 0.46 | |||||
| S. Lin et al. (31) | 2024 | RCT | China | Nerve block | Supraorbital nerve block | 30 | 12 (40%) | 69.20 ± 11.80 | 6 M | 7.41 ± 0.41 | VAS, PSQI, Adverse events | ||
| Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 30 | 14 (47%) | 68.40 ± 12.30 | 3 M | Subacute herpes zoster pain | Cranial, Cervical | 7.10 ± 1.60 | |||||
| Z.-W. Zhang et al. (32) | 2023 | RCT | China | Sham treatment | Sham treatment | 30 | 16 (53%) | 65.47 ± 4.49 | 6 M | 6.90 ± 1.80 | VAS, PSQI, Adverse events | ||
| Minimally invasive peripheral nerve modulation + Nerve block | Pulsed radiofrequency + Spinal nerve block | 30 | 17 (57%) | 67.42 ± 5.93 | 6 M | Acute herpes zoster pain, Subacute herpes zoster pain | Truncal | 7.32 ± 2.31 | |||||
| Physically-ablative neuromodulation + Nerve block | Low-temperature plasma ablation of the DRG + Spinal nerve block | 30 | 12 (40%) | 65.97 ± 2.97 | 3 M | 7.32 ± 2.31 | |||||||
| H. Sun et al. (33) | 2023 | RCT | China | Nerve block | Spinal nerve block | 82 | 32 (39%) | 66.02 ± 2.97 | 3 M | 7.11 ± 2.98 | VAS | ||
| Physical therapy and energy medicine | Extracorporeal shock wave treatment | 82 | 28 (34%) | 63.84 ± 10.20 | 3 M | Postherpetic neuralgia | Cranial, Cervical, Truncal | 7.17 ± 0.49 | |||||
| M. Sollie et al. (34) | 2023 | RCT | Denmark | Standard treatment | Standard oral therapy | 23 | / | 63.70 ± 3.10 | 3 M | 7.22 ± 0.47 | NRS | ||
| Biological therapy | Autologous fat grafting | 23 | / | 63.00 ± 4.30 | 1 M | Postherpetic neuralgia | / | 7.40 ± 0.40 | |||||
| S. Lin et al. (35) | 2023 | RCT | China | Sham treatment | Sham treatment | 30 | 12 (40%) | 61.33 ± 7.96 | 3 M | 7.30 ± 0.40 | VAS, PSQI | ||
| Minimally invasive peripheral nerve modulation | Bipolar high-voltage pulsed radiofrequency | 30 | 14 (47%) | 59.00 ± 7.60 | 6 M | Acute herpes zoster pain | Cranial, Cervical, Upper Limbs | 7.10 ± 1.60 | |||||
| W. Zhang et al. (36) | 2022 | RCT | China | Sham treatment | Sham treatment | 32 | 17 (53%) | / | 6 M | 6.90 ± 1.80 | VAS, PSQI, adverse events | ||
| Minimally invasive peripheral nerve modulation + Systemic pharmacological analgesia | Pulsed radiofrequency + Intravenous lidocaine infusion | 32 | 14 (44%) | / | 1 M | Subacute herpes zoster pain | / | 7.46 ± 1.07 | |||||
| C. Wang et al. (37) | 2022 | RCT | China | Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 12 | / | 56.80 ± 3.10 | 6 M | 7.21 ± 1.15 | VAS | ||
| Nerve block | Stellate ganglion block | 12 | / | 56.20 ± 3.70 | 2 M | Postherpetic neuralgia | / | 7.80 ± 1.30 | |||||
| Physical therapy and energy medicine | Extracorporeal shock wave therapy | 12 | / | 53.14 ± 4.64 | 1 M | 7.90 ± 0.90 | |||||||
| L. Sheng et al. (38) | 2022 | RCT | China | Physical therapy and energy medicine + Nerve block | Extracorporeal shock wave therapy + Stellate ganglion block | 38 | 19 (50%) | 54.50 ± 4.85 | 12 M | 7.80 ± 1.00 | VAS, Adverse events | ||
| Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 29 | 14 (48%) | 73.14 ± 6.64 | / | Postherpetic neuralgia | Cervical, Truncal | 6.66 ± 1.81 | |||||
| X. Li et al. (39) | 2022 | RCT | China | Minimally invasive central nervous system neuromodulation | Short-term spinal cord stimulation | 20 | 9 (45%) | 71.18 ± 7.30 | / | 7.21 ± 1.78 | VAS, SIS | ||
| Minimally invasive central nervous system neuromodulation | Short-term spinal cord stimulation | 20 | 10 (50%) | 73.20 ± 10.50 | / | Postherpetic neuralgia | Cervical, Truncal, Upper Limbs, Lower Limbs | 8.11 ± 0.24 | |||||
| M. Ji et al. (40) | 2022 | RCT | China | Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 36 | 16 (44%) | 77.50 ± 8.20 | / | 8.29 ± 0.96 | VAS, ISI | ||
| Minimally invasive peripheral nerve modulation + Nerve block | Pulsed radiofrequency + Methylene blue paravertebral nerve block | 36 | 18 (50%) | 66.82 ± 7.78 | 3 M | Postherpetic neuralgia | / | 6.50 ± 0.81 | |||||
| M. M. Eid et al. (41) | 2022 | RCT | Egypt | Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 28 | 20 (71%) | 66.75 ± 10.98 | 6 M | 6.50 ± 0.74 | VAS | ||
| Superficial electrical neuromodulation | Transcutaneous electrical nerve stimulation | 28 | 18 (64%) | 67.21 ± 9.37 | 6 M | Postherpetic neuralgia | / | 8.71 ± 0.93 | |||||
| L. Chen et al. (42) | 2022 | RCT | China | Physical therapy and energy medicine | Pulsed electromagnetic field therapy | 50 | 24 (48%) | 60.60 ± 2.20 | 6 M | 8.60 ± 0.78 | NRS, SQS, Adverse events | ||
| Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 50 | 24 (48%) | 59.60 ± 3.20 | 2 M | Postherpetic neuralgia | / | 6.00 ± 1.01 | |||||
| L. Chen et al. (43) | 2022 | RCT | China | Topical and peripheral chemical interventions | Botulinum Toxin A subcutaneous injection | 34 | 14 (41%) | 69.20 ± 11.80 | 2 M | 6.12 ± 1.10 | NRS, PSQI, Adverse events | ||
| Physical therapy and energy medicine | Extracorporeal shockwave therapy | 35 | 20 (57%) | 68.40 ± 12.30 | 6 M | Postherpetic neuralgia | Multiple sites | 7.18 ± 1.35 | |||||
| E. H. Abdelwahab et al. (44) | 2022 | RCT | Egypt | Standard treatment | Standard oral therapy | 30 | 16 (53%) | 65.47 ± 4.49 | 7.62 ± 1.35 | NRS | |||
| Nerve block | Erector spinae plane block | 30 | 17 (57%) | 67.42 ± 5.93 | Postherpetic neuralgia | Truncal | 7.00 ± 1.56 | ||||||
| C. F. Wan et al. (45) | 2021 | RCT | China | Nerve block | Thoracic paravertebral block | 46 | 26 (57%) | 65.97 ± 2.97 | 6 M | 7.36 ± 2.44 | NRS, Adverse events | ||
| Standard treatment | Standard oral therapy | 45 | 24 (53%) | 66.02 ± 2.97 | 1 M | 7.00 ± 1.56 | |||||||
| A. K. Saxena et al. (46) | 2021 | RCT | India | Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 20 | / | 63.84 ± 10.20 | 1 M | Acute herpes zoster pain, Subacute herpes zoster pain | Cervical, Truncal | 7.48 ± 2.91 | VAS, NRS-Sleep |
| Minimally invasive central nervous system neuromodulation | Short-term spinal cord stimulation | 20 | / | 63.70 ± 3.10 | 6 M | 7.39 ± 2.73 | |||||||
| J. Liu et al. (47) | 2021 | RCT | China | Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 80 | 36 (45%) | 63.00 ± 4.30 | 6 M | Postherpetic neuralgia | Truncal | 6.80 ± 1.50 | VAS, PSQI |
| Standard treatment | Standard oral therapy | 80 | 38 (48%) | 61.33 ± 7.96 | 12 M | 5.90 ± 1.10 | |||||||
| Y. Huang et al. (48) | 2021 | RCT | China | Minimally invasive central nervous system neuromodulation | Short-term spinal cord stimulation | 96 | 50 (52%) | 59.00 ± 7.60 | 6 M | Postherpetic neuralgia | Cervical, Truncal, Upper Limbs, Lower Limbs | 7.39 ± 0.88 | NRS, PSQI, Adverse events |
| Nerve block | Nerve block | 97 | 46 (47%) | / | 3 M | 7.48 ± 0.82 | |||||||
| M. A. El-Sayed et al. (49) | 2021 | RCT | Egypt | Systemic pharmacological analgesia | Hydromorphone intravenous patient-controlled analgesia | 20 | / | / | 3 M | Postherpetic neuralgia | Cranial, Cervical, Truncal, Lower Limbs | 6.90 ± 1.50 | VAS |
| Sham treatment | Sham treatment | 20 | / | 56.80 ± 3.10 | 3 M | 6.50 ± 1.60 | |||||||
| X. Dong et al. (50) | 2021 | RCT | China | Nerve block | Erector spinae plane block | 48 | 20 (42%) | 56.20 ± 3.70 | 1 M | Acute herpes zoster pain | Truncal | 8.89 ± 1.13 | VAS, Adverse events |
| Standard treatment | Standard oral therapy | 48 | 22 (46%) | 53.14 ± 4.64 | 1 M | 9.05 ± 1.52 | |||||||
| Z.-H. Xiong et al. (51) | 2020 | RCT | China | Nerve block | Continuous epidural block | 39 | 20 (51%) | 54.50 ± 4.85 | 3 M | Postherpetic neuralgia | Truncal, Upper Limbs, Lower Limbs | 9.00 ± 0.85 | NRS, Sleep disturbance scores |
| Standard treatment | Standard oral therapy | 39 | 22 (56%) | 73.14 ± 6.64 | 3 M | 9.00 ± 0.73 | |||||||
| B. Liu et al. (52) | 2020 | RCT | China | Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 31 | 18 (58%) | 71.18 ± 7.30 | 6 M | Postherpetic neuralgia | Truncal | 7.50 ± 1.40 | NRS, Adverse events |
| Standard treatment | Standard oral therapy | 32 | 14 (44%) | 73.20 ± 10.50 | 6 M | 7.60 ± 1.20 | |||||||
| S. Zheng et al. (53) | 2019 | RCT | China | Minimally invasive central nervous system neuromodulation | Short-term spinal cord stimulation | 70 | 41 (59%) | 77.50 ± 8.20 | 6 M | Subacute herpes zoster pain, Postherpetic neuralgia | Cervical, Truncal, Upper Limbs, Lower Limbs | 8.13 ± 1.07 | ZBPI |
| Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 70 | 39 (56%) | 66.82 ± 7.78 | 6 M | 7.73 ± 1.31 | |||||||
| P. Zhao et al. (54) | 2019 | RCT | China | Nerve block | Cervical nerve root block | 43 | 20 (47%) | 66.75 ± 10.98 | 1 M | Acute herpes zoster pain | Upper Limbs | 7.37 ± 1.76 | VAS, QS |
| Sham treatment | Sham treatment | 44 | 21 (48%) | 67.21 ± 9.37 | 1 M | 7.40 ± 1.75 | |||||||
| C. Wang et al. (55) | 2019 | RCT | China | Nerve block | Paraspinal nerve block | 46 | 21 (46%) | 60.60 ± 2.20 | 6 M | Subacute herpes zoster pain | / | 7.61 ± 0.53 | VAS |
| Standard treatment | Standard oral therapy | 47 | 20 (43%) | 59.60 ± 3.20 | 6 M | 7.59 ± 0.75 | |||||||
| Q. Pei et al. (56) | 2019 | RCT | China | Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 20 | 11 (55%) | 69.20 ± 11.80 | 2 M | Acute herpes zoster pain, Subacute herpes zoster pain | Cranial | 7.32 ± 2.33 | VAS, SQ |
| Sham treatment | Sham treatment | 20 | 9 (45%) | 68.40 ± 12.30 | 2 M | 7.31 ± 2.39 | |||||||
| J. Zhang et al. (57) | 2018 | RCT | China | Non-invasive central nervous system neuromodulation | 5-Hz Repetitive transcranial magnetic stimulation | 25 | 15 (60%) | 65.47 ± 4.49 | 1 M | Postherpetic neuralgia | / | 6.90 ± 1.10 | VAS, SIS, Adverse events |
| Non-invasive central nervous system neuromodulation | 10-Hz Repetitive transcranial magnetic stimulation | 25 | 13 (52%) | 67.42 ± 5.93 | 1 M | 6.30 ± 1.70 | |||||||
| M. Y. Makharita et al. (58) | 2018 | RCT | Egypt | Sham treatment | Sham treatment | 21 | 9 (43%) | 65.97 ± 2.97 | 12 M | 6.80 ± 1.60 | VAS, Adverse events | ||
| Nerve block | Epidural block of lidocaine | 22 | 10 (45%) | 66.02 ± 2.97 | 12 M | Postherpetic neuralgia | Truncal | 8.07 ± 0.97 | |||||
| D. Li et al. (59) | 2018 | RCT | China | Topical and peripheral chemical interventions | Intradermal injection of lidocaine | 15 | / | 63.84 ± 10.20 | / | 8.14 ± 0.56 | VAS | ||
| Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 15 | / | 63.70 ± 3.10 | / | Postherpetic neuralgia | Truncal | 7.31 ± 1.25 | |||||
| Sham treatment | Sham treatment | 15 | / | 63.00 ± 4.30 | / | 7.23 ± 1.29 | |||||||
| Sham treatment | Sham treatment | 15 | / | 61.33 ± 7.96 | / | Postherpetic neuralgia | Cervical, Truncal | 7.73 ± 0.88 | |||||
| B. Hu et al. (60) | 2018 | RCT | China | Nerve block | Nerve block | 46 | 18 (39%) | 59.00 ± 7.60 | 3 M | 8.07 ± 0.96 | VAS | ||
| Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 45 | 20 (44%) | / | 3 M | 7.80 ± 0.94 | |||||||
| J.-z. Cui et al. (61) | 2018 | RCT | China | Minimally invasive peripheral nerve modulation + Nerve block | Pulsed radiofrequency + Nerve block | 49 | 28 (57%) | / | 6 M | 7.80 ± 0.86 | VAS | ||
| Medical oxidant therapy | Ozone autohemotherapy | 48 | 28 (58%) | 56.80 ± 3.10 | 6 M | Postherpetic neuralgia | Cranial, Cervical, Truncal, Upper Limbs | 6.50 ± 1.40 | |||||
| J. Ni et al. (62) | 2017 | RCT | China | Standard treatment | Standard oral therapy | 50 | 26 (52%) | 56.20 ± 3.70 | 6 M | 6.20 ± 1.30 | NRS | ||
| Topical and peripheral chemical interventions | Intracutaneous injection of ropivacaine plus methylprednisolone | 50 | 27 (54%) | 53.14 ± 4.64 | 6 M | Acute herpes zoster pain | Truncal | 7.00 ± 1.20 | |||||
| J. Z. Cui et al. (63) | 2017 | RCT | China | Sham treatment | Sham treatment | 47 | 26 (55%) | 54.50 ± 4.85 | 6 M | 7.10 ± 1.40 | VAS | ||
| Topical and peripheral chemical interventions | Subcutaneous injection of triamcinolone and lidocaine | 46 | 27 (59%) | 73.14 ± 6.64 | 6 M | Acute herpes zoster pain | Cranial, Cervical, Truncal | 7.16 ± 1.22 | |||||
| A. K. Saxena et al. (64) | 2016 | RCT | India | Standard treatment | Standard oral therapy | 30 | 13 (43%) | 71.18 ± 7.30 | 2 M | 6.64 ± 1.44 | VAS, NRS-sleep | ||
| Topical and peripheral chemical interventions | Repetitive intracutaneous injections of local anesthetic plus steroid | 30 | 13 (43%) | 73.20 ± 10.50 | 2 M | Acute herpes zoster pain | Truncal | 7.50 ± 1.70 | |||||
| R. Meize-Grochowski et al. (65) | 2015 | RCT | USA | Standard treatment | Standard oral therapy | 13 | 6 (46%) | 77.50 ± 8.20 | 2 M | 7.50 ± 1.40 | SF-MPQ-2 | ||
| Minimally invasive peripheral nerve modulation | Pulsed radiofrequency | 14 | 9 (64%) | 66.82 ± 7.78 | 2 M | Postherpetic neuralgia | Truncal | 6.87 ± 0.73 | |||||
| M. Y. Makharita et al. (66) | 2015 | RCT | Egypt | Sham treatment | Sham treatment | 70 | 36 (51%) | 66.75 ± 10.98 | 6 M | 7.10 ± 0.80 | VAS, Adverse events | ||
| Complementary and alternative medicine | Mindfulness meditation | 68 | 37 (54%) | 67.21 ± 9.37 | 6 M | Postherpetic neuralgia | Multiple sites | 3.50 ± 2.20 | |||||
| K. Y. Park et al. (68) | 2013 | RCT | Korea | Standard treatment | Usual care | 14 | 7 (50%) | 60.60 ± 2.20 | / | 2.40 ± 1.50 | VAS, Adverse events | ||
| Nerve block | Paravertebral block | 14 | 6 (43%) | 59.60 ± 3.20 | / | Acute herpes zoster pain, Subacute herpes zoster pain | Truncal | 7.37 ± 1.57 | |||||
| K. Ma et al. (69) | 2013 | RCT | China | Sham treatment | Sham treatment | 48 | 23 (48%) | 69.20 ± 11.80 | 6 M | 7.34 ± 1.17 | VAS, Adverse events | ||
| Physical therapy and energy medicine | 830 nm Light-emitting diode therapy | 48 | 26 (54%) | 68.40 ± 12.30 | 6 M | Acute herpes zoster pain | Cranial, Cervical | 6.78 ± 0.87 | |||||
| Z. Apalla et al. (70) | 2013 | RCT | Greece | Standard treatment | Standard oral therapy | 15 | 7 (47%) | 65.47 ± 4.49 | 1 M | 6.64 ± 0.69 | VAS, 5-Item Sleep Questionnaire Score, Adverse events | ||
| Minimally invasive peripheral nerve modulation | Pulsed Radiofrequency | 15 | 5 (33%) | 67.42 ± 5.93 | 1 M | Postherpetic neuralgia | Truncal | 6.10 ± 0.08 | |||||
| G. Xu et al. (67) | 2013 | RCT | China | Sham treatment | Sham treatment | 33 | 18 (55%) | 65.97 ± 2.97 | 1 M | 6.36 ± 0.09 | NRS | ||
| Topical and peripheral chemical interventions | Botulinum Toxin A local injection | 66.02 ± 2.97 | Postherpetic neuralgia | Truncal, Upper Limbs, Lower Limbs | 8.80 ± 1.00 | ||||||||
| Sham treatment | Sham treatment | 33 | 16 (48%) | 63.84 ± 10.20 | 1 M | 8.70 ± 0.80 | |||||||
| M. Y. Makharita et al. (71) | 2012 | RCT | Egypt | Topical and peripheral chemical interventions | Local methylcobalamin injection | 31 | 18 (58%) | 63.70 ± 3.10 | 6 M | Subacute herpes zoster pain | Truncal | 6.90 ± 1.50 | VAS |
| Topical and peripheral chemical interventions | Subcutaneous lidocaine injection | 30 | 16 (53%) | 63.00 ± 4.30 | 6 M | 7.10 ± 1.60 | |||||||
| C.-j. He et al. (72) | 2012 | RCT | China | Standard treatment | Standard oral therapy | 36 | 25 (69%) | 61.33 ± 7.96 | 6 M | 6.90 ± 1.10 | VAS, Adverse events | ||
| Nerve block | Stellate ganglion block | 36 | 22 (61%) | 59.00 ± 7.60 | 6 M | Acute herpes zoster pain | Cranial | 7.00 ± 0.90 | |||||
| G. Ji et al. (73) | 2009 | RCT | China | Sham treatment | Sham treatment | 64 | 36 (56%) | / | 12 M | 7.10 ± 1.10 | VAS, Adverse events | ||
| Chemical selective neurolysis | Dorsal root ganglion destruction by adriamycin | 68 | 38 (56%) | / | 12 M | Postherpetic neuralgia | Truncal | 7.64 ± 1.19 | |||||
| A. J. van Wijck et al. (74) | 2006 | RCT | Netherlands | Nerve block | Transforaminal injection of dexamethasone plus lidocain | 301 | 183 (61%) | 56.80 ± 3.10 | 6 M | 7.55 ± 1.44 | VAS, Adverse events | ||
| Nerve block | Repetitive paravertebral injections of local anesthetics and steroids | 297 | 181 (61%) | 56.20 ± 3.70 | 6 M | Acute herpes zoster pain | Cervical, Truncal, Upper Limbs, Lower Limbs | 7.59 ± 1.07 |
Baseline characteristics of the included studies (serves to assess the transitivity assumption of the network meta-analysis by demonstrating the distributional balance of potential effect modifiers across the different intervention groups).
The risk of bias assessment for the included studies is illustrated in Figure 2. Of the 53 studies included, 23 (43.4%) were judged as low risk, 25 (47.2%) raised some concerns, and five (9.4%) were rated as high risk of bias. The main reasons for the ‘some concerns’ or ‘high risk’ ratings, analyzed by assessment domain, are shown below. In Domain 1 (D1: Bias arising from the randomization process), four studies received a high-risk rating due to an insufficient description of both random sequence generation and allocation concealment. An additional 12 studies were assessed as having ‘some concerns’ due to inadequate reporting of randomization-related details. Domain 2 (D2: Bias due to deviations from intended interventions) presented inherent methodological limitations. Fundamental differences in the nature of various interventions (e.g., comparisons between invasive procedures, such as neuromodulation and nerve blocks, and standard oral pharmacotherapy) precluded blinding of participants and study personnel in several studies. Regarding Domain 3 (D3: Bias due to missing outcome data), one study raised concerns due to a high attrition rate (> 10%) and the absence of an intention-to-treat analysis. The remaining studies exhibited a low risk given the minimal, balanced missing data across groups. For Domain 4 (D4: Bias in measurement of the outcome), the primary outcome (pain intensity) was patient-reported. In studies where participant blinding was not feasible, the potential for subjective expectation to influence reporting was a recognized source of bias. This is common in interventional research utilizing subjective endpoints. All studies were rated as low risk in Domain 5 (D5: Bias in selection of the reported result), indicating a low likelihood of selective outcome reporting. In summary, despite limitations regarding blinding and reporting randomization procedures, the overall risk of bias for most included studies remained within an acceptable range.
Figure 2

Bias assessment graph. (A) Summary chart of bias risk of included studies. (B) Risk of bias for each included study.
3.3 Results of the NMA
3.3.1 Network characteristics and model assessment
In the network diagram, each node corresponded to a distinct intervention, and the size of each node was proportional to the number of studies evaluating that intervention. Lines connecting nodes indicated the presence of direct comparative evidence. The thickness of the lines was proportional to the number of studies contributing to each direct comparison (Figure 3).
Figure 3

Network evidence diagram: (A) pain relief; (B) sleep quality improvement; (C) adverse events.
Evaluation of local heterogeneity revealed differential levels across the three outcomes. For the primary outcome (pain relief), most direct comparisons exhibited moderate to high heterogeneity (I2 ≥ 50%). Conversely, comparisons for the sleep quality and AEs were predominantly characterized by low heterogeneity (I2 < 50%). For all outcomes, certain comparisons lacked I2 calculations due to the inclusion of only one study (Supplementary Figures S1–S3).
Assessment of local inconsistency via the node-splitting method detected no significant divergence between direct and indirect evidence for any comparison related to pain relief, sleep quality, or AEs (all p > 0.05) (Supplementary Figures S4–S6). Global consistency was further evaluated by comparing the goodness-of-fit between the consistency and node-splitting inconsistency models. For both pain intensity (ΔDIC = −0.336) and sleep quality (ΔDIC = −0.396), the minimal absolute ΔDIC values were negative, indicating a superior fit for the consistency model and strongly supporting global consistency across the network. Regarding AEs, the value below five (ΔDIC = 4.390) did not suggest compelling evidence for substantial global inconsistency (Supplementary Table S1).
All models demonstrated a PSRF approaching 1.00 (< 1.05), indicating sufficient convergence of the Markov chains (Supplementary Figures S7–S9). Trace plots illustrated excellent mixing between chains for all parameters, further validating convergence (Supplementary Figures S10–S12).
3.3.2 Pooled results for each outcome measure
3.3.2.1 Pain relief
The present NMA synthesized data from all 53 included studies that reported pain scores. The results demonstrated that several intervention strategies, whether used alone or in combination, produced statistically significant pain reduction compared to standard oral treatment alone. Interventions associated with significantly lower pain scores included nerve block (SMD = 0.79, 95% CrI: 0.19 to 1.39), minimally invasive peripheral nerve modulation (SMD = 1.13, 95% CrI: 0.32 to 1.93), minimally invasive central nervous system neuromodulation (SMD = 2.48, 95% CrI: 1.37 to 3.59), topical and peripheral chemical interventions (SMD = 1.13, 95% CrI: 0.33 to 1.93), physical therapy and energy medicine (SMD = 1.81, 95% CrI: 0.75 to 2.87), chemical selective neurolysis (SMD = 4.34, 95% CrI: 2.18 to 6.49), minimally invasive peripheral nerve modulation + nerve block (SMD = 2.01, 95% CrI: 0.65 to 3.37), minimally invasive peripheral nerve modulation + systemic pharmacological analgesia (SMD = 2.96, 95% CrI: 0.71 to 5.18), and minimally invasive central nervous system neuromodulation + topical and peripheral chemical interventions (SMD = 3.41, 95% CrI: 1.08 to 5.73) (Table 2).
Table 2
| ST | |||||||||||||||||||||
| 0.79 (0.19, 1.39) | NB | ||||||||||||||||||||
| 1.13 (0.32, 1.93) | 0.34 (−0.43, 1.1) | MI-PNM | |||||||||||||||||||
| −0.05 (−0.82, 0.7) | −0.84 (−1.5, −0.19) | −1.18 (−1.83, −0.53) | Sham | ||||||||||||||||||
| 2.48 (1.37, 3.59) | 1.7 (0.63, 2.75) | 1.36 (0.5, 2.21) | 2.53 (1.51, 3.56) | MI-CNS-NM | |||||||||||||||||
| 1.13 (0.33, 1.93) | 0.34 (−0.52, 1.21) | 0 (−0.93, 0.95) | 1.18 (0.3, 2.08) | −1.36 (−2.57, −0.13) | TPCI | ||||||||||||||||
| 1.81 (0.75, 2.87) | 1.02 (−0.12, 2.16) | 0.68 (−0.61, 1.98) | 1.86 (0.61, 3.12) | −0.68 (−2.17, 0.82) | 0.68 (−0.62, 1.98) | PTEM | |||||||||||||||
| 0.9 (−1.27, 3.05) | 0.11 (−2.03, 2.23) | −0.23 (−2.37, 1.89) | 0.95 (−1.08, 2.97) | −1.59 (−3.87, 0.69) | −0.23 (−2.46, 1.97) | −0.91 (−3.3, 1.47) | SPA | ||||||||||||||
| 1.58 (−0.01, 3.17) | 0.79 (−0.73, 2.31) | 0.46 (−1.07, 1.97) | 1.63 (0.07, 3.2) | −0.9 (−2.61, 0.82) | 0.46 (−1.24, 2.13) | −0.22 (−2.09, 1.64) | 0.69 (−1.88, 3.25) | T-PNES | |||||||||||||
| 2.25 (−0.08, 4.57) | 1.46 (−0.91, 3.82) | 1.12 (−1.32, 3.57) | 2.3 (−0.12, 4.73) | −0.24 (−2.79, 2.33) | 1.12 (−1.34, 3.55) | 0.44 (−1.64, 2.51) | 1.35 (−1.8, 4.49) | 0.67 (−2.13, 3.46) | SEN | ||||||||||||
| 0.95 (−0.27, 2.18) | 0.17 (−1.14, 1.47) | −0.17 (−1.55, 1.2) | 1 (−0.3, 2.31) | −1.53 (−3.11, 0.04) | −0.17 (−1.59, 1.23) | −0.85 (−2.46, 0.75) | 0.06 (−2.35, 2.48) | −0.63 (−2.58, 1.32) | −1.29 (−3.91, 1.33) | BioTx | |||||||||||
| 1.58 (−0.12, 3.26) | 0.79 (−0.86, 2.43) | 0.45 (−1.19, 2.09) | 1.63 (0.13, 3.13) | −0.9 (−2.73, 0.91) | 0.45 (−1.31, 2.19) | −0.23 (−2.2, 1.72) | 0.68 (−1.83, 3.21) | 0 (−2.17, 2.17) | −0.67 (−3.53, 2.18) | 0.63 (−1.37, 2.62) | NI-CNS-NM | ||||||||||
| 0.66 (−0.84, 2.17) | −0.13 (−1.75, 1.5) | −0.47 (−2.16, 1.25) | 0.71 (−0.96, 2.41) | −1.82 (−3.68, 0.05) | −0.47 (−2.18, 1.23) | −1.15 (−2.98, 0.7) | −0.24 (−2.86, 2.41) | −0.92 (−3.1, 1.27) | −1.59 (−4.36, 1.18) | −0.29 (−2.23, 1.65) | −0.92 (−3.17, 1.35) | MOT | |||||||||
| 0.33 (−1.82, 2.48) | −0.46 (−2.68, 1.77) | −0.8 (−3.08, 1.49) | 0.38 (−1.88, 2.66) | −2.16 (−4.56, 0.27) | −0.8 (−3.09, 1.48) | −1.48 (−3.86, 0.92) | −0.56 (−3.59, 2.47) | −1.25 (−3.9, 1.42) | −1.92 (−5.07, 1.25) | −0.62 (−3.09, 1.85) | −1.25 (−3.98, 1.47) | −0.33 (−2.93, 2.29) | CAM | ||||||||
| 4.34 (2.18, 6.49) | 3.55 (1.49, 5.62) | 3.21 (1.01, 5.41) | 4.39 (2.23, 6.56) | 1.85 (−0.46, 4.17) | 3.21 (0.97, 5.44) | 2.53 (0.16, 4.89) | 3.44 (0.5, 6.4) | 2.76 (0.19, 5.32) | 2.09 (−1.07, 5.23) | 3.39 (0.94, 5.81) | 2.76 (0.13, 5.4) | 3.68 (1.05, 6.3) | 4.01 (0.96, 7.04) | CSN | |||||||
| 2.01 (0.65, 3.37) | 1.22 (−0.06, 2.51) | 0.88 (−0.4, 2.17) | 2.06 (0.79, 3.34) | −0.47 (−1.98, 1.05) | 0.88 (−0.58, 2.33) | 0.2 (−1.47, 1.89) | 1.11 (−1.27, 3.52) | 0.43 (−1.48, 2.34) | −0.24 (−2.91, 2.43) | 1.06 (−0.69, 2.82) | 0.43 (−1.54, 2.41) | 1.35 (−0.68, 3.38) | 1.68 (−0.85, 4.21) | −2.33 (−4.76, 0.1) | MI-PNM + NB | ||||||
| 1.59 (−0.57, 3.75) | 0.8 (−1.27, 2.88) | 0.46 (−1.74, 2.67) | 1.64 (−0.52, 3.82) | −0.9 (−3.21, 1.43) | 0.46 (−1.79, 2.7) | −0.21 (−2.6, 2.16) | 0.7 (−2.28, 3.67) | 0.01 (−2.56, 2.57) | −0.66 (−3.83, 2.5) | 0.64 (−1.82, 3.07) | 0.01 (−2.62, 2.67) | 0.93 (−1.71, 3.55) | 1.26 (−1.78, 4.3) | −2.75 (−5.68, 0.17) | −0.42 (−2.86, 2.01) | PAN + NB | |||||
| 2.96 (0.71, 5.18) | 2.17 (−0.05, 4.38) | 1.83 (−0.27, 3.92) | 3.01 (0.82, 5.19) | 0.47 (−1.78, 2.73) | 1.83 (−0.46, 4.11) | 1.15 (−1.3, 3.6) | 2.06 (−0.91, 5.04) | 1.38 (−1.21, 3.95) | 0.71 (−2.49, 3.91) | 2 (−0.48, 4.51) | 1.38 (−1.27, 4.02) | 2.3 (−0.41, 4.98) | 2.63 (−0.48, 5.7) | −1.38 (−4.41, 1.64) | 0.95 (−1.51, 3.39) | 1.37 (−1.68, 4.4) | MI-PNM + SPA | ||||
| 1.52 (−0.66, 3.7) | 0.74 (−1.43, 2.89) | 0.4 (−1.62, 2.43) | 1.58 (−0.55, 3.71) | −0.96 (−3.16, 1.25) | 0.4 (−1.85, 2.62) | −0.28 (−2.69, 2.11) | 0.63 (−2.32, 3.57) | −0.06 (−2.58, 2.48) | −0.72 (−3.9, 2.44) | 0.57 (−1.88, 3.01) | −0.06 (−2.65, 2.56) | 0.87 (−1.79, 3.5) | 1.19 (−1.86, 4.25) | −2.82 (−5.8, 0.18) | −0.49 (−2.88, 1.91) | −0.07 (−3.07, 2.92) | −1.43 (−4.35, 1.48) | MI-PNM + MOT | |||
| 3.41 (1.08, 5.73) | 2.62 (0.31, 4.92) | 2.28 (0.06, 4.5) | 3.46 (1.17, 5.75) | 0.92 (−1.13, 2.97) | 2.28 (−0.11, 4.65) | 1.6 (−0.93, 4.14) | 2.51 (−0.54, 5.57) | 1.83 (−0.85, 4.49) | 1.16 (−2.11, 4.43) | 2.46 (−0.13, 5.03) | 1.83 (−0.9, 4.57) | 2.75 (−0.03, 5.5) | 3.08 (−0.08, 6.22) | −0.93 (−4.03, 2.16) | 1.4 (−1.15, 3.93) | 1.82 (−1.28, 4.91) | 0.44 (−2.59, 3.52) | 1.89 (−1.12, 4.89) | MI-CNS-NM + TPCI | ||
| 0.8 (−1.25, 2.87) | 0.02 (−2.12, 2.16) | −0.32 (−2.53, 1.89) | 0.86 (−1.33, 3.06) | −1.68 (−4.01, 0.67) | −0.32 (−2.53, 1.88) | −1 (−3.31, 1.31) | −0.09 (−3.06, 2.92) | −0.78 (−3.37, 1.82) | −1.45 (−4.55, 1.65) | −0.15 (−2.55, 2.25) | −0.78 (−3.44, 1.91) | 0.15 (−2.41, 2.69) | 0.48 (−2.48, 3.44) | −3.53 (−6.51, −0.56) | −1.21 (−3.66, 1.27) | −0.78 (−3.76, 2.21) | −2.15 (−5.19, 0.89) | −0.72 (−3.71, 2.29) | −2.6 (−5.7, 0.49) | MOT + NB | |
| 1.86 (−0.39, 4.11) | 1.07 (−1.1, 3.26) | 0.73 (−1.57, 3.04) | 1.91 (−0.35, 4.2) | −0.63 (−3.04, 1.8) | 0.73 (−1.6, 3.07) | 0.05 (−2.4, 2.51) | 0.96 (−2.08, 4.01) | 0.28 (−2.37, 2.93) | −0.39 (−3.61, 2.83) | 0.9 (−1.63, 3.46) | 0.28 (−2.44, 3.01) | 1.19 (−1.52, 3.9) | 1.53 (−1.6, 4.62) | −2.48 (−5.48, 0.53) | −0.16 (−2.68, 2.38) | 0.27 (−2.73, 3.29) | −1.1 (−4.2, 2.02) | 0.33 (−2.72, 3.42) | −1.55 (−4.7, 1.63) | 1.05 (−1.99, 4.1) | PTEM + NB |
League table of pain relief outcomes.
Values represent the standardized mean difference (SMD) and 95% credible interval (CrI) for the comparison of the row intervention versus the column intervention in terms of pain relief outcomes. A positive SMD indicates that the row intervention is superior to the column intervention, while a negative SMD indicates the opposite. Statistically significant comparisons (95% CrI excluding 0) are presented in boldface. Full intervention names corresponding to the abbreviations used can be found in Supplementary Table S5.
Based on the SUCRA values, chemical selective neurolysis had the highest probability (95.96%) of being the most effective analgesic strategy. The next highest probabilities were for minimally invasive central nervous system neuromodulation + topical and peripheral chemical interventions (87.63%) and minimally invasive peripheral nerve modulation + systemic pharmacological analgesia (81.79%) (Figure 4A).
Figure 4

Rankograms of cumulative ranking probabilities. (A) Pain relief. The larger the area under each curve, the better the effect of treating and relieving pain. (B) Sleep quality improvement. The larger the area under each curve, the better the effect on improving sleep quality. (C) Adverse events. The larger the area under each curve, the lower the incidence of adverse reactions.
3.3.2.2 Sleep quality
Twenty studies reported on sleep quality outcomes. The NMA showed that several intervention strategies, whether used alone or in combination, were associated with significantly better sleep quality than standard treatment (oral conventional medications alone). These strategies included nerve block (SMD = 1.19, 95% CrI: 0.13 to 2.29), minimally invasive central nervous system neuromodulation (SMD = 2.43, 95% CrI: 1.27 to 3.78), minimally invasive peripheral nerve modulation + nerve block (SMD = 1.59, 95% CrI: 0.20 to 2.99), physically-ablative neuromodulation + nerve block (SMD = 2.12, 95% CrI: 0.28 to 4.04), minimally invasive peripheral nerve modulation + systemic pharmacological analgesia (SMD = 3.68, 95% CrI: 1.80 to 5.50), and minimally invasive central nervous system neuromodulation + topical and peripheral chemical interventions (SMD = 3.71, 95% CrI: 1.86 to 5.77) (Table 3).
Table 3
| ST | |||||||||||||
| 1.19 (0.13, 2.29) | NB | ||||||||||||
| 0.83 (−0.14, 1.74) | −0.36 (−1.38, 0.56) | MI-PNM | |||||||||||
| −0.37 (−1.62, 0.81) | −1.56 (−2.82, −0.41) | −1.2 (−2.01, −0.4) | Sham | ||||||||||
| 2.43 (1.27, 3.78) | 1.24 (0.23, 2.4) | 1.61 (0.75, 2.69) | 2.81 (1.69, 4.17) | MI-CNS-NM | |||||||||
| 1.24 (−0.02, 2.53) | 0.06 (−1.07, 1.16) | 0.42 (−0.54, 1.45) | 1.62 (0.56, 2.76) | −1.19 (−2.56, 0.02) | TPCI | ||||||||
| 0.18 (−1.32, 1.68) | −1.01 (−2.88, 0.81) | −0.65 (−2.39, 1.15) | 0.55 (−1.35, 2.51) | −2.25 (−4.3, −0.39) | −1.06 (−3.05, 0.89) | PTEM | |||||||
| 1.07 (−0.87, 2.96) | −0.11 (−2.06, 1.73) | 0.25 (−1.44, 1.92) | 1.44 (−0.01, 2.92) | −1.36 (−3.41, 0.43) | −0.17 (−2.05, 1.62) | 0.89 (−1.57, 3.3) | SPA | ||||||
| 1.82 (−0.03, 3.72) | 0.63 (−0.9, 2.16) | 0.99 (−0.76, 2.86) | 2.19 (0.31, 4.19) | −0.61 (−2.56, 1.19) | 0.58 (−1.31, 2.48) | 1.64 (−0.73, 4.08) | 0.75 (−1.63, 3.24) | T-PNES | |||||
| −0.11 (−1.78, 1.51) | −1.29 (−2.98, 0.27) | −0.94 (−2.31, 0.42) | 0.27 (−0.84, 1.36) | −2.55 (−4.32, −1.01) | −1.35 (−2.95, 0.16) | −0.29 (−2.54, 1.9) | −1.18 (−3.02, 0.65) | −1.93 (−4.23, 0.24) | NI-CNS-NM | ||||
| 1.59 (0.2, 2.99) | 0.4 (−0.81, 1.55) | 0.76 (−0.39, 1.96) | 1.96 (0.59, 3.39) | −0.84 (−2.35, 0.46) | 0.34 (−1.09, 1.76) | 1.41 (−0.64, 3.46) | 0.51 (−1.48, 2.57) | −0.23 (−2.19, 1.67) | 1.7 (−0.05, 3.51) | MI-PNM + NB | |||
| 2.12 (0.28, 4.04) | 0.94 (−0.6, 2.47) | 1.29 (−0.45, 3.16) | 2.49 (0.62, 4.51) | −0.31 (−2.26, 1.49) | 0.88 (−0.98, 2.78) | 1.94 (−0.43, 4.36) | 1.05 (−1.33, 3.54) | 0.3 (−1.86, 2.46) | 2.23 (0.07, 4.52) | 0.53 (−1.36, 2.49) | PAN + NB | ||
| 3.68 (1.8, 5.5) | 2.49 (0.57, 4.29) | 2.85 (1.26, 4.44) | 4.05 (2.28, 5.83) | 1.24 (−0.73, 2.98) | 2.44 (0.52, 4.26) | 3.5 (1.1, 5.85) | 2.61 (0.31, 4.92) | 1.86 (−0.61, 4.21) | 3.79 (1.71, 5.89) | 2.09 (0.1, 4.04) | 1.56 (−0.91, 3.9) | MI-PNM + SPA | |
| 3.71 (1.86, 5.77) | 2.52 (0.76, 4.43) | 2.88 (1.23, 4.79) | 4.09 (2.28, 6.15) | 1.28 (−0.21, 2.77) | 2.47 (0.59, 4.52) | 3.53 (1.16, 6.11) | 2.64 (0.34, 5.19) | 1.89 (−0.43, 4.35) | 3.82 (1.73, 6.18) | 2.12 (0.18, 4.27) | 1.59 (−0.72, 4.06) | 0.04 (−2.22, 2.55) | MI-CNS-NM + TPCI |
League table of sleep quality improvement outcomes.
Values represent the standardized mean difference (SMD) and 95% credible interval (CrI) for the comparison of the row intervention versus the column intervention in terms of sleep quality improvement outcomes. A positive SMD indicates that the row intervention is superior to the column intervention, while a negative SMD indicates the opposite. Statistically significant comparisons (95% CrI excluding 0) are presented in boldface. Full intervention names corresponding to the abbreviations used can be found in Supplementary Table S5.
SUCRA ranking probabilities revealed the following order of effectiveness: minimally invasive central nervous system neuromodulation + topical and peripheral chemical interventions (94.57%), minimally invasive peripheral nerve modulation + systemic pharmacological analgesia (93.71%), and minimally invasive central nervous system neuromodulation (79.69%). Minimally invasive central nervous system neuromodulation + topical and peripheral chemical interventions demonstrated the greatest reduction in sleep quality scores (Figure 4B).
3.3.2.3 AEs
Data on AEs were available from 24 studies. Most AEs were mild to moderate and transient, and severe events (e.g., pneumothorax) were rare. The NMA found no statistically significant differences in AE incidence between any intervention and standard oral treatment (as all 95% CrIs for the RR included 1). However, the wide range of RR estimates and their broad CrIs, however, suggest considerable uncertainty in these comparative safety profiles (Table 4).
Table 4
| ST | |||||||||||||||
| 1.29 (0.24, 6.42) | NB | ||||||||||||||
| 0.82 (0.03, 20.59) | 0.63 (0.03, 10.74) | MI-PNM | |||||||||||||
| 1.1 (0.05, 20.17) | 0.86 (0.06, 9.99) | 1.35 (0.15, 12.16) | Sham | ||||||||||||
| 0.49 (0.01, 26.77) | 0.38 (0.01, 14.94) | 0.61 (0.05, 6.47) | 0.45 (0.02, 11.71) | MI-CNS-NM | |||||||||||
| 0.48 (0.02, 11.22) | 0.37 (0.02, 5.76) | 0.58 (0.03, 10.83) | 0.43 (0.03, 6.93) | 0.96 (0.02, 43.08) | TPCI | ||||||||||
| 1 (0.04, 24.85) | 0.77 (0.02, 28.74) | 1.23 (0.01, 133.82) | 0.9 (0.01, 80.2) | 2.03 (0.01, 396.54) | 2.11 (0.02, 211.53) | PTEM | |||||||||
| 0.12 (0, 13.93) | 0.09 (0, 8.4) | 0.15 (0, 11.93) | 0.11 (0, 4.87) | 0.24 (0, 36.89) | 0.25 (0, 27.46) | 0.12 (0, 37.58) | SPA | ||||||||
| 0.56 (0.02, 14.46) | 0.43 (0.02, 7.54) | 0.68 (0.03, 18.5) | 0.5 (0.02, 15.08) | 1.12 (0.02, 68.28) | 1.17 (0.03, 48.41) | 0.55 (0.01, 53.7) | 4.61 (0.03, 1199.88) | T-PNES | |||||||
| 1.21 (0.04, 34.15) | 0.94 (0.02, 39.68) | 1.5 (0.02, 176.09) | 1.09 (0.01, 108.66) | 2.47 (0.01, 510.81) | 2.56 (0.03, 289.55) | 1.23 (0.01, 123.81) | 10.24 (0.03, 5847.25) | 2.2 (0.02, 245.93) | BioTx | ||||||
| 0.75 (0.01, 84.92) | 0.58 (0, 89.03) | 0.92 (0, 319.22) | 0.68 (0, 204.78) | 1.53 (0, 839.25) | 1.59 (0.01, 518.35) | 0.76 (0, 224.42) | 6.5 (0.01, 8025.7) | 1.36 (0, 437.01) | 0.61 (0, 198.55) | MOT | |||||
| 32.05 (0.57, 3326.64) | 24.52 (0.66, 1949.93) | 39.81 (0.4, 7591.93) | 29.34 (0.37, 4853.61) | 67.11 (0.38, 20912.83) | 68.6 (0.68, 12837.07) | 33.33 (0.19, 8572.82) | 284.04 (0.84, 223147.73) | 58.71 (0.55, 10813.61) | 27.38 (0.14, 7458.42) | 44.23 (0.09, 30501.13) | CSN | ||||
| 1.28 (0.03, 49.72) | 0.99 (0.04, 26.64) | 1.57 (0.02, 127.73) | 1.15 (0.02, 78.69) | 2.61 (0.02, 402.25) | 2.72 (0.04, 215.84) | 1.29 (0.01, 163.21) | 10.78 (0.04, 4739.76) | 2.32 (0.03, 185.82) | 1.06 (0.01, 141.58) | 1.71 (0, 640.44) | 0.04 (0, 5.49) | MI-PNM + NB | |||
| 1.54 (0.04, 58.51) | 1.19 (0.04, 32.11) | 1.88 (0.03, 153.39) | 1.38 (0.02, 93.62) | 3.1 (0.02, 474.89) | 3.22 (0.04, 258.88) | 1.54 (0.01, 189.38) | 12.85 (0.05, 5607.07) | 2.79 (0.03, 226.09) | 1.27 (0.01, 169.63) | 2.05 (0.01, 787.31) | 0.05 (0, 6.42) | 1.21 (0.01, 127.77) | PAN + NB | ||
| 3.54 (0.02, 832.23) | 2.72 (0.02, 511.17) | 4.2 (0.09, 379.44) | 3.16 (0.04, 475.64) | 7.1 (0.08, 1152.06) | 7.45 (0.06, 1506.78) | 3.59 (0.01, 1896.67) | 30.42 (0.09, 21759.82) | 6.39 (0.04, 1512.36) | 2.92 (0.01, 1686.26) | 4.83 (0, 6184.35) | 0.11 (0, 63.4) | 2.81 (0.01, 1329.45) | 2.32 (0.01, 1082.88) | MI-PNM + SPA | |
| 0.89 (0.01, 105.23) | 0.69 (0, 108.83) | 1.1 (0, 390.01) | 0.81 (0, 250) | 1.83 (0, 1034.81) | 1.87 (0.01, 656.9) | 0.89 (0, 272.06) | 7.6 (0.01, 9887.92) | 1.61 (0, 541.6) | 0.73 (0, 250.37) | 1.19 (0, 965.16) | 0.03 (0, 14.38) | 0.7 (0, 293.35) | 0.58 (0, 236.69) | 0.25 (0, 294.6) | MOT + NB |
League table of adverse event incidence.
Values represent the relative risk (RR) and 95% credible interval (CrI) for the comparison of the row intervention versus the column intervention in terms of adverse event incidence. An RR > 1 indicates that the row intervention is associated with a higher adverse event incidence than the column intervention, while an RR < 1 indicates the opposite. No comparisons showed statistical significance, as all 95% CrIs include 1. Full intervention names corresponding to the abbreviations used can be found in Supplementary Table S5.
SUCRA ranking probabilities indicated that minimally invasive central nervous system neuromodulation, after oral conventional medication, had the lowest probability of AEs (63.24%), followed by medical oxidant therapy plus nerve block (52.27%) and physical therapy and energy medicine (50.62%). In contrast, chemical selective neurolysis was ranked the lowest (7.97%), indicating the highest probability of AE incidence among all interventions (Figure 4C).
3.4 Bayesian meta-regression analysis
An assessment of transitivity (Table 1) revealed that, except for disease stage and lesion location, which exhibited the expected clinical variations, other factors, including age, sex, and baseline pain score, exhibited highly overlapping distributions across different comparison groups. These findings support the transitivity assumption underlying the NMA.
Bayesian meta-regression identified several significant effect modifiers. Regarding the pain relief outcome, the 95% CrIs for the regression coefficients of sample size, baseline pain score, disease stage, follow-up duration, age, and the proportion of female participants all excluded zero. This indicated their significant modifying effects on the treatment efficacy. No significant modifiers were detected for the sleep quality outcome. Regarding AEs, the 95% CrI for the regression coefficients of baseline pain score and sample size excluded zero. This suggested their significant influence on this outcome. After including these covariates, all models exhibited minimal residual heterogeneity (residual I2 < 10%). This finding implied a good model fit and that the majority of the heterogeneity had been accounted for. Crucially, despite these modifying effects, the relative ranking of intervention efficacy remained substantially unchanged, confirming the robustness of the primary analysis conclusions (Supplementary Table S2).
3.5 Publication bias
Publication bias was evaluated using contour-enhanced funnel plots and Egger’s test. The funnel plots demonstrated a symmetrical distribution (Figure 5), suggesting no substantial publication bias. This visual assessment was corroborated by Egger’s test. The results of the test indicated no significant funnel plot asymmetry for any of the three outcomes: pain relief (p = 0.32), sleep quality (p = 0.57), and AEs (p = 0.41) (Supplementary Table S3).
Figure 5
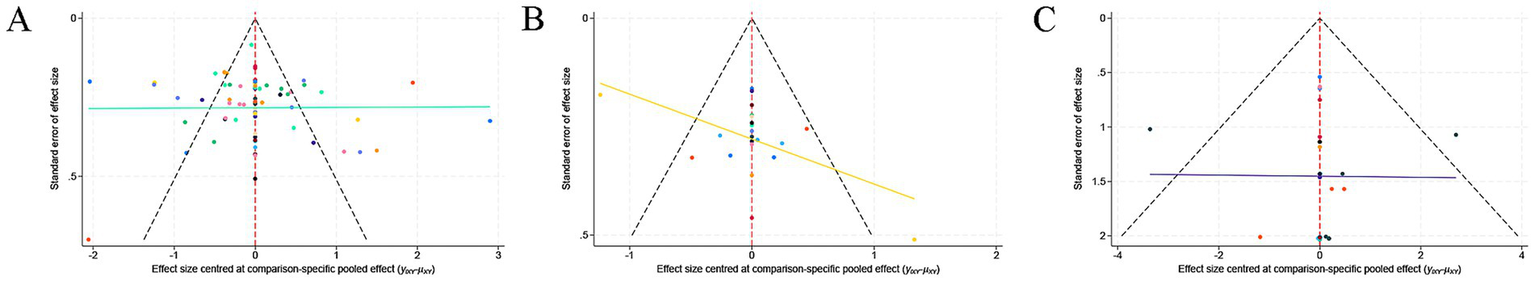
Comparison-adjusted funnel plot for publication bias assessment: (A) pain relief, (B) sleep quality improvement, and (C) adverse events.
3.6 CINeMA assessment
The CINeMA framework evaluation indicated an overall limited certainty of evidence for the three primary outcomes. Most comparisons for both pain relief and AEs were graded as “low” or “very low” certainty. The sleep quality outcome had the most favorable evidence profile, with 12 comparisons rated as “high” certainty. Nevertheless, over two-thirds (68.1%) of its comparisons had “low” or “very low” certainty. The primary reasons for downgrading the evidence were imprecision (wide CrIs) and within-study bias. The complete assessment results are available in Supplementary Table S4.
4 Discussion
This NMA revealed that chemical selective neurolysis had the greatest potential to alleviate ZAP. Two combination therapies, minimally invasive central nervous system neuromodulation + topical and peripheral chemical interventions, and minimally invasive peripheral nerve modulation + systemic pharmacological analgesia, demonstrated significant benefits for both pain relief and sleep improvement. A safety analysis suggested that minimally invasive central nervous system neuromodulation was associated with the lowest probability of AEs. Furthermore, most interventions exhibited a favorable safety profile. AEs were typically mild and transient.
Chemical selective neurolysis (doxorubicin-mediated DRG destruction) ranked first in the SUCRA for pain relief. However, it is important to note that SUCRA values reflect probabilistic ranking among interventions rather than definitive clinical superiority. Previous studies have confirmed the efficacy of doxorubicin-mediated selective DRG destruction in alleviating PHN and postherpetic trigeminal neuralgia. This suggests a potential approach to neuropathic pain management (75, 76). Doxorubicin is a broad-spectrum anthracycline antibiotic with cytotoxic properties that diffuses through the vascular pores of the DRG following paravertebral injection. It then undergoes retrograde axonal transport and accumulates selectively in the nuclei and nucleoli of sensory neurons (77). This accumulation induces chromatin condensation, nuclear membrane damage, and structural alterations in the nuclear pore complex, thereby inhibiting protein synthesis. The ultimate consequence is the necrosis of sensory neurons and Wallerian degeneration of associated nerve fibers, which effectively blocks pain signal transmission (78). Notably, this agent minimally impacts motor nerve roots, enabling the selective treatment of neuropathic pain in targeted areas (79). Regarding safety, doxorubicin therapy is associated with a relatively high incidence of adverse reactions, though severe complications are uncommon. The most frequent adverse effects are local numbness and hypoesthesia, which are primarily caused by ganglion cell necrosis, mild lymphocyte infiltration, and subsequent cellular loss (79). These symptoms are generally well-tolerated and seldom require specific intervention. Despite these findings, the clinical adoption of this therapy is limited by a lack of evidence and ethical considerations. First, the current evidence base is exceedingly limited: only a single-center randomized controlled trial (RCT) has evaluated the efficacy and safety of doxorubicin-mediated DRG destruction for PHN, and its follow-up duration was short. Consequently, the long-term benefits and potential risks must be validated through larger, multicenter studies. Second, as an irreversible, destructive intervention, this procedure causes permanent sensory loss and other irreversible neurological damage, raising significant ethical concerns (80). Currently, clinical practice guidelines for ZAP do not widely recommend such destructive procedures. The absence of robust, long-term safety data further restricts its clinical application scenarios (81).
Beyond pain, ZAP often presents with sleep disturbances, anxiety, depression, and impaired concentration. These symptoms substantially impair health-related quality of life in daily activities, work, and social functioning (82). Prior studies have suggested that minimally invasive central nervous system neuromodulation is more effective than minimally invasive peripheral nerve modulation in reducing pain, improving sleep, and reducing AEs. This supports the use of single-intervention strategies (83, 84). Nevertheless, the comparative effects and safety of different combination therapies have not yet been fully quantified using Bayesian NMA. The present results indicate that minimally invasive central nervous system neuromodulation combined with topical and peripheral chemical interventions, as well as minimally invasive peripheral nerve modulation combined with systemic pharmacological analgesia, yield favorable outcomes consistent with synergistic mechanisms. Neuromodulation inhibits central and peripheral pain signaling. For example, minimally invasive central nervous system neuromodulation activates A-β fibers, which suppresses C-fiber transmission. Conversely, minimally invasive peripheral nerve modulation targets nociceptive fibers (7, 85). Chemical and pharmacological interventions block sodium channels or reduce inflammation (86). This synergy may explain why combination therapies outperform monotherapies, as observed in sleep quality outcomes (87).
In this NMA, minimally invasive central nervous system neuromodulation, medical oxidant therapy plus nerve block, and physical therapy and energy medicine demonstrated favorable safety profiles, with a low incidence of AEs. The AEs reported in the included studies were predominantly mild to moderate and transient. Common reactions included local symptoms (e.g., injection-site pain, numbness, and mild hematoma) and systemic effects (e.g., nausea, dizziness, and somnolence). Although serious complications were uncommon, specific events such as pneumothorax, catheter dislodgement, and transient visual impairment were occasionally observed. Therefore, clinicians should still perform individualized assessments when selecting treatment strategies. According to relevant medical guidelines and clinical experience, this type of interventional therapy is not recommended for patients with puncture-site infections or skin ulcers, severe coagulation disorders, poor cooperation or inability to tolerate the procedure, significant anatomical abnormalities, or known drug hypersensitivity.
This review strictly adhered to the PRISMA and PRISMA-NMA reporting standards. Four major English databases, including Embase and PubMed, were searched thoroughly, and only high-quality RCTs were included. High-quality RCTs have a robust design and implementation, which effectively minimizes bias and ensures evidence homogeneity and strength of inference. Furthermore, this NMA provides a highly comprehensive assessment of ZAP management, covering a wider array of interventions than previous reports. This NMA uniquely synthesizes evidence from various categories, including physical, interventional, and alternative approaches, while examining the efficacy of combination regimens. Thus, it overcomes the limitations of previous studies that focused on isolated treatments.
Some limitations should be acknowledged. First, although key effect modifiers for different outcomes were identified via Bayesian meta-regression and core treatment rankings were maintained, considerable heterogeneity was observed among the included studies. Local inconsistency was detected within certain closed loops, which may affect the reliability of the associated effect size estimates. Second, the CINeMA evaluation revealed that the certainty of the evidence for most comparisons was low or very low, primarily due to imprecision (i.e., wide CrIs) and risks of bias within the original studies (e.g., lack of blinding). Third, the evidence base for key interventions remains limited. For example, data on the efficacy and safety of chemical selective neurolysis were derived from only a single, small-sample RCT. Additionally, the insufficient reporting of patient-important outcomes, such as anxiety, depression, and quality of life, constrained a comprehensive assessment of therapeutic benefits. Fourth, restricting the analysis to English-language publications may introduce language bias and limit the generalizability of the findings. Finally, the Bayesian meta-regression was conducted using aggregate study-level data, which limits the ability to make causal inferences.
4.1 Future prospects
Future research should include more high-quality, multicenter RCTs with long-term follow-up to verify the present findings, while also reporting multidimensional outcomes beyond pain intensity, including sleep quality, psychosocial function, and quality of life. From a methodological perspective, more emphasis should be placed on blinding procedures, such as using blinded outcome assessors and conducting sham-controlled interventions, to mitigate performance and detection biases. From a mechanistic research perspective, future investigations should extend beyond animal models and integrate multi-omics approaches to explore human biochemical markers and network mechanisms. Elucidating the foundational actions of different therapies will concurrently provide deeper evidence of their clinical value. Furthermore, current interventional research predominantly focuses on monotherapies and lacks in-depth exploration of combined and comprehensive treatment strategies. There is an urgent need to conduct RCTs specifically designed for combination regimens. These trials are essential for clarifying the relative efficacy of these regimens, optimizing treatment pathways, and identifying the patient subgroups that are most likely to benefit.
5 Conclusion
Based on the present analysis, minimally invasive central nervous system neuromodulation combined with topical and peripheral chemical interventions, as well as minimally invasive peripheral nerve modulation combined with systemic pharmacological analgesia, are relatively safe and effective options for treating ZAP. These options demonstrate favorable performance in terms of both pain relief and sleep quality improvement. For refractory and intractable ZAP, chemical selective neurolysis (doxorubicin-mediated dorsal root ganglion destruction) may be considered as a potential alternative, though its use requires careful consideration of the limited supporting evidence and lack of long-term safety data, as well as the ethical implications. Due to the low to very low certainty of the overall evidence, study heterogeneity, and short follow-up durations in the included trials, these findings should be interpreted with caution. Clinical practice should prioritize individualized treatment decisions based on patient characteristics and disease severity. Further large-scale, multicenter RCTs with long-term follow-up are warranted to validate the efficacy and safety of these interventions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YH: Conceptualization, Data curation, Methodology, Software, Writing – original draft. XL: Investigation, Visualization, Writing – review & editing. XM: Software, Validation, Writing – review & editing. TS: Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2026.1711536/full#supplementary-material
References
1.
Gershon AA Gershon MD . Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev. (2013) 26:728–43. doi: 10.1128/cmr.00052-13,
2.
Adriaansen EJM Jacobs JG Vernooij LM van Wijck AJM Cohen SP Huygen FJPM et al . 8. Herpes zoster and post herpetic neuralgia. Pain Pract. (2024) 25:e13423. doi: 10.1111/papr.13423,
3.
Jianbo W Koshy E Mengting L Kumar H . Epidemiology, treatment and prevention of herpes zoster: a comprehensive review. Indian J Dermatol Venereol Leprol. (2018) 84:251–62. doi: 10.4103/ijdvl.IJDVL_1021_16,
4.
Lin CS Lin YC Lao HC Chen CC . Interventional treatments for postherpetic neuralgia: a systematic review. Pain Physician. (2019) 22:209–28.
5.
Dworkin RH O'Connor AB Audette J Baron R Gourlay GK Haanpää ML et al . Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. (2010) 85:S3–S14. doi: 10.4065/mcp.2009.0649
6.
Kukkar A Bali A Singh N Jaggi AS . Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res. (2013) 36:237–51. doi: 10.1007/s12272-013-0057-y,
7.
Voute M Morel V Pickering G . Topical lidocaine for chronic pain treatment. Drug Des Devel Ther. (2021) 15:4091–103. doi: 10.2147/dddt.S328228,
8.
Soliman N Moisset X Ferraro MC de Andrade DC Baron R Belton J et al . Pharmacotherapy and non-invasive neuromodulation for neuropathic pain: a systematic review and meta-analysis. Lancet Neurol. (2025) 24:413–28. doi: 10.1016/s1474-4422(25)00068-7,
9.
Wu C-Y Lin H-C Chen S-F Chang W-P Wang C-H Tsai J-C et al . Efficacy of pulsed radiofrequency in herpetic neuralgia. Clin J Pain. (2020) 36:887–95. doi: 10.1097/ajp.0000000000000867,
10.
Liu Z Weng Y Liu F Jiang D Wu C Chen Y et al . Efficacy and safety of short-term spinal cord stimulation and pulsed radiofrequency in the treatment of postherpetic neuralgia: a meta-analysis. Front Neurol. (2025) 16:1586995. doi: 10.3389/fneur.2025.1586995,
11.
Dworkin RH O’Connor AB Kent J Mackey SC Raja SN Stacey BR et al . Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. (2013) 154:2249–61. doi: 10.1016/j.pain.2013.06.004,
12.
Song Y Yu Z Guan J Wu H Zhang J Qiaoling L et al . Efficacy of high-voltage pulsed radiofrequency in zoster-associated pain: a meta-analysis and systematic review. Anesthesiol Res Pract. (2023) 2023:1–11. doi: 10.1155/2023/8479293,
13.
Jiang X Li Y Chen N Zhou M He L . Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. (2023) 12:CD005582. doi: 10.1002/14651858.CD005582.pub5,
14.
Lu Y Liu K Liang Y Zhang X Liu Y Huang F et al . Should we prescribe anticonvulsants for acute herpes zoster neuralgia and to prevent postherpetic neuralgia?Medicine. (2021) 100:e24343. doi: 10.1097/md.0000000000024343,
15.
Li Z She Y Luo Z Liu Z Pei W Zeng J et al . Efficacy of thermotherapy for herpes zoster and postherpetic neuralgia. Medicine. (2021) 100:e23823. doi: 10.1097/md.0000000000023823,
16.
Liu Y Xiao S Li J Long X Zhang Y Li X . A network meta-analysis of randomized clinical trials to assess the efficacy and safety of antiviral agents for immunocompetent patients with herpes zoster-associated pain. Pain Physician. (2023) 26:337–46.
17.
Jansen J Naci H . Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. (2013) 11:159. doi: 10.1186/1741-7015-11-159,
18.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71,
19.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366. doi: 10.1136/bmj.l4898
20.
van Valkenhoef G Lu G de Brock B Hillege H Ades AE Welton NJ . Automating network meta-analysis. Res Synth Methods. (2012) 3:285–99. doi: 10.1002/jrsm.1054
21.
Liu Y Béliveau A Wei Y Chen MY Record-Lemon R Kuo P-L et al . A gentle introduction to Bayesian network meta-analysis using an automated R package. Multivar Behav Res. (2022) 58:706–22. doi: 10.1080/00273171.2022.2115965,
22.
Zheng C Xie Y Li H Zhang B Chen S Han W et al . Effectiveness and safety of stellate ganglion block with trioxygen autologous blood retransfusion therapy for facial postherpetic neuralgia in elderly patients. Sci Rep. (2025) 15:8025. doi: 10.1038/s41598-025-91847-7,
23.
Wu Y Gan M Wu J . Effect and immune mechanism of BCG-PSN on Postherpetic neuralgia: a single-masked, randomised controlled trial. J Coll Physicians Surg Pak. (2025) 35:590–5. doi: 10.29271/jcpsp.2025.05.590,
24.
Wu C Zhou Q Zhang Y Ren C Ou C . Comparison of two different neuromodulation treatments in patients with acute zoster-related trigeminal neuropathic pain and pain catastrophizing. Neuromodulation. (2025) 28:567–74. doi: 10.1016/j.neurom.2025.01.010,
25.
Li Y Wang J Chen Y Qiu F Sun T Zhao X . Comparative long-term efficacy of short-term spinal cord stimulation versus bipolar pulsed radiofrequency for refractory postherpetic neuralgia: a 24 month prospective study. Eur J Med Res. (2025) 30:272. doi: 10.1186/s40001-025-02560-0,
26.
Li Y Hao C Wang S Qiu F Zhao X Sun T . Temporary spinal cord stimulation combined with lidocaine patch for postherpetic neuralgia in the elderly: a controlled study. Front Neurol. (2025) 16:1529673. doi: 10.3389/fneur.2025.1529673,
27.
Zhou Z Hu X Yan F Zhou Y He R Ye X et al . Observation on the effect of platelet-rich plasma combined with drugs in the treatment of herpes zoster neuralgia. Int J Neurosci. (2024) 134:628–34. doi: 10.1080/00207454.2022.2138381,
28.
Wang R Xia Z Ma Y Huang B Yao M Ma L . Computed tomography-guided dorsal root ganglion ozone injection combined with pulsed radiofrequency for acute herpes zoster neuralgia treatment of middle-aged and elderly people. Clin J Pain. (2024) 40:469–77. doi: 10.1097/ajp.0000000000001226
29.
Patil A Vyshnavi S Raja T Shastry V Thammaiah SH Archana KN . A randomized clinical trial comparing the efficacy of ultrasound-guided erector spinae block and paravertebral block in preventing postherpetic neuralgia in patients with zoster-associated pain. J Anaesthesiol Clin Pharmacol. (2024) 40:510–5. doi: 10.4103/joacp.joacp_82_23,
30.
Liu Y Yan H Wan C Xi Q Huang M Wang Y et al . Short-term supraorbital nerve stimulation and pain relief for acute and subacute ophthalmic herpetic neuralgia: a randomized controlled crossover trial. Pain Physician. (2024) 27:203–12.
31.
Lin S Lin M Wang F Zhuo Y Lin K Wang J . Novel ultrasound-guided cervical sympathetic chain pulsed radiofrequency for subacute herpes zoster neuralgia. J Pain Res. (2024) 17:3627–37. doi: 10.2147/jpr.S470758,
32.
Zhang Z-W Zhao Y Du T-Y Zhang J Wu Q Wang Z-Y . A clinical study of C arm-guided selective spinal nerve block combined with low-temperature plasma radiofrequency ablation of dorsal root ganglion in the treatment of zoster-related neuralgia. Front Neurol. (2023) 14:1122538. doi: 10.3389/fneur.2023.1122538,
33.
Sun H Yu Z . Effect of extracorporeal shock wave combined with pregabalin on patients with post-herpetic neuralgia. Medicine. (2023) 102:e34361. doi: 10.1097/md.0000000000034361
34.
Sollie M Thomsen JB Sorensen JA . Autologous fat grafting is not superior to placebo as treatment of postherpetic neuralgia: a double-blind randomized clinical trial. Plast Reconstr Surg. (2023) 152:1053e–62e. doi: 10.1097/PRS.0000000000010462
35.
Lin S Lin M Dai Z Wang F Lin K Liu R . Novel bipolar high-voltage pulsed radiofrequency targeting the cervical sympathetic chain for treating acute herpetic neuralgia. Neuromodulation. (2023) 26:1808–16. doi: 10.1016/j.neurom.2021.12.003,
36.
Zhang W He C . Clinical efficacy of pulsed radiofrequency combined with intravenous lidocaine infusion in the treatment of subacute herpes zoster neuralgia. Pain Res Manag. (2022) 2022:5299753. doi: 10.1155/2022/5299753,
37.
Wang C Yuan F Cai L Lu H Chen G Zhou J . Ultrasound-guided stellate ganglion block combined with extracorporeal shock wave therapy on postherpetic neuralgia. J Healthc Eng. (2022) 2022:9808994. doi: 10.1155/2022/9808994,
38.
Sheng L Liu Z Zhou W Li X Wang X Gong Q . Short-term spinal cord stimulation or pulsed radiofrequency for elderly patients with postherpetic neuralgia: a prospective randomized controlled trial. Neural Plast. (2022) 2022:7055697. doi: 10.1155/2022/7055697,
39.
Li X Chen P He J Huang X Tang D Chen L et al . Comparison of the efficacy and safety of temporary spinal cord stimulation versus pulsed radiofrequency for postherpetic neuralgia: a prospective randomized controlled trial. Pain Res Manag. (2022) 2022. doi: 10.1155/2022/3880424,
40.
Ji M Yao P Han Z Zhu D . Pulsed radiofrequency combined with methylene blue paravertebral nerve block effectively treats thoracic postherpetic neuralgia. Front Neurol. (2022) 13:811298. doi: 10.3389/fneur.2022.811298,
41.
Eid MM Hamed NS Abdelbasset WK Elkholi SM Eladl HM El-Deen HAB . A comparative study between transcutaneous electrical nerve stimulation and pulsed electromagnetic field therapy in the management of post-herpetic neuralgia of the sciatic nerve. Medicine. (2022) 101:e31433. doi: 10.1097/md.0000000000031433,
42.
Chen L Zhang Y Chen Y Wang T Sun K Tang H et al . Efficacy and safety of botulinum toxin a and pulsed radiofrequency on postherpetic neuralgia: a randomized clinical trial. Contrast Media Mol Imaging. (2022) 2022:1579937. doi: 10.1155/2022/1579937,
43.
Chen L Qing A Zhu T Yang P Ye L . Effect and safety of extracorporeal shockwave therapy for postherpetic neuralgia: a randomized single-blind clinical study. Front Neurol. (2022) 13:948024. doi: 10.3389/fneur.2022.948024,
44.
Abdelwahab EH Hodeib AA Marof HM Fattooh NH Afandy ME . Ultrasound-guided erector spinae block versus ultrasound-guided thoracic paravertebral block for pain relief in patients with acute thoracic herpes zoster: a randomized controlled trial. Pain Physician. (2022) 25:E977–85.
45.
Wan CF Song T . Efficacy of pulsed radiofrequency or short-term spinal cord stimulation for acute/subacute zoster-related pain: a randomized, double-blinded, controlled trial. Pain Physician. (2021) 24:215–22.
46.
Saxena AK Singh A Chilkoti GT Sharma T Banerjee BD Das S et al . Modulation of signal transduction gene expression following pulsed radiofrequency in dorsal root ganglia and pregabalin therapy. Pain Manag. (2021) 12:347–56. doi: 10.2217/pmt-2021-0057,
47.
Liu J Zhang A Ye X Hu X He R Jiang Z . The effect of short-term spinal cord electrical stimulation on patients with postherpetic neuralgia and its effect on sleep quality. Neuro Endocrinol Lett. (2021) 42:81–6.
48.
Huang Y Xu C Zeng T Li Z Xia Y Tao G et al . Intravenous patient-controlled analgesia hydromorphone combined with pregabalin for the treatment of postherpetic neuralgia: a multicenter, randomized controlled study. Korean J Pain. (2021) 34:210–6. doi: 10.3344/kjp.2021.34.2.210,
49.
El-Sayed MA Zanaty O Shafshak W Abdelmaksoud R Gamal Eldine H . Role of ultrasound guided erector spinae plane block in management of acute herpes zoster pain and incidence of post-herpetic neuralgia. Egypt J Anaesth. (2021) 37:343–8. doi: 10.1080/11101849.2021.1955517
50.
Dong X Liu Y Yang Q Liu Z Zhang Z . Comparison of therapeutic effects of continuous epidural nerve block combined with drugs on postherpetic neuralgia. Int J Neurosci. (2021) 131:191–5. doi: 10.1080/00207454.2020.1736583,
51.
Xiong Z-H Tang X-F Huang L-T Yue L-R . A clinical study on the treatment of postherpetic neuralgia with pulsed radiofrequency of the dorsal root ganglion with pain management. Neurol Asia. (2020) 25:313–7.
52.
Liu B Yang Y Zhang Z Wang H Fan B Sima L . Clinical study of spinal cord stimulation and pulsed radiofrequency for management of herpes zoster-related pain persisting beyond acute phase in elderly patients. Pain Physician. (2020) 23:263–70.
53.
Zheng S Li X Yang X He L Xue Y Yang Z . Ultrasound-guided cervical nerve root block for the treatment of acute cervical herpes zoster: a randomized controlled clinical study. Pain Pract. (2019) 19:500–9. doi: 10.1111/papr.12770,
54.
Zhao P Mei L . A clinical study of paraspinal nerve block on treatment of herpes zoster under ultrasonic guidance. Neuro-Chirurgie. (2019) 65:382–6. doi: 10.1016/j.neuchi.2019.06.007,
55.
Wan C Dong D-s Song T . High-voltage, long-duration pulsed radiofrequency on Gasserian ganglion improves acute/subacute zoster-related trigeminal neuralgia: a randomized, double-blinded, controlled trial. Pain Physician. (2019) 22:361–8.
56.
Pei Q Wu B Tang Y Yang X Song L Wang N et al . Repetitive transcranial magnetic stimulation at different frequencies for postherpetic neuralgia: a double-blind, sham-controlled, randomized trial. Pain Physician. (2019) 22:E303–13.
57.
Zhang J Zhang L Peng Y Jin Y Lin H . A comparison of analgesic effects between intradermal injection and epidural block of lidocaine for postherpetic neuralgia. Int J Clin Exp Med. (2018) 11:9694.
58.
Makharita MY El Bendary HM Sonbul ZM Ahmed SES Latif MA . Ultrasound-guided pulsed radiofrequency in the management of thoracic postherpetic neuralgia: a randomized, double-blinded, controlled trial. Clin J Pain. (2018) 34:1017–24. doi: 10.1097/ajp.0000000000000629,
59.
Li D Sun G Sun H Wang Y Wang Z Yang J . Combined therapy of pulsed radiofrequency and nerve block in postherpetic neuralgia patients: a randomized clinical trial. PeerJ. (2018) 6:e4852. doi: 10.7717/peerj.4852,
60.
Hu B Zheng J Liu Q Yang Y Zhang Y . The effect and safety of ozone autohemotherapy combined with pharmacological therapy in postherpetic neuralgia. J Pain Res. (2018) 11:1637–43. doi: 10.2147/jpr.S154154,
61.
Cui J-z Zhang J-w Yan F Yang X-n Wang X-l Zhao Z-b et al . Effect of single intra-cutaneous injection for acute thoracic herpes zoster and incidence of postherpetic neuralgia. Pain Manag Nurs. (2018) 19:186–94. doi: 10.1016/j.pmn.2017.09.002,
62.
Ni J Wang X Tang Y Yang L Zeng Y Guo Y . Subcutaneous injection of triamcinolone and lidocaine to prevent postherpetic neuralgia. Pain Physician. (2017) 20:397–403.
63.
Cui JZ Zhang XB Zhu P Zhao ZB Geng ZS Zhang YH et al . Effect of repetitive Intracutaneous injections with local anesthetics and steroids for acute thoracic herpes zoster and incidence of Postherpetic neuralgia. Pain Med. (2017) 18:1566–72. doi: 10.1093/pm/pnw190,
64.
Saxena AK Lakshman K Sharma T Gupta N Banerjee BD Singal A . Modulation of serum BDNF levels in postherpetic neuralgia following pulsed radiofrequency of intercostal nerve and pregabalin. Pain Manag. (2016) 6:217–27. doi: 10.2217/pmt.16.3,
65.
Meize-Grochowski R Shuster G Boursaw B DuVal M Murray-Krezan C Schrader R et al . Mindfulness meditation in older adults with postherpetic neuralgia: a randomized controlled pilot study. Geriatr Nurs. (2015) 36:154–60. doi: 10.1016/j.gerinurse.2015.02.012,
66.
Makharita MY Amr YM El-Bayoumy Y . Single paravertebral injection for acute thoracic herpes zoster: a randomized controlled trial. Pain Pract. (2015) 15:229–35. doi: 10.1111/papr.12179,
67.
Xu G Lv ZW Feng Y Tang WZ Xu GX . A single-center randomized controlled trial of local methylcobalamin injection for subacute herpetic neuralgia. Pain Med. (2013) 14:884–94. doi: 10.1111/pme.12081,
68.
Park KY Han TY Kim IS Yeo IK Kim BJ Kim MN . The effects of 830 nm light-emitting diode therapy on acute herpes zoster ophthalmicus: a pilot study. Ann Dermatol. (2013) 25:163–7. doi: 10.5021/ad.2013.25.2.163,
69.
Ma K Fan Y Jin Y Huang X Liu X Cheng Z et al . Efficacy of pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia from the angulus costae: a randomized, double-blinded, controlled trial. Pain Physician. (2013) 16:15–25.
70.
Apalla Z Sotiriou E Lallas A Lazaridou E Ioannides D . Botulinum toxin a in postherpetic neuralgia: a parallel, randomized, double-blind, single-dose, placebo-controlled trial. Clin J Pain. (2013) 29:857–64. doi: 10.1097/AJP.0b013e31827a72d2,
71.
Makharita MY Amr YM El-Bayoumy Y . Effect of early stellate ganglion blockade for facial pain from acute herpes zoster and incidence of postherpetic neuralgia. Pain Physician. (2012) 15:467–74.
72.
He C-j Luo Y-r Nie H-x . Effects of dorsal root ganglion destruction by adriamycin in patients with postherpetic neuralgia. Acta Cir Bras. (2012) 27:404–9. doi: 10.1590/s0102-86502012000600008,
73.
Ji G Niu J Shi Y Hou L Lu Y Xiong L . The effectiveness of repetitive paravertebral injections with local anesthetics and steroids for the prevention of postherpetic neuralgia in patients with acute herpes zoster. Anesth Analg. (2009) 109:1651–5. doi: 10.1213/ANE.0b013e3181b79075,
74.
van Wijck AJ Opstelten W Moons KG van Essen GA Stolker RJ Kalkman CJ et al . The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: a randomised controlled trial. Lancet. (2006) 367:219–24. doi: 10.1016/S0140-6736(06)68032-X,
75.
Zheng B Song L Liu H . Gasserian ganglion injected with Adriamycin successfully relieves intractable trigeminal nerve postherpetic neuralgia for an elderly patient. Medicine. (2018) 97:e12388. doi: 10.1097/md.0000000000012388,
76.
Lu F Zhong J Liu H Xiao H . CT-guided paravertebral injection of doxorubicin for treatment of postherpetic neuralgia: a database-based retrospective stratified study. Front Neurol. (2023) 14:1258464. doi: 10.3389/fneur.2023.1258464,
77.
England JD Asbury AK Rhee EK Sumner AJ . Lethal retrograde axoplasmic transport of doxorubicin (adriamycin) to motor neurons. A toxic motor neuronopathy. Brain. (1988) 111:915–26. doi: 10.1093/brain/111.4.915,
78.
Xie G-l Guo D-p Li Z-g Liu C Zhang W . Application of radiofrequency thermocoagulation combined with adriamycin injection in dorsal root ganglia for controlling refractory pain induced by rib metastasis of lung cancer (a STROBE-compliant article). Medicine. (2016) 95:e4785. doi: 10.1097/md.0000000000004785
79.
Kamińska K Cudnoch-Jędrzejewska A . A review on the neurotoxic effects of doxorubicin. Neurotox Res. (2023) 41:383–97. doi: 10.1007/s12640-023-00652-5,
80.
Cohen SP Mao J . Neuropathic pain: mechanisms and their clinical implications. BMJ. (2014) 348:f7656. doi: 10.1136/bmj.f7656
81.
Deer TR Pope JE Lamer TJ Grider JS Provenzano D Lubenow TR et al . The neuromodulation appropriateness consensus committee on best practices for dorsal root ganglion stimulation. Neuromodulation. (2019) 22:1–35. doi: 10.1111/ner.12845,
82.
Van Oorschot D McGirr A Goulet P Koochaki P Pratiwadi R Shah S et al . A cross-sectional concept elicitation study to understand the impact of herpes zoster on patients’ health-related quality of life. Infect Dis Ther. (2022) 11:501–16. doi: 10.1007/s40121-021-00581-w,
83.
Xue S Yang W-j Cao Z-x Sun T . Comparing the efficacy and safety of short-term spinal cord stimulation and pulsed radiofrequency for zoster-related pain. Medicine. (2022) 101:e29073. doi: 10.1097/md.0000000000029073,
84.
Feng F Long Z Qiu L Zhang Y Ren C Ou C et al . Comparative efficacy and safety of pulsed radiofrequency versus spinal cord stimulation in thoracic herpes zoster pain: a retrospective study. Sci Rep. (2025) 15:23280. doi: 10.1038/s41598-025-06289-y,
85.
Joosten EA Franken G . Spinal cord stimulation in chronic neuropathic pain: mechanisms of action, new locations, new paradigms. Pain. (2020) 161:S104–13. doi: 10.1097/j.pain.0000000000001854,
86.
Finnerup NB Kuner R Jensen TS . Neuropathic pain: from mechanisms to treatment. Physiol Rev. (2021) 101:259–301. doi: 10.1152/physrev.00045.2019,
87.
Dağıstan G Erdine S Valeriani M . Efficacy and safety of pulsed radiofrequency of dorsal root ganglion in elderly patient population with acute and subacute zoster-related pain. Pain Res Manag. (2024) 2024:6586167. doi: 10.1155/2024/6586167,
Summary
Keywords
chemical selective neurolysis, combination therapy, dorsal root ganglion destruction, minimally invasive neuromodulation, network meta-analysis, non-oral therapeutic interventions, zoster-associated pain
Citation
Hao Y, Liu X, Ma X and Sun T (2026) Comparison of efficacy and safety of non-oral therapeutic interventions for zoster-associated pain: a systematic review and network meta-analysis. Front. Neurol. 17:1711536. doi: 10.3389/fneur.2026.1711536
Received
23 September 2025
Revised
04 December 2025
Accepted
05 January 2026
Published
27 January 2026
Volume
17 - 2026
Edited by
Marcelo M. Valença, Federal University of Pernambuco, Brazil
Reviewed by
Phillip R. Kramer, Texas A&M University, United States
Junpeng Yuan, The First Affiliated Hospital of Soochow University, China
Vahid Mohabbati, University of New South Wales, Australia
Updates
Copyright
© 2026 Hao, Liu, Ma and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Sun, suntaosdph@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.