Abstract
Background:
Traditional Chinese medicine (TCM) has been widely applied in the management of perimenopausal insomnia (PMI), yet comprehensive evidence on its efficacy and safety remains insufficient. We aimed to systematically evaluate the therapeutic effects and safety of TCM interventions in PMI treatment.
Methods:
Randomized controlled trials (RCTs) that compared TCM interventions with Western medicine for patients with PMI were searched across eight databases from inception to August 1, 2025. Methodological quality, risk of bias, and certainty of evidence were assessed using the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines and Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. Meta-analysis was performed using RevMan 5.4 and R 4.4.2 software.
Results:
A total of 48 RCTs involving 5,037 patients were included. Meta-analysis showed that TCM was superior to Western medicine in overall efficacy (RR = 1.20, 95% CI [1.17, 1.23]) and had fewer adverse reactions (RR = 0.30, 95% CI [0.24, 0.38]). Besides, TCM interventions significantly improved Pittsburgh Sleep Quality Index (PSQI) score (MD = −2.57, 95% CI [−3.01, −2.14]), reduced luteinizing hormone (LH) (MD = −4.51, 95% CI [−6.15, −2.87]) and follicle-stimulating hormone (FSH) (MD = −8.67, 95% CI [−10.96, −6.38]), increased estradiol (E2) level (MD = 9.64, 95% CI [7.45, 11.82]), and decreased Kupperman Menopausal Index (KMI) score (MD = −6.01, 95% CI [−8.56, −3.47]), Traditional Chinese Medicine Syndrome (TCMS) score (SMD = −2.27, 95% CI [−3.49, −1.05]), Self-Rating Anxiety Scale (SAS) score (MD = −4.77, 95% CI [−5.77, −3.76]), and Self-Rating Depression Scale (SDS) score (MD = −2.96, 95% CI [−5.80, −0.12]).
Conclusion:
TCM interventions demonstrate notable efficacy and safety in managing PMI by improving sleep quality, hormonal balance, and mental health. However, methodological limitations and heterogeneity warrant further validation through large-scale, multicenter, rigorously designed RCTs.
Systematic review registration:
PROSPERO registration number CRD420251127129.
1 Introduction
Perimenopause, the transitional phase from the reproductive period to menopause, is marked by fluctuations in sex hormone levels caused by declining ovarian function and is often accompanied by various physiological and psychological disturbances. Among these disturbances, the prevalence of insomnia is reported to be as high as 40–60% (1, 2). Perimenopausal insomnia (PMI) is mainly characterized by difficulty falling asleep, sleep maintenance disorders, early awakening, and impaired daytime function. Persistent insomnia not only reduces quality of life but may also exacerbate emotional disorders such as anxiety and depression and increase the risk of comorbidities, including cardiovascular disease and metabolic syndrome. Consequently, PMI has emerged as a significant public health issue affecting women’s health (3, 4).
Currently, the treatment of PMI primarily relies on hormone replacement therapy (HRT) and sedative-hypnotic medications, including benzodiazepines and non-benzodiazepines (5, 6). Although these therapies demonstrate clear short-term efficacy, their long-term use may cause adverse effects such as breast tenderness, endometrial hyperplasia, hepatic and renal impairment, and drug dependence. Additionally, some patients show low compliance due to contraindications to hormone use or concerns about drug adverse reactions (7). Therefore, exploring safe, effective, and patient-acceptable alternative therapies has become an important focus of clinical research.
Traditional Chinese Medicine (TCM) has a long history in the treatment of PMI. Clinically, doctors will adopt the principles of syndrome differentiation to select different TCM interventions including classical prescriptions, proprietary formulations, and acupuncture (8, 9). In recent years, several studies have shown that classic TCM prescriptions such as Chaihu Jia Longgu Muli Decoction, Huanglian Ejiao Decoction, and Suanzaoren Decoction have shown positive effects in alleviating insomnia symptoms and regulating sex hormone levels (10–12). However, existing studies vary in sample size, apply inconsistent outcome evaluation criteria, and yield controversial findings. Therefore, this study aims to evaluate the effectiveness and safety of TCM interventions in the treatment of PMI through systematic review and Meta-analysis, so as to provide high-level evidence-based basis for clinical decision-making.
2 Methods
This systematic review and Meta-analysis were conducted in strict accordance with the latest PRISMA guidelines. A complete scheme for this study was registered in the Prospective Register of Systematic Reviews (PROSPERO): no. CRD420251127129.
2.1 Search strategy and study selection
Systematic searches were conducted in Chinese and English databases to obtain randomized controlled trials related to the treatment of PMI with TCM interventions. Chinese databases included China National Knowledge Infrastructure (CNKI), China Biomedical Literature Database (CBM), CQVIP database, and Wanfang database. English databases included PubMed, Web of Science, Embase, and Cochrane Library. The search time span was limited from the establishment of each database to August 1, 2025. The search terms and keywords included “traditional Chinese medicine”, “perimenopause”, “menopausal”, “insomnia”, “sleep disorder”, “sleeplessness”, “RCTs”, etc. Some of the search strategies are documented in Supplementary Table 1.
After excluding duplicate studies, two researchers excluded studies that were not related to the treatment of PMI with TCM by reading the titles and abstracts. Then, the full text of the selected studies was further read, and the final included studies were determined with reference to the inclusion and exclusion criteria. If the two researchers had inconsistent opinions on study selection, it would be discussed and decided by the third researcher.
2.2 Inclusion and exclusion criteria
Inclusion criteria: (a) included studies were designed as randomized controlled trials (RCTs); (b) the language of studies was restricted to Chinese and English; (c) participants were Chinese individuals diagnosed with PMI, regardless of their ethnicity or disease duration; (d) the experimental group received TCM interventions, which could be administered as a single TCM therapy, a combination of multiple TCMs, TCM combined with Western medicine, or TCM combined with acupuncture; (e) the control group was required to receive conventional Western medicine therapy for PMI; (f) outcome measures included at least one of the primary outcomes and one secondary outcomes. Specifically, primary outcome measures of interests were overall efficiency and adverse reactions, and secondary outcome measures of interests were Pittsburgh Sleep Quality Index (PSQI), Estradiol (E2), Follicle-stimulating hormone (FSH), Luteinizing hormone (LH), Kupperman Menopausal Index (KMI), Traditional Chinese Medicine Syndrome (TCMS) score, Self-Rating Anxiety Scale (SAS), and Self-Rating Depression Scale (SDS).
Studies were excluded if it met the following criteria: (a) studies that do not match the target literature type, such as reviews, animal experiments, trials comparing different dosages of TCM, or non-randomized controlled trials; (b) studies with inconsistent research diseases or medication methods; (c) literature with incomplete data records, insufficient outcome measures, or inaccessible data extraction; (d) duplicate publications; (e) studies where the control group used interventions such as TCM, acupuncture, or other TCM interventions.
2.3 Data extraction and risk of bias assessment
Two independent reviewers used the Cochrane Risk of Bias Tool (Version 2.0) to screen and evaluate the quality of the included studies respectively, and simultaneously collected and verified the relevant outcome measures data. In case of disagreements between the two researchers, a third researcher would participate in the discussion to reach a consensus ultimately. The quality assessment criteria included seven items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias, with three evaluation levels: high risk, unclear risk, and low risk. Specifically, the judgment of “low risk” required the included studies to provide explicit and detailed descriptions of the corresponding methodological design without any identified flaws; “unclear risk” was assigned when the study report lacked sufficient information to permit a definitive judgment; and “high risk” was defined when the study design or implementation had obvious defects that could lead to significant bias (13).
2.4 Statistical analysis
Data analysis was performed using RevMan 5.4 software. For continuous variables: The mean difference (MD) was selected as the effect measure for continuous variables with uniform measurement units; The standard mean difference (SMD) was used as the effect measure for continuous variables with non-uniform measurement units, continuous variables measured by different scales, or continuous variables with a numerical difference greater than 10 times between different studies. For dichotomous variables, the Risk Ratio (RR) and its 95% Confidence Interval (CI) were used for evaluation (14, 15).
Heterogeneity testing was conducted for the included studies. If the heterogeneity was low (p > 0.10 and I2 ≤ 50%), a fixed-effects model was adopted for analysis; otherwise, a random-effects model was used for analysis. Subgroup analysis was performed based on differences of interventions. When an outcome measure included more than 10 studies, funnel plots were constructed and Egger’s test and Peter’s test were performed by R 4.4.2 software to assess potential publication bias (16, 17). When necessary, sensitivity analysis and regression analysis were conducted to analyze the sources of heterogeneity. p < 0.05 indicates that the difference is statistically significant.
3 Results
3.1 Study selection
In this study, a total of 1,418 articles were initially retrieved from 8 Chinese and English databases. After excluding duplicate articles, 662 articles remained. Based on the inclusion and exclusion criteria and the completeness of literature information, 48 articles were finally included for Meta-analysis. The literature screening process and results were shown in Figure 1.
Figure 1

Flow diagram of the literature search and study selection process.
3.2 Characteristics of included studies
This study summarized 48 clinical randomized controlled trials from mainland China, covering 17 provinces or autonomous regions. Among them, Henan Province (12/48), Zhejiang Province (8/48), and Beijing (6/48) were the most common. A total of 5,037 patients participated in these studies, with 2,547 in the experimental group and 2,490 in the control group. The sample size of the included studies ranged from 37 to 820. In addition, considering that 3 articles (18–20) contained multiple experimental groups or control groups, according to the regulations in the Cochrane Handbook for Systematic Reviews of Interventions (21), these studies were split into Study a and Study b.
A total of 42 TCM prescriptions or patent medicines for the treatment of PMI were included. Among them, Chaihu Jia Longgu Muli Decoction (4/42) and Huanglian Ejiao Decoction (4/42) were the most frequently used TCM interventions. Based on the different types of treatments, the study set up three subgroups for analysis: (1) The TCM group (TCM), where the experimental group used TCM alone. (2) The TCM-Western medicine group (TCM-WM), where the experimental group used TCM combined with Western medicine. (3) The TCM-Acupuncture group (TCM-A), where the experimental group used TCM combined with acupuncture. Therefore, subsequent subgroup analyses will be conducted according to these three intervention types. In the control groups of the included studies, all patients with PMI received conventional Western medical treatments, such as HRT or benzodiazepine sedative-hypnotics, among which estazolam and zopiclone were the two most commonly used medications. Additionally, the treatment duration of most studies ranged from 4 to 12 weeks. For detailed information on the basic characteristics of the included studies (please refer to Table 1).
Table 1
| Study ID | Region | Experimental treatment | Control treatment | Sample size (E/C) | Age (years, E/C) | Outcome measures | Duration |
|---|---|---|---|---|---|---|---|
| Chen et al. (12) | Zhejiang | Jiawei Suanzaoren Decoction | Lorazepam | 40/38 | 48.95 ± 2.45/47.82 ± 2.87 | ①②③ | 4 weeks |
| Qiao (18) | Henan | Kuntai Capsule | Femara | 30/30 | 47.9 ± 2.3/47.0 ± 2.4 | ①②③④⑤⑥⑩ | 12 weeks |
| Liu et al. (52) | Beijing | Chaihu Guizhi Longgu Muli Decoction combined with Ganmai Dazao Decoction | Estazolam | 68/68 | 51 ± 4/50 ± 4 | ①②③⑦⑧⑨⑩ | 4 weeks |
| Yao et al. (53) | Zhejiang | Zishen Jieyu Ningxin Formula | Estazolam | 60/53 | 48.5 ± 5.2/49.0 ± 6.0 | ①②③④⑤⑥⑧ | 8 weeks |
| Sun et al. (54) | Sichuan | Suanzaoren Decoction combined with Xiaoyao San | Alprazolam | 30/30 | 48.97 ± 2.83/49.07 ± 2.07 | ①②③⑥ | 4 weeks |
| Zhang et al. (55) | Beijing | Liandi Jiaotai Decoction | Lorazepam | 30/30 | 51.9 ± 2.4/52.3 ± 2.5 | ①②③⑦⑩ | 8 weeks |
| Zhu and Wang (25) | Anhui | Buxin Xiaoyao Yin | Zopiclone | 31/31 | 48.6 ± 4.9/48.3 ± 4.8 | ①②③ | 4 weeks |
| Du et al. (19) | Tianjin | Jiawei Wumei Wan | Estazolam | 41/41 | 49.76 ± 3.05/50.45 ± 3.19 | ②③⑤⑥⑦ | 4 weeks |
| Wang and Ma (26) | Beijing | Liuwei Dihuang Tang combined with Xiaoyao San | Sex hormones | 30/30 | 48.87 ± 3.85/48.33 ± 3.35 | ①③⑤⑥⑦⑧ | 3 months |
| Xiao and Niu (56) | Henan | Chaihu Jia Longgu Muli Decoction | Zopiclone and Paroxetine Hydrochloride Tablets | 410/410 | 52.10 ± 2.01/51.82 ± 2.13 | ①②③⑧ | 4 weeks |
| Mao (27) | Zhejiang | Huanglian Ejiao Decoction | Alprazolam | 45/45 | 50.82 ± 3.26/50.30 ± 3.41 | ①④⑤⑥ | 1 month |
| Mo et al. (28) | Henan | Chai Shao Yangxue Jieyu Decoction | Estazolam | 45/44 | 49.43 ± 3.02/49.56 ± 3.19 | ①④⑤⑥ | 12 weeks |
| Xie (57) | Jiangsu | Chaihu Guizhi Longgu Muli Decoction | Estazolam | 30/30 | 52.1 ± 4.2/51.5 ± 3.3 | ①③⑦⑧⑨⑩ | 4 weeks |
| Jia et al. (29) | Beijing | Gengxin Decoction | Zopiclone | 20/17 | 47.65 ± 3.83/49.71 ± 3.84 | ①③⑦⑧ | 4 weeks |
| Qian et al. (58) | Zhejiang | Danzhi Xiaoyao Wan combined with Kuntai Capsule | Estazolam | 120/80 | 45.16 ± 3.86/46.07 ± 2.89 | ①②③⑦ | 30 days |
| Chen et al. (20) | Hubei | Huanglian Ejiao Decoction | Alprazolam | 28/30 | 46.07 ± 3.50/47.03 ± 3.84 | ①④⑤⑥ | 30 days |
| Qi and Kang (30) | Zhejiang | Zishen Ningshen Decoction | Estazolam and oryzanol | 48/47 | 49.4 ± 4.1/49.6 ± 4.3 | ①②③④⑤⑥⑦⑧ | 8 weeks |
| Qiao et al. (31) | Heilongjiang | Tianwang Buxin Dan combined with Jiaotai Wan and conventional Western medicine | Estazolam combined with Sex hormones | 29/29 | 51.00 ± 2.79/49.63 ± 3.35 | ①②③④⑤⑥ | 4 weeks |
| Qiao (18) | Henan | Kuntai Capsule combined with Femara | Femara | 30/30 | 48.0 ± 2.6/47.0 ± 2.4 | ①②③④⑤⑥⑩ | 12 weeks |
| Wu et al. (23) | Jiangxi | Xiangshao Granules combined with Paroxetine | Paroxetine | 35/35 | 46.32 ± 2.74/45.12 ± 2.34 | ①④⑤⑥ | 8 weeks |
| Zhou et al. (32) | Jiangsu | Ziyin Anshen Gao combined with Zopiclone | Zopiclone | 30/29 | 49.38 ± 2.19/49.37 ± 2.17 | ①③④⑤⑥ | 1 month |
| Zhang et al. (33) | Fujian | Songyu Yinxu Formula combined with Estazolam | Estazolam | 58/58 | 51(48,53)/50(47,53) | ②③④⑤⑥⑨⑩ | 4 weeks |
| Zhang et al. (59) | Zhejiang | Erxian Tang combined with Suanzaoren Decoction and conventional Western medicine | Estazolam combined with Femara | 75/75 | 49.5 ± 2.7/50.1 ± 2.7 | ①③④⑤⑥ | 4 weeks |
| Li et al. (34) | Hubei | Banxia Xiexin Decoction combined with Zopiclone | Zopiclone | 73/72 | 50.42 ± 2.34/50.03 ± 2.01 | ①②③⑤ | 4 weeks |
| Li (60) | Henan | Zixin Yangshen Decoction combined with Estazolam | Estazolam | 49/49 | 48.77 ± 4.21/49.28 ± 4.57 | ①②③⑦ | 30 days |
| Du et al. (35) | Shaanxi | Roukou Wuwei Wan combined with Trazodone Hydrochloride | Paroxetine | 60/60 | 46.70 ± 3.74/46.48 ± 3.70 | ①③⑤⑥ | 8 weeks |
| Lin et al. (36) | Fujian | Zishen Shugan Anshen Decoction combined with Estazolam | Estazolam | 32/32 | 49.16 ± 2.07/49.06 ± 2.26 | ①②③⑧ | 4 weeks |
| Liang (37) | Shanghai | Wuling Capsule combined with Zopiclone | Zopiclone | 40/40 | 50.94 ± 5.22/50.82 ± 4.62 | ①②③ | 4 weeks |
| Liang and Zheng (61) | Henan | Baihe Dihuang Decoction combined with Zopiclone | Zopiclone | 41/41 | 52.54 ± 2.43/52.63 ± 2.52 | ①④⑤⑥ | 4 weeks |
| Wang and Wang (10) | Beijing | Chaihu Jia Longgu Muli Decoction combined with Estazolam | Estazolam | 30/30 | 49.6 ± 2.1/49.8 ± 2.2 | ①②③④⑤⑥⑦⑧ | 8 weeks |
| Wang et al. (38) | Anhui | Yiganxue combined with Suanzaoren Decoction and Estazolam | Estazolam | 69/69 | 49.12 ± 2.20/48.98 ± 2.45 | ①②③④⑤⑥ | 4 weeks |
| Dou et al. (24) | Henan | Yishen Anshen Decoction combined with conventional Western medicine | Zopiclone combined with Sex hormones | 36/36 | 50.33 ± 3.27/50.56 ± 1.52 | ①②④⑤⑥ | 4 weeks |
| Shao et al. (39) | Hebei | Chaiqi Ningshen Anmian Decoction combined with Estazolam | Estazolam | 30/30 | 51.62 ± 5.47/50.88 ± 5.78 | ①③⑧ | 4 weeks |
| Zheng (40) | Jilin | Guizhi Jia Longgu Muli Decoction combined with Alprazolam | Alprazolam | 30/30 | 43.21 ± 7.67/41.97 ± 11.25 | ①③ | 4 weeks |
| Chen (41) | Zhejiang | Guipi Tang combined with Estazolam | Estazolam | 30/30 | 48.23 ± 3.16/48.15 ± 3.26 | ①②③④⑤⑥ | 8 weeks |
| Chen et al. (42) | Guangdong | Guanlong Compound combined with Eszopiclone | Zopiclone | 26/26 | 49.04 ± 2.91/48.31 ± 2.45 | ②③④⑤⑥ | 2 months |
| Chen et al. (43) | Fujian | Kuntai Capsule combined with Estrogen and Progesterone | Sex hormones | 41/41 | 50.15 ± 3.1/50.23 ± 3.2 | ①③④⑤⑥⑦ | 3 months |
| Ran and Wang (44) | Henan | Modified Suanzaoren Decoction combined with Acupuncture | Estazolam | 43/43 | 50.63 ± 7.59/50.51 ± 7.57 | ②③④⑤⑥ | 4 weeks |
| Wu et al. (22) | Sichuan | Acupuncture combined with Zhumin Decoction | Sex hormones | 48/48 | 49.67 ± 2.76/49.80 ± 2.79 | ①③④⑤⑥ | 3 weeks |
| Sun et al. (45) | Henan | Acupuncture combined with Qingre Anshen Decoction | Estazolam | 53/53 | 51.62 ± 3.11/51.66 ± 3.12 | ①③④⑤⑥ | 2 months |
| Zuo and Jin (62) | Shanghai | Acupuncture combined with Xiaoyao Decoction | Estazolam | 38/38 | 52.15 ± 2.40/52.20 ± 2.54 | ①②③ | 4 weeks |
| Kang (46) | Henan | Acupuncture combined with Xiangfu Decoction | Estazolam | 43/43 | 50.45 ± 3.92/49.30 ± 3.15 | ①③④⑤⑥ | 1 month |
| Zhang and Fan (63) | Henan | Acupuncture combined with Huanglian Ejiao Decoction | Estazolam combined with oryzanol | 23/23 | 52.24 ± 1.86/50.06 ± 1.78 | ①③ | 1 month |
| Zhang and Zhou (47) | Beijing | Acupuncture combined with Baihe Dihuang Decoction | Estazolam | 39/39 | 52.76 ± 2.81/52.14 ± 2.63 | ①②③④⑤⑥ | 4 weeks |
| Xu and Zhao (48) | Zhejiang | Acupuncture combined with Wen’an Shenyangxue Decoction | Estazolam | 53/53 | 51.01 ± 5.22/51.24 ± 5.33 | ①②③④⑤⑥⑦ | 3 months |
| Du2017b (19) | Liaoning | Acupuncture combined with Jiawei Wumei Wan | Estazolam | 42/41 | 50.61 ± 2.62/50.45 ± 3.19 | ②③⑤⑥⑦ | 4 weeks |
| Yang and Liu (49) | Shandong | Acupuncture combined with Huanglian Wendan Decoction | Alprazolam | 30/30 | 49.06 ± 2.56/49.02 ± 2.31 | ①④⑤⑥⑦⑧ | 4 weeks |
| Yan et al. (50) | Henan | Acupuncture combined with Xiangfu Decoction | Estazolam | 59/57 | 50.8 ± 7.6/49.6 ± 7.2 | ①②③④⑤⑥⑧ | 16 weeks |
| Chen et al. (20) | Hubei | Acupuncture combined with Jiawei Huanglian Ejiao Tang | Alprazolam | 30/30 | 47.87 ± 4.12/47.03 ± 3.84 | ①④⑤⑥ | 30 days |
| Lu et al. (64) | Henan | Acupuncture combined with Baizi Yangxin Tang | Estazolam | 46/46 | 40–52/40–52 | ①②③⑤⑥ | 4 weeks |
| Huang et al. (51) | Fujian | Acupuncture combined with Sun’s Anshen Formula | Estazolam combined with Tibolone | 50/50 | 50.42 ± 5.90/50.38 ± 5.20 | ①②③⑨⑩ | 3 months |
Basic characteristics of included literature.
E: Experimental Group; C: Control Group; ①Overall Efficiency; ②Adverse Reactions; ③Pittsburgh Sleep Quality Index; ④Luteinizing Hormone; ⑤Follicle-Stimulating Hormone; ⑥Estradiol; ⑦Kupperman Menopausal Index; ⑧Traditional Chinese Medicine Syndrome; ⑨Self-Rating Anxiety Scale; ⑩Self-Rating Depression Scale.
Regarding diagnostic criteria, seven studies lacked specific criteria for perimenopause. The remaining studies provided clear diagnostic criteria for both perimenopause and insomnia. Among them, four studies adopted Western medical diagnostic criteria, six applied Traditional Chinese Medicine criteria, and the remaining 31 studies used both TCM and Western medical diagnostic criteria. Specifically, most studies diagnosed perimenopause based on Obstetrics and Gynecology, while the diagnosis of insomnia was primarily guided by the Chinese Classification of Mental Disorders, Third Edition (CCMD-3), as details in the Supplementary Table 2.
3.3 Risk of bias assessment
Among the 48 included studies, a total of 34 studies were judged as low risk, among which 2 studies (12, 22) used computer-generated random numbers, 1 study (23) adopted simple random grouping, 1 study (24) conducted random grouping by the draw-ball method, and the remaining 30 studies (10, 18, 19, 25–51) used random number tables to generate random sequences. In addition, 14 studies (20, 52–64) stated random grouping in the text but did not specify the randomization method, so their risk was judged as unclear risk. However, only 2 studies (22, 31) adopted the envelope method for allocation concealment, so they were rated as low risk; the other studies did not implement allocation concealment and were rated as high risk. Almost all included clinical randomized controlled trials did not mention blinding in the text, except for 1 study (12) that applied blinding to outcome assessors and data analysts. All randomized controlled trials had no missing data, or the missing data did not affect the result analysis, so they were assessed as low bias risk in this dimension. All randomized controlled trials reported all outcomes described in the study design or methodology, so they were assessed as low risk of selective reporting bias. Further details are provided in Figure 2, and the bias risk of each included study is shown in Supplementary Figure 1.
Figure 2

Risk of bias summary for included studies.
3.4 Primary outcomes
3.4.1 Overall efficiency
A total of 4,609 patients in 46 studies reported the overall efficiency in treating PMI. Since the heterogeneity was low (p = 0.80 > 0.10, I2 < 50%), a fixed-effects model was used for analysis. The extracted data revealed that TCM interventions were more effective than conventional Western medicine in treating PMI (RR = 1.20, 95% CI [1.17, 1.23], p < 0.00001). Subgroup analysis indicated that TCM combined with Western medicine may achieve higher efficacy (RR = 1.22, 95% CI [1.17, 1.28], p < 0.00001). However, no statistical significance was observed in the overall efficiency among the subgroups (p = 0.37). The results are shown in Figure 3.
Figure 3

Forest plot of overall efficacy rate.
3.4.2 Adverse reactions
A total of 2,486 patients in 29 studies reported the incidence of adverse events in treating PMI. Since the heterogeneity was low (p > 0.10, I2 < 50%), so a fixed-effects model was used for analysis. The extracted data indicated that patients treated with TCM interventions had a lower incidence of adverse reactions (RR = 0.30, 95% CI [0.24, 0.38], p < 0.00001). Subgroup analysis revealed that patients treated with TCM alone were associated with the lower incidence of adverse reactions (RR = 0.20, 95% CI [0.13, 0.31], p < 0.00001), and there was statistical significance in the incidence of adverse reactions among the subgroups (p = 0.001). The results are shown in Figure 4.
Figure 4

Forest plot of adverse reaction incidence.
3.5 Secondary outcomes
3.5.1 PSQI score
A total of 4,456 patients in 43 studies reported the PSQI score in treating PMI. The extracted data showed high heterogeneity across all subgroups (p < 0.10, I2 > 50%), so a random-effects model was used for analysis. The results indicated that patients with PMI who received TCM interventions had a lower PSQI score after treatment than those who received Western medicine treatment (MD = −2.57, 95% CI [−3.01, −2.14], p < 0.00001). Subgroup analysis revealed that the PSQI score of the experimental group was lower than that of the control group in all subgroups (all p < 0.05). However, there was no statistical significance among the subgroups (p = 0.25). The results are shown in Figure 5. Sensitivity analysis, as shown in the Supplementary Figure 2, suggested that the results were generally robust. Systematically removing studies one at a time fails to make heterogeneity reduce significantly.
Figure 5
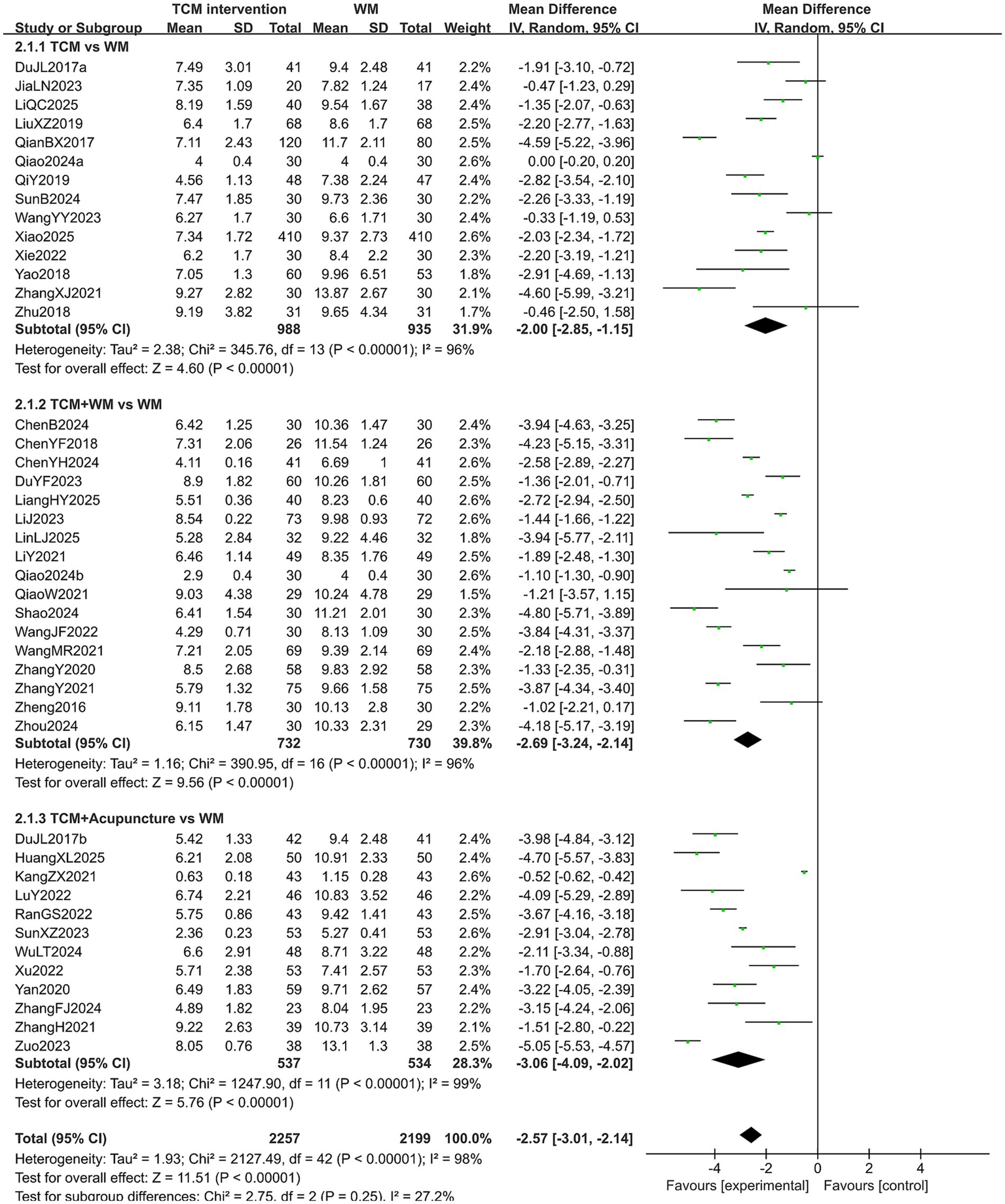
Forest plot of PSQI score.
3.5.2 LH level
A total of 2,358 patients in 36 studies reported the LH level in treating PMI. The heterogeneity test results showed high heterogeneity across all subgroups (p < 0.10, I2 > 50%), so a random-effects model was used for analysis. The extracted results indicated that patients with PMI who received TCM interventions had a lower LH level than those who received Western medicine (MD = −4.51, 95% CI [−6.15, −2.87], p < 0.00001). Subgroup analysis results showed that the LH level of the experimental group was lower than that of the control group in all subgroups (all p < 0.05). However, no statistical difference was observed in the LH level among the subgroups (p = 0.29). The results are shown in Figure 6. Sensitivity analysis, as shown in the Supplementary Figure 3, suggested that the results were generally robust. Systematically removing studies one at a time fails to make heterogeneity reduce significantly.
Figure 6

Forest plot of LH level.
3.5.3 FSH level
A total of 34 studies reported the post-treatment FSH level of 2,940 patients. The heterogeneity test results showed high heterogeneity across all subgroups (p < 0.10, I2 > 50%), so a random-effects model was used for analysis. The extracted data showed that patients treated with TCM interventions had a lower FSH level than those treated with Western medicine (MD = −8.67, 95% CI [−10.96, −6.38], p < 0.00001). Subgroup analysis indicated that the FSH level of the experimental group was lower than that of the control group in all subgroups (all p < 0.05). In addition, a statistical difference was observed in the FSH level among the subgroups (p = 0.02). Specifically, patients treated with TCM combined with acupuncture had the lower FSH level after treatment (MD = −14.09, 95% CI [−19.28, −8.90], p < 0.00001). The results are shown in Figure 7. Sensitivity analysis, as shown in the Supplementary Figure 4, suggested that the results were generally robust. Systematically removing studies one at a time fails to make heterogeneity reduce significantly.
Figure 7

Forest plot of FSH level.
3.5.4 E2 level
A total of 2,801 patients in 33 studies reported the E2 level in treating PMI. The heterogeneity test results showed high heterogeneity across all subgroups (p < 0.10, I2 > 50%), so a random-effects model was used for analysis. The extracted results showed that patients with PMI who received TCM interventions had a higher E2 level after treatment than those who received Western medicine treatment (MD = 9.64, 95% CI [7.45, 11.82], p < 0.00001). Subgroup analysis revealed that the E2 level of the experimental group was higher than that of the control group in all subgroups (all p < 0.05). In addition, a statistical difference was observed in the E2 level among the subgroups (p = 0.04). Specifically, patients treated with TCM combined with acupuncture had the higher E2 level after treatment (MD = 13.27, 95% CI [8.52, 18.01], p < 0.00001). The results are shown in Figure 8. Sensitivity analysis, as shown in the Supplementary Figure 5, suggested that the results were generally robust. Systematically removing studies one at a time fails to make heterogeneity reduce significantly.
Figure 8

Forest plot of E2 level.
3.5.5 KMI score
A total of 1,219 patients in 14 studies reported the KMI score in treating PMI. The heterogeneity test results showed high heterogeneity across all subgroups (p < 0.10, I2 > 50%), so a random-effects model was used for analysis. The extracted data showed that patients with PMI who received TCM interventions had a lower KMI score than those who received Western medicine (MD = −6.01, 95% CI [−8.56, −3.47], p < 0.00001). Subgroup analysis revealed that the KMI score of the experimental group was lower than that of the control group in all subgroups (all p < 0.05). However, no statistical difference was observed in the post-treatment KMI score among the subgroups (p = 0.57). The results are shown in Figure 9. Sensitivity analysis, as shown in the Supplementary Figure 6, suggested that the results were generally robust. Systematically removing studies one at a time fails to make heterogeneity reduce significantly.
Figure 9
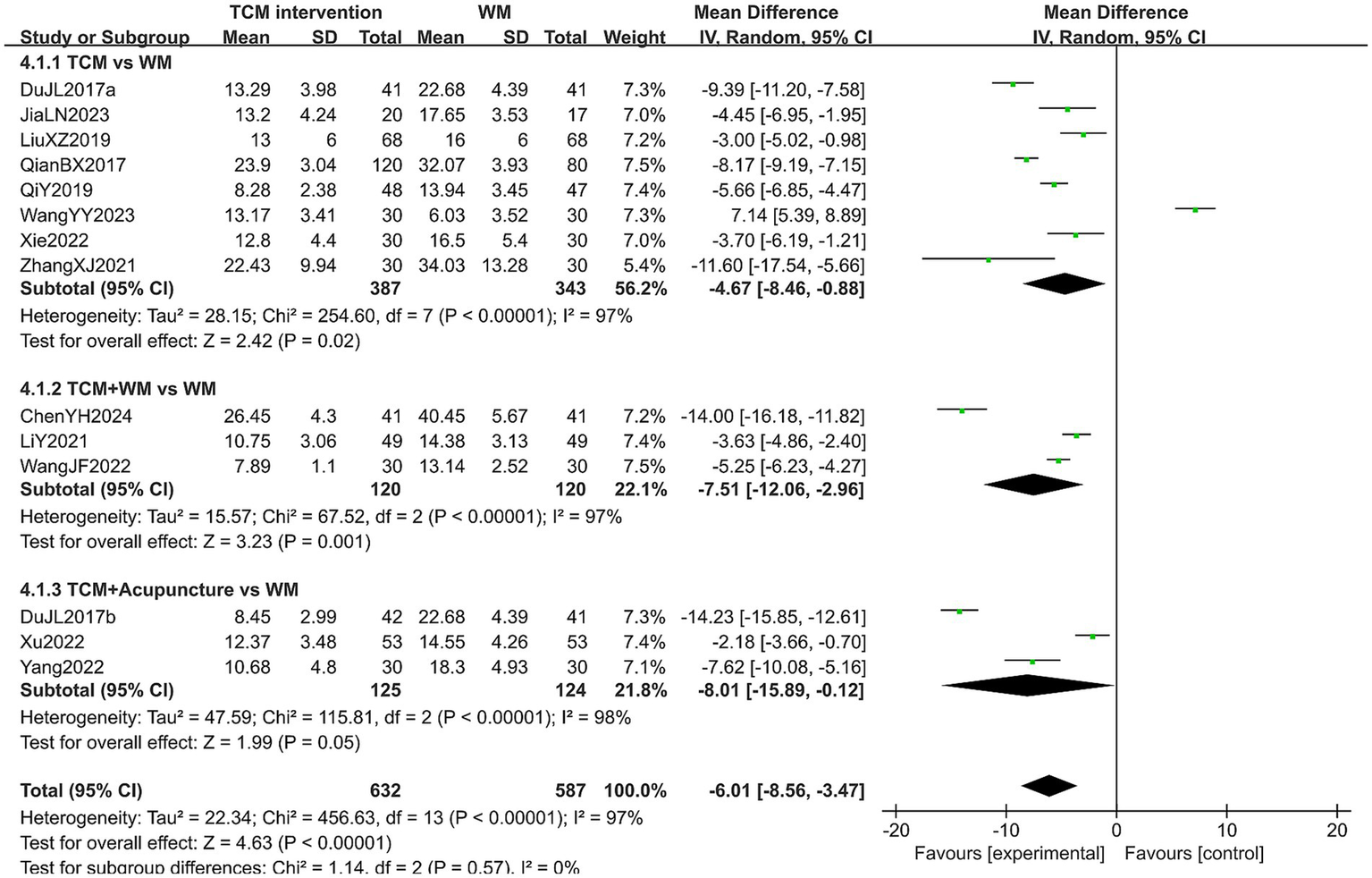
Forest plot of KMI score.
3.5.6 TCMS score
A total of 1,621 patients in 11 studies reported the TCMS score in treating PMI. Considering that some studies adopted different TCM symptoms as evaluation criteria, the SMD was used as the effect size. The heterogeneity test results showed high heterogeneity (p < 0.10, I2 > 50%), so a random-effects model was used for analysis. The extracted data indicated that patients with PMI who received TCM interventions had a lower TCMS score after treatment than those who received Western medicine treatment (SMD = −2.27, 95% CI [−3.49, −1.05], p < 0.00001). Subgroup analysis results showed that: In the TCM group and TCM-WM group, the TCMS score of patients in the experimental group was lower (all p < 0.05). However, no statistical difference was observed in the TCMS score in the TCM-A group (p = 0.23). This result may be related to the small number of included studies (n = 2) in this subgroup, which may lead to insufficient statistical power to detect potential differences. In addition, there was no statistical significance among the subgroups (p = 0.30). The results are shown in Figure 10. Sensitivity analysis, as shown in the Supplementary Figure 7, suggested that the results were generally robust. Systematically removing studies one at a time fails to make heterogeneity reduce significantly.
Figure 10

Forest plot of TCMS score.
3.5.7 SAS score
A total of 412 patients in 4 studies reported the SAS score in treating PMI. The heterogeneity test results showed low heterogeneity (p > 0.10, I2 < 50%), so a fixed-effects model was used for analysis. The extracted data showed that patients with PMI who received TCM interventions had a lower SAS score after treatment than those treated with Western medicine (MD = −4.77, 95% CI [−5.77, −3.76], p < 0.00001). Subgroup analysis indicated that patients treated with TCM alone may have lower SAS score after treatment (MD = −5.59, 95% CI [−7.16, −4.02], p < 0.00001), but this advantage did not reach a significant difference due to small sample sizes. The results are shown in Figure 11.
Figure 11

Forest plot of SAS score.
3.5.8 SDS score
A total of 592 patients in 7 studies reported the SDS score in treating PMI. The heterogeneity test results showed high heterogeneity (p < 0.10, I2 > 50%), so a random-effects model was used for analysis. The extracted data showed that patients with PMI who received TCM interventions had a lower SDS score than those who received Western medicine (MD = −2.96, 95% CI [−5.80, −0.12], p = 0.04). Subgroup analysis results indicated that: In the TCM group and TCM-WM group, no statistical difference was observed in the SDS score between the experimental group and the control group (both p > 0.05). In the TCM-A group, although the SDS score of the experimental group was lower, this subgroup only included one study, so the result needs to be interpreted with caution. In addition, no statistical difference was observed in the post-treatment SDS score among the subgroups (p = 0.26), as detailed in Figure 12. Sensitivity analysis, as shown in the Supplementary Figure 8, suggested that the overall results were generally robust, with consistent effect directions across leave-one-out analyses. However, the statistical significance of the pooled estimate was influenced by several studies (18, 51, 52, 57), suggesting some dependency on these trials. Although excluding certain studies could reduce heterogeneity, the heterogeneity after reduction remained substantial (I2 > 50%).
Figure 12

Forest plot of SDS score.
3.6 Subgroup analysis, sensitivity analysis and regression analysis
There subgroups were created based on the different interventions: TCM, TCM combined with WM and TCM combined with acupuncture. For outcome measures with significant heterogeneity remaining after subgroup analysis, sensitivity analysis was conducted to assess robustness, and studies were excluded one by one to identify the source of heterogeneity. The detailed results can be found in the Supplementary Figures 2–8. For the seven outcome indicators (PSQI, LH, FSH, E2, KMI, TCMS, and SDS) that still displayed high heterogeneity after subgroup analysis, we performed regression analysis to explore potential sources of heterogeneity. The results demonstrated that for the TCMS, both sample size and follow-up duration were identified as significant moderators of heterogeneity (both p < 0.05); in contrast, none of sample size, age, or follow-up duration exerted a significant moderating effect on the heterogeneity of the remaining six indicators (all p > 0.05). The detailed regression results can be found in Supplementary Table 3.
3.7 Publication bias
Publication bias analysis was conducted for outcome measures with more than 10 included studies. Results of the funnel plot and bias test revealed that for overall efficiency, adverse reactions, LH level, KMI score, TCMS score, SAS score, and SDS score, the possibility of publication bias was low. In contrast, for PSQI score, FSH level, and E2 level, the possibility of publication bias was high. The results are shown in Figure 13.
Figure 13

Funnel plot of the outcomes. (A) OE; (B) AE; (C) PSQI; (D) LH; (E) FSH; (F) E2; (G) KMI; (H) TCMS.
3.8 Quality of evidence
The quality of the evidence was evaluated using the GRADE profiler, the assessment results showed that the quality of evidence is relatively moderate. Further details are provided in the Supplementary Table 4.
4 Discussion
Nearly 50% of women in China experience insomnia during the perimenopausal period, which markedly interfere with daily functioning and overall well-being. As a result, PMI has emerged as a significant public health concern warranting increased clinical attention and research (65). The etiology of perimenopausal insomnia is highly complex. Modern clinical studies indicate that it is associated with multiple factors, including aging, psychological stress, neuroendocrine changes, and hormonal fluctuations (66–68). To date, there is no consensus on the precise pathophysiological mechanisms. According to Western medical theory, the primary mechanism involves ovarian function decline, which disrupts the hypothalamic–pituitary-ovarian (HPO) axis, leading to hormonal fluctuations, particularly in estrogen and progesterone, which play critical roles in maintaining neurotransmitter balance, regulating circadian rhythms, modulating sleep architecture, and indirectly influencing mood (69). Hormonal imbalances during the perimenopausal period can therefore perturb sleep–wake cycles and emotional regulation, contributing to the development of insomnia, mood disturbances, irritability, and other associated symptoms (70, 71). The treatment of PMI can be nonpharmacologic, pharmacologic, or both. Non-pharmacological interventions include aromatherapy, hypnosis, psychotherapy, cognitive behavioral therapy (CBT), and music therapy (72). In China, however, pharmacological treatment with Western medicine remains the primary approach for managing PMI. Conventional pharmacological treatments, such as benzodiazepine hypnotics, non–benzodiazepine receptor agonists and HRT, have shown efficacy in alleviating insomnia but remain limited by adverse events and drug dependence (6, 73). Consequently, there has been increasing interest in complementary and integrative approaches, with TCM emerging as a potential therapeutic option for PMI. According to TCM theory, PMI is associated with multiple factors, including liver and kidney deficiency, yin-yang imbalance, dual deficiency of the heart and spleen, as well as disharmony of qi and blood. Such deficiencies and imbalances are believed to disrupt the smooth flow and transformation of qi and blood, impair the nourishment of the shen (spirit), and compromise the body’s ability to regulate emotions and maintain physiological homeostasis, leading to heat rash, night sweats, emotional instability, and sleep disturbances (74). Various TCM interventions, including herbal medicine, acupuncture, and combined use of Chinese and Western medicine, have demonstrated the therapeutic advantages of TCM in the management of PMI. Herbal formulations such as Chaihu Jia Longgu Muli decoction and Kuntai capsule can regulate the hormonal levels in patients with PMI to alleviate insomnia symptoms (75, 76). Acupuncture is another widely utilized TCM therapy for treating PMI, with advantages including broad indications, significant efficacy, high cost-effectiveness, and few adverse reactions. Accumulating evidence from previous clinical and experimental studies indicates that acupuncture exerts its therapeutic effects on insomnia through multiple neurobiological pathway, including vagal stimulation, enhancement of 5-HT neurotransmission, and modulation of HPO axis (77–79). In addition, combination therapy has emerged as an increasingly prevalent strategy in the treatment of PMI. Integrating traditional Chinese and Western medicines, or combining acupuncture with TCM, not only facilitate the rapid alleviation of diverse symptoms in patients with PMI, but also may mitigate the adverse effects often associated with long-term use of synthetic hormones or sedative-hypnotic drugs (80, 81). Moreover, TCM can alleviate patients’ depression and anxiety by enhancing monoamine neurotransmitter levels, suppressing hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis, modulating hippocampal neurons and neurotrophic factors, regulating immune cytokines, counteracting excitatory amino acid toxicity, and modulating the microbiota–gut–brain axis (82–84).
Our systematic review and meta-analysis comprehensively synthesized available evidence from 48 RCTs encompassing a total of 5,037 participants, thereby providing robust and quantitatively rigorous insights into the therapeutic efficacy and safety profile of TCM in the management of PMI. The results demonstrated that TCM interventions, whether administered as monotherapy or integrated with conventional pharmacological or acupuncture, exhibited statistically significant superiority over Western medicines in enhancing multiple clinically relevant outcomes. Specifically, TCM was associated with a greater improvement in overall treatment efficiency along with significant amelioration in sleep quality indicated by reductions in PSQI scores, favorable modulation of key endocrine markers such as E2, FSH, and LH, and notable alleviation of menopausal symptoms reflected by decreased KMI scores. Additionally, psychological parameters including anxiety and depression levels, as assessed by the SAS score and SDS score, showed more pronounced improvement in the TCM groups. While enhancements in TCMS scores and SDS scores were also observed following TCM treatment, these effects were accompanied by considerable heterogeneity, and subsequent subgroup analyses revealed inconsistent findings, suggesting the need for cautious interpretation and further investigation. Of particular clinical significance was the safety profile of TCM interventions, which were consistently associated with a markedly lower incidence of adverse reactions compared to control groups, indicating a favorable risk–benefit ratio.
Notably, although subgroup analysis was conducted by therapeutic type, several outcome measures in this meta-analysis still exhibited substantial heterogeneity (I2 > 50%). To further identify potential sources of heterogeneity, we performed regression analysis targeting these indicators, with sample size, age of participants, and follow-up duration as the explanatory variables. The regression analysis results revealed that sample size and follow-up duration were statistically significant factors explaining the high heterogeneity of TCMS (both p < 0.05), while none of the three factors exerted a significant explanatory effect on the heterogeneity of the remaining six indicators (all p > 0.05). The result suggested that this substantial residual heterogeneity may be attributed to other unexamined factors, including differences in TCM syndrome differentiation, different diagnostic criteria for PMI across studies, diverse symptom presentations of PMI patients, or inconsistencies in outcome assessment tools. Moreover, the subgroup analysis results indicated that no statistically significant differences were observed among subgroups in most outcome measures. This suggests that the choice of TCM intervention may have less impact on outcomes. Sensitivity analyses conducted for each outcome measure confirmed the stability and reliability of our results. However, further studies with larger sample sizes are needed to confirm these findings.
Compared to previous meta-analyses (80, 81, 85), this study has the following advantages: (1) it included a larger number of clinical randomized controlled trials (48 in total) and patients (5,037 cases). (2) It included more comprehensive outcome measures. (3) We conducted detailed subgroup analyses to assess the efficacy and safety of three common TCM therapies for PMI. (4) We rated the certainty of evidence using the latest GRADE approach.
This study has the following limitations: (1) although most trials reported improvements favoring TCM, methodological quality was generally moderate. Notably, all included studies were single-center investigations, with a distinct paucity of multi-center RCTs in the current literature. Additionally, many studies failed to provide detailed descriptions of randomization procedures, allocation concealment, or blinding methods. (2) The control groups were limited to conventional Western medicine, which may limit the generalizability of the results. (3) Some outcome measures could not be extracted, which may lead to bias in some results. (4) The heterogeneity of some outcome indicators was high, which may affect the accuracy of the results. (5) All included studies were conducted in mainland China, where participants share specific genetic backgrounds, lifestyle patterns, and local TCM clinical practice norms. This regional restriction potentially limits the generalizability of our findings to broader populations, especially those from other countries or regions with distinct ethnic compositions and medical care systems. In conclusion, this meta-analysis provides compelling evidence that TCM interventions represent a safe, well-tolerated, and effective therapeutic option for patients suffering from PMI, with the potential to improve both physiological and psychological dimensions of the disorder, thus warranting consideration in integrative clinical management strategies and further validation through large-scale, high-quality randomized trials.
5 Conclusion
This systematic review and meta-analysis provide compelling evidence that TCM appears to be a promising and relatively safe option for patients with PMI. Clinically, these findings offer robust evidence-based support for integrating TCM into the standardized management of PMI patients, to alleviate symptoms and improve quality of life without severe adverse reactions. However, future research should prioritize rigorous methodological designs, larger sample sizes, longer follow-up, objective sleep measures and international collaborations to further substantiate these findings and guide evidence-based integration of TCM into clinical practice.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DY: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft. DC: Data curation, Formal analysis, Methodology, Writing – original draft. JP: Data curation, Formal analysis, Writing – original draft. YP: Conceptualization, Data curation, Writing – original draft. CY: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. JS: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. Funding for this research was provided by the Key Research and Development Project of the Sichuan Provincial Department of Science and Technology (grant number: 2024YFFK0087).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2026.1749660/full#supplementary-material
References
1.
Li L Wu J Pu D Zhao Y Wan C Sun L et al . Factors associated with the age of natural menopause and menopausal symptoms in chinese women. Maturitas. (2012) 73:354–60. doi: 10.1016/j.maturitas.2012.09.008,
2.
Troìa L Garassino M Volpicelli AI Fornara A Libretti A Surico D et al . Sleep disturbance and perimenopause: a narrative review. J Clin Med. (2025) 14:1479. doi: 10.3390/jcm14051479,
3.
Sander B Gordon JL . Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. (2021) 23:73. doi: 10.1007/s11920-021-01285-1,
4.
Sun D Shao H Li C Tao M . An analysis of the main reasons that perimenopausal and postmenopausal women in China have for seeking outpatient treatment and factors influencing their symptoms: a single-center survey. Clin Exp Obstet Gynecol. (2015) 42:146–51. doi: 10.12891/ceog1744.2015,
5.
Bondy E Prim J Rubinow D Schiff L Dichter GS Schiller CE . Effects of combined estrogen and bazedoxifene (CEB) on depressive symptoms in perimenopause. J Affect Disord. (2025) 390:119853. doi: 10.1016/j.jad.2025.119853,
6.
Morin CM Drake CL Harvey AG Krystal AD Manber R Riemann D et al . Insomnia disorder. Nat Rev Dis Primers. (2015) 1:15026. doi: 10.1038/nrdp.2015.26,
7.
Qaseem A Kansagara D Forciea MA Cooke M Denberg TD . Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. (2016) 165:125–33. doi: 10.7326/M15-2175,
8.
Li Z Yin S Feng J Gao X Yang Q Zhu F . Acupuncture combined with chinese herbal medicine in the treatment of perimenopausal insomnia: a systematic review and meta-analysis. Medicine. (2023) 102:e35942. doi: 10.1097/MD.0000000000035942,
9.
Peng W Sibbritt DW Hickman L Kong X Yang L Adams J . A critical review of traditional chinese medicine use amongst women with menopausal symptoms. Climacteric: J Int Menopause Soc. (2014) 17:635–44. doi: 10.3109/13697137.2014.904850,
10.
Wang JF Wang DH . Effects of modified Chaihu Jia Longgu Muli decoction on sleep quality, negative emotions, and endocrine hormones in patients with perimenopausal insomnia. Mod J Integr Tradit Chin West Med. (2022) 31:1842–5. doi: 10.3969/j.issn.1008-8849.2022.13.019
11.
Liang Y Huo Q . Research progress on Huanglian Ejiao decoction in the treatment of perimenopausal insomnia. Chin J Mod Distance Educ Tradit Chin Med. (2024) 22:188–90. doi: 10.3969/j.issn.1672-2779.2024.15.060
12.
Chen L-Q Zhou Y-C Ning F Zhu M-J . Jiawei suanzaoren decoction for the treatment of perimenopausal insomnia: clinical observation and experimental study. Front Pharmacol. (2025) 15:1495957. doi: 10.3389/fphar.2024.1495957,
13.
Higgins JP Savović J Page MJ Elbers RG Sterne JA . "Assessing risk of bias in a randomized trial" In: ChandlerJCumpstonMLiT, editors. Cochrane handbook for systematic reviews of interventions. New York, NY: John Wiley & Sons, Ltd (2019). 205–28.
14.
Higgins JP Li T Deeks JJ . "Choosing effect measures and computing estimates of effect" In: Cochrane handbook for systematic reviews of interventions. New York, NY: John Wiley & Sons, Ltd (2019). 143–76.
15.
Deeks JJ Higgins JP Altman DG Group on behalf of the CSM. "Analysing data and undertaking meta-analyses" In: Cochrane handbook for systematic reviews of interventions. New York, NY: John Wiley & Sons, Ltd (2019). 241–84.
16.
Peters JL Sutton AJ Jones DR Abrams KR Rushton L . Comparison of two methods to detect publication bias in meta-analysis. JAMA. (2006) 295:676–80. doi: 10.1001/jama.295.6.676,
17.
Sterne JAC Sutton AJ Ioannidis JPA Terrin N Jones DR Lau J et al . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002,
18.
Qiao W . Effects of Kuntai capsule combined with Fenmaotong in menopausal insomnia patients with depression. Chin J Drugs Clin. (2024) 24:1497–500. doi: 10.11655/zgywylc2024.23.001
19.
Du JL Fan WJ Du HJ . Clinical observation on Jin's three-needle therapy combined with Jiawei Wumei pill in treatment of perimenopausal insomnia. Chin J Pharm. (2017) 28:1104–7. doi: 10.6039/j.issn.1001-0408.2017.08.27
20.
Chen Y Liu HY Yang BX Tang L . Analysis of therapeutic effect of acupuncture combined with modified Huanglian Ejiao decoction on perimenopausal insomnia of heart-kidney disharmony type. Mod J Integr Tradit Chin West Med. (2015) 24:2902–4. doi: 10.3969/j.issn.1008-8849.2015.26.018
21.
Li T Higgins JP Deeks JJ . "Collecting data" In: ChandlerJCumpstonMLiT, editors. Cochrane handbook for systematic reviews of interventions. New York, NY: John Wiley & Sons, Ltd (2019). 109–41.
22.
Wu LT Zhou H Huang ZB Wang QQ Sun JG . Study on the clinical efficacy of acupuncture combined with Zumin decoction for perimenopausal insomnia and its effect on serum estrogen levels. Sichuan J Tradit Chin Med. (2024) 42:206–9. doi: 10.3969/j.issn.1000-3649.2024.9.sczy202409053
23.
Wu JY Zhou ZL Yang QM . Clinical observation on Xiangshao granules combined with paroxetine in treating perimenopausal depression accompanied by sleep disorders. J Pract Tradit Chin Med. (2023) 39:2185–7. doi: 10.3969/j.issn.1004-2814.syzyyzz202311040
24.
Dou CH Fu LR Wang HQ Peng SL . Effects of Yishen Anshen decoction as an adjunctive treatment on TCM symptom scores and sleep quality in perimenopausal insomnia patients of liver-kidney yin deficiency type. Chin J Tradit Chin Med Res. (2023) 36:39–43. doi: 10.3969/j.issn.1001-6910.2023.09.11
25.
Zhu JJ Wang YX . Clinical study of Buxin Xiaoyao drink in treating perimenopausal insomnia. Chin J Basic Med Tradit Chin Med. (2020) 26:1121–99. doi: 10.3969/j.issn.1006-3250.2020.08.026
26.
Wang YY Ma K . Clinical study on Liuwei Dihuang decoction combined with modified Xiaoyao powder in the treatment of perimenopausal sleep disorder of kidney deficiency and liver depression. Shaanxi J Tradit Chin Med. (2023) 44:584–611. doi: 10.3969/j.issn.1000-7369.2023.05.009
27.
Mao F . Effects of modified Huanglian Ejiao decoction on sleep quality and sex hormone levels in patients with perimenopausal insomnia. Chin J Mater Child Health. (2020) 35:4297–300. doi: 10.19829/j.zgfybj.issn.1001-4411.2020.22.046
28.
Mo MS Ren CF Lü YH . Effects of Chishao Yangxue Jieyu decoction on sleep quality and serum sex hormone levels in patients with perimenopausal insomnia. J Guangxi Univ Chin Med. (2024) 27:17–20. doi: 10.3969/j.issn.2095-4441.2024.03.007
29.
Jia LN Tang L Liu QJ Wang YX Ma HT . Clinical effect of Gengxin decoction on perimenopausal insomnia with heart⁃kidney non⁃interaction syndrome. World J Tradit Chin Med. (2023) 18:839–43. doi: 10.3969/j.issn.1673-7202.2023.06.018
30.
Qi Y Kang JY . Efficacy of Zishen Ningshen decoction in treating insomnia due to heart-kidney disharmony in perimenopausal syndrome. Mod Pract Med. (2019) 31:872–5. doi: 10.3969/j.issn.1671-0800.2019.07.010
31.
Qiao W Gao Y Sun KF Ding JJ Wang D Chen HJ et al . Clinical study of modified treatment with Tianwang Buxin pills plus Jiaotai pills for perimenopausal hypertension accompanied by insomnia. J Guangzhou Univ Tradit Chin Med. (2021) 38:1840–6. doi: 10.13359/j.cnki.gzxbtcm.2021.09.012
32.
Zhou Y Ding CH Huang F Zhan C . Improvement effect of nourishing Yin paste for tranquillization on sleep quality of patients with perimenopausal insomnia. Smart Health. (2024) 10:90–92, 99. doi: 10.19335/j.cnki.2096-1219.2024.24.030
33.
Zhang Y Huang JS Zhang M Zhang YF Gao YD Deng JJ et al . Clinical study on Songyu Yinxu decoction in treating perimenopausal insomnia patients with kidney deficiency and liver stagnation syndrome. Chin J Tradit Chin Med. (2020) 35:2102–5.
34.
Li J Xia XF Chu XT Liu DN Gong YL . Clinical observation of Banxia Xiexin decoction combined with zopiclone tablets in the treatment of perimenopausal insomnia patients. World Clin Drugs. (2023) 44:608–13. doi: 10.13683/j.wph.2023.06.016
35.
Du YF Li YY Zhang D Liu CZ Guo XB . Clinical study on Roukou Wuwei pills combined with trazodone hydrochloride in treating perimenopausal patients with insomnia accompanied by anxiety and depression. Mod Tradit Chin Med. (2023) 43:92–6. doi: 10.13424/j.cnki.mtcm.2023.05.018
36.
Lin LJ Mao XX Jiang PL Huang ZX Ding ZH . Clinical study of Zishen Shugan Anshen decoction in treating perimenopausal insomnia of kidney deficiency and liver stagnation type. J Tradit Chin Med Inform. (2025) 24:29–33. doi: 10.14046/j.cnki.zyytb2002.2025.04.012
37.
Liang HY . Therapeutic effect of Wuling capsules combined with Dexzopiclone tablets in the treatment of patients with perimenopausal insomnia. Med Front. (2025) 15:14–8. doi: 10.20235/j.issn.2095-1752.2025.03.005
38.
Wang MR Fang CH Han H Ding XJ . Effect of modified Yiganxue Suanzaoren decoction on levels of sex hormones and sleep quality of perimenopausal women with insomnia. J Hunan Norm Univ Med Sci. (2021) 18:9–13. doi: 10.3969/j.issn.1673-016X.2021.04.003
39.
Shao WJ Wu S Wang WL Ma YB Wang HL . Study on the effect of Chaiqi Ningshen Anmian decoction combined with Yi Mian pillow on perimenopausal insomnia. J Changchun Univ Chin Med. (2024) 40:1347–50. doi: 10.13463/j.cnki.cczyy.2024.12.011
40.
Zheng YH . Observation on the efficacy of Guizhi Jia Longgu Muli decoction in treating perimenopausal patients with anxiety-related insomnia disorder. Chin Prim Health Care. (2016) 30:59–60. doi: 10.3969/j.issn.1001-568X.2016.11.0023
41.
Chen B . Clinical study on modified Guipi decoction combined with esazolam tablets for perimenopausal insomnia of dual deficiency of the heart-spleen type. New Chin Med. (2024) 56:30–4. doi: 10.13457/j.cnki.jncm.2024.14.006
42.
Chen YF Lu J Zhang BT Lin YJ Lin YQ . Clinical study on Guanlong mixture combined with eszopiclone in treating perimenopausal insomnia of liver stagnation transforming into fire. Chin J Mod Distance Educ Tradit Chin Med. (2018) 16:117–9. doi: 10.3969/j.issn.1672-2779.2018.24.050
43.
Chen YH Wang JL Lin XY . Analysis of the effects of Kuntai capsule combined with sequential therapy of estrogenĉprogesterone on hormone levels and sleep quality in patients with perimenopausal syndrome and insomnia. World J Sleep Med. (2024) 11:2490–6. doi: 10.3969/j.issn.2095-7130.2024.11.027
44.
Ran GS Wang Y . Clinical study on supplemented sour jujube decoction combined with acupuncture and moxibustion in treating perimenopausal insomnia. Henan Tradit Chin Med. (2022) 42:1644–7. doi: 10.16367/j.issn.1003-5028.2022.11.0348
45.
Sun XJ Zhao KN Liu BX Chen L . Clinical observation on acupuncture and moxibustion combined with Qingre Anshen decoction for perimenopausal insomnia. Guangxi J Tradit Chin Med. (2023) 46:27–9. doi: 10.3969/j.issn.1003-0719.2023.05.007
46.
Kang ZX . Clinical study of acupuncture combined with modified Xiangfu decoction in treating perimenopausal insomnia with liver qi stagnation syndrome. Pract Clin J Integr Tradit Chin West Med. (2021) 21:35–6. doi: 10.13638/j.issn.1671-4040.2021.01.014
47.
Zhang H Zhou Q . Efficacy of Baihe Dihuang decoction combined with Zhenjing Anshen acupuncture in the treatment of female menopausal insomnia and influence on anxiety and depression. World J Integr Tradit Chin West Med. (2021) 16:405–9. doi: 10.13935/j.cnki.sjzx.210303
48.
Xu LY Zhao YR . Therapeutic observation of wen'an Shenyangxue decoction combined with Jinsan needle in the treatment of perimenopausal insomnia patients. Clin Educ Gen Med. (2022) 20:425–8. doi: 10.13558/j.cnki.issn1672-3686.2022.005.012
49.
Yang DL Liu WQ . Clinical study on Kaihe Liuqi acupuncture combined with modified Huanglian Wendan decoction for phlegm-heat disturbing type of perimenopausal insomnia. Hubei J Tradit Chin Med. (2022) 44:52–6.
50.
Yan XL Yu YD Yang DD . Clinical efficacy of acupuncture combined with modified Xiangfu decoction in treatment of menopausal insomnia cause by liver Qi stagnation. Chin J Chin Mater Med. (2020) 45:1460–4. doi: 10.19540/j.cnki.cjcmm.20191010.501,
51.
Huang XL Liu XP Lu LF Wang L Li J Wu MP . Clinical observation on Sun's Anshen recipe combined with plum blossom needle meridian tapping for perimenopausal insomnia. Chin J Folk Ther. (2025) 33:57–61. doi: 10.19621/j.cnki.11-3555/r.2025.0918
52.
Liu XZ Lin FB Ma LR . Clinical efficacy of Chaihu Guizhi Longgu Muli decoction combined with Ganmai Dazao decoction in treatment of peri-menopausal anxiety and insomnia. Chin J Drugs Clin. (2019) 19:1418–20. doi: 10.11655/zgywylc2019.09.008
53.
Yao ZX Chen H Zhu LP . Clinical observation on modified Zishen Jieyu Ningxin recipe for perimenopausal insomnia. Chin Tradit Pat Med. (2018) 40:240–2. doi: 10.3969/j.issn.1001-1528.2018.01.054
54.
Sun B Ding HR Zhou WS . Clinical observation of modified Suanzaoren decoction combined with Xiaoyao powder in the treatment of perimenopausal insomnia. Guangming J Chin Med. (2024) 39:4123–5. doi: 10.3969/j.issn.1003-8914.2024.20.028
55.
Zhang XJ Zhang Q Yang M Liu C Huang ZA . Observation on curative effect of modified Lian Di Jiao tai decoction on perimenopausal insomnia with psychosomatic disorder. Mod J Integr Tradit Chin West Med. (2021) 30:3222–6. doi: 10.3969/j.issn.1008-8849.2021.29.007
56.
Xiao HQ Niu JW . Observation on the efficacy of modified Chaihu Jia Longgu Muli decoction in treating perimenopausal insomnia of liver qi stagnation type. J Pract Tradit Chin Med. (2025) 41:63–5. doi: 10.3969/j.issn.1004-2814.2025.01.026
57.
Xie YZ . Clinical efficacy of Chaihu Guizhi Longgu Muli decoction in treatment of Peri-menopausal liver depression and insomnia. Chin J Ethnomed Ethnopharm. (2022) 31:114–8. doi: 10.3969/j.issn.1007-8517.2022.24.zgmzmjyyzz202224029
58.
Qian BX Liu J Lei XL Jiang SP . Clinical observation of Danzhi Xiaoyao pills combined with Kuntai capsules for perimenopause sleep disorders with syndrome of stagnant liver Qi turning into fire. New Chin Med. (2017) 49:91–3. doi: 10.13457/j.cnki.jncm.2017.09.029
59.
Zhang Y Liu L Yin Q . Efficacy of Erxian decoction combined with Suanzaoren decoction for perimenopausal insomnia and its effect on endocrine hormones. Mod J Integr Tradit Chin West Med. (2021) 30:3605–8. doi: 10.3969/j.issn.1008-8849.2021.32.015
60.
Li Y . Observation on the efficacy of Zixin Yangshen decoction in treating perimenopausal insomnia with heart-kidney disharmony syndrome. Mod Diagn Treat. (2021) 32:3044–6.
61.
Liang Y Zheng LJ . Observation on the efficacy of Baihe Dihuang decoction combined with conventional western medicine in treating insomnia in perimenopausal women. Mod Med Health. (2022) 38:2451–4. doi: 10.3969/j.issn.1009-5519.2022.14.025
62.
Zuo SL Jin FR . Study on the efficacy of modified Xiaoyao decoction combined with Zhenjing Anshen acupuncture in treating perimenopausal insomnia in women and its effect on anxiety and depression. Guizhou Med J. (2023) 47:1935–6. doi: 10.3969/j.issn.1000-744X.2023.12.052
63.
Zhang FJ Fan XH . Yingi Guiyuan acupuncture combined with Huanglian Ejiao decoction in treatment of perimenopausal insomnia with heart-kidney non-communication syndrome. Chin J Tradit Chin Med. (2024) 39:2256–60. doi: 10.16368/j.issn.1674-8999.2024.10.369
64.
Lu Y Li CC Gao T Guo YY . Clinical observation on treatment of perimenopausal insomnia with Baizi Yangxin decoction and acupuncture. J Pract Tradit Chin Intern Med. (2022) 36:8–10. doi: 10.13729/j.issn.1671-7813.Z20210186
65.
Song S Chen H Fu H . Systematic review and meta-analysis on the efficacy and safety of acupuncture for perimenopausal insomnia. Front Neurol. (2025) 16:1649856. doi: 10.3389/fneur.2025.1649856,
66.
Soares CN Frey BN . Challenges and opportunities to manage depression during the menopausal transition and beyond. Psychiatr Clin North Am. (2010) 33:295–308. doi: 10.1016/j.psc.2010.01.007,
67.
de Zambotti M Colrain IM Baker FC . Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. (2015) 100:1426–33. doi: 10.1210/jc.2014-3892,
68.
Moline ML Broch L Zak R Gross V . Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev. (2003) 7:155–77. doi: 10.1053/smrv.2001.0228,
69.
Lin Y Chen Y Lin Y Xin S Ren A Zhou X et al . Association between sleep quality and ovarian reserve in women of reproductive age: a cross-sectional study. Fertil Steril. (2025) 123:520–8. doi: 10.1016/j.fertnstert.2024.09.018,
70.
Kang W Malvaso A Bruno F Chan C-K . Psychological distress and myocardial infarction (MI): a cross-sectional and longitudinal UK population-based study. J Affect Disord. (2025) 384:47–52. doi: 10.1016/j.jad.2025.05.016,
71.
Freeman EW Sammel MD Liu L Gracia CR Nelson DB Hollander L . Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. (2004) 61:62–70. doi: 10.1001/archpsyc.61.1.62,
72.
Sutton EL . Insomnia. Ann Intern Med. (2021) 174:ITC33–48. doi: 10.7326/AITC202103160,
73.
Riemann D Nissen C Palagini L Otte A Perlis ML Spiegelhalder K . The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. (2015) 14:547–58. doi: 10.1016/S1474-4422(15)00021-6,
74.
Zhao F-Y Fu Q-Q Spencer SJ Kennedy GA Conduit R Zhang W-J et al . Acupuncture: a promising approach for comorbid depression and insomnia in perimenopause. Nat Sci Sleep. (2021) 13:1823–63. doi: 10.2147/NSS.S332474,
75.
Wang Y Guan R Zhong J Shi Q Ye Z Pan L . Research progress on the treatment of perimenopausal insomnia with chaihu jia longgu muli decoction based on brain-intestine-bacteria axis: a review. Medicine. (2023) 102:e36537. doi: 10.1097/MD.0000000000036537,
76.
Sun A Wang Y Gu B Zheng T Lin S Bai W et al . A multi-center, randomized, controlled and open clinical trial of heyan kuntai capsule (和颜坤泰胶囊) and hormone therapy in perimenopausal women. Chin J Integr Med. (2018) 24:487–93. doi: 10.1007/s11655-016-2266-y,
77.
Wu X Zhang W Qin Y Liu X Wang Z . Effect of acupuncture and its influence on cerebral activity in perimenopausal insomniacs: study protocol for a randomized controlled trial. Trials. (2017) 18:377. doi: 10.1186/s13063-017-2072-7,
78.
Kim S-A Lee S-H Kim J-H van den Noort M Bosch P Won T et al . Efficacy of acupuncture for insomnia: a systematic review and meta-analysis. Am J Chin Med. (2021) 49:1135–50. doi: 10.1142/S0192415X21500543,
79.
Zhao F-Y Spencer SJ Kennedy GA Zheng Z Conduit R Zhang W-J et al . Acupuncture for primary insomnia: effectiveness, safety, mechanisms and recommendations for clinical practice. Sleep Med Rev. (2024) 74:101892. doi: 10.1016/j.smrv.2023.101892,
80.
Yang B Jiang S Teng Y Wang Y Zhang J Gao C et al . Integrated acupuncture-pharmacotherapy for perimenopausal insomnia: a systematic review and meta-analysis. Front Neurol. (2025) 16:1633794. doi: 10.3389/fneur.2025.1633794,
81.
Jiang S Zhang Y Sun Y . The effectiveness and safety of acupuncture combined with medication in the treatment of perimenopausal insomnia: a systematic review and meta-analysis. Front Neurol. (2025) 16:1476719. doi: 10.3389/fneur.2025.1476719,
82.
Hu J Teng J Wang W Yang N Tian H Zhang W et al . Clinical efficacy and safety of traditional Chinese medicine xiao Yao san in insomnia combined with anxiety. Medicine. (2021) 100:e27608. doi: 10.1097/MD.0000000000027608,
83.
Zhuang W Liu S-L Xi S-Y Feng Y-N Wang K Abduwali T et al . Traditional Chinese medicine decoctions and chinese patent medicines for the treatment of depression: efficacies and mechanisms. J Ethnopharmacol. (2023) 307:116272. doi: 10.1016/j.jep.2023.116272,
84.
Wang L Liu Y Qi X Luo H . Perimenopausal depression: etiology, clinical characteristics, and the role of traditional Chinese medicine. Int J Psychiatry Clin Pract. (2025) 29:110–6. doi: 10.1080/13651501.2025.2501250,
85.
Zhang X Luo L Wang C Lv W Duan Y Kong L . Research progress on chaihu shugan san in treating perimenopausal syndrome: a review. Medicine. (2024) 103:e41044. doi: 10.1097/MD.0000000000041044,
Summary
Keywords
insomnia, meta-analysis, perimenopause, randomized controlled trial, sleep disorder, traditional Chinese medicine
Citation
Yang D, Chen D, Peng J, Peng Y, Yang C and Sun J (2026) Efficacy and security of traditional Chinese medicine in the treatment of perimenopausal insomnia in the Chinese population: a systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 17:1749660. doi: 10.3389/fneur.2026.1749660
Received
19 November 2025
Revised
28 January 2026
Accepted
04 February 2026
Published
19 February 2026
Volume
17 - 2026
Edited by
Iván Pérez-Neri, National Institute of Rehabilitation Luis Guillermo Ibarra Ibarra, Mexico
Reviewed by
Paulo Sargento, Lusofona University, Portugal
Jieying Zhang, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, China
Updates
Copyright
© 2026 Yang, Chen, Peng, Peng, Yang and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jungang Sun, 32298854@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.