- 1Clinical-based Human Research Department, Centre for Osteopathic Medicine Collaboration, Pescara, Italy
- 2Accademia Italiana Osteopatia Tradizionale, Pescara, Italy
- 3Department of Neuroscience, Imaging and Clinical Sciences “G. D'Annunzio” University of Chieti-Pescara, Pescara, Italy
- 4ITAB-Institute for Advanced Biomedical Technologies, “G. D'Annunzio” University of Chieti-Pescara, Pescara, Italy
- 5Department of Biomedical and Neuromotor Sciences, Bellaria Hospital, University of Bologna, Bologna, Italy
- 6IRCCS Istituto delle Scienze Neurologiche di Bologna, AUSL di Bologna, Bologna, Italy
Historically, approaches used in manual medicine to explain patient reported symptoms have been focused on the so-called exteroceptive paradigm. Arguably, this mindset lacks an appropriate “reading system” able to interpret musculoskeletal disorders from a different perspective, where the properties of the nervous system are embraced into a more holistic and functional-related context. Interestingly, if the underpinning mechanisms of a given treatment scenario/effect are taking into account, the majority of research outcomes focuses on a proprioceptive/exteroceptive explanation, leaving ting aside the additional or even central role of interoception. Currently, to date, the application of theoretical knowledge acquired on the relatively recent neuroscientific concepts and evidence concerning of interoception, sensitization, touch, autonomic functions, inflammation, and pain into a clinical/research manual medicine scenario is lacking, even if theoretically, the impact on the possible etiological mechanisms and treatment effects seems to be important. Here, we propose the conceptual foundations for a new way of interpreting and reading patients' clinical reported outcomes scenario based on interoception and sensitization. We argue that this will provide a foundation to create the ground for future research focusing on the hypotheses that manual therapies, specifically osteopathy, can intercede with sensitization states, at all levels, using interoceptive pathways.
Introduction: Input—Central Elaboration—Output
Interoception can be described as the moment-to-moment representation process of body sensations coming from the body itself (Craig, 2002). A broader definition considers interoception as a multi-dimensional construct, which includes how people evaluate and react to these sensations (Cameron, 2001). Interestingly, several health problems involve altered interoceptive processes, including chronic pain (Schmidt et al., 1989), post-traumatic stress disorder (Wald and Taylor, 2008), affective disorders (Paulus and Stein, 2010), addiction (Naqvi and Bechara, 2010), eating disorders (Pollatos et al., 2008; Herbert and Pollatos, 2014), somatoform disorders (Mirams et al., 2012; Schaefer et al., 2012), and dissociative disorders (Hankin, 2012; Michal et al., 2014; Sedeno et al., 2014).
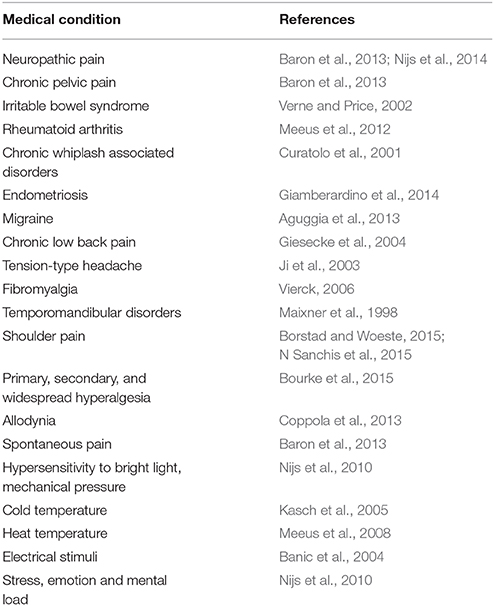
Sensitization is defined as the neurologically-based amplification response produced by repeated stimuli. To date, evidence consistently highlights that several subgroup of patients, with or without pain-related syndromes, exhibit a documented sensitization (Table 1). Notwithstanding this, it is still unclear how to recognize a sensitization state using clinical objective measure and tests (see Nijs et al., 2010, for guidelines).
The close link between interoception and sensitization (Flor et al., 2004), the individual time-course responses to sensitization (Baron et al., 2013) and the interpersonal clinical variability, create a scenario in which practitioners have to deal with a range of clinical circumstances possibly characterized by the following:
1. unexplainable symptoms, i.e., chest pain may depend upon aberrant pain processing from the esophagus due to sensitization of spinal dorsal horn cells and supraspinal centers (Mertz et al., 1998);
2. indecipherable pathogenesis, i.e., angina attack could be referred to the site of an old vertebral fracture (Henry and Montuschi, 1978);
3. clinical heterogeneity, considering the autonomic effects as relevant co-aspect of patient's clinical manifestations;
4. causal clinical validity of instrumental exams, i.e., the disease progression of osteoarthitis seems to be better associated with sensitization than with the actual joint destruction assessed by radiological scorings (Arendt-Nielsen et al., 2010);
5. unintelligible treatment outcomes, i.e., referred muscle hyperalgesia could persist even long time after the disappearance of primary focus in the viscous (Giamberardino, 2003);
6. unforeseeable prognosis, i.e., after an initial whiplash trauma, the presence of a sensitization process is important to predict the development of chronicity (Sterling et al., 2003);
7. clinical vs. scientific uncertainty of effects and mechanisms of therapies.
The seven above-mentioned points could highlight common heterogeneous scenarios in daily clinical practice, which in turn could impair the practitioners' ability to achieve better health outcomes to their patients. However, the scientific neurological underpinnings, mainly based on interoception and sensitization, found in the last two decades of research, may arguably revolutionize the way in which practitioners could “interact” with their patient in the clinical context.
The aim of the present review is to introduce, discuss and transfer the emerging concepts of interoception and sensitization to the context of manual medicine, specifically osteopathy. To this end, we propose an interdisciplinary, and innovative paradigm (the “interoceptive paradigm”) to interpret patients' signs and symptoms as well as patient's phenomenological changes and possibly use it for further clinical- and lab- based research.
The Afferent System: The Input Stimuli to the CNS
The modern classification of the sensory system evolved from the work of Sir Charles Sherrington who codified the senses into teloreceptive (vision and hearing), proprioceptive (limb position), exteroceptive (touch, including temperature and pain), chemoreceptive (smell and taste), and interoceptive (visceral) modalities (Sherrington, 1906). However, in the light of recent findings on neurofunctional anatomy, the sensory system can be divided into: teloreceptive, exteroceptive/proprioceptive (also classified as A/sensory system), and interoceptive/nociceptive (otherwise named B/sensory system; Craig, 2002). The differences between A and B system arealso embryologically proven: the development of small-diameter interoceptive afferents originating from small (B) cells is coordinated with the development of lamina 1 cells and represents a well distinct entity from the large-diameter exteroceptive afferents originating from large (A) cells that project to the deep dorsal horn, not connecting with lamina I neurons (Prechtl and Powley, 1990; Woodbury et al., 2001). This embryological difference implies not only different anatomical compounds (i.e., type and distribution of receptors, type of primary afferent nerve fibers, type of central afferent pathways), but also unique functional and physiological features (i.e., label lines, type of sensation—epicritic vs. protopathic, fiber threshold, habituation process, distinct phycophysically feelings). These characteristics generate two clear and distinct roles in decoding external and internal stimuli.
The stimuli can be diverse and organized by type—metabolic, physics, chemical, mechanical, and fluidic—upon the function of time—acute or chronic—frequency and intensity—low vs. high. Therefore, the ability of the two sensory pathways to detect variations (the major characteristics of any receptors) producing action potentials, initiates the process of selectively decoding stimuli coming from the outside and from the inside. Consequently, animals have the ability to decode, process, perceive the external (mainly using the teloreceptive and exteroceptive that is the A/sensory system) and the internal milieu (typically utilizing the interoceptive B/sensory system).
Interoception
Interoception has been recently reinterpreted by Craig as “the sense of the physiological condition of the entire body” (Craig, 2002), not merely the input coming from the viscera as historically described by Sherrington. This system is an ongoing homeostatic afferent pathway, argued as the sensory complement of the ANS (Craig, 2013), that carries signals from small-diameter Adelta and C primary afferent fibers that represent the physiological status of every tissue of the body. Once homeostatic information from tissues are decoded, then they are conveyed up to the anterior insula after making synaptic relays at different levels (spinal cord–lamina I and II, brainstem–homeostatic regions, thalamus). At the insular level, a meta-representation of the perception of self-emerged as a feeling (sentient) entity, which is a pre-stage for emotional awareness. Detailed reviews of the interoceptive evidence are available elsewhere (Craig, 2002, 2003, 2009).
Converging neurobiological evidence pointed out that the insular cortex (IC) is a critical hub for multimodal interoceptive integration. Thus, the IC has been implicated in interoceptive processes, such as awareness of bodily sensations (Khalsa et al., 2009), but also exteroceptive processes, such as perception of pain (Brooks et al., 2002; Gramsch et al., 2014), taste (Gagnon et al., 2014; Iannilli et al., 2014; Parabucki and Netser, 2014; van den Bosch et al., 2014), smell (Kurth et al., 2010), and touch (McGlone et al., 2014). Furthermore, emotional domains overlap in the anterior insula together with the interoceptive and exteroceptive scenario (Kurth et al., 2010), suggesting an underlying commonality (Critchley et al., 2002). As matter of fact, the insula has been proposed a convergence point between internal and external milieus (Azanon and Soto-Faraco, 2008; Mazzola et al., 2009; Azanon et al., 2010; Ibanez and Manes, 2012). In addition, it has been showed that external signals might also be considered as body-mapped signals of an interoceptive peripersonal space (Couto et al., 2015), particularly in the context of pain where afferent signals could be conceived as an extension of interoceptive processing to peripersonal space (Ferri et al., 2013). Notably, based on new evidence emerging from the field of touch in relation to interoception, it can be proposed the existence of an “interoceptive touch” (also referred as gentle/affective touch), which is mediated by low mechanical threshold C fibers (named C-tactile fibers or CTs), and whose analyses take place in the interoceptive stations, that is, lamina II of the spinal cord, thalamus, insular cortex (McGlone et al., 2014).
Taken together, available evidence indicates that sensory information (like nociception and touch) may be integrated by insular networks in a peripersonal-like fashion way and then further processed by emotional awareness and social behavior mechanisms.
Central Elaboration: The Sensitization State
Sensitization is generally defined as a non-associative learning process in which repeated stimuli bring to a progressive amplification of a response (Ursin, 2014). Sensitization has been considered a form of “nociceptive” memory because of similarity between its mechanisms with memory mechanisms (Ji et al., 2003). Considering the neuroanatomical and neurophysiological compound, it is possible to distinguish peripheral sensitization (PS) from central sensitization (CS). PS is defined as an increased responsiveness and, therefore, reduced threshold of nociceptors to stimulation (Sandkuhler, 2009). It has been argued that it has an protective role (Nijs et al., 2014) as increasing pain sensitivity into the site of inflammation (Ji et al., 2003) can prevent further damages (Sandkuhler, 2007). Indeed, PS is characteristic of tissue where inflammatory mediators such as prostaglandin E2, bradikinin, nerve growth factor, substance P (SP) are released altering, in turn, threshold and kinetics of receptors and ion channels of the nociceptive Adelta and C fiber nerve endings (receptor' sensitization). PS is clinically expressed through primary hyperalgesia (increased pain sensitivity at site of injury; Cervero, 2009; Sandkuhler, 2009) and allodynia (pain in response to a non-nociceptive stimulus; Sandkuhler, 2009). CS is a cellular process of increased excitability (Sandkuhler, 2007) that occurs within the CNS. CS includes altered sensory processing in the CNS, such as: (1) alterations of the descending inhibitory pathways arising from the periacqueductal gray matter and the rostral ventral medulla (Meeus et al., 2008), (2) temporal summation of second pain (wind-up; Arendt-Nielsen et al., 1994).
Historically, along the CNS, the spinal cord is the first station in which CS was found. Spinal cord sensitization is characterized by: (1) reduced threshold, (2) increased receptive field sizes, and (3) greater evoked responses in hyperexcitable spinal neurons of dorsal horn as a result of a short barrage of nociceptor input (Woolf and Wiesenfeld-Hallin, 1986; Woolf, 1993). The phenomenon is known as “activity-dependent CS” (Ji et al., 2003) or “homosynaptic facilitation,” and is characterized by the release of several neurotransmitters including SP (see Ji et al., 2003; Sandkuhler, 2009 for reviews). Spinal cord sensitization is a multifaceted phenomenon that has at least three, albeit secondary, actors: (1) A-afferent system, (2) spinal glial cells, and (3) ventral horn motoneurons. (1) Following both peripheral inflammation and nerve injury, it has been showed a phenotypic switch in some large A-system (non-nociceptive), DRG neurons that begin to express key molecules typical of CS, that is SP and BDNF (Neumann et al., 1996; Mannion et al., 1999). (2) Spinal glial cells have an intermediary role between the initial insult and neuronal plastic changes leading to pain amplification (Sandkuhler, 2009), and microglia in dorsal horn seems to have a particular role in inducing neuropathic pain (Watkins et al., 2001). Notwithstanding this, after a peripheral injury or inflammation, microglia (Aldskogius and Kozlova, 1998), and astrocytes (Lee et al., 2009) in spinal dorsal horn rise in number. (3) Although research on CS only indirectly focuses on ventral horn motoneurons by using the withdrawal reflex—a surrogate of enhanced nociception (Sandkuhler, 2009)—, recently it has been described a direct bradykinin-mediated sensitization of spinal lumbar motoneurons of rats (Bouhadfane et al., 2015).
Although, the precise mechanisms remain still understudied, it appears that a mixed of events is necessary to start and sustain the sensitization state in the spinal cord, clinically mainly showed with secondary hyperalgesia, that is an increased pain sensitivity in an area adjacent to the site of injury (Sandkuhler, 2009).
Several research studies have been conducted to examine neuronal sensitization at higher levels along the interoceptive pathway, in particular: (a) spinothalamic tract (Simone et al., 1991; Willis, 2002); (b) brainstem: rostroventral medulla (Porreca et al., 2002); and trigeminal nuclei (Hu et al., 1992)—especially trigeminal subnucleus caudalis (Cao et al., 2013; Wang et al., 2013); (c) diencephalum, thalamic neurons (Park et al., 2006; Kaneko et al., 2011), in the thalamic-anterior cingulate pathway (Shyu and Vogt, 2009), hypothalamic neurons (Peng et al., 2011; Daviu et al., 2014; Donnerer and Liebmann, 2015), in the hypothalamic-pituitary-adrenal axis (Daviu et al., 2014); (d) telencephalic level, including the anterior cingulate cortex (Wei and Zhuo, 2001), amygdala (Neugebauer and Li, 2003), and insular cortex (Qiu et al., 2014).
Collectively, the studies on sensitization begin to show that the PS is a well-studied phenomenon with clearly identified biological pathways. On the contrary, a part from spinal cord sensitization, CS is still an on-going area for research, which showed several characteristics according to the neuronal level considered, but a unified central sensitization state scenario is still lacking.
The Efferent System: The Vegetative-Somatic Dichotomy
The efferent pathway might be divided into a “somatic” and “vegetative” systems throughout the central (upper neuron) and peripheral (lower neuron) nervous system. The “somatic” system is composed by all those tracts which control the motor movements, the “vegetative” pathway generates control on all the functions out of the control of the conscious self.
Vegetative Output
John N. Langley coined the terms “autonomic nervous system” and “parasympathetic nervous system” about the turn of the twentieth century (Langley, 1921), to describe a system that is autonomous, involuntary, and regulates the body's “inner world.” However, the classical distinction between sympathetic and parasympathetic has been recently reviewed in the light of differential responses to stressors and differential involvement in pathophysiological states (Buijs et al., 2003; Buijs, 2013) between the various parts of the autonomic nervous system (ANS). Goldstein proposed that the ANS has at least five components with specific functions: the sympathetic noradrenergic system, the sympathetic cholinergic system, the parasympathetic cholinergic system, the sympathetic adrenergic system, and the enteric nervous system (see Goldstein, 2001, 2006, 2013b; Goldstein and McEwen, 2002, for reviews). In addition, the ANS should not only be seen as a system merely carrying out the commands of the brain; it also functions as a reflex circuit, using the sensory feedback of the organs, to change and precisely adapt the its output in order to adjust the physiological state of the body.
Although much is known about the organization of the ANS output to organs, there is relatively little knowledge regarding the feedback of organs to the brain. It is safe to assume that every organ has the capacity to reach the brain, i.e., via the release of hormones, and thus to provide feedback to the control center of the ANS, mainly using the interoceptive pathway. Most of these metabolic signals may be aimed at regulating the function of the organ in a “reflex” manner, but there is also evidence that this feedback may influence the function of other organs, or behavior, via neuronal sensory feedback (Uno et al., 2006; Warne et al., 2007). This mechanism has been also described in the context of inflammation as neurogenic inflammatory mechanism or more recently “neurogenic neuroinflammation” (Xanthos and Sandkühler, 2014) to define the participation of afferent nerve fibers (mainly amyelinated afferent C fibers–B/afferent system), using an antidromic transmission, to local inflammatory reaction in response to local metabolic modifications due, for example, to infection, trauma, stress, hormonal changes, thus variations in the interoceptive milieu. The aim is to maintain the integrity of the conditions of life within the internal environment,—originally the “milieu intérieur” (Bernard, 1912) then extended to “homeostasis” (Cannon, 1929)—through a mechanism of allostatic adaptation (McEwen, 2007), which can involve the release of key substances like SP, glucocorticoids, catecholamines, and different cytokines (McEwen, 2007; Goldstein and Kopin, 2008).
The active process of responding to challenges is called “allostasis.” This involves several mediators, including autonomic, cortisol, immune/inflammatory, metabolic, and neuromodulators within the brain, that interact non-linearly and promote tuning adaptation in the short term. Overuse (i.e., too much stress) or dysregulation among the mediators (e.g., too much or too little cortisol; too much or too little inflammatory cytokines) can produce cumulative changes that is referred to as “allostatic load and overload” (McEwen, 1998), which in turn can produce sensitization state. This allostatic load, a wear and tear response, produced by the repeated activation of adaptive mechanisms, can last for long and eventually result in a significant alteration of physiological resilience systems (McEwen et al., 2015a,b), which can produce exacerbation of clinical symptoms, including chronic pain.
In addition, several studies demonstrated as the neurogenic inflammation may be evident also at a distant site from the original exposure (Black, 2002). This can be established through the use of different but specific metabolites (i.e., SP), mechanisms (i.e., axon reflex, reverberation), and systems (i.e., immune system; see Xanthos and Sandkühler, 2014, for a review). Furthermore, recent available evidence suggests that neuronal activity in primary afferent peptide C nerve fibers or higher-order neurons is sufficient to elicit neurons in the spinal cord, vascular cells and innate and adaptive immune cells (Xanthos and Sandkühler, 2014).
Interestingly, the central control of the outflow of sympathetic nervous information is strategically organized as a long chain of motor neurons in the intermediolateral column of the spinal cord. This segmental organization allows the ACh-producing motor neurons to establish synapses with different and multiple ganglions along the spinal cord which contain neurons that use different neurotransmitters (i.e., noradrenaline/norepinephrine, neuropeptide Y; Lundberg et al., 1983). This anatomical and functional scenario permits to widen the vegetative output responses to different organs and tissues, creating the neurological ground for modifying the functions of distant bodily sites.
Muscle-Skeletal Output (Alfa Gamma Precision and Strength)
The second output pathway is the somatic efferent that is characterized by a central and peripheral component. The former is based on a series of tracts, which transport different information from different brain areas (i.e., motor cortex, cerebellum, basal ganglia, forebrain, midbrain) addressing different functions. The latter is characterized by specific efferent somatic neurons (alfa, beta, and gamma motorneurons) interconnected in a fashion-like function (i.e., alfa-gamma coactivation mechanism) controlling the striate muscles of the all body for precision and strength (cortico-spinal tract from the motor cortex), adjustment of head position in response to visual/auditory information (tetto-spinal tract from the superior/inferior colliculi), balance adjustment (cerebellum-spinal tract–cerebellum, rubro-spinal tract–red nucleus, reticulo-spinal tract–reticular formation).
Integration Between the Two Systems
The interconnection between the two systems is clear both from a neurological and metabolic perspective. Neurologically, vegetative output, and muscle-skeletal output are centrally integrated and reciprocally modulated in many areas of neural axis. Furthermore, are both integrated with the neuroendocrine system, allowing a high-complex level of integration, crucial to reach a coordinated response to ensure homeostasis (Jänig, 2006). Metabolically, nerves, both somatic and autonomic, are intimately associated with inflammatory cells; this is especially true of mast cells which resemble nerve cells in many respects (Purcell and Atterwill, 1995) and confirmed in the bradykinin-induced plasma extravasation inflammation model (Janig and Green, 2014). Moreover, recent evidence showed a mutual somato-vegetative relationship through the activation of an immune-mediated pathway (Sankowski et al., 2015).
Clinical Applications and Implications
“Scientific integrative medicine is not a treatment method or discipline but a way of thinking that applies systems concepts to understand normal physiology and clinical disorders, providing a framework for understanding complex and dynamic challenges to our integrity as organisms and, in turn, for developing novel treatments based on this complexity and dynamism” (Goldstein, 2013b, p. 16).
According to the homeostasis theory, stress is considered a state or a condition, in which expectations mismatch the perceptions of the external or internal environment (Goldstein and McEwen, 2002). This incongruity produces patterned and compensatory responses that can change not only the physiology of a target organ but also the general bodily reaction. Stress can be interpreted in terms of an error signal, due by different sources or triggers (i.e., traumatic injury, psychological condition, genetic and/or acquired diseases), which can reflect the difference between input information as felt, neural central “multimodal” elaboration and a series of effects determined by a regulator, possibly the ANS (Goldstein, 2013a). This concept has an intuitive clinical practice application for interpreting the patient's clinical history and treatment effects.
Implications in Manual Therapy, Specifically in Osteopathy
As general rule, the above-mentioned concepts can be applied at any (para-) medical approach including those methods that use a touch-based practice. For the purpose of this review, the following section will focus more on the translation of sensitization and interoception concepts into the field of osteopathy, a drug-free manual medicine approach, which uses touch and manipulation as procedures to diagnose, evaluate and treat (Cerritelli et al., 2015a,b). Osteopathic procedures include a structural evaluation followed by a treatment. The structural evaluation aims to diagnose somatic dysfunctions. It includes an accurate manual assessment of the skull, spine, pelvis, abdomen, upper, and lower limbs to locate bodily areas with an alteration of specific tissue parameters. The treatment includes the application of a range of manipulative techniques aimed at relieving the somatic dysfunctions. Notwithstanding the osteopathic interest, one of the goals of the current review is to propose a modern clinical neuroscience-based praxis to “read and interpret” patients' signs and symptoms, which can be widely shared across disciplines.
Very little research explored the effect of osteopathic manipulation on brain functions. Fryer et al. pointed out that the application of a single high-velocity low-amplitude lumbosacral joint osteopathic manipulation decreases the corticospinal and spinal reflex excitability measured with TMS and EMG suggesting an inhibitory effect at the level of the spinal cord (Fryer and Pearce, 2012).
Moreover, OMT seems to be associated with a reduction of pro-inflammatory substances both in vitro (Meltzer and Standley, 2007) and in vivo (Licciardone et al., 2012, 2013) hypothesizing an anti-inflammatory role of OMT, although only partially confirmed by recent clinical-based research (Degenhardt et al., 2014).
Osteopathic manipulations, therefore, could reduce the release of cytokines and the sympathetic activity creating a cascade of biological and neurological events that modulate the inflammatory and ANS mechanisms. The application of OMT was demonstrated to influence the ANS, producing a parasympathetic effect (Henley et al., 2008; Giles et al., 2013; Ruffini et al., 2015) and leading, therefore, toward a trophotropic tuning state (Ruffini et al., 2015). Remarkably, no differences were found in sham light-touch control in which only a simple touch was used. This might imply that a “technical” touch must be administered to produce effects. Thus, the operator and its education have a central role.
More recently, starting lab-based evidence showed the effect of specific osteopathic techniques on the enhancement of the lymphatic and immune system (Schander et al., 2012, 2013) by improving the leukocytes count and interleukin-8 (IL-8). Findings were confirmed by a recent 2014 paper where significant differences were detected in the levels of immune molecules, including IL-8, between OMT and sham light-touch control (Walkowski et al., 2014). OMT, therefore, could also have an effect on the immunological profile of specific circulating cytokines and leukocytes. As suggested by Xanthos and Sandkühler, treatments and interventions that are targeted at various levels to inhibit the source of inflammation and neuroinflammatory processes, or to promote the resolution of inflammation, would be recommended to interrupt the neurogenic neuroinflammatory vicious cycle (Xanthos and Sandkühler, 2014). Hypothesising that the osteopathic treatment would fulfill those requirements, in particular the anti-inflammatory action, it could be argued that being exposed to osteopathy can terminate neuroinflammation and reduce pathological outcomes.
However, although starting evidence tried to explore how osteopathy might work, there is no consensus regarding which “channels” osteopathy uses to produce its effects. In fact, the diagnostic knowledge of manual medicine historically is based and built on the prevalently exteroceptive consideration of symptom (i.e., postural interpretation, muscular chains) through the pure “muscle-skeletal paradigm” or “exteroceptive paradigm” in which (1) proprioceptive/exteroceptive afferent activity is integrated in (2) the central motor systems and (3) the output goes out through the Sherrington's final common pathway (alfa-gamma motor neurons of anterior horn of spinal cord). This kind of “exteroceptive paradigm” has been used, for example, in Korr's hypothesis of hyperactive monosynaptic stretch reflex as explanation for the reduction of range of motion (ROM) (Korr, 1975; Howell et al., 2006). However, considering the routinely clinical practice, it is important for clinicians to recognize that feelings from the body, such as pain, are neurologically distinct from tactile mechanoreception and proprioception at all levels. In fact, osteopathic medicine (OM) practitioners face daily clinical cases that cannot be fully explained by the “exteroceptive paradigm” as it lacks of a “clinical reading system” which is able to consider the patient as a whole and not merely a muscle-skeletal body entity. Thus, a wider approach, able to possibly better explain, evaluate, link and predict patients' signs and symptoms is recommended.
Here we propose the “interoceptive paradigm” in which (1) altered (acutely and chronically) interoceptive information lead to (2) neurological “sensitization states” (SS) that express their dysfunction through (3) an altered firing of the autonomic nervous system (ANS), which in turn (4) brings the peripheral tissue to an hypersensitivity state and, thus, creating the ground for a (5) vicious metabolic and neurologic cycle (positive feedback loop) and rapid system failure (Figure 1). The recognition of this paradigm will impact the clinical practice with several advantages/benefits:
• Appropriate clinical interpretation of symptoms with respect to the causal and pathogenetic aspects;
• Pertinent ability to “read” and “elucidate” the clinical history, linking aspects related to organs functions, neurology, and pathophysiological adaptation/compensation;
• Adequate comprehension of roles in the mutual doctor-patient relationship.
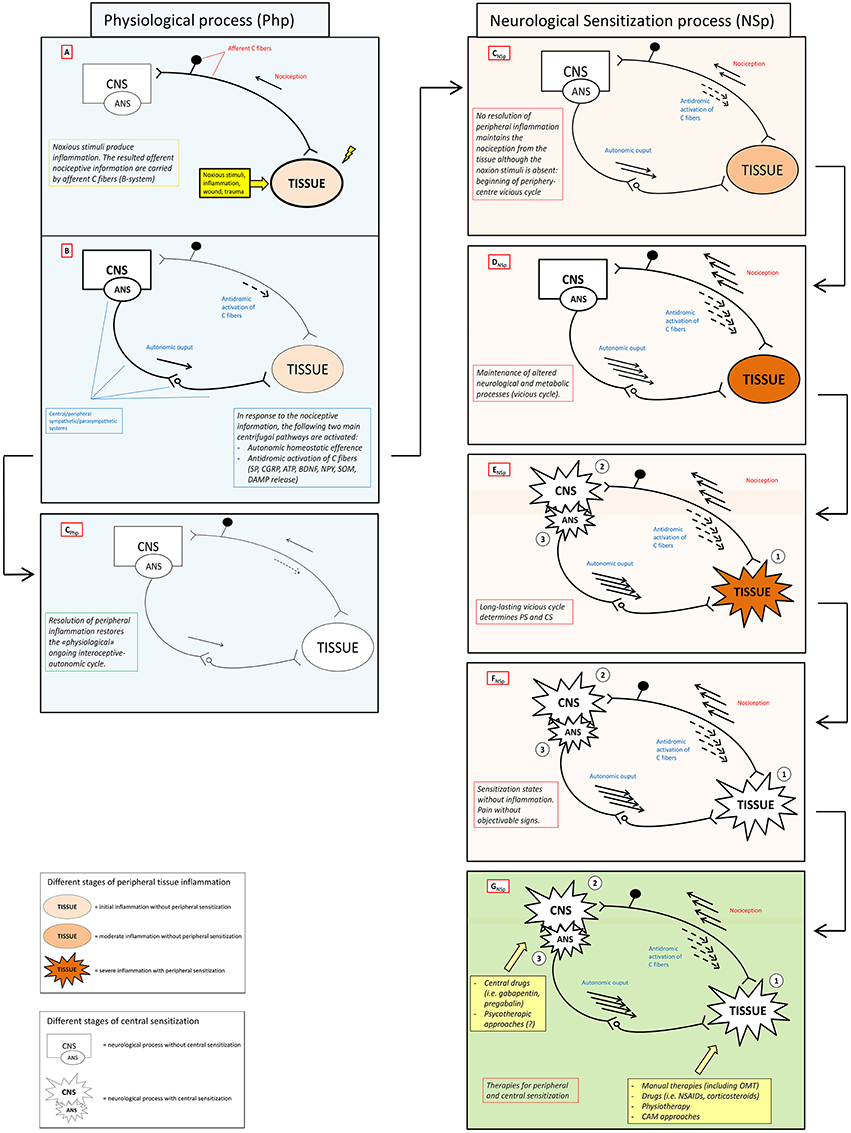
Figure 1. Neuronal activity in physiological and sensitization processes. The figure schematically shows the antithetic processes and relative outcomes occurring in physiological (Php) and neurological sensitization process (NSp) after that a stimulus (noxious stimuli, inflammation, wound, trauma) activates the nociceptive afference (box A). Physiologically, antidromic activation of C fibers, the so-called neuorogenic inflammation, and specific autonomic efferences (box B) sustain peripheral healing process restoring both homeostasis and physiological ongoing interoceptive-centrifugal communication between periphery and CNS (box CPhp). In the right column (pink boxes) lacking of resolution of peripheral inflammation sustains nociceptive afference with a consecutive amplification of centrifugal phenomena (boxes CNSp – DNSp) that can become maladaptive or neurotoxic, see Xanthos and Sandkuhler for details (Xanthos and Sandkühler, 2014). Maintenance of this metabolic-neurological TISSUE-CNS vicious cycle (box ENSp)could bring to PS (1) and CS (2) as well as to a never-ending self-maintenance inflammation state (3) (box FNSp). Several therapies (box FNSp), including OMT, could be theoretically administered to solve neurological sensitization in different clinical conditions where PS and/or CS are present. CNS, central nervous system; ANS, autonomic nervous system; PS, peripheral sensitization; CS central sensitization; SP, substance P; CGRP, calcitonin gene-related peptide; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; NPY, neuropeptide Y; SOM, somatostatin; CB, cannabinoid; DAMP, danger associated molecular patterns; OMT, osteopathic manipulative treatment; NSAIDs, non-steoridal anti-inflammatory drugs; CAM, complementary alternative medicine.
Although, according to some authors (Sandkuhler, 2007; Cervero, 2009), sensitization is a status of increased excitability at the cellular level, it can be also used in a broader perspective, at clinical and behavioral levels (Coppola et al., 2013; Ursin, 2014), to describe an increased sensitivity to pain or an increased excitability of the central nervous system (CNS). It has been shown that an input is necessary to start, sustain, or impair the sensitization process (Melzack et al., 2001; Affaitati et al., 2011; Baron et al., 2013). Being stimulus-dependent, sensitization can be seen has an adapting response of CNS to environmental challenges presenting through the nociceptive afference that not necessarily has to become subjectively perceived (Treede et al., 1999; Kidd and Urban, 2001; Sandkuhler, 2009). This is remarkable from a case history taking and, more generally, a diagnostic point of view. From a therapeutic perspective, it is important to consider that touch could be a potential input able to modify the sensitization state. Indeed, emerging evidence showed the relevance of a gentle/affective touch to elicit CT fibers and therefore modulate the interoceptive pathway. This produces a central reaction which in turn evokes a series of neurological events that brings the ANS to respond to a given stimulus. This type of touch is different from the well-known “exteroceptive touch” that is mediated by the low-threshold mechanoreceptors (LTMs) innervated by Abeta afferents. This “exteroceptive touch” is able to rapidly detect, discriminate and identify external stimuli to prepare an appropriate sensorimotor transformation. On the contrary, CTs, found only in hairy but not glabrous skin, respond to slow (1–10 cm/s), weak (0.3–2.5 mN), mechanical stimuli (Perini et al., 2015). It has been shown that CTs are also temperature tuned at 32°C and are associated to sensual touch (Ackerley et al., 2014; Perini et al., 2015) suggesting not only a socially-relevant function (Perini et al., 2015) but also a possible role in the neurodevelopment during the perinatal period (Bystrova et al., 2009).
Translating this evidence into a clinical touch-based manual medicine perspective, although the role of CTs in pain modulation (especially allodynic experience) remains an open question (Nagi et al., 2011; Delfini et al., 2013), the interoceptive affinity of a well determined type of touch able to activate CTs provide a rational basis for complementary medical approaches like therapeutic touch (Craig, 2013). In addition, these findings reveal that feelings from the body, such as pain, are inherently linked with autonomic conditions, such as plasma extravasation or cardiac rhythmicity, because they are, respectively, sensory and motor aspects of the same homeostatic system. Moreover it is important to consider that also the exteroceptive touch mediated by low-threshold-mechanoceptors could modulate efferent activity of the ANS, especially locally (Jänig, 2006; Craig, 2014). Thus, the relationship between manual therapies effects, specifically osteopathy, and interoception implications can be argued at the light of the prevailing neuroscience literature, although not formally tested.
Continuing to translate neuroscientific paradigms into osteopathy, interestingly, Livingstone (1943) proposed that the afferent activity produced by injured peripheral nerves elicits an abnormal firing pattern within the spinal cord. The author argued that a disturbance occurs in an internuncial pool of dorsal horn interneurons resulting in reverberatory activity that spreads to various areas of the spinal cord, including the sympathetic chain. Increased activity in sympathetic output would interfere with vasoregulation and induce further hypersensitivity of peripheral tissue, leading to increased afferent input and a vicious circle of peripheral-central activity. If this process lasts for long, then a sensitization state is produced, as described above. Therefore, if the osteopathic touch is supposed to produce an anti-inflammatory and hyper-parasympathetic effect, it can be argued that, potentially, modulating the vegetative firing, it can produce positive feedback effects on the sensitization state.
As final remarks, it is paradoxical the difference between the presence of interoceptive and sensitization phenomena in osteopathic clinical practice, and the almost absence of these concepts in the philosophical, diagnostic, and therapeutic body of OM. This paradox becomes dramatic considering the neurological “qualitative” nature (intero/nociceptive) of the ultimate symptom faced in medical setting: the pain. As Craig argued, from a therapeutic perspective it is relevant to consider that when patients report their symptomatology they are possibly giving a description of the condition of homeostatic systems (Craig, 2013). However it is uncertain patients' accuracy in describing internal states (Petersen et al., 2015). As a matter of fact, it could be important listening to and reporting the spontaneous patients' feelings during the treatment phase. It might represent a potential online homeostatic/allostatic feedback, which can be used for optimizing the treatment plan. In addition, it is important to consider that patients emotional/psychological status (i.e., anxiety or fear) is recognized as part of the perceptual process. This is particularly relevant when given self-rated symptoms (i.e., pain and dyspnea) are described (Petersen et al., 2015), generating over- or under- estimation of interoceptive/nociceptive reporting.
Furthermore, the importance of the interaction between brain and body in order to maintain homeostasis has been emphasized. This is not just a matter of a top- down- or reflex regulation, it is also a matter of signals from the organs influencing the functioning of the brain. The output of the CNS to control its autonomic output shows an amazing differentiation; not only there are different neurons, which may influence selectively the parasympathetic or sympathetic motor neurons, there are also different neurons that project to different body compartments. Based on all that information, the brain sets the balance of the different parts of the ANS, changing the emphasis of the ANS output depending on the situation. If that balance is disturbed, either by behavior or by disease of the organ/tissue, this may lead to pathology that may affect the functioning of the whole individual. Several research studies, indeed, support the hypothesis that lack of balance in the autonomic output to a single organ may have effects not only on the organ itself but also on the entire body physiology.
Conclusions
The current review presented the “interoceptive paradigm” as a theoretical framework to explain how patient's signs, symptoms, and clinical history can be mutually related in clinical practice. Moreover, it suggested that touch-based manual practices, in particular osteopathy that seems to produce anti-inflammatory and hyper-parasympathetic effects, can offer an alternative but unique way to modify temporary or permanent sensitization states throughout the interaction with (treatment of) peripheral tissues. This is supposed to produce a biological and neurological cascade of events that change the interoceptive processes, breaking the vicious cycle of an on-going low threshold inflammatory condition. Therefore, this work proposes the conceptual foundations for a new way of interpreting and reading patients' clinical scenario based on up-to-date neuroscientific concepts. This will possibly create the ground for future research focusing on the concrete possibility of manual therapies, specifically osteopathy, to interactively modify the sensitization states, at all levels, using interoceptive pathways.
Author Contributions
GD, FC, PC conceived the idea and drafted the first version of the paper. PC supervised the manuscript and reviewed the paper for intellectual content. All authors approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors sincerely thank Dr. Jorge Esteves for reviewing the paper.
References
Ackerley, R., Backlund Wasling, H., Liljencrantz, J., Olausson, H., Johnson, R. D., and Wessberg, J. (2014). Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J. Neurosci. 34, 2879–2883. doi: 10.1523/JNEUROSCI.2847-13.2014
Affaitati, G., Costantini, R., Fabrizio, A., Lapenna, D., Tafuri, E., and Giamberardino, M. A. (2011). Effects of treatment of peripheral pain generators in fibromyalgia patients. Eur. J. Pain. 15, 61–69. doi: 10.1016/j.ejpain.2010.09.002
Aguggia, M., Saracco, M. G., Cavallini, M., Bussone, G., and Cortelli, P. (2013). Sensitization and pain. Neurol. Sci. 34(Suppl. 1), S37–S40. doi: 10.1007/s10072-013-1382-0
Aldskogius, H., and Kozlova, E. N. (1998). Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 55, 1–26. doi: 10.1016/S0301-0082(97)00093-2
Arendt-Nielsen, L., Brennum, J., Sindrup, S., and Bak, P. (1994). Electrophysiological and psychophysical quantification of temporal summation in the human nociceptive system. Eur. J. Appl. Physiol. Occup. Physiol. 68, 266–273. doi: 10.1007/BF00376776
Arendt-Nielsen, L., Nie, H., Laursen, M. B., Laursen, B. S., Madeleine, P., Simonsen, O. H., et al. (2010). Sensitization in patients with painful knee osteoarthritis. Pain 149, 573–581. doi: 10.1016/j.pain.2010.04.003
Azanon, E., Longo, M. R., Soto-Faraco, S., and Haggard, P. (2010). The posterior parietal cortex remaps touch into external space. Curr. Biol. 20, 1304–1309. doi: 10.1016/j.cub.2010.05.063
Azanon, E., and Soto-Faraco, S. (2008). Changing reference frames during the encoding of tactile events. Curr Biol. 18, 1044–1049. doi: 10.1016/j.cub.2008.06.045
Banic, B., Petersen-Felix, S., Andersen, O. K., Radanov, B. P., Villiger, P. M., Arendt-Nielsen, L., et al. (2004). Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 107, 7–15. doi: 10.1016/j.pain.2003.05.001
Baron, R., Hans, G., and Dickenson, A. H. (2013). Peripheral input and its importance for central sensitization. Ann. Neurol. 74, 630–636. doi: 10.1002/ana.24017
Black, P. H. (2002). Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav. Immun. 16, 622–653. doi: 10.1016/S0889-1591(02)00021-1
Borstad, J., and Woeste, C. (2015). The role of sensitization in musculoskeletal shoulder pain. Braz. J. Phys. Ther. 19, 251–257. doi: 10.1590/bjpt-rbf.2014.0100
Bouhadfane, M., Kaszas, A., Rozsa, B., Harris-Warrick, R. M., Vinay, L., and Brocard, F. (2015). Sensitization of neonatal rat lumbar motoneuron by the inflammatory pain mediator bradykinin. Elife 4:e06195. doi: 10.7554/eLife.06195
Bourke, J. H., Langford, R. M., and White, P. D. (2015). The common link between functional somatic syndromes may be central sensitisation. J. Psychosom. Res. 78, 228–236. doi: 10.1016/j.jpsychores.2015.01.003
Brooks, J. C., Nurmikko, T. J., Bimson, W. E., Singh, K. D., and Roberts, N. (2002). fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage 15, 293–301. doi: 10.1006/nimg.2001.0974
Buijs, R. M. (2013). The autonomic nervous system: a balancing act. Handb. Clin. Neurol. 117, 1–11. doi: 10.1016/B978-0-444-53491-0.00001-8
Buijs, R. M., La Fleur, S. E., Wortel, J., Van Heyningen, C., Zuiddam, L., Mettenleiter, T. C., et al. (2003). The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 464, 36–48. doi: 10.1002/cne.10765
Bystrova, K., Ivanova, V., Edhborg, M., Matthiesen, A. S., Ransjo-Arvidson, A. B., Mukhamedrakhimov, R., et al. (2009). Early contact versus separation: effects on mother-infant interaction 1 year later. Birth 36, 97–109. doi: 10.1111/j.1523-536X.2009.00307.x
Cameron, O. G. (2001). Interoception: the inside story–a model for psychosomatic processes. Psychosom. Med. 63, 697–710. doi: 10.1097/00006842-200109000-00001
Cao, Y., Li, K., Fu, K. Y., Xie, Q. F., Chiang, C. Y., and Sessle, B. J. (2013). Central sensitization and MAPKs are involved in occlusal interference-induced facial pain in rats. J. Pain 14, 793–807. doi: 10.1016/j.jpain.2013.02.005
Cerritelli, F., Ginevri, L., Messi, G., Caprari, E., Di Vincenzo, M., Renzetti, C., et al. (2015a). Clinical effectiveness of osteopathic treatment in chronic migraine: 3-Armed randomized controlled trial. Complement. Ther. Med. 23, 149–156. doi: 10.1016/j.ctim.2015.01.011
Cerritelli, F., Pizzolorusso, G., Renzetti, C., Cozzolino, V., D'orazio, M., Lupacchini, M., et al. (2015b). A multicenter, randomized, controlled trial of osteopathic manipulative treatment on preterms. PLoS ONE 10:e0127370. doi: 10.1371/journal.pone.0127370
Cervero, F. (2009). Spinal cord hyperexcitability and its role in pain and hyperalgesia. Exp. Brain Res. 196, 129–137. doi: 10.1007/s00221-009-1789-2
Coppola, G., Di Lorenzo, C., Schoenen, J., and Pierelli, F. (2013). Habituation and sensitization in primary headaches. J. Headache Pain 14:65. doi: 10.1186/1129-2377-14-65
Couto, B., Adolfi, F., Sedeno, L., Salles, A., Canales-Johnson, A., Alvarez-Abut, P., et al. (2015). Disentangling interoception: insights from focal strokes affecting the perception of external and internal milieus. Front. Psychol. 6:503. doi: 10.3389/fpsyg.2015.00503
Craig, A. D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. doi: 10.1038/nrn894
Craig, A. D. (2003). Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 13, 500–505. doi: 10.1016/S0959-4388(03)00090-4
Craig, A. D. (2009). How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. doi: 10.1038/nrn2555
Craig, A. D. (2013). Cooling, pain, and other feelings from the body in relation to the autonomic nervous system. Handb. Clin. Neurol. 117, 103–109. doi: 10.1016/B978-0-444-53491-0.00009-2
Craig, A. D. (2014). How do you Feel? An Interoceptive Moment with Your Neurobiological Self. Princeton, NJ: Princeton University Press.
Critchley, H. D., Mathias, C. J., and Dolan, R. J. (2002). Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33, 653–663. doi: 10.1016/S0896-6273(02)00588-3
Curatolo, M., Petersen-Felix, S., Arendt-Nielsen, L., Giani, C., Zbinden, A. M., and Radanov, B. P. (2001). Central hypersensitivity in chronic pain after whiplash injury. Clin. J. Pain 17, 306–315. doi: 10.1097/00002508-200112000-00004
Daviu, N., Andero, R., Armario, A., and Nadal, R. (2014). Sex differences in the behavioural and hypothalamic-pituitary-adrenal response to contextual fear conditioning in rats. Horm. Behav. 66, 713–723. doi: 10.1016/j.yhbeh.2014.09.015
Degenhardt, B. F., Johnson, J. C., Fossum, C., Andicochea, C. T., and Stuart, M. K. (2014). Changes in cytokines, sensory tests, and self-reported pain levels after manual treatment of low back pain. J. Spinal Disord. Tech. doi: 10.1097/BSD.0000000000000231. [Epub ahead of print].
Delfini, M. C., Mantilleri, A., Gaillard, S., Hao, J., Reynders, A., Malapert, P., et al. (2013). TAFA4, a chemokine-like protein, modulates injury-induced mechanical and chemical pain hypersensitivity in mice. Cell Rep. 5, 378–388. doi: 10.1016/j.celrep.2013.09.013
Donnerer, J., and Liebmann, I. (2015). pERK1/2 immunofluorescence in rat dorsal horn and paraventricular nucleus neurons as a marker for sensitization and inhibition in the pain pathway. Tissue Cell 47, 55–60. doi: 10.1016/j.tice.2014.11.003
Ferri, F., Ardizzi, M., Ambrosecchia, M., and Gallese, V. (2013). Closing the gap between the inside and the outside: interoceptive sensitivity and social distances. PLoS ONE 8:e75758. doi: 10.1371/journal.pone.0075758
Flor, H., Diers, M., and Birbaumer, N. (2004). Peripheral and electrocortical responses to painful and non-painful stimulation in chronic pain patients, tension headache patients and healthy controls. Neurosci. Lett. 361, 147–150. doi: 10.1016/j.neulet.2003.12.064
Fryer, G., and Pearce, A. J. (2012). The effect of lumbosacral manipulation on corticospinal and spinal reflex excitability on asymptomatic participants. J. Manipulative Physiol. Ther. 35, 86–93. doi: 10.1016/j.jmpt.2011.09.010
Gagnon, L., Vestergaard, M., Madsen, K., Karstensen, H. G., Siebner, H., Tommerup, N., et al. (2014). Neural correlates of taste perception in congenital olfactory impairment. Neuropsychologia 62, 297–305. doi: 10.1016/j.neuropsychologia.2014.07.018
Giamberardino, M. A. (2003). Referred muscle pain/hyperalgesia and central sensitisation. J. Rehabil. Med. 41(Suppl.), 85–88. doi: 10.1080/16501960310010205
Giamberardino, M. A., Tana, C., and Costantini, R. (2014). Pain thresholds in women with chronic pelvic pain. Curr. Opin. Obstet. Gynecol. 26, 253–259. doi: 10.1097/GCO.0000000000000083
Giesecke, T., Gracely, R. H., Grant, M. A., Nachemson, A., Petzke, F., Williams, D. A., et al. (2004). Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 50, 613–623. doi: 10.1002/art.20063
Giles, P. D., Hensel, K. L., Pacchia, C. F., and Smith, M. L. (2013). Suboccipital decompression enhances heart rate variability indices of cardiac control in healthy subjects. J. Altern. Complement. Med. 19, 92–96. doi: 10.1089/acm.2011.0031
Goldstein, D. S. (2001). The Autonomic Nervous System in Health and Disease. New York, NY: Marcel Dekker.
Goldstein, D. S. (2006). Adrenaline and the Inner World: An Introduction to Scientific Integrative Medicine. Baltimore, MD: Johns Hopkins University Press.
Goldstein, D. S. (2013a). Concepts of scientific integrative medicine applied to the physiology and pathophysiology of catecholamine systems. Compr. Physiol. 3, 1569–1610. doi: 10.1002/cphy.c130006
Goldstein, D. S. (2013b). Differential responses of components of the autonomic nervous system. Handb. Clin. Neurol. 117, 13–22. doi: 10.1016/B978-0-444-53491-0.00002-X
Goldstein, D. S., and Kopin, I. J. (2008). Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr. Regul. 42, 111–119.
Goldstein, D. S., and McEwen, B. (2002). Allostasis, homeostats, and the nature of stress. Stress 5, 55–58. doi: 10.1080/102538902900012345
Gramsch, C., Kattoor, J., Icenhour, A., Forsting, M., Schedlowski, M., Gizewski, E. R., et al. (2014). Learning pain-related fear: neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain. Neurobiol. Learn. Mem. 116, 36–45. doi: 10.1016/j.nlm.2014.08.003
Hankin, B. L. (2012). Future directions in vulnerability to depression among youth: integrating risk factors and processes across multiple levels of analysis. J. Clin. Child Adolesc. Psychol. 41, 695–718. doi: 10.1080/15374416.2012.711708
Henley, C. E., Ivins, D., Mills, M., Wen, F. K., and Benjamin, B. A. (2008). Osteopathic manipulative treatment and its relationship to autonomic nervous system activity as demonstrated by heart rate variability: a repeated measures study. Osteopath. Med. Prim. Care 2:7. doi: 10.1186/1750-4732-2-7
Henry, J. A., and Montuschi, E. (1978). Cardiac pain referred to site of previously experienced somatic pain. Br. Med. J. 2, 1605–1606. doi: 10.1136/bmj.2.6152.1605-a
Herbert, B. M., and Pollatos, O. (2014). Attenuated interoceptive sensitivity in overweight and obese individuals. Eat. Behav. 15, 445–448. doi: 10.1016/j.eatbeh.2014.06.002
Howell, J. N., Cabell, K. S., Chila, A. G., and Eland, D. C. (2006). Stretch reflex and Hoffmann reflex responses to osteopathic manipulative treatment in subjects with Achilles tendinitis. J. Am. Osteopath. Assoc. 106, 537–545.
Hu, J. W., Sessle, B. J., Raboisson, P., Dallel, R., and Woda, A. (1992). Stimulation of craniofacial muscle afferents induces prolonged facilitatory effects in trigeminal nociceptive brain-stem neurones. Pain 48, 53–60. doi: 10.1016/0304-3959(92)90131-T
Iannilli, E., Noennig, N., Hummel, T., and Schoenfeld, A. M. (2014). Spatio-temporal correlates of taste processing in the human primary gustatory cortex. Neuroscience 273, 92–99. doi: 10.1016/j.neuroscience.2014.05.017
Ibanez, A., and Manes, F. (2012). Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology 78, 1354–1362. doi: 10.1212/WNL.0b013e3182518375
Jänig, W. (2006). The Integrative Action of the Autonomic Nervous System. Neurobiology of Homeostasis. Cambridge: Cambridge University Press.
Janig, W., and Green, P. G. (2014). Acute inflammation in the joint: its control by the sympathetic nervous system and by neuroendocrine systems. Auton. Neurosci. 182, 42–54. doi: 10.1016/j.autneu.2014.01.001
Ji, R. R., Kohno, T., Moore, K. A., and Woolf, C. J. (2003). Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705. doi: 10.1016/j.tins.2003.09.017
Kaneko, M., Kaneko, T., Kaneko, R., Chokechanachaisakul, U., Kawamura, J., Sunakawa, M., et al. (2011). The role of N-methyl-D-aspartate receptor subunits in the rat thalamic mediodorsal nucleus during central sensitization. Brain Res. 1371, 16–22. doi: 10.1016/j.brainres.2010.11.054
Kasch, H., Qerama, E., Bach, F. W., and Jensen, T. S. (2005). Reduced cold pressor pain tolerance in non-recovered whiplash patients: a 1-year prospective study. Eur. J. Pain 9, 561–569. doi: 10.1016/j.ejpain.2004.11.011
Khalsa, S. S., Rudrauf, D., Feinstein, J. S., and Tranel, D. (2009). The pathways of interoceptive awareness. Nat. Neurosci. 12, 1494–1496. doi: 10.1038/nn.2411
Kidd, B. L., and Urban, L. A. (2001). Mechanisms of inflammatory pain. Br. J. Anaesth. 87, 3–11. doi: 10.1093/bja/87.1.3
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R., and Eickhoff, S. B. (2010). A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214, 519–534. doi: 10.1007/s00429-010-0255-z
Lee, J. W., Siegel, S. M., and Oaklander, A. L. (2009). Effects of distal nerve injuries on dorsal-horn neurons and glia: relationships between lesion size and mechanical hyperalgesia. Neuroscience 158, 904–914. doi: 10.1016/j.neuroscience.2008.10.010
Licciardone, J. C., Kearns, C. M., Hodge, L. M., and Bergamini, M. V. (2012). Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: results from the OSTEOPATHIC Trial. J. Am. Osteopath. Assoc. 112, 596–605.
Licciardone, J. C., Kearns, C. M., Hodge, L. M., and Minotti, D. E. (2013). Osteopathic manual treatment in patients with diabetes mellitus and comorbid chronic low back pain: subgroup results from the OSTEOPATHIC Trial. J. Am. Osteopath. Assoc. 113, 468–478.
Lundberg, J. M., Terenius, L., Hokfelt, T., and Goldstein, M. (1983). High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci. Lett. 42, 167–172. doi: 10.1016/0304-3940(83)90401-9
Maixner, W., Fillingim, R., Sigurdsson, A., Kincaid, S., and Silva, S. (1998). Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain 76, 71–81. doi: 10.1016/S0304-3959(98)00028-1
Mannion, R. J., Costigan, M., Decosterd, I., Amaya, F., Ma, Q. P., Holstege, J. C., et al. (1999). Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc. Natl. Acad. Sci. U.S.A. 96, 9385–9390. doi: 10.1073/pnas.96.16.9385
Mazzola, L., Isnard, J., Peyron, R., Guenot, M., and Mauguiere, F. (2009). Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain 146, 99–104. doi: 10.1016/j.pain.2009.07.014
McEwen, B. S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N.Y. Acad. Sci. 840, 33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x
McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904. doi: 10.1152/physrev.00041.2006
McEwen, B. S., Bowles, N. P., Gray, J. D., Hill, M. N., Hunter, R. G., Karatsoreos, I. N., et al. (2015a). Mechanisms of stress in the brain. Nat. Neurosci. 18, 1353–1363. doi: 10.1038/nn.4086
McEwen, B. S., Gray, J., and Nasca, C. (2015b). Recognizing resilience: learning from the effects of stress on the brain. Neurobiol. Stress 1, 1–11. doi: 10.1016/j.ynstr.2014.09.001
McGlone, F., Wessberg, J., and Olausson, H. (2014). Discriminative and affective touch: sensing and feeling. Neuron 82, 737–755. doi: 10.1016/j.neuron.2014.05.001
Meeus, M., Nijs, J., Van De Wauwer, N., Toeback, L., and Truijen, S. (2008). Diffuse noxious inhibitory control is delayed in chronic fatigue syndrome: an experimental study. Pain 139, 439–448. doi: 10.1016/j.pain.2008.05.018
Meeus, M., Vervisch, S., De Clerck, L. S., Moorkens, G., Hans, G., and Nijs, J. (2012). Central sensitization in patients with rheumatoid arthritis: a systematic literature review. Semin. Arthritis Rheum. 41, 556–567. doi: 10.1016/j.semarthrit.2011.08.001
Meltzer, K. R., and Standley, P. R. (2007). Modeled repetitive motion strain and indirect osteopathic manipulative techniques in regulation of human fibroblast proliferation and interleukin secretion. J. Am. Osteopath. Assoc. 107, 527–536.
Melzack, R., Coderre, T. J., Katz, J., and Vaccarino, A. L. (2001). Central neuroplasticity and pathological pain. Ann. N.Y. Acad. Sci. 933, 157–174. doi: 10.1111/j.1749-6632.2001.tb05822.x
Mertz, H., Fullerton, S., Naliboff, B., and Mayer, E. A. (1998). Symptoms and visceral perception in severe functional and organic dyspepsia. Gut 42, 814–822. doi: 10.1136/gut.42.6.814
Michal, M., Reuchlein, B., Adler, J., Reiner, I., Beutel, M. E., Vogele, C., et al. (2014). Striking discrepancy of anomalous body experiences with normal interoceptive accuracy in depersonalization-derealization disorder. PLoS ONE 9:e89823. doi: 10.1371/journal.pone.0089823
Mirams, L., Poliakoff, E., Brown, R. J., and Lloyd, D. M. (2012). Interoceptive and exteroceptive attention have opposite effects on subsequent somatosensory perceptual decision making. Q. J. Exp. Psychol. (Hove). 65, 926–938. doi: 10.1080/17470218.2011.636823
Nagi, S. S., Rubin, T. K., Chelvanayagam, D. K., Macefield, V. G., and Mahns, D. A. (2011). Allodynia mediated by C-tactile afferents in human hairy skin. J. Physiol. (Lond). 589, 4065–4075. doi: 10.1113/jphysiol.2011.211326
Naqvi, N. H., and Bechara, A. (2010). The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct. Funct. 214, 435–450. doi: 10.1007/s00429-010-0268-7
Neugebauer, V., and Li, W. (2003). Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J. Neurophysiol. 89, 716–727. doi: 10.1152/jn.00799.2002
Neumann, S., Doubell, T. P., Leslie, T., and Woolf, C. J. (1996). Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384, 360–364. doi: 10.1038/384360a0
Nijs, J., Torres-Cueco, R., Van Wilgen, C. P., Girbes, E. L., Struyf, F., Roussel, N., et al. (2014). Applying modern pain neuroscience in clinical practice: criteria for the classification of central sensitization pain. Pain Phys. 17, 447–457.
Nijs, J., Van Houdenhove, B., and Oostendorp, R. A. (2010). Recognition of central sensitization in patients with musculoskeletal pain: application of pain neurophysiology in manual therapy practice. Man. Ther. 15, 135–141. doi: 10.1016/j.math.2009.12.001
N Sanchis, M., Lluch, E., Nijs, J., Struyf, F., and Kangasperko, M. (2015). The role of central sensitization in shoulder pain: a systematic literature review. Semin. Arthritis Rheum. 44, 710–716. doi: 10.1016/j.semarthrit.2014.11.002
Parabucki, A., and Netser, S. (2014). Origin of palatability coding in medial prefrontal cortex. J. Neurosci. 34, 4121–4122. doi: 10.1523/JNEUROSCI.0362-14.2014
Park, S. J., Zhang, S., Chiang, C. Y., Hu, J. W., Dostrovsky, J. O., and Sessle, B. J. (2006). Central sensitization induced in thalamic nociceptive neurons by tooth pulp stimulation is dependent on the functional integrity of trigeminal brainstem subnucleus caudalis but not subnucleus oralis. Brain Res. 1112, 134–145. doi: 10.1016/j.brainres.2006.06.115
Paulus, M. P., and Stein, M. B. (2010). Interoception in anxiety and depression. Brain Struct. Funct. 214, 451–463. doi: 10.1007/s00429-010-0258-9
Peng, J. M., Xu, L. S., Zhu, Q., Gong, S., Yu, X. M., Guo, S. Y., et al. (2011). Enhanced NMDA receptor NR1 phosphorylation and neuronal activity in the arcuate nucleus of hypothalamus following peripheral inflammation. Acta Pharmacol. Sin. 32, 160–166. doi: 10.1038/aps.2010.190
Perini, I., Olausson, H., and Morrison, I. (2015). Seeking pleasant touch: neural correlates of behavioral preferences for skin stroking. Front. Behav. Neurosci. 9:8. doi: 10.3389/fnbeh.2015.00008
Petersen, S., Von Leupoldt, A., and Van Den Bergh, O. (2015). Interoception and the uneasiness of the mind: affect as perceptual style. Front. Psychol. 6:1408. doi: 10.3389/fpsyg.2015.01408
Pollatos, O., Kurz, A. L., Albrecht, J., Schreder, T., Kleemann, A. M., Schopf, V., et al. (2008). Reduced perception of bodily signals in anorexia nervosa. Eat. Behav. 9, 381–388. doi: 10.1016/j.eatbeh.2008.02.001
Porreca, F., Ossipov, M. H., and Gebhart, G. F. (2002). Chronic pain and medullary descending facilitation. Trends Neurosci. 25, 319–325. doi: 10.1016/S0166-2236(02)02157-4
Prechtl, J. C., and Powley, T. L. (1990). The fiber composition of the abdominal vagus of the rat. Anat. Embryol. 181, 101–115. doi: 10.1007/BF00198950
Purcell, W. M., and Atterwill, C. K. (1995). Mast cells in neuroimmune function: neurotoxicological and neuropharmacological perspectives. Neurochem. Res. 20, 521–532. doi: 10.1007/BF01694534
Qiu, S., Zhang, M., Liu, Y., Guo, Y., Zhao, H., Song, Q., et al. (2014). GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. J. Neurosci. 34, 13505–13515. doi: 10.1523/JNEUROSCI.1431-14.2014
Ruffini, N., D'alessandro, G., Mariani, N., Pollastrelli, A., Cardinali, L., and Cerritelli, F. (2015). Variations of high frequency parameter of heart rate variability following osteopathic manipulative treatment in healthy subjects compared to control group and sham therapy: randomized controlled trial. Front. Neurosci. 9:272. doi: 10.3389/fnins.2015.00272
Sandkuhler, J. (2007). Understanding LTP in pain pathways. Mol. Pain 3, 9. doi: 10.1186/1744-8069-3-9
Sandkuhler, J. (2009). Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 89, 707–758. doi: 10.1152/physrev.00025.2008
Sankowski, R., Mader, S., and Valdes-Ferrer, S. I. (2015). Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front. Cell. Neurosci. 9:28. doi: 10.3389/fncel.2015.00028
Schaefer, M., Egloff, B., and Witthoft, M. (2012). Is interoceptive awareness really altered in somatoform disorders? Testing competing theories with two paradigms of heartbeat perception. J. Abnorm. Psychol. 121, 719–724. doi: 10.1037/a0028509
Schander, A., Downey, H. F., and Hodge, L. M. (2012). Lymphatic pump manipulation mobilizes inflammatory mediators into lymphatic circulation. Exp. Biol. Med. (Maywood). 237, 58–63. doi: 10.1258/ebm.2011.011220
Schander, A., Padro, D., King, H. H., Downey, H. F., and Hodge, L. M. (2013). Lymphatic pump treatment repeatedly enhances the lymphatic and immune systems. Lymphat. Res. Biol. 11, 219–226. doi: 10.1089/lrb.2012.0021
Schmidt, A. J., Gierlings, R. E., and Peters, M. L. (1989). Environmental and interoceptive influences on chronic low back pain behavior. Pain 38, 137–143. doi: 10.1016/0304-3959(89)90231-5
Sedeno, L., Couto, B., Melloni, M., Canales-Johnson, A., Yoris, A., Baez, S., et al. (2014). How do you feel when you can't feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PLoS ONE 9:e98769. doi: 10.1371/journal.pone.0098769
Sherrington, C. S. (1906). The Integrative Action of the Nervous System. Cambridge: Cambridge University Press.
Shyu, B. C., and Vogt, B. A. (2009). Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway. Mol. Pain. 5:51. doi: 10.1186/1744-8069-5-51
Simone, D. A., Sorkin, L. S., Oh, U., Chung, J. M., Owens, C., Lamotte, R. H., et al. (1991). Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J. Neurophysiol. 66, 228–246.
Sterling, M., Jull, G., Vicenzino, B., and Kenardy, J. (2003). Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain 104, 509–517. doi: 10.1016/S0304-3959(03)00078-2
Treede, R. D., Kenshalo, D. R., Gracely, R. H., and Jones, A. K. (1999). The cortical representation of pain. Pain 79, 105–111. doi: 10.1016/S0304-3959(98)00184-5
Uno, K., Katagiri, H., Yamada, T., Ishigaki, Y., Ogihara, T., Imai, J., et al. (2006). Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science 312, 1656–1659. doi: 10.1126/science.1126010
Ursin, H. (2014). Brain sensitization to external and internal stimuli. Psychoneuroendocrinology 42, 134–145. doi: 10.1016/j.psyneuen.2014.01.008
van den Bosch, I., Dalenberg, J. R., Renken, R., Van Langeveld, A. W., Smeets, P. A., Griffioen-Roose, S., et al. (2014). To like or not to like: neural substrates of subjective flavor preferences. Behav. Brain Res. 269, 128–137. doi: 10.1016/j.bbr.2014.04.010
Verne, G. N., and Price, D. D. (2002). Irritable bowel syndrome as a common precipitant of central sensitization. Curr. Rheumatol. Rep. 4, 322–328. doi: 10.1007/s11926-002-0041-x
Vierck, C. J. Jr. (2006). Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia). Pain 124, 242–263. doi: 10.1016/j.pain.2006.06.001
Wald, J., and Taylor, S. (2008). Responses to interoceptive exposure in people with posttraumatic stress disorder (PTSD): a preliminary analysis of induced anxiety reactions and trauma memories and their relationship to anxiety sensitivity and PTSD symptom severity. Cogn. Behav. Ther. 37, 90–100. doi: 10.1080/16506070801969054
Walkowski, S., Singh, M., Puertas, J., Pate, M., Goodrum, K., and Benencia, F. (2014). Osteopathic manipulative therapy induces early plasma cytokine release and mobilization of a population of blood dendritic cells. PLoS ONE 9:e90132. doi: 10.1371/journal.pone.0090132a
Wang, H., Xie, Y. F., Chiang, C. Y., Dostrovsky, J. O., and Sessle, B. J. (2013). Central alpha-adrenoceptors contribute to mustard oil-induced central sensitization in the rat medullary dorsal horn. Neuroscience 236, 244–252. doi: 10.1016/j.neuroscience.2013.01.016
Warne, J. P., Foster, M. T., Horneman, H. F., Pecoraro, N. C., Ginsberg, A. B., Akana, S. F., et al. (2007). Afferent signalling through the common hepatic branch of the vagus inhibits voluntary lard intake and modifies plasma metabolite levels in rats. J. Physiol. (Lond). 583, 455–467. doi: 10.1113/jphysiol.2007.135996
Watkins, L. R., Milligan, E. D., and Maier, S. F. (2001). Glial activation: a driving force for pathological pain. Trends Neurosci. 24, 450–455. doi: 10.1016/S0166-2236(00)01854-3
Wei, F., and Zhuo, M. (2001). Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J. Physiol. (Lond). 532, 823–833. doi: 10.1111/j.1469-7793.2001.0823e.x
Willis, W. D. (2002). Long-term potentiation in spinothalamic neurons. Brain Res. Brain Res. Rev. 40, 202–214. doi: 10.1016/S0165-0173(02)00202-3
Woodbury, C. J., Ritter, A. M., and Koerber, H. R. (2001). Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. J. Comp. Neurol. 436, 304–323. doi: 10.1002/cne.1069
Woolf, C. J. (1993). The pathophysiology of peripheral neuropathic pain–abnormal peripheral input and abnormal central processing. Acta Neurochir. Suppl. (Wien) 58, 125–130. doi: 10.1007/978-3-7091-9297-9_29
Woolf, C., and Wiesenfeld-Hallin, Z. (1986). Substance P and calcitonin gene-related peptide synergistically modulate the gain of the nociceptive flexor withdrawal reflex in the rat. Neurosci. Lett. 66, 226–230. doi: 10.1016/0304-3940(86)90195-3
Keywords: osteopathic medicine, autonomic nervous system, interoceptive paradigm, allostasis, homeostasis, inflammation, nociception
Citation: D'Alessandro G, Cerritelli F and Cortelli P (2016) Sensitization and Interoception as Key Neurological Concepts in Osteopathy and Other Manual Medicines. Front. Neurosci. 10:100. doi: 10.3389/fnins.2016.00100
Received: 10 December 2015; Accepted: 26 February 2016;
Published: 10 March 2016.
Edited by:
Yoko Nagai, Brighton and Sussex Medical School, UKReviewed by:
Bruno Bonaz, Grenoble Faculty of Medicine and Hospital, FranceAntonio Martocchia, S. Andrea Hospital - Sapienza University of Rome, Italy
Copyright © 2016 D'Alessandro, Cerritelli and Cortelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Cerritelli, ZnJhbmNlc2NvLmNlcnJpdGVsbGlAZ21haWwuY29t
 Giandomenico D'Alessandro
Giandomenico D'Alessandro Francesco Cerritelli
Francesco Cerritelli Pietro Cortelli5,6
Pietro Cortelli5,6