- Laboratory of Respiratory Pathophysiology, Respiratory Division, Department of Medicine, Federal University of Mato Grosso do Sul, Campo Grande, Brazil
Purpose: There is evidence of complex interaction between vitamin B12 (vB12) level, hyperhomocysteinemia (HyCy), and natriuretic peptide secretion. Exercise training could also modulate such interaction. In this secondary analysis of a Randomized Clinical Trial performed in a chronic obstructive pulmonary disease (COPD) rehabilitation setting, our primary objective was to investigate the interaction between vB12 supplementation, exercise training, and changes in NT-proBNP levels after 8 weeks of intervention. Secondary objectives were to explore the correlations between acute changes in NT-proBNP levels with (i) acute exercise and (ii) oxygen uptake (V’O2) kinetics during rest-to-exercise transition.
Methods: Thirty-two subjects with COPD were randomized into four groups: Rehabilitation+vB12 (n = 8), Rehabilitation (n = 8), vB12 (n = 8), or Maltodextrin(n = 8). They were evaluated at baseline and after 8 weeks, during resting and immediately after maximal exercise constant work-rate tests (CWTs, Tlim), for NT-proBNP plasmatic levels.
Results: After interaction analysis, the supplementation with vB12 significantly changed the time course of NT-proBNP responses during treatment (p = 0.048). However, the final analysis could not support a significant change in NT-proBNP levels owing to high-intensity constant work-rate exercise (p-value > 0.05). There was a statistically significant correlation between V’O2 time constant and ΔNT-proBNP values (Tlim – rest) at baseline (p = 0.049) and 2 months later (p = 0.015), considering all subjects (n = 32).
Conclusion: We conclude that vB12 supplementation could modulate NT-proBNP secretion. Moreover, possibly, the slower the initial V’O2 adjustments toward a steady-state during rest-to-exercise transitions, the more severe the ventricular chamber volume/pressure stress recruitment, expressed through higher NT-proBNP secretion in subjects with larger V’O2 time constants, despite unchanged final acute exercise-induced neurohormone secretion.
Introduction
In a recent Randomized Controlled Trial (RCT), we showed a slight but significant increase in maximal exercise tolerance (Tlim) in patients with chronic obstructive pulmonary disease (COPD) supplemented with vitamin B12 during physical training, but without effects on oxygen uptake (V’O2) kinetics beyond training alone (Paulin et al., 2017). Individuals with COPD are at risk of vitamin B12 deficiency (Solomon, 2016) and hyperhomocysteinemia (HyCy) (Seemungal et al., 2007; Fimognari et al., 2009). Accordingly, HyCy is linked to numerous cardiovascular alteration (Ganguly and Alam, 2015) including histological changes in the heart (Piquereau et al., 2017), impaired global and segmental cardiac contractility (Kaya et al., 2014), or increased N-terminal-pro-B-type natriuretic peptide (NT-proBNP) secretion (Herrmann et al., 2007; Guéant Rodriguez et al., 2013). This prepropeptide is synthesized and stored as a high molecular weight mass propeptide from both the atria and ventricles, and released mainly under pressure/volume overload of the cardiac chambers, after cleavage of the active form of BNP, inducing natriuresis and vasodilatation (Calzetta et al., 2016). However, NT-proBNP has a longer plasma half-life and attains larger concentrations, besides being described as a significant marker of prognosis in heart failure (HF) (Cipriano et al., 2014). Moreover, vitamin B12 or folic acid supplementation reduced NT-proBNP levels after 2 months of supplementation in subjects with NT-proBNP > 40 pg/mL (Herrmann et al., 2007), mitigated HyCy-induced cardiac dysfunction (Jeremic et al., 2018), and proved to be protective for mitochondrial function and cardiac contractile properties in a murine model, with lessening of upregulated atrial brain natriuretic peptide (ANP) (Piquereau et al., 2017). In contrast, an experimental study was negative for cardiac morphological alterations during HyCy induction (Taban-Shomal et al., 2009).
It is recognized that physical training causes a reduction in cardiac natriuretic peptides in HF (Cipriano et al., 2014) as well as which, a beneficial interaction between physical training and vitamin B12 or folate supplementation in reducing HyCy has been suggested (König et al., 2003; Tyagi and Joshua, 2014). Of note, there is evidence of increased secretion of BNP associated with increased pulmonary vascular resistance during acute exercise in COPD (Fujii et al., 1999); a mechanism which demonstrates potential for attenuation through physical training. Thus, this secondary analysis aims primarily to explore the interaction between vitamin B12 supplementation, physical training, and resting/exercise levels for NT-proBNP in a stable population of COPD patients. In addition, we sought to analyze possible associations between acute changes in NT-proBNP levels during exercise with Tlim, delivered power (watts, w), and oxygen uptake kinetics (V’O2 time constant) on an ergometer, in order to explore the determinants of these possible changes. The central hypothesis was that there would be attenuation of neurohormone alterations with supplementation alone or combined with physical training.
Materials and Methods
As this is a secondary study of an already published RCT, the entire methodology has been previously described in detail (Paulin et al., 2017). Similarly, ethical considerations and consent details are published and recorded in the Brasilian Clinical Trials Registry (ReBEC number RBR-55f97c/2014). Additional unpublished methods will be considered in this exploratory study.
Participants and Study Design
In the final analysis, 32 stable COPD patients were consecutively randomized to four groups: (1) 8-weeks physical rehabilitation (REHA) group, (2) 8-weeks physical rehabilitation group with daily vitamin B12 supplementation of 500 mg (REHA+B12), (3) supplementation group as stand-alone with daily vitamin B12 supplementation of 500 mg (B12), and (4) placebo group (maltodextrin 500 mg) (P). All groups continued with their usual optimized pharmacological treatment for COPD. Among the subjects who completed the study, 28/32 were already being followed up at the specialized COPD clinic and had an echocardiogram performed within the previous 6 months. Mild mitral or aortic reflux and ventricular hypertrophy were accepted in the inclusion criteria. Patient history and further detailed physical examinations did not show signs of associated heart disease in the remaining subjects recruited without echocardiography.
Standard Doppler Echocardiography
All transthoracic echocardiography followed standard guidelines (Lang et al., 2005). Measurements of the cardiac cavities, interventricular septum, and left ventricular posterior wall thickness were collected by M-mode and two-dimensional analysis. Left atrial volume used the biplane Simpson method and was indexed by body surface. The ejection fraction was measured by the Teichholz method. The tricuspid reflux velocity was obtained by continuous Doppler in the right ventricle inlet, and the sPAP value was calculated by adding 10 mmHg of pressure in the right atrium.
Cardiopulmonary Exercise Testing (CPET)
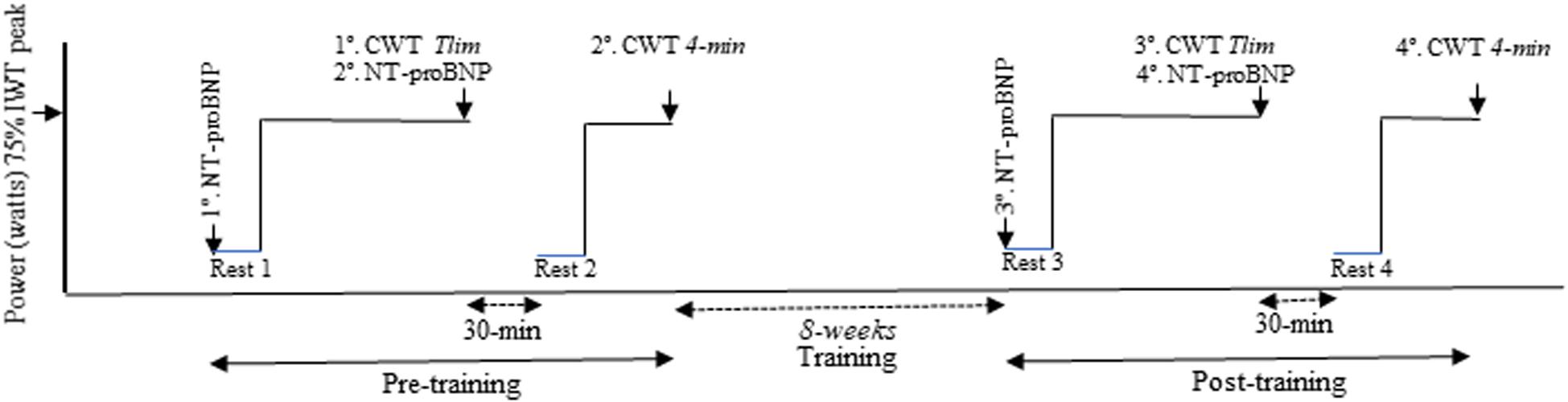
All subjects were invited to perform an incremental cardiopulmonary exercise testing (CPET) and two equal constant work-rate tests (CWTs, 75% of the maximum incremental CPET load) to calculate time constants (tau, τ) for V’O2 during the rest-to-exercise transition. Detailed CWT methods and oxygen uptake kinetics analysis were previously published (Paulin et al., 2017; Müller et al., 2019). Blood samples were collected at rest and Tlim during the first CWT, before and after 8 weeks, for NT-proBNP plasmatic level analysis (Figure 1).
NT-proBNP Analysis
After a suitable rest period, before the CWT, a polyurethane 22G catheter (Injex-Cath, Ourinhos, Brazil) was inserted on the back of the patient’s right hand for venous blood sample collections at rest and during exercise. The material was collected in tubes containing a mixture of plasma-lithium, as recommended by the manufacturer, and incubated at -20°C for 30 min. The electrochemiluminescence sandwich immunoassay by COBAS e602® system (Roche Diagnostics, Germany) measurement method was used, with a measurement range of 5–35,000 pg/mL and predicted coefficient of variation < 3.1%.
Data Analysis and Statistics
Data are expressed as mean ± standard deviation (SD) or median (IQR), mirroring, respectively, Gaussian or non-Gaussian distribution after the Shapiro–Wilk test. As τ (time constant, tau)—representing the time to attain 63% of the steady-state V’O2 during a CWT—demonstrates marked skewness, we performed a reciprocal transformation and used k (k = 1/τ) instead of τ. In exponential growth, k is equal to the reciprocal of the time constant tau, i.e., k = 1/τ and represents the rate of increase in V’O2 toward a steady-state plateau. Hence, the faster the rate of adjustment (k) of V’O2 toward the steady-state during rest-to-exercise constant work-rate exercise transitions, the lower the time constant tau. Thus, we chose to perform Pearson product-moment coefficients for correlations using k, as k generates a more robust Gaussian distribution (Bland and Altman, 1996) and would imply similarly to a plausible physiological significance, as we recently suggested (Müller et al., 2019). The slopes of linear regression for pre- and post-training moments were compared with Univariate ANCOVA. In the temporal analysis of the effects of training and supplementation, we performed three-way repeated measures (RM) ANOVA with two between-subjects factors (training and supplementation) and four within-subjects RM (NT-proBNP level at rest/Tlim at baseline and rest/Tlim after 2 months), taking into account the sphericity standard and the Greenhouse-Geisser correction. In addition, one-way ANOVA for groups was performed for comparison of baseline characteristics and one-way RM ANOVA for time-dependent within-group NT-proBNP level changes. The calculated study power > 0.8, with an alpha risk of 0.05 (two-tailed), was calculated from the placebo Group with their respective SD differences. Thus, we considered the average within-subjects SD difference of 1 pg/mL for NT-proBNP and, two between-subjects factors, B12 and exercise, with measured SD of the difference of 103 pg/mL and 100 s, respectively, in a three-way RM ANOVA design. We defined a p-value < 0.05 as statistically significant. The statistical program SPSS 20.0 was used for all statistical analysis (SPSS, IBM Corp, United States, 2011).
Results
Selected baseline characteristics are described in Table 1 and detailed data have been previously published (Paulin et al., 2017). The groups were relatively balanced, with a significant difference only for Tlim at baseline (p = 0.041, Table 1). Despite a similar baseline PaO2, the groups as a whole (n = 32) presented significantly reduced hemoglobin saturation through peripheral oximetry post-exercise (p < 0.0001). After interaction analysis, the supplementation with vitamin B12 significantly changed the time course of NT-proBNP level during treatment (p = 0.048, Table 2 and Figure 2A). In addition, the final analysis did not support a significant change in NT-proBNP levels owing to high-intensity constant work-rate exercise (three-way RM ANOVA analysis and one-way RM ANOVA analysis with p-value > 0.05 for both, Table 2). A statistically significant correlation was observed only between V’O2 time constant and ΔNT-proBNP level (Tlim – rest) at baseline (p = 0.049, Figure 2B) and 2 months later (p = 0.015, Figure 2C), considering all subjects (n = 32), with an absence of significant correlations between ΔNT-proBNP level and Tlim or delivered Power on the cycle ergometer (p > 0.05 for both). The pre- and post-training slopes representing the correlation between the V’O2 time constant and ΔNT-proBNP (Figures 2B,C) were not significantly different for the groups as a whole (p = 0.259).
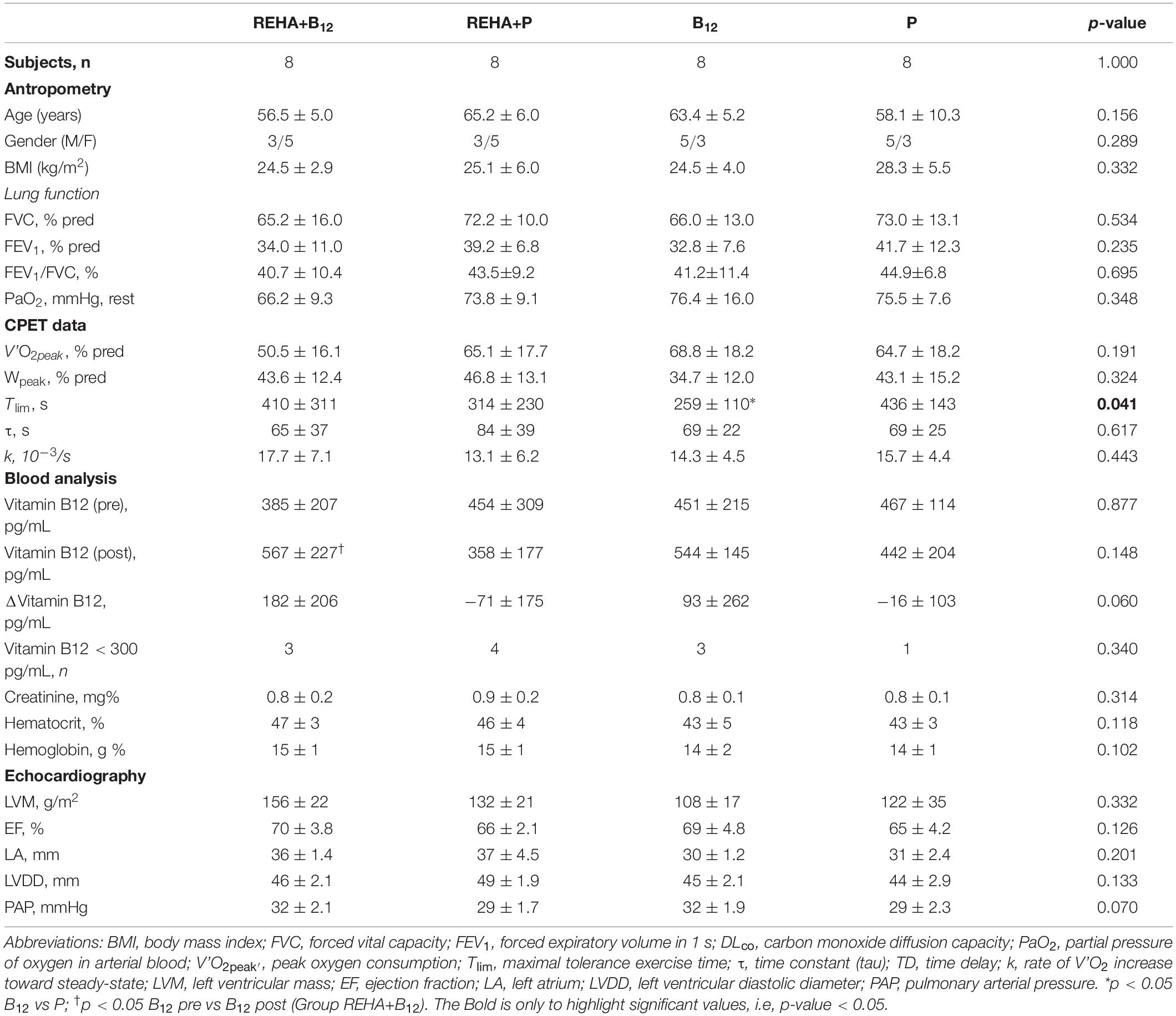
Table 1. Selected baseline clinical and physiological characteristic of the four groups of COPD patients.
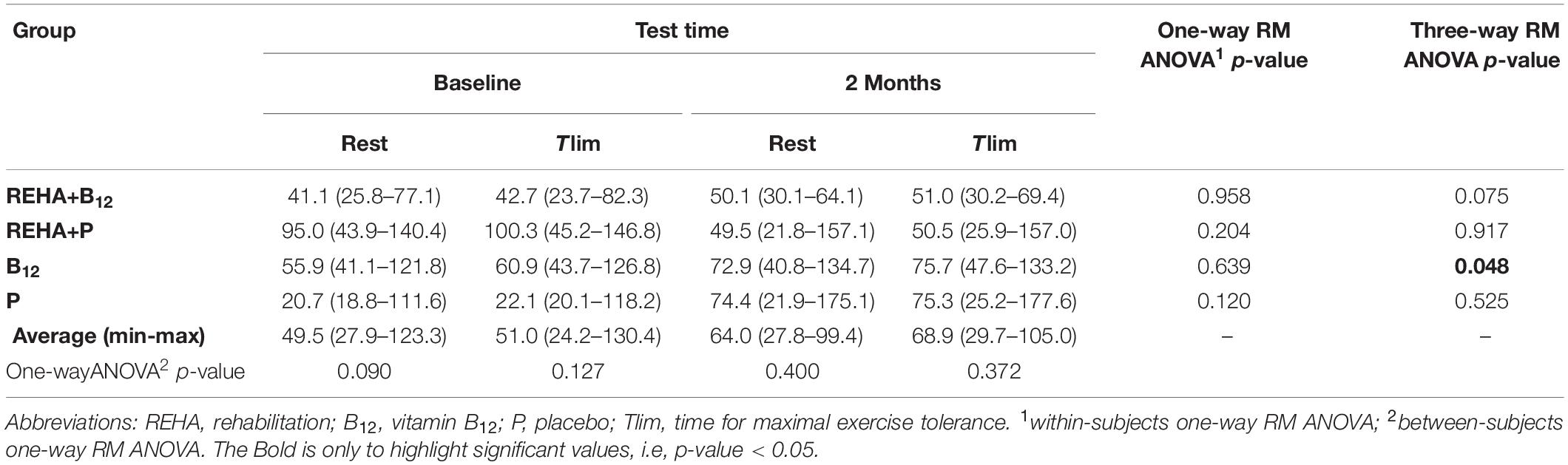
Table 2. NT-proBNP levels during acute exercise (rest and Tlim) evaluated at baseline and after 2 months of intervention.
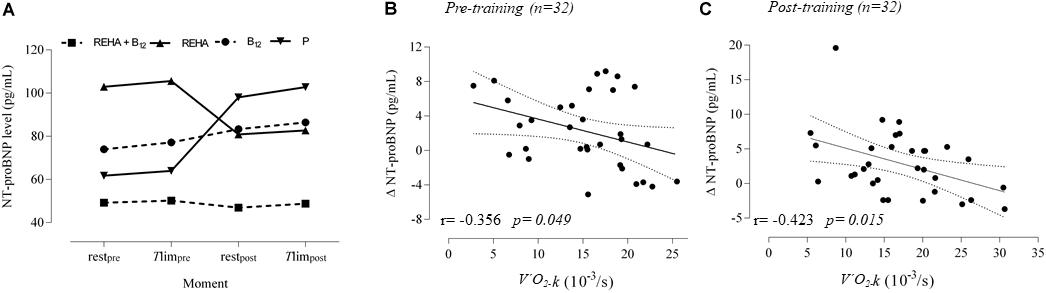
Figure 2. NT-proBNP levels at rest and Tlim, before and after 2 months. The time course is depicted in (A) (interrupted lines were constructed only to show the time course of NT-proBNP level under vitamin B12 influence compared to control groups). Correlations for acute responses (Tlim – rest, Δ) vs individual time constant for V’O2 (k or 1/tau) at baseline and 2 months later are represented in (B) and (C). During the rest-to-exercise transition following constant work-rate exercise, there is an oxygen deficit that is inversely related to the rate of increase inV’O2 (k) toward the steady-state condition.
Discussion
This exploratory study suggests that B12 measurements and/or supplementation should be considered in future studies on cardiovascular morbidity in COPD subjects. This is in line with the overall pathophysiology of cardiovascular morbidity in COPD which has not yet been totally unraveled. In addition, we did not detect acute changes in plasma levels for NT-proBNP during high-intensity constant work-rate exercise. However, during the rest-to-exercise transition, patients with slower V’O2 adjustments toward a steady-state appeared to secrete higher NT-proBNP levels.
Normal levels of vitamin B12 do not rule out the possibility of cobalamin deficiency, and low-normal levels (200–300 pg/mL), when associated with risk factors for increased oxidative stress, demonstrate metabolic evidence of deficiency, with high levels of HyCy or methylmalonic acid (MMA), since this vitamin is inactivated by oxidation (Solomon, 2016). Our study population contained at least three subjects in each group with levels < 300 pg/mL of vitamin B12 and supplementation significantly increased their levels after 8 weeks for the REHA+B12 group and not significantly for the B12 group (Paulin et al., 2017).
Several previously cited animal and human studies have described a relationship between vitamin B12 or folic acid level, HyCy, and NT-proBNP levels. Although preliminary, our study points to an attenuating effect on NT-proBNP secretion with vitamin B12 supplementation, in agreement with previous studies (Herrmann et al., 2007; Guéant Rodriguez et al., 2013), despite weak evidence. In monitoring the evolution of HF, attenuated or stable levels of NT-proBNP secretion were associated with fewer total cardiovascular events (Franke et al., 2011). Although the mechanisms underpinning this small but significant effect are largely unexplored, downregulation of natriuretic peptide production by reducing homocysteine and MMA accumulation under certain conditions is possible (Piquereau et al., 2017).
Two important secondary findings were described. Although we did not find an acute change in NT-proBNP owing to acute exercise during high-intensity CWT, there was a small but significant relationship between an acute change in this neurohormone (Δ) and oxygen uptake kinetics. Our study differs from a previous study, which showed increased secretion of BNP after exercise in a population similar to ours during CWT (Fujii et al., 1999). These contradictory results occurred despite both presenting significant hemoglobin desaturation during exercise. Arterial hypoxemia is a known trigger for natriuretic peptide secretion (Fujii et al., 1999). Surprisingly, our study agrees with another study for NT-proBNP also under CWT in mild-to-moderate COPD (Wang et al., 2011). Differences may reflect other factors, such as the secretion-to-metabolization ratio of natriuretic peptides and differences in exercise protocol. Our data suggest that much of the secretion of natriuretic peptides during CWT occurs at the time of initial adjustment during rest-to-exercise transitions, where the sudden change in the cardiac chamber pressure/volume condition is additionally stressed by the direct effect of transmural pressure, due to the abrupt increase in intrathoracic respiratory pressure swings, following the kinetics of the increase in pulmonary arterial pressure during the first minute and posterior decay during exercise (Lonsdorfer-Wolf et al., 2004). In this sense, our study is in agreement with a previous study that showed a direct relationship between BNP secretion and oxygen deficit during constant work-rate exercise in HF (Brunner-La Rocca et al., 1999).
As a limitation of this study, we cite the small number of individuals, notwithstanding this being partially tempered by the strong study design. In addition, we did not measure homocysteine and MMA levels. Four subjects were not evaluated by echocardiography; however, clinical data, NT-proBNP levels, and CPET analysis were not compatible with major heart disease.
Conclusion
We suggest that vitamin B12 supplementation could modulate NT-proBNP secretion, but the effects are small and further studies are needed. Moreover, we did not find an increase in neurohormone level caused by acute exercise per se; however, there was an association with slower V’O2 adjustment during the rest-to-exercise transition. The significance of this neurohormone dynamic warrants more detailed studies, considering different modalities of exercise training, e.g., high-intensity 1-min bouts, with unexplored cardiac neuro- hormone signaling and unknown cardiovascular consequences.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee, affiliated to Brazilian Clinical Trial Registry and Federal University of Mato Grosso Do Sul. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
PM contributed to study design, literature search, data collection, analysis of data, and manuscript preparation and review. FP contributed to literature search, data collection, analysis of data, and manuscript preparation and review. LG contributed to study design, literature search, data collection, analysis of data, and manuscript review. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Post-Graduate Program on Health and Development in West Central Region at Federal University of Mato Grosso do Sul (Brazil) and was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil—Finance Code 001.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the technical support team for their help during collection and processing of blood analysis for NT-proBNP.
References
Brunner-La Rocca, H. P., Weilenmann, D., Follath, F., Schlumpf, M., Rickli, H., Schalcher, C., et al. (1999). Oxygen uptake kinetics during low level exercise in patients with heart failure: relation to neurohormones, peak oxygen consumption, and clinical findings. Heart 81, 121–127. doi: 10.1136/hrt.81.2.121
Calzetta, L., Orlandi, A., Page, C., Paola, R., Barbara, R., Giuseppe, R., et al. (2016). Brain natriuretic peptide: much more than a biomarker. Int. J. Cardiol. 221, 1031–1038. doi: 10.1016/j.ijcard.2016.07.109
Cipriano, G., Cipriano, V. T., da Silva, V. Z., Cipriano, G. F., Chiappa, G. R., de Lima, A. C., et al. (2014). Aerobic exercise effect on prognostic markers for systolic heart failure patients: a systematic review and meta-analysis. Heart Fail. Rev. 19, 655–667. doi: 10.1007/s10741-013-9407-6
Fimognari, F. L., Loffredo, L., Di Simone, S., Sampietro, F., Pastorelli, R., Monaldo, M., et al. (2009). Hyperhomocysteinaemia and poor vitamin B status in chronic obstructive pulmonary disease. Nutr. Metab. Cardiovasc. Dis. 19, 654–659. doi: 10.1016/j.numecd.2008.12.006
Franke, J., Frankenstein, L., Schellberg, D., Bajrovic, A., Wolter, J. S., Ehlermann, P., et al. (2011). Is there an additional benefit of serial NT-proBNP measurements in patients with stable chronic heart failure receiving individually optimized therapy? Clin. Res. Cardiol. 100, 1059–1067. doi: 10.1007/s00392-011-0340-1
Fujii, T., Otsuka, T., Tanaka, S., Kanazawa, H., Hirata, K., Kohno, M., et al. (1999). Plasma endothelin-1 level in chronic obstructive pulmonary disease: relationship with natriuretic peptide. Respiration 66, 212–219. doi: 10.1159/000029380
Ganguly, P., and Alam, S. F. (2015). Role of homocysteine in the development of cardiovascular disease. Nutr. J. 14:6. doi: 10.1186/1475-2891-14-6
Guéant Rodriguez, R. M., Spada, R., Pooya, S., Jeannesson, E., Moreno Garcia, M. A., Anello, G., et al. (2013). Homocysteine predicts increased NT-pro-BNP through impaired fatty acid oxidation. Int. J. Cardiol. 167, 768–775. doi: 10.1016/j.ijcard.2012.03.047
Herrmann, M., Stanger, O., Paulweber, B., Hufnagl, C., and Herrmann, W. (2007). Effect of folate supplementation on N-terminal pro-brain natriuretic peptide. Int. J. Cardiol. 118, 267–269. doi: 10.1016/j.ijcard.2006.07.034
Jeremic, J., Nikolic Turnic, T., Zivkovic, V., Jeremic, N., Milosavljevic, I., Srejovic, I., et al. (2018). Vitamin B complex mitigates cardiac dysfunction in high-methionine diet-induced hyperhomocysteinemia. Clin. Exp. Pharmacol. Physiol. 45, 683–693. doi: 10.1111/1440-1681.12930
Kaya, Z., Bicer, A., Yalcin, F., Er, A., Camuzcuoglu, A., Korkmaz, N., et al. (2014). Impaired global and segmental myocardial deformation assessed by two-dimensional speckle tracking echocardiography in patients with vitamin B12 deficiency. Cardiol. J. 21, 60–66. doi: 10.5603/CJ.a2013.0059
König, D., Bissé, E., Deibert, P., Müller, H. M., Wieland, H., and Berg, A. (2003). Influence of training volume and acute physical exercise on the homocysteine levels in endurance-trained men: interactions with plasma folate and vitamin B12. Ann. Nutr. Metab. 47, 114–118. doi: 10.1159/000070032
Lang, R. M., Bierig, M., Devereux, R. B., Flachskampf, F. A., Foster, E., Pellikka, P. A., et al. (2005). Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 18, 1440–1463. doi: 10.1016/j.echo.2005.10.005
Lonsdorfer-Wolf, E., Bougault, V., Doutreleau, S., Charloux, A., Lonsdorfer, J., and Oswald-Mammosser, M. (2004). Intermittent exercise test in chronic obstructive pulmonary disease patients: how do the pulmonary hemodynamics adapt? Med. Sci. Sports Exerc. 36, 2032–2039. doi: 10.1249/01.mss.0000147631.59070.7d
Müller, P. T., Nogueira, J. Z., Augusto, T. R., and Chiappa, G. R. (2019). Faster oxygen uptake, heart rate and ventilatory kinetics in Stepping compared to cycle ergometry in COPD patients during moderate intensity exercise. Appl. Physiol. Nutr. Metab. 44, 879–885. doi: 10.1139/apnm-2018-0662
Paulin, F. V., Zagatto, A. M., Chiappa, G. R., and Müller, P. T. (2017). Addition of vitamin B12 to exercise training improves cycle ergometer endurance in advanced COPD patients: a randomized and controlled study. Respir. Med. 122, 23–29. doi: 10.1016/j.rmed.2016.11.015
Piquereau, J., Moulin, M., Zurlo, G., Mateo, P., Gressette, M., Paul, J. L., et al. (2017). Cobalamin and folate protect mitochondrial and contractile functions in a murine model of cardiac pressure overload. J. Mol. Cell Cardiol. 102, 34–44. doi: 10.1016/j.yjmcc.2016.11.010
Seemungal, T. A., Lun, J. C., Davis, G., Neblett, C., Chinyepi, N., Dookhan, C., et al. (2007). Plasma homocysteine is elevated in COPD patients and is related to COPD severity. Int. J. Chron. Obstruct. Pulmon. Dis. 2, 313–321. doi: 10.2147/copd.s2147
Solomon, L. R. (2016). Low cobalamin levels as predictors of cobalamin deficiency: importance of comorbidities associated with increased oxidative stress. Am. J. Med. 129, 115.e9–115.e16. doi: 10.1016/j.amjmed.2015.07.017
Taban-Shomal, O., Kilter, H., Wagner, A., Schorr, H., Umanskaya, N., Hübner, U., et al. (2009). The cardiac effects of prolonged vitamin B12 and folate deficiency in rats. Cardiovasc. Toxicol. 9, 95–102. doi: 10.1007/s12012-009-9038-2
Tyagi, S. C., and Joshua, I. G. (2014). Exercise and nutrition in myocardial matrix metabolism, remodeling, regeneration, epigenetics, microcirculation, and muscle. Can. J. Physiol. Pharmacol. 92, 521–523. doi: 10.1139/cjpp-2014-0197
Keywords: COPD, exercise training, hyperhomocysteinemia, natriuretic peptides, vitamin B12
Citation: Paulin FV, Goelzer LS and Müller PT (2020) Vitamin B12 Supplementation and NT-proBNP Levels in COPD Patients: A Secondary Analysis of a Randomized and Controlled Study in Rehabilitation. Front. Neurosci. 14:740. doi: 10.3389/fnins.2020.00740
Received: 09 January 2020; Accepted: 23 June 2020;
Published: 14 July 2020.
Edited by:
Tijana Bojić, University of Belgrade, SerbiaReviewed by:
Moacir Fernandes Godoy, Faculty of Medicine of São José do Rio Preto, BrazilVlasta Bari, IRCCS Policlinico San Donato, Italy
Copyright © 2020 Paulin, Goelzer and Müller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paulo de Tarso Müller, cGF1bG8ubXVsbGVyQHVmbXMuYnI=
 Fernanda Viana Paulin
Fernanda Viana Paulin Leandro Steinhorst Goelzer
Leandro Steinhorst Goelzer Paulo de Tarso Müller
Paulo de Tarso Müller