- Nutritional Neuroscience Lab, Department of Health Studies, Center for Neuroscience and Behavior, American University, Washington, DC, United States
Excitotoxicity has been implicated in many neurological disorders and is a leading cause of oxidative stress and neuroinflammation in the nervous system. Most of the research to date has focused on each of these conditions individually; however, excitotoxicity, oxidative stress, and neuroinflammation have the ability to influence one another in a self-sustaining manner, thus functioning as a “neurotoxic triad.” This perspective article re-introduces the concept of the neurotoxic triad and reviews how specific dietary micronutrients have been shown to protect against not only oxidative stress, but also excitotoxicity and neuroinflammation. Future dietary interventions for neurological disorders could focus on the effects on all three aspects of the neurotoxic triad.
Introduction
Excitotoxicity has been implicated in many neurological disorders including, but not limited to, Alzheimer’s (Wang and Reddy, 2017), Parkinson’s (Iovino et al., 2020), amyotrophic lateral sclerosis (ALS; King et al., 2016), multiple sclerosis (MS; Kostic et al., 2013), epilepsy (Vishnoi et al., 2016), migraine (Longoni and Ferrarese, 2006), chronic pain (Inquimbert et al., 2018), and psychiatric conditions (Olloquequi et al., 2018). Excitotoxicity is characterized by over-excitation of neurons which leads to neuronal cell death, and this process is primarily mediated by high amounts of the excitatory neurotransmitter glutamate (Olney, 1990). This process potentiates oxidative stress in the nervous system (Bondy and LeBel, 1993) and the interplay between excitotoxicity, oxidative stress, and neuroinflammation is thought to be associated with neurodegenerative conditions; thus, being an important target for future therapies (Chamorro et al., 2016; Kamal et al., 2020). These three biological states appear to be able to influence one another in a self-sustaining cycle (Nguyen et al., 2011) forming a “neurotoxic triad.” Figure 1 illustrates the self-sustaining interaction between excitotoxicity, oxidative stress, and inflammation (which are reviewed below), while also presenting some nutritional deficiencies which can fuel the triad.
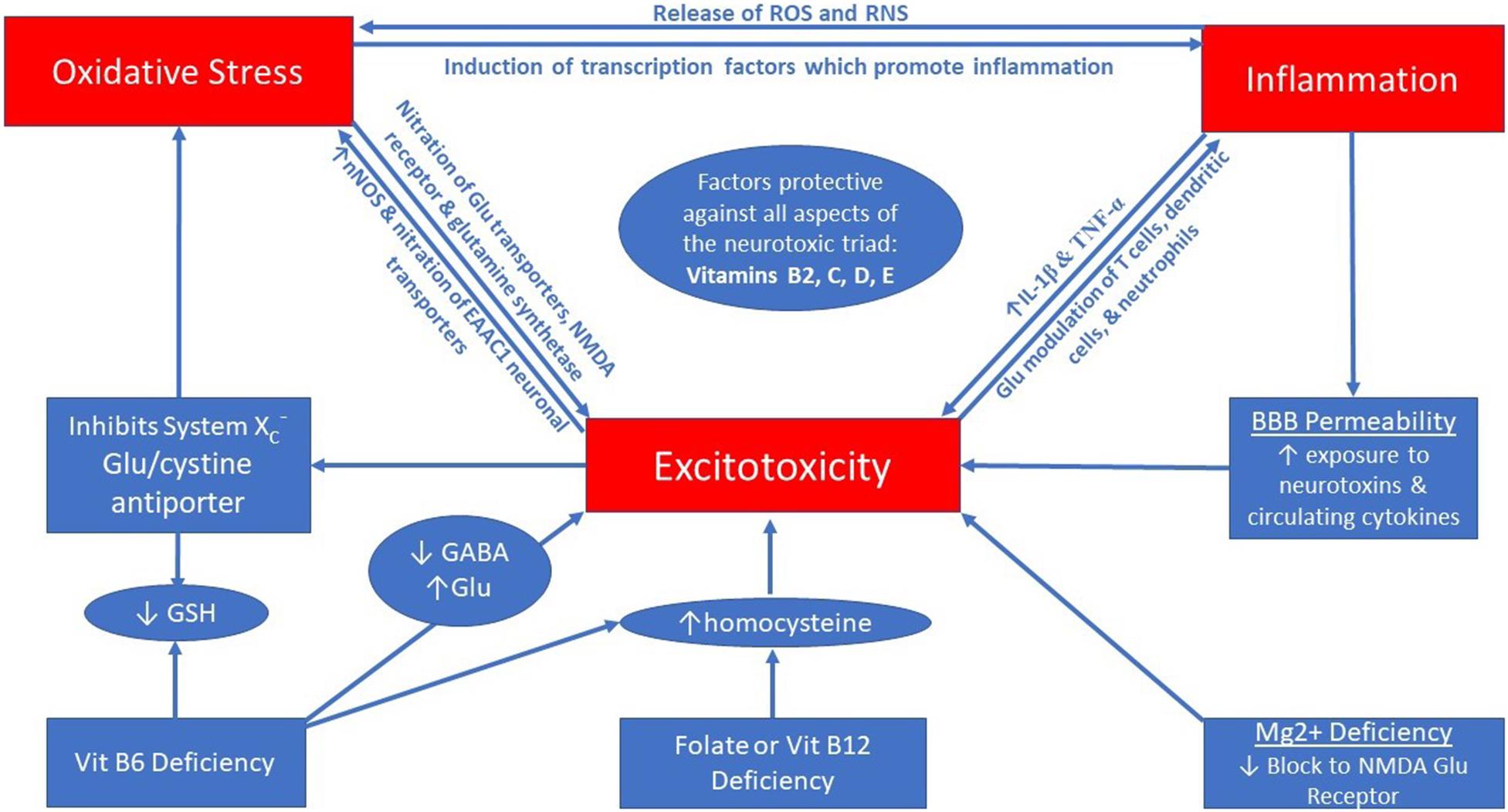
Figure 1. The neurotoxic triad is a self-perpetuating cycle between excitotoxicity, oxidative stress, and neuroinflammation.
The objective of this perspective article is to re-introduce the concept of the neurotoxic triad with a focus on the self-sustaining interaction between excitotoxicity, oxidative stress, and neuroinflammation; and to examine why dietary micronutrients could play a central role in the treatment of disorders characterized by the neurotoxic triad.
Current Status of Knowledge
Most of the research to date has evaluated each aspect of the neurotoxic triad individually, in regards to one or more neurological/psychiatric conditions, or has focused on the intersection of only two components. A few authors have written about the co-occurrence of excitotoxicity, oxidative stress, and neuroinflammation (Chamorro et al., 2016; Ambrogini et al., 2019; Kamal et al., 2020), but to date, little attention has been paid to the reinforcing properties of these three detrimental states. Nguyen et al. (2011) have illustrated the cyclical properties of excitotoxicity, oxidative stress, and changes in mitochondrial function in mice, but this work did not evaluate inflammation. The novel perspective presented herein aims to bridge the current knowledge base to demonstrate the intricate and reinforcing connection between excitotoxicity, oxidative stress, and neuroinflammation while presenting an example of how specific micronutrients may have the unique ability to protect against all three aspects of the neurotoxic triad.
Neurotoxic Triad – Excitotoxicity, Oxidative Stress and Neuroinflammation
Excitotoxicity occurs when abnormally high levels of glutamate are in the synaptic cleft between neurons, leading to prolonged activation of glutamate receptors, such as the NMDA receptor, resulting in increased calcium influx into the postsynaptic neuron and resultant cell death (Olney, 1990). Glutamate excitotoxicity can lead to increased production of neuronal nitrogen oxide synthase (nNOS) which in turn can lead to higher production of reactive nitrogen species, damage to cellular components, and ultimately, cell death (Chen et al., 2017). Excitotoxicity can impair mitochondrial function, leading to altered cellular energy production (Sullivan and Brown, 2005), and this is thought to be mediated by the oxidative stress triggered by excitotoxicity (Nguyen et al., 2011). Additionally, mitochondrial dysfunction has been associated with increased expression of NMDA receptors (Nguyen et al., 2011). An excellent review of this interaction has been published please see Gillessen et al. (2002). Glutamate release can also directly affect inflammation through its ability to stimulate immune cells. For example, T cells are well known for their ability to respond to glutamate concentration, as they contain both ionotropic and metabotropic glutamate receptors (Ganor and Levite, 2014). In disorders such as multiple sclerosis, glutamate is thought to stimulate production of autoreactive T cells (Ganor and Levite, 2014), which may be due to stimulation of AMPA glutamate receptors on the cell surface (Ganor et al., 2007).
Oxidative stress also has the ability to directly affect excitotoxicity and neuroinflammation. An excellent example of this is the action of reactive nitrogen species (RNS) on glutamate transporters. Glutamate transporters are necessary for clearing excess glutamate from the synaptic cleft to prevent excitotoxicity (Martinez-Lozada et al., 2016; Rose et al., 2018). Some glutamate transporters also facilitate the uptake of cysteine, which is the rate limiting factor in the production of the endogenous antioxidant glutathione (Lu, 2009). Nitration of glutamate transporters can inhibit their function, increasing the likelihood of excitotoxicity (Chen et al., 2010). Similarly, nitration of glutamine synthetase (GS) can also increase neuronal excitation via decreased conversion of excitatory glutamate to non-toxic glutamine before transport between astrocytes and neurons (Muscoli et al., 2013). Additionally, this can cause astrocytic swelling, which leads to channel opening that has been associated with astrocytic release of glutamate, which would further support excitotoxicity (Kimelberg et al., 1990). Peroxynitrite (combination of a superoxide radical with nitric oxide) has the ability to interact with NMDA glutamate receptors, leading to the nitration of NR1 receptor subunits (Lafon-Cazal et al., 1993). This change supports activation of NMDARs ultimately leading to excitotoxicity (Mishra and Delivoria-Papadopoulos, 1999; Zanelli et al., 2000). Moreover, peroxynitrite has been implicated in the nitration of the EAAC1 neuronal transporters, reducing the cell’s ability to take up cysteine and inhibiting the intracellular neuronal production of the endogenous antioxidant glutathione, which can also lead to cell death due to the inability to counter intracellular oxidative stress (Aoyama et al., 2008). Thus, peroxynitrite has the ability to further potentiate oxidative stress by reducing the rate-limiting substrate for the production of glutathione, in addition to being able to increase excitotoxicity, making it especially detrimental for the nervous system. Additionally, oxidative stress can also stimulate neuroinflammation directly (Bulua et al., 2011) and scavenging of ROS has been shown to effectively reduce neuroinflammation induced by seizures in an animal model (McElroy et al., 2017).
Not only can excitotoxicity and oxidative stress lead to neuroinflammation, but neuroinflammation caused by trauma, stress, or infiltration of peripheral cytokines, can also independently cause both excitotoxicity and oxidative stress (Hewett et al., 1994; Poletti et al., 2018). Microglia are the main immune cells of the nervous system, and these mediate neuroinflammation through the release of inflammatory cytokines such as IL-6, TNF-α, and IL-1β, in addition to chemokines and secondary messengers such as nitric oxide (NO; Becher et al., 2017). Two of these cytokines, TNF-α and IL-1β, have been shown to increase excitatory neurotransmission via glutamate (Kawasaki et al., 2008; Olmos and Llado, 2014), thereby allowing these two cytokines to influence excitotoxicity. Neuroinflammation can lead to the release of ROS as well as RNS, thereby independently increasing oxidative stress in the nervous system (Brown and Neher, 2010; Kempuraj et al., 2016). During pathological conditions, cytokines can also stimulate inducible nitrogen oxide synthase (iNOS) which results in the production of NO in astrocytes and microglial cells (Murphy, 2000), which can then combine with superoxide radicals to form the aforementioned peroxynitrite, facilitating excitotoxicity (Leist et al., 1997) and DNA damage, ultimately leading to cell death (Xia et al., 1996).
In summary, the neurotoxic triad is self-perpetuating, with each component able to support the propagation of the others. Thus, any disorder for which excitotoxicity has been implicated, will have a high likelihood of oxidative stress and neuroinflammation also being present. Optimal treatment options may need to address all three aspects of the neurotoxic triad, thereby stopping this self-perpetuating cycle. Interestingly, micronutrients may be a leading treatment contender due to their ability to protect against excitotoxicity, oxidative stress, and neuroinflammation concurrently. Below, we review micronutrients of particular interest due to their ability to protect against all three aspects of the neurotoxic triad, as well as a few micronutrients which can protect against two aspects of the triad, with potential indirect effects on the third. Effects of these micronutrients on each aspect of the neurotoxic triad, in addition to food sources, and some clinical findings are listed in Table 1.
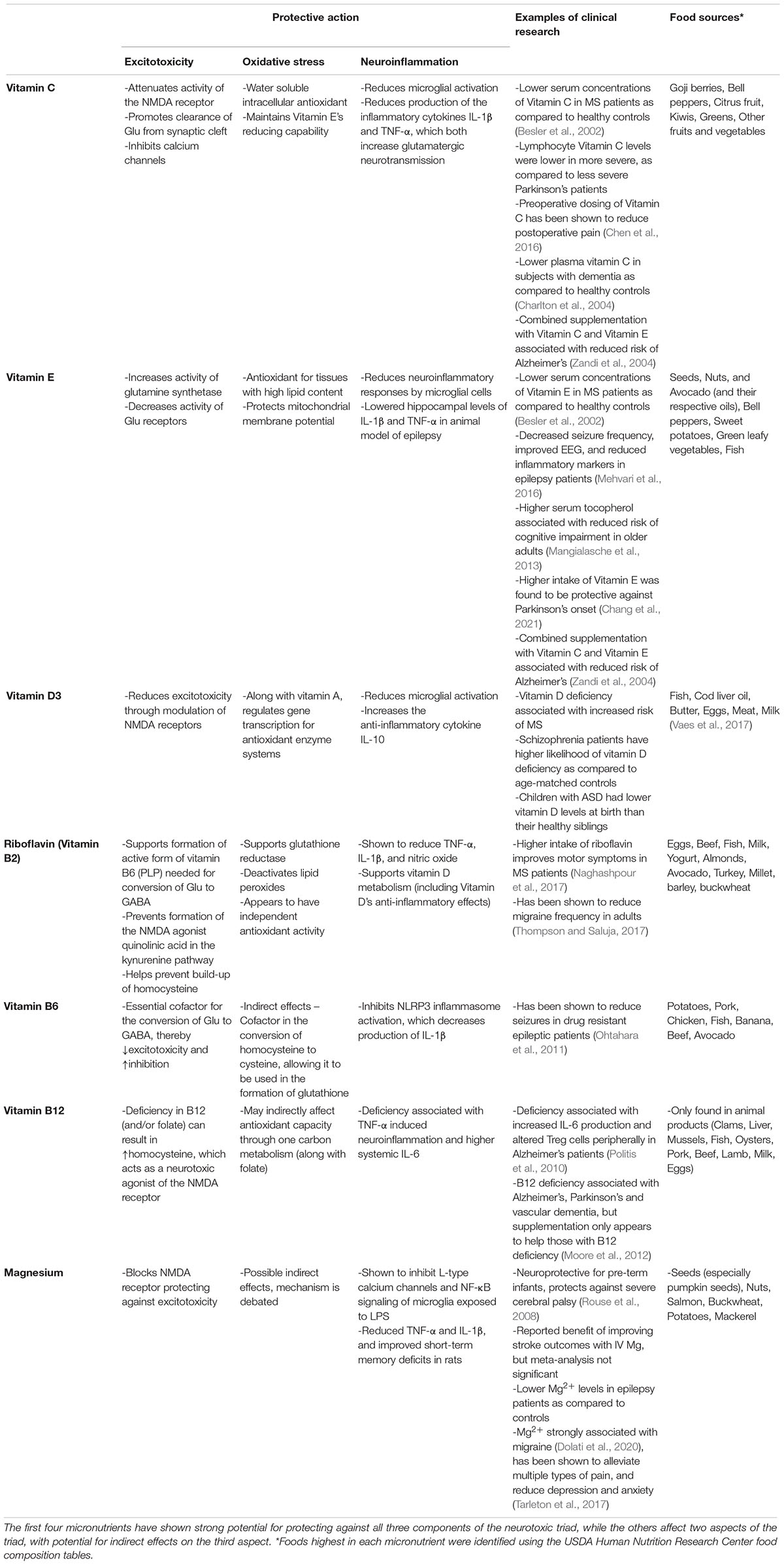
Table 1. Micronutrients which protect against excitotoxicity, oxidative stress, and/or neuroinflammation.
Vitamins C and E
Two vitamins which are well known for their antioxidant capabilities, vitamin C and vitamin E, have been shown to also be protective against excitotoxicity and neuroinflammation. Vitamin C (ascorbic acid) is distributed throughout the body, but is found in highest concentration in the brain (Smythies, 1996). In the central nervous system, vitamin C acts as a cofactor in monoamine neurotransmitter production for dopamine and norepinephrine (May et al., 2012, 2013; Meredith and May, 2013), contributes to myelinization of axons (Olsen and Bunge, 1986; Eldridge et al., 1987), and to prenatal (Meredith and May, 2013) and postnatal (Pastor et al., 2013) brain development, in addition to its very important role as an intracellular antioxidant (Padayatty et al., 2003). Vitamin C plays a major antioxidant role in the aqueous parts of the cell and also helps maintain the reducing capability of vitamin E for protection of cell membranes (Beyer, 1994; May, 1999; Getoff, 2013). Additionally, vitamin C has been shown to be protective against glutamate induced excitotoxicity (Majewska et al., 1990; MacGregor et al., 1996; Matarredona et al., 1997). It can attenuate activity of the NMDA receptor (Majewska et al., 1990), enhance the uptake of glutamate from the synaptic cleft (Lane and Lawen, 2013), and directly inhibit calcium channels, thereby modulating neuronal excitability (Nelson et al., 2007). Additionally, reductions in neuroinflammation have been observed in animal models. Pretreatment with vitamin C has been shown to reduce microglial activation and production of the proinflammatory cytokines TNF-α and IL-1β in an LPS model in mice (Zhang et al., 2018). Similarly, vitamin C was shown to reduce microglial activation in a rat model of ethanol induced neuroinflammation (Ahmad et al., 2016). Additionally, double deficiency in vitamins C and E have been shown to cause increased neuroinflammation, while also impairing conditioned fear memory, in mice (Takahashi et al., 2019).
Similar to vitamin C, vitamin E also appears to affect every aspect of the neurotoxic triad (Ambrogini et al., 2019). Vitamin E is well known for its chain breaking antioxidant function where it has the ability to stop the perpetuating cycle of oxidation of cell membranes (Gonzalez Flecha et al., 1991; Serbinova et al., 1991). As a fat-soluble vitamin, it has the ability to protect tissues with high lipid content (Lee and Han, 2018). Due to the large amount of polyunsaturated fatty acids in the brain, this organ is at high risk for oxidative damage (Cobley et al., 2018), making vitamin E essential for brain health. Animal seizure models have demonstrated that pharmacological doses of vitamin E administered after a kainate-induced seizure, can almost completely restore function of glutamine synthase, the enzyme responsible for converting excitatory glutamate, into non-toxic glutamine, for transport between astrocytes and neurons (Ambrogini et al., 2019). There is some in vitro data which suggests that vitamin E may be able to reduce activity of the ionotropic AMPA, kainate, and NMDA glutamate receptors, in addition to protecting mitochondrial membrane potential through its antioxidant function (Abedi et al., 2017). Additionally, there is in vitro and in vivo evidence that vitamin E can reduce neuroinflammatory responses mediated by microglial cells (Li et al., 2001; Gonzalez-Perez et al., 2002; Godbout et al., 2004; Stolzing et al., 2006; Annahazi et al., 2007), including studies showing that treatment with alpha-tocopherol lowered hippocampal levels of IL-1β and TNF-α in an animal model of epilepsy (Ambrogini et al., 2019).
As mentioned earlier, antioxidants work together to maintain themselves in their reduced form. Vitamin E can be reduced to its active form by vitamin C and the ascorbate form of vitamin C can be regenerated by thiols such as glutathione (Packer et al., 2001). Thus, these three antioxidants have synergistic action to protect against oxidative stress in the nervous system.
Glutathione
Glutathione (GSH) is an extremely important endogenous antioxidant that is a tripeptide formed from the combination of glutamate, cysteine, and glycine (Huang et al., 1993). The availability of cysteine is the rate limiting factor in its production (Huang et al., 1993). Approximately 10–15% of intracellular GSH can be found in the mitochondria, and reduced GSH levels have been associated with mitochondrial dysfunction, including low production of ATP (Enns et al., 2014). In order for GSH to be produced and maintained in its reduced state, a few vitamins are needed, including vitamin D and riboflavin. Vitamin D plays an important supportive role in antioxidant function through its modulation of gene transcription for antioxidant enzyme systems like glutathione peroxidase (Bhat and Ismail, 2015; Tohari et al., 2016; Masjedi et al., 2020). Vitamin D can also upregulate production of the enzymes needed for GSH synthesis and reduction, thereby upregulating its production and antioxidant activity (Jain and Micinski, 2013). Of important note, vitamin D may also be able to reduce excitotoxicity through its action on L-type calcium channels which are thought to modulate NMDA receptors (Brewer et al., 2001), to reduce microglial activation in an LPS model of inflammation (Hur et al., 2014), and to increase release of the anti-inflammatory cytokine, IL-10 (Boontanrart et al., 2016), making it of even greater importance. In addition to vitamin D, riboflavin is also necessary for glutathione function as it serves as a cofactor for glutathione reductase, which is the enzyme responsible for maintaining GSH in its reduced form (Beutler, 1969). Similarly, vitamins B6, folate, and B12 work together in one carbon metabolism (Selhub, 2002) to provide cysteine for the production of glutathione (Dalto and Matte, 2017), and this also allows glutamate to be used in the production of glutathione, thereby limiting glutamate availability for excitotoxicity (Wu et al., 2004).
Riboflavin (Vitamin B2)
In addition to vitamin C, vitamin E, and glutathione, riboflavin (vitamin B2) also appears to play an important role in affecting redox activity in vivo, countering excitotoxicity, and protecting against neuroinflammation. In addition to the supportive role of riboflavin for glutathione reductase mentioned above (Beutler, 1969), riboflavin also has the ability to affect ROS in multiple ways. First, riboflavin has been shown to have its own antioxidant activity, where the reduced form of riboflavin was able to deactivate lipid peroxides (Ashoori and Saedisomeolia, 2014). Other research has demonstrated riboflavin’s activity against mutagen produced free radicals (Durusoy et al., 2002). Second, early data suggests that riboflavin may also have the ability to affect superoxide dismutase (SOD) and catalase (Huang et al., 2010). Thus, it appears that riboflavin may be a key micronutrient for supporting antioxidant activity, though more research in humans is needed.
Riboflavin also protects against excitotoxicity in multiple ways. First, riboflavin is required for the production of the active form of vitamin B6, pyridoxal phosphate (PLP; Adelekan et al., 1987), which is necessary for the formation of many neurotransmitters in the nervous system (Spinneker et al., 2007). One of the neurotransmitter pathways where PLP is active, is as a cofactor for glutamate decarboxylase, which converts glutamate into gamma aminobutyric acid (GABA). GABA is the major inhibitory neurotransmitter in the nervous system and ideally needs to be in balance with glutamate for optimal neuronal functioning (Petroff, 2002). A deficiency in riboflavin can lead to reduced production of PLP [essentially a vitamin B6 deficiency (Madigan et al., 1998)], preventing it from serving as a cofactor in the production of GABA (Spinneker et al., 2007); meaning that riboflavin deficiency can cause increased glutamate and deceased GABA levels, thereby contributing to excitotoxicity. Riboflavin and PLP are also essential cofactors in the kynurenine pathway (Marashly and Bohlega, 2017), where adequate intake of these vitamins has been associated with production of kynurenic acid (Theofylaktopoulou et al., 2014), rather than quinolinic acid, which acts as an agonist to the NMDA receptor, thereby potentiating excitotoxicity (Zinger et al., 2011; Curto et al., 2015). Riboflavin may also be able to directly affect excitotoxicity by inhibiting the exocytosis of glutamate vesicles in presynaptic neurons (Wang et al., 2008). Finally, riboflavin (along with PLP, folate, and vitamin B12) also has the ability to help protect against homocysteine build-up (Rozycka et al., 2013). Homocysteine has been shown to be neurotoxic via its ability to act as an agonist at the NMDA receptor (Deep et al., 2019), making riboflavin’s ability to reduce homocysteine very valuable.
Riboflavin additionally has the ability to protect against neuroinflammation. First, riboflavin was shown to effectively reduce TNF-α, IL-1β, and nitric oxide (NO) in a staphylococcus infection model (Dey and Bishayi, 2016). Riboflavin also plays an indirect role for opposing inflammation through its effects on vitamin D metabolism. Multiple enzymes in the biosynthetic pathway of vitamin D are dependent on riboflavin for their action (Pinto and Cooper, 2014). Animal models have been able to induce vitamin D deficiency from riboflavin deficiency due to this effect on the internal synthesis of vitamin D (Pinto and Cooper, 2014). Since vitamin D has anti-inflammatory effects (as detailed above), riboflavin deficiency may inhibit this protective function of vitamin D.
Other Micronutrients of Interest
In addition to the above micronutrients, there are a few others which also affect two aspects of the neurotoxic triad. In addition to vitamin B6’s role in glutathione production, it also acts as cofactor in the conversion of glutamate to GABA (Martin, 1987), with deficiency leading to increased excitation and decreased inhibition, thereby potentiating effects of excitotoxicity (Baumeister et al., 1994). Moreover, it has the ability, along with folate and vitamin B12, to limit the production of homocysteine (Zaric et al., 2019), which can act as a neurotoxic agonist of the NMDA receptor (Deep et al., 2019). There is also some data to suggest that vitamin B6 and B12 deficiency may be associated with increased inflammation (Al-Daghri et al., 2016; Ueland et al., 2017). Finally, the mineral magnesium serves as a block of the NMDA receptor, helping to protect against excitotoxicity (Nikolaev et al., 2012), and also appears to reduce microglial activation and downstream production of inflammatory cytokines (Maier et al., 2021).
Potential for Deficiencies
Food is very impactful for human health in general, and the dietary components reviewed above are great examples of how important food may be for optimizing health of the nervous system and for treating neurological conditions which are characterized by the neurotoxic triad. Food sources for each of the micronutrients is included in Table 1 for easy reference. Deficiencies in many of these micronutrients are common. Inadequate consumption of vitamin C is thought to be common in the United States (Hampl et al., 2004). Similarly, current estimates suggest that the majority of the population may not be receiving adequate quantities of vitamin E (McBurney et al., 2015). Vitamin D deficiency is also very common in populations living at more extreme latitudes due to reduced exposure to UV light, and is further compounded in those who do not eat fish frequently (Holick, 2004) and who do not compensate in some manner such as taking cod liver oil during the colder months. Finally, riboflavin has also been shown to be under-consumed, with estimates suggesting that 10–15% of the global population may be deficient, and over 50% of non-elderly British adults were estimated to have borderline deficiency based on glutathione reductase activation in erythrocytes (Kennedy, 2016). Deficiency of vitamin B12 is common in vegetarians and vegans, as well as populations suffering from protein malnutrition (Stabler and Allen, 2004), and 45% of Americans are thought to be deficient in magnesium (Workinger et al., 2018). Therefore, increased research attention on the effects of these micronutrients is warranted.
Conclusion
The neurotoxic triad is characterized by excitotoxicity, oxidative stress, and neuroinflammation, with each aspect of the triad being able to perpetuate the others inside of the nervous system. It has been suggested in the literature that to adequately address oxidative stress as a contributor to neurodegeneration, that we must also simultaneously address excitotoxicity (Li et al., 2013), but dietary micronutrients may offer an even better solution by reducing all three aspects of the neurotoxic triad, including neuroinflammation. Vitamin C, vitamin E, vitamin D, and riboflavin are key dietary antioxidants which simultaneously protect against excitotoxicity, oxidative stress, and neuroinflammation. Similarly, glutathione also appears to directly affect all three aspects of the neurotoxic triad. Future dietary research should examine how increased intake of these micronutrients, along with other nutrients like vitamins B6 and B12, and magnesium, may be protective against excitotoxicity, oxidative stress, and neuroinflammation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
KH was responsible for the design, research, writing, and final content of this manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abedi, Z., Khaza’ai, H., Vidyadaran, S., and Mutalib, M. S. A. (2017). The Modulation of NMDA and AMPA/Kainate Receptors by Tocotrienol-Rich Fraction and Alpha-Tocopherol in Glutamate-Induced Injury of Primary Astrocytes. Biomedicines 5:68 doi: 10.3390/biomedicines5040068
Adelekan, D. A., Adekile, A. D., and Thurnham, D. I. (1987). Dependence of pyridoxine metabolism on riboflavin status in sickle cell patients. Am. J. Clin. Nutr. 46, 86–90. doi: 10.1093/ajcn/46.1.86
Ahmad, A., Shah, S. A., Badshah, H., Kim, M. J., Ali, T., Yoon, G. H., et al. (2016). Neuroprotection by Vitamin C Against Ethanol-Induced Neuroinflammation Associated Neurodegeneration in the Developing Rat Brain. CNS Neurol. Disord. Drug Targets 15, 360–370.
Al-Daghri, N. M., Rahman, S., Sabico, S., Yakout, S., Wani, K., Al-Attas, O. S., et al. (2016). Association of Vitamin B12 with Pro-Inflammatory Cytokines and Biochemical Markers Related to Cardiometabolic Risk in Saudi Subjects. Nutrients 8:460 doi: 10.3390/nu8090460
Ambrogini, P., Torquato, P., Bartolini, D., Albertini, M. C., Lattanzi, D., Di Palma, M., et al. (2019). Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: the role of vitamin E. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1098–1112. doi: 10.1016/j.bbadis.2019.01.026
Annahazi, A., Mracsko, E., Sule, Z., Karg, E., Penke, B., Bari, F., et al. (2007). Pre-treatment and post-treatment with alpha-tocopherol attenuates hippocampal neuronal damage in experimental cerebral hypoperfusion. Eur. J. Pharmacol. 571, 120–128. doi: 10.1016/j.ejphar.2007.05.048
Aoyama, K., Matsumura, N., Watabe, M., and Nakaki, T. (2008). Oxidative stress on EAAC1 is involved in MPTP-induced glutathione depletion and motor dysfunction. Eur. J. Neurosci. 27, 20–30. doi: 10.1111/j.1460-9568.2007.05979.x
Ashoori, M., and Saedisomeolia, A. (2014). Riboflavin (vitamin B(2)) and oxidative stress: a review. Br. J. Nutr. 111, 1985–1991. doi: 10.1017/s0007114514000178
Baumeister, F. A., Gsell, W., Shin, Y. S., and Egger, J. (1994). Glutamate in pyridoxine-dependent epilepsy: neurotoxic glutamate concentration in the cerebrospinal fluid and its normalization by pyridoxine. Pediatrics 94, 318–321.
Becher, B., Spath, S., and Goverman, J. (2017). Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 17, 49–59. doi: 10.1038/nri.2016.123
Besler, H. T., Comoðlu, S., and Okçu, Z. (2002). Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr. Neurosci. 5, 215–220. doi: 10.1080/10284150290029205
Beutler, E. (1969). Glutathione reductase: stimulation in normal subjects by riboflavin supplementation. Science 165, 613–615. doi: 10.1126/science.165.3893.613
Beyer, R. E. (1994). The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and coenzyme Q. J. Bioenerg. Biomembr. 26, 349–358. doi: 10.1007/bf00762775
Bhat, M., and Ismail, A. (2015). Vitamin D treatment protects against and reverses oxidative stress induced muscle proteolysis. J. Steroid Biochem. Mol. Biol. 152, 171–179. doi: 10.1016/j.jsbmb.2015.05.012
Bondy, S. C., and LeBel, C. P. (1993). The relationship between excitotoxicity and oxidative stress in the central nervous system. Free Radic. Biol. Med. 14, 633–642. doi: 10.1016/0891-5849(93)90144-j
Boontanrart, M., Hall, S. D., Spanier, J. A., Hayes, C. E., and Olson, J. K. (2016). Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J. Neuroimmunol. 292, 126–136. doi: 10.1016/j.jneuroim.2016.01.015
Brewer, L. D., Thibault, V., Chen, K. C., Langub, M. C., Landfield, P. W., and Porter, N. M. (2001). Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 21, 98–108. doi: 10.1523/jneurosci.21-01-00098.2001
Brown, G. C., and Neher, J. J. (2010). Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 41, 242–247. doi: 10.1007/s12035-010-8105-9
Bulua, A. C., Simon, A., Maddipati, R., Pelletier, M., Park, H., Kim, K. Y., et al. (2011). Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208, 519–533. doi: 10.1084/jem.20102049
Chamorro, A., Dirnagl, U., Urra, X., and Planas, A. M. (2016). Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 15, 869–881. doi: 10.1016/s1474-4422(16)00114-9
Chang, M. C., Kwak, S. G., and Kwak, S. (2021). Effect of dietary vitamins C and E on the risk of Parkinson’s disease: a meta-analysis. Clin. Nutr. 40, 3922–3930. doi: 10.1016/j.clnu.2021.05.011
Charlton, K. E., Rabinowitz, T. L., Geffen, L. N., and Dhansay, M. A. (2004). Lowered plasma vitamin C, but not vitamin E, concentrations in dementia patients. J. Nutr. Health Aging 8, 99–107.
Chen, R., Gong, P., Tao, T., Gao, Y., Shen, J., Yan, Y., et al. (2017). O-GlcNAc Glycosylation of nNOS Promotes Neuronal Apoptosis Following Glutamate Excitotoxicity. Cell. Mol. Neurobiol. 37, 1465–1475. doi: 10.1007/s10571-017-0477-1
Chen, S., Roffey, D. M., Dion, C. A., Arab, A., and Wai, E. K. (2016). Effect of Perioperative Vitamin C Supplementation on Postoperative Pain and the Incidence of Chronic Regional Pain Syndrome: a Systematic Review and Meta-Analysis. Clin. J. Pain 32, 179–185. doi: 10.1097/ajp.0000000000000218
Chen, Z., Muscoli, C., Doyle, T., Bryant, L., Cuzzocrea, S., Mollace, V., et al. (2010). NMDA-receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain 149, 100–106. doi: 10.1016/j.pain.2010.01.015
Cobley, J. N., Fiorello, M. L., and Bailey, D. M. (2018). 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 15, 490–503. doi: 10.1016/j.redox.2018.01.008
Curto, M., Lionetto, L., Negro, A., Capi, M., Fazio, F., Giamberardino, M. A., et al. (2015). Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 17:47.
Dalto, D. B., and Matte, J. J. (2017). Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 9:189 doi: 10.3390/nu9030189
Deep, S. N., Mitra, S., Rajagopal, S., Paul, S., and Poddar, R. (2019). GluN2A-NMDA receptor-mediated sustained Ca(2+) influx leads to homocysteine-induced neuronal cell death. J. Biol. Chem. 294, 11154–11165. doi: 10.1074/jbc.ra119.008820
Dey, S., and Bishayi, B. (2016). Riboflavin along with antibiotics balances reactive oxygen species and inflammatory cytokines and controls Staphylococcus aureus infection by boosting murine macrophage function and regulates inflammation. J. Inflamm. 13:36.
Dolati, S., Rikhtegar, R., Mehdizadeh, A., and Yousefi, M. (2020). The Role of Magnesium in Pathophysiology and Migraine Treatment. Biol. Trace Elem. Res. 196, 375–383. doi: 10.1007/s12011-019-01931-z
Durusoy, M., Karagoz, E., and Ozturk, K. (2002). Assessment of the relationship between the antimutagenic action of riboflavin and glutathione and the levels of antioxidant enzymes. J. Nutr. Biochem. 13, 598–602.
Eldridge, C. F., Bunge, M. B., Bunge, R. P., and Wood, P. M. (1987). Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J. Cell Biol. 105, 1023–1034.
Enns, G. M., Moore, T., Le, A., Atkuri, K., Shah, M. K., Cusmano-Ozog, K., et al. (2014). Degree of glutathione deficiency and redox imbalance depend on subtype of mitochondrial disease and clinical status. PLoS One 9:e100001. doi: 10.1371/journal.pone.0100001
Ganor, Y., and Levite, M. (2014). The neurotransmitter glutamate and human T cells: glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J. Neural. Transm. 121, 983–1006. doi: 10.1007/s00702-014-1167-5
Ganor, Y., Teichberg, V. I., and Levite, M. (2007). TCR activation eliminates glutamate receptor GluR3 from the cell surface of normal human T cells, via an autocrine/paracrine granzyme B-mediated proteolytic cleavage. J. Immunol. 178, 683–692. doi: 10.4049/jimmunol.178.2.683
Getoff, N. (2013). Vitamin C: electron emission, free radicals and biological versatility. In Vivo 27, 565–570.
Gillessen, T., Budd, S. L., and Lipton, S. A. (2002). Excitatory amino acid neurotoxicity. Adv. Exp. Med. Biol. 513, 3–40.
Godbout, J. P., Berg, B. M., Kelley, K. W., and Johnson, R. W. (2004). alpha-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J. Neuroimmunol. 149, 101–109. doi: 10.1016/j.jneuroim.2003.12.017
Gonzalez Flecha, B. S., Repetto, M., Evelson, P., and Boveris, A. (1991). Inhibition of microsomal lipid peroxidation by alpha-tocopherol and alpha-tocopherol acetate. Xenobiotica 21, 1013–1022. doi: 10.3109/00498259109039541
Gonzalez-Perez, O., Gonzalez-Castaneda, R. E., Huerta, M., Luquin, S., Gomez-Pinedo, U., Sanchez-Almaraz, E., et al. (2002). Beneficial effects of alpha-lipoic acid plus vitamin E on neurological deficit, reactive gliosis and neuronal remodeling in the penumbra of the ischemic rat brain. Neurosci. Lett. 321, 100–104. doi: 10.1016/s0304-3940(02)00056-3
Hampl, J. S., Taylor, C. A., and Johnston, C. S. (2004). Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am. J. Public Health 94, 870–875.
Hewett, S. J., Csernansky, C. A., and Choi, D. W. (1994). Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron 13, 487–494. doi: 10.1016/0896-6273(94)90362-x
Holick, M. F. (2004). Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 80, 1678S–1688S.
Huang, C. S., Chang, L. S., Anderson, M. E., and Meister, A. (1993). Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J. Biol. Chem. 268, 19675–19680. doi: 10.1016/s0021-9258(19)36569-x
Huang, J., Tian, L., Wu, X., Yang, H., and Liu, Y. (2010). Effects of dietary riboflavin levels on antioxidant defense of the juvenile grouper Epinephelus coioides. Fish Physiol. Biochem. 36, 55–62. doi: 10.1007/s10695-008-9279-1
Hur, J., Lee, P., Kim, M. J., and Cho, Y. W. (2014). Regulatory Effect of 25-hydroxyvitamin D3 on Nitric Oxide Production in Activated Microglia. Korean J. Physiol. Pharmacol. 18, 397–402. doi: 10.4196/kjpp.2014.18.5.397
Inquimbert, P., Moll, M., Latremoliere, A., Tong, C. K., Whang, J., Sheehan, G. F., et al. (2018). NMDA Receptor Activation Underlies the Loss of Spinal Dorsal Horn Neurons and the Transition to Persistent Pain after Peripheral Nerve Injury. Cell Rep. 23, 2678–2689.
Iovino, L., Tremblay, M. E., and Civiero, L. (2020). Glutamate-induced excitotoxicity in Parkinson’s disease: the role of glial cells. J. Pharmacol. Sci. 144, 151–164. doi: 10.1016/j.jphs.2020.07.011
Jain, S. K., and Micinski, D. (2013). Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 437, 7–11.
Kamal, H., Tan, G. C., Ibrahim, S. F., Shaikh, M. F., Mohamed, I. N., Mohamed, R. M. P., et al. (2020). Alcohol Use Disorder, Neurodegeneration, Alzheimer’s and Parkinson’s Disease: interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity. Front. Cell. Neurosci. 14:282. doi: 10.3389/fncel.2020.00282
Kawasaki, Y., Zhang, L., Cheng, J. K., and Ji, R. R. (2008). Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 28, 5189–5194. doi: 10.1523/jneurosci.3338-07.2008
Kempuraj, D., Thangavel, R., Natteru, P. A., Selvakumar, G. P., Saeed, D., Zahoor, H., et al. (2016). Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 1:1003
Kennedy, D. O. B. (2016). Vitamins and the Brain: mechanisms, Dose and Efficacy–A Review. Nutrients 8:68. doi: 10.3390/nu8020068
Kimelberg, H. K., Anderson, E., and Kettenmann, H. (1990). Swelling-induced changes in electrophysiological properties of cultured astrocytes and oligodendrocytes. II. Whole-cell currents. Brain Res. 529, 262–268.
King, A. E., Woodhouse, A., Kirkcaldie, M. T., and Vickers, J. C. (2016). Excitotoxicity in ALS: overstimulation, or overreaction? Exp. Neurol. 275, 162–171. doi: 10.1016/j.expneurol.2015.09.019
Kostic, M., Zivkovic, N., and Stojanovic, I. (2013). Multiple sclerosis and glutamate excitotoxicity. Rev. Neurosci. 24, 71–88.
Lafon-Cazal, M., Culcasi, M., Gaven, F., Pietri, S., and Bockaert, J. (1993). Nitric oxide, superoxide and peroxynitrite: putative mediators of NMDA-induced cell death in cerebellar granule cells. Neuropharmacology 32, 1259–1266. doi: 10.1016/0028-3908(93)90020-4
Lane, D. J., and Lawen, A. (2013). The glutamate aspartate transporter (GLAST) mediates L-glutamate-stimulated ascorbate-release via swelling-activated anion channels in cultured neonatal rodent astrocytes. Cell Biochem. Biophys. 65, 107–119. doi: 10.1007/s12013-012-9404-8
Lee, G. Y., and Han, S. N. (2018). The Role of Vitamin E in Immunity. Nutrients 10:1614 doi: 10.3390/nu10111614
Leist, M., Fava, E., Montecucco, C., and Nicotera, P. (1997). Peroxynitrite and nitric oxide donors induce neuronal apoptosis by eliciting autocrine excitotoxicity. Eur. J. Neurosci. 9, 1488–1498. doi: 10.1111/j.1460-9568.1997.tb01503.x
Li, J., O, W., Li, W., and Jiang, Z. G. (2013). Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 14, 24438–24475. doi: 10.3390/ijms141224438
Li, Y., Liu, L., Barger, S. W., Mrak, R. E., and Griffin, W. S. (2001). Vitamin E suppression of microglial activation is neuroprotective. J. Neurosci. Res. 66, 163–170. doi: 10.1002/jnr.1208
Longoni, M., and Ferrarese, C. (2006). Inflammation and excitotoxicity: role in migraine pathogenesis. Neurol. Sci. 27, S107–S110.
Lu, S. C. (2009). Regulation of glutathione synthesis. Mol. Aspects Med. 30, 42–59. doi: 10.1016/j.mam.2008.05.005
MacGregor, D. G., Higgins, M. J., Jones, P. A., Maxwell, W. L., Watson, M. W., Graham, D. I., et al. (1996). Ascorbate attenuates the systemic kainate-induced neurotoxicity in the rat hippocampus. Brain Res. 727, 133–144. doi: 10.1016/0006-8993(96)00362-9
Madigan, S. M., Tracey, F., McNulty, H., Eaton-Evans, J., Coulter, J., McCartney, H., et al. (1998). Riboflavin and vitamin B-6 intakes and status and biochemical response to riboflavin supplementation in free-living elderly people. Am. J. Clin. Nutr. 68, 389–395. doi: 10.1093/ajcn/68.2.389
Maier, J. A., Castiglioni, S., Locatelli, L., Zocchi, M., and Mazur, A. (2021). Magnesium and inflammation: advances and perspectives. Semin. Cell Dev. Biol. 115, 37–44. doi: 10.1016/j.semcdb.2020.11.002
Majewska, M. D., Bell, J. A., and London, E. D. (1990). Regulation of the NMDA receptor by redox phenomena: inhibitory role of ascorbate. Brain Res. 537, 328–332. doi: 10.1016/0006-8993(90)90379-p
Mangialasche, F., Solomon, A., Kåreholt, I., Hooshmand, B., Cecchetti, R., Fratiglioni, L., et al. (2013). Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp. Gerontol. 48, 1428–1435. doi: 10.1016/j.exger.2013.09.006
Marashly, E. T., and Bohlega, S. A. (2017). Riboflavin Has Neuroprotective Potential: focus on Parkinson’s Disease and Migraine. Front. Neurol. 8:333. doi: 10.3389/fneur.2017.00333
Martin, D. L. (1987). Regulatory properties of brain glutamate decarboxylase. Cell. Mol. Neurobiol. 7, 237–253. doi: 10.1007/bf00711302
Martinez-Lozada, Z., Guillem, A. M., and Robinson, M. B. (2016). Transcriptional Regulation of Glutamate Transporters: from Extracellular Signals to Transcription Factors. Adv. Pharmacol. 76, 103–145.
Masjedi, F., Keshtgar, S., Zal, F., Talaei-Khozani, T., Sameti, S., Fallahi, S., et al. (2020). Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulosa cells of normal and polycystic ovaries. J. Steroid Biochem. Mol. Biol. 197:105521. doi: 10.1016/j.jsbmb.2019.105521
Matarredona, E. R., Santiago, M., Machado, A., and Cano, J. (1997). Lack of involvement of glutamate-induced excitotoxicity in MPP+ toxicity in striatal dopaminergic terminals: possible involvement of ascorbate. Br. J. Pharmacol. 121, 1038–1044. doi: 10.1038/sj.bjp.0701216
May, J. M. (1999). Is ascorbic acid an antioxidant for the plasma membrane? FASEB J. 13, 995–1006. doi: 10.1096/fasebj.13.9.995
May, J. M., Qu, Z. C., and Meredith, M. E. (2012). Mechanisms of ascorbic acid stimulation of norepinephrine synthesis in neuronal cells. Biochem. Biophys. Res. Commun. 426, 148–152. doi: 10.1016/j.bbrc.2012.08.054
May, J. M., Qu, Z. C., Nazarewicz, R., and Dikalov, S. (2013). Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res. Bull. 90, 35–42. doi: 10.1016/j.brainresbull.2012.09.009
McBurney, M. I., Yu, E. A., Ciappio, Bird, J. K., Eggersdorfer, M., and Mehta, S. (2015). Suboptimal Serum alpha-Tocopherol Concentrations Observed among Younger Adults and Those Depending Exclusively upon Food Sources, NHANES 2003-20061-3. PLoS One 10:e0135510. doi: 10.1371/journal.pone.0135510
McElroy, P. B., Liang, L. P., Day, B. J., and Patel, M. (2017). Scavenging reactive oxygen species inhibits status epilepticus-induced neuroinflammation. Exp. Neurol. 298, 13–22. doi: 10.1016/j.expneurol.2017.08.009
Mehvari, J., Motlagh, F. G., Najafi, M., Ghazvini, M. R., Naeini, A. A., and Zare, M. (2016). Effects of Vitamin E on seizure frequency, electroencephalogram findings, and oxidative stress status of refractory epileptic patients. Adv. Biomed. Res. 5:36. doi: 10.4103/2277-9175.178780
Meredith, M. E., and May, J. M. (2013). Regulation of embryonic neurotransmitter and tyrosine hydroxylase protein levels by ascorbic acid. Brain Res. 1539, 7–14. doi: 10.1016/j.brainres.2013.09.040
Mishra, O. P., and Delivoria-Papadopoulos, M. (1999). Cellular mechanisms of hypoxic injury in the developing brain. Brain Res. Bull. 48, 233–238. doi: 10.1016/s0361-9230(98)00170-1
Moore, E., Mander, A., Ames, D., Carne, R., Sanders, K., and Watters, D. (2012). Cognitive impairment and vitamin B12: a review. Int. Psychogeriatr. 24, 541–556. doi: 10.1017/s1041610211002511
Murphy, S. (2000). Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia 29, 1–13. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n
Muscoli, C., Dagostino, C., Ilari, S., Lauro, F., Gliozzi, M., Bardhi, E., et al. (2013). Posttranslational nitration of tyrosine residues modulates glutamate transmission and contributes to N-methyl-D-aspartate-mediated thermal hyperalgesia. Mediators Inflamm. 2013:950947.
Naghashpour, M., Jafarirad, S., Amani, R., Sarkaki, A., and Saedisomeolia, A. (2017). Update on riboflavin and multiple sclerosis: a systematic review. Iran. J. Basic Med. Sci. 20, 958–966.
Nelson, M. T., Joksovic, P. M., Su, P., Kang, H. W., Van Deusen, A., Baumgart, J. P., et al. (2007). Molecular mechanisms of subtype-specific inhibition of neuronal T-type calcium channels by ascorbate. J. Neurosci. 27, 12577–12583. doi: 10.1523/jneurosci.2206-07.2007
Nguyen, D., Alavi, M. V., Kim, K. Y., Kang, T., Scott, R. T., Noh, Y. H., et al. (2011). A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis. 2:e240.
Nikolaev, M. V., Magazanik, L. G., and Tikhonov, D. B. (2012). Influence of external magnesium ions on the NMDA receptor channel block by different types of organic cations. Neuropharmacology 62, 2078–2085. doi: 10.1016/j.neuropharm.2011.12.029
Ohtahara, S., Yamatogi, Y., and Ohtsuka, Y. (2011). Vitamin B(6) treatment of intractable seizures. Brain Dev. 33, 783–789. doi: 10.1016/j.braindev.2011.01.010
Olloquequi, J., Cornejo-Cordova, E., Verdaguer, E., Soriano, F. X., Binvignat, O., Auladell, C., et al. (2018). Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: therapeutic implications. J. Psychopharmacol. 32, 265–275. doi: 10.1177/0269881118754680
Olmos, G., and Llado, J. (2014). Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014:861231.
Olsen, C. L., and Bunge, R. P. (1986). Requisites for growth and myelination of urodele sensory neurons in tissue culture. J. Exp. Zool. 238, 373–384. doi: 10.1002/jez.1402380310
Packer, L., Kraemer, K., and Rimbach, G. (2001). Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 17, 888–895. doi: 10.1016/s0899-9007(01)00658-x
Padayatty, S. J., Katz, A., Wang, Y., Eck, P., Kwon, O., Lee, J. H., et al. (2003). Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. Am. Coll. Nutr. 22, 18–35. doi: 10.1080/07315724.2003.10719272
Pastor, P., Cisternas, P., Salazar, K., Silva-Alvarez, C., Oyarce, K., Jara, N., et al. (2013). SVCT2 vitamin C transporter expression in progenitor cells of the postnatal neurogenic niche. Front. Cell. Neurosci. 7:119. doi: 10.3389/fncel.2013.00119
Pinto, J. T., and Cooper, A. J. (2014). From cholesterogenesis to steroidogenesis: role of riboflavin and flavoenzymes in the biosynthesis of vitamin D. Adv. Nutr. 5, 144–163. doi: 10.3945/an.113.005181
Poletti, S., Myint, A. M., Schuetze, G., Bollettini, I., Mazza, E., Grillitsch, D., et al. (2018). Kynurenine pathway and white matter microstructure in bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 268, 157–168. doi: 10.1007/s00406-016-0731-4
Politis, A., Olgiati, P., Malitas, P., Albani, D., Signorini, A., Polito, L., et al. (2010). Vitamin B12 levels in Alzheimer’s disease: association with clinical features and cytokine production. J. Alzheimers Dis. 19, 481–488. doi: 10.3233/jad-2010-1252
Rose, C. R., Ziemens, D., Untiet, V., and Fahlke, C. (2018). Molecular and cellular physiology of sodium-dependent glutamate transporters. Brain Res. Bull. 136, 3–16. doi: 10.1016/j.brainresbull.2016.12.013
Rouse, D. J., Hirtz, D. G., Thom, E., Varner, M. W., Spong, C. Y., Mercer, B. M., et al. (2008). A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N. Engl. J. Med. 359, 895–905.
Rozycka, A., Jagodzinski, P. P., Kozubski, W., Lianeri, M., and Dorszewska, J. (2013). Homocysteine Level and Mechanisms of Injury in Parkinson’s Disease as Related to MTHFR, MTR, and MTHFD1 Genes Polymorphisms and L-Dopa Treatment. Curr. Genomics 14, 534–542. doi: 10.2174/1389202914666131210210559
Selhub, J. (2002). Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J. Nutr. Health Aging 6, 39–42.
Serbinova, E., Kagan, V., Han, D., and Packer, L. (1991). Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 10, 263–275. doi: 10.1016/0891-5849(91)90033-y
Smythies, J. R. (1996). The role of ascorbate in brain: therapeutic implications. J. R. Soc. Med. 89:241. doi: 10.1177/014107689608900501
Spinneker, A., Sola, R., Lemmen, V., Castillo, M. J., Pietrzik, K., and Gonzalez-Gross, M. (2007). Vitamin B6 status, deficiency and its consequences–an overview. Nutr. Hosp. 22, 7–24.
Stabler, S. P., and Allen, R. H. (2004). Vitamin B12 deficiency as a worldwide problem. Annu. Rev. Nutr. 24, 299–326. doi: 10.1146/annurev.nutr.24.012003.132440
Stolzing, A., Widmer, R., Jung, T., Voss, P., and Grune, T. (2006). Tocopherol-mediated modulation of age-related changes in microglial cells: turnover of extracellular oxidized protein material. Free Radic. Biol. Med. 40, 2126–2135. doi: 10.1016/j.freeradbiomed.2006.02.011
Sullivan, P. G., and Brown, M. R. (2005). Mitochondrial aging and dysfunction in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 407–410.
Takahashi, K., Yanai, S., Takisawa, S., Kono, N., Arai, H., Nishida, Y., et al. (2019). Vitamin C and vitamin E double-deficiency increased neuroinflammation and impaired conditioned fear memory. Arch. Biochem. Biophys. 663, 120–128. doi: 10.1016/j.abb.2019.01.003
Tarleton, E. K., Littenberg, B., MacLean, C. D., Kennedy, A. G., and Daley, C. (2017). Role of magnesium supplementation in the treatment of depression: a randomized clinical trial. PLoS One 12:e0180067. doi: 10.1371/journal.pone.0180067
Theofylaktopoulou, D., Ulvik, A., Midttun, O., Ueland, P. M., Vollset, S. E., Nygard, O., et al. (2014). Vitamins B2 and B6 as determinants of kynurenines and related markers of interferon-gamma-mediated immune activation in the community-based Hordaland Health Study. Br. J. Nutr. 112, 1065–1072. doi: 10.1017/s0007114514001858
Thompson, D. F., and Saluja, H. S. (2017). Prophylaxis of migraine headaches with riboflavin: a systematic review. J. Clin. Pharm. Ther. 42, 394–403. doi: 10.1111/jcpt.12548
Tohari, A. M., Zhou, X., and Shu, X. (2016). Protection against oxidative stress by vitamin D in cone cells. Cell Biochem. Funct. 34, 82–94. doi: 10.1002/cbf.3167
Ueland, P. M., McCann, A., Midttun, Ø, and Ulvik, A. (2017). Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 53, 10–27. doi: 10.1016/j.mam.2016.08.001
Vaes, A. M. M., Brouwer-Brolsma, E. M., van der Zwaluw, N. L., van Wijngaarden, J. P., Berendsen, A. A. M., van Schoor, N., et al. (2017). Food sources of vitamin D and their association with 25-hydroxyvitamin D status in Dutch older adults. J. Steroid Biochem. Mol. Biol. 173, 228–234. doi: 10.1016/j.jsbmb.2016.10.004
Vishnoi, S., Raisuddin, S., and Parvez, S. (2016). Glutamate Excitotoxicity and Oxidative Stress in Epilepsy: modulatory Role of Melatonin. J. Environ. Pathol. Toxicol. Oncol. 35, 365–374. doi: 10.1615/jenvironpatholtoxicoloncol.2016016399
Wang, R., and Reddy, P. H. (2017). Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 57, 1041–1048. doi: 10.3233/jad-160763
Wang, S. J., Wu, W. M., Yang, F. L., Hsu, G. S., and Huang, C. Y. (2008). Vitamin B2 inhibits glutamate release from rat cerebrocortical nerve terminals. Neuroreport 19, 1335–1338. doi: 10.1097/wnr.0b013e32830b8afa
Workinger, J. L., Doyle, R. P., and Bortz, J. (2018). Challenges in the Diagnosis of Magnesium Status. Nutrients 10:1202. doi: 10.3390/nu10091202
Wu, G., Fang, Y. Z., Yang, S., Lupton, J. R., and Turner, N. D. (2004). Glutathione metabolism and its implications for health. J. Nutr. 134, 489–492. doi: 10.1093/jn/134.3.489
Xia, Y., Dawson, V. L., Dawson, T. M., Snyder, S. H., and Zweier, J. L. (1996). Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc. Natl. Acad. Sci. U. S. A. 93, 6770–6774. doi: 10.1073/pnas.93.13.6770
Zandi, P. P., Anthony, J. C., Khachaturian, A. S., Stone, S. V., Gustafson, D., Tschanz, J. T., et al. (2004). Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch. Neurol. 61, 82–88. doi: 10.1001/archneur.61.1.82
Zanelli, S. A., Ashraf, Q. M., Delivoria-Papadopoulos, M., and Mishra, O. P. (2000). Peroxynitrite-induced modification of the N-methyl-D-aspartate receptor in the cerebral cortex of the guinea pig fetus at term. Neurosci. Lett. 296, 5–8. doi: 10.1016/s0304-3940(00)01608-6
Zaric, B. L., Obradovic, M., Bajic, V., Haidara, M. A., Jovanovic, M., and Isenovic, E. R. (2019). Homocysteine and Hyperhomocysteinaemia. Curr. Med. Chem. 26, 2948–2961.
Zhang, X. Y., Xu, Z. P., Wang, W., Cao, J. B., Fu, Q., Zhao, W. X., et al. (2018). Vitamin C alleviates LPS-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 65, 438–447. doi: 10.1016/j.intimp.2018.10.020
Keywords: excitotoxicity, antioxidants, oxidative stress, neuroinflammation, neurotoxic triad, vitamins, glutathione
Citation: Holton KF (2021) Micronutrients May Be a Unique Weapon Against the Neurotoxic Triad of Excitotoxicity, Oxidative Stress and Neuroinflammation: A Perspective. Front. Neurosci. 15:726457. doi: 10.3389/fnins.2021.726457
Received: 16 June 2021; Accepted: 31 August 2021;
Published: 22 September 2021.
Edited by:
Jong-Min Kim, Seoul National University Bundang Hospital, South KoreaReviewed by:
Brian M. Polster, University of Maryland, Baltimore, United StatesReza Rastmanesh, Independent Researcher, Tehran, Iran
Copyright © 2021 Holton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathleen F. Holton, SG9sdG9uQGFtZXJpY2FuLmVkdQ==
 Kathleen F. Holton
Kathleen F. Holton