Abstract
The gut microbiota (GM) plays an important role in the physiology and pathology of the host. Microbiota communicate with different organs of the organism by synthesizing hormones and regulating body activity. The interaction of the central nervous system (CNS) and gut signaling pathways includes chemical, neural immune and endocrine routes. Alteration or dysbiosis in the gut microbiota leads to different gastrointestinal tract disorders that ultimately impact host physiology because of the abnormal microbial metabolites that stimulate and trigger different physiologic reactions in the host body. Intestinal dysbiosis leads to a change in the bidirectional relationship between the CNS and GM, which is linked to the pathogenesis of neurodevelopmental and neurological disorders. Increasing preclinical and clinical studies/evidence indicate that gut microbes are a possible susceptibility factor for the progression of neurological disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS) and autism spectrum disorder (ASD). In this review, we discuss the crucial connection between the gut microbiota and the central nervous system, the signaling pathways of multiple biological systems and the contribution of gut microbiota-related neurological disorders.
1. Introduction
Human health is seriously threatened by the dramatic environmental and lifestyle changes of the modern era. An unprecedented rise in a diverse range of neurological disorders is one of the major global challenges. Since the last decade, it has been evident that the gut microbiota has a potential role in brain function by mediating signaling pathways through microbial metabolites (Grochowska et al., 2019; Iannone et al., 2019). At the connection of neuroscience and microbiology, groundbreaking studies, largely conducted over the past ten years, have revealed active relations between animals and the microbial populations that live inside them that support the development and operation of neurological systems. These interactions, which involve immunological, neural, and chemical communication, are complex, but they are vital to the health of individuals and our understanding of neurological disorders (Morais et al., 2021). The gut microbiota residing in the gastrointestinal (GI) tract plays an important role in the health status of the host by regulating cells in local and distant organs, including the brain. Bidirectional transmission occurs in the gut–brain axis (GBA) in the form of a two-way communication mechanism between the gut and the neurological system of the host. This information can be transferred through brain networks, hormones, and the immune system, which facilitate the intestinal microbiota. Bidirectional transmission in the GBA regulates brain dysfunction mechanistically, maintains a mutualistic association with the host and regulates the innate and adaptive immune systems (Collins et al., 2012; Carabotti et al., 2015). This axis involves different pathways, such as the autonomic and enteric nervous system, the endocrine system, the hypothalamic–pituitary–adrenal axis (HPA), the immune system, and the microbiota and its metabolites (Blaser, 2017; Burberry et al., 2020). A healthy gut microbiota benefits the host by producing microbial metabolites and neurotransmitters for communication with host cells, such as intestinal epithelial cells (IECs) and immune cells. Alterations in the gut microbiota and microbial metabolite production have been linked to a wide range of immune-related neurological disorders, including developmental disorders, neurodegeneration, and emotional dysregulation. The brain is the organ responsible for all of an individual’s behavior and for controlling it. It is composed of many diverse populations of neuronal and nonneuronal cells that are connected by incredibly sophisticated structural networks (Deidda and Biazzo, 2021). The digestive tract (GI) is the habitat for more than 98% of the bacteria in our bodies. The term “gut microbiota” refers to the particular microorganisms that are present and reside in the gut (Ma et al., 2019).
The development of omics techniques has contributed to the understanding of the gut microbiota as one of the key regulators of the interactions between the gut and the brain (Bhattarai et al., 2021; Zhu et al., 2022). Animal and human research has provided evidence that the gut microbiota might influence brain behavior and cognitive development by producing hormones, immunological factors, and metabolites, which also suggests that changing the gut microbiome may improve or potentially treat brain disorders (Lee et al., 2011; Braniste et al., 2014; Jasarevic et al., 2015; Ogbonnaya et al., 2015; Yano et al., 2015; Wang and Wang, 2016). Signals from the brain can affect the sensorimotor and secretory functions of the stomach through intricate neurohumoral networks, and likewise, visceral afferent signals coming in the gastrointestinal tract can affect brain function (Cryan and Dinan, 2012). The gut-brain axis has recently emerged as a key participant in the regulation of normal brain functioning under physiologically normal conditions as well as in the development of neuropathological diseases as a risk factor or condition (Ma et al., 2019).
However, there is a lack of widespread confirmation of the mechanisms underlying links between the gut microbiota and brain disorders (Martin et al., 2018). New technologies are being created to go beyond correlative research and find and validate biological mechanisms of action that have the real potential to treat human disease. In this review, we discuss the interaction between the gut and brain and their signaling pathways. Furthermore, we discuss the function of the microbiota and neurological disorders such as neuropsychiatric disorders (schizophrenia and ADS), mood disorders (anxiety and depression), and neurodegenerative disorders (PD, AD, and MS).
2. Gut microbiota-brain axis
The gut microbiome consists of bacteria, archaea, viruses, and eukaryotic microbes that colonize the digestive tract. The gut microbiota, which comprises approximately 100–150 times more genes than the human genome, is found in the human intestines and includes approximately 1,000 species and 7,000 types of bacteria, gram-positive or gram-negative Firmicutes (including the species Lactobacillus, Eubacterium, and Clostridium), and gram-negative Bacteroidetes form the majority of the bacteria (containing Bacteroides and Prevotella) (Flowers and Ellingrod, 2015; Blaser, 2017; Askarova et al., 2020; Tarawneh and Penhos, 2022). The following five phyla make up the majority of the gut microbial community: Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobia (The Human Microbiome Project Consortium, 2012). Individuals’ diet, age, gender, environment, and genes had an impact on the composition of their gut microbiota (Takagi et al., 2019). Dysbiosis of the human gut microbiome has been associated with various pathologies (Perry et al., 2016). Gut dysbiosis, as shown by variations in the diversity and frequency of the microbial community (overall taxa and species) that comprise the gut flora, has been connected in both animal and human studies to abnormal brain protein aggregation, inflammation, immune dysregulation, and reduced neuronal and synaptic activity studies of AD (Cryan et al., 2020; Gubert et al., 2020).
The capability of the gut microbiota to affect brain-related activities suggests that it triggers the production of immune factors that target both the CNS and the enteric nervous system (ENS), such as cytokines and inflammatory mediators (Wood and Galligan, 2004). The autonomic nervous system, a component of the peripheral nervous system, regulates physiological processes not subject to conscious control. It controls vital visceral functions by coordinating complimentary responses between the sympathetic and parasympathetic nervous systems. Understanding the bidirectional communication between the CNS and the digestive tract was greatly advanced by the discovery of the ENS, a branch of the autonomic nervous system. The ENS, sometimes known as the “second brain in the body,” is maintained in a healthy state by the cooperation of enteric neurons and connections to the CNS (Rao and Gershon, 2018). The ENS is made up of millions of neurons that are found in the mucosa of the digestive tract. These neurons are responsible for maintaining the equilibrium of intestinal activities. The most direct route of communication between the gut and the brain is the vagus nerve (de la Fuente-Nunez et al., 2018). A deeper understanding of the gut-brain connection showed a complex communication pathway that not only maintains the health of the gastrointestinal system but is also likely to have a variety of consequences on how the brain functions as a whole, including higher cognitive function and motivation (Rhee et al., 2009). The gut-brain axis (GBA), which is a sophisticated bidirectional communication network between the intestine and the CNS, is where communication occurs between the CNS and intestine (Figure 1; Sudo et al., 2004; Rao and Gershon, 2018; Skonieczna Zydecka et al., 2018). The routes of communication involve the autonomic nervous system [for example, the enteric nervous system (ENS) and the vagus nerve], the neuroendocrine system, the hypothalamic–pituitary–adrenal (HPA) axis, the immune system and metabolic pathways (Duvallet et al., 2017; Blacher et al., 2019; Burberry et al., 2020). Several neurotransmitters (Yano et al., 2015; O'Keefe, 2016) and metabolites, including short-chain fatty acids, secondary bile acids, vital vitamins, and amino acids (Ellwardt et al., 2016; Engelhardt et al., 2016; Mittal et al., 2017), modulate many immune system pathways (Baj et al., 2019; Dalile et al., 2019), which in turn affect cognition, behavior, learning, movement, and neurodegenerative diseases (Jenkins et al., 2016; Kennedy et al., 2017; Feng et al., 2020). The gut-brain axis has been termed the GMB axis since it appears to regulate the immune system, digestive tract, behavior, stress response, and CNS processes (Savignac et al., 2011; Collins et al., 2012; De Palma et al., 2014; Fond et al., 2015; Pirbaglou et al., 2016; Rincel and Darnaudery, 2020). Notably, advancements in gut microbiota sequencing have revealed a strong relationship between the complex ecosystem and the CNS (Knight et al., 2018). In recent years, there has been increasing interest in studying interactions between the brain, gastrointestinal microbiome and their bidirectional relationship.
Figure 1

The physiological homeostasis attained during typical brain functioning is a result of the interactions between the brain and the gut-brain (gut microbiota). Several brain disorders, including Parkinson’s disease, neurodegenerative diseases, depression, stress, Alzheimer’s disease, and neurodevelopmental disorders, have been linked to altered gut microbiota or gut dysbiosis.
3. How the gut microbiota affects the brain
The CNS and ENS communicate with one another using a number of chemical signaling mechanisms, including direct neuronal, immune, and endocrine pathways (Yoo and Mazmanian, 2017). The gut-brain axis is a network of connections involving multiple biological systems that facilitates bidirectional communication between gut bacteria and the brain and is vital for maintaining the gastrointestinal, neurological, and microbial systems of animals (Martin et al., 2018; Cryan et al., 2019). In addition to the neurological system, the gut microbiota also affects the brain through the endocrine, immunological, and metabolic systems (the gut-brain neuroanatomical pathway) (Cryan and Dinan, 2012; Montiel-Castro et al., 2013). In the gut microbiota-brain axis, more emphasis is placed on the involvement of bacteria because the gut microbiota can be used as an independent variable and modified intentionally (Al Omran and Aziz, 2014). Microbes can affect how the nervous system develops, matures, ages, and maintains homeostasis, for example, by altering how neurotrophic factors and N-methyl D-aspartate (NMDA) receptor subunits in the hippocampus are expressed (Bercik et al., 2011a; Heijtz et al., 2011). The main ways that the microbiota can influence the development and function of the nervous system are biological networks, including direct and indirect transmission via chemical transmitters, the immune system, neuronal pathways, and endocrine pathways, as shown in Figure 2.
Figure 2
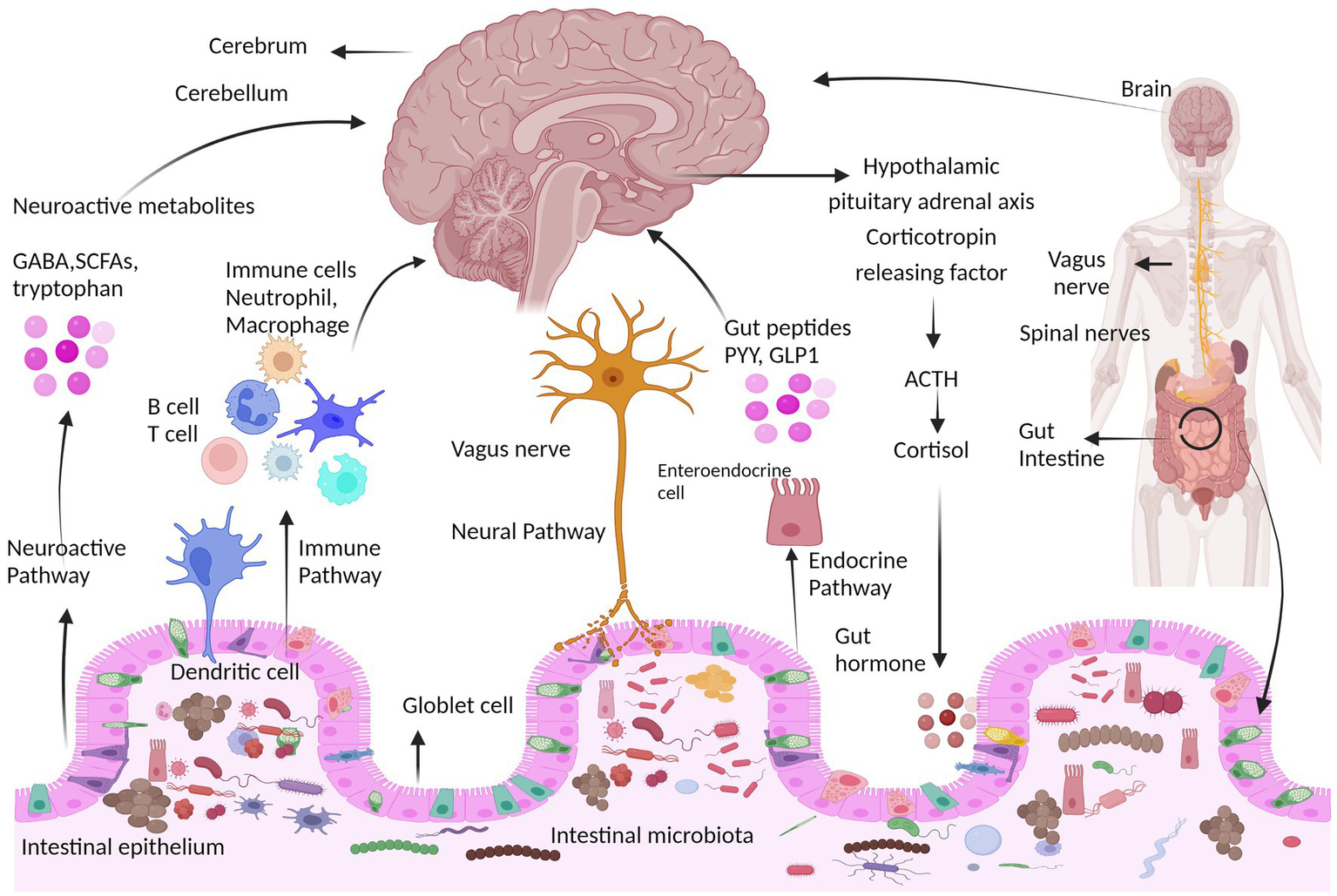
Communication pathways between the brain and gut microbiota. The interaction between the central nervous system (CNS) and gut microorganisms is mediated via several direct and indirect gut-brain axis mechanisms. They include the immune pathway (including cytokines), short-chain fatty acids and microbial metabolites; the neuroactive pathway, including neurotransmitters and neuroactive metabolites; the neural pathway [enteric nervous system, vagus nerve, and spinal nerves (Sgritta et al., 2019); and the endocrine pathway, hypothalamic pituitary adrenal axis (HPA) (Lyte, 2014b)]. HPA axis response that involves neurons of the hypothalamus that release hormones such as corticotropin receptor hormone (CRH) into the portal circulation or the brain, causing the release of the hormone adrenocorticotropic hormone (ACTH), which starts the production of cortisol and its release. The neuroimmune signaling reactions are regulated by cortisol.
3.1. Microbiota and neurotransmitters
Gut microbes can help regulate bodily functions and alter behavior in their animal host through chemical interactions with the nervous system, including both “direct” and “indirect” communication (Morais et al., 2021). Microorganisms have the ability to synthesize some of the neuroactive compounds themselves as well as stimulate the production of other metabolites and neurotransmitters by the host that regulate gut-brain signaling (Poutahidis et al., 2013). The microbiota is also required for the normal maturation, activation, and development of microglia, which are innate immune cells in the brain (Zheng et al., 2020). It seems that immune programming by microglia is regulated by signals from microbial metabolism because administering bacterial-derived short-chain fatty acids (SCFAs) to germ-free (GF) mice restores microglial shape and function (Erny et al., 2015). Microbial-derived molecules signaling to the brain. Neurotransmitters such as dopamine, serotonin, norepinephrine, glycine, and gamma-aminobutyric acids are produced by the intestinal microbiota, and each has a specific impact on brain γ-aminobutyric acid (GABA). Imbalances in these neurotransmitters can lead to disorders such as AD, PD, autism spectrum disorder, anxiety disorders, and depressive disorders (Chen et al., 2021).
For example, Bifidobacterium infantis increases blood plasma tryptophan levels, which affects central serotonin transmission; GABA can be produced by Lactobacillus and Bifidobacterium; noradrenaline can be produced by Escherichia, Bacillus, and Saccharomyces species; serotonin can be produced by Streptococcus, Candida, Escherichia, and Enterococcus species; dopamine can be produced by bacteria; and acetylcholine can be produced by Lactobacillus (Lyte, 2014a). SCFAs, a type of direct signaling, are lipids generated by intestinal microbes through the fermentation of dietary fiber that have the ability to influence the immune system, epigenetics, and neuroplasticity in the CNS (Dalile et al., 2019). The brain, energy balance, and metabolism are all impacted by SCFAs, which include butyrate, propionate, and acetate and are vital metabolic byproducts of gut microbial activity (Dinan et al., 2015). Additionally, SCFAs serve as endogenous ligands for orphan G protein–coupled receptors (GPCRs), and intracellular SCFAs regulate gene expression by preventing histone deacetylases. In addition, SCFAs interact with vagal afferents, which affects inflammation and hormone regulation. The interactions of SCFAs with particular cellular systems and gut-brain signaling pathways support the idea that SCFAs can play a significant role in GMB communication (Dalile et al., 2019). Through indirect chemical communication, the microbiota also affects the neurological system and behavior, as evidenced by the microbial regulation of the neuroendocrine system (Sudo et al., 2004). Gut microbiota can affect their host’s appetite and eating patterns by changing the endocrine signals produced by enteroendocrine cells (EECs) in the gut epithelium, which involves the production of the hormone glucagon-like peptide 1 (GLP1) (Aresti Sanz and El Aidy, 2019). The microbiota in the gut can produce neurotransmitters on their own and can also stimulate the creation of these chemicals in their animal hosts. For example, a number of microbes, including Escherichia spp., Bacteroides, Bifidobacterium, and its species, are known to generate the neurotransmitter GABA (Strandwitz et al., 2019). Bacteria have been demonstrated to be essential for the production of the neurotransmitter serotonin 5-hydroxytryptamine (5-HT) in animal mouse model systems (Clarke et al., 2013). Microbial metabolites such as indole, SCFAs, secondary bile acids, α-tocopherol, p-aminobenzoate, and tyramine have an impact on the generation and secretion of 5-HT by enteroendocrine cells (EECs) (Yano et al., 2015; Morris et al., 2017). Gut microbes synthesize SCFAs, 5-HT, dopamine, butyric acid, gamma amino acids, and gamma amino acids (Forsythe et al., 2014; Lyte, 2014b), and these substances are accessible between microbial cells (Forsythe et al., 2014). The gut, particularly intestinal cells, can synthesize large amounts of 5-HT, which affects the brain. Additionally, microbial enzymes can manufacture neurotoxins such D-lactic acid and ammonia (Manicassamy et al., 2010; Smith, 2015). These neuroactive metabolites, such as the neurotransmitters GABA, dopamine, noradrenaline and serotonin, amino acids (for example, tryptophan and tyramine) T lipopolysaccharide (LPS), short-chain fatty acids (SCFAs), long-chain fatty acids (LCFAs), trimethylamine-N-oxide (TAMO), and polysaccharide A (PSA), either directly or indirectly induce the migration of peripheral immune cells to the brain and are thought to cause neuroinflammation and influence CNS functions (Harms et al., 2018; Morais et al., 2021). Microbial-associated molecular patterns (MAMPs), which are released, also connect the CNS to the microbiota (Sampson and Mazmanian, 2015). MAMPs are molecules produced by gut microbes, such as double-stranded RNA, lipopolysaccharides, and lipoproteins, that are identified by a variety of receptors, especially Toll-like receptors (Akira and Hemmi, 2003; Schachtle and Rosshart, 2021). However, 5-HT and its metabolic precursor tryptophan concentrations in the hippocampus were decreased in germ-free mice, indicating a possible role for the gut microbiota in regulating 5-HT signaling pathways in the CNS (Clarke et al., 2013). In fact, it is difficult to evaluate how much microbial metabolism directly affects CNS activity, in part because we do not fully understand the average rate of transport for numerous microbial metabolites into the brain (Muller et al., 2020).
3.2. Endocrine pathway
SCFAs can alter the function of the gut-brain axis by regulating the production of gut hormones. The activation of G protein-coupled receptors (GPCRs) by SCFAs in the colon is the mechanism underlying the production of these gut hormones, which enhances the release of peptide YY (PYY) and glucagon-like peptide 1 (GLP1) from enteroendocrine L cells (Tolhurst et al., 2012; Psichas et al., 2015; Larraufie et al., 2018). These hormones in turn have the power to affect mood, memory, and learning. Through the use of free fatty acid receptors (FFARs), SCFAs can signal to the brain by directly activating vagal afferents (Dalile et al., 2019). GLP1 has many receptors throughout the body and can affect brain functions via both humoral and neuronal routes, including the CNS and PNS, as well as the heart, lungs, intestines and pancreas (Alvarez et al., 2005; Katsurada and Yada, 2016). GLP1 is involved in enhanced memory and learning in mice (Isacson et al., 2011), enhanced neuroplasticity and neuroprotection in the hippocampus (McClean et al., 2011; Porter et al., 2011), in animal models of AD, and in reduced βamyloid plaques and microglia activation (McClean et al., 2011). Another anorexic neuropeptide, PYY, reduces appetite and prevents gastric motility. In addition to the distal gastrointestinal tract’s L cells secreting it (colon and ileum), the hypothalamus and pituitary gland have the highest levels of PYY expression in the human brain, which is expressed throughout the brain (Morimoto et al., 2008). The most common form of circulating PYY is PYY3–36, a truncated form of the protein that preferentially interacts with the Y2 neuropeptide Y receptor (Murphy and Bloom, 2006). According to research conducted on animals, PYY affects both appetite and brain activity by either mechanisms that cross the blood–brain barrier (BBB) (Nonaka et al., 2003) or by activating vagal afferent pathways that connect to the gut wall’s lamina propria and myenteric plexus and transmitting to the brainstem (Koda et al., 2005; Waise et al., 2018). Other metabolic hormones that affect brain function and are influenced by SCFAs include ghrelin, leptin, and insulin; however, research on these hormones has been less focused than that on PYY and GLP1. Leptin is a hormone that induces weight loss that is mostly produced by adipose cells (Hube et al., 1996), and it is well known for regulating the body’s energy balance by activating its hypothalamic receptors to express orexigenic and anorexigenic neuropeptides such as neuropeptide Y and α melanoma-stimulating hormone, which reduces appetite (Elias et al., 1999).
3.3. Immune pathway
The immune system is influenced and directly affected by both the CNS and the gut microbiome. The gut microbiota has a significant impact on the development and function of the peripheral immune system (Zheng et al., 2020). The microbiota is necessary for the development and activation of innate immune cells in the brain (Abdel-Haq et al., 2019). The pathophysiology of psychiatric disorders may involve immune responses and inflammation (Miller and Raison, 2016). CNS-cytokine interactions affect brain functions and have an effect on neurocircuits that regulate motivation, motor activity, and mood (Capuron and Miller, 2011). Additionally, through the systemic immune system and circulating cytokines, the gut microbiota and the brain communicate (Hsiao et al., 2013). Immune cells directly penetrate the BBB and reach the CNS, or they can produce cytokines and chemokines in the brain (Morais et al., 2021). Cytokines are substances made in the intestine that can travel through the bloodstream and, under certain conditions, have an effect on the hypothalamus and other areas of the brain (El Aidy et al., 2014). The BBB is a physical barrier that separates the brain microenvironment from the rest of the body. It is made of tight junction proteins that connect the mural and microvascular endothelial cells (Morais et al., 2021). The BBB regulates the movement of molecules between the bloodstream and the cerebrospinal fluid of the CNS. Permeability of the BBB is influenced by the gut microbiota, as some reports show that GF mice have increased BBB permeability relative to control mice, partially due to reduced expression of tight-junction proteins such as occludin and claudin 5 (Braniste et al., 2014). The BBB allows it to effectively control the flow of chemicals, ions, and cells between the body’s environment and the brain (Engelhardt and Liebner, 2014). The BBB is important because it protects the brain against pathogens and unfavorable immune responses that could harm the neurons and the connections between them (Daneman and Prat, 2015). Many psychiatric disorders, such as major depression, schizophrenia, autism spectrum disorder, and obsessive–compulsive disorder, have been linked to microglial dysregulation (Frick et al., 2013). SCFAs have a direct impact on immune cells and immunological modulators to maintain homeostasis. The influence of SCFAs on intestinal mucosal immunity is well described by Corrêa-Oliveira et al. (2016). However, SCFAs may also have an impact on the peripheral immune system, modulating brain activity. By increasing the intestinal barrier and inhibiting the transfer of bacteria and bacterial metabolites or by direct contact between SCFAs and immune cells, which could decrease neuroinflammation in the brain, systemic inflammation may be reduced (Dalile et al., 2019). SCFAs regulate the maturation and activation of T lymphocytes, macrophages, dendritic cells (DCs), and neutrophils (Corrêa-Oliveira et al., 2016). Neutrophils, the most prevalent granulocyte type, are an essential part of the innate immune system and are produced in the bone marrow. They are the first to appear at the site of inflammation, and they exploit the production of cytokines to draw in other cells, such macrophages (Rodrigues et al., 2016). SCFAs have an immediate effect on neutrophils by regulating the production of proinflammatory cytokines such as tumor necrosis factor (TNF), possibly through histone deacetylase (HDAC) inhibition. By regulating the synthesis of chemokines such as CXC motif chemokine ligand 1 (CXCL1) and CXC motif ligand 8 (CXCL8), they also function as neutrophil chemoattractants. SCFAs affect neutrophil chemotaxis by causing free fatty acid receptor 2 (FFAR2) in these cells to become active (Rodrigues et al., 2016). SCFAs can affect adaptive immune responses by directly or indirectly affecting T-cell development and proliferation (Kim et al., 2014).
3.4. Neuronal pathways for gut–brain interactions
The gut and brain are physically linked through neurological connections. The most significant of these neural networks is the vagus nerve, which emerges from the brainstem and innervates the gastrointestinal tract and ENS (Yoo and Mazmanian, 2017). The most direct and well-studied link between the gut and the CNS, the vagus nerve, is another pathway through which gut microbes communicate with the brain (Fülling et al., 2019). Almost the whole digestive system is innervated by the vagus nerve, which has 80% afferent and 20% efferent fibers. The vagal afferent nerve terminals innervate multiple layers of the digestive wall, while the mucosal afferents end within the lamina propria of the intestinal mucosa (Waise et al., 2018). Vagal receptors sense inflammatory chemicals, dietary elements, bacterial metabolites, and regulatory gut peptides to transfer signals to the central nervous system (De Lartigue et al., 2011). However, there is some evidence that the bacteria in the gut can directly activate neurons. Toll-like receptors 3 and 7, which detect viral RNA, as well as Toll-like receptors 2 and 4, which detect peptidoglycan and lipopolysaccharide, are present in the enteric nervous systems of both mice and humans (Brun et al., 2013; Martin et al., 2018). Bacteroides fragilis, Lactobacillus rhamnosus (JB-1), and isolated polysaccharide A of B. fragilis have all been demonstrated to stimulate intestine afferent neurons ex vivo (Mao et al., 2013). Chronic treatment with Bifidobacterium longum NCC3001 reduced the symptoms of anxiety induced by persistent gut inflammation (Bercik et al., 2011b). The effects seen in these trials were eliminated when the vagus nerve’s integrity was compromised through vagotomy (Bravo et al., 2011; Bercik et al., 2011b). Additionally, microbial metabolites have the capacity to directly activate neurons. The receptors FXR and TGR5 are expressed in brain neurons, although healthy individuals have low or undetectable bile acid concentrations in these organs (Huang et al., 2016). Various studies have identified the superior cervical ganglion as the location of G protein-coupled receptor 41 (GPR41) and free fatty acid receptor 3 (FFAR3) receptors (Kimura et al., 2011), prevertebral ganglia (Won et al., 2013), submucosal and myenteric ganglia neurons (Nohr et al., 2013), sympathetic ganglia of the thoracic and lumbar sympathetic trunks, and vagal ganglion (Nohr et al., 2015), suggesting neuronal activation by microbially derived SCFAs. Neuronal innervation of the colonic epithelium is reduced in GF mice and restored by microbial colonization (De Vadder et al., 2018). Gut bacteria also aid in the development of enteric glial cells in mice, which are essential for maintaining neuronal networks and controlling gut homeostasis (Kabouridis et al., 2015; Aktar et al., 2020). The activity of enteric neurons can be influenced by the gut microbiota through chemical communication, according to a recent study showing that activating aryl hydrocarbon receptors in adult mice can affect gut motility by affecting the ENS (Obata et al., 2020).
4. Gut microbiota and neurological disorders
Neurological and neuropsychiatric disorders are associated with changes in the composition of the gut microbiota (Cryan et al., 2019; Tian et al., 2023). Neurological disorders are ailments of the central and peripheral nervous system that may harm the brain, spinal cord, cranial and peripheral nerves, autonomous nervous system, nerve roots, and neuromuscular plaque. Numerous conditions can lead to brain bleeding, including diseases of the blood vessels, disorders caused by issues with nervous system development, injuries to the spinal cord or brain, and brain tumors (Dugger and Dickson, 2017). A wide variety of neurological diseases are connected to dysbiosis of the human gut microbiome (Frank et al., 2007; Bibbo et al., 2017; Gavin et al., 2018; Kasselman et al., 2018; Duan et al., 2019). In contrast, patients with neurological diseases and healthy controls have dramatically different microbiota compositions (Sampson et al., 2016; Blacher et al., 2019; Valles-Colomer et al., 2019). Importantly, communication along the gut microbiota–brain axis occurs throughout life, as seen in diseases of neurodevelopment (for example, ASD), neurodegeneration (for example, PD and AD) and behavior (for example, depression and anxiety) (Figure 1). According to some recent studies in animals and humans, most of which were association studies, modifications in microbial diversity are linked to negative health outcomes and may cause alterations in the CNS (Table 1); these alterations are associated with ASD, depression, and anxiety (Felice and O'Mahony, 2017). Other studies have reported additional links between the microbiota composition and depression, anxiety, and ASD (Bercik et al., 2010; Sekirov et al., 2010; Claesson et al., 2012). Thus, the composition of the microbiota, which evolves over time, may have implications in brain function. In this Perspective, we review recent developments in the field of neuromicrobiology, particularly the links between the gut microbiota and neurological disease. In exploring the role that gut microbes play in neurological disorders, we specifically focused on ASD, AD, PD, depression, and anxiety disorders.
Table 1
| Neurological disorders | Animal model | Causation vs. association | Changes in microbiota | Reference |
|---|---|---|---|---|
| Alzheimer’s disease | Human | The first evidence that the bacterial population, viral load, and progress of AD symptoms may be related. Each pathogen effects cognitive decline | Associated with infectious load, being viral (HSV-1 and CMV) or bacterial Helicobacter pylori Chlamydia pneumoniae, and Borrelia burgdorferi, | Bu et al. (2015) |
| Alzheimer’s disease | Human | Probiotic treatment did not meaningfully change other factors including oxidative stress and inflammation, but it may have a good impact on AD patients’ cognitive function. | probiotic supplementation containing: Bifidobacterium bifidum, Lactobacillus casei, Lactobacillus fermentum, and Lactobacillus acidophilus | Akbari et al. (2016) |
| Parkinson’s disease | Human | When mucosal and stool samples were analyzed with Parkinson’s disease were studied, several genes were shown to be downregulated in the stool microbiota of these people; the microbiota composition of the mucosal and stool samples was linked to substantial changes in patients with PD. | Bacterial increase: Proteobacteria, Betaproteobacteria, Coprococcus, Blautia, Akkermansia, Oscillospira, Roseburia, Bacteroides; bacterial decrease: Faecalibacterium, Firmicutes, class Clostridia | Keshavarzian et al. (2015) |
| Parkinson’s disease | Human | change in the fecal microbiota may contribute to the development of PD; Prevotellaceae was decreased in people with Parkinson’s disease, and a high abundance of this genus was not indicative of having PD; Prevotellaceae may serve as a biomarker to rule out PD because of their great abundance. | Bacteria decrease, Provotellaceae; the abundance of Ruminococcaceae could be associated with levels of Provotellaceae | Scheperjans et al. (2015) |
| Autism | Human | Autism symptoms and gastrointestinal (GI) alterations are related, and the development of autism may be influenced by an imbalance of bacteria associated with a healthy state. | Bacteria increase: Lactobacillus, Bacteria decrease: Bifidobacterium and Enterococcus; autism group more probable to have increased levels of Bacillus and reduced Klebsiella oxytoca | Adams et al. (2011) |
| Autism | Human | Children with autism have higher concentrations of Suterella spp. in their feces, and Ruminococcus torques is also more prevalent and may be linked to GI issues in these kids. | Bacteria increase: Ruminococcus torques and Suterella | Wang et al. (2013) |
| Autism | Human | A less diversified microbiome was found in autistic children, and the intestinal microbiota was linked to GI problems. | Bacteria reduction: Veillonellaceae, Coprococcus, and Prevotella; main phyla in microbiota of patients with autism: Bacteroidetes and Firmicutes; most rich genera: Akkermansia, Bifidobacterium, Bacteroides, Faecalibacterium, and Subdoligranulum | Kang et al. (2013) |
| Autism | Human | Detected a connection between bacterial populations and genes expressed in the colon of autistic children; the source of these intestinal abnormalities is still under investigation. | Bacteria increase; Bacteroidetes to Firmicutes ratio Lachnospiraceae and Ruminococcaceae, Betaproteobacteria, Bacteria decrease: Bacteroidetes | Williams et al. (2011) |
| Depression | Human | Bifidobacterium and Lactobacillus are less prevalent in people with major depressive disorder; fecal samples were examined to visualize the relationship between the bacterial population and irritable bowel syndrome (IBS). | Bacteria decrease: Bifidobacterium and Lactobacillus | Aizawa et al. (2016) |
| Depression | Rat | Probiotics normalized the immunological response, improved behavioral issues, and balanced noradrenaline levels in addition to reducing depressive symptoms. | Probiotic supplement comprising Bifidobacterium infantis 35,624 | Desbonnet et al. (2010) |
| Depression | Mice | GF animals colonized with a “depression microbiota” had additional symptoms compared to control GF animals | Bacteria growth: Lactobacillaceae Coriobacterineae, Clostridiales, Streptococcaceae, Actinomycineae Lachnospiraceae Erysipelotrichaceae, Ruminococcaceae, and Eubacteriaceae: Bacteria reduction: Acidaminococcaceae, Rikenellaceae, Lachnospiraceae, Veillonellaceae, Bacteroidaceae, and Sutterellaceae | Zheng et al. (2016) |
| Depression | An improved understanding of the association between the microbiota and occurrence of particular bacteria with symptoms associated with depression resulted from examination of fecal samples from people with and without depression. | Bacteria increase: Rikenellaceae Enterobacteriaceae, Acidaminococcaceae, Porphyromonadaceae, and Fusobacteriaceae, Bacteria lessening: Prevotellaceae Erysipelotrichaceae, Lachnospiraceae., Veillonellaceae Bacteroidaceae, and Ruminococcaceae | Jiang et al. (2015) | |
| Anxiety | Humans | Probiotic administration has been linked to better mental health; nevertheless, this probiotic combination had no negative effects on the hypothalamic–pituitary–adrenal (HPA) axis. | Probiotic supplement Lactobacillus casei, Bifidobacterium logum, LA5 and Bifidobacterium lactisBB12, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus thermophilus, bulgaricus, Streptococcus | Mohammadi et al. (2016) |
| Anxiety | Rats and humans | In rats, the probiotic complex was linked to decreased anxiety, and healthy humans showed improved psychological effects. | Probiotic supplement comprising Lactobacillus helveticus R0052, and Bifidobacterium longumR0175 | Messaoudi et al. (2011) |
Relationship between neurological disorders and gut microbiota.
4.1. Alzheimer’s disease
Alzheimer’s disease (AD) affects approximately 50 million people globally and is the most frequent cause of progressive chronic and irreversible neurological disease and the most common type of dementia in elderly individuals. As the condition progresses, symptoms that impair thinking and memory can seriously compromise even the most basic daily activities (Scheltens et al., 2016; Rutsch et al., 2020). Loss of neurons and progressively worsening synaptic dysfunction are symptoms of AD (Tiraboschi et al., 2004; Alzheimer's, 2016). AD is caused by the formation of aggregates of polymerized forms of β-amyloid precursor protein (Aβ) in soluble multimeric and/or insoluble amyloid deposits in the brain that trigger a cascade of pathological events leading to neurofibrillary tangles, aggregates of hyperphosphorylated tau proteins, formation of neurofibrillary lesions, and ultimately dementia (Scheltens et al., 2016). The inflammasome and its products have been connected to the pathogenesis of AD since a greater expression of IL-1β and IL-18 has been observed in the microglia, astrocytes, and neurons that surround Ab plaques or in the plasma of AD patients (Malaguarnera et al., 2006; Ojala et al., 2009). Peripheral blood mononuclear cells (PBMCs) from AD patients also showed greater expression of NLRP3, ASC, caspase-1, caspase-5, IL-1β, and IL-18 (Saresella et al., 2016). Patients with tauopathies, which are neurodegenerative diseases characterized by the accumulation of aberrant tau protein in the brain, typically exhibit increased levels of cleaved caspase-1 and ASC as well as mature IL-1β in the cortex (Ising et al., 2019). Important evidence links neuroinflammation caused by the NLRP3 inflammasome to the development and progression of AD. AD pathogenesis has been associated with a number of microbiological causes (Atarashi et al., 2011; Geuking et al., 2011). Compared to controls, AD patients’ stool samples showed higher levels of Bacteroidetes and lower levels of Firmicutes and Actinobacteria. Ruminococcaceae, Turicibacteraceae, and Clostridiaceae were all Firmicutes families where AD patients had lower abundances (Vogt et al., 2017). According to several studies, there may be mechanistic links between the pathophysiology of AD and other microbes, such spirochaetes, fungi, and Chlamydia pneumoniae (Lim et al., 2014; Stojkovi et al., 2020). In recent studies, the gut microbiota has also been connected to the etiology of AD. A metabolite microbiota-derived protein found in the cerebral fluid of AD patients and connected to two disease-related biomarkers (phosphorylated tau and phosphorylated tau/A-42) raises the possibility that the gut microbiome plays a role in the etiology of AD (Vogt et al., 2018). When comparing fecal microbiomes and fecal SCFAs between AD-affected mice and wild-type mice at various ages, dramatic increases in Proteobacteria and Verrucomicrobia and marked decreases in Butyricicoccus and Ruminococcus were observed in AD mice, indicating altered microbiota composition and diversity. The decreased level of SCFAs further indicates alterations in many metabolic pathways (Zhang et al., 2017). It was demonstrated that, compared to non-transgenic wild-type mice, the gut microbiota diversity of the commonly utilized APP/PS1 double transgenic mice—which produce a chimeric mouse/human amyloid precursor protein (APP) and a mutant human presenilin 1 (PS1)—was markedly changed. Additionally, compared to healthy control mice with gut microbiota, germ-free APP/PS1 transgenic animals show a striking reduction in the degree of cerebral β-amyloid pathology (Harach et al., 2017). Bäuerl et al. (2018) reported similar findings about the shift in microbiota composition in the transgenic APP/PS1 mouse model, which shows increased numbers of the closely related inflammatory Erysipelotrichaceae family. Furthermore, germ-free APP/PS1 mice showed decreased amyloid pathology compared to conventional mice (Radde et al., 2006).
4.2. Parkinson’s disease
Parkinson’s disease (PD), which affects more than 1% of the elderly population and 0.3% of the general population worldwide, is the second most prevalent neurodegenerative condition after AD (Tysnes and Storstein, 2017). PD is a progressive neurodegenerative disorder characterized by the inability to control voluntary movements as a result of severe alterations in the function of the substantia nigra and striatum. The degradation of dopaminergic neurons, the accumulation of phosphorylated versions of the neuronal protein α-synuclein (αSyn), mitochondrial malfunction, an excess of reactive oxygen species, and a rise in microglia activation are some of these alterations (Blandini et al., 2000). Inflammation and α-synuclein misfolding are both key pathological mechanisms underlying α-synucleinopathies such as PD (Lema Tom et al., 2013). The pathogenesis of PD largely depends on the accumulation of α-synuclein. The gene for α-synuclein has five exons and is located on chromosome 4q21.3-q22. -synuclein is a protein with 140 amino acids (Mehra et al., 2019). PD symptoms include tremors, trouble walking, a hunched posture, and muscle rigidity. Gastrointestinal issues, most frequently constipation, may affect up to 80% of patients with Parkinson’s disease (Chen et al., 2015) and can precede PD diagnoses by many years (Cersosimo et al., 2013). Growing evidence suggests that gut dysbiosis contributes to the onset, development, and progression of PD (Zhu et al., 2022). Comparing patients with prodromal and/or clinically diagnosed PD to subjects under control, we found that these patients had dysbiosis of the gut microbiota. The general organization and composition of the gut microbiota associated with PD have been examined using culture-independent high-throughput sequencing techniques, and features of the altered microbiota profiles in PD patients have been found (Zhu et al., 2022). Numerous earlier studies found that PD patients had higher α-diversity but lower bacterial diversity than healthy people (Qian et al., 2018; Barichella et al., 2019). Additionally, one study revealed that there were differences in β-diversity (between samples) between PD patients and controls (Boertien et al., 2019). There has been a connection between the clinical characteristics of PD and the decline in bacterial diversity, which is primarily assessed using α-diversity indexes such as Shannon and Simpson. According to a recent study by Heinzel et al. (2021), certain symptoms of PD may be particularly related to the prodromal microbiome, including constipation, possible rapid eye movement sleep behavior disorder (RBD), physical inactivity, smoking, urate levels, and subthreshold parkinsonism. Contrary to sex, inactivity, suspected RBD, constipation, and smoking, which were all connected to β-diversity, were constipation, occupational solvent exposure, and the three previously mentioned variables. Age and medications that reduce urate were linked to both α and β-diversity (Heinzel et al., 2021). However, research by Plassais et al. (2021) revealed that the gut microbiome’s α-diversity is not a biomarker of PD. The intestinal permeability and inflammation caused by the gut dysbiosis associated with PD, such as increased Akkermansia and decreased SCFA-producing bacteria, can facilitate the exposure of the intestinal neural plexus to toxins such as lipopolysaccharide (LPS) and pesticides, which can cause abnormal α-synuclein fibril aggregation and the development of Lewy bodies (Hirayama and Ohno, 2021). Despite people with other diseases, people with PD have a different microbiome composition from people who are healthy or have other neurological disorders (Hasegawa et al., 2015; Keshavarzian et al., 2015; Scheperjans et al., 2015). The intestinal flora in PD patients is lacking in bacteria that produce SCFAs (mostly butyrate), such as taxa from the Lachnospiraceae family (Hill-Burns et al., 2017; Petrov et al., 2017; Barichella et al., 2019) and Faecalibacterium prausnitzii (Keshavarzian et al., 2015; Unger et al., 2016), which have known anti-inflammatory properties. Additionally, certain bacterial species, such as Proteus mirabilis, which causes mice to develop motor impairments, may be the cause of PD-like disease (Choi et al., 2018). Prospective long-term longitudinal microbiome investigations are required to track the development of the disease and characterize changes in the microbiome’s taxonomic composition that contributed to or may potentially have defined the disease state. Uncertainty persists regarding the precise way in which the gut microbiome may affect PD-related symptoms.
4.3. Multiple sclerosis
Multiple sclerosis (MS) is a neurological and inflammatory condition that affects over two million individuals worldwide. The main symptoms of this condition include demyelination, axonal loss, lymphocyte infiltration into the CNS, and neuroinflammation. Some of the clinical signs of MS include ataxia, poor coordination, hyperreflexia, stiffness, visual and sensory impairment, fatigue, and cognitive deficits. The majority of patients suffer a kind of disease known as relapsing–remitting, which is characterized by a gradual but significant deterioration in neurological function and a progressive reappearance of symptoms (McFarland and Martin, 2007). Most patients have brain lesions or lesions in the brain and spinal cord; however, some people only have lesions in the spinal cord (McFarland and Martin, 2007; Rutsch et al., 2020). Microbes (and the substances they secrete or toxins they produce) are a significant contributor to the pathophysiology of MS among environmental variables (Ronchi et al., 2016; Rutsch et al., 2020). MS patients have a different microbiome composition than healthy individuals (Oleskin and Shenderov, 2016). It is interesting to note that even MS patients with active disease have a different microbiome from those who are in remission, whose microbiota is more comparable to that of healthy controls (Bhargava and Mowry, 2014; Chen et al., 2016; Jangi et al., 2016; Pröbstel and Baranzini, 2018). Greater Firmicutes abundance and the absence of Fusobacteria in pediatric MS patients were associated with a shorter time to relapse (Tremlett et al., 2017). Compared to healthy people, fecal samples from people with MS show alterations in the richness of Mycoplana, Dorea, Pseudomonas, Blautia, and Akkermansia species (Mangalam et al., 2017). Attenuated multiple sclerosis-like disease appears in preclinical models in GF mice (Lee et al., 2011), and mice receiving the intestinal microbiota of MS patients experienced more severe experimental autoimmune encephalomyelitis and had lower proportions of anti-inflammatory regulatory T cells than mice receiving the microbiome of healthy individuals (Berer et al., 2017; Cekanaviciute et al., 2017). The remarkable finding was that transplanting the intestinal microbes of the MS twins into GF animals, which are genetically predisposed to developing experimental autoimmune encephalomyelitis (EAE), was sufficient to promote the illness in vivo with a significantly higher incidence than transplanting the microbes of the healthy twins (Berer et al., 2017). Interestingly, immune cells from mice that received MS-derived samples produced less IL-10 than cells from animals that had their microbiota from healthy twins colonize (Berer et al., 2017). In mice inoculated with healthy fecal samples, the neutralization of IL-10, one of the main regulatory cytokines, increased the incidence of disease (Berer et al., 2017). This important finding demonstrated how the human microbiome may produce particular immune system changes that may be the cause or consequence of the onset of MS. Uncertainty persists regarding whether this role plays a crucial part in the beginning and development of the disease. In light of this, there is considerable interest in the variations in the microbiota of MS patients compared to healthy controls.
4.4. Autism spectrum disorder
Autism spectrum disorder (ASDs) are a set of neurological development changes marked by difficulties with social interaction and communication as well as stereotyped and repetitive conduct (Maiuolo et al., 2021). Constipation, diarrhea, abdominal pain, flatulence, and intestinal gas are common among people with ASD problems (23–70%) and are frequently comorbid with gastrointestinal diseases (Mulle et al., 2013). The gut microbiota mediates the levels of chemical transmitters such as GABA, glutamate, oxytocin and serotonin 5-HT complex in ASD. Due to the low-grade inflammation that ASD patients experience, microbial influences on the immune system may also be very important in determining neuroimmune responses in ASD. New technologies are being applied in this rapidly expanding field of research as it becomes obvious how much microbial metabolites, including taurine, bile acid metabolites, SCFAs, and 5-aminovaleric acid, affect ASD symptoms (Morais et al., 2021). There are few and generally inconsistent ASD studies that highlight the role of the microbiome in pathogenesis. However, there are a few that highlight the differences in bacteria such as Firmicutes, Clostridiales, Prevotella, Bifidobacterium, and Clostridium perfringens, species that are seen between ASD patients and controls (Ho et al., 2020). This results in a change in the composition of the gut microbiota, a reduction in dietary quality, and a deficiency in nutrients (Berding and Donovan, 2016). The scientific literature data generally show a reduction of Bacteroides with a ratio (% ASD child/% control children) equal to 0.71; a reduction of Bifidobacterium with a ratio (% ASD child/% control children) equal to 0.52; a reduction of Escherichia coli with a ratio (%) equal to 0.3; an increase in Faecalibacterium with a ratio (%) equal to 1.32; and an increase in Lactobacillus Clostridium is still present in a largely unchanged amount (Tomova et al., 2015). It is evident that these neurological disorders are accompanied by reduced amounts of beneficial bacteria and larger levels of deadly bacteria, even though it cannot be said that certain bacteria are compatible and connected with the start of ASD (Iglesias-Vázquez et al., 2020). The gut microbiota and its metabolites may be crucially significant in the pathophysiology of ASD (Xu et al., 2019).
4.5. Anxiety and depression
Anxiety and depression are mental and neurological disorders that affect 25% of the global population. These two pathological conditions appear to be intimately related: in fact, 85% of people with depression and 90% of people with anxiety disorders both experience considerable anxiety (Bui and Fava, 2017; Maiuolo et al., 2021). Early and late stages of these pathologies have significantly different clinical signs (Groeneweg-Koolhoven et al., 2017). Teenage suicide deaths have increased in recent decades as a result of the rise in depressed symptoms (Jorm et al., 2017; Matsumoto et al., 2017; Weinberger et al., 2018; Twenge et al., 2019). The relationship between anxiety and depression and changes in the stability and composition of the gut microbiota has been thoroughly investigated (Tognini, 2016; Zhao et al., 2018; Bastiaanssen et al., 2019). Numerous studies have recently focused on the relationship between the intestinal microbiota and people who suffer from anxiety and mood disorders. In particular, evidence from human research has demonstrated that when taking into account microbial diversity and taxonomic compositions, there is frequently some variation in the fecal microbiota between patients and healthy controls. Additionally, it was revealed that certain bacteria were linked to clinical traits and metabolic or inflammatory profiles (Huang et al., 2019). There have been some studies on human microbial diversity, but the majority of them have been unable to show a connection between low microbial diversity and depressive disorders (Chen et al., 2014; Naseribafrouei et al., 2014; Zheng et al., 2016). Despite the fact that only one study found that individuals with major depressive disorder (MDD) had a higher alpha diversity of the gut microbiota than healthy subjects, alpha diversity is the number of species that can be detected in a microbial ecosystem (Jiang et al., 2015). Comparing patients with MDD to drug-responders with healthy controls, patients with MDD showed higher fecal a-diversity (higher levels of Enterobacteriaceae and Alistipes but lower levels of Faecalibacterium). Because of this, the authors reported a link between Faecalibacterium and the intensity of depression symptoms that was negative (Jiang et al., 2015). Interesting changes in the fecal microbiota have also been found in patients with anxiety disorders. They observed that patients with generalized anxiety disorder (GAD) had lower levels of microbial diversity and richness, which was correlated with lower levels of short-chain fatty acid producers such as Eubacterium rectale and Fecalibacterium and higher levels of Ruminococcus, Escherichia, Shigella, and Fusobacterium (Jiang et al., 2018). According to another study, probiotics (Bifidobacterium bifidum, Lactobacillus acidophilus, and Lactobacillus casei) administered to MDD patients dramatically reduced depression symptoms when compared to a placebo (Akkasheh et al., 2016). The potential of bacteria to produce 3,4-dihydroxyphenylacetic acid, a metabolite of dopamine, correlates favorably with mental health according to fecal metagenomic data, which raises the possibility that microbes play a role in the production of different neuroactive molecules during depression than under normal conditions (Valles-Colomer et al., 2019). Lactobacillus rhamnosus releases GABA and activates GABA receptors in the brain (that is, GABA Aα2 and GABA B1b receptors) and has been revealed to attenuate depression and anxiety-like behaviors in mice (Bravo et al., 2011).
4.6. Stroke
Stroke is the second leading cause of death worldwide. The morbidity and mortality of stroke grow in many countries, contributing to financial burden and loss of life quality and thus diminishing the national happiness index. Approximately 15 million people around the world are victims of a stroke every year (Feigin et al., 2017). They may occur due to modifications in various diseases, such as cerebrovascular disease, atherosclerosis, dyslipidemia, diabetes, and arterial hypertension (Goldman et al., 2022). However, to date, few studies have focused on exploring the correlation between hemorrhagic stroke and the gut microbiota. GM microflora may be involved in the development of stroke and/or brain injuries (Singh et al., 2016). Studies have reported that ischemic stroke accounts for ~80% of all strokes (Sadler et al., 2020), and the gut microbiota plays an essential role in the pathogenesis and prognosis of ischemic stroke. Multiple studies have shown that ischemic stroke significantly changes the gut microbiota composition (Ling et al., 2020; Xiang et al., 2020; Xu et al., 2021). Patients suffering from transient ischemic attack or stroke have been found to have opportunistic pathogens such as Desulfovibrio, Enterobacter, Megasphaera, and Osicillibacter, as well as fewer beneficial or commensal pathogens such as Bacteroides, Fecalibacterium, and Prevotella (Yin et al., 2015). The abundance of Peptococcaceae and Prevotellaceae is linked to stroke severity (Tiwari et al., 2023). Recently, a preclinical study also suggested that alterations in the gut microbiota were associated with hemorrhagic transformation (HT). The relative abundance of Proteobacteria and Actinobacteria was significantly increased in HT rats after experimental stroke, indicating that the gut microbiota is involved in the progression of ischemic stroke (Huang et al., 2022). The precise role and mechanism of GM in the onset and progression of stroke and brain injury remain unknown. Although animal models have yielded fascinating results, more clinical research is needed to fully elucidate the potential of such microbial therapeutic modalities.
5. Conclusion and future directions
The gut microbiome is important for the host’s health and disease states, and most of the research on this subject to date has only revealed associations between certain clinical disorders and bacterial profiles. The gut microbiota has a substantial impact on both the physiology and pathophysiology of the brain due to the interaction between the intestine and neurological system in both directions. This communication takes place via a variety of pathways and involves the vagal nerve, neuroendocrine systems, neurotransmitters of the CNS, and inflammatory substances. The discussed evidence is accumulated from preclinical and clinical studies on gut microbiota, its dysbiosis and association with the development and progression of neurological disorder neurodevelopmental abnormalities to depression and Parkinson’s diseases, even if determining their exact mode of action requires more research, and probiotic supplement therapies are useful with promising therapeutic possibilities for neurological diseases. Probiotic supplement therapies are effective tools with considerable therapeutic potential for neurological disorders, even though determining their precise mode of action requires more research. Future studies in this field may provide insight into the connection between the microbiota and the CNS and developments in the treatment of neurological disorders. The fields of microbiology and neuroscience, as well as other disciplines, must proceed to work together to develop thorough and pertinent methods to ascertain mechanisms of action for outcomes that are currently observational, along with responsible efforts in translating these discoveries to improve human health. World’s major populations are suffering from neurological disorders, which are expected to rise by 13% by 2030. Hence, there is an urgency to develop more reliable biomarkers and feasible therapeutic options in view of the diseases’ pathogenicity. Multiple studies have shown that the GM is critical for brain development and function. In a number of preclinical and clinical research studies, the GIT microbiome in the GBA has been reviewed for its association with multiple neurological disorders, such as AD, MS, PD, ASD, epilepsy, stroke, and brain injury. However, deeper research is needed to understand the mechanism of action and function of GM in disease pathogenesis and its further applicability for therapeutic or prognostic purposes. However, the impact on the GM and the composition of their beneficial species in the GBA still need to be elucidated in future studies. Because many patients are given multiple medications, more research is needed to clarify any potential GM–drug interactions. The GM is a new line that separates human health from a variety of disorders, and future neurotherapeutic research will provide critical information on this topic. Despite recent developments in our understanding of the GBA, further research is required to determine whether this knowledge can be helpful in a clinical environment. Future studies must clarify the underlying links between the GM and various neurological diseases and determine whether treating the microbiota is a safe and effective course of treatment. It may be possible to develop techniques that target the gut microbiota to offer innovative, safe, and efficient therapy options for neurodegenerative disorders if traditional brain disorders are viewed comprehensively and now as entire conditions with a significant role for the gastrointestinal tract.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
HU and SA: original draft preparation and conceptualization. YT, C-qL, YC, LQ, MK, and IH: methodology. HU: review and editing of the manuscript. KL: supervision. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We would like to thank American Journal Experts (AJEs) for proofreading the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- GM
gut microbiota
- GBA
gut-brain axis
- CNS
central nervous system
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- GI
digestive tract
- MS
multiple sclerosis
- ASD
autism spectrum disorder
- ENS
enteric nervous system
- GBA
gut-brain axis
- HPA
hypothalamic–pituitary–adrenal axis
- NMDA
N-methyl-d-aspartate
- GABA
gamma-aminobutyric acid
- EECs
enteroendocrine cells
- GLP1
glucagon-like peptide 1
- 5-HT
5-hydroxytryptamine
- LPS
T lipopolysaccharide
- LCFAs
long-chain fatty acids
- TAMO
trimethylamine-N-oxide
- PSA
polysaccharide A
- MAMPs
microbial-associated molecular patterns
- GPCRs
G protein-coupled receptors
- PYY
peptide YY
- GLP1
glucagon-like peptide 1
- FFARs
free fatty acid receptors
- BBB
blood–brain barrier
- TNF
tumor necrosis factor
- DCs
dendritic cells
- HDAC
histone deacetylase
- CXCL1
CXC motif chemokine ligand 1
- CXCL8
CXC motif ligand 8
- FFAR2
free fatty acid receptor 2
- GPR41
G protein-coupled receptor 41
- FFAR3
free fatty acid receptor 3
- PBMCs
peripheral blood mononuclear cells
- APP
amyloid precursor protein
- PS1
presenilin 1
- αSyn
α-synuclein
- GAD
generalized anxiety disorder
- EAE
experimental autoimmune encephalomyelitis
Abbreviations
References
1
Abdel-Haq R. Schlachetzki J. C. M. Glass C. K. Mazmanian S. K. (2019). Microbiome microglia connections via the gutâ brain axis. J. Exp. Med.216, 41–59. doi: 10.1084/jem.20180794
2
Adams J. B. Johansen L. J. Powell L. D. Quig D. Rubin R. A. (2011). Gastrointestinal flora and gastrointestinal status in children with autism comparisons to typical children and correlation with autism severity. BMC Gastroenterol.11, 1–13. doi: 10.1186/1471-230X-11-22
3
Aizawa E. Tsuji H. Asahara T. Takahashi T. Teraishi T. Yoshida S. et al . (2016). Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord.202, 254–257. doi: 10.1016/j.jad.2016.05.038
4
Akbari E. Asemi Z. Daneshvar Kakhaki R. Bahmani F. Kouchaki E. Tamtaji O. R. et al . (2016). Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front. Aging Neurosci.8:256. doi: 10.3389/fnagi.2016.00256
5
Akira S. Hemmi H. (2003). Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett.85, 85–95. doi: 10.1016/S0165-2478(02)00228-6
6
Akkasheh G. Kashani-Poor Z. Tajabadi-Ebrahimi M. Jafari P. Akbari H. Taghizadeh M. et al . (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition32, 315–320. doi: 10.1016/j.nut.2015.09.003
7
Aktar R. Parkar N. Stentz R. Baumard L. Parker A. Goldson A. et al . (2020). Human resident gut microbe Bacteroides thetaiotaomicron regulates colonic neuronal innervation and neurogenic function. Gut Microbes11, 1745–1757. doi: 10.1080/19490976.2020.1766936
8
Al Omran Y. Aziz Q. (2014). The brain-gut axis in health and disease. Microbial Endocrinol, 817, 135–153. doi: 10.1007/978-1-4939-0897-4_6
9
Alvarez E. Martinez M. D. Roncero I. Chowen J. A. Garcia-Cuartero B. Gispert J. D. et al . (2005). The expression of GLP1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem.92, 798–806. doi: 10.1111/j.1471-4159.2004.02914.x
10
Alzheimer's A. (2016). Alzheimer's disease facts and figures. Alzheimers Dement.12, 459–509. doi: 10.1016/j.jalz.2016.03.001
11
Aresti Sanz J. El Aidy S. (2019). Microbiota and gut neuropeptides: a dual action of antimicrobial activity and neuroimmune response. Psychopharmacology236, 1597–1609. doi: 10.1007/s00213-019-05224-0
12
Askarova S. Umbayev B. Masoud A. R. Kaiyrlykyzy A. Safarova Y. Tsoy A. et al . (2020). The links between the gut microbiome, aging, modern lifestyle and Alzheimer's disease. Front. Cell. Infect. Microbiol.10:104. doi: 10.3389/fcimb.2020.00104
13
Atarashi K. Tanoue T. Shima T. Imaoka A. Kuwahara T. Momose Y. et al . (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science331, 337–341. doi: 10.1126/science.1198469
14
Baj A. Moro E. Bistoletti M. Orlandi V. Crema F. Giaroni C. (2019). Glutamatergic signaling along the microbiota-gut-brain axis. Int. J. Mol. Sci.20:1482. doi: 10.3390/ijms20061482
15
Barichella M. Severgnini M. Cilia R. Cassani E. Bolliri C. Caronni S. et al . (2019). Unraveling gut microbiota in Parkinson's disease and atypical parkinsonism. Mov. Disord.34, 396–405. doi: 10.1002/mds.27581
16
Bastiaanssen T. F. S. Cowan C. S. M. Claesson M. J. Dinan T. G. Cryan J. F. (2019). Making sense of the microbiome in psychiatry. Int. J. Neuropsychopharmacol.22, 37–52. doi: 10.1093/ijnp/pyy067
17
Bäuerl C. Collado M. C. Diaz Cuevas A. Viña J. Martínez G. P. (2018). Shifts in gut microbiota composition in an APP/PSS 1 transgenic mouse model of Alzheimer's disease during lifespan. Lett Appl Microbiol.66, 464–471. doi: 10.1111/lam.12882
18
Bercik P. Denou E. Collins J. Jackson W. Lu J. Jury J. et al . (2011a). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology141:e593, 599–609.e3. doi: 10.1053/j.gastro.2011.04.052
19
Bercik P. Park A. J. Sinclair D. Khoshdel A. Lu J. Huang X. et al . (2011b). The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. J Gastrointestinal Motility23, 1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x
20
Bercik P. Verdu E. F. Foster J. A. Macri J. Potter M. Huang X. et al . (2010). Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology139:e2101. doi: 10.1053/j.gastro.2010.06.063
21
Berding K. Donovan S. M. (2016). Microbiome and nutrition in autism spectrum disorder: current knowledge and research needs. Nutr. Rev.74, 723–736. doi: 10.1093/nutrit/nuw048
22
Berer K. Gerdes L. A. Cekanaviciute E. Jia X. Xiao L. Xia Z. et al . (2017). Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. PNAS114, 10719–10724. doi: 10.1073/pnas.1711233114
23
Bhargava P. Mowry E. M. (2014). Gut microbiome and multiple sclerosis. Curr. Neurol. Neurosci. Rep.14, 1–8. doi: 10.1007/s11910-014-0492-2
24
Bhattarai Y. Si J. Pu M. Ross O. A. McLean P. J. Till L. et al . (2021). Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson’s disease. Gut Microbes13:1866974. doi: 10.1080/19490976.2020.1866974
25
Bibbo S. Dore M. P. Pes G. M. Delitala G. Delitala A. P. (2017). Is there a role for gut microbiota in type 1 diabetes pathogenesis?Ann. Med.49, 11–22. doi: 10.1080/07853890.2016.1222449
26
Blacher E. Bashiardes S. Shapiro H. Rothschild D. Mor U. Dori-Bachash M. et al . (2019). Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature572, 474–480. doi: 10.1038/s41586-019-1443-5
27
Blandini F. Nappi G. Tassorelli C. Martignoni E. (2000). Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog. Neurobiol.62, 63–88. doi: 10.1016/S0301-0082(99)00067-2
28
Blaser M. J. (2017). The theory of disappearing microbiota and the epidemics of chronic diseases. Nat. Rev. Immunol.17, 461–463. doi: 10.1038/nri.2017.77
29
Boertien J. M. Pereira P. A. B. Aho V. T. E. Scheperjans F. (2019). Increasing comparability and utility of gut microbiome studies in Parkinson 's disease: a systematic review. J. Parkinsons Dis.9, S297–S312. doi: 10.3233/JPD-191711
30
Braniste V. al-Asmakh M. Kowal C. Anuar F. Abbaspour A. Tóth M. et al . (2014). The gut microbiota influences blood–brain barrier permeability in mice. Sci. Transl. Med.6:263ra158. doi: 10.1126/scitranslmed.3009759
31
Bravo J. A. Forsythe P. Chew M. V. Escaravage E. Savignac H. M. Dinan T. G. et al . (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. PNAS108, 16050–16055. doi: 10.1073/pnas.1102999108
32
Brun P. Giron M. C. Qesari M. Porzionato A. Caputi V. Zoppellaro C. et al . (2013). Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology145, 1323–1333. doi: 10.1053/j.gastro.2013.08.047
33
Bu X. L. Yao X. Q. Jiao S. S. Zeng F. Liu Y. H. Xiang Y. et al . (2015). A study on the association between infectious burden and Alzheimer's disease. Eur. Neurol.22, 1519–1525. doi: 10.1111/ene.12477
34
Bui E. Fava M. (2017). From depression to anxiety, and back. Acta Psychiatr. Neurol. Scand.136, 341–342. doi: 10.1111/acps.12801
35
Burberry A. Wells M. F. Limone F. Couto A. Smith K. S. Keaney J. et al . (2020). C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature582, 89–94. doi: 10.1038/s41586-020-2288-7
36
Capuron L. Miller A. H. (2011). Immune system to brain signaling: neuropsychopharmacological implications. Pharm. Therap.130, 226–238. doi: 10.1016/j.pharmthera.2011.01.014
37
Carabotti M. Scirocco A. Maselli M. A. Severi C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals Gastroenterol28:203.
38
Cekanaviciute E. Yoo B. B. Runia T. F. Debelius J. W. Singh S. Nelson C. A. et al . (2017). Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. PNAS114, 10713–10718. doi: 10.1073/pnas.1711235114
39
Cersosimo M. G. Raina G. B. Pecci C. Pellene A. Calandra C. R. Gutiérrez C. et al . (2013). Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J. Neurol260, 1332–1338. doi: 10.1007/s00415-012-6801-2
40
Chen J. Chia N. Kalari K. R. Yao J. Z. Novotna M. Paz Soldan M. M. et al . (2016). Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep.6, 1–10. doi: 10.1038/srep28484
41
Chen Y. Xu J. Chen Y. (2021). Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients13:2099. doi: 10.3390/nu13062099
42
Chen H. Zhao E. J. Zhang W. Lu Y. Liu R. Huang X. et al . (2015). Meta-analyses on prevalence of selected Parkinson's nonmotor symptoms before and after diagnosis. Transl. Neurodegen.4, 1–8. doi: 10.1186/2047-9158-4-1
43
Chen J. Zheng P. Liu Y. Zhong X. Wang H. Guo Y. et al . (2014). Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr. Dis. Treat.14:647.
44
Choi J. G. Kim N. Ju I. G. Eo H. Lim S. M. Jang S. E. et al . (2018). Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci. Rep.8, 1–13. doi: 10.1038/s41598-018-19646-x
45
Claesson M. J. Jeffery I. B. Conde S. Power S. E. O’Connor E. M. Cusack S. et al . (2012). Gut microbiota composition correlates with diet and health in the elderly. Nature488, 178–184. doi: 10.1038/nature11319
46
Clarke G. Grenham S. Scully P. Fitzgerald P. Moloney R. D. Shanahan F. et al . (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molec Psychiat18, 666–673. doi: 10.1038/mp.2012.77
47
Collins S. M. Surette M. Bercik P. (2012). The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol.10, 735–742. doi: 10.1038/nrmicro2876
48
Corrêa-Oliveira R. Fachi J. L. Vieira A. Sato F. T. Vinolo M. A. (2016). Regulation of immune cell function by short-chain fatty acids. CTI5:e73. doi: 10.1038/cti.2016.17
49
Cryan J. F. Dinan T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behavior. Nat. Rev. Neurosci.13, 701–712. doi: 10.1038/nrn3346
50
Cryan J. F. O'Riordan K. J. Cowan C. S. M. Sandhu K. V. Bastiaanssen T. F. S. Boehme M. et al . (2019). The microbiota-gut-brain axis. Physiol. Rev.99, 1877–2013. doi: 10.1152/physrev.00018.2018
51
Cryan J. F. O'Riordan K. J. Sandhu K. Peterson V. Dinan T. G. (2020). The gut microbiome in neurological disorders. Lancet Neurol.19, 179–194. doi: 10.1016/S1474-4422(19)30356-4
52
Dalile B. Van Oudenhove L. Vervliet B. Verbeke K. (2019). The role of short-chain fatty acids in microbiota gut brain communication. Nat. Rev. Gastroenterol. Hepatol.16, 461–478. doi: 10.1038/s41575-019-0157-3
53
Daneman R. Prat A. (2015). The blood–brain barrier. Cold Spring Harb Perspect Biol7:a020412. doi: 10.1101/cshperspect.a020412
54
De la Fuente-Nunez C. Meneguetti B. T. Franco O. L. Lu T. K. (2018). Neuromicrobiology: how microbes influence the brain. ACS Chem. Neurosci.9, 141–150. doi: 10.1021/acschemneuro.7b00373
55
De Lartigue G. de La Serre C. B. Raybould H. E. (2011). Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav.105, 100–105. doi: 10.1016/j.physbeh.2011.02.040
56
De Palma G. Collins S. M. Bercik P. Verdu E. F. (2014). The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both?J Physiol (London)592, 2989–2997. doi: 10.1113/jphysiol.2014.273995
57
de Vadder F. Grasset E. Mannerås Holm L. Karsenty G. Macpherson A. J. Olofsson L. E. et al . (2018). Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. PNAS115, 6458–6463. doi: 10.1073/pnas.1720017115
58
Deidda G. Biazzo M. (2021). Gut and Brain: Investigating physiological and pathological interactions between microbiota and brain to gain new therapeutic avenues for brain diseases. Front. Neurosci15:753915. doi: 10.3389/fnins.2021.753915
59
Desbonnet L. Garrett L. Clarke G. Kiely B. Cryan J. F. Dinan T. G. (2010). Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neurosci. J.170, 1179–1188. doi: 10.1016/j.neuroscience.2010.08.005
60
Dinan T. G. Stilling R. M. Stanton C. Cryan J. F. (2015). Collective unconscious: how gut microbes shape human behavior. J. Psychiatr. Res.63, 1–9. doi: 10.1016/j.jpsychires.2015.02.021
61
Duan Y. Prasad R. Feng D. Beli E. Li Calzi S. Longhini A. L. F. et al . (2019). Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ. Res.125, 969–988. doi: 10.1161/CIRCRESAHA.119.315743
62
Dugger B. N. Dickson D. W. (2017). Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol.9:a028035. doi: 10.1101/cshperspect.a028035
63
Duvallet C. Gibbons S. M. Gurry T. Irizarry R. A. Alm E. J. (2017). Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun.8, 1–10. doi: 10.1038/s41467-017-01973-8
64
El Aidy S. Dinan T. G. Cryan J. F. (2014). Immune modulation of the brain-gut-microbe axis. Front Media:146.
65
Elias C. F. Aschkenasi C. Lee C. Kelly J. Ahima R. S. Bjorbæk C. et al . (1999). Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron23, 775–786. doi: 10.1016/S0896-6273(01)80035-0
66
Ellwardt E. Walsh J. T. Kipnis J. Zipp F. (2016). Understanding the role of T cells in CNS homeostasis. Trends Immunol.37, 154–165. doi: 10.1016/j.it.2015.12.008
67
Engelhardt B. Carare R. O. Bechmann I. Laman J. D. Weller R. O. (2016). Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol.132, 317–338. doi: 10.1007/s00401-016-1606-5
68
Engelhardt B. Liebner S. (2014). Novel insights into the development and maintenance of the blood- brain barrier. Cell Tissue Res.355, 687–699. doi: 10.1007/s00441-014-1811-2
69
Erny D. Hrabě de Angelis A. L. Jaitin D. Wieghofer P. Staszewski O. David E. et al . (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci.18, 965–977. doi: 10.1038/nn.4030
70
Feigin V. L. Norrving B. Mensah G. A. (2017). Global burden of stroke. Circ. Res.120, 439–448. doi: 10.1161/CIRCRESAHA.116.308413
71
Felice V. D. O'Mahony S. M. (2017). The microbiome and disorders of the central nervous system. Pharmacol. Biochem. Behav.160, 1–13. doi: 10.1016/j.pbb.2017.06.016
72
Feng Y. Zhou Z. Zheng C. Feng F. Xie F. Wu Z. (2020). Interleukin 17-producing´ T-cell induced demyelination of the brain in angiostrongylus cantonensis infection.
73
Flowers S. A. Ellingrod V. L. (2015). The microbiome in mental health: potential contribution of gut microbiota in disease and pharmacotherapy management. Pharmacotherapy35, 910–916.
74
Fond G. Boukouaci W. Chevalier G. Regnault A. Eberl G. Hamdani N. et al . (2015). The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: a systematic review. Pathol. Biol.63, 35–42. doi: 10.1016/j.patbio.2014.10.003
75
Forsythe P. Bienenstock J. Kunze W. A. (2014). Vagal pathways for microbiome-brain-gut axis communication. Microbial Endocrinol.817, 115–133. doi: 10.1007/978-1-4939-0897-4_5
76
Frank D. N. St. Amand A. L. Feldman R. A. Boedeker E. C. Harpaz N. Pace N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. PNAS104, 13780–13785. doi: 10.1073/pnas.0706625104
77
Frick L. R. Williams K. Pittenger C. (2013). Microglial dysregulation in psychiatric disease. Clin. Develop. Immunol.2013:608654. doi: 10.1155/2013/608654
78
Fülling C. Dinan T. G. Cryan J. F. (2019). Gut microbe to brain signaling: what happens in vagus¦. Neuron101, 998–1002. doi: 10.1016/j.neuron.2019.02.008
79
Gavin P. G. Mullaney J. A. Loo D. Cao K. L. Gottlieb P. A. Hill M. M. et al . (2018). Intestinal metaproteomics reveals host-microbiota interactions in subjects at risk for type 1 diabetes. Diabetes Care41, 2178–2186. doi: 10.2337/dc18-0777
80
Geuking M. B. Cahenzli J. Lawson M. A. E. Ng D. C. K. Slack E. Hapfelmeier S. M. et al . (2011). Intestinal bacterial colonization induces mutualistic regulatory T-cell responses. Immunity34, 794–806. doi: 10.1016/j.immuni.2011.03.021
81
Goldman L. Siddiqui E. M. Khan A. Jahan S. Rehman M. U. Mehan S. et al . (2022). Understanding acquired brain injury: a review. Biomedicine10:2167. doi: 10.3390/biomedicines10092167
82
Grochowska M. Laskus T. Radkowski M. (2019). Gut microbiota in neurological disorders. Arch. Immunol. Ther.67, 375–383. doi: 10.1007/s00005-019-00561-6
83
Groeneweg-Koolhoven I. Ploeg M. Comijs H. C. WJH Penninx B. van der Mast R. C. Schoevers R. A. et al . (2017). Apathy in early and late-life depression. J. Affect. Disord.223, 76–81. doi: 10.1016/j.jad.2017.07.022
84
Gubert C. Kong G. Renoir T. Hannan A. J. (2020). Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis.134:104621. doi: 10.1016/j.nbd.2019.104621
85
Harach T. Marungruang N. Duthilleul N. Cheatham V. Mc Coy K. D. Frisoni G. et al . (2017). Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep.7, 1–15. doi: 10.1038/srep41802
86
Harms A. S. Thome A. D. Yan Z. Schonhoff A. M. Williams G. P. Li X. et al . (2018). Peripheral monocyte entry is required for alpha-synuclein induced inflammation and neurodegeneration in a model of Parkinson disease. Exp. Neurol.300, 179–187. doi: 10.1016/j.expneurol.2017.11.010
87
Hasegawa S. Goto S. Tsuji H. Okuno T. Asahara T. Nomoto K. et al . (2015). Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One10:e0142164. doi: 10.1371/journal.pone.0142164
88
Heijtz R. D. Wang S. Anuar F. Qian Y. Björkholm B. Samuelsson A. et al . (2011). Normal gut microbiota modulates brain development and behavior. PNAS108, 3047–3052. doi: 10.1073/pnas.1010529108
89
Heinzel S. Aho V. T. E. Suenkel U. von Thaler A. K. Schulte C. Deuschle C. (2021). Gut microbiome signatures of risk and prodromal markers of Parkinson disease. Ann. Neurol.90, E1–E12. doi: 10.1002/ana.26128
90
Hill-Burns E. M. Debelius J. W. Morton J. T. Wissemann W. T. Lewis M. R. Wallen Z. D. et al . (2017). Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov. Disord.32, 739–749. doi: 10.1002/mds.26942
91
Hirayama M. Ohno K. (2021). Parkinson 's disease and gut microbiota. Ann. Nutr. Metab.77, 28–35. doi: 10.1159/000518147
92
Ho L. K. H. Tong V. J. W. Syn N. Nagarajan N. Tham E. H. Tay S. K. et al . (2020). Gut microbiota changes in children with autism spectrum disorder: a systematic review. Gut Pathogens12, 1–18. doi: 10.1186/s13099-020-0346-1
93
Hsiao E. Y. McBride S. W. Hsien S. Sharon G. Hyde E. R. McCue T. et al . (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cells155, 1451–1463. doi: 10.1016/j.cell.2013.11.024
94
Huang Q. Di L. Yu F. Feng X. Liu Z. Wei M. et al . (2022). Alterations in the gut microbiome with hemorrhagic transformation in experimental stroke. CNS Neurosci. Ther.28, 77–91. doi: 10.1111/cns.13736
95
Huang T. T. Lai J. B. Du Y. L. Xu Y. Ruan L. M. Hu S. H. (2019). Current understanding of gut microbiota in mood disorders: an update of human studies. Front. Genet.10:98. doi: 10.3389/fgene.2019.00098
96
Huang C. Wang J. Hu W. Wang C. Lu X. Tong L. et al . (2016). Identification of functional farnesoid X receptors in brain neurons. FEBS Lett.590, 3233–3242. doi: 10.1002/1873-3468.12373
97
Hube F. Lietz U. Igel M. Jensen P. B. Tornqvist H. Joost H. G. et al . (1996). Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm. Metab. Res.28, 690–693. doi: 10.1055/s-2007-979879
98
Iannone L. F. Preda A. Blottière H. M. Clarke G. Albani D. Belcastro V. et al . (2019). Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert. Rev. Neurother.19, 1037–1050. doi: 10.1080/14737175.2019.1638763
99
Iglesias-Vázquez L. Van Ginkel Riba G. Arija V. Canals J. (2020). Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients12:792. doi: 10.3390/nu12030792
100
Isacson R. Nielsen E. Dannaeus K. Bertilsson G. Patrone C. Zachrisson O. et al . (2011). The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur. J. Pharmacol.650, 249–255. doi: 10.1016/j.ejphar.2010.10.008
101
Ising C. Venegas C. Zhang S. Scheiblich H. Schmidt S. V. Vieira-Saecker A. et al . (2019). NLRP3 inflammasome activation drives tau pathology. Nature575, 669–673. doi: 10.1038/s41586-019-1769-z
102
Jangi S. Gandhi R. Cox L. M. Li N. von Glehn F. Yan R. et al . (2016). Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun.7, 1–11. doi: 10.1038/ncomms12015
103
Jasarevic E. Howerton C. L. Howard C. D. Bale T. L. (2015). Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology156, 3265–3276. doi: 10.1210/en.2015-1177
104
Jenkins T. A. Nguyen J. C. D. Polglaze K. E. Bertrand P. P. (2016). Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients8:56. doi: 10.3390/nu8010056
105
Jiang H. Ling Z. Zhang Y. Mao H. Ma Z. Yin Y. et al . (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun.48, 186–194. doi: 10.1016/j.bbi.2015.03.016
106
Jiang H. Zhang X. Yu Z. Zhang Z. Deng M. Zhao J. et al . (2018). Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res.104, 130–136. doi: 10.1016/j.jpsychires.2018.07.007
107
Jorm A. F. Patten S. B. Brugha T. S. Mojtabai R. (2017). Has increased provision of treatment reduced the prevalence of common mental disorders? Review of the evidence from four countries. World Psychiatry16, 90–99. doi: 10.1002/wps.20388
108
Kabouridis P. S. Lasrado R. McCallum S. Chng S. H. Snippert H. J. Clevers H. et al . (2015). Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron85, 289–295. doi: 10.1016/j.neuron.2014.12.037
109
Kang D. W. Park J. G. Ilhan Z. E. Wallstrom G. LaBaer J. Adams J. B. et al . (2013). Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One8:e68322. doi: 10.1371/journal.pone.0068322
110
Kasselman L. J. Vernice N. A. DeLeon J. Reiss A. B. (2018). The gut microbiome and elevated cardiovascular risk in obesity and autoimmunity. Atherosclerosis271, 203–213. doi: 10.1016/j.atherosclerosis.2018.02.036
111
Katsurada K. Yada T. (2016). Neural effects of gut-and brain derived glucagonâ like peptideâ-1 and its receptor agonist. J. Diabetes Investig.7, 64–69. doi: 10.1111/jdi.12464
112
Kennedy P. J. Cryan J. F. Dinan T. G. Clarke G. (2017). Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology112, 399–412. doi: 10.1016/j.neuropharm.2016.07.002
113
Keshavarzian A. Green S. J. Engen P. A. Voigt R. M. Naqib A. Forsyth C. B. (2015). Colonic bacterial composition in Parkinson's disease. Move. Disord.30, 1351–1360. doi: 10.1002/mds.26307
114
Kim C. H. Park J. Kim M. (2014). Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw.14, 277–288. doi: 10.4110/in.2014.14.6.277
115
Kimura I. Inoue D. Maeda T. Hara T. Ichimura A. Miyauchi S. et al . (2011). Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). PNAS108, 8030–8035. doi: 10.1073/pnas.1016088108
116
Knight R. Vrbanac A. Taylor B. C. Aksenov A. Callewaert C. Debelius J. et al . (2018). Best practices for analysing microbiomes. Nat. Rev. Microbiol.16, 410–422. doi: 10.1038/s41579-018-0029-9
117
Koda S. Date Y. Murakami N. Shimbara T. Hanada T. Toshinai K. (2005). The role of the vagal nerve in peripheral PYY3â€36-induced feeding reduction in rats. Endocrinology146, 2369–2375. doi: 10.1210/en.2004-1266
118
Larraufie P. Martin-Gallausiaux C. Lapaque N. Dore J. Gribble F. M. R. Reimann F. et al . (2018). SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep.8:74. doi: 10.1038/s41598-017-18259-0
119
Lee Y. K. Menezes J. S. Umesaki Y. Mazmanian S. K. (2011). Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. PNAS108, 4615–4622. doi: 10.1073/pnas.1000082107
120
Lema Tom C. M. Tyson T. Rey N. L. Grathwohl S. Britschgi M. Brundin P. (2013). Inflammation and α-synuclein’s prion-like behavior in Parkinson's disease—is there a link. Mol. Neurobiol.47, 561–574. doi: 10.1007/s12035-012-8267-8
121
Lim C. Hammond C. J. Hingley S. T. Balin B. J. (2014). Chlamydia pneumoniae infection of monocytes in vitro stimulates innate and adaptive immune responses relevant to those in Alzheimer's disease. J. Neuroinflammation11, 1–11. doi: 10.1186/s12974-014-0217-0
122
Ling Y. Gong T. Zhang J. Gu Q. Gao X. Weng X. et al . (2020). Gut microbiome signatures are biomarkers for cognitive impairment in patients with ischemic stroke. Front. Aging Neurosci.12:511562. doi: 10.3389/fnagi.2020.511562
123
Lyte M. (2014a). Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol.817, 3–24. doi: 10.1007/978-1-4939-0897-4_1
124
Lyte M. (2014b). Microbial endocrinology: host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes5, 381–389. doi: 10.4161/gmic.28682
125
Ma Q. Xing C. Long W. Wang H. Y. Liu Q. Wang R. F. (2019). Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J. Neuroinflammation16, 1–14. doi: 10.1186/s12974-019-1434-3
126
Maiuolo J. Gliozzi M. Musolino V. Carresi C. Scarano F. Nucera S. et al . (2021). The contribution of gut microbiota- brain axis in the development of brain disorders. Front. Neurosci.15:616883. doi: 10.3389/fnins.2021.616883
127
Malaguarnera L. Motta M. Di Rosa M. Anzaldi M. Malaguarnera M. (2006). Interleukin†18 and transforming growth factor beta 1 plasma levels in Alzheimer's disease and vascular dementia. Neuropathology26, 307–312. doi: 10.1111/j.1440-1789.2006.00701.x
128
Mangalam A. Shahi S. K. Luckey D. Karau M. Marietta E. Luo N. et al . (2017). Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep.20, 1269–1277. doi: 10.1016/j.celrep.2017.07.031
129
Manicassamy S. Reizis B. Ravindran R. Nakaya H. Salazar-Gonzalez R. M. (2010). Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science329, 849–853. doi: 10.1126/science.1188510
130
Mao Y. K. Kasper D. L. Wang B. Forsythe P. Bienenstock J. Kunze W. A. (2013). Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat. Commun.4, 1–10. doi: 10.1038/ncomms2478
131
Martin C. R. Osadchiy V. Kalani A. Mayer E. A. (2018). The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol.6, 133–148. doi: 10.1016/j.jcmgh.2018.04.003
132
Matsumoto M. Ooga T. Kibe R. Aiba Y. Koga Y. Benno Y. (2017). Colonic absorption of low-molecular-weight metabolites influenced by the intestinal microbiome: a pilot study. PLoS One12:e0169207. doi: 10.1371/journal.pone.0169207
133
McClean P. L. Parthsarathy V. Faivre E. Halscher C. (2011). The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J. Neurosci.31, 6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011
134
McFarland H. F. Martin R. (2007). Multiple sclerosis: a complicated picture of autoimmunity. Nature Immunol.8, 913–919. doi: 10.1038/ni1507
135
Mehra S. Sahay S. Maji S. K. (2019). α-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta Rev. Cancer1867, 890–908. doi: 10.1016/j.bbapap.2019.03.001
136
Messaoudi M. L. Lalonde R. Violle N. Javelot H. Desor D. Nejdi A. (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr.105, 755–764. doi: 10.1017/S0007114510004319
137
Miller A. H. Raison C. L. (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol.16, 22–34. doi: 10.1038/nri.2015.5
138
Mittal R. Debs L. H. Patel A. P. Nguyen D. Patel K. O'Connor G. et al . (2017). Neurotransmitters: the critical modulators regulating gut-brain axis. J. Cell. Physiol.232, 2359–2372. doi: 10.1002/jcp.25518
139
Mohammadi A. A. Jazayeri S. Khosravi-Darani K. Solati Z. Mohammadpour N. Asemi Z. et al . (2016). The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci.19, 387–395. doi: 10.1179/1476830515Y.0000000023
140
Montiel-Castro A. J. Gonzlez-Cervantes R. M. Bravo-Ruiseco G. Pacheco-Lapez G. (2013). The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front. Integr. Neurosci.7:70. doi: 10.3389/fnint.2013.00070
141
Morais L. H. Schreiber H. L. Mazmanian S. K. (2021). The gut microbiota-brain axis in behavior and brain disorders. Nat. Rev. Microbiol.19, 241–255. doi: 10.1038/s41579-020-00460-0
142
Morimoto R. Satoh F. Murakami O. Totsune K. Saruta M. Suzuki T. et al . (2008). Expression of peptide YY in human brain and pituitary tissues. Nutrition24, 878–884. doi: 10.1016/j.nut.2008.06.011
143
Morris G. Berk M. Carvalho A. Caso J. R. Sanz Y. Walder K. et al . (2017). The role of the microbial metabolites including tryptophan catabolites and short-chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol. Neurobiol.54, 4432–4451. doi: 10.1007/s12035-016-0004-2
144
Mulle J. G. Sharp W. G. Cubells J. F. (2013). The gut microbiome: a new frontier in autism research. Curr. Psychiatry Rep.15, 1–9. doi: 10.1007/s11920-012-0337-0
145
Muller P. A. Schneeberger M. Matheis F. Wang P. Kerner Z. Ilanges A. et al . (2020). Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature583, 441–446. doi: 10.1038/s41586-020-2474-7
146
Murphy K. G. Bloom S. R. (2006). Gut hormones and the regulation of energy homeostasis. Nature444, 854–859. doi: 10.1038/nature05484
147
Naseribafrouei A. Hestad K. Avershina E. Sekelja M. Linlkken A. Wilson R. (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil.26, 1155–1162. doi: 10.1111/nmo.12378
148
Nohr M. K. Egerod K. L. Christiansen S. H. Gille A. Offermanns S. Schwartz T. W. (2015). Expression of the short-chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience290, 126–137. doi: 10.1016/j.neuroscience.2015.01.040
149
Nohr M. K. Pedersen M. H. Gille A. Egerod K. L. Engelstoft M. S. Husted A. S. (2013). GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology154, 3552–3564. doi: 10.1210/en.2013-1142
150
Nonaka N. Shioda S. Niehoff M. L. Banks W. A. (2003). Characterization of blood–brain barrier permeability to PYY3-36 in the mouse. J. Pharmacol. Exp. Th.306, 948–953. doi: 10.1124/jpet.103.051821
151
Obata Y. Castaño Á. Boeing S. Bon-Frauches A. C. Fung C. Fallesen T. et al . (2020). Neuronal programming by microbiota regulates intestinal physiology. Nature578, 284–289. doi: 10.1038/s41586-020-1975-8
152
Ogbonnaya E. S. Clarke G. Shanahan F. Dinan T. G. Cryan J. F. O. Leary O. F. (2015). Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry78, e7–e9. doi: 10.1016/j.biopsych.2014.12.023
153
Ojala J. Alafuzoff I. Herukka S. van Groen T. Tanila H. Pirttil T. (2009). Expression of interleukin-18 is increased in the brains of Alzheimer's disease patients. Neurobiol. Aging30, 198–209. doi: 10.1016/j.neurobiolaging.2007.06.006
154
O'Keefe S. J. D. (2016). Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol.13, 691–706. doi: 10.1038/nrgastro.2016.165
155
Oleskin A. V. Shenderov B. A. (2016). Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis.27:30971. doi: 10.3402/mehd.v27.30971
156
Perry R. J. Peng L. Barry N. A. Cline G. W. Zhang D. Cardone R. L. et al . (2016). Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature534, 213–217. doi: 10.1038/nature18309
157
Petrov V. A. Saltykova I. V. Zhukova I. A. Alifirova V. M. Zhukova N. G. Dorofeeva Y. B. et al . (2017). Analysis of gut microbiota in patients with Parkinsons disease. Bull. Exp. Biol. Med.162, 734–737. doi: 10.1007/s10517-017-3700-7
158
Pirbaglou M. Katz J. de Souza R. J. Stearns J. C. Motamed M. Ritvo P. (2016). Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr Res36, 889–898.
159
Plassais J. Gbikpi-Benissan G. Figarol M. Scheperjans F. Gorochov G. Derkinderen P. et al . (2021). Gut microbiome alpha-diversity is not a marker of Parkinson’s disease and multiple sclerosis. Brain Commun.3:fcab113. doi: 10.1093/braincomms/fcab113
160
Porter D. W. Irwin N. Flatt P. R. Hscher C. Gault V. A. (2011). Prolonged GIP receptor activation improves cognitive function, hippocampal synaptic plasticity and glucose homeostasis in high-fat fed mice. Eur. J. Pharmacol.650, 688–693. doi: 10.1016/j.ejphar.2010.10.059
161
Poutahidis T. Kearney S. M. Levkovich T. Qi P. Varian B. J. Lakritz J. R. et al . (2013). Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One8:e78898. doi: 10.1371/journal.pone.0078898
162
Pröbstel A.-K. Baranzini S. E. (2018). The role of the gut microbiome in multiple sclerosis risk and progression: towards characterization of the “MS Microbiome”. Neurotherapeutics15, 126–134. doi: 10.1007/s13311-017-0587-y
163
Psichas A. Sleeth M. L. Murphy K. G. Brooks L. Bewick G. A. Hanyaloglu A. C. (2015). The short-chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes.39, 424–429. doi: 10.1038/ijo.2014.153
164
Qian Y. Yang X. Xu S. Wu C. Song Y. Qin N. et al . (2018). Alteration of the fecal microbiota in Chinese patients with Parkinson 's disease. Brain Behav. Immun.70, 194–202. doi: 10.1016/j.bbi.2018.02.016
165
Radde R. Bolmont T. Kaeser S. A. Coomaraswamy J. Lindau D. Stoltze L. et al . (2006). Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep.7, 940–946. doi: 10.1038/sj.embor.7400784
166
Rao M. Gershon M. D. (2018). Enteric nervous system development: what could possibly go wrong?Nat. Rev. Neurosci.19, 552–565. doi: 10.1038/s41583-018-0041-0
167
Rhee S. H. Pothoulakis C. Mayer E. A. (2009). Principles and clinical implications of the brainngut enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol.6, 306–314. doi: 10.1038/nrgastro.2009.35
168
Rincel M. Darnaudery M. (2020). Maternal separation in rodents: a journey from gut to brain and nutritional perspectives. Proc. Nutr. Soc.79, 113–132. doi: 10.1017/S0029665119000958
169
Rodrigues H. G. Sato F. T. Curi R. Vinolo M. A. R. (2016). Fatty acids as modulators of neutrophil recruitment, function and survival. Eur. J. Pharmacol.785, 50–58. doi: 10.1016/j.ejphar.2015.03.098
170
Ronchi F. Basso C. Preite S. Reboldi A. Baumjohann D. Perlini L. et al . (2016). Experimental priming of encephalitogenic Th1/Th17 cells requires pertussis toxin-driven IL-1β production by myeloid cells. Nat. Commun.7, 1–11. doi: 10.1038/ncomms11541
171
Rutsch A. Kantsj J. B. Ronchi F. (2020). The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol.11:604179. doi: 10.3389/fimmu.2020.604179
172
Sadler R. Cramer J. V. Heindl S. Kostidis S. Betz D. Zuurbier K. R. et al . (2020). Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J. Neurosci.40, 1162–1173. doi: 10.1523/JNEUROSCI.1359-19.2019
173
Sampson T. R. Debelius J. W. Thron T. Janssen S. Shastri G. G. Ilhan Z. E. et al . (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cells167, 1469–1480.e12. doi: 10.1016/j.cell.2016.11.018
174
Sampson T. R. Mazmanian S. K. (2015). Control of brain development, function, and behavior by the microbiome. Cell Host Microbe17, 565–576. doi: 10.1016/j.chom.2015.04.011
175
Saresella M. la Rosa F. Piancone F. Zoppis M. Marventano I. Calabrese E. et al . (2016). The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer's disease. Mol. Neurodegener.11, 1–14. doi: 10.1186/s13024-016-0088-1
176
Savignac H. M. Dinan T. G. Cryan J. F. (2011). Resistance to early-life stress in mice: effects of genetic background and stress duration. Front. Behav. Neurosci.5:13. doi: 10.3389/fnbeh.2011.00013
177
Schachtle M. A. Rosshart S. P. (2021). The microbiota-gut-brain axis in health and disease and its implications for translational research. Front. Cell. Neurosci15:698172. doi: 10.3389/fncel.2021.698172
178
Scheltens P. Blennow K. Breteler M. M. de Strooper B. Frisoni G. B. Salloway S. et al . (2016). Alzheimer's disease. Lancet (London, England)388, 505–517. doi: 10.1016/S0140-6736(15)01124-1
179
Scheperjans F. Aho V. Pereira P. A. B. Koskinen K. Paulin L. Pekkonen E. et al . (2015). Gut microbiota are related to Parkinson's disease and clinical phenotype. Movement. Disord. Clin. Pract.30, 350–358. doi: 10.1002/mds.26069
180
Sekirov I. Russell S. L. Antunes L. C. M. Finlay B. B. (2010). Gut microbiota in health and disease. Physiol. Rev.90, 859–904. doi: 10.1152/physrev.00045.2009
181
Sgritta M. Dooling S. W. Buffington S. A. Momin E. N. Francis M. B. Britton R. A. et al . (2019). Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron101:e246, –259.e6. doi: 10.1016/j.neuron.2018.11.018
182
Singh V. Roth S. Llovera G. Sadler R. Garzetti D. Stecher B. R. et al . (2016). Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci.36, 7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016
183
Skonieczna Zydecka K. Marlicz W. Misera A. Koulaouzidis A. Aoniewski I. (2018). Microbiome the missing link in the gut-brain axis: focus on its role in gastrointestinal and mental health. J. Clin. Med.7:521. doi: 10.3390/jcm7120521
184
Smith P. A. (2015). The tantalizing links between gut microbes and the brain. Nature526, 312–314. doi: 10.1038/526312a
185
Stojkovi D. Kosti M. Smiljkovi M. Aleksi M. Vasiljevi P. Nikoli M. et al . (2020). Linking antimicrobial potential of natural products derived from aquatic organisms and microbes involved in Alzheimers disease-a review. Curr. Med. Chem.27, 4372–4391. doi: 10.2174/0929867325666180309103645
186
Strandwitz P. Kim K. H. Terekhova D. Liu J. K. Sharma A. Levering J. et al . (2019). GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol.4, 396–403. doi: 10.1038/s41564-018-0307-3
187
Sudo N. Chida Y. Aiba Y. Sonoda J. Oyama N. Yu X. N. et al . (2004). Postnatal microbial colonization programs the hypothalamic pituitary adrenal system for stress response in mice. J. Physiol.558, 263–275. doi: 10.1113/jphysiol.2004.063388
188
Takagi T. Naito Y. Inoue R. Kashiwagi S. Uchiyama K. Mizushima K. et al . (2019). Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J. Gastroenterol.54, 53–63. doi: 10.1007/s00535-018-1488-5
189
Tarawneh R. Penhos E. (2022). The gut microbiome and Alzheimer’s disease: complex and bidirectional interactions. Neurosci. Biobehav. Rev.141:104814. doi: 10.1016/j.neubiorev.2022.104814
190
The Human Microbiome Project Consortium (2012). Structure, function and diversity of the healthy human microbiome. Nature486, 207–214. doi: 10.1038/nature11234
191
Tian Y. Ullah H. Gu J. Li K. (2023). Immune-metabolic mechanisms of posttraumatic stress disorder and atherosclerosis. Front. Physiol.14:1123692. doi: 10.3389/fphys.2023.1123692
192
Tiraboschi P. Hansen L. A. Thal L. J. Corey-Bloom J. (2004). The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology62, 1984–1989. doi: 10.1212/01.WNL.0000129697.01779.0A
193
Tiwari P. Dwivedi R. Bansal M. Tripathi M. Dada R. (2023). Role of gut microbiota in neurological disorders and its therapeutic significance. J. Clin. Med.12:1650. doi: 10.3390/jcm12041650
194
Tognini P. (2016). Gut microbiota: a potential regulator of neurodevelopment. Front. Cell. Neurosci.11:25.
195
Tolhurst G. Heffron H. Lam Y. S. Parker H. E. Habib A. M. Diakogiannaki E. et al . (2012). Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein coupled receptor FFAR2. Diabetes61, 364–371. doi: 10.2337/db11-1019
196
Tomova A. Husarova V. Lakatosova S. Bakos J. Vlkova B. Babinska K. et al . (2015). Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav.138, 179–187. doi: 10.1016/j.physbeh.2014.10.033
197
Tremlett H. Bauer K. C. Appel-Cresswell S. Finlay B. B. Waubant E. (2017). The gut microbiome in human neurological disease: a review. Ann. Neurol.81, 369–382. doi: 10.1002/ana.24901
198
Twenge J. M. Cooper A. B. Joiner T. E. Duffy M. E. Binau S. G. (2019). Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005-2017. J. Abnorm. Psychol.128:185. doi: 10.1037/abn0000410
199
Tysnes O. B. Storstein A. (2017). Epidemiology of Parkinson's disease. J. Neural Transm.124, 901–905. doi: 10.1007/s00702-017-1686-y
200
Unger M. M. Spiegel J. Dillmann K.-U. Grundmann D. Philippeit H. Bürmann J. et al . (2016). Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat. Disord.32, 66–72. doi: 10.1016/j.parkreldis.2016.08.019
201
Valles-Colomer M. Falony G. Darzi Y. Tigchelaar E. F. Wang J. Tito R. Y. et al . (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol.4, 623–632. doi: 10.1038/s41564-018-0337-x
202
Vogt N. M. Kerby R. L. Dill-McFarland K. A. Harding S. J. Merluzzi A. P. Johnson S. C. et al . (2017). Gut microbiome alterations in Alzheimer' s disease. Sci. Reports.7, 1–11.
203
Vogt N. M. Romano K. A. Darst B. F. Engelman C. D. Johnson S. C. Carlsson C. M. et al . (2018). The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res. Ther.10, 1–8. doi: 10.1186/s13195-018-0451-2
204
Waise T. M. Dranse H. J. Lam T. K. T. (2018). The metabolic role of vagal afferent innervation. Nat. Rev. Gastroenterol. Hepatol.15, 625–636. doi: 10.1038/s41575-018-0062-1
205
Wang L. Christophersen C. T. Sorich M. J. Gerber J. P. Angley M. T. Conlon M. A. (2013). Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism.4, 1–4. doi: 10.1186/2040-2392-4-42
206
Wang H.-X. Wang Y. P. (2016). Gut microbiota-brain axis. Chin. Med.129, 2373–2380. doi: 10.4103/0366-6999.190667
207
Weinberger A. H. Gbedemah M. Martinez A. M. Nash D. Galea S. Goodwin R. D. (2018). Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychol. Med.48, 1308–1315. doi: 10.1017/S0033291717002781
208
Williams B. L. Hornig M. Buie T. Bauman M. L. Cho Paik M. Wick I. et al . (2011). Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One6:e24585. doi: 10.1371/journal.pone.0024585
209
Won Y.-J. Lu V. B. Puhl H. L. Ikeda S. R. (2013). b-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J. Neurosci.33, 19314–19325. doi: 10.1523/JNEUROSCI.3102-13.2013
210
Wood J. D. Galligan J. J. (2004). Function of opioids in the enteric nervous system. Neurogastroenterol. Motilit.16, 17–28. doi: 10.1111/j.1743-3150.2004.00554.x
211
Xiang L. Lou Y. Liu L. Liu Y. Zhang W. Deng J. et al . (2020). Gut microbiotic features aiding the diagnosis of acute ischemic stroke. Front. Cell. Infect.10:587284. doi: 10.3389/fcimb.2020.587284
212
Xu D. J. Wang K. C. Yuan L. B. Li H. F. Xu Y. Y. Wei L. Y. et al . (2021). Compositional and functional alterations of gut microbiota in patients with stroke. Nutr. Metab. Cardiovasc. Dis.31, 3434–3448. doi: 10.1016/j.numecd.2021.08.045
213
Xu M. Xu X. Li J. Li F. (2019). Association between gut microbiota and autism spectrum disorder: a systematic review and meta-analysis. Front. Psych.10:473. doi: 10.3389/fpsyt.2019.00473
214
Yano J. M. Yu K. Donaldson G. P. Shastri G. G. Ann P. Ma L. et al . (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cells161, 264–276. doi: 10.1016/j.cell.2015.02.047
215
Yin J. Liao S. X. He Y. Wang S. Xia G. H. Liu F. T. et al . (2015). Dysbiosis of gut microbiota with reduced trimethylamine‐n‐oxide level in patients with large‐artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc.4:e002699. doi: 10.1161/JAHA.115.002699
216
Yoo B. B. Mazmanian S. K. (2017). The enteric network: interactions between the immune and nervous systems of the gut. Immunity46, 910–926. doi: 10.1016/j.immuni.2017.05.011
217
Zhang L. Wang Y. Xiayu X. Shi C. Chen W. Song N. et al . (2017). Altered gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimers Dis.60, 1241–1257. doi: 10.3233/JAD-170020
218
Zhao Z. X. Fu J. Ma S. R. Peng R. Yu J. B. Cong L. et al . (2018). Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics8, 5945–5959. doi: 10.7150/thno.28068
219
Zheng D. Liwinski T. Elinav E. (2020). Interaction between microbiota and immunity in health and disease. Cell Res.30, 492–506. doi: 10.1038/s41422-020-0332-7
220
Zheng P. Zeng B. Zhou C. Liu M. Fang Z. Xu X. et al . (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry21, 786–796. doi: 10.1038/mp.2016.44
221
Zhu M. Liu X. Ye Y. Yan X. Cheng Y. Zhao L. Chen F. Ling Z. (2022). Gut Microbiota: A novel therapeutic target for Parkinson's disease. Front. Immunol. 3123.
Summary
Keywords
microbiota, neurological disorders, gut-brain axis, signaling pathways, gut dysbiosis
Citation
Ullah H, Arbab S, Tian Y, Liu C-q, Chen Y, Qijie L, Khan MIU, Hassan IU and Li K (2023) The gut microbiota–brain axis in neurological disorder. Front. Neurosci. 17:1225875. doi: 10.3389/fnins.2023.1225875
Received
20 May 2023
Accepted
07 July 2023
Published
04 August 2023
Volume
17 - 2023
Edited by
Daniele Lana, University of Florence, Italy
Reviewed by
Guoxue Zhu, Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, China; Sahibzada Waheed Abdullah, Chinese Academy of Agricultural Sciences, China
Updates
Copyright
© 2023 Ullah, Arbab, Tian, Liu, Chen, Qijie, Khan, Hassan and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ka Li, lika127@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.