- 1Department of Neurology, Shandong Provincial Hospital, Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 2Department of Neurology, Shandong First Medical University, Jinan, Shandong, China
- 3Department of Ultrasound, Shandong Provincial Hospital, Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 4Department of Ultrasound, Shandong First Medical University, Jinan, Shandong, China
As a common neurodegenerative disease, Parkinson’s disease (PD) is typified by α-synuclein (α-syn) aggregation and progressive degeneration of dopaminergic neurons within the substantia nigra. Clinical manifestations encompass motor symptoms and non-motor aspects that severely impair quality of life. Existing treatments mainly address symptoms, with no effective disease-modifying therapies available. The gut microbiota refers to the community of microorganisms that colonize the intestinal tract. The gut microbiota, gut, and brain are all connected via a complicated, mutual communication pathway known as the “gut microbiota-gut-brain axis.” Gut microbiota dysbiosis is strongly linked to the onset and course of PD, according to growing data. In individuals with PD, gut dysbiosis correlates with clinical phenotype, disease duration, severity, and progression rates. Mechanistically, gut dysbiosis contributes to PD through enhanced intestinal permeability, increased intestinal inflammation and neuroinflammation, abnormal α-syn aggregation, oxidative stress, and reduced neurotransmitter synthesis. Therefore, focusing on the gut microbiota is regarded as a potentially effective treatment strategy. Fecal microbiota transplantation (FMT) is an emerging approach to modulate gut microbiota, with the goal of recovering microbiota diversity and function by transferring functional intestinal flora from healthy individuals into patients’ gastrointestinal tracts. FMT is expected to become a promising therapy of PD and has a broad research and application prospect. Evidence suggests that FMT may restore gut microbiota, ease clinical symptoms, and provide potential neuroprotective benefits. However, the precise therapeutic mechanisms of FMT in PD remain uncertain, necessitating further research to clarify its effectiveness. This review examines alterations in gut microbiota linked to PD, mechanisms through which gut dysbiosis influences the disease, and the latest advancements in FMT research for treating PD, setting the stage for its clinical application.
1 Introduction
As a prevalent neurodegenerative illness, Parkinson’s disease (PD) is typified by aberrant α-synuclein (α-syn) folding and aggregation, as well as dopaminergic neurons degenerating and losing within the substantia nigra (SN; Lajoie et al., 2021). It has a complicated and multifaceted etiology that includes environmental, genetic, and other variables. Its pathogenesis mainly involves α-syn abnormal aggregation (Tofaris, 2022), neuroinflammation (Marogianni et al., 2020), oxidative stress (Ding et al., 2018), and mitochondrial dysfunction (Malpartida et al., 2021).
According to statistics, in 2015, the number of PD cases worldwide was approximately 6.2 million, and by 2040, that figure is predicted to reach 12.9 million (Dorsey and Bloem, 2018). The incidence of PD is significantly related to age (Qi et al., 2021). The typical course is insidious, slow, and progressive. Clinical manifestations encompass motor aspects like bradykinesia, resting tremor, and rigidity, along with non-motor aspects like gastrointestinal dysfunction and sleep disturbances, which often more significantly affect the quality of life. According to statistical data, from 1999 to 2019, the death rate for PD rose from 5.4 per 100,000 to 8.8 per 100,000 (Rong et al., 2021). The growth rate of PD-related disabilities and deaths is faster than that of any other neurological disease (The Lancet, 2022).
Currently, the treatment of PD is mainly symptomatic, comprising pharmacotherapy and surgical interventions. However, effective disease-modifying therapies are still lacking. Commonly utilized medications in clinical treatment include the dopamine (DA) precursor levodopa, catechol-O-methyltransferase inhibitors, monoamine oxidase B inhibitors, anticholinergic drugs, and DA receptor agonists. Among these, DA replacement therapy remains the first-line intervention for PD, but it only partially improves motor symptoms, without alleviating non-motor symptoms or delaying disease progression.
Furthermore, its efficacy tends to decline after 3–5 years, and prolonged use may produce adverse effects such as the wearing-off phenomenon, the on–off phenomenon, and dyskinesia, resulting in poor patient tolerance (Armstrong and Okun, 2020). Deep brain stimulation, the main surgical treatment for PD, alleviates symptoms by implanting electrodes into specific brain nuclei and delivering microcurrents to stimulate target areas, thereby modifying electrical signals in related neural circuits (Sharma et al., 2020). However, deep brain stimulation controls symptoms without curing the disease. Patients still require long-term medication post-surgery, and the associated medical costs remain high. Currently, no existing therapy has shown significant efficacy in reversing α-syn aggregation, restoring DA neuron degeneration, or delaying disease progression (Suarez-Cedeno et al., 2017).
Recent research has introduced a new perspective: the central nervous system (CNS) may be impacted by the gut microbiota. Gut microbiota is known as the collection of bacteria, viruses, fungi, and other microorganisms inhabiting the intestinal tract, representing the largest and most intricate microflora in the human body. It includes approximately 50 bacterial phyla. Bacteroidetes and Firmicutes comprise more than 90% of the total (Ding et al., 2021).
Under normal circumstances, the relative abundance and diversity of gut microbiota are dynamically balanced, influenced by variables including diet, stress and antibiotics (Sun et al., 2022). Many physiological processes, including nutrient digestion and absorption, energy metabolism, immune function regulation, neural function modulation, and intestinal barrier maintenance, are influenced by the gut microbiota. It is necessary for the immune, endocrine, and nervous systems to grow and mature. Dysbiosis of gut microbiota refers to disturbances in overall microbial composition and the relative abundance of specific flora, disrupting the body’s homeostasis. In preclinical studies, gut dysbiosis has been linked to the pathophysiological mechanisms of intestinal diseases (e.g., inflammatory bowel disease, irritable bowel syndrome), mental disorders (e.g., anxiety, depression, autism spectrum disorder), as well as neurological diseases (e.g., multiple sclerosis, Alzheimer’s disease [AD], PD, amyotrophic lateral sclerosis; Mou et al., 2022; Sorboni et al., 2022; Kujawa et al., 2023; Solanki et al., 2023; Loh et al., 2024).
Modifications to gut microbiota abundance or diversity and its metabolites have been observed in animal models and clinical cases of PD, highlighting the significance in the onset and progression of the disease. Addressing gut dysbiosis offers PD patients a potential treatment strategy. This article reviews the characteristic changes in gut microbiota among PD patients, the potential mechanisms by which gut dysbiosis may contribute to PD pathogenesis, and recent advancements in fecal microbiota transplantation (FMT) for PD treatment, providing a scientific foundation for future clinical applications.
2 Gut microbiota and the connection with PD
2.1 Gut microbiota-gut-brain axis
Recent research has revealed a sophisticated bidirectional communication pathway connecting the gut microbiota, the gut, and the brain, termed the “gut microbiota-gut-brain axis” (MGBA; Cryan and Dinan, 2012). This axis enables crosstalk between the enteric nervous system (ENS) and the CNS by means of neurological, immunological, endocrine, and metabolic signaling pathways (Matheson and Holsinger, 2023). Dysregulation of this axis has been associated with the pathophysiology of PD.
Braak et al. (2006) first proposed the hypothesis that the origins of PD may be in the gastrointestinal tract. They proposed that a neurotropic pathogen crossing the gastric epithelium could induce α-syn misfolding in the ENS, then propagate to the brain via retrograde axonal transport through a chain of interconnected neurons, driving PD pathology. This hypothesis aligns with clinical observations that gastrointestinal symptoms frequently appear before motor problems in PD patients. According to epidemiological research, patients with inflammatory bowel disease are more likely to acquire PD than people without inflammatory bowel disease (Brudek and van Laar, 2019). Furthermore, vagotomy performed to treat peptic ulcers has been shown to reduce PD risk (Sun et al., 2022). These findings underscore the strong association between PD and the gastrointestinal tract, indirectly supporting the hypothesis of intestinal origin.
Preclinical studies provide additional evidence. Kim et al. (2019) found that α-syn was transferred from the gut, initially appearing in the vagus nerve’s dorsal motor nucleus nerve and eventually reaching the SN compacta via sequential diffusion and transmission. Importantly, vagotomy effectively prevented α-syn transfer from the colon to the brain, confirming the role of the vagus nerve. Similarly, Bhattarai et al. (2021) administered rotenone to both germ-free and conventionally raised mice for 6 weeks. Although tyrosine hydroxylase neurons were lost in each group, only conventionally raised mice exhibited decreased motor strength and coordination, emphasizing the importance of gut microbiota in PD etiology. Collectively, these findings highlight the critical function of this axis in the onset and course of PD.
2.2 Dysbiosis of gut microbiota in PD patients
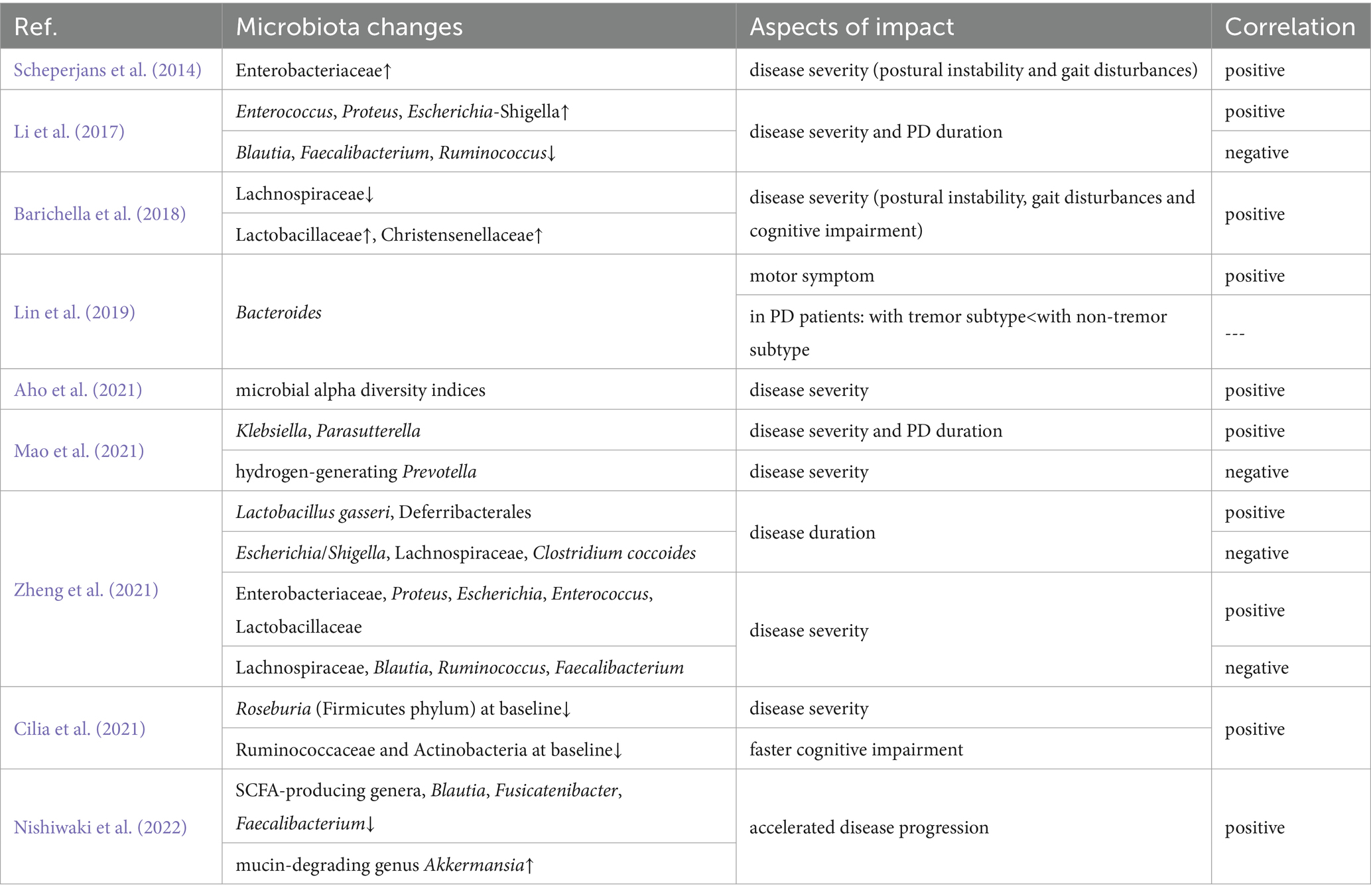
Numerous case–control investigations have researched the gut microbiota composition of PD patients. While findings vary due to differences in sample size, inclusion and exclusion criteria, experimental design, and individual factors (e.g., age, diet, geography, and genetic background; Li Z. et al., 2022), certain consistent trends have emerged. For example, compared with healthy controls, PD patients exhibit higher abundances of certain genera, including Bilophila, Akkermansia, Verrucomicrobia, Lactobacillus, and Parabacteroides, alongside lower abundances of beneficial bacteria, especially Lachnospiraceae, Roseburia, Faecalibacterium, Blautia, and Prevotella (Scheperjans et al., 2014; Li et al., 2017; Barichella et al., 2018; Lin et al., 2019; Lubomski et al., 2019; Cirstea et al., 2020; Nishiwaki et al., 2020a; Yu et al., 2023). Furthermore, specific microbial taxa correlate with clinical phenotypes, disease duration, severity, and progression rate. Table 1 summarizes these associations, illustrating the complicated link between microbiota changes and PD pathology.
2.3 Potential mechanisms of gut microbiota dysbiosis in PD pathogenesis
In recent years, research has increasingly supported the hypothesis that gut microbiota dysbiosis acts as a triggering factor for PD (Costa et al., 2022). Dysbiosis and its metabolites are thought to affect the onset and progression via several interconnected mechanisms, including raised intestinal permeability, exacerbated intestinal inflammation and neuroinflammation, aberrant α-syn aggregation, elevated oxidative stress, and reduced neurotransmitter manufacture (Figure 1).
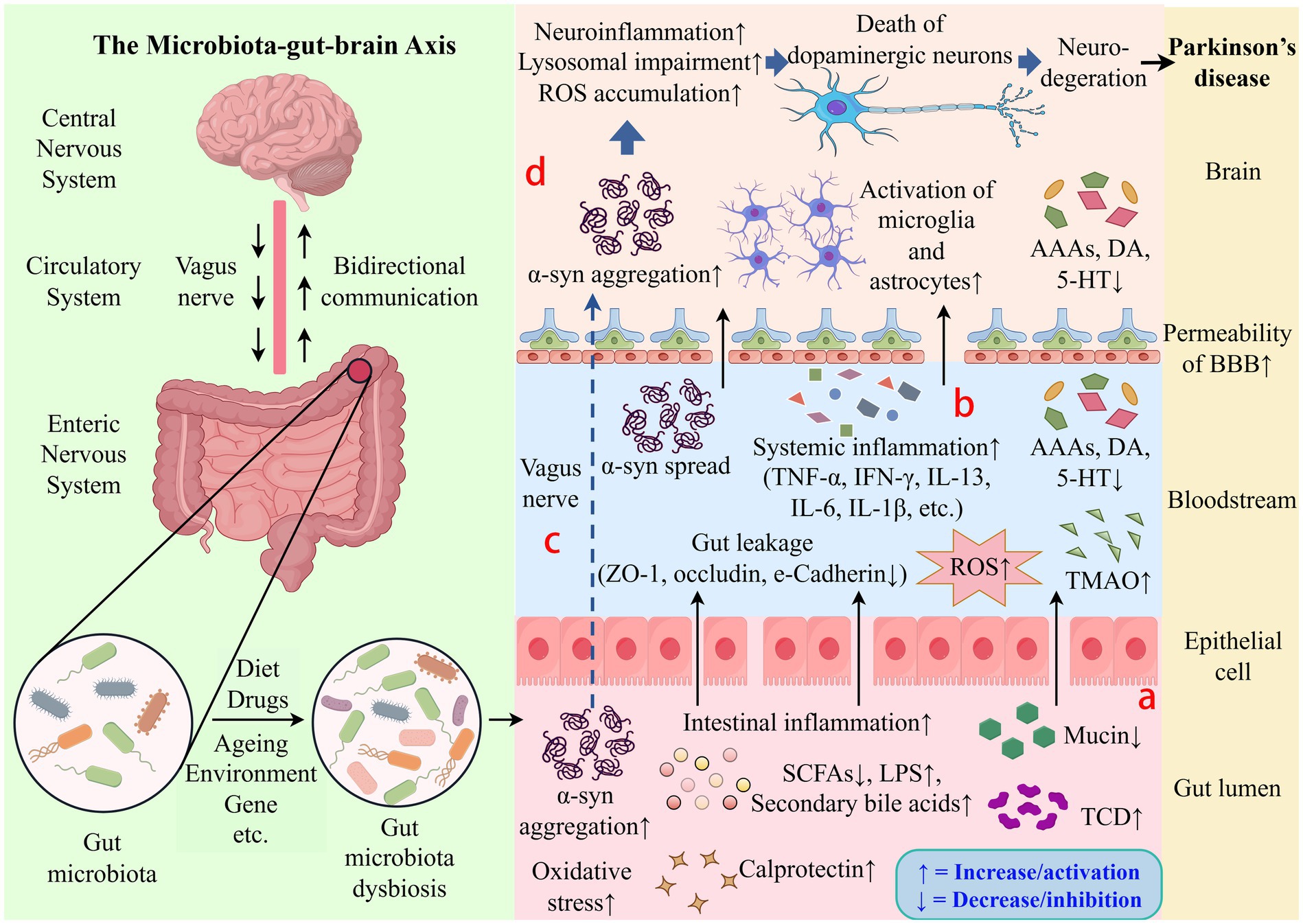
Figure 1. Schematic diagram of potential mechanisms by which gut microbiota influences Parkinson’s disease. The gut microbiota-gut-brain axis can affect the onset and course of PD. (a) Dysbiosis is associated with increased intestinal permeability, dysregulated gut microbiota metabolites, exacerbated oxidative stress and intestinal inflammation. (b) Gut leakage can trigger inflammatory cytokines to be released, resulting in systemic inflammation. (c) The vagus nerve allows abnormal α-syn aggregation to spread from the ENS to the CNS. (d) Pro-inflammatory cytokines can penetrate the blood–brain barrier, cause the activation of microglia and astrocytes, and aggravate neuroinflammation, which results in dopaminergic neuron loss and degeneration. ROS, Reactive Oxygen Species; AAAs, Aromatic Amino Acids; DA, Dopamine; 5-HT, 5-Hydroxytryptamine; α-syn, α-synuclein; BBB, Blood–Brain Barrier; TNF-α, Tumor Necrosis Factor-alpha; IFN-γ, Interferon-gamma; IL-13, Interleukin-13; IL-6, Interleukin-6; IL-1β, Interleukin-1 beta; ZO-1, Zonula Occludens-1; TMAO, Trimethylamine N-Oxide; SCFAs, Short-Chain Fatty Acids; LPS, Lipopolysaccharide; TCD, Total Cumulative Dose.
2.3.1 Increase in intestinal permeability
Dysbiosis of gut microbiota can compromise the intestinal epithelial barrier, leading to increased penetration. This “leaky gut” condition permits neuroactive small molecules, including potentially toxic metabolites derived from bacteria and microbial sources, to translocate into systemic circulation, accelerating pathological processes and elevating PD risk. Mucin, a key structural component of gastrointestinal mucosa, is essential for preserving barrier integrity. In PD patients, Prevotella deficiency is correlated to impaired mucin production, increased gut permeability, and disease progression (Scheperjans et al., 2014). Fang et al. (2024) demonstrated that chronic rotenone administration significantly reduced colonic mucus thickness and downregulated the expression of tight junction proteins (e.g., Zonula Occludens-1, occludin), confirming the essential role of gut microbiota in maintaining intestinal barrier integrity. Notably, FMT effectively alleviated rotenone-induced intestinal barrier impairment. The study further revealed that gut microbiota dysbiosis promotes excessive hydrogen sulfide production by sulfate-reducing bacteria, which subsequently degrades the mucus layer, disrupts the intestinal epithelial barrier, enhances intestinal permeability, and ultimately contribute to gut leakage (Munteanu et al., 2024). Short-chain fatty acids (SCFAs) contribute to intestinal barrier maintenance. According to studies, PD patients have much less bacteria that produce SCFA, resulting in lower fecal SCFA levels (Bisaglia, 2022). Experimental evidence suggests that administering butyrate to PD animal models can delay disease progression by improving motor function, preserving intestinal barrier integrity, reducing intestinal leakage, and secondary translocation of intestinal contents (Zheng et al., 2021).
2.3.2 Aggravation of intestinal inflammation and neuroinflammation
According to compelling data, chronic intestinal inflammation and neuroinflammation are exacerbated by pro-inflammatory dysbiosis of the gut microbiota, which are thought to potentially contribute to the PD pathophysiology. Lin et al. (2019) reported elevated concentrations of pro-inflammatory cytokines, including Tumor Necrosis Factor-alpha (TNF-α), Interferon-gamma, and Interleukin-13, in the plasma of PD patients. There were positive correlations between the levels of TNF-α and Interferon-gamma and the abundance of Bacteroides and Verrucomicrobia, respectively. Additionally, fecal calprotectin, a hallmark of intestinal inflammation, was considerably increased in PD patients (Weis et al., 2019). Keshavarzian et al. (2015) used high-throughput ribosomal RNA sequencing to reveal that PD patients had lower “anti-inflammatory” bacteria, including Blautia, Coprococcus, and Roseburia, alongside more “pro-inflammatory” bacteria like Faecalibacterium. Preclinical studies demonstrate that dysbiosis exacerbates neuroinflammation through pathways such as Toll-like receptor 4 (TLR4)/Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which raises the production of inflammatory markers, such as Glycogen synthase kinase 3 beta, inducible nitric oxide synthase, and Interleukin-1 beta, along with activating microglia and astrocytes in the SN (Varesi et al., 2022).
2.3.3 Abnormal α-syn aggregation
α-syn aggregation, a characteristic of PD pathophysiology, might be impacted by gut dysbiosis. Altered microbiota promotes α-syn misfolding in the ENS and gastrointestinal epithelial cells, promoting pro-inflammatory immune activation and facilitating its spread to the CNS (Sterling et al., 2022). On the one hand, it has been shown that certain gut bacteria or their secreted metabolites promote the aggregation and dissemination of α-syn. In mice with leucine-rich repeat kinase 2 gene, Liang et al. (2023) demonstrated that giving them Escherichia coli by mouth caused curli-mediated phosphorylation and accumulation of α-syn in the colon, which then spread along the gut-brain axis to the CNS. Additionally, hemolysin A secreted by Proteus mirabilis triggers α-syn oligomerization via activation of Mechanistic Target of Rapamycin-dependent autophagy signaling pathways in intestinal cells, ultimately inducing motor deficits and neurodegeneration (Huh et al., 2023). Notably, Dubosiella has been implicated in α-syn aggregation via the suppression of branched-chain amino acid catabolism, leading to the peripheral accumulation of valine and isoleucine, which disrupts lysosomal function and hinders α-syn clearance (Wu et al., 2025). On the other hand, Wang et al. (2022) discovered that the probiotic Lactobacillus plantarum DP189 could suppress oxidative stress, restore microbial diversity, and decrease α-syn aggregation in the SN of PD mice, thus delaying disease progression. The findings indicate that targeted modulation of gut microbiota could be a possible therapeutic approach to reduce α-syn aggregation in PD pathogenesis.
2.3.4 Increase in oxidative stress
Dysbiosis can worsen oxidative stress by changing microbial metabolism and decreasing antioxidant metabolite production. This promotes neuronal damage and α-syn misfolding in the ENS, which subsequently spreads to the CNS (Bullich et al., 2019; Shandilya et al., 2022). Studies have shown that Akkermansia increases intestinal permeability, exposing neurons to oxidative conditions that favor α-syn aggregation (Nishiwaki et al., 2020b). Yu et al. (2023) found that gut dysbiosis aggravated oxidative stress responses and neurobehavioral impairments by downregulating Nicotinamide mononucleotide adenylyltransferase 2, a gene involved in NAD+ synthesis, in PD rat models. Emerging evidence indicates that the modulation of gut microbiota can reduce oxidative stress responses. Zhu et al. (2025) demonstrated that sleep deprivation promotes the synthesis of microbiota-derived adenosine, which elevates the production of reactive oxygen species by upregulating the pro-oxidant enzyme NADPH oxidase 4 and inhibiting the antioxidant factor Nuclear factor erythroid 2-related factor 2, consequently exacerbating oxidative damage to dopaminergic neurons. Probiotic supplements significantly mitigated these effects. Nurrahma et al. (2022) revealed that a high dose of the mangosteen pericarp, abundant in antioxidants, restores gut microbiota balance by diminishing pro-inflammatory bacterial genera (e.g., Sutterella, Rothia, Aggregatibacter), which exhibited a negative correlation with antioxidant gene expression. This improves antioxidant levels and alleviates PD motor deficits. Additionally, Gao et al. (2024) demonstrated that in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice, administration of ginkgolide C could restore gut microbiota homeostasis, exert antioxidant effects by activating the Protein Kinase B/Nuclear factor erythroid 2-related factor 2/Heme oxygenase-1 pathway in SN4741 neuronal cells, and alleviate pathological damage in mice.
2.3.5 Reduction in production of neurotransmitters
It has been shown that the gut microbiota synthesize numerous neurotransmitters that are present in the human brain, such as DA, serotonin, γ-aminobutyric acid, and noradrenaline. Gut microbiota dysbiosis may disrupt neurotransmitters synthesis, perturbing the CNS homeostasis through gut-brain axis signaling pathways, which may contribute to the pathological progression of PD neurological dysfunction (Strandwitz, 2018; Wang et al., 2021). Research by Gao et al. (2018) revealed that when gut microbiota in experimental animals was changed through antibiotic infusion, there were significant reductions in the concentrations of serotonin, DA, and aromatic amino acids in their blood and hypothalamus compared to a control group infused with normal saline. This discovery emphasizes how vital the gut bacteria is to preserving neurotransmitter and precursor levels. Similarly, van Kessel et al. (2019) investigated the impact of microbial tyrosine decarboxylase in the proximal small intestine—a primary area for Levodopa absorption—in PD patients. They observed an increase in tyrosine decarboxylase activity, which led to premature transformation of L-dopa, significantly reducing its plasma levels and bioavailability. This, in turn, increased therapeutic dose requirements and reduced drug efficacy. According to these findings, gut dysbiosis may directly or indirectly influence the pharmacokinetics, bioavailability, and side effects of medications used to treat PD.
Overall, the findings presented underscore the role that gut microbiota plays in regulating neurotransmitter production and its profound implications for PD pathophysiology as well as the optimization of therapy approaches.
2.4 Metabolites of gut microbiota
The metabolites produced by the gut microbiota play a critical role in regulating neurodegenerative diseases, such as PD, via the MGBA. The pathological mechanisms mediated by four key metabolite classes are outlined.
2.4.1 SCFAs
SCFAs, including acetate, propionate, and butyrate, are microbial metabolites derived from anaerobic fermentation of dietary fibers. Emerging evidence establishes a link between SCFA homeostasis disruption and neurodegenerative pathogenesis. In PD, reduced fecal SCFA levels compromise the structural integrity of the intestinal barrier and the blood–brain barrier (BBB), promote α-syn pathological aggregation, and exacerbate intestinal inflammation and neuroinflammation (Chen et al., 2022; Duan et al., 2023). Preclinical studies demonstrate that SCFA supplementation attenuates dopaminergic neurodegeneration and alleviates motor impairments in PD mice by inhibiting NF-κB/mitogen-activated protein kinase pathway inhibition in the SN and reducing α-syn aggregation (Hou Y. F. et al., 2021; Hou Y. et al., 2021). The neuroprotective effects of SCFAs extend to AD pathophysiology by modulating synaptic plasticity, amyloid-β (Aβ) and tau pathology, and neuroinflammation (Tang et al., 2022). Clinically, mild cognitive impairment patients exhibit a significant reduction of fecal SCFAs that inversely correlates with Aβ burden in cognition-associated brain regions (Gao et al., 2023). Notably, in models of amyotrophic lateral sclerosis, the abundance of butyrate-producing bacteria decreases in SOD1G93A mice, whereas butyrate supplementation enhances gut barrier integrity, reduces SOD1G93A aggregates, decelerates motor neuron degeneration, and prolongs survival of these mice (Loh et al., 2024).
2.4.2 Secondary bile acids
Intestinal microbiota mediate the biotransformation of primary to secondary bile acids. In PD patients, increased levels of deoxycholic acid and lithocholic acid in the cecum are closely related to increased abundance of bile acid-synthesizing microbiota. These secondary bile acids induce pathologic α-syn aggregation and propagation through exerting pro-inflammatory and cytotoxic effects while simultaneously impairing mitochondrial function and autophagy regulation, contributing to neurodegenerative disease pathology (Castro-Caldas et al., 2012; Kiriyama and Nochi, 2023). Notably, taurodeoxycholic acid, a neuroprotective bile acid, demonstrates therapeutic potential across neurodegenerative models. In PD mice, taurodeoxycholic acid administration significantly delays dopaminergic neurodegeneration by inhibiting the c-Jun N-terminal kinase apoptosis pathway, reducing mitochondrial reactive oxygen species, and activating the Protein Kinase B survival pathway (Li et al., 2021). AD rodent models further reveal the capacity of taurodeoxycholic acid to reduce Aβ deposition in the hippocampus and prefrontal cortex and rescue cognitive deficits in spatial, recognition, and contextual memory domains (Lo et al., 2013).
2.4.3 Trimethylamine N-oxide
The gut microbiota mediates enzymatic conversion of dietary choline and carnitine to trimethylamine, which undergoes hepatic oxidation to generate TMAO, a compound implicated in neurodegeneration through various mechanisms (Caradonna et al., 2025). Clinical metabolomic profiling reveals elevated circulating TMAO concentrations in PD patients, though independent of disease progression (Voigt et al., 2022). Mechanistically, TMAO promotes abnormal α-syn conformational changes and pathological aggregation and activates pro-inflammatory signaling pathways, such as NF-κB. Additionally, TMAO penetrates the BBB, exacerbating neuroinflammation and neuronal damage (Caradonna et al., 2024). Lee et al. (2022) demonstrated that TMAO-treated midbrain organoids showed impaired brain-derived neurotrophic factor signaling, loss of dopaminergic neurons, astrocyte activation, and neuromelanin accumulation. Furthermore, TMAO induced the pathological phosphorylation of α-syn and tau proteins, facilitating their aggregation. Vogt et al. (2018) identified a correlation between elevated TMAO levels and AD pathology and markers of neuronal degeneration in the cerebrospinal fluid. Individuals with mild cognitive impairment and AD dementia exhibited higher TMAO levels in the cerebrospinal fluid compared to cognitively normal individuals.
2.4.4 Lipopolysaccharide
LPS, an endotoxin produced by Gram-negative bacteria, plays a multifaceted role in neurodegenerative pathology. Gorecki et al. (2019) found that PD patients had a significantly higher abundance of LPS-producing Gammaproteobacteria in the gut compared to healthy controls, and LPS reduces the expression and disrupts the distribution of intestinal epithelial tight junction markers (e.g., Zonula Occludens-1, e-Cadherin). A clinical study indicated that plasma LPS rose with cognitive decline, and in non-dementia participants, high plasma LPS was independently linked to mild cognitive impairment (Saji et al., 2022). LPS activates TLR4 receptors, triggering downstream Myeloid differentiation primary response protein 88 and TIR-domain-containing adapter-inducing interferon-β pathways. It also induces the release of pro-inflammatory cytokines (e.g., TNF-α, Interleukin-1 beta) from microglia and astrocytes, causes oxidative stress and mitochondrial dysfunction, promotes Aβ deposition, Tau hyperphosphorylation, and α-syn aggregation, and results in neuronal and synaptic damage, thus driving neurodegeneration (Batista et al., 2019; Kesika et al., 2021; Brown et al., 2023).
In summary, the metabolites of gut microbiota regulate organismal homeostasis through complex mechanisms, and their dysregulation may raise the risk of neurodegenerative diseases. Thus, targeting the generation or signaling pathways of these metabolites may provide potential therapeutic strategies for PD and other neurodegenerative conditions.
3 FMT treatment for PD
For PD, the gut microbiota has become a potential treatment focus. Restoring gut microbiota balance to delay or prevent neurodegeneration in PD represents a novel treatment strategy. Interventions that target the gut microbiota include antibiotics, probiotics, prebiotics, dietary modifications, and FMT. Among these, FMT has drawn a lot of interest as a novel and promising approach for treating PD (Varesi et al., 2022).
3.1 Definition and application of FMT
To restore gut microbiota diversity and function, FMT entails transplanting functional intestinal flora from healthy donors’ feces into the patients’ gastrointestinal tract. This procedure aims to increase beneficial bacteria, reduce harmful bacterial populations, and re-establish gut homeostasis, thereby mitigating disease progression. According to data from ClinicalTrials.gov, more than 400 FMT-related clinical trials have been registered worldwide, underscoring its growing prominence in medical research.
FMT can be administered through two primary methods: capsule transplantation and bacterial liquid transplantation. Bacterial liquid transplantation is further divided into three pathways: (1) the upper gastrointestinal tract route, using nasogastric or nasojejunal tubes or a gastroscope to introduce the transplant; (2) the colonoscope route, involving the insertion of a colonoscope to deliver fecal bacteria to the ileum; and (3) the enema route (König et al., 2016). Donor selection for FMT requires stringent screening criteria, including eight dimensions of assessments. Standardized effectiveness criteria for FMT are currently lacking.
Existing evidence demonstrates the short-term safety of FMT, with most adverse events being mild, self-limiting gastrointestinal symptoms such as abdominal discomfort, diarrhea, constipation, borborygmi, bloating, nausea, and vomiting. Serious adverse events are rare but warrant investigation to improve safety protocols. Notably, the US Food and Drug Administration has approved FMT for the treatment of Clostridium difficile infection, achieving cure rates of approximately 90% (Carlucci et al., 2016).
Beyond Clostridium difficile infection, FMT holds potential for a variety of diseases linked to gut microbiota dysbiosis, including ulcerative colitis, irritable bowel syndrome, sepsis, depression, type 2 diabetes, autism spectrum disorder, multiple sclerosis, PD, AD, epilepsy, Guillain-Barré syndrome, and amyotrophic lateral sclerosis (Li et al., 2019; Kim et al., 2020b; Kim et al., 2020a; Vendrik et al., 2020; Wang et al., 2020; Cui et al., 2021; Chen et al., 2023). This broad applicability underscores the promising future of FMT in both intestinal and systemic diseases linked to microbiota dysregulation.
3.2 FMT and PD
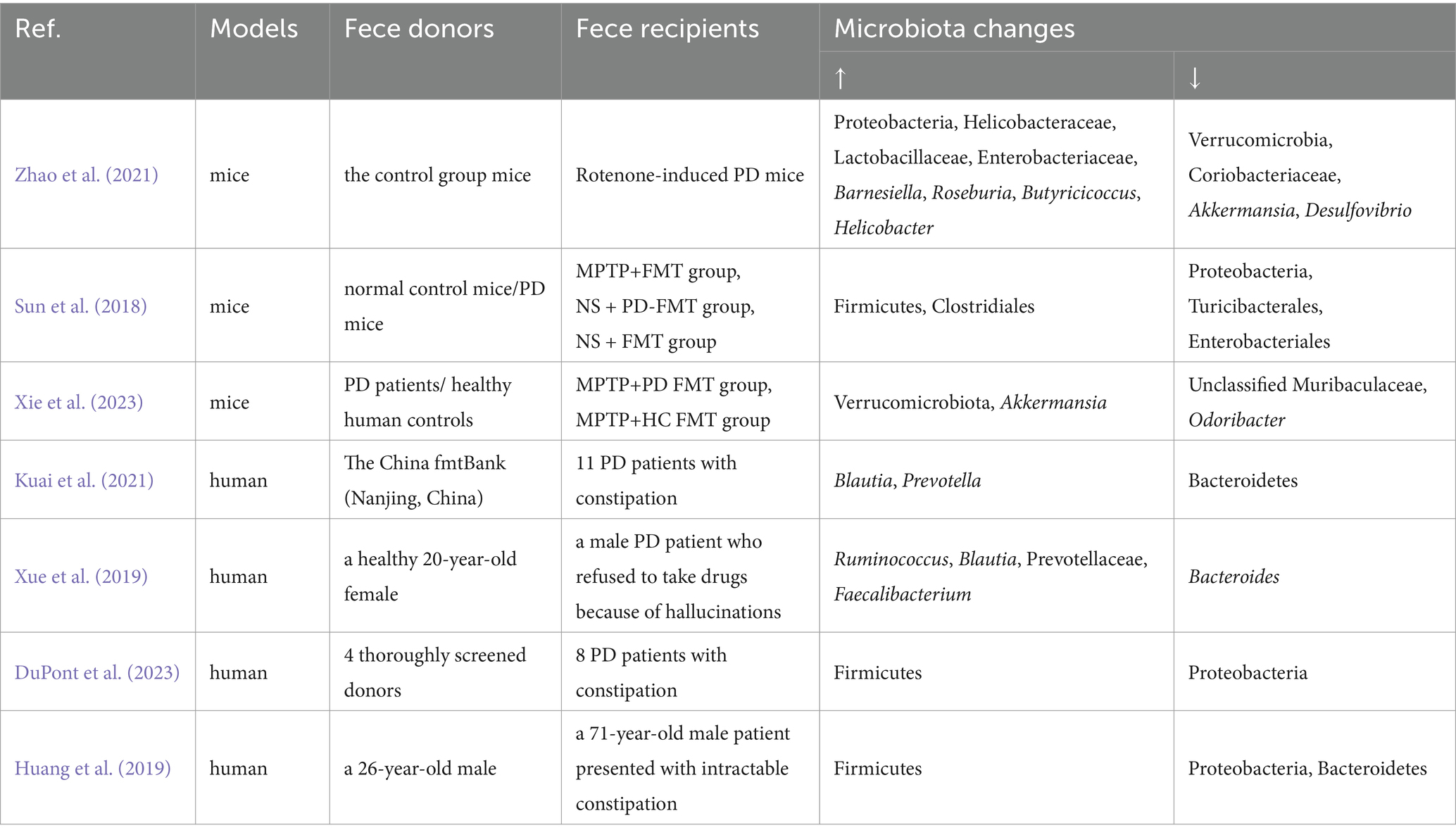
FMT has been investigated in preclinical and clinical research for PD. As these studies consistently demonstrate, FMT can effectively restore gut microbiota dysbiosis associated with PD (Table 2).
3.2.1 Preclinical studies
Preclinical evidence reveals several key mechanisms through which FMT improves gastrointestinal function, alleviates motor symptoms, and delays neurodegeneration in PD (Figure 2):
1. Reduction of Inflammatory Effects and Oxidative Stress: FMT relieves the neurotoxic effects of microglia and astrocytes, lowers LPS in the colon and SN, and reduces the secretion of pro-inflammatory cytokines while elevating anti-inflammatory factors. Moreover, FMT modulates inflammatory signaling pathways, including TLR4/TANK-binding kinase 1/NF-κB/TNF-α (Sun et al., 2018), TLR4/Phosphatidylinositol 3-kinase/Protein Kinase B/NF-κB (Zhong et al., 2021), and TLR4/Myeloid differentiation primary response protein 88/NF-κB (Zhao et al., 2021). In addition, Xie et al. (2023) confirmed that FMT activates the AMP-activated protein kinase/Superoxide dismutase 2 pathway, mitigating mitochondrial damage and enhancing mitochondrial antioxidative capacity. Studies have indicated that FMT reduced oxidative stress induced by 6-Hydroxydopamine in PD rat models, a known contributor to PD progression (Yu et al., 2023).
2. Reduction of α -syn Aggregation: Transplantation of fecal microbiota from PD patients into mice has been shown to promote microglial activation and α-syn aggregation by modulating metabolites such as SCFAs, which exacerbates motor dysfunction (Sampson et al., 2016). In PD mouse models, FMT has been reported to restore gut microbiota diversity, elevate SCFA levels (especially butyrate), and reduce pathological α-syn aggregation in both the ENS and SN, ultimately ameliorating motor dysfunction (Sun et al., 2018; Liang et al., 2023). Ni et al. (2025) demonstrated that FMT may regulate SCFA levels by upregulating SCFA receptors Free Fatty Acid Receptor 2 and Free Fatty Acid Receptor 3, thereby mitigating pathological features. Fang et al. (2024) found that rotenone-induced gut dysbiosis promotes α-syn transcription via activation of the CCAAT/Enhancer-Binding Protein Beta/Asparagine Endopeptidase pathway, while FMT alleviates this pathological damage.
3. Restoration of BBB Integrity: FMT has been demonstrated to enhance BBB integrity and mitigate dopaminergic neuronal damage, thereby exerting neuroprotective effects. Studies have revealed that compared to normal controls, germ-free mice and antibiotic-treated mice with gut microbiota depletion exhibit significantly increased BBB permeability. FMT can upregulate the expression of tight junction proteins in the CNS, including Zonula Occludens-1, Zonula Occludens-2, occludin, and claudin-5, thereby restoring BBB integrity and reducing its permeability (Braniste et al., 2014; Sun N. et al., 2021). In PD mouse models, Zhao et al. (2021) found that FMT treatment ameliorated the tight junction structure defects in the SN, alleviated endothelial cell damage, and significantly upregulated the Messenger RNA levels of tight junction proteins.
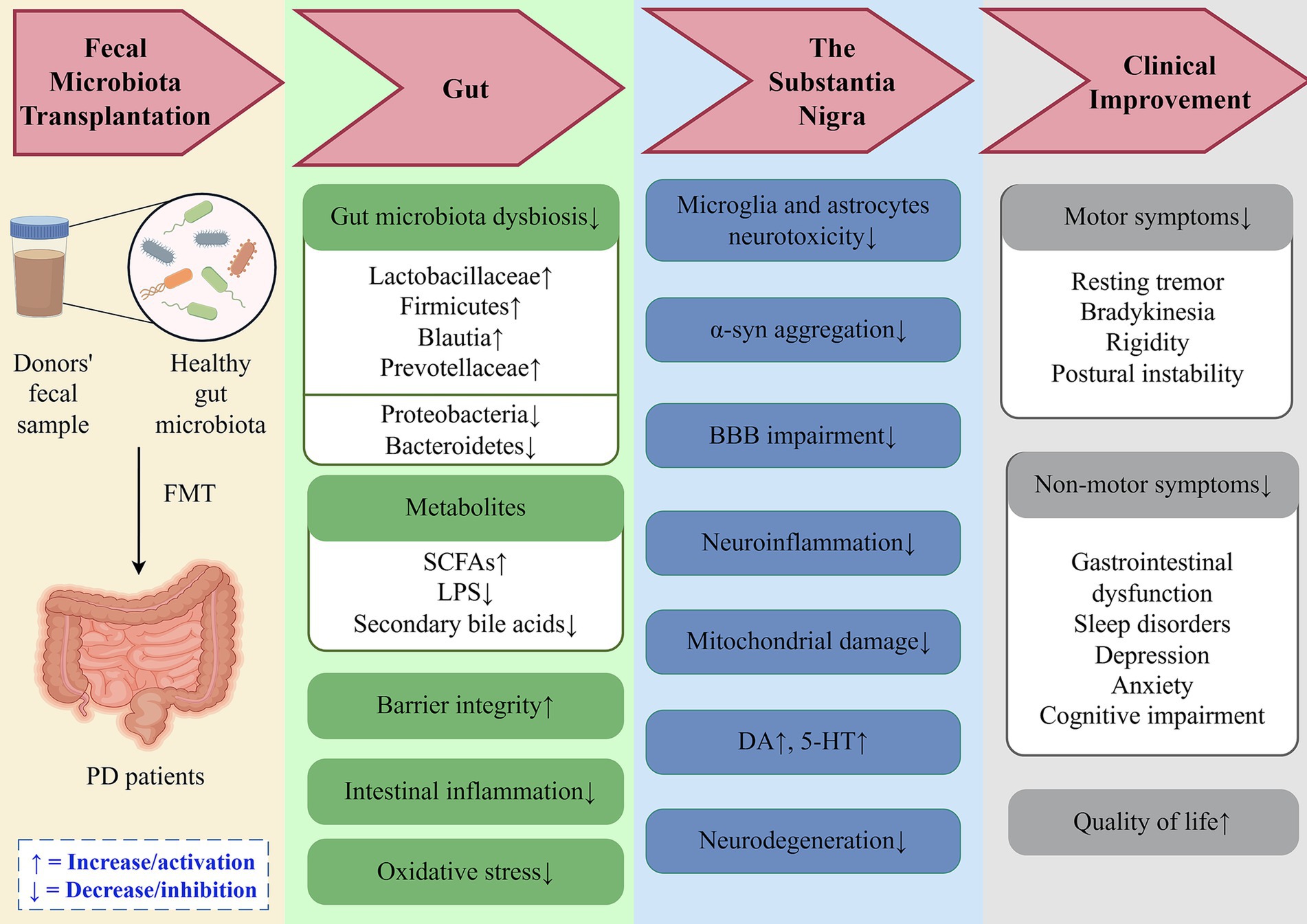
Figure 2. Potential mechanisms of fecal microbiota transplantation in PD treatment. FMT restores gut microbial homeostasis in PD patients by correcting dysbiosis, enhancing intestinal barrier integrity, and reducing oxidative stress and inflammatory responses. These effects mitigate neurodegeneration through modulation of the gut-brain axis, ultimately improving clinical manifestations and quality of life. DA, Dopamine; 5-HT, 5-Hydroxytryptamine; α-syn, α-synuclein; BBB, Blood–Brain Barrier; SCFAs, Short-Chain Fatty Acids; LPS, Lipopolysaccharide.
3.2.2 Clinical investigations
Clinical trials involving FMT in PD patients further support these preclinical findings. Fecal samples collected before and after FMT have undergone microbiota sequencing, revealing significant restoration of gut microbiota composition. Symptom assessments using scales such as the Unified Parkinson’s Disease Rating Scale, Non-Motor Symptoms Scale, and Parkinson’s Disease Questionnaire-39 indicate improvements in motor symptoms, constipation, anxiety, depression, sleep, and cognitive function, all of which improve overall quality of life. Furthermore, adverse events are less common and generally self-limiting (Xue et al., 2019; Kuai et al., 2021; Segal et al., 2021; Cheng et al., 2023; DuPont et al., 2023; Liu et al., 2023).
However, FMT’s therapeutic effects appear time-dependent. Research indicates that microbiota composition and related motor and non-motor symptoms, except constipation, may partially revert after a certain period post-transplantation (Huang et al., 2019; Xue et al., 2019). Further investigation is needed to determine long-term efficacy and stability. Additionally, transplantation methods may impact therapeutic outcomes. For instance, Xue et al. (2020) compared colonoscope-administered FMT with nasojejunal tube administration and found that the former yielded superior clinical benefits.
3.2.3 Single-strain microbiota transplantation
Recent studies highlight the promising potential of single-strain microbiota transplantation in PD treatment through modulation of the MGBA. Lactobacillus plantarum PS128, a probiotic strain, has been shown to alleviate motor deficits in PD mice through multi-target mechanisms. Specifically, PS128 restores gut microbiota homeostasis, diminishes neuroinflammation via the microRNA-155-5p/Suppressor of Cytokine Signaling 1 pathway, inhibits the neurotoxic activation of microglia and astrocytes, alleviates oxidative stress damage, protects dopaminergic neurons, and ultimately mitigates neurodegeneration (Liao et al., 2020; Lee et al., 2023). Clinical trials further support its therapeutic efficacy, with PS128 supplementation demonstrating significant improvements in motor symptoms and quality of life in PD patients (Lu et al., 2021).
Additionally, other microbial strains have shown promise in the treatment of PD. For example, Bifidobacterium breve (CCFM1067, Bif11) and Lacticaseibacillus rhamnosus E9 demonstrate neuroprotective effects in PD mouse models by enhancing intestinal barrier integrity and alleviating pathological progression (Li T. et al., 2022; Aktas et al., 2024; Valvaikar et al., 2024). Oral administration of Clostridium butyricum has been shown to restore colonic Glucagon-Like Peptide-1 (GLP-1) and G Protein-Coupled Receptor 41/43 levels, along with cerebral GLP-1 Receptor expression in PD mice, thereby mediating neuroprotection via the GLP-1/GLP-1 Receptor pathway (Sun J. et al., 2021).
These findings collectively emphasize the therapeutic potential of single-strain microbiota transplantation in mitigating PD progression. Future studies should aim to elucidate the molecular mechanisms underlying single-strain interventions and validate the long-term safety and therapeutic efficacy through rigorous clinical trials.
4 Discussion
In summary, the bidirectional regulation and communication through the MGBA provide an innovative framework for investigating the pathological processes underlying PD. There is much evidence now available linking gut microbiota dysbiosis, its metabolites, and PD initiation and progression. While characteristic alterations in the composition have been shown in PD, inconsistencies across studies suggest that a consensus on specific microbial alterations has yet to be reached. Nevertheless, FMT has demonstrated potential in alleviating clinical symptoms and delaying PD progression.
Despite the fact that several research studies have verified the short-term effectiveness and safety of FMT, the field remains in its early stages with limited clinical trials. Most existing research comprises cross-sectional comparisons between PD patients and healthy people, with limited follow-up investigations tracking long-term outcomes. The long-term safety and sustained effectiveness thus require further exploration.
In PD, the gut microbiota represents an emerging potential therapeutic target. However, to fully assess the clinical utility, future research should focus on (1) elucidating the molecular mechanisms underlying gut dysbiosis in PD; (2) conducting rigorous, high-quality clinical trials to validate the efficacy and safety of FMT; and (3) optimizing FMT protocols by determining the optimal transplantation routes, dosing regimens, and administration frequencies. The development of standardized treatment guidelines would facilitate the responsible translation of FMT into clinical practice. Provided that ongoing research continues to demonstrate both safety and efficacy, FMT may potentially emerge as an adjunctive approach in PD management.
Author contributions
MG: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. HG: Writing – original draft. YW: Funding acquisition, Supervision, Writing – review & editing. YX: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is supported by the Youth Science Foundation of Shandong First Medical University (No. 202201-068) and Postdoctoral Innovation Project of Shandong Province (SDCX-ZG-202303095).
Acknowledgments
The authors are grateful to all subjects for participation in our study. Figure 1 (export ID: YWSAS475b5), Figure 2 (export ID: YTAPT1d1b1) were created by Figdraw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aho, V. T. E., Houser, M. C., Pereira, P. A. B., Chang, J., Rudi, K., Paulin, L., et al. (2021). Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 16:6. doi: 10.1186/s13024-021-00427-6
Aktas, B., Aslim, B., and Ozdemir, D. A. (2024). A neurotherapeutic approach with Lacticaseibacillus rhamnosus E9 on gut microbiota and intestinal barrier in MPTP-induced mouse model of Parkinson’s disease. Sci. Rep. 14:15460. doi: 10.1038/s41598-024-65061-w
Armstrong, M. J., and Okun, M. S. (2020). Diagnosis and treatment of Parkinson disease. JAMA 323:548. doi: 10.1001/jama.2019.22360
Barichella, M., Severgnini, M., Cilia, R., Cassani, E., Bolliri, C., Caronni, S., et al. (2018). Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 34, 396–405. doi: 10.1002/mds.27581
Batista, C. R. A., Gomes, G. F., Candelario-Jalil, E., Fiebich, B. L., and de Oliveira, A. C. P. (2019). Lipopolysaccharide-induced Neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 20:2293. doi: 10.3390/ijms20092293
Bhattarai, Y., Si, J., Pu, M., Ross, O. A., McLean, P. J., Till, L., et al. (2021). Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson’s disease. Gut Microbes 13:1866974. doi: 10.1080/19490976.2020.1866974
Bisaglia, M. (2022). Mediterranean diet and Parkinson’s disease. Int. J. Mol. Sci. 24:42. doi: 10.3390/ijms24010042
Braak, H., de Vos, R. A. I., Bohl, J., and Del Tredici, K. (2006). Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci. Lett. 396, 67–72. doi: 10.1016/j.neulet.2005.11.012
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Tóth, M., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6:263ra158. doi: 10.1126/scitranslmed.3009759
Brown, G. C., Camacho, M., and Williams-Gray, C. H. (2023). The endotoxin hypothesis of Parkinson’s disease. Mov. Disord. 38, 1143–1155. doi: 10.1002/mds.29432
Brudek, T., and van Laar, T. (2019). Inflammatory bowel diseases and Parkinson’s disease. J. Parkinsons Dis. 9, S331–S344. doi: 10.3233/jpd-191729
Bullich, C., Keshavarzian, A., Garssen, J., Kraneveld, A., and Perez-Pardo, P. (2019). Gut vibes in Parkinson’s disease: the microbiota-gut-brain Axis. Movement Disor. Clin. Prac. 6, 639–651. doi: 10.1002/mdc3.12840
Caradonna, E., Abate, F., Schiano, E., Paparella, F., Ferrara, F., Vanoli, E., et al. (2025). Trimethylamine-N-oxide (TMAO) as a rising-star metabolite: implications for human health. Meta 15:220. doi: 10.3390/metabo15040220
Caradonna, E., Nemni, R., Bifone, A., Gandolfo, P., Costantino, L., Giordano, L., et al. (2024). The brain–gut axis, an important player in Alzheimer and Parkinson disease: a narrative review. J. Clin. Med. 13:4130. doi: 10.3390/jcm13144130
Carlucci, C., Petrof, E. O., and Allen-Vercoe, E. (2016). Fecal microbiota-based therapeutics for recurrent Clostridium difficile infection, ulcerative colitis and obesity. EBioMedicine 13, 37–45. doi: 10.1016/j.ebiom.2016.09.029
Castro-Caldas, M., Carvalho, A. N., Rodrigues, E., Henderson, C. J., Wolf, C. R., Rodrigues, C. M. P., et al. (2012). Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Mol. Neurobiol. 46, 475–486. doi: 10.1007/s12035-012-8295-4
Chen, S.-J., Chen, C.-C., Liao, H.-Y., Lin, Y.-T., Wu, Y.-W., Liou, J.-M., et al. (2022). Association of Fecal and Plasma Levels of short-chain fatty acids with gut microbiota and clinical severity in patients with Parkinson disease. Neurology 98, e848–e858. doi: 10.1212/wnl.0000000000013225
Chen, Q., Fan, Y., Zhang, B., Yan, C., Chen, Z., Wang, L., et al. (2023). Specific fungi associated with response to capsulized fecal microbiota transplantation in patients with active ulcerative colitis. Front. Cell. Infect. Microbiol. 12:1086885. doi: 10.3389/fcimb.2022.1086885
Cheng, Y., Tan, G., Zhu, Q., Wang, C., Ruan, G., Ying, S., et al. (2023). Efficacy of fecal microbiota transplantation in patients with Parkinson’s disease: clinical trial results from a randomized, placebo-controlled design. Gut Microbes 15:2284247. doi: 10.1080/19490976.2023.2284247
Cilia, R., Piatti, M., Cereda, E., Bolliri, C., Caronni, S., Ferri, V., et al. (2021). Does gut microbiota influence the course of Parkinson’s disease? A 3-year prospective exploratory study in de novo patients. J. Parkinsons Dis. 11, 159–170. doi: 10.3233/jpd-202297
Cirstea, M. S., Yu, A. C., Golz, E., Sundvick, K., Kliger, D., Radisavljevic, N., et al. (2020). Microbiota composition and metabolism are associated with gut function in Parkinson’s disease. Mov. Disord. 35, 1208–1217. doi: 10.1002/mds.28052
Costa, H. N., Esteves, A. R., Empadinhas, N., and Cardoso, S. M. (2022). Parkinson’s disease: a multisystem disorder. Neurosci. Bull. 39, 113–124. doi: 10.1007/s12264-022-00934-6
Cryan, J. F., and Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712. doi: 10.1038/nrn3346
Cui, J., Lin, Z., Tian, H., Yang, B., Zhao, D., Ye, C., et al. (2021). Long-term follow-up results of fecal microbiota transplantation for irritable bowel syndrome: a single-center, retrospective study. Front. Med. 8:710452. doi: 10.3389/fmed.2021.710452
Ding, M., Lang, Y., Shu, H., Shao, J., and Cui, L. (2021). Microbiota–gut–brain Axis and epilepsy: a review on mechanisms and potential therapeutics. Front. Immunol. 12:742449. doi: 10.3389/fimmu.2021.742449
Ding, Y., Xin, C., Zhang, C.-W., Lim, K.-L., Zhang, H., Fu, Z., et al. (2018). Natural molecules from Chinese herbs protecting against Parkinson’s disease via anti-oxidative stress. Front. Aging Neurosci. 10:246. doi: 10.3389/fnagi.2018.00246
Dorsey, E. R., and Bloem, B. R. (2018). The Parkinson pandemic—a call to action. JAMA Neurol. 75, 9–10. doi: 10.1001/jamaneurol.2017.3299
Duan, W.-X., Wang, F., Liu, J.-Y., and Liu, C.-F. (2023). Relationship between short-chain fatty acids and Parkinson’s disease: a review from pathology to clinic. Neurosci. Bull. 40, 500–516. doi: 10.1007/s12264-023-01123-9
DuPont, H. L., Suescun, J., Jiang, Z.-D., Brown, E. L., Essigmann, H. T., Alexander, A. S., et al. (2023). Fecal microbiota transplantation in Parkinson’s disease—a randomized repeat-dose, placebo-controlled clinical pilot study. Front. Neurol. 14:1104759. doi: 10.3389/fneur.2023.1104759
Fang, X., Liu, S., Muhammad, B., Zheng, M., Ge, X., Xu, Y., et al. (2024). Gut microbiota dysbiosis contributes to α-synuclein-related pathology associated with C/EBPβ/AEP signaling activation in a mouse model of Parkinson's disease. Neural Regen. Res. 19, 2081–2088. doi: 10.4103/1673-5374.391191
Gao, X., Fu, S., Wen, J., Yan, A., Yang, S., Zhang, Y., et al. (2024). Orally administered Ginkgolide C alleviates MPTP-induced neurodegeneration by suppressing Neuroinflammation and oxidative stress through microbiota-gut-brain Axis in mice. J. Agric. Food Chem. 72, 22115–22131. doi: 10.1021/acs.jafc.4c03783
Gao, C., Li, B., He, Y., Huang, P., Du, J., He, G., et al. (2023). Early changes of fecal short-chain fatty acid levels in patients with mild cognitive impairments. CNS Neurosci. Ther. 29, 3657–3666. doi: 10.1111/cns.14252
Gao, K., Pi, Y., Mu, C. L., Peng, Y., Huang, Z., and Zhu, W. Y. (2018). Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J. Neurochem. 146, 219–234. doi: 10.1111/jnc.14333
Gorecki, A. M., Preskey, L., Bakeberg, M. C., Kenna, J. E., Gildenhuys, C., MacDougall, G., et al. (2019). Altered gut microbiome in Parkinson’s disease and the influence of lipopolysaccharide in a human α-Synuclein over-expressing mouse model. Front. Neurosci. 13:839. doi: 10.3389/fnins.2019.00839
Hou, Y., Li, X., Liu, C., Zhang, M., Zhang, X., Ge, S., et al. (2021). Neuroprotective effects of short-chain fatty acids in MPTP induced mice model of Parkinson’s disease. Exp. Gerontol. 150:111376. doi: 10.1016/j.exger.2021.111376
Hou, Y. F., Shan, C., Zhuang, S.-y., Zhuang, Q.-q., Ghosh, A., Zhu, K.-c., et al. (2021). Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease. Microbiome 9:34. doi: 10.1186/s40168-020-00988-6
Huang, H., Xu, H., Luo, Q., He, J., Li, M., Chen, H., et al. (2019). Fecal microbiota transplantation to treat Parkinson’s disease with constipation. Medicine 98:e16163. doi: 10.1097/md.0000000000016163
Huh, E., Choi, J. G., Choi, Y., Ju, I. G., Kim, B., Shin, Y.-J., et al. (2023). P. mirabilis-derived pore-forming haemolysin, HpmA drives intestinal alpha-synuclein aggregation in a mouse model of neurodegeneration. EBioMedicine 98:104887. doi: 10.1016/j.ebiom.2023.104887
Keshavarzian, A., Green, S. J., Engen, P. A., Voigt, R. M., Naqib, A., Forsyth, C. B., et al. (2015). Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 30, 1351–1360. doi: 10.1002/mds.26307
Kesika, P., Suganthy, N., Sivamaruthi, B. S., and Chaiyasut, C. (2021). Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 264:118627. doi: 10.1016/j.lfs.2020.118627
Kim, S. M., DeFazio, J. R., Hyoju, S. K., Sangani, K., Keskey, R., Krezalek, M. A., et al. (2020a). Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat. Commun. 11:2354. doi: 10.1038/s41467-020-15545-w
Kim, S. M., Kim, Y., Choi, H., Kim, W., Park, S., Lee, D., et al. (2020b). Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 69, 283–294. doi: 10.1136/gutjnl-2018-317431
Kim, S., Kwon, S.-H., Kam, T.-I., Panicker, N., Karuppagounder, S. S., Lee, S., et al. (2019). Transneuronal propagation of pathologic α-Synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641.e7. doi: 10.1016/j.neuron.2019.05.035
Kiriyama, Y., and Nochi, H. (2023). Role of microbiota-modified bile acids in the regulation of intracellular organelles and neurodegenerative diseases. Gene 14:825. doi: 10.3390/genes14040825
König, J., Siebenhaar, A., Högenauer, C., Arkkila, P., Nieuwdorp, M., Norén, T., et al. (2016). Consensus report: faecal microbiota transfer – clinical applications and procedures. Aliment. Pharmacol. Ther. 45, 222–239. doi: 10.1111/apt.13868
Kuai, X., Yao, X., Xu, L., Zhou, Y., Zhang, L., Liu, Y., et al. (2021). Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb. Cell Factories 20:98. doi: 10.1186/s12934-021-01589-0
Kujawa, D., Laczmanski, L., Budrewicz, S., Pokryszko-Dragan, A., and Podbielska, M. (2023). Targeting gut microbiota: new therapeutic opportunities in multiple sclerosis. Gut Microbes 15:2274126. doi: 10.1080/19490976.2023.2274126
Lajoie, A. C., Lafontaine, A.-L., and Kaminska, M. (2021). The Spectrum of sleep disorders in Parkinson disease. Chest 159, 818–827. doi: 10.1016/j.chest.2020.09.099
Lee, Y. Z., Cheng, S.-H., Chang, M.-Y., Lin, Y.-F., Wu, C.-C., and Tsai, Y.-C. (2023). Neuroprotective effects of Lactobacillus plantarum PS128 in a mouse model of Parkinson’s disease: the role of gut microbiota and MicroRNAs. Int. J. Mol. Sci. 24:6794. doi: 10.3390/ijms24076794
Lee, Y., Kang, J. S., Ham, O.-J., Son, M.-Y., and Lee, M.-O. (2022). Gut metabolite trimethylamine N-oxide induces aging-associated phenotype of midbrain organoids for the induced pluripotent stem cell-based modeling of late-onset disease. Front. Aging Neurosci. 14:925227. doi: 10.3389/fnagi.2022.925227
Li, T., Chu, C., Yu, L., Zhai, Q., Wang, S., Zhao, J., et al. (2022). Neuroprotective effects of Bifidobacterium breve CCFM1067 in MPTP-induced mouse models of Parkinson’s disease. Nutrients 14:4678. doi: 10.3390/nu14214678
Li, P., Killinger, B. A., Ensink, E., Beddows, I., Yilmaz, A., Lubben, N., et al. (2021). Gut microbiota Dysbiosis is associated with elevated bile acids in Parkinson’s disease. Meta 11:29. doi: 10.3390/metabo11010029
Li, Z., Liang, H., Hu, Y., Lu, L., Zheng, C., Fan, Y., et al. (2022). Gut bacterial profiles in Parkinson’s disease: a systematic review. CNS Neurosci. Ther. 29, 140–157. doi: 10.1111/cns.13990
Li, N., Tian, H., Chen, Q., Yang, B., Ma, C., Lin, Z., et al. (2019). Efficacy analysis of fecal microbiota transplantation in the treatment of 2010 patients with intestinal disorders. Chin. J. Gastrointest. Surg. 22, 861–868. doi: 10.3760/cma.j.issn.1671⁃0274.2019.09.011
Li, W., Wu, X., Hu, X., Wang, T., Liang, S., Duan, Y., et al. (2017). Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 60, 1223–1233. doi: 10.1007/s11427-016-9001-4
Liang, D., Liu, H., Jin, R., Feng, R., Wang, J., Qin, C., et al. (2023). Escherichia coli triggers α-synuclein pathology in the LRRK2 transgenic mouse model of PD. Gut Microbes 15:2276296. doi: 10.1080/19490976.2023.2276296
Liao, J.-F., Cheng, Y.-F., You, S.-T., Kuo, W.-C., Huang, C.-W., Chiou, J.-J., et al. (2020). Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models of Parkinson’s disease. Brain Behav. Immun. 90, 26–46. doi: 10.1016/j.bbi.2020.07.036
Lin, C.-H., Chen, C.-C., Chiang, H.-L., Liou, J.-M., Chang, C.-M., Lu, T.-P., et al. (2019). Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation 16:129. doi: 10.1186/s12974-019-1528-y
Liu, J., Chen, Q., Yang, B., Wang, L., Lv, X., Li, N., et al. (2023). Fecal microbiota transplantation for constipation in patients with Parkinson disease: clinical efficacy observation. Chinese J. Integrated Trad. Western Med. Digestion 31, 874–878. doi: 10.3969/j.issn.1671-038X.2023.11.10
Lo, A. C., Callaerts-Vegh, Z., Nunes, A. F., Rodrigues, C. M. P., and D'Hooge, R. (2013). Tauroursodeoxycholic acid (TUDCA) supplementation prevents cognitive impairment and amyloid deposition in APP/PS1 mice. Neurobiol. Dis. 50, 21–29. doi: 10.1016/j.nbd.2012.09.003
Loh, J. S., Mak, W. Q., Tan, L. K. S., Ng, C. X., Chan, H. H., Yeow, S. H., et al. (2024). Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 9:37. doi: 10.1038/s41392-024-01743-1
Lu, C.-S., Chang, H.-C., Weng, Y.-H., Chen, C.-C., Kuo, Y.-S., and Tsai, Y.-C. (2021). The add-on effect of Lactobacillus plantarum PS128 in patients with Parkinson’s disease: a pilot study. Front. Nutr. 8:650053. doi: 10.3389/fnut.2021.650053
Lubomski, M., Tan, A. H., Lim, S.-Y., Holmes, A. J., Davis, R. L., and Sue, C. M. (2019). Parkinson’s disease and the gastrointestinal microbiome. J. Neurol. 267, 2507–2523. doi: 10.1007/s00415-019-09320-1
Malpartida, A. B., Williamson, M., Narendra, D. P., Wade-Martins, R., and Ryan, B. J. (2021). Mitochondrial dysfunction and Mitophagy in Parkinson’s disease: from mechanism to therapy. Trends Biochem. Sci. 46, 329–343. doi: 10.1016/j.tibs.2020.11.007
Mao, L., Zhang, Y., Tian, J., Sang, M., Zhang, G., Zhou, Y., et al. (2021). Cross-sectional study on the gut microbiome of Parkinson’s disease patients in Central China. Front. Microbiol. 12:728479. doi: 10.3389/fmicb.2021.728479
Marogianni, C., Sokratous, M., Dardiotis, E., Hadjigeorgiou, G. M., Bogdanos, D., and Xiromerisiou, G. (2020). Neurodegeneration and inflammation—An interesting interplay in Parkinson’s disease. Int. J. Mol. Sci. 21:8421. doi: 10.3390/ijms21228421
Matheson, J.-A. T., and Holsinger, R. M. D. (2023). The role of fecal microbiota transplantation in the treatment of neurodegenerative diseases: a review. Int. J. Mol. Sci. 24:1001. doi: 10.3390/ijms24021001
Mou, Y., Du, Y., Zhou, L., Yue, J., Hu, X., Liu, Y., et al. (2022). Gut microbiota interact with the brain through systemic chronic inflammation: implications on Neuroinflammation, neurodegeneration, and aging. Front. Immunol. 13:796288. doi: 10.3389/fimmu.2022.796288
Munteanu, C., Onose, G., Rotariu, M., Postaru, M., Turnea, M., and Galaction, A. I. (2024). Role of microbiota-derived hydrogen sulfide (H₂S) in modulating the gut-brain axis: implications for Alzheimer’s and Parkinson’s disease pathogenesis. Biomedicine 12:2670. doi: 10.3390/biomedicines12122670
Ni, Y., Tong, Q., Xu, M., Gu, J., and Ye, H. (2025). Gut microbiota-induced modulation of the central nervous system function in Parkinson’s disease through the gut-brain Axis and short-chain fatty acids. Mol. Neurobiol. 62, 2480–2492. doi: 10.1007/s12035-024-04370-7
Nishiwaki, H., Hamaguchi, T., Ito, M., Ishida, T., Maeda, T., Kashihara, K., et al. (2020a). Short-chain fatty acid-producing gut microbiota is decreased in Parkinson’s disease but not in rapid-eye-movement sleep behavior disorder. mSystems 5, 00797–00720. doi: 10.1128/mSystems.00797-20
Nishiwaki, H., Ito, M., Hamaguchi, T., Maeda, T., Kashihara, K., Tsuboi, Y., et al. (2022). Short chain fatty acids-producing and mucin-degrading intestinal bacteria predict the progression of early Parkinson’s disease. NPJ Parkinsons Dis. 8:65. doi: 10.1038/s41531-022-00328-5
Nishiwaki, H., Ito, M., Ishida, T., Hamaguchi, T., Maeda, T., Kashihara, K., et al. (2020b). Meta-analysis of gut dysbiosis in Parkinson’s disease. Mov. Disord. 35, 1626–1635. doi: 10.1002/mds.28119
Nurrahma, B. A., Yeh, T.-H., Hsieh, R.-H., Tsao, S.-P., Chen, C.-W., Lee, Y.-P., et al. (2022). Mangosteen pericarp extract supplementation boosts antioxidant status via rebuilding gut microbiota to attenuate motor deficit in 6-OHDA-induced Parkinson’s disease. Antioxidants 11:2396. doi: 10.3390/antiox11122396
Qi, S., Yin, P., Wang, L., Qu, M., Kan, G. L., Zhang, H., et al. (2021). Prevalence of Parkinson’s disease: a community-based study in China. Mov. Disord. 36, 2940–2944. doi: 10.1002/mds.28762
Rong, S., Xu, G., Liu, B., Sun, Y., Snetselaar, L. G., Wallace, R. B., et al. (2021). Trends in mortality from Parkinson disease in the United States, 1999–2019. Neurology 97, e1986–e1993. doi: 10.1212/wnl.0000000000012826
Saji, N., Saito, Y., Yamashita, T., Murotani, K., Tsuduki, T., Hisada, T., et al. (2022). Relationship between plasma lipopolysaccharides, gut microbiota, and dementia: a cross-sectional study. J. Alzheimers Dis. 86, 1947–1957. doi: 10.3233/jad-215653
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and Neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12. doi: 10.1016/j.cell.2016.11.018
Scheperjans, F., Aho, V., Pereira, P. A. B., Koskinen, K., Paulin, L., Pekkonen, E., et al. (2014). Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 30, 350–358. doi: 10.1002/mds.26069
Segal, A., Zlotnik, Y., Moyal-Atias, K., Abuhasira, R., and Ifergane, G. (2021). Fecal microbiota transplant as a potential treatment for Parkinson’s disease – a case series. Clin. Neurol. Neurosurg. 207:106791. doi: 10.1016/j.clineuro.2021.106791
Shandilya, S., Kumar, S., Kumar Jha, N., Kumar Kesari, K., and Ruokolainen, J. (2022). Interplay of gut microbiota and oxidative stress: perspective on neurodegeneration and neuroprotection. J. Adv. Res. 38, 223–244. doi: 10.1016/j.jare.2021.09.005
Sharma, V. D., Patel, M., and Miocinovic, S. (2020). Surgical treatment of Parkinson’s disease: devices and lesion approaches. Neurotherapeutics 17, 1525–1538. doi: 10.1007/s13311-020-00939-x
Solanki, R., Karande, A., and Ranganathan, P. (2023). Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front. Neurol. 14:1149618. doi: 10.3389/fneur.2023.1149618
Sorboni, S. G., Moghaddam, H. S., Jafarzadeh-Esfehani, R., and Soleimanpour, S. (2022). A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin. Microbiol. Rev. 35:e0033820. doi: 10.1128/cmr.00338-20
Sterling, K. G., Dodd, G. K., Alhamdi, S., Asimenios, P. G., Dagda, R. K., De Meirleir, K. L., et al. (2022). Mucosal immunity and the gut-microbiota-brain-Axis in Neuroimmune disease. Int. J. Mol. Sci. 23:13328. doi: 10.3390/ijms232113328
Strandwitz, P. (2018). Neurotransmitter modulation by the gut microbiota. Brain Res. 1693, 128–133. doi: 10.1016/j.brainres.2018.03.015
Suarez-Cedeno, G., Suescun, J., and Schiess, M. C. (2017). Earlier intervention with deep brain stimulation for Parkinson’s disease. Parkinson's Dis. 2017, 1–9. doi: 10.1155/2017/9358153
Sun, N., Hu, H., Wang, F., Li, L., Zhu, W., Shen, Y., et al. (2021). Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav. Immun. 92, 102–114. doi: 10.1016/j.bbi.2020.11.032
Sun, J., Li, H., Jin, Y., Yu, J., Mao, S., Su, K.-P., et al. (2021). Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav. Immun. 91, 703–715. doi: 10.1016/j.bbi.2020.10.014
Sun, X., Xue, L., Wang, Z., and Xie, A. (2022). Update to the treatment of Parkinson’s disease based on the gut-brain Axis mechanism. Front. Neurosci. 16:878239. doi: 10.3389/fnins.2022.878239
Sun, M., Zhu, Y., Zhou, Z., Jia, X., Xu, Y., Yang, Q., et al. (2018). Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 70, 48–60. doi: 10.1016/j.bbi.2018.02.005
Tang, H.-d., Chen, S.-d., Liu, X.-l., Xie, R.-y., and Qian, X.-h. (2022). Mechanisms of short-chain fatty acids derived from gut microbiota in Alzheimer’s disease. Aging Dis. 13, 1252–1266. doi: 10.14336/ad.2021.1215
The Lancet (2022). Parkinson’s disease needs an urgent public health response. Lancet Neurol. 21:759. doi: 10.1016/s1474-4422(22)00312-x
Tofaris, G. K. (2022). Initiation and progression of α-synuclein pathology in Parkinson’s disease. Cell. Mol. Life Sci. 79:210. doi: 10.1007/s00018-022-04240-2
Valvaikar, S., Vaidya, B., Sharma, S., Bishnoi, M., Kondepudi, K. K., and Sharma, S. S. (2024). Supplementation of probiotic Bifidobacterium breve Bif11 reverses neurobehavioural deficits, inflammatory changes and oxidative stress in Parkinson’s disease model. Neurochem. Int. 174:105691. doi: 10.1016/j.neuint.2024.105691
van Kessel, S. P., Frye, A. K., El-Gendy, A. O., Castejon, M., Keshavarzian, A., van Dijk, G., et al. (2019). Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 10:310. doi: 10.1038/s41467-019-08294-y
Varesi, A., Campagnoli, L. I. M., Fahmideh, F., Pierella, E., Romeo, M., Ricevuti, G., et al. (2022). The interplay between gut microbiota and Parkinson’s disease: implications on diagnosis and treatment. Int. J. Mol. Sci. 23:12289. doi: 10.3390/ijms232012289
Vendrik, K. E. W., Ooijevaar, R. E., de Jong, P. R. C., Laman, J. D., van Oosten, B. W., van Hilten, J. J., et al. (2020). Fecal microbiota transplantation in neurological disorders. Front. Cell. Infect. Microbiol. 10:98. doi: 10.3389/fcimb.2020.00098
Vogt, N. M., Romano, K. A., Darst, B. F., Engelman, C. D., Johnson, S. C., Carlsson, C. M., et al. (2018). The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res. Ther. 10:124. doi: 10.1186/s13195-018-0451-2
Voigt, R. M., Wang, Z., Brown, J. M., Engen, P. A., Naqib, A., Goetz, C. G., et al. (2022). Gut microbial metabolites in Parkinson’s disease: association with lifestyle, disease characteristics, and treatment status. Neurobiol. Dis. 170:105780. doi: 10.1016/j.nbd.2022.105780
Wang, Y., Peng, L., Song, J., Bai, Y., Sun, Y., and Wang, F. (2020). The effects of fecal microbiota transplantation on chronic unpredictable mild stress induced depression model rats. Chin. J. Clin. Psychol. 28, 1083–1093. doi: 10.16128/j.cnki.1005-3611.2020.06.002
Wang, Y., Tong, Q., Ma, S.-R., Zhao, Z.-X., Pan, L.-B., Cong, L., et al. (2021). Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 6:77. doi: 10.1038/s41392-020-00456-5
Wang, L., Zhao, Z., Zhao, L., Zhao, Y., Yang, G., Wang, C., et al. (2022). Lactobacillus plantarum DP189 reduces α-SYN aggravation in MPTP-induced Parkinson’s disease mice via regulating oxidative damage, inflammation, and gut microbiota disorder. J. Agric. Food Chem. 70, 1163–1173. doi: 10.1021/acs.jafc.1c07711
Weis, S., Schwiertz, A., Unger, M. M., Becker, A., Faßbender, K., Ratering, S., et al. (2019). Effect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota. NPJ Parkinsons Dis. 5:28. doi: 10.1038/s41531-019-0100-x
Wu, J., Li, C.-S., Huang, W.-Y., Zhou, S.-Y., Zhao, L.-P., Li, T., et al. (2025). Gut microbiota promote the propagation of pathologic α-syn from gut to brain in a gut-originated mouse model of Parkinson’s disease. Brain Behav. Immun. 128, 152–169. doi: 10.1016/j.bbi.2025.04.001
Xie, Z., Zhang, M., Luo, Y., Jin, D., Guo, X., Yang, W., et al. (2023). Healthy human fecal microbiota transplantation into mice attenuates MPTP-induced neurotoxicity via AMPK/SOD2 pathway. Aging Dis. 14, 2193–2214. doi: 10.14336/ad.2023.0309
Xue, L., Ou, Z., Wang, L., Wei, M., Yang, X., Zheng, J., et al. (2019). A case analyse of Parkinson’s disease treated with fecal microbiota transplantation instead of dopaminergic drugs. J. Clin. Neurol. 32, 329–332. doi: 10.3969/j.issn.1004-1648.2019.05.006
Xue, L., Yang, X., Tong, Q., Shen, P., Ma, S., Wu, S., et al. (2020). Fecal microbiota transplantation therapy for Parkinson’s disease. Medicine 99:e22035. doi: 10.1097/md.0000000000022035
Yu, J., Meng, J., Qin, Z., Yu, Y., Liang, Y., Wang, Y., et al. (2023). Dysbiosis of gut microbiota inhibits NMNAT2 to promote neurobehavioral deficits and oxidative stress response in the 6-OHDA-lesioned rat model of Parkinson’s disease. J. Neuroinflammation 20:117. doi: 10.1186/s12974-023-02782-1
Zhao, Z., Ning, J., Bao, X.-q., Shang, M., Ma, J., Li, G., et al. (2021). Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 9:226. doi: 10.1186/s40168-021-01107-9
Zheng, S., Li, H., Xu, R., Miao, W., Dai, M., Ding, S., et al. (2021). Potential roles of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res. Rev. 69:101347. doi: 10.1016/j.arr.2021.101347
Zhong, Z., Chen, W., Gao, H., Che, N., Xu, M., Yang, L., et al. (2021). Fecal microbiota transplantation exerts a protective role in MPTP-induced Parkinson’s disease via the TLR4/PI3K/AKT/NF-κB pathway stimulated by α-Synuclein. Neurochem. Res. 46, 3050–3058. doi: 10.1007/s11064-021-03411-0
Keywords: Parkinson’s disease, gut microbiota, gut-brain axis, fecal microbiota transplantation, gut dysbiosis
Citation: Guo M, Gao H, Wang Y and Xiang Y (2025) Exploring the role of gut microbiota in Parkinson’s disease: insights from fecal microbiota transplantation. Front. Neurosci. 19:1574512. doi: 10.3389/fnins.2025.1574512
Edited by:
Zuleide Maria Ignácio, Federal University of the Southern Frontier, BrazilReviewed by:
Rajesh Singh Yadav, Dr. Hari Singh Gour University, IndiaXie Zhenchao, Southern Medical University, China
Copyright © 2025 Guo, Gao, Wang and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Wang, MTUxNjg4Njc1MzlAMTYzLmNvbQ==; Yuanyuan Xiang, MTUxNjg4ODkzODNAMTI2LmNvbQ==
 Mengqi Guo
Mengqi Guo Han Gao
Han Gao Yuan Wang
Yuan Wang Yuanyuan Xiang
Yuanyuan Xiang