- 1Institute of Flight Technology, Civil Aviation Flight University of China, Guanghan, Sichuan, China
- 2Aviation Health Department, Southwest Regional Administration of Civil Aviation Administration of China, Chengdu, China
- 3Hospital of Civil Aviation Flight University of China, Civil Aviation Flight University of China, Guanghan, Sichuan, China
- 4Key Laboratory for Neuroinformation of Ministry of Education, School of Life Sciences and Technology, University of Electronic Science and Technology of China, Chengdu, China
- 5CAAC Academy, Civil Aviation Flight University of China, Guanghan, China
Background: Investigating the neural mechanisms underlying pilots’ brains is crucial for enhancing aviation safety. However, prior research has predominantly focused on identifying structural and functional differences in the brain, while the relationship between structure and function remains insufficiently elucidated.
Methods: This study collected T1-weighted structural magnetic resonance imaging (MRI), resting-state functional MRI (rs-fMRI), and diffusion tensor imaging (DTI) data from 47 pilots and 38 matched controls. Structural–functional coupling (SFC) strength was quantified using the Structural Decoupling Index (SDI) based on graph signal processing (GSP). Functional connectivity was further decomposed into structurally coupled and decoupled components, with subsequent group comparisons conducted at the regional brain level.
Results: Compared to controls, pilots exhibited significantly higher SDI values in several brain regions, including the left and right middle frontal gyri, left precentral gyrus, inferior temporal gyrus, left posterior superior temporal sulcus, right superior and inferior parietal lobules, left visual cortex, and right basal ganglia, indicating reduced SFC in these areas. In contrast, enhanced coupling was observed in the bilateral inferior frontal gyri, left paracentral lobule, and left insula. Notably, pilots showed increased decoupled functional connectivity (d-FC) between the left cuneus and right insula, as well as between the right insula and the left medial occipital cortex, accompanied by a reduction in coupled functional connectivity (c-FC). Importantly, the strength of decoupled functional connectivity between the right insula and the left medial occipital cortex was positively correlated with total flight hours.
Conclusion: These findings suggest that prolonged flight experience may induce neuroplastic changes in regional SFC within the brains of pilots. This work provides novel insights into the neural adaptations associated with flight training and may contribute to the refinement of pilot selection and training protocols aimed at improving aviation safety.
1 Introduction
Pilots are professionals who operate under high levels of stress and must respond rapidly to constantly changing flight environments. In recent years, the proportion of aviation accidents attributed to human factors has increased significantly (Wang H. et al., 2020). Although extensive research has been dedicated to reducing human errors caused by pilots’ psychological traits, such as hazardous attitudes and risk tolerance (Ji et al., 2011; Lee and Park, 2016), as well as cognitive deficiencies, including limited working memory, improper attention allocation, and spatial disorientation (Bałaj et al., 2019; Seyfzadehdarabad et al., 2023), human factors remain a critical contributor to compromised flight safety (Kharoufah et al., 2018). Therefore, in-depth research on the neural mechanisms underlying pilot brain function is essential to improve flight safety.
Magnetic resonance imaging (MRI) has recently emerged as an indispensable tool for exploring brain structure and functional plasticity due to the modality’s non-invasive nature and high spatial resolution. Neuroplasticity has demonstrated that skill acquisition and experiential learning can induce plastic changes in both brain structure and function (Chen et al., 2006; Bezzola et al., 2011; May, 2011). For instance, functional imaging studies have demonstrated that pilots display distinct activation patterns during sensorimotor tasks, especially in regions related to observational learning and motor simulation, differentiating them from non-pilots (Callan et al., 2013). Furthermore, flight training can increase the degree of centrality in the prefrontal and occipital cortices, potentially improving executive functions (Chen et al., 2023). Studies have observed structural changes, indicating that airline pilots have greater gray and white matter volumes in the visual, sensorimotor, and prefrontal parietal regions compared to the general population (Qiu et al., 2021). Our previous research also suggests that flight training can increase the gray matter volume (GMV) of the lingual and fusiform gyri (Xu et al., 2023). Although these findings highlight significant brain changes in pilots, current methods are still not fully elucidating the influence of anatomical constraints on functional brain activity.
Understanding the structural constraints of functional brain activity is a significant area of research in cognitive neuroscience (Stiso and Bassett, 2018). Structural–functional coupling (SFC) reflects the extent to which structural connectivity (SC) supports functional connectivity (Baum et al., 2020). A deep understanding of SFC is essential to clarify how white matter structures promote sensory integration and executive functions (Rajesh et al., 2024). Early studies typically employed simple correlation analyses to explore this relationship (Honey et al., 2009; Mišić et al., 2016; Amico and Goñi, 2018). However, as the field has advanced, more complex approaches, such as communication and biophysical models, have emerged (Deco et al., 2011; Cabral et al., 2017; Mišić et al., 2018; Seguin et al., 2018). Recently, graph signal processing (GSP) has provided a novel framework for analyzing brain imaging data (Huang et al., 2018). GSP-based methods introduce a Structural Decoupling Index (SDI) to reflect the alignment of each brain region with its SC. This approach projects functional signals onto the structural harmonic space via the eigendecomposition of SC, decomposing graph signals into low-frequency and high-frequency components. The ratio of the energies of these components constitutes the SDI (Preti and Van De Ville, 2019). Existing studies have used GSP to investigate how dance and musical training affect the SFC in the brains of dancers and musicians (Gao et al., 2024).
To assess the influence of flight experience on structural–functional relationships in the brains of pilots, we recruited a group of professional pilots with extensive flight experience and a matched control group comprising individuals from various occupational backgrounds, who had no prior flight experience. The two groups were carefully matched for age, gender, and educational background. First, we applied GSP to examine the coupling between functional and structural signals at the regional level. Next, we explored how the coupling and decoupling components of functional signals contribute to interregional functional connectivity. Finally, we evaluated whether these metrics were related to the training experience. This study aimed to explore the neural mechanisms of pilots’ brains to provide a scientific basis for optimizing pilot training and selection programs.
2 Materials and methods
2.1 Methods outline
We first collected T1-weighted structural images, resting-state functional MRI (rs-fMRI), and diffusion tensor imaging (DTI), followed by preprocessing procedures. SFC strength was quantified using the SDI, within the framework of GSP (Preti and Van De Ville, 2019). Specifically, a graph Laplacian matrix was constructed to represent the brain’s structural network, and eigendecomposition was performed to obtain eigenvectors serving as harmonic components of the connectome. Functional signals were then decomposed into coupled and decoupled components via the Graph Fourier transform (GFT). SDI was defined as the log-ratio of the energy in the decoupled to coupled components (Figure 1).
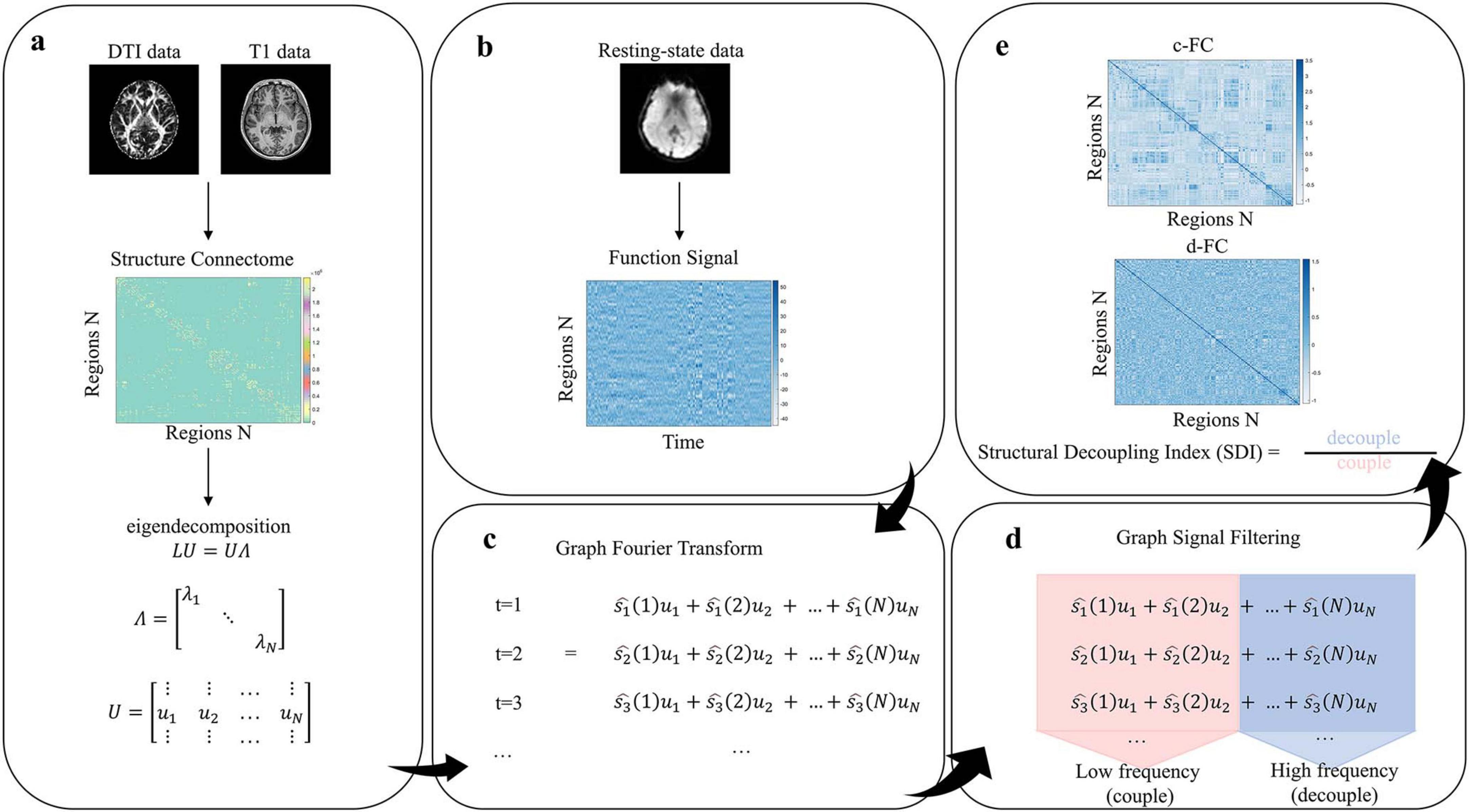
Figure 1. Methods pipeline (a) Construct structural connectivity matrix and perform eigen-decomposition. (b) Construct functional signal matrix. (c) Graph Fourier Transform (GFT). (d) Decompose functional signals via graph signal filtering. (e) Calculate structural decoupling index (SDI) and construct coupled/decoupled functional connectivity matrices.
2.2 Participants
This study was approved by the Ethics Committee of the University of Electronic Science and Technology of China (Chengdu) (Approval Number: 1420200408-07) and conducted in strict accordance with the principles outlined in the 1964 Declaration of Helsinki. Prior to participating in the experiment, all participants signed an informed consent form. The pilot group consisted of 47 experienced male airline pilots with flight hours ranging from 350 to 19,000. The control group comprised 38 flight-naïve individuals from various occupational backgrounds, matched to the pilot group by age, gender, handedness, and years of education. Exclusion criteria included: (1) claustrophobia, (2) history of psychiatric or neurological disorders, and (3) substance dependence.
2.3 MRI data acquisition
All imaging was performed on a 3T MRI scanner at the University of Electronic Science and Technology of China. T1-weighted images were acquired using a three-dimensional (3D) fast spoiled gradient recalled echo sequence. The parameters were as follows: 156 slices, TR = 6.012 ms, TE = 1.9872 ms, FOV = 256 mm × 256 mm, FA = 9°, matrix = 256 × 256, slice thickness = 1 mm. DTI data were acquired using a diffusion-weighted spin echo EPI sequence with the following parameters: TR = 8500 ms, TE = 67 ms, FOV = 256 mm × 256 mm, matrix = 128 × 128, slice thickness = 2 mm, 78 slices, b = 1000 s/mm. rs-fMRI data were acquired using a gradient echo planar imaging (EPI) sequence with an eight-channel phased-array head coil. The scanning parameters were as follows: TR = 2000 ms, TE = 30 ms, FA = 90°, matrix = 64 × 64, FOV = 240 mm × 240 mm, slice thickness = 4 mm.
During scanning, foam pads were used to minimize head motion. Participants were instructed to keep their eyes closed, remain relaxed, stay awake, and refrain from engaging in deliberate thinking.
2.4 MRI data preprocessing
Functional MRI data were preprocessed using the SPM12 toolbox in MATLAB R2018b. To allow for signal stabilization, the first five volumes were discarded. The remaining 250 volumes were subjected to slice timing correction and realignment for head motion correction. To minimize potential artifacts caused by head motion, participants with head motion exceeding 2.5 mm of translation or 2.5° of rotation were excluded to reduce motion-related artifacts. Subsequently, functional images were coregistered with corresponding T1-weighted structural images, normalized to the Montreal Neurological Institute (MNI) space (voxel size = 3 mm × 3 mm × 3 mm), and spatially smoothed using an 8 mm full width at half maximum (FWHM) Gaussian kernel. Additionally, nuisance regression was performed to remove confounding signals, including Friston-24 motion parameters, whole-brain mean, white matter, and cerebrospinal fluid (CSF) signals. Finally, linear detrending and band-pass filtering (0.01–0.08 Hz) were applied.
Diffusion MRI data were preprocessed using FSL. First, non-brain tissues were removed from the b0 image using the Brain Extraction Tool (BET) to generate a brain mask. Head motion and eddy current distortions were corrected using the eddy tool. Diffusion tensors were estimated using DTIFIT to compute scalar maps such as fractional anisotropy (FA). Voxel-wise fiber orientation distributions were then modeled using BEDPOSTX, which applies Markov Chain Monte Carlo (MCMC) sampling to account for crossing fibers. Finally, probabilistic tractography was performed using PROBTRACKX2, with tracking parameters set to 5,000 samples per seed voxel, a step length of 0.5 mm, and a curvature threshold of 0.2.
T1-weighted images were preprocessed using the Computational Anatomy Toolbox (CAT12.7, r1742) implemented in SPM12, running on MATLAB R2018b. Images were first reoriented to align with the anterior commissure–posterior commissure (AC–PC) plane, followed by bias field correction. The images were then segmented into gray matter (GM), white matter (WM), and CSF. Subsequently, the segmented images were normalized to MNI space (voxel size = 1.5 mm × 1.5 mm × 1.5 mm), modulated to preserve volume information, and smoothed using a 4 mm FWHM Gaussian kernel.
2.5 Structural connectivity matrix and functional signal matrix
The brain was first parcellated into 246 regions based on the Human Brainnetome Atlas, which comprises 210 cortical regions and 36 subcortical regions (Fan et al., 2016). Then, white matter fiber connectivity between the 246 brain regions defined by the Human Brainnetome Atlas was estimated using the PROBTRACKX2 tool in FSL. Subsequently, the GMV of each region was extracted from the T1-weighted images. The number of fiber tracts between any two regions was normalized by the sum of their GMVs to produce a 246 × 246 structural connectivity matrix for each participant. Finally, the mean BOLD signal within each brain region was calculated by averaging across all voxels within the region, resulting in a 246 × 250 functional signal matrix, where 250 refers to the number of time points remaining after removing the first five volumes of rs-fMRI data.
2.6 Structural connectivity harmonics and GFT
First, the SC matrix was defined as an adjacency matrix A. A symmetric normalized graph Laplacian matrix L was then computed as L = I−D−1/2AD−1/2, where D is the degree matrix, and I is the identity matrix. The matrix Lcharacterizes the topological structure and connectivity between brain regions. Eigendecomposition was applied to obtain the eigenvectors and eigenvalues of L, denoted as LU = UΛ, where U contains the eigenvectors and Λ is a diagonal matrix of eigenvalues. The eigenvalues λk are interpreted as frequencies, and the corresponding eigenvectors uk serve as the SC harmonics. Eigenvectors associated with low-frequency eigenvalues represent smooth variations over the network structure. Functional signals were then projected into the spectral domain using the GFT: .
2.7 Measurement of SDI
Graph signal filtering was applied to decompose the functional signal into two components: one that aligns closely with the brain’s structural topology (coupled), and one that diverges from it (decoupled). The cut-off frequency C was determined to divide the average spectral energy density into two equal parts. The coupled and decoupled signals were obtained using the following equations: and , where Ulow and Uhigh contain the eigenvectors associated with the low and high frequencies, respectively. These filtered signals structure-aligned and structure-independent components of the functional signal. For each brain region, the SDI was defined as the base-2 logarithmic ratio of the L2 norms of the decoupled and coupled components: . A negative SDI indicates strong alignment (coupling) between structure and function in that region, while a positive SDI suggests relative independence (decoupling). Additionally, we calculated the Pearson’s correlation coefficients between across regions, resulting in two 246 × 246 functional connectivity matrices: the coupled functional connectivity (c-FC) matrix and the decoupled functional connectivity (d-FC) matrix. c-FC refers to components of functional connectivity that are highly consistent with SC, reflecting functional activities that rely on stable anatomical pathways; whereas d-FC refers to components of functional connectivity that are independent of SC, potentially reflecting greater flexibility or adaptability (Griffa et al., 2022).
2.8 Statistical analysis
Two-sample t-tests were first conducted to compare regional differences in SDI between the pilot and control groups. Statistical significance was assessed using a non-parametric permutation test with 5,000 iterations. A two-tailed test was applied, and the significance threshold was set at p < 0.05. Subsequently, Fisher’s Z-transformation was applied to the c-FC and d-FC matrices, followed by a 2 × 2 repeated-measures analysis of variance (ANOVA). To control for multiple comparisons, family-wise error (FWE) correction was applied, with the significance threshold set at p < 0.01. Age, years of education, total intracranial volume (TIV), and mean framewise displacement (mFD) were included as covariates in all statistical analyses. Finally, the effects of age, years of education, TIV, and mFD were regressed out from the data, and outliers were subsequently removed from the residuals. Spearman’s correlation analysis was then performed to examine the relationship between coupling and decoupling functional connectivity among the brain regions showing significant interaction effects in the repeated-measures ANOVA and total flight hours.
3 Results
3.1 Participant demographic information
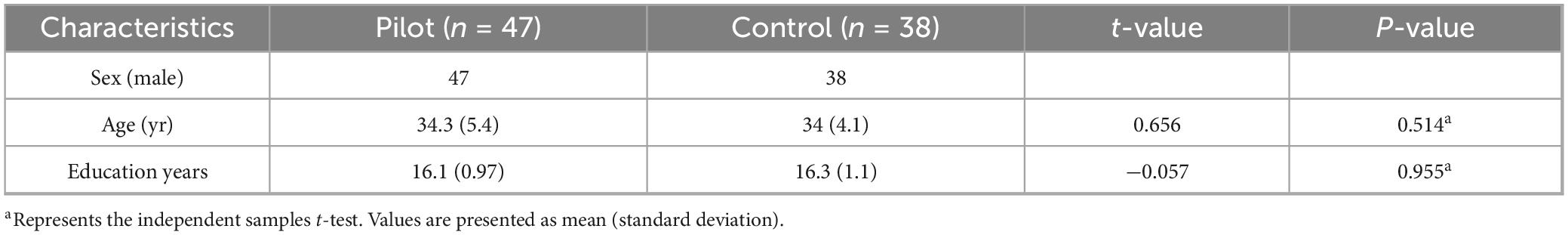
A total of 47 male airline pilots (mean age: 34.3 ± 5.4 years; mean education: 16.1 ± 0.97 years) and 38 male flight-naïve controls (mean age: 34.0 ± 4.1 years; mean education: 16.3 ± 1.1 years) were included in the analysis (Table 1). Independent samples t-tests revealed no significant group differences in age (t = 0.656, p = 0.514) or years of education (t = −0.057, p = 0.955).
3.2 Whole-brain distribution of structural–functional coupling
As illustrated in Figure 2, our findings reveal a hierarchical distribution pattern consistent with previous research. In unimodal cortex (including primary sensory and motor regions), SFC is generally higher (i.e., more negative SDI values), indicating that functional activity in these areas is heavily reliant on stable structural connections. In contrast, transmodal cortex exhibits lower levels of SFC (i.e., more positive SDI values).
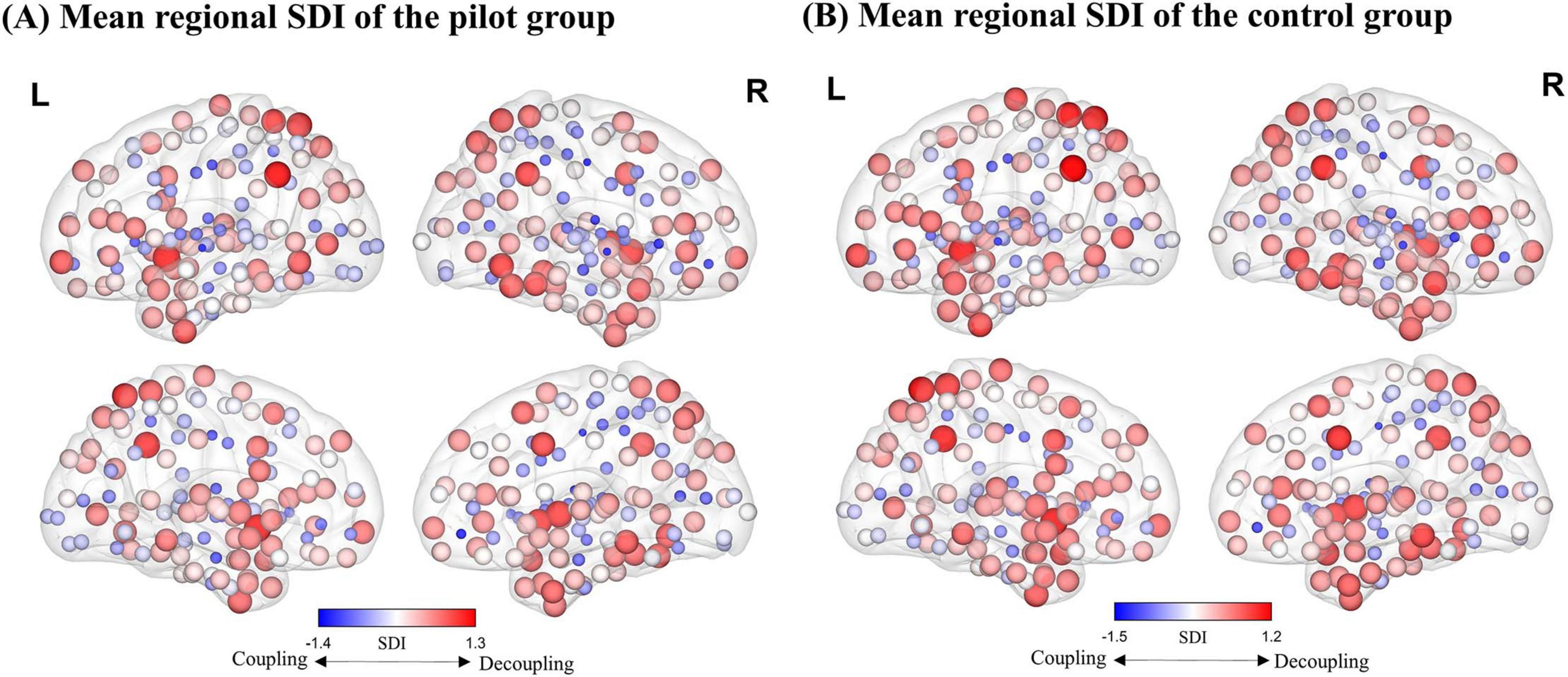
Figure 2. Distribution map of the regional average Structure-Decoupling Index (SDI). (A) Average SDI map for the pilot group. (B) Average SDI map for the control group. Node size is proportional to SDI values, and node color indicates SDI magnitude. Larger positive SDI values indicate greater decoupling, while smaller negative SDI values reflect stronger coupling.
3.3 Group differences in regional structural-functional coupling
Two-sample t-tests identified significant differences in SDI across 16 regions of the brain (p < 0.05). Compared to controls, pilots exhibited significantly higher SDI values in the left and right middle frontal gyri, left precentral gyrus, inferior temporal gyrus, left posterior superior temporal sulcus, right superior and inferior parietal lobules, left visual cortex, and right basal ganglia, indicating reduced SFC in these areas. In contrast, increased coupling was observed in the bilateral inferior frontal gyri, left paracentral lobule, and left insula in the pilot group (Figure 3 and Supplementary Table 1).
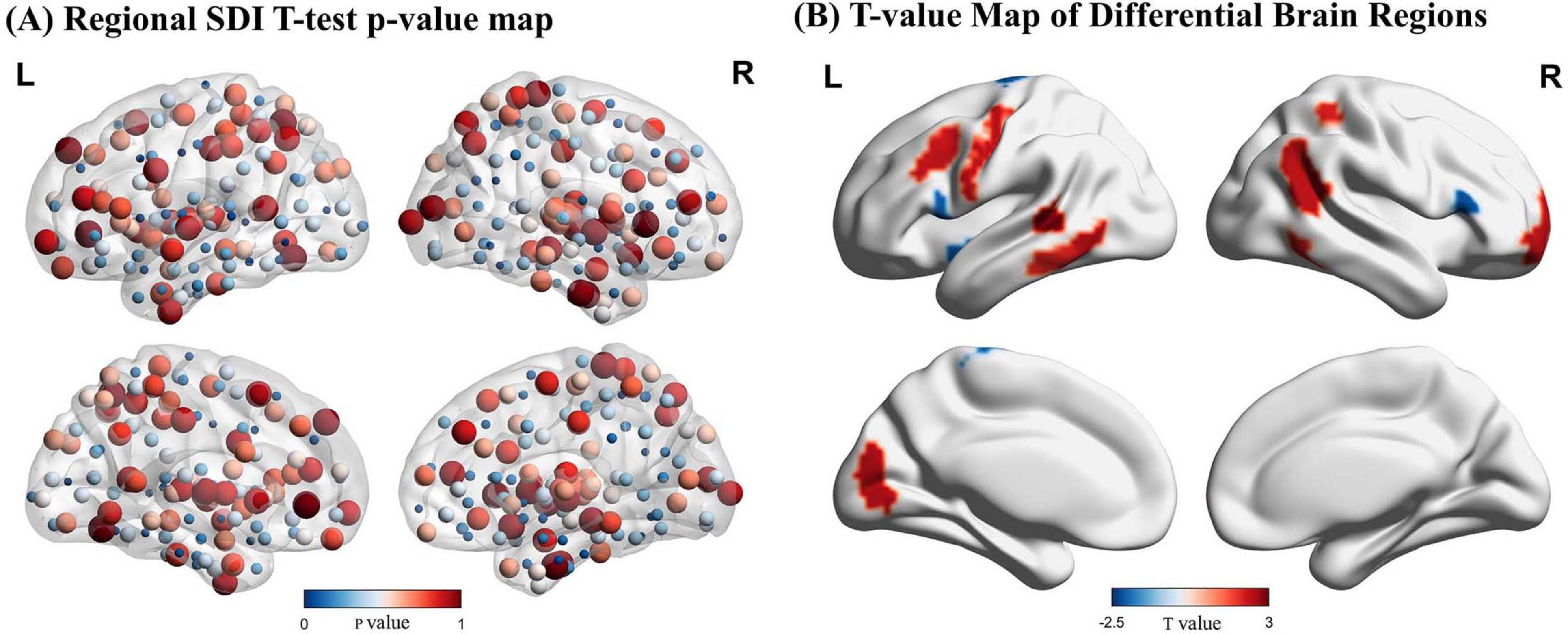
Figure 3. Results of the Structural Decoupling Index (SDI) difference analysis. (A) Regional SDI differences represented by node color based on p-values from t-tests. Larger nodes correspond to larger p-values. (B) T-value map illustrating brain regions with significant SDI differences. Positive t-values (red) indicate regions with greater decoupling (higher SDI values) in pilots, while negative t-values (blue) represent regions with greater coupling (lower SDI values) relative to controls. GFT, Graph Fourier Transform.
3.4 Interaction effects in functional connectivity
Repeated-measures ANOVA revealed significant interaction effects in functional connectivity between the left cuneus and right insula, as well as between the right insula and left medial occipital cortex (FWE-corrected p < 0.01). Simple effects analysis showed that c-FC between these regions was significantly reduced, while decoupled connectivity was increased (Figure 4).
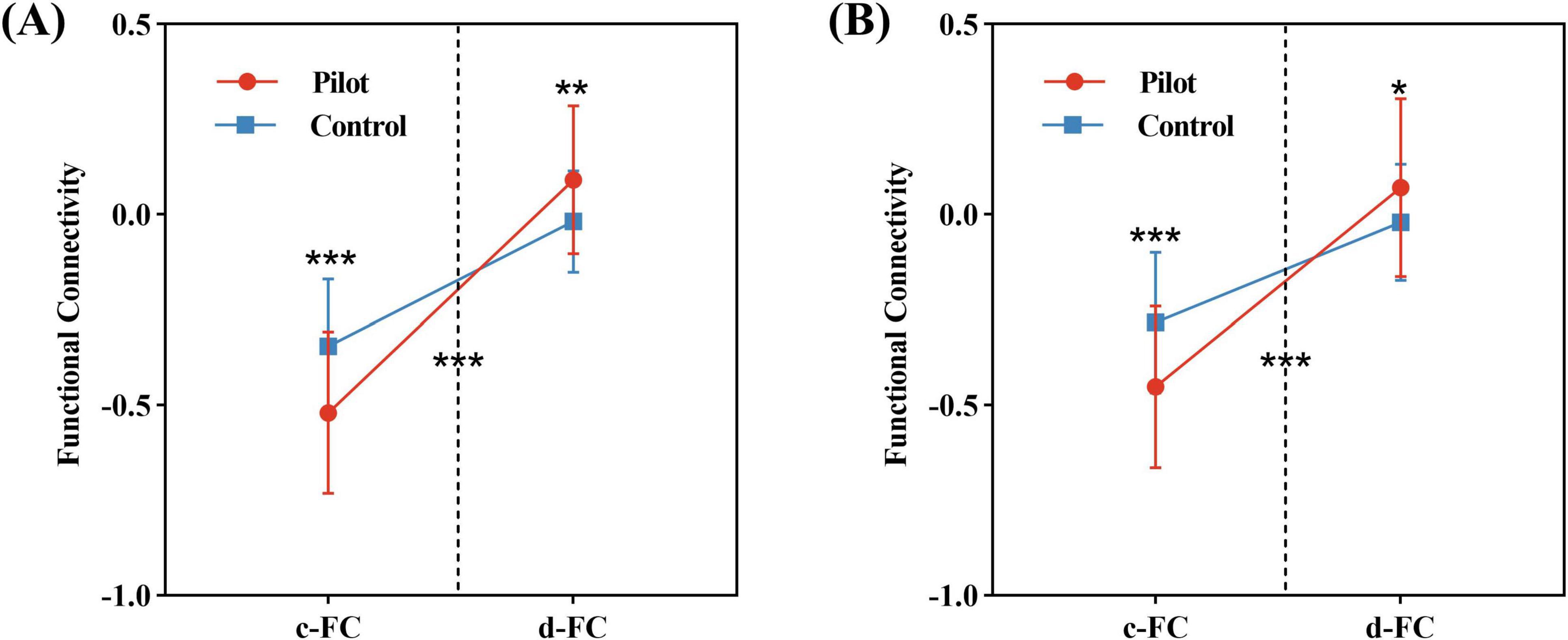
Figure 4. Results of the repeated-measures ANOVA. (A) Significant interaction effect between FC components (c-FC and d-FC) and group in connectivity between the left cuneus and the right insula. (B) Significant interaction effect for connectivity between the right insula and the left medial occipital cortex. Statistical significance indicated as: *P < 0.05, **P < 0.01, ***P < 0.001 (FWE-corrected P < 0.01).
3.5 Correlation of d-FC with behavioral measures
We examined the correlation between functional connectivity and flight experience. Spearman’s correlation analysis revealed a significant positive relationship between d-FC of the right insula and the left medial occipital cortex and total flight hours (r2 = 0.155, p = 0.009; Figure 5).
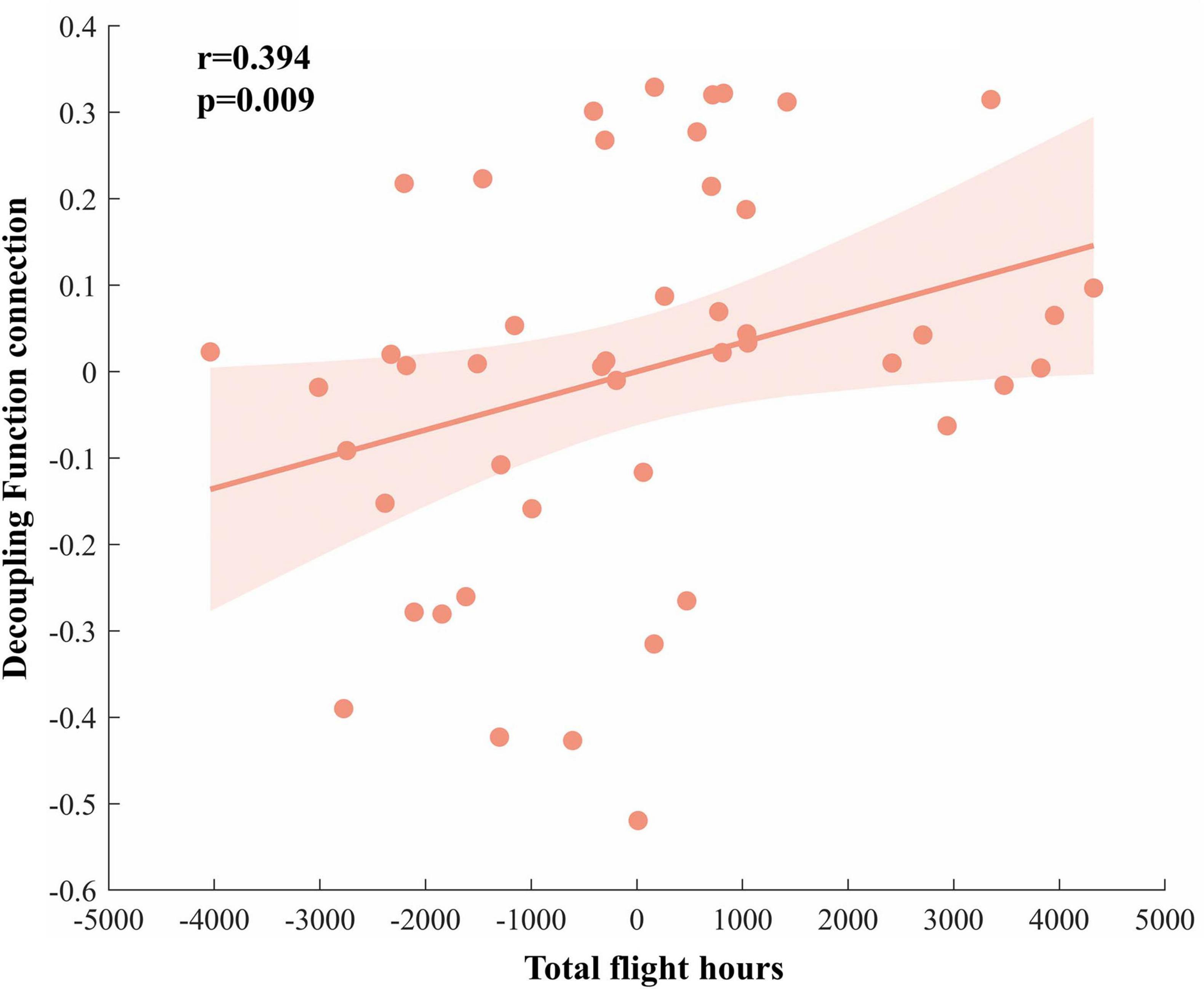
Figure 5. Correlation analysis results. The decoupling functional connectivity between the right insula and left medial occipital cortex in the pilot group is positively correlated with total flight hours.
4 Discussion
This study employed a GSP framework to investigate differences in SFC between airline pilots and matched controls, aiming to elucidate neuroplastic changes associated with long-term flight experience. The results revealed altered SFC patterns in several brain regions among pilots. Specifically, reduced coupling was observed in the bilateral middle frontal gyri, inferior temporal gyri, right inferior and superior parietal lobules, and left visual cortex. In contrast, increased coupling was identified in the bilateral inferior frontal gyri, left paracentral lobule, and left insula. Furthermore, pilots demonstrated significantly increased d-FC between the left cuneus and right insula, as well as between the right insula and the left medial occipital cortex, accompanied by a reduction in c-FC. Notably, the strength of d-FC between the right insula and the left medial occipital cortex was positively correlated with total flight hours, suggesting experience-dependent modulation of structure-independent functional interactions.
This study found that both pilots and controls exhibited a hierarchical distribution of whole-brain SFC, consistent with previous findings. Notably, the pilot group demonstrated significantly reduced SFC in several transmodal association cortical regions, including the middle frontal gyrus, inferior temporal gyrus, and both the superior and inferior parietal lobules. Previous neuroimaging studies have highlighted the importance of reduced SFC in transmodal association cortices for cognitive flexibility and adaptability (Vázquez-Rodríguez et al., 2019). The parietal cortex is considered a “flexible hub” for multitask control, playing a key role in task switching and integration under complex environmental conditions (Cole et al., 2013). The inferior temporal gyrus is primarily associated with complex visual representations and semantic processing, forming a critical basis for high-level cognitive functions (Lin et al., 2020). Prior research suggests that cognitive flexibility depends on the multimodal integration capacity of prefrontal and parietal regions (Uddin, 2021). In general, reduced SFC implies that functional activity is less constrained by anatomical architecture, which may reflect enhanced flexibility and adaptability (Baum et al., 2020). Moreover, a healthy brain can dynamically adjust its level of flexibility in response to changing environmental demands (Egner and Siqi-Liu, 2024). Based on these findings and prior literature, we speculate that the reduced SFC observed in these regions among pilots may reflect neuroadaptive remodeling driven by long-term engagement in complex flight tasks. These changes may support superior cognitive flexibility and help reduce operational risk. This hypothesis is consistent with prior studies linking lower SFC to greater cognitive flexibility and more effective task performance (Wu et al., 2020; Rajesh et al., 2024). Our earlier research also found that pilots exhibited increased inter-network connectivity, decreased intra-network connectivity, and increased brain-state transition frequency, which may underlie their cognitive flexibility (Chen et al., 2020). Moreover, a recent study based on the triple-network model demonstrated enhanced inter-network connectivity in pilot trainees during dynamic functional connectivity analysis, suggesting superior cognitive flexibility in managing complex tasks (Ye et al., 2025). However, it should be noted that direct behavioral evidence supporting these neural mechanisms is still lacking. Future studies should incorporate task paradigms to further elucidate the functional implications and behavioral correlates of altered SFC in pilots.
Moreover, we observed increased SFC in the inferior frontal gyrus (IFG) among pilots. Neuroimaging evidence suggests that the IFG plays a critical role in various cognitive processes, including language processing, attentional capture, and response inhibition (Wang J. et al., 2020; Rivas-Fernández et al., 2021; Wang, 2025). It is also implicated in multisensory integration, particularly exhibiting significant integrative functions during category learning tasks (Li et al., 2020). From a neurobiological perspective, enhanced SFC is generally regarded as a marker of increased neural pathway stability and regional functional specialization (Baum et al., 2020). A previous study on fighter pilots found reduced functional connectivity strength of the IFG within whole-brain networks compared to the general population, suggesting a potentially higher degree of specialized processing in this region (Radstake et al., 2023). Therefore, we speculate that the increased SFC in the IFG may reflect the development of more stable and specialized neural pathways resulting from long-term engagement in complex perception and action environments, thereby supporting rapid detection and efficient regulation of linguistic cues and attentional resources during high-demand task execution. It is worth noting, however, that there remains debate as to whether the IFG is specifically involved in semantic and language processing or whether it serves broader domain-general cognitive functions (Yeo et al., 2015). Accordingly, future studies should incorporate well-designed behavioral paradigms and cognitive assessments to further elucidate the functional significance of the observed increase in coupling—namely, whether it represents enhanced functional specialization or a general improvement in cognitive capacity.
For pilots, spatial navigation is a highly complex cognitive task that requires continuous monitoring of flight instruments and directional judgment, particularly in the absence of external postural or motion cues (Dahlstrom and Nahlinder, 2009; Bałaj et al., 2019). Previous studies have shown that pilots exhibit significant activation in the frontal and parietal cortices during stressful situations, observational learning tasks, and spatial cognition assessments (Sladky et al., 2016; Causse et al., 2019, 2024). As a key region involved in higher-order cognitive processes, the prefrontal cortex plays a critical role in spatial working memory and spatial attention tasks (Funahashi, 2017). The inferior parietal lobule is involved in the integration of sensory information, spatial localization, and sustained attention (Wang et al., 2001; Salgado-Pineda et al., 2003; Behrmann et al., 2004; Buchsbaum et al., 2005), while the superior parietal lobule primarily integrates auditory and visual inputs and supports goal-directed spatial orienting (Shomstein et al., 2010). These two regions operate in close coordination during spatial attention and orienting tasks, performing essential functions (Vandenberghe et al., 2012). In the present study, we observed significantly reduced SFC in the middle frontal gyrus, superior parietal lobule, and inferior parietal lobule in the pilot group. According to existing literature, weakened SFC in specific brain regions may be associated with enhanced executive function and memory performance (Griffa et al., 2022). Furthermore, flight experience has been shown to improve cognitive capacity and mitigate the effects of cognitive aging (Causse et al., 2011). Based on these findings, we speculate that pilots undergo specific neuroadaptive remodeling processes as a result of prolonged engagement in cognitively demanding tasks. These neural changes may enhance executive control and memory functions, thereby reducing operational errors and improving aviation safety. This interpretation is also consistent with our previous findings. We previously demonstrated increased degree centrality in the left middle frontal gyrus following flight training, and this change was significantly associated with better executive function performance (Chen et al., 2023).
The insula is a core brain region responsible for multimodal information integration, with widespread connections to various cortical and subcortical areas. It plays a vital role in integrating sensory, affective, and cognitive information (Evrard, 2019). In addition to its involvement in spatial orientation, the insula also contributes to higher-order spatial navigation processes (Qiu et al., 2019; Baier et al., 2021; Bleau et al., 2022). The precuneus is critical for spatial orientation and visual task processing (Grill-Spector and Malach, 2004; Parker et al., 2014). Recent studies have identified the precuneus as a key connectivity hub, characterized by dense temporo-occipital and extensive long-range connections (Palejwala, 2021), further underscoring its role in visual information processing. Increased white matter volume in the precuneus among pilots has been reported, which may reflect the high demand for visual processing during flight tasks (Qiu et al., 2021). In addition, the middle occipital gyrus (MOG) is involved in higher-level visual processing and plays an important role in rapid decision-making and task execution (Aberbach-Goodman and Mukamel, 2023; Wang et al., 2024). Coordinated activity among these regions provides essential neural support for pilots operating under high-demand conditions, particularly in spatial orientation and visual processing. In the present study, we found that pilots exhibited enhanced d-FC between the left insula and the left cuneus, as well as between the left insula and the left MOG. The functional decoupling is thought to support an individual’s capacity to integrate novel information, and previous studies have shown that decoupled connectivity is closely related to sustained attention (Griffa et al., 2022). Further analysis revealed that the degree of functional decoupling between the insula and the MOG was positively correlated with total flight hours among pilots. This finding suggests that long-term flight experience may induce adaptive remodeling of the connectivity patterns between the insula and visual cortices, thereby enhancing pilots’ performance during complex flight operations. Our previous research also demonstrated that flight training enhances functional connectivity between parietal and occipital cortices in pilot trainees (Chen et al., 2024). In addition, other studies have indicated that exposure to different gravity environments can significantly affect functional brain networks. For instance, short-term high-G acceleration experienced frequently by fighter pilots (Radstake et al., 2023) and prolonged microgravity exposure in astronauts (Jillings et al., 2023) have both been associated with extensive reorganization of functional brain networks. The present findings extend this line of research, jointly supporting the notion that flight experience and exposure to extreme environments exert profound effects on large-scale brain functional architecture.
5 Limitation
This study has several limitations: (1) We did not control for the type of education received by participants. Although most individuals in the control group were employed in science- and engineering-related fields, variations in educational content and training approaches may still influence brain function. Therefore, future studies should carefully control for this factor. (2) The use of resting-state fMRI in this study may underestimate brain network reorganization during actual task execution in pilots. Future research should incorporate task-based fMRI or flight simulation paradigms to more comprehensively elucidate the underlying functional mechanisms. (3) This study lacks a longitudinal design, thus limiting our ability to establish causality. Future research should adopt a longitudinal approach to better elucidate how flight experience impacts the brain’s structure-function relationships. (4) This study did not include direct assessments of cognitive function in pilots. Including such measures, particularly those assessing cognitive flexibility, would help clarify the behavioral relevance of the observed neural differences and provide further insight into their functional implications.
6 Conclusion
In conclusion, this study investigated the structural-functional coupling of the pilot’s brains within a novel research framework. We noted that prolonged flight experience can induce changes in the structural-functional coupling strength, thus enhancing our understanding of the effects of flight on neural plasticity. The results may provide insights for optimizing future pilot selection and training programs. However, further research is needed to validate these findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://osf.io/xhqdk/.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the University of Electronic Science and Technology of China (Chengdu) (Approval Number: 1420200408-07). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XC: Writing – original draft, Methodology, Conceptualization, Formal Analysis, Project administration, Funding acquisition, Writing – review and editing. QM: Writing – review and editing, Writing – original draft, Visualization, Data curation, Validation. QS: Resources, Writing – review and editing. PX: Writing – review and editing, Investigation. SZ: Writing – review and editing, Investigation. DH: Writing – review and editing, Resources. QC: Writing – review and editing, Investigation. JF: Software, Writing – review and editing. CL: Writing – review and editing, Supervision. XL: Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Nature Science Foundation of China (No. U2033209).
Acknowledgments
We would like to thank the Institute of Aviation Human Factors and Cognitive Neuroscience in Civil Aviation Flight University of China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MY declared a shared parent affiliation with the authors XC, QM, PX, SZ, DH, QC, XL to the handling editor at the time of review.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2025.1608739/full#supplementary-material
References
Aberbach-Goodman, S., and Mukamel, R. (2023). Temporal hierarchy of observed goal-directed actions. Sci. Rep. 13:19701. doi: 10.1038/s41598-023-46917-z
Amico, E., and Goñi, J. (2018). Mapping hybrid functional-structural connectivity traits in the human connectome. Netw. Neurosci. 2, 306–322. doi: 10.1162/netn_a_00049
Baier, B., Cuvenhaus, H. S., Müller, N., Birklein, F., and Dieterich, M. (2021). The importance of the insular cortex for vestibular and spatial syndromes. Eur. J. Neurol. 28, 1774–1778. doi: 10.1111/ene.14660
Bałaj, B., Lewkowicz, R., Francuz, P., Augustynowicz, P., Fudali-Czyż, A., Stróżak, P., et al. (2019). Spatial disorientation cue effects on gaze behaviour in pilots and non-pilots. Cogn. Tech. Work 21, 473–486. doi: 10.1007/s10111-018-0534-7
Baum, G. L., Cui, Z., Roalf, D. R., Ciric, R., Betzel, R. F., Larsen, B., et al. (2020). Development of structure–function coupling in human brain networks during youth. Proc. Natl. Acad. Sci. U.S.A. 117, 771–778. doi: 10.1073/pnas.1912034117
Behrmann, M., Geng, J. J., and Shomstein, S. (2004). Parietal cortex and attention. Curr. Opin. Neurobiol. 14, 212–217. doi: 10.1016/j.conb.2004.03.012
Bezzola, L., Mérillat, S., Gaser, C., and Jäncke, L. (2011). Training-induced neural plasticity in golf novices. J. Neurosci. 31, 12444–12448. doi: 10.1523/JNEUROSCI.1996-11.2011
Bleau, M., Paré, S., Chebat, D.-R., Kupers, R., Nemargut, J. P., and Ptito, M. (2022). Neural substrates of spatial processing and navigation in blindness: An activation likelihood estimation meta-analysis. Front. Neurosci. 16:1010354. doi: 10.3389/fnins.2022.1010354
Buchsbaum, B. R., Greer, S., Chang, W., and Berman, K. F. (2005). Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting task and component processes. Hum. Brain Mapp. 25, 35–45. doi: 10.1002/hbm.20128
Cabral, J., Kringelbach, M. L., and Deco, G. (2017). Functional connectivity dynamically evolves on multiple time-scales over a static structural connectome: Models and mechanisms. Neuroimage 160, 84–96. doi: 10.1016/j.neuroimage.2017.03.045
Callan, D. E., Terzibas, C., Cassel, D. B., Callan, A., Kawato, M., and Sato, M. (2013). Differential activation of brain regions involved with error-feedback and imitation based motor simulation when observing self and an expert’s actions in pilots and non-pilots on a complex glider landing task. Neuroimage 72, 55–68. doi: 10.1016/j.neuroimage.2013.01.028
Causse, M., Chua, Z. K., and Rémy, F. (2019). Influences of age, mental workload, and flight experience on cognitive performance and prefrontal activity in private pilots: A fNIRS study. Sci. Rep. 9:7688. doi: 10.1038/s41598-019-44082-w
Causse, M., Dehais, F., Arexis, M., and Pastor, J. (2011). Cognitive aging and flight performances in general aviation pilots. Aging Neuropsychol. Cogn. 18, 544–561. doi: 10.1080/13825585.2011.586018
Causse, M., Mouratille, D., Rouillard, Y., El Yagoubi, R., Matton, N., and Hidalgo-Muñoz, A. (2024). How a pilot’s brain copes with stress and mental load? Insights from the executive control network. Behav. Brain Res. 456:114698. doi: 10.1016/j.bbr.2023.114698
Chen, F., Hu, Z., Zhao, X., Wang, R., Yang, Z., Wang, X., et al. (2006). Neural correlates of serial abacus mental calculation in children: A functional MRI study. Neurosci. Lett. 403, 46–51. doi: 10.1016/j.neulet.2006.04.041
Chen, X., Jiang, H., Meng, Y., Xu, Z., and Luo, C. (2024). Increased Functional connectivity between the parietal and occipital modules among flight cadets. Aeros. Med. Hum. Perform. 95, 375–380. doi: 10.3357/AMHP.6370.2024
Chen, X., Wang, Q., Luo, C., Yang, Y., Jiang, H., Guo, X., et al. (2020). Increased functional dynamics in civil aviation pilots: Evidence from a neuroimaging study. PLoS One 15:e0234790. doi: 10.1371/journal.pone.0234790
Chen, X., Wang, Z., Jiang, H., Meng, Y., Wang, H., Li, Y., et al. (2023). Flight training changes the brain functional pattern in cadets. Front. Neurosci. 17:1120628. doi: 10.3389/fnins.2023.1120628
Cole, M. W., Reynolds, J. R., Power, J. D., Repovs, G., Anticevic, A., and Braver, T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 16, 1348–1355. doi: 10.1038/nn.3470
Dahlstrom, N., and Nahlinder, S. (2009). Mental workload in aircraft and simulator during basic civil aviation training. Int. J. Aviat. Psychol. 19, 309–325. doi: 10.1080/10508410903187547
Deco, G., Jirsa, V. K., and McIntosh, A. R. (2011). Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 12, 43–56. doi: 10.1038/nrn2961
Egner, T., and Siqi-Liu, A. (2024). Insights into control over cognitive flexibility from studies of task-switching. Curr. Opin. Behav. Sci. 55:101342. doi: 10.1016/j.cobeha.2023.101342
Evrard, H. C. (2019). The Organization of the primate insular cortex. Front. Neuroanat. 13:43. doi: 10.3389/fnana.2019.00043
Fan, L., Li, H., Zhuo, J., Zhang, Y., Wang, J., Chen, L., et al. (2016). The human brainnetome atlas: A new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526. doi: 10.1093/cercor/bhw157
Funahashi, S. (2017). Working Memory in the prefrontal cortex. Brain Sci. 7:49. doi: 10.3390/brainsci7050049
Gao, K., He, H., Lu, B., Xie, Q., Lu, J., Yao, D., et al. (2024). Discrepant changes in structure–function coupling in dancers and musicians. Cereb. Cortex 34:bhae068. doi: 10.1093/cercor/bhae068
Griffa, A., Amico, E., Liégeois, R., Van De Ville, D., and Preti, M. G. (2022). Brain structure-function coupling provides signatures for task decoding and individual fingerprinting. Neuroimage 250:118970. doi: 10.1016/j.neuroimage.2022.118970
Grill-Spector, K., and Malach, R. (2004). The human visual cortex. Annu. Rev. Neurosci. 27, 649–677. doi: 10.1146/annurev.neuro.27.070203.144220
Honey, C. J., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., et al. (2009). Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U.S.A. 106, 2035–2040. doi: 10.1073/pnas.0811168106
Huang, W., Bolton, T. A. W., Medaglia, J. D., Bassett, D. S., Ribeiro, A., and Van De Ville, D. (2018). A Graph signal processing perspective on functional brain imaging. Proc. IEEE 106, 868–885. doi: 10.1109/JPROC.2018.2798928
Ji, M., You, X., Lan, J., and Yang, S. (2011). The impact of risk tolerance, risk perception and hazardous attitude on safety operation among airline pilots in China. Saf. Sci. 49, 1412–1420. doi: 10.1016/j.ssci.2011.06.007
Jillings, S., Pechenkova, E., Tomilovskaya, E., Rukavishnikov, I., Jeurissen, B., Van Ombergen, A., et al. (2023). Prolonged microgravity induces reversible and persistent changes on human cerebral connectivity. Commun. Biol. 6:46. doi: 10.1038/s42003-022-04382-w
Kharoufah, H., Murray, J., Baxter, G., and Wild, G. (2018). A review of human factors causations in commercial air transport accidents and incidents: From to 2000–2016. Prog. Aeros. Sci. 99, 1–13. doi: 10.1016/j.paerosci.2018.03.002
Lee, H.-B., and Park, J.-W. (2016). Comparative study on hazardous attitudes and safe operational behavior in airline pilots. J. Air Trans. Manag. 54, 70–79. doi: 10.1016/j.jairtraman.2016.03.024
Li, Y., Seger, C., Chen, Q., and Mo, L. (2020). Left inferior frontal gyrus integrates multisensory information in category learning. Cereb. Cortex 30, 4410–4423. doi: 10.1093/cercor/bhaa029
Lin, Y.-H., Young, I. M., Conner, A. K., Glenn, C. A., Chakraborty, A. R., Nix, C. E., et al. (2020). Anatomy and white matter connections of the inferior temporal gyrus. World Neurosurg. 143, e656–e666. doi: 10.1016/j.wneu.2020.08.058
May, A. (2011). Experience-dependent structural plasticity in the adult human brain. Trends Cogn. Sci. 15, 475–482. doi: 10.1016/j.tics.2011.08.002
Mišić, B., Betzel, R. F., De Reus, M. A., Van Den Heuvel, M. P., Berman, M. G., McIntosh, A. R., et al. (2016). Network-level structure-function relationships in human neocortex. Cereb. Cortex 26, 3285–3296. doi: 10.1093/cercor/bhw089
Mišić, B., Betzel, R. F., Griffa, A., De Reus, M. A., He, Y., Zuo, X.-N., et al. (2018). Network-Based asymmetry of the human auditory system. Cereb. Cortex 28, 2655–2664. doi: 10.1093/cercor/bhy101
Palejwala, A. H. (2021). Anatomy and White matter connections of the lingual gyrus and cuneus. World Neurosurg. 151, e426–e437. doi: 10.1016/j.wneu.2021.04.050
Parker, J. G., Zalusky, E. J., and Kirbas, C. (2014). Functional MRI mapping of visual function and selective attention for performance assessment and presurgical planning using conjunctive visual search. Brain Behav. 4, 227–237. doi: 10.1002/brb3.213
Preti, M. G., and Van De Ville, D. (2019). Decoupling of brain function from structure reveals regional behavioral specialization in humans. Nat. Commun. 10:4747. doi: 10.1038/s41467-019-12765-7
Qiu, C., Zhao, C., Hu, G., Zhang, Y., Zhu, Y., Wu, X., et al. (2021). Brain structural plasticity in visual and sensorimotor areas of airline pilots: A voxel-based morphometric study. Behav. Brain Res. 411:113377. doi: 10.1016/j.bbr.2021.113377
Qiu, Y., Wu, Y., Liu, R., Wang, J., Huang, H., and Huang, R. (2019). Representation of human spatial navigation responding to input spatial information and output navigational strategies: An ALE meta-analysis. Neurosci. Biobehav. Rev. 103, 60–72. doi: 10.1016/j.neubiorev.2019.06.012
Radstake, W. E., Jillings, S., Laureys, S., Demertzi, A., Sunaert, S., Van Ombergen, A., et al. (2023). Neuroplasticity in F16 fighter jet pilots. Front. Physiol. 14:1082166. doi: 10.3389/fphys.2023.1082166
Rajesh, A., Seider, N. A., Newbold, D. J., Adeyemo, B., Marek, S., Greene, D. J., et al. (2024). Structure–function coupling in highly sampled individual brains. Cereb. Cortex 34:bhae361. doi: 10.1093/cercor/bhae361
Rivas-Fernández, M. Á, Varela-López, B., Cid-Fernández, S., and Galdo-Álvarez, S. (2021). Functional Activation and connectivity of the left inferior frontal gyrus during lexical and phonological retrieval. Symmetry 13:1655. doi: 10.3390/sym13091655
Salgado-Pineda, P., Baeza, I., Pérez-Gómez, M., Vendrell, P., Junqué, C., Bargalló, N., et al. (2003). Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage 19, 365–375. doi: 10.1016/S1053-8119(03)00094-6
Seguin, C., Van Den Heuvel, M. P., and Zalesky, A. (2018). Navigation of brain networks. Proc. Natl. Acad. Sci. U.S.A. 115, 6297–6302. doi: 10.1073/pnas.1801351115
Seyfzadehdarabad, F., Sadeghi-Firoozabadi, V., Shokri, O., Bagheri, M., and Sadeghi Firoozabadi, A. (2023). Cognitive correlates of maritime pilots’ human errors. Saf. Sci. 165:106196. doi: 10.1016/j.ssci.2023.106196
Shomstein, S., Lee, J., and Behrmann, M. (2010). Top-down and bottom-up attentional guidance: Investigating the role of the dorsal and ventral parietal cortices. Exp. Brain Res. 206, 197–208. doi: 10.1007/s00221-010-2326-z
Sladky, R., Stepniczka, I., Boland, E., Tik, M., Lamm, C., Hoffmann, A., et al. (2016). Neurobiological differences in mental rotation and instrument interpretation in airline pilots. Sci. Rep. 6:28104. doi: 10.1038/srep28104
Stiso, J., and Bassett, D. S. (2018). Spatial embedding imposes constraints on neuronal network architectures. Trends Cogn. Sci. 22, 1127–1142. doi: 10.1016/j.tics.2018.09.007
Uddin, L. Q. (2021). Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 22, 167–179. doi: 10.1038/s41583-021-00428-w
Vandenberghe, R., Molenberghs, P., and Gillebert, C. R. (2012). Spatial attention deficits in humans: The critical role of superior compared to inferior parietal lesions. Neuropsychologia 50, 1092–1103. doi: 10.1016/j.neuropsychologia.2011.12.016
Vázquez-Rodríguez, B., Suárez, L. E., Markello, R. D., Shafiei, G., Paquola, C., Hagmann, P., et al. (2019). Gradients of structure–function tethering across neocortex. Proc. Natl. Acad. Sci. U.S.A. 116, 21219–21227. doi: 10.1073/pnas.1903403116
Wang, G., Jiang, N., Liu, T., Wang, L., Suo, D., Chen, D., et al. (2024). Using unsupervised capsule neural network reveal spatial representations in the human brain. Hum. Brain Mapp. 45:e26573. doi: 10.1002/hbm.26573
Wang, H., Pan, T., Si, H., Li, Y., and Jiang, N. (2020). Research on influencing factor selection of pilot’s intention. Int. J. Aeros. Eng. 2020, 1–13. doi: 10.1155/2020/4294538
Wang, J., Yang, Y., Zhao, X., Zuo, Z., and Tan, L.-H. (2020). Evolutional and developmental anatomical architecture of the left inferior frontal gyrus. Neuroimage 222:117268. doi: 10.1016/j.neuroimage.2020.117268
Wang, L., Kakigi, R., and Hoshiyama, M. (2001). Neural activities during wisconsin card sorting test — MEG observation. Cogn. Brain Res. 12, 19–31. doi: 10.1016/S0926-6410(01)00022-2
Wang, Y. (2025). Neural representation of response inhibition and attentional capture in the right inferior frontal gyrus. Eur. J Neurosci. 61:e70048. doi: 10.1111/ejn.70048
Wu, D., Fan, L., Song, M., Wang, H., Chu, C., Yu, S., et al. (2020). Hierarchy of connectivity–function relationship of the human cortex revealed through predicting activity across functional domains. Cereb. Cortex 30, 4607–4616. doi: 10.1093/cercor/bhaa063
Xu, K., Liu, R., Chen, X., Yang, Y., and Wang, Q. (2023). Brain structure variability study in pilots based on VBM. PLoS One 18:e0276957. doi: 10.1371/journal.pone.0276957
Ye, L., Ba, L., and Yan, D. (2025). A study of dynamic functional connectivity changes in flight trainees based on a triple network model. Sci. Rep. 15:7828. doi: 10.1038/s41598-025-89023-y
Keywords: structural-functional coupling, flying experience, neuroplasticity, function connectivity, graph signal processing
Citation: Chen X, Meng Q, Song Q, Xu P, Zhang S, Huang D, Chu Q, Fan J, Luo C and Li X (2025) Neuroadaptive changes in brain structural–functional coupling among pilots. Front. Neurosci. 19:1608739. doi: 10.3389/fnins.2025.1608739
Received: 09 April 2025; Accepted: 04 July 2025;
Published: 24 July 2025.
Edited by:
Yilong Ma, Feinstein Institutes for Medical Research, United StatesReviewed by:
Elizabeth Hernández-Echeagaray, National Autonomous University of Mexico, MexicoFloris L. Wuyts, University of Antwerp, Belgium
Gilberto Uriel Rosas Sánchez, Universidad Veracruzana, Mexico
Meng Yu, Civil Aviation Flight University of China, China
Copyright © 2025 Chen, Meng, Song, Xu, Zhang, Huang, Chu, Fan, Luo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donglin Huang, aHVhbmdkb25nbGluMjAyNEAxNjMuY29t
 Xi Chen
Xi Chen Qingbin Meng
Qingbin Meng Qingsong Song2
Qingsong Song2 Shicong Zhang
Shicong Zhang Cheng Luo
Cheng Luo