Abstract
Subjective tinnitus is defined as the perception of sound in the absence of an external acoustic source, characterized by the phantom and persistent experience of noise or indistinct, internally generated non-verbal tones. The prevalence of chronic tinnitus is estimated to range from 5 to 42% of the global population. As a significant individual and societal issue, preclinical and clinical studies have been conducted to investigate this condition and explore potential therapeutic approaches. However, a comprehensive understanding of tinnitus and a definitive treatment remain elusive. In most cases, tinnitus arises from acquired and sustained hearing loss. However, the precise mechanisms and neuronal network models responsible for the perceptual generation and persistence of tinnitus are not yet fully understood. Animal studies have demonstrated that tinnitus is associated with synaptic plasticity dysfunction in multiple brain regions, including the auditory and limbic systems. Attention has been devoted to the contribution of inflammatory cytokines and deregulated levels of Brain-Derived Neurotrophic Factor (BDNF) to the pathogenesis of tinnitus. This narrative review aims to elucidate the functional structures and biological mechanisms underlying tinnitus and propose alternative novel therapeutic approaches.
1 Introduction
Tinnitus is the perception of sound in the absence of an external acoustic source, representing a phantom auditory experience without a corresponding acoustic signal or mechanism in the cochlea (Han et al., 2021; Messina et al., 2022). The prevalence of tinnitus varies across countries, largely due to differing definitions of the condition and challenges in measuring its severity (Han et al., 2021). A recent meta-analysis revealed that tinnitus affects 14% of adults globally, rising to 24% in older adults (Jarach et al., 2022). 10–60% of individuals with chronic tinnitus experience depressive disorders, and 28–45% exhibit significant symptoms of anxiety (Patil et al., 2023). Severe tinnitus is strongly associated also with insomnia, difficulty concentrating, reduced emotional and psychological wellbeing, and poor quality of life. In extreme cases, it can lead to functional disability and cognitive impairment (Blazer and Tucci, 2019). The unclear pathogenesis of tinnitus makes accurate diagnosis and effective treatment challenging. To date, no medications have been specifically approved by the U.S. Food and Drug Administration for the treatment of tinnitus (Kim et al., 2021).
This narrative review provides a concise overview of the mechanisms underlying tinnitus and the key molecular players involved, with a particular emphasis on Brain-Derived Neurotrophic Factor (BDNF). We conducted a comprehensive review of studies across three electronic medical databases: PubMed, EMBASE, and Web of Science. The keywords used were “Tinnitus,” and “Central auditory system,” “Limbic System,” “Inflammatory cytokines,” “Neuroplasticity,” “BDNF,” “Low-Dose Medicine.” We included studies published in English with available abstracts and excluded case reports. We meticulously analyzed the full-text articles, selecting the most pertinent studies for inclusion in this review (Figure 1). The narrative review refers to papers searched up to February 22, 2025.
Figure 1

Diagram of the literature selection process. The search has been conducted from PubMed, EMBASE, and Web of Science. No relevant records were obtained from other sources. After exclusion of duplicates (11), 43 studies were excluded for the following reasons: editorial letters, book articles and not relevant topics. Therefore, 164 studies were eligible for full test screening. After exclusion of 71 studies for unrelated and redundant topics, a total of 93 studies were included in our study.
2 Understanding tinnitus: emerging insights into etiopathogenesis
The precise etiopathogenesis of tinnitus remains incompletely understood, with highly diverse underlying causes, as summarized in Figure 2. Tinnitus frequently occurs following damage to the auditory system, particularly due to exposure to excessive sound pressure levels. Evidence from both animal models and human studies has established a strong association between noise-induced hearing loss and tinnitus development (Henton and Tzounopoulos, 2021). Indeed, cochlear hair cells injured by loud sounds as well as by aging convert the aberrant sound vibrations into abnormal electrical signals, which the brain misinterprets as sound, leading to the perception of tinnitus (Fettiplace, 2017). This highlights the necessity of addressing hearing loss as a key factor in tinnitus prevention and management. Also, metabolic dysfunctions can lead to hearing loss and contribute to the generation or exacerbation of tinnitus through various pathways, including microvascular damage, neuropathy, direct effects on cellular metabolism within the inner ear, and systemic inflammation. Recently, hypertriglyceridemia and high total cholesterol/high-density lipoprotein cholesterol ratio were associated with increased risk of tinnitus in a Korean population (Lee et al., 2024). In addition, diabetes is strongly associated with an increased risk of tinnitus (Mousavi et al., 2021) through various mechanisms among which vascular damage, nerve damage, oxidative stress and low-grade systemic inflammation. Of interest, functional magnetic resonance imaging in type 2 diabetic individuals underscored a relation between the levels of Hemoglobin A1c, used to monitor glucose control, and decreased cerebral blood flow patterns in the right medial prefrontal gyrus, a region that is implicated not only in the auditory perception of tinnitus but also in how individuals experience and cope with it emotionally and cognitively (Xia et al., 2021).
Figure 2

Risk factors contributing to tinnitus. Tinnitus induction is heterogeneous in nature as the onset can occur due to psychological and neurological issues, physical trauma, lifestyle and genetic susceptibility, metabolic dysfunctions, sensorineural hearing loss, conductive hearing loss, cochlear vascular dysfunction, ototoxicity, and otosclerosis.
Other contributors to tinnitus include environmental toxins, ototoxic medications (e.g., aminoglycosides, cisplatin, ethacrynic acid, furosemide, and salicylates) (Campo et al., 2013), and pathological conditions such as Ménière’s disease (Yoshida et al., 2011) and vestibular schwannoma (King et al., 2024). Risk factors for tinnitus extend beyond direct damage to the auditory system to include head trauma, ear infections, lifestyle habits, psychological problems, affective and psycho-emotional disorders, multiple physical or mental health conditions, and aging (Fagelson, 2022). Tinnitus may also be triggered by adverse life events such as grief, divorce, job loss, unemployment, unwanted retirement, and emotional stress (Han et al., 2021). Among these, post-traumatic stress disorder is particularly notable, affecting hundreds of millions globally, including approximately 1.45 billion people who survived conflicts between 1989 and 2015 (Patil et al., 2023).
Questions have arisen regarding a possible correlation between COVID-19 vaccination and the development of tinnitus (Ahmed et al., 2022). A systematic review by Arabzadeh Bahri et al. (2024) reported that patients who received COVID-19 vaccines occasionally experienced sensory disturbances, including smell or taste disorders, aural fullness, tinnitus, and headaches following the first or second vaccine dose. Conversely, a retrospective study by Aldè et al. (2023) demonstrated the efficacy of the COVID-19 vaccine in reducing vestibular and auditory disorders, including tinnitus, in a pediatric population with normal hearing or unilateral hearing loss. Among vaccinated children, the incidence of tinnitus was 1.4%, with symptoms resolving spontaneously within 24 h. In contrast, children who contracted SARS-CoV-2 showed a higher tinnitus incidence of 8.3%, with symptoms persisting for up to 5 days.
While no single “tinnitus gene” has been conclusively identified, evidence suggests that certain genetic variations may contribute to its development. Genetic predisposition appears to play a role in tinnitus severity, with specific genes linked to increased susceptibility (Amanat et al., 2020). Notably, a potential association has been identified between tinnitus and the BDNF Val66Met polymorphism, which introduces a functional missense mutation that impairs BDNF secretion from synapses (Coskunoglu et al., 2017; Yuksel et al., 2023). The BDNF gene is regulated by the BDNF antisense RNA gene (BDNF-AS), located downstream of BDNF. Polymorphisms in BDNF-AS are known to influence the auditory pathway and may increase the risk of chronic tinnitus (Yuksel et al., 2023). As a result, BDNF and BDNF-AS are emerging as candidate genes with the potential to significantly impact auditory performance.
3 Tinnitus: a multifaceted neurological phenomenon
The initial theory regarding the origin of tinnitus suggested a cochlear basis. However, this perspective has evolved over time, with current understanding pointing to the involvement of not only the peripheral but also the central auditory system (Figure 3), and beyond to involve areas integrating auditory perception with emotional, attentional, and memory processes.
Figure 3

Central auditory system. The central auditory system originates from the cochlear nerve, which transmits auditory information from the cochlea to higher-order auditory structures in the brain for processing.
Many researchers support the hypothesis that cochlear damage triggers tinnitus by inducing abnormal hyperactivity in key areas, including the dorsal cochlear nucleus, thought to be a tinnitus-generating site, the inferior colliculus (IC), which integrates auditory pathway signals, the medial geniculate nucleus in the thalamus, a crucial hub for processing auditory signals, and the auditory cortex (Xu et al., 2019). This process is accompanied by reorganization of the central auditory system’s tonotopic map, disrupting the balance between excitatory and inhibitory neurotransmission across associated networks. Further research has demonstrated that tinnitus also engages non auditory brain areas, particularly those associated with attention, memory, emotional regulation, and cognitive processing such as the limbic system (Henton and Tzounopoulos, 2021; Singh et al., 2023; Xu et al., 2019; Davis and Johnsrude, 2003; Watanabe et al., 2008), the prefrontal cortex, the parietal lobes, the Default Mode Network (DMN) (Smitha et al., 2017; Shao et al., 2019) and the Dorsal Attention Network (DAN) (Rosemann and Rauschecker, 2023).
Hints about the involvement of some of these areas derive from clinical data. Notably, clinical cases of tinnitus cessation following cerebrovascular trauma affecting the caudate nucleus and putamen (Lowry et al., 2004) suggest the potential of striatal neuromodulation in tinnitus treatment. Abnormal excitability in the nucleus accumbens has been associated with emotional responses to sound and the severity of tinnitus and hyperacusis (Mahoney et al., 2011; Asokan et al., 2018). The cingulum cortex, integral to emotional reactivity, processing, and inhibitory control (Fan et al., 2024), has been linked to discomfort experienced by tinnitus patients (Singh et al., 2023). In tinnitus, disrupted functional connectivity within the auditory cortex, thalamic, limbic, and prefrontal regions, and networks like the DMN and DAN, has been observed (Rosemann and Rauschecker, 2023). Studies reveal reduced connectivity between DMN regions, such as the precuneus and superior parietal lobe, in patients with bothersome tinnitus (Schmidt et al., 2017). Conversely, long-term tinnitus has been associated with increased connectivity within the DAN.
Animal models of tinnitus are commonly induced using salicylates or prolonged exposure to high-intensity noise, both of which primarily affect outer hair cells by reducing their electromotility and leading to hyperactivity in the auditory cortex (Henton and Tzounopoulos, 2021). Dynamic imaging of cerebral microvascularization has identified the anterior cingulate cortex as a critical region involved in salicylate-induced tinnitus (Fan et al., 2024). Animal studies have demonstrated that tinnitus is closely associated with alterations in the synaptic plasticity of the limbic areas, particularly the hippocampus. This altered plasticity, which refers to the brain’s ability to strengthen or weaken connections between neurons, may impair the auditory gating mechanism.
Dysregulated neuroplasticity is mediated by the imbalance between various neurotransmitters. We rapidly overview the role of excitatory/inhibitory balance (glutamate vs. GABA) and neuromodulators like dopamine and endocannabinoids in tinnitus. In rats with noise-induced tinnitus, whole-cell patch-clamp recordings show that prolonged exposure to high-intensity sound does not change glutamatergic excitatory transmission while increasing the amplitude of inhibitory currents mediated by Gamma-Amino Butyric Acid (GABA), an inhibitory neurotransmitter which is present throughout multiple levels of the auditory pathway (Cunha et al., 2019). In the same experimental model, magnetic resonance spectroscopy (Brozoski et al., 2012; Isler et al., 2022) reveals decreased GABA levels in the medial geniculate body and systemic treatment with GABA agonists effectively suppresses behavioral evidence of tinnitus (Brozoski et al., 2007). These studies underscore the relevance of integrating various technical approaches to investigate various phenomena in the brains. In individuals with tinnitus, a human MRS study identified a deficiency of GABA in the right auditory cortex compared to healthy controls, and indicate that loud sounds may disrupt inhibitory transmission with consequent increased excitability of the auditory pathway (Sedley et al., 2015) (Figure 4).
Figure 4

Dysregulation of neurotransmitter systems in the auditory pathway leading to tinnitus. Impaired GABAergic transmission, including altered GABA levels and inhibitory currents, leads to increased neuronal excitability in the auditory pathway. NMDA receptor dysfunction enhances glutamatergic activity and synaptic plasticity, while disruption of endocannabinoid signaling reduces inhibitory control over both GABA and glutamate release. Dopaminergic neurons in the ventral tegmental area (VTA) send projections to limbic structures, where dopamine modulates the processing of auditory signals. Together, these alterations contribute to a hyperexcitable auditory network, which underlies aberrant auditory processing.
Glutamate is the most common excitatory neurotransmitter in the auditory system and N-methyl-d-aspartate receptors (NMDARs) are the most widely distributed glutamate receptors in the auditory system. In animal models of tinnitus NMDARs are overactivated and tinnitus is relieved by NMDAR antagonists (Ruel et al., 2008; Ralli et al., 2014) (Figure 4). Surprisingly, reduced Glutamate concentration in the right auditory cortex was measured in humans with tinnitus without clinical relevant hearing loss (Isler et al., 2022). Clinical trials with NMDAR antagonists to treat tinnitus yielded contrasting results (Figueiredo et al., 2008; Suckfüll et al., 2011), probably because tinnitus is multifactorial and heterogeneous. We anticipate that future studies should stratify patients based on etiology to assess the pharmacological efficacy of NMDAR antagonists for tinnitus.
Endocannabinoid signaling also plays a critical role in modulating synaptic plasticity (Viveros et al., 2007). CB1 receptors, which are highly expressed in brain regions such as the auditory cortex, neocortex, hippocampus, basal ganglia, cerebellum, and brainstem, influence auditory function by modulating glutamatergic, glycinergic, and cholinergic neurotransmission (Bhat et al., 2023). In an experimental model of salicylate-induced tinnitus, CB1 receptors contribute to enhanced excitation in the dorsal cochlear nucleus circuitry (Smith and Zheng, 2016) (Figure 4). Research on the link between tinnitus and cannabinoid use in humans has yielded inconsistent results (Perin et al., 2020).
Dopamine, a key neurotransmitter in the basal ganglia, has been implicated in tinnitus development by modulating auditory pathway neurotransmission between the limbic system and the cochlea (Lopez-Gonzalez and Esteban-Ortega, 2005). Dopaminergic neurons in the ventral tegmental area project to the limbic structures, where dopamine influences auditory signal processing. Notably, injecting dopaminergic agonists into the nucleus accumbens has been shown to significantly reduce auditory gating in the hippocampus, suggesting that dopaminergic neurotransmission within the limbic system may contribute to tinnitus perception at the conscious level (Singh et al., 2023).
3.1 Tinnitus: the role of inflammatory cytokines
A systematic review of nine animal and 20 human studies reported changes in the primary and secondary auditory cortices, where increases in Tumor Necrosis Factor (TNF)-α and Interleukin (IL)-1β are accompanied by reductions in Interferon (IFN)-γ and astrocytic microglial activation, while IL-6 levels remain unchanged (Mennink et al., 2022). Tinnitus inducing noise triggers inflammasome activation, particularly NLRP3, within hours and sustained IL-18 elevation up to 10 days after noise exposure (Wang et al., 2019). In rodents, increased levels of TNF-α and IL-1β in the cochlea and dorsal cochlear nucleus, accompanied by astrocytic activation, were described (Mennink et al., 2022). Elevated levels of pro-inflammatory cytokines such as TNF-α and IL-1β have also been observed in the IC and medial geniculate body (MGB), a structure that integrates auditory inputs from the IC, reticular nucleus, and limbic structures while projecting to the auditory cortex, and TNF-α knock-out mice do not develop noise-induced tinnitus (Henton and Tzounopoulos, 2021). Of note, TNF-α and IL-1β directly affect synaptic plasticity along the auditory pathway. TNF-α induces endocytosis of GABA-A receptors, reducing inhibitory synapses and enhancing excitatory neurotransmission (Pribiag and Stellwagen, 2013), and IL-1β suppresses GABAergic currents (Wang et al., 2000). Severe tinnitus generates a vicious cycle because, as a source of stress, it activates the Hypothalamus-Pituitary–Adrenal axis, leading to increased levels of cortisol, reduced BDNF, and elevated norepinephrine, serotonin, and 5-hydroxyindoleacetic acid (Patil et al., 2023). Cortisol release may exacerbate the activity of the glutamate receptors NMDA and Amino-Methyl Phosphonic Acid (AMPA), contributing to worsen tinnitus (Patil et al., 2023).
Clinical studies have yielded mixed results regarding cytokine levels in individuals with tinnitus. Weber et al. reported significantly elevated IL-6 levels in tinnitus patients (Weber et al., 2002), while Heider et al. found no changes in IL-6 but observed reduced IL-10 levels (Haider et al., 2020). Interestingly, IL-10 levels increased with the chronicization of tinnitus, while levels of IL-1α, IL-1β, IL-2, IFN-γ, TNF-α, and Transforming Growth Factor (TGF)-β remained unchanged (Weber et al., 2002; Haider et al., 2020). Discrepancies between animal and human studies could arise from differences in measurement timing relative to tinnitus onset. Animal studies often measure TNF-α immediately after tinnitus induction, while clinical studies assess cytokines in chronic tinnitus patients, potentially after TNF-α levels have normalized. Moreover, human studies measure cytokine levels in blood, which may not reflect localized changes in auditory structures.
Mediators released by platelets appear to play a role in tinnitus onset. Notably, increased mean platelet volume and platelet distribution width have been observed in tinnitus patients (Yazici and Cihan, 2023) and may result in impaired perfusion of the inner ear and tinnitus (Kemal et al., 2016). Moreover, larger platelets are metabolically and enzymatically more active, contain more granules and produce higher levels of biologically active factors, including vasoactive and thrombotic molecules (Bekler et al., 2015). Activated platelets release cytokines like IL-6, IL-8, and TNF-α within minutes, potentially contributing to prothrombotic conditions or thrombotic events in the internal auditory artery, leading to cochlear hypoperfusion and tinnitus. Additionally, platelets, which have glutamate transporters, remove glutamate from the bloodstream and a reduced glutamate uptake, as reported in aging, may exacerbate tinnitus by increasing excitotoxicity.
3.2 Tinnitus: the role of BDNF
BDNF (Brain-Derived Neurotrophic Factor) plays a crucial role in maintaining a healthy brain. It is essential for neurogenesis, synaptic plasticity, and neuronal survival. These functions are vital for cognitive abilities, emotional regulation, and even resilience to stress. Given its importance, when BDNF function is disrupted, it can lead to cognitive impairment and is implicated in various mental illnesses, including depression (Numakawa and Kajihara, 2025).
As mentioned above, genetic studies suggest that dysregulated BDNF may play a role in the development of tinnitus. BDNF, the most prevalent neurotrophin in mammals’ brain, is widely expressed in the central and peripheral nervous systems. It plays a crucial role in various biological processes (Table 1), including neuronal survival, growth, differentiation, and synaptic plasticity also in auditory system. BDNF supports the survival and growth of afferent fibers that connect the vestibular organ to the cochlear regions responsible for mapping low-frequency sound information to central auditory nuclei and higher auditory centers (Singer et al., 2014).
Table 1
| Brain-Derived Neurotrophic Factor activity | |
|---|---|
| Neuronal survival | Promotes survival of existing neurons and prevents cell death. |
| Neurogenesis | Stimulates the growth and differentiation of new neurons from neural stem cells. |
| Axonal and dendritic growth | Enhances outgrowth and branching of axons and dendrites, potentiating connectivity. |
| Synaptogenesis and synaptic stability | Stimulates generation of new synapses and enhances synaptic strength and stability. |
| Synaptic plasticity | Improves synaptic plasticity, essential for learning and memory. |
| Anti-inflammatory activity | Modulates release of proinflammatory cytokines. |
| Anti-Reactive Oxygen Species (ROS) activity | Counteracts ROS production and maintains redox homeostasis. |
| Anti-degenerative and anti-aging activity | Reduces Tau phosphorylation and promotes β-Amyloid protein uptake and degradation thus supporting brain health during aging. |
| Modulation dopaminergic, serotoninergic, cholinergic and GABAergic signaling | Modulates dopaminergic, serotoninergic, cholinergic circuits and GABAergic signaling. |
| Development of neuroprotective mechanisms. | Restores long-term-potentiation and activates CREB leading to increased expression of genes encoding proteins involved in neuroprotection. |
Main activities of Brain-Derived Neurotrophic Factor.
BDNF is initially synthesized as a precursor protein, proBDNF, which is cleaved into its mature form (mBDNF). These two forms exert distinct and sometimes opposing effects on neuronal function. mBDNF primarily signals through the tropomyosin-related kinase B (TrkB) receptor, triggering pathways involved in neuronal survival, differentiation, and synaptic plasticity (Brigadski and Leßmann, 2020). In contrast, proBDNF binds to the p75 neurotrophin receptor (p75NTR), typically promoting neuronal apoptosis, synaptic pruning, and neurodevelopmental remodeling (Ali et al., 2024). During early development, proBDNF plays a key role in neuro-glial interactions, synaptogenesis, apoptosis regulation, and the elimination of improperly formed connections. In adulthood, mBDNF contributes to neurotransmission efficiency by promoting neuritic growth, dendritic arborization, dendritic spine development, and the downregulation of GABA receptor expression, reducing GABAergic interneuron excitability. Additionally, mBDNF enhances neuroprotection by mitigating glutamatergic excitotoxicity, reducing Ca2+ influx, and activating cAMP response element-binding protein (CREB), which promotes the expression of neuroprotective genes. Furthermore, BDNF has been shown to prevent cellular damage and neuronal loss following permanent sensorineural hearing impairment (Schimmang et al., 2003; Barclay et al., 2011; Wong and Ryan, 2015).
As a key modulator of neuroplasticity, BDNF counteracts the effects of pro-inflammatory cytokines (Charlton et al., 2023), which are critical mediators of neurodegeneration. BDNF also serves as a biomarker of stress-related responses; acute stress elevates BDNF levels (Linz et al., 2019), whereas chronic stress leads to BDNF downregulation (Robinson et al., 2021). Cortisol appears to regulate BDNF expression (Issa et al., 2010), with its daily fluctuation mirroring the cortisol circadian rhythm—peaking at approximately 8:00 a.m. and gradually decreasing throughout the day (Ehrhardt et al., 2024). Notably, physical exercise enhances BDNF release in the brain (Sleiman et al., 2016).
In healthy individuals, average plasma BDNF levels are approximately 92.5 pg/mL (range: 8.0–927.0 pg/mL), with higher concentrations observed in women. However, BDNF levels decline with advancing age and increased body weight in both sexes (Lommatzsch et al., 2005; Roh et al., 2022). Reduced circulating BDNF is associated with the onset of several neurodegenerative diseases, including Alzheimer’s, Parkinson’s, Multiple Sclerosis, and Huntington’s disease (Ibrahim et al., 2022), as well as psychiatric disorders such as major depressive disorder (Wang et al., 2011; Mojtabavi et al., 2020). Also, tinnitus patients exhibit decreased serum and hair levels of BDNF (Coskunoglu et al., 2017; Ranjbar et al., 2023), suggesting a potential link between tinnitus pathophysiology and BDNF signaling. Furthermore, specific single nucleotide polymorphisms (SNPs) in the BDNF gene, including rs6265 (Val66Met), rs2030324, and rs1491850, have been associated with altered BDNF serum levels and brainstem auditory evoked response (BAER) test results (Yuksel et al., 2023).
Experimental studies indicate that changes in BDNF expression influence tinnitus-like behavior. Noise-induced hearing loss, a common precursor to tinnitus, is associated with altered BDNF levels in the auditory cortex and other brain regions. Moreover, the limbic system, which plays a crucial role in the emotional and cognitive aspects of tinnitus, is particularly susceptible to BDNF dysregulation (Camuso et al., 2022). Altered BDNF signaling in limbic structures such as the hippocampus and amygdala may contribute to the heightened distress often experienced by individuals with tinnitus. The role of reduced levels of BDNF in the pathophysiology of tinnitus are summarized in Figure 5.
Figure 5
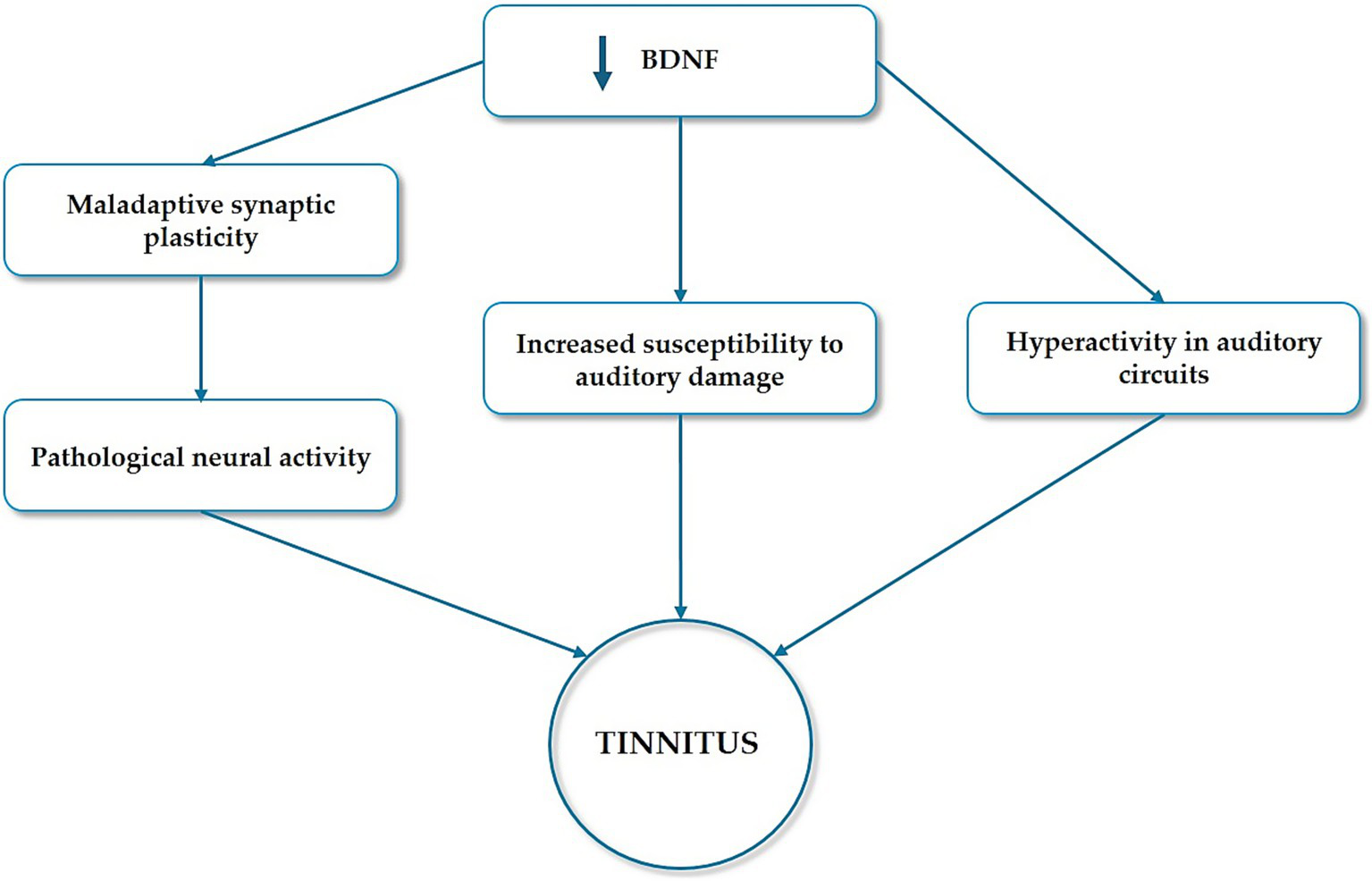
The role of BDNF dysregulation in tinnitus pathophysiology. Tinnitus is believed to result from maladaptive synaptic plasticity in the auditory and limbic systems. As a key regulator of synaptic plasticity, BDNF dysregulation may contribute to the pathological neural activity underlying tinnitus. Moreover, BDNF supports the survival and function of neurons in the auditory pathway. Reduced levels of BDNF have been implicated in increased susceptibility to auditory damage and the subsequent development of tinnitus. Additionally, tinnitus is often associated with hearing loss, during which BDNF-mediated compensatory mechanisms in the auditory system may lead to hyperactivity in auditory circuits, a hallmark of tinnitus.
4 BDNF as a potential therapeutic strategy for tinnitus
The regulation of BDNF levels presents a promising avenue for tinnitus treatment. Notably, physical exercise and specific auditory training protocols have been shown to elevate BDNF levels, potentially mitigating tinnitus severity (Carpenter-Thompson et al., 2015). However, effective delivery of BDNF to targeted brain regions remains a significant challenge due to its short half-life. Interestingly, circulating BDNF is capable of crossing the Blood–Brain Barrier (BBB) via a rapid, saturable transport system (Pan et al., 1998). This efficient unidirectional transport suggests that peripheral administration of BDNF could facilitate its entry into the Central Nervous System (CNS), thereby promoting neuronal regeneration and repair.
In recent years, the administration of exogenous BDNF has been explored as a therapeutic strategy for neurodegenerative diseases. Both human and animal studies have demonstrated that BDNF supplementation is associated with improved cognitive function and reduced synaptic dysfunction. In developing neural circuits, exogenous BDNF has been shown to enhance dendritic growth, thereby increasing the complexity of pyramidal neurons, particularly in the visual cortex (Bathina and Das, 2015). Additionally, exogenous BDNF administration has been found to prevent and mitigate neurodegenerative changes associated with brain aging, restoring long-term potentiation and spatial memory in aged animal models (Nagahara et al., 2009).
Despite its therapeutic potential, the administration of exogenous BDNF presents several challenges (Table 2), primarily related to its bioavailability in the brain. If the delivered concentration is insufficient, it may fail to induce the desired neuroprotective effects. Conversely, excessive BDNF levels could have paradoxical consequences, including the downregulation of TrkB receptors and a subsequent reduction in intracellular signaling pathways crucial for neuronal function and plasticity. These limitations underscore the need for novel strategies to optimize BDNF delivery and regulation, potentially enhancing its therapeutic utility for tinnitus and other neurodegenerative conditions. In rodents and primates, oral administration of BDNF has yielded limited success due to its molecular properties, including its moderate size and charge, which hinder its transport across the intestinal barrier and BBB (Nagahara and Tuszynski, 2011). Previous studies have identified additional limitations associated with neurotrophin administration in humans, particularly concerning dosage and pharmacokinetics (Thoenen and Sendtner, 2002). A promising approach to achieving precise physiological regulation involves the use of low-dose substances, as supported by extensive literature (Gariboldi et al., 2009; Uberti et al., 2018). Low Dose Medicine (LDM) emerged from the intersection of molecular biology and psycho-neuro-endocrine-immunology, initially evolving from research in nanopharmacology (Fioranelli and Roccia, 2014; Bernasconi, 2018). The mechanism of action of low-dose cytokines, hormones, neuropeptides, and growth factors is based on the sensitization or activation of cellular and plasma receptors, driven by their high dilution. These molecules operate within a physiological range, from approximately 10−6 M (micrograms) for hormones to 10−15 M (femtograms) for other signaling molecules (Castiglioni et al., 2017; Cazzaniga et al., 2024). Nearly two decades of research on LDM has validated its conceptual foundation and demonstrated the efficacy and safety of therapeutic interventions using orally administered sub-nanomolar doses of signaling molecules, as exemplified by low-dose IL-2 therapy. Low-dose IL-2 therapy, a novel immunomodulatory strategy that re-establish immune homeostasis, is an emerging and promising treatment strategy for various autoimmune, inflammatory, and, increasingly, neurodegenerative diseases (He et al., 2020; Yuan et al., 2023; Zhang et al., 2024). This approach specifically targets and expands regulatory T cells (Matsuoka et al., 2013), thus offering a more precise therapeutic intervention with a promising efficacy and safety profile across a growing spectrum of immune-mediated and neurodegenerative disorders.
Table 2
| Aspect | Challenge | Possible solution |
|---|---|---|
| Delivery to CNS | Limited blood–brain barrier permeability of BDNF | Potential use of low-dose BDNF? |
| Pharmacokinetics | Short half-life of BDNF | Use of low-dose, high-frequency regimens to maintain stable levels |
| Dosage optimization | High doses may cause receptor desensitization or paradoxical effects | Potential use of low dose BDNF? |
| Heterogeneity of tinnitus | Multifactorial etiology complicates treatment response | Stratified clinical trials based on genetic, neurophysiological, and psychological profiles |
| Clinical evidence | Only preclinical data | Planned clinical trials approved by AIFA/EMA |
Challenges and opportunities of BDNF-based therapy in tinnitus.
Low-dose BDNF may represent a novel therapeutic avenue in tinnitus management. Recently, low-dose BDNF has been approved by the Agenzia Italiana del Farmaco-AIFA for therapeutic use, and clinical trials are planned in the near future. In an experimental model, the evidence that low-dose BDNF exhibits a high capacity to traverse the enterohepatic circulation and remains detectable in the bloodstream for at least 24 h (Molinari et al., 2020) suggests that it may represent a promising candidate for therapeutic supplementation. This study also shows that in the brain low-dose BDNF maintains redox homeostasis, limits the effects of pro-inflammatory cytokines, significantly increases astrocytic vitality, reduces Tau phosphorylation and promotes β-Amyloid protein uptake and degradation. Additionally, low-dose BDNF treatment for 24 h led to an upregulation of endogenous BDNF expression, indicating its potential to enhance endogenous BDNF production through stimulation and induction mechanisms. Randomized controlled clinical trials are necessary to validate the efficacy of low dose BDNF in restoring impaired functions and modulating neuroplasticity.
5 Conclusion
Tinnitus is characterized by dysfunctions in synaptic plasticity across multiple brain regions. Understanding this widespread neural involvement is crucial for developing effective therapeutic strategies. Given the central role of BDNF in regulating neuroplasticity, alterations in BDNF levels may contribute to the pathogenesis of tinnitus. Consequently, the administration of exogenous BDNF—already investigated in the context of neurodegenerative disorders—could represent a promising approach for tinnitus treatment. While challenges in its clinical translation remain, preliminary evidence supports the potential of low-dose BDNF therapy as a novel intervention. Rigorous, well-designed research focusing on mechanistic insights, optimized delivery, and stratified clinical trials is crucial to unlock the full therapeutic potential of low-dose BDNF for individuals suffering from this debilitating condition.
Statements
Author contributions
VM: Conceptualization, Writing – original draft, Writing – review & editing, Validation. SC: Writing – review & editing, Validation. JM: Writing – review & editing, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank Dr. Michele Nichelatti for his suggestions in elaborating Figure 1.
Conflict of interest
VM was employed by GUNA S.p.a.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ahmed S. H. Waseem S. Shaikh T. G. Qadir N. A. Siddiqui S. A. Ullah I. et al . (2022). SARS-CoV-2 vaccine-associated-tinnitus: a review. Ann. Med. Surg.75:103293. doi: 10.1016/j.amsu.2022.103293
2
Aldè M. Di Berardino F. Ambrosetti U. Barozzi S. Piatti G. Zanetti D. et al . (2023). Audiological and vestibular symptoms following SARS-CoV-2 infection and COVID-19 vaccination in children aged 5–11 years. Am. J. Otolaryngol.44:103669. doi: 10.1016/j.amjoto.2022.103669
3
Ali N. H. Al-kuraishy H. M. Al-Gareeb A. I. Alnaaim S. A. Saad H. M. Batiha G. E.-S. (2024). The molecular pathway of p75 Neurotrophin receptor (p75NTR) in Parkinson’s disease: the way of new inroads. Mol. Neurobiol.61, 2469–2480. doi: 10.1007/s12035-023-03727-8
4
Amanat S. Gallego-Martinez A. Lopez-Escamez J. A. (2020). Genetic inheritance and its contribution to tinnitus. Curr. Top Behav. Neurosci., 29–47. doi: 10.1007/7854_2020_155
5
Arabzadeh Bahri R. Esmaeilpur Abianeh F. Peisepar M. Hadi R. Basti F. A. Maleki S. et al . (2024). Anosmia or Ageusia following COVID-19 vaccination: a systematic review. Ear Nose Throat J.103, 164S–170S. doi: 10.1177/01455613241233098
6
Asokan M. M. Williamson R. S. Hancock K. E. Polley D. B. (2018). Sensory overamplification in layer 5 auditory corticofugal projection neurons following cochlear nerve synaptic damage. Nat. Commun.9:2468. doi: 10.1038/s41467-018-04852-y
7
Barclay M. Ryan A. F. Housley G. D. (2011). Type I vs type II spiral ganglion neurons exhibit differential survival and neuritogenesis during cochlear development. Neural Dev.6:33. doi: 10.1186/1749-8104-6-33
8
Bathina S. Das U. N. (2015). Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci.6, 1164–1178. doi: 10.5114/aoms.2015.56342
9
Bekler A. Ozkan M. T. A. Tenekecioglu E. Gazi E. Yener A. U. Temiz A. et al . (2015). Increased platelet distribution width is associated with severity of coronary artery disease in patients with acute coronary syndrome. Angiology66, 638–643. doi: 10.1177/0003319714545779
10
Bernasconi S. (2018). Low dose medicine: theoretical background and scientific evidence. Ital. J. Pediatr.44:23. doi: 10.1186/s13052-018-0460-6
11
Bhat V. Onaivi E. S. Sharma V. (2023). Endocannabinoid system components as potential neuroimmune therapeutic targets in tinnitus. Front. Neurol.14:1148327. doi: 10.3389/fneur.2023.1148327
12
Blazer D. G. Tucci D. L. (2019). Hearing loss and psychiatric disorders: a review. Psychol. Med.49, 891–897. doi: 10.1017/S0033291718003409
13
Brigadski T. Leßmann V. (2020). The physiology of regulated BDNF release. Cell Tissue Res.382, 15–45. doi: 10.1007/s00441-020-03253-2
14
Brozoski T. J. Spires T. J. D. Bauer C. A. (2007). Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J. Assoc. Res. Otolaryngol.8, 105–118. doi: 10.1007/s10162-006-0067-2
15
Brozoski T. Odintsov B. Bauer C. (2012). Gamma-aminobutyric acid and glutamic acid levels in the auditory pathway of rats with chronic tinnitus: a direct determination using high resolution point-resolved proton magnetic resonance spectroscopy (1H-MRS). Front. Syst. Neurosci.6:9. doi: 10.3389/fnsys.2012.00009
16
Campo P. Morata T. C. Hong O. (2013). Chemical exposure and hearing loss. Dis. Mon.59, 119–138. doi: 10.1016/j.disamonth.2013.01.003
17
Camuso S. La Rosa P. Fiorenza M. T. Canterini S. (2022). Pleiotropic effects of BDNF on the cerebellum and hippocampus: implications for neurodevelopmental disorders. Neurobiol. Dis.163:105606. doi: 10.1016/j.nbd.2021.105606
18
Carpenter-Thompson J. R. McAuley E. Husain F. T. (2015). Physical activity, tinnitus severity, and improved quality of life. Ear Hear.36, 574–581. doi: 10.1097/AUD.0000000000000169
19
Castiglioni S. Miranda V. Cazzaniga A. Campanella M. Nichelatti M. Andena M. et al . (2017). Femtograms of interferon-γ suffice to modulate the behavior of Jurkat cells: a new light in immunomodulation. Int. J. Mol. Sci.18:2715. doi: 10.3390/ijms18122715
20
Cazzaniga A. Miranda V. Castiglioni S. Maier J. (2024). The different effects of pharmacological or low-doses of IFN-γ on endothelial cells are mediated by distinct intracellular signalling pathways. J. Biol. Regul. Homeost. Agents38, 111–122. doi: 10.23812/j.biol.regul.homeost.agents.20243801.8
21
Charlton T. Prowse N. McFee A. Heiratifar N. Fortin T. Paquette C. et al . (2023). Brain-derived neurotrophic factor (BDNF) has direct anti-inflammatory effects on microglia. Front. Cell. Neurosci.17:1188672. doi: 10.3389/fncel.2023.1188672
22
Coskunoglu A. Orenay-Boyacioglu S. Deveci A. Bayam M. Onur E. Onan A. et al . (2017). Evidence of associations between brain-derived neurotrophic factor (BDNF) serum levels and gene polymorphisms with tinnitus. Noise Health19, 140–148. doi: 10.4103/nah.NAH_74_16
23
Cunha A. O. S. de Deus J. L. Ceballos C. C. Leão R. M. (2019). Increased hippocampal GABAergic inhibition after long-term high-intensity sound exposure. PLoS One14:e0210451. doi: 10.1371/journal.pone.0210451
24
Davis M. H. Johnsrude I. S. (2003). Hierarchical Processing in Spoken Language Comprehension. The Journal of Neuroscience23, 3423–3431. doi: 10.1523/JNEUROSCI.23-08-03423.2003
25
Ehrhardt M. Schreiber S. Duderstadt Y. Braun-Dullaeus R. Borucki K. Brigadski T. et al . (2024). Circadian rhythm of brain-derived neurotrophic factor in serum and plasma. Exp. Physiol.109, 1755–1767. doi: 10.1113/EP091671
26
Fagelson M. (2022). Tinnitus and traumatic memory. Brain Sci.12:1585. doi: 10.3390/brainsci12111585
27
Fan T. Guan P. Zhong X. Xiang M. Peng Y. Zhou R. et al . (2024). Functional connectivity alterations and molecular characterization of the anterior cingulate cortex in tinnitus pathology without hearing loss. Adv. Sci.11:e2304709. doi: 10.1002/advs.202304709
28
Fettiplace R. (2017). Hair cell transduction, tuning, and synaptic transmission in the mammalian cochlea. Compr. Physiol.7, 1197–1227. doi: 10.1002/cphy.c160049
29
Figueiredo R. R. Langguth B. de Oliveira P. M. Azevedo A. A. (2008). Tinnitus treatment with memantine. Otolaryngol. Head Neck Surg.138, 492–496. doi: 10.1016/j.otohns.2007.11.027
30
Fioranelli M. Roccia M. (2014). The history of low dose medicine research review of preclinical and clinical studies with low dose SKA cytokines since 2009. Interdis. J. Microinflamm.1. doi: 10.4172/ijm.1000115
31
Gariboldi S. Palazzo M. Zanobbio L. Dusio G. F. Mauro V. Solimene U. et al . (2009). Low dose oral administration of cytokines for treatment of allergic asthma. Pulm. Pharmacol. Ther.22, 497–510. doi: 10.1016/j.pupt.2009.05.002
32
Haider H. F. Ribeiro S. F. Martins C. Ribeiro D. Trigueiros N. Szczepek A. J. et al . (2020). Tinnitus, hearing loss and inflammatory processes in an older Portuguese population. Int. J. Audiol.59, 323–332. doi: 10.1080/14992027.2019.1698775
33
Han B. I. Lee H. W. Ryu S. Kim J. S. (2021). Tinnitus update. J. Clin. Neurol.17, 1–10. doi: 10.3988/jcn.2021.17.1.1
34
He J. Zhang R. Shao M. Zhao X. Miao M. Chen J. et al . (2020). Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis.79, 141–149. doi: 10.1136/annrheumdis-2019-215396
35
Henton A. Tzounopoulos T. (2021). What’s the buzz? The neuroscience and the treatment of tinnitus. Physiol. Rev.101, 1609–1632. doi: 10.1152/physrev.00029.2020
36
Ibrahim A. M. Chauhan L. Bhardwaj A. Sharma A. Fayaz F. Kumar B. et al . (2022). Brain-derived neurotropic factor in neurodegenerative disorders. Biomedicines10:1143. doi: 10.3390/biomedicines10051143
37
Isler B. von Burg N. Kleinjung T. Meyer M. Stämpfli P. Zölch N. et al . (2022). Lower glutamate and GABA levels in auditory cortex of tinnitus patients: a 2D-JPRESS MR spectroscopy study. Sci. Rep.12:4068. doi: 10.1038/s41598-022-07835-8
38
Issa G. Wilson C. Terry A. V. Pillai A. (2010). An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol. Dis.39, 327–333. doi: 10.1016/j.nbd.2010.04.017
39
Jarach C. M. Lugo A. Scala M. Van Den Brandt P. A. Cederroth C. R. Odone A. et al . (2022). Global prevalence and incidence of tinnitus: a systematic review and meta-analysis. JAMA Neurol.79, 888–900. doi: 10.1001/jamaneurol.2022.2189
40
Kemal O. Müderris T. Başar F. Kutlar G. Gül F. (2016). Prognostic value of mean platelet volume on tinnitus. J. Laryngol. Otol.130, 162–165. doi: 10.1017/S0022215115003254
41
Kim S. H. Kim D. Lee J.-M. Lee S. K. Kang H. J. Yeo S. G. (2021). Review of pharmacotherapy for tinnitus. Healthcare9:779. doi: 10.3390/healthcare9060779
42
King A. M. Cooper J. N. Oganezova K. Mittal J. McKenna K. Godur D. A. et al . (2024). Vestibular schwannoma and tinnitus: a systematic review of microsurgery compared to gamma knife radiosurgery. J. Clin. Med.13:3065. doi: 10.3390/jcm13113065
43
Lee H. J. Lee D. C. Kim C. O. (2024). The association between serum lipid levels and tinnitus prevalence and severity in Korean elderly: a nationwide population-based cross-sectional study. Yonsei Med. J.65, 156–162. doi: 10.3349/ymj.2022.0626
44
Linz R. Puhlmann L. M. C. Apostolakou F. Mantzou E. Papassotiriou I. Chrousos G. P. et al . (2019). Acute psychosocial stress increases serum BDNF levels: an antagonistic relation to cortisol but no group differences after mental training. Neuropsychopharmacology44, 1797–1804. doi: 10.1038/s41386-019-0391-y
45
Lommatzsch M. Zingler D. Schuhbaeck K. Schloetcke K. Zingler C. Schuff-Werner P. et al . (2005). The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging26, 115–123. doi: 10.1016/j.neurobiolaging.2004.03.002
46
Lopez-Gonzalez M. A. Esteban-Ortega F. (2005). Tinnitus dopaminergic pathway. Ear noises treatment by dopamine modulation. Med. Hypotheses65, 349–352. doi: 10.1016/j.mehy.2005.02.016
47
Lowry L. D. Eisenman L. M. Saunders J. C. (2004). An absence of tinnitus. Otol. Neurotol.25, 474–478. doi: 10.1097/00129492-200407000-00013
48
Mahoney C. J. Rohrer J. D. Goll J. C. Fox N. C. Rossor M. N. Warren J. D. (2011). Structural neuroanatomy of tinnitus and hyperacusis in semantic dementia. J. Neurol. Neurosurg. Psychiatry82, 1274–1278. doi: 10.1136/jnnp.2010.235473
49
Matsuoka K. Koreth J. Kim H. T. Bascug G. McDonough S. Kawano Y. et al . (2013). Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci. Transl. Med.5:179ra43. doi: 10.1126/scitranslmed.3005265
50
Mennink L. M. Aalbers M. W. van Dijk P. van Dijk J. M. C. (2022). The role of inflammation in tinnitus: a systematic review and meta-analysis. J. Clin. Med.11:1000. doi: 10.3390/jcm11041000
51
Messina A. Corvaia A. Marino C. (2022). Definition of tinnitus. Audiol. Res.12, 281–289. doi: 10.3390/audiolres12030029
52
Mojtabavi H. Saghazadeh A. van den Heuvel L. Bucker J. Rezaei N. (2020). Peripheral blood levels of brain-derived neurotrophic factor in patients with post-traumatic stress disorder (PTSD): a systematic review and meta-analysis. PLoS One15:e0241928. doi: 10.1371/journal.pone.0241928
53
Molinari C. Morsanuto V. Ruga S. Notte F. Farghali M. Galla R. et al . (2020). The Role of BDNF on Aging-Modulation Markers. Brain Sci10, 285. doi: 10.3390/brainsci10050285
54
Mousavi S. H. G. Sajadinejad B. Khorsandi S. Farhadi A. (2021). Diabetes mellitus and tinnitus: an epidemiology study. Maedica J. Clin. Med.16, 580–584. doi: 10.26574/maedica.2021.16.4.580
55
Nagahara A. H. Merrill D. A. Coppola G. Tsukada S. Schroeder B. E. Shaked G. M. et al . (2009). Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med.15, 331–337. doi: 10.1038/nm.1912
56
Nagahara A. H. Tuszynski M. H. (2011). Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov.10, 209–219. doi: 10.1038/nrd3366
57
Numakawa T. Kajihara R. (2025). The role of brain-derived neurotrophic factor as an essential mediator in neuronal functions and the therapeutic potential of its mimetics for neuroprotection in neurologic and psychiatric disorders. Molecules30:848. doi: 10.3390/molecules30040848
58
Pan W. Banks W. A. Fasold M. B. Bluth J. Kastin A. J. (1998). Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology37, 1553–1561. doi: 10.1016/S0028-3908(98)00141-5
59
Patil J. D. Alrashid M. A. Eltabbakh A. Fredericks S. (2023). The association between stress, emotional states, and tinnitus: a mini-review. Front. Aging Neurosci.15:1131979. doi: 10.3389/fnagi.2023.1131979
60
Perin P. Mabou Tagne A. Enrico P. Marino F. Cosentino M. Pizzala R. et al . (2020). Cannabinoids, inner ear, hearing, and tinnitus: a Neuroimmunological perspective. Front. Neurol.11:505995. doi: 10.3389/fneur.2020.505995
61
Pribiag H. Stellwagen D. (2013). TNF-α downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA A receptors. J. Neurosci.33, 15879–15893. doi: 10.1523/JNEUROSCI.0530-13.2013
62
Ralli M. Troiani D. Podda M. Paciello F. Eramo S. Corso D. et al . (2014). The effect of the NMDA channel blocker memantine on salicylate-induced tinnitus in rats. Acta Otorhinolaryngol. Ital.34, 198–204.
63
Ranjbar N. Shahbazi A. Nourizadeh N. Namvar Arefi H. Kheirkhah M. T. (2023). Relationship between serum levels of brain-derived neurotrophic factor (BDNF) and hearing loss and tinnitus. Indian J. Otolaryngol. Head Neck Surg.75, 507–513. doi: 10.1007/s12070-023-03600-z
64
Robinson S. Mogul A. S. Taylor-Yeremeeva E. M. Khan A. Tirabassi A. D. Wang H.-Y. (2021). Stress diminishes BDNF-stimulated TrkB signaling, TrkB-NMDA receptor linkage and neuronal activity in the rat brain. Neuroscience473, 142–158. doi: 10.1016/j.neuroscience.2021.07.011
65
Roh E. Hwang S. Y. Song E. Park M. J. Yoo H. J. Baik S. H. et al . (2022). Association of plasma brain-derived neurotrophic factor levels and frailty in community-dwelling older adults. Sci. Rep.12:18605. doi: 10.1038/s41598-022-19706-3
66
Rosemann S. Rauschecker J. P. (2023). Disruptions of default mode network and precuneus connectivity associated with cognitive dysfunctions in tinnitus. Sci. Rep.13:5746. doi: 10.1038/s41598-023-32599-0
67
Ruel J. Chabbert C. Nouvian R. Bendris R. Eybalin M. Leger C. L. et al . (2008). Salicylate enables Cochlear arachidonic-acid-sensitive NMDA receptor responses. J. Neurosci.28, 7313–7323. doi: 10.1523/JNEUROSCI.5335-07.2008
68
Schimmang T. Tan J. Müller M. Zimmermann U. Rohbock K. Köpschall I. et al . (2003). Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development130, 4741–4750. doi: 10.1242/dev.00676
69
Schmidt S. A. Carpenter-Thompson J. Husain F. T. (2017). Connectivity of precuneus to the default mode and dorsal attention networks: a possible invariant marker of long-term tinnitus. Neuroimage Clin.16, 196–204. doi: 10.1016/j.nicl.2017.07.015
70
Sedley W. Parikh J. Edden R. A. E. Tait V. Blamire A. Griffiths T. D. (2015). Human auditory cortex neurochemistry reflects the presence and severity of tinnitus. J. Neurosci.35, 14822–14828. doi: 10.1523/JNEUROSCI.2695-15.2015
71
Shao M. Lin H. Yin D. Li Y. Wang Y. Ma J. et al . (2019). Learning to play badminton altered resting-state activity and functional connectivity of the cerebellar sub-regions in adults. PLoS One14:e0223234. doi: 10.1371/journal.pone.0223234
72
Singer W. Panford-Walsh R. Knipper M. (2014). The function of BDNF in the adult auditory system. Neuropharmacology76, 719–728. doi: 10.1016/j.neuropharm.2013.05.008
73
Singh A. Smith P. F. Zheng Y. (2023). Targeting the limbic system: insights into its involvement in tinnitus. Int. J. Mol. Sci.24:9889. doi: 10.3390/ijms24129889
74
Sleiman S. F. Henry J. Al-Haddad R. El Hayek L. Abou Haidar E. Stringer T. et al . (2016). Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife5:e15092. doi: 10.7554/eLife.15092
75
Smith P. F. Zheng Y. (2016). Cannabinoids, cannabinoid receptors and tinnitus. Hear. Res.332, 210–216. doi: 10.1016/j.heares.2015.09.014
76
Smitha K. Akhil Raja K. Arun K. Rajesh P. Thomas B. Kapilamoorthy T. et al . (2017). Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J30, 305–317. doi: 10.1177/1971400917697342
77
Suckfüll M. Althaus M. Ellers-Lenz B. Gebauer A. Görtelmeyer R. Jastreboff P. J. et al . (2011). A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord.11:1. doi: 10.1186/1472-6815-11-1
78
Thoenen H. Sendtner M. (2002). Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat. Neurosci.5, 1046–1050. doi: 10.1038/nn938
79
Uberti F. Morsanuto V. Ghirlanda S. Ruga S. Clemente N. Boieri C. et al . (2018). Highly diluted acetylcholine promotes wound repair in an in vivo model. Adv. Wound Care (New Rochelle)7, 121–133. doi: 10.1089/wound.2017.0766
80
Viveros M.-P. Marco E.-M. Llorente R. López-Gallardo M. (2007). Endocannabinoid system and synaptic plasticity: implications for emotional responses. Neural Plast.2007, 1–12. doi: 10.1155/2007/52908
81
Wang H. Brozoski T. J. Ling L. Hughes L. F. Caspary D. M. (2011). Impact of sound exposure and aging on brain-derived neurotrophic factor and tyrosine kinase B receptors levels in dorsal cochlear nucleus 80 days following sound exposure. Neuroscience172, 453–459. doi: 10.1016/j.neuroscience.2010.10.056
82
Wang S. Cheng Q. Malik S. Yang J. (2000). Interleukin-1beta inhibits gamma-aminobutyric acid type a (GABA(A)) receptor current in cultured hippocampal neurons. J. Pharmacol. Exp. Ther.292, 497–504.
83
Wang W. Zhang L. S. Zinsmaier A. K. Patterson G. Leptich E. J. Shoemaker S. L. et al . (2019). Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biol.17:e3000307. doi: 10.1371/journal.pbio.3000307
84
Watanabe T. Yagishita S. Kikyo H. (2008). Memory of music: Roles of right hippocampus and left inferior frontal gyrus. Neuroimage39, 483–491. doi: 10.1016/j.neuroimage.2007.08.024
85
Weber C. Arck P. Mazurek B. Klapp B. F. (2002). Impact of a relaxation training on psychometric and immunologic parameters in tinnitus sufferers. J. Psychosom. Res.52, 29–33. doi: 10.1016/S0022-3999(01)00281-1
86
Wong A. C. Y. Ryan A. F. (2015). Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci.7:58. doi: 10.3389/fnagi.2015.00058
87
Xia W. Cui J. Luo Y. Xu J.-J. Chen H. Yin X. et al . (2021). Glucose control has an impact on cerebral blood flow alterations in chronic tinnitus patients. Front. Neurosci.14:623520. doi: 10.3389/fnins.2020.623520
88
Xu J.-J. Cui J. Feng Y. Yong W. Chen H. Chen Y.-C. et al . (2019). Chronic Tinnitus Exhibits Bidirectional Functional Dysconnectivity in Frontostriatal Circuit. Front Neurosci.13. doi: 10.3389/fnins.2019.01299
89
Yazici D. Cihan M. C. (2023). Correlation of tinnitus severity index and tinnitus handicap inventory with hematological parameters in patients with subjective tinnitus. Int. Arch. Otorhinolaryngol.27, e608–e612. doi: 10.1055/s-0042-1758216
90
Yoshida T. Stephens D. Kentala E. Levo H. Auramo Y. Poe D. et al . (2011). Tinnitus complaint behaviour in long-standing Menière’s disorder: its association with the other cardinal symptoms. Clin. Otolaryngol.36, 461–467. doi: 10.1111/j.1749-4486.2011.02381.x
91
Yuan L. Xie L. Zhang H. Zhang Y. Wei Y. Feng J. et al . (2023). Low-dose IL-2 treatment rescues cognitive deficits by repairing the imbalance between Treg and Th17 cells at the middle Alzheimer’s disease stage. J. Neuroimmune Pharmacol.18, 674–689. doi: 10.1007/s11481-023-10090-x
92
Yuksel B. Dogan M. Boyacioglu O. Sahin M. Orenay-Boyacioglu S. (2023). Association between chronic tinnitus and brain-derived neurotrophic factor antisense RNA polymorphisms linked to the Val66Met polymorphism in BDNF. Gene875:147507. doi: 10.1016/j.gene.2023.147507
93
Zhang R. Zhao Y. Chen X. Zhuang Z. Li X. Shen E. (2024). Low-dose IL-2 therapy in autoimmune diseases: an update review. Int. Rev. Immunol.43, 113–137. doi: 10.1080/08830185.2023.2274574
Summary
Keywords
tinnitus, BDNF, inflammatory cytokines, synaptic plasticity, low-dose medicine
Citation
Miranda V, Castiglioni S and Maier JA (2025) Neuroplasticity and tinnitus: the role of Brain-Derived Neurotrophic Factor in pathogenesis and treatment. Front. Neurosci. 19:1620894. doi: 10.3389/fnins.2025.1620894
Received
30 April 2025
Accepted
13 June 2025
Published
08 July 2025
Volume
19 - 2025
Edited by
Holger Schulze, University of Erlangen Nuremberg, Germany
Reviewed by
Stephanie Rosemann, University of Oldenburg, Germany
Tadeas Mares, Charles University, Czechia
Updates
Copyright
© 2025 Miranda, Castiglioni and Maier.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Castiglioni, sara.castiglioni@unimi.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.