Abstract
Objective:
This study utilized resting-state functional magnetic resonance imaging (fMRI) to examine changes in brain functional activity following acupuncture treatment for acute Bell’s palsy (BP) and to investigate the potential central regulatory mechanisms involved.
Methods:
A total of 55 patients with acute Bell’s facial paralysis (within 1–7 days of onset) were enrolled in the patient group, while 48 individuals without the condition were included as the healthy control group. The patient group received acupuncture therapy at EX-HN16 (Qianzheng), SJ17 (Yifeng), ST2 (Sibai), GB14 (Yangbai), EX-HN4 (Yuyao), SI18 (Quanliao), ST6 (Jiache), ST4 (Dicang), ST8 (Touwei), and bilateral LI4 (Hegu) points on the affected side. Each session lasted 30 min and was administered three times a week (Wednesday, Friday, and Sunday) until day 28 of the disease course. The patient group underwent fMRI scans, House–Brackmann (H-B) grading, Sunnybrook scale evaluation, and facial disability index (FDI) assessment both prior to the initial treatment and on the 28th day. The healthy group received a single fMRI scan after enrollment. MATLAB R2017 software was used to calculate the fractional amplitude of low-frequency fluctuation (fALFF) and regional homogeneity (ReHo) in patients before and after treatment, as well as in healthy controls.
Results:
Following treatment, the patient group showed significant improvements in H-B, Sunnybrook, and FDI scores compared to pretreatment levels (P < 0.05), with an overall effective rate of 96.4% (53/55). Prior to treatment, compared to healthy controls, patients exhibited decreased fALFF in the right posterior cingulate gyrus, increased fALFF in the right postcentral gyrus, left and right middle frontal gyri, and increased ReHo in the left precentral gyrus, right postcentral gyrus, and left middle occipital gyrus. After treatment, when compared to healthy controls, patients showed decreased fALFF in the left and right medial superior frontal gyri, and increased fALFF in the right postcentral gyrus, left precentral gyrus, and bilateral lingual gyri, and increased ReHo in the right precentral gyrus, bilateral transverse temporal gyri, right lingual gyrus, and right thalamus, and decreased ReHo in the right middle frontal gyrus. Relative to pretreatment values, patients displayed decreased fALFF in the left medial superior frontal gyrus and increased fALFF in the left precentral gyrus. Additionally, ReHo decreased the right and left medial superior frontal gyri, while it increased in the right inferior parietal angular gyrus, right precentral gyrus, and left superior parietal gyrus.
Conclusion:
Acupuncture demonstrates a clear therapeutic effect on acute BP and contribute to clinical symptom improvement. Marked differences in brain functional activity were observed between patients and healthy individuals. The therapeutic effect of acupuncture may be linked to its ability to facilitate functional reorganization in brain regions associated with sensation, movement, and emotion.
Clinical trial registration:
1 Introduction
Bell’s palsy (BP) is an acute idiopathic peripheral facial paralysis characterized by a sudden onset of weakness or paralysis on one side of the face. It is among the most frequently encountered facial nerve disorders in clinical settings, accounting for approximately 60%–75% of all facial paralysis cases (Peitersen, 2002). Although its exact cause remains unclear, it is generally thought to be associated with inflammation, edema, and compression of the facial nerve triggered by viral infection (Mu et al., 2022), such as the reactivation of herpes simplex virus type 1. Regarding treatment (Gagyor et al., 2019), glucocorticoids are considered the first-line therapy due to their effectiveness in reducing nerve swelling, and their combination with antiviral medications may further enhance patient outcomes. Additionally, physical therapy and surgical decompression should be tailored based on individual patient conditions. Acupuncture therapy (An et al., 2025) for BP has been shown to support the recovery of facial nerve function, alleviate facial symptoms, improve therapeutic outcomes, and has relatively few adverse effects. Nonetheless, the underlying mechanisms by which acupuncture exerts these effects remain unclear (Ma et al., 2021), necessitating further investigation.
Magnetic resonance imaging (MRI) serves as one of the objective indicators for evaluating BP (Wang Y. et al., 2021). Functional MRI (fMRI) is a key technique for investigating brain activity in humans (Glover, 2011). In recent years, fMRI has proven particularly valuable for studying the dynamic interactions within brain networks, neural plasticity, and mechanisms underlying traditional medical treatments (Li and Liu, 2010). It is a useful tool for exploring the central mechanisms of acupuncture. Moreover, the effects of acupuncture are closely linked to the central nervous system (Cai et al., 2018; You et al., 2013). Some researchers have utilized fMRI to investigate the mechanism of BP and have suggested that it can dynamically monitor central compensatory processes (Calistri et al., 2021). Fractional amplitude of low-frequency fluctuation (fALFF) and regional homogeneity (ReHo) are analytical methods based on resting-state fMRI used to assess localized brain function. Together, these methods offer a more comprehensive evaluation of functional alterations in specific brain areas. Therefore, building on the assessment of acupuncture’s clinical efficacy in treating BP, this study employed fALFF and ReHo to examine central nervous system dysfunction and its underlying mechanisms in BP, aiming to investigate how acupuncture influences brain function in BP patients and to provide an objective foundation for its clinical application.
2 Materials and methods
2.1 Research design
This study was conducted as an experimental intervention. Individuals with acute BP, recruited from the general population, were included in the BP group (BP patients). Concurrently, healthy controls (HCs) were recruited, matched to the BP group in terms of age, sex, and handedness. None of the HC participants had immediate family members with a history of BP.
All participants underwent an initial fMRI examination with typical findings and provided written informed consent in accordance with the guidelines set by the Ethics Committee of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (Ethical Approval No.: 2022AH-22). The clinical trial was registered under ChiCTR2200065223. Participants were enrolled only after registration, ethics approval, and a thorough explanation of the study protocol. Personal information was kept confidential, and participants retained the right to withdraw from the study at any point. Because of the nature of acupuncture treatment, blinding of acupuncturists was not feasible. However, outcome assessors were blinded to group allocation and treatment status. They received standardized training to ensure scoring consistency and were instructed not to ask patients about treatment details. Additionally, the fMRI data analysts were blinded to group allocation and time points during preprocessing and statistical analysis.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
1. Acute BP patients: Diagnosed with Bell’s facial paralysis based on established diagnostic criteria (Jia, 2013); first-time unilateral BP with an H-B facial nerve grade higher than I but lower than VI, with symptom onset within the past 7 days; no acupuncture treatment received in the past 3 months; aged between 16 and 65 years, of any sex, right-handed; years of education ≥12 years; routine MRI scans show no significant intracranial abnormalities; eligible for MRI scanning (i.e., no metal dental work, pacemakers, stents, or claustrophobia); normal mental and behavioral status, without major systemic diseases; and provided voluntary informed consent and were able to comply with and complete the acupuncture treatment.
2. Healthy subjects: Healthy individuals matched in age, sex, years of education, and other characteristics with the BP patients, right-handed; no acupuncture treatment within the past 3 months; normal neurological examination findings, no evident abnormalities on routine MRI scans, and no personal or family history of neurological or psychiatric disorders; eligible for MRI scanning (i.e., no metal dental work, pacemakers, stents, or claustrophobia); and voluntarily signed informed consent.
2.2.2 Exclusion criteria:
1. Acute BP patients: Individuals with facial paralysis secondary to other conditions (e.g., tumors, trauma, or otitis media); those with comorbid underlying diseases, severe organic disorders of major organs, or mental disorders; pregnant or breastfeeding women; and those with poor compliance or inability to complete the study.
2. Healthy subjects: Individuals with underlying diseases, severe organic disorders of major organs, or mental disorders; pregnant or lactating women; those with a history of facial trauma, otitis media, or viral infection (e.g., herpes simplex) within the past 3 months; those with a family history of neurological diseases (not limited to BP); and individuals currently taking medications that affect central nervous system function (e.g., antidepressants, corticosteroids).
2.3 Clinical evaluation scale
2.3.1 H-B scale
Grade I: normal function
Grade II: mild dysfunction
Grade III: moderate dysfunction
Grade IV: moderately severe dysfunction
Grade V: severe dysfunction
Grade VI: complete paralysis (House and Brackmann, 1985)
2.3.2 Sunnybrook facial nerve rating scale
The static assessment evaluates three regions: the eye, cheek, and mouth. Scoring ranges are as follows: eye 0–3 points, cheek 0–4 points, and mouth 0–2 points. The static score is calculated as total score × 5.
Dynamic assessment includes five facial movements: raising the forehead, gently closing the eyes, smiling with an open mouth, wrinkling the nose, and puckering the lips. This part evaluates both voluntary movement and synkinesis. Voluntary movement is scored from 1 to 5 points per movement, with the voluntary movement score computed as total score × 4. Synkinesis is graded from 0 to 3 points, with the synkinesis score being the total of these values. The dynamic assessment score is calculated as voluntary movement score–static score–synkinesis score. The total possible score ranges from 0 to 100, with higher scores indicating better facial nerve function (Li, 2005).
2.3.3 Facial disability index (FDI) score
This index includes two components: physical function (FDIP) and social/well-being function (FDIS). FDIP has a total score ranging from 0 to 25, where higher scores reflect better physical motor function. FDIS scores range from 5 to 30 points, with lower scores indicating better social function and quality of life (Deng et al., 2017).
2.4 Evaluation criteria of curative effect
Based on the H-B scale (House and Brackmann, 1985), treatment outcomes were categorized as follows: Cured: H-B grade I; Markedly effective: H-B grade II; Effective: H-B grade III; Ineffective: H-B grades IV–VI.
2.5 Acupuncture intervention measures
Patients in the BP group received standard acupuncture treatment.
Acupoint selection: In accordance with the guidelines outlined in Acupuncture (Liang and Wang, 2016), the selected acupoints on the affected side included ST2 (Sibai), GB14 (Yangbai), ST8 (Touwei), EX-HN16 (Qianzheng), SJ17 (Yifeng), SI18 (Quanliao), ST6 (Jiache), ST4 (Dicang), EX-HN4 (Yuyao) and bilateral LI4 (Hegu) (Figure 1).
FIGURE 1

Distribution of acupoints on the face and hands.
Procedure: After disinfecting the local skin, patients were placed in a supine position. Disposable acupuncture needles (0.25 mm × 25–40 mm; Wujiang Yunlong Medical Equipment Co., Ltd., Su Jizhun 20142200226) were used. ST2: inserted perpendicularly 10–15 mm. GB14: inserted 20–25 mm toward the EX-HN4 region. ST8: inserted horizontally 10–15 mm in an upward direction. SI18: inserted obliquely 10–15 mm toward the anterior aspect of the lower root of the ear on the affected side. SJ17: inserted perpendicularly 12–20 mm. SI18: inserted perpendicularly 12–20 mm. ST6 and ST4: inserted bidirectionally 15–20 mm. LI4: inserted perpendicularly 12–20 mm.
Criteria for assessing “Deqi” (Liang and Wang, 2016): “Deqi” was defined as the simultaneous occurrence of two conditions: (1) needle sensations reported by the patient (e.g., soreness, numbness, distension, heaviness) and (2) a tight, rough sensation under the needle perceived by the acupuncturist (i.e., “needle sensation”). Once the arrival of qi was achieved, the needles were retained for 30 min. Treatment sessions were conducted three times per week (Wednesday, Friday, and Sunday) and continued until the 28th day from the onset of symptoms. If a patient was cured within the 28-day period, treatment was concluded early; if not, treatment continued beyond that point. All acupuncture procedures were carried out by the same practitioner, who had more than 3 years of clinical experience.
2.6 fMRI data acquisition
All fMRI data in this study were collected using an 8-channel head coil on a 3.0T Discovery MR750 scanner (GE, USA) at the Imaging Center of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine. These scans were performed by a trained technician. The BP group underwent two fMRI scans: one before the first acupuncture treatment and another on the 28th day of the disease course. The HC group underwent a single fMRI scan after enrollment. Before scanning, participants removed all metal and magnetic objects in the preparation room. After sufficient relaxation, they entered the scanning room in a calm state and wore earplugs to reduce noise. Subjects were instructed to lie flat with their eyes closed; keep their entire body, especially the head, still; remain awake without engaging in any active thinking; and breathe evenly. The fMRI procedure included both structural and functional imaging.
Structural image parameters: Structural images were obtained using a 3D T1 BRAVO sequence with the following parameters: repetition time (TR) = 8.2 ms, echo time (TE) = 3.2 ms, inversion time = 450 ms, flip angle = 12°, scanning field = 256 mm × 256 mm, matrix size = 256 × 256, slice thickness = 1.0 mm, slice gap = 1.0 mm, total of 200 slices acquired in the sagittal plane. The total scan duration was 5 min and 36 s.
Functional imaging parameters: Functional images were acquired using a gradient echo planar imaging sequence with TR = 2000 ms, TE = 30 ms, flip angle = 90°, scanning field = 224 mm × 224 mm, matrix size = 64 × 64, slice thickness = 3.0 mm, voxel size = 3 mm × 3 mm × 3 mm, total of 36 slices obtained axially. The total acquisition time was 6 min and 10 s.
2.7 fMRI data preprocessing
Imaging data in this study were processed using dcm2niigui and the DPABI software package within MATLAB R2017b. First, the data in DICOM format were converted to NIFTI format. The “co-” data (with the neck removed) were selected, and each dataset was checked for artifacts or excessive head movement. Next, resting-state fMRI data were preprocessed using DPABI software with the following steps: (1) Time correction by discarding the first 10 time points. (2) Head motion correction: Head motion was quantified using framewise displacement (FD). Time points with FD > 0.5 mm were excluded, and participants with >20% of time points discarded were removed from further analysis. Both mean FD and maximum rotation angle were calculated, and datasets with head motion exceeding 3 mm or 3° were excluded. (3) Spatial normalization by registering data to the Montreal Institute of Neuroscience template and resampling to a voxel size of 3 mm × 3 mm × 3 mm. (4) Regression of nuisance signals, including head motion parameters, white matter, and cerebrospinal fluid signals. (5) Spatial smoothing using a Gaussian kernel with a full width at half maximum of 6 mm × 6 mm × 6 mm. (6) Temporal filtering to retain low-frequency signals with the range 0.01–0.08 Hz.
2.8 Statistical analysis
SPSS 25.0 software was used to analyze the general data and clinical scale data of the BP and HC groups. Measurement data were tested for normality and homogeneity of variance. If both assumptions were met, independent sample t-tests and paired t-tests were applied. If these conditions were not met, the Wilcoxon rank sum test was used. Categorical data were analyzed using the chi-squared test. A P-value of < 0.05 was considered statistically significant. DPABI software was utilized to conduct two-sample independent t-tests comparing brain imaging indicators between BP patients before and after acupuncture and HCs. Paired t-tests were conducted to compare brain imaging indicators before and after acupuncture treatment in patients. The final results were corrected using Gaussian random field (GRF) theory with thresholds set at voxel-level P < 0.001 and cluster-level P < 0.01.
3 Results
3.1 Demographic characteristics of participants
Between September 2023 and September 2024, a total of 103 participants were recruited from the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, including 55 patients in the BP group and 48 in the HC group. None of the participants dropped out. All subjects completed the fMRI scans, and all 55 patients underwent acupuncture treatment without any serious adverse effects. There were no significant differences in age, sex, or years of education between the two groups. The demographic details of all participants are presented in Table 1.
TABLE 1
| Group | n | Sex/n | Age/years | Years of education | |
| Male | Female | Average () | Average () | ||
| BP | 55 | 32 | 23 | 37.38 ± 10.35 | 13.89 ± 2.57 |
| HC | 48 | 23 | 25 | 37.65 ± 11.14 | 13.63 ± 2.13 |
| t/x 2 /Z | 1.085 | −0.056 | −0.443 | ||
| P | 0.298 | 0.963 | 0.658 | ||
Comparison of general data between the BP and HC.
BP, Bell’s palsy; HC, healthy control. Compared with BP and HC, P > 0.05.
3.2 Comparison of H-B scale, Sunnybrook, and FDI (including FDIP and FDIS) scores before and after treatment in the BP group
Following treatment, the H-B scale score in the BP group showed significant improvement compared to before treatment (P < 0.05), as presented in Table 2. Additionally, the Sunnybrook and FDIP scores of patients in the acute phase of BP were higher than those before treatment (P < 0.05), while the FDIS scores were lower than before treatment (P < 0.05), as shown in Table 3.
TABLE 2
| Time | n | H-B | |||||
| Grade I | Grade II | Grade III | Grade IV | Grade V | Grade VI | ||
| Before treatment | 55 | 0 | 4 | 22 | 24 | 5 | 0 |
| After treatment | 55 | 27 | 23 | 3 | 2 | 0 | 0 |
Comparison of H-B scale grading before and after treatment in the BP group.
BP, Bell’s palsy; H-B, House–Brackmann. Compared with before treatment, P < 0.05.
TABLE 3
| Time | n | Sunnybrook | FDIP | FDIS |
| Before treatment | 55 | 38.73 ± 10.35 | 19.47 ± 2.31 | 10.38 ± 1.45 |
| After treatment | 55 | 83.22 ± 13.551 | 24.29 ± 1.151 | 9.82 ± 0.431 |
| t/Z | −24.112 | −16.804 | −2.566 | |
| P | 0.000 | 0.000 | 0.010 |
Comparison of Sunnybrook, FDIP, and FDIS scores before and after treatment in the BP group.
FDIP, facial disability index-physical function; FDIS, facial disability index-social/well-being function. Compared with before treatment,
1 P < 0.05.
3.3 Clinical efficacy of BP group
Among the 55 patients with acute BP treated, 27 were cured, 23 showed marked improvement, 3 were effective, and 2 were ineffective, resulting in a total effective rate of 96.4%.
3.4 fMRI results
Before treatment, compared to the HC group, the BP group showed a decreased fALFF value in the right posterior cingulate gyrus and increased fALFF value in the right postcentral gyrus, left middle frontal gyrus, and right middle frontal gyrus, as detailed in Table 4 and Figure 2. The ReHo values increased in the left precentral gyrus, right postcentral gyrus, and left middle occipital gyrus, as shown in Table 5 and Figure 3. These results were corrected using GRF, with voxel-level P < 0.001 and cluster-level P < 0.01.
TABLE 4
| Brain Regions | MNI coordinate | Voxel | t | ||
| X | Y | Z | |||
| Right postcentral gyrus | 51 | –24 | 42 | 71 | 5.679 |
| Left middle frontal gyrus | –27 | 9 | 51 | 46 | 4.981 |
| Right middle frontal gyrus | 36 | 12 | 48 | 30 | 5.319 |
| Right posterior cingulate gyrus | 3 | –43 | 8 | 54 | –3.205 |
Significant differences in fALFF between before acupuncture treatment BP patients and HC.
BP, Bell’s palsy; fALFF, fractional amplitude of low-frequency fluctuation; HC, healthy control; MNI, Montreal Institute of Neuroscience.
FIGURE 2

Significant differences in fALFF between before acupuncture treatment BP patients and HC. The result was corrected for GRF with voxel P < 0.001, cluster P < 0.05. ① Right postcentral gyrus, ② Left middle frontal gyrus, ③ Right middle frontal gyrus Left middle frontal gyrus, ④ Right posterior cingulate gyrus.
TABLE 5
| Brain regions | MNI coordinate | Voxel | t | ||
| X | Y | Z | |||
| Left precentral gyrus | –51 | –3 | 33 | 98 | 4.245 |
| Right postcentral gyrus | 48 | –24 | 42 | 69 | 5.367 |
| Left middle occipital gyrus | –33 | –99 | 0 | 101 | 5.004 |
Significant differences in ReHo between before acupuncture treatment BP patients and HC.
BP, Bell’s palsy; HC, healthy control; MNI, Montreal Institute of Neuroscience; ReHo, regional homogeneity.
FIGURE 3

Significant differences in ReHo between before acupuncture treatment BP patients and HC. The result was corrected for GRF with voxel P < 0.001, cluster P < 0.05. ① Left precentral gyrus, ② Right postcentral gyrus, ③ Left middle occipital gyrus.
After treatment, compared to the HC group, the BP group exhibited decreased fALFF values in the left and right medial superior frontal gyri and increased fALFF values in the right postcentral gyrus, left precentral gyrus, right lingual gyrus, and left lingual gyrus, as shown in Table 6 and Figure 4. The ReHo values were increased in the right precentral gyrus, right lingual gyrus, right transverse temporal gyrus, left transverse temporal gyrus, and right thalamus, as presented in Table 7 and Figure 5. GRF correction was applied with voxel-level P < 0.001 and cluster-level P < 0.01.
TABLE 6
| Brain regions | MNI coordinate | Voxel | t | ||
| X | Y | Z | |||
| Right postcentral gyrus | 51 | –24 | 39 | 476 | 6.335 |
| Left precentral gyrus | –27 | –6 | 54 | 353 | 6.776 |
| Left medial superior frontal gyrus | –3 | 54 | 36 | 174 | –3.393 |
| Right medial superior frontal gyrus | 27 | 63 | 15 | 116 | –3.391 |
| Right lingual gyrus | 18 | –78 | 0 | 72 | 4.890 |
| Left lingual gyrus | –12 | –72 | –3 | 61 | 5.031 |
Significant differences in fALFF between after acupuncture treatment BP patients and HC.
BP, Bell’s palsy; fALFF, fractional amplitude of low-frequency fluctuation; HC, healthy control; MNI, Montreal Institute of Neuroscience.
FIGURE 4

Significant differences in fALFF between after acupuncture treatment BP patients and HC. The result was corrected for GRF with voxel P < 0.001, cluster P < 0.05. ① Right postcentral gyrus, ② Left precentral gyrus, ③ Left medial superior frontal gyrus, ④ Right medial superior frontal gyrus, ⑤ Right lingual gyrus, ⑥ Left lingual gyrus.
TABLE 7
| Brain regions | MNI coordinate | Voxel | t | ||
| X | Y | Z | |||
| Right precentral gyrus | 33 | –6 | 54 | 606 | 6.363 |
| Right lingual gyrus | 18 | –51 | –12 | 464 | 5.714 |
| Right transverse temporal gyrus | 48 | –15 | 9 | 252 | 6.015 |
| Left transverse temporal gyrus | –36 | –30 | 15 | 168 | 5.071 |
| Right thalamus | 12 | –21 | 3 | 74 | 5.146 |
| Right middle frontal gyrus | 39 | 42 | 33 | 1984 | –3.389 |
Significant differences in ReHo between after acupuncture treatment BP patients and HC.
BP, Bell’s palsy; HC, healthy control; MNI, Montreal Institute of Neuroscience; ReHo, regional homogeneity.
FIGURE 5
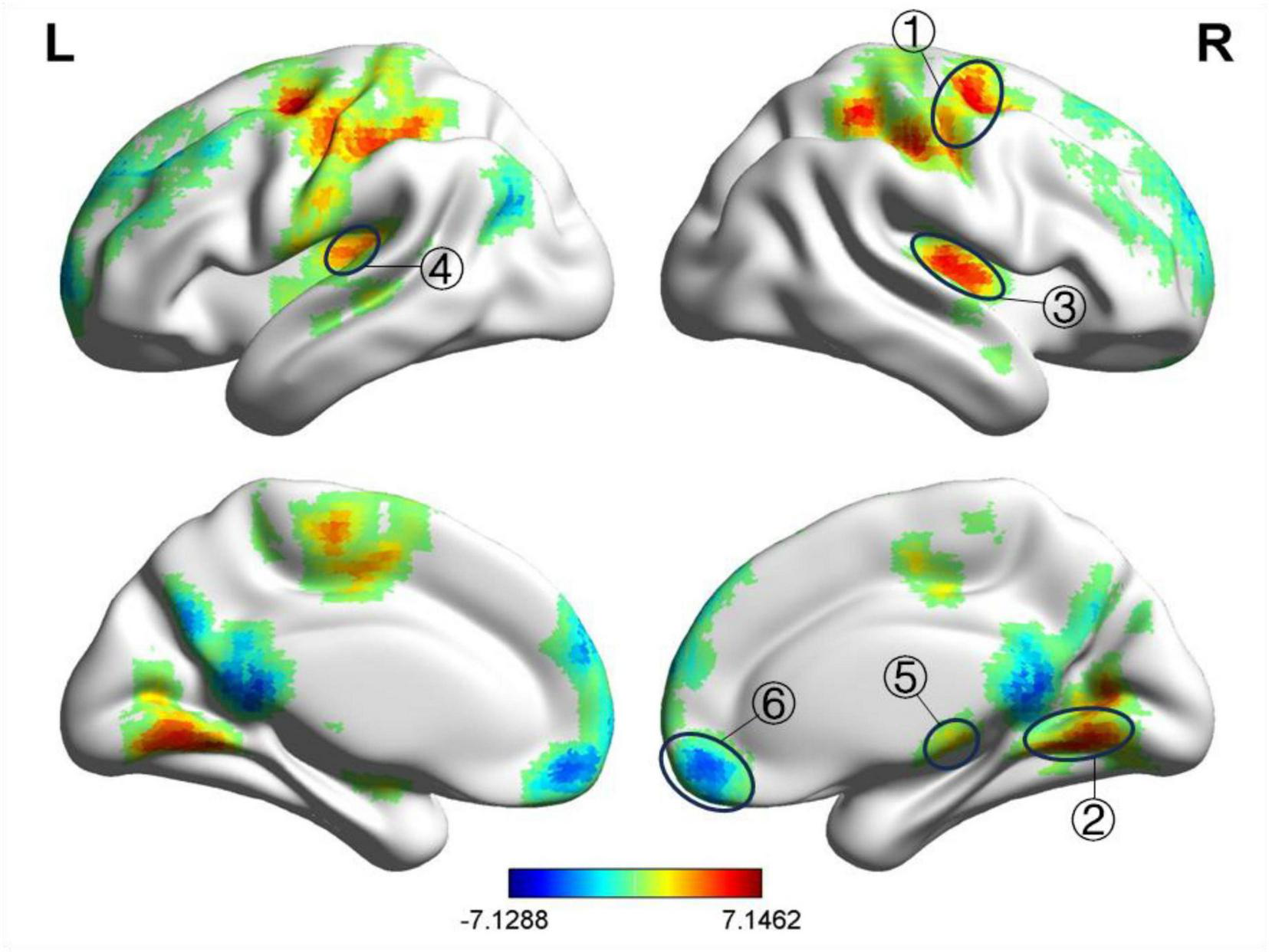
Significant differences in ReHo between after acupuncture treatment BP patients and HC. The result was corrected for GRF with voxel P < 0.001, cluster P < 0.05. ① Right precentral gyrus, ② Right lingual gyrus, ③ Right transverse temporal gyrus, ④ Left transverse temporal gyrus, ⑤ Right thalamus, ⑥ Right middle frontal gyrus.
Compared with before treatment, the fALFF value of the left medial superior frontal gyrus in the BP group decreased after treatment, while the fALFF value of the left precentral gyrus and the right angular gyrus increased, as shown in Table 8 and Figure 6. The ReHo values of the right and left medial superior frontal gyrus decreased, whereas the ReHo values of the right inferior parietal angular gyrus, right precentral gyrus, and left superior parietal gyrus increased, as shown in Table 9 and Figure 7. GRF correction was applied, with voxel-level P < 0.001 and cluster-level P < 0.01.
TABLE 8
| Brain regions | MNI coordinate | Voxel | t | ||
| X | Y | Z | |||
| Left medial superior frontal gyrus | –3 | 54 | 45 | 43 | –3.266 |
| Left precentral gyrus | –30 | –3 | 57 | 32 | 4.084 |
Significant differences in fALFF between after and before acupuncture treatment BP patients.
BP, Bell’s palsy; fALFF, fractional amplitude of low-frequency fluctuation; MNI, Montreal Institute of Neuroscience.
FIGURE 6

Significant differences in fALFF between after and before acupuncture treatment BP patients. The result was corrected for GRF with voxel P < 0.001, cluster P < 0.05. ① Left medial superior frontal gyrus, ② Left precentral gyrus, ③ Right angular gyrus.
TABLE 9
| Brain regions | MNI coordinate | Voxel | t | ||
| X | Y | Z | |||
| Right medial superior frontal gyrus | 6 | 50 | 39 | 136 | –3.480 |
| Left medial superior frontal gyrus | –3 | 63 | 9 | 121 | –3.480 |
| Right inferior parietal angular gyrus | 30 | –45 | 51 | 74 | 6.228 |
| Right precentral gyrus | 15 | –24 | 69 | 61 | 4.442 |
| Left superior parietal gyrus | –18 | –57 | 48 | 52 | 4.924 |
Significant differences in ReHo between after and before acupuncture treatment BP patients.
BP, Bell’s palsy; MNI, Montreal Institute of Neuroscience; ReHo, regional homogeneity.
FIGURE 7

Significant differences in ReHo between after and before acupuncture treatment BP patients. The result was corrected for GRF with voxel P < 0.001, cluster P < 0.05. ① Right medial superior frontal gyrus, ② Left medial superior frontal gyrus, ③ Right inferior parietal lobule (angular gyrus), ④ Right precentral gyrus, ⑤ Left superior parietal gyrus.
4 Discussion
Using resting-state fMRI combined with two indicators, including fALFF and ReHo, this study systematically explored the clinical efficacy and the underlying central regulatory mechanism of acupuncture in the treatment of acute BP. Our analyses revealed that acupuncture treatment resulted in improved H-B scale grade, Sunnybrook scale score, and FDI score in BP patients, with an overall treatment effective rate of 96.4%. This suggests that acupuncture can significantly improve the patients’ clinical symptoms and quality of life, achieving remarkable efficacy in the treatment of BP. Moreover, our fMRI analyses indicated that there were significant differences in brain functional activities between BP patients and healthy individuals and that acupuncture treatment may improve abnormal brain functional activities by promoting functional reorganization of relevant brain regions.
Fractional amplitude of low-frequency fluctuation measures localized, spontaneous neuronal activity and can significantly inhibit non-specific signaling components in resting-state MRI, increasing sensitivity to local spontaneous brain activity (Wang Y. et al., 2021). ReHo measures the uniformity of brain functional regions under specific conditions, which can reflect the synchronization of spontaneous neural activity in brain regions, and higher ReHo values indicated higher consistency and centrality of the activity cadence of adjacent voxels within brain regions (Lv et al., 2018; Zang et al., 2004).
In the present study, the fALFF and ReHo values in the central posterior gyrus, the fALFF values in the middle frontal gyrus, the ReHo values in the left central anterior gyrus, and the ReHo values in the middle occipital gyrus increased with acupuncture treatment whereas the fALFF values in the right posterior cingulate gyrus decreased with acupuncture treatment, compared with the pretreatment values. The posterior central gyrus (Smith et al., 2009) is an important part of the cerebral cortex and is the main somatosensory area of the brain, which is also responsible for spatial orientation of sensations in various parts of the body. Changes in the central posterior gyrus in BP patients mean that this area not only has an increased level of spontaneous activity but also that these activities exhibit higher local synchrony. This change may reflect the brain’s adaptive response to sensory and motor impairment caused by facial paralysis, or it may be that the brain is trying to compensate for the loss of sensory and motor control caused by facial paralysis; activity and synchronicity in specific brain regions are increased to adjust and improve facial nerve dysfunction, with the observed changes reflecting the adaptive ability of the central nervous system. This finding may also indicate the underlying neuroplasticity, i.e., the ability to adjust brain structures and function, as a coping mechanism in BP. Whereas the frontal lobe (Sohn et al., 2004) is the cortical center of upper facial movement, the middle frontal gyrus is the core component of the premotor area, participating in motor planning and executive function (Niendam et al., 2012), and the anterior central gyrus is the primary motor cortex, which directly regulates somatic movement (Grefkes et al., 2008). The increased fALFF values found in the middle frontal gyrus and the increased ReHo values found in the anterior central gyrus indicate that the middle frontal gyrus strengthens the accuracy of facial movement planning by enhancing spontaneous activity, while the anterior central gyrus optimizes the efficiency of movement execution by improving internal synchronization; the two regions are linked to maintain the basic motor functions of the face to the greatest extent possible. The middle occipital gyrus is an important part of the optic cortex, involved in facial expression recognition and visual information processing (Fang et al., 2007; Gong et al., 2015; Kosslyn and Ochsner, 1994; Zhang et al., 2018), whereas the posterior cingulate gyrus is involved in self-awareness, emotional regulation, and sensory integration (Smith et al., 2009). Changes in the middle occipital gyrus may reflect the brain’s adaptive or compensatory mechanisms in pathological states, possibly related to changes in visual processing, facial expression recognition, or other tasks related to the occipital lobe function. This change is likely the brain’s response to injury, trying to restore or optimize function by increasing functional synchronicity in certain areas. The weakening of activity in the posterior cingulate gyrus may be associated with psychologic states, such as anxiety and social avoidance, caused by changes in facial appearance in patients with BP.
The results of this study showed that, compared with the HCs, BP patients had higher fALFF and ReHo values in the anterior central and lingual gyri, higher fALFF values in the central posterior gyrus, lower fALFF values in the superior frontal gyrus, higher ReHo values in the temporal transverse gyrus and thalamus, and lower ReHo values in the right middle frontal gyrus after acupuncture treatment. These changes suggest that acupuncture may be involved in modulating neuronal activity in these brain regions, thereby alleviating symptoms of facial paralysis and facial sensory and motor dysfunction in BP patients. Studies have shown (Yang et al., 2012) that acupuncture treatment in patients with BP results in enhanced connectivity between the primary sensory cortex, primary motor cortex, superior frontal gyrus, and premotor area of the middle frontal gyrus in their brain regions. The possible mechanism is that due to the loss of facial muscle function, BP patients are unable to fully execute the neural instructions sent by the primary sensory cortex and primary motor cortex. Consequently, higher-level central premotor areas are required to play a role in motor coordination, thereby strengthening the linkage between the primary sensory cortex and the primary motor cortex. This study found that acupuncture enhances the activity of the precentral gyrus, postcentral gyrus, lingual gyrus, transverse temporal gyrus, and thalamus. These brain regions are mainly responsible for regulating somatic motor function, sensory function, visual information processing, emotional regulation, and other aspects. Using resting-state fMRI, Kong et al. (2015) observed that acupuncture at Hegu (LI4) and Jiache (ST6) acupoints in BP patients could activate brain regions such as the precentral gyrus, postcentral gyrus, and middle frontal gyrus, which correspond to the somatosensory cortex, motor cortex, and prefrontal cortex. Additionally, a study by Liu et al. (2014) also showed that acupuncture treatment in BP patients results in functional reorganization in the premotor cortex and supplementary motor area of the cerebral cortex. These findings are largely consistent with the results of this study. Taken together, we speculate that acupuncture can regulate brain functional plasticity and enhance the functional connectivity between relevant brain regions by activating areas such as the somatosensory cortex, motor cortex, and prefrontal cortex of the brain. As a key region for functions like emotional regulation (Wang et al., 2017), the superior frontal gyrus exhibits reduced activity, suggesting that acupuncture alleviates negative emotions, such as anxiety and inferiority, by improving the facial appearance of patients, thereby restoring emotion-related cortical regions from a state of overactivation to a stable level, consistent with the decreased FDIS scores in patients. The decreased ReHo values in the middle frontal gyrus indicate a reduction in the compensatory overactivation of the middle frontal gyrus in BP patients and a return of brain function to a normal level; therefore, this finding should be considered a potential imaging manifestation of therapeutic efficacy. The present study reveals that BP patients still experience functional abnormalities in multiple brain regions after acupuncture treatment, suggesting that even after clinical recovery, BP patients still exhibit partial brain functional reorganization. A study by Klingner et al. (2011) corroborates this finding.
In the present study, the fALFF values in the right angular gyrus, the ReHo values in the right inferior parietal lobule (angular gyrus) and the left superior parietal gyrus, and the fALFF and ReHo values in the precentral gyrus increased whereas the fALFF and ReHo values in the superior frontal gyrus decreased after acupuncture treatment compared with the pretreatment values in BP patients. The parietal lobe (Yang and Gang, 2015; Zhang et al., 2019)—including the postcentral, superior parietal, and angular gyri and the inferior parietal lobule—is a critical brain region that participates in motor planning and coordination by interacting with the motor cortex; this is crucial in relearning fine motor control of the face in BP patients. Facial paralysis can impair patients’ facial perception, and the parietal lobe can aid in adjusting and adapting to these changes. The increases in the values of indicators related to the parietal lobe observed after treatment suggest that acupuncture enhances the neural activity capacity and synchrony in this region, which may strengthen functional coordination within and across distinct brain regions. The superior frontal gyrus (Wang et al., 2017) is involved in emotional regulation. The decreased fALFF and ReHo values observed in this region after acupuncture treatment may be associated with the improvement of emotional function observed following acupuncture treatment in BP patients Reduced brain regional activity may reflect a stabilization of patients’ emotional state following acupuncture treatment. The precentral gyrus (Song et al., 2024) is responsible for motor control and sensory information integration, particularly the fine movements of the face and other body parts. The increased fALFF and ReHo values detected in this gyrus after treatment indicate that acupuncture enhances the neural activity and functional coordination in this region. In BP patients, the enhanced function of the precentral gyrus may promote the repair of damaged nerves or improve the efficiency of neural signal transmission, thereby facilitating the recovery of facial motor function. The increased ReHo and fALFF values may also reflect the reorganization or optimization of brain functional networks after acupuncture treatment. Such changes may contribute to improved regulation of facial motor control by the brain, demonstrating the plasticity and self-repair capacity of the brain.
However, this study did not include a sham acupuncture control group; therefore, interference of natural disease recovery and psychological factors cannot be completely ruled out. A further analysis combining the disease characteristics and data features is as follows: on the one hand, although BP demonstrates natural recovery, the high recovery rate (27/55) of patients within 28 days in this study and the strong correlation between brain function changes and the acupuncture intervention cycle cannot be fully explained by “spontaneous recovery.” Peitersen (2002) pointed out that most patients with natural recovery require several weeks to months, and approximately 10%–15% of may have residual sequelae. In contrast, most patients in this study recovered faster and more completely, suggesting that acupuncture may have accelerated the recovery process. On the other hand, although psychological factors may affect the scores of subjective scales, the physiological responses induced by “Deqi” (needling sensation) during acupuncture and the improvement of objective brain function data from fMRI all indicate specific physiological regulation, rather than mere psychological suggestion.
Although this study provides preliminary evidence for the central mechanism underlying acupuncture treatment in BP patients, several study limitations should be addressed. First, the present study did not include a sham acupuncture group as a control; therefore, the interference of the natural course of recovery and the placebo effect cannot be completely ruled out. Although the impact of acupuncture treatment was evaluated by clinical and objective imaging indicators, future studies should include a sham acupuncture group as a control to further clarify the contributions of acupuncture’s “acupoint specificity” and the “Deqi effect.” Second, the study included a small sample size of 55 BP patients and 48 HCs and was conducted in a single center, introducing a potential selection bias. Future investigations should include multi-center studies with large cohorts and should consider relaxing the inclusion criteria to improve the generalizability of our findings. Third, the patients were followed for up to 28 days after treatment, with no long-term follow-up evaluations conducted every 3–6 months; therefore, we could not evaluate the persistence of brain functional changes or the stability of the clinical efficacy of acupuncture treatment. Furthermore, correlation analyses exploring associations between the indicators of brain function and clinical scores were not conducted, hindering our ability to identify specific brain regional changes that are directly associated with the observed therapeutic effects. Subsequent studies should conduct longitudinal fMRI follow-up evaluations in combination with correlation analysis to screen imaging biomarkers for the prediction of therapeutic efficacy. Finally, the study only used resting-state fMRI and did not include other techniques, such as diffusion tensor imaging or task-state fMRI. Therefore, we were not able to fully reveal the regulatory effect of acupuncture on brain structure–function networks. Future studies should employ multimodal imaging to analyze the mechanism of acupuncture in multiple dimensions.
In conclusion, this study provides preliminary evidence that acupuncture regulates brain function in BP patients. Changes in brain function emphasize that acupuncture treatment may exert a positive effect in BP patients by promoting better coordination between brain regions and enhancing the intensity of regional activity.
5 Conclusion
Significant differences were observed in brain functional activity, particularly in motor and sensory functions, between BP patients and healthy individuals. Acupuncture treatment may improve abnormal brain function activity while alleviating patients’ clinical symptoms by facilitating functional reorganization of the sensory cortex, motor cortex, and emotion-related brain regions.
Statements
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (Ethical Approval No.: 2022AH-22). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
X-sX: Visualization, Writing – original draft, Writing – review & editing. Y-tZ: Formal analysis, Writing – review & editing. X-wL: Investigation, Writing – review & editing. Y-lS: Investigation, Writing – review & editing. J-cZ: Writing – review & editing. T-tM: Investigation, Writing – review & editing. Y-yY: Data curation, Writing – review & editing. JY: Conceptualization, Resources, Writing – review & editing. H-pS: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Collaborative Innovation Project of Universities in Anhui Province: GXXT-2021-083 (ChiCTR2200065223), and the Special Project for Clinical Medicine Research and Transformation of Anhui Province, Anhui Provincial Department of Science and Technology: 202304295107020062.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BP, Bell’s palsy; fALFF, fractional amplitude of low-frequency fluctuation; FD, framewise displacement; FDI, facial disability index; fMRI, functional magnetic resonance imaging; GRF, Gaussian random field; H-B, House–Brackmann; HCs, healthy controls; MRI, magnetic resonance imaging; ReHo, regional homogeneity; TE, echo time; TR, repetition time.
References
1
An J. Li H. Kan R. H. Wang L. J. Yuan Y. H. Pei M. Y. (2025). Efficacy of acupuncture combined with fire-dragon cupping therapy for Bell’s palsy and its effect on facial nerve function.Shanghai J. Acupuncture Moxibust44213–218. 10.13460/j.issn.1005-0957.2025.02.0213
2
Cai R. L. Shen G. M. Wang H. Guan Y. Y. (2018). Brain functional connectivity network studies of acupuncture: A systematic review on resting-state fMRI.J. Integr. Med.1626–33. 10.1016/j.joim.2017.12.002
3
Calistri V. Mancini P. Raz E. Nicastri M. Tinelli E. Russo F. Y. et al (2021). fMRI in Bell’s palsy: Cortical activation is associated with clinical status in the acute and recovery phases.J. Neuroimag.3190–97. 10.1111/jon.12798
4
Deng Y. A. Guo J. K. Yu J. D. Wang D. P. Sun X. G. Lin C. R. (2017). Research progress on clinical assessment methods for facial paralysis.Chin. J. Rehabil. Theory Pract.231407–1410. 10.3969/j.issn.1006-9771.2017.12.010
5
Fang H. H. Wang M. Cao P. W. Zhang D. P. (2007). Language function and working mechanisms of the occipital lobe.Neural Injury Funct. Reconstr.3184–186.
6
Gagyor I. Madhok V. B. Daly F. Sullivan F. (2019). Antiviral treatment for Bell’s palsy (idiopathic facial paralysis).Cochrane Datab. Syst. Rev.9:CD001869. 10.1002/14651858.cd001869.pub5
7
Glover G. H. (2011). Overview of functional magnetic resonance imaging.Neurosurg. Clin. N. Am.22133–139. 10.1016/j.nec.2010.11.001
8
Gong L. Wang J. Feng L. Wang M. Li X. Hu J. et al (2015). Explicit memory and implicit memory in occipital lobe stroke patients.J. Stroke Cerebrovasc. Dis.24663–667. 10.1016/j.jstrokecerebrovasdis.2014.10.018
9
Grefkes C. Nowak D. A. Eickhoff S. B. Dafotakis M. Küst J. Karbe H. et al (2008). Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging.Ann. Neurol.63236–246. 10.1002/ana.21228
10
House J. W. Brackmann D. E. (1985). Facial nerve grading system.Otolaryngol. Head Neck Surg.93146–147. 10.1177/019459988509300202
11
Jia J. P. (2013). Neurology.Beijing: People’s Medical Publishing House.
12
Klingner C. M. Volk G. F. Maertin A. Brodoehl S. Burmeister H. P. Guntinas-Lichius O. et al (2011). Cortical reorganization in Bell’s palsy.Restor. Neurol. Neurosci.29203–214. 10.3233/RNN-2011-0592
13
Kong S.-P. Tan Q.-W. Liu Y. Jing X.-H. Zhu B. Huo Y.-J. et al (2015). Specific correlation between the Hegu point (LI4) and the orofacial part: Evidence from an fMRI study.Evid. Based Compl. Alternat. Med.2015:585493. 10.1155/2015/585493
14
Kosslyn S. M. Ochsner K. N. (1994). In search of occipital activation during visual mental imagery.Trends Neurosci.17290–292. 10.1016/0166-2236(94)90059-0
15
Li K. C. Liu J. T. (2010). Ten-year progress in neuroimaging.Chin. J. Contemp. Neurol. Neurosurg.10123–126. 10.3969/j.issn.1672-6731.2010.01.028
16
Li J. D. (2005). Grading criteria for facial nerve function.For. Med. Sci.6:73.
17
Liang F. R. Wang H. (2016). Acupuncture and moxibustion.Beijing: China Press of Traditional Chinese Medicine.
18
Liu J. P. Xu C. S. Lu Q. Li C. F. Yang J. (2014). Resting-state functional MRI study of acupuncture treatment for peripheral facial paralysis based on regional homogeneity algorithm.Magn. Reson. Imag.5430–435. 10.3969/j.issn.1674-8034.2014.06.005
19
Lv H. Wang Z. Tong E. Williams L. M. Zaharchuk G. Zeineh M. et al (2018). Resting-state functional MRI: Everything that nonexperts have always wanted to know.AJNR Am. J. Neuroradiol.391390–1399. 10.3174/ajnr.A5527
20
Ma C. Li J. Yu F. (2021). Visual analysis of knowledge mapping on acupuncture treatment for peripheral facial paralysis based on CiteSpace.J. Clin. Acupuncture Moxibust3757–62. 10.19917/j.cnki.1005-0779.021119
21
Mu H. Liu J. Mao Y. Han Y. Xu L. Zhang D. et al (2022). The alterations and significance of intercellular adhesion molecule-1 in mouse brainstem during herpes simplex virus type 1–induced facial palsy.Appl. Biochem. Biotechnol.1943483–3493. 10.1007/s12010-022-03837-4
22
Niendam T. A. Laird A. R. Ray K. L. Dean Y. M. Glahn D. C. Carter C. S. (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions.Cogn. Affect. Behav. Neurosci.12241–268. 10.3758/s13415-011-0083-5
23
Peitersen E. (2002). Bell’s palsy: The spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies.Acta Otolaryngol.1224–30. 10.1080/000164802760370736
24
Smith S. M. Fox P. T. Miller K. L. Glahn D. C. Fox P. M. Mackay C. E. et al (2009). Correspondence of the brain’s functional architecture during activation and rest.Proc. Natl. Acad. Sci. U. S. A.10613040–13045. 10.1073/pnas.0905267106
25
Sohn Y. H. Voller B. Dimyan M. St Clair, Gibson A. Hanakawa T. et al (2004). Cortical control of voluntary blinking: A transcranial magnetic stimulation study.Clin. Neurophysiol.115341–347. 10.1016/j.clinph.2003.10.035
26
Song G. J. Xu G. Q. Liu J. W. Zhou Z. W. Wu Q. (2024). Magnetic resonance study on resting-state brain functional connectivity strength in hemiplegic patients with ischemic stroke.J. Zunyi Med. Univ.4776–82. 10.14169/j.cnki.zunyixuebao.2024.0012
27
Wang J. Xie S. Guo X. Becker B. Fox P. T. Eickhoff S. B. et al (2017). Correspondent functional topography of the human left inferior parietal lobule at rest and under task revealed using resting-state fMRI and coactivation based parcellation.Hum. Brain Mapp.381659–1675. 10.1002/hbm.23488
28
Wang S. Rao J. Yue Y. Xue C. Hu G. Qi W. et al (2021). Altered frequency-dependent brain activation and white matter integrity associated with cognition in characterizing preclinical Alzheimer’s disease stages.Front. Hum. Neurosci.15:625232. 10.3389/fnhum.2021.625232
29
Wang Y. Tang W. Chai Y. Zhu W. Li X. Wang Z. (2021). Diagnostic value of dynamic contrast-enhanced MRI in Bell’s palsy: Initial experience.Clin. Radiol.76237.e9–237.e14. 10.1016/j.crad.2020.10.001
30
Yang J. Li C. F. Zhang Q. P. Yuan A. H. Xu C. S. Zhu Y. F. et al (2012). Preliminary analysis of brain functional imaging in patients with peripheral facial paralysis receiving acupuncture at Hegu (LI4).J. Changchun Univ. Chin. Med.28608–610. 10.13463/j.cnki.cczyy.2012.04.019
31
Yang D. Gang B. Z. (2015). Parietal lobe and cognitive dysfunction.Med. Recapitulate214106–4108. 10.3969/j.issn.1006-2084.2015.22.028
32
You Y. Bai L. Dai R. Cheng H. Liu Z. Wei W. et al (2013). Altered hub configurations within default mode network following acupuncture at ST36: A multimodal investigation combining fMRI and MEG.PLoS One8:64509. 10.1371/journal.pone.0064509
33
Zang Y. Jiang T. Lu Y. He Y. Tian L. (2004). Regional homogeneity approach to fMRI data analysis.Neuroimage22394–400. 10.1016/j.neuroimage.2003.12.030
34
Zhang B. He S. Weng X. (2018). Localization and functional characterization of an occipital visual word form sensitive area.Sci. Rep.8:6723. 10.1038/s41598-018-25029-z
35
Zhang X. Guo T. T. Wang Y. J. (2019). Parietal lobe stroke and motor system dysfunction.Chin. J. Geriatr. Heart Brain Vessel Dis.21891–892. 10.3969/j.issn.1009-0126.2019.08.028
Summary
Keywords
Bell’s palsy, acupuncture, functional magnetic resonance imaging (fMRI), fractional amplitude of low-frequency fluctuation (fALFF), regional homogeneity (ReHo)
Citation
Xu X-s, Zhang Y-t, Li X-w, Shu Y-l, Zhang J-c, Miao T-t, Yang Y-y, Yang J and Shi H-p (2025) Exploring the potential central regulatory mechanisms of acupuncture for acute-stage Bell’s palsy: an fMRI-based investigation. Front. Neurosci. 19:1647538. doi: 10.3389/fnins.2025.1647538
Received
15 June 2025
Accepted
08 September 2025
Published
24 September 2025
Volume
19 - 2025
Edited by
Kathrin Machetanz, Tübingen University Hospital, Germany
Reviewed by
Peng Cao, University of Science and Technology of China, China
Anastasia Urbanelli, University of Turin, Italy
Updates
Copyright
© 2025 Xu, Zhang, Li, Shu, Zhang, Miao, Yang, Yang and Shi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-ping Shi, shihaipingdyx@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.