- 1Ocugen India, Hyderabad, Telangana, India
- 2Ocugen, Malvern, PA, United States
Autism spectrum disorder is a neurological and developmental condition known to impact a person's learning, communication, and interpersonal interactions. Recent research has highlighted the role of nuclear hormone receptors (NHRs) in neurodevelopment and synaptic function, suggesting their potential involvement in ASD pathophysiology. NHRs regulate gene expression that are critical for neural differentiation, plasticity, and metabolic processes. Dysregulation of these receptors can lead to altered neural circuit formation and neurotransmitter imbalances, which are commonly observed in ASD. Understanding the interplay between NHRs and ASD could open new avenues for therapeutic interventions, providing hope for more personalized approaches to managing the disorder. One key receptor is retinoic acid-related orphan receptor-alpha (RORA), which was shown to be reduced in individuals with ASD. Among its numerous functions during development, RORA was shown to regulate the transcription of genes involved in neuronal differentiation, synaptic function, and neuroprotection. Studies have identified that RORA expression is reduced in individuals with ASD, particularly in the prefrontal cortex and cerebellum, affecting transcription of multiple ASD-associated genes. In the present review, we discuss the underlying mechanisms leading to ASD pathophysiology, various treatment modalities, the prospects of the RORA gene therapy approach, and future perspectives.
Introduction
Autism is referred to as a “neurodevelopmental disorder” since symptoms often manifest in the first 2 years of life, even though a diagnosis can be made at any age. Differences in social and communicative behaviors, intellectual difficulties, and other physical and mental health concerns are just a few of the many symptoms that people with autism may experience, hence, it is considered a spectrum disorder. Autism has a heterogeneous diagnosis that may undergo developmental changes over time and an array of epidemiologic, genetic, epigenetic, and environment-related factors has been associated with the condition. Among the additional characteristics are atypical patterns of activities and behaviors, as demonstrated by challenges in switching between activities, a strong focus on minute details, and atypical responses to sensory input. Intellectual disability [currently estimated at around 30% of cases (Christensen et al., 2012); historically estimated at around 70%] and attention deficits (occurring in approximately 30%−40% of cases, though estimates outside of this range are common) are common impairments that correspond to autism spectrum disorder (ASD). Other common impairments include sensory sensitivity, gastrointestinal issues, immunological deficiencies, anxiety and depression, sleep disturbances, and a variety of comorbid medical conditions (Croen et al., 2015; Matson and Cervantes, 2014). Although some people with autism are capable of leading independent lives, others require lifelong care and support. Opportunities in education and employment are often impacted by autism. Early intervention and tailored support can significantly improve outcomes for individuals with the condition. It is therefore crucial to recognize and accommodate the unique strengths and challenges of each person, promoting inclusion and understanding in all aspects of life.
Based on research on autism prevalence around the world, considering how socioeconomic, racial, and geographic factors affect prevalence estimates, worldwide, about 1 in 100 children receive a diagnosis of autism spectrum condition. According to estimates in the US, one in 36, 8-year-old children (about 4% of boys and 1% of girls) will have ASD in 2020 (Maenner et al., 2023). Over the past three decades, the prevalence of ASD has gradually but significantly risen across Europe, North America, and Oceania (Anorson et al., 2021). For children of about 4 years, the prevalence increased by 26% from 2018 to 2020 (Shaw et al., 2023). The prevalence estimate in 2020 for children in the age group of 8 years was 27.6% (Maenner et al., 2023). Sex related bias has been observed in the prevalence of ASD where there exist roughly four males for every girl with ASD. This “male to female” sex ratio seemed to decline with increasing severity of ASD (Werling, 2013). Females often camouflage their autistic traits by mimicking social behaviors, suppressing stimming, or rehearsing conversations, which can lead to underdiagnosis (Wood-Downie et al., 2021), and thereby significantly impacts the male-to-female incidence ratio (Cancino-Barros and Villacura-Herrera, 2025). Historically, there has been a substantial sex gap evident in all populations under study (Dworzynski et al., 2012), the variations in how symptoms appear in females and the potential diagnostic biases (Dworzynski et al., 2012) need further research.
In this review, we discuss the key characteristic features of ASD, genetic and molecular pathways, the role of nuclear hormone receptors, aspects related to gen therapy, and other therapeutic interventions.
Characteristic features and pathophysiology
A higher incidence of ASD in the offspring has been linked to maternal use of pharmaceutical medications, particularly in the first trimester of pregnancy, such as thalidomide, valproic acid, and antidepressants (primarily selective serotonin reuptake inhibitors; Croen et al., 2011; Christensen et al., 2013). Distinguishing the effects of medicine from those of the mother's underlying disease, which may potentially affect autism risk, can be challenging (Lyall et al., 2013). Particularly for those who are genetically predisposed, and have exposure to a variety of toxicants, such as pesticides, polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs), can negatively impact developmental processes. In addition to their neurotoxic and endocrine-disrupting properties, PCBs and PBDEs also bioaccumulate in the food chain and remain in the environment (Newschaffer et al., 2007). Furthermore, several neurotoxic substances may disrupt neurotransmitter systems linked to ASD (Quaak et al., 2013). Pregnancy-related ASD risk has been linked to maternal residential proximity to agricultural pesticide applications; however, this situation might indicate abnormally high exposure levels (Ornoy et al., 2015). Additionally, these substances may be immunotoxic, which could result in the changed cytokine production commonly seen in ASD (Goines and Ashwood, 2013). A recent worldwide study confirmed that older parents are known risk factors for autism, but it also indicated that parents who are not of the same age increase the likelihood of developing ASD (Sandin et al., 2016). It is believed that methylation abnormalities in gametes, which can be brought on by elevated oxidative stress that damages and fragments DNA, are a result of older parents (Menezo et al., 2015). Obesity, diabetes, and folic acid insufficiency are maternal metabolic and nutritional risk factors. Zinc deficiency has been observed in autistic children and may play a role in pathogenesis (Lyall et al., 2013; Grabrucker, 2013; Ornoy et al., 2015).
Intellectual disability, motor impairment, attention issues, sensory differences, sleep disorders, seizures, and externalizing behaviors like violence and affective disorders are among the co-morbid conditions that are linked to ASD (Maski et al., 2011; Devnani and Hegde, 2015). Rett syndrome, Asperger syndrome, autism, and pervasive developmental disorder-not otherwise specified are among the many complex and varied conditions that are included in the category of autism spectrum disorders (Barrett et al., 1996). Although monogenic syndromes do not always have traits of autism spectrum disorder (ASD), many do have a high prevalence of ASD diagnoses or traits. The expression of ASD traits depends on the specific syndrome, the gene involved, and personal traits such as sex and mutation type (Lord et al., 2000). Studies in toddlers infer that a deficiency in social interaction, communication, and behavior may indicate early signs of autism as early as 14 months of age, and clinical signs appear at 3 years (Landa et al., 2007). Numerous studies show that some disorders with predisposing genetic syndromes are closely associated with ASD (Jedele, 2007; Autism Research Institute, 2023, 2022; White et al., 2020; Ornoy et al., 2016; Genovese and Butler, 2023). Both genetic and environmental factors contribute to the pathophysiology of ASD, even if the causes of these anomalies are still being studied (Herbert et al., 2006; Minshew and Williams, 2007; Persico and Bourgeron, 2006).
Although the etiologies of most patients are still unknown, ASD exhibits clinical variability and can co-occur in up to 10% of cases with well-characterized neurological and genetic abnormalities, including co-occurring syndromes (Rapin, 2002). Some academics support using the term “autism” rather than a single disorder because of the clinical unpredictability and heterogeneity linked to autism (Geschwind and Levitt, 2007). Though monogenic pathologies such as Angelman syndrome, Rett syndrome, tuberous sclerosis, or Phelan–McDermid syndrome, etc. have different pathologies, researchers argue that mutation discovery of this sort offers an important opportunity to identify neurodevelopmental mechanisms of the disease. They hope that these mechanisms will show some degree of convergence that may be amenable to treatment intervention (Gamsiz et al., 2015).
Autism is linked to disruptions in neurotransmitter systems, including those involving glutamate, GABA, serotonin (5-hydroxytryptamine, 5-HT; Pagan et al., 2014), melatonin (Melke et al., 2008), dopamine (DA; Farook et al., 2012) and arginine vasopressin (AVP; Figure 1). New behavioral neuroscience, chemical genetics, optogenetics, and electrophysiology methods have aided in developing connections between different social behaviors and cerebral circuit activity as shown in Figure 1.
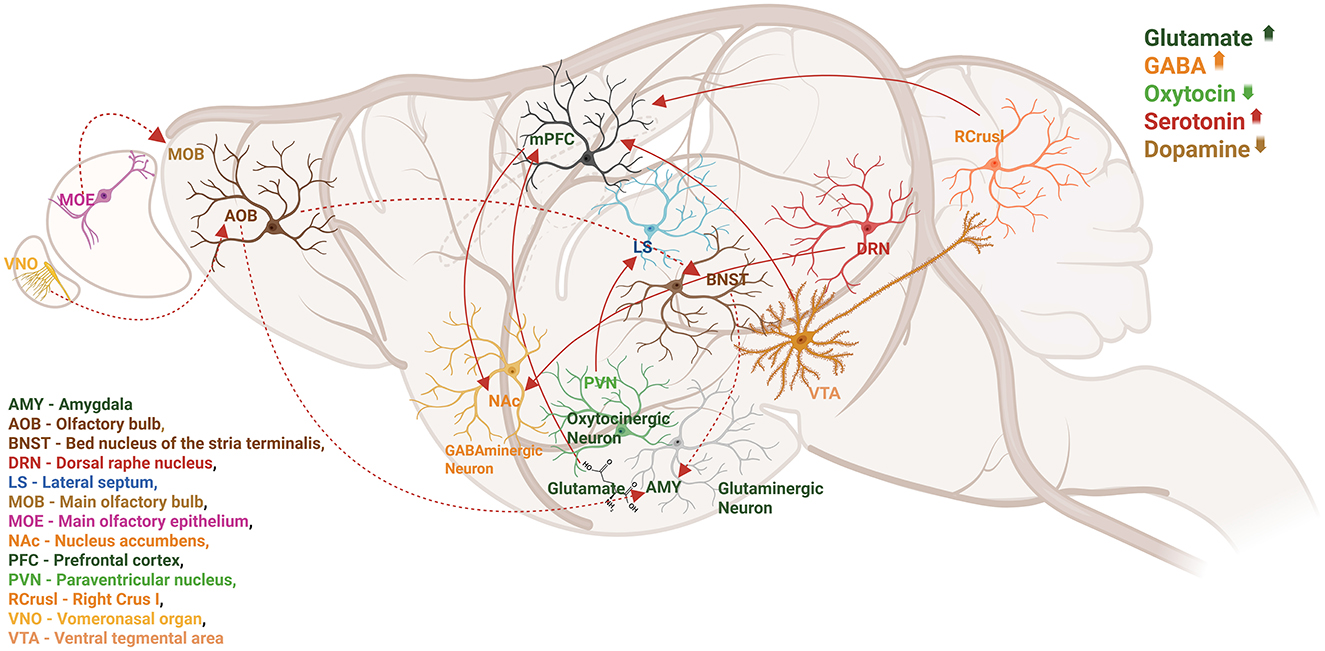
Figure 1. Local and distal circuits involved in social behaviors are depicted in a sagittal image of the rodent brain. Numerous neurotransmitters and neuromodulators collaborate to coordinate the activity of brain regions and neural circuits. Studies indicate that autism is linked to disruptions in neurotransmitter systems, including glutamate, GABA, serotonin (5-hydroxytryptamine, 5-HT), melatonin, dopamine (DA), OT, and arginine vasopressin (AVP). Therefore, dynamic variations in neurotransmitter concentration, release, and receptor density may directly impact behavioral performance and neuronal circuit function.
Although they only make up a small percentage of cases, several genetic disorders are known to have strong correlations with autism. Monozygotic twins had approximately 91% autism heritability rate, which drops to 53% in dizygotic twins (Tick et al., 2016). Numerous associations between non-genetic factors and ASD have been found, which paved the way for additional research to examine mechanisms for the development of the disorder (Grabrucker, 2013).
Genetic factors associated with ASD
Autism is the most heritable psychiatric illness (Hawi et al., 2018). Apart from genomic screening and candidate gene studies, the identification of chromosomal abnormalities and Mendelian syndromes in individuals with autism has helped refine our understanding of the complex genetics underlying these conditions. In a case series study, when only cases without minor congenital anomalies were included, the male-to-female sex ratio was nearly 10:1, and there was a much higher rate of autism cases in the families (Miles and Hillman, 2000). Though modern studies exclude cases with known etiologies, but do not exclude mild, dysmorphic features of unknown origin. For autism that the examiners judge to be idiopathic, the most parsimonious genetic model is one in which several genes interact with one another to produce the autism phenotype. A cytogenetic analysis of 67 people who had previously been diagnosed with mental retardation and autistic characteristics showed that 3 (4.5%) had autosome abnormalities and one person (1.5%) had the fragile X chromosome [fra(X); Cantú et al., 1990]. Autism, along with atypical bipolar disorder associated with 15ql2 deletion, was first reported by Kerbeshian et al. (1990). Partial trisomy of chromosome 15 may be linked to a distinct condition that includes mild-to-moderate physical stigma, autistic behavior, and moderate-to-severe mental impairment. Kyphoscoliosis, epilepsy, and muscular hypotonia seem to be related characteristics (Gillberg et al., 1991). The region 15pter-ql3, which has a molecular border at locus D15S24, has one or more genes that are predisposed to behavioral issues, including infantile autism or autistic disorder (Martinsson et al., 1996). ASD is linked to the chromosomal abnormality of an extra marker chromosome with a duplication of 15q11-13 and the clinical symptoms of epilepsy and ataxia (Bundey et al., 1994). The GABRA5 and GABRB3 genes on the maternally derived chromosome are duplicated, and an interstitial duplication of 15q (Bundey et al., 1994). Siblings with ASDs had mutations in the neuroligins NLGN3 and NLGN4, the two X-linked genes, which impact synapse-localized cell adhesion molecules, indicating synaptogenesis breakdown may be a risk factor for autism (Jamain et al., 2003). In addition to duplication, which also seems to be a high-penetrance risk factor, five cases of a de novo deletion of 593 kb on chromosomes 16p11.2 were found among Autism Genetic Resource Exchange (AGRE) families (Weiss et al., 2008). A comparative genomic hybridization, a reciprocal microduplication, and a unique, recurring microdeletion carry a significant risk of autism and are be responsible for around 1% of cases (Weiss et al., 2008). Durand and colleagues provide insight into one gene dosage-sensitive synaptic pathway implicated in autism spectrum disorders, although only a small percentage of people can develop language and/or social communication impairments due to a mutation of a single copy of SHANK3 on chromosome 22q13 (Durand et al., 2007). Following data analysis using an affected sib-pair (ASP) approach, the whole-genome microsatellite marker analysis of 110 multiplex families using 335 microsatellite markers produced multipoint maximum LOD scores (MLS) that surpass the recognized threshold for suggestive linkage on chromosomes 5, X, and 19 (Liu et al., 2001). The significance of peak LOD scores based on statistical evidence at adjacent marker loci was also evaluated using scan statistics to increase the power to detect linkage. The results showed impressive evidence for linkage to autism and autism-spectrum disorders, with significant genome-wide P values < 0.05 for markers on chromosomes 5 and 8 and suggestive linkage evidence for a marker on chromosome 19 (Liu et al., 2001). In 427 unrelated ASD cases, karyotyping and single-nucleotide polymorphism microarrays were performed to evaluate the genome for structural abnormalities. In 44% of ASD families, 277 imbalanced copy number variants (CNV), (i.e., the number of copies of a particular gene varies from one individual to the next) were identified using microarrays that were absent in 500 controls and additional balanced alterations were discovered using karyotyping where 27 cases with de novo modifications, and in three (11%) of these individuals, two or more new variations were recorded, though the majority of variants were inherited, additional genes (DPP6, DPP10 PCDH9, RPS6KA2, and RET); other hereditary CNVs (IDS, IL1RAPL1, and TSPAN7), MR loci (15q24, 16p11.2) implicate the SHANK3, NLGN4, and NRXN1-PSD genes (Marshall et al., 2008). Genetic abnormalities in ASD include de novo mutations (Sanders et al., 2012; O'Roak et al., 2012), Multiple genes are implicated in synaptic formation, transcriptional regulation, and chromatin-remodeling pathways (De Rubeis et al., 2014). Mutations in FMR1, SHANKs, NRXNs, NLGNs, SYNGAP1, CHD2, SCN2A, POGZ, ADNP, and DYRK1A share pathways with altered NMDAR (glutamate receptors; Zhang et al., 2024).
Molecular pathways involved in ASD
Dysregulated signaling and metabolic pathways that are indicative of a systemic illness that may be treated once identified are at least partially responsible for the neurological manifestations of ASD. The distinct biological phenotypes of ASD are corroborated by pathway analyses, which also distinguish several types of idiopathic autism symptoms. Studies have identified several pathways related to ASD, some of which are listed below.
There are several phases of cerebellar development when Wnt signaling pathways are implicated. Specifically, Wnt1 contributes to the preservation of the midbrain-hindbrain area during the early stages of cerebellar anlage formation (Brault et al., 2001). E12.5 also expresses Wnt1 from migratory granule cell progenitors (Shimamura et al., 1994). Wnt7a, which enhances afferent mossy fiber synapses, is also expressed by mature granule cells (Hall et al., 2000). Disruptions in non-canonical Wnt genes, such as PRICKLE2 may exacerbate the synaptic defects that underlie ASDs. ASD patients' PRICKLE2 variations result in a loss of PRICKLE2 protein function, and may be a contributing factor to neurological dysfunction in disorders like ASD (Sowers et al., 2013; Figure 2A). The WNT “off state” is triggered, and the cytosolic β-catenin is attached to its destruction complex, which is made up of CK-1, APC, AXIN, and GSK-3β. Following CK1's phosphorylation of the Ser45 residue, GSK-3β phosphorylates β-catenin on the Thr41, Ser37, and Ser33 residues. The proteasome then breaks down phosphorylated β-catenin. Thus, in the absence of WNT ligands, the cytosolic level of β-catenin is maintained at a low level. The TCF/LEF complex cannot activate the target genes if β-catenin is not translocated to the nucleus (Figures 2B, C).
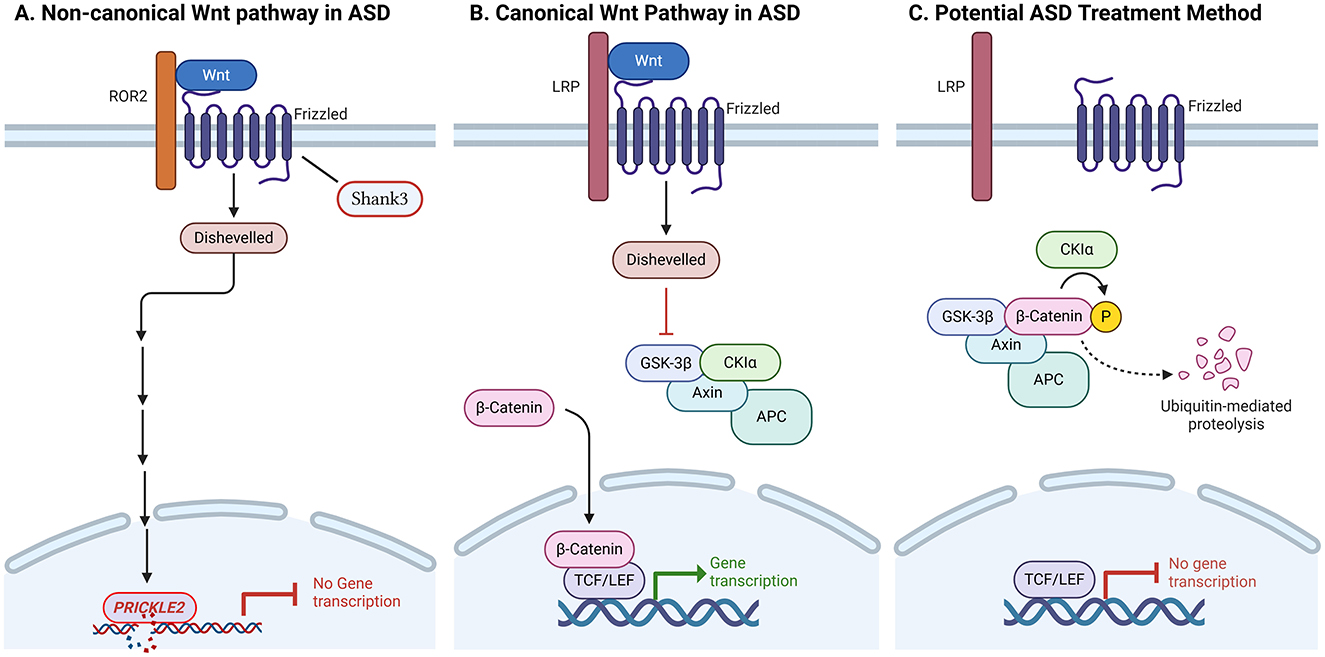
Figure 2. Anomalies in the canonical and non-canonical Wnt signaling pathways are the cause of autism spectrum disorders (ASDs). (A) Disruptions in non-canonical Wnt genes, such as PRICKLE2, may exacerbate the synaptic defects that underlie ASDs. Mice with PRICKLE2 disruption exhibit atypical behavior, such as impaired social interaction, improper learning, and rigid conduct. In mouse hippocampal neurons, PRICKLE2 disruption resulted in decreases in post-synaptic density size, synapse number, and dendritic branching. (B) The canonical Wnt pathway is β-catenin-mediated. Wnt target genes associated with ASD can be activated by stabilized β-catenin entering the nucleus and dislodging Goucho/transducin-like enhancer of split (TLE) repressors from T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors. (C) The destruction complex, which includes Axin, adenomatous polyposis coli (APC), casein kinase 1 alpha (CK1α), and glycogen synthase kinase 3 beta (GSK3β), constitutively breaks down β-catenin in the absence of Wnt activation or due to the presence of an inhibitor. The breakdown of β-catenin through the ubiquitin-mediated proteasome pathway is facilitated by phosphorylation by GSK3β and CK1α. Deactivation of Wnt could be used as a potential ASD treatment method.
An elevated JAK/STAT signaling induction is observed in ASD. According to preliminary data, neurodevelopmental problems are associated with the JAK/STAT pathway (Mount et al., 2007). The JAK/STAT pathway is essential for the survival, proliferation, and differentiation of various cell types (Adamson et al., 2009). According to research, STAT5 is crucial for the development of cortical interneurons in the developing brain (Markham et al., 2007). Significantly, target modulators have been shown to reduce neuronal issues linked to both acute and long-term neurological impairments. Modulators of JAK-STAT, mTOR, and PPARγ signaling have therapeutic significance against neuronal dysfunctions (Kumar et al., 2023).
PPAR-gamma agonists could be used along with JAK-STAT inhibitors as target therapeutic interventions for autism. There is involvement and mutual regulation of JAK-STAT and PPAR-gamma signaling in controlling multiple pathological factors associated with autism (Khera et al., 2022). ASD pathologies may be lessened by enhancing cytokine and JAK/STAT signaling. mTOR-dependent increased spine density is associated with ASD-like stereotypies and cortico-striatal hyperconnectivity in Tsc2 haplo-insufficient mice (Pagani et al., 2021). An analogous cortical-striatal hyperconnectivity is observed in children with idiopathic ASD, which can be used as a connectivity fingerprint for ASD-dysregulated genes interacting with mTOR or Tsc2 (Pagani et al., 2021). The mTOR and the MAPK pathways showed increased activity, which are primary regulators of protein synthesis and synaptogenesis. Based on their clinical diagnosis, rpS6, p-eIF4E, TSC1, and p-MNK1 expression levels varied, inferring that various components of signaling pathways form a molecular signature for the severity of autism (Rosina et al., 2019). Loss of CHD8-mediated regulatory control may perturb normal proliferation and differentiation of neuronal progenitors, given the functions of the genes strongly affected by CHD8 knockdown in human neural stem cells (hNSCs), which may result in altered numbers or relative proportions of neuronal populations derived later in cortical development (Cotney et al., 2015). Transcriptional and splicing dysregulation are the underlying mechanisms of neuronal dysfunction that provide strong evidence for convergent molecular abnormalities in ASD (Voineagu et al., 2011; Yizhar et al., 2011).
Epigenetics of ASD
Investigating epigenetic pathways in idiopathic autism is intriguing not only because it provides insight into higher-order regulation of gene expression but also because exposure to biological modulators and environmental variables might impact these alterations. Therefore, the relationship between genotype and extrinsic (environmental) or intrinsic (biological) factors that contribute to ASDs may be mediated by epigenetics (Nguyen et al., 2010). DNA methylation is identified as a potential mechanism for epigenetic regulation in idiopathic autism by global methylation profiling of lymphoblastoid cell lines from phenotypically discordant monozygotic twins and non-autistic siblings (Nguyen et al., 2010). Key targets, such as increased levels of chemokine receptors and ZIC family genes, have been identified by recent studies on autism spectrum disorder (ASD).
Nuclear hormone receptors
Nuclear hormone receptors (NHRs) are transcription factors that interact with co-repressors and co-activators to control the expression of various genes. A combination of functional and computational research has established the evolutionary alterations of NR1H and NR1I receptors across vertebrates and their potential divergence from ancestral receptors that initially appeared in invertebrates (Krasowski et al., 2011). Several studies have linked defects in NHRs to autism in humans (Kong et al., 2020; Olivares et al., 2016). To date, the human genome has been identified as having 48 NHRs, while the mouse genome has 49 (Zhang et al., 2004). Nuclear receptors play important roles in reproduction, development, and physiology by controlling gene expression. This, in turn, affects the growth (Fu et al., 2019), differentiation (Ma et al., 2022), and death (Tschuck et al., 2023; Lambrecht et al., 2023) of various cell types. Specific roles for NHRs linked to ASD are described below.
NHR structure and regulation
NHRs are built with conserved domain organization, typically including a hinge region, a ligand-binding domain (LBD) with a second transactivation domain, a variable amino-terminal domain (A/B), and a DNA-binding domain (DBD) with zinc finger motifs (De Bosscher et al., 2020; Pavek, 2016; Lavery and McEwan, 2005). As NHRs are natural ligand-activated transcription factors, endogenous substances such as bile acids, retinoids, steroid hormones, thyroid hormones, and vitamin D act as their ligands (Tao et al., 2020). PXR and CAR are examples of these promiscuous receptors for the structurally diverse class of ligands that function as agonists (Bwayi et al., 2022). When ligands bind to specific hormone response regions on DNA, they cause changes in shape that enable nuclear translocation, dimerization, and the regulation of gene expression (Li et al., 2019). NHRs can control gene expression by directly binding to target genes and interacting with other nuclear receptors (Aranda and Pascual, 2001; Ogawa et al., 2005; Béchet, 1980; Huggins and Greene, 2023; Jiang et al., 2024; Ratman et al., 2016; Wang et al., 2023; Miao et al., 2019).
Role of NHRs in ASD
Because of their important roles in regulating transcription, brain development, controlling gene expression, and their connection to known risk factors for ASD, NHRs are increasingly recognized as key target genes in ASD. Histone deacetylases (HDACs) change chromatin structure and gene expression. They are linked to nuclear receptor corepressors (NCORs), which play a vital role as transcriptional co-regulators. The NCOR complex connects gene-environment interactions and changes in gene regulation in the context of ASD by interacting with well-known ASD-related genes like MECP2 and TBL1XR1 (Mastrototaro et al., 2021). For instance, Rett syndrome, is associated with mutations that disrupt MECP2-NCOR interactions (Lyst et al., 2013). Prenatal exposure to HDAC inhibitors like valproic acid (VPA), which is an environmental risk factor for ASD, interferes with NHR-related mechanisms of gene repression (Kong et al., 2020). Genes linked to neurotransmitter systems that are disrupted in ASD include glutamate receptors (NMDA and mGluR receptors; Lee et al., 2025), genes from the GABAergic system (Zhao et al., 2022), and synaptic scaffolding (SHANK3; Mihalj et al., 2024), are affected by NHRs. Key features of ASD are the imbalance between excitatory and inhibitory signals and problems with synapses, which worsen due to the disruption of these genes caused by faulty NHR corepressor activity. Like in other ASD models, mouse models that lack NCOR components show problems with excitability, changes in glutamate receptor expression, and issues with cognitive and social functions (Lim et al., 2022; Kong et al., 2020). Hormones and vitamins activate NHRs (Krasowski et al., 2011), which are ligand-dependent transcription factors that control gene networks important for synapse formation, brain growth, and neuronal differentiation (Olivares et al., 2016). They also change epigenetic markers that are crucial for maintaining a stable gene expression during development. For instance, members of the NHR family, such as retinoic acid receptors and thyroid hormone receptors, influence the maturation of brain circuits connected to social behaviors and cognitive impairment in ASD (Olivares et al., 2016; Kong et al., 2020). NHRs play an important role in controlling cell fate, hence 15–20% of all pharmaceutical medications target them (Kenda and Sollner Dolenc, 2020; Pal et al., 2023). Tiny compounds like synthetic ligands or HDAC inhibitors can change their activity, making NHRs appealing targets for therapy (De Bosscher et al., 2020; Pal et al., 2023). Specific NR-targeting drugs are often used to treat various diseases. For example, raloxifene and tamoxifen target the estrogen receptor (ER), and are used to treat breast cancer and osteoporosis.
We may be able to restore disrupted transcriptional and epigenetic patterns in ASD by focusing on NHR pathways. For example, in mouse models of ASD linked to failures in NHR-related genes, researchers have shown that modifying histone acetylation with HDAC inhibitors leads to behavioral recovery (Dash et al., 2009). Additionally, given the neuroinflammatory factors of ASD, the anti-inflammatory roles of specific NHRs, such as Retinoic acid-related orphan receptor-alpha (RORA) and peroxisome proliferator-activated receptors (PPARs), may present new treatment options (Jiang et al., 2022).
Retinoic acid-related orphan receptor-alpha (RORA)
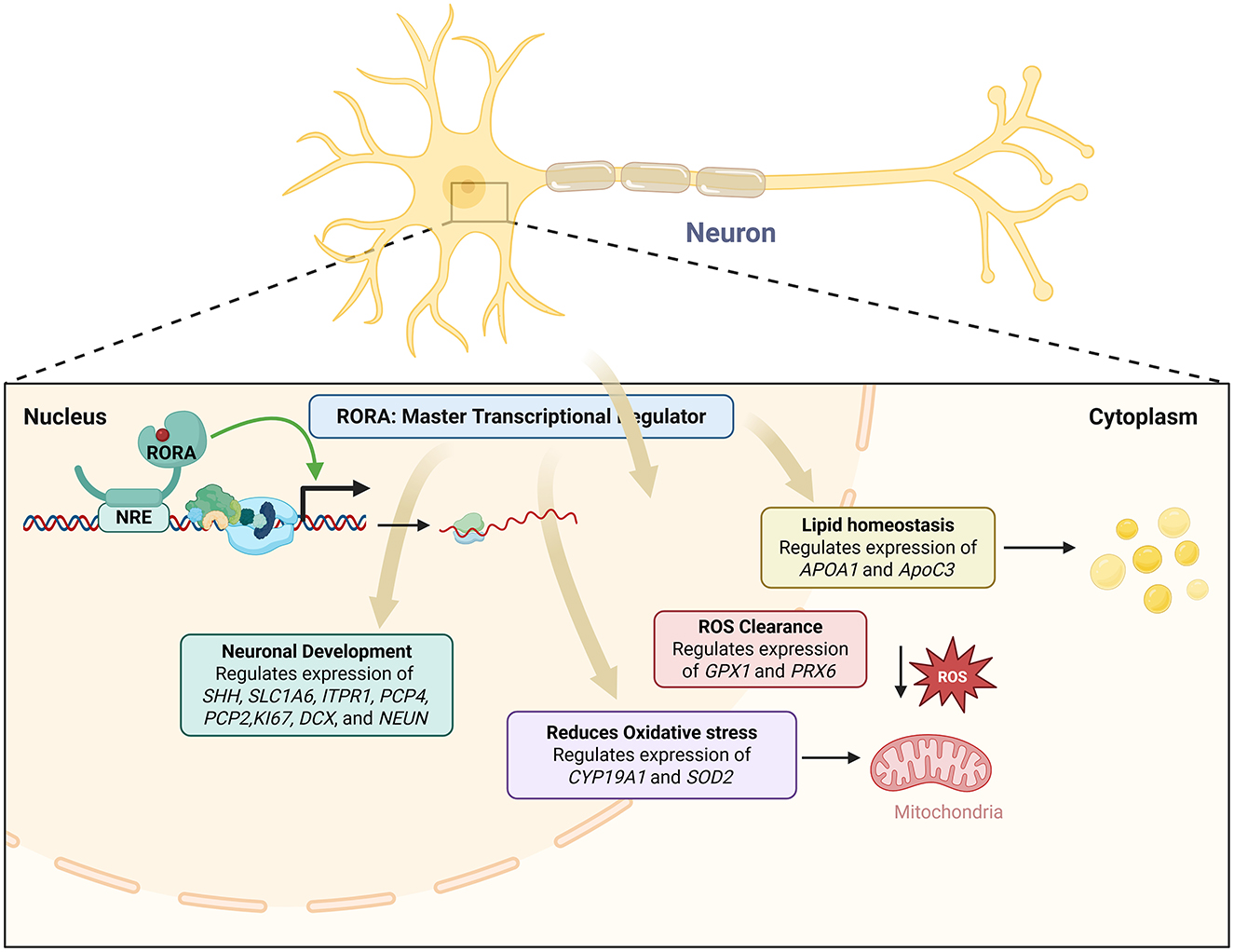
ROR alpha 1 belongs to the unique NR1 subfamily of monomeric orphan nuclear receptors. These receptors use a variety of ways to accomplish high-affinity and selective DNA binding and hence can act as master transcription regulators (Giguère et al., 1995). RORA activates transcription from only a subset of sites to which it binds strongly as a monomer (Harding et al., 1997). RORA also selectively binds as a homodimer to a direct repeat of this monomer site with a 2-bp spacing between the AGGTCA sequences (RevDR2 site) and is a much more potent transcriptional activator on this site than on monomer sites or other direct repeats (Harding et al., 1997). Mutational analysis revealed that RORA contains both transcriptional activation and transcriptional repression domains, with the repression domain being more active in some cell types (Harding et al., 1997). The abilities of RORA polypeptides to repress transcription correlate with their abilities to interact with the nuclear receptor corepressors N-CoR and SMRT in vitro (Harding et al., 1997). Transcriptional regulation by RORA is complex and likely to be regulated in a cell-type and target gene-specific manner (Harding et al., 1997). RORα polymorphisms (rs11639084 and rs4774388) have been associated with ASD risk (Sayad et al., 2017). Studying twins where one sibling has autism and the other does not, revealed increased CpG island methylation at the upstream RORα promoter sites of the twin with ASD (Nguyen et al., 2010). Global methylation profiling revealed that the RORα protein levels were significantly reduced in the brains of individuals with ASD due to epigenetic alterations at the RORα gene (Nguyen et al., 2010). Experimental correlation of methylation with gene expression data revealed that RORA exhibited methylation-specific gene silencing in conjunction with enhanced methylation of particular CpG sites in corresponding upstream regulatory CpG islands (Nguyen et al., 2010). Perhaps likely not all populations carry methylation of the RORA promoter region as an epigenetic risk factor for autism (Salehi et al., 2017). Post-mortem examination of age-matched case-control individuals also showed decreased expression of RORα protein in the prefrontal cortex and the cerebellum of autistic individuals (Hu et al., 2009). These findings are significant because studies on the RORαsg mice indicate that RORα protein is involved in several processes relevant to autism including Purkinje cell differentiation (Hadj-Sahraoui et al., 2001; Boukhtouche et al., 2006b; Chen et al., 2013), cerebellar development (Gold et al., 2003; Harding et al., 1997), brain lipid homeostasis (Chen et al., 2020), protection against oxidative stress and inflammation (Boukhtouche et al., 2006c; Delerive et al., 2001; Nejati Moharrami et al., 2018; Yue et al., 2020), and the circadian rhythm (Fatemi et al., 2012).
Role of RORA in CNS development
During CNS development and in mature neurons, modifications in the dendritic architecture refine the function of neural circuits (Bottjer and Arnold, 1997). RORA was shown to regulate dendritic remodeling during CNS development (Boukhtouche et al., 2006c). The deficiency of Purkinje cells is a consistently identified neuroanatomical abnormality in the brains of individuals with ASD (Palmen et al., 2004; Polleux and Lauder, 2004) and RORα is critical in the development of Purkinje cells (Hadj-Sahraoui et al., 2001; Boukhtouche et al., 2006a; Doulazmi et al., 1999; Hamilton et al., 1996). Purkinje cells inhibit the action potentials from deep cerebellar nuclei and vestibular neurons in the cerebellum. By regulating the rate at which signals fire in the cerebellum, Purkinje cells produce precise motor coordination. Disruption of Purkinje function causes multiple motor defects associated with ASD. Purkinje cells express RORα very early in development which continues during adulthood (Ino, 2004). In RORαsg mice, most of the Purkinje cells die within the first month of life (Herrup and Mullen, 1979; Dussault et al., 1998; Vogel et al., 2000; Doulazmi et al., 2001). The surviving Purkinje cells fail to mature and develop spiny branchlets (Landis and Sidman, 1978; Sotelo, 1978). RORα is necessary for the retraction of transient dendrites in the early development of Purkinje cells to establish a mature dendritic tree, an essential step in Purkinje cell maturation (Boukhtouche et al., 2006a). RORα deficiency in adult mice (Chen et al., 2013) also creates defects in Purkinje cells such as premature dendritic atrophy and death (Hadj-Sahraoui et al., 2001), Therefore RORα is a terminal differentiation gene that defines the functional properties of a mature Purkinje cell from development to maintenance, throughout its life. The genetic programs in developing Purkinje cells, analyzed daily during mouse prenatal development revealed that RORα bound to the promoter sites and controlled the expression of Shh, Slc1a6, Itpr1, Pcp4, and Pcp2 (Gold et al., 2003). These RORα target genes provide mitogenic drive and are also required for reciprocal signals between Purkinje, granule, and molecular cells in cerebellar development. Studies in RORαsg mice suggest a possible role of RORα in the expression of cell proliferation, neuronal differentiation, and mature neuron markers (Ki67, DCX, and NeuN, respectively) in the dentate gyrus (Yi et al., 2010). The dentate gyrus is the first region where all sensory modalities converge to form unique representations that bind the different sensory stimuli together, thereby playing a critical role in learning and memory. Liver X-receptor (LXR)β, a nuclear receptor closely related to RORα has been linked to dentate gyrus development abnormalities and autism spectrum disorders (Cai et al., 2018). Exogenous RORα expression in RORαsg mice partially restored the normal cerebellum Purkinje cell count and neuronal architecture (Iizuka et al., 2016). The RORA-related neurodevelopmental disorder triad comprises developmental retardation, cerebellar features, and a spectrum of myoclonic epilepsy (Talarico et al., 2025). In a study, out of 40 individuals carrying RORA pathogenic/likely pathogenic variants collected through an international collaboration, 32/40 presented with developmental delay, 25/34 with cerebellar signs and 22/32 with intellectual disability. Cerebellar symptoms were divided into early-onset, late-onset, and progressive subgroups. Cerebellar hypoplasia, atrophy, or both (16/25) were more frequent in individuals with missense variants in the DNA-binding domain. Epilepsy (18/38), with prominent myoclonic seizure types (11/18), was classified in Nationa Library of Medicine (2024) genetic generalized epilepsy (10/18); National Institute of Mental Health (2022) developmental and epileptic encephalopathy (5/18) and (3) unclassified (n = 3/18). A participant with rapid deterioration of visual acuity and cone/rod dystrophy was also reported (Talarico et al., 2025). Furthermore, RORA mutations cause “Intellectual developmental disorder with or without epilepsy or cerebellar ataxia” syndrome (IDDECA). While de novo dominant toxic variants cause intellectual disability, ataxia, and cerebellar atrophy, de novo or dominantly inherited loss-of-function variants cause intellectual disability with autistic features. These two different phenotypes show what kind of functional effect the variant has (Guissart et al., 2018). Twenty-five of the 53 RORA gene variants found so far are classified as pathogenic or possibly pathogenic whose allelic frequencies range from low to very low (Leiden Open Variation, n.d.).
RORA and neuronal gene regulation
Multiple genes associated with ASD are direct RORα targets and reduction of RORα expression results in reduced expression of these genes leading to ASD (Sarachana and Hu, 2013). Individuals with ASD show significant disruptions in their circadian cycles (Hu et al., 2009; Bourgeron, 2007; Melke et al., 2008; Nicholas et al., 2007), and RORα plays a critical role in the regulation of the circadian rhythm (Jetten, 2009). Environmental and metabolic factors of ASD have also been reported to affect RORα expression levels (Kanlayaprasit et al., 2021; Xiao et al., 2022; Yu et al., 2022). Sex differences in the expression of RORα and its target genes in the brain have been investigated as a potential contributor to the sex bias in autism (Hu et al., 2015). A strong correlation between RORα and aromatase levels was observed in males with ASD but not in females, suggesting that females might have compensatory mechanisms to offset RORα deficiency. Additionally, the RORαsg mice display behaviors associated with autism including abnormal spatial learning, reduced exploration, limited maze patrolling, and increased perseverative behavior relative to WT mice (Lalonde and Strazielle, 2008; Goodall and Gheusi, 1987; Lalonde, 1987; Lalonde et al., 1987, 1988). This is especially true when examining dysregulated genes with systemic functions like apoptosis and circadian rhythm (Nguyen et al., 2010). By augmenting the generation of the antioxidant proteins glutathione peroxidase 1 and peroxiredoxin 6, the overexpression of human RORA1 offers neuroprotection by reducing the buildup of reactive oxygen species (ROS) brought on by stress (Boukhtouche et al., 2006c; Figure 3). Glutathione peroxidase 1 and peroxiredoxin 6 are the main mediators of RORA's neuroprotective action (Boukhtouche et al., 2006c; Figure 3). Gpx1 and Prx6, two crucial enzymes in ROS clearance, were shown to be overexpressed in hRORa1-overexpressing cells by a gene candidate analysis employing real-time RT-PCR (Boukhtouche et al., 2006c). Through its ability to degrade hydrogen peroxide, the enzyme primarily responsible for peroxide detoxification in mammalian cells is encoded by the Gpx1 gene (Boukhtouche et al., 2006c). It has been demonstrated that its overexpression via viral vector infection protects against cell death brought on by experimental stroke in vivo (Hoehn et al., 2003). T regulatory cells that reside in tissues like the gut and express RORA may be able to reduce allergic inflammation in organs other than the skin (Schiering et al., 2014; Figure 3). RORA expression was noticeably higher in human skin T regulatory cells than in blood T regulatory cells, indicating that the results are relevant to humans (Malhotra et al., 2018; Figure 3). A positive circuit for hypoxia signaling is activated when ROR-alpha and its ligands activate HIF-1-alpha (Kim et al., 2008; Figure 3). RORα is a key regulator of TH17 cell growth, autoimmune, and chronic inflammation, as shown by a mix of genetic, molecular biology, and pharmacological methods (Wang et al., 2021). By controlling oxidative stress, cellular quality control procedures, and cell death mechanisms, RORs play important protective roles in the onset and progression of several human diseases (Nematisouldaragh et al., 2024). Given that it induces cerebellar developmental abnormalities that impact downstream targets and regulate extracerebral systems like inflammation, circadian rhythms, and sexual dimorphism, RORα deficiency is probably a major contributor to neuro-developmental disorders (Ribeiro and Sherrard, 2023). Similar to the androgen receptor complex that is thought to develop on the promoter and enhancer of the prostate-specific antigen gene, RORα-dependent complexes can form at independent locations or communicate between proximal and distal sites by looping (Shang et al., 2002). The level of expression of some genes relates directly to the severity of the phenotype and may serve as useful candidates for eQTL analyses (Hu et al., 2009). Network analysis of genes that are shared between the severely language-impaired and mild ASD subgroups reveals a set of genes that are probably critical concerning the neurological and metabolic abnormalities of ASD (Hu et al., 2009). A set of differentially expressed genes, most of which lie in non-coding regions, are shared among all three ASD subgroups examined (Hu et al., 2009). Differences between affected functions and pathways among the different phenotypic groups may be responsible for the differences in symptom severity observed in autism (Hu et al., 2009). Sonic Hedgehog is a direct transcriptional target of RORα based on the array data's decreased expression of multiple EGL-expressed cell cycle and proliferation marker genes. By stimulating Amyc, its binding partner Baf53, and different cyclins (Kenney et al., 2003; Kenney and Rowitch, 2000), Purkinje cell-derived SHH has been demonstrated to be both sufficient to induce granule precursor proliferation in postnatal explant cultures in a dose-responsive manner and required for normal levels of granule cell genesis (Wechsler-Reya and Scott, 1999). While various nuclear receptors' cofactors can interact with RORα in vitro (Atkins et al., 1999; Delerive et al., 2002; Lau et al., 1999), particular DNA factors that are recruited to the promoters of RORα target genes in a manner that is dependent on RORα. Tip60, SRC-1, and β-catenin are among the factors that RORα recruits and play a functional role in RORα-dependent transcriptional activation. It has recently been demonstrated that Tip60 recruitment is a necessary coactivator for particular NF-κB gene targets (Baek et al., 2002). Tip60 is recruited to each of three RORα-responsive promoters in a manner dependent on RORα. Coactivators are recruited to target gene promoters uniquely and distinctly, that is, dependent on RORα, and numerous recruited cofactors exhibit functional activity. The calmodulin inhibitor Pcp4, the IP3 receptor and calmodulin target Itpr1, the Itpr1 binding partner Cals1, and the calcium buffer Calb1 are among the calcium signal transduction genes that exhibit RORα-dependent expression. The array data's time course indicates that these genes' expression is drastically decreased by E17.5, before Purkinje cell number loss (Vogel et al., 2000). This suggests that the variations in expression are due to a modified regulatory mechanism rather than subsequent disease. RORA1 and NGFI-B utilize distinct subdomains of the DBD carboxy-terminal extension and therefore reveal a novel strategy by which monomeric nuclear receptors recognize their cognate HREs. Male and female hormones differentially regulate the expression of a novel autism candidate gene, retinoic acid-related orphan receptor-alpha (RORA), in a neuronal cell line, SH-SY5Y (Sarachana et al., 2011). RORA transcriptionally regulates aromatase, an enzyme that converts testosterone to estrogen (Sarachana et al., 2011). Aromatase protein is significantly reduced in the frontal cortex of autistic subjects relative to sex- and age-matched controls and is strongly correlated with RORA protein levels in the brain (Sarachana et al., 2011). RORA has the potential to be under both negative and positive feedback regulation by male and female hormones, respectively, through one of its transcriptional targets, aromatase, suggesting a mechanism for introducing sex bias in autism (Sarachana et al., 2011).
RORA's regulation of brain lipid metabolism
Polyunsaturated fatty acids (PUFA), such as omega-6 and omega-3 fatty acids, are critical for early brain development (Crawford et al., 2003; Haag, 2003; Lauritzen et al., 2001; Martinez, 1992). The predominant PUFA species, arachidonic acid (n-6) and docosahexaenoic acid (DHA; n-3; Brenna and Diau, 2007; Diau et al., 2005), are enriched for neuronal growth, synaptogenesis, neuronal survival, and modulation of neurotransmitters (Darios and Davletov, 2006; Kim and Spector, 2018; Kuperstein et al., 2008). Although abnormal neural lipid metabolism in individuals with ASD has not been extensively investigated, abnormal lipid metabolism has been reported as one of the plasma biomarkers (Das, 2013; Tamiji and Crawford, 2010) and PUFA interventions in animal models may alleviate autistic-like cognitive and social behaviors (Weiser et al., 2016; Shultz et al., 2008, 2009). RORα regulates lipoprotein homeostasis (Vu-Dac et al., 1997) and RORαsg mice exhibit aberrant lipid metabolism (reduced serum cholesterol and triglycerides) due to reduced expressions of ApoA1 and ApoC3, respectively (Raspé et al., 2001; Mamontova et al., 1998). RORα may regulate lipogenesis and mitochondrial fatty acid oxidation by suppressing expressions of peroxisome proliferator-activated receptor-γ (PPARγ), its co-activator PGC1α and lipin1 (Lau et al., 2008; Kim K. et al., 2017). Recent reports suggest that RORα deficiency delays all fatty acid accretions during critical periods of brain development, however, deficiency in the omega-3 PUFA species—DHA, persists in adult RORαsg mice and is not rescued by dietary DHA supplementation (Chen et al., 2020). Similarly, a meta-analysis of case-control cohorts found selectively lower DHA levels in the blood of ASD children (≤12 years) despite no differences in reported dietary intakes with the control group (Mazahery et al., 2017). Although DHA supplementation reversed some impairments in mouse models of ASD (BTBR and serotonin transporter knockout; Matsui et al., 2018; van Elst et al., 2019), human trials reporting an increase in blood DHA after dietary supplementation failed to observe improvements of social behavior in children with ASD (Bent et al., 2011; Voigt et al., 2014). This provides additional evidence that RORα deficiency may affect the efficacy of dietary DHA supplementation either by slowing DHA incorporation into, and/or accelerating loss of DHA from brain phospholipids. Therefore, in humans, RORα upregulation by gene therapy could be a viable option for individuals with ASD (Figure 4) in combination with supplements and other medical options for symptom management.
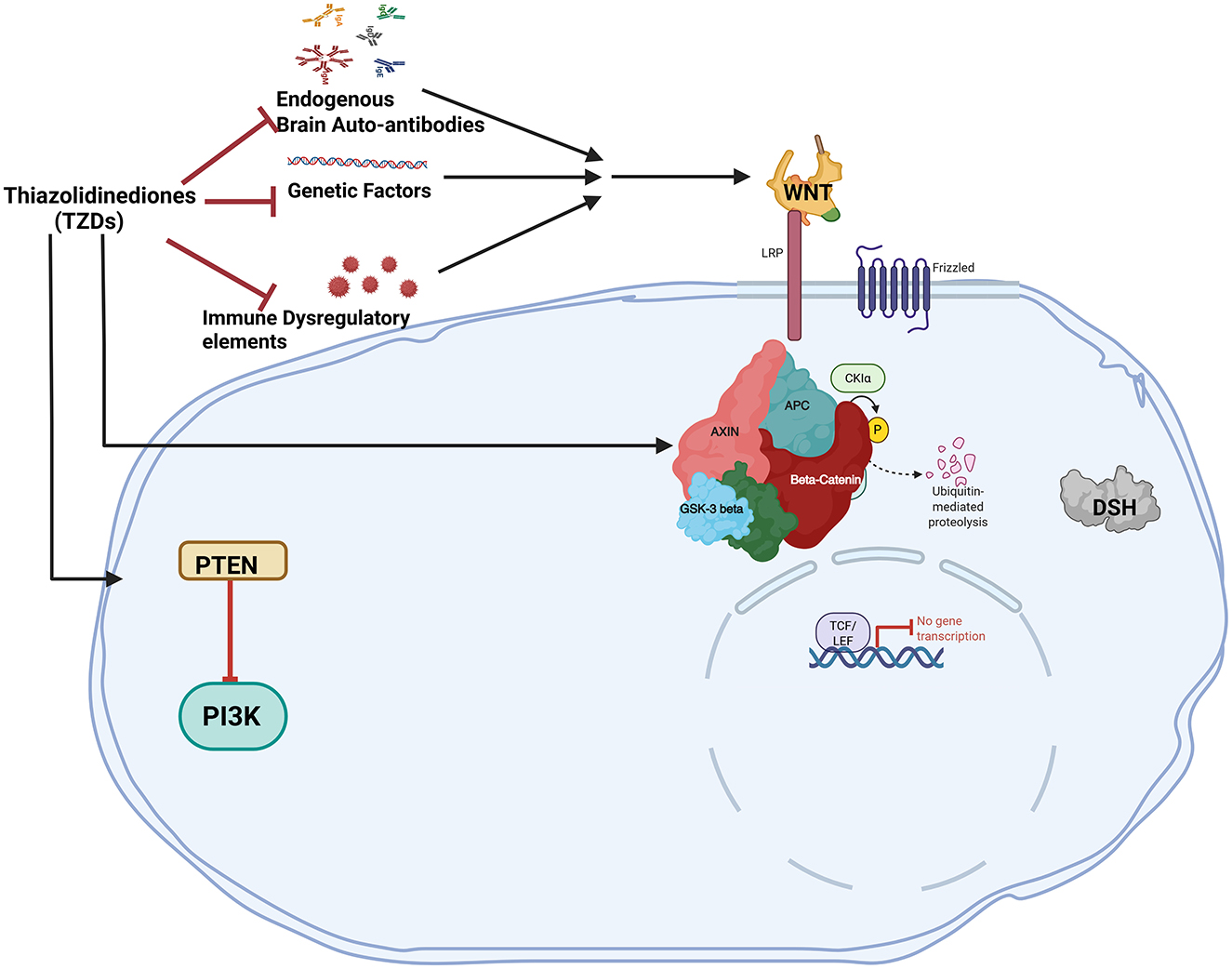
Figure 4. The canonical WNT/β-catenin pathway and PPARγ have an antagonistic relationship in ASD. By preventing the release of inflammatory factors and cytokines. Thiazolidinediones act as PPARγ agonists which can directly prevent neuroinflammation. PPARγ agonists directly activate DKK1 to block the WNT pathway. When PPARγ agonists are administered, the WNT pathway is downregulated due to the antagonistic relationship between PPARγ and the canonical WNT/β-catenin pathway.
RORA protects neurons from oxidative stress
Oxidative stress is a common feature in autism cases, which may be further exacerbated due to the presence of genetically susceptible alleles (Chauhan and Chauhan, 2006, 2009; Deth et al., 2008; Kern and Jones, 2006; Wells et al., 2009). Limited antioxidant capacity, high energy requirement, and high levels of iron and PUFA in the brain increase its vulnerability to oxidative stress. Postmortem studies on brain tissues of individuals with ASD have shown elevated levels of oxidative damage and reduced antioxidant capacity as compared to age-matched control subjects. Individuals with ASD show higher levels of metabolites such as lipid hydroperoxide (from fatty acid oxidation; Chauhan et al., 2011), malonyl dialdehyde (from lipid peroxidation; Chauhan et al., 2012), 8-hydroxy-2′-deoxyguanosine (from oxidative DNA damage; Chauhan et al., 2010; Sajdel-Sulkowska et al., 2009), protein carbonyl (from protein oxidation; Chauhan et al., 2010), 3-nitrotyrosine (from protein nitration; Sajdel-Sulkowska et al., 2008), carboxyethyl pyrrole (a lipid-derived oxidative protein modification; Evans et al., 2008). Overexpression of human RORα1 in cultured mouse cortical neurons increases the expression of the antioxidant proteins glutathione peroxidase 1 (Gpx1) and peroxiredoxin 6 (Prx6), decreases the levels of reactive oxygen species (OS; Figure 4), and protects neurons from apoptosis due to oxidative stressors such as β-amyloid peptide, c2-ceramide, and H2O2 (Boukhtouche et al., 2006c). Another study reported that maternal diabetes in mice induced oxidative stress in the brains of their offspring and led to autism-like behavior (Pagani et al., 2021). Both the oxidative stress and ALB in the mice offspring were accompanied by downregulation of RORα and its target genes CYP19A1 (aromatase) and Sod2 (superoxide dismutase). Post-natal overexpression of RORα in the offspring rescued both ALB and neuronal oxidative stress, while sh-RNA knockdown had the reverse effect and worsened the ALB (Yu et al., 2022).
RORA protects neurons from neuroinflammation
A striking feature common to individuals with ASD is the presence of ongoing neuroinflammation over a broad age range (Vargas et al., 2005) with elevated levels of cytokines and chemokines such as IL-6, TGFβ1, TNFα, CCL2, and CCL17 in the cerebellum and other areas of the brain (Vargas et al., 2005; Chez et al., 2007; Li et al., 2009; Wei et al., 2011). Transcriptome organization patterns in the brain of individuals with ASD show abnormalities in gene co-expression networks associated with immune activation (Voineagu et al., 2011). Disrupted monocyte/macrophage function under resting conditions is reported in ASD (Ashwood et al., 2009; Enstrom et al., 2010) with decreased production of regulatory (anti-inflammatory) cytokines TGFβ1 and IL-10 (Ashwood et al., 2004, 2008), and elevated levels of antibodies against cerebellar proteins (Goines et al., 2011), all of which are associated with worsening behavioral phenotype. Astrocytes are multifunctional macroglial cells that provide neurons with structural and metabolic support (Volterra and Meldolesi, 2005; Marty et al., 2005), absorb neurotransmitters, modulate ion concentration, and synaptic transmission (Haydon and Carmignoto, 2006; Parpura and Haydon, 2000; Fellin et al., 2006). They also maintain the blood-brain barrier (Parri and Crunelli, 2003), act as chemosensors (McDougal et al., 2011), promote myelination (Ishibashi et al., 2006), axon regeneration (Anderson et al., 2016), and drive the molecular oscillations in the circadian clock (Brancaccio et al., 2019). Astrocytes' role in neuroinflammation is becoming apparent (Volterra and Meldolesi, 2005; Song et al., 2002; Farina et al., 2007). As effectors of innate immunity in the brain, astrocytes are principally activated by the NF-κB signaling pathway and produce high levels of IL-6 (Van Wagoner and Benveniste, 1999) by a RORα-dependent mechanism (Delerive et al., 2001; Journiac et al., 2009). Although astrocytes from RORαsg mice were reported to have lower resting IL-6 levels than WT mice, upon stimulation by pro-inflammatory cytokines IL-1β and TNFα, the IL-6 levels were significantly higher in RORαsg astrocytes indicative of a pro-neuroinflammatory drive in the absence of RORα (Journiac et al., 2009).
Peroxisome proliferator-activated receptor gamma (PPARγ)
Studies demonstrated an involvement and mutual regulation of PPAR-gamma and JAK-STAT signaling in controlling multiple pathological factors associated with autism (Khera et al., 2022). A direct repeat (DR) of a hexanucleotide sequence [5′-TGA (A/C/T) CT] divided by a single nucleotide (DR1) makes up the binding motif of the known PPREs. Several other nuclear receptor response elements, including the TR, RAR, VDR, COUP transcriptional factor, and retinoid X receptor (RXR), also contain this type of sequence (Umesono et al., 1991; Yu et al., 1991). The positioning and spacing of the half-site motifs allow these receptors to differentiate between different target elements even though they may all determine the identical half-site motif. Diverse receptors that can operate either positively or negatively control a target gene's expression may also target the same location. For example, retinoid receptors and homomeric and heteromeric complexes of the transcription factors RXR and COUP detect the DR1 (Kliewer et al., 1992b,a). Additionally, it has recently been established that for the TR, RAR, and VDR to bind to their respective target DNA sequences effectively, they all need an auxiliary factor (RXR; Yu et al., 1991; Kliewer et al., 1992a,b; Bugge et al., 1992; Leid et al., 1992). In particular, PPARγ: (1) causes adipogenesis in healthy SAT but not in unhealthy VAT (Mayerson et al., 2002; Miyazaki et al., 2002; Adams et al., 1997); (2) increases the expression of uncoupling protein 1 in BAT (Cao et al., 2004; Zhang et al., 2014); (3) raises serum adiponectin levels (Hirose et al., 2002); (4) inhibits inflammation (Pascual et al., 2005); and (5) encourages mitochondrial biogenesis and lowers reactive oxygen species production in the mitochondria (Fujisawa et al., 2009). Although TZDs are complete agonists of PPARγ, as was previously mentioned, they have unfavorable side effects that limit or prohibit their clinical use. A new generation of agonists with improved safety profiles and wider applicability may be developed due to a growing understanding of PPARγ signaling.
Two different signaling pathways are triggered when TZDs and other ligands directly bind to PPARγ. Through the recruitment of co-activators like PPARγ co-activator-1α (PGC-1α), PPARγ dimerization with the retinoid X receptor (RXR; Spiegelman, 1998), agonist-dependent conformational changes in the activation function 2 (AF-2) domain of PPARγ, and the binding of this complex to peroxisome proliferator response elements (PPREs) in the promoters of target genes, cis-activation drives transcription. Alternatively, it has been established that ligand-bound PPARγ decreases inflammation by a process known as trans-repression. The PPARγ C126A/E127A mutant, which is unable to cis-activate but still able to repress lipopolysaccharide-induced genes, demonstrates that trans-repression is independent of PPARγ's ability to bind DNA (Li et al., 2000). RXR dimerization is also not necessary for this mode of repression; however, the small ubiquitin-like modifier must sumoylate PPARγ2 at K395 and then bind PPARγ, nuclear receptor co-repressor, and histone deacetylase to NFκB and AP-1 complexes that control the transcription of inflammatory response genes (Pascual et al., 2005).
Other NHRs
Liver X receptors (LXRs) are important modulators of lipid and cholesterol metabolism (Kalaany and Mangelsdorf, 2006). More recently, LXRs have been demonstrated to adversely affect the Hedgehog signaling pathway, which is implicated in cancer and embryonic development (Gill et al., 2008; Kim et al., 2009). LXRα is expressed at similar levels in a wide range of tissues, which is the foundation for an alternate term for this receptor called “ubiquitous receptor” (Song et al., 1995). A study revealed that the brain's Aβ generation was reduced by LXRβ through inhibition and degradation, further triggering the expression of autophagy-related proteins by decreasing the expression of proteins related to Ras/Raf/Erkl/2 signaling pathway, thereby improving autism behaviors (Zhang et al., 2016).
Therapeutic options for ASD
Drug molecules under investigation
ASD related irritability can be treated by using antipsychotics such as risperidone and aripiprazole, which are approved by the US FDA (Lamy and Erickson, 2018). ASD patients have a greater risk of side effects with methylphenidate, such as depressive mood disorder symptoms, low appetite, insomnia, irritability, increased levels of social withdrawal, and lower treatment response rates compared to patients with ADHD alone (Research Units on Pediatric Psychopharmacology Autism Network, 2005; Reichow et al., 2013). Atomoxetine moderately improved ADHD related symptoms despite frequent adverse events such as fatigue, nausea, low appetite, and early morning awakening (Harfterkamp et al., 2012). Extended-release Guanfacine as an alpha-2 agonist was effective for ADHD in children with ASD (Scahill et al., 2015). Randomized, placebo-controlled trials in ASD youth showed an over 50% reduction in the irritability score when treated with Risperidone (Levine et al., 2016). Another antipsychotic drug approved by the FDA, aripiprazole, for treating irritability associated with autism showed lower severity scores on the ABC-I and the CGI-I scales, but with weight gain being a common side effect (Wink et al., 2014). Topiramate (Mazzone and Ruta, 2006) and Divalproex (Hollander et al., 2010), the anticonvulsant drugs, are effective for treating irritability associated with ASD. The repetitive behaviors in adults with ASD treated with Fluoxetine were improved (Hollander et al., 2012). Some of the over-the-counter supplements, such as exogenous melatonin in different formulations, were effective and safe in improving the sleep patterns in children with ASD (Souders et al., 2017). A study to assess whether response to the selective serotonin reuptake inhibitor (SSRI) escitalopram in the treatment of ASD was associated with symptom improvement, observed over 6 weeks of open-label treatment (Najjar et al., 2015). Cell therapies for people with ASD have shown positive outcomes in nine out of 10 clinical trials, with no significant side effects (Nabetani et al., 2023). Certain neurobehavioral abnormalities observed in Slc7a5 mutants mice could be rescued by intracerebroventricular injections administration of leucine and isoleucine in adulthood (Tărlungeanu et al., 2016).
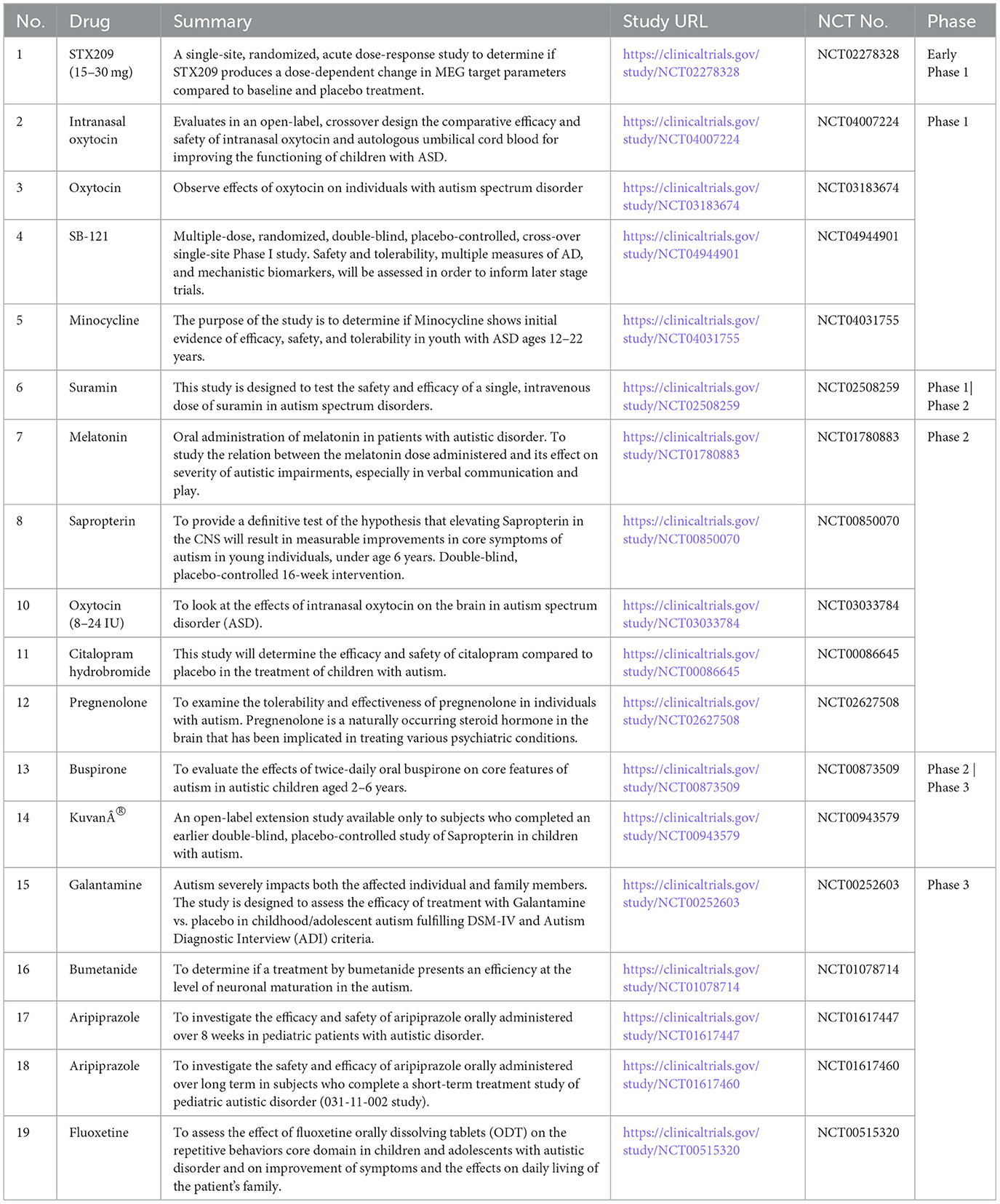
Treatment with Oxytocin plays a prominent role in setting social behaviors just after birth. It could be a novel therapeutic approach for the treatment of neurological diseases such as autism (Meziane et al., 2015). In a cohort of 19 children with ASD, Clonidine was found effective in decreasing night awakening, sleep initiation latency, mood instability, and improving attention deficit hyperactivity and aggressiveness (Ming et al., 2008). Aripiprazole (or Memantine) combination increased the protein levels of BDNF, p-CREB, and Glt-1 gene expression with restoration of GABA/glutamate balance, attenuation of VPA-induced neurodegenerative changes and autistic-like symptoms, and improvement of cognitive performance (Zohny et al., 2023). Maternal diabetes–mediated oxidative stress is diminished due to hematopoietic stem cells transplantation with increased Sod2 expression, apart from triggering epigenetic changes in neurons, thereby impairing autism-like behavior in autism-like offspring (Zeng et al., 2022). Mannose-PEG600-lipoic acid (Man-LA), a brain-targeted H2S donor cross-linked nano micelle, interacts with glucose transporter 1 (GLUT1) in astrocytes, facilitating a gradual release of H2S that is modulated by glutathione (Hirsch and Pringsheim, 2016; Zhang et al., 2025). Man-LA alleviates symptoms of ASD, correlating with increased expression of aerobic glycolysis enzymes, elevated lactate production, and higher H2S levels, while preventing damage to hippocampal neurons under in-vivo conditions (Zhang et al., 2025). Man-LA tightly binds to aldehyde dehydrogenase family three member B1 (Aldh3b1) in astrocytes in in-vitro conditions, upregulating its expression (Zhang et al., 2025). Within the glycolytic pathway, the Aldh3b1 gene is a potential target for ASD (Zhang et al., 2025). The exogenous melatonin administration for ASD related abnormal sleep parameters is based on evidence (Rossignol and Frye, 2014). Various clinical studies for the investigation of novel drugs are stated in Table 1.
Challenges in the current state of drug treatments for ASD
Integrating clinical and translational treatment studies, the United States has invested more than USD 110 million in ASD research networks and institutes over the past 20 years (excluding individual initiatives). Risperidone (McCracken et al., 2002), Methylphenidate (Kim S. J. et al., 2017; Joshi et al., 2020), Guanfacine (Politte et al., 2018; Scahill et al., 2015), Secretin (Levy et al., 2003; Williams et al., 2012), Aripiprazole (Hirsch and Pringsheim, 2016), plus language intervention (Williams et al., 2024), Citalopram (Wichers et al., 2019), Fluoxetine, Intranasal Oxytocin (Sikich et al., 2021), Escitalopram (Owley et al., 2010), Buspirone (Chugani et al., 2016; Ghanizadeh and Ayoobzadehshirazi, 2015), are some of the molecules which were investigated. Integrating clinical and translational treatment studies, the United States has invested more than USD 110 million in ASD research networks and institutes over the past 20 years (excluding individual initiatives). There are still more clinical trials going on. The Autism Centers of Excellence (ACE) Centers and Networks programs continue these extensive programmatic NIH ASD research activities. The Innovative Medicines Initiative 2 (IMI2)-funded Multicenter Study for Developing New Medications (EU-AIMS; http://www.eu-aims.eu) is well underway, focusing on biomarker identification and individualized treatment regimens. Coupled with initiatives from privately financed organizations and industry, other cross-collaborative options, including Canada's POND network, have complemented and greatly aided the endeavor to collect complementary data. At the same time, smaller controlled studies of Oxytocin (Lemprière, 2022), Vasopressin (Parker et al., 2019; Bolognani et al., 2019), D-Cycloserine (Urbano et al., 2014; Minshawi et al., 2016; Wink et al., 2017), N-Acetylcysteine (Wink et al., 2016; Ghanizadeh and Moghimi-Sarani, 2013), CX516 (Berry-Kravis et al., 2006), Donepezil (Gabis et al., 2019; Handen et al., 2011; Buckley et al., 2011), and Fluoxetine (Lucchelli and Bertschy, 2018) have been completed, along with pharma-supported Phase II and III ASD. An increasing number of randomized controlled trials (RCTs) have focused on fundamental impairments, such as social withdrawal, in individuals with Arbaclofen treated for autism spectrum disorder (ASD) and fragile X syndrome (FXS). Additionally, many of these trials have targeted related symptoms, including irritability, hyperactivity, and anxiety, as their primary objectives. Although some businesses have deemed drug development for neurodevelopmental disorders (NDDs) to be too risky, a growing understanding of the neurobiology and genetics of ASD is causing this perspective to shift, thereby paving the way for the discovery of medicines for various behavioral endpoints in people with ASD (Ameis et al., 2018). Only three EMA and two US FDA indications have been approved thus far, and they are all for “irritability” or sleeplessness related to ASD.
Many molecules with different modes of action at different stages of clinical development are represented among the compounds now in development. A more comprehensive perspective that incorporates preclinical development of drugs is needed. Though Gene therapeutic models are picking up pace, they still have some bottlenecks that need to be addressed. Gene therapeutic agents need to be tested thoroughly at pre-clinical levels before being taken to clinical confirmatory trials. Optimum dose concentrations and copy number variants of genes must be investigated. Modes of gene regulation have to be examined, and inducers or gene modulators have to be utilized as and when required.
Challenges in ASD clinical trials
Among the current difficulties in clinical trials for ASD are addressing the variability of ASD, failures to translate targeted medicines from preclinical to clinical, the absence of objective, validated biomarkers for target mechanism engagement, early change detection, therapy prediction, diagnosis, stratification, and pertinent brain circuit modulation, better clinical endpoints are required, setting molecular target priorities, developing stronger trial designs, managing the legal criteria for novel therapeutic uses, establishing priorities for comorbidity-focused therapies, including the viewpoints of participants and caregivers, and addressing potential ethical problems in research.
Gene therapy
With just one or a few therapeutic interventions, gene therapies that permanently alter the genome could be a game-changing novel therapy for monogenic ASD. Transient gene therapies like RNA-editing, ncRNA, and ASOs leave the genome unblemished and will need repeated dosage, but it might have the benefit of being reversible and controlled (Weuring et al., 2021). These variations draw attention to the crucial questions surrounding gene therapies, specifically those related to dosage, delivery, and safety. However, better viral and non-viral vectors, such as delivery nanoparticles, more precise gene editors with minimal off-target effects, and regulated transgene expression, are made possible by the rapid speed of technological advancement (Weuring et al., 2021). The efficiency of a neuronal gene therapeutic strategy will rely on directing disease-modifying substances to the right positions, which incorporates both intracellular localization and transduction of the proper cells and circuits. Although liposomes or nanoparticle-mediated delivery are other options, a viral approach is most likely to be used to introduce a target gene or gene-editing tools into a neuron (Naldini, 2015). Leveraging the biology of the virus to express transgenes and altering or minimizing the residual viral genome in a way that inhibits pathogenic features like viral replication following host transduction are essential components of creating an efficient viral vector. Several genetically modified viruses under development can safely introduce heterologous genes or DNA sequences into neurons. Currently, lentiviruses and adeno-associated viruses (AAVs) are the two most common classes. Other viruses, such as HSV, rabies, and Semliki forest virus, can cause immunological reactions, transient transgene expression, or unacceptable toxicity in addition to transducing neurons (Manfredsson and Mandel, 2010).
AAV vectors' low toxicity, long-term gene expression, capacity to productively infect both dividing and non-dividing cells in a wide variety of host tissues and organs, and lack of pathogenesis are only a few of their many alluring qualities for safe and effective gene therapy (Berns, 1999; Kay et al., 2001). Several genetically modified viruses under development for the treatment of CNS disorders, AAVs, are currently the recommended gene delivery vector (Blessing and Déglon, 2016; Choudhury et al., 2017). They offer minimally harmful transgene expression over an extended period. Numerous AAV serotypes exist, and their distributions and tropisms can vary (Wu et al., 2006). Although it can occasionally integrate into the genome, AAV-delivered DNA is typically found in extrachromosomal episomes (High and Aubourg, 2011). Thus, insertional mutagenesis is possible, just like with lentiviruses. According to Zaiss and Muruve (2005), certain AAV serotypes exhibit a comparatively high level of natural immunity. AAV serotypes with low or no prevalence in the human population will be needed because neutralizing antibodies can stop brain transduction (Boutin et al., 2010). Investigations into using this vector should consider that the high levels of neutralizing antibodies (Nab) against wild-type AAV can decrease recombinant AAV (rAAV) mediated transduction in the brain (Peden et al., 2004). Anti-AAV antibody assay harmonization offers a chance to integrate immunogenicity data from multiple research projects, raise the degree of dependability for a crucial methodology, and generally enhance our capacity to forecast the effectiveness of viral vector-based gene therapies (GTx)—based therapy (Gorovits et al., 2021). The use of different analytical platforms and assay formats, the variety of reagents, and the semi-quantitative nature of total binding antibodies (Leiden Open Variation, n.d.) tests due to the absence of a real calibration standard are some of the difficulties that now impede standardization (Gorovits et al., 2021).
Several potential therapies are under investigation for ASD. A mouse model established the feasibility of using gene therapy to combat the loss of function of Scn2a channels and is under investigation as a therapeutic strategy for patients with ASD (Weuring et al., 2021). A hereditary form of ASD that has a SHANK3 mutation and deletion is the focus of JAG201, the gene therapy product which delivers a functional SHANK3 minigene, straight to CNS neurons via an adeno-associated virus serotype 9 (AAV9) vector is also under investigation (Nationa Library of Medicine, 2024).
As mentioned in the earlier section, overexpressing the human RORA protects neurons from death caused by oxidative stress (Boukhtouche et al., 2006c). Study shows that A2BP1, CYP19A1, HSD17B10, ITPR1, NLGN1, and NTRK2 are all regulated by RORA and when RORA levels are cut in half, the expression of all six genes are reduced in ASD (Sarachana and Hu, 2013). Additionally, RORA-deficient postmortem brain tissues from autistic individuals show lower expression levels of these six genes compared to age-matched, unaffected controls (Valerie, 2013). Furthermore, RORA alpha's transcriptional targets affect several directly regulated genes, including NLGN1 and NTRK2, as well as pathways that are disrupted in ASD (Devanna and Vernes, 2014). Recent research has linked the biology of autism to mutations in the X-linked neuroligin genes NLGN3 and NLGN4 (Ylisaukko-oja et al., 2005). There is evidence that NTRK2 gene is a susceptibility factor for autism and a disruption of the BDNF/TrkB signaling pathway is associated with autism (Correia et al., 2010). Considering the above evidences showing the involvement of RORA in several pathways, AAV-hRORA-mediated gene therapy could hold promise to treat and minimize the effects of ASD as RORA transcriptionally regulates expression levels of several proteins, including aromatase (Sarachana et al., 2011), whose expression is reduced in ASD subjects. Pharmacologically stimulating RORA with synthetic agonists has demonstrated promising results in reducing repetitive behaviors in animal models. This suggests that restoring RORA function may be a part of treating ASD (Wang et al., 2016; Benger et al., 2018; Weuring et al., 2021). Although specific AAV-mediated hRORA gene therapy in humans is not yet well-documented in clinical studies, the efficacy of AAV-mediated gene therapies in other neurological illnesses and gene replacement models supports this approach as a reasonable future strategy (Ramachandran et al., 2025; Manini et al., 2022; Ye et al., 2024). Hence, further research is warranted on these grounds to optimize various studies at pre-clinical and clinical levels in ASD subjects, apart from understanding the mechanism of action in detail.
New perspectives in NHR therapy: challenges and limitations
The importance of cellular context in NHR activity is shown by tissue-specific expression patterns and ligand availability (Evans and Mangelsdorf, 2014). Selective modulation techniques, like selective receptor modulators, may improve therapeutic specificity. Since the current medications often lack specificity and have serious side effects, like severe heart failure, researchers are currently developing new compounds with stronger binding affinities and improved specificity as novel NR-targeted medications (Huss and Kelly, 2004; Vega and Kelly, 2017). The NHRs, due to the effects that depend on tissue and context, as well as their varied functions, make it hard to carry out targeted treatments without causing unwanted side effects. Hepatotoxicity, cardiotoxicity, hormone disruption, cancer risk, metabolic disorders, and neurotoxicity are some of the dangers linked to unintended or prolonged regulation of NHRs (Rao et al., 2024). These harmful effects are particularly related to receptors such as glucocorticoid receptors, androgen receptors, estrogen receptors, and peroxisome proliferator-activated receptors (PPARs; Rao et al., 2024). To fully tap into the potential of NHRs, it is essential to understand their functions in different tissues, their connections with co-regulators, and their structural details. The current challenges in NHR research and drug development involve balancing their strong regulatory ability with concerns about specificity and safety (Schulman, 2010). NHR activation depends on specific ligands, which provide flexibility but also present risks of unwanted effects and multiple outcomes (Kanner, 1968). The lack of known endogenous ligands for several NHRs makes them “orphan receptors” (Rao et al., 2024). This complicates our understanding of their physiological roles and possible treatments. The natural disorder in certain NHR domains hampers full structural characterization and limits detailed understanding of their mechanisms. Drug targeting is also harder because the variable ligand affinity and competition among NHRs for co-regulators unpredictably affect receptor function. One of the most pressing scientific challenges in biomedicine is understanding how NHR genes are selected and creating specific inhibitors that target nuclear receptors, this warrants more structured studies.
Conclusions
ASD is a serious condition that requires innovative treatment because of its complex physiology and lack of definitive cure. Understanding of ASD neurobiology, genetics, early identification, and early intervention have advanced significantly over the past 70 years since Leo Kanner initially identified autism (Kanner, 1943). With just one or a few treatments, gene therapies can potentially reduce the symptoms of the disorder. Antisense oligonucleotides, non-coding RNA, and RNA editing are examples of temporary gene treatments that leave the genome intact and necessitate repeated dosages, although they may have the benefit of being reversible and controllable. These variations draw attention to the crucial questions surrounding gene treatments, specifically the dose, delivery, and safety concerns, which should be thoroughly investigated for individual gene therapeutic drugs. There is an immense need to develop better viral and non-viral vectors for safe and effective delivery of gene therapy products. These include controlled transgene expression, more precise gene editors with minimal off-target effects, and nanoparticles for delivery. The speed at which accurate gene editing of a single-nucleotide gene mutation is getting close to therapeutic use is astounding. In vitro and in vivo testing of both temporary and permanent gene treatments for ASD has already been completed, paving the way for future drug development. Since structural variants necessitate a different strategy than missense mutations, the method of gene therapy is primarily determined by the precise genetic etiology. For the safe use of gene treatments in the future, accurate DNA diagnostics and accurate prediction of the functional effects of mutation will probably be equally crucial. In recent years, we have seen previously unheard-of breakthroughs in our understanding of neurology, genetics, early detection, and early intervention of ASD. Recent increases in ASD prevalence estimates underscore the critical need for continued work to translate new findings about ASD into effective interventions for all individuals with ASD. In this article, we highlight promising areas for new and existing research to speed scientific discovery and ultimately translate research findings into understood and empirically validated interventions for people with ASD. The relevance of RORA in ASD conditions can be corroborated through the fact that overexpression of human RORA confers neuroprotection by reducing the accumulation of ROS triggered by stress by increasing the production of the antioxidant proteins glutathione peroxidase 1 and peroxiredoxin 6, and the primary mediators of RORA's neuroprotective effects were glutathione peroxidase 1 and peroxiredoxin 6. Gene expression analysis of lymphoblastoid cell lines (LCLs) from people with and without ASD has confirmed that expression of RORA decreased in ASD, especially in the subtype linked to severe language impairment (Hu et al., 2006). Interestingly, exposure of cells to a methylation inhibitor can correct reduced expression, which is linked to increased methylation of the RORA promoter in LCL from people with ASD (Nguyen et al., 2010; Hu et al., 2006). Compared to sex- and age-matched controls, the post-mortem cerebellum and frontal cortex of people with ASD had lower levels of RORA protein. Further, male and female hormones regulate RORA in different ways, with estrogen raising RORA expression and dihydrotestosterone decreasing it (Sarachana et al., 2011). More importantly, RORA in turn controls the transcription of aromatase, an enzyme that changes testosterone into estrogen, offering a tenable explanation for the higher testosterone levels linked to a higher risk of ASD (Baron-Cohen et al., 2005), which brings in more strength to the theory of RORA being a formidable candidate in the treatment of ASD. Apart from the above facts, it was found that in the postmortem frontal brain of people with ASD, both RORA and aromatase proteins are significantly lower than in controls; a correlation coefficient of 0.91 indicates that there may be a causative link between RORA decrease and aromatase deficiency (Sarachana et al., 2011). And as per preliminary confocal immunofluorescence investigations, the frontal cortex of unaffected males has a 15–20% lower RORA protein level than that of age-matched females. This suggests sexual dimorphism in RORA expression. Based on the above facts, we tried to address autism spectrum disease and different therapeutic approaches, emphasizing the significance of AAV-hRORA-mediated gene therapy. Early diagnosis and personalized treatment for a variety of neurodegenerative diseases have improved due to the identification of novel biomarkers, such as cerebrospinal fluid (CSF), genetic markers, and developments in artificial intelligence (AI) and bioinformatics. Increased research focus in these areas may speed up the translation of knowledge about the etiological mechanisms of ASD to biological and psychological therapies to reduce the burden of ASD on affected individuals and their families.
Future directions
In summary, recent increases in ASD prevalence highlight the urgent need to transform scientific progress into accessible, effective therapies. Future research must address barriers by exploring psychiatric comorbidities and overlaps with other neurodevelopmental disorders. Integrating behavioral, brain imaging, and genetic methods will help uncover mechanisms and individual differences within the ASD population. Emphasis should shift toward developmental trajectories and personalized interventions that respect neurodiversity and family needs. Additionally, identifying disease-modifying genes like NHRs could open new avenues for targeted treatments.
Author contributions
SC: Writing – review & editing, Writing – original draft. KR: Writing – original draft, Writing – review & editing. AU: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
SC was employed by company Ocugen India. KR and AU were employed by company Ocugen.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, M., Montague, C. T., Prins, J. B., Holder, J. C., Smith, S. A., Sanders, L., et al. (1997). Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J. Clin. Invest. 100, 3149–3153. doi: 10.1172/JCI119870
Adamson, A. S., Collins, K., Laurence, A., and O'Shea, J. J. (2009). The Current STATus of lymphocyte signaling: new roles for old players. Curr. Opin. Immunol. 21, 161–166. doi: 10.1016/j.coi.2009.03.013
Ameis, S. H., Kassee, C., Corbett-Dick, P., Cole, L., Dadhwal, S., Lai, M. C., et al. (2018). Systematic review and guide to management of core and psychiatric symptoms in youth with autism. Acta Psychiatr. Scand. 138, 379–400. doi: 10.1111/acps.12918
Anderson, M. A., Burda, J. E., Ren, Y., Ao, Y., O'Shea, T. M., Kawaguchi, R., et al. (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200. doi: 10.1038/nature17623
Anorson, N., Male, I., Farr, W., and Memon, A. (2021). Prevalence of autism in Europe, North America and Oceania, 2000-2020: a systematic review. Euro. J. Public Health 31:786. doi: 10.1093/eurpub/ckab164.786
Aranda, A., and Pascual, A. (2001). Nuclear hormone receptors and gene expression. Physiol. Rev. 81, 1269–1304. doi: 10.1152/physrev.2001.81.3.1269
Ashwood, P., Anthony, A., Torrente, F., and Wakefield, A. J. (2004). Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J. Clin. Immunol. 24, 664–673. doi: 10.1007/s10875-004-6241-6
Ashwood, P., Enstrom, A., Krakowiak, P., Hertz-Picciotto, I., Hansen, R. L., Croen, L. A., et al. (2008). Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J. Neuroimmunol. 204, 149–153. doi: 10.1016/j.jneuroim.2008.07.006
Ashwood, P., Schauer, J., Pessah, I. N., and Van de Water, J. (2009). Preliminary evidence of the in vitro effects of BDE-47 on innate immune responses in children with autism spectrum disorders. J. Neuroimmunol. 208, 130–135. doi: 10.1016/j.jneuroim.2008.12.012
Atkins, G. B., Hu, X., Guenther, M. G., Rachez, C., Freedman, L. P., and Lazar, M. A. (1999). Coactivators for the orphan nuclear receptor RORalpha. Mol. Endocrinol. 13, 1550–1557. doi: 10.1210/mend.13.9.0343
Autism Research Institute (2022). Related Disorders. Available online at: https://autism.org/related-conditions/ (accessed October 10, 2025).
Autism Research Institute (2023). Co-Occurring Conditions and Autism. Available online at: https://autism.org/comorbidities-of-autism/ (Accessed October 10, 2025).
Baek, S. H., Ohgi, K. A., Rose, D. W., Koo, E. H., Glass, C. K., and Rosenfeld, M. G. (2002). Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell 110, 55–67. doi: 10.1016/S0092-8674(02)00809-7
Baron-Cohen, S., Knickmeyer, R. C., and Belmonte, M. K. (2005). Sex differences in the brain: implications for explaining autism. Science 310, 819–823. doi: 10.1126/science.1115455
Barrett, P. M., Dadds, M. R., and Rapee, R. M. (1996). Family treatment of childhood anxiety: a controlled trial. J. Consult. Clin. Psychol. 64, 333–342. doi: 10.1037/0022-006X.64.2.333
Béchet, D. (1980). Control of gene expression by steroid hormones. Reprod. Nutr. Dev. 26, 1025–1055. doi: 10.1051/rnd:19860701
Benger, M., Kinali, M., and Mazarakis, N. D. (2018). Autism spectrum disorder: prospects for treatment using gene therapy. Mol. Autism 9:39. doi: 10.1186/s13229-018-0222-8
Bent, S., Bertoglio, K., Ashwood, P., Bostrom, A., and Hendren, R. L. (2011). A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. J. Autism Dev. Disord. 41, 545–554. doi: 10.1007/s10803-010-1078-8
Berns, K. I. (1999). The Gordon Wilson lecture. From basic virology to human gene therapy. Trans. Am. Clin. Climatol. Assoc. 110, 75–85.
Berry-Kravis, E., Krause, S. E., Block, S. S., Guter, S., Wuu, J., Leurgans, S., et al. (2006). Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a controlled trial. J. Child Adolesc. Psychopharmacol. 16, 525–540. doi: 10.1089/cap.2006.16.525
Blessing, D., and Déglon, N. (2016). Adeno-associated virus and lentivirus vectors: a refined toolkit for the central nervous system. Curr. Opin. Virol. 21, 61–66. doi: 10.1016/j.coviro.2016.08.004
Bolognani, F., Del Valle Rubido, M., Squassante, L., Wandel, C., Derks, M., Murtagh, L., et al. (2019). A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci. Transl. Med. 11:eaat7838. doi: 10.1126/scitranslmed.aat7838
Bottjer, S. W., and Arnold, A. P. (1997). Developmental plasticity in neural circuits for a learned behavior. Annu. Rev. Neurosci. 20, 459–481. doi: 10.1146/annurev.neuro.20.1.459
Boukhtouche, F., Doulazmi, M., Frederic, F., Dusart, I., Brugg, B., and Mariani, J. (2006a). RORalpha, a pivotal nuclear receptor for Purkinje neuron survival and differentiation: from development to ageing. Cerebellum 5, 97–104. doi: 10.1080/14734220600750184
Boukhtouche, F., Janmaat, S., Vodjdani, G., Gautheron, V., Mallet, J., Dusart, I., et al. (2006b). Retinoid-related orphan receptor alpha controls the early steps of Purkinje cell dendritic differentiation. J. Neurosci. 26, 1531–1538. doi: 10.1523/JNEUROSCI.4636-05.2006
Boukhtouche, F., Vodjdani, G., Jarvis, C. I., Bakouche, J., Staels, B., Mallet, J., et al. (2006c). Human retinoic acid receptor-related orphan receptor alpha1 overexpression protects neurones against oxidative stress-induced apoptosis. J. Neurochem. 96, 1778–1789. doi: 10.1111/j.1471-4159.2006.03708.x
Bourgeron, T. (2007). The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb. Symp. Quant. Biol. 72, 645–654. doi: 10.1101/sqb.2007.72.020
Boutin, S., Monteilhet, V., Veron, P., Leborgne, C., Benveniste, O., Montus, M. F., et al. (2010). Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21, 704–712. doi: 10.1089/hum.2009.182
Brancaccio, M., Edwards, M. D., Patton, A. P., Smyllie, N. J., Chesham, J. E., Maywood, E. S., et al. (2019). Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 363, 187–192. doi: 10.1126/science.aat4104
Brault, V., Moore, R., Kutsch, S., Ishibashi, M., Rowitch, D. H., McMahon, A. P., et al. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264. doi: 10.1242/dev.128.8.1253
Brenna, J. T., and Diau, G. Y. (2007). The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot. Essent. Fatty Acids 77, 247–250. doi: 10.1016/j.plefa.2007.10.016
Buckley, A. W., Sassower, K., Rodriguez, A. J., Jennison, K., Wingert, K., Buckley, J., et al. (2011). An open label trial of donepezil for enhancement of rapid eye movement sleep in young children with autism spectrum disorders. J. Child Adolesc. Psychopharmacol. 21, 353–357. doi: 10.1089/cap.2010.0121
Bugge, T. H., Pohl, J., Lonnoy, O., and Stunnenberg, H. G. (1992). RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 11, 1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x
Bundey, S., Hardy, C., Vickers, S., Kilpatrick, M. W., and Corbett, J. A. (1994). Duplication of the 15q11-13 region in a patient with autism, epilepsy and ataxia. Dev. Med. Child Neurol. 36, 736–742. doi: 10.1111/j.1469-8749.1994.tb11916.x
Bwayi, M. N., Garcia-Maldonado, E., Chai, S. C., Xie, B., Chodankar, S., Huber, A. D., et al. (2022). Molecular basis of crosstalk in nuclear receptors: heterodimerization between PXR and CAR and the implication in gene regulation. Nucleic Acids Res. 50, 3254–3275. doi: 10.1093/nar/gkac133
Cai, Y., Tang, X., Chen, X., Li, X., Wang, Y., Bao, X., et al. (2018). Liver X receptor β regulates the development of the dentate gyrus and autistic-like behavior in the mouse. Proc. Natl. Acad. Sci. U.S.A. 115, E2725–E2733. doi: 10.1073/pnas.1800184115
Cancino-Barros, I., and Villacura-Herrera, C. (2025). A meta-analytic review of quantification methods for camouflaging behaviors in autistic and neurotypical individuals. Sci. Rep. 15:22885. doi: 10.1038/s41598-025-06137-z
Cantú, E. S., Stone, J. W., Wing, A. A., Langee, H. R., and Williams, C. A. (1990). Cytogenetic survey for autistic fragile X carriers in a mental retardation center. Am. J. Ment. Retard. 94, 442–447.
Cao, W., Daniel, K. W., Robidoux, J., Puigserver, P., Medvedev, A. V., Bai, X., et al. (2004). p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 24, 3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004
Chauhan, A., Audhya, T., and Chauhan, V. (2012). Brain region-specific glutathione redox imbalance in autism. Neurochem. Res. 37, 1681–1689. doi: 10.1007/s11064-012-0775-4
Chauhan, A., and Chauhan, V. (2006). Oxidative stress in autism. Pathophysiology 13, 171–181. doi: 10.1016/j.pathophys.2006.05.007
Chauhan, A., and Chauhan, V. (2009). Autism: Oxidative Stress, Inflammation, and Immune Abnormalities, 1st Edn. CRC Press. doi: 10.1201/9781420068870
Chauhan, A., Essa, M., Muthaiyah, B., Brown, W., Wegiel, J., Chauhan, V., et al. (2010). Increased Protein Oxidation in Cerebellum, Frontal and Temporal Cortex in Autism, International Meeting for Autism Research. Available online at: https://insar.confex.com/imfar/2010/webprogram/Paper7165.html (Accessed May 14, 2025).
Chauhan, A., Gu, F., Essa, M. M., Wegiel, J., Kaur, K., Brown, W. T., et al. (2011). Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J. Neurochem. 117, 209–220. doi: 10.1111/j.1471-4159.2011.07189.x
Chen, C. T., Schultz, J. A., Haven, S. E., Wilhite, B., Liu, C. H., Chen, J., et al. (2020). Loss of RAR-related orphan receptor alpha (RORα) selectively lowers docosahexaenoic acid in developing cerebellum. Prostaglandins Leukot. Essent. Fatty Acids 152:102036. doi: 10.1016/j.plefa.2019.102036
Chen, X. R., Heck, N., Lohof, A. M., Rochefort, C., Morel, M. P., Wehrlé, R., et al. (2013). Mature Purkinje cells require the retinoic acid-related orphan receptor-α (RORα) to maintain climbing fiber mono-innervation and other adult characteristics. J. Neurosci. 33, 9546–9562. doi: 10.1523/JNEUROSCI.2977-12.2013
Chez, M. G., Dowling, T., Patel, P. B., Khanna, P., and Kominsky, M. (2007). Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr. Neurol. 36, 361–365. doi: 10.1016/j.pediatrneurol.2007.01.012
Choudhury, S. R., Hudry, E., Maguire, C. A., Sena-Esteves, M., Breakefield, X. O., and Grandi, P. (2017). Viral vectors for therapy of neurologic diseases. Neuropharmacology 120, 63–80. doi: 10.1016/j.neuropharm.2016.02.013
Christensen, D. L., Braun, K. V. N., Baio, J., Bilder, D., Charles, J., Constantino, J. N., et al. (2012). Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill. Summ. 65, 1–23. doi: 10.15585/mmwr.ss6513a1
Christensen, J., Grønborg, T. K., Sørensen, M. J., Schendel, D., Parner, E. T., Pedersen, L. H., et al. (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309, 1696–1703. doi: 10.1001/jama.2013.2270
Chugani, D. C., Chugani, H. T., Wiznitzer, M., Parikh, S., Evans, P. A., Hansen, R. L., et al. (2016). Efficacy of low-dose buspirone for restricted and repetitive behavior in young children with autism spectrum disorder: a randomized trial. J. Pediatr. 170, 45–53.e534. doi: 10.1016/j.jpeds.2015.11.033
Correia, C. T., Coutinho, A. M., Sequeira, A. F., Sousa, I. G., Lourenço Venda, L., Almeida, J. P., et al. (2010). Increased BDNF levels and NTRK2 gene association suggest a disruption of BDNF/TrkB signaling in autism. Genes Brain Behav. 9, 841–848. doi: 10.1111/j.1601-183X.2010.00627.x
Cotney, J., Muhle, R. A., Sanders, S. J., Liu, L., Willsey, A. J., Niu, W., et al. (2015). The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat. Commun. 6:6404. doi: 10.1038/ncomms7404
Crawford, M. A., Golfetto, I., Ghebremeskel, K., Min, Y., Moodley, T., Poston, L., et al. (2003). The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants. Lipids 38, 303–315. doi: 10.1007/s11745-003-1065-1
Croen, L. A., Grether, J. K., Yoshida, C. K., Odouli, R., and Hendrick, V. (2011). Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch. Gen. Psychiatry 68, 1104–1112. doi: 10.1001/archgenpsychiatry.2011.73
Croen, L. A., Zerbo, O., Qian, Y., Massolo, M. L., Rich, S., Sidney, S., et al. (2015). The health status of adults on the autism spectrum. Autism 19, 814–823. doi: 10.1177/1362361315577517
Darios, F., and Davletov, B. (2006). Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature 440, 813–817. doi: 10.1038/nature04598
Das, U. N. (2013). Autism as a disorder of deficiency of brain-derived neurotrophic factor and altered metabolism of polyunsaturated fatty acids. Nutrition 29, 1175–1185. doi: 10.1016/j.nut.2013.01.012
Dash, P. K., Orsi, S. A., and Moore, A. N. (2009). Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience 163, 1–8. doi: 10.1016/j.neuroscience.2009.06.028
De Bosscher, K., Desmet, S. J., Clarisse, D., Estébanez-Perpiña, E., and Brunsveld, L. (2020). Nuclear receptor crosstalk - defining the mechanisms for therapeutic innovation. Nat. Rev. Endocrinol. 16, 363–377. doi: 10.1038/s41574-020-0349-5
De Rubeis, S., He, X., Goldberg, A. P., Poultney, C. S., Samocha, K., Cicek, A. E., et al. (2014). Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215. doi: 10.1038/nature13772
Delerive, P., Chin, W. W., and Suen, C. S. (2002). Identification of Reverb(alpha) as a novel ROR(alpha) target gene. J. Biol. Chem. 277, 35013–35018. doi: 10.1074/jbc.M202979200
Delerive, P., Monté, D., Dubois, G., Trottein, F., Fruchart-Najib, J., Mariani, J., et al. (2001). The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. EMBO Rep. 2, 42–48. doi: 10.1093/embo-reports/kve007
Deth, R., Muratore, C., Benzecry, J., Power-Charnitsky, V. A., and Waly, M. (2008). How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. Neurotoxicology 29, 190–201. doi: 10.1016/j.neuro.2007.09.010
Devanna, P., and Vernes, S. C. (2014). A direct molecular link between the autism candidate gene RORa and the schizophrenia candidate MIR. Sci. Rep. 4:3994. doi: 10.1038/srep03994
Devnani, P. A., and Hegde, A. U. (2015). Autism and sleep disorders. J. Pediatr. Neurosci. 10, 304–307. doi: 10.4103/1817-1745.174438
Diau, G. Y., Hsieh, A. T., Sarkadi-Nagy, E. A., Wijendran, V., Nathanielsz, P. W., and Brenna, J. T. (2005). The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 3:11. doi: 10.1186/1741-7015-3-11
Doulazmi, M., Frédéric, F., Capone, F., Becker-André, M., Delhaye-Bouchaud, N., and Mariani, J. (2001). A comparative study of Purkinje cells in two RORalpha gene mutant mice: staggerer and RORalpha(-/-). Brain Res. Dev. Brain Res. 127, 165–174. doi: 10.1016/S0165-3806(01)00131-6
Doulazmi, M., Frédéric, F., Lemaigre-Dubreuil, Y., Hadj-Sahraoui, N., Delhaye-Bouchaud, N., and Mariani, J. (1999). Cerebellar Purkinje cell loss during life span of the heterozygous staggerer mouse (Rora(+)/Rora(sg)) is gender-related. J. Comp. Neurol. 411, 267–273. doi: 10.1002/(SICI)1096-9861(19990823)411:2<267::AID-CNE7>3.0.CO;2-4
Durand, C. M., Betancur, C., Boeckers, T. M., Bockmann, J., Chaste, P., Fauchereau, F., et al. (2007). Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 39, 25–27. doi: 10.1038/ng1933
Dussault, I., Fawcett, D., Matthyssen, A., Bader, J. A., and Giguère, V. (1998). Orphan nuclear receptor ROR alpha-deficient mice display the cerebellar defects of staggerer. Mech. Dev. 70, 147–153. doi: 10.1016/S0925-4773(97)00187-1
Dworzynski, K., Ronald, A., Bolton, P., and Happé, F. (2012). How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child Adolesc. Psychiatry 51, 788–797. doi: 10.1016/j.jaac.2012.05.018
Enstrom, A. M., Onore, C. E., Van de Water, J. A., and Ashwood, P. (2010). Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav. Immun. 24, 64–71. doi: 10.1016/j.bbi.2009.08.001
Evans, R. M., and Mangelsdorf, D. J. (2014). Nuclear receptors, RXR, and the big bang. Cell 157, 255–266. doi: 10.1016/j.cell.2014.03.012
Evans, T. A., Siedlak, S. L., Smith, M. A., Perry, G., Zhu, X., Lu, L., et al. (2008). The autistic phenotype exhibits a remarkably localized modification of brain protein by products of free radical-induced lipid oxidation. Am. J. Biochem. Biotechnol. 4, 61–72. doi: 10.3844/ajbbsp.2008.61.72
Farina, C., Aloisi, F., and Meinl, E. (2007). Astrocytes are active players in cerebral innate immunity. Trends Immunol. 28, 138–145. doi: 10.1016/j.it.2007.01.005
Farook, M. F., DeCuypere, M., Hyland, K., Takumi, T., LeDoux, M. S., and Reiter, L. T. (2012). Altered serotonin, dopamine and norepinepherine levels in 15q duplication and Angelman syndrome mouse models. PLoS ONE 7:e43030. doi: 10.1371/journal.pone.0043030
Fatemi, S. H., Aldinger, K. A., Ashwood, P., Bauman, M. L., Blaha, C. D., Blatt, G. J., et al. (2012). Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11, 777–807. doi: 10.1007/s12311-012-0355-9
Fellin, T., Sul, J. Y., D'Ascenzo, M., Takano, H., Pascual, O., and Haydon, P. G. (2006). Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP. Novartis Found. Symp. 276, 208–281. doi: 10.1002/9780470032244.ch16
Fu, T., Coulter, S., Yoshihara, E., Oh, T. G., Fang, S., Cayabyab, F., et al. (2019). FXR regulates intestinal cancer stem cell proliferation. Cell 176, 1098–1112.e18. doi: 10.1016/j.cell.2019.01.036
Fujisawa, K., Nishikawa, T., Kukidome, D., Imoto, K., Yamashiro, T., Motoshima, H., et al. (2009). TZDs reduce mitochondrial ROS production and enhance mitochondrial biogenesis. Biochem. Biophys. Res. Commun. 379, 43–48. doi: 10.1016/j.bbrc.2008.11.141
Gabis, L. V., Ben-Hur, R., Shefer, S., Jokel, A., and Shalom, D. B. (2019). Improvement of language in children with autism with combined donepezil and choline treatment. J. Mol. Neurosci. 69, 224–234. doi: 10.1007/s12031-019-01351-7
Gamsiz, E. D., Sciarra, L. N., Maguire, A. M., Pescosolido, M. F., van Dyck, L. I., and Morrow, E. M. (2015). Discovery of rare mutations in autism: elucidating neurodevelopmental mechanisms. Neurotherapeutics 12, 553–571. doi: 10.1007/s13311-015-0363-9
Genovese, A., and Butler, M. G. (2023). The autism spectrum: behavioral, psychiatric and genetic associations. Genes 14:677. doi: 10.3390/genes14030677
Geschwind, D. H., and Levitt, P. (2007). Autism spectrum disorders: developmental disconnection syndromes. Curr. Opin. Neurobiol. 17, 103–111. doi: 10.1016/j.conb.2007.01.009
Ghanizadeh, A., and Ayoobzadehshirazi, A. (2015). A randomized double-blind placebo-controlled clinical trial of adjuvant buspirone for irritability in autism. Pediatr. Neurol. 52, 77–81. doi: 10.1016/j.pediatrneurol.2014.09.017
Ghanizadeh, A., and Moghimi-Sarani, E. (2013). A randomized double blind placebo controlled clinical trial of N-Acetylcysteine added to risperidone for treating autistic disorders. BMC Psychiatry 13:196. doi: 10.1186/1471-244X-13-196
Giguère, V., McBroom, L. D., and Flock, G. (1995). Determinants of target gene specificity for ROR alpha 1: monomeric DNA binding by an orphan nuclear receptor. Mol. Cell. Biol. 15, 2517–2526. doi: 10.1128/MCB.15.5.2517
Gill, S., Chow, R., and Brown, A. J. (2008). Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog. Lipid Res. 47, 391–404. doi: 10.1016/j.plipres.2008.04.002
Gillberg, C., Steffenburg, S., Wahlström, J., Gillberg, I. C., Sjöstedt, A., Martinsson, T., et al. (1991). Autism associated with marker chromosome. J. Am. Acad. Child Adolesc. Psychiatry 30, 489–494. doi: 10.1097/00004583-199105000-00022
Goines, P., Haapanen, L., Boyce, R., Duncanson, P., Braunschweig, D., Delwiche, L., et al. (2011). Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav. Immun. 25, 514–523. doi: 10.1016/j.bbi.2010.11.017
Goines, P. E., and Ashwood, P. (2013). Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol. Teratol. 36, 67–81. doi: 10.1016/j.ntt.2012.07.006
Gold, D. A., Baek, S. H., Schork, N. J., Rose, D. W., Larsen, D. D., Sachs, B. D., et al. (2003). RORalpha coordinates reciprocal signaling in cerebellar development through sonic hedgehog and calcium-dependent pathways. Neuron 40, 1119–1131. doi: 10.1016/S0896-6273(03)00769-4
Goodall, G., and Gheusi, G. (1987). Abnormal patterns of maze patrolling in the mutant mouse staggerer. Behav. Neural Biol. 47, 307–320. doi: 10.1016/S0163-1047(87)90422-5
Gorovits, B., Azadeh, M., Buchlis, G., Harrison, T., Havert, M., Jawa, V., et al. (2021). Evaluation of the humoral response to adeno-associated virus-based gene therapy modalities using total antibody assays. AAPS J. 23:108. doi: 10.1208/s12248-021-00628-3
Grabrucker, A. M. (2013). Environmental factors in autism. Front. Psychiatry 3:118. doi: 10.3389/fpsyt.2012.00118
Guissart, C., Latypova, X., Rollier, P., Khan, T. N., Stamberger, H., McWalter, K., et al. (2018). Dual molecular effects of dominant RORA mutations cause two variants of syndromic intellectual disability with either autism or cerebellar ataxia. Am. J. Hum. Genet. 102, 744–759. doi: 10.1016/j.ajhg.2018.02.021
Haag, M. (2003). Essential fatty acids and the brain. Can. J. Psychiatry 48, 195–203. doi: 10.1177/070674370304800308
Hadj-Sahraoui, N., Frederic, F., Zanjani, H., Delhaye-Bouchaud, N., Herrup, K., and Mariani, J. (2001). Progressive atrophy of cerebellar Purkinje cell dendrites during aging of the heterozygous staggerer mouse (Rora(+/sg)). Brain Res. Dev. Brain Res. 126, 201–209. doi: 10.1016/S0165-3806(01)00095-5
Hall, A. C., Lucas, F. R., and Salinas, P. C. (2000). Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100, 525–535. doi: 10.1016/S0092-8674(00)80689-3
Hamilton, B. A., Frankel, W. N., Kerrebrock, A. W., Hawkins, T. L., FitzHugh, W., Kusumi, K., et al. (1996). Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature 379, 736–739. doi: 10.1038/379736a0
Handen, B. L., Johnson, C. R., McAuliffe-Bellin, S., Murray, P. J., and Hardan, A. Y. (2011). Safety and efficacy of donepezil in children and adolescents with autism: neuropsychological measures. J. Child Adolesc. Psychopharmacol. 21, 43–50. doi: 10.1089/cap.2010.0024
Harding, H. P., Atkins, G. B., Jaffe, A. B., Seo, W. J., and Lazar, M. A. (1997). Transcriptional activation and repression by RORalpha, an orphan nuclear receptor required for cerebellar development. Mol. Endocrinol. 11, 1737–1746. doi: 10.1210/mend.11.11.0002
Harfterkamp, M., van de Loo-Neus, G., Minderaa, R. B., van der Gaag, R. J., Escobar, R., Schacht, A., et al. (2012). A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 51, 733–741. doi: 10.1016/j.jaac.2012.04.011
Hawi, Z., Tong, J., Dark, C., Yates, H., Johnson, B., and Bellgrove, M. A. (2018). The role of cadherin genes in five major psychiatric disorders: a literature update. Am. J. Med. Genet. B Neuropsychiatr. Genet. 177, 168–180. doi: 10.1002/ajmg.b.32592
Haydon, P. G., and Carmignoto, G. (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 86, 1009–1031. doi: 10.1152/physrev.00049.2005
Herbert, M. R., Russo, J. P., Yang, S., Roohi, J., Blaxill, M., Kahler, S. G., et al. (2006). Autism and environmental genomics. Neurotoxicology 27, 671–684. doi: 10.1016/j.neuro.2006.03.017
Herrup, K., and Mullen, R. J. (1979). Regional variation and absence of large neurons in the cerebellum of the staggerer mouse. Brain Res. 172, 1–12. doi: 10.1016/0006-8993(79)90891-6
High, K. A., and Aubourg, P. (2011). rAAV human trial experience. Methods Mol. Biol. 807, 429–457. doi: 10.1007/978-1-61779-370-7_18
Hirose, H., Kawai, T., Yamamoto, Y., Taniyama, M., Tomita, M., Matsubara, K., et al. (2002). Effects of pioglitazone on metabolic parameters, body fat distribution, and serum adiponectin levels in Japanese male patients with type 2 diabetes. Metab. Clin. Exp. 51, 314–317. doi: 10.1053/meta.2002.30506
Hirsch, L. E., and Pringsheim, T. (2016). Aripiprazole for autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2016:CD009043. doi: 10.1002/14651858.CD009043.pub3
Hoehn, B., Yenari, M. A., Sapolsky, R. M., and Steinberg, G. K. (2003). Glutathione peroxidase overexpression inhibits cytochrome C release and proapoptotic mediators to protect neurons from experimental stroke. Stroke 34, 2489–2494. doi: 10.1161/01.STR.0000091268.25816.19
Hollander, E., Chaplin, W., Soorya, L., Wasserman, S., Novotny, S., Rusoff, J., et al. (2010). Divalproex sodium vs placebo for the treatment of irritability in children and adolescents with autism spectrum disorders. Neuropsychopharmacology 35, 990–998. doi: 10.1038/npp.2009.202
Hollander, E., Soorya, L., Chaplin, W., Anagnostou, E., Taylor, B. P., Ferretti, C. J., et al. (2012). A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am. J. Psychiatry 169, 292–299. doi: 10.1176/appi.ajp.2011.10050764
Hu, V. W., Frank, B. C., Heine, S., Lee, N. H., and Quackenbush, J. (2006). Gene expression profiling of lymphoblastoid cell lines from monozygotic twins discordant in severity of autism reveals differential regulation of neurologically relevant genes. BMC Genom. 7:118. doi: 10.1186/1471-2164-7-118
Hu, V. W., Sarachana, T., Kim, K. S., Nguyen, A., Kulkarni, S., Steinberg, M. E., et al. (2009). Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2, 78–97. doi: 10.1002/aur.73
Hu, V. W., Sarachana, T., Sherrard, R. M., and Kocher, K. M. (2015). Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol. Autism 6:7. doi: 10.1186/2040-2392-6-7
Huggins, R. J., and Greene, G. L. (2023). ERα/PR crosstalk is altered in the context of the ERα Y537S mutation and contributes to endocrine therapy-resistant tumor proliferation. NPJ Breast Cancer. 9:96. doi: 10.1038/s41523-023-00601-7
Huss, J. M., and Kelly, D. P. (2004). Nuclear receptor signaling and cardiac energetics. Circ. Res. 95, 568–578. doi: 10.1161/01.RES.0000141774.29937.e3
Iizuka, A., Matsuzaki, Y., Konno, A., and Hirai, H. (2016). Plasticity of the developmentally arrested staggerer cerebellum in response to exogenous RORα. Brain Struct. Funct. 221, 2879–2889. doi: 10.1007/s00429-015-1077-9
Ino, H. (2004). Immunohistochemical characterization of the orphan nuclear receptor ROR alpha in the mouse nervous system. J. Histochem. Cytochem. 52, 311–323. doi: 10.1177/002215540405200302
Ishibashi, T., Dakin, K. A., Stevens, B., Lee, P. R., Kozlov, S. V., Stewart, C. L., et al. (2006). Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832. doi: 10.1016/j.neuron.2006.02.006
Jamain, S., Quach, H., Betancur, C., Råstam, M., Colineaux, C., Gillberg, I. C., et al. (2003). Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 34, 27–29. doi: 10.1038/ng1136
Jedele, K. B. (2007). The overlapping spectrum of Rett and Angelman syndromes: a clinical review. Semin. Pediatr. Neurol. 14, 108–117. doi: 10.1016/j.spen.2007.07.002
Jetten, A. M. (2009). Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 7:e003. doi: 10.1621/nrs.07003
Jiang, C. C., Lin, L. S., Long, S., Ke, X. Y., Fukunaga, K., Lu, Y. M., et al. (2022). Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications. Signal Transduct. Target Ther. 7:229. doi: 10.1038/s41392-022-01081-0
Jiang, L., Liu, X., Liang, X., Dai, S., Wei, H., Guo, M., et al. (2024). Structural characterization of the DNA binding mechanism of retinoic acid-related orphan receptor gamma. Structure 32, 467–475.e3. doi: 10.1016/j.str.2024.01.004
Joshi, G., DiSalvo, M., Wozniak, J., Ceranoglu, T. A., Yule, A., Surman, C., et al. (2020). A prospective open-label trial of long-acting liquid methylphenidate for the treatment of attention deficit/hyperactivity disorder in intellectually capable adults with autism spectrum disorder. World J. Biol. Psychiatry 21, 274–290. doi: 10.1080/15622975.2019.1679392
Journiac, N., Jolly, S., Jarvis, C., Gautheron, V., Rogard, M., Trembleau, A., et al. (2009). The nuclear receptor ROR(alpha) exerts a bi-directional regulation of IL-6 in resting and reactive astrocytes. Proc. Natl. Acad. Sci. U.S.A. 106, 21365–21370. doi: 10.1073/pnas.0911782106
Kalaany, N. Y., and Mangelsdorf, D. J. (2006). LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 68, 159–191. doi: 10.1146/annurev.physiol.68.033104.152158
Kanlayaprasit, S., Thongkorn, S., Panjabud, P., Jindatip, D., Hu, V. W., Kikkawa, T., et al. (2021). Autism-related transcription factors underlying the sex-specific effects of prenatal bisphenol a exposure on transcriptome-interactome profiles in the offspring prefrontal cortex. Int. J. Mol. Sci. 22:13201. doi: 10.3390/ijms222413201
Kay, M. A., Glorioso, J. C., and Naldini, L. (2001). Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat. Med. 7, 33–40. doi: 10.1038/83324
Kenda, M., and Sollner Dolenc, M. (2020). Computational study of drugs targeting nuclear receptors. Molecules 25:1616. doi: 10.3390/molecules25071616
Kenney, A. M., Cole, M. D., and Rowitch, D. H. (2003). Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development 130, 15–28. doi: 10.1242/dev.00182
Kenney, A. M., and Rowitch, D. H. (2000). Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol. Cell. Biol. 20, 9055–9067. doi: 10.1128/MCB.20.23.9055-9067.2000
Kerbeshian, J., Burd, L., Randall, T., Martsolf, J., and Jalal, S. (1990). Autism, profound mental retardation and atypical bipolar disorder in a 33-year-old female with a deletion of 15q12. J. Ment. Defic. Res. 34, 205–210. doi: 10.1111/j.1365-2788.1990.tb01530.x
Kern, J. K., and Jones, A. M. (2006). Evidence of toxicity, oxidative stress, and neuronal insult in autism. J. Toxicol. Environ. Health B Crit. Rev. 9, 485–499. doi: 10.1080/10937400600882079
Khera, R., Mehan, S., Kumar, S., Sethi, P., Bhalla, S., and Prajapati, A. (2022). Role of JAK-STAT and PPAR-gamma signalling modulators in the prevention of autism and neurological dysfunctions. Mol. Neurobiol. 59, 3888–3912. doi: 10.1007/s12035-022-02819-1
Kim, E. J., Yoo, Y. G., Yang, W. K., Lim, Y. S., Na, T. Y., Lee, I. K., et al. (2008). Transcriptional activation of HIF-1 by RORalpha and its role in hypoxia signaling. Arterioscler. Thromb. Vasc. Biol. 28, 1796–1802. doi: 10.1161/ATVBAHA.108.171546
Kim, H. Y., and Spector, A. A. (2018). N-Docosahexaenoylethanolamine: a neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol. Aspects Med. 64, 34–44. doi: 10.1016/j.mam.2018.03.004
Kim, K., Boo, K., Yu, Y. S., Oh, S. K., Kim, H., Jeon, Y., et al. (2017). RORα controls hepatic lipid homeostasis via negative regulation of PPARγ transcriptional network. Nat. Commun. 8:162. doi: 10.1038/s41467-017-00215-1
Kim, S. J., Shonka, S., French, W. P., Strickland, J., Miller, L., and Stein, M. A. (2017). Dose-response effects of long-acting liquid methylphenidate in children with attention deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD): a pilot study. J. Autism Dev. Disord. 47, 2307–2313. doi: 10.1007/s10803-017-3125-1
Kim, W. K., Meliton, V., Park, K. W., Hong, C., Tontonoz, P., Niewiadomski, P., et al. (2009). Negative regulation of Hedgehog signaling by liver X receptors. Mol. Endocrinol. 23, 1532–1543. doi: 10.1210/me.2008-0453
Kliewer, S. A., Umesono, K., Heyman, R. A., Mangelsdorf, D. J., Dyck, J. A., and Evans, R. M. (1992a). Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc. Natl. Acad. Sci. U.S.A. 89, 1448–1452. doi: 10.1073/pnas.89.4.1448
Kliewer, S. A., Umesono, K., Mangelsdorf, D. J., and Evans, R. M. (1992b). Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature 355, 446–449. doi: 10.1038/355446a0
Kong, Y., Zhou, W., and Sun, Z. (2020). Nuclear receptor corepressors in intellectual disability and autism. Mol. Psychiatry 25, 2220–2236. doi: 10.1038/s41380-020-0667-y
Krasowski, M. D., Ni, A., Hagey, L. R., and Ekins, S. (2011). Evolution of promiscuous nuclear hormone receptors: LXR, FXR, VDR, PXR, and CAR. Mol. Cell. Endocrinol. 334, 39–48. doi: 10.1016/j.mce.2010.06.016
Kumar, S., Mehan, S., and Narula, A. S. (2023). Therapeutic modulation of JAK-STAT, mTOR, and PPAR-γ signaling in neurological dysfunctions. J. Mol. Med. 101, 9–49. doi: 10.1007/s00109-022-02272-6
Kuperstein, F., Eilam, R., and Yavin, E. (2008). Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J. Neurochem. 106, 662–671. doi: 10.1111/j.1471-4159.2008.05418.x
Lalonde, R. (1987). Exploration and spatial learning in staggerer mutant mice. J. Neurogenet. 4, 285–291. doi: 10.3109/01677068709167189
Lalonde, R., Botez, M. I., and Boivin, D. (1987). Object exploration in staggerer mutant mice. Physiol. Behav. 41, 115–117. doi: 10.1016/0031-9384(87)90139-9
Lalonde, R., Manseau, M., and Botez, M. I. (1988). Spontaneous alternation and exploration in staggerer mutant mice. Behav. Brain Res. 27, 273–276. doi: 10.1016/0166-4328(88)90124-6
Lalonde, R., and Strazielle, C. (2008). Discrimination learning in Rora(sg) and Grid2(ho) mutant mice. Neurobiol. Learn. Mem. 90, 472–474. doi: 10.1016/j.nlm.2008.05.004
Lambrecht, R., Delgado, M. E., Gloe, V., Schuetz, K., Plazzo, A. P., Franke, B., et al. (2023). Liver receptor homolog-1 (NR5A2) orchestrates hepatic inflammation and TNF-induced cell death. Cell Rep. 42:113513. doi: 10.1016/j.celrep.2023.113513
Lamy, M., and Erickson, C. A. (2018). Pharmacological management of behavioral disturbances in children and adolescents with autism spectrum disorders. Curr. Probl. Pediatr. Adolesc. Health Care 48, 250–264. doi: 10.1016/j.cppeds.2018.08.015
Landa, R. J., Holman, K. C., and Garrett-Mayer, E. (2007). Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch. Gen. Psychiatry 64, 853–864. doi: 10.1001/archpsyc.64.7.853
Landis, D. M., and Sidman, R. L. (1978). Electron microscopic analysis of postnatal histogenesis in the cerebellar cortex of staggerer mutant mice. J. Comp. Neurol. 179, 831–863. doi: 10.1002/cne.901790408
Lau, P., Bailey, P., Dowhan, D. H., and Muscat, G. E. (1999). Exogenous expression of a dominant negative RORalpha1 vector in muscle cells impairs differentiation: RORalpha1 directly interacts with p300 and myoD. Nucleic Acids Res. 27, 411–420. doi: 10.1093/nar/27.2.411
Lau, P., Fitzsimmons, R. L., Raichur, S., Wang, S. C., Lechtken, A., and Muscat, G. E. (2008). The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J. Biol. Chem. 283, 18411–18421. doi: 10.1074/jbc.M710526200
Lauritzen, L., Hansen, H. S., Jørgensen, M. H., and Michaelsen, K. F. (2001). The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 40, 1–94. doi: 10.1016/S0163-7827(00)00017-5
Lavery, D. N., and McEwan, I. J. (2005). Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem. J. 391, 449–464. doi: 10.1042/BJ20050872
Lee, S., Moon, H., and Kim, E. (2025). NMDAR dysfunction in autism spectrum disorders: lessons learned from 10 years of study. Curr. Opin. Neurobiol. 92:103023. doi: 10.1016/j.conb.2025.103023
Leid, M., Kastner, P., Lyons, R., Nakshatri, H., Saunders, M., Zacharewski, T., et al. (1992). Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell 68, 377–395. doi: 10.1016/0092-8674(92)90478-U
Leiden Open Variation (n.d.). Database. LOVD software ©2004–2024 Leiden University Medical Center. Available online at: https://databases.lovd.nl/shared/variants/RORA (Accessed September 9, 2025).
Lemprière, S. (2022). Novel oxytocin spray is beneficial in ASD. Nat. Rev. Neurol. 18:127. doi: 10.1038/s41582-022-00627-8
Levine, S. Z., Kodesh, A., Goldberg, Y., Reichenberg, A., Furukawa, T. A., Kolevzon, A., et al. (2016). Initial severity and efficacy of risperidone in autism: results from the RUPP trial. Eur. Psychiatry 32, 16–20. doi: 10.1016/j.eurpsy.2015.11.004
Levy, S. E., Souders, M. C., Wray, J., Jawad, A. F., Gallagher, P. R., Coplan, J., et al. (2003). Children with autistic spectrum disorders. I: comparison of placebo and single dose of human synthetic secretin. Arch. Dis. Child. 88, 731–736. doi: 10.1136/adc.88.8.731
Li, A., Zhang, H., Han, H., Zhang, W., Yang, S., Huang, Z., et al. (2019). LC3 promotes the nuclear translocation of the vitamin D receptor and decreases fibrogenic gene expression in proximal renal tubules. Metab. Clin. Exp. 98, 95–103. doi: 10.1016/j.metabol.2019.06.008
Li, M., Pascual, G., and Glass, C. K. (2000). Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol. Cell. Biol. 20, 4699–4707. doi: 10.1128/MCB.20.13.4699-4707.2000
Li, X., Chauhan, A., Sheikh, A. M., Patil, S., Chauhan, V., Li, X. M., et al. (2009). Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 207, 111–116. doi: 10.1016/j.jneuroim.2008.12.002
Lim, H. K., Yoon, J. H., and Song, M. (2022). Autism spectrum disorder genes: disease-related networks and compensatory strategies. Front. Mol. Neurosci. 15:922840. doi: 10.3389/fnmol.2022.922840
Liu, J., Nyholt, D. R., Magnussen, P., Parano, E., Pavone, P., Geschwind, D., et al. (2001). A genomewide screen for autism susceptibility loci. Am. J. Hum. Genet. 69, 327–340. doi: 10.1086/321980
Lord, C., Cook, E. H., Leventhal, B. L., and Amaral, D. G. (2000). Autism spectrum disorders. Neuron 28, 355–363. doi: 10.1016/S0896-6273(00)00115-X
Lucchelli, J. P., and Bertschy, G. (2018). Low-dose fluoxetine in four children with autistic spectrum disorder improves self-injurious behavior, ADHD-like symptoms, and irritability. Case Rep. Psychiatry 2018:6278501. doi: 10.1155/2018/6278501
Lyall, K., Schmidt, R., and Hertz-Picciotto, I. (2013). “Chapter 2.7-The environment in autism spectrum disorders,” in The Neuroscience of Autism Spectrum Disorders (Academic Press/Elsevier), 203–214. doi: 10.1016/B978-0-12-391924-3.00014-4
Lyst, M. J., Ekiert, R., Ebert, D. H., Merusi, C., Nowak, J., Selfridge, J., et al. (2013). Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat. Neurosci. 16, 898–902. doi: 10.1038/nn.3434
Ma, H., de Zwaan, E., Guo, Y. E., Cejas, P., Thiru, P., van de Bunt, M., et al. (2022). The nuclear receptor THRB facilitates differentiation of human PSCs into more mature hepatocytes. Cell Stem Cell 29, 795–809.e11. doi: 10.1016/j.stem.2022.03.015
Maenner, M. J., Warren, Z., Williams, A. R., Amoakohene, E., Bakian, A. V., Bilder, D. A., et al. (2023). Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill. Summ. 72, 1–14. doi: 10.15585/mmwr.ss7202a1
Malhotra, N., Leyva-Castillo, J. M., Jadhav, U., Barreiro, O., Kam, C., O'Neill, N. K., et al. (2018). RORα-expressing T regulatory cells restrain allergic skin inflammation. Sci. Immunol. 3:eaao6923. doi: 10.1126/sciimmunol.aao6923
Mamontova, A., Séguret-Macé, S., Esposito, B., Chaniale, C., Bouly, M., Delhaye-Bouchaud, N., et al. (1998). Severe atherosclerosis and hypoalphalipoproteinemia in the staggerer mouse, a mutant of the nuclear receptor RORalpha. Circulation 98, 2738–2743. doi: 10.1161/01.CIR.98.24.2738
Manfredsson, F. P., and Mandel, R. J. (2010). Development of gene therapy for neurological disorders. Discov. Med. 9, 204–211. Available online at: https://www.discoverymedicine.com/Fredric-P-Manfredsson/2010/03/08/development-of-gene-therapy-for-neurological-disorders/ (Accessed April 21, 2025).
Manini, A., Abati, E., Nuredini, A., Corti, S., and Comi, G. P. (2022). Adeno-associated virus (AAV)-mediated gene therapy for duchenne muscular dystrophy: the issue of transgene persistence. Front. Neurol. 12:814174. doi: 10.3389/fneur.2021.814174
Markham, K., Schuurmans, C., and Weiss, S. (2007). STAT5A/B activity is required in the developing forebrain and spinal cord. Mol. Cell. Neurosci. 35, 272–282. doi: 10.1016/j.mcn.2007.03.001
Marshall, C. R., Noor, A., Vincent, J. B., Lionel, A. C., Feuk, L., Skaug, J., et al. (2008). Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 82, 477–488. doi: 10.1016/j.ajhg.2007.12.009
Martinez, M. (1992). Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 120, S129–S138. doi: 10.1016/S0022-3476(05)81247-8
Martinsson, T., Johannesson, T., Vujic, M., Sjöstedt, A., Steffenburg, S., Gillberg, C., et al. (1996). Maternal origin of inv dup(15) chromosomes in infantile autism. Eur. Child Adolesc. Psychiatry 5, 185–192. doi: 10.1007/BF00538845
Marty, N., Dallaporta, M., Foretz, M., Emery, M., Tarussio, D., Bady, I., et al. (2005). Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J. Clin. Invest. 115, 3545–3553. doi: 10.1172/JCI26309
Maski, K. P., Jeste, S. S., and Spence, S. J. (2011). Common neurological co-morbidities in autism spectrum disorders. Curr. Opin. Pediatr. 23, 609–615. doi: 10.1097/MOP.0b013e32834c9282
Mastrototaro, G., Zaghi, M., Massimino, L., Moneta, M., Mohammadi, N., Banfi, F., et al. (2021). TBL1XR1 ensures balanced neural development through NCOR complex-mediated regulation of the MAPK pathway. Front. Cell. Dev. Biol. 9:641410. doi: 10.3389/fcell.2021.641410
Matson, J. L., and Cervantes, P. E. (2014). Commonly studied comorbid psychopathologies among persons with autism spectrum disorder. Res. Dev. Disabil. 35, 952–962. doi: 10.1016/j.ridd.2014.02.012
Matsui, F., Hecht, P., Yoshimoto, K., Watanabe, Y., Morimoto, M., Fritsche, K., et al. (2018). DHA mitigates autistic behaviors accompanied by dopaminergic change in a gene/prenatal stress mouse model. Neuroscience 371, 407–419. doi: 10.1016/j.neuroscience.2017.12.029
Mayerson, A. B., Hundal, R. S., Dufour, S., Lebon, V., Befroy, D., Cline, G. W., et al. (2002). The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 51, 797–802. doi: 10.2337/diabetes.51.3.797
Mazahery, H., Stonehouse, W., Delshad, M., Kruger, M. C., Conlon, C. A., Beck, K. L., et al. (2017). Relationship between long chain n-3 polyunsaturated fatty acids and autism spectrum disorder: systematic review and meta-analysis of case-control and randomised controlled trials. Nutrients 9:155. doi: 10.3390/nu9020155
Mazzone, L., and Ruta, L. (2006). Topiramate in children with autistic spectrum disorders. Brain Dev. 28:668. doi: 10.1016/j.braindev.2006.05.004
McCracken, J. T., McGough, J., Shah, B., Cronin, P., Hong, D., Aman, M. G., et al. (2002). Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med. 347, 314–321. doi: 10.1056/NEJMoa013171
McDougal, D. H., Hermann, G. E., and Rogers, R. C. (2011). Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J. Neurosci. 31, 14037–14045. doi: 10.1523/JNEUROSCI.2855-11.2011
Melke, J., Goubran Botros, H., Chaste, P., Betancur, C., Nygren, G., Anckarsäter, H., et al. (2008). Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatry 13, 90–98. doi: 10.1038/sj.mp.4002016
Menezo, Y. J., Elder, K., and Dale, B. (2015). Link between increased prevalence of autism spectrum disorder syndromes and oxidative stress, DNA methylation, and imprinting: the impact of the environment. JAMA Pediatr. 169, 1066–1067. doi: 10.1001/jamapediatrics.2015.2125
Meziane, H., Schaller, F., Bauer, S., Villard, C., Matarazzo, V., Riet, F., et al. (2015). An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism. Biol. Psychiatry 78, 85–94. doi: 10.1016/j.biopsych.2014.11.010
Miao, L., Yang, Y., Liu, Y., Lai, L., Wang, L., Zhan, Y., et al. (2019). Glycerol kinase interacts with nuclear receptor NR4A1 and regulates glucose metabolism in the liver. FASEB J. 33, 6736–6747. doi: 10.1096/fj.201800945RR
Mihalj, D., Borbelyova, V., Pirnik, Z., Bacova, Z., Ostatnikova, D., and Bakos, J. (2024). Shank3 deficiency results in a reduction in GABAergic postsynaptic puncta in the olfactory brain areas. Neurochem. Res. 49, 1008–1016. doi: 10.1007/s11064-023-04097-2
Miles, J. H., and Hillman, R. E. (2000). Value of a clinical morphology examination in autism. Am. J. Med. Genet. 91, 245–253. doi: 10.1002/(SICI)1096-8628(20000410)91:4<245::AID-AJMG1>3.0.CO;2-2
Ming, X., Gordon, E., Kang, N., and Wagner, G. C. (2008). Use of clonidine in children with autism spectrum disorders. Brain Dev. 30, 454–460. doi: 10.1016/j.braindev.2007.12.007
Minshawi, N. F., Wink, L. K., Shaffer, R., Plawecki, M. H., Posey, D. J., Liu, H., et al. (2016). A randomized, placebo-controlled trial of D-cycloserine for the enhancement of social skills training in autism spectrum disorders. Mol. Autism 7:2. doi: 10.1186/s13229-015-0062-8
Minshew, N. J., and Williams, D. L. (2007). The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch. Neurol. 64, 945–950. doi: 10.1001/archneur.64.7.945
Miyazaki, Y., Mahankali, A., Matsuda, M., Mahankali, S., Hardies, J., Cusi, K., et al. (2002). Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 87, 2784–2791. doi: 10.1210/jcem.87.6.8567
Mount, M. P., Lira, A., Grimes, D., Smith, P. D., Faucher, S., Slack, R., et al. (2007). Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J. Neurosci. 27, 3328–3337. doi: 10.1523/JNEUROSCI.5321-06.2007
Nabetani, M., Mukai, T., and Taguchi, A. (2023). Cell therapies for autism spectrum disorder based on new pathophysiology: a review. Cell Transplant. 32:9636897231163217. doi: 10.1177/09636897231163217
Najjar, F., Owley, T., Mosconi, M. W., Jacob, S., Hur, K., Guter, S. J., et al. (2015). Pharmacogenetic study of serotonin transporter and 5HT2A genotypes in autism. J. Child Adolesc. Psychopharmacol. 25, 467–474. doi: 10.1089/cap.2014.0158
Naldini, L. (2015). Gene therapy returns to centre stage. Nature 526, 351–360. doi: 10.1038/nature15818
Nationa Library of Medicine (2024). A Phase 1/2, Multicenter, Open-Label, Dose-Escalation, Safety, Tolerability, and Clinical Activity Study of a Single Dose of JAG201 Gene Therapy Delivered Via Intracerebroventricular Administration in Participants with SHANK3 Haploinsufficiency. NCT06662188. Available online at: https://clinicaltrials.gov/study/NCT06662188 (Accessed April 21, 2025).
National Institute of Mental Health (2022). Autism Spectrum Disorder. NIH Publication No. 22-MH-8084. Available online at: https://www.nimh.nih.gov/health/publications/autism-spectrum-disorder (Accessed April 14, 2025).
Nejati Moharrami, N., Bjørkøy Tande, E., Ryan, L., Espevik, T., and Boyartchuk, V. (2018). RORα controls inflammatory state of human macrophages. PLoS ONE 13:e0207374. doi: 10.1371/journal.pone.0207374
Nematisouldaragh, D., Kirshenbaum, E., Uzonna, M., Kirshenbaum, L., and Rabinovich-Nikitin, I. (2024). The role of retinoic-acid-related orphan receptor (RORs) in cellular homeostasis. Int. J. Mol. Sci. 25:11340. doi: 10.3390/ijms252111340
Newschaffer, C. J., Croen, L. A., Daniels, J., Giarelli, E., Grether, J. K., Levy, S. E., et al. (2007). The epidemiology of autism spectrum disorders. Annu. Rev. Public Health 28, 235–258. doi: 10.1146/annurev.publhealth.28.021406.144007
Nguyen, A., Rauch, T. A., Pfeifer, G. P., and Hu, V. W. (2010). Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J. 24, 3036–3051. doi: 10.1096/fj.10-154484
Nicholas, B., Rudrasingham, V., Nash, S., Kirov, G., Owen, M. J., and Wimpory, D. C. (2007). Association of Per1 and Npas2 with autistic disorder: support for the clock genes/social timing hypothesis. Mol. Psychiatry 12, 581–592. doi: 10.1038/sj.mp.4001953
Ogawa, S., Lozach, J., Benner, C., Pascual, G., Tangirala, R. K., Westin, S., et al. (2005). Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122, 707–721. doi: 10.1016/j.cell.2005.06.029
Olivares, A. M., Moreno-Ramos, O. A., and Haider, N. B. (2016). Role of nuclear receptors in central nervous system development and associated diseases. J. Exp. Neurosci. 9, 93–121. doi: 10.4137/JEN.S25480
Ornoy, A., Weinstein-Fudim, L., and Ergaz, Z. (2015). Prenatal factors associated with autism spectrum disorder (ASD). Reprod. Toxicol. 56, 155–169. doi: 10.1016/j.reprotox.2015.05.007
Ornoy, A., Weinstein-Fudim, L., and Ergaz, Z. (2016). Genetic syndromes, maternal diseases and antenatal factors associated with autism spectrum disorders (ASD). Front. Neurosci. 10:316. doi: 10.3389/fnins.2016.00316
O'Roak, B. J., Vives, L., Fu, W., Egertson, J. D., Stanaway, I. B., Phelps, I. G., et al. (2012). Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338, 1619–1622. doi: 10.1126/science.1227764
Owley, T., Brune, C. W., Salt, J., Walton, L., Guter, S., Ayuyao, N., et al. (2010). A pharmacogenetic study of escitalopram in autism spectrum disorders. Autism Res. 3, 1–7. doi: 10.1002/aur.109
Pagan, C., Delorme, R., Callebert, J., Goubran-Botros, H., Amsellem, F., Drouot, X., et al. (2014). The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Transl. Psychiatry 4:e479. doi: 10.1038/tp.2014.120
Pagani, M., Barsotti, N., Bertero, A., Trakoshis, S., Ulysse, L., Locarno, A., et al. (2021). mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat. Commun. 12:6084. doi: 10.1038/s41467-021-26131-z
Pal, D., Raj, K., Nandi, S. S., Sinha, S., Mishra, A., Mondal, A., et al. (2023). Potential of synthetic and natural compounds as novel histone deacetylase inhibitors for the treatment of hematological malignancies. Cancers 15:2808. doi: 10.3390/cancers15102808
Palmen, S. J., van Engeland, H., Hof, P. R., and Schmitz, C. (2004). Neuropathological findings in autism. Brain 127, 2572–22583. doi: 10.1093/brain/awh287
Parker, K. J., Oztan, O., Libove, R. A., Mohsin, N., Karhson, D. S., Sumiyoshi, R. D., et al. (2019). A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med. 11:eaau7356. doi: 10.1126/scitranslmed.aau7356
Parpura, V., and Haydon, P. G. (2000). Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc. Natl. Acad. Sci. U.S.A. 97, 8629–8634. doi: 10.1073/pnas.97.15.8629
Parri, R., and Crunelli, V. (2003). An astrocyte bridge from synapse to blood flow. Nat. Neurosci. 6, 5–6. doi: 10.1038/nn0103-5
Pascual, G., Fong, A. L., Ogawa, S., Gamliel, A., Li, A. C., Perissi, V., et al. (2005). A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437, 759–763. doi: 10.1038/nature03988
Pavek, P. (2016). Pregnane X receptor (PXR)-mediated gene repression and cross-talk of PXR with other nuclear receptors via coactivator interactions. Front. Pharmacol. 7:456. doi: 10.3389/fphar.2016.00456
Peden, C. S., Burger, C., Muzyczka, N., and Mandel, R. J. (2004). Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 78, 6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004
Persico, A. M., and Bourgeron, T. (2006). Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 29, 349–358. doi: 10.1016/j.tins.2006.05.010
Politte, L. C., Scahill, L., Figueroa, J., McCracken, J. T., King, B., and McDougle, C. J. (2018). A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology 43, 1772–1778. doi: 10.1038/s41386-018-0039-3
Polleux, F., and Lauder, J. M. (2004). Toward a developmental neurobiology of autism. Ment. Retard. Dev. Disabil. Res. Rev. 10, 303–317. doi: 10.1002/mrdd.20044
Quaak, I., Brouns, M. R., and Van de Bor, M. (2013). The dynamics of autism spectrum disorders: how neurotoxic compounds and neurotransmitters interact. Int. J. Environ. Res. Public Health 10, 3384–3408. doi: 10.3390/ijerph10083384
Ramachandran, S., Ardinger, J., Bu, J., Ramos, M., Guo, L., Ghosh, D., et al. (2025). Cross-species efficacy of AAV-mediated ARSA replacement for metachromatic leukodystrophy. J. Clin. Invest. 135:e185001. doi: 10.1172/JCI185001
Rao, M., McDuffie, E., Srivastava, S., Plaisted, W., and Sachs, C. (2024). Safety implications of modulating nuclear receptors: a comprehensive analysis from non-clinical and clinical perspectives. Pharmaceuticals 17:875. doi: 10.3390/ph17070875
Rapin, I. (2002). The autistic-spectrum disorders. N. Engl. J. Med. 347, 302–303. doi: 10.1056/NEJMp020062
Raspé, E., Duez, H., Gervois, P., Fiévet, C., Fruchart, J. C., Besnard, S., et al. (2001). Transcriptional regulation of apolipoprotein C-III gene expression by the orphan nuclear receptor RORalpha. J. Biol. Chem. 276, 2865–2871. doi: 10.1074/jbc.M004982200
Ratman, D., Mylka, V., Bougarne, N., Pawlak, M., Caron, S., Hennuyer, N., et al. (2016). Chromatin recruitment of activated AMPK drives fasting response genes co-controlled by GR and PPARα. Nucleic Acids Res. 44, 10539–10553. doi: 10.1093/nar/gkw742
Reichow, B., Volkmar, F. R., and Bloch, M. H. (2013). Systematic review and meta-analysis of pharmacological treatment of the symptoms of attention-deficit/hyperactivity disorder in children with pervasive developmental disorders. J. Autism Dev. Disord. 43, 2435–2441. doi: 10.1007/s10803-013-1793-z
Research Units on Pediatric Psychopharmacology Autism Network (2005). Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch. Gen. Psychiatry 62, 1266–1274. doi: 10.1001/archpsyc.62.11.1266
Ribeiro, S., and Sherrard, R. M. (2023). Cerebellum and neurodevelopmental disorders: RORα is a unifying force. Front. Cell. Neurosci. 17:1108339. doi: 10.3389/fncel.2023.1108339
Rosina, E., Battan, B., Siracusano, M., Di Criscio, L., Hollis, F., Pacini, L., et al. (2019). Disruption of mTOR and MAPK pathways correlates with severity in idiopathic autism. Transl. Psychiatry 9:50. doi: 10.1038/s41398-018-0335-z
Rossignol, D. A., and Frye, R. E. (2014). Melatonin in autism spectrum disorders. Curr. Clin. Pharmacol. 9, 326–334. doi: 10.2174/15748847113086660072
Sajdel-Sulkowska, E. M., Lipinski, B., Windom, H., Audhya, T., and McGinnis, W. (2008). Oxidative stress in autism: elevated cerebellar 3-nitrotyrosine levels. Am. J. Biochem. Biotechnol. 4, 73–84. doi: 10.3844/ajbbsp.2008.73.84
Sajdel-Sulkowska, E. M., Xu, M., and Koibuchi, N. (2009). Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum 8, 366–372. doi: 10.1007/s12311-009-0105-9
Salehi, M., Kamali, E., Karahmadi, M., and Mousavi, S. M. (2017). RORA and autism in the isfahan population: is there an epigenetic relationship. Cell J. 18, 540–546. doi: 10.22074/cellj.2016.4720
Sanders, S. J., Murtha, M. T., Gupta, A. R., Murdoch, J. D., Raubeson, M. J., Willsey, A. J., et al. (2012). De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241. doi: 10.1038/nature10945
Sandin, S., Schendel, D., Magnusson, P., Hultman, C., Surén, P., Susser, E., et al. (2016). Autism risk associated with parental age and with increasing difference in age between the parents. Mol. Psychiatry 21, 693–700. doi: 10.1038/mp.2015.70
Sarachana, T., and Hu, V. W. (2013). Genome-wide identification of transcriptional targets of RORA reveals direct regulation of multiple genes associated with autism spectrum disorder. Mol. Autism 4:14. doi: 10.1186/2040-2392-4-14
Sarachana, T., Xu, M., Wu, R. C., and Hu, V. W. (2011). Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS ONE 6:e17116. doi: 10.1371/journal.pone.0017116
Sayad, A., Noroozi, R., Omrani, M. D., Taheri, M., and Ghafouri-Fard, S. (2017). Retinoic acid-related orphan receptor alpha (RORA) variants are associated with autism spectrum disorder. Metab. Brain Dis. 32, 1595–1601. doi: 10.1007/s11011-017-0049-6
Scahill, L., McCracken, J. T., King, B. H., Rockhill, C., Shah, B., Politte, L., et al. (2015). Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am. J. Psychiatry 172, 1197–1206. doi: 10.1176/appi.ajp.2015.15010055
Schiering, C., Krausgruber, T., Chomka, A., Fröhlich, A., Adelmann, K., Wohlfert, E. A., et al. (2014). The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568. doi: 10.1038/nature13577
Schulman, I. G. (2010). Nuclear receptors as drug targets for metabolic disease. Adv. Drug Deliv. Rev. 62, 1307–1315. doi: 10.1016/j.addr.2010.07.002
Shang, Y., Myers, M., and Brown, M. (2002). Formation of the androgen receptor transcription complex. Mol. Cell 9, 601–610. doi: 10.1016/S1097-2765(02)00471-9
Shaw, K. A., Bilder, D. A., McArthur, D., Williams, A. R., Amoakohene, E., Bakian, A. V., et al. (2023). Early identification of autism spectrum disorder among children aged 4 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill. Summ. 72, 1–15. doi: 10.15585/mmwr.ss6903a1
Shimamura, K., Hirano, S., McMahon, A. P., and Takeichi, M. (1994). Wnt-1-dependent regulation of local E-cadherin and alpha N-catenin expression in the embryonic mouse brain. Development 120, 2225–2234. doi: 10.1242/dev.120.8.2225
Shultz, S. R., Macfabe, D. F., Martin, S., Jackson, J., Taylor, R., Boon, F., et al. (2009). Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behav. Brain Res. 200, 33–41. doi: 10.1016/j.bbr.2008.12.023
Shultz, S. R., MacFabe, D. F., Ossenkopp, K. P., Scratch, S., Whelan, J., Taylor, R., et al. (2008). Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology 54, 901–911. doi: 10.1016/j.neuropharm.2008.01.013
Sikich, L., Kolevzon, A., King, B. H., McDougle, C. J., Sanders, K. B., Kim, S. J., et al. (2021). Intranasal oxytocin in children and adolescents with autism spectrum disorder. N. Engl. J. Med. 385, 1462–1473. doi: 10.1056/NEJMoa2103583
Song, C., Hiipakka, R. A., Kokontis, J. M., and Liao, S. (1995). Ubiquitous receptor: structures, immunocytochemical localization, and modulation of gene activation by receptors for retinoic acids and thyroid hormones. Ann. N.Y. Acad. Sci. 761, 38–49. doi: 10.1111/j.1749-6632.1995.tb31367.x
Song, H., Stevens, C. F., and Gage, F. H. (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39–44. doi: 10.1038/417039a
Sotelo, C. (1978). Purkinje cell ontogeny: formation and maintenance of spines. Prog. Brain Res. 48, 149–170. doi: 10.1016/S0079-6123(08)61021-3
Souders, M. C., Zavodny, S., Eriksen, W., Sinko, R., Connell, J., Kerns, C., et al. (2017). Sleep in children with autism spectrum disorder. Curr. Psychiatry Rep. 19:34. doi: 10.1007/s11920-017-0782-x
Sowers, L. P., Loo, L., Wu, Y., Campbell, E., Ulrich, J. D., Wu, S., et al. (2013). Disruption of the non-canonical Wnt gene PRICKLE2 leads to autism-like behaviors with evidence for hippocampal synaptic dysfunction. Mol. Psychiatry 18, 1077–1089. doi: 10.1038/mp.2013.71
Spiegelman, B. M. (1998). PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 47, 507–514. doi: 10.2337/diabetes.47.4.507
Talarico, M., de Bellescize, J., De Wachter, M., Le Guillou, X., Le Meur, G., Egloff, M., et al. (2025). RORA-neurodevelopmental disorder: a unique triad of developmental disabilities, cerebellar anomalies, and myoclonic seizures. Genet. Med. 27:101347. doi: 10.1016/j.gim.2024.101347
Tamiji, J., and Crawford, D. A. (2010). The neurobiology of lipid metabolism in autism spectrum disorders. Neurosignals 18, 98–112. doi: 10.1159/000323189
Tao, L. J., Seo, D. E., Jackson, B., Ivanova, N. B., and Santori, F. R. (2020). Nuclear hormone receptors and their ligands: metabolites in control of transcription. Cells 9:2606. doi: 10.3390/cells9122606
Tărlungeanu, D. C., Deliu, E., Dotter, C. P., Kara, M., Janiesch, P. C., Scalise, M., et al. (2016). Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell 167, 1481–1494.e18. doi: 10.1016/j.cell.2016.11.013
Tick, B., Bolton, P., Happé, F., Rutter, M., and Rijsdijk, F. (2016). Heritability of autism spectrum disorders: a meta-analysis of twin studies. J. Child Psychol. Psychiatry 57, 585–595. doi: 10.1111/jcpp.12499
Tschuck, J., Theilacker, L., Rothenaigner, I., Weiß, S. A. I., Akdogan, B., Lam, V. T., et al. (2023). Farnesoid X receptor activation by bile acids suppresses lipid peroxidation and ferroptosis. Nat. Commun. 14:6908. doi: 10.1038/s41467-023-42702-8
Umesono, K., Murakami, K. K., Thompson, C. C., and Evans, R. M. (1991). Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell 65, 1255–1266. doi: 10.1016/0092-8674(91)90020-Y
Urbano, M., Okwara, L., Manser, P., Hartmann, K., Herndon, A., and Deutsch, S. I. (2014). A trial of D-cycloserine to treat stereotypies in older adolescents and young adults with autism spectrum disorder. Clin. Neuropharmacol. 37, 69–72. doi: 10.1097/WNF.0000000000000033
Valerie, H. U. (2013). GW Researcher Discovers New Regulatory Autism Gene: Press Release, GW School of Medicine and Health Sciences, Washington. Available online at: https://smhs.gwu.edu/news/gw-researcher-discovers-new-regulatory-autism-gene (Accessed October 10, 2025).
van Elst, K., Brouwers, J. F., Merkens, J. E., Broekhoven, M. H., Birtoli, B., Helms, J. B., et al. (2019). Chronic dietary changes in n-6/n-3 polyunsaturated fatty acid ratios cause developmental delay and reduce social interest in mice. Eur. Neuropsychopharmacol. 29, 16–31. doi: 10.1016/j.euroneuro.2018.11.1106
Van Wagoner, N. J., and Benveniste, E. N. (1999). Interleukin-6 expression and regulation in astrocytes. J. Neuroimmunol. 100, 124–139. doi: 10.1016/S0165-5728(99)00187-3
Vargas, D. L., Nascimbene, C., Krishnan, C., Zimmerman, A. W., and Pardo, C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 57, 67–81. doi: 10.1002/ana.20315
Vega, R. B., and Kelly, D. P. (2017). Cardiac nuclear receptors: architects of mitochondrial structure and function. J. Clin. Invest. 127, 1155–1164. doi: 10.1172/JCI88888
Vogel, M. W., Sinclair, M., Qiu, D., and Fan, H. (2000). Purkinje cell fate in staggerer mutants: agenesis versus cell death. J. Neurobiol. 42, 323–337. doi: 10.1002/(SICI)1097-4695(20000215)42:3<323::AID-NEU4>3.0.CO;2-2
Voigt, R. G., Mellon, M. W., Katusic, S. K., Weaver, A. L., Matern, D., Mellon, B., et al. (2014). Dietary docosahexaenoic acid supplementation in children with autism. J. Pediatr. Gastroenterol. Nutr. 58, 715–722. doi: 10.1097/MPG.0000000000000260
Voineagu, I., Wang, X., Johnston, P., Lowe, J. K., Tian, Y., Horvath, S., et al. (2011). Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384. doi: 10.1038/nature10110
Volterra, A., and Meldolesi, J. (2005). Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 6, 626–640. doi: 10.1038/nrn1722
Vu-Dac, N., Gervois, P., Grötzinger, T., De Vos, P., Schoonjans, K., Fruchart, J. C., et al. (1997). Transcriptional regulation of apolipoprotein A-I gene expression by the nuclear receptor RORalpha. J. Biol. Chem. 272, 22401–22404. doi: 10.1074/jbc.272.36.22401
Wang, C., Gao, Y., Luan, C., Sun, W., Ge, S., Li, Y., et al. (2023). Zinc finger protein ZBTB17 controls cellular senescence via interacting with nuclear receptor RXRA and its downstream calcium signaling. FASEB J. 37:e23193. doi: 10.1096/fj.202301050R
Wang, R., Campbell, S., Amir, M., Mosure, S. A., Bassette, M. A., Eliason, A., et al. (2021). Genetic and pharmacological inhibition of the nuclear receptor RORα regulates TH17 driven inflammatory disorders. Nat. Commun. 12:76. doi: 10.1038/s41467-020-20385-9
Wang, Y., Billon, C., Walker, J. K., and Burris, T. P. (2016). Therapeutic effect of a synthetic RORα/γ agonist in an animal model of autism. ACS Chem. Neurosci. 7, 143–148. doi: 10.1021/acschemneuro.5b00159
Wechsler-Reya, R. J., and Scott, M. P. (1999). Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22, 103–114. doi: 10.1016/S0896-6273(00)80682-0
Wei, H., Zou, H., Sheikh, A. M., Malik, M., Dobkin, C., Brown, W. T., et al. (2011). IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J. Neuroinflammation. 8:52. doi: 10.1186/1742-2094-8-52
Weiser, M. J., Mucha, B., Denheyer, H., Atkinson, D., Schanz, N., Vassiliou, E., et al. (2016). Dietary docosahexaenoic acid alleviates autistic-like behaviors resulting from maternal immune activation in mice. Prostaglandins Leukot. Essent. Fatty Acids 106, 27–37. doi: 10.1016/j.plefa.2015.10.005
Weiss, L. A., Shen, Y., Korn, J. M., Arking, D. E., Miller, D. T., Fossdal, R., et al. (2008). Association between microdeletion and microduplication at 16p11.2 and autism. N. Engl. J. Med. 358, 667–675. doi: 10.1056/NEJMoa075974
Wells, P. G., McCallum, G. P., Chen, C. S., Henderson, J. T., Lee, C. J., Perstin, J., et al. (2009). Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol. Sci. 108, 4–18. doi: 10.1093/toxsci/kfn263
Werling, D. M. (2013). Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 26, 146–153. doi: 10.1097/WCO.0b013e32835ee548
Weuring, W., Geerligs, J., and Koeleman, B. P. C. (2021). Gene therapies for monogenic autism spectrum disorders. Genes 12:1667. doi: 10.3390/genes12111667
White, S. W., Maddox, B. B., and Mazefsky, C. A. (2020). The Oxford Handbook of Autism and Co-Occurring Psychiatric Conditions, Online Edn. Oxford Library of Psychology, Oxford Academic. doi: 10.1093/oxfordhb/9780190910761.001.0001
Wichers, R. H., Findon, J. L., Jelsma, A., Giampietro, V., Stoencheva, V., Robertson, D. M., et al. (2019). Modulation of brain activation during executive functioning in autism with citalopram. Transl. Psychiatry 9:286. doi: 10.1038/s41398-019-0641-0
Williams, K., Wray, J. A., and Wheeler, D. M. (2012). Intravenous secretin for autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2012:CD003495. doi: 10.1002/14651858.CD003495.pub3
Williams, K., Wray, J. A., and Wheeler, D. M. (2024). Pharmacological and non-pharmacological interventions for irritability in autism spectrum disorder: a systematic review and meta-analysis with the GRADE assessment. Mol. Autism 15:7. doi: 10.1186/s13229-024-00585-6
Wink, L. K., Adams, R., Wang, Z., Klaunig, J. E., Plawecki, M. H., Posey, D. J., et al. (2016). A randomized placebo-controlled pilot study of N-acetylcysteine in youth with autism spectrum disorder. Mol. Autism 7:26. doi: 10.1186/s13229-016-0088-6
Wink, L. K., Early, M., Schaefer, T., Pottenger, A., Horn, P., McDougle, C. J., et al. (2014). Body mass index change in autism spectrum disorders: comparison of treatment with risperidone and aripiprazole. J. Child Adolesc. Psychopharmacol. 24, 78–82. doi: 10.1089/cap.2013.0099
Wink, L. K., Minshawi, N. F., Shaffer, R. C., Plawecki, M. H., Posey, D. J., Horn, P. S., et al. (2017). d-Cycloserine enhances durability of social skills training in autism spectrum disorder. Mol. Autism 8:2. doi: 10.1186/s13229-017-0116-1
Wood-Downie, H., Wong, B., Kovshoff, H., Mandy, W., and Hull, L. (2021). Sex/gender differences in camouflaging in children and adolescents with autism. J. Autism Dev. Disord. 51, 1353–1364. doi: 10.1007/s10803-020-04615-z
Wu, Z., Asokan, A., and Samulski, R. J. (2006). Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 14, 316–327. doi: 10.1016/j.ymthe.2006.05.009
Xiao, L., Wang, M., Zhang, W., Song, Y., Zeng, J., Li, H., et al. (2022). Maternal diabetes-mediated RORA suppression contributes to gastrointestinal symptoms in autism-like mouse offspring. BMC Neurosci. 23:8. doi: 10.1186/s12868-022-00693-0
Ye, D., Chukwu, C., Yang, Y., Hu, Z., and Chen, H. (2024). Adeno-associated virus vector delivery to the brain: technology advancements and clinical applications. Adv. Drug Deliv. Rev. 211:115363. doi: 10.1016/j.addr.2024.115363
Yi, S. S., Hwang, I. K., Shin, J. H., Baek, S. H., Yoon, Y. S., and Seong, J. K. (2010). Neuronal differentiation and developmental characteristics in the dentate gyrus of staggerer mutant mice. BMB Rep. 43, 122–126. doi: 10.5483/BMBRep.2010.43.2.122
Yizhar, O., Fenno, L. E., Prigge, M., Schneider, F., Davidson, T. J., O'Shea, D. J., et al. (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178. doi: 10.1038/nature10360
Ylisaukko-oja, T., Rehnström, K., Auranen, M., Vanhala, R., Alen, R., Kempas, E., et al. (2005). Analysis of four neuroligin genes as candidates for autism. Eur. J. Hum. Genet. 13, 1285–1292. doi: 10.1038/sj.ejhg.5201474
Yu, H., Niu, Y., Jia, G., Liang, Y., Chen, B., Sun, R., et al. (2022). Maternal diabetes-mediated RORA suppression in mice contributes to autism-like offspring through inhibition of aromatase. Commun. Biol. 5:51. doi: 10.1038/s42003-022-03005-8
Yu, V. C., Delsert, C., Andersen, B., Holloway, J. M., Devary, O. V., Näär, A. M., et al. (1991). RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell 67, 1251–1266. doi: 10.1016/0092-8674(91)90301-E
Yue, D., Zhao, J., Chen, H., Guo, M., Chen, C., Zhou, Y., et al. (2020). MicroRNA-7, synergizes with RORα, negatively controls the pathology of brain tissue inflammation. J. Neuroinflamm. 17:28. doi: 10.1186/s12974-020-1710-2
Zaiss, A. K., and Muruve, D. A. (2005). Immune responses to adeno-associated virus vectors. Curr. Gene Ther. 5, 323–331. doi: 10.2174/1566523054065039
Zeng, J., Liang, Y., Sun, R., Huang, S., Wang, Z., Xiao, L., et al. (2022). Hematopoietic stem cell transplantation ameliorates maternal diabetes-mediated gastrointestinal symptoms and autism-like behavior in mouse offspring. Ann. N.Y. Acad. Sci. 1512, 98–113. doi: 10.1111/nyas.14766
Zhang, C., Yang, L., Wang, F., Liu, M., Liu, Z., Zou, M., et al. (2025). Therapeutic efficacy of a synthetic brain-targeted H2S donor cross-linked nanomicelle in autism spectrum disorder rats through aerobic glycolysis. ACS Appl. Mater. Interfaces 17, 157–173. doi: 10.1021/acsami.4c11663
Zhang, J. X., Zhang, J., and Li, Y. (2016). Liver X receptor-β improves autism symptoms via downregulation of β-amyloid expression in cortical neurons. Ital. J. Pediatr. 42:46. doi: 10.1186/s13052-016-0249-4
Zhang, Y., Guo, H., Deis, J. A., Mashek, M. G., Zhao, M., Ariyakumar, D., et al. (2014). Lipocalin 2 regulates brown fat activation via a nonadrenergic activation mechanism. J. Biol. Chem. 289, 22063–22077. doi: 10.1074/jbc.M114.559104
Zhang, Y., Tang, R., Hu, Z. M., Wang, X. H., Gao, X., Wang, T., et al. (2024). Key synaptic pathology in autism spectrum disorder: genetic mechanisms and recent advances. J. Integr. Neurosci. 23:184. doi: 10.31083/j.jin2310184
Zhang, Z., Burch, P. E., Cooney, A. J., Lanz, R. B., Pereira, F. A., Wu, J., et al. (2004). Genomic analysis of the nuclear receptor family: new insights into structure, regulation, and evolution from the rat genome. Genome Res. 14, 580–590. doi: 10.1101/gr.2160004
Zhao, H., Mao, X., Zhu, C., Zou, X., Peng, F., Yang, W., et al. (2022). GABAergic system dysfunction in autism spectrum disorders. Front. Cell. Dev. Biol. 9:781327. doi: 10.3389/fcell.2021.781327
Zohny, S. M., Habib, M. Z., Mohamad, M. I., Elayat, W. M., Elhossiny, R. M., El-Salam, M. F. A., et al. (2023). Memantine/aripiprazole combination alleviates cognitive dysfunction in valproic acid rat model of autism: hippocampal CREB/BDNF signaling and glutamate homeostasis. Neurotherapeutics 20, 464–483. doi: 10.1007/s13311-023-01360-w
Keywords: autism spectrum disorder, NHRs, RORA, adeno-associated viral vectors, gene therapy
Citation: Chintalapally S, Rajanala K and Upadhyay A (2025) Autism spectrum disorder and the role of nuclear hormone receptors: insights and therapeutic implications. Front. Neurosci. 19:1655348. doi: 10.3389/fnins.2025.1655348
Received: 03 July 2025; Accepted: 01 October 2025;
Published: 30 October 2025.
Edited by:
Kazuhiko Sawada, Tsukuba International University, JapanCopyright © 2025 Chintalapally, Rajanala and Upadhyay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kalpana Rajanala, a2FscGFuYS5yYWphbmFsYUBvY3VnZW4uY29t; Arun Upadhyay, YXJ1bkBvY3VnZW4uY29t
 Shivakanth Chintalapally
Shivakanth Chintalapally Kalpana Rajanala
Kalpana Rajanala Arun Upadhyay
Arun Upadhyay