- Centre for Cognition and Decision Making, Institute for Cognitive Neuroscience, HSE University, Moscow, Russia
Introduction: Understanding the neural mechanisms underlying decision-making under uncertainty represents a fundamental challenge in cognitive neuroscience. This meta-analysis aimed to identify the consistent neural correlates of uncertainty processing specifically during decision-making tasks.
Methods: We synthesized findings from 76 fMRI studies (N = 4,186 participants). Using the Activation Likelihood Estimation (ALE) method, we performed a voxel-wise meta-analysis of activation foci to identify brain regions consistently activated across studies.
Results: The analysis revealed nine distinct activation clusters, revealing a comprehensive neural network involved in uncertainty processing. Key findings demonstrated predominant activations in the anterior insula (up to 63.7% representation), inferior frontal gyrus (up to 40.7%), and inferior parietal lobule (up to 78.1%). We found a functional specialization between emotional-motivational processes (clusters 1–5) and cognitive processes (clusters 6–9), with notable hemispheric asymmetries. The left anterior insula was more strongly associated with reward evaluation, while the right was involved in learning and cognitive control. Similarly, the right inferior frontal gyrus was linked to impulse control, and the left to motor planning.
Discussion: Our findings extend the current understanding of the neural architecture of decision-making under uncertainty. The comprehensive mapping of neural signatures advances our knowledge of the distinct roles of key brain regions and provides insights into potential clinical applications, particularly for developing interventions for uncertainty-related anxiety. The study highlights important directions for future research in cognitive neuroscience and clinical practice.
Introduction
Under uncertainty, making decisions is considered as a primary cognitive process which influences how we behave as humans and allow us navigate through unpredictable environment by estimating risks and reward. This capability solely relies on a network of neural activities at certain brain regions, including the dorsolateral prefrontal cortex (dlPFC), anterior insula, and the anterior cingulate cortex (ACC), which are considered essential in integrating sensory information, appraising risk, and modulating emotional responses, crucial for decision-making across different contexts (Morriss et al., 2019) from simple, everyday decisions to complex, high-stake problem despite the lack of complete information (Craig, 2009; Shenhav et al., 2013). These regions of the brain are constantly in the investigation of uncertain processes in neuroimaging research for fields in neuroeconomics, psychology and behavioral neuroscience (Bechara et al., 2000; Kahneman and Tversky, 2013).
Morriss et al. (2019), through a comprehensive coordinate-based meta-analysis, provided foundational insights into the neural circuitry involved in general uncertainty processing. Their findings emphasized the anterior cingulate cortex (ACC) and anterior insula as integrative hubs for cognitive and emotional signals during uncertainty. However, a critical limitation they acknowledged was the broad scope of uncertainty types examined, without isolating those specifically linked to decision-making processes. The present meta-analysis addresses this gap by focusing exclusively on decision-related uncertainty to identify brain regions consistently recruited when individuals evaluate incomplete or ambiguous information in the context of choice. Unlike previous meta-analyses that examined neural correlates of uncertainty across broad contexts (e.g., Morriss et al., 2019), the present study specifically focuses on active decision-making paradigms to identify the core neural substrates of choice under conditions of incomplete information.
Our review contributes to the field with the updated framework based on data from recently published neuroimaging studies by Spohrs et al. (2021) and Savage et al. (2020) illustrating the pivotal roles of the ACC and the anterior insula in tuning emotional responses amid decision-making and Gorka et al. (2015), highlighting the association of subcortical structures like the amygdala in decision making processes that are based on reward under uncertainty, to provide an updated outline of the neural mechanisms activated in the presence of incomplete information and when outcomes are unpredictable (Lissek et al., 2014; Gorka et al., 2016; Visalli et al., 2019). For example, Canessa et al. (2013) discovered that the disparities in decision-making approach when faced with uncertainty are corresponding to contrasting activation patterns in the ACC and the anterior insula. By merging these findings, this paper explores to augment our comprehension of the neural mechanisms of facilitating decision making processed in uncertain situations. This updated composite of neural signatures will propel the field forward by elucidating the distinct roles of the key brain regions activated during decision making processes under uncertainty, underlining future research directions.
To contextualize the expected findings, the cascade model of prefrontal executive function (Koechlin et al., 2003) offers a compelling theoretical framework. This model proposes that the prefrontal cortex supports hierarchical and parallel control processes during decision-making. Medial structures—such as the dorsal anterior cingulate cortex (dACC)—evaluate ongoing strategy reliability, while lateral prefrontal regions, including frontopolar cortex, support the generation and maintenance of alternative strategies. Applying this model to uncertainty may help clarify how the brain dynamically integrates emotional evaluation and cognitive control to optimize decisions in unpredictable environments.
This meta-analysis systematically maps the current landscape of fMRI research on decision-making under uncertainty through Activation Likelihood Estimation (ALE). While advanced methods like meta-analytic connectivity modeling (MACM) or subgroup analyses could offer deeper mechanistic insights, our primary objective is to establish a foundational synthesis of reported neural activations across studies. By focusing on spatial concordance rather than connectivity or subpopulation effects, we provide a comprehensive reference for: (1) identifying consistently reported activation patterns, (2) highlighting methodological and conceptual trends in the literature, and (3) guiding future hypothesis-driven investigations with more complex analytical approaches.
Materials and methods
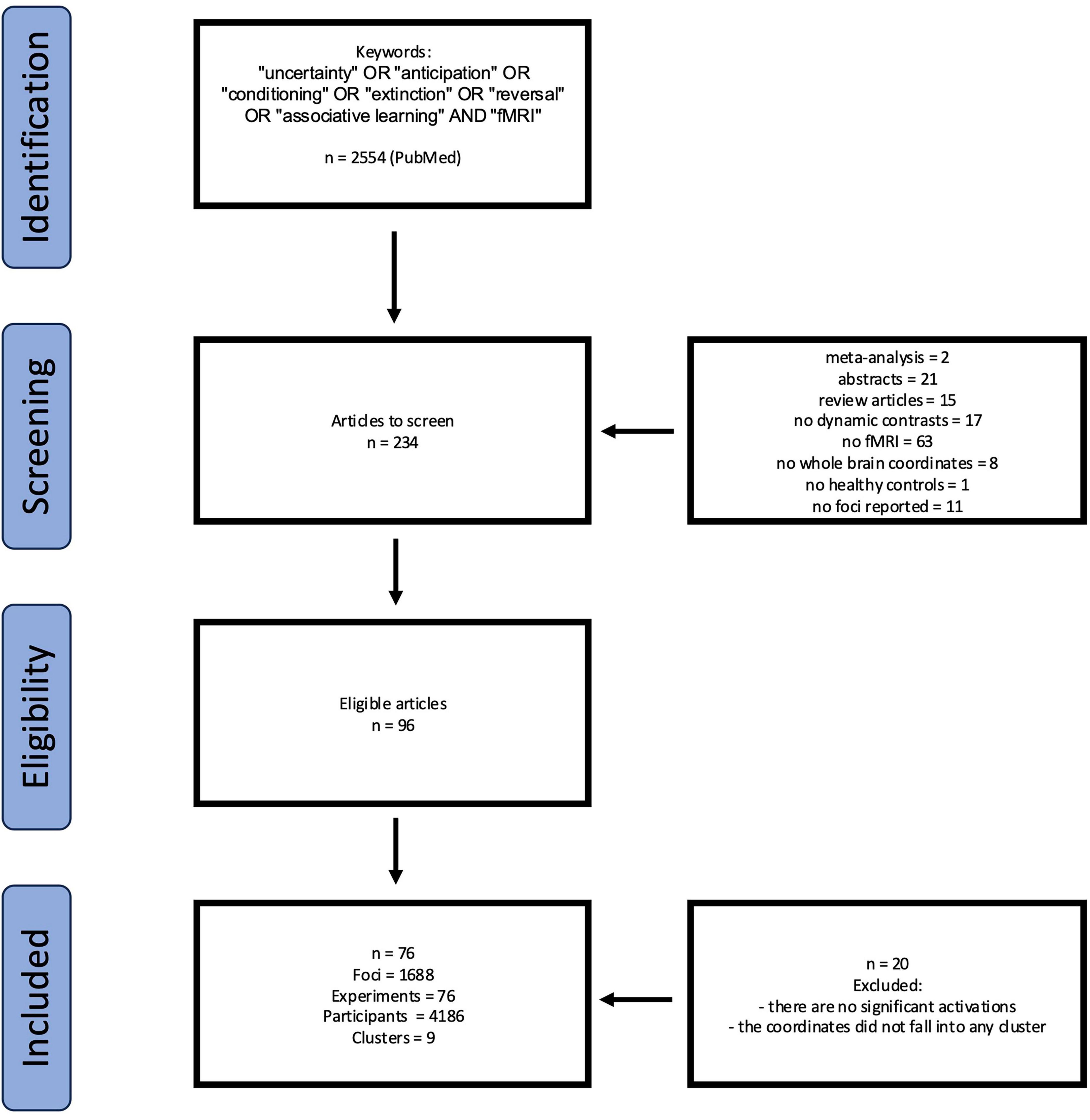
Literature search
The initial literature search was conducted in July 2024, with the additional search in January 2025 to account for newly published studies. The literature search was conducted using PubMed database. Articles were selected using the following keywords: “uncertainty,” “anticipation,” “conditioning,” “extinction,” “reversal,” “fMRI” and “associative learning,” years 1995–2024. Initially, 2,554 articles were identified. Following preliminary screening for duplicates and required data (fMRI data and decision-making paradigms under uncertainty), 234 articles were selected for further analysis and subsequent clustering resulting in 76 eligible studies with the data collected on healthy adults, random-effects model. PRISMA chart represents the stages of data collection and selection (Figure 1). Studies were included if they: (1) used fMRI; (2) involved healthy adult participants; (3) employed a task explicitly involving active decision-making under conditions of uncertainty (e.g., risk, ambiguity, unpredictable threat, reversal learning); (4) reported whole-brain coordinates of significant activation contrasts related to uncertainty processing. Studies were excluded if they focused solely on perceptual uncertainty without a decision component or studied exclusively clinical populations.
ALE approach
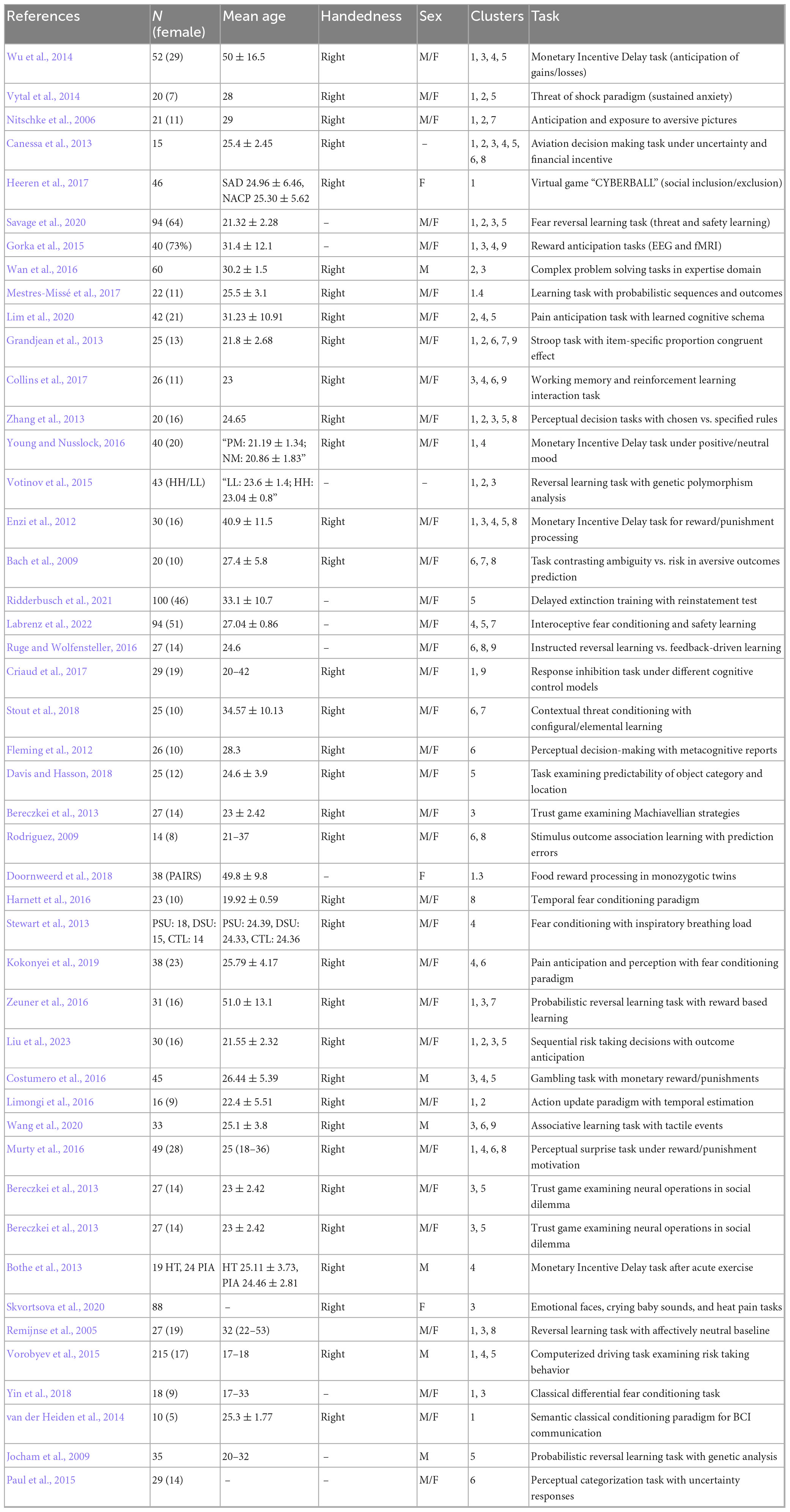
This study employed the Activation Likelihood Estimation (ALE) method, which enables voxel-wise analysis of images with random effects. GingerALE 3.0.2 was utilized in the current investigation. The software allows the use of coordinates to create a probabilistic activation map using ALE values. These values provide information about statistical maps of probable activation in relation to the tasks included in the analysis. For data consistency, coordinates presented in Montreal Neurological Institute (MNI) format were converted to Talairach space. Statistical significance was assessed using cluster-level correction for multiple comparisons at p = 0.05 and a cluster-forming threshold of p < 0.001 (Eickhoff et al., 2012, 2017). The following are the articles included in the final list of publications included in the results of the analysis (Table 1).
Results
Based on the meta-analysis, which included 1,688 activation foci from 76 experiments with a total of 4,186 participants, key brain structures involved in decision-making processes were identified. The results demonstrate that decision-making engages an extensive neural network encompassing both cortical and subcortical structures in both hemispheres of the brain.
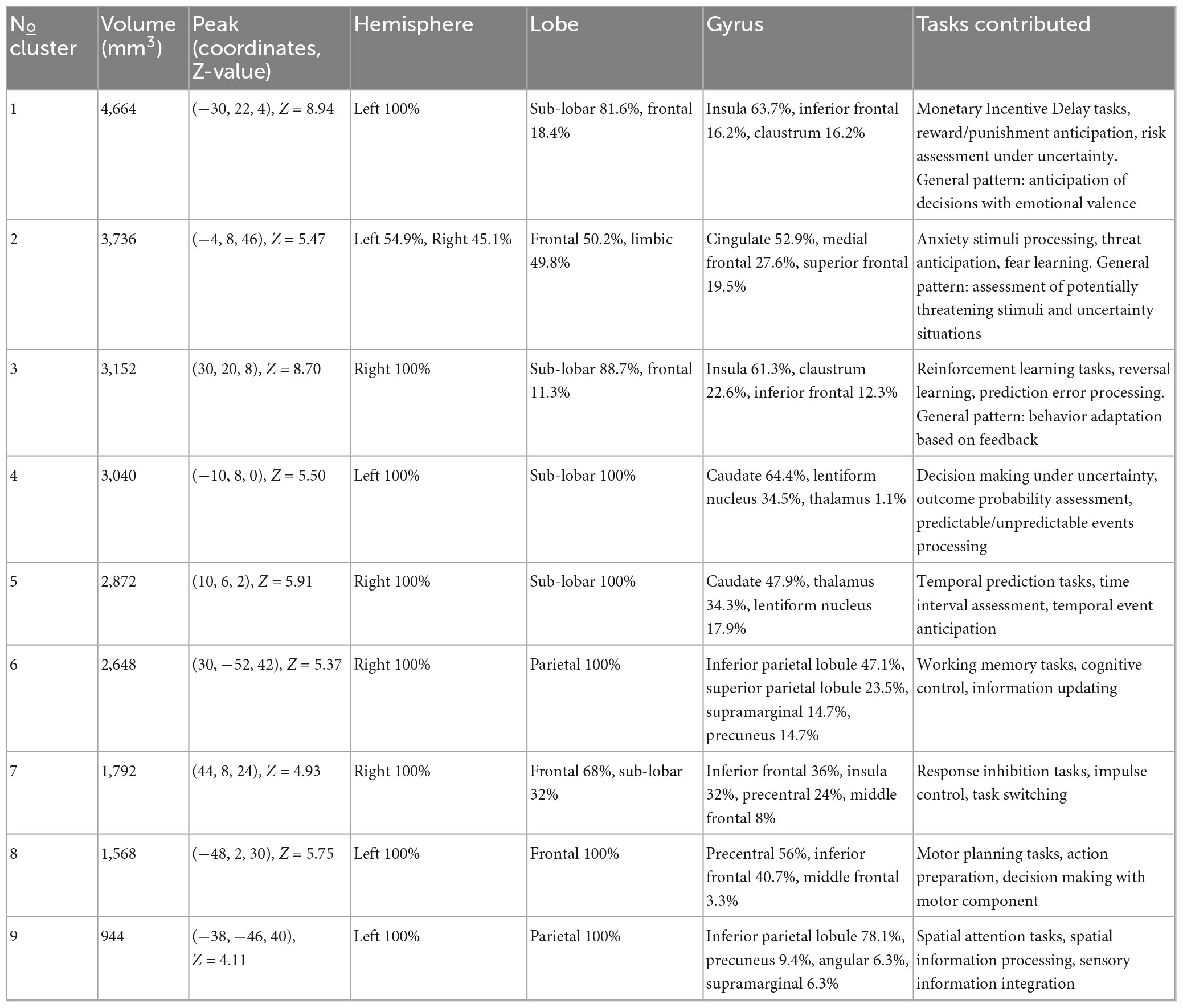
We identified 9 statistically significant activation clusters (Table 2). Figure 2 shows a brain activation map. The image was obtained using the Mango program v.4.1.
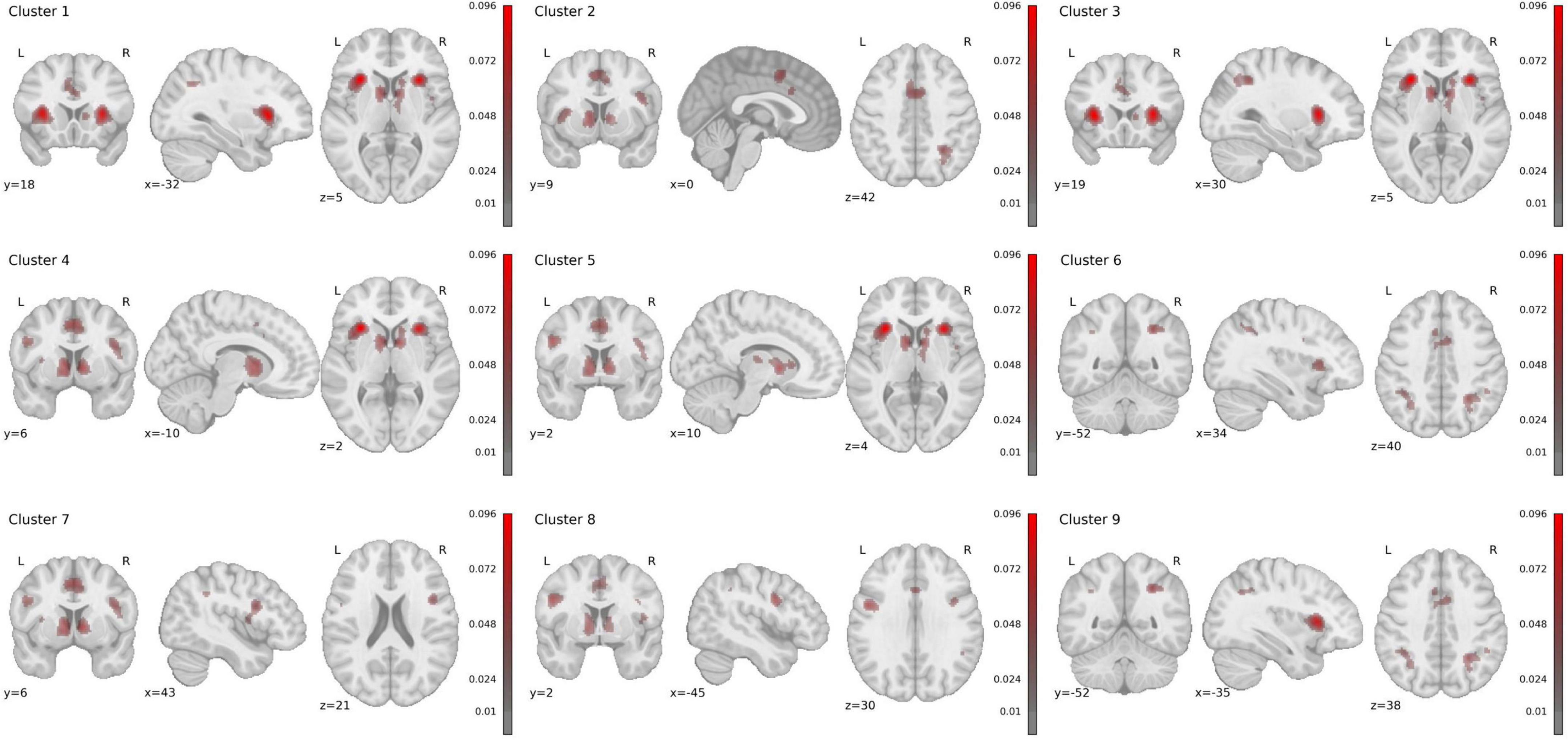
Figure 2. Rendered ALE maps showing significant concordance across studies for uncertainty processing.
Cluster 1 has a volume of 4,664 mm3 and is entirely located in the left hemisphere. The majority (81.6%) consists of subcortical structures, while the remaining 18.4% is in the prefrontal cortex. The anterior insula has the largest representation at 63.7%. Additional activations are present in the inferior frontal gyrus (16.2%) and claustrum (16.2%). The activations correspond to Brodmann areas 13 (59.8%), 47 (10.6%), and 45 (3.9%). Studies in this cluster employed paradigms involving monetary reward and punishment (Monetary Incentive Delay tasks), anticipation of gains and losses, and risk assessment under uncertainty. General pattern: anticipation of decision outcomes with emotional valence.
Cluster 2 has a volume of 3,736 mm3 with predominant activation in the left hemisphere (54.9% left, 45.1% right). Activation topography is distributed between the frontal region (50.2%) and limbic system (49.8%). Maximum activity was recorded in the cingulate gyrus (52.9%), medial frontal gyrus (27.6%), and superior frontal gyrus (19.5%). Activations correspond to Brodmann areas 32 (43.6%), 6 (33.5%), and 24 (21.8%). Studies included tasks involving anxiety-provoking stimuli processing, threat anticipation, and fear learning. General pattern: assessment of potentially threatening stimuli and uncertainty situations.
Cluster 3, with a volume of 3,152 mm3, is localized in the right hemisphere. It predominantly includes subcortical structures (88.7%) and partially frontal cortex (11.3%). Dominant activity is observed in the anterior insula (61.3%), claustrum (22.6%), and inferior frontal gyrus (12.3%), with inclusion of the lentiform nucleus (2.8%). Activations correspond to Brodmann areas 13 (53.8%), 47 (11.3%), and 45 (2.8%). Studies included reinforcement learning tasks, reversal learning, and prediction error processing. General pattern: behavior adaptation based on feedback. While Clusters 1 and 3 both involve the anterior insula and inferior frontal gyrus, they demonstrate clear hemispheric specialization and distinct anatomical profiles. Cluster 1 (left) shows additional activation in the claustrum, while Cluster 3 (right) includes the lentiform nucleus. Functionally, they are dissociable: Cluster 1 is predominantly associated with motivational anticipation, while Cluster 3 is linked to behavioral adaptation. This pattern suggests complementary rather than identical roles in uncertainty processing.
Cluster 4, with a volume of 3,040 mm3, is entirely localized in the left hemisphere within subcortical structures (100%). Primary activations are in the caudate (64.4%), lentiform nucleus (34.5%), and thalamus (1.1%). Detailed analysis revealed activations in the caudate head (34.5%), caudate body (30%), and putamen (12.7%). Studies included decision-making tasks under uncertainty, outcome probability assessment, and processing of predictable and unpredictable events. General pattern: probabilistic information processing.
Cluster 5, with a volume of 2,872 mm3, is entirely located in the right hemisphere within subcortical structures (100%). It includes the caudate (47.9%), thalamus (34.3%), and lentiform nucleus (17.9%). Detailed analysis showed activations in the caudate head (26.1%), caudate body (21.8%), and medial dorsal Nucleus (8.9%). Studies included temporal prediction tasks, temporal interval estimation, and temporal event anticipation. General pattern: temporal information processing and prediction. Clusters 4 and 5, while both involving basal ganglia structures, exhibit different anatomical distributions and functional specializations. Cluster 4 (left) is predominantly located in the caudate nucleus, while Cluster 5 (right) shows greater thalamic involvement. Correspondingly, Cluster 4 is associated with probabilistic information processing, whereas Cluster 5 is linked to temporal prediction. These findings indicate distinct subcortical contributions to different dimensions of uncertainty.
Cluster 6, with a volume of 2,648 mm3, is localized in the right hemisphere within the parietal region (100%). Primary activations are in the inferior parietal lobule (47.1%), superior parietal lobule (23.5%), and supramarginal gyrus (14.7%). It corresponds to Brodmann areas 40 (54.4%), 7 (36.8%), and 19 (8.8%). Studies included working memory tasks, cognitive control, and information updating. General pattern: maintenance and manipulation of information in working memory.
Cluster 7, with a volume of 1,792 mm3, is localized in the right hemisphere. It includes frontal cortex regions (68%) and subcortical structures (32%). Primary activations are in the inferior frontal gyrus (36%), anterior insula (32%), and precentral gyrus (24%). It corresponds to Brodmann areas 9 (44%), 13 (32%), 44 (12%), and 6 (12%). Studies included response inhibition tasks, impulse control, and task switching. General pattern: cognitive control and inhibition.
Cluster 8, with a volume of 1,568 mm3, is located in the left hemisphere within the frontal cortex (100%). It includes the precentral Gyrus (56%) and inferior frontal gyrus (40.7%). Activations correspond to Brodmann areas 6 (61.5%) and 9 (38.5%). Studies included motor planning tasks, action preparation, and decision-making with motor components. General pattern: motor planning and execution. Clusters 7 and 8 demonstrate clear hemispheric and anatomical differentiation. Cluster 7 (right) encompasses the inferior frontal gyrus and anterior insula, regions central to inhibitory control. In contrast, Cluster 8 (left) is centered on the precentral gyrus, supporting motor planning functions. This dissociation aligns with established models of lateralized frontal cortex organization, where right-sided regions specialize in control processes and left-sided regions in action implementation.
Cluster 9, with a volume of 944 mm3, is localized in the left hemisphere within the parietal region (100%). Dominant activations are in the inferior parietal lobule (78.1%) and precuneus (9.4%). It corresponds to Brodmann areas 40 (81.3%), 39 (12.5%), and 19 (6.3%). Studies included spatial attention tasks, spatial information processing, and sensory information integration. General pattern: spatial processing and attention.
Discussion
Our meta-analysis of 76 fMRI studies (N = 4,186) reveals that decision-making under uncertainty engages a distributed network of nine cortical and subcortical clusters. The most consistent activations occur in the anterior insula (up to 63.7% representation), inferior frontal gyrus (up to 40.7%), and inferior parietal lobule (up to 78.1%), with distinct functional specialization between emotional-motivational processes (clusters 1–5) and cognitive processes (clusters 6–9). These findings align with and extend previous meta-analyses by Morriss et al. (2019) and Feng et al. (2022), who similarly identified anterior insula and anterior cingulate cortex as core hubs for uncertainty processing. Our results provide novel insights into hemispheric specialization, with left anterior insula’s involvement in reward evaluation and right anterior insula’s engagement in learning and cognitive control.
The observed functional dissociation between ventral emotional-motivational networks and dorsal cognitive-control systems aligns with hierarchical models of prefrontal cortex organization–the cascade model of cognitive control (Koechlin et al., 2003; Collins and Koechlin, 2012; Donoso et al., 2014) which posits two coordinated tracks within the prefrontal cortex: a medial track, including the vmPFC and dACC, responsible for evaluating the reliability of ongoing behavioral strategies, and a lateral track, encompassing frontopolar and lateral PFC regions, which facilitates the exploration and implementation of alternative strategies. This model offers a structured account of how the brain manages uncertainty by balancing stability and flexibility in decision-making (Vassena et al., 2014).
While the Cascade Model provides a compelling framework for our findings, other prominent models offer complementary perspectives. For instance, the expected value of control theory (Shenhav et al., 2013) conceptualizes the anterior cingulate cortex as a region computing the value of deploying cognitive control, which aligns with the role of dACC (Cluster 2) in monitoring strategy reliability under uncertainty. Similarly, the Salience Network perspective (Seeley et al., 2007)—comprising the anterior insula and dACC—emphasizes the detection and integration of salient stimuli to guide behavior, a process central to decision-making under uncertainty. Our findings of consistent co-activations in these regions strongly support this network view. The cascade model was chosen for its specific focus on hierarchical control organization, which effectively parses the functional dissociation between medial (evaluative) and lateral (cognitive control) systems observed in our clusters.
Beyond the hierarchical organization within the PFC, which is described by the Cascade Model, our findings can be fruitfully interpreted through the lens of large-scale brain networks. The consistent co-activation of the anterior insula and dorsal anterior cingulate cortex (dACC) (Clusters 1, 2, 3) aligns them with the core of the Salience Network (SN), which is dedicated to detecting and integrating behaviorally relevant stimuli (Seeley et al., 2007). Concurrently, activations in the dorsolateral prefrontal cortex (dlPFC) and inferior parietal lobule (IPL) (Clusters 6, 7, 8, 9) correspond to the Central Executive Network (CEN), which subserves working memory, cognitive control, and goal-directed attention (Vincent et al., 2008; Worthy et al., 2016).
The Triple Network Model (Menon, 2011) posits that the SN plays a crucial role in dynamic switching between the CEN and the Default Mode Network (DMN). We propose that uncertainty represents a potent salient signal that engages the SN. This engagement may facilitate the attenuation of DMN activity (associated with internal mentation) and the subsequent recruitment of the CEN to implement cognitive control strategies necessary for decision-making in unpredictable environments. Thus, the functional dissociation observed in our ALE maps likely reflects the coordinated yet distinct contributions of the SN (for appraisal and alerting) and the CEN (for implementation and control) during uncertainty processing. This network perspective complements the cascade model by describing the broader architectural context in which these medial and lateral PFC systems are embedded.
Medial track engagement is supported by the activation pattern observed in Cluster 2, which includes the dorsal ACC and medial frontal gyrus—regions implicated in conflict detection and appraisal of strategy failure. This mirrors the cascade model’s conceptualization of dACC as a monitor of strategy reliability, particularly during threat anticipation and emotionally salient uncertainty. These findings also align with Morriss et al.’s (2019) finding that ACC integrates cognitive and emotional information during uncertainty.
Lateral track involvement is evident in right-lateralized clusters (e.g., Clusters 3, 6, and 7), encompassing the inferior frontal gyrus and inferior parietal lobule. These areas are involved in response inhibition, working memory, and adaptive control—functions associated with evaluating and switching between alternative strategies, as outlined in the cascade model’s lateral system. The inferior parietal lobule activations (Cluster 6) during working memory tasks further reflect the cascade model’s proposed monitoring of the counterfactual strategy. These mappings not only support the cascade model’s architecture but also underscore how emotional and cognitive circuits are dynamically recruited in parallel to guide behavior under uncertainty.
The bilateral activation patterns reveal complementary processing streams. Left anterior insula’s dominance in reward evaluation (Cluster 1) corresponds to the cascade model’s actor reliability monitoring, while right anterior insula’s involvement in learning (Cluster 3) reflects hypothesis testing for alternative strategies. Similarly, the lateralization in inferior frontal gyrus–right-sided for impulse control (Cluster 7) and left-sided for motor planning (Cluster 8)–suggests parallel uncertainty resolution mechanisms. These findings extend Feng et al.’s (2022) network approach by demonstrating how hemispheric specialization facilitates concurrent strategy evaluation. The prominence of anxiety-related activations in Cluster 2 (cingulate gyrus, medial frontal gyrus) suggests that uncertainty processing abnormalities may stem from disrupted reliability monitoring (Stout et al., 2019). As Morriss et al. (2021) noted, individuals with high intolerance of uncertainty show altered prefrontal activation patterns. Our Cluster 2 findings provide specific neural targets for interventions, particularly for conditions like generalized anxiety disorder where dorsal ACC hyperactivity during threat appraisal may reflect maladaptive hypothesis generation (Buff et al., 2016; Klass et al., 2021; Spohrs et al., 2022).
While our search strategy prioritized terms that allowed for direct comparison with prior meta-analytic work (Morriss et al., 2019), future studies might benefit from incorporating additional keywords such as “cognitive flexibility” and “exploration-exploitation” to capture an even broader spectrum of decision-making under uncertainty. Furthermore, while ALE approach establishes spatial convergence, it cannot assess dynamic interactions central to the cascade model. Future research should employ dynamic causal modeling to test information flow between medial and lateral prefrontal pathways and examine how individual differences in metacognitive ability (Fleming et al., 2012; Jiang et al., 2022) modulate these networks. The convergence between our meta-analytic findings and the cascade model suggests promising avenues for theoretical integration with explicitly test model predictions about dACC-triggered exploration and frontopolar-mediated strategy maintenance using paradigm-driven fMRI designs. Such work would bridge meta-analytic patterns with computational accounts of prefrontal function.
Several limitations of the ALE methodology should be considered. First, ALE relies on reported peak coordinates, which may introduce a bias toward statistically strong foci. Second, the spatial smoothing inherent to the method can inflate the apparent overlap between activations. Third, ALE identifies consistency, not magnitude, of activation across studies. Finally, our approach assumes a degree of homogeneity across studies in design and analysis, which is an inherent challenge in meta-analysis.
It is noteworthy that our meta-analysis did not identify significant convergence in the cerebellum, despite its established role in various cognitive functions. This is likely attributable to the specific nature of the included paradigms. Our analysis focused on value-based and emotional-motivational appraisal under uncertainty, which engages a cortico-striato-thalamic network. In contrast, the cerebellum’s contribution may be more critical for processing uncertainty in contexts that require explicit probabilistic reasoning and the construction of internal models to guide predictions, as demonstrated in tasks of inductive inference (Blackwood et al., 2004). Furthermore, its role is well-established in domains requiring sensorimotor prediction and adaptive behavioral updating (Blakemore et al., 2001; Kobayashi and Hsu, 2017; Johnson et al., 2019). The paradigms in our final sample were not optimized to capture this specific computational demand of the cerebellum. Future research employing paradigms that directly contrast these different forms of uncertainty could help delineate the distinct and shared neural systems involved.
Conclusion
Current study provides significant insights into the neural mechanisms underlying decision-making under uncertainty. By identifying nine distinct activation clusters, we highlighted the critical roles of the anterior insula, inferior frontal Gyrus, and inferior parietal lobule in uncertainty processing. Our findings confirm previous research regarding the anterior insula’s prominence in reward evaluation and emotional regulation, while also revealing functional specialization within the identified clusters. The observed bilateral activation patterns underscore the complexity of neural networks involved in decision-making, with distinct roles attributed to each hemisphere.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AT: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Investigation, Methodology. SA: Data curation, Formal analysis, Investigation, Writing – original draft. OZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare financial support was received for the research and/or publication of this article. This article was an output of a research project implemented as part of the Basic Research Program at the National Research University Higher School of Economics (HSE University).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bach, D., Seymour, B., and Dolan, R. (2009). Neural activity associated with the passive prediction of ambiguity and risk for aversive events. J. Neurosci. 29, 1648–1656. doi: 10.1523/JNEUROSCI.4578-08.2009
Bechara, A., Damasio, H., and Damasio, A. (2000). Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex 10, 295–307. doi: 10.1093/cercor/10.3.295
Bereczkei, T., Deak, A., Papp, P., Perlaki, G., and Orsi, G. (2013). Neural correlates of Machiavellian strategies in a social dilemma task. Brain Cogn. 82, 108–116. doi: 10.1016/j.bandc.2013.02.012
Blackwood, N., Simmons, A., Bentall, R., Murray, R., and Howard, R. (2004). The cerebellum and decision making under uncertainty. Cogn. Brain Res. 20, 46–53. doi: 10.1016/j.cogbrainres.2003.12.009
Blakemore, S. J., Frith, C. D., and Wolpert, D. M. (2001). The cerebellum is involved in predicting the sensory consequences of action. Neuroreport 12, 1879–1884. doi: 10.1097/00001756-200107030-00023
Bothe, N., Zschucke, E., Dimeo, F., Heinz, A., Wüstenberg, T., and Ströhle, A. (2013). Acute exercise influences reward processing in highly trained and untrained men. Med. Sci. Sports Exerc. 45, 583–591. doi: 10.1249/MSS.0b013e318275306f
Buff, C., Brinkmann, L., Neumeister, P., Feldker, K., Heitmann, C., Gathmann, B., et al. (2016). Specifically altered brain responses to threat in generalized anxiety disorder relative to social anxiety disorder and panic disorder. NeuroImage Clin. 12, 698–706. doi: 10.1016/j.nicl.2016.09.023
Canessa, N., Crespi, C., Motterlini, M., Baud-Bovy, G., Chierchia, G., Pantaleo, G., et al. (2013). The functional and structural neural basis of individual differences in loss aversion. J. Neurosci. 33, 14307–14317. doi: 10.1523/JNEUROSCI.0497-13.2013
Collins, A., and Koechlin, E. (2012). Reasoning, learning, and creativity: frontal lobe function and human decision-making. PLoS Biol. 10:e1001293. doi: 10.1371/journal.pbio.1001293
Collins, A., Ciullo, B., Frank, M., and Badre, D. (2017). Working memory load strengthens reward prediction errors. J. Neurosci. 37, 4332–4342. doi: 10.1523/JNEUROSCI.2700-16.2017
Costumero, V., Barrós-Loscertales, A., Fuentes, P., Rosell-Negre, P., Bustamante, J., and Ávila, C. B. A. S. - (2016). drive trait modulates dorsomedial striatum activity during reward response-outcome associations. Brain Imaging Behav. 10, 869–879. doi: 10.1007/s11682-015-9466-5
Craig, A. (2009). How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. doi: 10.1038/nrn2555
Criaud, M., Longcamp, M., Anton, J., Nazarian, B., Roth, M., Sescousse, G., et al. (2017). Testing the physiological plausibility of conflicting psychological models of response inhibition: a forward inference fMRI study. Behav. Brain Res. 333, 192–202. doi: 10.1016/j.bbr.2017.06.030
Davis, B., and Hasson, U. (2018). Predictability of what or where reduces brain activity, but a bottleneck occurs when both are predictable. NeuroImage 167, 224–236. doi: 10.1016/j.neuroimage.2016.06.001
Donoso, M., Collins, A., and Koechlin, E. (2014). Foundations of human reasoning in the prefrontal cortex. Science 344, 1481–1486. doi: 10.1126/science.1252254
Doornweerd, S., De Geus, E., Barkhof, F., Van Bloemendaal, L., Boomsma, D., Van Dongen, J., et al. (2018). Brain reward responses to food stimuli among female monozygotic twins discordant for BMI. Brain Imaging Behav. 12, 718–727. doi: 10.1007/s11682-017-9711-1
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., and Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017
Eickhoff, S. B., Laird, A. R., Fox, P. M., Lancaster, J. L., and Fox, P. T. (2017). Implementation errors in the GingerALE Software: Description and recommendations. Hum. Brain Mapp. 38, 7–11. doi: 10.1002/hbm.23342
Enzi, B., Edel, M., Lissek, S., Peters, S., Hoffmann, R., Nicolas, V., et al. (2012). Altered ventral striatal activation during reward and punishment processing in premanifest Huntington’s disease: a functional magnetic resonance study. Exp. Neurol. 235, 256–264. doi: 10.1016/j.expneurol.2012.02.003
Feng, S., Zhang, M., Peng, Y., Yang, S., Wang, Y., Wu, X., et al. (2022). Brain networks under uncertainty: a coordinate-based meta-analysis of brain imaging studies. J. Affect. Disord. 319, 627–637. doi: 10.1016/j.jad.2022.09.099
Fleming, S., Huijgen, J., and Dolan, R. (2012). Prefrontal contributions to metacognition in perceptual decision making. J. Neurosci. 32, 6117–6125. doi: 10.1523/JNEUROSCI.6489-11.2012
Gorka, S., Nelson, B., Phan, K., and Shankman, S. (2016). Intolerance of uncertainty and insula activation during uncertain reward. Cogn. Affect. Behav. Neurosci. 16, 929–939. doi: 10.3758/s13415-016-0443-2
Gorka, S., Phan, K., and Shankman, S. (2015). Convergence of EEG and fMRI measures of reward anticipation. Biol. Psychol. 112, 12–19. doi: 10.1016/j.biopsycho.2015.09.007
Grandjean, J., d’Ostilio, K., Fias, W., Phillips, C., Balteau, E., Degueldre, C., et al. (2013). Exploration of the mechanisms underlying the ISPC effect: Evidence from behavioral and neuroimaging data. Neuropsychologia 51, 1040–1049. doi: 10.1016/j.neuropsychologia.2013.02.015
Harnett, N., Shumen, J., Wagle, P., Wood, K., Wheelock, M., Baños, J., et al. (2016). Neural mechanisms of human temporal fear conditioning. Neurobiol. Learn. Mem. 136, 97–104. doi: 10.1016/j.nlm.2016.09.019
Heeren, A., Dricot, L., Billieux, J., Philippot, P., Grynberg, D., De Timary, P., et al. (2017). Correlates of social exclusion in social anxiety disorder: an fMRI study. Sci. Rep. 7:260. doi: 10.1038/s41598-017-00310-9
Jiang, S., Wang, S., and Wan, X. (2022). Metacognition and mentalizing are associated with distinct neural representations of decision uncertainty. PLoS Biol. 20:e3001301. doi: 10.1371/journal.pbio.3001301
Jocham, G., Klein, T., Neumann, J., von Cramon, D., Reuter, M., and Ullsperger, M. (2009). Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J. Neurosci. 29, 3695–3704. doi: 10.1523/JNEUROSCI.5195-08.2009
Johnson, J. F., Belyk, M., Schwartze, M., Pinheiro, A. P., and Kotz, S. A. (2019). The role of the cerebellum in adaptation: ALE meta-analyses on sensory feedback error. Hum. Brain Mapp. 40, 3966–3981. doi: 10.1002/hbm.24681
Kahneman, D., and Tversky, A. (2013). “Prospect theory: an analysis of decision under risk,” in In: Handbook of the Fundamentals of Financial Decision Making, eds LC Maclean and WT Ziemba, (London: World Scientific). doi: 10.1142/9789814417358_0006
Klass, A., Otto, T., Tegenthoff, M., and Lissek, S. (2021). The DA-antagonist Tiapride affects context-related extinction learning in a predictive learning task, but not initial forming of associations, or renewal. Neurobiol. Learn. Mem. 183:107465. doi: 10.1016/j.nlm.2021.107465
Kobayashi, K., and Hsu, M. (2017). Neural mechanisms of updating under reducible and irreducible uncertainty. J. Neurosci. 37, 6972–6982. doi: 10.1523/JNEUROSCI.0535-17.2017
Koechlin, E., Ody, C., and Kouneiher, F. (2003). The architecture of cognitive control in the human prefrontal cortex. Science 302, 1181–1185. doi: 10.1126/science.1088545
Kokonyei, G., Galambos, A., Edes, A., Kocsel, N., Szabo, E., Pap, D., et al. (2019). Anticipation and violated expectation of pain are influenced by trait rumination: An fMRI study. Cogn. Affect. Behav. Neurosci. 19, 56–72. doi: 10.3758/s13415-018-0644-y
Labrenz, F., Spisak, T., Ernst, T., Gomes, C., Quick, H., Axmacher, N., et al. (2022). Temporal dynamics of fMRI signal changes during conditioned interoceptive pain-related fear and safety acquisition and extinction. Behav. Brain Res. 427:113868. doi: 10.1016/j.bbr.2022.113868
Lim, M., O’Grady, C., Cane, D., Goyal, A., Lynch, M., Beyea, S., et al. (2020). Threat prediction from schemas as a source of bias in pain perception. J. Neurosci. 40, 1538–1548. doi: 10.1523/jneurosci.2104-19.2019
Limongi, R., Pérez, F., Modroño, C., and González-Mora, J. (2016). Temporal uncertainty and temporal estimation errors affect insular activity and the frontostriatal indirect pathway during action update: A predictive coding study. Front. Hum. Neurosci. 10:276. doi: 10.3389/fnhum.2016.00276
Lissek, S., Bradford, D., Alvarez, R., Burton, P., Espensen-Sturges, T., Reynolds, R., et al. (2014). Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc. Cogn. Affect. Neurosci. 9, 1134–1142. doi: 10.1093/scan/nst096
Liu, S., Li, S., Jiang, H., Zhang, Z., Gong, Y., Guo, X., et al. (2023). The neural correlates underlying the regulation of anticipation on regret. Behav. Brain Res. 436:114075. doi: 10.1016/j.bbr.2022.114075
Menon, V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506. doi: 10.1016/j.tics.2011.08.003
Mestres-Missé, A., Trampel, R., Turner, R., and Kotz, S. (2017). Uncertainty and expectancy deviations require cortico-subcortical cooperation. NeuroImage 144, 23–34. doi: 10.1016/j.neuroimage.2016.05.069
Morriss, J., Bell, T., Biagi, N., Johnstone, T., and Van Reekum, C. (2021). Intolerance of uncertainty is associated with heightened responding in the prefrontal cortex during cue-signalled uncertainty of threat. Cogn. Affect. Behav. Neurosci. 21, 269–283. doi: 10.3758/s13415-021-00932-7
Morriss, J., Gell, M., and van Reekum, C. (2019). The uncertain brain: A co-ordinate based meta-analysis of the neural signatures supporting uncertainty during different contexts. Neurosci. Biobehav. Rev. 96, 241–249. doi: 10.1016/j.neubiorev.2018.12.013
Murty, V., LaBar, K., and Adcock, R. (2016). Distinct medial temporal networks encode surprise during motivation by reward versus punishment. Neurobiol. Learn. Mem. 134, 55–64. doi: 10.1016/j.nlm.2016.01.018
Nitschke, J., Sarinopoulos, I., Mackiewicz, K., Schaefer, H., and Davidson, R. (2006). Functional neuroanatomy of aversion and its anticipation. Neuroimage 29, 106–116. doi: 10.1016/j.neuroimage.2005.06.068
Paul, E., Smith, J., Valentin, V., Turner, B., Barbey, A., and Ashby, F. (2015). Neural networks underlying the metacognitive uncertainty response. Cortex 71, 306–322. doi: 10.1016/j.cortex.2015.07.028
Remijnse, P., Nielen, M., Uylings, H., and Veltman, D. (2005). Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage 26, 609–618. doi: 10.1016/j.neuroimage.2005.02.009
Ridderbusch, I., Wroblewski, A., Yang, Y., Richter, J., Hollandt, M., Hamm, A., et al. (2021). Neural adaptation of cingulate and insular activity during delayed fear extinction: A replicable pattern across assessment sites and repeated measurements. NeuroImage 237:118157. doi: 10.1016/j.neuroimage.2021.118157
Rodriguez, P. (2009). Stimulus-outcome learnability differentially activates anterior cingulate and hippocampus at feedback processing. Learn. Mem. 16, 324–331. doi: 10.1101/lm.1191609
Ruge, H., and Wolfensteller, U. (2016). Distinct contributions of lateral orbito-frontal cortex, striatum, and fronto-parietal network regions for rule encoding and control of memory-based implementation during instructed reversal learning. NeuroImage 125, 1–12. doi: 10.1016/j.neuroimage.2015.10.005
Savage, H., Davey, C., Fullana, M., and Harrison, B. (2020). Clarifying the neural substrates of threat and safety reversal learning in humans. NeuroImage 207:116427. doi: 10.1016/j.neuroimage.2019.116427
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007
Shenhav, A., Botvinick, M., and Cohen, J. (2013). The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79, 217–240. doi: 10.1016/j.neuron.2013.07.007
Skvortsova, A., Veldhuijzen, D., de Rover, M., Pacheco-Lopez, G., Bakermans-Kranenburg, M., van IJzendoorn, M., et al. (2020). Effects of oxytocin administration and conditioned oxytocin on brain activity: An fMRI study. PLoS One 15:e0229692. doi: 10.1371/journal.pone.0229692
Spohrs, J., Ulrich, M., Grön, G., Plener, P., and Abler, B. (2022). FAAH polymorphism (rs324420) modulates extinction recall in healthy humans: an fMRI study. Eur. Arch. Psychiatry Clin. Neurosci. 272, 1495–1504. doi: 10.1007/s00406-021-01367-4
Spohrs, J., Ulrich, M., Grön, G., Prost, M., Plener, P., Fegert, J., et al. (2021). Fear extinction learning and anandamide: an fMRI study in healthy humans. Transl. Psychiatry 11:161. doi: 10.1038/s41398-020-01177-7
Stewart, J., Parnass, J., May, A., Davenport, P., and Paulus, M. (2013). Altered frontocingulate activation during aversive interoceptive processing in young adults transitioning to problem stimulant use. Front. Syst. Neurosci. 7:89. doi: 10.3389/fnsys.2013.00089
Stout, D., Glenn, D., Acheson, D., Simmons, A., and Risbrough, V. (2019). Characterizing the neural circuitry associated with configural threat learning. Brain Res. 1719, 225–234. doi: 10.1016/j.brainres.2019.06.003
Stout, D., Glenn, D., Acheson, D., Spadoni, A., Risbrough, V., and Simmons, A. (2018). Neural measures associated with configural threat acquisition. Neurobiol. Learn. Mem. 150, 99–106. doi: 10.1016/j.nlm.2018.03.012
van der Heiden, L., Liberati, G., Sitaram, R., Kim, S., Jaśkowski, P., Raffone, A., et al. (2014). Insula and inferior frontal triangularis activations distinguish between conditioned brain responses using emotional sounds for basic BCI communication. Front. Behav. Neurosci. 8:247. doi: 10.3389/fnbeh.2014.00247
Vassena, E., Silvetti, M., Boehler, C., Achten, E., Fias, W., and Verguts, T. (2014). Overlapping neural systems represent cognitive effort and reward anticipation. PLoS One 9:e91008. doi: 10.1371/journal.pone.0091008
Vincent, J. L., Kahn, I., Snyder, A. Z., Raichle, M. E., and Buckner, R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 100, 3328–3342. doi: 10.1152/jn.90355.2008
Visalli, A., Capizzi, M., Ambrosini, E., Mazzonetto, I., and Vallesi, A. (2019). Bayesian modeling of temporal expectations in the human brain. Neuroimage 202:116097. doi: 10.1016/j.neuroimage.2019.116097
Vorobyev, V., Kwon, M., Moe, D., Parkkola, R., and Hämäläinen, H. (2015). Risk-taking behavior in a computerized driving task: Brain activation correlates of decision-making, outcome, and peer influence in male adolescents. PLoS One 10:e0129516. doi: 10.1371/journal.pone.0129516
Votinov, M., Pripfl, J., Windischberger, C., Moser, E., Sailer, U., and Lamm, C. (2015). A functional polymorphism in the prodynorphin gene affects cognitive flexibility and brain activation during reversal learning. Front. Behav. Neurosci. 9:172. doi: 10.3389/fnbeh.2015.00172
Vytal, K., Overstreet, C., Charney, D., Robinson, O., and Grillon, C. (2014). Sustained anxiety increases amygdala–dorsomedial prefrontal coupling: A mechanism for maintaining an anxious state in healthy adults. J. Psychiatry Neurosci. 39, 321–329. doi: 10.1503/jpn.130145
Wan, X., Cheng, K., and Tanaka, K. (2016). The neural system of postdecision evaluation in rostral frontal cortex during problem-solving tasks. eNeuro 3:10.1523/ENEURO.0188-16.2016. doi: 10.1523/ENEURO.0188-16.2016
Wang, B., Schlaffke, L., and Pleger, B. (2020). Modulations of insular projections by prior belief mediate the precision of prediction error during tactile learning. J. Neurosci. 40, 3827–3837. doi: 10.1523/JNEUROSCI.2904-19.2020
Worthy, D., Davis, T., Gorlick, M., Cooper, J., Bakkour, A., Mumford, J., et al. (2016). Neural correlates of state-based decision-making in younger and older adults. Neuroimage 130, 13–23. doi: 10.1016/j.neuroimage.2015.12.004
Wu, C., Samanez-Larkin, G., Katovich, K., and Knutson, B. (2014). Affective traits link to reliable neural markers of incentive anticipation. NeuroImage 84, 279–289. doi: 10.1016/j.neuroimage.2013.08.055
Yin, S., Liu, Y., Petro, N., Keil, A., and Ding, M. (2018). Amygdala adaptation and temporal dynamics of the salience network in conditioned fear: A single-trial fMRI study. eNeuro 5:10.1523/ENEURO.0445-17.2018. doi: 10.1523/ENEURO.0445-17.2018
Young, C., and Nusslock, R. (2016). Positive mood enhances reward-related neural activity. Soc. Cogn. Affect. Neurosci. 11, 934–944. doi: 10.1093/scan/nsw012
Zeuner, K., Knutzen, A., Granert, O., Sablowsky, S., Götz, J., Wolff, S., et al. (2016). Altered brain activation in a reversal learning task unmasks adaptive changes in cognitive control in writer’s cramp. Neuroimage Clin. 10, 63–70. doi: 10.1016/j.nicl.2015.11.006
Keywords: uncertainty, decision-making, meta-analysis, fMRI, cascade model, ALE
Citation: Timashkov A, Anderson S and Zinchenko O (2025) Neural correlates of uncertainty processing: meta-analysis of fMRI studies. Front. Neurosci. 19:1662272. doi: 10.3389/fnins.2025.1662272
Received: 08 July 2025; Accepted: 23 October 2025;
Published: 11 November 2025.
Edited by:
Hanliang Fu, Xi’an University of Architecture and Technology, ChinaReviewed by:
Sandra Chanraud, Université Paris Sciences et Lettres, FranceCaroline Demro, University of Minnesota Twin Cities, United States
Copyright © 2025 Timashkov, Anderson and Zinchenko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrey Timashkov, YXl0aW1hc2hrb3ZAaHNlLnJ1
 Andrey Timashkov
Andrey Timashkov Sarah Anderson
Sarah Anderson Oksana Zinchenko
Oksana Zinchenko