- 1China Tobacco Zhejiang Industrial Co., Ltd., Hangzhou, China
- 2Shanghai New Tobacco Product Research Institute Co., Ltd., Shanghai, China
- 3Department of Pharmacology, Hangzhou City University School of Medicine, Hangzhou, China
- 4Department of Pharmacology, Zhejiang University School of Medicine, Hangzhou, China
- 5China National Tobacco Quality Supervision and Test Center, Zhengzhou, China
Nicotine, recognized as the principal addictive component in tobacco, is mechanistically linked to its interaction with neuronal nicotinic acetylcholine receptors (nAChRs). nAChRs are ligand-gated ion channels composed of five transmembrane subunits, with the α4β2 receptor subtype being the most common in the brain, playing a crucial role in the behavioral effects of nicotine. When nicotine binds to α4β2 nAChR, it significantly enhances the firing rate and burst firing of dopamine neurons in the brain, thereby activating the mesolimbic dopamine system. This system promotes the formation of nicotine addiction in the early stages of addiction through rewarding sensory stimulation and associative learning. The α4β2 nAChR subunit has been identified as the principal subtype implicated in the pathogenesis of nicotine addiction. However, other nAChRs subtypes also play important roles in the onset and maintenance of nicotine addiction. Understanding the relationship between nicotine addiction and nAChR subtypes is crucial for fully uncovering the neurobiological mechanism behind its addictive properties and lays the foundation for developing more targeted smoking cessation strategies.
Highlights
• This review delineates the subtype-specific roles of nAChRs—such as α4β2, α6β2*, and α5-containing subtypes—in mediating nicotine rewards and aversion via distinct neural circuits.
• The VTA-NAc pathway is recognized for its dopaminergic mechanisms underlying rewards, whereas the MHb-IPN circuit is implicated in nicotine aversion through glutamatergic and GABAergic signaling.
• Genetic variants like CHRNA5 rs16969968 and stoichiometric differences among nAChR subtypes are identified as critical determinants of individual susceptibility to nicotine dependence.
• Integrating multi-system neurotransmitter interactions—including dopamine, glutamate, GABA, and GLP-1—offers a more comprehensive model of nicotine addiction that extends beyond traditional rewards pathways.
1 Introduction
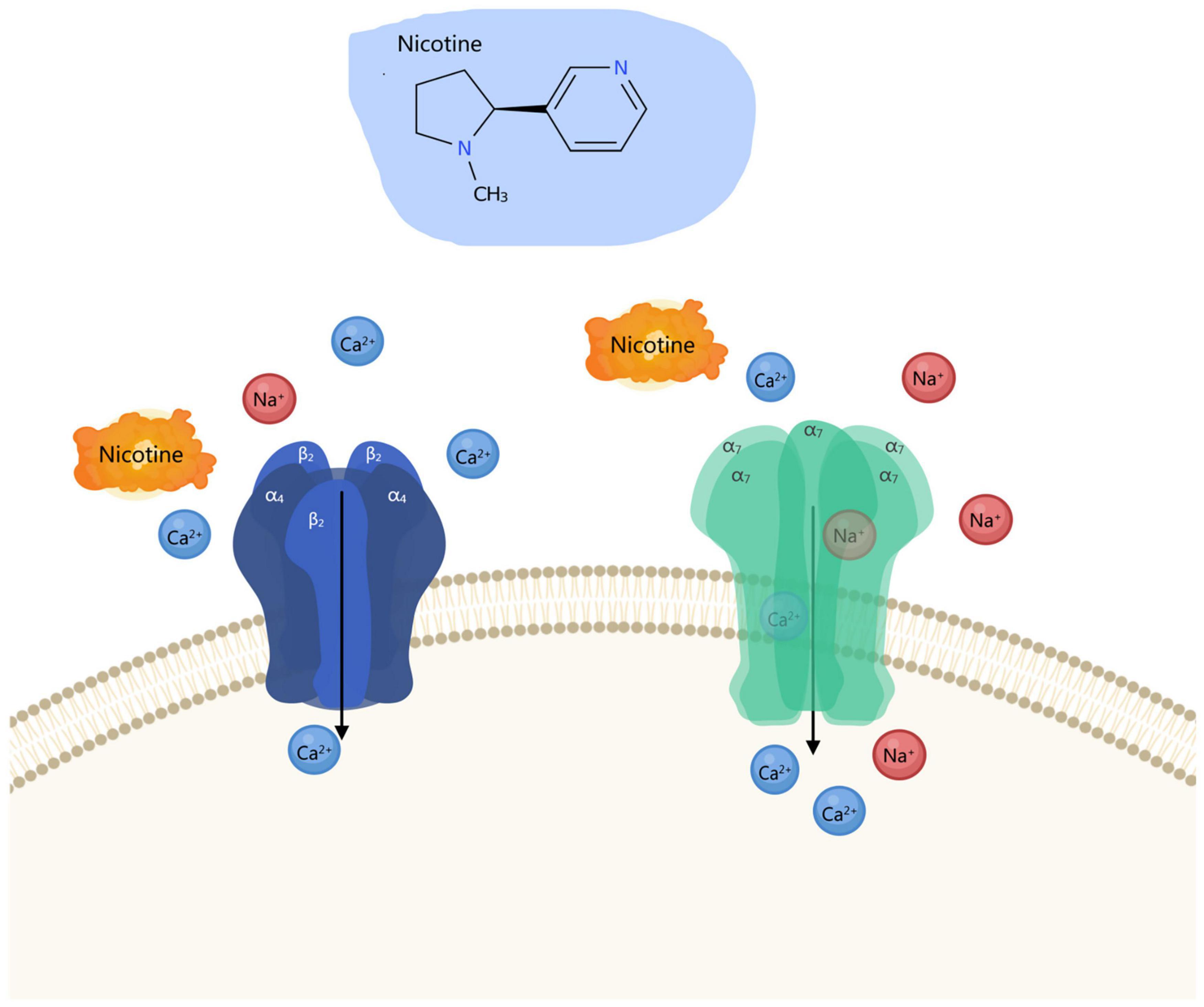
Nicotine is the primary active component in tobacco products that causes addiction. Nicotine exerts both strong rewards and aversive effects in the central nervous system through its interaction with nicotinic acetylcholine receptors (nAChRs) (Maskos et al., 2005; Picciotto et al., 1998; Tolu et al., 2013). This process not only endows nicotine with high pharmacological activity in addiction but also complicates the mechanisms of its physiological and pathological effects resulting from direct or indirect activation of multiple intracellular signaling pathways. Nicotinic acetylcholine receptors are ligand-gated ion channels composed of five subunits, each containing an extracellular ligand-binding domain and four transmembrane regions (Le Novère et al., 2002; Lindstrom, 1997). In mammals, nAChRs subtypes are highly diverse, such as the most common α4β2 heteropentamer and α7 homopentamer in the brain (Maskos et al., 2005; Wooltorton et al., 2003). A comprehensive understanding of nAChR function is essential for analyzing the mechanisms of nicotine addiction. On one front, the elucidation of dynamic regulatory mechanisms governing nAChR structural plasticity and functional modulation promises to yield a more comprehensive understanding of molecular addiction processes. On another front, research into the distribution of nAChRs and their systemic impacts will help elucidate the broader physiological consequences of nicotine addiction. This review synthesizes current evidence on the relationship between nicotine addiction and nAChRs, and clarify the neurobiological basis of nicotine addiction.
2 Neurobiological basis of nicotine addiction
The rewarding mechanism of nicotine addiction exhibits complex biphasic regulatory characteristics, where the dynamic equilibrium between positive reinforcement (such as euphoria) and negative regulation (such as aversive reactions) constitutes the neurobiological basis for the formation of addictive behaviors. The rewarding effects of nicotine typically manifest as sensations of “lightheaded euphoria” or “excitement” post-consumption, while aversive effects are reflected in discomfort reactions such as nausea and dizziness (Corrigall et al., 1992; Koob, 1992; Villanueva et al., 1989). The balance of these effects is closely associated with individual-specific factors, including dosage, personal sensitivity, and tolerance development. The addictive properties of nicotine are predominantly mediated by the integration of interactive signaling processing rewards and aversion across multiple brain regions. As the central hub of the mesolimbic dopamine system, dopaminergic neurons in the ventral tegmental area (VTA) form critical neural circuits through their projections to the nucleus accumbens (NAc) and prefrontal cortex, constituting the neural substrate for nicotine’s rewarding effects (Clarke et al., 1988; Corrigall et al., 1994; Nisell et al., 1996).
2.1 Rewarding mechanisms in nicotine addiction
The core pathological mechanism of nicotine addiction involves the rewards modulation system of the mesolimbic pathway. This system generates positive reinforcement signals primarily through dopaminergic transmission within the mesolimbic circuit, mediated by dynamic interactions between the ventral tegmental area (VTA) and the nucleus accumbens (NAc).(Fu et al., 2003; Mansvelder and McGehee, 2000; Rice and Cragg, 2004; Schwartz et al., 1984). Research has suggests that nicotine produces rewarding effects not through a single neurotransmitter system, but through the integrated actions of dopaminergic, GABAergic, glutamatergic systems and atypical rewarding pathways, which together facilitate spatiotemporally specific neuroplastic adaptations (Wooltorton et al., 2003; Grieder et al., 2019; Mansvelder et al., 2002). This multidimensional regulatory mechanism explains how nicotine induces rapid addiction.
As a high-affinity agonist of nicotinic acetylcholine receptors (nAChRs), nicotine directly activates VTA dopamine neurons through β2 subunit-containing receptors, inducing Na+/Ca2+ influx that causes membrane depolarization. This enhances the firing frequency of dopaminergic neurons and triggers transient surges of dopamine release in the NAc. This process is completely abolished in β2 subunit knockout mice, confirming its role as the molecular basis of rewarding effects (Maskos et al., 2005; Picciotto et al., 1998; Tolu et al., 2013; Dani and Bertrand, 2007; De Biasi and Dani, 2011; Mao et al., 2011; Pidoplichko et al., 1997). Nicotine not only directly acts on dopaminergic neurons, but also transiently enhances GABAergic neurons’ inhibitory drive on dopaminergic neurons by binding to nAChRs within GABAergic neurons in the VTA (Wooltorton et al., 2003; Grieder et al., 2019; Mansvelder et al., 2002). In the initial stage, nicotine activates GABAergic interneurons through α4β2-nAChRs, increasing their spontaneous discharge frequency and thereby augmenting inhibitory inputs to dopaminergic neurons; However, with rapid receptor desensitization, the GABAergic inhibitory effects attenuate following sustained exposure, forming a “disinhibition-excitation potentiation” delayed reinforcement pattern. This temporal dissociation characteristic may underlie the dual-phase reinforcement properties of nicotine rewards. Due to the rapid desensitization of α4β2 nAChRs, when exposed to sustained low concentrations of nicotine, the GABAergic drive gradually diminishes over time, thereby relieving inhibition on dopaminergic neurons and ultimately enhancing their excitability. This phenomenon regulates the activity states of VTA dopamine neurons through dual mechanisms, playing a critical role in the process of nicotine-induced rewarding effects (Mansvelder et al., 2002; Yan et al., 2019). In the context of long-term effects, presynaptic α7nAChRs further promote the long-term excitability of dopaminergic neurons by enhancing glutamatergic inputs (Mao et al., 2011; Ostroumov and Dani, 2018; Pidoplichko et al., 2004). The combination of enhanced synaptic input and removal of inhibitory constraints constitutes a critical step in the initiation of nicotine addiction.
The nicotine rewarding effect is further complicated by its direct regulation of dopamine release within the striatum. In the nucleus accumbens core and dorsal striatum, dopamine release is regulated by presynaptically expressed heteromeric nAChRs, particularly those mediated by receptors containing α6, α4, β2, and β3 subunits (Salminen et al., 2004; Zoli et al., 2002). Nicotine activates these receptors to physically enhance dopamine release while concurrently reducing basal dopamine levels. Although this rapid desensitization phenomenon appears paradoxical to the rewarding mechanism, it effectively enhances the signal-to-noise ratio of dynamic rewarding signals by reducing background dopamine “noise,” thereby strengthening the coupling between nicotine rewarding and environmental cues (Rice and Cragg, 2004; Threlfell and Cragg, 2011; Zhang et al., 2009). This signal optimization mechanism makes nicotine a particularly substance prone to induce addiction.
Studies further reveal that nicotine’s rewarding effects are not limited to the regulation of the dopamine system. Nicotine can directly act on brain regions outside the mesolimbic dopamine system, such as the central linear nucleus and parabrachial nucleus, and manifests rewarding effects independent of dopamine through interactions with opioid receptors or other neuropeptides, suggesting a multi-system interactive rewarding integration mechanism (Ikemoto et al., 2006; Neugebauer et al., 2011; Trigo et al., 2009).
In summary, the core of the nicotine rewarding mechanism lies in its profound impact on dopaminergic transmission in the mesolimbic system, achieved through complex direct and indirect pathways. Starting with high-affinity β2 subunit-containing nAChRs, nicotine directly activates VTA dopamine neurons; concurrently, it enhances phasic dopaminergic signaling and optimizes the signal-to-noise ratio of rewarding signals by modulating GABAergic and glutamatergic inputs. These synergistic neuroregulatory mechanisms collectively establish nicotine as a substance with potent reinforcing properties, cementing its central role in addictive behaviors.
2.2 Aversive effects of nicotine addiction
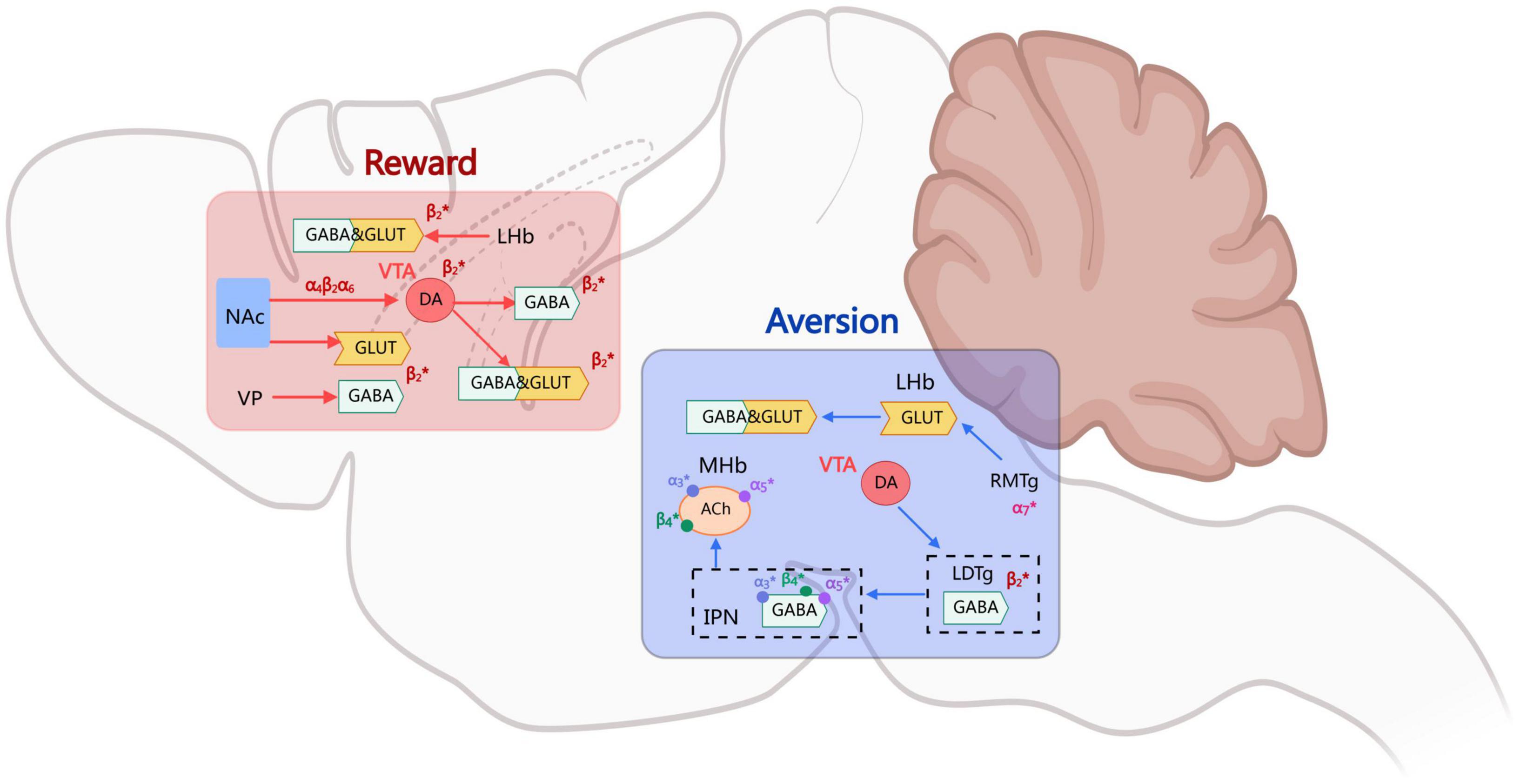
The formation and maintenance of nicotine addiction fundamentally constitutes a neurobiological process involving dynamic interactions between rewarding and punishment mechanisms. Within this framework, punishment mechanisms exert critical constraining effects on nicotine-seeking behavior through negative reinforcement effects mediated by specific neural circuits. Recent studies have revealed that the medial habenula (MHb)-interpeduncular nucleus (IPN) pathway serves as the central hub mediating nicotine’s aversive effects (Fowler and Kenny, 2011; Jensen et al., 2015; Sartor et al., 2010).
Through optogenetics, chemogenetics, and molecular imaging techniques, researchers have systematically elucidated the aversion signaling pathway mediated by α3/α5/β4 nicotinic acetylcholine receptors (nAChRs) within this neural circuit (Girod et al., 2000; Grady et al., 2001, 2009; Ren et al., 2011). The high-affinity binding of nicotine to α5 subunit-containing receptors on MHb neurons triggers Ca2+-dependent burst firing, which induces the axonal terminal release of glucagon-like peptide-1 (GLP-1). This subsequently activates GLP-1 receptors in the IPN to promote cAMP production, significantly enhancing the excitability of glutamatergic neurons in the IPN (Hussain et al., 2008; Sherafat et al., 2020; Tuesta et al., 2017). Studies on gene knockout mice have demonstrated that deletion of the α5 nAChR subunit significantly reduces nicotine’s aversive effects, enabling animals to tolerate higher doses of nicotine (Fowler et al., 2011). The missense mutation rs16969968 in the CHRNA5 gene results in the substitution of aspartic acid with asparagine at position 398 of the encoded α5 subunit. This variant exhibits decreased sensitivity to nicotine agonists and reduced calcium permeability, substantially elevating the risk of nicotine addiction (Bierut, 2010; Breetvelt et al., 2012; Buczkowski et al., 2015; Liu et al., 2010).
The IPN acts as a relay station for aversive signals, regulating downstream neural activity through dual projection pathways: its GABAergic fibers directly inhibit cholinergic neurons in the laterodorsal tegmental nucleus (LDTg), while glutamatergic projections activate an NMDA receptor-dependent negative regulatory network within the VTA (Ables et al., 2017; Dautan et al., 2016; Lima et al., 2017; Liu et al., 2022; Quina et al., 2017; Wolfman et al., 2018). Optogenetics experiments demonstrate that specific activation of the IPN→LDTg GABAergic pathway induces robust place avoidance behavior, while inhibition of this pathway completely abolishes the aversive effects of high-dose nicotine (Wolfman et al., 2018; Alderson et al., 2005; Ishibashi et al., 2009; Maskos, 2008). The LDTg reduces glutamatergic input strength to VTA dopamine neurons through GABAB receptor-mediated presynaptic inhibition mechanisms, establishing functional antagonism against the rewarding system (Melani et al., 2019). This bidirectional regulation manifests behaviorally as dose-dependent biphasic effects: low-dose nicotine induces reward via VTA dopaminergic activation, while high-dose nicotine elicits aversion through the MHb-IPN-LDTg pathway, with the critical dose threshold being regulated by α5 subunit expression levels.
Besides regions like the VTA and the MHb-IPN pathway, the process of nicotine addiction also involves a range of other brain areas and related neurotransmitter systems. For example, the insular cortex is a key region in regulating nicotine intake and seeking behavior. Damage to this area can significantly reduce an individual’s craving for nicotine, making it a potential target for withdrawal and relapse interventions (Naqvi et al., 2007). Other studies have shown that certain cortical areas of brain, such as the prefrontal cortex and basolateral amygdala, are essential in strengthening the memory and relapse in the addiction process by integrating rewarding and emotional information (Forget et al., 2010; Kodas et al., 2007; Le Foll et al., 2008). Thus, nicotine is not merely a rewards-promoting substance that simply activates nAChRs, but rather a complex modulator capable of dynamically regulating dopamine, glutamate, GABA, and other neurotransmitters.
Overall, the integration of rewarding and aversive signals in the process of nicotine addiction depends on the dynamic interactions of multiple brain regions and neural circuits. The VTA-NAc pathway dominates the rewarding mechanism, while the MHb-IPN pathway regulates the aversive effect. At the same time, other regions of the brain, such as the insular cortex, also play a significant role in nicotine addiction and withdrawal (Figure 1).
3 Core mechanistic roles of nAChRs in nicotine addiction
As pivotal members of the ligand-gated cation channel superfamily, nicotinic acetylcholine receptors (nAChRs) serve as the cornerstone of nicotine addiction neurobiology, with the complexity of their molecular architecture and functional modulation forming its fundamental basis (Le Novère et al., 2002; Lindstrom, 1997). Since the groundbreaking discovery of acetylcholine as a neurotransmitter by Dale (1935), our understanding of nAChRs has evolved from a simple neuromuscular junction signaling apparatus to a molecular nexus mediating cross-system neural plasticity (Changeux, 2020; Hulme et al., 1990). nAChRs are composed of five subunits (α and β subunits) assembled into a pentameric structure, forming a central water-filled ion channel. To date, nine α subunits (α2–α10) and 3 β subunits (β2–β4) have been identified. These subunits combine in various configurations to form functionally diverse receptor subtypes, with the α4β2 and α7 subtypes being the most representative in the central nervous system (CNS). As an exogenous agonist, nicotine binds with high affinity to the ligand-binding site within the extracellular domain of receptors, triggering conformational changes that open ion channels. This facilitates transmembrane flow of Na+, K+, and Ca2+, inducing cell membrane depolarization and activating downstream signaling cascades (Figure 2) (Changeux, 2018; McKay et al., 2007). nAChRs are ubiquitously distributed across virtually all anatomical brain regions, including presynaptic and postsynaptic membranes, axonal terminals, and somatic compartments. Within the brain, nAChRs demonstrate remarkable heterogeneity, with distinct subtypes executing specialized functional roles in specific brain regions. For instance, α4β2 receptors are widely distributed in the VTA, NAc, and prefrontal cortex, playing a central role in regulating dopamine release and reinforcement learning (Maskos et al., 2005; Picciotto et al., 1998). In contrast, α7 receptors are primarily localized in dopaminergic neurons and participate in modulating long-term potentiation of glutamatergic neurons (Wooltorton et al., 2003; Mansvelder et al., 2002). Within the VTA, dopaminergic neurons exhibit highly heterogeneous expression of nAChR subtypes, predominantly α4β2 and α6β2β3 complexes. Activation of these receptors enhances burst firing in dopaminergic neurons, increases dopamine release in the nucleus accumbens, thereby forming rewarding signals for external stimuli such as nicotine (Lindstrom, 1997; Exley et al., 2011). On the other hand, in the MHb, the α5, α3, and β4 subtypes similarly play negative regulatory roles in nicotine uptake and aversive effects (De Biasi and Salas, 2008; Sheffield et al., 2000). This bidirectional regulatory mechanism reflects the integrative role of nAChRs within complex interregional brain networks.
The rewarding mechanism of nicotine addiction is closely linked to the subtype-specific functions and molecular diversity of nicotinic acetylcholine receptors (nAChRs), the core of which lies in the spatiotemporal regulation of neurotransmitter release by distinct nAChR subtypes within the mesolimbic dopamine system. As the most abundant subtype in the central nervous system, the stoichiometric ratio differences between (α4β2)2β2 and (α4β2)2α4 of α4β2 nAChR determine receptor sensitivity and functional characteristics toward nicotine: The (α4β2)2β2 subtype exhibits high agonist affinity, whereas the (α4β2)2α4 subtype demonstrates 3–4-fold enhanced activation efficacy despite lower affinity, establishing a dual regulatory paradigm of “high-affinity” and “high-efficacy” (Gotti et al., 2009). Gene knockout experiments confirm that deletion of α4 or β2 subunits completely blocks nicotine-induced burst firing of VTA dopamine neurons and dopamine release in the nucleus accumbens, while mice expressing hypersensitive α4 mutants exhibit exaggerated rewarding responses to low-dose nicotine (Maskos et al., 2005; Picciotto et al., 1998; Mameli-Engvall et al., 2006; McGranahan et al., 2011; Naudé et al., 2016; Peng et al., 2017; Tapper et al., 2004). Pharmacological studies further reveal that the α4β2* nAChR partial agonist varenicline significantly reduces nicotine self-administration by competitively inhibiting nicotine binding and attenuating dopamine release. Concurrently, dihydro-β-erythroidine (DHβE), a selective antagonist of β2 nAChR, also inhibits nicotine addiction (Coe et al., 2005; Ivanová and Greenshaw, 1997; Reperant et al., 2010). The upregulation of α4β2 receptor expression in VTA GABAergic neurons shows high correlation with nicotine addiction susceptibility. Positron emission tomography (PET) studies demonstrate a positive correlation between α4β2 receptor density and withdrawal difficulty in smokers, indicating its central role in the dynamic regulation of addiction progression (Brody et al., 2004). The functional differentiation of α6β2* nAChR subtypes within the mesolimbic system further enriches the complexity of nicotine rewarding mechanisms. The α6 subunit exhibits specific expression in VTA dopamine neurons and their striatal terminals, co-assembling with the β3 subunit to form high calcium permeability complexes: (α6β2)2β3 and (α4β2)(α6β2)β3 (Salminen et al., 2004; Zoli et al., 2002). These receptors demonstrate significantly higher nicotine sensitivity compared to other subtypes. Notably, α6 knockout mice exhibit abolished motivation for nicotine intake in both acute nicotine self-administration and two-bottle choice paradigms (Bagdas et al., 2019; Liu et al., 2012). The β3 subunit serves as an auxiliary component of α6-containing receptors, enhancing nicotine’s regulation of striatal dopamine release by promoting receptor maturation and membrane localization (Gotti et al., 2009; Moen et al., 2021). Knockout of the β3 subunit reduces α6 receptor expression in the striatum and attenuates nicotine-induced dopamine release, while allelic variations in the CHRNB3 gene cluster show significant association with nicotine addiction risk (Bierut et al., 2007; Gotti et al., 2005; Thorgeirsson et al., 2010; Wen et al., 2016). The α5 subunit incorporates into α4β2 receptors as a non-ligand-binding auxiliary subunit, forming (α4β2)2α5 complexes that exhibit enhanced calcium permeability compared to classical α4β2 receptors and demonstrate resistance to agonist-induced desensitization (Chatterjee et al., 2013). The loss-of-function mutation (D398N) at the CHRNA5 gene rs16969968 locus increases nicotine addiction risk by reducing receptor calcium permeability, while VTA dopamine neurons in α5-knockout mice exhibit attenuated responsiveness to nicotine (Kuryatov et al., 2008; Morel et al., 2014; Sciaccaluga et al., 2015; Tapia et al., 2007). The α5 receptor primarily regulates dopamine release in the dorsal striatum, demonstrating spatial differentiation from α6* receptor function in the nucleus accumbens. This anatomical specificity may explain nicotine’s differential regulation of distinct behavioral paradigms (Exley et al., 2012). In contrast, α7 homomeric nAChRs play a relatively limited role in nicotine rewarding mechanisms. Although it mediates enhanced glutamatergic inputs to the VTA, α7 knockout mice exhibited no behavioral differences in nicotine self-administration and conditioned place preference tests. Only female individuals showed reduced nicotine intake during chronic oral administration (Bagdas et al., 2019; Pons et al., 2008). While methyllycaconitine (MLA) demonstrates efficacy in attenuating nicotine self-administration behaviors, its non-selective pharmacological actions coupled with null results observed in α7-nAChR knockout models collectively suggest a dissociation from canonical α7-mediated pathways in eliciting this behavioral modulation (Bryant et al., 2002; Markou and Paterson, 2001; Salas et al., 2007).
In conclusion, the molecular structure, diverse combinations, widespread distribution, and functional variety of nAChRs collectively underpin their central role in nicotine addiction (Table 1).
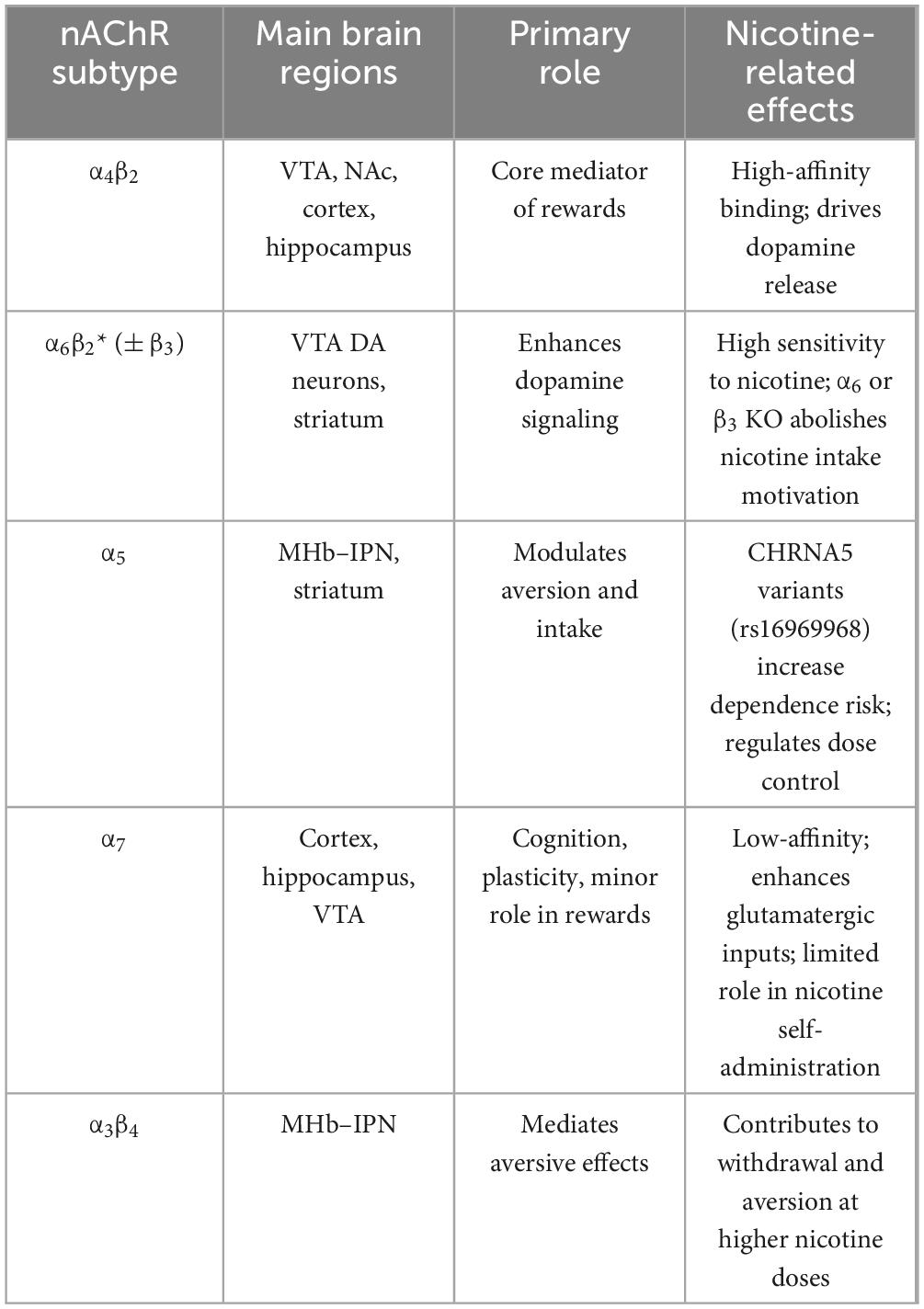
Table 1. Major nicotinic acetylcholine recepto (nAChR) subtypes and their roles in nicotine addiction.
4 Discussion
This article provides a detailed overview of the mechanisms underlying nicotine addiction and the roles of various nicotinic acetylcholine receptor (nAChR) subtypes in this process. Nevertheless, the mechanisms of nicotine addiction remain only partially understood. As discussed above, most studies have concentrated on α4, α5, α6, α7, and β2 nAChR subunits (Braunscheidel et al., 2024; Gu et al., 2019; Huang et al., 2022, 2025; Jackson et al., 2017; Rigotti et al., 2023; Sakkiah et al., 2020; Yang et al., 2023), whereas others—such as α3 (Icick et al., 2020), which is densely expressed in the mHb—have received far less attention. Evidence indicates that allelic variations in the CHRNA3 gene, which encodes the α3 subunit, are associated with an elevated risk of nicotine addiction, although the precise mechanisms remain unclear (Elayouby et al., 2021). Therefore, future mechanistic studies should not only focus on well-studied subunits but also expand to include understudied nAChRs, thereby enabling a more comprehensive understanding of how nicotine induces addiction.
In addition, emerging technologies are reshaping our understanding of nicotine addiction. For example, omics approaches are playing an increasingly significant role in mechanistic studies. Several groups have applied single-nucleus transcriptomics (snRNA-seq) to ventral tegmental area (VTA) neurons and glial cells across three stages—pre-addiction, addiction, and post-addiction—yielding deeper insights into nicotine-induced changes (Fan et al., 2024). Looking ahead, single-cell ATAC-seq (Kimbrough et al., 2021; Jackson et al., 2024), spatial transcriptomics (Scott et al., 2024), proteomics (Lee et al., 2021), and metabolomics (Lian et al., 2024) are expected to further advance nicotine addiction research. These methods can unravel molecular mechanisms across multiple levels: epigenetic regulation (gene switching), spatial organization (regional and cellular interactions), protein function (receptors and signaling pathways), and metabolic states (energy balance and neurotransmission). Integrating multi-omics data will enable construction of a complete causal chain—from chromatin remodeling → gene transcription → protein function → metabolic alterations → behavioral phenotypes. This systems-level framework will provide valuable resources for identifying biomarkers and therapeutic targets, ultimately laying the groundwork for personalized smoking cessation strategies.
5 Conclusion
Nicotine addiction arises from the diversity and dynamic regulation of nAChR subtypes, which shape the balance between rewards and aversion in mesolimbic circuits. Recent evidence highlights the critical role of α4β2 and α7 receptors in modulating dopamine release through subtype-specific stoichiometry and calcium permeability, while α5-containing assemblies have been identified as genetic determinants of addiction vulnerability. At the same time, the MHb–IPN pathway, mediated by α3α5β4 receptors, has been increasingly recognized as a central hub for aversive modulation, expanding the traditional dopamine-centered framework. Technological advances in single-cell transcriptomics and spatial multi-omics now allow unprecedented resolution of subtype distribution and plasticity. This review argues that future research should integrate molecular, circuit, and behavioral perspectives, with emphasis on cell-type–specific receptor dynamics, adaptive plasticity under different nicotine exposure conditions, and individual genetic risk factors. Taken together, these insights suggest that nAChR subtype heterogeneity is not only fundamental to the mechanisms of nicotine addiction but also provides a foundation for precision strategies in smoking cessation.
Author contributions
JJ: Project administration, Writing – original draft, Investigation. XL: Conceptualization, Writing – original draft. A-fH: Investigation, Writing – original draft. G-jZ: Investigation, Writing – original draft. Y-hG: Investigation, Writing – original draft. CX: Investigation, Writing – original draft. X-mW: Supervision, Writing – review & editing. H-JW: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by Key R&D Program of China National Tobacco Corporation (110202202019).
Conflict of interest
JJ, XL, A-fH, G-jZ were employed by China Tobacco Zhejiang Industrial Co., Ltd. Y-hG was employed by Shanghai New Tobacco Product Research Institute Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. During the preparation of this work, the authors used ChatGPT and DeepSeek to improve the English language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ables, J., Görlich, A., Antolin-Fontes, B., Wang, C., Lipford, S., Riad, M., et al. (2017). Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference. Proc. Natl. Acad. Sci. U.S.A. 114, 13012–13017. doi: 10.1073/pnas.1717506114
Alderson, H., Latimer, M., and Winn, P. (2005). Involvement of the laterodorsal tegmental nucleus in the locomotor response to repeated nicotine administration. Neurosci. Lett. 380, 335–339. doi: 10.1016/j.neulet.2005.01.067
Bagdas, D., Diester, C., Riley, J., Carper, M., Alkhlaif, Y., AlOmari, D., et al. (2019). Assessing nicotine dependence using an oral nicotine free-choice paradigm in mice. Neuropharmacology 157:107669. doi: 10.1016/j.neuropharm.2019.107669
Bierut, L. (2010). Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24-25. Trends Pharmacol. Sci. 31, 46–51. doi: 10.1016/j.tips.2009.10.004
Bierut, L., Madden, P., Breslau, N., Johnson, E., Hatsukami, D., Pomerleau, O., et al. (2007). Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum. Mol. Genet. 16, 24–35. doi: 10.1093/hmg/ddl441
Braunscheidel, K., Voren, G., Fowler, C., Lu, Q., Kuryatov, A., Cameron, M., et al. (2024). SR9883 is a novel small-molecule enhancer of α4β2* nicotinic acetylcholine receptor signaling that decreases intravenous nicotine self-administration in rats. Front. Mol. Neurosci. 17:1459098. doi: 10.3389/fnmol.2024.1459098
Breetvelt, E., Numans, M., Aukes, M., Hoeben, W., Strengman, E., Luykx, J., et al. (2012). The association of the alpha-5 subunit of the nicotinic acetylcholine receptor gene and the brain-derived neurotrophic factor gene with different aspects of smoking behavior. Psychiatr. Genet. 22, 96–98. doi: 10.1097/YPG.0b013e32834c0c75
Brody, A., Olmstead, R., London, Farahi, J., Meyer, J., Grossman, P., et al. (2004). Smoking-induced ventral striatum dopamine release. Am. J. Psychiatry 161, 1211–1218. doi: 10.1176/appi.ajp.161.7.1211
Bryant, D., Free, R., Thomasy, S., Lapinsky, D., Ismail, K., McKay, S., et al. (2002). Structure-activity studies with ring E analogues of methyllycaconitine on bovine adrenal alpha3beta4* nicotinic receptors. Neurosci. Res. 42, 57–63. doi: 10.1016/s0168-0102(01)00304-2
Buczkowski, K., Sieminska, A., Linkowska, K., Czachowski, S., Przybylski, G., Jassem, E., et al. (2015). Association between genetic variants on chromosome 15q25 locus and several nicotine dependence traits in Polish population: A case-control study. Biomed. Res. Int. 2015:350348. doi: 10.1155/2015/350348
Changeux, J. (2018). Structural identification of the nicotinic receptor ion channel. Trends Neurosci. 41, 67–70. doi: 10.1016/j.tins.2017.11.003
Changeux, J. (2020). Discovery of the first neurotransmitter receptor: The acetylcholine nicotinic receptor. Biomolecules 10:547. doi: 10.3390/biom10040547
Chatterjee, S., Santos, N., Holgate, J., Haass-Koffler, C., Hopf, F., Kharazia, V., et al. (2013). The α5 subunit regulates the expression and function of α4*-containing neuronal nicotinic acetylcholine receptors in the ventral-tegmental area. PLoS One 8:e68300. doi: 10.1371/journal.pone.0068300
Clarke, P., Fu, D., Jakubovic, A., and Fibiger, H. (1988). Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J. Pharmacol. Exp. Ther. 246, 701–708.
Coe, J., Brooks, P., Vetelino, M., Wirtz, M., Arnold, E., Huang, J., et al. (2005). Varenicline: An alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J. Med. Chem. 48, 3474–3477. doi: 10.1021/jm050069n
Corrigall, W., Coen, K., and Adamson, K. (1994). Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 653, 278–284. doi: 10.1016/0006-8993(94)90401-4
Corrigall, W., Franklin, K., Coen, K., and Clarke, P. (1992). The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology 107, 285–289. doi: 10.1007/BF02245149
Dale, H. (1935). Pharmacology and nerve-endings (Walter ernest dixon memorial lecture): (Section of therapeutics and pharmacology). Proc. R. Soc. Med. 28, 319–332. doi: 10.1177/003591573502800330
Dani, J., and Bertrand, D. (2007). Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 47, 699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214
Dautan, D., Souza, A., Huerta-Ocampo, I., Valencia, M., Assous, M., Witten, I., et al. (2016). Segregated cholinergic transmission modulates dopamine neurons integrated in distinct functional circuits. Nat. Neurosci. 19, 1025–1033. doi: 10.1038/nn.4335
De Biasi, M., and Dani, J. (2011). Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci. 34, 105–130. doi: 10.1146/annurev-neuro-061010-113734
De Biasi, M., and Salas, R. (2008). Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp. Biol. Med. 233, 917–929. doi: 10.3181/0712-MR-355
Elayouby, K., Ishikawa, M., Dukes, A., Smith, A., Lu, Q., Fowler, C., et al. (2021). α3* nicotinic acetylcholine receptors in the habenula-interpeduncular nucleus circuit regulate nicotine intake. J. Neurosci. 41, 1779–1787. doi: 10.1523/JNEUROSCI.0127-19.2020
Exley, R., Maubourguet, N., David, V., Eddine, R., Evrard, A., Pons, S., et al. (2011). Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc. Natl. Acad. Sci. U.S.A. 108, 7577–7582. doi: 10.1073/pnas.1103000108
Exley, R., McIntosh, J., Marks, M., Maskos, U., and Cragg, S. (2012). Striatal α5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum. J. Neurosci. 32, 2352–2356. doi: 10.1523/JNEUROSCI.4985-11.2012
Fan, L., Liu, B., Yao, R., Gao, X., Wang, H., Jiang, S., et al. (2024). Nicotine-induced transcriptional changes and mitochondrial dysfunction in the ventral tegmental area revealed by single-nucleus transcriptomics. J. Genet. Genom. 51, 1237–1251. doi: 10.1016/j.jgg.2024.08.009
Forget, B., Wertheim, C., Mascia, P., Pushparaj, A., Goldberg, S., and Le Foll, B. (2010). Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35, 1751–1760. doi: 10.1038/npp.2010.42
Fowler, C., and Kenny, P. (2011). Intravenous nicotine self-administration and cue-induced reinstatement in mice: Effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology 61, 687–698. doi: 10.1016/j.neuropharm.2011.05.012
Fowler, C., Lu, Q., Johnson, P., Marks, M., and Kenny, P. (2011). Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601. doi: 10.1038/nature09797
Fu, Y., Matta, S., Kane, V., and Sharp, B. (2003). Norepinephrine release in amygdala of rats during chronic nicotine self-administration: An in vivo microdialysis study. Neuropharmacology 45, 514–523. doi: 10.1016/s0028-3908(03)00201-6
Girod, R., Barazangi, N., McGehee, D., and Role, L. (2000). Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors. Neuropharmacology 39, 2715–2725. doi: 10.1016/s0028-3908(00)00145-3
Gotti, C., Clementi, F., Fornari, A., Gaimarri, A., Guiducci, S., Manfredi, I., et al. (2009). Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 78, 703–711. doi: 10.1016/j.bcp.2009.05.024
Gotti, C., Moretti, M., Clementi, F., Riganti, L., McIntosh, J., Collins, A., et al. (2005). Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol. Pharmacol. 67, 2007–2015. doi: 10.1124/mol.105.011940
Grady, S., Meinerz, N., Cao, J., Reynolds, A., Picciotto, M., Changeux, J., et al. (2001). Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: A function mediated by a different nAChR than dopamine release from striatum. J. Neurochem. 76, 258–268. doi: 10.1046/j.1471-4159.2001.00019.x
Grady, S., Moretti, M., Zoli, M., Marks, M., Zanardi, A., Pucci, L., et al. (2009). Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci. 29, 2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009
Grieder, T., Besson, M., Maal-Bared, G., Pons, S., Maskos, U., and van der Kooy, D. (2019). β2* nAChRs on VTA dopamine and GABA neurons separately mediate nicotine aversion and reward. Proc. Natl. Acad. Sci. U.S.A. 116, 25968–25973. doi: 10.1073/pnas.1908724116
Gu, S., Matta, J., Davini, W., Dawe, G., Lord, B., and Bredt, D. (2019). α6-containing nicotinic acetylcholine receptor reconstitution involves mechanistically distinct accessory components. Cell Rep. 26:866–874.e3. doi: 10.1016/j.celrep.2018.12.103
Huang, J., Meyer, P., Guerrero, I., Becker, G., Rullmann, M., Mauche, N., et al. (2025). The relationship between α4β2-nicotinic acetylcholine receptor availability and brain arousal regulation as assessed by 2-[18F]F-A85380 PET and EEG following nicotine cessation in male individuals with nicotine dependence. Psychiatry Res. Neuroimaging 352:112035. doi: 10.1016/j.pscychresns.2025.112035
Huang, Y., Ma, Z., Zheng, C., Ma, X., Taylor, D., Gao, M., et al. (2022). Levo-tetrahydropalmatine inhibits α4β2 nicotinic receptor response to nicotine in cultured SH-EP1 cells. Acta Pharmacol. Sin. 43, 889–896. doi: 10.1038/s41401-021-00709-1
Hulme, E., Birdsall, N., and Buckley, N. (1990). Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 30, 633–673. doi: 10.1146/annurev.pa.30.040190.003221
Hussain, R., Taraschenko, O., and Glick, S. (2008). Effects of nicotine, methamphetamine and cocaine on extracellular levels of acetylcholine in the interpeduncular nucleus of rats. Neurosci. Lett. 440, 270–274. doi: 10.1016/j.neulet.2008.06.001
Icick, R., Forget, B., Cloëz-Tayarani, I., Pons, S., Maskos, U., and Besson, M. (2020). Genetic susceptibility to nicotine addiction: Advances and shortcomings in our understanding of the CHRNA5/A3/B4 gene cluster contribution. Neuropharmacology 177, 108234. doi: 10.1016/j.neuropharm.2020.108234
Ikemoto, S., Qin, M., and Liu, Z. (2006). Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J. Neurosci. 26, 723–730. doi: 10.1523/JNEUROSCI.4542-05.2006
Ishibashi, M., Leonard, C., and Kohlmeier, K. (2009). Nicotinic activation of laterodorsal tegmental neurons: Implications for addiction to nicotine. Neuropsychopharmacology 34, 2529–2547. doi: 10.1038/npp.2009.82
Ivanová, S., and Greenshaw, A. (1997). Nicotine-induced decreases in VTA electrical self-stimulation thresholds: Blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology 134, 187–192. doi: 10.1007/s002130050441
Jackson, A., Bagdas, D., Muldoon, P., Lichtman, A., Carroll, F., Greenwald, M., et al. (2017). In vivo interactions between α7 nicotinic acetylcholine receptor and nuclear peroxisome proliferator-activated receptor-α: Implication for nicotine dependence. Neuropharmacology 118, 38–45. doi: 10.1016/j.neuropharm.2017.03.005
Jackson, D., Burgon, R., Thompson, S., and Sudweeks, S. (2024). Single-cell quantitative expression of nicotinic acetylcholine receptor mRNA in rat hippocampal interneurons. PLoS One 19:e0301592. doi: 10.1371/journal.pone.0301592
Jensen, K., DeVito, E., Herman, A., Valentine, G., Gelernter, J., and Sofuoglu, M. A. (2015). CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology 40, 2813–2821. doi: 10.1038/npp.2015.131
Kimbrough, A., Kallupi, M., Smith, L., Simpson, S., Collazo, A., and George, O. (2021). Characterization of the brain functional architecture of psychostimulant withdrawal using single-cell whole-brain imaging. eNeuro 8:0208–19.2021. doi: 10.1523/ENEURO.0208-19.2021
Kodas, E., Cohen, C., Louis, C., and Griebel, G. (2007). Cortico-limbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology 194, 161–171. doi: 10.1007/s00213-007-0813-0
Koob, G. (1992). Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 13, 177–184. doi: 10.1016/0165-6147(92)90060-j
Kuryatov, A., Onksen, J., and Lindstrom, J. (2008). Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol. Pharmacol. 74, 132–143. doi: 10.1124/mol.108.046789
Le Foll, B., Forget, B., Aubin, H., and Goldberg, S. (2008). Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: Insights from pre-clinical and clinical studies. Addict. Biol. 13, 239–252. doi: 10.1111/j.1369-1600.2008.00113.x
Le Novère, N., Corringer, P., and Changeux, J. (2002). The diversity of subunit composition in nAChRs: Evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 53, 447–456. doi: 10.1002/neu.10153
Lee, A., Mansuri, M., Wilson, R., Lam, T., Nairn, A., and Picciotto, M. (2021). Sex differences in the ventral tegmental area and nucleus accumbens proteome at baseline and following nicotine exposure. Front. Mol. Neurosci. 14:657064. doi: 10.3389/fnmol.2021.657064
Lian, X., Liu, Z., Liu, S., Jin, L., Wu, T., Chen, Y., et al. (2024). Alterations in serum metabolomics during the first seizure and after effective control of epilepsy. Sci. Rep. 14:19180. doi: 10.1038/s41598-024-68966-8
Lima, L., Bueno, D., Leite, F., Souza, S., Gonçalves, L., Furigo, I., et al. (2017). Afferent and efferent connections of the interpeduncular nucleus with special reference to circuits involving the habenula and raphe nuclei. J. Comp. Neurol. 525, 2411–2442. doi: 10.1002/cne.24217
Lindstrom, J. (1997). Nicotinic acetylcholine receptors in health and disease. Mol. Neurobiol. 15, 193–222. doi: 10.1007/BF02740634
Liu, C., Tose, A., Verharen, J., Zhu, Y., Tang, L., de Jong, J., et al. (2022). An inhibitory brainstem input to dopamine neurons encodes nicotine aversion. Neuron 110: 3018–3035.e7. doi: 10.1016/j.neuron.2022.07.003
Liu, J., Tozzi, F., Waterworth, D., Pillai, S., Muglia, P., Middleton, L., et al. (2010). Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat. Genet. 42, 436–440. doi: 10.1038/ng.572
Liu, L., Zhao-Shea, R., McIntosh, J., Gardner, P., and Tapper, A. (2012). Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing α4 and α6 subunits. Mol. Pharmacol. 81, 541–548. doi: 10.1124/mol.111.076661
Mameli-Engvall, M., Evrard, A., Pons, S., Maskos, U., Svensson, T., Changeux, J., et al. (2006). Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50, 911–921. doi: 10.1016/j.neuron.2006.05.007
Mansvelder, H., and McGehee, D. (2000). Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27, 349–357. doi: 10.1016/s0896-6273(00)00042-8
Mansvelder, H., Keath, J., and McGehee, D. (2002). Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33, 905–919. doi: 10.1016/s0896-6273(02)00625-6
Mao, D., Gallagher, K., and McGehee, D. (2011). Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J. Neurosci. 31, 6710–6720. doi: 10.1523/JNEUROSCI.5671-10.2011
Markou, A., and Paterson, N. (2001). The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob. Res. 3, 361–373. doi: 10.1080/14622200110073380
Maskos, U. (2008). The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: Relevance to drugs of abuse and pathology. Br. J. Pharmacol. 153, S438–S445. doi: 10.1038/bjp.2008.5
Maskos, U., Molles, B., Pons, S., Besson, M., Guiard, B., Guilloux, J., et al. (2005). Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–107. doi: 10.1038/nature03694
McGranahan, T., Patzlaff, N., Grady, S., Heinemann, S., and Booker, T. (2011). α4β2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J. Neurosci. 31, 10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011
McKay, B., Placzek, A., and Dani, J. (2007). Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 74, 1120–1133. doi: 10.1016/j.bcp.2007.07.001
Melani, R., Von Itter, R., Jing, D., Koppensteiner, P., and Ninan, I. (2019). Opposing effects of an atypical glycinergic and substance P transmission on interpeduncular nucleus plasticity. Neuropsychopharmacology 44, 1828–1836. doi: 10.1038/s41386-019-0396-6
Moen, J., DeBaker, M., Myjak, J., Wickman, K., and Lee, A. (2021). Bidirectional sex-dependent regulation of α6 and β3 nicotinic acetylcholine receptors by protein kinase Cε. Addict. Biol. 26:e12954. doi: 10.1111/adb.12954
Morel, C., Fattore, L., Pons, S., Hay, Y., Marti, F., Lambolez, B., et al. (2014). Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol. Psychiatry 19, 930–936. doi: 10.1038/mp.2013.158
Naqvi, N., Rudrauf, D., Damasio, H., and Bechara, A. (2007). Damage to the insula disrupts addiction to cigarette smoking. Science 315, 531–534. doi: 10.1126/science.1135926
Naudé, J., Tolu, S., Dongelmans, M., Torquet, N., Valverde, S., Rodriguez, G., et al. (2016). Nicotinic receptors in the ventral tegmental area promote uncertainty-seeking. Nat. Neurosci. 19, 471–478. doi: 10.1038/nn.4223
Neugebauer, N., Henehan, R., Hales, C., and Picciotto, M. (2011). Mice lacking the galanin gene show decreased sensitivity to nicotine conditioned place preference. Pharmacol. Biochem. Behav. 98, 87–93. doi: 10.1016/j.pbb.2010.12.015
Nisell, M., Nomikos, G., Hertel, P., Panagis, G., and Svensson, T. (1996). Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 22, 369–381. doi: 10.1002/(SICI)1098-2396(199604)22:4&<369::AID-SYN8&>3.0.CO;2-9
Ostroumov, A., and Dani, J. (2018). Convergent neuronal plasticity and metaplasticity mechanisms of stress, nicotine, and alcohol. Annu. Rev. Pharmacol. Toxicol. 58, 547–566. doi: 10.1146/annurev-pharmtox-010617-052735
Peng, C., Engle, S., Yan, Y., Weera, M., Berry, J., Arvin, M., et al. (2017). Altered nicotine reward-associated behavior following α4 nAChR subunit deletion in ventral midbrain. PLoS One 12:e0182142. doi: 10.1371/journal.pone.0182142
Picciotto, M., Zoli, M., Rimondini, R., Léna, C., Marubio, L., Pich, E., et al. (1998). Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177. doi: 10.1038/34413
Pidoplichko, V., DeBiasi, M., Williams, J., and Dani, J. (1997). Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390, 401–404. doi: 10.1038/37120
Pidoplichko, V., Noguchi, J., Areola, O., Liang, Y., Peterson, J., Zhang, T., et al. (2004). Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn. Mem. 11, 60–69. doi: 10.1101/lm.70004
Pons, S., Fattore, L., Cossu, G., Tolu, S., Porcu, E., McIntosh, J., et al. (2008). Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J. Neurosci. 28, 12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008
Quina, L., Harris, J., Zeng, H., and Turner, E. (2017). Specific connections of the interpeduncular subnuclei reveal distinct components of the habenulopeduncular pathway. J. Comp. Neurol. 525, 2632–2656. doi: 10.1002/cne.24221
Ren, J., Qin, C., Hu, F., Tan, J., Qiu, L., Zhao, S., et al. (2011). Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron 69, 445–452. doi: 10.1016/j.neuron.2010.12.038
Reperant, C., Pons, S., Dufour, E., Rollema, H., Gardier, A., and Maskos, U. (2010). Effect of the alpha4beta2* nicotinic acetylcholine receptor partial agonist varenicline on dopamine release in beta2 knock-out mice with selective re-expression of the beta2 subunit in the ventral tegmental area. Neuropharmacology 58, 346–350. doi: 10.1016/j.neuropharm.2009.10.007
Rice, M., and Cragg, S. (2004). Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 7, 583–584. doi: 10.1038/nn1244
Rigotti, N., Benowitz, N., Prochaska, J., Leischow, S., Nides, M., Blumenstein, B., et al. (2023). Cytisinicline for smoking cessation: A randomized clinical trial. JAMA 330, 152–160. doi: 10.1001/jama.2023.10042
Sakkiah, S., Leggett, C., Pan, B., Guo, W., Valerio, L., and Hong, H. (2020). Development of a nicotinic acetylcholine receptor nAChR α7 binding activity prediction model. J. Chem. Inf. Model. 60, 2396–2404. doi: 10.1021/acs.jcim.0c00139
Salas, R., Main, A., Gangitano, D., and De Biasi, M. (2007). Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology 53, 863–869. doi: 10.1016/j.neuropharm.2007.08.017
Salminen, O., Murphy, K., McIntosh, J., Drago, J., Marks, M., Collins, A., et al. (2004). Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol. Pharmacol. 65, 1526–1535. doi: 10.1124/mol.65.6.1526
Sartor, C., Lessov-Schlaggar, C., Scherrer, J., Bucholz, K., Madden, P., Pergadia, M., et al. (2010). Initial response to cigarettes predicts rate of progression to regular smoking: Findings from an offspring-of-twins design. Addict. Behav. 35, 771–778. doi: 10.1016/j.addbeh.2010.03.004
Schwartz, R., Lehmann, J., and Kellar, K. (1984). Presynaptic nicotinic cholinergic receptors labeled by [3H]acetylcholine on catecholamine and serotonin axons in brain. J. Neurochem. 42, 1495–1498. doi: 10.1111/j.1471-4159.1984.tb02818.x
Sciaccaluga, M., Moriconi, C., Martinello, K., Catalano, M., Bermudez, I., Stitzel, J., et al. (2015). Crucial role of nicotinic α5 subunit variants for Ca2+ fluxes in ventral midbrain neurons. FASEB J. 29, 3389–3398. doi: 10.1096/fj.14-268102
Scott, E., Safarian, N., Casasbuenas, D., Dryden, M., Tockovska, T., Ali, S., et al. (2024). Integrating single-cell and spatially resolved transcriptomic strategies to survey the astrocyte response to stroke in male mice. Nat. Commun. 15:1584. doi: 10.1038/s41467-024-45821-y
Sheffield, E., Quick, M., and Lester, R. (2000). Nicotinic acetylcholine receptor subunit mRNA expression and channel function in medial habenula neurons. Neuropharmacology 39, 2591–2603. doi: 10.1016/s0028-3908(00)00138-6
Sherafat, Y., Bautista, M., Fowler, J., Chen, E., Ahmed, A., and Fowler, C. (2020). The interpeduncular-ventral hippocampus pathway mediates active stress coping and natural reward. eNeuro 7:0191–20.2020. doi: 10.1523/ENEURO.0191-20.2020
Tapia, L., Kuryatov, A., and Lindstrom, J. (2007). Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol. Pharmacol. 71, 769–776. doi: 10.1124/mol.106.030445
Tapper, A., McKinney, S., Nashmi, R., Schwarz, J., Deshpande, P., Labarca, C., et al. (2004). Nicotine activation of alpha4* receptors: Sufficient for reward, tolerance, and sensitization. Science 306, 1029–1032. doi: 10.1126/science.1099420
Thorgeirsson, T., Gudbjartsson, D., Surakka, I., Vink, J., Amin, N., Geller, F., et al. (2010). Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 42, 448–453. doi: 10.1038/ng.573
Threlfell, S., and Cragg, S. (2011). Dopamine signaling in dorsal versus ventral striatum: The dynamic role of cholinergic interneurons. Front. Syst. Neurosci. 5:11. doi: 10.3389/fnsys.2011.00011
Tolu, S., Eddine, R., Marti, F., David, V., Graupner, M., Pons, S., et al. (2013). Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol. Psychiatry 18, 382–393. doi: 10.1038/mp.2012.83
Trigo, J., Zimmer, A., and Maldonado, R. (2009). Nicotine anxiogenic and rewarding effects are decreased in mice lacking beta-endorphin. Neuropharmacology 56, 1147–1153. doi: 10.1016/j.neuropharm.2009.03.013
Tuesta, L., Chen, Z., Duncan, A., Fowler, C., Ishikawa, M., Lee, B., et al. (2017). GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat. Neurosci. 20, 708–716. doi: 10.1038/nn.4540
Villanueva, H., James, J., and Rosecrans, J. (1989). Evidence of pharmacological tolerance to nicotine. NIDA Res. Monogr. 95, 349–350.
Wen, L., Yang, Z., Cui, W., and Li, M. (2016). Crucial roles of the CHRNB3-CHRNA6 gene cluster on chromosome 8 in nicotine dependence: Update and subjects for future research. Transl. Psychiatry 6:e843. doi: 10.1038/tp.2016.103
Wolfman, S., Gill, D., Bogdanic, F., Long, K., Al-Hasani, R., McCall, J., et al. (2018). Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum. Nat. Commun. 9:2710. doi: 10.1038/s41467-018-04654-2
Wooltorton, J., Pidoplichko, V., Broide, R., and Dani, J. (2003). Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 23, 3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003
Yan, Y., Beckley, N., Kim, V., and Drenan, R. (2019). Differential nicotinic modulation of glutamatergic and GABAergic VTA microcircuits. eNeuro 6:0298–19.2019. doi: 10.1523/ENEURO.0298-19.2019
Yang, K., McLaughlin, I., Shaw, J., Quijano-Cardé, N., Dani, J., and De Biasi, M. (2023). CHRNA5 gene variation affects the response of VTA dopaminergic neurons during chronic nicotine exposure and withdrawal. Neuropharmacology 235:109547. doi: 10.1016/j.neuropharm.2023.109547
Zhang, T., Zhang, L., Liang, Y., Siapas, A., Zhou, F., and Dani, J. (2009). Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J. Neurosci. 29, 4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009
Keywords: nicotine, nAChRs, VTA-NAc, MHb-IPN, addiction
Citation: Jiang J, Li X, Hu A-f, Zhou G-j, Gao Y-h, Xu C, Wu X-m and Wang H-J (2025) Nicotine and neuronal nicotinic acetylcholine receptors: unraveling the mechanisms of nicotine addiction. Front. Neurosci. 19:1670883. doi: 10.3389/fnins.2025.1670883
Received: 22 July 2025; Accepted: 30 September 2025;
Published: 17 October 2025.
Edited by:
Lei-Yun Wang, Wuhan No.1 Hospital, ChinaReviewed by:
Styliani (Stella) Vlachou, Dublin City University, IrelandFatimah Almahasneh, Yarmouk University, Jordan
Copyright © 2025 Jiang, Li, Hu, Zhou, Gao, Xu, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Juan Wang, cmVkYnJpMjAxM0AxNjMuY29t
 Jian Jiang1
Jian Jiang1 Hong-Juan Wang
Hong-Juan Wang