- 1Department of Robotics, Nazarbayev University, Astana, Kazakhstan
- 2Department of Computer Science, Nazarbayev University, Astana, Kazakhstan
Deep learning has emerged as a transformative tool for the automated detection and classification of seizure events from intracranial EEG (iEEG) recordings. In this review, we synthesize recent advancements in deep learning techniques including convolutional neural networks (CNN), recurrent neural networks (RNN) with long short term memory (LSTM) units, and transformer based architectures that enable accurate localization of epileptogenic zones (EZ) in drug resistant epilepsy. These approaches effectively extract spatial and temporal features from raw iEEG signals to detect epileptiform discharges (ED) including seizures alongside other electro-physiological biomarkers such as high-frequency oscillations (HFO). Importantly, beyond relying solely on these traditional markers, several studies have indicated direct seizure detection by modeling ictal and preictal dynamics. Such methods capture alternative biomarkers including spectral changes, connectivity patterns, and complex temporal signatures that directly reflect seizure activity. Although deep learning models often achieve high accuracy, they continue to face several challenges due to data scarcity, heterogeneity in iEEG acquisition, inconsistent preprocessing protocols, and limited model interpretability. We also highlight emerging integrative strategies that combine multimodal neuroimaging data with deep learning analyses as well as neuromorphic computing techniques designed for real-time clinical application. Addressing these limitations has significant potential for surgical planning, reducing diagnostic subjectivity, and ultimately enhancing patient outcomes in epilepsy care.
1 Introduction
Epilepsy is a major neurological disorder affecting approximately 65 million people worldwide, imposing a substantial global health burden (Dash et al., 2022; Milligan, 2021). Characterized by recurrent, unprovoked seizures, this chronic condition disrupts daily functioning and adversely impacts cognitive performance, psychosocial integration, and overall quality of life. Although anti-seizure medications (ASM) serve as the basis of treatment, about one-third of patients show drug resistant epilepsy (DRE) experiencing persistent seizures despite appropriate pharmacological intervention (Worrell and Gotman, 2011; Enatsu and Mikuni, 2016). Such people face limited therapeutic alternatives which leads to rising morbidity and highlights the necessity for alternative techniques. In cases of drug resistant epilepsy, surgical intervention can be curative, although its effectiveness depends on accurately identifying and completely resecting the epileptogenic zone (EZ), defined as the smallest cortical region whose removal yields seizure freedom (Frauscher, 2020; Jobst et al., 2020). Achieving this precision means finding a balance between increasing seizure control and avoiding disturbance of healthy functionally necessary brain tissue.
Traditionally, EZ localization has depended predominantly on expert visual inspection of intracranial electroencephalography (iEEG) recordings (Flanary et al., 2022; Sperling, 1997). Although this procedure is an established clinical standard, it requires considerable effort and expertise due to the size and complexity of the data. Moreover, the process can be highly subjective. Multiple studies have identified significant inter-expert variability in labeling iEEG patterns potentially leading to inconsistent outcomes in surgical planning and patient care (Flanary et al., 2022; Weiss et al., 2019). Such variability highlights the need for computational tools particularly deep learning (DL) methods that can reduce subjectivity, enhance reproducibility, and improve clinical decision making.
While automated seizure detection excels at identifying ictal events in long-term iEEG recordings reducing review time for clinicians, its primary clinical value lies in supporting the localization of the epileptogenic zone (EZ) and seizure onset zone (SOZ). The primary goal of epilepsy surgery is to identify the smallest cortical area whose removal eliminates seizures, while balancing effectiveness with the preservation of critical tissue (Nissen et al., 2021). Deep learning addresses this by extracting spatiotemporal patterns that associate detected events with EZ boundaries. Nevertheless, detection alone does not determine surgical margins.
In response, researchers have increasingly developed automated and semi-automated approaches to facilitate EZ localization (Bernabei et al., 2023a; Ahmedt-Aristizabal et al., 2017). Advances in computational neuroscience have accelerated this shift, showing the potential of deep learning in processing complex iEEG signals. Specifically, deep learning enables automated feature extraction from raw input data, thus showing the complex spatiotemporal dynamics of neural activity (Mirchi et al., 2022). Transformer based architectures in particular have started to gain attention for seizure detection and iEEG classification (Potter et al., 2022; Wang et al., 2021; Zhang X. et al., 2024; Sun et al., 2022; Yang et al., 2024) extending the capabilities of more traditional CNN and RNN based methods (Antoniades et al., 2017, 2016; Liu et al., 2019; Zuo et al., 2019; Ren et al., 2021; Lai et al., 2019).
Among the most notable applications of deep learning in this domain is the automated detection and classification of seizure events. Such events are believed to represent key pathophysiological processes leading to irregular neural activity and the formation and spreading of epileptic seizures. Their correlation with the epileptogenic zone has caused an increased focus on deploying deep learning techniques not only for accurate seizure identification but also for differentiating pathological signals from normal background patterns (Lai et al., 2019; Liu et al., 2016). Seizure signals show significant heterogeneity causing substantial difficulties for conventional signal processing methods. Modern deep learning architectures including transformer based networks provide an effective solution to these challenges by directly modeling complex temporal dependencies (Wang et al., 2021; Potter et al., 2022; Zhang X. et al., 2024). Although traditional methods using Long Short Term Memory (LSTM) networks or CNN have exceeded 90% accuracy in identifying epileptiform activity (Medvedev et al., 2019), emerging alternatives ranging from neuromorphic systems to hyperdimensional computing are also showing potential for real-time, low-power detection, potentially suitable for implantable surgical devices (Schindler and Rahimi, 2021; Burrello et al., 2018, 2019). Deep Learning not only detects events but simulates epileptogenicity indices (EI) by scoring channel contributions to EZ propagation, guiding resection margins and predicting postoperative seizure reduction (Yamamoto et al., 2021; Zhang et al., 2021).
Nevertheless, recent studies highlight the importance of standardizing automated seizure detection methods and performing strict clinical validation (Bernabei et al., 2023a; Abibullaev et al., 2010; Ahmedt-Aristizabal et al., 2017; Sindhu et al., 2020; Dimakopoulos et al., 2022). These initiatives highlight the challenges in selecting and validating appropriate algorithms for clinical use while also providing guidelines for safely integrating advanced computational methods into epilepsy surgery workflows. This review offers a structured examination of methodologies for seizure detection and classification including transformer based models, RNN, CNN, and emerging alternative architectures. We discuss the limitations of conventional iEEG analysis and automated approaches, propose potential solutions, and outline future research directions to strengthen clinical implementation and improve surgical planning outcomes. Figure 1 shows the whole workflow from iEEG acquisition to surgical decision making. The subsequent sections explore the significance of seizure detection (Section 3), review current deep learning strategies (Section 4), current challenges and ethical considerations (Section 5), and conclude by outlining prospective developments (Section 6).
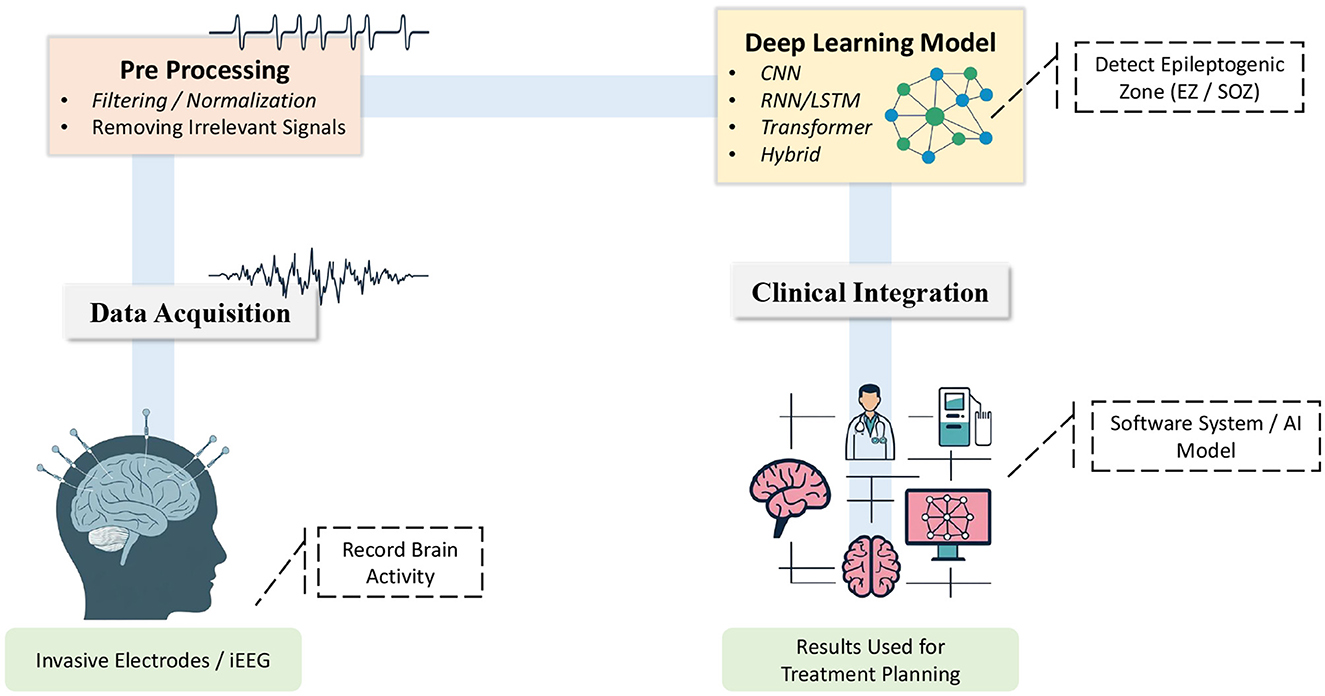
Figure 1. An overview of the workflow for detecting epileptogenic zones using deep learning. (1) Data Acquisition: Brain signals are recorded through electrodes. (2) Preprocessing: Noise is removed and signals are downsampled to retain clinically relevant information. (3) Deep Learning Model: A neural network architecture is applied to detect epileptogenic zones (EZ/SOZ). (4) Clinical Integration: The model results inform treatment planning enabling clinicians to utilize the identified zones to direct therapeutic actions.
2 Literature search methodology and strategy
A systematic literature review was undertaken to identify and analyze studies applying deep learning (DL) methods to seizure detection within the context of epilepsy surgery. The review specifically targeted peer-reviewed journal articles and conference proceedings that utlized intracranial EEG (iEEG) data to develop, evaluate, or clinically integrate DL based techniques. Comprehensive searches were conducted across multiple academic databases, including PubMed, IEEE Xplore, Scopus, and Web of Science using various keyword combinations coupled with Boolean operators focusing on literature published in the last five years. Principal search queries included:
• “Deep Learning” AND “Epilepsy Surgery”
• “Intracranial EEG (iEEG)” AND “Deep Learning”
• “Epileptiform” AND “Seizure” AND “iEEG” AND “Deep Learning”
• “High Frequency Oscillation (HFO)” AND “Epilepsy” AND “iEEG” AND “Deep Learning”
Additional targeted terms such as “CNN”, “RNN”, “LSTM”, “Transformer”, and “Neuromorphic Computing” were also used to refine the scope. Articles referenced within the bibliographies of selected studies were subsequently examined to ensure a comprehensive collection of relevant literature.
The screening and selection process followed explicit inclusion and exclusion criteria (Figure 2) and involved multiple stages. Initially, titles and abstracts were inspected to exclude clearly unrelated works. Next, the full texts of potentially relevant papers were examined to confirm their alignment with the review objectives. Finally, only those that met all inclusion criteria were retained for detailed comparative analysis. From each selected study, key methodological and outcome details were extracted including the size and characteristics of the dataset, the type of deep learning architecture utilized (CNN, RNN/LSTM, Transformer based models), and performance metrics such as accuracy, sensitivity, specificity, or area under the curve (AUC). This structured approach enabled a thorough synthesis of recent advancements, ongoing methodological challenges, and future research prospects in deep learning based seizure analysis for epilepsy surgery.
3 Electrophysiological biomarkers in epilepsy
High frequency oscillations (HFO) which are considered a distinct type of epileptiform discharge (ED) along with other ED patterns are important electrophysiological biomarkers in current epilepsy research and clinical practice (Krikid et al., 2023; Smith et al., 2022).
HFO, typically defined as transient events in the 80–500 Hz range, are thought to result from synchronized neuronal firing within the epileptogenic zone (EZ) (Worrell and Gotman, 2011; Kobayashi et al., 2017). These oscillations can appear during interictal and ictal periods and are usually classified into ripples (80–250 Hz) and fast ripples (250–500 Hz) (Jacobs et al., 2012; Lee et al., 2019; Ye et al., 2023). More evidence suggests that HFO rich regions overlap significantly with the EZ making these oscillations valuable indicators for localizing seizure onset zones in surgical candidates (Zhang et al., 2021; Qing et al., 2024).
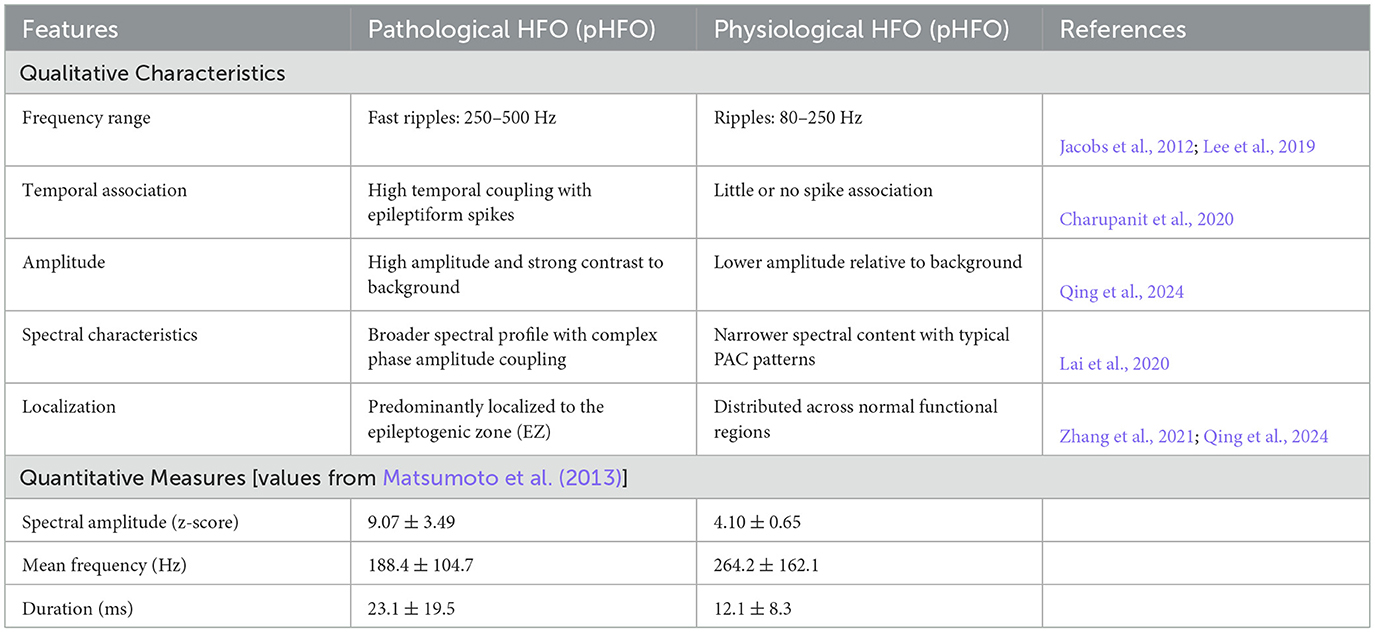
An ongoing challenge in applying HFO to clinical practice involves distinguishing pathological HFO (pHFO) from physiological HFO (pHO) (Cimbalnik et al., 2018). Both share similar frequency ranges although originate from different underlying processes (Weiss et al., 2019; Zhang et al., 2022). Table 1 summarizes the commonly used morphological (e.g., amplitude, duration) and spectral (e.g., power distribution, phase amplitude coupling) features utilized to differentiate pHFO from pHO (Weiss et al., 2019; Sindhu et al., 2024; Lai et al., 2020). This differentiation is essential for ensuring that surgical resections target only epileptogenic tissue thereby minimizing the risk of normal cognitive functions.
To complement the quantitative summary in Table 1 and Figure 3 provides a visual overview of how high-frequency oscillations (HFO) are characterized in epilepsy research. It highlights typical ripple and fast ripple waveforms, contrasts pathological and physiological HFO morphologies, and shows their expression in time–frequency space and through phase—amplitude coupling (PAC). The lower panels summarize key quantitative metrics reported in the literature, including spectral amplitude, mean frequency, and event duration, which help distinguish pathological from physiological activity.
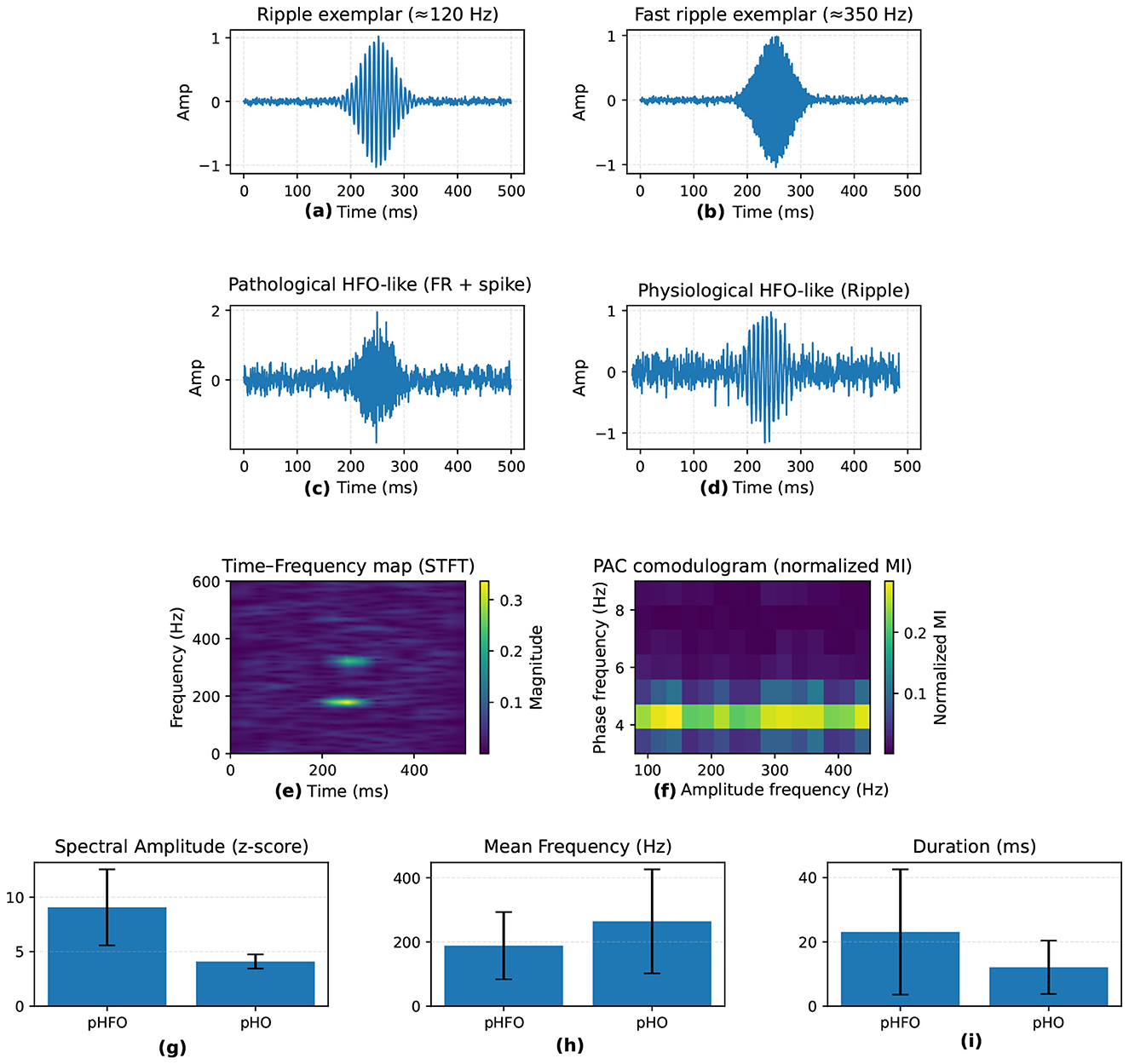
Figure 3. Visual summary of HFO in epilepsy. (a, b) Time-domain examples of ripples (80–250 Hz) and fast ripples (250–500 Hz). (c, d) Pathological HFO (pHFO) show spike association and higher amplitude, while physiological HFO (pHO) appear in normal networks. (e) Time-frequency map with transient HFO bursts; (f) phase-amplitude coupling (PAC). (g–i) Quantitative feature comparisons (spectral amplitude, mean frequency, duration).
The EZ includes the seizure onset zone (SOZ; early ictal involvement), regions prominent in high-frequency oscillations (HFO; indicative of hypersynchronous firing), and irritative zones (interictal spike generators that may extend beyond the SOZ) (Cai et al., 2021). Deep learning identifies epileptogenic zones (EZ) by integrating them through multi-task models: for instance, convolutional neural networks (CNN) extract spatial high-frequency oscillation (HFO) patterns, while transformers assess the temporal propagation of the seizure onset zone (SOZ) in relation to irritative activity, producing channel-specific scores (e.g., through attention maps) that identify resectable tissue. This reduces qualitative variability in visual inspection; however, validation against postoperative outcomes is important for clinical confidence (Broti et al., 2025).
Epileptiform discharges (ED) including spikes, sharp waves, and spike-and-wave complexes are likewise fundamental to understanding epilepsy pathophysiology and guiding treatment strategies (Zhao et al., 2018; Jaoude et al., 2019; Kural et al., 2020). ED generally present as temporary high amplitude waveforms (approximately 20–70 ms in duration) sometimes followed by slow-wave components (Falach et al., 2024). These events are examined in terms of morphological, temporal, and spectral properties to determine their clinical significance. Table 2 outlines qualitative and quantitative measures that assist in differentiating pathological ED from benign variants (Zhao et al., 2018; Yamamoto et al., 2021). Although manual iEEG analysis remains a clinical benchmark, emerging automated tools utilizing deep learning show potential in reducing manual work and lead to more consistent evaluations (Kolodziej et al., 2023; Zhao et al., 2022b).
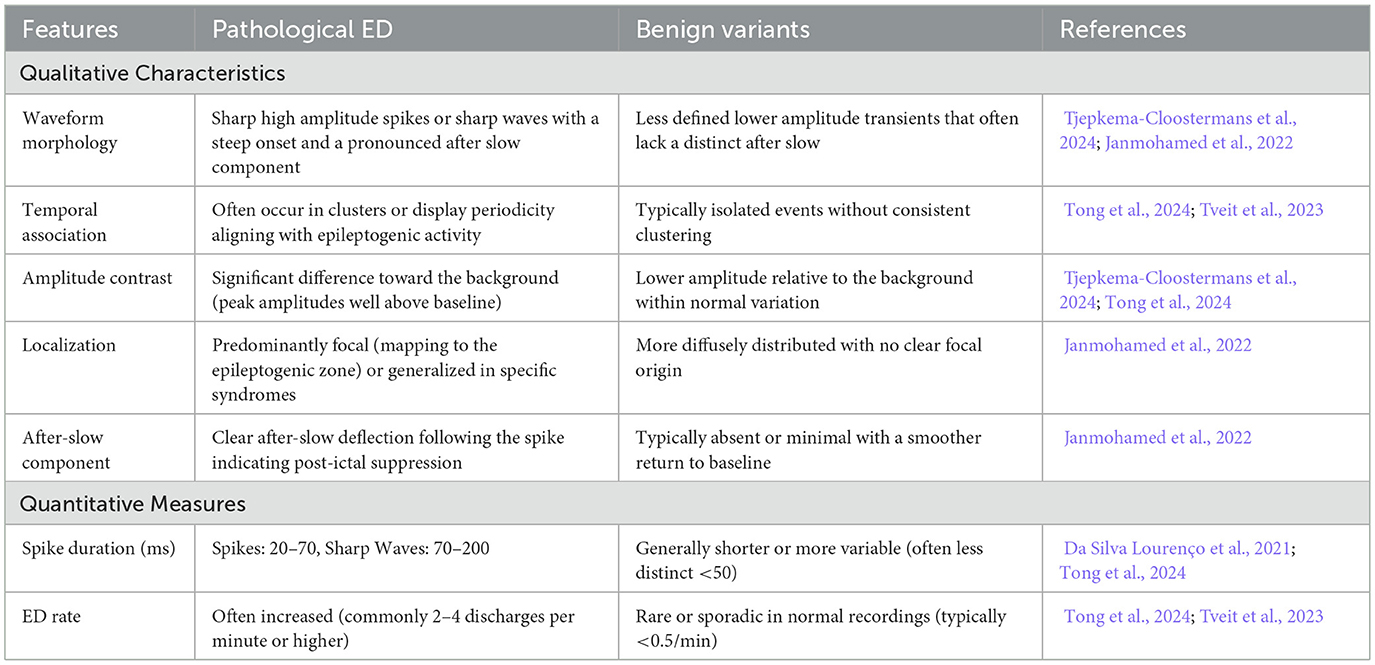
Table 2. Morphological and quantitative distinctions between pathological and benign epileptiform discharges.
Overall, reliable detection and differentiation of HFO and ED are essential for refining epilepsy diagnostics and surgical interventions. Advances in computational neuroscience and large scale data analytics continue to drive improvements in the accuracy, consistency, and clinical relevance of these electrophysiological biomarkers.
4 Deep learning for detection of HFO, epileptiform discharge, and seizure dynamics
Traditional machine learning and signal processing techniques have significantly advanced automated seizure detection, particularly in identifying high-frequency oscillations (HFO) and epileptiform discharges (ED) (Abibullaev et al., 2009; Weiss et al., 2019; Sharifshazileh et al., 2021; Burelo et al., 2022; Burrello et al., 2019; Schindler and Rahimi, 2021; Krikid et al., 2021; Lachner-Piza et al., 2019; Guo et al., 2021; Sciaraffa et al., 2020; Sindhu et al., 2020; Dimakopoulos et al., 2022; Wong et al., 2020; Donos et al., 2020; Sindhu et al., 2024; Li et al., 2021b). However, these approaches face persistent challenges. They typically depend on manual feature extraction and extensive parameter tuning which limit robustness and generalizability across heterogeneous iEEG datasets. In addition, balancing sensitivity and specificity often requires complex post-processing or careful recalibration for different seizure biomarkers.
Recent advances in deep learning (DL) represent a paradigm shift by enabling end-to-end feature learning directly from raw iEEG signals (Hoogteijling and Zijlmans, 2021). Unlike traditional methods that rely on manual features, DL architectures learn rich, nonlinear representations that can capture complex seizure related patterns including HFO, ED, and subtler biomarkers (Wang et al., 2021). Various architectures have been explored, including convolutional neural networks (CNN), recurrent neural networks (RNN), and more recently transformer based models which can effectively model the spatio-temporal dynamics of epileptic events (Geng et al., 2021; Singh and Malhotra, 2022; Wang et al., 2024b; Ma et al., 2018; Zuo et al., 2019; Ma et al., 2019; Sadek et al., 2023; Liu et al., 2019; Zhao B. et al., 2020; Ren et al., 2021; Zanghieri et al., 2021; Wang et al., 2020; Abderrahim et al., 2023). Transformers are particularly promising due to their self-attention mechanisms which model long-range dependencies and contextual relationships while supporting parallel processing (Guo et al., 2022; Vaswani et al., 2017). To improve clinical applicability, several studies have proposed lightweight CNN and transformer networks optimized for patient-independent real-time seizure detection (Si et al., 2023; Yang et al., 2024; Sun et al., 2022; Potter et al., 2022). Hybrid frameworks that combine CNN with RNN or transformers aim to utilize each model strengths, CNN for spatial representation and RNN or transformers for temporal dependencies resulting in more robust and adaptable detection systems (Ouichka et al., 2022; Sun L. et al., 2024).
Recent DL approaches have also focused on localizing the epileptogenic zone (EZ) through probabilistic mapping. Hybrid CNN-LSTM models, for instance, assign EZ likelihood scores to individual channels by linking HFO/ED density with seizure onset zone (SOZ) propagation, often analyzed via Granger causality (Wang et al., 2024b). Attention mechanisms further refine these models by reducing the influence of non-propagating spikes, generating saliency heatmaps to guide surgical inspection (Gunnarsdottir et al., 2022; Nejedly et al., 2025). Transformer based models extend the classic epileptogenicity index (EI) by learning dynamic, channel-wise margins: multi-head attention layers capture HFO-SOZ correlations, while adaptive thresholds minimize irritative zone spillover (Yamamoto et al., 2021). Such techniques enable predictive modeling of surgical outcomes in drug-resistant epilepsy by identifying interictal epileptogenic networks, guiding resections to maximize overlap with these networks and reduce distance from resected tissue. Reported performance reaches an AUC of 0.85-0.86 for predicting ≥50% seizure reduction and Engel Class I/II outcomes (Partamian et al., 2025). Key challenges remain in validating these models for multifocal epilepsy where resection margins are inherently ambiguous.
Beyond HFO and ED, DL has expanded to capture ictal dynamics directly, offering insight into seizure onset and progression critical for surgical planning (Ma et al., 2018; Singh and Malhotra, 2022; Dwaraka et al., 2021; Sun Y. et al., 2024; Zhao X. et al., 2020; Zhang and Zhou, 2023). For example, time-aware RNN effectively model the temporal evolution of ictal states (Palanichamy and Ahamed, 2022) while autoencoder based frameworks have distinguished focal from non-focal epilepsy biomarkers (Jangde et al., 2023). Advanced time-frequency methods such as spectral envelope analysis further enrich representations by characterizing power changes during seizures (Zhang and Zhou, 2023). Ensemble models that integrate CNN, SVM, and LSTM outputs have achieved superior accuracy by combining complementary strengths (Usman et al., 2021). Other hybrid designs for example, reconstruction independent component analysis (R-ICA) combined with LSTM networks reduce computational complexity while maintaining performance (Dwaraka et al., 2021).
Finally, DL based architectures contribute to EZ/SOZ localization by clustering high-density biomarkers (e.g., HFO during seizures) to specific channels, thereby supporting surgical decision-making (see Section 5). Figure 4 summarizes common deep learning paradigms for seizure detection and classification, illustrating how CNN, RNN/LSTM, and transformers are utilized to model the complex spatio-temporal structure of iEEG.
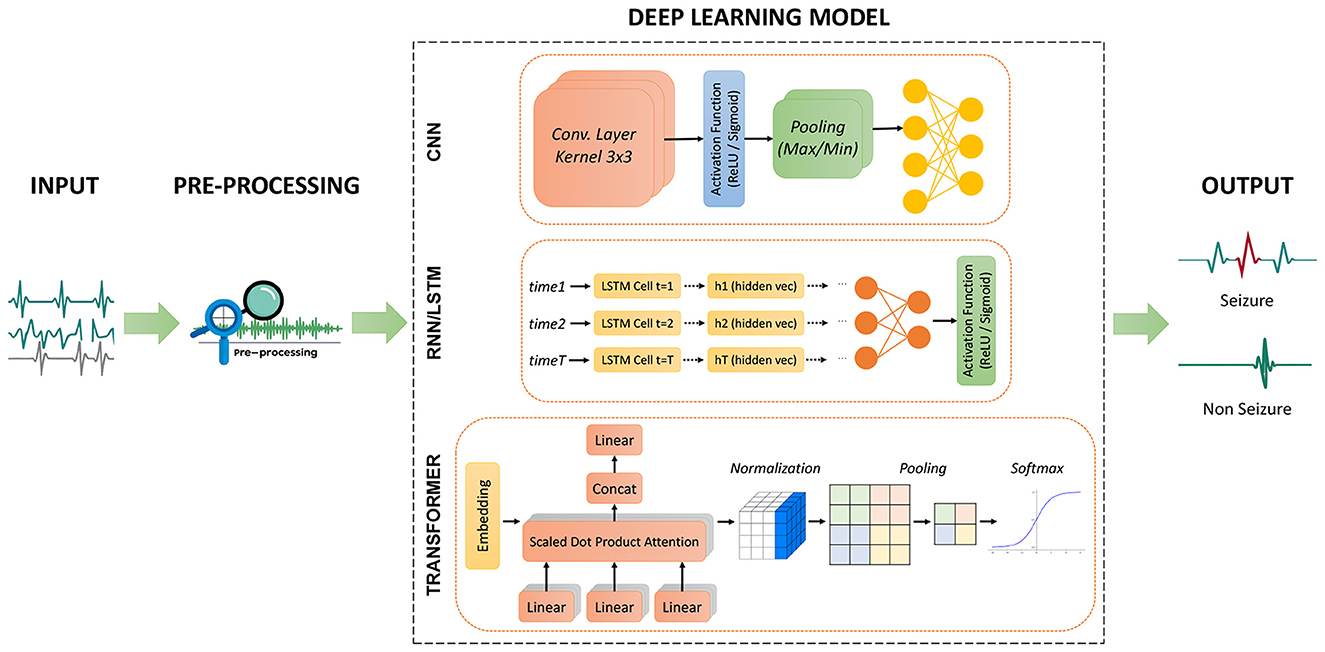
Figure 4. Overview of three deep learning architectures for seizure detection and classification. CNN: Convolutional layers extract features from raw signals, followed by activation, pooling, and fully connected layers for classification. RNN/LSTM: Sequential modeling of preprocessed iEEG captures temporal dependencies before final classification. Transformer: Self-attention layers model long-range dependencies followed by normalization and softmax classification.
4.1 CNN: advanced architectures for spatiotemporal feature extraction
CNN have gained significant attention for their efficacy in analyzing iEEG data due to their capacity for extracting both spatial and temporal features (Lai et al., 2019; Ren et al., 2021; Zuo et al., 2019). By stacking convolutional and pooling layers, CNN can learn complex representations directly from raw signals. These representations are important for detecting HFO, ED, and related biomarkers essential to localizing the epileptogenic zone (EZ). Additionally, the high sensitivity and specificity that CNN based models achieved make them suitable for real-time clinical applications. Many recent studies have refined CNN based algorithms for seizure detection and classification as summarized in Tables 3, 4.
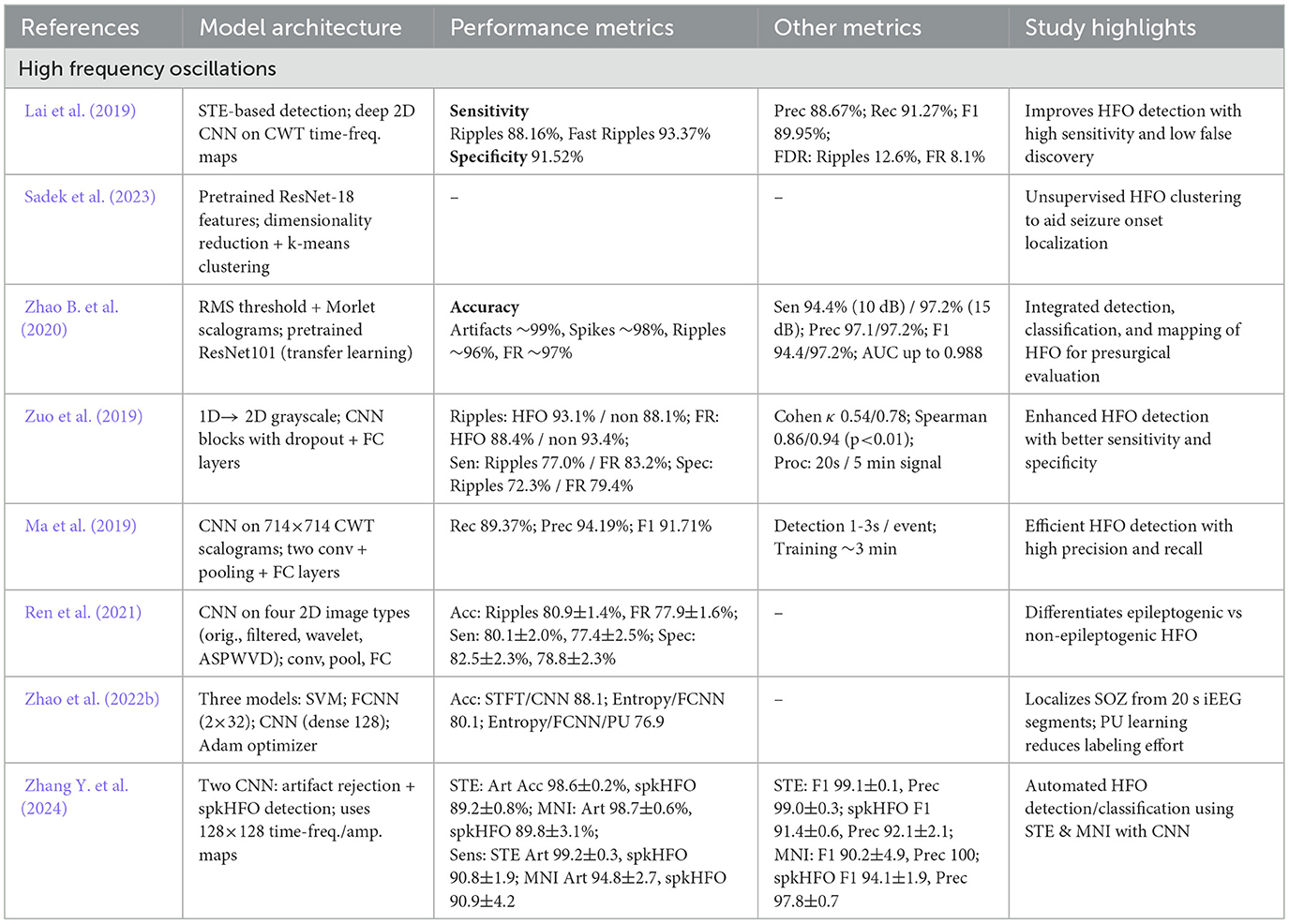
Table 3. Overview and performance comparison of CNN based deep learning architectures for seizure detection and classification.
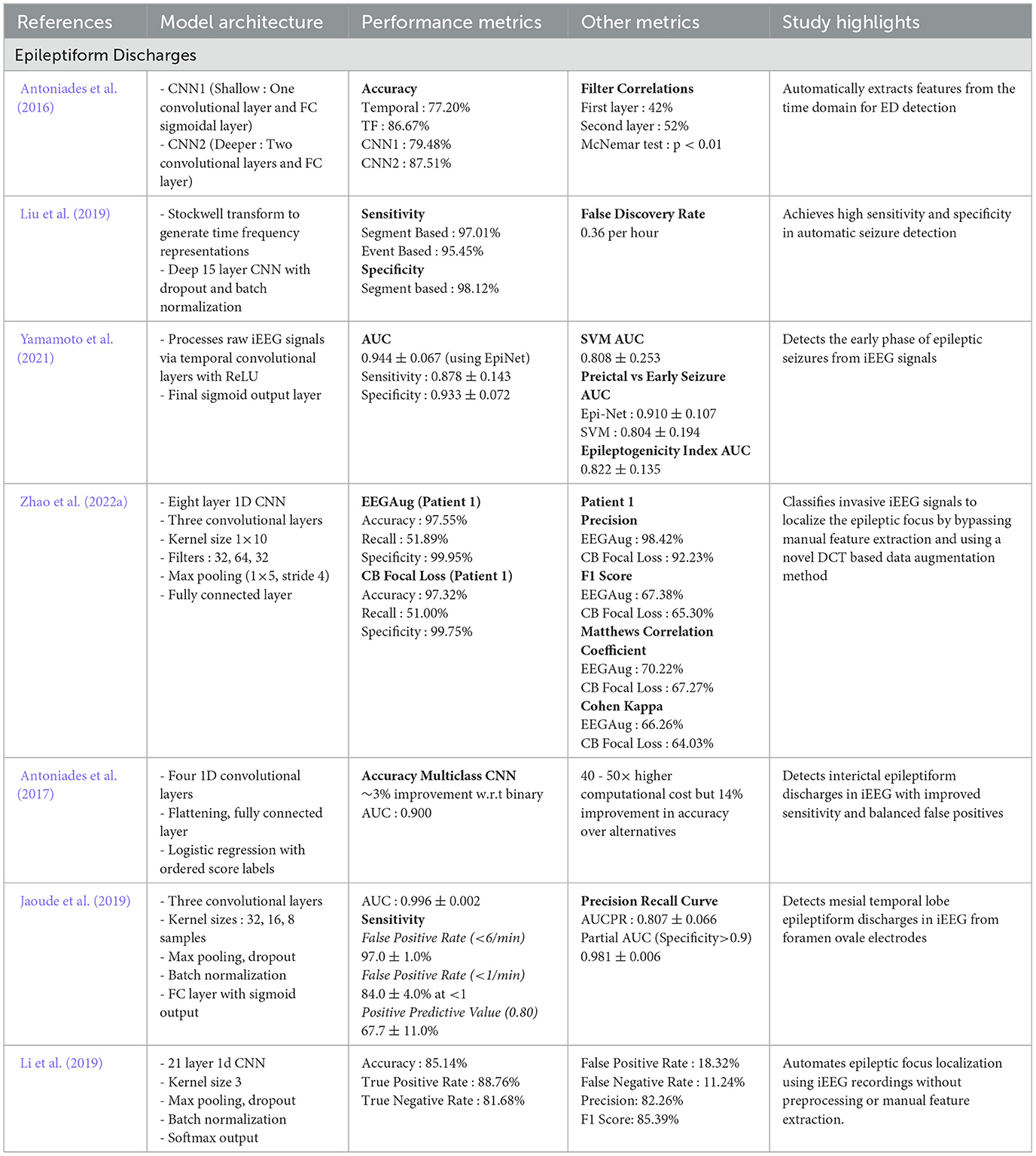
Table 4. Overview and perfromance comparison of CNN based deep learning architectures for seizure detection and classification.
4.1.1 HFO detection via CNN
In a study, Ma et al. (2019) proposed a CNN based framework for HFO detection in iEEG by converting 80 - 500 Hz signals into time-frequency scalograms. Trained on 18,000 labeled samples, their five-layer CNN achieved 94.19% precision. Sadek et al. (2023) proposed an unsupervised clustering framework using a temporal basis set, dimensionality reduction, and a deep cluster module to separate HFO from artifacts and ripple peaks, successfully identifying the seizure onset zone (SOZ) for potential surgical guidance. Zhao B. et al. (2020) developed a model combining bandpass filtering, amplitude thresholding, and time-frequency scalograms, classified by four ResNet101 CNN into artifacts, spikes, ripples, and fast ripples. Similarly, Zuo et al. (2019) transformed 1D iEEG signals into 2D grayscale images and used a pyramidal CNN to classify ripples and fast ripples from non-HFO.
Furthermore, Zhao et al. (2022b) extended CNN for SOZ localization by segmenting iEEG into 20s intervals, extracting spectral and entropy features, and comparing classifiers (CNN, SVM, FCNN). Using positive-unlabeled learning to limit labeling needs, PU learning achieved 76.91% with only 15.87% labeled data. More recently, Ren et al. (2021) proposed a multi-stream CNN that processes raw and filtered waveforms, wavelet spectrograms, and smoothed pseudo Wigner-Ville distributions to distinguish EZ from non-EZ HFO. Lai et al. (2019) similarly combined short time energy (STE) estimation with a CNN architecture using filters of various sizes. Expanding on these advances, Zhang Y. et al. (2024) introduced PyHFO, an open-source platform with CNN based classifiers and fast detection algorithms to distinguish artifacts from true HFO and separate spike-associated from non-spike HFO. Tested on iEEG datasets, PyHFO achieved up to 50x faster performance than MATLAB tools like RIPPLELAB.
4.1.2 Epileptiform discharge analysis via CNN
Antoniades et al. (2017) introduced a CNN based approach for detecting ED. By relying on continuous score labels rather than binary classifications, they improved both sensitivity and the ability to capture salient ED morphologies thereby enhancing clinical interpretability. In an earlier study, Antoniades et al. (2016) showed that CNN could effectively learn features for subject independent ED classification training on data from 25 patients without manual feature engineering. Similarly, Jaoude et al. (2019) achieved area under the curve (AUC = 0.996) in detecting mesial temporal lobe ED using a CNN trained on large augmented datasets from 46 patients.
In related work, Li et al. (2019) presented a one dimensional CNN that classifies focal versus non-focal signals directly from raw iEEG recordings achieving 85.14% accuracy. Meanwhile, Liu et al. (2019) combined Stockwell transform (S-transform) with a 15-layer CNN achieved high sensitivity (97.01%) and specificity (98.12%) across a public database of 21 patients. Yamamoto et al. (2021) developed EpiNet, a CNN architecture that outperformed an SVM baseline with 0.944 AUC, and introduced a data driven epileptogenicity index (d-EI) correlating with surgical outcomes. Further simplifying the approach, Zhao et al. (2022a) proposed a one dimensional CNN that operates directly on raw iEEG data combining a novel discrete cosine transform (DCT) based data augmentation method to address limited labeled data achieving high classification performance on the Juntendo iEEG dataset.
4.2 RNN and LSTM: modeling temporal dependencies
RNN have an important role for modeling the complex temporal dependencies of iEEG signals with LSTM architectures being particularly effective in addressing seizure detection challenges (Medvedev et al., 2019). By maintaining internal states and combining of memory cells through feedback loops, RNN offer a robust mechanism to capture transient neural events including HFO and ED (Rezk et al., 2020). LSTM networks, a specialized variant of RNN, minimize the problem of vanishing gradients through gating mechanisms (input, forget, and output gates) thus preserving both short and long term temporal dependencies (Lindemann et al., 2021). Table 5 present an overview of RNN/LSTM based architectures for seizure detection across different iEEG biomarkers.
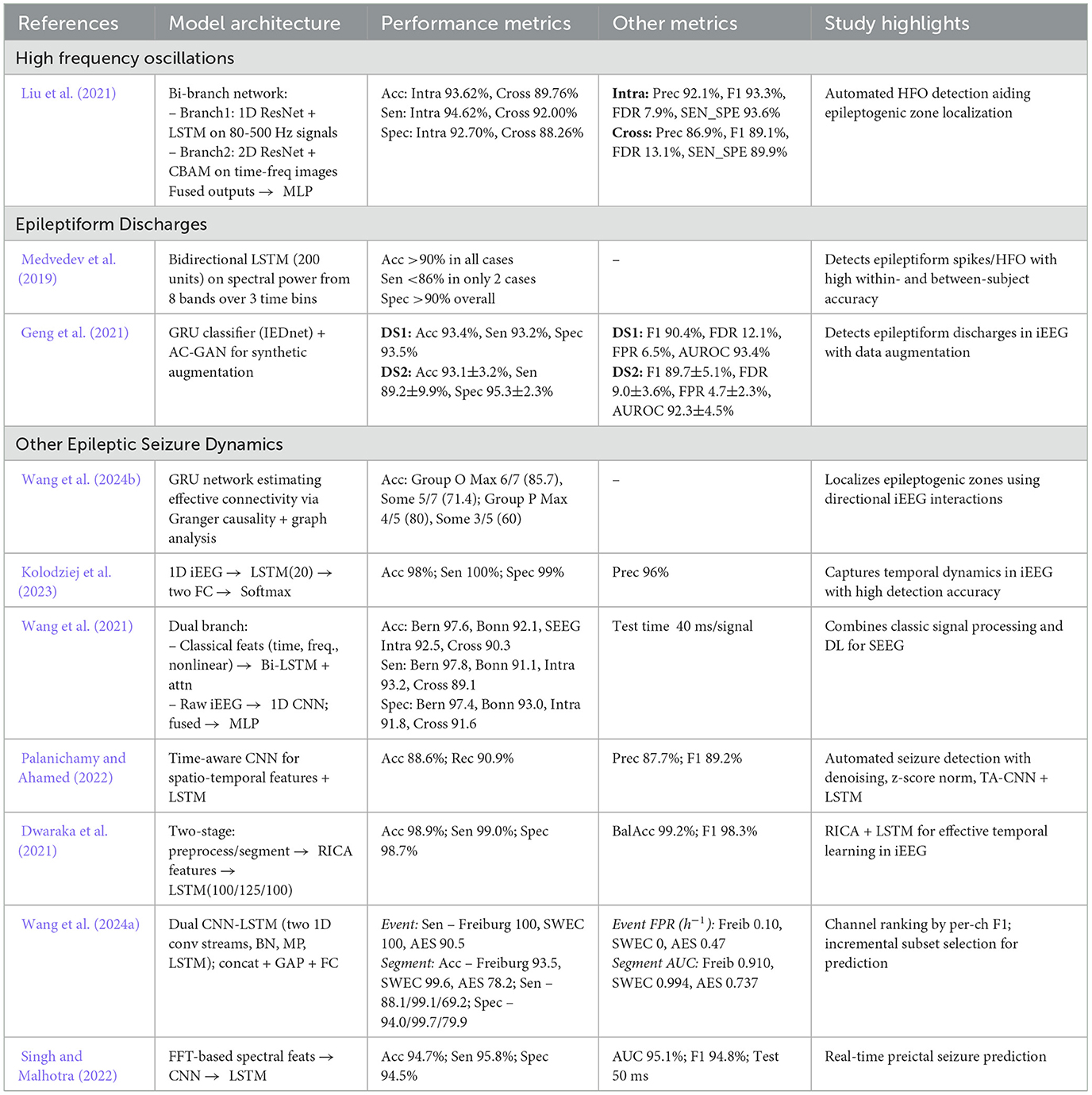
Table 5. Overview and performance comparison of RNN/LSTM based deep learning architectures for seizure detection and classification.
4.2.1 HFO and ED analysis via RNN/LSTM
In a study, Liu et al. (2021) proposed a hybrid bi-branch neural network for HFO detection, combining a 1D ResNet-LSTM on bandpass-filtered signals with a 2D ResNet-CBAM on wavelet transforms. A multilayer perceptron integrated these features, achieving high sensitivity, specificity, and accuracy in both intra and cross subject tests. In another study, Geng et al. (2021) presented IEDNet which integrates LSTM, GRU, and an auxiliary classifier GAN to detect interictal epileptiform discharges (ED). Synthetic data augmentation further boosts detection robustness. Medvedev et al. (2019) similarly demonstrated the efficacy of bi-directional LSTM networks in capturing multiple forms of epileptiform activity including spikes and ripples achieving overall accuracies exceeding 90%.
4.2.2 Seizure dynamics modeling via RNN/LSTM
Wang et al. (2021) proposed a multi-branch fusion network with a Bi-LSTM and attention mechanism, utilizing bidirectional temporal context and feature weighting to outperform single direction LSTM in distinguishing epileptogenic from non-epileptogenic signals. Kolodziej et al. (2023) combined wavelet based higher order statistics and chaos theory measures with an LSTM classifier achieving seizure detection accuracies exceeding 98%. Singh and Malhotra (2022) proposed a frequency band decomposition method that feeds iEEG signals into both CNN and LSTM architectures for seizure prediction. Wang et al. (2024b) utilized Gated Recurrent Units (GRUs) within a Granger causality framework to localize ictal onsets and analyze network connectivity patterns in iEEG data.
Similarly, Wang et al. (2024a) introduced a dual CNN-LSTM model that utilizes a channel reordering approach achieving 100% event based sensitivity on two standard iEEG datasets. Other hybrid systems include TA-CNN-RNN (Palanichamy and Ahamed, 2022) where a time-aware CNN integrates spatial and temporal feature extraction before passing outputs to an LSTM based classifier achieving accuracies of 88.6% on the Bonn dataset. Dwaraka et al. (2021) combined Reconstruction Independent Component Analysis (RICA) with LSTM layers (RICA-LSTM) attaining an accuracy of 98.92%. These results collectively highlights the capacity of LSTM based models to precisely capture short and long-term dependencies in iEEG data thereby facilitating more reliable detection of diverse seizure biomarkers. RNN and LSTM have shown their effectiveness in identifying temporal patterns in iEEG recordings enhancing accuracy in detecting HFO, ED, and other seizure related events. Although modern LSTM implementations are sufficiently optimized for potential real-time applications, further work remains necessary to optimize deployment in resource constrained clinical environments particularly where on-device processing or ultra-low-latency inference is critical.
4.3 Transformer models: capturing long-range temporal context
Transformer based architectures originally developed for natural language processing (Vaswani et al., 2017; Kumar and Khan, 2023; Anwar et al., 2024) are increasingly being adopted in biomedical signal analysis for their capability to model extensive temporal dependencies in parallel. Unlike RNN which process data sequentially, transformers utilize a multi head self-attention mechanism to capture complex relationships across entire sequences supported by positional encodings that preserve temporal order (Lin et al., 2022). These properties are particularly advantageous for seizure detection where ictal events and interictal patterns may span variable durations and occur at multiple time scales.
The success of transformer models for seizure analysis comes from key architectural advantages. First, multi-head attention enables the model to attend to different time-scale dependencies concurrently (Li et al., 2021a). Second, positional encodings ensure that sequence order is effectively maintained during parallel processing (Li et al., 2023). Third, feed-forward layers within transformer blocks facilitate complex, nonlinear transformations of attention weighted representations (Gillioz et al., 2020). Table 6 summarizes recent developments in transformer based frameworks for iEEG analysis.
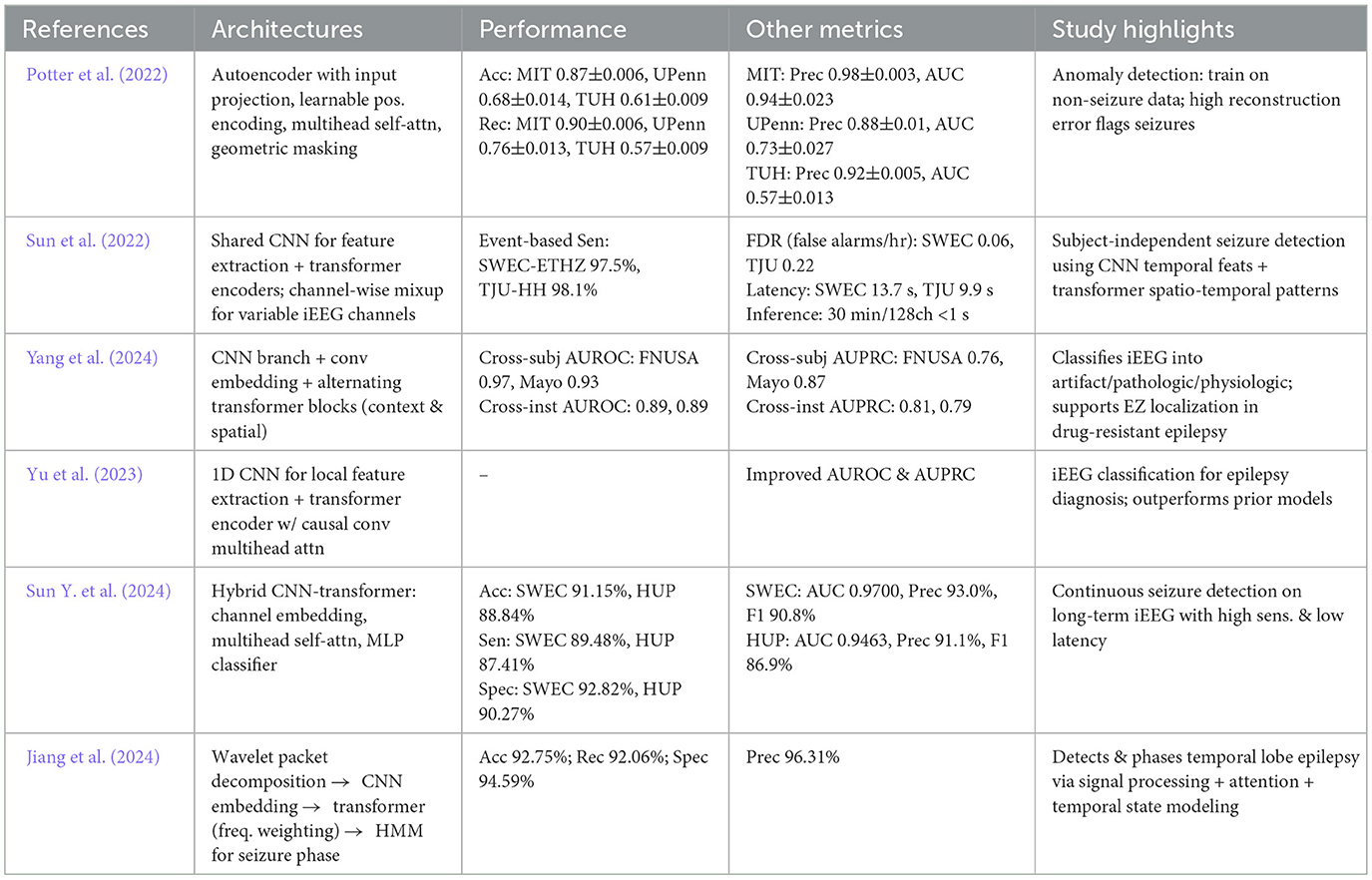
Table 6. Overview and performance comparison of transformer based deep learning architectures for seizure detection and classification.
In a study, Potter et al. (2022) developed an unsupervised anomaly detection approach using transformers, training an encoder on non-seizure data and identifying seizures through enhanced reconstruction errors. Sun et al. (2022) introduced a combined Transformer-CNN method for raw multichannel iEEG data. Similarly, Yang et al. (2024) utilized a hierarchical structure which combines local features (extracted by CNN) with global representations (extracted by transformer layers) enhancing classification performance. Yu et al. (2023) presented IEEG-CT which integrates a 1D CNN and a transformer encoder with convolutional multi-head attention outperforming prior models.
Further advancements include Sun Y. et al. (2024) proposed a subject-independent seizure detection model combining channel-wise mixup with multi-task transformer learning, achieving high AUC and improving generalization across patients and electrode setups. Jiang et al. (2024) extended this concept by merging wavelet packet decomposition with a convolution based embedding module and transformer blocks in a hybrid HMM-Wavformer design achieving 92.75% seizure detection accuracy while identifying crucial frequency bands.
Transformer based methods showed an exceptional ability to capture extensive contextual information in iEEG recordings while preserving critical temporal patterns (Wang et al., 2021). Despite these advancements, other deep learning architectures such as CNN and LSTM remain competitive particularly when combined with advanced signal representations. Ongoing research thus focuses on hybrid architectures, optimizing real-time performance and enhancing clinical interpretability. Comparisons indicate that transformer based models frequently outperform conventional CNN and RNN on long-sequence tasks (Potter et al., 2022; Sun et al., 2022; Yang et al., 2024; Yu et al., 2023) further emphasizing their potential for real-time seizure detection where parallel processing and precise segmentation of critical neurological events is important.
4.4 Alternative deep learning architectures
Although CNN, RNN, and transformer based methods dominate the area of deep learning for seizure detection, several alternative architectures have also gained attention by providing unique benefits and methodological innovations (Wu et al., 2021; Li et al., 2022; Jangde et al., 2023). These architectures utilize autoencoders, variational approaches, or graph based neural networks to capture complex iEEG patterns beyond the capabilities of more conventional techniques. By focusing on unsupervised or semi-supervised learning structures, they often reduce reliance on large labeled datasets while maintaining robust performance.
Li et al. (2022) introduced a convolutional variational autoencoder (CVAE) based approach for unsupervised HFO detection. In their method, time-frequency maps of candidate HFO (ripples and fast ripples) were derived from continuous wavelet transforms with only the red channel retained to highlight dominant frequency components. Similarly, Wu et al. (2021) proposed a similarly multi-stage strategy. First, they identified events of interest using a Hilbert envelope based threshold, converting these segments into time-frequency representations via an optimized wavelet transform (SE-CMWT). They then utilized a stacked denoising autoencoder (SDAE) to extract robust features before classifying events with an AdaBoost based SVM ensemble enhanced by sample weight adjusting factors (SWAF-ABSVM). Gharebaghi and Sardouie (2024) also applied advanced signal modeling to HFO detection by converting raw iEEG segments into graph structures. Each time sample was treated as a node, embedding energy-based features (RMS, short-time energy) within a learnable adjacency matrix. A Deep Graph CNN then classified HFO events, reporting a sensitivity of 90.7%. Table 7 summarizes these diverse architectures and their respective outcomes in seizure detection tasks.
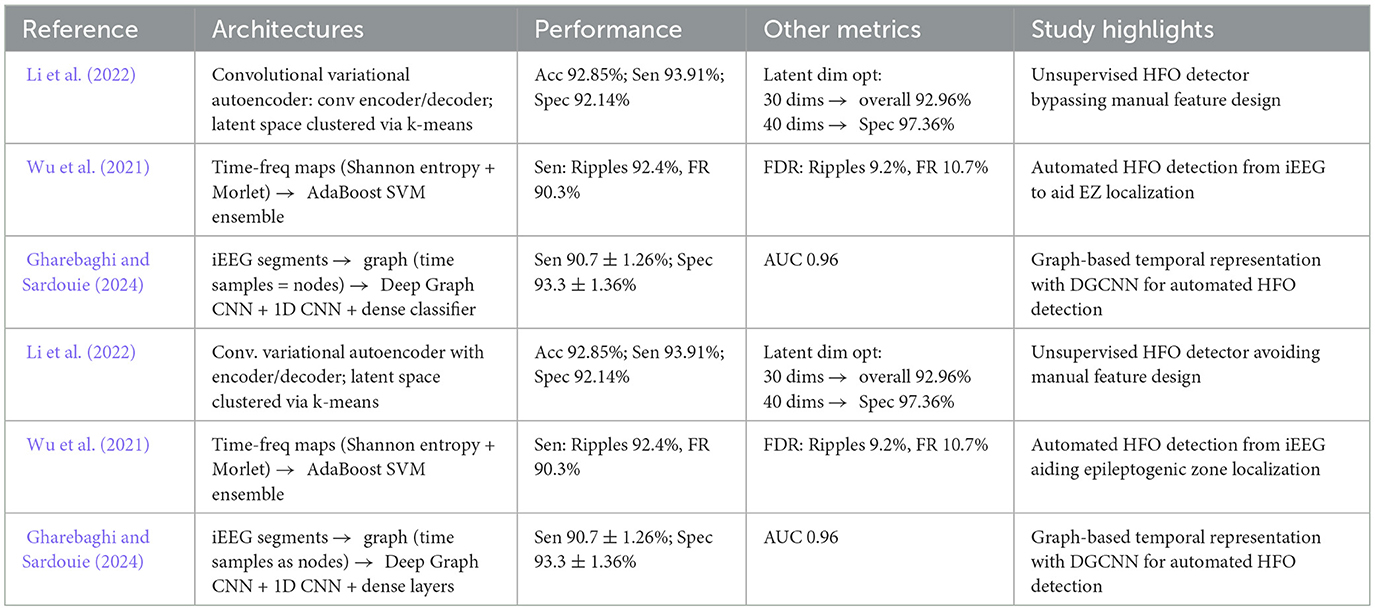
Table 7. Overview and performance comparison of alternate deep learning architectures for seizure detection and classification.
While many deep learning studies focus on seizure detection, it is important to distinguish this from the clinically critical task of EZ/SOZ localization. Seizure detection primarily assists in efficiently screening long-term iEEG recordings, whereas localization links pathological patterns such as HFO and ED to their spatial and temporal distributions, which directly informs surgical planning. Thus, CNN, RNN/LSTM, and Transformer based approaches should not only be viewed as tools for automated detection but also as additional techniques that help in identifying epileptogenic regions for resection.
To complement the detailed tables, Figure 5 presents radar charts summarizing the performance profiles of the main deep learning model families (CNN, RNN/LSTM, Transformers, and hybrid architectures). This visualization enables a quick comparison of accuracy, sensitivity, AUROC, and F1-score across methods. CNN show strong and well-balanced performance; RNN/LSTM achieve high sensitivity but struggle with cross-patient generalization; Transformers combine high accuracy with strong AUROC, indicating better scalability; hybrid models remain promising but inconsistent. These patterns provide a clear, visual guide for selecting architectures and designing future seizure detection systems.
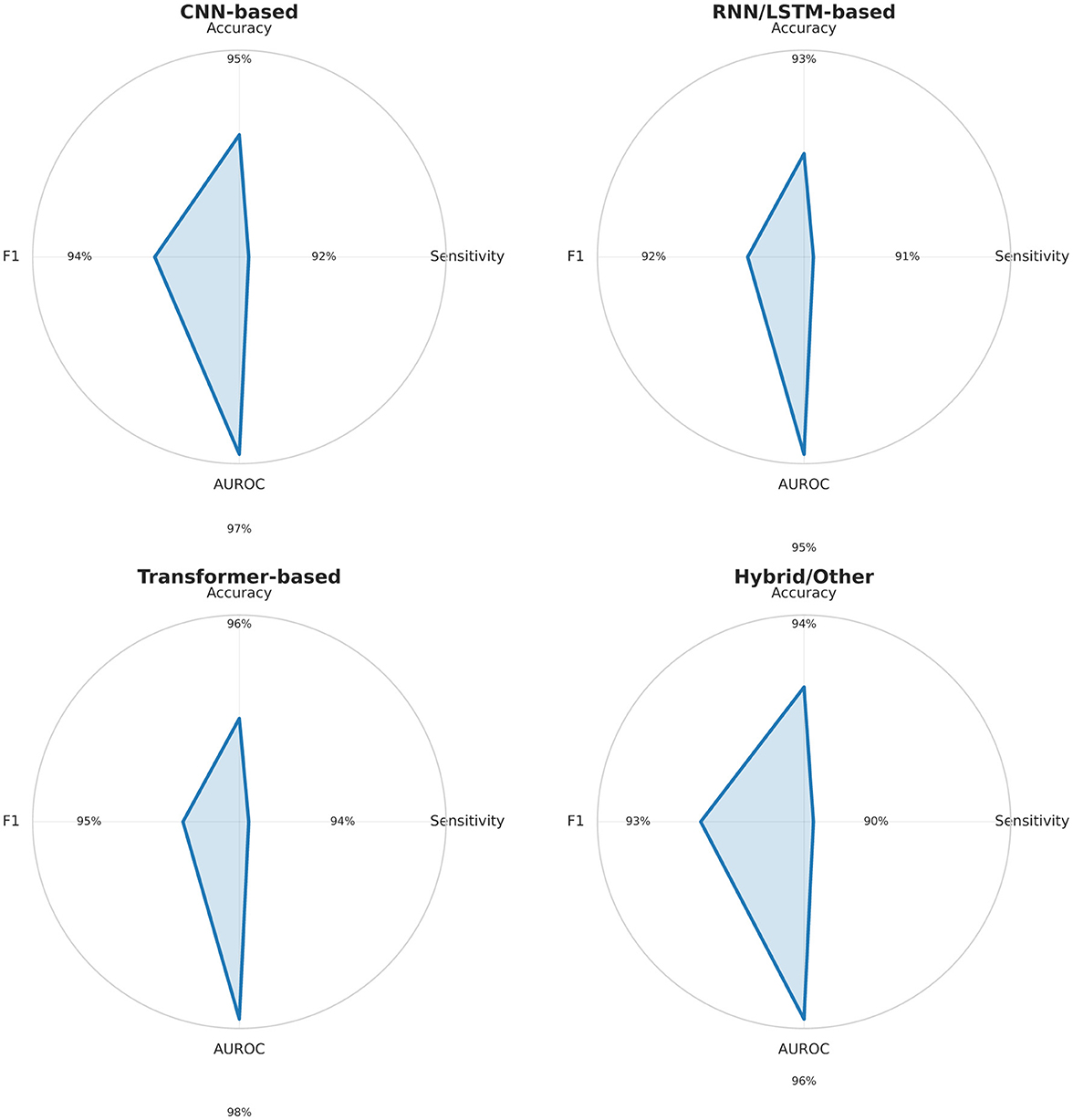
Figure 5. Radar charts summarizing Accuracy, Sensitivity, AUROC, and F1-score (0–100%) for major deep learning model families (CNN, RNN/LSTM, Transformer, Hybrid) based on Tables 3–7. CNN and Transformer models show balanced, high performance; RNN/LSTM models excel in sensitivity but vary in AUROC; hybrid methods remain heterogeneous.
5 Methodological challenges and limitations
Despite the potential of deep learning in automating seizure detection, the field currently faces several methodological challenges including data availability, preprocessing protocols, and technical constraints. Such challenges affect model robustness, generalizability, and clinical adoption. Key among these issues are limitations in dataset size and representativeness, lack of standardized preprocessing pipelines, data scarcity and heterogeneity, and the complexity of deep learning algorithms which complicates model interpretability. Table 8 summarizes these fundamental barriers.
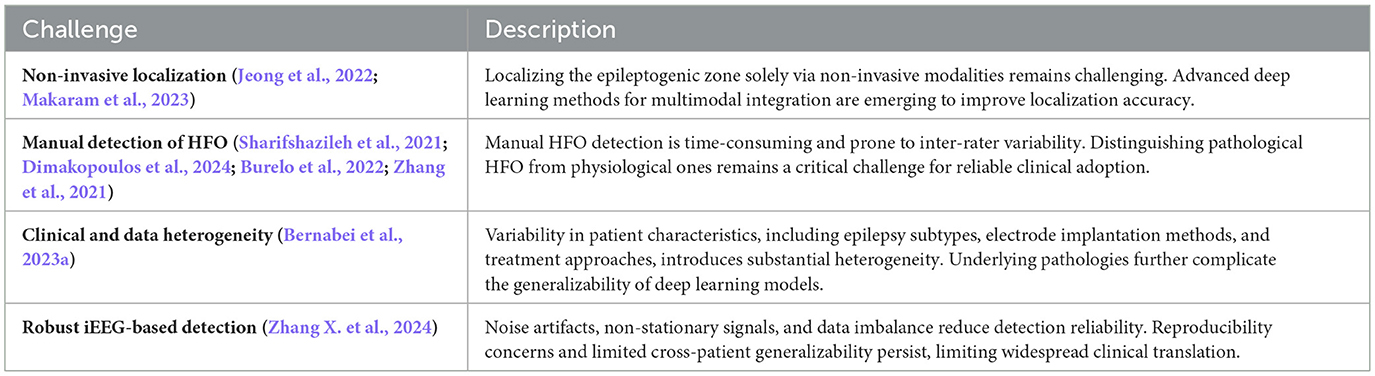
Table 8. Methodological challenges in deep learning based seizure detection and classification methods.
5.1 Preprocessing and feature extraction
Studies in seizure detection frequently utilizes various signal preprocessing and feature extraction methodologies which can lead to inconsistent or non-replicable findings (Bernabei et al., 2023a; Zhang X. et al., 2024). These inconsistencies become visible in diverse artifact removal practices, variance in filtering parameters, and signal normalization schemes each of which can significantly affect model performance (Bernabei et al., 2023a; Zhang X. et al., 2024). Consequently, comparing results across different research groups or replicating methodologies becomes challenging as different methods obscure whether performance gains arise from genuine algorithmic improvements or insignificant differences in data handling.
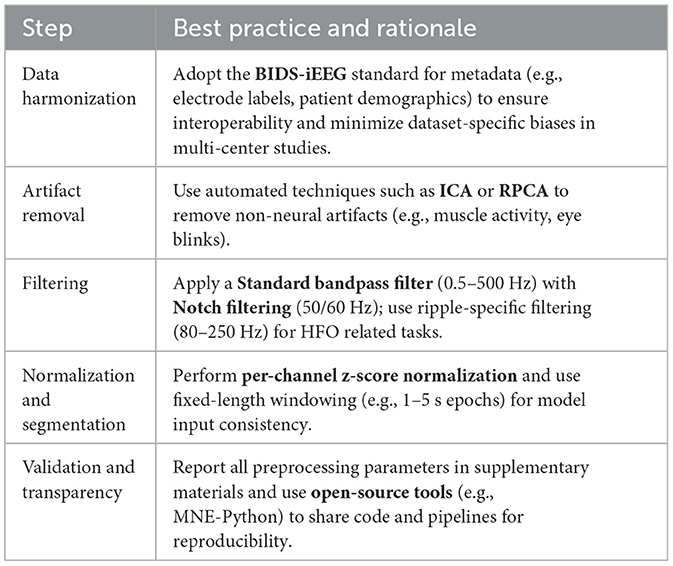
The issue extends beyond basic signal conditioning to include feature selection. Different studies may choose distinct sets of signal characteristics (e.g., temporal, spectral, or higher order measures) thereby affecting detection rates and challenging cross-validation efforts (Bernabei et al., 2023a; Zhang X. et al., 2024). As such, establishing standardized protocols for preprocessing and feature extraction remains a high priority. Initiatives that develop and provide comprehensive recommendations similar to standardized frameworks in other areas of biomedical signal processing are essential for sustaining meaningful comparisons, ensuring reproducibility, and ultimately advancing the reliability of deep learning based seizure detection. Table 9 describes best practices for data harmonization and standardized preprocessing pipelines to enhance reproducibility in iEEG based deep learning studies.
5.2 Data scarcity and heterogeneity
Data scarcity and heterogeneity present significant challenges to developing robust, generalizable deep learning models (Bernabei et al., 2023a; Zhang X. et al., 2024; Keutayeva and Abibullaev, 2024). Despite significant progress having been made in releasing more comprehensive iEEG datasets, publicly available resources still tend to be small and lack uniform annotation and quality control protocols. The problem is made worse by the high variability in recording conditions, patient demographics, and seizure types.
In clinical practice, epilepsy takes multiple forms and requires different electrode placements and recording methodologies. As a result, data from different sources show significant heterogeneity, complicating cross-institutional model evaluations (Bernabei et al., 2023a). Moreover, limited data often lead to overfitting wherein models perform better during training but fail to generalize effectively across broader patient data. Tables 10, 11 detail the inconsistencies in sample size, annotation quality, and public accessibility among the commonly utilized iEEG datasets. Addressing these limitations will require continuous community wide collaboration, including the development of larger, more diverse datasets and the adoption of shared annotation protocols.
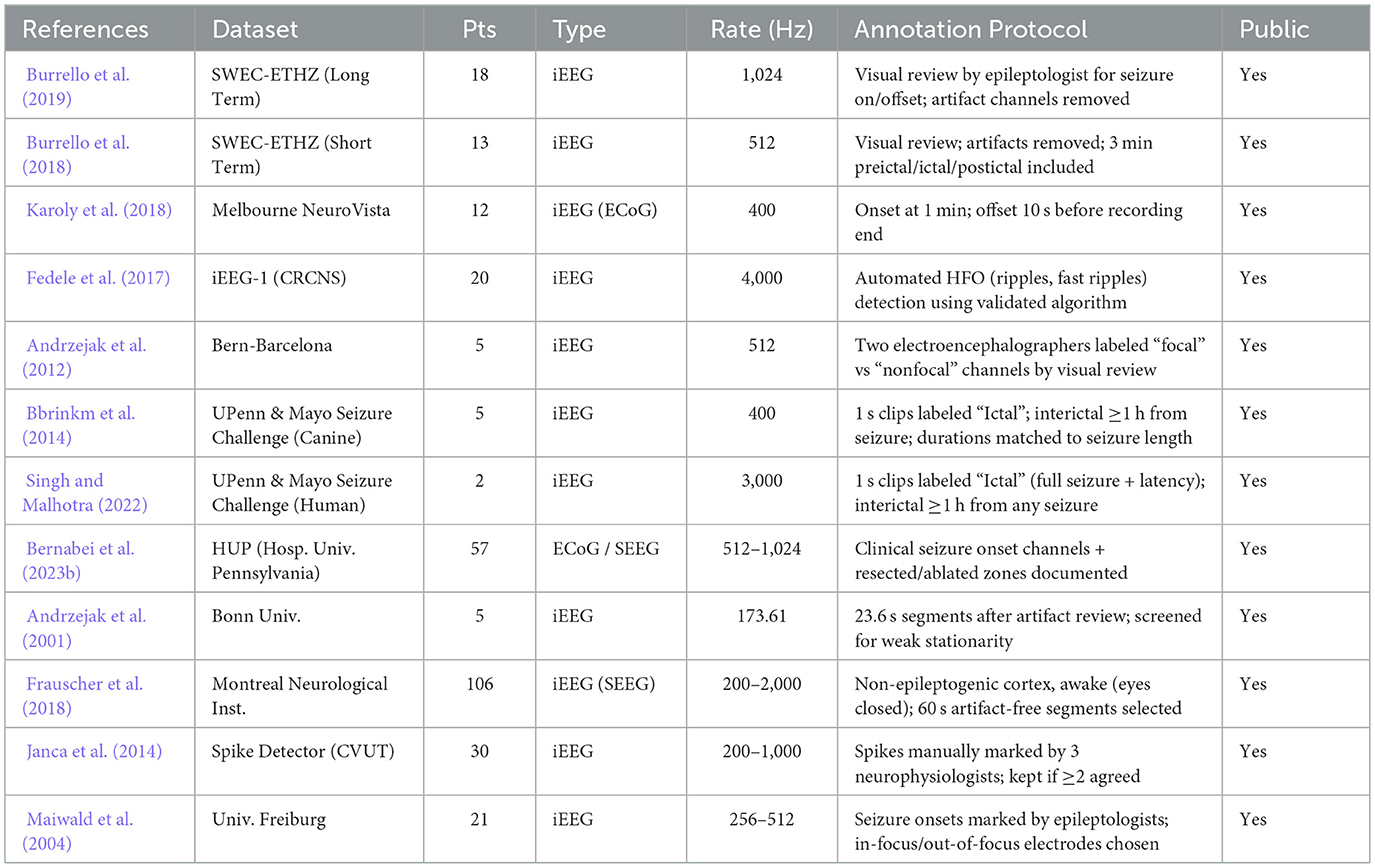
Table 10. Comparison of datasets used in deep learning based seizure detection and classification methods.
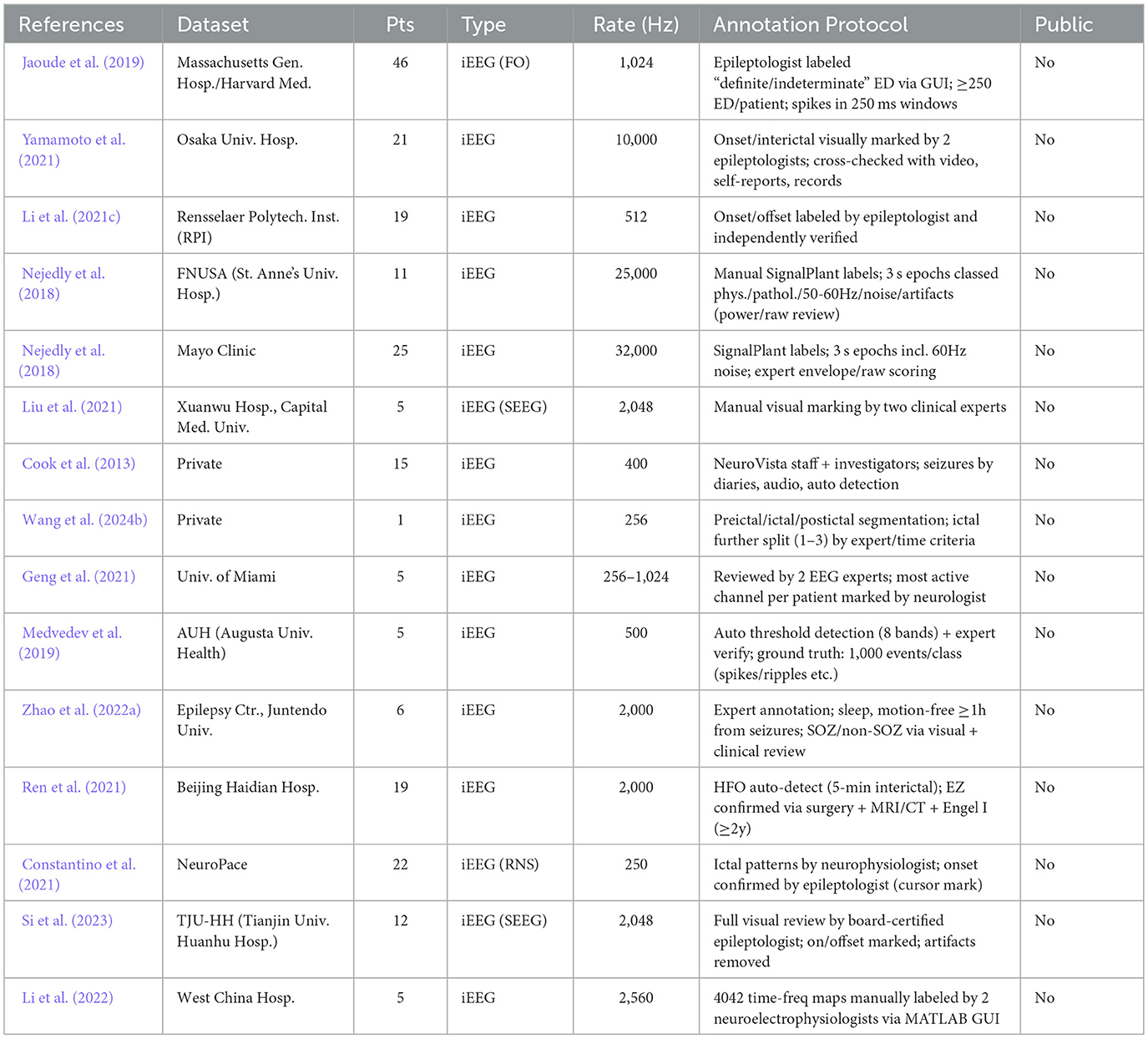
Table 11. Comparison of datasets used in deep learning based seizure detection and classification methods.
5.3 Interpretability and explainability
A critical concern for the clinical adoption of deep learning based seizure detection systems is their black-box nature (Zhang et al., 2021). While such models can achieve high accuracy, the complex decision making processes limit clinical trust and acceptance. This challenge has started discussions about model agnostic versus architecture specific interpretability strategies (Stiglic et al., 2020). Model agnostic methods such as local surrogate models or feature importance scores offer broad applicability but may lack the granularity of architecture specific approaches which provide deeper insights by utilizing essential network components (e.g., attention weights, filter visualizations).
To address these limitations, several explainable AI (XAI) techniques have been systematically applied to deep learning models in iEEG analysis to enhance the interpretability of seizure detection and epileptogenic zone (EZ) localization (Patil and Patil, 2023). Gradient-weighted Class Activation Mapping (Grad-CAM) generates class-specific heatmaps by weighting convolutional layer activations with gradients from the target class, highlighting critical regions in input signals such as high-amplitude spikes in iEEG recordings associated with epileptic seizures (Kolodziej et al., 2023).
However, a central interpretability challenge lies in how models integrate multiple electrophysiological biomarkers into a unified EZ representation. While CNN can capture spatial HFO patterns and transformers highlight temporal propagation within the SOZ, the implicit weighting of SOZ, HFO, and irritative activity often remains opaque (Sun Y. et al., 2024; Yi et al., 2025). Attention maps and probabilistic EZ heatmaps partially address this issue but without explicit fusion strategies, clinicians may struggle to reconcile model outputs with established EZ definitions. Validation against postoperative outcomes therefore becomes essential to ensure clinical trust in such designations.
Recent findings highlight that combining multiple biomarkers such as pathological patterns alongside baseline activity can create more accurate insights into epileptogenic regions (Ye et al., 2023). This highlights the need for interpretable systems capable of contextualizing detection decisions based on these diverse signal elements. Although reverse engineering methods, including visualization and saliency mapping represent initial steps (Zhang et al., 2021), more advanced approaches that can explain how deep networks synthesize multi-modal iEEG information remain an active area of research. Achieving clinically relevant interpretability not only involves computational innovation but also collaborative input from clinicians, ensuring that model explanations align with established medical knowledge and practical decision making processes.
5.4 Technical details and hardware constraints
Implementing deep learning for seizure detection in real-world clinical environments imposes strict requirements on both data acquisition and computational infrastructure. First, sampling rates must be sufficiently high to capture the rapid transient events often characterizing seizure onset or HFO (Lee et al., 2019). While moderate sampling frequencies (e.g., 500 Hz to 1 kHz) may be sufficient for slower seizure activities, detecting higher frequency phenomena (e.g., fast ripples at 250–500 Hz) typically requires sampling rates exceeding 2 kHz, and very high frequency oscillations (VHFO) require sampling above 5 kHz (Lee et al., 2019). Lower sampling rates increase the possibility of noise and the potential loss of crucial temporal details thereby reducing the model accuracy and reliability.
Second, deploying deep learning models in real-time clinical settings is challenged by limited hardware resources and the energy constraints related to neuromorphic and embedded platforms (Xu et al., 2025; Schuman et al., 2022; Van Albada et al., 2018). These systems often have restricted memory footprints and computational throughput, necessitating careful optimization of network architectures (Li and Meng, 2023). Techniques such as model quantization and custom architecture design can reduce computational cost and memory usage while maintaining robust detection performance (Liang et al., 2021). Consequently, the choice of hardware platform combined with algorithmic efficiency remains critical for achieving real-time seizure detection and classification in clinical practice.
6 Future directions
Ongoing advances in deep learning for iEEG analysis have greatly expanded the range of computational tools available for epilepsy diagnosis and surgical planning. Addressing persistent methodological challenges such as robust algorithmic performance, model interpretability, and standardization of evaluation remains a priority. Table 12 outlines the open questions and future research directions in the field of deep learning based seizure detection.
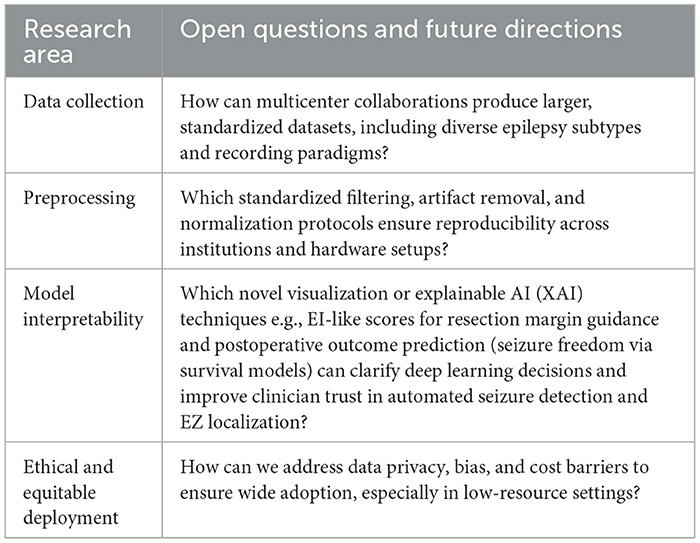
Table 12. Future research directions and open questions in deep learning based seizure detection and classification methods.
One promising research focus lies in predictive modeling and outcome predictions. Although current deep learning systems can reliably detect and classify seizures, they increasingly aim to predict them and guide surgical interventions. By examining diverse iEEG biomarkers including HFO, ED, and connectivity metrics models can identify diagnostic signals associated with either favorable or unfavorable surgical outcomes (Zhang et al., 2021; Qing et al., 2024; Dimakopoulos et al., 2024). This capacity to predict postsurgical outcomes is especially valuable for refining patient selection and customizing resection strategies as shown by Dimakopoulos et al. (2024) who reported significant gains in seizure freedom rates when the epileptogenic zone was fully removed.
Multimodal data integration represents an additional approach for improving the sensitivity and specificity of automated seizure detection and localization. Incorporating MRI, fMRI, and MEG data provides a more deeper insight on the cortical and subcortical networks that cause seizure generation (Jeong et al., 2022; Makaram et al., 2023). However, the integration of multimodal data and neuromorphic computing introduces several practical limitations that must be addressed for widespread clinical adoption. High-resolution neuroimaging modalities such as fMRI and MEG are resource intensive with significant costs associated with acquisition equipment and specialized personnel, limiting accessibility in low-resource or rural settings (Ooi et al., 2025). Moreover, the computational demands of fusing heterogeneous data streams iEEG with volumetric MRI or dynamic fMRI require substantial processing power, potentially necessitating cloud based infrastructure that raises data privacy concerns and latency issues in real-time applications. Neuromorphic hardware while promising for low-power edge computing in implantable devices remains in early stages with limited scalability, high development costs, and compatibility challenges with existing clinical workflows (Sun et al., 2025). Future efforts should prioritize cost-effective hybrid solutions such as federated learning for distributed multimodal training to overcome these challenges and enhance equitable deployment. As these innovations move closer to clinical implementation, ethical, practical, and equity concerns demand careful attention. Ensuring patient privacy in the era of cloud based analytics is essential while issues such as algorithmic bias and uneven data representation can adversely impact diagnostic outcomes for specific demographic groups (Singh et al., 2023; Fong et al., 2024). Efforts to expand data diversity and establish transparent, fair model training protocols must align with ongoing initiatives to standardize clinical validation. Moreover, cost-effectiveness analyses, regulatory pathways, and user acceptance among clinicians and patients are all crucial elements in determining whether new AI driven solutions will be widely adopted.
Table 13 summarizes several ethical and clinical considerations important for effective integration of deep learning in epilepsy care. Ultimately, the way forward necessitates establishing closer relationships between engineers, clinicians, and other stakeholders. By coordinating strict technical development with robust patient centered evaluation, the next generation of deep learning based seizure detection and localization tools can be helpful in enhancing results for individuals with drug resistant epilepsy.
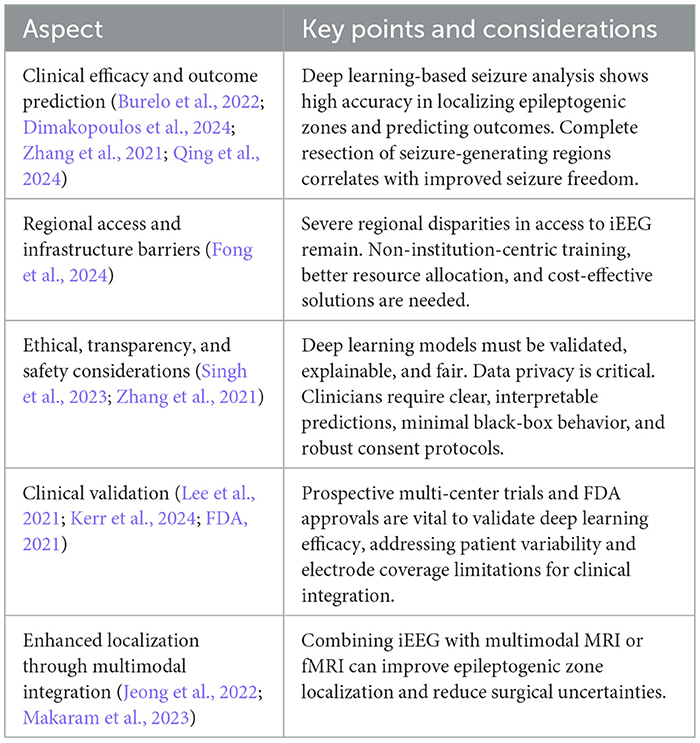
Table 13. Clinical implications and ethical considerations in deep learning based seizure detection and classification methods.
7 Discussion and conclusion
In this study, we reviewed recent advances in deep learning for the analysis of intracranial EEG (iEEG) data, focusing on seizure detection and epileptogenic zone (EZ) localization. We studied many different types of deep learning architectures including convolutional neural networks (CNN), recurrent neural networks (RNN), and transformer based models highlighting their ability to automate feature extraction and improve classification accuracy. Although deep learning architectures are proficient in seizure detection and offer temporal anchors for iEEG analysis, their clinical efficacy depends on the incorporation of localization metrics (such as HFO and SOZ mapping) to inform surgical decisions, as inaccurate EZ delineation may lead to incomplete resection or functional impairments. Additionally, we discussed the integration of hybrid models that combine CNN for spatial feature extraction with LSTM for temporal modeling enhancing the performance of seizure detection systems.
Despite these advancements, several challenges remain for the clinical adoption of deep learning based seizure detection systems. One of the primary limitations is the heterogeneity of the data as iEEG signals vary significantly between patients due to differences in electrode placement, acquisition protocols, and epilepsy pathology. The performance of deep learning models often declines when applied to unseen datasets, emphasizing the need for cross-institutional validation and improved generalization techniques.
Another critical challenge is the interpretability of deep learning models in clinical decision making. Unlike traditional machine learning approaches that rely on explicitly defined features, deep networks function as black-box models, making it difficult for clinicians to trust their predictions. Techniques such as Grad-CAM and saliency mapping have been explored to improve interpretability but further work is needed to ensure that these methods align with clinical expectations.
Realistically, deep learning can guide resectable areas by generating epileptogenicity index (EI) heatmaps that delineate SOZ and HFO rich regions from margin buffers, addressing localization ambiguity in epilepsy cases. By utilizing federated learning across multi-center datasets, these models predict postoperative seizure freedom aligning with the primary iEEG objective of optimizing surgical resection while minimizing functional deficits.
Additionally, real-time application limitations present an additional significant challenge. Many state-of-the-art deep learning models require substantial computational resources, making them impractical for real-time seizure monitoring in implantable or portable devices. Exploring lightweight architectures, neuromorphic computing strategies, and hardware optimization techniques could bridge this gap and facilitate real-time clinical deployment.
Future research should focus on improving the diversity and standardization of the data sets. The development of large-scale publicly available datasets that combine multiple recording environments, seizure types, and demographic variations is essential to improve model robustness. Transfer learning and domain adaptation techniques may also help reduce inter-subject variability and facilitate generalization across different datasets.
Another promising direction involves multi-modal data integration, combining iEEG with additional neuroimaging modalities such as functional MRI (fMRI), diffusion tensor imaging (DTI), and magnetoencephalography (MEG). These approaches have demonstrated potential in refining EZ localization by providing additional spatial and functional context to iEEG derived features. Furthermore, ethical and regulatory considerations surrounding deep learning in epilepsy care must be addressed. Ensuring data privacy, developing explainable AI frameworks, and establishing standardized clinical validation protocols are necessary steps for regulatory approval and adoption in clinical practice.
Deep learning has emerged as a transformative tool for seizure detection and EZ localization, offering significant advantages over traditional methods. However, challenges related to model generalization, interpretability, and real-time deployment must be overcome to enable successful clinical integration. Future research should emphasize cross-institutional validation, multi-modal approaches, and neuromorphic computing solutions to advance AI-driven epilepsy diagnostics. With continued collaboration between machine learning researchers and clinicians, deep learning holds the potential to improve epilepsy care.
Author contributions
WQ: Writing – review & editing, Writing – original draft, Visualization. M-HL: Supervision, Writing – original draft, Writing – review & editing. BA: Funding acquisition, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP27510939).
Acknowledgments
We would like to thank Nazarbayev University for providing access to essential online resources which greatly supported our research efforts.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abderrahim, N., Echtioui, A., Khemakhem, R., Zouch, W., Ghorbel, M., and Hamida, A. B. (2023). Epileptic seizures detection using ieeg signals and deep learning models. Circuits Syst. Signal Proc. 43, 1597–1626. doi: 10.1007/s00034-023-02527-8
Abibullaev, B., Kim, M. S., and Seo, H. D. (2009). Seizure detection in temporal lobe epileptic eegs using the best basis wavelet functions. J. Med. Syst. 34, 755–765. doi: 10.1007/s10916-009-9290-9
Abibullaev, B., Seo, H. D., and Kim, M. S. (2010). Epileptic spike detection using continuous wavelet transforms and artificial neural networks. Int. J. Wavel. Multiresolut. Inform. Proc. 8, 33–48. doi: 10.1142/S0219691310003341
Ahmedt-Aristizabal, D., Fookes, C., Dionisio, S., Nguyen, K., Cunha, J. P. S., and Sridharan, S. (2017). Automated analysis of seizure semiology and brain electrical activity in presurgery evaluation of epilepsy: a focused survey. Epilepsia 58, 1817–1831. doi: 10.1111/epi.13907
Andrzejak, R. G., Lehnertz, K., Mormann, F., Rieke, C., David, P., and Elger, C. E. (2001). Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: dependence on recording region and brain state. Phys. Rev. E: Statistical Physics, Plasmas, Fluids Related Interdiscipl. Topics 64:6. doi: 10.1103/PhysRevE.64.061907
Andrzejak, R. G., Schindler, K., and Rummel, C. (2012). Nonrandomness, nonlinear dependence, and nonstationarity of electroencephalographic recordings from epilepsy patients. Phys. Rev. E 86:4. doi: 10.1103/PhysRevE.86.046206
Antoniades, A., Spyrou, L., Martin-Lopez, D., Valentin, A., Alarcon, G., and Sanei, S. (2017). Detection of interictal discharges with convolutional neural networks using discrete ordered multichannel intracranial EEG. IEEE Trans. Neural Syst. Rehabilitat. Eng. 25, 2285–2294. doi: 10.1109/TNSRE.2017.2755770
Antoniades, A., Spyrou, L., Took, C. C., and Sanei, S. (2016). “Deep learning for epileptic intracranial EEG data,” in Proceedings of the International Workshop on Machine Learning for Signal Processing (Vietri sul Mare: IEEE), 1–6.
Anwar, A., Khalifa, Y., Coyle, J. L., and Sejdic, E. (2024). Transformers in biosignal analysis: a review. Inform. Fusion 114:102697. doi: 10.1016/j.inffus.2024.102697
Bbrinkm, S., and Cukierski, W. (2014). Upenn and Mayo Clinic's Seizure Detection Challenge. Chicago, IL: American Epilepsy Society. Available online at: https://kaggle.com/competitions/seizure-detection (Accessed March 10, 2025).
Bernabei, J. M., Li, A., Revell, A. Y., Smith, R. J., Gunnarsdottir, K. M., Ong, I. Z., et al. (2023a). Quantitative approaches to guide epilepsy surgery from intracranial EEG. Brain 146, 2248–2258. doi: 10.1093/brain/awad007
Bernabei, J. M., Li, A., Revell, A. Y., Smith, R. J., Gunnarsdottir, K. M., Ong, I. Z., et al. (2023b). Hup IEEG Epilepsy Dataset.
Broti, N. M., Iwasaki, M., and Ono, Y. (2025). Machine learning detection of epileptic seizure onset zone from IEEG. Biomed. Eng. Lett. 15, 677–692. doi: 10.1007/s13534-025-00480-w
Burelo, K., Sharifshazileh, M., Indiveri, G., and Sarnthein, J. (2022). Automatic detection of high-frequency oscillations with neuromorphic spiking neural networks. Front. Neurosci. 16:861480. doi: 10.3389/fnins.2022.861480
Burrello, A., Schindler, K., and Benini, L. (2018). “One-shot learning for ieeg seizure detection using end-to-end binary operations: local binary patterns with hyperdimensional computing,” in 2022 IEEE Biomedical Circuits and Systems Conference (BioCAS) (Cleveland, OH: IEEE).
Burrello, A., Schindler, K., Benini, L., and Rahimi, A. (2019). Hyperdimensional computing with local binary patterns: one-shot learning of seizure onset and identification of ictogenic brain regions using short-time ieeg recordings. IEEE Trans. Biomed. Eng. 67, 601–613. doi: 10.1109/TBME.2019.2919137
Cai, Z., Sohrabpour, A., Jiang, H., Ye, S., Joseph, B., Brinkmann, B. H., et al. (2021). Noninvasive high-frequency oscillations riding spikes delineates epileptogenic sources. Proc. Nat. Acad. Sci. 118:e2011130118. doi: 10.1073/pnas.2011130118
Charupanit, K., Sen-Gupta, I., Lin, J. J., and Lopour, B. A. (2020). Detection of anomalous high-frequency events in human intracranial eeg. Epilepsia Open 5, 263–273. doi: 10.1002/epi4.12397
Cimbalnik, J., Brinkmann, B., Kremen, V., Jurak, P., Berry, B., Van Gompel, J., et al. (2018). Physiological and pathological high frequency oscillations in focal epilepsy. Ann. Clini. Transl. Neurol. 5, 1062–1076. doi: 10.1002/acn3.618
Constantino, A. C., Sisterson, N. D., Zaher, N., Urban, A., Richardson, R. M., and Kokkinos, V. (2021). Expert-level intracranial electroencephalogram ictal pattern detection by a deep learning neural network. Front. Neurol. 12:603868. doi: 10.3389/fneur.2021.603868
Cook, M. J., O'Brien, T. J., Berkovic, S. F., Murphy, M., Morokoff, A., Fabinyi, G., et al. (2013). Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 12, 563–571. doi: 10.1016/S1474-4422(13)70075-9
Da Silva Lourenço, C., Tjepkema-Cloostermans, M. C., and Van Putten, M. J. (2021). Machine learning for detection of interictal epileptiform discharges. Clini. Neurophysiol. 132, 1433–1443. doi: 10.1016/j.clinph.2021.02.403
Dash, D. P., Kolekar, M., Chakraborty, C., and Khosravi, M. R. (2022). Review of machine and deep learning techniques in epileptic seizure detection using physiological signals and sentiment analysis. ACM Trans, Asian Low-Resource Lang. Inform. Proc. 23, 1–29. doi: 10.1145/3552512
Dimakopoulos, V., Gotman, J., Klimes, P., von Ellenrieder, N., Tan, S. B., Smith, G., et al. (2024). Multicentre analysis of seizure outcome predicted by removal of high frequency oscillations. Brain 148, 1769–1777. doi: 10.1093/brain/awae361
Dimakopoulos, V., Gotman, J., Stacey, W., Ellenrieder, N., Jacobs, J., Papadelis, C., et al. (2022). Protocol for multicentre comparison of interictal high-frequency oscillations as a predictor of seizure freedom. Brain Commun. 4:3. doi: 10.1093/braincomms/fcac151
Donos, C., Mîndruta, I., and Barborica, A. (2020). Unsupervised detection of high-frequency oscillations using time-frequency maps and computer vision. Front. Neurosci. 14:183. doi: 10.3389/fnins.2020.00183
Dwaraka, P. H., Subhas, C., and Rama Naidu, K. (2021). IEEG based epileptic seizure detection using reconstruction independent component analysis and long short term memory network. Int. J. Comp. Commun. Cont. 16:4268. doi: 10.15837/ijccc.2021.5.4268
Enatsu, R., and Mikuni, N. (2016). Invasive evaluations for epilepsy surgery: a review of the literature. Neurol. Med. Chir. 56, 221–227. doi: 10.2176/nmc.ra.2015-0319
Falach, R., Geva-Sagiv, M., Eliashiv, D., Goldstein, L., Budin, O., Gurevitch, G., et al. (2024). Annotated interictal discharges in intracranial EEG sleep data and related machine learning detection scheme. Scientific Data 11:1. doi: 10.1038/s41597-024-04187-y
FDA (2021). Artificial Intelligence and Machine Learning in Software as a Medical Device. Silver Spring, MD: Food and Drug Administration.
Fedele, T., Burnos, S., Boran, E., Krayenbühl, N., Hilfiker, P., Grunwald, T., et al. (2017). Resection of high frequency oscillations predicts seizure outcome in the individual patient. Sci. Rep. 7:1. doi: 10.1038/s41598-017-13064-1
Flanary, J., Daly, S. R., Bakker, C., Herman, A., Park, M., McGovern, R., et al. (2022). Reliability of visual review of intracranial electroencephalogram in identifying the seizure onset zone: a systematic review and implications for the accuracy of automated methods. Epilepsia 64, 6–16. doi: 10.1111/epi.17446
Fong, M. W. K., Lim, K. S., Fong, S. L., Chen, C., Kwan, S. Y., Lee, C. C., et al. (2024). The development of stereo-electroencephalography (SEEG) in southeast asia and oceania: Challenges to equity across the region. Neurophysiologie Clinique 55:103033. doi: 10.1016/j.neucli.2024.103033
Frauscher, B. (2020). Localizing the epileptogenic zone. Curr. Opin. Neurol. 33, 198–206. doi: 10.1097/WCO.0000000000000790
Frauscher, B., Von Ellenrieder, N., Zelmann, R., Doležalová, I., Minotti, L., Olivier, A., et al. (2018). Atlas of the normal intracranial electroencephalogram: neurophysiological awake activity in different cortical areas. Brain 141, 1130–1144. doi: 10.1093/brain/awy035
Geng, D., Alkhachroum, A., Bicchi, M. A. M., Jagid, J. R., Cajigas, I., and Chen, Z. S. (2021). Deep learning for robust detection of interictal epileptiform discharges. J. Neural Eng. 18:056015. doi: 10.1088/1741-2552/abf28e
Gharebaghi, F., and Sardouie, S. H. (2024). “HFO detection from ieeg signals in epilepsy using time-trained graphs and deep graph convolutional neural network,” in 2024 32nd International Conference on Electrical Engineering (ICEE) (Tehran: IEEE), 1–7.
Gillioz, A., Casas, J., Mugellini, E., and Khaled, O. A. (2020). Overview of the transformer-based models for nlp tasks. Ann. Comp. Sci. Inform. Syst. 21, 179–183. doi: 10.15439/2020F20
Gunnarsdottir, K. M., Li, A., Smith, R. J., Kang, J., Korzeniewska, A., Crone, N. E., et al. (2022). Source-sink connectivity: a novel interictal eeg marker for seizure localization. Brain 145, 3901–3915. doi: 10.1093/brain/awac300
Guo, J., Li, H., Sun, X., Qi, L., Qiao, H., and Pan, Y. (2021). Detecting high frequency oscillations for stereoelectroencephalography in epilepsy via hypergraph learning. IEEE Trans. Neural Syst. Rehabilit. Eng. 29, 587–596. doi: 10.1109/TNSRE.2021.3056685
Guo, J., Xiao, N., Li, H., He, L., Li, Q., Wu, T., et al. (2022). Transformer-based high-frequency oscillation signal detection on magnetoencephalography from epileptic patients. Front. Mol. Biosci. 9:822810. doi: 10.3389/fmolb.2022.822810
Hoogteijling, S., and Zijlmans, M. (2021). Deep learning for epileptogenic zone delineation from the invasive EEG: challenges and lookouts. Brain Commun. 4:fcab307. doi: 10.1093/braincomms/fcab307
Jacobs, J., Staba, R., Asano, E., Otsubo, H., Wu, J. Y., Zijlmans, M., et al. (2012). High-frequency oscillations (hfos) in clinical epilepsy. Prog. Neurobiol. 98, 302–315. doi: 10.1016/j.pneurobio.2012.03.001
Janca, R., Jezdik, P., Cmejla, R., Tomasek, M., Worrell, G. A., Stead, M., et al. (2014). Detection of interictal epileptiform discharges using signal envelope distribution modelling: application to epileptic and non-epileptic intracranial recordings. Brain Topogr. 28, 172–183. doi: 10.1007/s10548-014-0379-1
Jangde, A. S., Anuragi, A., and Sisodia, D. S. (2023). “Autoencoder-based IEEG signal classification for accurate focal and non-focal epilepsy detection,” in International Conference on Electronics and Sustainable Communication Systems (ICESC), 262–267.
Janmohamed, M., Nhu, D., Kuhlmann, L., Gilligan, A., Tan, C. W., Perucca, P., et al. (2022). Moving the field forward: detection of epileptiform abnormalities on scalp electroencephalography using deep learning–clinical application perspectives. Brain Commun. 4:fcac218. doi: 10.1093/braincomms/fcac218
Jaoude, M. A., Jing, J., Sun, H., Jacobs, C. S., Pellerin, K. R., Westover, M. B., et al. (2019). Detection of mesial temporal lobe epileptiform discharges on intracranial electrodes using deep learning. Clini. Neurophysiol. 131, 133–141. doi: 10.1016/j.clinph.2019.09.031
Jeong, J.-W., Lee, M. H., Kuroda, N., Sakakura, K., O'Hara, N., Juhasz, C., et al. (2022). Multi-scale deep learning of clinically acquired multi-modal mri improves the localization of seizure onset zone in children with drug-resistant epilepsy. IEEE J. Biomed. Health Inform. 26, 5529–5539. doi: 10.1109/JBHI.2022.3196330
Jiang, W., Lin, Z., Wang, K., Ding, S., Fang, C., Bo, H., et al. (2024). “Exploring the neural dynamics in temporal lobe epilepsy: a study using transformer and hidden markov models,” in 2021 IEEE International Conference on Bioinformatics and Biomedicine (BIBM) (Lisbon: IEEE), 1526–1531. doi: 10.1109/BIBM62325.2024.10822005
Jobst, B. C., Bartolomei, F., Gotman, J., Jobst, B., Bartolomei, F., Diehl, B., et al. (2020). Intracranial EEG in the 21st century. Epilepsy Curr. 20, 180–188. doi: 10.1177/1535759720934852
Karoly, P., Cook, M., Kuhlmmann, L., Freestone, D., Grayden, D., Nurse, E., et al. (2018). Melbourne University Neurovista Seizure Prediction Data, Version 3. Melbourne, VIC: The University of Melbourne.
Kerr, W. T., McFarlane, K. N., and Pucci, G. F. (2024). The present and future of seizure detection, prediction, and forecasting with machine learning, including the future impact on clinical trials. Front. Neurol. 15:1425490. doi: 10.3389/fneur.2024.1425490
Keutayeva, A., and Abibullaev, B. (2024). Data constraints and performance optimization for transformer-based models in EEG-based brain-computer interfaces: a survey. IEEE Access 12, 62628–62647. doi: 10.1109/ACCESS.2024.3394696
Kobayashi, K., Akiyama, T., Agari, T., Sasaki, T., Shibata, T., Hanaoka, Y., et al. (2017). Significance of high-frequency electrical brain activity. PubMed 71, 191–200. doi: 10.18926/amo/55201
Kolodziej, M., Majkowski, A., and Rysz, A. (2023). Implementation of machine learning and deep learning techniques for the detection of epileptic seizures using intracranial electroencephalography. Appl. Sci. 13:8747. doi: 10.3390/app13158747
Krikid, F., Karfoul, A., Chaibi, S., Kachenoura, A., Nica, A., Kachouri, A., et al. (2021). Classification of high frequency oscillations in intracranial EEG signals based on coupled time-frequency and image-related features. Biomed. Signal Process. Control 73:103418. doi: 10.1016/j.bspc.2021.103418
Krikid, F., Karfoul, A., Chaibi, S., Kachenoura, A., Nica, A., Kachouri, A., et al. (2023). Multi-classification of high-frequency oscillations in intracranial EEG signals based on cnn and data augmentation. Signal Image Video Proc. 18, 1099–1109. doi: 10.1007/s11760-023-02808-4
Kumar, S., and Khan, Y. U. (2023). “Biomedical signals classification with transformer based model,” in International Conference on Power, Instrumentation, Energy and Control (PIECON) (Aligarh: IEEE), 1–5.
Kural, M. A., Duez, L., Hansen, V. S., Larsson, P. G., Rampp, S., Schulz, R., et al. (2020). Criteria for defining interictal epileptiform discharges in EEG. Neurology 94, e2139–e2147. doi: 10.1212/WNL.0000000000009439
Lachner-Piza, D., Jacobs, J., Bruder, J. C., Schulze-Bonhage, A., Stieglitz, T., and Dümpelmann, M. (2019). Automatic detection of high-frequency-oscillations and their sub-groups co-occurring with interictal-epileptic-spikes. J. Neural Eng. 17:016030. doi: 10.1088/1741-2552/ab4560
Lai, D., Zhang, X., Chan, W., Zhang, H., Kang, T., Yuan, H., et al. (2020). Channel-wise characterization of high frequency oscillations for automated identification of the seizure onset zone. IEEE Access 8, 45531–45543. doi: 10.1109/ACCESS.2020.2978290
Lai, D., Zhang, X., Ma, K., Chane, Z., Chan, W., Zhang, H., et al. (2019). Automated detection of high frequency oscillations in intracranial eeg using the combination of short-time energy and convolutional neural networks. IEEE Access 7, 82501–82511. doi: 10.1109/ACCESS.2019.2923281
Lee, A. Y., Yanagihara, R. T., Lee, C. S., Blazes, M., Jung, H. C., Chee, Y. E., et al. (2021). Multicenter, head-to-head, real-world validation study of seven automated artificial intelligence diabetic retinopathy screening systems. Diabetes Care 44, 1168–1175. doi: 10.2337/dc20-1877
Lee, S., Issa, N. P., Rose, S., Tao, J. X., Warnke, P. C., Towle, V. L., et al. (2019). Dc shifts, high frequency oscillations, ripples and fast ripples in relation to the seizure onset zone. Seizure 77, 52–58. doi: 10.1016/j.seizure.2019.05.001
Li, B., Zhao, X., Zhao, Q., Tanaka, T., and Cao, J. (2019). “A one-dimensional convolutional neural network model for automated localization of epileptic foci,” in 2022 Asia-Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA ASC) (Lanzhou: IEEE), 741–744.
Li, H., and Meng, L. (2023). Hardware-aware approach to deep neural network optimization. Neurocomputing 559:126808. doi: 10.1016/j.neucom.2023.126808
Li, J., Wang, X., Tu, Z., and Lyu, M. R. (2021a). On the diversity of multi-head attention. Neurocomputing 454, 14–24. doi: 10.1016/j.neucom.2021.04.038
Li, W., Zhong, L., Xiang, W., Kang, T., and Lai, D. (2022). “A novel unsupervised autoencoder-based hfos detector in intracranial EEG signals,” in ICASSP 2022 - 2022 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) (Singapore: IEEE), 1426–1430. doi: 10.1109/ICASSP43922.2022.9746014
Li, X., Yu, T., Ren, Z., Wang, X., Yan, J., Chen, X., et al. (2021b). Localization of the epileptogenic zone by multimodal neuroimaging and high-frequency oscillation. Front. Hum. Neurosci. 15:677840. doi: 10.3389/fnhum.2021.677840
Li, Y., Miao, N., Ma, L., Shuang, F., and Huang, X. (2023). Transformer for object detection: Review and benchmark. Eng. Appl. Artif. Intell. 126:107021. doi: 10.1016/j.engappai.2023.107021
Li, Z., Fields, M., Panov, F., Ghatan, S., Yener, B., and Marcuse, L. (2021c). Deep learning of simultaneous intracranial and scalp EEG for prediction, detection, and lateralization of mesial temporal lobe seizures. Front. Neurol. 12:705119. doi: 10.3389/fneur.2021.705119
Liang, T., Glossner, J., Wang, L., Shi, S., and Zhang, X. (2021). Pruning and quantization for deep neural network acceleration: a survey. Neurocomputing 461, 370–403. doi: 10.1016/j.neucom.2021.07.045
Lin, T., Wang, Y., Liu, X., and Qiu, X. (2022). A survey of transformers. AI Open 3, 111–132. doi: 10.1016/j.aiopen.2022.10.001
Lindemann, B., Müller, T., Vietz, H., Jazdi, N., and Weyrich, M. (2021). A survey on long short-term memory networks for time series prediction. Procedia CIRP 99:650–655. doi: 10.1016/j.procir.2021.03.088
Liu, G., Zhou, W., and Geng, M. (2019). Automatic seizure detection based on s-transform and deep convolutional neural network. Int. J. Neural Syst. 30:1950024. doi: 10.1142/S0129065719500242
Liu, S., Sha, Z., Sencer, A., Aydoseli, A., Bebek, N., Abosch, A., et al. (2016). Exploring the time–frequency content of high frequency oscillations for automated identification of seizure onset zone in epilepsy. J. Neural Eng. 13:026026. doi: 10.1088/1741-2560/13/2/026026
Liu, Z., Wei, P., Wang, Y., Yang, Y., Dai, Y., Cao, G., et al. (2021). Automatic detection of high-frequency oscillations based on an end-to-end bi-branch neural network and clinical cross-validation. Comput. Intell. Neurosci. 2021:1. doi: 10.1155/2021/7532241
Ma, K., Lai, D., Chen, Z., Zeng, Z., Zhang, X., Chen, W., et al. (2019). “Automatic detection of high frequency oscillations (80-500hz) based on convolutional neural network in human intracerebral electroencephalogram,” in Annual International Conference of the IEEE Engineering in Medicine and Biology Society (Berlin: IEEE), 5133–5136.
Ma, X., Qiu, S., Zhang, Y., Lian, X., and He, H. (2018). “Predicting epileptic seizures from intracranial eeg using lstm-based multi-task learning,” in Lecture Notes in Computer Science (Guangzhou: Springer), 157–167.
Maiwald, T., Winterhalder, M., Aschenbrenner-Scheibe, R., Voss, H. U., Schulze-Bonhage, A., and Timmer, J. (2004). Comparison of three nonlinear seizure prediction methods by means of the seizure prediction characteristic. Physica D: Nonlinear Phenomena 194, 357–368. doi: 10.1016/j.physd.2004.02.013
Makaram, N., Gupta, S., Pesce, M., Bolton, J., Stone, S., Haehn, D., et al. (2023). Deep learning-based visual complexity analysis of electroencephalography time-frequency images: can it localize the epileptogenic zone in the brain? Algorithms 16:567. doi: 10.3390/a16120567
Matsumoto, A., Brinkmann, B. H., Stead, S. M., Matsumoto, J., Kucewicz, M. T., Marsh, W. R., et al. (2013). Pathological and physiological high-frequency oscillations in focal human epilepsy. J. Neurophysiol. 110, 1958–1964. doi: 10.1152/jn.00341.2013
Medvedev, A. V., Agoureeva, G. I., and Murro, A. M. (2019). A long short-term memory neural network for the detection of epileptiform spikes and high frequency oscillations. Sci. Rep. 9:1. doi: 10.1038/s41598-019-55861-w
Milligan, T. A. (2021). Epilepsy: a clinical overview. Am. J. Med. 134, 840–847. doi: 10.1016/j.amjmed.2021.01.038
Mirchi, N., Karwowski, W., Farahani, F., Flok, K., Taiar, R., Hancock, P. A., et al. (2022). Decoding intracranial eeg with machine learning: a systematic review. Front. Hum. Neurosci. 16:913777. doi: 10.3389/fnhum.2022.913777
Nejedly, P., Cimbalnik, J., Klimes, P., Plesinger, F., Halamek, J., Kremen, V., et al. (2018). Intracerebral EEG artifact identification using convolutional neural networks. Neuroinformatics 17, 225–234. doi: 10.1007/s12021-018-9397-6
Nejedly, P., Hrtonova, V., Pail, M., Cimbalnik, J., Daniel, P., Travnicek, V., et al. (2025). Leveraging interictal multimodal features and graph neural networks for automated planning of epilepsy surgery. Brain Commun. 7:fcaf140. doi: 10.1093/braincomms/fcaf140
Nissen, I. A., Millán, A. P., Stam, C. J., Van Straaten, E. C. W., Douw, L., Pouwels, P. J. W., et al. (2021). Optimization of epilepsy surgery through virtual resections on individual structural brain networks. Sci. Rep. 11:98046. doi: 10.1038/s41598-021-98046-0
Ooi, L. Q. R., Orban, C., Zhang, S., Nichols, T. E., Tan, T. W. K., Kong, R., et al. (2025). Longer scans boost prediction and cut costs in brain-wide association studies. Nature 644, 731–740. doi: 10.1038/s41586-025-09250-1
Ouichka, O., Echtioui, A., and Hamam, H. (2022). Deep learning models for predicting epileptic seizures using ieeg signals. Electronics 11:605. doi: 10.3390/electronics11040605
Palanichamy, I., and Ahamed, F. B. B. (2022). Prediction of seizure in the eeg signal with time aware recurrent neural network. Revue D Intelligence Artificielle 36, 717–724. doi: 10.18280/ria.360508
Partamian, H., Jahromi, S., Corona, L., Perry, M. S., Tamilia, E., Madsen, J. R., et al. (2025). Machine learning on interictal intracranial EEG predicts surgical outcome in drug resistant epilepsy. NPJ Digit. Med. 8:1531. doi: 10.1038/s41746-025-01531-3
Patil, A., and Patil, M. (2023). Performance analysis of deep-learning and explainable ai techniques for detecting and predicting epileptic seizures. Int. J. Recent Innovat. Trends Comp. Commun. 11, 314–327. doi: 10.17762/ijritcc.v11i9.8357
Potter, I. Y., Zerveas, G., Eickhoff, C., and Duncan, D. (2022). “Unsupervised multivariate time-series transformers for seizure identification on EEG,” in 21st IEEE International Conference on Machine Learning and Applications (ICMLA) (Nassau: IEEE), 1304–1311.
Qing, K., Von Stein, E., Yamada, L., Fogarty, A., and Nuyujukian, P. (2024). Classifying high-frequency oscillations by morphologic contrast to background, with surgical outcome correlates. J. Clini. Neurophysiol. 42, 294–303. doi: 10.1097/WNP.0000000000001121
Ren, G., Sun, Y., Wang, D., Ren, J., Dai, J., Mei, S., et al. (2021). Identification of epileptogenic and non-epileptogenic high-frequency oscillations using a multi-feature convolutional neural network model. Front. Neurol. 12:640526. doi: 10.3389/fneur.2021.640526
Rezk, N. M., Purnaprajna, M., Nordstrom, T., and Ul-Abdin, Z. (2020). Recurrent neural networks: an embedded computing perspective. IEEE Access 8, 57967–57996. doi: 10.1109/ACCESS.2020.2982416
Sadek, Z., Hadriche, A., and Jmail, N. (2023). “Clustering of high frequency oscillations HFO in epilepsy using pretrained neural networks,” in Lecture Notes in Networks and Systems, 100–107.
Schindler, K. A., and Rahimi, A. (2021). A primer on hyperdimensional computing for IEEG seizure detection. Front. Neurol. 12:701791. doi: 10.3389/fneur.2021.701791
Schuman, C. D., Kulkarni, S. R., Parsa, M., Mitchell, J. P., Date, P., and Kay, B. (2022). Opportunities for neuromorphic computing algorithms and applications. Nature Comp. Sci. 2, 10–19. doi: 10.1038/s43588-021-00184-y
Sciaraffa, N., Klados, M. A., Borghini, G., Di Flumeri, G., Babiloni, F., and Aricò, P. (2020). Double-step machine learning based procedure for HFOs detection and classification. Brain Sci. 10:220. doi: 10.3390/brainsci10040220
Sharifshazileh, M., Burelo, K., Sarnthein, J., and Indiveri, G. (2021). An electronic neuromorphic system for real-time detection of high frequency oscillations (HFO) in intracranial EEG. Nat. Commun. 12:1. doi: 10.1038/s41467-021-23342-2
Si, X., Yang, Z., Zhang, X., Sun, Y., Jin, W., Wang, L., et al. (2023). Patient-independent seizure detection based on long-term IEEG and a novel lightweight CNN. J. Neural Eng. 20:016037. doi: 10.1088/1741-2552/acb1d9
Sindhu, K. R., Orellana, M., Ombao, H., Riba, A., Philips, D., et al. (2024). Electrode surface area impacts measurement of high frequency oscillations in human intracranial EEG. IEEE Trans. Biomed. Eng. 71, 3283–3292. doi: 10.1109/TBME.2024.3416440
Sindhu, K. R., Staba, R., and Lopour, B. A. (2020). Trends in the use of automated algorithms for the detection of high-frequency oscillations associated with human epilepsy. Epilepsia 61, 1553–1569. doi: 10.1111/epi.16622
Singh, A., Velagala, V. R., Kumar, T., Dutta, R. R., and Sontakke, T. (2023). The application of deep learning to electroencephalograms, magnetic resonance imaging, and implants for the detection of epileptic seizures: a narrative review. Cureus 15:e42460. doi: 10.7759/cureus.42460
Singh, K., and Malhotra, J. (2022). Prediction of epileptic seizures from spectral features of intracranial eeg recordings using deep learning approach. Multimed. Tools Appl. 81, 28875–28898. doi: 10.1007/s11042-022-12611-x
Smith, E. H., Liou, J., Merricks, E. M., Davis, T., Thomson, K., Greger, B., et al. (2022). Human interictal epileptiform discharges are bidirectional traveling waves echoing ictal discharges. Elife 11:73541. doi: 10.7554/eLife.73541
Sperling, M. R. (1997). Clinical challenges in invasive monitoring in epilepsy surgery. Epilepsia 38:s4. doi: 10.1111/j.1528-1157.1997.tb04541.x
Stiglic, G., Kocbek, P., Fijacko, N., Zitnik, M., Verbert, K., and Cilar, L. (2020). Interpretability of machine learning-based prediction models in healthcare. Wiley Interdisciplinary Reviews: Data Mining and Knowledge Discov. 10(5). doi: 10.1002/widm.1379
Sun, J., Sun, J., Li, X., Sun, Y., Hong, Q., and Wang, C. (2025). A review of recent developments in neuromorphic computing based on emerging memory devices. Nonlinear Dynam. doi: 10.1007/s11071-025-11727-7
Sun, L., Feng, C., Zhang, E., Chen, H., Jin, W., Zhu, J., et al. (2024). High-performance prediction of epilepsy surgical outcomes based on the genetic neural networks and hybrid ieeg marker. Sci. Rep. 14:1. doi: 10.1038/s41598-024-56827-3
Sun, Y., Cheng, L., Si, X., He, R., Pereira, T., Pang, M., et al. (2024). Multi-task transformer network for subject-independent ieeg seizure detection. Expert Syst. Appl. 2024:126282. doi: 10.1016/j.eswa.2024.126282
Sun, Y., Jin, W., Si, X., Zhang, X., Cao, J., Wang, L., et al. (2022). Continuous seizure detection based on transformer and long-term IEEG. IEEE J. Biomed. Health Inform. 26, 5418–5427. doi: 10.1109/JBHI.2022.3199206
Tjepkema-Cloostermans, M. C., Tannemaat, M. R., Wieske, L., Van Rootselaar, A., Stunnenberg, B. C., Keijzer, H. M., et al. (2024). Expert level of detection of interictal discharges with a deep neural network. Epilepsia. 66, 184–194. doi: 10.1111/epi.18164
Tong, P. F., Dong, B., Zeng, X., Chen, L., and Chen, S. X. (2024). Detection of interictal epileptiform discharges using transformer based deep neural network for patients with self-limited epilepsy with centrotemporal spikes. Biomed. Signal Process. Control 101:107238. doi: 10.1016/j.bspc.2024.107238
Tveit, J., Aurlien, H., Plis, S., Calhoun, V. D., Tatum, W. O., Schomer, D. L., et al. (2023). Automated interpretation of clinical electroencephalograms using artificial intelligence. JAMA Neurol. 80:805. doi: 10.1001/jamaneurol.2023.1645
Usman, S. M., Khalid, S., and Bashir, S. (2021). A deep learning based ensemble learning method for epileptic seizure prediction. Comput. Biol. Med. 136:104710. doi: 10.1016/j.compbiomed.2021.104710
Van Albada, S. J., Rowley, A. G., Senk, J., Hopkins, M., Schmidt, M., Stokes, A. B., et al. (2018). Performance comparison of the digital neuromorphic hardware spinnaker and the neural network simulation software nest for a full-scale cortical microcircuit model. Front. Neurosci. 12:291. doi: 10.3389/fnins.2018.00291
Vaswani, A., Shazeer, N., Parmer, N., Uszkoreit, J., Jones, L., Gomez, A., et al. (2017). Attention is all you need. arXiv [preprint] arXiv:1706.03762. doi: 10.48550/arXiv.1706.03762
Wang, G., Wang, D., Du, C., Li, K., Zhang, J., Liu, Z., et al. (2020). Seizure prediction using directed transfer function and convolution neural network on intracranial EEG. IEEE Trans. Neural Syst. Rehabilitat. Eng. 28, 2711–2720. doi: 10.1109/TNSRE.2020.3035836
Wang, X., Gao, Z., Zhang, M., Wang, Y., Yang, L., Lin, J., et al. (2024a). Combination of channel reordering strategy and dual cnn-lstm for epileptic seizure prediction using three ieeg datasets. IEEE J. Biomed. Health Inform. 28, 6557–6567. doi: 10.1109/JBHI.2024.3438829
Wang, X., Liu, Y., and Yang, C. (2024b). Ictal-onset localization through effective connectivity analysis based on RNN-GC with intracranial eeg signals in patients with epilepsy. Brain Inform. 11:1. doi: 10.1186/s40708-024-00233-y
Wang, Y., Dai, Y., Liu, Z., Guo, J., Cao, G., Ouyang, M., et al. (2021). Computer-aided intracranial eeg signal identification method based on a multi-branch deep learning fusion model and clinical validation. Brain Sci. 11:615. doi: 10.3390/brainsci11050615
Weiss, S. A., Waldman, Z., Raimondo, F., Slezak, D., Donmez, M., Worrell, G., et al. (2019). Localizing epileptogenic regions using high-frequency oscillations and machine learning. Biomark. Med. 13, 409–418. doi: 10.2217/bmm-2018-0335
Wong, S. M., Arski, O., Workewych, A., Donner, E., Ochi, A., Otsuobo, H., et al. (2020). Detection of high-frequency oscillations in electroencephalography: a scoping review and an adaptable open-source framework. Seizure 84, 23–33. doi: 10.1016/j.seizure.2020.11.009
Worrell, G., and Gotman, J. (2011). High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomark. Med. 5, 557–566. doi: 10.2217/bmm.11.74
Wu, M., Qin, H., Wan, X., and Du, Y. (2021). HFO detection in epilepsy: a stacked denoising autoencoder and sample weight adjusting factors-based method. IEEE Trans. Neural Syst. Rehabilitat. Eng. 29, 1965–1976. doi: 10.1109/TNSRE.2021.3113293
Xu, Y., Khan, T. M., Song, Y., and Meijering, E. (2025). Edge deep learning in computer vision and medical diagnostics: a comprehensive survey. Artif. Intellig. Rev. 58:3. doi: 10.1007/s10462-024-11033-5
Yamamoto, S., Yanagisawa, T., Fukuma, R., Oshino, S., Tani, N., Khoo, H. M., et al. (2021). Data-driven electrophysiological feature based on deep learning to detect epileptic seizures. J. Neural Eng. 18:056040. doi: 10.1088/1741-2552/ac23bf
Yang, M., Wang, K., Li, C., Qian, R., and Chen, X. (2024). Ieeg-hct: a hierarchical cnn-transformer combined network for intracranial eeg signal identification. IEEE Sens. Letters 8, 1–4. doi: 10.1109/LSENS.2024.3351913
Ye, H., Chen, C., Weiss, S. A., and Wang, S. (2023). Pathological and physiological high-frequency oscillations on electroencephalography in patients with epilepsy. Neurosci. Bull. 40, 609–620. doi: 10.1007/s12264-023-01150-6
Yi, G., Song, J., Zhang, W., Wang, J., Li, S., and Cai, L. (2025). Cnn with double-side weighted visibility graph for automated classification of high-frequency oscillations in epilepsy. Biomed. Signal Process. Cont. 110:108178. doi: 10.1016/j.bspc.2025.108178
Yu, M., Zhang, Y., Liu, H., Wu, X., Du, M., and Liu, X. (2023). “IEEG-CT: a CNN and transformer based method for intracranial eeg signal classification,” in International Conference on Neural Information Processing, Lecture Notes in Computer Science (Changsha: Springer), 549–563.
Zanghieri, M., Burrello, A., Benatti, S., Schindler, K., and Benini, L. (2021). “Low-latency detection of epileptic seizures from ieeg with temporal convolutional networks on a low-power parallel MCU,” in IEEE Sensors Applications Symposium (SAS) (Sundsvall: IEEE), 1–6.
Zhang, P., and Zhou, S. (2023). “Detecting epileptic seizures from ieeg with spectral envelope analysis and deep CNNS,” in Global Conference on Information Technologies and Communications (GCITC) (Bangalore: IEEE), 1–6.
Zhang, X., Zhang, X., Huang, Q., and Chen, F. (2024). A review of epilepsy detection and prediction methods based on EEG signal processing and deep learning. Front. Neurosci. 18:1468967. doi: 10.3389/fnins.2024.1468967
Zhang, Y., Chung, H., Ngo, J. P., Monsoor, T., Hussain, S. A., Matsumoto, J. H., et al. (2022). Characterizing physiological high-frequency oscillations using deep learning. J. Neural Eng. 19:066027. doi: 10.1088/1741-2552/aca4fa
Zhang, Y., Liu, L., Ding, Y., Chen, X., Monsoor, T., Daida, A., et al. (2024). Pyhfo: lightweight deep learning-powered end-to-end high-frequency oscillations analysis application. J. Neural Eng. 21:036023. doi: 10.1088/1741-2552/ad4916
Zhang, Y., Lu, Q., Monsoor, T., Hussain, S. A., Qiao, J. X., Salamon, N., et al. (2021). Refining epileptogenic high-frequency oscillations using deep learning: a reverse engineering approach. Brain Commun. 4:1. doi: 10.1101/2021.08.31.458385
Zhao, B., Hu, W., Zhang, C., Wang, X., Wang, Y., Liu, C., et al. (2020). Integrated automatic detection, classification and imaging of high frequency oscillations with stereoelectroencephalography. Front. Neurosci. 14:546. doi: 10.3389/fnins.2020.00546
Zhao, X., Sole-Casals, J., Li, B., Huang, Z., Wang, A., Cao, J., et al. (2020). “Classification of epileptic ieeg signals by cnn and data augmentation,” in ICASSP 2022 - 2022 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) (Barcelona: IEEE), 926–930. doi: 10.1109/ICASSP40776.2020.9052948
Zhao, X., Sole-Casals, J., Sugano, H., and Tanaka, T. (2022a). Seizure onset zone classification based on imbalanced ieeg with data augmentation. J. Neural Eng. 19:065001. doi: 10.1088/1741-2552/aca04f
Zhao, X., Zhao, Q., Tanaka, T., Cao, J., Kong, W., Sugano, H., et al. (2018). “Detection of epileptic foci based on interictal ieeg by using convolutional neural network,” in International Conference on Digital Signal Processing (DSP) (Shanghai: IEEE), 1–5.
Zhao, X., Zhao, Q., Tanaka, T., Solé-Casals, J., Zhou, G., Mitsuhashi, T., et al. (2022b). Classification of the epileptic seizure onset zone based on partial annotation. Cogn. Neurodyn. 17, 703–713. doi: 10.1007/s11571-022-09857-4
Keywords: clinical neurophysiology, high frequency oscillations (HFO), intracranial EEG (iEEG), epileptiform discharges, machine learning in healthcare, deep learning, neural signal analysis, signal processing
Citation: Qamar WUR, Lee M-H and Abibullaev B (2025) Deep learning in intracranial EEG for seizure detection: advances, challenges, and clinical applications. Front. Neurosci. 19:1677898. doi: 10.3389/fnins.2025.1677898
Received: 01 August 2025; Accepted: 06 October 2025;
Published: 30 October 2025.
Edited by:
Mikhail A. Lebedev, Lomonosov Moscow State University, RussiaReviewed by:
Donghwa Yang, Korea University Guro Hospital, Republic of KoreaRehab Naily, Gabes University, Tunisia
Copyright © 2025 Qamar, Lee and Abibullaev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Berdakh Abibullaev, YmVyZGFraC5hYmlidWxsYWV2QG51LmVkdS5reg==
 Wasi Ur Rehman Qamar
Wasi Ur Rehman Qamar Min-Ho Lee
Min-Ho Lee Berdakh Abibullaev
Berdakh Abibullaev