Abstract
Depression, one of the most prevalent neuropsychiatric disorders, continues to pose escalating global health challenges. Despite the discovery of numerous antidepressants, their clinical applications are limited, and there is still an urgent need to identify more effective antidepressant drugs and their molecular targets. In recent years, sigma receptors have garnered considerable interest among depression researchers because of their diverse biological functions and significant roles in the central nervous system. This review provides a comprehensive summary of the potential roles of sigma receptors and their ligands in the pathogenesis of depression and the course of antidepressant treatment, with the aim of offering insights into further research and potential therapeutic development.
Graphical Abstract
1 Introduction
Depression is a widespread mental disorder characterized by persistent sadness, lack of interest, low energy, concentration issues, and changes in appetite and sleep (Smith, 2014; Alexopoulos, 2005). It affects mental health, social life, and overall quality of life, with complex causes, including genetic, environmental, psychological, and biochemical factors (Monroe and Harkness, 2022; Rakel, 1999). Globally, over 300 million people are affected by this disease, making it a major contributor to the global disease burden (Lee et al., 2023). Its prevalence varies by country and is influenced by cultural, economic, and social factors (Malhi and Mann, 2018).
The etiology of depression is multifactorial and involves a complex interplay between genetic and environmental factors. Dysfunction of the brain’s monoamine neurotransmitter systems [e.g., 5-hydroxytryptamine (5-HT), norepinephrine (NE), and dopamine (DA)] (Shao and Zhu, 2020), immune-inflammatory responses (Yin et al., 2024; Han and Yu, 2014), imbalances in the hypothalamic–pituitary–adrenal (HPA) axis (Normann and Buttenschon, 2019), and dysregulation of the endorphin/κ-opioid receptor system (Wang et al., 2023) are considered critical contributors to depression. Despite advancements in the understanding of the pathological mechanisms of depression and the development and application of numerous new treatments, pharmacotherapy remains the primary modality for managing this condition (Sinyor, 2019). As the primary pharmacological agents of choice in clinical practice, selective serotonin reuptake inhibitors (SSRIs) represent a prevalent class of antidepressant medications that alleviate depressive symptoms by augmenting serotonin levels in the brain (Fu et al., 2024). Nevertheless, its clinical applicability is constrained by its adverse effects, but is not limited to sexual dysfunction, hyponatremia, cardiovascular events, elevated risk of fractures, and dermatological reactions (Zahiroddin et al., 2015; Varela Pinon and Adan-Manes, 2017; Kim et al., 2019; Khanassov et al., 2018; Masuka et al., 2022). Other frequently used pharmacological agents, including tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and monoamine oxidase inhibitors (MAOIs), have limited efficacy and delayed therapeutic action onset (Wang et al., 2022).
Recently, emerging studies have revealed that sigma receptors, a class of receptors extensively distributed throughout the central nervous system, are involved in the pathogenesis of depression by interacting with monoaminergic and glutamatergic pathways (Kulkarni and Dhir, 2009; Ren et al., 2025; Fujii et al., 2024; Izumi et al., 2024). They are promising therapeutic targets for the treatment of depression, attributable to their diverse structures and high binding affinities (Ren et al., 2025; Hashimoto, 2015; Garces-Ramirez et al., 2011). Drawing on preclinical studies and clinical trials, we provide a comprehensive review of the underlying mechanisms by which sigma receptors are involved in the pathogenesis of depression and emphasize the potential of sigma receptor ligands as therapeutic targets for depression.
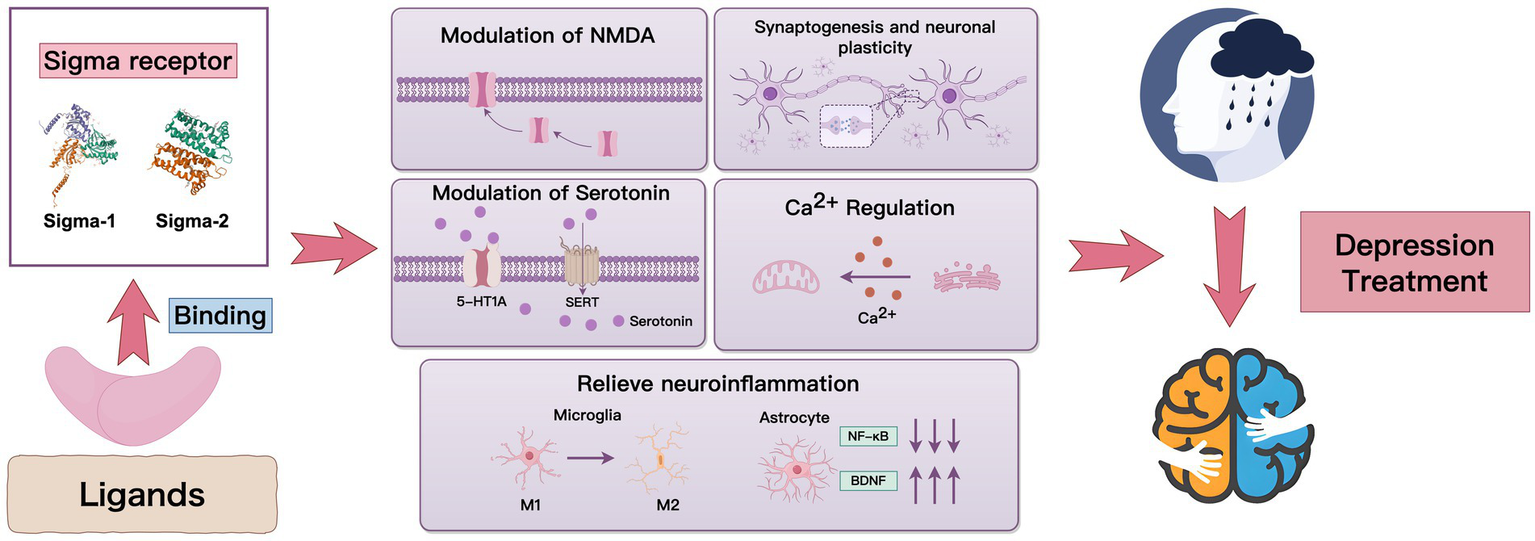
2 Sigma receptors
Sigma receptors were originally proposed as a subtype of opioid receptors (Martin et al., 1976); however, further investigations have shown that they are specialized proteins that remain highly conserved among various species, cell types, and organelles (Hanner et al., 1996; Kekuda et al., 1996; Seth et al., 1997; Seth et al., 1998; Mei and Pasternak, 2001). In particular, the brain exhibits notable concentrations of sigma receptors within the hippocampus, frontal cortex, hypothalamus, olfactory bulb, and depression-associated regions such as the limbic and endocrine (Drevets et al., 2008; aan het Rot et al., 2009; Belmaker and Agam, 2008; Alonso et al., 2000). Sigma receptors are predominantly categorized as sigma-1 and sigma-2 receptors, which exhibit distinct structural and functional characteristics. The sigma-1 receptor (σ1R) is a pharmacologically regulated integral membrane protein predominantly located in the endoplasmic reticulum-mitochondrial-associated membrane (MAM). It is a highly conserved 223 amino acid protein with two transmembrane domains, including amino acid sequences 11–29 (transmembrane domain I) and 92–112 (transmembrane domain II) (Hanner et al., 1996; Kekuda et al., 1996; Su et al., 2016). In the central nervous system, the sigma-1 receptor has been reported to regulate various depression-associated processes, including synaptic plasticity, endoplasmic reticulum stress, neuroinflammation, and calcium homeostasis (Yang et al., 2019; Su et al., 2010; Hayashi and Su, 2007; Mori et al., 2013; Jia et al., 2018), by partially interacting with G protein-coupled receptors and ion channels. The sigma-2 receptor (σ2R) was recently characterized as a transmembrane protein 97 (TMEM97) (Alon et al., 2017). Extensive research has indicated that the sigma-2 receptor is overexpressed in various tumor cell lines, and its ligands demonstrate substantial efficacy in inhibiting cancer cell proliferation and survival (Oyer et al., 2019). Notably, a recent study reported that the activation of the sigma-2 receptor exhibits antidepressant activity comparable to that of citalopram and imipramine in a chronic mild stress model in mice (Sanchez and Papp, 2000), pointing to a novel direction investigating sigma-2 receptors in nervous system diseases.
3 Sigma receptor ligands
In 1994, Glennon et al. investigated the structure-affinity relationships of phenylalkylamine derivatives with respect to sigma-1 receptor binding. They identified super-potent sigma-1 ligands characterized by a pharmacophoric binding mode that includes a central basic amine nitrogen atom flanked by two hydrophobic features (Glennon et al., 1994). Since then, various unrelated and structurally different ligands have been found to exhibit high affinities for the sigma-1 receptor. These substances include anti-Parkinsonian amantadine (Kornhuber et al., 1993), analgesic pentazocine (Zhao et al., 2014), and antidepressant agents such as fluoxetine (Safrany and Brimson, 2016) (Table 1). To address this diversity, it has been proposed that sigma-1 receptors possess adaptable structures that enable binding to a wide range of structurally diverse compounds. However, the absence of a specific methodology has hindered our understanding of the mechanisms underlying these diverse ligand-receptor interactions. To address this issue, a new technique was recently developed. A novel set of BRET (Bioluminescence Resonance Energy Transfer) assays was developed to analyze how ligands induce multimerization of the sigma-1 receptor and its interaction with the immunoglobulin heavy chain-binding protein (BiP). The interaction between the heteromeric sigma-1 receptor and BiP, as examined using BRET assays, demonstrated that (+)-pentazocine and haloperidol caused opposite signal patterns (Yano et al., 2018).
Table 1
| Compounds | Ki (nM) | 1/2 ratio | Chemical class | ||
|---|---|---|---|---|---|
| Sigma | Sigma-1 | Sigma-2 | |||
| Desipramin (Narita et al., 1996) | 343 | 2,107 | 0.163 | TCAs | |
| Imipramine (Narita et al., 1996) | 1,987 | 11,430 | 0.174 | TCAs | |
| Opipramol (Rao et al., 1990) | 50 | TCAs | |||
| Sertraline (Narita et al., 1996) | 57 | 5,297 | 0.095 | SSRI | |
| Citalopram (Narita et al., 1996) | 292 | 5,410 | 0.054 | SSRI | |
| Paroxetine (Narita et al., 1996) | 1,893 | 22,870 | 0.083 | SSRI | |
| Fluvoxamine (Martin et al., 2020) | 36 | 8,439 | 0.004 | SSRIs | |
| Fluoxetine (Narita et al., 1996) | 120 | 5,480 | 0.022 | SSRIs | |
| Clorgyline (Itzhak et al., 1991) | 2.9 | 505 | 0.006 | MAOIs | |
| (+)Deprenyl (Itzhak et al., 1991) | 82 | 1880 | 0.044 | MAOIs | |
| Harmaline (Itzhak et al., 1991) | 310 | 2,100 | 0.148 | MAOIs | |
| Ro11-1163 (Itzhak et al., 1991) | 860 | >50,000 | <0.0172 | MAOIs | |
| Progesterone (McCann et al., 1994) | 246 | 15,700 | 0.016 | Steroids | |
| Deoxycorticorsterone (Su et al., 1988) | 938 | Steroids | |||
| Dehydroepiandrosterone (Takebayashi et al., 2004) | 3,700 | Steroids | |||
| Donepezil (Kato et al., 1999) | 14.6 | Acetylcholinesterase inhibitor | |||
| 4-IBP (Megalizzi et al., 2007) | 1.7 | 25.2 | 0.067 | Derivatives of benzamide | |
| PD144418 (Akunne et al., 1997) | 0.08 | 1,377 | 0.00005 | Oxalate | |
| DTG (Lever et al., 2006) | 35.45 | 39.87 | 0.889 | Guanidines | |
| SA4503 (Lever et al., 2006) | 4.6 | 63.1 | 0.073 | Piperazines | |
| Igmesine (Earley et al., 1991) | 39 | 390 | 0.100 | Benzenamine hydrochloride | |
| YL-0919 (Ren et al., 2023) | 175.1 | 38,200 | 0.005 | Pyridinone hydrochloride | |
| BD 1047 (Matsumoto et al., 1995) | 0.93 | 47 | 0.019 | Derivatives of dichlorophenyl | |
| BD 1063 (Matsumoto et al., 1995) | 9.15 | 449 | 0.020 | Derivatives of methylpiperazine | |
| PRE-084 (Su et al., 1991) | 2.2 | 13,091 | 0.0002 | Phenylalkyl esters | |
| PB190 (Skuza et al., 2014) | 0.42 | 36.3 | 0.012 | Derivatives of naphthalene | |
| PB212 (Skuza et al., 2014) | 0.03 | 17.9 | 0.002 | Derivatives of naphthalene | |
| Haloperidol (Lever et al., 2006) | 0.90 | 7.93 | 0.113 | Butyrophenones | |
| NE-100 (Nakazato et al., 1999) | 1.5 | 84.6 | 0.018 | Propylamine hydrochloride | |
| EST73502 (Garcia et al., 2020) | 118 | 4-aryl analogs | |||
| EST64454 (Diaz et al., 2020) | 22 | Derivatives of pyrazolone | |||
| SI 1/28 (Wilson et al., 2022b) | 6.1 | 583 | 0.010 | Derivatives of methylpiperazine | |
| LMH-2 (Deciga-Campos et al., 2020) | 17 | 6 | Butyrophenones | ||
| Dextromethorphan (Musacchio et al., 1989) | 43 | 1,100 | 0.039 | Methylmethylphosphates | |
| (+)-Pentazocine (PTZ) (Lever et al., 2006) | 1.62 | 728.4 | 0.002 | Opioids | |
| TS-157 (Shi et al., 2021) | 1.8 | 304 | 0.006 | Alkoxyisoxazole | |
| Fenfluramine (Martin et al., 2020) | 266 | Amphetamines | |||
| SCH 23390 (Zhang G. F. et al., 2024) | 3.16 | Benzazepines | |||
| WLB-87848 (Christmann et al., 2024) | 9 | >1,000 | <0.009 | Thienopyrimidines | |
| AF710B (Fisher et al., 2016) | 250 | >10,000 | <0.025 | Derivatives of diazaspiro | |
| LS-1-137 (Malik et al., 2015) | 3.2 | 256 | 0.013 | Benzylpiperidine | |
| RC-752 (Rossino et al., 2023) | 6.2 | 360 | 0.017 | Arylbutylamines | |
| PB-28 (Berardi et al., 2009) | 0.68 | 0.38 | 0.179 | Cyclohexylpiperazines | |
| Lu 28–179 (Perregaard et al., 1995) | 17 | 0.12 | 141.667 | Aminomethylindoles | |
| CM572 (Nicholson et al., 2015) | >10,000 | 14.6 | >684.93 | Isosulfocyanides | |
| CM398 (Wilson et al., 2022a) | >430 | 0.43 | >1,000 | Benzimidazolone | |
| BS148 (Franchini et al., 2017) | 62.8 | 2.5 | 25.12 | Disulfiramocyclopentane | |
| UKH-1114 (Sahn et al., 2017) | 1,379 | 64 | 21.547 | Methanobenzazocine | |
| DKR-1005 (Sahn et al., 2017) | 2,224 | 157 | 36.459 | Methanobenzazocine | |
| DKR-1051 (Sahn et al., 2017) | 556 | 61 | 9.115 | Methanobenzazocines | |
| SAS-0132 (Sahn et al., 2017) | 396 | 90 | 4.400 | Norbenzomorphan | |
| CT1812 (Rishton et al., 2021) | 8.5 | Isoindoline | |||
| 2-Aminopyridine derivatives (Abate et al., 2012) | 68.0 | 16.1 | 4.224 | 2-Aminopyridine | |
| SM21 (Matsumoto et al., 2007) | 1,050 | 145 | 7.241 | Agmatine | |
| AC927 (Matsumoto et al., 2007) | 0.34 | Phenethylpiperidines | |||
| UMB24 (Matsumoto et al., 2007) | 322 | 170 | 1.894 | Phenethylpiperazines | |
| AD164 (Dichiara et al., 2022) | 94 | 1,125 | 0.083 | – | |
| Memantine (Peeters et al., 2004) | 2,600 | Adamantanes | |||
| Amantadine (Peeters et al., 2004) | 7,440 | Adamantanes | |||
| Dimemorfan (Chou et al., 1999) | 151 | 4,421 | 0.034 | Methyl morpholans | |
Representative compounds with affinity for sigma receptors.
*Only opipramol has been approved for the clinical treatment of depression (Le et al., 2024); the other ligands are currently in experimental stages.
4 Sigma receptors in depression: key mechanisms
4.1 Sigma receptors modulate glutamatergic dysfunction in depression
4.1.1 σ1R-NMDA interactions
Dysfunction in glutamatergic neurotransmission has been widely implicated in the pathogenesis of depression (Fan et al., 2023; De Berardis et al., 2020; Ferrero and Cereseto, 2004; Chu et al., 2021; Panczyszyn-Trzewik et al., 2023; Bagot et al., 2015; Shen et al., 2019). Compounds that modulate glutamatergic neurotransmission, including N-methyl-D-aspartic acid (NMDA) receptor antagonists, present a promising avenue for more effective and rapid treatment of depression compared to conventional monoaminergic antidepressants (Kadriu et al., 2019; Iosifescu et al., 2022; Tabuteau et al., 2022). Recent studies have revealed that sigma receptors can regulate neural activity mediated by NMDA receptors upon modulation by ligands (Pabba et al., 2014; Rodriguez-Munoz et al., 2018; Martina et al., 2007; Wang et al., 2007). For example, activation of the sigma-1 receptor can increase the expression of NMDA receptor subunit (NR2A and NR2B), promote NMDA receptor translocation to the cell surface, restore NR2B subunit phosphorylation in NMDA receptors by boosting the neuronal nitric oxide synthase (nNOS)—nitric oxide (NO)—cAMP-response element binding protein (CREB) signaling pathway and upregulate the phencyclidine (PCP)/NMDA receptor complex (Pabba et al., 2014; Zhang S. et al., 2017; Itzhak, 1994). In electrophysiology, activating the sigma-1 receptor can enhance NMDAR responses and long-term potentiation (LTP) by inhibiting Ca2+-activated K+ channels (SK channels), highlighting their role in synaptic transmission (Martina et al., 2007). Consistently, sigma-1 receptor knockout in mice impairs NMDA receptor-dependent LTP and independent long-term depression (LTD) in the basolateral amygdala, potentially leading to depression-like behavior (Zhang B. et al., 2017). These findings suggest that sigma receptor modulation of NMDA responses may contribute to the antidepressant-like effects (Figure 1).
Figure 1

Intracellular signaling pathways in depression and the antidepressant effects mediated by sigma receptors.
4.1.2 Ketamine synergy
Ketamine, a non-competitive NMDA receptor antagonist, is well known for its rapid antidepressant effects in patients with treatment-resistant depression. Some preliminary evidence suggests that activation of the sigma-1 receptor, in conjunction with ketamine, could produce significant outcomes that manifest within hours post-administration, whereas inhibiting the sigma-1 receptor significantly impairs ketamine’s ability to aid recovery in medial prefrontal cortex (mPFC) pyramidal neurons (Salvadore and Singh, 2013), indicating that the sigma-1 receptor may be involved in the lasting antidepressant effect of ketamine, and using ketamine with sigma-1 receptor agonists might improve the treatment for depression (Ma et al., 2024).
4.2 Sigma receptors interplay serotonergic system in depression
Evidence indicates that chronic stress impairs serotonin neurotransmission and reduces neurogenesis in the hippocampus, both of which are associated with the development of depressive symptoms. A diminished capacity for serotonin release in individuals undergoing major depressive episodes has been observed using positron emission tomography (PET) (Erritzoe et al., 2023). Currently, serotonergic neurotransmission is considered to be fundamental to the therapeutic efficacy of SSRI, a widely used treatment for major depressive disorder (MDD) treatment (Fried et al., 2015; Hale et al., 2013; Vahid-Ansari et al., 2019; Roberts et al., 2020), which mitigates depressive symptoms by reinstating serotonin levels and enhancing neurogenesis (Mahar et al., 2014). Interestingly, the effects of serotonergic modulators on depression are influenced by sigma receptors, although the effects of sigma receptors are pleiotropic. For example, in dizocilpine-induced cognitive impairment models, inhibition of sigma-1 receptors is reported to reduce the neuroprotective effect of a neuronal serotonin releaser, fenfluramine (N-ethyl-α-methyl-3-(trifluoromethyl) phenethylamine) (Martin et al., 2022). Consistently, activation of both sigma-1 and 5-HT1A receptors in a forced swimming test produced antidepressant-like effects, which were not observed when each receptor was activated individually. Inhibiting these receptors partially reduced the combined effect, indicating a synergistic antidepressant effect from simultaneous activation (Skuza and Rogoz, 2007). The sigma-1 receptor may enhance the membrane transport and uptake functions of the serotonin transporter (SERT) and its C-terminal deletion mutant (SERTΔCT) (Asano et al., 2019). These results indicate that sigma receptors function as neuroprotectants. Nevertheless, in DBA/2 mice, the sigma-1 receptor inhibition counteracted the reduction in fluvoxamine-induced immobility and functioned as an SSRI, consistent with the activation of the sigma-1 receptor resulting in a dose-dependent reduction in immobility (Sugimoto et al., 2012). These results indicate that sigma receptors may be neurotoxic. Additionally, modulating sigma receptors can impact serotonin-related gene transcription, influence stress responses, and possibly enhance the effectiveness of antidepressants (Skuza et al., 2011). These findings underscore the key role of sigma receptors in serotonin regulation, suggesting new pathways for antidepressant development via 5HT1A and sigma receptor activation.
4.3 Sigma receptors regulates Ca2+ responses in depression
Multiple Ca2+-dependent processes have been shown to contribute to the mechanisms underlying the antidepressant effects in animal behavioral models. The antidepressant effects of igmesine rely on both intracellular and extracellular calcium, consistent with the observation that the calcium chelator EGTA inhibited the antidepressant effects of igmesine and demethylimidazole. A recent study also suggested that mice lacking Orai1, a calcium channel, exhibit decreased inflammation-related Ca2+ signaling in astrocytes, inhibited neurotransmission in the hippocampus, and improvements in depression-like behaviors, including anhedonia and helplessness (Novakovic et al., 2023). Additionally, Kun Li et al. observed a significant increase in the levels of βCaMKII (calmodulin-dependent protein kinases that are essential for Ca2+ signaling) in the lateral habenula (LHb) in depression models. Lowering βCaMKII levels or inhibiting its activity can alleviate depression symptoms, making βCaMKII vital for LHb function and a significant factor in depression (Li et al., 2013).
Previous studies have shown that Ca2+-responses in depression are regulated by sigma receptors. Hayashi and Su (2007) showed that the sigma-1 receptor acts as a Ca2+-sensitive chaperone, aiding Ca2+ signaling between the endoplasmic reticulum (ER) and mitochondria during ER calcium depletion or ligand stimulation, thereby affecting cell survival. Moreover, sigma receptors also modulate various types of calcium channels, including (1) N-type calcium channel, the sigma-1 receptor is co-expressed with the N-type calcium channel in cholinergic interneurons of the rat striatum, and activation of the sigma-1 receptor significantly inhibited the N-type calcium current, whereas a sigma-1 receptor antagonist completely reversed this effect (Zhang K. et al., 2017); (2) L-type calcium currents (ICA-L), sigma-1 receptor activation inhibits ICA-L, which is blocked by sigma-1 receptor inhibition (Guo Y. et al., 2021; Chen et al., 2020); (3) voltage-gated ion channels (VGICs) within the Ca2+ superfamily, sigma-1 can directly modulate VGICs within the Ca2+ superfamily (Aishwarya et al., 2021), thereby regulating neuronal activity, including synaptic transmission and intrinsic excitability; (4) non-voltage-gated Ca2+-permeable channels that are regulated by sigma receptors through direct protein–protein interactions, including interactions with inositol 1,4,5-trisphosphate (IP3) receptors located in the endoplasmic reticulum, as well as with acid-sensing ion channel 1a (ASIC1a) in the plasma membrane (Mari et al., 2015). Prolonged activation of this sigma-1 receptor improves depression-like behaviors in CAMKIV null mice, increases hippocampal brain-derived neurotrophic factor (BDNF) mRNA, and enhances LTP, suggesting that sigma-1 antidepressant effects may involve the regulation of intracellular Ca2+ levels (Moriguchi et al., 2015). Overall, the direct modulation of calcium ion channels by sigma receptors represents a significant mechanism by which these receptors act as therapeutic targets for depression.
4.4 Sigma receptors regulate neuroinflammation in depression
Persistent neuroinflammation within the central nervous system (CNS), a multifaceted process involving various cellular entities, including microglia and astrocytes, plays a substantial role in the development of MDD (Woelfer et al., 2019; Liu C. H. et al., 2019; Zhang Y. et al., 2024; Kwon and Koh, 2020). Microglia are immune cells in the CNS that control neuroinflammation (Salter and Beggs, 2014). Previous studies have shown that activating sigma receptors may reduce M1 microglial polarization and neuroinflammation by influencing ER-mitochondrial interactions and function in models of stress-induced hypertensive rats and valproic acid-induced autistic rats (Ooi et al., 2021; Mohamed et al., 2025). Consistently, exogenous activation of sigma receptors significantly reduces microglial activation following traumatic brain injury (TBI) (Shi et al., 2022). Surprisingly, the sigma-1 receptor inhibition significantly reduced cortical microglial proliferation in a mouse model of chronic osteoarthritis pain, thereby alleviating depression-like behaviors (Carcole et al., 2019). This demonstrates that the role of sigma receptors in microglia may vary depending on the context of depression. In addition to microglia, the effects of sigma receptors on astrocytes have been observed. In mouse models with depression-like symptoms, blocking the sigma-1 receptor in astrocytes triggers depression-related behaviors through NF-κB-driven neuroinflammation, which is alleviated by sigma-1 receptor agonists (Wang et al., 2024). Coincidentally, activating the astrocytic sigma-1 receptor significantly alleviates lipopolysaccharide (LPS)-induced depression-like behaviors (Guo L. et al., 2021). These findings indicate the potential of sigma receptors as therapeutic targets for neuroinflammation in depression.
4.5 Synaptogenesis/neuronal plasticity: implications for antidepressant efficacy
Depression is a complex mental disorder associated with changes in synaptic plasticity and synaptogenesis, particularly in the prefrontal cortex and hippocampus. Reduction of synapses in brain regions related to mood and cognition is linked to depression, and fast-acting antidepressants such as ketamine could promote synapse formation and repair stress-related synaptic damage (Duman and Aghajanian, 2012). Additionally, changes in spinogenesis in the basolateral amygdala and medial prefrontal cortex contribute to the onset and resolution of depressive episodes as well as the sustained effects of antidepressants such as ketamine and imipramine (Moda-Sava et al., 2019; Leem et al., 2020). Consistently, the expression of BDNF, a protein crucial for synaptic plasticity, is lower in the hippocampus and prefrontal cortex of depressed mice, and antidepressants, such as ketamine, can increase BDNF levels and counteract stress (Duman et al., 2021). These results indicate that targeting synaptic plasticity may be effective in treating depression.
Interestingly, recent studies have highlighted sigma-1 receptor agonists as promising treatments for depression by boosting neuroplasticity. Activation of the sigma-1 receptor improved anxiety and depression-like behaviors within a week by reversing estrogen withdrawal-induced reductions in hippocampal dendritic complexity and spine density (Ren et al., 2024). Consistently, co-administration of selective sigma-1 and 5-HT1A receptor agonists enhances neurogenesis and synaptic plasticity in the dorsal hippocampal dentate gyrus, potentially producing an antidepressant effect in chronically stressed mice (Ren et al., 2025). Additionally, several studies have demonstrated the role of sigma receptors in LTP, a crucial representation of synaptic plasticity. A study showed that sigma receptor activation might counteract the impaired LTP induced by corticosterone, olfactory bulb resection, or CaMKIV-deficiency and have antidepressant benefits, in which the Akt/GSK-3β/β-catenin pathway enhanced neurogenesis and increased phosphorylation of CaMKII and GluA1 might be involved (Moriguchi et al., 2015; Kaminska et al., 2000; Moriguchi et al., 2013). Consistently, male mice lacking the sigma-1 receptor show depression-like symptoms (Zhang B. et al., 2017; Voronin et al., 2020; Salaciak and Pytka, 2022), reduced field excitatory postsynaptic potential (fEPSP) slope, impairments in LTP and LTD, and decreased NMDAR NR2B phosphorylation. Administration of an NMDA agonist improved LTD-related behavior and depressive symptoms. Indicating that sigma-1 receptor deficiency affects nNOS activity and NO production via NMDA receptor dysfunction, reducing GABAAR-mediated inhibition and leading to depression-like phenotypes (Zhang B. et al., 2017; Qin et al., 2022). Taken together, sigma receptor modulators hold considerable promise for enhancing depression treatment by facilitating synaptogenesis and neuronal plasticity (Yang et al., 2019; Hashimoto, 2013; Klawonn et al., 2017; Ishima et al., 2014).
5 Ligand classes and clinical progress
5.1 Clinically approved antidepressants with sigma receptor activity
5.1.1 Tricyclic antidepressants
TCAs are pharmacological agents that inhibit norepinephrine and serotonin reuptake, thereby increasing the concentration of these neurotransmitters at synaptic junctions. Previous studies have shown that TCAs interact with sigma receptors (Paschos et al., 2009). Opipramol, a compound approved in Europe for the treatment of depression and anxiety, has a high affinity for sigma-1 receptors and a low affinity for sigma-2 receptors. Subchronic administration of opipramol reduced sigma-2 receptor expression, whereas prolonged administration of opipramol resulted in a reduction in sigma-1 receptor mRNA in the nucleus accumbens (Holoubek and Muller, 2003). Additionally, the antidepressant effects of another antidepressant, igmesine, depend on sigma receptors, as evidenced by its ability to reduce immobility time in the forced swim test (FST) and chronic forced swim (CFS) tests, effects that are inhibited by Sigma-1 receptor antagonists and sigma-1 knockout mice (Villard et al., 2011). Desipramine also exerts modulatory effects on sigma receptors by affecting their expression in glial cells (Barg et al., 1991).
5.1.2 Selective serotonin reuptake inhibitors
SSRIs are a class of antidepressants that inhibit serotonin reuptake, aligning with the monoamine hypothesis that depression is linked to reduced monoamine levels (Skuza et al., 2011). Fluvoxamine, a notable SSRI that shows significant binding to sigma receptors (Hindmarch and Hashimoto, 2010), alleviates methamphetamine-induced anxiety behaviors through sigma-1 receptor activation (Zhang et al., 2023). In rats with chronic mild unpredictable stress (CUMS)-induced MDD, fluvoxamine exhibits antiarrhythmic effects via sigma-1 receptor-dependent mechanisms (Guo Y. et al., 2021). Other SSRIs that interact with sigma receptors include, sertraline which inhibits LTP in rat hippocampal slices through inverse agonism at the sigma-1 receptor (Izumi et al., 2024); fluoxetine which shows high affinity for sigma receptors and enhances nerve growth factor (NGF)-induced neurite outgrowth (Ishima et al., 2014); and citalopram which modulates the response of embryonic thalamic axons to Netrin-1 through sigma-1 receptor activation (Bonnin et al., 2012). Notably, paroxetine does not exhibit sigma-1 receptor agonistic effects (Sugimoto et al., 2012). It is interesting to identify the SSRIs that functions through sigma receptors and analyze the common structural features of these SSRIs, which may help developing novel SSRIs with higher selectivity or efficacy.
5.1.3 Monoamine oxidase inhibitors
MAOIs are recognized for their effectiveness in treating depression by increasing brain monoamines through the inhibition of their degradation (Yang et al., 2019). Compounds such as selegiline and rasagiline display neuroprotective properties by stabilizing mitochondrial function, inhibiting apoptotic pathways, and promoting neurotrophic factor expression. These effects are partly mediated by sigma receptors (Behl et al., 2021; Naoi and Maruyama, 2010). Clorgyline shows a high affinity for the sigma binding site in the C57BL/6 J mouse brain (Itzhak and Kassim, 1990), whereas deprenyl reduces sigma-1 receptor-mediated behavioral sensitization to cocaine (Tsai et al., 2015). Elucidating the roles of sigma receptors in MAOIs may help in developing novel MAOIs with minimal adverse effects, such as the “cheese effect” while enhancing neuroprotective outcomes (Finberg, 2014; Schapira, 2011).
5.1.4 Neurosteroid and acetylcholinesterase inhibitor
Neurosteroids have emerged as promising antidepressant agents, especially for the treatment of postpartum depression (McEvoy et al., 2018; Rupprecht et al., 2023; Kargbo, 2023). Crystallographic studies have revealed that progesterone and dehydroepiandrosterone sulfate (DHEAS) interact with the sigma-1 receptor through hydrophobic interactions, which might contribute to DHEAS-enhanced presynaptic glutamate release in the prefrontal cortex (Fu et al., 2024; Chen et al., 2017). Another neurosteroid, dehydroepiandrosterone (DHEA), increases GLT-1 activity and promotes the migration of hippocampal astrocytes through sigma-1 receptor-mediated PKC activation, thereby providing protection against excitotoxicity (Dong et al., 2007). Donepezil, a central acetylcholinesterase inhibitor that acts as a sigma-1 receptor agonist, has also been reported to alleviate depression in patients with Alzheimer’s disease, probably due to its direct binding to sigma-1 receptors in the human brain, as evidenced by PET scans (Carotenuto et al., 2022; Devanand et al., 2018).
5.2 Novel sigma receptor ligands in preclinical development
5.2.1 Sigma-1 receptor ligands
YL-0919 is chemically known as 1-(1-benzyl-4-hydroxypiperidin-4-ylmethyl)-2(1H)-pyridinone hydrochloride. Intragastric administration of YL-0919 alleviates depressive symptoms, as assessed by the FST and Tail Suspension Test (TST), without increasing suicide risk or causing weight gain (Sun et al., 2019). Consistently, YL-0919 could enhance the firing of glutamatergic pyramidal neurons in the mPFC (Yan et al., 2024), highlighting the necessity of considering cell type and/or brain region when studying the mechanisms underlying YL-0919 therapy. Additionally, in the mPFC, YL-0919 promotes neuronal remodeling and restores mitochondrial function (Li J. F. et al., 2023) and reduces NF-κB-induced neuroinflammation in depressed mice with sigma-1 receptor knockdown (Wang et al., 2024). In the hippocampus, YL-0919 boosts cyclic adenosine monophosphate (cAMP) signaling (Ran Y. H. et al., 2018) and activated mTOR pathway, which contributes to its effects of decreasing swimming retention time and feeding latency (Ran Y. et al., 2018). However, to the best of our knowledge, whether sigma receptors in different cell types or brain regions function differently during YL-0919 therapy is largely unknown.
Interestingly, sigma receptors in astrocytes may be potential targets for YL-0919 therapy. For example, inhibiting sigma-1 receptors on ventral hippocampal astrocytes led to anxiety and depression-like behaviors and blocked YL-0919’s antidepressant effects in stressed mice. Consistently, knockdown of sigma-1 receptors hinders the ability of YL-0919 to improve mitochondrial function and BDNF expression in astrocytes (Li et al., 2025). As for microglia, previous studies showed that the administration of YL-0919 partially reversed PLX3397-induced microglial depletion in the mPFC of mice and alleviated depressive symptoms (Chang et al., 2023); however, whether these effects were attributed to sigma receptors on microglia needs further exploration.
SA-4503, chemically known as 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl) piperazine dihydrochloride, acts as a sigma-1 receptor agonist (Matsuno et al., 1996; Kobayashi et al., 1996; Senda et al., 1996). SA-4503 exhibits antidepressant effects through various mechanisms, including (1) formation of sigma-1 receptor-5-HT1A complexes, showing neuroprotective effects that were inhibited by sigma-1 receptor antagonist (Ren et al., 2025; Skuza and Rogoz, 2007); (2) synergistic interactions with NMDA receptor antagonists (Wang et al., 2007; Skuza and Rogoz, 2006), including enhancement of the antidepressant effects of ketamine, which were partially counteracted by BD1407 (Ma et al., 2024); (3) improvement of hippocampal LTP (Moriguchi et al., 2015); and (4) enhancement of cardiac function, showing its efficacy in treating depressive-behavior associated with heart failure (Liu X. et al., 2019; Liu et al., 2018; Shinoda et al., 2016). Current research has predominantly focused on the adjunctive use of SA-4503 with antidepressants, whereas studies on its independent neurobiological mechanisms remain limited, thus offering avenues for future research.
SKF-83959, chemically known as 6-Chloro-7,8-dihydroxy-3-methyl-1-(3-methylphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine, is a triple reuptake inhibitor with antidepressant properties (Liu et al., 2009; Jiang et al., 2014; Fang et al., 2013). As a potent allosteric modulator of the sigma-1 receptor, SKF-83959 inhibits the calcineurin/GSK3β pathway in the hippocampus, thereby alleviating depression-like symptoms in mice with epilepsy (Guo et al., 2022). SOMCL-668, a novel sigma-1 receptor modulator derived from SKF-83959, also showed rapid antidepressant effects in the CUMS model. However, in contrast to the inhibition of SKF-83959 on hippocampal GSK3β signaling, SOMCL-668 is reported to simultaneously increase GSK3β phosphorylation in the hippocampus (Guo et al., 2022), indicating the distinct mechanisms underlying SOMCL-668 and SKF-83959 therapy. However, their effects were inhibited by sigma-1 receptor antagonists. SCH-23390, an analog of SKF-83959, serves as a positive allosteric modulator of the sigma-1 receptor (Zhang G. F. et al., 2024), although its antidepressant effects require further validation. Understanding the mechanisms of action of SKF-83959, SCH-23390, and SOMCL-668, along with their structural frameworks, may inform future antidepressant drug development.
Other sigma-1 receptor agonists, such as SKF-10047, PRE-084, and igmesine (Zhang K. et al., 2017; Wang et al., 2019; Skuza and Rogoz, 2009; Ito et al., 2012; Urani et al., 2001), have shown antidepressant effects in behavioral paradigms, although additional laboratory data are needed. Sigma-1 receptor antagonists, including PD-144418, BD-1047, and BD-1063, have been found to mitigate “mania-like” behaviors without causing depression-like behaviors (Sanchez-Blazquez et al., 2018), and the sigma-1 receptor antagonist E-52862 has demonstrated antidepressant effects in osteoarthritis mouse models, which may be mediated by the inhibition of cortical microgliosis (Carcole et al., 2019). In pharmaceutical research, newly synthesized pyrimidine thioethers (5c, 5e, and 5f) have shown antidepressant and cognitive enhancement properties, potentially through interactions with sigma receptors (Fioravanti et al., 2023). Understanding the structure–activity relationships and employing computer-aided design can aid in the discovery of novel sigma receptor-binding agents and the mechanisms underlying their antidepressant effects.
5.2.2 Sigma-2 receptor ligands
The sigma-2 receptor complex has been implicated in autophagy, cholesterol synthesis, progesterone signaling, and receptor stabilization, all of which are associated with depression (Izzo et al., 2020). Consistently, mice deficient in the sigma-2 receptor/Tmem97 exhibit reduced depression-like behavior, particularly in females (Hong et al., 2024). However, research on sigma-2 receptors and their ligands in the context of depression is relatively limited compared to studies on sigma-1 receptors. Lu 28–179, a selective sigma-2 receptor ligand, demonstrated antidepressant effects at a dosage of 1.0 mg/kg administered subcutaneously per day in a rat model of chronic mild stress-induced depression (Sanchez and Papp, 2000). Ibogaine, a compound derived from African psychedelic flora, shows a high affinity for sigma-2 receptors (Ki: 90.4 and 250 nM) but a lower affinity for sigma-1 receptors (Ki: 9310 nM) (Mach et al., 1995; Bowen et al., 1995). A single intraperitoneal administration of ibogaine (20–40 mg/kg) has been shown to produce antidepressant-like effects in rats, which are attributed to increased BDNF mRNA levels in the prefrontal cortex (Rodri Guez et al., 2020). Based on the pharmacophore of ibogaine, several novel small-molecule compounds have been discovered and shown to be effective in alleviating depression-like symptoms in murine models (Singh et al., 2023; Cameron et al., 2021). Importantly, a case report suggested the effectiveness of ibogaine in reducing depressive symptoms in bipolar depression (Fernandes-Nascimento et al., 2022). These results suggest that the sigma-2 receptor is a promising therapeutic target for depression and merits further investigation for drug development.
Despite the identification of promising ligands, the sigma-2 receptor remains underexplored, a paradox largely due to several interconnected limitations. First, the sigma-2 receptor had not been cloned for an extended period and was believed to be an 18–22 kDa protein enriched in lipid rafts (Crawford et al., 2002). It was not until 2017 that the sigma-2 receptor was identified as TMEM97, a protein involved in cholesterol regulation (Alon et al., 2017). This delayed validation of the sigma-2 receptor molecular identity significantly hindered early progress. Consequently, initial studies on sigma-2 receptor ligands primarily relied on in vitro cell lines (Barg et al., 1994), as researchers lacked genetic tools (e.g., knockout models) to investigate the roles of sigma-2 receptors and their ligands in depression, which were assessed through behavioral testing. Furthermore, the expression of the sigma-2 receptor varies across heterogeneous cell lines (Barg et al., 1994; John et al., 1998; John et al., 1999), and this inconsistency has also impeded consensus on the therapeutic potential of the sigma-2 receptor in depression, even for well-characterized ligands. Third, the sigma-2 receptor is part of the progesterone receptor membrane component (PRMC) family, which shares structural homology with other cholesterol-sensing proteins (Chu et al., 2015; Hiranita, 2016). Consequently, sigma-2 receptor ligands may cross-react with other PRMC family members, such as PGRMC1, or with cholesterol transporters, potentially resulting in off-target effects that obscure sigma-2 receptor-specific signaling (Zeng et al., 2019; Riad et al., 2020). Until recently, no sigma-2 receptor ligands with absolute selectivity were available, thereby limiting researchers’ ability to ascertain whether the observed antidepressant effects are attributable to sigma-2 receptor activity rather than off-target binding.
Additionally, sigma receptor ligands can also bind to multiple targets. As previously discussed, numerous sigma receptor ligands, particularly those developed in the early stages, including fenfluramine, opipramol, and donepezil, often exhibit limited selectivity (Rao et al., 1990; Martin et al., 2020; Kato et al., 1999). For example, (1) Donepezil is known to interact with acetylcholinesterase and α-synuclein, which enhances LTP (Mandai et al., 2020); (2) Opipramol has the potential to engage with dopamine receptor, thereby enhancing dopamine metabolism in the striatum and other brain regions (Rao et al., 1990); (3) Ketamine could directly bind to NMDA receptor to activate the BDNF-mTOR pathway (Kim et al., 2024); and (4) Sertraline could directly bind to SERT, which could in turn enhances synaptic plasticity (Ferres-Coy et al., 2016). The off-target effects of these ligands make it difficult to precisely determine the role(s) of the sigma receptors in these biological processes. Additional experiments base on advanced methodologies such as network pharmacology, computational modeling and omics technologies may help us understand whether the observed effect of sigma receptor ligand attributes to its action on sigma receptors, other targets or both.
5.3 Sigma ligands in clinical trials
Currently, sigma ligands have attracted considerable interest in clinical trials in treating depression, including:
SA-4503, a sigma-1 receptor agonist, has shown antidepressant effects in animal studies. In 2007, a Phase II clinical trial assessed the safety and efficacy of SA-4503 in individuals with major depression, involving 150 participants over an eight-week period. However, the results of this trial have not yet been published (ClinicalTrials.gov Identifier: NCT00551109). Opipramol, which binds to both sigma-1 and sigma-2 receptors (Volz and Stoll, 2004), has been found to alleviate hot flashes and depressive symptoms in postmenopausal women compared to placebo (van Lith and Motke, 1983). In a double-blind study with 17 participants aged 28–60 years, the combination of opipramol and baclofen significantly reduced polysubstance use disorder and depressive symptoms while also increasing DHEA-S levels and the DHEA-S to cortisol ratio, indicating potential therapeutic benefits for depression in patients with substance use disorder (Bareli et al., 2021). Fluvoxamine, a selective serotonin reuptake inhibitor, exhibits agonistic effects on sigma-1 receptors. A two-month study involving patients with MDD found that fluvoxamine alleviated depressive symptoms and lowered interleukin-6 (IL-6) levels, with these effects partially mediated by sigma receptor activity (ClinicalTrials.gov Identifier: NCT04160377) (Li X. et al., 2023). A study on fluvoxamine maleate for the treatment of depression in children remains unpublished [ClinicalTrials.gov Identifier: NCT00353028]. AXS-05, a combination of dextromethorphan and bupropion, also acts as a sigma-1 receptor agonist. In a Phase III trial with 327 patients with MDD, a 6-week treatment with AXS-05 proved superior to placebo in the Montgomery-Asberg Depression Rating Scale (MADRS) scores, with 39.5% of participants achieving remission (ClinicalTrials.gov Identifier: NCT04019704) (Iosifescu et al., 2022). Another trial also showed that AXS-05 had greater efficacy than bupropion in reducing MADRS scores over 6 weeks (ClinicalTrials.gov Identifier: NCT03595579) (Tabuteau et al., 2022). A trial comparing AXS-05 with a placebo for depression recurrence is forthcoming (ClinicalTrials.gov Identifier: NCT04608396). Indeed, the efficacy of AXS-05 in treating depression has been reviewed in a previous systematic review of five studies, demonstrating that AXS-05 significantly reduced depression severity within 2 weeks, with effects lasting for 12 months, suggesting that it is a rapid-acting option for MDD (Akbar et al., 2023). Dextromethorphan, a component of AXS-05, exhibits rapid antidepressant effects via sigma receptors in animal models (Nguyen et al., 2014). A phase IIa clinical trial with 20 patients with treatment-resistant depression assessed the combination of dextromethorphan and quinidine. Over a 10-week period, the Montgomery-Åsberg Depression Rating Scale (MADRS) scores decreased by 13.0 points, and the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) scores decreased by 5.9 points, indicating preliminary efficacy (Murrough et al., 2017). An ongoing study is currently recruiting participants to investigate depression-related neuropsychiatric changes in patients with HIV who are starting treatment with dolutegravir in combination with TDF/FTC (ClinicalTrials.gov: NCT06787976). YL-0919 is in phase 2 trials, showing antidepressant effects and multitarget properties in preclinical studies (ClinicalTrials.gov: NCT03404466, NCT03739632, and NCT04598607) (Table 2).
Table 2
| Compounds | Clinical phase | Patients No. | Condition | Outcome | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| SA4503 | Phase II | 150 | MDD | Unreleased | NCT00551109 |
| Opipramol | Pilot study | 17 | Polysubstance use disorder (PSUD) | Significant improvement in depressive symptoms | Not registered |
| Fluvoxamine | Phase II | 150 | Depression | Relieved | NCT04160377 |
| Phase IV | 90 | MDD | Unreleased | NCT00353028 | |
| AXS-05 | Phase III | 327 | MDD | Partial remission | NCT04019704 |
| Phase II | 97 | MDD | Decrease in MADRS | NCT03595579 | |
| Phase II | 44 | MDD | Unreleased | NCT03595579 | |
| Dextromethorphan | Phase IIa | 20 | Treatment-resistant depression | Decrease in MADRS and QIDS-SR | Not registered |
| Phase IV | 140 | Patients with HIV | Unreleased | NCT06787976 | |
| YL-0919 | Phase I/II | 42 | MDD | Unreleased | NCT03404466 |
| Phase II | 240 | MDD | Unreleased | NCT03739632 | |
| Phase I | 36 | MDD | Unreleased | NCT04598607 |
Sigma receptor ligands in clinical trials.
6 Conclusion and prospect
Sigma receptors play crucial roles in the development of depression. Recent studies on sigma receptor ligands have made significant progress, with sigma-1 receptor agonists demonstrating antidepressant effects. Many therapeutic benefits of antidepressants are linked to sigma receptors, and drugs targeting these receptors are currently undergoing clinical trials. Sigma receptor ligands affect monoaminergic neurotransmission and synaptic plasticity through intracellular translocation. Differences in binding affinities may explain the varied antidepressant effects, highlighting the need for personalized treatment strategies. The complex nature of sigma receptors offers the potential for depression treatment, and the development of high-affinity ligands could effectively address depression and its comorbidities. Although research on sigma receptor ligands is promising, there are still significant gaps. The lack of large-scale clinical trials limits the confirmation of efficacy and safety in patients with depression, as most evidence comes from preclinical studies. Research must clarify the long-term effects on the body and the mechanisms of action, especially regarding neurotransmitter interactions. Although research on sigma-2 receptors is limited, previous studies may guide the development of pharmacological agents. The development of specific sigma receptor ligands is essential, as existing ligands exhibit off-target effects. Future research should focus on developing high-specificity ligands using computational methods and high-throughput screenings.
Statements
Author contributions
PZ: Writing – original draft, Writing – review & editing. ZN: Formal analysis, Investigation, Project administration, Validation, Writing – review & editing. HC: Writing – original draft. JC: Writing – review & editing. ZL: Writing – review & editing. FX: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant Nos. 82271212, 82371476, 82030038, and 82293644), Major Project of TCM of Shaanxi Province (2021-ZXY-SYS-001) and the Discipline Boost Program of Xijing Hospital (grant Nos. XJZT24JC21, LHJJ24WQ09).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
aan het Rot M. Mathew S. J. Charney D. S. (2009). Neurobiological mechanisms in major depressive disorder. CMAJ180, 305–313. doi: 10.1503/cmaj.080697
2
Abate C. Ferorelli S. Niso M. Lovicario C. Infantino V. Convertini P. et al . (2012). 2-aminopyridine derivatives as potential sigma(2) receptor antagonists. ChemMedChem7, 1847–1857. doi: 10.1002/cmdc.201200246
3
Aishwarya R. Abdullah C. S. Morshed M. Remex N. S. Bhuiyan M. S. (2021). Sigmar1's molecular, cellular, and biological functions in regulating cellular pathophysiology. Front. Physiol.12:705575. doi: 10.3389/fphys.2021.705575
4
Akbar D. Rhee T. G. Ceban F. Ho R. Teopiz K. M. Cao B. et al . (2023). Dextromethorphan-bupropion for the treatment of depression: a systematic review of efficacy and safety in clinical trials. CNS Drugs37, 867–881. doi: 10.1007/s40263-023-01032-5
5
Akunne H. C. Whetzel S. Z. Wiley J. N. Corbin A. E. Ninteman F. W. Tecle H. et al . (1997). The pharmacology of the novel and selective sigma ligand, PD 144418. Neuropharmacology36, 51–62. doi: 10.1016/s0028-3908(96)00161-x
6
Alexopoulos G. S. (2005). Depression in the elderly. Lancet365, 1961–1970. doi: 10.1016/S0140-6736(05)66665-2
7
Alon A. Schmidt H. R. Wood M. D. Sahn J. J. Martin S. F. Kruse A. C. (2017). Identification of the gene that codes for the sigma(2) receptor. Proc. Natl. Acad. Sci. USA114, 7160–7165. doi: 10.1073/pnas.1705154114
8
Alonso G. Phan V. L. Guillemain I. Saunier M. Legrand A. Anoal M. et al . (2000). Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience97, 155–170. doi: 10.1016/s0306-4522(00)00014-2
9
Asano M. Motoike S. Yokota C. Usuki N. Yamamoto H. Urabe T. et al . (2019). SKF-10047, a prototype Sigma-1 receptor agonist, augmented the membrane trafficking and uptake activity of the serotonin transporter and its C-terminus-deleted mutant via a Sigma-1 receptor-independent mechanism. J. Pharmacol. Sci.139, 29–36. doi: 10.1016/j.jphs.2018.11.005
10
Bagot R. C. Parise E. M. Peña C. J. Zhang H. X. Maze I. Chaudhury D. et al . (2015). Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun.6:7062. doi: 10.1038/ncomms8062
11
Bareli T. Ahdoot H. L. Ben Moshe H. Barnea R. Warhaftig G. Gispan I. et al . (2021). Novel Opipramol-baclofen combination alleviates depression and craving and facilitates recovery from substance use disorder-an animal model and a human study. Front. Behav. Neurosci.15:788708. doi: 10.3389/fnbeh.2021.788708
12
Barg J. Belcheva M. M. Bem W. T. Lambourne B. McLachlan J. A. Tolman K. C. et al . (1991). Desipramine modulation of sigma and opioid peptide receptor expression in glial cells. Peptides12, 845–849. doi: 10.1016/0196-9781(91)90144-e
13
Barg J. Thomas G. E. Bem W. T. Parnes M. D. Ho A. M. Belcheva M. M. et al . (1994). In vitro and in vivo expression of opioid and sigma receptors in rat C6 glioma and mouse N18TG2 neuroblastoma cells. J. Neurochem.63, 570–574. doi: 10.1046/j.1471-4159.1994.63020570.x
14
Behl T. Kaur D. Sehgal A. Singh S. Sharma N. Zengin G. et al . (2021). Role of monoamine oxidase activity in Alzheimer's disease: an insight into the therapeutic potential of inhibitors. Molecules26:3724. doi: 10.3390/molecules26123724
15
Belmaker R. H. Agam G. (2008). Major depressive disorder. N. Engl. J. Med.358, 55–68. doi: 10.1056/NEJMra073096
16
Berardi F. Abate C. Ferorelli S. Uricchio V. Colabufo N. A. Niso M. et al . (2009). Exploring the importance of piperazine N-atoms for sigma(2) receptor affinity and activity in a series of analogs of 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28). J. Med. Chem.52, 7817–7828. doi: 10.1021/jm9007505
17
Bonnin A. Zhang L. Blakely R. D. Levitt P. (2012). The SSRI citalopram affects fetal thalamic axon responsiveness to netrin-1 in vitro independently of SERT antagonism. Neuropsychopharmacology37, 1879–1884. doi: 10.1038/npp.2012.35
18
Bowen W. D. Vilner B. J. Williams W. Bertha C. M. Kuehne M. E. Jacobson A. E. (1995). Ibogaine and its congeners are sigma 2 receptor-selective ligands with moderate affinity. Eur. J. Pharmacol.279, R1–R3. doi: 10.1016/0014-2999(95)00247-i
19
Cameron L. P. Tombari R. J. Lu J. Pell A. J. Hurley Z. Q. Ehinger Y. et al . (2021). A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature589, 474–479. doi: 10.1038/s41586-020-3008-z
20
Carcole M. Zamanillo D. Merlos M. Fernández-Pastor B. Cabañero D. Maldonado R. et al . (2019). Blockade of the Sigma-1 receptor relieves cognitive and emotional impairments associated to chronic osteoarthritis pain. Front. Pharmacol.10:468. doi: 10.3389/fphar.2019.00468
21
Carotenuto A. Fasanaro A. M. Manzo V. Amenta F. Traini E. (2022). Association between the cholinesterase inhibitor donepezil and the cholinergic precursor choline Alphoscerate in the treatment of depression in patients with Alzheimer's disease. J. Alzheimers Dis. Rep.6, 235–243. doi: 10.3233/ADR-200269
22
Chang H. X. Dai W. Bao J. H. Li J. F. Zhang J. G. Li Y. F. (2023). Essential role of microglia in the fast antidepressant action of ketamine and hypidone hydrochloride (YL-0919). Front. Pharmacol.14:1122541. doi: 10.3389/fphar.2023.1122541
23
Chen T. Tanaka M. Wang Y. Sha S. Furuya K. Chen L. et al . (2017). Neurosteroid dehydroepiandrosterone enhances activity and trafficking of astrocytic GLT‐1viaσ1receptor‐mediated PKC activation in the hippocampal dentate gyrus of rats. Glia65, 1491–1503. doi: 10.1002/glia.23175
24
Chen X. Zhang C. Guo Y. Liu X. Ye T. Fo Y. et al . (2020). Chronic stimulation of the sigma-1 receptor ameliorates ventricular ionic and structural remodeling in a rodent model of depression. Life Sci.257:118047. doi: 10.1016/j.lfs.2020.118047
25
Chou Y. C. Liao J. F. Chang W. Y. Lin M. F. Chen C. F. (1999). Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan. Brain Res.821, 516–519. doi: 10.1016/s0006-8993(99)01125-7
26
Christmann U. Garriga L. Llorente A. V. Díaz J. L. Pascual R. Bordas M. et al . (2024). WLB-87848, a selective σ1 receptor agonist, with an unusually positioned NH group as positive Ionizable moiety and showing neuroprotective activity. J. Med. Chem.67, 9150–9164. doi: 10.1021/acs.jmedchem.4c00288
27
Chu U. B. Mavlyutov T. A. Chu M. L. Yang H. Schulman A. Mesangeau C. et al . (2015). The Sigma-2 receptor and progesterone receptor membrane component 1 are different binding sites derived from independent genes. EBioMedicine2, 1806–1813. doi: 10.1016/j.ebiom.2015.10.017
28
Chu S. F. Zhang Z. Zhou X. He W. B. Yang B. Cui L. Y. et al . (2021). Low corticosterone levels attenuate late life depression and enhance glutamatergic neurotransmission in female rats. Acta Pharmacol. Sin.42, 848–860. doi: 10.1038/s41401-020-00536-w
29
Crawford K. W. Coop A. Bowen W. D. (2002). Sigma(2) receptors regulate changes in sphingolipid levels in breast tumor cells. Eur. J. Pharmacol.443, 207–209. doi: 10.1016/s0014-2999(02)01581-9
30
De Berardis D. Tomasetti C. Pompili M. Serafini G. Vellante F. Fornaro M. et al . (2020). An update on glutamatergic system in suicidal depression and on the role of Esketamine. Curr. Top. Med. Chem.20, 554–584. doi: 10.2174/1568026620666200131100316
31
Deciga-Campos M. Melo-Hernández L. A. Torres-Gómez H. Wünsch B. Schepmann D. González-Trujano M. E. et al . (2020). Design and synthesis of N-(benzylpiperidinyl)-4-fluorobenzamide: a haloperidol analog that reduces neuropathic nociception via sigma(1) receptor antagonism. Life Sci.245:117348. doi: 10.1016/j.lfs.2020.117348
32
Devanand D. P. Pelton G. H. D'Antonio K. Ciarleglio A. Scodes J. Andrews H. et al . (2018). Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am. J. Geriatr. Psychiatry26, 1050–1060. doi: 10.1016/j.jagp.2018.05.008
33
Diaz J. L. García M. Torrens A. Caamaño A. M. Enjo J. Sicre C. et al . (2020). EST64454: a highly soluble sigma(1) receptor antagonist clinical candidate for pain management. J. Med. Chem.63, 14979–14988. doi: 10.1021/acs.jmedchem.0c01575
34
Dichiara M. Artacho-Cordón A. Turnaturi R. Santos-Caballero M. González-Cano R. Pasquinucci L. et al . (2022). Dual Sigma-1 receptor antagonists and hydrogen sulfide-releasing compounds for pain treatment: design, synthesis, and pharmacological evaluation. Eur. J. Med. Chem.230:114091. doi: 10.1016/j.ejmech.2021.114091
35
Dong L. Y. Cheng Z. X. Fu Y. M. Wang Z. M. Zhu Y. H. Sun J. L. et al . (2007). Neurosteroid dehydroepiandrosterone sulfate enhances spontaneous glutamate release in rat prelimbic cortex through activation of dopamine D1 and sigma-1 receptor. Neuropharmacology52, 966–974. doi: 10.1016/j.neuropharm.2006.10.015
36
Drevets W. C. Price J. L. Furey M. L. (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct.213, 93–118. doi: 10.1007/s00429-008-0189-x
37
Duman R. S. Aghajanian G. K. (2012). Synaptic dysfunction in depression: potential therapeutic targets. Science338, 68–72. doi: 10.1126/science.1222939
38
Duman R. S. Deyama S. Fogaca M. V. (2021). Role of BDNF in the pathophysiology and treatment of depression: activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci.53, 126–139. doi: 10.1111/ejn.14630
39
Earley B. Burke M. Leonard B. E. Gouret C. J. Junien J. L. (1991). Evidence for an anti-amnesic effect of JO 1784 in the rat: a potent and selective ligand for the sigma receptor. Brain Res.546, 282–286. doi: 10.1016/0006-8993(91)91492-j
40
Erritzoe D. Godlewska B. R. Rizzo G. Searle G. E. Agnorelli C. Lewis Y. et al . (2023). Brain serotonin release is reduced in patients with depression: a [(11)C]Cimbi-36 positron emission tomography study with a d-amphetamine challenge. Biol. Psychiatry93, 1089–1098. doi: 10.1016/j.biopsych.2022.10.012
41
Fan J. Guo F. Mo R. Chen L. Y. Mo J. W. Lu C. L. et al . (2023). O-GlcNAc transferase in astrocytes modulates depression-related stress susceptibility through glutamatergic synaptic transmission. J. Clin. Invest.133:e160016. doi: 10.1172/JCI160016
42
Fang X. Guo L. Jia J. Jin G. Z. Zhao B. Zheng Y. Y. et al . (2013). SKF83959 is a novel triple reuptake inhibitor that elicits anti-depressant activity. Acta Pharmacol. Sin.34, 1149–1155. doi: 10.1038/aps.2013.66
43
Fernandes-Nascimento M. H. Viana-Ferreira K. Chaves B. D. R. Negrão A. B. Wang Y. P. (2022). Ibogaine microdosing in a patient with bipolar depression: a case report. Braz. J. Psychiatry44, 462–463. doi: 10.47626/1516-4446-2021-2359
44
Ferrero A. Cereseto M. (2004). Glutamatergic neurotransmission, depression and antidepressants. Vertex15, 91–98.
45
Ferres-Coy A. Galofré M. Pilar-Cuéllar F. Vidal R. Paz V. Ruiz-Bronchal E. et al . (2016). Therapeutic antidepressant potential of a conjugated siRNA silencing the serotonin transporter after intranasal administration. Mol. Psychiatry21, 328–338. doi: 10.1038/mp.2015.80
46
Finberg J. P. (2014). Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: focus on modulation of CNS monoamine neurotransmitter release. Pharmacol. Ther.143, 133–152. doi: 10.1016/j.pharmthera.2014.02.010
47
Fioravanti R. Proia E. Tyurenkov I. N. Kurkin D. V. Bakulin D. A. Kovalev N. S. et al . (2023). Pyrimidine thioethers: a novel class of antidepressant agents, endowed with anxiolytic, performance enhancing and nootropic activity. Eur. J. Med. Chem.245:114902. doi: 10.1016/j.ejmech.2022.114902
48
Fisher A. Bezprozvanny I. Wu L. Ryskamp D. A. Bar-Ner N. Natan N. et al . (2016). AF710B, a novel M1/sigma1 agonist with therapeutic efficacy in animal models of Alzheimer's disease. Neurodegener Dis16, 95–110. doi: 10.1159/000440864
49
Franchini S. Sorbi C. Battisti U. M. Tait A. Bencheva L. I. Cichero E. et al . (2017). Structure-activity relationships within a series of sigma(1) and sigma(2) receptor ligands: identification of a sigma(2) receptor agonist (BS148) with selective toxicity against metastatic melanoma. ChemMedChem12, 1893–1905. doi: 10.1002/cmdc.201700427
50
Fried E. I. Boschloo L. van Borkulo C. Schoevers R. A. Romeijn J. W. Wichers M. et al . (2015). Commentary: "consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression". Front. Psych.6:117. doi: 10.3389/fpsyt.2015.00117
51
Fu C. H. Y. Antoniades M. Erus G. Garcia J. A. Fan Y. Arnone D. et al . (2024). Neuroanatomical dimensions in medication-free individuals with major depressive disorder and treatment response to SSRI antidepressant medications or placebo. Nat. Ment. Health2, 164–176. doi: 10.1038/s44220-023-00187-w
52
Fu C. Xiao Y. Zhou X. Sun Z. (2024). Insight into binding of endogenous neurosteroid ligands to the sigma-1 receptor. Nat. Commun.15:5619. doi: 10.1038/s41467-024-49894-7
53
Fujii C. Zorumski C. F. Izumi Y. (2024). Endoplasmic reticulum stress, autophagy, neuroinflammation, and sigma 1 receptors as contributors to depression and its treatment. Neural Regen. Res.19, 2202–2211. doi: 10.4103/1673-5374.391334
54
Garces-Ramirez L. Green J. L. Hiranita T. Kopajtic T. A. Mereu M. Thomas A. M. et al . (2011). Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biol. Psychiatry69, 208–217. doi: 10.1016/j.biopsych.2010.07.026
55
Garcia M. Virgili M. Alonso M. Alegret C. Farran J. Fernández B. et al . (2020). Discovery of EST73502, a dual mu-opioid receptor agonist and sigma(1) receptor antagonist clinical candidate for the treatment of pain. J. Med. Chem.63, 15508–15526. doi: 10.1021/acs.jmedchem.0c01127
56
Glennon R. A. Ablordeppey S. Y. Ismaiel A. M. el-Ashmawy M. B. Fischer J. B. Howie K. B. (1994). Structural features important for sigma 1 receptor binding. J. Med. Chem.37, 1214–1219. doi: 10.1021/jm00034a020
57
Guo L. Gao T. Gao C. Jia X. Ni J. Han C. et al . (2021). Stimulation of astrocytic sigma-1 receptor is sufficient to ameliorate inflammation- induced depression. Behav. Brain Res.410:113344. doi: 10.1016/j.bbr.2021.113344
58
Guo L. Gao T. Jia X. Gao C. Tian H. Wei Y. et al . (2022). SKF83959 attenuates memory impairment and depressive-like behavior during the latent period of epilepsy via allosteric activation of the Sigma-1 receptor. ACS Chem. Neurosci.13, 3198–3209. doi: 10.1021/acschemneuro.2c00629
59
Guo Y. Zhang C. Chen X. Liu X. Ye T. Fo Y. et al . (2021). Sigma-1 receptor ligands improves ventricular repolarization-related ion remodeling in rats with major depression disorder. Psychopharmacology238, 487–499. doi: 10.1007/s00213-020-05697-4
60
Hale M. W. Raison C. L. Lowry C. A. (2013). Integrative physiology of depression and antidepressant drug action: implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacol. Ther.137, 108–118. doi: 10.1016/j.pharmthera.2012.09.005
61
Han Q. Q. Yu J. (2014). Inflammation: a mechanism of depression?Neurosci. Bull.30, 515–523. doi: 10.1007/s12264-013-1439-3
62
Hanner M. Moebius F. F. Flandorfer A. Knaus H. G. Striessnig J. Kempner E. et al . (1996). Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. USA93, 8072–8077. doi: 10.1073/pnas.93.15.8072
63
Hashimoto K. (2013). Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog. Neurobiol.100, 15–29. doi: 10.1016/j.pneurobio.2012.09.001
64
Hashimoto K. (2015). Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J. Pharmacol. Sci.127, 6–9. doi: 10.1016/j.jphs.2014.11.010
65
Hayashi T. Su T. P. (2007). Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate ca(2+) signaling and cell survival. Cell131, 596–610. doi: 10.1016/j.cell.2007.08.036
66
Hindmarch I. Hashimoto K. (2010). Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Hum. Psychopharmacol.25, 193–200. doi: 10.1002/hup.1106
67
Hiranita T. (2016). Identification of the Sigma-2 receptor: distinct from the progesterone receptor membrane component 1 (PGRMC1). J. Alcohol. Drug Depend.4:10.4172/2329-6488.1000e130. doi: 10.4172/2329-6488.1000e130
68
Holoubek G. Muller W. E. (2003). Specific modulation of sigma binding sites by the anxiolytic drug opipramol. J. Neural Transm. (Vienna)110, 1169–1179. doi: 10.1007/s00702-003-0019-5
69
Hong V. M. Rade A. D. Yan S. M. Bhaskara A. Yousuf M. S. Chen M. et al . (2024). Loss of Sigma-2 receptor/TMEM97 is associated with neuropathic injury-induced depression-like behaviors in female mice. eNeuro11, ENEURO.0488–ENEU23.2024. doi: 10.1523/ENEURO.0488-23.2024
70
Iosifescu D. V. Jones A. O’Gorman C. Streicher C. Feliz S. Fava M. et al . (2022). Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J. Clin. Psychiatry83:21m14345. doi: 10.4088/JCP.21m14345
71
Ishima T. Fujita Y. Hashimoto K. (2014). Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur. J. Pharmacol.727, 167–173. doi: 10.1016/j.ejphar.2014.01.064
72
Ito K. Hirooka Y. Matsukawa R. Nakano M. Sunagawa K. (2012). Decreased brain sigma-1 receptor contributes to the relationship between heart failure and depression. Cardiovasc. Res.93, 33–40. doi: 10.1093/cvr/cvr255
73
Itzhak Y. (1994). Modulation of the PCP/NMDA receptor complex and sigma binding sites by psychostimulants. Neurotoxicol. Teratol.16, 363–368. doi: 10.1016/0892-0362(94)90024-8
74
Itzhak Y. Kassim C. O. (1990). Clorgyline displays high affinity for sigma binding sites in C57BL/6 mouse brain. Eur. J. Pharmacol.176, 107–108. doi: 10.1016/0014-2999(90)90139-w
75
Itzhak Y. Stein I. Zhang S. H. Kassim C. O. Cristante D. (1991). Binding of sigma-ligands to C57BL/6 mouse brain membranes: effects of monoamine oxidase inhibitors and subcellular distribution studies suggest the existence of sigma-receptor subtypes. J. Pharmacol. Exp. Ther.257, 141–148. doi: 10.1016/S0022-3565(25)24697-3
76
Izumi Y. Reiersen A. M. Lenze E. J. Mennerick S. J. Zorumski C. F. (2024). Sertraline modulates hippocampal plasticity via sigma 1 receptors, cellular stress and neurosteroids. Transl. Psychiatry14:474. doi: 10.1038/s41398-024-03185-3
77
Izzo N. J. Colom-Cadena M. Riad A. A. Xu J. Singh M. Abate C. et al . (2020). Proceedings from the fourth international symposium on sigma-2 receptors: role in health and disease. eNeuro7:ENEURO.0317-20.2020. doi: 10.1523/ENEURO.0317-20.2020
78
Jia J. Cheng J. Wang C. Zhen X. (2018). Sigma-1 receptor-modulated Neuroinflammation in neurological diseases. Front. Cell. Neurosci.12:314. doi: 10.3389/fncel.2018.00314
79
Jiang B. Wang F. Yang S. Fang P. Deng Z. F. Xiao J. L. et al . (2014). SKF83959 produces antidepressant effects in a chronic social defeat stress model of depression through BDNF-TrkB pathway. Int. J. Neuropsychopharmacol.18:pyu096. doi: 10.1093/ijnp/pyu096
80
John C. S. Bowen W. D. Fisher S. J. Lim B. B. Geyer B. C. Vilner B. J. et al . (1999). Synthesis, in vitro pharmacologic characterization, and preclinical evaluation of N-[2-(1′-piperidinyl)ethyl]-3-[125I]iodo-4-methoxybenzamide (P[125I]MBA) for imaging breast cancer. Nucl. Med. Biol.26, 377–382. doi: 10.1016/s0969-8051(98)00104-8
81
John C. S. Gulden M. E. Li J. Bowen W. D. McAfee J. G. Thakur M. L. (1998). Synthesis, in vitro binding, and tissue distribution of radioiodinated 2-[125I]N-(N-benzylpiperidin-4-yl)-2-iodo benzamide, 2-[125I]BP: a potential sigma receptor marker for human prostate tumors. Nucl. Med. Biol.25, 189–194. doi: 10.1016/s0969-8051(97)00168-6
82
Kadriu B. Musazzi L. Henter I. D. Graves M. Popoli M. Zarate C. A. Jr. (2019). Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int. J. Neuropsychopharmacol.22, 119–135. doi: 10.1093/ijnp/pyy094
83
Kaminska M. Harris J. Gijsbers K. Dubrovsky B. (2000). Dehydroepiandrosterone sulfate (DHEAS) counteracts decremental effects of corticosterone on dentate gyrus LTP. Implications for depression. Brain Res. Bull.52, 229–234. doi: 10.1016/s0361-9230(00)00251-3
84
Kargbo R. B. (2023). Neurosteroids and postpartum depression: the mechanism, efficacy, and approval of Brexanolone and Zurzuvae. ACS Med. Chem. Lett.14, 1326–1328. doi: 10.1021/acsmedchemlett.3c00388
85
Kato K. Hayako H. Ishihara Y. Marui S. Iwane M. Miyamoto M. (1999). TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons. Neurosci. Lett.260, 5–8. doi: 10.1016/s0304-3940(98)00943-4
86
Kekuda R. Prasad P. D. Fei Y. J. Leibach F. H. Ganapathy V. (1996). Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem. Biophys. Res. Commun.229, 553–558. doi: 10.1006/bbrc.1996.1842
87
Khanassov V. Hu J. Reeves D. van Marwijk H. (2018). Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int. J. Geriatr. Psychiatry33, 1688–1708. doi: 10.1002/gps.4974
88
Kim Y. Lee Y. S. Kim M. G. Song Y.-K. Jang H. Kim J. H. et al . (2019). The effect of selective serotonin reuptake inhibitors on major adverse cardiovascular events: a meta-analysis of randomized-controlled studies in depression. Int. Clin. Psychopharmacol.34, 9–17. doi: 10.1097/YIC.0000000000000238
89
Kim J. W. Suzuki K. Kavalali E. T. Monteggia L. M. (2024). Ketamine: mechanisms and relevance to treatment of depression. Annu. Rev. Med.75, 129–143. doi: 10.1146/annurev-med-051322-120608
90
Klawonn A. M. Nilsson A. Rådberg C. F. Lindström S. H. Ericson M. Granseth B. et al . (2017). The Sigma-2 receptor selective agonist Siramesine (Lu 28-179) decreases cocaine-reinforced Pavlovian learning and alters glutamatergic and dopaminergic input to the striatum. Front. Pharmacol.8:714. doi: 10.3389/fphar.2017.00714
91
Kobayashi T. Matsuno K. Nakata K. Mita S. (1996). Enhancement of acetylcholine release by SA4503, a novel sigma 1 receptor agonist, in the rat brain. J. Pharmacol. Exp. Ther.279, 106–113. doi: 10.1016/S0022-3565(25)20943-0
92
Kornhuber J. Schoppmeyer K. Riederer P. (1993). Affinity of 1-aminoadamantanes for the sigma binding site in post-mortem human frontal cortex. Neurosci. Lett.163, 129–131. doi: 10.1016/0304-3940(93)90362-o
93
Kulkarni S. K. Dhir A. (2009). Sigma-1 receptors in major depression and anxiety. Expert. Rev. Neurother.9, 1021–1034. doi: 10.1586/ern.09.40
94
Kwon H. S. Koh S. H. (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener.9:42. doi: 10.1186/s40035-020-00221-2
95
Le G. H. Wong S. Kwan A. T. H. Rosenblat J. D. Mansur R. B. Teopiz K. M. et al . (2024). Association of antidepressants with cataracts and glaucoma: a disproportionality analysis using the reports to the United States Food and Drug Administration adverse event reporting system (FAERS) pharmacovigilance database. CNS Spectr.29, 682–696. doi: 10.1017/S1092852924002360
96
Lee J. Choi K. S. Yun J. A. (2023). The effects of sociodemographic factors on help-seeking for depression: based on the 2017-2020 Korean community health survey. PLoS One18:e0280642. doi: 10.1371/journal.pone.0280642
97
Leem Y. H. Yoon S. S. Jo S. A. (2020). Imipramine ameliorates depressive symptoms by blocking differential alteration of dendritic spine structure in amygdala and prefrontal cortex of chronic stress-induced mice. Biomol. Ther. (Seoul)28, 230–239. doi: 10.4062/biomolther.2019.152
98
Lever J. R. Gustafson J. L. Xu R. Allmon R. L. Lever S. Z. (2006). Sigma1 and sigma2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse59, 350–358. doi: 10.1002/syn.20253
99
Li J. F. Chang H. X. Zhao J. N. Bao J. H. Dai W. Li Y. F. (2025). The astrocytic sigma-1 receptor constitutes in the fast antidepressant action of hypidone hydrochloride (YL-0919) in rodents. Front. Pharmacol.16:1564851. doi: 10.3389/fphar.2025.1564851
100
Li J. F. Hu W. Y. Chang H. X. Bao J. H. Kong X. X. Ma H. et al . (2023). Astrocytes underlie a faster-onset antidepressant effect of hypidone hydrochloride (YL-0919). Front. Pharmacol.14:1175938. doi: 10.3389/fphar.2023.1175938
101
Li X. Yan D. Liao M. Zhang L. Li Z. X. Liu B. et al . (2023). Effect of fluvoxamine on plasma interleukin-6 in patients with major depressive disorder: a prospective follow-up study. Front. Psych.14:1163754. doi: 10.3389/fpsyt.2023.1163754
102
Li K. Zhou T. Liao L. Yang Z. Wong C. Henn F. et al . (2013). BetaCaMKII in lateral habenula mediates core symptoms of depression. Science341, 1016–1020. doi: 10.1126/science.1240729
103
Liu X. Qu C. Shi S. Ye T. Wang L. Liu S. et al . (2019). The reversal effect of Sigma-1 receptor (S1R) agonist, SA4503, on atrial fibrillation after depression and its underlying mechanism. Front. Physiol.10:1346. doi: 10.3389/fphys.2019.01346
104
Liu X. Qu C. Yang H. Shi S. Zhang C. Zhang Y. et al . (2018). Chronic stimulation of the sigma-1 receptor ameliorates autonomic nerve dysfunction and atrial fibrillation susceptibility in a rat model of depression. Am. J. Physiol. Heart Circ. Physiol.315, H1521–H1531. doi: 10.1152/ajpheart.00607.2017
105
Liu J. Wang W. Wang F. Cai F. Hu Z. L. Yang Y. J. et al . (2009). Phosphatidylinositol-linked novel D(1) dopamine receptor facilitates long-term depression in rat hippocampal CA1 synapses. Neuropharmacology57, 164–171. doi: 10.1016/j.neuropharm.2009.05.001
106
Liu C. H. Zhang G.-Z. Li B. Li M. Woelfer M. Walter M. et al . (2019). Role of inflammation in depression relapse. J. Neuroinflammation16:90. doi: 10.1186/s12974-019-1475-7
107
Ma H. Li J. F. Qiao X. Zhang Y. Hou X. J. Chang H. X. et al . (2024). Sigma-1 receptor activation mediates the sustained antidepressant effect of ketamine in mice via increasing BDNF levels. Acta Pharmacol. Sin.45, 704–713. doi: 10.1038/s41401-023-01201-8
108
Mach R. H. Smith C. R. Childers S. R. (1995). Ibogaine possesses a selective affinity for sigma 2 receptors. Life Sci.57, PL57–PL62. doi: 10.1016/0024-3205(95)00301-l
109
Mahar I. Bambico F. R. Mechawar N. Nobrega J. N. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev.38, 173–192. doi: 10.1016/j.neubiorev.2013.11.009
110
Malhi G. S. Mann J. J. (2018). Depression. Lancet392, 2299–2312. doi: 10.1016/S0140-6736(18)31948-2
111
Malik M. Rangel-Barajas C. Sumien N. Su C. Singh M. Chen Z. et al . (2015). The effects of sigma (sigma1) receptor-selective ligands on muscarinic receptor antagonist-induced cognitive deficits in mice. Br. J. Pharmacol.172, 2519–2531. doi: 10.1111/bph.13076
112
Mandai T. Sako Y. Kurimoto E. Shimizu Y. Nakamura M. Fushimi M. et al . (2020). T-495, a novel low cooperative M(1) receptor positive allosteric modulator, improves memory deficits associated with cholinergic dysfunction and is characterized by low gastrointestinal side effect risk. Pharmacol. Res. Perspect.8:e00560. doi: 10.1002/prp2.560
113
Mari Y. Katnik C. Cuevas J. (2015). σ-1 receptor inhibition of ASIC1a channels is dependent on a pertussis toxin-sensitive G-protein and an AKAP150/Calcineurin complex. Neurochem. Res.40, 2055–2067. doi: 10.1007/s11064-014-1324-0
114
Martin P. de Witte P. A. M. Maurice T. Gammaitoni A. Farfel G. Galer B. (2020). Fenfluramine acts as a positive modulator of sigma-1 receptors. Epilepsy Behav.105:106989. doi: 10.1016/j.yebeh.2020.106989
115
Martin W. R. Eades C. G. Thompson J. A. Huppler R. E. Gilbert P. E. (1976). The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther.197, 517–532. doi: 10.1016/S0022-3565(25)30536-7
116
Martin P. Maurice T. Gammaitoni A. Farfel G. Boyd B. Galer B. (2022). Fenfluramine modulates the anti-amnesic effects induced by sigma-1 receptor agonists and neuro(active)steroids in vivo. Epilepsy Behav.127:108526. doi: 10.1016/j.yebeh.2021.108526
117
Martina M. Turcotte M.‐. E. B. Halman S. Bergeron R. (2007). The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J. Physiol.578, 143–157. doi: 10.1113/jphysiol.2006.116178
118
Masuka J. T. Mchunu N. Mkhize Z. Thandar Y. Mosam A. (2022). Selective serotonin reuptake inhibitor and serotonin-norepinephrine reuptake inhibitor associated cutaneous adverse drug reactions: a systematic review of case reports and case series. Australas. J. Dermatol.63, e13–e20. doi: 10.1111/ajd.13780
119
Matsumoto R. R. Bowen W. D. Tom M. A. Vo V. N. Truong D. D. de Costa B. R. (1995). Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur. J. Pharmacol.280, 301–310. doi: 10.1016/0014-2999(95)00208-3
120
Matsumoto R. R. Pouw B. Mack A. L. Daniels A. Coop A. (2007). Effects of UMB24 and (+/−)-SM 21, putative sigma2-preferring antagonists, on behavioral toxic and stimulant effects of cocaine in mice. Pharmacol. Biochem. Behav.86, 86–91. doi: 10.1016/j.pbb.2006.12.011
121
Matsuno K. Nakazawa M. Okamoto K. Kawashima Y. Mita S. (1996). Binding properties of SA4503, a novel and selective sigma 1 receptor agonist. Eur. J. Pharmacol.306, 271–279. doi: 10.1016/0014-2999(96)00201-4
122
McCann D. J. Weissman A. D. Su T. P. (1994). Sigma-1 and sigma-2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns. Synapse17, 182–189. doi: 10.1002/syn.890170307
123
McEvoy K. Payne J. L. Osborne L. M. (2018). Neuroactive steroids and perinatal depression: a review of recent literature. Curr. Psychiatry Rep.20:78. doi: 10.1007/s11920-018-0937-4
124
Megalizzi V. Mathieu V. Mijatovic T. Gailly P. Debeir O. De Neve N. et al . (2007). 4-IBP, a sigma1 receptor agonist, decreases the migration of human cancer cells, including glioblastoma cells, in vitro and sensitizes them in vitro and in vivo to cytotoxic insults of proapoptotic and proautophagic drugs. Neoplasia9, 358–369. doi: 10.1593/neo.07130
125
Mei J. Pasternak G. W. (2001). Molecular cloning and pharmacological characterization of the rat sigma1 receptor11Abbreviations: DTG, N,N′-di(o-tolyl-guanidine); HA, hemagglutinin; RT-PCR, reverse transcriptase-polymerase chain reaction; RACE, rapid amplification of cDNA ends; and CHO, Chinese hamster ovary. Biochem. Pharmacol.62, 349–355. doi: 10.1016/s0006-2952(01)00666-9
126
Moda-Sava R. N. Murdock M. H. Parekh P. K. Fetcho R. N. Huang B. S. Huynh T. N. et al . (2019). Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science364:eaat8078. doi: 10.1126/science.aat8078
127
Mohamed A. F. El-Gammal M. A. EL-Yamany M. F. Khodeir A. E. (2025). Sigma-1 receptor modulation by fluvoxamine ameliorates valproic acid-induced autistic behavior in rats: involvement of chronic ER stress modulation, enhanced autophagy and M1/M2 microglia polarization. Prog. Neuro-Psychopharmacol. Biol. Psychiatry136:111192. doi: 10.1016/j.pnpbp.2024.111192
128
Monroe S. M. Harkness K. L. (2022). Major depression and its recurrences: life course matters. Annu. Rev. Clin. Psychol.18, 329–357. doi: 10.1146/annurev-clinpsy-072220-021440
129
Mori T. Hayashi T. Hayashi E. Su T. P. (2013). Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One8:e76941. doi: 10.1371/journal.pone.0076941
130
Moriguchi S. Sakagami H. Yabuki Y. Sasaki Y. Izumi H. Zhang C. et al . (2015). Stimulation of Sigma-1 receptor ameliorates depressive-like behaviors in CaMKIV null mice. Mol. Neurobiol.52, 1210–1222. doi: 10.1007/s12035-014-8923-2
131
Moriguchi S. Shinoda Y. Yamamoto Y. Sasaki Y. Miyajima K. Tagashira H. et al . (2013). Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS One8:e60863. doi: 10.1371/journal.pone.0060863
132
Murrough J. W. Stade E. Sayed S. Ahle G. Kiraly D. D. Welch A. et al . (2017). Dextromethorphan/quinidine pharmacotherapy in patients with treatment resistant depression: a proof of concept clinical trial. J. Affect. Disord.218, 277–283. doi: 10.1016/j.jad.2017.04.072
133
Musacchio J. M. Klein M. Canoll P. D. (1989). Dextromethorphan and sigma ligands: common sites but diverse effects. Life Sci.45, 1721–1732. doi: 10.1016/0024-3205(89)90510-9
134
Nakazato A. Ohta K. Sekiguchi Y. Okuyama S. Chaki S. Kawashima Y. et al . (1999). Design, synthesis, structure-activity relationships, and biological characterization of novel arylalkoxyphenylalkylamine sigma ligands as potential antipsychotic drugs. J. Med. Chem.42, 1076–1087. doi: 10.1021/jm980212v
135
Naoi M. Maruyama W. (2010). Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr. Pharm. Des.16, 2799–2817. doi: 10.2174/138161210793176527
136
Narita N. Hashimoto K. Tomitaka S. I. Minabe Y. (1996). Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur. J. Pharmacol.307, 117–119. doi: 10.1016/0014-2999(96)00254-3
137
Nguyen L. Robson M. J. Healy J. R. Scandinaro A. L. Matsumoto R. R. (2014). Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PLoS One9:e89985. doi: 10.1371/journal.pone.0089985
138
Nicholson H. Comeau A. Mesangeau C. McCurdy C. R. Bowen W. D. (2015). Characterization of CM572, a selective irreversible partial agonist of the Sigma-2 receptor with antitumor activity. J. Pharmacol. Exp. Ther.354, 203–212. doi: 10.1124/jpet.115.224105
139
Normann C. Buttenschon H. N. (2019). Gene-environment interactions between HPA-axis genes and stressful life events in depression: a systematic review. Acta Neuropsychiatr.31, 186–192. doi: 10.1017/neu.2019.16
140
Novakovic M. M. Korshunov K. S. Grant R. A. Martin M. E. Valencia H. A. Budinger G. R. S. et al . (2023). Astrocyte reactivity and inflammation-induced depression-like behaviors are regulated by Orai1 calcium channels. Nat. Commun.14:5500. doi: 10.1038/s41467-023-40968-6
141
Ooi K. Hu L. Feng Y. Han C. Ren X. Qian X. et al . (2021). Sigma-1 receptor activation suppresses microglia M1 polarization via regulating endoplasmic reticulum-mitochondria contact and mitochondrial functions in stress-induced hypertension rats. Mol. Neurobiol.58, 6625–6646. doi: 10.1007/s12035-021-02488-6
142
Oyer H. M. Sanders C. M. Kim F. J. (2019). Small-molecule modulators of Sigma1 and Sigma2/TMEM97 in the context of Cancer: foundational concepts and emerging themes. Front. Pharmacol.10:1141. doi: 10.3389/fphar.2019.01141
143
Pabba M. Wong A. Y. C. Ahlskog N. Hristova E. Biscaro D. Nassrallah W. et al . (2014). NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J. Neurosci.34, 11325–11338. doi: 10.1523/JNEUROSCI.0458-14.2014
144
Panczyszyn-Trzewik P. Czechowska E. Stachowicz K. Sowa-Kućma M. (2023). The importance of alpha-klotho in depression and cognitive impairment and its connection to glutamate neurotransmission-an up-to-date review. Int. J. Mol. Sci.24:15268. doi: 10.3390/ijms242015268
145
Paschos K. A. Veletza S. Chatzaki E. (2009). Neuropeptide and sigma receptors as novel therapeutic targets for the pharmacotherapy of depression. CNS Drugs23, 755–772. doi: 10.2165/11310830-000000000-00000
146
Peeters M. Romieu P. Maurice T. Su T. P. Maloteaux J. M. Hermans E. (2004). Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. Eur. J. Neurosci.19, 2212–2220. doi: 10.1111/j.0953-816X.2004.03297.x
147
Perregaard J. Moltzen E. K. Meier E. Sanchez C. (1995). Sigma ligands with subnanomolar affinity and preference for the sigma 2 binding site. 1. 3-(omega-aminoalkyl)-1H-indoles. J. Med. Chem.38, 1998–2008. doi: 10.1021/jm00011a019
148
Qin Y. Xu W. Li K. Luo Q. Chen X. Wang Y. et al . (2022). Repeated inhibition of sigma-1 receptor suppresses GABA(a) receptor expression and long-term depression in the nucleus accumbens leading to depressive-like behaviors. Front. Mol. Neurosci.15:959224. doi: 10.3389/fnmol.2022.959224
149
Rakel R. E. (1999). Depression. Prim. Care26, 211–224. doi: 10.1016/s0095-4543(08)70003-4
150
Ran Y. H. Hu X. X. Wang Y. L. Zhao N. Zhang L. M. Liu H. X. et al . (2018). YL-0919, a dual 5-HT(1A) partial agonist and SSRI, produces antidepressant- and anxiolytic-like effects in rats subjected to chronic unpredictable stress. Acta Pharmacol. Sin.39, 12–23. doi: 10.1038/aps.2017.83
151
Ran Y. Jin Z. Chen X. Zhao N. Fang X. Zhang L. et al . (2018). Hypidone hydrochloride (YL-0919) produces a fast-onset reversal of the behavioral and synaptic deficits caused by chronic stress exposure. Front. Cell. Neurosci.12:395. doi: 10.3389/fncel.2018.00395
152
Rao T. S. Rao T. Cler J. Mick S. Dilworth V. Contreras P. et al . (1990). Neurochemical characterization of dopaminergic effects of opipramol, a potent sigma receptor ligand, in vivo. Neuropharmacology29, 1191–1197. doi: 10.1016/0028-3908(90)90044-r
153
Ren P. Wang J. Y. Chen H. L. Chang H. X. Zeng Z. R. Li G. X. et al . (2023). Sigma-1 receptor agonist properties that mediate the fast-onset antidepressant effect of hypidone hydrochloride (YL-0919). Eur. J. Pharmacol.946:175647. doi: 10.1016/j.ejphar.2023.175647
154
Ren P. Wang J. Y. Chen H. L. Wang Y. Cui L. Y. Duan J. Y. et al . (2024). Activation of σ-1 receptor mitigates estrogen withdrawal-induced anxiety/depressive-like behavior in mice via restoration of GABA/glutamate signaling and neuroplasticity in the hippocampus. J. Pharmacol. Sci.154, 236–245. doi: 10.1016/j.jphs.2024.02.003
155
Ren P. Wang J. Y. Xu M. J. Chen H. L. Duan J. Y. Li Y. F. (2025). Sigma-1 receptor activation produces faster antidepressant-like effect through enhancement of hippocampal neuroplasticity: focus on sigma-1-5-HT1A heteroreceptor complex. Neurochem. Int.184:105937. doi: 10.1016/j.neuint.2025.105937
156
Riad A. Lengyel-Zhand Z. Zeng C. Weng C. C. Lee V. M. Trojanowski J. Q. et al . (2020). The Sigma-2 receptor/TMEM97, PGRMC1, and LDL receptor complex are responsible for the cellular uptake of Aβ42 and its protein aggregates. Mol. Neurobiol.57, 3803–3813. doi: 10.1007/s12035-020-01988-1
157
Rishton G. M. Look G. C. Ni Z. J. Zhang J. Wang Y. Huang Y. et al . (2021). Discovery of investigational drug CT1812, an antagonist of the Sigma-2 receptor complex for Alzheimer's disease. ACS Med. Chem. Lett.12, 1389–1395. doi: 10.1021/acsmedchemlett.1c00048
158
Roberts C. Sahakian B. J. Robbins T. W. (2020). Psychological mechanisms and functions of 5-HT and SSRIs in potential therapeutic change: lessons from the serotonergic modulation of action selection, learning, affect, and social cognition. Neurosci. Biobehav. Rev.119, 138–167. doi: 10.1016/j.neubiorev.2020.09.001
159
Rodri Guez P. Urbanavicius J. Prieto J. P. Fabius S. Reyes A. L. Havel V. et al . (2020). A single administration of the atypical psychedelic Ibogaine or its metabolite Noribogaine induces an antidepressant-like effect in rats. ACS Chem. Neurosci.11, 1661–1672. doi: 10.1021/acschemneuro.0c00152
160
Rodriguez-Munoz M. Onetti Y. Cortés-Montero E. Garzón J. Sánchez-Blázquez P. (2018). Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor. Mol. Brain11:51. doi: 10.1186/s13041-018-0395-2
161
Rossino G. Marra A. Listro R. Peviani M. Poggio E. Curti D. et al . (2023). Discovery of RC-752, a novel Sigma-1 receptor antagonist with Antinociceptive activity: a promising tool for fighting neuropathic pain. Pharmaceuticals (Basel)16:962. doi: 10.3390/ph16070962
162
Rupprecht R. Pradhan A. K. Kufner M. Brunner L. M. Nothdurfter C. Wein S. et al . (2023). Neurosteroids and translocator protein 18 kDa (TSPO) in depression: implications for synaptic plasticity, cognition, and treatment options. Eur. Arch. Psychiatry Clin. Neurosci.273, 1477–1487. doi: 10.1007/s00406-022-01532-3
163
Safrany S. T. Brimson J. M. (2016). Are fluoxetine's effects due to sigma-1 receptor agonism?Pharmacol. Res.113, 707–708. doi: 10.1016/j.phrs.2016.05.031
164
Sahn J. J. Mejia G. L. Ray P. R. Martin S. F. Price T. J. (2017). Sigma 2 receptor/Tmem97 agonists produce long lasting Antineuropathic pain effects in mice. ACS Chem. Neurosci.8, 1801–1811. doi: 10.1021/acschemneuro.7b00200
165
Salaciak K. Pytka K. (2022). Revisiting the sigma-1 receptor as a biological target to treat affective and cognitive disorders. Neurosci. Biobehav. Rev.132, 1114–1136. doi: 10.1016/j.neubiorev.2021.10.037
166
Salter M. W. Beggs S. (2014). Sublime microglia: expanding roles for the guardians of the CNS. Cell158, 15–24. doi: 10.1016/j.cell.2014.06.008
167
Salvadore G. Singh J. B. (2013). Ketamine as a fast acting antidepressant: current knowledge and open questions. CNS Neurosci. Ther.19, 428–436. doi: 10.1111/cns.12103
168
Sanchez C. Papp M. (2000). The selective sigma2 ligand Lu 28-179 has an antidepressant-like profile in the rat chronic mild stress model of depression. Behav. Pharmacol.11, 117–124. doi: 10.1097/00008877-200004000-00003
169
Sanchez-Blazquez P. Cortés-Montero E. Rodríguez-Muñoz M. Garzón J. et al . (2018). Sigma 1 receptor antagonists inhibit manic-like behaviors in two congenital strains of mice. Int. J. Neuropsychopharmacol.21, 938–948. doi: 10.1093/ijnp/pyy049
170
Schapira A. H. (2011). Monoamine oxidase B inhibitors for the treatment of Parkinsonʼs disease. CNS Drugs25, 1061–1071. doi: 10.2165/11596310-000000000-00000
171
Senda T. Matsuno K. Okamoto K. Kobayashi T. Nakata K. Mita S. (1996). Ameliorating effect of SA4503, a novel sigma 1 receptor agonist, on memory impairments induced by cholinergic dysfunction in rats. Eur. J. Pharmacol.315, 1–10. doi: 10.1016/s0014-2999(96)00572-9
172
Seth P. Fei Y. J. Li H. W. Huang W. Leibach F. H. Ganapathy V. (1998). Cloning and functional characterization of a sigma receptor from rat brain. J. Neurochem.70, 922–931. doi: 10.1046/j.1471-4159.1998.70030922.x
173
Seth P. Leibach F. H. Ganapathy V. (1997). Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem. Biophys. Res. Commun.241, 535–540. doi: 10.1006/bbrc.1997.7840
174
Shao X. Zhu G. (2020). Associations among monoamine neurotransmitter pathways, personality traits, and major depressive disorder. Front. Psych.11:381. doi: 10.3389/fpsyt.2020.00381
175
Shen C. J. Zheng D. Li K. X. Yang J. M. Pan H. Q. Yu X. D. et al . (2019). Cannabinoid CB(1) receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med.25, 337–349. doi: 10.1038/s41591-018-0299-9
176
Shi J. J. Jiang Q. H. Zhang T. N. Sun H. Shi W. W. Gunosewoyo H. et al . (2021). Sigma-1 receptor agonist TS-157 improves motor functional recovery by promoting neurite outgrowth and pERK in rats with focal cerebral ischemia. Molecules26:1212. doi: 10.3390/molecules26051212
177
Shi M. Liu L. Min X. Mi L. Chai Y. Chen F. et al . (2022). Activation of Sigma-1 receptor alleviates ER-associated cell death and microglia activation in traumatically injured mice. J. Clin. Med.11:2348. doi: 10.3390/jcm11092348
178
Shinoda Y. Tagashira H. Bhuiyan M. S. Hasegawa H. Kanai H. Zhang C. et al . (2016). Corticosteroids mediate heart failure-induced depression through reduced sigma1-receptor expression. PLoS One11:e0163992. doi: 10.1371/journal.pone.0163992
179
Singh I. Seth A. Billesbølle C. B. Braz J. Rodriguiz R. M. Roy K. et al . (2023). Structure-based discovery of conformationally selective inhibitors of the serotonin transporter. Cell186, 2160–2175.e17. doi: 10.1016/j.cell.2023.04.010
180
Sinyor M. (2019). Universal access to cognitive behavioral therapy and antidepressants is necessary for all patients with major depressive disorder. Ann. Intern. Med.171, 849–850. doi: 10.7326/M19-2623
181
Skuza G. Rogoz Z. (2006). The synergistic effect of selective sigma receptor agonists and uncompetitive NMDA receptor antagonists in the forced swim test in rats. J. Physiol. Pharmacol.57, 217–229.
182
Skuza G. Rogoz Z. (2007). Antidepressant-like effect of combined treatment with selective sigma receptor agonists and a 5-HT1A receptor agonist in the forced swimming test in rats. Pharmacol. Rep.59, 773–777
183
Skuza G. Rogoz Z. (2009). Antidepressant-like effect of PRE-084, a selective sigma1 receptor agonist, in albino Swiss and C57BL/6J mice. Pharmacol. Rep.61, 1179–1183. doi: 10.1016/s1734-1140(09)70181-1
184
Skuza G. Sadaj W. Kabziński M. Cassano G. Gasparre G. Abate C. et al . (2014). The effects of new sigma (σ) receptor ligands, PB190 and PB212, in the models predictive of antidepressant activity. Pharmacol. Rep.66, 320–324. doi: 10.1016/j.pharep.2013.12.002
185
Skuza G. Szymańska M. Budziszewska B. Abate C. Berardi F. (2011). Effects of PB190 and PB212, new σ receptor ligands, on glucocorticoid receptor-mediated gene transcription in LMCAT cells. Pharmacol. Rep.63, 1564–1568. doi: 10.1016/s1734-1140(11)70722-8
186
Smith K. (2014). Mental health: a world of depression. Nature515:181. doi: 10.1038/515180a
187
Su T. P. Hayashi T. Maurice T. Buch S. Ruoho A. E. (2010). The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci.31, 557–566. doi: 10.1016/j.tips.2010.08.007
188
Su T. P. London E. D. Jaffe J. H. (1988). Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science240, 219–221. doi: 10.1126/science.2832949
189
Su T. P. Su T. C. Nakamura Y. Tsai S. Y. (2016). The Sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol. Sci.37, 262–278. doi: 10.1016/j.tips.2016.01.003
190
Su T. P. Wu X. Z. Cone E. J. Shukla K. Gund T. M. Dodge A. L. et al . (1991). Sigma compounds derived from phencyclidine: identification of PRE-084, a new, selective sigma ligand. J. Pharmacol. Exp. Ther.259, 543–550. doi: 10.1016/S0022-3565(25)20283-X
191
Sugimoto Y. Tagawa N. Kobayashi Y. Mitsui-Saito K. Hotta Y. Yamada J. (2012). Involvement of the sigma1 receptor in the antidepressant-like effects of fluvoxamine in the forced swimming test in comparison with the effects elicited by paroxetine. Eur. J. Pharmacol.696, 96–100. doi: 10.1016/j.ejphar.2012.09.030
192
Sun L. J. Zhang L. M. Liu D. Xue R. Liu Y. Q. Li L. et al . (2019). The faster-onset antidepressant effects of hypidone hydrochloride (YL-0919). Metab. Brain Dis.34, 1375–1384. doi: 10.1007/s11011-019-00439-8
193
Tabuteau H. Jones A. Anderson A. Jacobson M. Iosifescu D. V. (2022). Effect of AXS-05 (dextromethorphan-bupropion) in major depressive disorder: a randomized double-blind controlled trial. Am. J. Psychiatry179, 490–499. doi: 10.1176/appi.ajp.21080800
194
Takebayashi M. Hayashi T. Su T. P. (2004). A perspective on the new mechanism of antidepressants: neuritogenesis through sigma-1 receptors. Pharmacopsychiatry37 Suppl 3, S208–S213. doi: 10.1055/s-2004-832679
195
Tsai S. Y. Chuang J. Y. Tsai M.-S. Wang X.-F. Xi Z.-X. Hung J.-J. et al . (2015). Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc. Natl. Acad. Sci. USA112, E6562–E6570. doi: 10.1073/pnas.1518894112
196
Urani A. Roman F. J. Phan V. L. Su T. P. Maurice T. (2001). The antidepressant-like effect induced by sigma(1)-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J. Pharmacol. Exp. Ther.298, 1269–1279. doi: 10.1016/S0022-3565(24)29502-1
197
Vahid-Ansari F. Zhang M. Zahrai A. Albert P. R. (2019). Overcoming resistance to selective serotonin reuptake inhibitors: targeting serotonin, serotonin-1A receptors and adult neuroplasticity. Front. Neurosci.13:404. doi: 10.3389/fnins.2019.00404
198
van Lith N. D. Motke J. C. (1983). Opipramol in the climacteric syndrome. A double-blind, placebo-controlled trial. Maturitas5, 17–23. doi: 10.1016/0378-5122(83)90017-8
199
Varela Pinon M. Adan-Manes J. (2017). Selective serotonin reuptake inhibitor-induced hyponatremia: clinical implications and therapeutic alternatives. Clin. Neuropharmacol.40, 177–179. doi: 10.1097/WNF.0000000000000221
200
Villard V. Meunier J. Chevallier N. Maurice T. (2011). Pharmacological interaction with the sigma1 (sigma1)-receptor in the acute behavioral effects of antidepressants. J. Pharmacol. Sci.115, 279–292. doi: 10.1254/jphs.10191fp
201
Volz H. P. Stoll K. D. (2004). Clinical trials with sigma ligands. Pharmacopsychiatry37, S214–S220. doi: 10.1055/s-2004-832680
202
Voronin M. V. Vakhitova Y. V. Seredenin S. B. (2020). Chaperone Sigma1R and antidepressant effect. Int. J. Mol. Sci.21:7088. doi: 10.3390/ijms21197088
203
Wang Y. Jiang H. F. Ni J. Guo L. (2019). Pharmacological stimulation of sigma-1 receptor promotes activation of astrocyte via ERK1/2 and GSK3β signaling pathway. Naunyn Schmiedeberg's Arch. Pharmacol.392, 801–812. doi: 10.1007/s00210-019-01632-3
204
Wang D. Noda Y. Tsunekawa H. Zhou Y. Miyazaki M. Senzaki K. et al . (2007). Role of N-methyl-D-aspartate receptors in antidepressant-like effects of sigma 1 receptor agonist 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine dihydrochloride (SA-4503) in olfactory bulbectomized rats. J. Pharmacol. Exp. Ther.322, 1305–1314. doi: 10.1124/jpet.107.124685
205
Wang J. Y. Ren P. Cui L. Y. Duan J. Y. Chen H. L. Zeng Z. R. et al . (2024). Astrocyte-specific activation of sigma-1 receptors in mPFC mediates the faster onset antidepressant effect by inhibiting NF-κB-induced neuroinflammation. Brain Behav. Immun.120, 256–274. doi: 10.1016/j.bbi.2024.06.008
206
Wang Y. M. Xia C. Y. Jia H. M. He J. Lian W. W. Yan Y. et al . (2022). Sigma-1 receptor: a potential target for the development of antidepressants. Neurochem. Int.159:105390. doi: 10.1016/j.neuint.2022.105390
207
Wang Y. J. Zan G. Y. Xu C. Li X. P. Shu X. Yao S. Y. et al . (2023). The claustrum-prelimbic cortex circuit through dynorphin/κ-opioid receptor signaling underlies depression-like behaviors associated with social stress etiology. Nat. Commun.14:7903. doi: 10.1038/s41467-023-43636-x
208
Wilson L. L. Alleyne A. R. Eans S. O. Cirino T. J. Stacy H. M. Mottinelli M. et al . (2022a). Characterization of CM-398, a novel selective Sigma-2 receptor ligand, as a potential therapeutic for neuropathic pain. Molecules27:3617. doi: 10.3390/molecules27113617
209
Wilson L. L. Eans S. O. Ramadan-Siraj I. Modica M. N. Romeo G. Intagliata S. et al . (2022b). Examination of the novel Sigma-1 receptor antagonist, SI 1/28, for antinociceptive and anti-allodynic efficacy against multiple types of nociception with fewer liabilities of use. Int. J. Mol. Sci.23:615. doi: 10.3390/ijms23020615
210
Woelfer M. Kasties V. Kahlfuss S. Walter M. (2019). The role of depressive subtypes within the Neuroinflammation hypothesis of major depressive disorder. Neuroscience403, 93–110. doi: 10.1016/j.neuroscience.2018.03.034
211
Yan J. Z. Li G. X. Sun S. R. Cui L. Y. Yin Y. Y. Li Y. F. (2024). A rate-limiting step in antidepressants onset: excitation of glutamatergic pyramidal neurons in medial prefrontal cortex of rodents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry130:110911. doi: 10.1016/j.pnpbp.2023.110911
212
Yang K. Wang C. Sun T. (2019). The roles of intracellular chaperone proteins, sigma receptors, in Parkinson's disease (PD) and major depressive disorder (MDD). Front. Pharmacol.10:528. doi: 10.3389/fphar.2019.00528
213
Yano H. Bonifazi A. Xu M. Guthrie D. A. Schneck S. N. Abramyan A. M. et al . (2018). Pharmacological profiling of sigma 1 receptor ligands by novel receptor homomer assays. Neuropharmacology133, 264–275. doi: 10.1016/j.neuropharm.2018.01.042
214
Yin Y. Ju T. Zeng D. Duan F. Zhu Y. Liu J. et al . (2024). "inflamed" depression: a review of the interactions between depression and inflammation and current anti-inflammatory strategies for depression. Pharmacol. Res.207:107322. doi: 10.1016/j.phrs.2024.107322
215
Zahiroddin A. Faridhosseini F. Zamani A. Shahini N. (2015). Comparing the efficacy of bupropion and amantadine on sexual dysfunction induced by a selective serotonin reuptake inhibitor. Iran Red Crescent Med J17:e24998. doi: 10.5812/ircmj.24998
216
Zeng C. Weng C. C. Schneider M. E. Jr. Puentes L. Riad A. Xu K. et al . (2019). TMEM97 and PGRMC1 do not mediate sigma-2 ligand-induced cell death. Cell Death Discov.5:58. doi: 10.1038/s41420-019-0141-2
217
Zhang K. Chen L. Yang J. Liu J. Li J. Liu Y. et al . (2023). Gut microbiota-derived short-chain fatty acids ameliorate methamphetamine-induced depression- and anxiety-like behaviors in a Sigmar-1 receptor-dependent manner. Acta Pharm. Sin. B13, 4801–4822. doi: 10.1016/j.apsb.2023.09.010
218
Zhang S. Hong J. Zhang T. Wu J. Chen L. (2017). Activation of Sigma-1 receptor alleviates postpartum estrogen withdrawal-induced "depression" through restoring hippocampal nNOS-NO-CREB activities in mice. Mol. Neurobiol.54, 3017–3030. doi: 10.1007/s12035-016-9872-8
219
Zhang B. Wang L. Chen T. Hong J. Sha S. Wang J. et al . (2017). Sigma-1 receptor deficiency reduces GABAergic inhibition in the basolateral amygdala leading to LTD impairment and depressive-like behaviors. Neuropharmacology116, 387–398. doi: 10.1016/j.neuropharm.2017.01.014
220
Zhang Y. Yang Y. Li H. Feng Q. Ge W. Xu X. (2024). Investigating the potential mechanisms and therapeutic targets of inflammatory cytokines in post-stroke depression. Mol. Neurobiol.61, 132–147. doi: 10.1007/s12035-023-03563-w
221
Zhang K. Zhao Z. Lan L. Wei X. Wang L. Liu X. et al . (2017). Sigma-1 receptor plays a negative modulation on N-type Calcium Channel. Front. Pharmacol.8:302. doi: 10.3389/fphar.2017.00302
222
Zhang G. F. Zhu K. L. Li Q. Zhang Y. Waddington J. L. du X. D. et al . (2024). The classical D1 dopamine receptor antagonist SCH23390 is a functional sigma-1 receptor allosteric modulator. Acta Pharmacol. Sin.45, 1582–1590. doi: 10.1038/s41401-024-01256-1
223
Zhao J. Ha Y. Liou G. I. Gonsalvez G. B. Smith S. B. Bollinger K. E. (2014). Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Invest. Ophthalmol. Vis. Sci.55, 3375–3384. doi: 10.1167/iovs.13-12823
Summary
Keywords
depression, sigma receptor, sigma receptor ligands, glutamatergic dysfunction, serotonergic system, Ca2+ responses, neuroinflammation, neuronal plasticity
Citation
Zhang P, Ning Z, Chen H, Chen J, Lu Z and Xu F (2025) Advancing depression treatment: the role of sigma receptor ligands. Front. Neurosci. 19:1691987. doi: 10.3389/fnins.2025.1691987
Received
25 August 2025
Revised
23 October 2025
Accepted
10 November 2025
Published
25 November 2025
Volume
19 - 2025
Edited by
Snezana Kusljic, The University of Melbourne, Australia
Reviewed by
Peng Ren, Shandong Provincial Hospital, China
Jing-Ya Wang, Academy of Military Medical Sciences (AMMS), China
Updates
Copyright
© 2025 Zhang, Ning, Chen, Chen, Lu and Xu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihong Lu, deerlu23@163.com; Feifei Xu, xufeifei0204@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.