- 1Department of Neurosurgery, Xuzhou Central Hospital, Southeast University Affiliated Xuzhou Central Hospital, Xuzhou Clinical School of Xuzhou Medical University, Xuzhou, China
- 2Department of Neurology, Xuzhou Central Hospital, Southeast University Affiliated Xuzhou Central Hospital, Xuzhou Clinical School of Xuzhou Medical University, Xuzhou, China
- 3Department of Neurology and Suzhou Clinical Research Center of Neurological Disease, The Second Affiliated Hospital of Soochow University, Suzhou, China
- 4Department of Radiotherapy, Xuzhou Cancer Hospital, Xuzhou, China
Background and purpose: Mechanical thrombectomy advances enable detection of thrombus composition changes post-intravenous thrombolysis, but evidence remains limited. This study retrospectively analyzed thrombi obtained from mechanical thrombectomy in patients with acute ischemic stroke to explore the impact of intravenous thrombolysis (IVT) on the composition of thrombi in patients with different TOAST classifications and the association between changes in thrombus components and clinical outcome data.
Materials and methods: Thrombus samples from 141 patients undergoing mechanical thrombectomy (n = 60) or bridging therapy (n = 81) at two hospitals between December 2020 and January 2024 were included. Baseline data and outcome data were collected. Components were analyzed via HE staining and immunohistochemistry for CD61 and FGB, quantifying area percentages of red blood cell (RBC), white blood cell (WBC), platelet-fibrin complexes (PLT + FIB), platelet (PLT), and fibrin (FIB). Thrombus components were compared between the mechanical thrombectomy and bridging therapy groups according to TOAST subtypes, and a combined analysis was conducted with clinical prognosis and surgical conditions.
Results: No significant baseline differences (p > 0.05). Significant component differences between mechanical thrombectomy and bridging therapy across subtypes: in large artery atherosclerotic (LAA), FIB higher in mechanical thrombectomy (p = 0.001); in cardioembolic (CE), PLT and FIB higher in mechanical thrombectomy (p = 0.003, p < 0.001). Subtype comparisons showed differences: LAA vs. CE in RBC, PLT + FIB, PLT, FIB (p < 0.05); LAA vs. stroke of undetermined source (SUE) in PLT and FIB (p < 0.05); CE vs. SUE in FIB (p < 0.05).
Conclusion: This study quantified the thrombus components of different etiologies and found that IVT could reduce the FIB content in thrombi of large artery occlusion cerebral infarction, especially in the LAA type. The platelet content in the LAA type was higher than that in the CE and the SUE types. The FIB level in the SUE type was higher than that in the CE type. FIB was not only present in the PLT area but also in the RBC area. IVT may improve thrombectomy success by disrupting thrombus structure, but it can increase fragment embolization and prolong procedure time.
Introduction
Prior to 2015, as the mechanical thrombectomy (MT) technology remained imperfect and its therapeutic effectiveness had not been extensively validated, there was a paucity of research focusing on the pathological characteristics of thrombi (Staessens et al., 2021). Along with the progress of technology and the publication of the results of pivotal clinical trials, MT has currently emerged as the mainstay treatment for acute large-vessel occlusive ischemic stroke (AIS-LVO). Furthermore, while MT exhibits a higher vascular recanalization rate compared to intravenous thrombolysis (IVT), IVT remains the preferred choice for patients who meet the time-window criteria, attributable to its operational simplicity and relatively low cost (National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995; Hacke et al., 2008). Nevertheless, a subset of patients with stroke resulting from large vessel occlusion (LVO) demonstrate a poor response to IVT and subsequently undergo MT treatment (Gong et al., 2019). This combined treatment modality is referred to as bridging therapy (BT). Several studies have compared the efficacy of MT and BT with the aim of investigating whether IVT exerts an influence. These studies reported that BT can enhance the vascular recanalization rate (Behme et al., 2016). However, the subgroup analyses of SWIFT and STAR, as well as the HERMES meta-analysis, did not lend support to this finding (Goyal et al., 2016; Coutinho et al., 2017). We considered that the inherent bias could potentially stem from the inclusion of MT patients who did not meet the eligibility criteria for IVT. Additionally, research has indicated that among patients who meet the IVT criteria, there are no significant differences in clinical outcomes between those who undergo MT and those who receive BT (Tsivgoulis et al., 2017). Therefore, the debate over whether IVT treatment is necessary before MT continues.
Although MT has increased the rate of vascular recanalization, there are still individual differences in clinical outcomes. Studies have shown that the histopathological composition of thrombi may be a key factor influencing the efficacy of thrombectomy (Yuki et al., 2012), and the composition of thrombi may also influence the thrombectomy strategy in endovascular treatment. In recent years, research on the association between thrombus components and the etiology of stroke has gradually deepened, especially in exploring the pathogenesis of intracranial AIS-LVO, significant progress has been made (Fitzgerald et al., 2021; Manisha et al., 2024). Staessens et al.’s (2020) research found that in AIS-LVO patients, the proportions of red blood cells, white blood cells, and fibrin in thrombi vary among different etiological subtypes. Moreover, thrombi with different components can affect the success rate of intravenous thrombolysis, the number of mechanical thrombectomy operations, the incidence of complications, and clinical prognosis. Niesten et al.’s (2014) research indicated that thrombi rich in fibrin are not only associated with a greater need for repeated recanalization operations during thrombectomy but also show greater resistance to thrombolytic therapy compared to those rich in red blood cells. Vandelanotte et al.’s (2024a) research found that thrombi rich in red blood cells are more sensitive to thrombolytic therapy. Zhang et al.’s (2024) research discovered that thrombi rich in red blood cells are associated with a shorter time interval from puncture to reperfusion in patients with acute ischemic stroke and better clinical outcomes.
Furthermore, the study by Rossi et al. (2021) found that the extracted thrombi from patients who received BT were significantly smaller than those from patients who only received MT, although the final results of vascular recanalization were not different. Currently, studies on the differences in thrombus composition between patients receiving MT and BT are relatively limited. Most previous studies only used one type of staining, such as hematoxylin–eosin staining (HE), Masson’s trichrome staining (MSB), or HE staining combined with immunohistochemistry (IHC). Few studies have simultaneously used multiple staining methods to investigate the components of thrombi (Aliena-Valero et al., 2021). There is a lack of research on the differences in thrombus composition among patients with different stroke subtypes who received MT and BT treatments. Data on the association between thrombus composition and stroke subtypes are still insufficient.
Therefore, this study aims to evaluate thrombus components in AIS-LVO patients using HE staining and IHC, assess the effect of IVT on thrombus composition, and correlate findings with clinical outcomes and surgical characteristics to provide insights into stroke pathogenesis.
Materials and methods
Enrollment of patients
This study was a retrospective one, consecutively enrolling AIS-LVO patients who were treated with MT at Xuzhou Central Hospital and the Second Affiliated Hospital of Soochow University from December 2020 to January 2024. Patients were divided into the BT group (n = 81) and the MT group (n = 60) based on whether they received IVT treatment or not.
Inclusion and exclusion criteria
Inclusion Criteria: (1) Age≥18 years old; (2) Time of onset≤24 h; (3) All received MT and successfully removed the thrombus; (4) Preoperative Modified Rankin Scale (mRS) score≤2 points; (5) Preoperative National Institutes of Health Stroke Scale (NIHSS) score≥6 points; (6) Preoperative Alberta Stroke Program Early CT Score (ASPECTS) ≥ 6 points.
Exclusion Criteria: (1) Preoperative CT indicated cerebral hemorrhage; (2) Patient lacks clinical or imaging data; (3) Patients with contraindications related to thrombolysis; (4) Patients with severe dysfunction of heart, liver, spleen, lung or kidney, or malignant tumors, making them ineligible for thrombectomy; (5) Patients not falling into any of the LAA, CE or SUE categories of the TOAST classification.
Collection of baseline data
Collect the baseline data of patients, including age, gender, vascular risk factors (previous history of coronary heart disease, atrial fibrillation), laboratory tests (coagulation indicators, blood lipids), etiological classification of stroke, preoperative NIHSS score, anterior and posterior circulation, preoperative mRS score, time from admission to femoral artery puncture (Door-to-puncture time, DPT), and time from onset to vascular recanalization (onset-to-recanalization time, ORT).
Collection of data on clinical outcomes and surgical characteristics
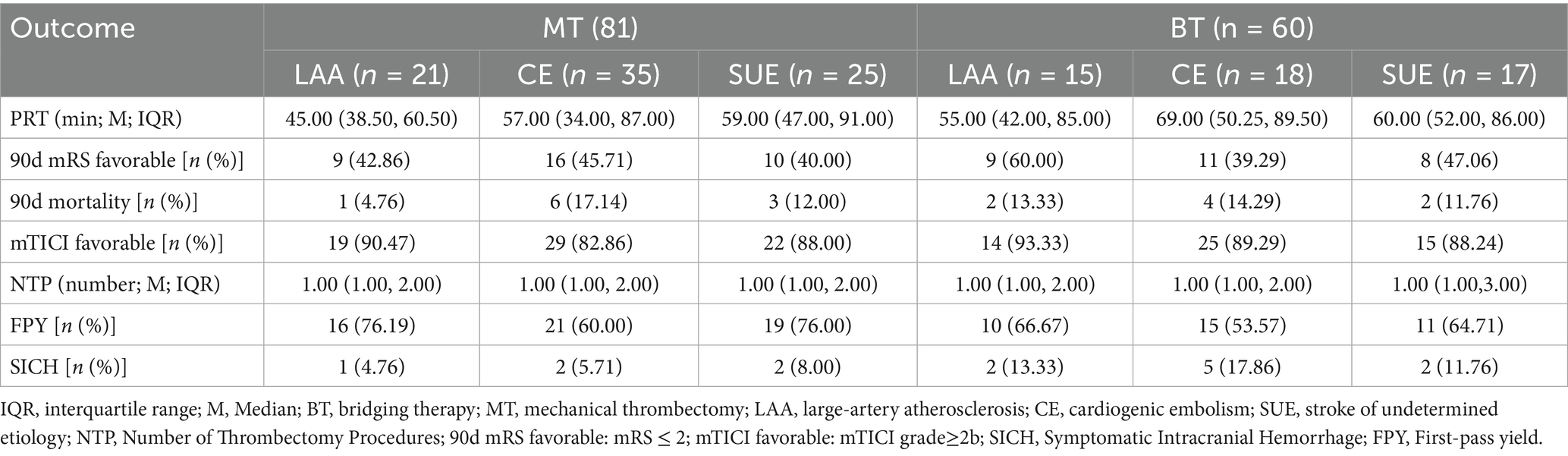
Data were collected on puncture-to-recanalization time (PRT), 90-day favorable functional outcome (defined as mRS ≤ 2), 90-day mortality, successful revascularization (mTICI≥2b), number of thrombectomy passes, first-pass efficacy, and symptomatic intracranial hemorrhage (SICH). SICH was defined as any parenchymal hematoma, subarachnoid hemorrhage or intraventricular hemorrhage associated with death, or a neurological deterioration of ≥ 4 points on the NIHSS within 24 h.
Ethics
All patients received informed consent signed by the patients or their legal representatives before intravenous thrombolysis or endovascular mechanical thrombectomy. This study was approved by the Ethics Committee of Xuzhou Central Hospital and the Second Affiliated Hospital of Soochow University (Ethics Number: XZXY-LK-20230517-074, JD-LK2023070-I01).
Intravenous thrombolysis and mechanical thrombectomy
Patients who met the indications for intravenous thrombolysis and gave informed consent were treated with 0.9 mg/kg alteplase (r-tPA) intravenously (Emberson et al., 2014). The patients underwent MT, and the thrombus was collected in a sterile manner.
Analysis of histological components
First, the thrombus was immediately placed in a universal tissue fixative (10% formalin solution) for more than 24 h after removal and then stored and transported at room temperature. The tissue was taken out of the fixative and trimmed flat with a scalpel in a fume hood at the target site. The trimmed tissue and corresponding labels were placed in embedding frames and put into a dehydrator for sequential dehydration with gradient alcohol.
Secondly, consecutive sections of thrombus with a thickness of 3 micrometers were used (i.e., obtained from the same thrombus sample) to ensure the comparability of staining results. These sections were stained with hematoxylin–eosin (HE) to observe the distribution and morphological characteristics of red blood cells (RBC), white blood cells (WBC), and platelet-fibrin complexes (PLT + FIB). Using CD61 IHC staining to observe platelets (PLT) alone, and use FGB IHC staining to observe fibrin (FIB) alone. For immunohistochemical staining, paraffin sections were deparaffinized and rehydrated through a gradient of alcohol. The following steps were then carried out in sequence: (1) Antigen retrieval specific to the tissue (preventing excessive evaporation of the buffer solution and avoiding dry sections); (2) Blocking with 3% H₂O₂ (25 min, protected from light); (3) Blocking with serum (30 min, at room temperature); (4) Incubation with primary antibody CD61 (integrin β3, 66,952-1-ig, Proteintech, 1:1000) overnight at 4 °C; (5) Incubation with HRP-labeled secondary antibody (Anti-FGB Antibody, M01204-1, BOSTER, 1:200) for 50 min at room temperature; (6) Color development with DAB chromogen from the immunohistochemical kit followed by washing with water to stop the reaction; (7) Counterstaining with hematoxylin (3 min) and bluing with hematoxylin bluing solution; (8) Dehydration through a gradient of alcohol and clearing with xylene; (9) Mounting with neutral gum; (10) Analysis under an optical microscope. Between all steps, sections were washed with PBS buffer (3 × 5 min each time), and the duration and concentration of each chemical treatment were carried out according to the standard protocol. It should be noted that the serum blocking solution should be selected based on the species of the primary antibody [if the primary antibody is from goat, use rabbit serum for blocking, otherwise use bovine serum albumin (BSA)].
Finally, after all sections were examined under an optical microscope, a full-field digital section was generated using a slide digital scanner (Pannoramic MIDI), and then the scanned images were converted to digital images (TIFF format) using CaseViewer (C.V2.4, 3DHISTECH, Hungary) for analysis of thrombus components. All the relevant images of HE-stained specimens were analyzed by a pathologist who was unaware of the patients’ clinical information, with the magnification adjusted to 100 × . The area percentages of all stained red blood cells, fibrin and platelet complexes, fibrin, platelets and white blood cells were batch-calculated by a researcher who was unaware of the patient’s clinical and image information using the Trainable Weka Segmentation plugin in Fiji software (2.14, https://imagej.net/software/fiji) in ijm format. When using the Trainable Weka Segmentation plugin to analyze stained images, four color categories are set for target recognition. By default, two categories with customizable chromaticity coordinates are provided, and the two newly added categories use the default values. In HE staining: green [51, 255, 0] represents the PLT and FIB complex, blue [0, 0, 204] represents WBC, purple (default) represents RBC, and yellow (default) represents the background; in CD61 staining (for PLT): green [51, 255, 0] represents other complexes, blue [0, 0, 204] represents WBC, purple (default) represents PLT, and yellow (default) represents the background; in FGB staining (for FIB): purple (default) represents FIB, and the rest are consistent with CD61 staining.
Criteria for the classification of stroke subtypes
The stroke subtypes were determined by two researchers based on The publications Committee for the Trial of ORG 10172 in acute stroke treatment (TOAST) investigators (1998) method, and they were blinded to each other during the assessment process. After the initial classification was completed, a third researcher checked the results. For cases with discrepancies, the final classification was determined by a senior physician. Large artery atherosclerosis (LAA) was defined when significant stenosis (>50%) was present in the large arteries associated with acute ischemic stroke and no evidence of potential cardiogenic embolism was found. Cardiogenic embolism (CE) was defined as the presence of at least one clear evidence of cardiogenic embolism source. Stroke cases with more than two causes or with an unclear cause despite patient evaluation were classified as stroke of undetermined source (SUE).
Statistical analysis
Statistical analysis was performed using SPSS 27.0. The Kolmogorov–Smirnov test was used for normality testing. For data that conformed to a normal distribution, the mean ± standard deviation ( ± s,) was used to represent the measurement data, and the independent samples t-test was used for comparison between two groups. For data that did not conform to a normal distribution, the median and interquartile range were used to represent the measurement data. The Mann–Whitney U test was used for statistical analysis in the comparison between different TOAST subtypes and within the MT group and BT group for each TOAST subtype. Count data were expressed as frequency and percentage, and the χ2 test or Fisher’s exact test was used for comparison between two groups. A p value < 0.05 was considered statistically significant.
Results
Baseline data
The baseline data of 141 patients included in the study are shown in Table 1. No statistically significant differences were found among the groups (p > 0.05). Among the 141 included patients, there were 36 cases (25.5%) of LAA, 63 cases (44.7%) of cardioembolic stroke, and 42 cases (29.8%) of undetermined etiology. The proportions of TOAST subtypes in the MT group and the BT group are shown in Table 1, and there was no significant difference in the composition of TOAST subtypes between the two groups (p = 0.915).
Pathology of thrombosis
According to HE and immunohistochemical staining, it can be seen that the thrombus is composed of RBC, a small amount of WBC, PLT and FIB. The distribution of each component in the thrombus varies depending on the stroke etiology classification. In LAA thrombus, RBC is aggregated in the center of the thrombus, with FIB and PLT distributed around the edge. It was also found that FIB is distributed in RBC aggregation area. While in CE, fibrin and platelets are evenly distributed throughout the thrombus. SUE shows similar characteristics to CE, as shown in Figure 1.

Figure 1. Comparison of three staining methods in different TOAST classification. We selected three representative thrombus slice samples and observed them under 100x and 400x magnification. By selecting a representative region and magnifying it 400 times. In HE staining, red represents RBC, blue represents WBC, and the rest are PLT + FIB complexes. In IHC staining, in CD61, brown indicates PLT, blue indicates WBC, and the rest are other complexes; in FGB, brown indicates FIB, blue indicates WBC, and the rest are other complexes; We found that fibrin was present in the areas rich in red blood cells. LAA, large-artery atherosclerosis; CE, cardiogenic embolism; SUE, stroke of undetermined etiology; CD61, cluster of differentiation 61; IHC, immunohistochemistry; RBC, red blood cell; PLT, platelet; FIB, fibrin; Original magnification: 100×, Scale bar = 200 μm; Total magnification: 400×, Scale bar = 50 μm.
Thrombus component analysis
The baseline data of 141 patients are shown in Table 1. There were differences in the ratios of PLT and FIB between the MT group and the BT group: PLT was [38.41% (34.67, 45.19) vs. 35.82% (29.33, 41.31), p = 0.006], FIB was [44.73% (40.66, 49.18) vs. 39.13% (36.70, 46.78), p < 0.001], and the immunohistochemical PLT + FIB was [85.11% (77.01, 92.48) vs. 74.88% (67.56, 83.90), p < 0.001], as shown in Figure 2. The comparison results of MT and BT under different TOAST classifications are presented in Table 2 and Figure 3. The comparison of different TOAST classifications within each group of MT and BT is detailed in Table 3 and Figure 4.

Figure 2. Thrombus component analysis using two distinct staining Methods in both MT group and BT group. *p < 0.05; ns: p > 0.05; IHC, immunohistochemistry; RBC, red blood cell; PLT, platelet; FIB, fibrin; WBC, white blood cell; BT, bridging therapy; MT, mechanical thrombectomy.

Table 2. Comparison of mechanical thrombectomy versus bridging Therapy across different TOAST subtypes.

Figure 3. Differences in thrombus composition among AIS subtypes: LAA, CE, and SUE. **p < 0.05; ns: p > 0.05; LAA, Large-artery Atherosclerosis; CE, Cardiogenic Embolism; SUE, Stroke of Undetermined Etiology; RBC, red blood cell; PLT, platelet; FIB, fibrin; WBC, white blood cell; r-tPA, Recombinant Tissue Plasminogen Activator.
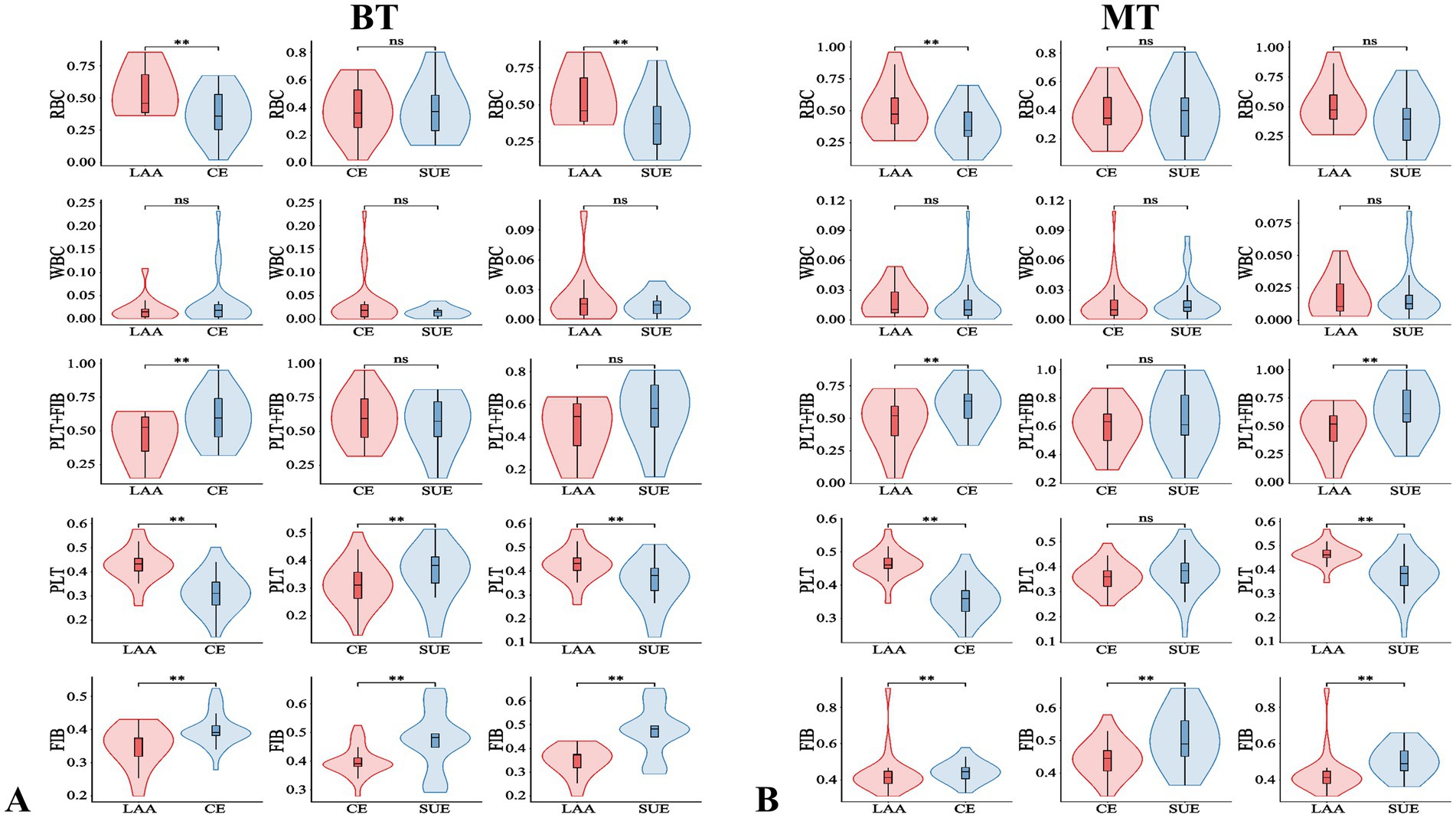
Figure 4. Comparison of different TOAST classifications in the BT and MT group. **p < 0.05; ns: p > 0.05; BT, bridging therapy; MT, mechanical thrombectomy; TOAST classification (each classification represents a group): LAA, large-artery atherosclerosis; CE, cardiogenic embolism; SUE, stroke of undetermined etiology; RBC, red blood cell; PLT, platelet; FIB, fibrin; WBC, white blood cell; PLT+FIB, PLT+FIB complex.
Data analysis of clinical outcomes and surgical characteristics
The comparison results of MT and BT in different TOAST subtypes are detailed in Table 4. Pairwise comparisons of different TOAST subtypes within each group of MT and BT did not reveal significant differences (p > 0.05). The specific data are presented in Table 5.
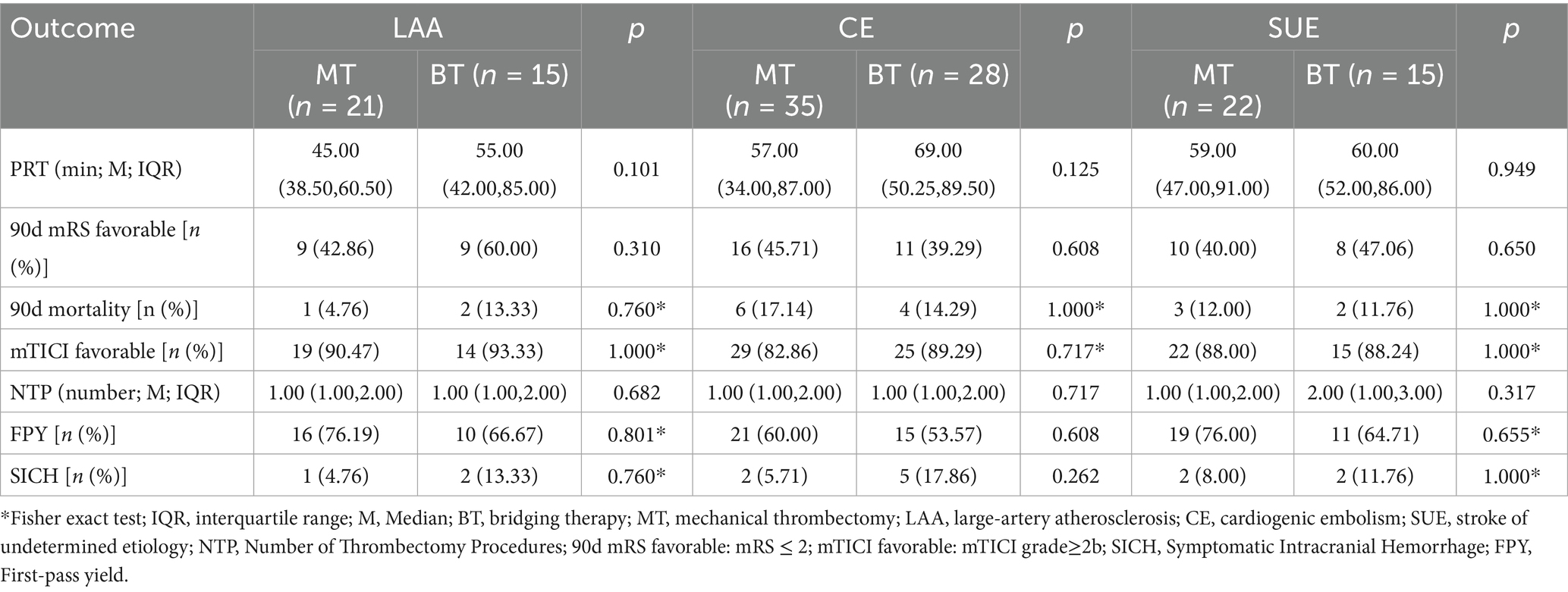
Table 4. Comparative outcomes of mechanical thrombectomy vs. bridging therapy across different TOAST subtypes.
Discussion
This study retrospectively analyzed thrombus samples from AIS patients undergoing MT, using HE staining and IHC to systematically characterize thrombus composition across TOAST subtypes (LAA, CE, SUE), assess the impact of IVT on clot composition, and evaluate clinical outcomes and surgical characteristics. The discussion focuses on key findings, underlying mechanisms, clinical implications, and research value.
Firstly, this study is the first to evaluate the impact of IVT on the composition of thrombus in different etiological subtypes by IHC labeling of PLT and FIB. The core findings are as follows: (1) IVT reduces the FIB content in LAA and CE thrombi; (2) the effect of IVT on PLT is only observed in CE thrombi; (3) there is no difference in the content of PLT and FIB in the SUE subgroup after IVT. In addition, in the LAA subtype, thrombi are characterized by a high content of RBC and a small amount of FIB and PLT. The density of the FIB network is lower than that in the CE subtype, allowing drugs to penetrate more easily (Kim Y. D. et al., 2015; Choi et al., 2018), making it more sensitive to rt-PA and leading to FIB dissolution, but no significant change was observed in the PLT component. However, this result is not entirely consistent with our expected hypothesis: we speculated that in the LAA subtype, due to the faster carotid blood flow, platelets might be washed away by the blood flow after fibrinolysis, but this phenomenon was not observed in this study, which might be related to the limited sample size. Notably, previous studies using HE staining failed to confirm a reduction in PLT + FIB in thrombus after thrombolysis. This study, through IHC technology, found that the effect of IVT on PLT may be underestimated by traditional HE staining-HE staining can only show PLT + FIB, while IHC can specifically distinguish PLT from FIB. The results show that the PLT content in CE thrombi significantly decreases after IVT. The above research results indicate that in CE-type thrombi, when exposed to r-tPA, fibrin in the marginal area (i.e., the side in contact with blood flow) dissolves first, thereby indirectly exposing and partially removing the surface platelets. This phenomenon is consistent with the findings of Doche et al. (2024). However, due to the existence of a densely cross-linked fibrin network within the thrombus (Jiang et al., 2021), its sensitivity to r-tPA is significantly reduced, showing a certain degree of resistance. This structural characteristic may be the main reason why the FIB content in CE-type thrombi in this study only decreased by 10–15%, with a limited reduction. In addition, the high FIB content and dense mesh structure of SUE thrombi may be the main reasons for their resistance to IVT, consistent with the reports of Vandelanotte et al. (2024a,b). A study (Marta-Enguita et al., 2024) has revealed that aged thrombi might develop a more compact fibrin network as a result of ongoing cross-linking reactions (such as the effect of FXIIIa). This, consequently, gives rise to resistance to thrombolysis.
Secondly, through quantitative IHC analysis, this study also found that LAA thrombi are characterized by RBC aggregation as the core feature, with RBCs mainly distributed in the central area of the thrombus, while FIB and PLT form a relatively simple structure at the periphery. Our findings are consistent with previous reports showing comparable thrombus composition patterns (Bembenek et al., 2017; Fitzgerald et al., 2021). This feature may be related to the formation mechanism of LAA thrombi - after the rupture of atherosclerotic plaques, local blood flow stasis leads to the retention and aggregation of RBC, while PLT and FIB mainly participate in the initial thrombus formation on the surface of the plaque. In contrast, CE thrombi present a “FIB-dominant” complex grid structure, with FIB and PLT widely interwoven throughout the thrombus, while RBC are scattered. This result supports the classic theory that LAA thrombi have a higher proportion of RBC and that cardiogenic emboli are mainly composed of PLT + FIB (Kim S. K. et al., 2015; Ahn et al., 2016; Maekawa et al., 2018; Fitzgerald et al., 2021), but it contradicts the earlier view that LAA thrombi are mainly composed of PLT + FIB (Packham and Mustard, 1986; White, 1994; Shin et al., 2018). Interestingly, we found that fibrin also exists in areas rich in red blood cells, which is consistent with previous studies (Staessens et al., 2020; Pir et al., 2022), suggesting that there may be interactions among these components. In RBC-dominated thrombi, a small amount of fibrin acts as a “scaffold” to wrap around red blood cells, maintaining the overall structural integrity of the thrombus. This mixed structure enhances stability (Doche et al., 2024). Previous studies only found that in HE staining, the FIB and RBC areas had relatively distinct manifestations (Staessens et al., 2020). Additionally, this study further quantified through immunohistochemistry that the PLT content in LAA thrombi was significantly higher than that in CE and SUE thrombi, which may be related to more intense local PLT activation after plaque rupture; these results are consistent with the conclusions of Fitzgerald and Brinjikji (2019) and Dargazanli et al. (2020). CE and SUE thrombi have a higher FIB content, suggesting that FIB plays a key role in the stability of the structure of CE and SUE thrombi. Moreover, we found that the component characteristics of SUE thrombi are between LAA and CE: their RBC, WBC, and PLT + FIB composition is similar to that of CE (Sporns et al., 2017; Nouh et al., 2020), but the FIB content differs between the MT and BT groups (higher in the SUE group), and only in the BT group is there a difference in PLT content between CE and SUE. This result suggests that SUE thrombi may have “mixed” pathological features, and the complexity of their etiology (such as cryptogenic embolism, small vessel disease, or multi-site embolism) may lead to the heterogeneity of thrombus components. However, the current understanding of the specific mechanisms (such as the thrombus formation microenvironment and dynamic evolution process) is still limited, and further subgroup analysis and mechanism exploration are needed. In patients with TOAST classification, the comparison between MT and BT showed that the PRT in the MT group tended to decrease, while FPY showed the opposite trend. We analyzed that the possible mechanism might be that intravenous thrombolysis led to partial fibrinolysis, damaged the structure of thrombus, produced thrombus fragments, and then thrombus escape might occur, making it impossible to completely remove the thrombus at one time, thus prolonging the operation time. Doche et al.’s (2024) study also pointed out a similar conclusion. In addition, the application of thrombolytic drugs may change the structural stability of thrombus, which is also an important factor affecting complete thrombus removal. Although Jadhav et al.’s (2022) study found that FPY was important for a good prognosis, no difference was found in our study, which might be related to the sample size. No difference was found in other prognostic indicators, suggesting that for patients with different TOAST classifications, the two treatment strategies of MT and BT did not show significant differences in overall efficacy. Previous studies (Doheim et al., 2025) did not involve the differences in clinical outcomes of TOAST subtype patients after receiving MT or BT. This study to some extent filled the gap in this field, but large-scale clinical trials are still needed to further verify the above conclusions.
Finally, the innovation of this study lies in the combined application of HE staining and IHC techniques, which overcomes the limitations of single staining methods: although HE staining can display PLT + FIB, it cannot precisely distinguish PLT from FIB (Sporns et al., 2017; Maekawa et al., 2018; Gong et al., 2019; Patel et al., 2021; Manisha et al., 2024); Masson staining has limited ability to differentiate the two; while IHC achieves precise quantification of components through specific labeling, providing a new tool for analyzing the microstructure of thrombi (Brinjikji et al., 2021). Meanwhile, systematically correlating the changes in thrombus composition with the effect of vascular recanalization, surgical conditions, and patient functional outcomes further enhanced the clinical depth of the research. It is worth noting that in the acquisition of thrombi, we selected the first thrombus from each case for study, and almost all of them were obtained through the first or second thrombectomy, reducing the potential changes in thrombus composition due to multiple thrombectomies. Many previous studies (Boeckh-Behrens et al., 2016; Vandelanotte et al., 2024a) did not describe the method of thrombus selection, such as whether the thrombi were randomly selected from different thrombectomy procedures or whether all thrombi from each patient were comprehensively quantified. In such cases, the results obtained might be biased. Of course, intravenous thrombolysis itself may affect the structure and composition of thrombi, and this confounding factor is difficult to completely avoid in clinical research. However, as a multi-center retrospective analysis, this study has the following limitations: (1) The sample size is small, which may reduce the statistical power of subgroup analysis (e.g., the IVT effect on SUE thrombi did not reach significance); (2) The retrospective design cannot completely eliminate selection bias (e.g., some patients did not receive IVT due to contraindications); (3) Thrombus samples were only from MT patients and did not include patients who received only IVT or did not receive thrombolysis, thus failing to fully reflect the dynamic evolution of thrombus components; (4) Potential thrombogenic components such as vWF and extracellular DNA were not stained, making it impossible to verify their association with IVT resistance [e.g., the hypothesis of elevated PAI-1 levels proposed by Jiang et al., 2021]. Future large-scale, multicenter prospective studies should integrate thrombus component parameters with multidimensional imaging features (e.g., thrombus volume, embolization site, structural stability) and key clinical indicators to build a multimodal prognostic prediction model. This model can guide clinical decisions—such as selecting thrombolytic therapy or prioritizing mechanical thrombectomy based on specific thrombus composition—enabling precise patient stratification and individualized treatment optimization. Additionally, further research is needed on the dynamic evolution of thrombus components and novel thrombolytic targets (e.g., enzymes that degrade dense fibrin networks) to improve recanalization rates and clinical outcomes in stroke patients.
In conclusion, this study precisely quantified the component characteristics of thrombi in different etiological subtypes and their associations with IVT and clinical outcomes, revealing the selective impact of IVT on thrombus composition. This provides important theoretical support for optimizing the combined strategy of intravenous thrombolysis and mechanical thrombectomy in AIS patients.
Conclusion
Overall, thrombus composition differs between LAA and CE regardless of IVT: LAA is red blood cell–rich, whereas CE is fibrin-dominant; SUE resembles CE but has higher FIB content. IVT benefits AIS-LVO patients by reducing FIB, particularly in LAA, while PLT levels remain higher in LAA than in CE or SUE. FIB is present in both PLT-rich and RBC-rich regions, suggesting multiple roles in thrombosis. By loosening the clot structure, IVT may improve thrombectomy success but could cause fragment embolization or prolong procedure time. Although no differences in MT vs. BT outcomes were observed across TOAST subtypes in this study, larger studies are warranted.
Data availability statement
The datasets supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Biomedical Research Ethics Review Committee of Xuzhou Central Hospital Ethics Committee of the Second Affiliated Hospital of Suzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CZ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. GC: Conceptualization, Data curation, Software, Validation, Writing – review & editing. ZG: Conceptualization, Data curation, Methodology, Software, Writing – review & editing. SC: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing. LP: Data curation, Writing – review & editing. QL: Data curation, Writing – review & editing. XZ: Data curation, Writing – review & editing. MD: Funding acquisition, Validation, Writing – review & editing. YZ: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was jointly funded by the following projects: General Project of Traditional Chinese Medicine Science and Technology Development Plan in Jiangsu Province (No. MS2023077, Beneficiary: YZ); New Round of Xuzhou “Pengcheng Talent Program” - High-level Healthcare Talent Recruitment and Development Project (No. 2025GG005, Beneficiary: MD); Development Fund of Affiliated Hospital of Xuzhou Medical University (No. XYFM202410, Beneficiary: YZ); Key Research and Development Project of Xuzhou Science and Technology Bureau (No. KC22169, Beneficiary: YZ); the Basic Research Projects of Xuzhou Science and Technology Bureau (No. KC23066, Beneficiary: MD).
Acknowledgments
The authors would like to express their gratitude to the stroke teams at Xuzhou Central Hospital and the Second Affiliated Hospital of Soochow University for their support and collaboration in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahn, S. H., Hong, R., Choo, I. S., Heo, J. H., Nam, H. S., Kang, H. G., et al. (2016). Histologic features of acute thrombi retrieved from stroke patients during mechanical reperfusion therapy. Int. J. Stroke 11, 1036–1044. doi: 10.1177/1747493016641965
Aliena-Valero, A., Baixauli-Martin, J., Torregrosa, G., Tembl, J. I., and Salom, J. B. (2021). Clot composition analysis as a diagnostic tool to gain insight into ischemic stroke etiology: a systematic review. J. Stroke 23, 327–342. doi: 10.5853/jos.2021.02306
Behme, D., Kabbasch, C., Kowoll, A., Dorn, F., Liebig, T., Weber, W., et al. (2016). Intravenous thrombolysis facilitates successful recanalization with stent-retriever mechanical Thrombectomy in middle cerebral artery occlusions. J. Stroke Cerebrovasc. Dis. 25, 954–959. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.007
Bembenek, J. P., Niewada, M., Siudut, J., Plens, K., Czlonkowska, A., and Undas, A. (2017). Fibrin clot characteristics in acute ischaemic stroke patients treated with thrombolysis: the impact on clinical outcome. Thromb. Haemost. 117, 1440–1447. doi: 10.1160/TH16-12-0954
Boeckh-Behrens, T., Kleine, J. F., Zimmer, C., Neff, F., Scheipl, F., Pelisek, J., et al. (2016). Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke 47, 1864–1871. doi: 10.1161/STROKEAHA.116.013105
Brinjikji, W., Nogueira, R. G., Kvamme, P., Layton, K. F., Delgado Almandoz, J. E., Hanel, R. A., et al. (2021). Association between clot composition and stroke origin in mechanical thrombectomy patients: analysis of the stroke thromboembolism registry of imaging and pathology. J. Neurointerv. Surg. 13, 594–598. doi: 10.1136/neurintsurg-2020-017167
Choi, M. H., Park, G. H., Lee, J. S., Lee, S. E., Lee, S. J., Kim, J. H., et al. (2018). Erythrocyte fraction within retrieved thrombi contributes to thrombolytic response in acute ischemic stroke. Stroke 49, 652–659. doi: 10.1161/STROKEAHA.117.019138
Coutinho, J. M., Liebeskind, D. S., Slater, L. A., Nogueira, R. G., Clark, W., Davalos, A., et al. (2017). Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: a pooled analysis of the SWIFT and STAR studies. JAMA Neurol. 74, 268–274. doi: 10.1001/jamaneurol.2016.5374
Dargazanli, C., Zub, E., Deverdun, J., Decourcelle, M., de Bock, F., Labreuche, J., et al. (2020). Machine learning analysis of the cerebrovascular thrombi proteome in human ischemic stroke: an exploratory study. Front. Neurol. 11:575376. doi: 10.3389/fneur.2020.575376
Doche, E., Sulowski, C., Guigonis, J. M., Graslin, F., Casolla, B., Hak, J. F., et al. (2024). How clot composition influences fibrinolysis in the acute phase of stroke: a proteomic study of cerebral thrombi. Stroke 55, 1818–1829. doi: 10.1161/STROKEAHA.124.047156
Doheim, M. F., Knapen, R., Staals, J., Schonewille, W. J., Dippel, D. W. J., van Es, A., et al. (2025). Direct endovascular versus bridging therapy in M2 segment occlusion of middle cerebral artery: a MR CLEAN registry study. Stroke 56, 2866–2878. doi: 10.1161/STROKEAHA.125.051967
Emberson, J., Lees, K. R., Lyden, P., Blackwell, L., Albers, G., Bluhmki, E., et al. (2014). Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384, 1929–1935. doi: 10.1016/S0140-6736(14)60584-5
Fitzgerald, S., and Brinjikji, W. (2019). Response by Fitzgerald and Brinjikji to letter regarding article, "platelet-rich emboli in cerebral large vessel occlusion are associated with a large artery atherosclerosis source". Stroke 50:e298. doi: 10.1161/STROKEAHA.119.026856
Fitzgerald, S., Rossi, R., Mereuta, O. M., Jabrah, D., Okolo, A., Douglas, A., et al. (2021). Per-pass analysis of acute ischemic stroke clots: impact of stroke etiology on extracted clot area and histological composition. J. Neurointerv. Surg. 13, 1111–1116. doi: 10.1136/neurintsurg-2020-016966
Gong, L., Zheng, X., Feng, L., Zhang, X., Dong, Q., Zhou, X., et al. (2019). Bridging therapy versus direct mechanical thrombectomy in patients with acute ischemic stroke due to middle cerebral artery occlusion: a clinical- histological analysis of retrieved thrombi. Cell Transplant. 28, 684–690. doi: 10.1177/0963689718823206
Goyal, M., Menon, B. K., van Zwam, W. H., Dippel, D. W., Mitchell, P. J., Demchuk, A. M., et al. (2016). Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387, 1723–1731. doi: 10.1016/S0140-6736(16)00163-X
Hacke, W., Kaste, M., Bluhmki, E., Brozman, M., Davalos, A., Guidetti, D., et al. (2008). Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 359, 1317–1329. doi: 10.1056/NEJMoa0804656
Jadhav, A. P., Desai, S. M., Zaidat, O. O., Nogueira, R. G., Jovin, T. G., Haussen, D. C., et al. (2022). First pass effect with Neurothrombectomy for acute ischemic stroke: analysis of the systematic evaluation of patients treated with stroke devices for acute ischemic stroke registry. Stroke 53, e30–e32. doi: 10.1161/STROKEAHA.121.035457
Jiang, Y., Liu, N., Han, J., Li, Y., Spencer, P., Vodovoz, S. J., et al. (2021). Diabetes mellitus/Poststroke hyperglycemia: a detrimental factor for tPA thrombolytic stroke therapy. Transl. Stroke Res. 12, 416–427. doi: 10.1007/s12975-020-00872-3
Kim, Y. D., Nam, H. S., Kim, S. H., Kim, E. Y., Song, D., Kwon, I., et al. (2015). Time-dependent Thrombus resolution after tissue-type plasminogen activator in patients with stroke and mice. Stroke 46, 1877–1882. doi: 10.1161/STROKEAHA.114.008247
Kim, S. K., Yoon, W., Kim, T. S., Kim, H. S., Heo, T. W., and Park, M. S. (2015). Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient-Echo MRI. AJNR Am. J. Neuroradiol. 36, 1756–1762. doi: 10.3174/ajnr.A4402
Maekawa, K., Shibata, M., Nakajima, H., Mizutani, A., Kitano, Y., Seguchi, M., et al. (2018). Erythrocyte-rich Thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical Thrombectomy. Cerebrovasc. Dis. Extra 8, 39–49. doi: 10.1159/000486042
Manisha, K. Y., Poyuran, R., Narasimhaiah, D., Kumar Paramasivan, N., Ramachandran, H., Erat Sreedharan, S., et al. (2024). Thrombus histology does not predict stroke etiological subtype but influences recanalization. J. Clin. Neurosci. 124, 54–59. doi: 10.1016/j.jocn.2024.04.013
Marta-Enguita, J., Navarro-Oviedo, M., Machado, F., Bermejo, R., Aymerich, N., Herrera, M., et al. (2024). Role of factor XIII in ischemic stroke: a key molecule promoting thrombus stabilization and resistance to lysis. J. Thromb. Haemost. 22, 1080–1093. doi: 10.1016/j.jtha.2023.12.029
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995). Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 333, 1581–1588. doi: 10.1056/NEJM199512143332401
Niesten, J. M., van der Schaaf, I. C., van Dam, L., Vink, A., Vos, J. A., Schonewille, W. J., et al. (2014). Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One 9:e88882. doi: 10.1371/journal.pone.0088882
Nouh, A., Mehta, T., Hussain, M., Song, X., and Ollenschleger, M. (2020). Clot composition of embolic strokes of undetermined source: a feasibility study. BMC Neurol. 20:383. doi: 10.1186/s12883-020-01969-w
Packham, M. A., and Mustard, J. F. (1986). The role of platelets in the development and complications of atherosclerosis. Semin. Hematol. 23, 8–26
Patel, T. R., Fricano, S., Waqas, M., Tso, M., Dmytriw, A. A., Mokin, M., et al. (2021). Increased perviousness on CT for acute ischemic stroke is associated with fibrin/platelet-rich clots. AJNR Am. J. Neuroradiol. 42, 57–64. doi: 10.3174/ajnr.A6866
Pir, G. J., Parray, A., Ayadathil, R., Pananchikkal, S. V., Mir, F. A., Muhammad, I., et al. (2022). Platelet-neutrophil association in NETs-rich areas in the retrieved AIS patient thrombi. Int. J. Mol. Sci. 23:14477. doi: 10.3390/ijms232214477
Rossi, R., Fitzgerald, S., Molina, S., Mereuta, O. M., Douglas, A., Pandit, A., et al. (2021). The administration of rtPA before mechanical thrombectomy in acute ischemic stroke patients is associated with a significant reduction of the retrieved clot area but it does not influence revascularization outcome. J. Thromb. Thrombolysis 51, 545–551. doi: 10.1007/s11239-020-02279-1
Shin, J. W., Jeong, H. S., Kwon, H. J., Song, K. S., and Kim, J. (2018). High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS One 13:e0197492. doi: 10.1371/journal.pone.0197492
Sporns, P. B., Hanning, U., Schwindt, W., Velasco, A., Buerke, B., Cnyrim, C., et al. (2017). Ischemic stroke: histological Thrombus composition and pre-interventional CT attenuation are associated with intervention time and rate of secondary embolism. Cerebrovasc. Dis. 44, 344–350. doi: 10.1159/000481578
Staessens, S., Denorme, F., Francois, O., Desender, L., Dewaele, T., Vanacker, P., et al. (2020). Structural analysis of ischemic stroke thrombi: histological indications for therapy resistance. Haematologica 105, 498–507. doi: 10.3324/haematol.2019.219881
Staessens, S., Francois, O., Brinjikji, W., Doyle, K. M., Vanacker, P., Andersson, T., et al. (2021). Studying stroke thrombus composition after thrombectomy: what can we learn? Stroke 52, 3718–3727. doi: 10.1161/STROKEAHA.121.034289
The publications Committee for the Trial of ORG 10172 in acute stroke treatment (TOAST) investigators (1998). Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. JAMA 279, 1265–1272.
Tsivgoulis, G., Katsanos, A. H., Mavridis, D., Alexandrov, A. W., Magoufis, G., Arthur, A., et al. (2017). Endovascular thrombectomy with or without systemic thrombolysis? Ther. Adv. Neurol. Disord. 10, 151–160. doi: 10.1177/1756285616680549
Vandelanotte, S., Francois, O., Desender, L., Staessens, S., Vanhoorne, A., Van Gool, F., et al. (2024a). R-tPA resistance is specific for platelet-rich stroke thrombi and can be overcome by targeting nonfibrin components. Stroke 55, 1181–1190. doi: 10.1161/STROKEAHA.123.045880
Vandelanotte, S., Staessens, S., Francois, O., De Wilde, M., Desender, L., De Sloovere, A. S., et al. (2024b). Association between thrombus composition and first-pass recanalization after thrombectomy in acute ischemic stroke. J. Thromb. Haemost. 22, 2555–2561. doi: 10.1016/j.jtha.2024.05.034
White, J. G. (1994). Platelets and atherosclerosis. Eur. J. Clin. Investig. 24, 25–29. doi: 10.1111/j.1365-2362.1994.tb02422.x
Yuki, I., Kan, I., Vinters, H. V., Kim, R. H., Golshan, A., Vinuela, F. A., et al. (2012). The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. AJNR Am. J. Neuroradiol. 33, 643–648. doi: 10.3174/ajnr.A2842
Keywords: ischemic stroke, thrombus, TOAST classification, mechanical thrombectomy, thrombolysis
Citation: Zhao C, Chen G, Guo Z, Chen S, Ping L, Li Q, Zhang X, Ding M and Zhang Y (2025) Histological analysis of thrombus composition in acute ischemic stroke with large vessel occlusion: impact of r-tPA treatment. Front. Neurosci. 19:1707934. doi: 10.3389/fnins.2025.1707934
Edited by:
Hongjian Pu, University of Pittsburgh, United StatesReviewed by:
Oana Madalina Mereuta, Mayo Clinic, United StatesGhulam Jeelani Pir, Hamad Medical Corporation, Qatar
Varsha Muddasani, University of Texas Health Science Center at Houston, United States
Fairoz Jayyusi, Sheba Medical Center, Israel
Copyright © 2025 Zhao, Chen, Guo, Chen, Ping, Li, Zhang, Ding and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Zhang, aHVheWluZ2xpZWh1b0AxNjMuY29t; Manhua Ding, ZGluZ21hbmh1YWJAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Changzhi Zhao
Changzhi Zhao Guofang Chen
Guofang Chen Zhiliang Guo
Zhiliang Guo Sha Chen
Sha Chen Lei Ping
Lei Ping Qiao Li
Qiao Li Xiaoying Zhang
Xiaoying Zhang Manhua Ding
Manhua Ding Yang Zhang
Yang Zhang