Explore article hub
- 1Cardiovascular Research Institute Maastricht (CARIM), Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands
- 2Mosa Meat B.V., Maastricht, Netherlands
A Viewpoint on the Frontiers in Science Lead Article
Hybrid alternative protein-based foods: designing a healthier and more sustainable food supply
Key points
- The numerous possibilities for the formulation of hybrid products—each involving distinct considerations related to functionality, nutrition, cost, sustainability, sensory attributes, and consumer acceptance—illustrate the vast opportunity for the development of hybrid protein alternatives, such as the cost reduction of hybrid cultivated meats.
- Innovating and developing successful hybrid products will require a high degree of creativity, interdisciplinary collaboration, and sustained investment from both public and private stakeholders.
- New product development should consider consumer perception, using intuitive labels like “blended” rather than “hybrid” to better convey the combination of multiple protein sources into a unified, palatable product.
- The very nature of hybrid products blurs the line between plant-based meat alternatives and conventional meat; this cultural shift could pave the way toward a plant-based diet.
Introduction
Alternative proteins such as mycelium-derived products, single-cell proteins, and cultivated meat have been introduced over the past few decades as standalone replacements for animal- or livestock-derived proteins. For instance, the first cultivated meat hamburger, presented in 2013, was composed entirely of cultivated protein; it was not a hybrid product (1). At the time, it cost €250,000 to produce and required large amounts of plastic for cell culture dishes, which was acceptable for a prototype. The philosophy among cultivated meat pioneers was to offer consumers a product identical to conventional meat, but without the negative environmental and animal welfare impacts. Achieving this was thought to require not only “pure” products but also high-quality ones composed of fully mature muscle and fat tissue indistinguishable from meat. While this level of fidelity may be less critical for ground products such as hamburgers, it is essential for whole-cut meat replacements such as ribeye steaks. A prevailing assumption was also that consumers would more readily accept pure meat alternatives than hybrid products, although no data were available to support that notion. Over time, however, the drive to develop affordable, market-ready products led to compromises in product purity. Abandoning purity led to the blending of plant protein-based products with cultivated cells, the latter being added to enhance taste. Furthermore, the level of muscle or fat differentiation was also reduced to meet cost and scalability targets. Some companies have even moved away from using meat-specific cells (2). For example, the observation that undifferentiated avian fibroblasts could enhance the flavor of a plant-protein base was unexpected but supports the case for hybrid approaches. With advancements in cell culture scale-up, reduced cost of medium components, and assuming a certain scale of production and inclusion rate of cultured avian cells in plant protein-based products, Pasitka et al. (2) demonstrated that a cost-effective hybrid product is now within reach.
Hybrid meat alternatives
In their thoughtful and comprehensive lead article in Frontiers in Science, Kaplan and McClements (3) explore the current landscape of hybrid products, evaluate their advantages and drawbacks, and outline pathways for future development. The review underscores the breadth of options that can and should be explored, given the diversity of plant-protein bases and alternative protein additives, and thus, they recommend leveraging artificial intelligence (AI) tools.
Formulating successful hybrid products requires a nuanced understanding of each alternative protein source. Kaplan and McClements (3) begin by reviewing the characteristics, benefits, and limitations of these ingredients. Clearly, plant-based sources offer the widest range of existing ingredient solutions by leveraging decades of industry experience and agricultural optimization. Not only do botanical sources differ widely, but different processing techniques yield a broad range of products with varying purities, polysaccharides and lipid content, flavors, pigments, and preservatives.
In contrast, novel mycelium-based ingredients remain much more limited, with only a few fungal strains commonly used to produce textured meat analogues (4). Most producers obtain mycelium from submerged fermentation (or liquid fermentation) practices, allowing for better control of the process. These processes yield a fibrous biomass often referred to as mycoprotein, which is nutritious and high in protein but has been observed to lack the techno-functional properties needed to structure a pure mycelium product with sensory properties like meat. Thus, the future of mycelium-derived meat analogues will almost certainly rely on hybridization with other protein sources, as reviewed by Kaplan and McClements (3). On the other hand, the fungal kingdom extends well beyond the handful of strains currently used by the food industry, probably due to regulatory constraints (4). Therefore, the possibilities for mycelium-based products have not been exhausted yet, and hyphae-related structural differences within different taxonomical groups of fungi may reveal new desirable functionalities.
Cultivated meat is the most recent addition to alternative proteins. The enthusiasm for cultivated meat is based on the belief that it can provide sustainable, ethical products that match meat nutritionally and organoleptically. Especially for ground meat products, taste is an important driver toward market adoption of cultivated meat that indeed can be replicated. The most secure method is to cultivate a product that has a similar composition and structure to conventional meat. This assumption has scientific support: meat flavor development is driven by complex reactions, but these are only partially understood. We do know, however, that non-odor active molecule precursors such as simple sugars, amino acids, and phospholipids are responsible for flavor development (5). Heat-induced oxidation of these active molecules, as well as a cascade of cross-reactions, leads to the formation of volatile products that induce human-specific perception of meat aroma. At the same time, the umami flavor, which originates from 5′-nucleotides, glutamic acid, and aspartic acid, is another important taste component strongly associated with this aroma.
Interestingly, recent findings suggest that full differentiation into muscle and fat cells is not necessary to replicate key flavor attributes. In fact, undifferentiated cells, and possibly even non meat-specific cells, can contribute to meat-like flavors. Unfortunately, this evidence largely comes from private companies only, and no independent academic confirmation has been published yet. Older studies report that phospholipids that make up the cell membrane play a crucial role in the formation of animal-specific flavor. This is caused by the unique composition of the animal cell membrane and the favorable oxidation of unsaturated fatty chains (6). However, the taxonomic kingdom specificity of these phospholipids and their role in flavor is still poorly understood. The complexity of meat flavor and our limited knowledge necessitate an empirical approach for developing meat-matching flavors. Starting with animal cells remains the most promising route, given their compositional similarity to the tissue being replaced. Moreover, cultivating cells at different levels of differentiation could help close knowledge gaps in meat flavor science.
Current cultivated meat products that have passed regulatory approval and reached small-scale markets are hybrid products with variable percentages of cultivated cells, mostly undifferentiated and composed of fibroblasts. Blending—and therefore diluting—cultured animal cells with plant-based substrates enables larger-scale introduction at relatively low prices and could be viewed as a strategic compromise. Hybrid products with structured and cultivated meat composed of fully differentiated muscle and fat have yet to receive regulatory approval and subsequent market introduction. There are no indications that these products face consumer acceptance issues, although surveys suggest that hybrid products are less accepted than pure plant-based meat substitutes and that cultivated meat hybrids score lower than plant-based hybrids (7). Nonetheless, hybrid products expand consumer choice. In fact, a desire for variety appears to be a key driver for consumers to purchase meat alternatives (8). We predict that an increasing array of hybrid products will further blur the cultural dichotomy between plants and meat, thereby weakening the symbolic associations with meat (9).
Among the other alternative proteins reviewed by Kaplan and McClements (3), recombinant proteins are particularly notable for their functional roles as ingredients within meat or hybrid products. Some, such as transglutaminase, are already used widely. These proteins typically add little nutritional or caloric value to the hybrid. Although currently expensive to produce, costs are expected to decrease once processes transition from pharmaceutical to food-grade conditions.
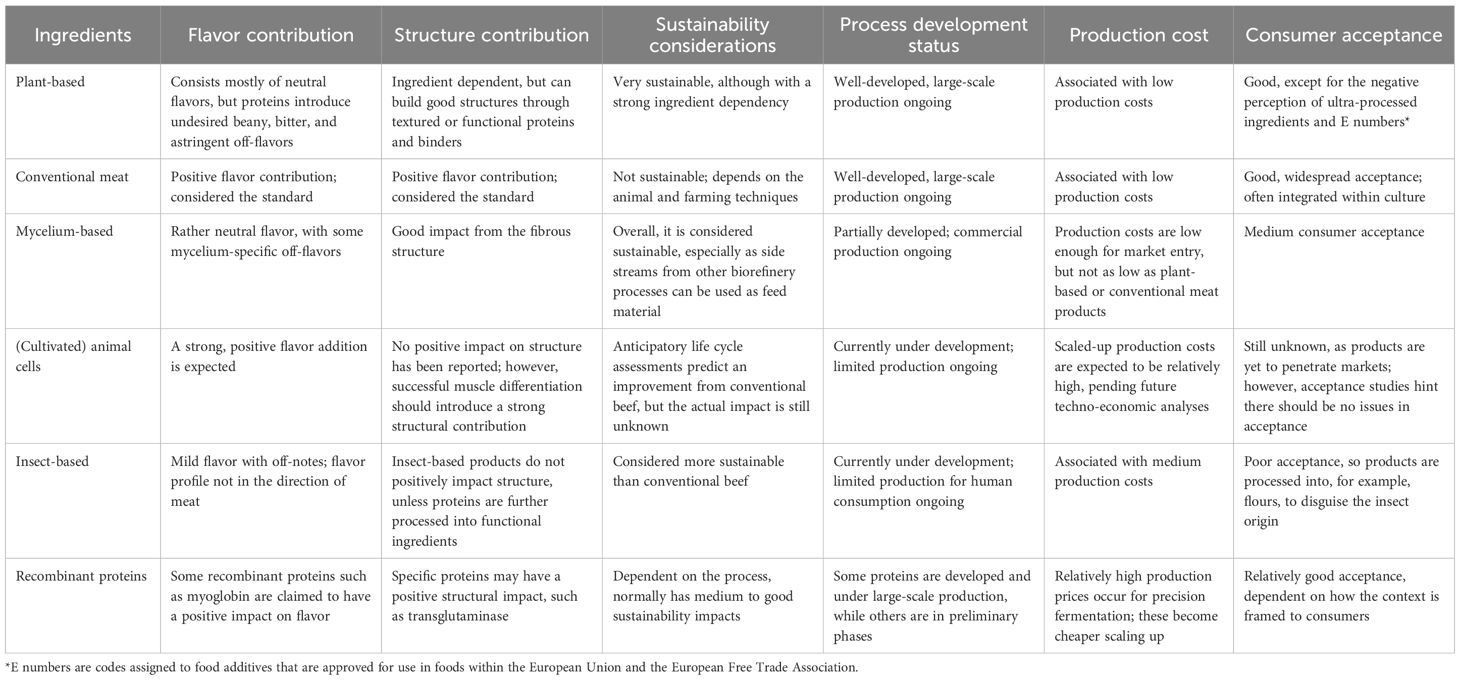
Kaplan and McClements (3) also highlight the potential for novel hybrid combinations that leverage complementary attributes of various alternative protein sources. Table 1 summarizes the organoleptic, sustainability, processing, and consumer acceptance characteristics of different sources: these can be combined strategically to optimize hybrid products. More complex hybrids, i.e., those integrating plant proteins for structure, mycelium for nutrition and neutral flavor, and cultivated meat for aroma, are plausible. The neutral taste profile of mycelium is particularly helpful in mitigating undesirable plant off-notes such as bitterness or beany flavors. One example of research in this area is the European Union-funded PLENITUDE project (10).
The role of AI
Kaplan and McClements (3) also emphasize the potential of data-driven tools in accelerating hybrid protein development. With growing datasets on individual components and their functional properties, AI can help predict successful combinations and even suggest novel hybrids not yet tested. Combining such a database with metabolic models and in silico digestion and absorption simulations could further refine formulation strategies. Two important factors must be considered to successfully create this hybrid component database. First, we must address knowledge gaps about the properties needed for flavor, structure, scalability, cost-efficiency, and consumer acceptance. Second, these data must be publicly accessible. Since most current data are proprietary, a shift toward more open, socially responsible data sharing is needed— one that also recognizes that collective progress can benefit individual companies as well.
Concluding remarks
The numerous possibilities for the formulation of hybrid products—each involving distinct considerations related to functionality, nutrition, cost, sustainability, sensory attributes, and consumer acceptance—highlight the vast area of opportunity in this field. Innovating and developing successful products will require a careful balancing of these factors, as well as a high degree of creativity, interdisciplinary collaboration, and sustained investment from both public and private stakeholders. More specifically, successful new product development also needs to engage with how consumers perceive these products. For example, the term “hybrid” may carry unintended connotations or cause confusion. A more intuitive label, such as “blended,” might better reflect the process of combining various protein sources into a cohesive and palatable product, at least for those that are truly blended after synthesis and not co-synthesized.
Statements
Author contributions
MJP: Visualization, Conceptualization, Project administration, Validation, Supervision, Writing – review & editing, Resources, Writing – original draft.
FZ: Writing – review & editing, Writing – original draft, Conceptualization.
Funding
The authors declared that financial support was received for this work and/or its publication. Mosa Meat B.V. received funding from the Circular Bio-Based Europe Joint Undertaking (CBE JU) flagship project PLENITUDE, a consortium that was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
MJP is a co-founder, shareholder, and Chief Scientific Officer of Mosa Meat B.V., a company that aims to commercialize cultivated meat. He has patents related to cultivated meat, but none for hybrid products.
FZ is employed as a Scientist by Mosa Meat B.V.
Generative AI statement
The authors declared that no generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Post MJ. Cultured beef: medical technology to produce food. J Sci Food Agric (2014) 94(6):1039–41. doi: 10.1002/jsfa.6474
2. Pasitka L, Wissotsky G, Ayyash M, Yarza N, Rosoff G, Kaminker R, et al. Empirical economic analysis shows cost-effective continuous manufacturing of cultivated chicken using animal-free medium. Nat Food (2024) 5(8):693–702. doi: 10.1038/s43016-024-01022-w
3. Kaplan DL and McClements DJ. Hybrid alternative protein-based foods: designing a healthier and more sustainable food supply. Front Sci (2025) 3:1599300. doi: 10.3389/fsci.2025.1599300
4. Holt RR, Munafo JP Jr, Salmen J, Keen CL, Mistry BS, Whiteley JM, et al. Mycelium: a nutrient-dense food to help address world hunger, promote health, and support a regenerative food system. J Agric Food Chem (2024) 72(5):2697–707. doi: 10.1021/acs.jafc.3c03307
5. Kosowska M, Majcher MA, and Fortuna T. Volatile compounds in meat and meat products. Food Sci Technol (2017) 37(1):1–7. doi: 10.1590/1678-457X.08416
6. Mottram DS. Flavour formation in meat and meat products: a review. Food Chem (1998) 62(4):415–24. doi: 10.1016/S0308-8146(98)00076-4
7. van Dijk B, Jouppila K, Sandell M, and Knaapila A. No meat, lab meat, or half meat? Dutch and Finnish consumers’ attitudes toward meat substitutes, cultured meat, and hybrid meat products. Food Qual Prefer (2023) 108:104886. doi: 10.1016/j.foodqual.2023.104886
8. Garwood G. Who’s buying alt-meat? Unpacking consumer motivations [online]. Food Institute (2023). Available at: https://foodinstitute.com/focus/whos-buying-alt-meat-unpacking-consumer-motivations/
9. Zaraska M. Meathooked: the history and science of our 2.5-million-year obsession with meat. New York: Basic Books (2016)
Keywords: cultivated meat, mycelium, alternative proteins, plant-based, novel food, consumer acceptance, techno-economic analysis
Citation: Post MJ and Zaccarian F. Hybrid protein alternatives: feeble compromise or food of the future? Front Sci (2025) 3:1683816. doi: 10.3389/fsci.2025.1683816
Received: 11 August 2025; Accepted: 16 September 2025;
Published: 30 September 2025.
Edited and reviewed by:
Lovedeep Kaur, Massey University, New ZealandCopyright © 2025 Post and Zaccarian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark J. Post, bS5wb3N0QG1hYXN0cmljaHR1bml2ZXJzaXR5Lm5s
 Mark J. Post
Mark J. Post Francesco Zaccarian
Francesco Zaccarian