- Department of Chemistry, University of Toronto, Toronto, ON, Canada
A biosensor device involves the transduction of a surface-oriented biochemical interaction into an electrical signal. This structure offers the potential for obtaining highly specific signals for the assay of bioanalytical targets in a rapid and cost-effective manner. These devices drive a rapidly expanding global market with notable contributions to analysis of blood glucose levels and great potential in the field of personalized medicine. However, some reluctance remains in the application of biosensors in clinical biochemistry laboratories. This article explores these challenges in attempts to highlight opportunities for improvement.
The biosensor and its marketplace
The biosensor device involves the transduction of a surface-oriented biochemical interaction into an electrical signal. The structure offers the potential for obtaining highly specific signals for the assay of bioanalytical targets in a rapid and cost-effective manner. A wide variety of technologies have been employed in terms of transduction including those based on electrochemistry (viz. amperometry, potentiometry, conductimetry, impedimetry, and voltammetry), optical systems such as surface plasmon resonance and chemiluminescent-based biosensors, and mass-sensitive biosensors including the piezoelectric quartz crystal microbalance and surface acoustic wave sensors. The method of detection is selected based on a variety of factors. These factors include the type of analyte, sensitivity requirements, risk of matrix interference, and desired speed of detection. A wide plethora of applications have been introduced including monitoring and assays in environmental analysis, food safety, industrial processes, drug discovery, and the subject matter of this opinion piece, medical science.
The scientific literature has shown a growing interest in biosensors over many years. As illustrated in Figure 1, the earliest publication on biosensors dates back to 1979. In 2024 alone, 4,967 papers related to biosensors were published and as of mid-2025, 3,871 articles on biosensors have been released. The data in Figure 1 reveals an average annual growth rate in publications of 21.3%, suggesting a steady expansion in the field of biosensors. According to Web of Science (Web of Science Clarivate, 2025), there are 18 journals dedicated to biosensor research, with Biosensors and Bioelectronics having the highest impact factor of 10.61 as of 2021. Beyond journal articles, biosensors are widely discussed in books and book chapters. One notable recent publication is Biosensors: Fundamentals, Emerging Technologies, and Applications by Özkan et al. (2022) published by Taylor & Francis in 2022. This book provides an in-depth exploration of the biosensor market, covering fundamental principles and advancements in the field.
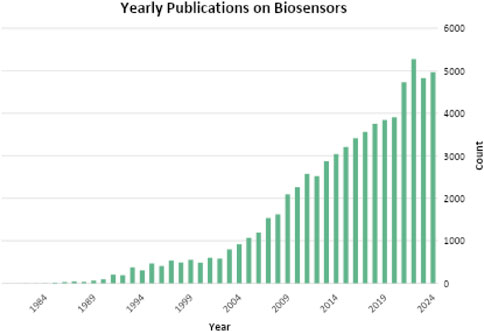
Figure 1. Yearly number of journal article publications on biosensors from 1979 to 2024, based on Web of Science dataset (Web of Science Clarivate, 2025).
With regard to its marketplace, the Biosensor Market Report dated April 2021 reveals the estimated market size of biosensors is 25.5 billion USD dollars with estimated growth of an additional 36.7 billion USD dollars by Biosensors Market Size et al. (2025). This data encompasses biosensor applications among point-of-care, environmental monitoring, food and beverage, and biodefense industries. The report suggests that there is some market reluctance in adopting new practices, more on this will be discussed in this paper. However, it is forecasted that wearable biosensor devices will continue to rise in popularity, promoting complete patient care and personalized medicine. As would be expected, developments in glucose detection would still appear to dominate the marketplace.
Applications of biosensor technology: point-of-care testing versus typical clinical biochemistry laboratories
In the medical sector, the biosensor device, in general terms, can be thought of being employed in two distinct arenas, that of point-of-care (POC) assays, and secondly clinical laboratory analysis. That said, some hospital facilities also conduct POC determinations. The former involves potential use in the home, hospital bedside, and doctor’s office. The legendary glucose assay, pregnancy, and COVID-19 tests are notable examples of successful commercial biosensors, enabling self-monitoring through their prompt response, ease-of-use, portability, low cost, small sample volume requirements, and disposable nature (Clark and Lyons, 1962; Andre et al., 2024; Flynn and Chang, 2024; Rusling and Forster, 2021; Justino et al., 2016; Zucolotto, 2020).
It is the potential application of biosensor devices in the clinical biochemistry operation that is the focus of this perspective. Here, we take a brief look at a typical laboratory which is based in a Province of Ontario-hospital, where it is considered that 75% of medical decisions are based on the results obtained. The laboratory is staffed as follows: 22 pathologists, 3 clinical biochemists, 1 mass spectrometry assay specialist, 1 molecular assay specialist, 4 microbiologists, 2 hematologists (one oversees transfusion medicine), 1 hemato-pathologist, 5 managers (one for each division plus quality), 70 medical laboratory technicians (MLAs for phlebotomy, etc.) and 140 medical laboratory technologists. This facility conducts approximately 5 million assays annually in the areas of biochemistry, transfusion medicine, hematology, microbiology, pathology, and molecular biology. Biochemistry incorporates both diagnostic and therapeutic assays in blood, urine, and other body fluids. Transfusion medicine deals with blood donation, hematology looks at cell blood disorders while microbiology examines the presence of bacteria, fungi, and viruses in biological fluid. Pathology involves histology and cytology, often for cancer determinations. Finally, molecular biology deals with DNA and RNA assays. The extensive variety of tests requested over recent years, as an example, are depicted in Figure 2. As expected, the bulk of these lies in the biochemistry area, which incredibly includes approximately 2000 different species, with thyroid stimulating hormone (TSH) being the “winner.”
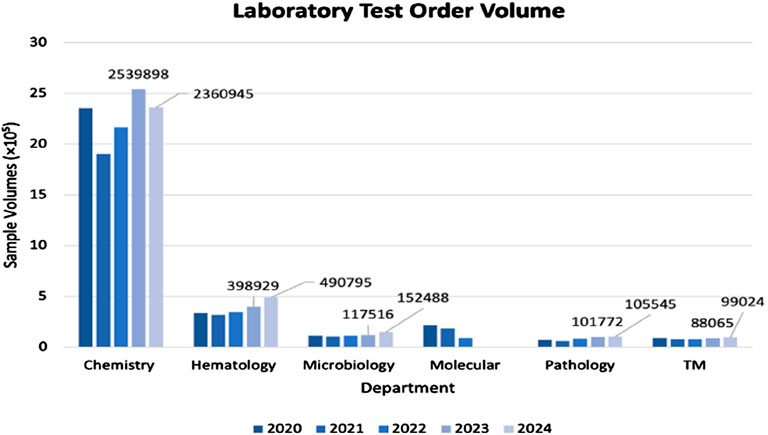
Figure 2. Sample volumes of laboratory tests ordered by a major hospital on an annual basis (TM–transfusion medicine).
Biosensor technology and the clinical biochemistry operation
As specified above, the typical clinical biochemistry laboratory is a dedicated space for analyzing a wide variety of biological samples involving highly trained personnel and specialized tools that are often automated or robotic (Thompson et al., 2013; Thurow, 2023; Brazaca and Sempionatto, 2024). Any new technology under consideration to improve and disrupt these existing methods must overcome the main challenges of validation, cost, and regulation (Figure 3). These considerable time-consuming challenges have clearly, in our opinion, been responsible for hindering the widespread adoption of biosensors in the typical hospital (or commercial private) clinical laboratory. We further consider these issues below.
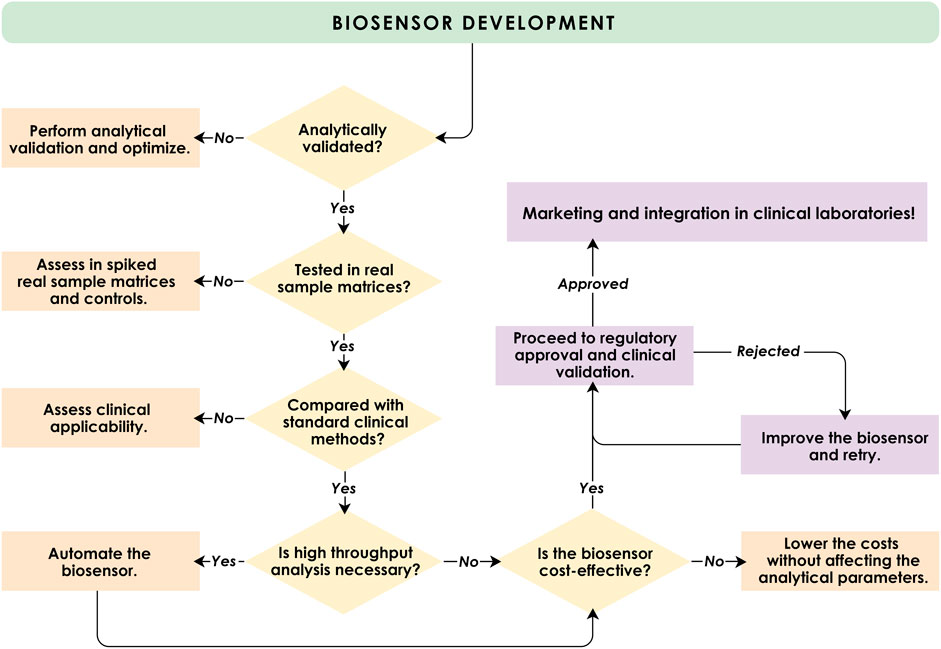
Figure 3. A suggested model for the process of transitioning a biosensor from research to clinical laboratories.
It is a reality of biosensor research, especially in academic laboratories, that there is a focus on sensitivity, selectivity, specificity, limits of detection and quantification, calibration curves, robustness, accuracy, precision, and stability (Justino et al., 2016; Romanholo and Sgobbi, 2024). However, “routine” use of biosensor technology to assay real clinical samples such as serum, saliva, and urine is far less common (Romanholo and Sgobbi, 2024). A major critical factor in ensuring the clinical relevance of a biosensor’s data is the minimization of interferences from non-specific adsorption (NSA) (Thompson et al., 2013; Pendley and Lindner, 2017) Avoidance of this phenomenon, often termed fouling by the engineering community, has not figured prominently at least as it pertains to operation on real clinical samples. A solution would clearly require tandem development of probe and anti-fouling surface chemistry.
An additional issue is the pragmatic question of whether introduced biosensor technology can replace entrenched, existing methodology from the cost per assay standpoint (Thompson et al., 2013; Romanholo and Sgobbi, 2024; Pitruzzello, 2024), rather than simply acting as a further analytical approach. In this regard, the biosensor must be capable of dealing with sample throughput, whether it means processing a high volume of samples daily or only a few per year. Since automation is common in clinical laboratories, substantial financial investment would be mandatory with respect to the incorporation of sensor signalling technology (Ebubekir et al., 2017). From a practical point of view, it may be advisable to introduce biosensors for detection and assay of less common or rare diseases, where routine analysis and automation are unnecessary and clinical diagnostic tools are currently limited or lacking. One relevant recent example of this approach would be the identification of microRNAs associated with early-stage diseases, which presents a promising opportunity for their clinical detection using biosensors (Belete et al., 2024; Mohseni et al., 2025; Frisk et al., 2023).
Assuming that a clinically acceptable biosensor device can be produced, there is the essential issue that the technology will require necessary regulatory approval from appropriate jurisdictions (Government of Canada, 2025; Mulla and Patel, 2025; Talreja et al., 2024). Regulatory requirements for clinical applications are far more stringent than those for commercial use, which may be a key factor contributing to the more rapid market adoption of point-of-care devices compared to biosensors designed for clinical laboratories. An interesting feature of this aspect is that the clinical community is regarded to be particularly conservative as it pertains to the introduction of new technologies.
Final comment
On a more optimistic note, the possibility of biosensors to offer rapid, automatable, real-time detection with high sensitivity and antifouling capability holds tremendous potential to play a transformative role in future clinical biochemistry laboratories. As they become ubiquitous across the healthcare industry, biosensors could disrupt traditional diagnostic and prognostic methods, bridging the gap between innovative technology and traditional centralized laboratory analysis. The growing adoption of commercial biosensors in the coming decades may drive greater interest into integrating biosensors in clinical laboratory operations. Further research advancements, coupled with collaborative efforts with regulatory agencies, can help establish biosensors as standard clinical tools. Biosensor technology will ultimately strengthen public health and healthcare systems, revolutionizing disease identification, monitoring, and treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KD: Writing – original draft, Investigation, Writing – review and editing, Formal Analysis, Conceptualization. LN: Conceptualization, Writing – original draft, Writing – review and editing, Formal Analysis. MT: Writing – original draft, Resources, Conceptualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andre, R. S., Sanfelice, R. C., da Costa, M. M., Mercante, L. A., Correa, D. S., and Pavinatto, A. (2024). “Commercial potential of biosensors,” in Biosensors in precision medicine: from fundamentals to future trends (Elsevier), 345–376. doi:10.1016/B978-0-443-15380-8.00012-6
Belete, M. A., Anley, D. T., Tsega, S. S., Moges, N., Anteneh, R. M., Zemene, M. A., et al. (2024). The potential of circulating MicroRNAs as novel diagnostic biomarkers of COVID-19: a systematic review and meta-analysis. BMC Infect. Dis. 24, 1011. doi:10.1186/s12879-024-09915-8
Biosensors Market Size, Share, Statistics (2025). Industry growth analysis report by type, product (wearable, non-wearable), technology, application (POC, home diagnostics, research lab, environmental monitoring, food & beverages, biodefense). Glob. Growth Driv. Industry Forecast 2030; SE 3097; Mark. Mark. 12.
Brazaca, L. C., and Sempionatto, J. R. (2024). “The application of biosensors in precision medicine,” in Biosensors in precision medicine: from fundamentals to future trends (Elsevier), 133–162. doi:10.1016/B978-0-443-15380-8.00006-0
Clark, L. C., and Lyons, C. (1962). Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. 102, 29–45. doi:10.1111/j.1749-6632.1962.tb13623.x
Ebubekir, B., Nurinnisa, O., and Nurcan, K. B. (2017). Automation in the clinical laboratory: integration of several analytical and intralaboratory Pre- and post-analytical systems. Turk. J. Biochem. 42 (1), 1–13. doi:10.1515/tjb-2016-0234
Flynn, C. D., and Chang, D. (2024). Artificial intelligence in point-of-care biosensing: challenges and opportunities. Diagnostics 14 (11), 1100. doi:10.3390/diagnostics14111100
Frisk, N. L. S., Sørensen, A. E., Pedersen, O. B. V., and Dalgaard, L. T. (2023). Circulating MicroRNAs for early diagnosis of ovarian cancer: a systematic review and meta-analysis. Biomol 13 (5), 871. doi:10.3390/biom13050871
Government of Canada (2025). Medical devices regulations, SOR/98-282. Justice Laws Website. Available online at: https://laws-lois.justice.gc.ca/eng/regulations/sor-98-282/FullText.html (Accessed July 18, 2025).
Justino, C. I. L., Duarte, A. C., and Rocha-Santos, T. A. P. (2016). Critical overview on the application of sensors and biosensors for clinical analysis. Trends Anal. Chem. 85, 36–60. doi:10.1016/j.trac.2016.04.004
Mohseni, M., Behzad, G., Farhadi, A., Behroozi, J., Mohseni, H., and Valipour, B. (2025). MicroRNA frontiers: illuminating early detection paths in multiple sclerosis. Mult. Scler. Relat. Disord. 95, 106237. doi:10.1016/j.msard.2024.106237
Mulla, S. J., and Patel, M. (2025). Global regulatory perspectives on sensor-enabled and biomaterial-integrated medical devices: a focus on India, Canada, and Australia. Biomed. Mater. Devices. doi:10.1007/s44174-025-00327-z
Özkan, S. A., Uslu, B., and Sezgintürk, M. K. (2022). Biosensors: fundamentals, emerging technologies, and applications. Biosens. Fundam. Emerg. Technol. Appl., 1–394. doi:10.1201/9781003189435
Pendley, B. D., and Lindner, E. (2017). Medical sensors for the diagnosis and management of disease: the physician perspective. ACS Sens. 2 (11), 1549–1552. doi:10.1021/acssensors.7b00642
Pitruzzello, G. (2024). Optical biosensors towards the Clinic. Nat. Photonics 18, 1126–1128. doi:10.1038/s41566-024-01559-z
Romanholo, P. V. V., and Sgobbi, L. F. (2024). “Validation of biosensors,” in Biosensors in precision medicine: from fundamentals to future trends (Elsevier), 105–131. doi:10.1016/B978-0-443-15380-8.00005-9
Rusling, J. F., and Forster, R. J. (2021). Biosensors designed for clinical applications. Biomedicines 9 (7), 702. doi:10.3390/biomedicines9070702
Talreja, R. K., Sable, H., Chaudhary, V., Kadian, S., Singh, M., Kumar, M., et al. (2024). Review—Challenges in lab-to-clinic translation of 5th/6th generation intelligent nanomaterial-enabled biosensors. ECS Sens. Plus 3, 041602. doi:10.1149/2754-2726/ad9f7e
Thompson, M., Sheikh, S., Blaszykowski, C., and Romaschin, A. (2013). “Biosensor technology and the clinical biochemistry laboratory-issue of signal interference from the biological matrix,” in Detection challenges in clinical diagnostics (Cambridge, UK: The Royal Society of Chemistry), 1–34. doi:10.1039/9781849737302-00001
Thurow, K. (2023). Strategies for automating analytical and bioanalytical laboratories. Anal. Bioanal. Chem. 415 (21), 5057–5066. doi:10.1007/s00216-023-04727-2
Web of Science Clarivate (2025). Web of science; clarivate. Available online at: https://www.webofscience.com (Accessed February 16, 2025).
Keywords: biosensors, clinical biochemistry, critical appraisal, public healh, non-specific adsorption
Citation: Davoudian K, Nemtsov L and Thompson M (2025) Challenges in the application of biosensor technology in the clinical biochemistry laboratory. Front. Sens. 6:1641221. doi: 10.3389/fsens.2025.1641221
Received: 04 June 2025; Accepted: 14 August 2025;
Published: 28 August 2025.
Edited by:
Raja Chinnappan, Alfaisal University, Saudi ArabiaReviewed by:
Mohana Marimuthu, Innovaspark, IndiaMuhammad Sheraz, Northwestern Polytechnical University, China
Copyright © 2025 Davoudian, Nemtsov and Thompson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Thompson, bS50aG9tcHNvbkB1dG9yb250by5jYQ==
 Katharina Davoudian
Katharina Davoudian Lidia Nemtsov
Lidia Nemtsov Michael Thompson
Michael Thompson