- 1Natural Resource Ecology Laboratory, Colorado State University, Fort Collins, CO, United States
- 2The Land Institute, Salina, KS, United States
Restoring soil organic matter (SOM) in arable land is considered one of the best natural solutions to sustain food production and mitigate climate change. With typically deep, robust root systems compared to annual grains, perennial systems are likely to promote soil organic carbon (C) sequestration while offering many ecosystem co-benefits. The intermediate wheatgrass domesticated for grain production as Kernza® (Thinopyrum intermedium) is the first perennial grain available to US growers. We quantified the formation of SOM over 2 years from the roots and shoots of Kernza grown alone and in an alfalfa (Medicago sativa) intercrop using continuously 13C- and 15N-labeled plant material. We compared SOM formation of the Kernza tissues under three contrasting agronomic environments: (1) unfertilized Kernza monoculture, (2) unfertilized Kernza biculture with nitrogen (N)-fixing alfalfa, and (3) fertilized (100 kg N ha−1 year−1) Kernza monoculture. We hypothesized that the management and plant tissues with higher N would enhance mineral associated organic matter (MAOM) formation by alleviating microbial N-limitation and leading to enhanced efficiency of microbial residue transformation. Furthermore, we hypothesized that root tissues would contribute to SOM formation primarily as occluded particulate organic matter (oPOM) due to their chemistry and interface with the soil matrix. We found that overall Kernza promoted new SOM formation with 14% of roots and 8% of shoot-derived C recovered in bulk soil after 27 months compared to 5% for alfalfa roots and shoots. There were no differences between the efficiency of MAOM formation of alfalfa vs. Kernza. The intercrop sustained similar C and N stocks to the fertilized treatment, although we found little evidence that N management was a major influence on SOM formation. Of the Kernza root tissue C incorporated into SOM, we found 3.5% in MAOM and 6% in oPOM, implying that 9.5% of root tissue C inputs may be stabilized in the soil. Legume intercrops can support Kernza cropping systems with minimal synthetic inputs, although in our study, they did not lead to enhanced SOM formation even with comparable levels of productivity.
1 Introduction
Restoring soil organic matter (SOM) in arable land is considered one of the best natural solutions to sustain food production (1), restore ecosystem function (2, 3), and mitigate climate change (2, 4, 5). Towards these goals, perennial grains hold many promises. With deep, robust root systems compared to annual grains, perennial systems are likely to promote soil organic carbon (C) accrual (6–10) and more efficient nutrient cycling (11). When perennial grains replace annuals, they result in increased soil C inputs through longer growing seasons and greater and deeper root growth while also reducing the frequency of disturbance (12, 13). The intermediate wheatgrass domesticated for grain production as Kernza® (Thinopyrum intermedium) is the first perennial grain available to US growers, and it has demonstrated many ecosystem co-benefits: Kernza reduces erosion and the need for weed management (14), has high water-use efficiency and known tendency to act as a C sink (15), and has been shown to have increased resilience and fewer synthetic inputs when intercropped with a legume (16). Previous studies have demonstrated the importance of Kernza roots in the formation of particulate organic matter (POM) (17), the fraction of SOM that is chiefly composed of structural, fragmented, and partially decomposed plant, and, in minor part, microbial materials (18). Since the POM fraction is more vulnerable to disturbance and decomposes on shorter timescales than mineral-associated organic matter (MAOM) (19–21) unless it is protected in aggregates (22–24), an unanswered question is how to regenerate both POM and MOAM in cropping systems, as these types of SOM perform different functions and form via different pathways (9, 25, 26).
Restoring these fractions of SOM has benefits to soil health in an agricultural context, as the continuous turnover and replenishment of free POM (fPOM) can stimulate nutrient cycling and microbial activity. The accumulation of aggregate-occluded POM (oPOM) can promote soil structure and water infiltration, crucial to sustain production, while the slower cycling of oPOM and MAOM fractions can lead to long-term SOC storage (27, 28).
In a perennial agricultural context, a fundamental management decision to achieve both increased SOM and grain production is controlling the inputs to the soil through crop(s) and nutrient selection, which will have implications for the soil input chemistry and overall productivity, drivers known to explain roughly half of the rate change in SOC (29). Intercropping perennial grains with species with contrasting chemistry such as legumes could achieve a system with chemically diverse litter, which could promote both POM and MOAM formation (21). Legume intercrops could also reduce the need for synthetic fertilizers due to their nitrogen (N) contributions from biological N fixation and the potential to adjust the rate that N becomes available during the growing season to the rate of decomposition (30, 31), minimizing N losses through volatilization and denitrification (32, 33) and improving nutrient cycling efficiency and system sustainability (34–36).
Litter chemistry influences SOM dynamics largely through the proportion of soluble compounds, nitrogen, and the acid unhydrolyzable fraction (AUR) (Talbot and Treseder, 2012), which is often used to describe complex, aromatic compounds such as lignin, cutins, tannin, and suberin that require specialized oxidation enzymes to decompose (37, 38). Litter tissues with a high proportion of AUR and low N such as is commonly observed in thick roots compared to aboveground plant tissues have long been thought to limit litter mass loss (39, 40). They can also lead to greater CO2 losses during decomposition, although, due to low microbial-use efficiency (41) [as low as 1% (42)–8% (43)] and contribute little to MAOM (44, 45). Litter with higher AUR/N would primarily contribute to particulate organic matter (POM) over time (25, 46). In contrast, litter with substrates easily metabolized by soil microbes; that is, compounds that are water soluble and/or high in N, might be most efficiently stabilized in soil as MAOM (47). This could occur either through direct association of the water-soluble compounds to fine minerals (48) via the ex vivo pathway (49) or through microbial assimilation and subsequent contribution of microbial necromass and extracellular metabolites to MAOM via the in vivo pathway (47, 49, 50).
While optimizing mixtures of litter chemistry for SOM formation could theoretically be achieved with a grass–legume intercrop, the net effect of incorporating a legume on SOM is unclear. Intercropping with a legume could reduce plant C inputs due to them having a reduced biomass compared to the grass species (51, 52) and lead to lower SOM values compared to monoculture grass crops (53, 54). To the extent legumes reduce plant C inputs, they may counteract potential benefits derived from increased C-use efficiency from their more readily digestible tissues (29, 41). Intercrops can also have variable effects on crop yield, often showing a net-competitive effect initially and a facilitative relationship once established (16), thus influencing plant C inputs. What is the net effect of intercrops on potential SOM formation, given the changes to litter chemistry, N availability, productivity, and soil C input? We lack mechanistic understanding and quantification of SOM formation from litter decomposition of perennial grain and perennial grain intercrops and how these systems might differ in their potential to form SOM based on contrasting C inputs and N management.
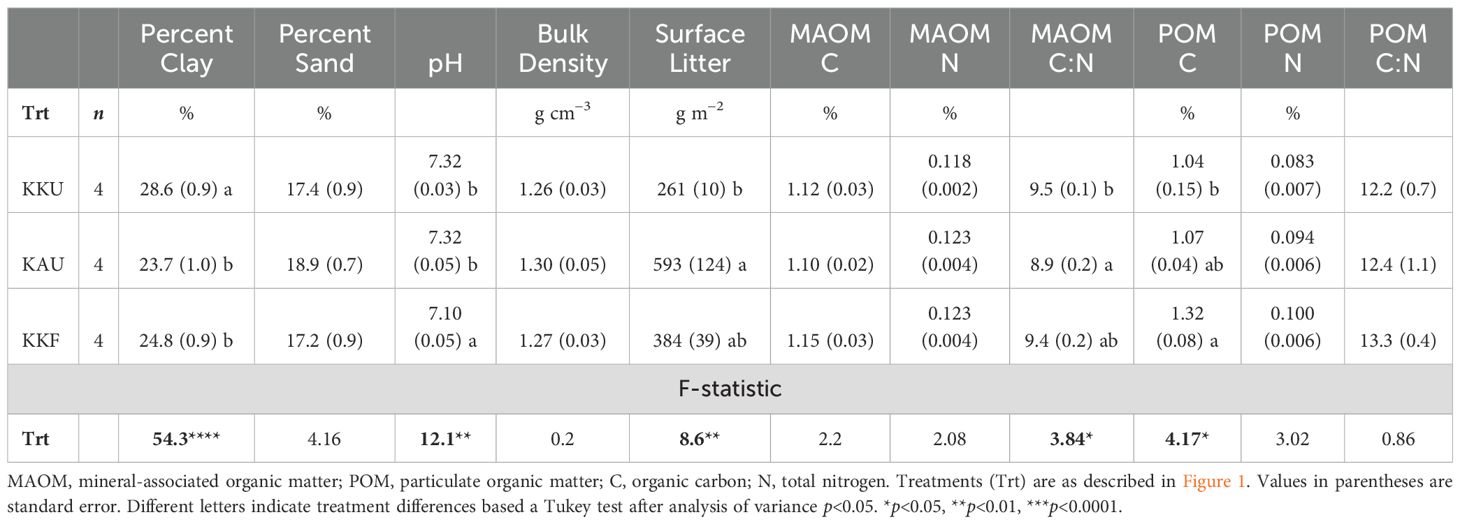
We thus set out to understand and quantify how litter from Kernza and legume intercrop alfalfa (Medicago sativa) form SOM as well as evaluate their potential for longer-term C storage under contrasting N management. To this end, we incubated continuously 13C- and 15N-labeled root and shoot residues from both species within field plots where Kernza was grown either with (1) no fertilization (KKU), (2) intercropped with alfalfa (KAU), or (3) with urea (100 kg N ha−1 year−1) (KKF) (Figure 1). During the 2-year incubation, we traced residues as they decomposed, were incorporated into microbial biomass, or formed chemically and functionally distinct pools of SOM, such as fPOM, oPOM, and MAOM (55). Through this experiment, we aimed to answer the questions: how does Kernza form SOM, and how does that compare to legume intercrop alfalfa?
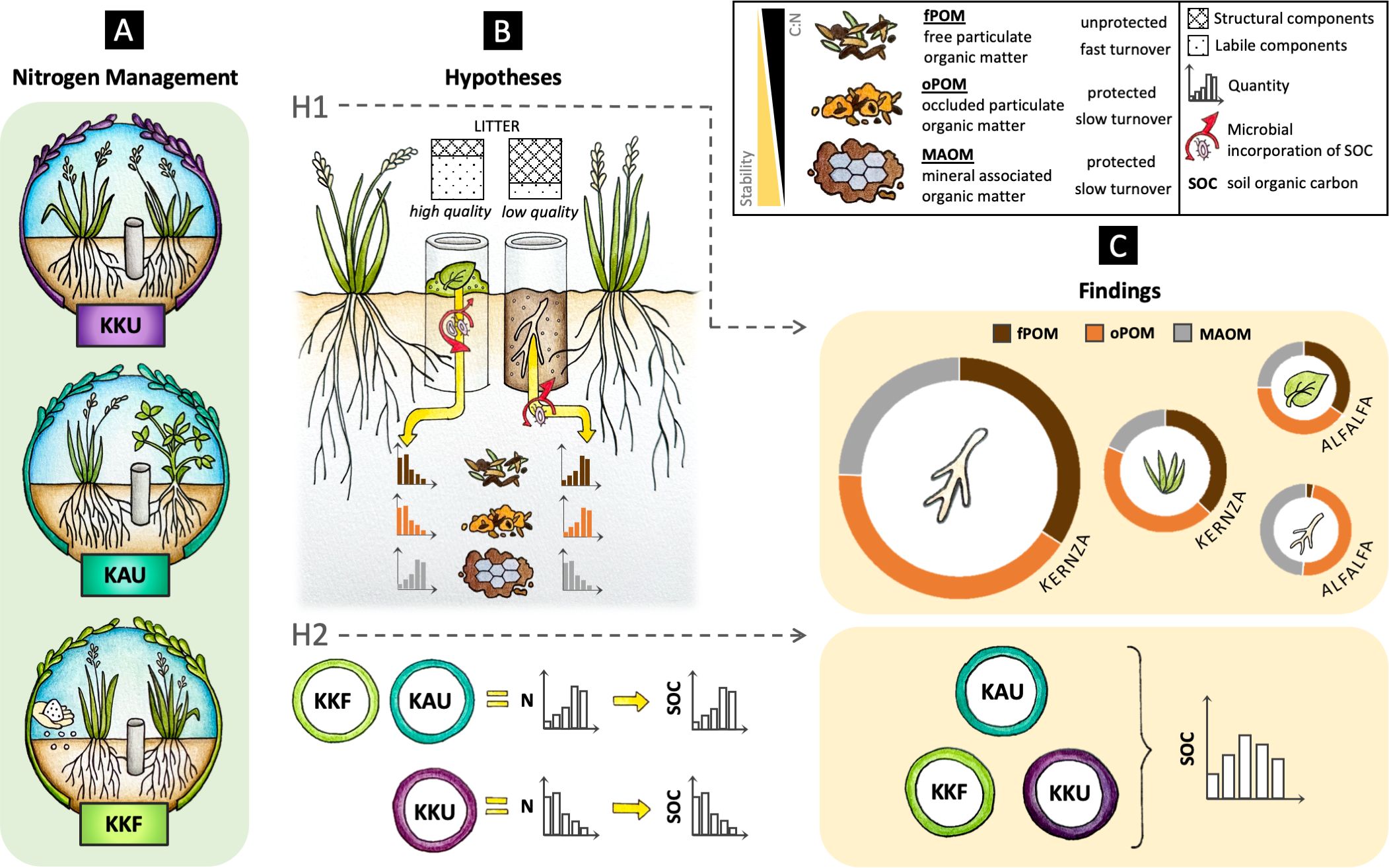
Figure 1. (A) Depiction of the nitrogen (N) management where the isotopically labeled litter incubation occurred. There were four replicated blocks of each Kernza monoculture unfertilized (KKU; purple), Kernza-alfalfa intercrop unfertilized (KAU; turquoise), and Kernza monoculture fertilized (KKF; light green). (B) Study hypotheses: Hypothesis 1 (H1) that the litter with greater soluble content and N would preferentially contribute to the formation of mineral-associated organic matter (MAOM; <53 μm) while structural components would primarily contribute to particulate organic matter (POM; (>53 μm) fractions that were occluded in aggregates (oPOM) or free (fPOM). Hypothesis 2 (H2) that the treatments with greater N would lead to enhanced soil organic carbon (SOC) through greater productivity (soil C inputs) and higher efficiency of C stabilization. (C) Findings of the proportion each labeled litter contributed to the soil fractions after 27 months of MAOM (gray), fPOM (brown), and oPOM (orange) where the size of the ring represents the overall amount of litter-incorporation and the area of the ring represents the proportion of SOC formed in each fraction.
We hypothesized that plant tissues would preferentially contribute to POM when composed of more structural components (i.e., higher AUR such as Kernza and alfalfa roots) and to MAOM when they had higher N and soluble content (i.e., higher HWE C and N) (Figure 1), due to differences in microbial C use efficiency (47) and formation pathways (25). Our experimental design also enabled us to address the question of how N management influenced SOM stocks and productivity. We hypothesized that treatments with greater N addition would have higher SOM stocks because they would support greater plant productivity and thus soil input. We further hypothesized that adding N through the steady decomposition of legume residues (vs. annual application of urea) may further enhance MAOM formation from reduced microbial N limitation (Figure 1) leading to greater microbial C-use efficiency (56) and thus SOM accrual. Overall, with this study, we aim to clarify the mechanisms of SOM formation under perennial grain crops and evaluate the potential for legume intercrop to replace synthetic N inputs while sustaining productivity and SOM.
2 Materials and methods
2.1 Field site description and management
This incubation took place in experimental research plots established in 2015 and maintained by The Land Institute near Salina, Kansas, USA (38.770284 N, −97.591795 W); this land was originally home to at least five indigenous nations including Kaw, Osage, Comanche, and Pawnee Nations (https://native-land.ca/). The site is located 370 m above sea level with annual precipitation averaging 737 mm (1-10th as snow). Rainfall is concentrated during the spring and fall with common summer droughts. The mean average temperature is 13.2°C with daily average lows of −6.6°C in January and average highs of 35°C in July. The experimental plots were situated in a transition zone of coarse-silty, mixed, mesic Fluventic Haplustolls in the Cozad series and fine-silty, mixed, mesic Cumulic Haplustolls in the Hord silt loam series (57).
Prior to use for research, the site was cultivated under annual wheat production until 1990 (58) and a series of cover crops, Kernza (Thynopyrum intermedium), and biennial sorghum (Sorghum bicolor) between 2002 and 2015. In 2015, a randomized block design consisting of four blocks with 16 treatment plots was established to compare Kernza–legume intercrop combinations, fertilizer management, and crop spacing of Kernza (The Land Institute, cycle 5). Each plot measured 3.6 × 3.6 m2 and was planted with a drill depth of 1.2–2.5 cm and a seeding rate of ~11–13 kg ha−1. Plots were weeded by hoe twice in the establishment year and spot-weeded occasionally as needed to remove individual weeds subsequently.
The treatments utilized in this study consisted of (1) unfertilized Kernza monoculture (KKU), (2) unfertilized Kernza biculture with alfalfa (Medicago sativa, StarSeed A100 variety; KAU), and (3) fertilized Kernza monoculture (KKF; 100 kg N ha−1 year−1 as urea) (Figure 1A). The row spacing in each plot was 30 cm with alfalfa rows replacing alternating rows of Kernza in KAU. In the KKF treatment, fertilizer was broadcast by hand each spring between April 15 and May 15. Alfalfa rows in plots were mowed two to four times each summer with the residue left on the soil surface after each cutting. There was a gopher infestation that targeted the KAU plots the summer of 2019; by 2020, there was little detectable alfalfa remaining in most plots. Kernza harvest took place in the third week of July using a rice binder.
The amount of surface litter varied by management but did not mirror the productivity, as the aboveground material for Kernza was removed each harvest and the alfalfa left in the field as a green manure. The biculture plots had the greatest surface litter at the time of sampling in May 2019 (Table 1).
2.2 Background soil analyses
At the time of setting up our experiment in May 2019 as described below, we collected samples from each plot for native litter and soil background characterization. Three points within each plot were randomly chosen for litter and soil core sampling. We quantified aboveground litter biomass by collecting and weighing aboveground litter within a 0.04-m2 square. We estimated bulk density (0–10 cm) using cores collected with a slide-hammer corer (6.4 cm diameter) by dividing fresh sample mass minus soil water content by the core volume; coarse fragments >2 mm were negligible. Average bulk density in the field was 1.28 g cm3. On the cores collected for bulk density, we measured soil pH using a 1:5 soil to water ratio and soil texture based on the hydrometer method (59). We tested for inorganic carbon presence at the 0–10 cm depth using an acid-fizz test with 1M hydrochloric acid and did not find any.
Additionally, we used these background soil samples to quantify and characterize the soil organic C (SOC) and soil N stocks for the different management in this study. We conducted physical fractionation of the bulk soil into two size fractions: POM (>53 μm) and MAOM (<53 μm) after dispersing aggregates by shaking ~8 g of 2-mm-sieved soil samples for 18 h with glass beads in a 0.5% sodium hexametaphosphate solution (60). Oven-dried (60°C, 72 h) fractions and bulk soil were ground to a powder using mortar and pestle and analyzed for total C and N by dry combustion using a LECO Tru-Spec CN gas analyzer (LECO Corp., St. Joseph, MI, USA).
Surprisingly, we found a small soil texture difference across blocks and treatments, as there was a 3% increase in clay and decrease in sand content from block one to four (p=0.01, F=8.6 for sand), such that the plots in blocks 3 and 4 were silty clay loam soils while all others in the treatment were silt loam. The KKU management tended to have ~4% more clay than the other managements (p<0.001, t>8; Table 1). The KKF treatment had a lower pH than the unfertilized treatments (Table 1; p=0.01, t=2).
2.3 Aboveground net primary productivity and nitrogen budget
We calculated aboveground net primary productivity (ANPP) following Crews et al. (16). Briefly, we calculated ANPP based on the dry weight biomass divided by the row length for each plot based on two harvested rows of Kernza (~2 m2) and five rows (e.g., all rows) of alfalfa (KAU only; 5.4 m2) per plot. Biomass was weighed using a field scale with a subsample bagged, dried at 60˚C for 48 h, and re-weighed to determine moisture content. Measurements were taken for 3 years (2016–2018) prior to the start of our experiment and 1 year during the experiment (2020). Notably, 2018 was a severe drought for the region (National Integrated Drought Information System (US) drought.gov).
We estimated the N balance for each year with ANPP data and the N inputs as fertilizer or alfalfa N-fixation minus the N exported by ANPP harvest. Aboveground N-fixation (Nfix) was estimated based on a linear regression equation (R2 = 0.91, n=120 farms in Europe) (61) for alfalfa based on the dry matter (DM) yield (kg ha−1 year−1):
To estimate the proportion of N from fixation in alfalfa roots, we used a root factor of 1.61 (16) to estimate belowground biomass and estimation that N-fixed in roots is 80% that of shoots (62).
We also calculated the relative yields (RY) to compare the ANPP of the intercrop and monocrops (16, 63) as follows:
RY values >1 indicate that productivity of the intercrop is greater than the monocrop in an equivalent area, implying facilitation, while RY<1 indicates lower productivity in the intercrop compared to monocrop and suggests competition (63).
2.4 Production of 13C, 15N continuously labeled litter
Kernza (The Land Institute, breeding cycle 5) and inoculated alfalfa (Millborn Seed Co) seedlings were grown in a continuous dual isotope labeling chamber, described by Soong et al. (64). Plants were grown in 12-L pots with a growing medium of 3:4 ratio of sand and ceramic clay, inoculated with soil from the Kernza-only plots. The air-tight chamber continuously received 13C enriched CO2 to achieve a concentration of 360–400 ppm and a target label of 4 atom% during the active photosynthetic period. Plants were watered with 15N-labeled Hoagland’s solution to achieve a target 15N label of 6 atom%, with increasing fertilizer quantities as plant biomass increased. Plants were grown for 3.5 months prior to harvest; alfalfa plants had begun to flower, and some Kernza plants had initiated grain production at that time. Upon removal from the labeling chamber, aboveground biomass was clipped at the surface of the growing medium and air-dried. Roots were shaken to remove bulk sand and clay and then rinsed on 2-mm sieves until free of the growing medium and air-dried. Air-dried plant material was composited by species and above versus below ground; it was well mixed and cut to 2 cm prior to being weighed into uniform aliquots for field application. A subsample of each plant species and above- and below-ground tissue was finely ground on a Wiley mill equipped with a 0.75-mm mesh screen and oven-dried at 40°C for chemical, elemental, and isotopic analysis as described below.
2.5 Litter chemistry analysis
To determine the representativeness of our litter, we compared the labeled litter material to the Kernza and alfalfa plants growing in the experimental plots. We analyzed litter chemistry for the field and chamber grown plant material with the following measures: (1) hot water extractable (HWE) C and N and (2) estimated proportions of acid hydrolysable (AHR, e.g., celluloses) and acid unhydrolyzable (AUR, e.g., lignins, tannins, cutins, and suberins) residues. At the time of the background sampling (May 2019), we collected representative tissue samples from five separate plants in each plot (roots to a depth of 10 cm). Additionally, we analyzed three lab replicate samples from the labeled litter. For the quantification of HWE, we digested 0.3 g of oven-dried (105°C) material for 3 h (65) as modified by Soong et al. (66) and measured C and N on a Shimadzu TOC-L/TNM-L Analyzer (Shimadzu Corporation, Kyoto, Japan). For the fiber analyses, we used the acid detergent fiber (ADF) method (67). The ADF digestion consisted of boiling 0.3 g of oven-dried (105°C) material for an hour in detergent solution to remove hemicellulose and non-structural carbohydrates and lipids (“weak acid-soluble”). We then removed the AHR by digesting the remaining material in 73% sulfuric acid. The remaining residue was considered the AUR after ash correction (68).
The isotopically labeled litter that we grew in the growth chamber differed from the plants grown in the field, having higher C and HWE N in roots and N concentrations in roots and shoots (Table 2) and ~17% more weak-acid soluble (p<0.001, t>6.6) than the field-grown plants. For the shoot tissues, labeled litter had ~25 mg N g−1 tissue lower HWE N than the field grown plants (p<0.001, t>8.0). The C:N of the labeled Kernza litter was 32% lower for shoots and 54% lower for roots than the plants in the field (p<0.001, t>4.2); labeled alfalfa litter also had a lower C:N, although these differences were less robust (40% shoots, p=0.07; 17% roots, p=0.9). The labeled Kernza roots contained ~10% less AHR than the corresponding roots in the KKF and KKU treatments (p<0.001, t>5.2). The AUR/N and LCI tended to be lower for the labeled litter overall, but it was only significantly different than the corresponding field-grown plant tissues for Kernza roots for AUR/N (p=0.04, t=3.8) and both Kernza and alfalfa for LCI (p<0.04, t>3.8).
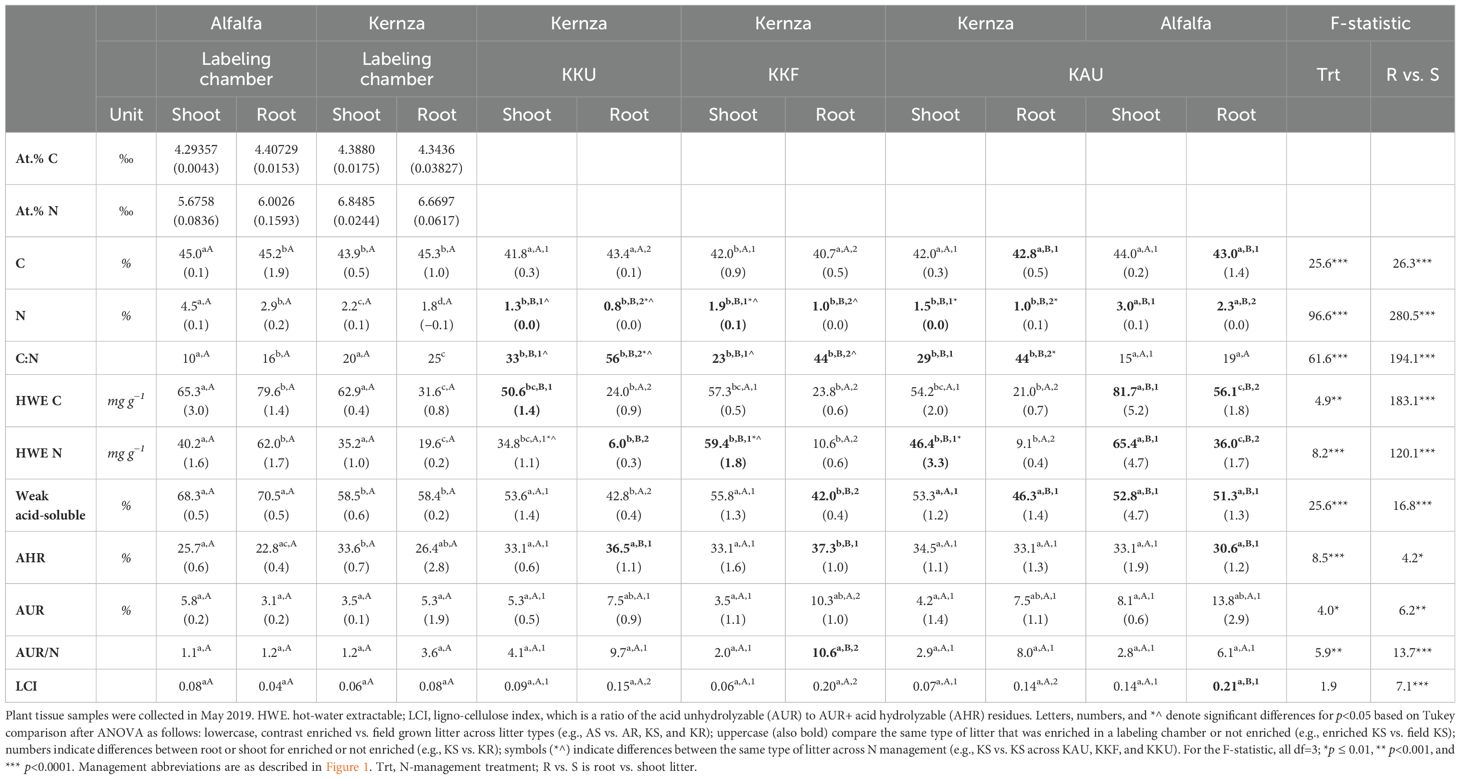
Table 2. Average litter chemistry values ( ± SE) for greenhouse-grown labeled litter (n=3) and plants from each management of the experimental plots (n=4) in Salina, KS.
2.6 Litter incubation experimental design
To quantify SOM formation from contrasting plant tissues, we compared the 13C and 15N concentrations (i.e., atom%) in SOM fractions of soil collars with and without added labeled litter to estimate the proportion of litter-derived (LD) C and N in SOM (46, 55, 69). The experimental block design consisted of: three managements (KKU, KAU, and KKF), three harvest times (3, 12, and 27 months after initial placement), two (roots and shoots from Kernza for KKU and KKF) or four (with also root and shoot from alfalfa for KAU), and two no-litter controls for either roots [“disturbed” (DC) to mimicking root mixing in the soil) or shoots (“undisturbed” (UC)], all in four replicates. No-litter controls were established to obtain natural abundance isotope end members for our mixing models as described below.
For the incubation, polyvinyl chloride (PVC) collars (15 cm height, 10 cm diameter) with 0.65 cm diameter holes drilled into the sides to allow root ingrowth were pounded into the ground to a depth of 10 cm. Any plants growing within the collar were clipped at the soil surface and placed within the collar. The labeled litter was added to the collars at a rate of 494 g m−2 (~229 g C m−2) for both Kernza and alfalfa roots and shoots (~4 g collar−1 or ~1.75 g C collar−1). This rate was a compromise between the measured surface litter, the estimated aboveground net primary production of similar 3-year-old stands of Kernza (470 ± 21 g m−2) and alfalfa (180 ± 19 g m−2), the amount of available labeled root and shoot material for the two crops, and the desire to minimize the proportion of soil C stock the labeled litter addition represented (15% ± 2% SD by mass). This application rate is comparable to other studies using this approach [~227 g C m−2 (46) and ~302 g C m−2 (69)]. Root litter was incorporated with the 0–10-cm soil depth by scooping soil within the collar and mixing it within a plastic bag, then returning the soil to the collar and compacting it to return to the original soil volume (see drawing Figure 1B). Disturbed control collars to pair with the root litter underwent the same procedure but without receiving litter addition. Shoot litter was applied to the soil surface; undisturbed control collars to pair with the shoot litter were clipped of any weeds at the soil surface but otherwise had nothing applied to them. Each collar was covered with a nylon, mesh screen with 2-cm square openings to contain the added litter; these screens were subsequently replaced (August 2020) with screens with a 1-cm square openings as the original mesh had the unfortunate consequence of trapping snakes.
2.6.1 Field collection of soil collars
Soil collars were first installed in May 2019 at a time when the site experienced record rainfall. Collars were destructively collected in August 2019 (3 months), May 2020 (12 months), and August 2021 (27 months). At each harvest, the live plants in the collars remaining in the field for later sampling points were clipped and the aboveground material placed inside the collar. Harvested collars were pulled from the ground intact, wrapped in foil, placed in plastic bags, and refrigerated at 4°C until processed. The excavated hole was measured at 5 points and averaged to obtain the average sample depth for bulk density determination.
Gophers present in half of the KAU plots buried three collars, resulting in deeper soil being deposited on top of the collars. The deposited soil could be easily distinguished by its color and reduced density and was collected and processed separately, but data are not included in the analysis. While the gophers did eliminate much of the alfalfa in the intercrop, alfalfa residue would have continued to decompose and contribute to soil N for the duration of the study.
2.7 Processing and analysis of soil
Each intact sample was weighed to obtain the total mass, minus the mass of the collar. Surface litter >2 mm and live plants clipped at the soil surface were carefully removed prior to processing the soil samples. Collars from undisturbed samples were split in half by depth (0–5 and 5–10 cm) to ensure that isotopic label could be traced in samples with surface litter application.
Soils were sieved to 8 mm, and a sub-sample for each was analyzed for gravimetric water content by mass loss after drying for 48 h at 105°C. A soil sub-sample was immediately sieved to 2 mm and frozen at −80°C for microbial biomass (see Supplementary Methods). The remainder of the sample was air-dried and then passed through a 2-mm sieve before further analysis. All plant materials including live plants, surface litter, and below-ground (root) litter were dried at 40°C for 48 h, weighed, and ground on a Wiley Mill with a mesh size of 0.75 mm for further analyses.
We estimated soil bulk density (BD) of each soil core as described above for the field samples and used an equivalent soil mass approach to calculate soil C and N stocks (70). The average soil mass sampled in the collars (0–10 cm) was 126.5 kg soil m−2 ± SD 1.2. Soil C and N stocks were calculated by normalizing all samples to the mean sample mass minus one standard deviation (114.0 kg m−2) to compare equivalent soil mass across soil collars over time.
2.8 Soil organic matter density and size fractionation
We used a density and size fractionation to quantify the formation of newly formed SOM into three chemically and physically distinct pools. The fractionation scheme that we followed was as described by Haddix et al. (55); however, while we isolated the dissolved organic matter, we elected to not analyze it, as it only made a negligible contribution to the total SOM. The measured fractions consisted of free light POM (fPOM), sand sized and occluded POM (oPOM), and silt and clay-sized, mineral-associated organic matter (MAOM). The sample was gently shaken in a solution of sodium polytungstate (SPT) at a density of 1.85 g cm−3, placed in a vacuum chamber for 10 min to remove air trapped within soil aggregates, and centrifuged at 1,069 gravitation for 30 min. We then aspirated off the fPOM and rinsed the remaining soil sample four times with DI water to remove the remaining SPT. We separated the oPOM from MAOM by wet-sieving the heavy fraction on a 53-μm sieve (oPOM >53 μm, MAOM <53 μm) after dispersing aggregates by shaking the samples for 18 h with glass beads in a 0.5% sodium hexametaphosphate solution. It is noteworthy that the fraction we here define as oPOM may also contain small amounts of organic matter associated with free sand particles (71). The fractions were finely ground by hand on a mortar and pestle for elemental and isotopic analysis measured on a Costech elemental combustion system coupled to a Thermo Scientific Delta V Advantage isotope ratio mass spectrometer for %C, %N, δ13C, and δ15N. Mass recovery after fractionation was on average 99% ± 1.5% SD. After fractionation, we achieved an average recovery (n=243) of 89% ± 11% SD for C and of 84% ± 6% SD for N. δ13C and δ15N values were converted to atom% for further calculation, following equation in Cotrufo and Pressler (72).
2.8.1 Calculation of SOM formation from litter decomposition
We quantified the litter-derived C (LDC) and N (LDN) in litter residue, bulk soil, and SOM fraction as described by Cotrufo et al. (25) and Soong and Cotrufo (46). We first calculated the relative contribution of labeled litter C (and N) to litter residue, bulk soil, and SOM fractions (FL) using the mixing model in Equation 3 (73).
FL is the fraction of labeled LDC for each replicate, atom‰13Cm is the atom percent 13C in the fraction for each replicate, atom‰13Ct0 is the average 13C in the corresponding control fraction, and atom‰ 13CL is the average percent 13C of the C in the initial labeled litter. The same equation was applied for N using the respective atom% 15N values.
Then, we calculated the amount of LDC (or N) in bulk soil by multiplying FL by the amount of C (or N) in the respective pool (i.e., litter residue, bulk soil, and SOM fraction). As done in previous studies (25, 46), to avoid the variability in C and N concentrations among replicate samples affecting the variability of the calculated LDC and N, we averaged the percent C and N for control soil and litter samples by fraction (or litter type) for each treatment. For the control soil samples, we additionally averaged the percent C and N across time. Furthermore, we calculated the SOM fractions C and N formation efficiency at each sampling point as the amount of LDC (or N) in the fraction divided by the amount of labeled litter C (or N) added minus the labeled litter that remained undecomposed (25). This value represents the proportion of SOM formed from the decomposition of the labeled litter.
2.9 Statistical analyses
We assessed the normality of the data residuals by Shapiro-Wilk test and visual assessment on QQplots. To determine the extent that management and litter treatment affected the litter mass loss and formation of LDC and N in bulk SOM, fPOM, oPOM, and MAOM, we ran the linear-regression analysis of variance (ANOVA) using R (74) version 4.4.2. We also tested for differences by management, litter type, plant species, and ANPP. Negative values (n=17/618) for the calculated proportion of LDC and N were converted to zero prior to analyses.
To account for sampling the same plots over time, we included a unique variable that combined the block and management in each analysis. Time, litter type, treatment, and soil fraction were treated as fixed variables, as was the treatment-block variable in cases where the mixed-effects model was singular. For each test of significance of litter type, we included an interaction between litter type, time, and, where applicable, soil fraction. We used Tukey-adjusted pairwise comparisons to evaluate the differences in management and litter treatment over time and by litter fraction using the dplyr (75), car (76), emmeans (77), and lmer (78) packages; data were processed and figures were made with several packages including tidyr (79), openxlsx (80), xplorerr (81), ggplot2 (82), and superb (83).
3 Results
3.1 Litter chemistry
The litter chemistry of the plants growing in the field under contrasting management were similar in the proportion of structural and metabolic components, %C, and HWE C (Table 2). Kernza tissues had higher N concentrations in the fertilized than the unfertilized plots (p<0.001, t>4.1); the KKF and KAU Kernza roots both contained more N than the KKU (p=0.009, t=4.0 KKF; p=0.01, t=4.0 KAU). Species differences between Kernza and alfalfa grown in the field were consistently higher C (2%) and N (1.5%) and HWE C and N in alfalfa compared to that in Kernza (p<0.0001, t>4.7). Alfalfa roots additionally had 9% more weak-acid soluble components than Kernza roots in the KKF and KKU treatments (p<0.01, t=4.2). While roots tended to have an AUR/N ~7 higher than shoots, the same tissue type across species were similar. The lignocellulose index (LCI), calculated as the ratio of AUR to AUR+AHR (84), was only greater for Kernza roots than shoots (p<0.04, t=3.8). Alfalfa and Kernza shoots contained similar proportions of weak-acid soluble components (p=0.8), although these more digestible components were ~9% higher in alfalfa roots than those of Kernza (p<0.01, t>4.3).
3.2 Soil carbon and yields across the contrasting Kernza N management
The soils in this experimental site had a large proportion of MAOM (74%–91%; median, 84% ± 0.2 SE) and very little POM (0.1%–1.9%; median, 0.4% ± 0.02 SE; Supplementary Figure S1). The soil in fertilized management contrasted with the unfertilized in a few ways prior to the start of the incubation experiment. Compared to the unfertilized treatments, soil in KKF had soil C stocks that were 1.1 and 1.4 Mg ha−1 greater than KAU and KKU, respectively (Supplementary Figure S1, Supplementary Table S1). The fertilized plots also had a tendency for higher C:N in the POM and MAOM fractions; KAU had similar N stocks to KKF (Supplementary Figure S1, Supplementary Table S1) but lower mean C:N in the SOM fractions (Table 1).
The aboveground net primary production (ANPP) varied significantly from year to year (Figure 2, Supplementary Table S2), with a significant decline (p<0.03, t> 2.91) in Kernza production each year measured across managements except for 2017 and 2020 (p>0.1, t>2.25). The KKF management had higher yields than both KAU and KKU in 2020 (p<0.02, t=2.81). KAU had the highest overall ANPP in 2018 (Figure 2, Supplementary Table S2; p ≤ 0.0001, t≥5.0).
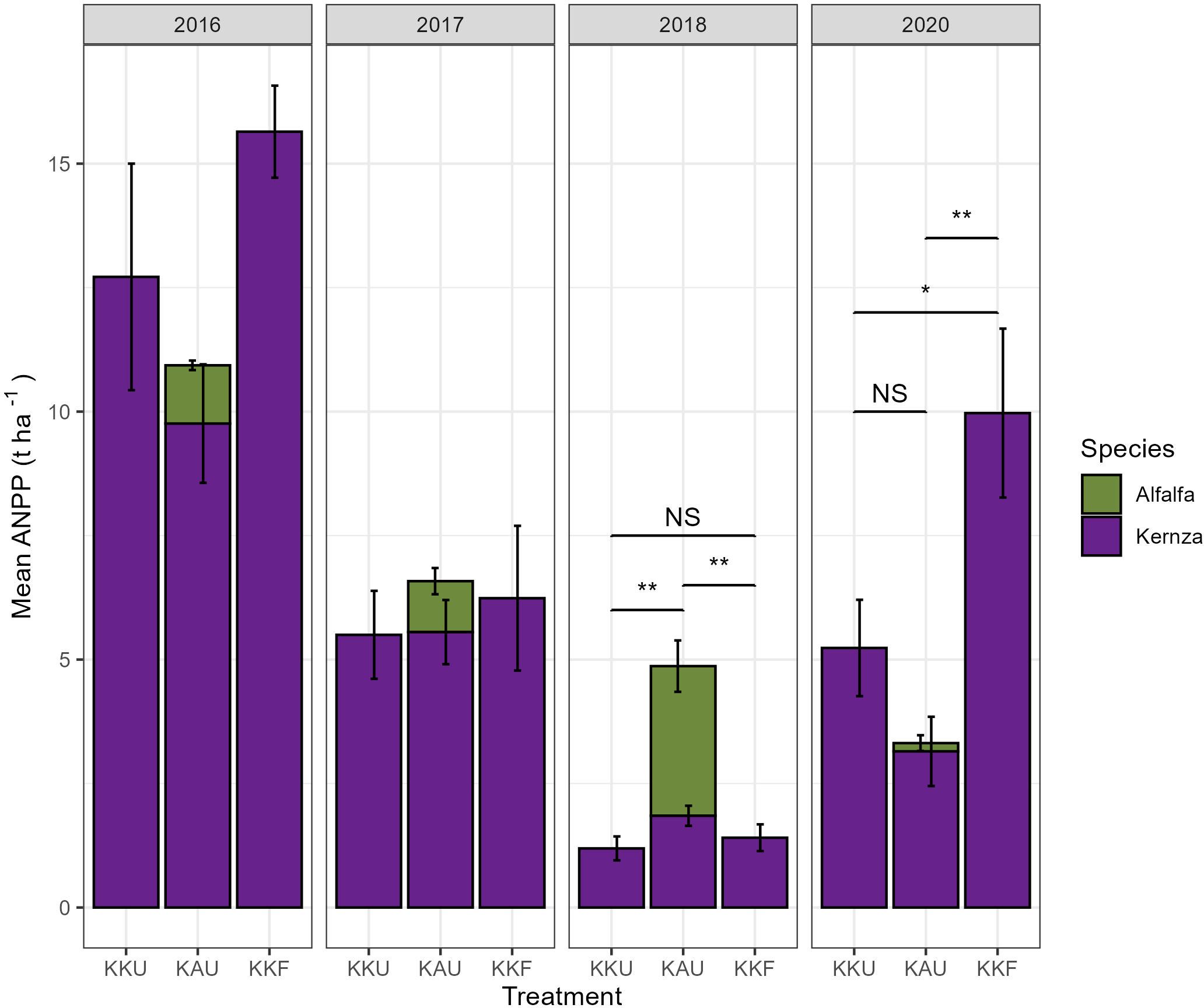
Figure 2. Annual net primary productivity (ANPP) of Kernza (purple) and alfalfa (green) by management (described in Figure 1) for 2016, 2017, 2018, and 2020. No data were collected in 2019. Kernza ANPP (grain and aboveground tissues) was measured at harvest each year for Kernza and at each mowing event (1–3× growing season) for alfalfa; whiskers represent standard error. Brackets with * indicate significant differences based on a Tukey comparison of means following ANOVA (Supplementary Table S2) where *p<0.05, **p<0.001, and NS means “not significant.” Panels without brackets indicate no significant differences between treatment.
Total (root+shoot) estimated fixation-derived N from the alfalfa intercrop (KAU) ranged from 39 (2020) to 129 (2018) kg N ha−1 year−1 (Equation 1). Considering the N balance (Supplementary Figure S2), we estimated that there were net N losses from each management and year, with losses averaging 39 kg N ha ha−1 year−1 ± 22 SE, except for 2018. The N supplied, in KAU from alfalfa N fixation and in KKF by fertilizer application, fell short of the Kernza ANPP export by 7–73 kg N ha−1 year−1and 18–122 kg N ha−1 year−1 (Supplementary Figure S2) for KAU and KKF, respectively. In 2018 (the drought year), both KAU and KKF managements had positive estimated N balances of ~100 and ~73 kg N ha−1, respectively. The relative yield (RY) ratios (16) (Equation 2) for the intercrop were >1 in 2018 compared to the KKF and in both 2017 and 2018 compared to KKU (Supplementary Table S3).
3.3 Litter decomposition and bulk soil organic matter formation
All labeled litter achieved almost full mass loss by the end of the incubation as 0.01%-5% of the initial litter C remained after 27 months of incubation (Figure 3). Below, we detail the results of SOM formation and litter decomposition considering N management treatments, type of litter (root vs. shoot), and plant species.
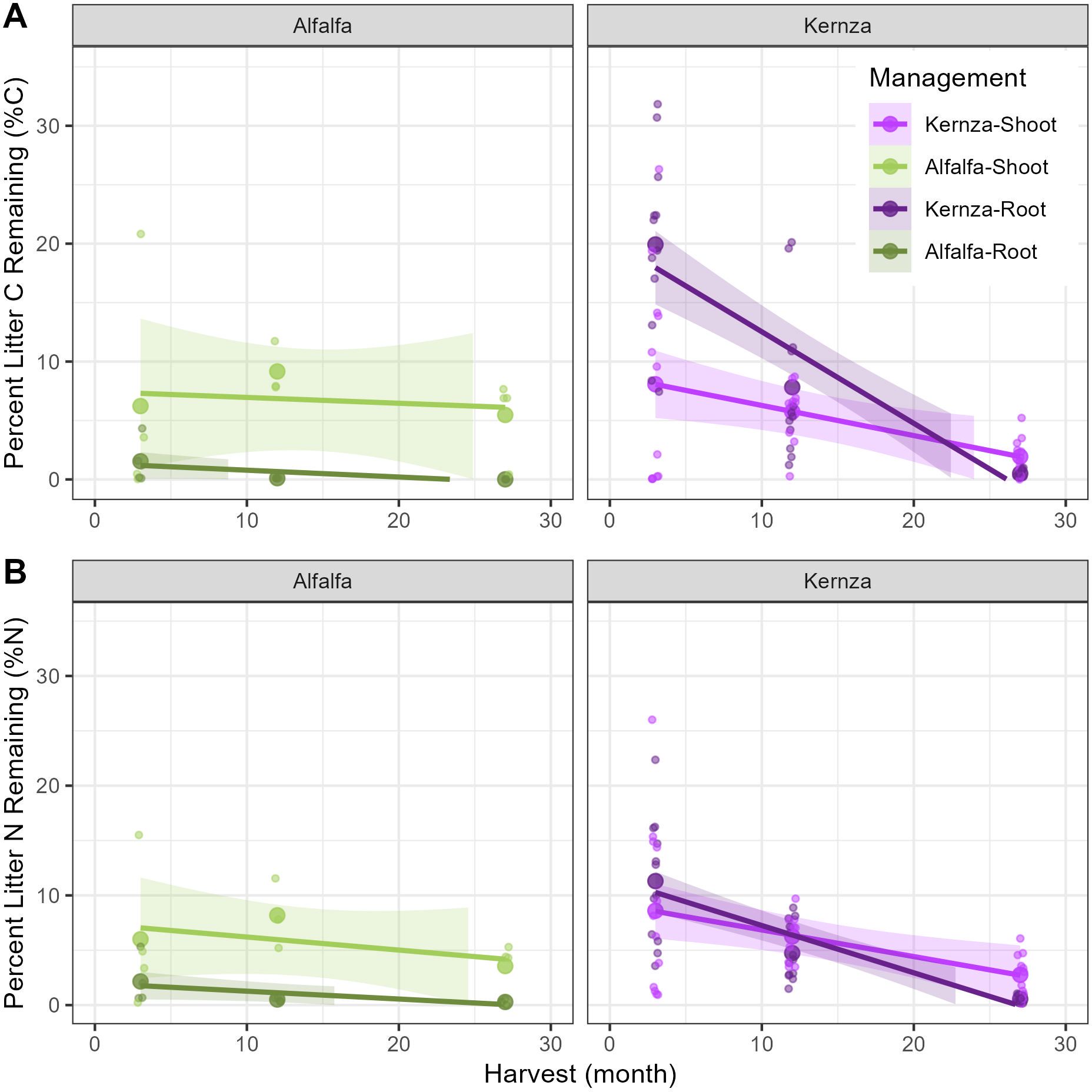
Figure 3. Percent litter of (A) carbon (C) and (B) nitrogen (N) remaining undecomposed at each harvest during the 27-month litter incubation. Undecomposed litter was the litter-derived C and N in the corresponding labeled litter for each soil collar. Small dots represent measured values for each soil collar (n=4 for alfalfa, n=12 for Kernza); larger dots represent the mean values. Darker colors represent root litter while lighter colors represent shoots. Shading represents the standard error.
3.3.1 N management
N management had only small effects (p=0.17, F=1.87) on litter-derived SOM formation (Supplementary Figure S3). The greatest instances where N-management may have influenced SOM formation was in the incorporation of labeled litter into microbial biomass C and N (p<0.001, F>15; Supplementary Figure S4, Supplementary Table S4) where management with higher N seemed to reduce litter-derived microbial C and N. Additionally, N management may have influenced the rate of decomposition at least initially, as there was greater litter-N remaining undecomposed after 3 months in the KKF treatment than the other two (p<0.03, t=2.6) and more apparent rapid decomposition by N management for C and N for KAU (Supplementary Figure S5), although these were not statistically meaningful (p=0.2). Along those lines, the C and N formation efficiency from Kernza shoots tended to be higher for the KKU and KAU especially for bulk soil and MAOM (Supplementary Figure S3; p=0.1, F=1.98 C; p=0.07, F=2.72, N). The root litter, in contrast, had higher SOM-C formation efficiency for the KKF and KAU treatments especially for the bulk and POM (fPOM and oPOM) fractions, although often similar (or higher) formation efficiency of SOM-N in the KKU and KAU treatments than the KKF (Supplementary Figure S3). Differences in SOM formation efficiency from root litter were slight, however, and did not emerge to have statistical significance (p>0.2). Since there otherwise were no significant N-management effects and results on litter decomposition and SOM formation, we averaged them across N-management.
3.3.2 Root vs. shoot litter
Independent of the plant species, shoots had higher relative mass remaining than roots (2%–5%), with <1% of roots remaining undecomposed at the end. The alfalfa roots exhibited the fastest decomposition, with only 1.5% initial litter C remaining by the first harvest at 3 months and 0.01% detected after 27 months (Figure 3).
Root and shoot litter showed distinct trends in how they were incorporated into soil over time (Figure 4, Table 3; p<0.001, F=6.2). Root litter of both Kernza and alfalfa, which was mixed into the soil matrix, quickly contributed to soil C and N with 25% ± 5 SE of root C and 54% ± 5 SE of root N recovered in SOM by month 3.
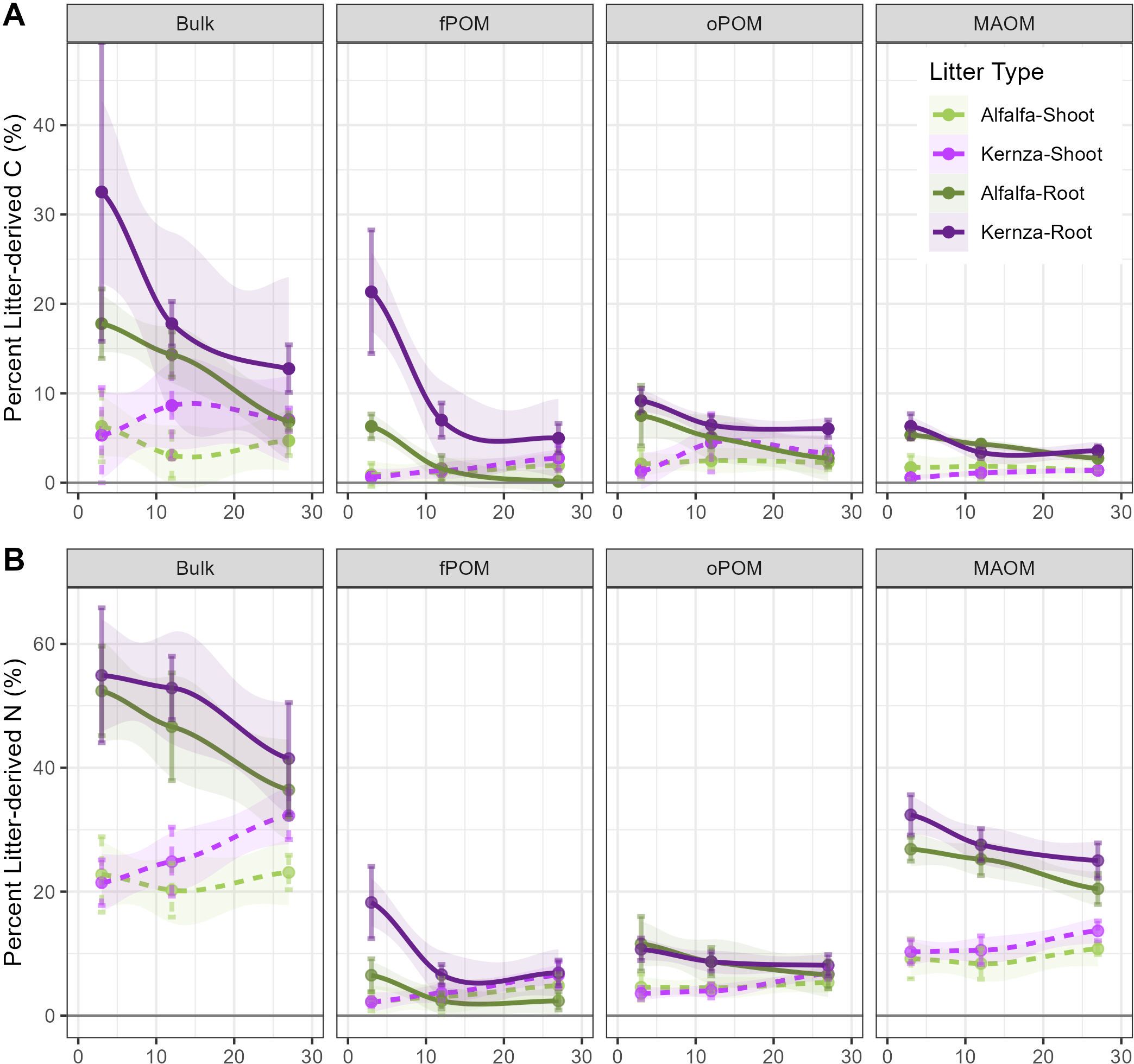
Figure 4. Percent litter-derived soil (A) carbon and (B) nitrogen in each measured soil and litter fraction over 27 months averaged across treatment (0-10 cm). Bars represent standard error. Root (solid) and shoot (dashed) refer to those respective litters in the soil collar; MAOM is mineral-associated organic matter (<53 μm); oPOM is occluded particulate organic matter; fPOM is free particulate organic matter.
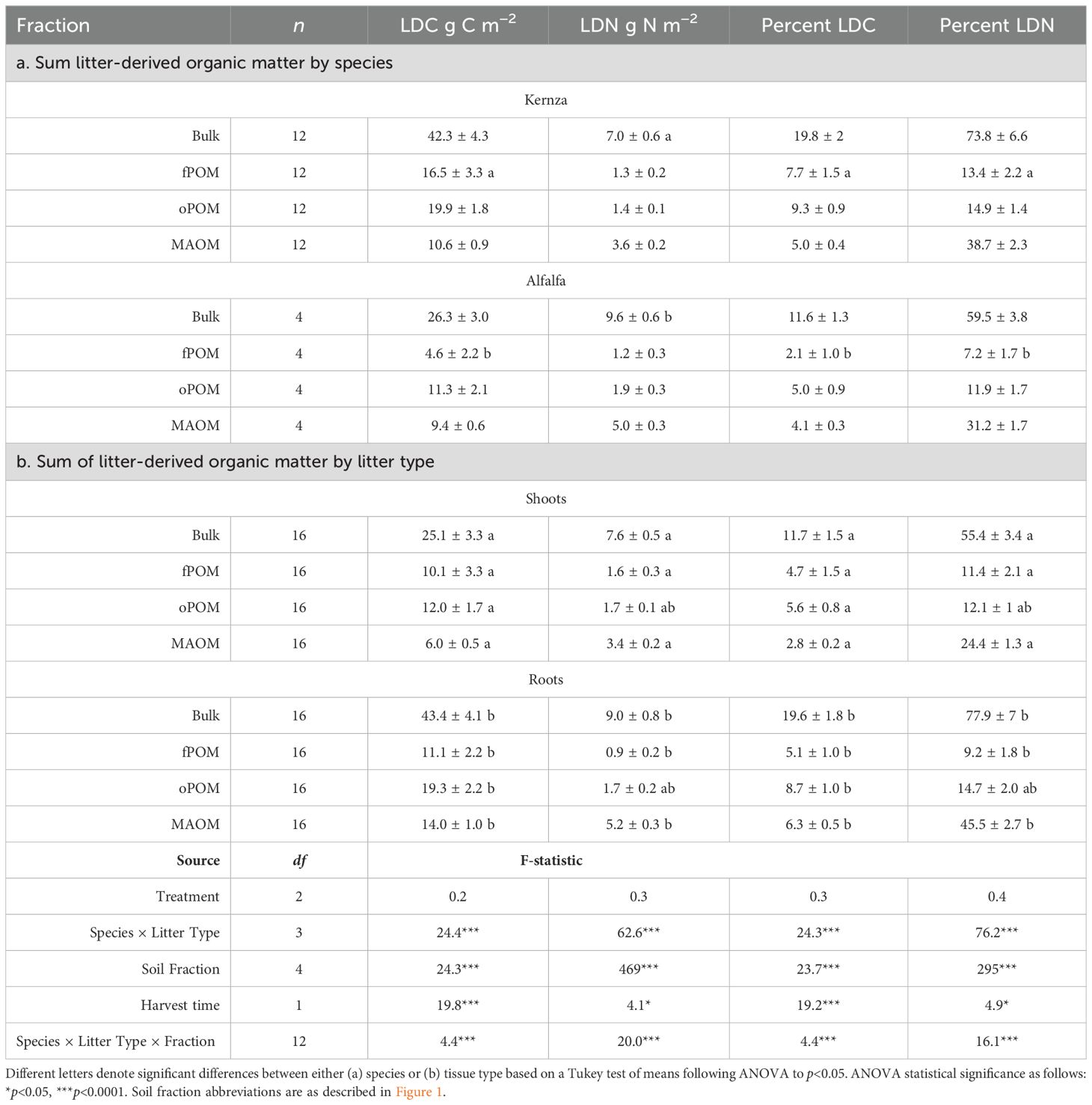
Table 3. Mean ( ± SE) litter-derived (LD) carbon (C) and nitrogen (N) by soil fractions by (a) plant species and (b) tissue type calculated using Equation 3 by soil fraction (0-10 cm).
The proportion of shoot-derived C and N recovered in bulk soil, in contrast, remained static or increased slightly over the course of the incubation (Figure 4, Supplementary Figure S3, Table 3). The formation efficiency of Kernza roots was higher than the other litter types in the bulk (~15% C; ~21% N) and fPOM (~11% C; ~7% N) fractions (Figure 1C; p<0.001, t>4.2). Root N was retained more efficiently in MAOM than the shoot litter for both species (+12.5% alfalfa; +18.3% Kernza; p<0.001, t>4.6), but there were no differences in C (Supplementary Figure S3).
Roots contributed roughly 50% more C and 30% more N to SOM than did the shoots (Table 3). The biggest differences were in the MAOM fraction, where roots added 8 g C m−2 ± 1.2 SE more than shoots and 18 g C m−2 ± 1.2 SE more overall (bulk). The only instance where shoots exceeded root contribution to soil fractions were for fPOM where greater portions of shoot-derived N were incorporated into this fraction (Table 3). Overall shoot litter C mostly became POM (~4:1 POM : MAOM), while shoot N was equally distributed across fractions (1:1 POM : MAOM) (Figure 1C, Table 3, Supplementary Table S5). Roots, in contrast, had greater contributions to MAOM where C was 2:1 (Figure 1C) and N was 1:2 POM : MAOM.
3.3.3 Kernza vs. alfalfa
Generally, Kernza contributed greater amounts of C and N to the soil than alfalfa, although these differences were only significant for fPOM (Figures 1C, 4, Table 3; p<0.05). Each species contributed similar proportions of C and N to oPOM (~43% LDC, ~23% LDN), with Kernza C forming fPOM more than MAOM (35% fPOM, 23% MAOM) and alfalfa tending to form more MAOM than fPOM (19% fPOM, 37% MAOM). N from both species chiefly contributed to MAOM (~60% of N). Notably, the alfalfa litter contributed greater amounts of N to MAOM than did Kernza (Table 3). The total amount of LDN from alfalfa was ~31% ± 8 SE (2.5 g N m−2 ± 0.9) higher than from Kernza, suggesting that proportionately more of the original alfalfa-N was lost, but a greater overall amount formed SOM-N than from Kernza.
4 Discussion
For a newly domesticated, perennial crop and with a goal of managing low-input, diverse systems for food production, we studied how Kernza as monoculture and intercrop with legume alfalfa form SOM and estimated the potential to form SOM. This study revealed the surprising lack of influence of N management influencing SOM formation and how the interaction of litter chemistry with the soil matrix shifts how SOM forms and ultimately persists. We synthesize these findings and their implications below.
4.1 How does N management affect SOM dynamics and productivity: N management important for C and N stocks but little effect on SOM formation
We tested the effects of N management on SOM dynamics with two overarching motivations: First, the source of N has implications for the overall sustainability of the system with potential feedback between productivity and soil C and N stocks. Second, we hypothesized that N introduced to the soil via legume decomposition may enhance SOM formation.
We examined whether the intercropped management (KAU) was comparable to the fertilized treatment (KKF) in terms of productivity and soil C and N stocks and found mixed results.
System ANPP was highest for KKF; as the KAU plots followed a replacement design (16), however, we would expect these plots to have a reduced above-ground biomass, since there are fewer Kernza plants per area (Figure 2). Intercrops of grass and legumes including Kernza and alfalfa (16, 85) often have 15%–30% greater productivity and yield resilience (86) compared to monocultures [summarized in Renard and Tilman (87)], however.
In this study, the intercrop did not match the productivity of the fertilized treatment likely due to combined N limitation and interspecies competition most years (Supplementary Table S3).
When examining the role of N management in SOM dynamics, we found surprisingly little effect. The contrasting management did lead to differences in productivity, plant N content (and C:N) (Table 2), and SOC and soil N stocks at the beginning of the incubation (Supplementary Figure S1) with the highest N content in plant and soil in the fertilized treatment. Resulting in greater soil inputs, the increased productivity of the KKF treatment explains how this treatment tended to have higher soil C and N stocks. That the KAU plots had similar SOC stocks as the KKF likely reflects the retention of alfalfa aboveground residues as soil inputs while the aboveground biomass in the monocultures are removed. The net trend of negative N balance may limit the capacity of these treatments to accrue SOM, particularly as MAOM, which has a lower C:N (<15) (88). A net SOM gain of 0.3 Mg C ha−1 over 5 years would require at least a positive N balance of 20 kg N ha−1 year−1 (16).
In terms of the new formation of SOM or distribution of C and N among soil fractions, we saw no effect of N source. The similarity of the KKF and KAU treatments supports the notion that the intercrop was able to maintain similar soil conditions as the fertilized treatment without reliance on synthetic inputs. Our hypothesis that the continuous decomposition and potential availability of N in the KAU plots would result in greater MAOM formation was not supported, however. Rather than promote more C-efficient microbial communities (89) as we hypothesized, the N made available from decomposing alfalfa residues may have promoted faster turnover of the soil microbes. The lower C:N of the KAU soil (Table 1) indicates a stronger microbial signature, potentially indicating an enhanced in vivo pathway of SOM formation (49). An accelerated microbial turnover may result in greater C losses and negate efficient new SOM formation even if the individual microbial C use efficiency was higher.
The absence of N inputs (KKU) did have some consequences for the SOM dynamics. The microbial biomass was similar for all treatments, but the microbes in the KKU plots incorporated significantly more of the labeled litter N initially than did the microbial community in KKF or KAU (Supplementary Figure S4, Supplementary Table S4). This implies a tendency for N immobilization, consistent also with the lower N content of the plant tissues in KKU. The KKU also had lower microbial incorporation of C by the end of the experiment (Supplementary Figure S4, Supplementary Table S4), suggesting lower C efficiency with greater N limitation.
Overall, the absence of N management effects on the SOM dynamics were surprising given the observed differences in productivity, plant tissue chemistry, and soil C and N stocks. The similarity of the KKF and KAU treatments, however, supports the intercrop’s ability to replace synthetic fertilizer with similar outcome for the soil.
4.2 Determinants of SOM formation: the role of litter chemistry vs. incorporation in the soil matrix
Comparing the formation of MAOM and POM helps us understand the stability of the newly formed SOM and its potential to contribute to longer-term soil C storage.
We hypothesized that the greater proportion of structural components in roots and Kernza tissues would result in greater POM formation (fPOM and oPOM) with roots having greater oPOM formation due to their presence in the soil matrix. We predicted that alfalfa, with a higher concentration of extractable components and N, would have more efficient MAOM formation than Kernza. Broadly, our hypotheses were supported with differences among litter types that highlight how litter chemistry influences SOM formation.
4.2.1 fPOM formation determined primarily by litter chemistry, MAOM promoted by incorporation in soil, and oPOM driven by soil
Our results shed some light on the relative importance of aspects of litter chemistry in contributing to SOM over time. Previous studies have estimated that roots contribute at least twice as much C to the soil than aboveground tissues (90–94) and often estimates are substantially greater (95, 96). Given their chemistry compared to aboveground materials, roots would more likely form POM (25, 97). In this study, the relative contribution of Kernza roots and shoots to SOM fractions were broadly similar, although the overall amount of SOM formed from Kernza roots was twice as high as from shoot tissues or alfalfa roots (Figure 1C, Table 3). The greater formation rate of Kernza roots underlines the importance of the fibrous components of plant litter in leading to greater SOM formation (98).
The relatively low SOM formation rate of alfalfa roots and that they formed SOM in equal parts MAOM and POM was surprising, given their placement in the soil matrix (Figure 1). The litter chemistry of the alfalfa roots was unusual, however, as it had the highest concentrations of HWE C and N and a ratio of AUR/N six times lower in the labeled litter than the field-grown plant tissues; the other litters had an AUR/N only three times lower (Table 2). The more labile composition of the labeled litter is an artifact of the young age and low stress environment from growing under greenhouse vs. field conditions. As alfalfa typically develops thick, lignified tap roots, early-stage development in a greenhouse may be more different for alfalfa than for the perennial grass. The HWE C and N can strongly influence the composition and dynamics of the microbial community (99, 100), potentially promoting rapid bacterial community growth. The AUR/N predicts direct plant contributions to SOM, especially as fPOM in early stages of decay (101). High AUR/N ratios also characterize litter with a slower decomposition rate (40, 102). The low AUR/N and location in the soil matrix of alfalfa roots likely resulted in greater microbial processing (97, 103) as suggested by the tendency for higher microbial biomass with alfalfa root litter (Supplementary Figure S4). This may have led to proportionately more MAOM formation but also may have resulted in greater total C loss from respiration (104, 105). Other studies have found that high concentrations of N-rich leachates may lead to reduced SOM formation, as they quickly but temporarily alleviate nutrient limitation and shift microbial community stoichiometry in a way that favors lignin decomposition early in the decomposition process, ultimately limiting SOM stabilization (97, 100). Had our labeled alfalfa root litter more closely resembled the chemistry of plants grown outdoors, it may have decomposed more slowly and had greater potential to form SOM as was the case with Kernza roots compared to the shoots.
4.2.2 MAOM formation influenced by litter chemistry and both oPOM and MAOM are promoted by incorporation in the soil matrix
What we can learn from this study is that incorporation into the soil matrix increases the likelihood of MAOM and, to a lesser extent oPOM, formation regardless of litter chemistry. This may be even more true for N than for C, as root-derived N recovery in MAOM was twice as large as shoot-derived N (Figure 1, Table 3). The oPOM formed was greater for roots than shoots (and for Kernza more than alfalfa), suggesting that incorporation in the soil results in greater oPOM formation. Another study that compared SOM formation from the same surface-applied and incorporated litter found 56% greater MAOM-C formation (and overall greater SOM formation) from litter incorporated in the soil (69), supporting the role of the soil matrix influencing SOM formation pathways (106). A study of root litter decomposition over multiple depths found SOM formation efficiency increased with depth and that there was greater SOM formation from root tissues as POM that started with a higher C:N (92). These findings seem also to be supported by incubation studies comparing litter with contrasting root and shoot chemistry that were uniformly mixed in the soil found no difference in MAOM-C formation (107) or preferential formation from incorporated litter with higher N content (108) between litter types. Similarly, a field study measuring oPOM found formation to be influenced by soil texture, although litter with higher N content having higher oPOM formation (109), which we did not observe in this study. Thus, this study supports the finding that broad differences in root vs. shoot SOM formation efficiency are driven by interaction with the soil matrix, while the litter chemistry strongly influences the form of SOM with higher C:N tissues preferentially forming POM and N-rich and soluble compounds contributing preferentially to MAOM.
4.3 Evaluation of observed formation rates compared to similar studies
Estimates of C inputs retained as SOM range widely from ~4% LDC (46) to 46% (69) after 1 year or even higher as reported in reviews (96, 110). Given the wide variation reported in the literature (110) and the longer incubation time of this experiment compared to many (27 vs. <12 months), the estimates in this study [5%-14% LDC, 12-31 g C m−2 (Table 3)] are within range although lower than some studies that reported 21%-46% formation efficiency after 2 years (92). Other studies have observed lower formation rates such as a 17-month pulse-labeling field study of SOM formation from cover crop residue where MAOM-C from roots and shoots was 7%-11% and POM ~2% (94). Unlike in this study, they found no difference in root and shoot SOM formation by the end of the experiment, although differences had been evident in the first year. Much of the variation in estimates could be attributed to the climate (55, 111) and soil minerology (106, 112). Given the difference in litter chemistry of the labeled litter and the plants grown in the field and the importance of the structural plant components to SOM formation, we might expect the formation rates in this study to be underestimated.
4.4 Evaluating the potential for low AUR/N litter to affect SOM accrual
We had hypothesized that the KAU treatment could potentially lead to greater SOM formation due to the steady release of N during the growing season through the decomposition of alfalfa tissues. Providing N at this slow but steady rate, we argued, could lead to microbial communities with greater C-use efficiency and ultimately accrue higher SOM overall as may have been observed in field studies with a legume in rotation with grains (28). Our preliminary analysis of the SOM across treatments showed this not to be the case, however (Supplementary Figure S3). The lower formation of SOM by alfalfa compared to Kernza, likewise, does not support the hypothesis that higher quality (i.e., lower AUR/N) litter would lead to greater SOM formation overall, although it did lead to a greater proportion of MAOM formation (Figure 1). Does this mean that the hypothesis that including legumes with a grass might increase SOM is flawed?
The question is relevant for how Kernza and Kernza-legume intercrops might alter SOM stocks and soil structure over time. Other studies that examined the role of plant diversity and legumes on increasing SOM have found no relationship between rate of C accrual in SOM and legumes (53, 113–116). A study that used isotope tracers to assess soil C dynamics found that plant diversity (but not legumes as a functional group) promoted SOM accrual primarily by increasing productivity as net primary production (NPP) and slowing the decomposition of the plant tissues (117). The legume intercrop in this study supported higher ANPP only 1 out of 4 years, meaning this management had lower soil inputs and litter likely to decompose more rapidly (Figure 2, Supplementary Table S2). Notably, the intercrop SOM stock, although lower than the fertilized treatment, was not significantly different (Supplementary Figure S1, Supplementary Table S1), suggesting that the reduced crop inputs to soil may have been partially compensated by the alfalfa. Further testing of this question should evaluate SOM formation and change to SOM over time using combinations of grass and legume tissues, holding the total amount of input constant.
Previous studies have found that fields under Kernza crops had significantly greater soil C in POM than in MAOM (17), suggesting that Kernza may enhance SOM initially and most easily detectably through the accumulation of POM from root tissues. Meta-analyses focused on perennials and cover crops have shown that POM increases proportionately to increases in root C inputs and that the degree of increased root C input from previous management may ultimately limit MAOM accumulation (9). Our findings support this conclusion, as the majority of Kernza tissues contributed to the POM fractions. This study does not support the idea that legumes would enhance SOM formation overall, however. While the alfalfa in this study did contribute similar amounts of N (Table 3) to SOM while supplying an important source of N to the system, three times as much legume residues are needed above or belowground to form a similar amount of SOM as the Kernza. Given the lack of synthetic inputs in the intercrop, however, management should be considered wholistically given the reduced reliance on fossil fuels (and subsequent emissions) when evaluating its overall effect in the climate and sustainability (118), although this type of evaluation was beyond the scope of this study.
There is likely a “sweet spot” of litter chemistry to optimize SOM formation in climates with grassland ecosystems. A meta-analysis comparing soil C sequestration and N losses based on C:N of residues found that only residues with C:N > 30 supported SOC accrual (104). Notably, all of the labeled litter in this study had a C:N < 25 (Table 2). A question worth exploring is whether there is evidence to support that the community-level average of C:N and AUR/N of the plants growing in close proximity or over time such as an intercrop or prairie can promote SOM formation (119–121) or if the chemistry of the individual plant is the best predictor of how it will contribute to SOM. Plant species may also alter the microclimate and thus influence decomposition rates in multiple ways (122), and this may vary depending on the underlying soil minerology (123). In our pursuit of this question, we have in a way assumed that the community average of litter traits is important, but the physical separation of litter especially in low-disturbance systems may mean that microbial communities derive little C-use efficiency benefit from the mixing of high and low C:N and AUR/N litters or that those effects may change over time (124, 125).
5 Conclusion
Perennial grains have the potential to restore many ecosystem services hampered by annual agriculture including increasing SOC. Roughly 15% of Kernza root tissue C incorporated into SOM after 27 months with 3.5% as MAOM and 6% as oPOM, implying that 9.5% of root tissue C may be stabilized in soil. We did not find evidence that the alfalfa tissues or intercrop management enhanced SOM formation, although it did sustain similar levels of SOM as the fertilized monoculture. Thus, we conclude that legume intercrops are unlikely to lead to enhanced SOM accumulation, although they may, as they did in this study, support similar levels of SOM as fertilized grass monocultures without synthetic inputs. Future studies should focus on the net energy balance associated with contrasting management to evaluate the energetic and greenhouse gas cost of potential SOM accumulation.
Since our labeled litter differed in its chemical composition significantly from the field-grown plants, future studies using greenhouse chamber-produced crop residues should use caution when extrapolating their results, as the more labile chamber-grown plants may be inherently more likely to contribute to the MAOM soil fraction and have accelerated decomposition rates. Introducing greater plant stress into the chamber environment such as deep, bottom-fed pots and vigorous fan cycles may reduce the discrepancy between field and greenhouse-grown plant chemistry, although these efforts are unlikely to eliminate these differences.
Finally, this study points to the role of litter chemistry and integration in the soil matrix being more influential in the form and stability of SOM than the N management. The N management seems to influence plant growth and tissue chemistry, thus potentially driving long-term trends in SOM formation. However, the N management had little effect at the fine scale of this experiment; there may be a threshold of labile:structural components that optimizes SOM formation and protection. Experimentation with the proportions of the structural and labile tissues in soils and soil N could identify the relevant thresholds for optimizing microbial litter transformation efficiency and aggregation for long-term SOM accumulation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LP: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. TC: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing. MC: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (Project 1015239) and The Land Institute in Salina, Kansas. LP was supported by the National Science Foundation (NSF) (Grant DGE-006784).
Acknowledgments
We thank Rebecca Even and Henry Krieger for their assistance collecting soil samples. A special thank you to Rebecca Even for her help with the microbial biomass analysis. Many thanks also to Kelli Hatch, Camden Meyer, Lauren Hibbard, Laramie Woods, and Em Walker who helped with sample processing and analyses, and thanks to Dr. Guy Beresford, Michelle Haddix, and Dan Reuss for their advice and training in laboratory analyses. Dr. Besiana Sinanaj co-designed and created Figure 1 of this manuscript for which we are so grateful. We would also like to thank the technical, administrative, and research staff at The Land Institute for their hospitality during sample collection and maintenance and harvest data collection of the experimental research plots where this experiment took place.
Conflict of interest
MC is a cofounder of Cquester Analytics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF or USDA.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsoil.2025.1548577/full#supplementary-material
References
1. Schipanski ME, MacDonald GK, Rosenzweig S, Chappell MJ, Bennett EM, Kerr RB, et al. Realizing resilient food systems. BioScience. (2016) 66(7):600–10. doi: 10.1093/biosci/biw052
2. Buma B, Gordon DR, Kleisner KM, Bartuska A, Bidlack A, DeFries R, et al. Expert review of the science underlying nature-based climate solutions. Nat Climate Change. (2024) 14:402–6. doi: 10.1038/s41558-024-01960-0
3. Kopittke PM, Berhe AA, Carrillo Y, Cavagnaro TR, Chen D, Chen Q-L, et al. Ensuring planetary survival: the centrality of organic carbon in balancing the multifunctional nature of soils. Crit Rev Environ Sci Technol. (2022) 52:4308–24. doi: 10.1080/10643389.2021.2024484
4. Paustian K, Larson E, Kent J, Marx E, and Swan A. Soil C sequestration as a biological negative emission strategy. Front Climate. (2019) 1:8. doi: 10.3389/fclim.2019.00008
5. Bossio DA, Cook-Patton SC, Ellis PW, Fargione J, Sanderman J, Smith P, et al. The role of soil carbon in natural climate solutions. Nat Sustainability. (2020) 3:391–98. doi: 10.1038/s41893-020-0491-z
6. McGowan AR, Roozeboom KL, and Rice CW. Nitrous oxide emissions from annual and perennial biofuel cropping systems. Agron J. (2019) 111:84–925. doi: 10.2134/agronj2018.03.0187
7. Soto-Gómez D and Pérez-Rodríguez P. Sustainable agriculture through perennial grains: wheat, rice, maize, and other species. A review. Agriculture Ecosystems Environ. (2022) 325:107747. doi: 10.1016/j.agee.2021.107747
8. Zhang S, Huang G, Zhang Y, Lv X, Wan K, Liang J, et al. Sustained productivity and agronomic potential of perennial rice. Nat Sustainability. (2022) 6:1–11. doi: 10.1038/s41893-022-00997-3
9. King AE, Amsili JP, Córdova SC, Culman S, Fonte SJ, Kotcon J, et al. Constraints on mineral-associated and particulate organic carbon response to regenerative management: carbon inputs and saturation deficit. Soil Tillage Res. (2024) 238:106008. doi: 10.1016/j.still.2024.106008
10. Siddique IA, Grados D, Chen J, Lærke PE, and Jørgensen U. Soil organic carbon stock change following perennialization: A meta-analysis. Agron Sustain Dev. (2023) 43:585. doi: 10.1007/s13593-023-00912-w
11. Asbjornsen H, Hernandez-Santana V, Liebman M, Bayala J, Chen J, Helmers M, et al. Targeting perennial vegetation in agricultural landscapes for enhancing ecosystem services. Renewable Agric Food Syst. (2014) 29:101–25. doi: 10.1017/S1742170512000385
12. Anderson-Teixeira KJ, Masters MD, Black CK, Zeri M, Hussain MZ, Bernacchi CJ, et al. Altered belowground carbon cycling following land-use change to perennial bioenergy crops. Ecosystems. (2013) 16:508–205. doi: 10.1007/s10021-012-9628-x
13. Crews TE and Rumsey B. What agriculture can learn from native ecosystems in building soil organic matter: A review. Sustainability. (2017) 9:5785. doi: 10.3390/su9040578
14. Crews TE, Carton W, and Olsson L. Is the future of agriculture perennial? Imperatives and opportunities to reinvent agriculture by shifting from annual monocultures to perennial polycultures. Global Sustainability. (2018) 1:e11. doi: 10.1017/sus.2018.11
15. de Oliveira G, Brunsell NA, Crews TE, DeHaan LR, and Vico G. Carbon and water relations in perennial kernza (Thinopyrum intermedium): an overview. Plant Science Food Secur under Climate Change. (2020) 295:110279. doi: 10.1016/j.plantsci.2019.110279
16. Crews TE, Kemp L, Bowden JH, and Murrell EG. How the nitrogen economy of a perennial cereal-legume intercrop affects productivity: can synchrony be achieved? Front Sustain Food Syst. (2022) 6:755548. doi: 10.3389/fsufs.2022.755548
17. van der Pol LK, Nester B, Schlautman B, Crews TE, and Cotrufo MF. Perennial Grain Kernza ® Fields Have Higher Particulate Organic Carbon at Depth than Annual Grain Fields. Can J Soil Sci. (2022), 1–5. doi: 10.1139/cjss-2022-0026
18. Lavallee JM, Soong JL, and Cotrufo MF. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Global Change Biol. (2020) 26:261–735. doi: 10.1111/gcb.14859
19. Cambardella CA and Elliott ET. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci Soc America J. (1992) 56:777–83. doi: 10.2136/sssaj1992.03615995005600030017x
20. Poeplau C, Don A, Six J, Kaiser M, Benbi D, Chenu C, et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils – A comprehensive method comparison. Soil Biol Biochem. (2018) 125:10–26. doi: 10.1016/j.soilbio.2018.06.025
21. Cotrufo MF and Lavallee JM. Chapter one - soil organic matter formation, persistence, and functioning: A synthesis of current understanding to inform its conservation and regeneration. In: . Sparks DL, editor. Advances in agronomy (2022) 172:1–66. doi: 10.1016/bs.agron.2021.11.002
22. Goebel M-O, Woche SK, and Bachmann J. Do soil aggregates really protect encapsulated organic matter against microbial decomposition? Biologia. (2009) 64:443–85. doi: 10.2478/s11756-009-0065-z
23. Besnard E, Chenu C, Balesdent J, Puget P, and Arrouays D. Fate of particulate organic matter in soil aggregates during cultivation. Eur J Soil Sci. (1996) 47:495–503. doi: 10.1111/j.1365-2389.1996.tb01849.x
24. Even RJ and Cotrufo MF. The ability of soils to aggregate, more than the state of aggregation, promotes protected soil organic matter formation. Geoderma. (2024) 442:116760. doi: 10.1016/j.geoderma.2023.116760
25. Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, et al. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci. (2015) 8:776–95. doi: 10.1038/ngeo2520
26. Fulton-Smith S, Even R, and Cotrufo MF. Depth impacts on the aggregate-mediated mechanisms of root carbon stabilization in soil: trade-off between MAOM and POM pathways. Geoderma. (2024) 452:117078. doi: 10.1016/j.geoderma.2024.117078
27. Janzen HH. The soil carbon dilemma: shall we hoard it or use it? Soil Biol Biochem. (2006) 38:419–24. doi: 10.1016/j.soilbio.2005.10.008
28. van der Pol LK, Robertson A, Schipanski M, Calderon FJ, Wallenstein MD, and Cotrufo MF. Addressing the soil carbon dilemma: legumes in intensified rotations regenerate soil carbon while maintaining yields in semi-arid dryland wheat farms. Agriculture Ecosystems Environ. (2022) 330:107906. doi: 10.1016/j.agee.2022.107906
29. Luo Z, Feng W, Luo Y, Baldock J, and Wang E. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Global Change Biol. (2017) 23:4430–395. doi: 10.1111/gcb.13767
30. Rodriguez C, Carlsson G, Englund J-E, Flöhr A, Pelzer E, Jeuffroy Marie-Hélène, et al. Grain legume-cereal intercropping enhances the use of soil-derived and biologically fixed nitrogen in temperate agroecosystems. A meta-analysis. Eur J Agron. (2020) 118:126077. doi: 10.1016/j.eja.2020.126077
31. Yan M, Pan G, Lavallee JM, and Conant RT. Rethinking sources of nitrogen to cereal crops. Global Change Biol. (2020) 26:191–995. doi: 10.1111/gcb.14908
32. Ladha JK, Pathak H, Krupnik TJ, Six J, and van Kessel C. Efficiency of fertilizer nitrogen in cereal production: retrospects and prospects.” In. Adv Agron. (2005) 87:85–156. doi: 10.1016/S0065-2113(05)87003-8
33. Li T, Wang Z, Wang C, Huang J, Feng Y, Shen W, et al. Ammonia volatilization mitigation in crop farming: A review of fertilizer amendment technologies and mechanisms. Chemosphere. (2022) 303:134944. doi: 10.1016/j.chemosphere.2022.134944
34. Peoples MB, Herridge DF, and Ladha JK. Biological nitrogen fixation: an efficient source of nitrogen for sustainable agricultural production? In: Ladha JK and Peoples MB, editors. Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems. Dordrecht: Springer Netherlands (1995), pp. 3–28. (Developments in Plant and Soil Sciences). doi: 10.1007/978-94-011-0055-7_1
35. Crews TE. Perennial crops and endogenous nutrient supplies. Renewable Agric Food Syst. (2005) 20:25–37. doi: 10.1079/RAF200497
36. Crews TE and Peoples M. Legume versus fertilizer sources of nitrogen: ecological tradeoffs and human needs. Agriculture Ecosystems Environ. (2004) 102:279–975. doi: 10.1016/j.agee.2003.09.018
37. Hammel KE, Kapich AN, Jensen KA, and Ryan ZC. Reactive oxygen species as agents of wood decay by fungi. Enzyme Microbial Technol. (2002) 30:445–53. doi: 10.1016/S0141-0229(02)00011-X. Recent Advances in Lignin Biodegradation.
38. Rasse DP, Rumpel C, and Dignac M-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil. (2005) 269:341–565. doi: 10.1007/s11104-004-0907-y
39. Berg B and Staaf H. Decomposition rate and chemical changes of scots pine needle litter. II. Influence of chemical composition. Ecol Bulletins. (1980) 32:373–90. Available online at: https://www.jstor.org/stable/20112825.
40. Melillo JM, Aber JD, and Muratore JF. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology. (1982) 63:621–265. doi: 10.2307/1936780
41. Roller BRK and Schmidt TM. The physiology and ecological implications of efficient growth. ISME J. (2015) 9:1481–875. doi: 10.1038/ismej.2014.235
42. Stott DE, Kassim G, Jarrell WM, Martin JP, and Haider K. Stabilization and Incorporation into Biomass of Specific Plant Carbons during Biodegradation in Soil. Plant Soil. (1983) 70:15–265. doi: 10.1007/BF02374746
43. Bahri H, Rasse DP, Rumpel C, Dignac M-F, Bardoux G, and Mariotti A. Lignin degradation during a laboratory incubation followed by 13C isotope analysis. Soil Biol Biochem. (2008) 40:1916–225. doi: 10.1016/j.soilbio.2008.04.002
44. Grandy AS and Neff JC. Molecular C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci Total Environ. (2008) 404:297–307. doi: 10.1016/j.scitotenv.2007.11.013
45. Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S, et al. Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. J Plant Nutr Soil Sci. (2008) 171:61–825. doi: 10.1002/jpln.200700048
46. Soong JL and Cotrufo MF. Annual burning of a tallgrass prairie inhibits C and N cycling in soil, increasing recalcitrant pyrogenic organic matter storage while reducing N availability. Global Change Biol. (2015) 21:2321–335. doi: 10.1111/gcb.12832
47. Cotrufo MF, Wallenstein MD, Boot CM, Denef K, and Paul E. The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Global Change Biol. (2013) 19:988–55. doi: 10.1111/gcb.12113
48. Islam ,Md.R, Singh B, and Dijkstra FA. Microbial carbon use efficiency of glucose varies with soil clay content: A meta-analysis. Appl Soil Ecol. (2023) 181:104636. doi: 10.1016/j.apsoil.2022.104636
49. Liang C, Schimel JP, and Jastrow JD. The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol. (2017) 2:17105. doi: 10.1038/nmicrobiol.2017.105
50. Bradford MA, Keiser AD, Davies CA, Mersmann CA, and Strickland MS. Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry. (2013) 113:271–815. doi: 10.1007/s10533-012-9822-0
51. Bolinder MA, Janzen HH, Gregorich EG, Angers DA, and VandenBygaart AJ. An approach for estimating net primary productivity and annual carbon inputs to soil for common agricultural crops in Canada. Agriculture Ecosystems Environ. (2007) 118:29–42. doi: 10.1016/j.agee.2006.05.013
52. Monfreda C, Ramankutty N, and Foley JA. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Global Biogeochemical Cycles. (2008) 22(1):GB1022. doi: 10.1029/2007GB002947
53. King AE and Blesh J. Crop rotations for increased soil carbon: perenniality as a guiding principle. Ecol Appl. (2018) 28:249–615. doi: 10.1002/eap.1648
54. Kong AYY, Six J, Bryant DC, Denison RF, and van Kessel C. The relationship between carbon input, aggregation, and soil organic carbon stabilization in sustainable cropping systems. Soil Sci Soc America J. (2005) 69:1078–85. doi: 10.2136/sssaj2004.0215
55. Haddix ML, Gregorich EG, Helgason BL, Janzen H, Ellert BH, and Cotrufo MF. Climate, carbon content, and soil texture control the independent formation and persistence of particulate and mineral-associated organic matter in soil. Geoderma. (2020) 363:114160. doi: 10.1016/j.geoderma.2019.114160
56. Riggs CE, Hobbie SE, Bach EM, Hofmockel KS, and Kazanski CE. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry. (2015) 125:203–195. doi: 10.1007/s10533-015-0123-2
57. Soil Survey Staff. Soil survey geographic database (SSURGO). Natural Resources Conservation Service (1992). Available online at: https://www.nrcs.usda.gov/resources/data-and-reports/soil-survey-geographic-database-ssurgo.
58. Barker AA and Piper JK. Growth and seed yield of three grassland perennials in monocultures and mixtures. In: Harnett DC, editor. Proceedings of the 14th North American Prairie Conference. Manhattan, KS, USA: Kansas State University Press (1994), 193–97.
59. Gee GW and Bauder JW. Particle‐size Analysis. In: Klute A, editor. Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods, Agronomy Monograph No 9, 2nd ed (SSSA Book Series). Madison, WI: Society of Agronomy/Soil Science Society of America (1986), 383–411. doi: 10.2136/sssabookser5.1.2ed.c15
60. Cotrufo MF, Ranalli MG, Haddix ML, Six J, and Lugato E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat Geosci. (2019) 12:989–45. doi: 10.1038/s41561-019-0484-6
61. Carlsson G and Huss-Danell K. Nitrogen fixation in perennial forage legumes in the field. Plant Soil. (2003) 253:353–72. doi: 10.1023/A:1024847017371
62. Walley FL, Tomm GO, Matus A, Slinkard AE, and van Kessel C. Allocation and cycling of nitrogen in an alfalfa-bromegrass sward. Agron J. (1996) 88:834–435. doi: 10.2134/agronj1996.00021962008800050025x
63. Vandermeer JH. Intercropping. In: Carroll CR, Vandermeer JH, and Rosset PM, editors. Agroecology. McGraw-Hill Inc, New York (1990). p. 481–516. Available online at: https://www.cabdirect.org/cabdirect/abstract/19920758895 (Accessed October 27, 2022).
64. Soong JL, Reuss D, Pinney C, Boyack Ty, Haddix ML, Stewart CE, et al. Design and operation of a continuous 13C and 15N labeling chamber for uniform or differential, metabolic and structural, plant isotope labeling. J Visualized Experiments : JoVE. (2014) 83:51117. doi: 10.3791/51117
66. Soong JL, Parton WJ, Calderon F, Campbell EE, and Cotrufo MF. A new conceptual model on the fate and controls of fresh and pyrolized plant litter decomposition. Biogeochemistry. (2015) 124:27–445. doi: 10.1007/s10533-015-0079-2
67. Van Soest PJ and Wine RH. Determination of lignin and cellulose in acid-detergent fiber with permanganate. J Assoc Off Analytical Chem. (1968) 51:780–85. doi: 10.1093/jaoac/51.4.780
68. Rowland AP and Roberts JD. Lignin and cellulose fractionation in decomposition studies using acid-detergent fibre methods. Commun Soil Sci Plant Anal. (1994) 25:269–77. doi: 10.1080/00103629409369035
69. Leichty S, Cotrufo MF, and Stewart CE. Less efficient residue-derived soil organic carbon formation under no-till irrigated corn. Soil Sci Soc America J. (2020) 84:1928–425. doi: 10.1002/saj2.20136
70. Ellert BH, Janzen HH, and Entz T. Assessment of a method to measure temporal change in soil carbon storage. Soil Sci Soc America J. (2002) 66:1687–95. doi: 10.2136/sssaj2002.1687
71. Leuthold SJ, Haddix ML, Lavallee J, and Cotrufo MF. Physical fractionation techniques. In: Reference module in earth systems and environmental sciences. Elsevier (2022). doi: 10.1016/B978-0-12-822974-3.00067-7
72. Cotrufo MF and Pressler Y. A primer on stable isotopes in ecology. Oxford, United Kingdom: Oxford University Press (2023), 128 p.
73. Balesdent Jérôme and Mariotti A. Measurement of soil organic matter turnover using 13C natural abundance. In: Mass spectrometry of soils. Institut National de la Recherche Agronomique, Université Pierre et Marie Curie, CNRS, Paris, France (1996). p. 83–111. doi: 10.5555/19961906961
74. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2024). Available online at: https://www.R-project.org/ (Accessed December 11, 2024).
75. Wickham H, François R, Henry L, and Müller K. Dplyr: A grammar of data manipulation (2022). Available online at: https://github.com/tidyverse/dplyr (Accessed October 4, 2022).
76. Fox JW. An R companion to applied regression. Thousand Oaks, CA: Sage (2019). Available online at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (Accessed October 4, 2022).
77. Lenth RV. Emmeans: Estimated Marginal Means, Aka Least-Squares Means.” R package version 1.10.1 (2024). Available online at: https://CRAN.R-project.org/package=emmeans (Accessed December 19, 2024).
78. Bates D, Mächler M, Bolker B, and Walker S. Fitting linear mixed-effects models using lme4. J Stat Software. (2015) 67(1):1–48. doi: 10.18637/jss.v067.i01
79. Wickham H, Vaughan D, and Girlich M. Tidyr: tidy messy data (2024). Available online at: https://CRAN.R-project.org/package=tidyr (Accessed December 19, 2024).
80. Schauberger P and Walker A. Openxlsx: read, write and edit xlsx files (2023). Available online at: https://CRAN.R-project.org/package=openxlsx (Accessed December 19, 2024).
81. Hebbali A. Xplorerr: tools for interactive data exploration (2021). Available online at: https://CRAN.R-project.org/package=xplorerr (Accessed December 19, 2024).
82. Wickham H. Ggplot2: elegant graphics for data analysis. New York, NY: Springer-Verlag New York (2016). Available online at: https://ggplot2.tidyverse.org.
83. Cousineau D, Goulet M-A, and Harding B. Summary plots with adjusted error bars: the superb framework with an implementation in {R}. Adv Methods Practices psychol Sci. (2021) 4:1–465. doi: 10.1177/25152459211035109
84. Melillo JM, Aber JD, Linkins AE, Ricca A, Fry B, and Nadelhoffer KJ. Carbon and nitrogen dynamics along the decay continuum: plant litter to soil organic matter. Plant Soil. (1989) 115:189–985. doi: 10.1007/BF02202587
85. Li S, Barreiro A, Jensen ES, Zhang Y, and Dimitrova Mårtensson L-M. Early interspecific dynamics, dry matter production and nitrogen use in kernza (Thinopyrum intermedium) – alfalfa (Medicago sativa L.) mixed intercropping. Soil Plant Sci. (2019) 70:165–755. doi: 10.1080/09064710.2019.1686164
86. Jungers J, Runck B, Ewing PM, Maaz T, Carlson C, Neyhart J, et al. Adapting perennial grain and oilseed crops for climate resiliency. Crop Sci. (2023) 63:1701–21. doi: 10.1002/csc2.20972
87. Renard D and Tilman D. Cultivate biodiversity to harvest food security and sustainability. Curr Biol. (2021) 31:R1154–58. doi: 10.1016/j.cub.2021.06.082
88. Crews TE, Blesh J, Culman SW, Hayes RC, Jensen ES, Mack MC, et al. Going where no grains have gone before: from early to mid-succession. Agriculture Ecosystems Environ. (2016) 223:223–38. doi: 10.1016/j.agee.2016.03.012
89. Zang H, Wang J, and Kuzyakov Y. N fertilization decreases soil organic matter decomposition in the rhizosphere. Appl Soil Ecol. (2016) 108:47–53. doi: 10.1016/j.apsoil.2016.07.021
90. Barber SA. Corn residue management and soil organic matter1. Agron J. (1979) 71:625–27. doi: 10.2134/agronj1979.00021962007100040025x
91. Puget P and Drinkwater LE. Short-term dynamics of root- and shoot-derived carbon from a leguminous green manure. Soil Sci Soc America J. (2001) 65:771–95. doi: 10.2136/sssaj2001.653771x
92. Cotrufo MF, Haddix ML, Mullen JL, Zhang Y, and McKay JK. Deepening root inputs: potential soil carbon accrual from breeding for deeper rooted maize. Global Change Biol. (2024) 30:e175915. doi: 10.1111/gcb.17591
93. Mendez-Millan M, Dignac M-F, Rumpel C, Rasse DP, and Derenne S. Molecular dynamics of shoot vs. Root biomarkers in an agricultural soil estimated by natural abundance 13C labelling. Soil Biol Biochem. (2010) 42:169–775. doi: 10.1016/j.soilbio.2009.10.010
94. Austin EE, Wickings K, McDaniel MD, Robertson GP, and Grandy AS. Cover crop root contributions to soil carbon in a no-till corn bioenergy cropping system. GCB Bioenergy. (2017) 9:1252–635. doi: 10.1111/gcbb.12428
95. Mazzilli SebastiánR, Kemanian AR, Ernst OR, Jackson RB, and Piñeiro G. Greater Humification of Belowground than Aboveground Biomass Carbon into Particulate Soil Organic Matter in No-till Corn and Soybean Crops. Soil Biol Biochem. (2015) 85:22–30. doi: 10.1016/j.soilbio.2015.02.014
96. Jackson RB, Lajtha K, Crow SE, Hugelius G, Kramer MG, and Piñeiro G. The ecology of soil carbon: pools, vulnerabilities, and biotic and abiotic controls. Annu Rev Ecology Evolution Systematics. (2017) 48:419–55. doi: 10.1146/annurev-ecolsys-112414-054234
97. Huys R, Poirier V, Bourget MY, Roumet C, Hättenschwiler S, Fromin N, et al. Plant litter chemistry controls coarse-textured soil carbon dynamics. J Ecol. (2022) 110:2911–28. doi: 10.1111/1365-2745.13997
98. Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, et al. Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Global Change Biol. (2008) 14:2636–605. doi: 10.1111/j.1365-2486.2008.01674.x
99. Breulmann M, Schulz E, Weißhuhn K, and Buscot F. Impact of the plant community composition on labile soil organic carbon, soil microbial activity and community structure in semi-natural grassland ecosystems of different productivity. Plant Soil. (2012) 352:253–655. doi: 10.1007/s11104-011-0993-6
100. Fanin N. An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter–microbe system - fanin - 2013 - ecology letters - wiley online library. Ecol Lett. (2013) 16(6):764–72. doi: 10.1111/ele.12108
101. Angst G, Mueller KE, Kögel-Knabner I, Freeman KH, and Mueller CW. Aggregation controls the stability of lignin and lipids in clay-sized particulate and mineral associated organic matter. Biogeochemistry. (2017) 132:307–245. doi: 10.1007/s10533-017-0304-2
102. Knorr M, Frey SD, and Curtis PS. Nitrogen additions and litter decomposition: A meta-analysis. Ecology. (2005) 86:3252–57. doi: 10.1890/05-0150
103. Miltner A, Bombach P, Schmidt-Brücken B, and Kästner M. SOM genesis: microbial biomass as a significant source. Biogeochemistry. (2012) 111:41–555. doi: 10.1007/s10533-011-9658-z
104. Xia L, Lam SK, Wolf B, Kiese R, Chen D, and Butterbach-Bahl K. Trade-offs between soil carbon sequestration and reactive nitrogen losses under straw return in global agroecosystems. Global Change Biol. (2018) 24:5919–325. doi: 10.1111/gcb.14466
105. Cotrufo MF, Lavallee JM, Zhang Y, Hansen PM, Paustian KH, Schipanski M, et al. In-N-out: A hierarchical framework to understand and predict soil carbon storage and nitrogen recycling. Global Change Biol. (2021) 27:4465–685. doi: 10.1111/gcb.15782
106. King AE, Amsili JP, Córdova SC, Culman S, Fonte SJ, Kotcon J, et al. A Soil Matrix Capacity Index to Predict Mineral-Associated but Not Particulate Organic Carbon across a Range of Climate and Soil pH. Biogeochemistry. (2023) 165:1–14. doi: 10.1007/s10533-023-01066-3
107. Steffens C, Helfrich M, Joergensen RG, Eissfeller V, and Flessa H. Translocation of 13C-labeled leaf or root litter carbon of beech (Fagus sylvatica L.) and ash (Fraxinus excelsior L.) during decomposition – A laboratory incubation experiment. Soil Biol Biochem. (2015) 83:125–37. doi: 10.1016/j.soilbio.2015.01.015
108. Lavallee JM, Conant RT, Paul EA, and Cotrufo MF. Incorporation of Shoot versus Root-Derived 13C and 15N into Mineral-Associated Organic Matter Fractions: Results of a Soil Slurry Incubation with Dual-Labelled Plant Material. Biogeochemistry. (2018) 137:379–935. doi: 10.1007/s10533-018-0428-z
109. von Haden AC, Kucharik CJ, Jackson RD, and Marín-Spiotta E. Litter quantity, litter chemistry, and soil texture control changes in soil organic carbon fractions under bioenergy cropping systems of the north central U.S. Biogeochemistry. (2019) 143:313–265. doi: 10.1007/s10533-019-00564-7
110. Castellano MJ, Mueller KE, Olk DC, Sawyer JE, and Six J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Global Change Biol. (2015) 21:3200–32095. doi: 10.1111/gcb.12982
111. Heckman K, Hicks Pries CE, Lawrence CR, Rasmussen C, Crow SE, Hoyt AM, et al. Beyond bulk: density fractions explain heterogeneity in global soil carbon abundance and persistence. Global Change Biol. (2022) 28:1178–96. doi: 10.1111/gcb.16023
112. Xu Y, Liu K, Yao S, Zhang Y, Zhang X, He H, et al. Formation efficiency of soil organic matter from plant litter is governed by clay mineral type more than plant litter quality. Geoderma. (2022) 412:115727. doi: 10.1016/j.geoderma.2022.115727
113. Carranca C, Torres MO, and Baeta J. White lupine as a beneficial crop in southern europe. II. Nitrogen recovery in a legume–oat rotation and a continuous oat–oat. Eur J Agron. (2009) 31:190–94. doi: 10.1016/j.eja.2009.05.010
114. Gentile R, Vanlauwe B, and Six J. Litter quality impacts short- but not long-term soil carbon dynamics in soil aggregate fractions. Ecol Appl. (2011) 21:695–7035. doi: 10.1890/09-2325.1
115. Mueller KE, Hobbie SE, Chorover J, Reich PB, Eisenhauer N, Castellano MJ, et al. Effects of litter traits, soil biota, and soil chemistry on soil carbon stocks at a common garden with 14 tree species. Biogeochemistry. (2015) 123:313–27. doi: 10.1007/s10533-015-0083-6
116. Chen J, Heiling M, Resch C, Mbaye M, Gruber R, and Dercon G. Does maize and legume crop residue mulch matter in soil organic carbon sequestration? Agriculture Ecosystems Environ. (2018) 265:123–31. doi: 10.1016/j.agee.2018.06.005
117. Lange M, Eisenhauer N, Sierra CA, Bessler H, Engels C, Griffiths RI, et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat Commun. (2015) 6:6707. doi: 10.1038/ncomms7707
118. Crews. Grain agriculture and the end of the fossil fuel era. J Agriculture Food Systems Community Dev. (2024) 13:55–60. doi: 10.5304/jafscd.2024.133.022
119. Chen H, Mommer L, van Ruijven J, de Kroon H, Fischer C, Gessler A, et al. Plant species richness negatively affects root decomposition in grasslands. J Ecol. (2017) 105:209–185. doi: 10.1111/1365-2745.12650
120. Gartner TB and Cardon ZG. Decomposition dynamics in mixed-species leaf litter. Oikos. (2004) 104:230–465. doi: 10.1111/j.0030-1299.2004.12738.x
121. Aerts R, De Caluwe H, and Beltman B. Plant community mediated vs. Nutritional controls on litter decomposition rates in grasslands. Ecology. (2003) 84:3198–32085. doi: 10.1890/02-0712
122. McLaren JR and Turkington R. Plant identity influences decomposition through more than one mechanism. PloS One. (2011) 6:e237025. doi: 10.1371/journal.pone.0023702
123. Tamura M, Suseela V, Simpson M, Powell B, and Tharayil N. Plant litter chemistry alters the content and composition of organic carbon associated with soil mineral and aggregate fractions in invaded ecosystems. Global Change Biol. (2017) 23:4002–185. doi: 10.1111/gcb.13751
124. Bray SR, Kitajima K, and Mack MC. Temporal dynamics of microbial communities on decomposing leaf litter of 10 plant species in relation to decomposition rate. Soil Biol Biochem. (2012) 49:30–7. doi: 10.1016/j.soilbio.2012.02.009
Keywords: intermediate wheatgrass, Thinopyrum intermedium, legume intercrop, Medicago sativa, stable isotopes 13C, 15N, soil organic carbon
Citation: van der Pol LK, Crews TE and Cotrufo MF (2025) Mechanisms of soil organic matter formation for perennial grain Kernza® under contrasting nitrogen management. Front. Soil Sci. 5:1548577. doi: 10.3389/fsoil.2025.1548577
Received: 19 December 2024; Accepted: 16 April 2025;
Published: 27 May 2025.
Edited by:
Melanie A. Mayes, Oak Ridge National Laboratory (DOE), United StatesReviewed by:
Eduardo Vázquez, Universidad Politécnica de Madrid, SpainGrazia Masciandaro, National Research Council (CNR), Italy
Copyright © 2025 van der Pol, Crews and Cotrufo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura K. van der Pol, bHZhbmRlcnBvbEBsYW5kaW5zdGl0dXRlLm9yZw==
 Laura K. van der Pol
Laura K. van der Pol Timothy E. Crews
Timothy E. Crews M. Francesca Cotrufo
M. Francesca Cotrufo