- Department of Biotechnology, GLA University, Mathura, India
Soil salinity is a significant global issue that adversely affects plant growth by reducing the availability of essential nutrients, leading to deficiencies. This presents challenges for the production of medicinal plants, as their value relies on nutrient-dependent metabolites. To address this, bioremediation strategies using living organisms have gained attention. Native bacteria in saline soils offer a sustainable way to restore soil health and mitigate salt stress. This study investigates the impact of native rhizosphere soil bacteria on the growth and nutritional value of Aloe vera. We screened four bacterial isolates from the rhizosphere of A. vera plants grown in saline soil in the Mathura region of Uttar Pradesh, India, focusing on their nutrient-solubilizing abilities. These bacterial strains demonstrated phosphate solubilization, potassium solubilization, siderophore production, indole-3-acetic acid (IAA) production, and protease activity. Using partial 16S rRNA gene sequencing, bacterial isolates were identified as Paenibacillus sp., Arthrobacter sp., Pseudomonas sp., and Bacillus sp. Subsequently, a pot experiment was conducted to augment the population of these bacteria in the soil and to evaluate their impact on A. vera’s growth and nutritional value. The bacteria were applied both individually and as a consortium. To assess the impact of these inoculations, the nutrient content of leaf gel and various soil health parameters were measured. The results showed that the application of the bacterial consortium yielded higher number of leaves (47%), leaf fresh weight (74%), gel content (33%), and nutritional properties as compared to control treatment (non-inoculated). Furthermore, bacterial inoculation significantly enhanced soil enzymatic activity and increased the soluble nitrate and phosphate content in the experimental soil. In conclusion, the presence of these bacteria in the rhizosphere of A. vera, along with their nutrient-solubilizing activities, enhances nutrient uptake and metabolite synthesis in the host plant under saline soil conditions.
1 Introduction
Aloe vera is a succulent plant belonging to the Asphodelaceae family. It is highly valued in the pharmaceutical, food, and cosmetic industries due to its numerous medicinal applications that have been recognized for thousands of years (1). A. vera is used worldwide in nutraceuticals and personal care products. It is believed to have originated from Africa and the Arabian Peninsula and possesses a unique ability to tolerate dry conditions through a process called Crassulacean acid metabolism (CAM). The plant propagates by producing shoots and has succulent leaves that can store water in their gelatinous pulp. A. vera has been extensively studied for its bioactive compounds, including polyphenols like aloin and aloe-emodin, flavonoids, and polysaccharides such as acemannan and fructan. These metabolite compounds have been associated with various biological effects, including anticancer, antioxidant, antimicrobial, anti-diabetic, and anti-inflammatory properties. The therapeutic potential of A. vera continues to attract researchers and health enthusiasts, encouraging further exploration into its medicinal uses (2).
The beneficial properties of A. vera leaf gel are attributed to its secondary metabolites. The gel consists of 98–99% water, with the remaining 1–2% made up of bioactive molecules such as vitamins, proteins, and phenolic compounds (3). These metabolites help plants to defend against environmental stress and have potential applications in pharmaceuticals and cosmetics. However, these compounds are present in low concentrations, and their production is influenced by environmental factors (4). Among environmental factors, soil nutrient availability is particularly crucial for synthesizing bioactive metabolites. Research has shown that A. vera is sensitive to salt stress and alkaline soils (5, 6). The uptake of key nutrients, particularly phosphorus (P) and potassium (K), is limited in alkaline soils due to reduced availability and the formation of nutrient complexes (Ca-P, Fe-P, Al-P precipitates, micas and feldspars) (7). As a result, there has been increased interest in methods to enhance the metabolites concentrations of A. vera in sustainable ways. Native rhizosphere bacteria in saline soils have gained attention to increases nutrient availability for plants under saline conditions.
Beneficial soil bacteria, known as plant growth-promoting rhizobacteria (PGPR), play a crucial role in nutrient acquisition, stress management, and activating systemic resistance against plant pathogens (8). These bacteria reside in the soil surrounding plant roots, which is rich in nutrients. Root exudates provide a source for carbon (C), N and P for these microorganisms. Additionally, plants release signals that promote the growth of specific microbial communities. In turn, these communities support plant growth and development under both normal and stress conditions (9). PGPRs play an essential role in transforming and acquiring nutrients such as nitrogen N, P, K, and iron (Fe). They solubilize nutrients from inorganic source and facilitate the release of organic sources, primarily through the production of organic acids, hydrogen ion excretion, and enzyme biosynthesis. Organic acids, including gluconic, citric, and acetic acids, help make insoluble nutrients available to plants (8). Bacteria from the Pseudomonas, Arthrobacter, Alcaligenes, Paenibacillus and Bacillus genera are particularly effective at converting soil nutrients into accessible forms to plants (10). Nutrient solubilization is essential for plants’ health and growth because it measures how rapidly plants can absorb these essential nutrients from the soil. Furthermore, PGPRs have also been shown to stimulate the synthesis of secondary metabolites (11–13). However, the interactions between plants and microbes can vary depending on environmental conditions and may be specific to certain plant species. Research on how native microbes in saline soil affect the synthesis of metabolites and the nutritional value of A. vera plants is still limited.
This study focused on the field soil of A. vera cultivated (at small scale) in the Mathura district of Uttar Pradesh, India, located at coordinates N 27° 36.3502’ and E 77° 35.6722’. This region falls within the Yamuna sub-basin, where irrigation predominantly relies on groundwater. Several research efforts have highlighted the high salt content present in both the soil and groundwater in this area (14, 15). The aim of our study was to isolate native rhizosphere bacteria from A. vera grown in saline soil and to characterize these bacteria for their nutrient-solubilizing and other plant growth-promoting (PGP) activities. We hypothesized that augmenting indigenous PGPR isolates, which possess nutrient-solubilizing capabilities, could effectively enhance nutrient uptake by plants, thereby improving metabolite content and nutrient quality in the host plant. To test this hypothesis, we conducted a pot trial using A. vera plants in the same field soil, inoculated with individual and consortium formulations of the isolated bacteria, along with a control group.
2 Materials and methods
2.1 Plant sample collection and isolation of rhizosphere bacteria
The soil samples used for bacterial isolation were collected from the rhizosphere of A. vera plants grown in a horticulture garden at GLA University, Mathura, India. A. vera have been cultivated in this location for many years, suggesting that the soil microbes have acclimatized to the host plants. Plant samples were collected in the first week of June 2022. The field soil type was silty with organic carbon (0.53%), pH (8.9), electrical conductivity (0.46 dS/m) and total dissolved solids in the saturated soil extract (387 ppm). Samples were collected in triplicate from a depth of 15 cm in the root zone. The soil adhered to the roots was obtained by shaking them and scraping off tightly adhering soil for rhizosphere sampling. The samples were stored in sterile plastic bags at 4°C overnight, and were then used for further analysis the next day. Dilution was done in eight clean test tubes (labelled 1 to 8), prefilled with 9 mL of 0.8% saline water. Samples were diluted from 10-1 to 10-8, with the 10-6 and 10-8 dilutions ultimately selected for spreading on agar media. Diluted samples were plated on Nutrient agar (NA), King’s B agar, and Tryptic Soy Agar, all of which were supplemented with cycloheximide to prevent fungal growth. Plates were incubated at 30°C for 48 hours. From these incubations, 33 distinct colonies were identified based on their different morphologies across all three media (data not shown). These colonies were further purified through repeated culturing and preserved in a 20% glycerol stock at −80°C. Finally, all colonies were screened for various PGP activities, and those exhibiting the highest levels of activity were selected for further analysis.
2.2 Evaluation of PGP traits in isolated bacterial strains
The production of indole-3-acetic acid (IAA) was measured using the method described by Gordon and Weber (16). The medium was supplemented with 0.1% tryptophan. A positive result for IAA production was indicated by the development of a pink color.
To assess nitrogen-fixing ability, a repetitive subculturing of bacterial isolates were done on nitrogen-free Jensen’s agar medium and incubated at 37°C until growth was observed (17). A non-nitrogen fixing bacterium was also inoculated on the same medium to serve as a negative control.
Siderophore production was evaluated using Chrome Azurol S (CAS) medium (in brief the CAS agar medium—CAS 0.06 g in 50 ml ddH2O, FeCl3 0.0027 g in 10 ml HCl (10 mM), HDTMA 0.073 g in 40 ml ddH2O and minimal media 9 (MM9) 100 ml, glucose 10 ml (20%), CAS Amino acid solution 30 ml, PIPES 32.24 g, Agar 15 g, and distilled water 750 ml). A yellow to orange halo around the colonies indicated a positive result for siderophore production (18). The iron-producing capacity of the strains was determined by the given formula:
where, D = zone diameter + colony diameter; and d = colony diameter.
P solubilization activity of the bacterial isolates were assessed on Pikovskaya’s agar medium following the method described by Pikovskaya (19). A positive indication for P solubilization was the development of the halo zone around the colonies. The phosphate solubilization index (SI) was determined using the widths of the colony and halo zone, calculated using the formula:
where ZD= zone diameter and CD = colony diameter (20).
K solubilizing activity of bacterial isolates was screened on Aleksandrov agar medium. A positive indication for K solubilization was the development of the halo zone around the colonies. K solubilization index (KSI) was calculated using the formula provided by Saheewala and coworker (21):
Bacterial isolates were also screened for their ability to produce hydrolytic enzymes such as cellulase, xylanase, amylase, pectinase, chitinase and protease. The bacteria were inoculated on a medium supplemented with different enzyme substrates in individual petri plates containing 1% (w/v) carboxymethylcellulose, 1% (w/v) xylan, 1% (w/v) starch, and 1% (w/v) pectin, and then incubated at 28 ± 2°C for 72 hours. After incubation, congo red and iodine solution were flooded on the medium, and the hydrolysis halo zone was identified in the respective enzyme assay (22). Chitinase activity of bacteria was screened on chitin agar plates and kept in the incubator at 35 ± 2°C for up to 6 days. A cleared zone surrounding the bacteria indicated the hydrolysis of chitin (23). Furthermore, the bacterial isolates were tested for protease enzyme activity in skimmed milk agar for 72 hours at 35°C. A characteristic clear zone surrounding the bacterial colony indicated the production of the proteolytic enzyme (24). The entire enzyme activities halo zone diameters were measured by the given formula.
2.3 Molecular characterization of bacterial isolates
Total four bacterial isolates were selected based on their maximum PGP traits and nutrient mineralizing ability for molecular identification through 16S rRNA gene sequencing. Bacterial DNA was extracted using the Nucleo-pore genomic DNA extraction mini kit (Genetix Biotech Asia Pvt. Ltd.). The purity and integrity of DNA was checked by 260/280 ratio and visualized on 1% agarose gel electrophoresis respectively. The molecular characterization of bacterial isolates was done by 16S rRNA gene sequencing using universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). Amplified gene was visualized on agarose gel electrophoresis (1%). The sequences were analyzed through a similarity search using the Nucleotide BLAST (BLASTn) tool. The closest percent similarity, strain and species similarity to the isolates were obtained from the BLAST result and similar sequences were subjected for the alignment in ClustalW in MEGA11 software. The phylogenetic tree was constructed using the neighbor-joining method in MEGA11 software using 1000 Bootstrap value (25). All four bacterial sequences were submitted to the GeneBank NCBI database for accession numbers.
2.4 Plant growth experiment with bacterial inoculation
The pot experiment aimed to augment the population of selected bacteria (GLAU-BT2, GLAU-BT16, GLAU-BT21, and GLAF3) in the rhizosphere and assess their role as individual and in consortium in the growth promotion and nutritional value of A. vera plants. For bacterial inoculum preparation, a 24-hour-old bacterial culture was used, and the cell pellet was resuspended in sterile water to achieve a final concentration of 108 CFU/mL for inoculation. Before development of consortium inoculum, a cross-compatibility test was performed with all four bacterial isolates to ensure their compatibility in terms of growth (Supplementary Figure S3). Two different bacterial strains were tested in various combinations across a total of six plates, along with one plate that contained all four strains together. These were streaked on NA medium and incubated for 48 hours at 35°C. All strains exhibited concurrent growth, suggesting compatibility with one another. Therefore, the four strains were combined to develop a consortium inoculum. For the consortium inoculum, the cell pellets of all four bacterial isolates were mixed to obtain the final concentration of 108 CFU/mL. All bacterial inoculums were then used to soak the pre-sterilized rhizomes for one hour before planting them in 5-liter earthen pots. The same field soil was used for the experiment without sterilization to maintain the original characteristics of the soil along with its native microflora. One plant was maintained per pot. The experiment consisted of six treatment groups: (T1)- Control (non-inoculated), (T2) GLAU-BT2 (Paenibacillus sp.), (T3) GLAU-BT16 (Arthrobacter sp.), (T4) GLAU-BT21 (Pseudomonas sp.), (T5) GLAF3 (Bacillus sp.), (T6) Consortium (T2 + T3 + T4 + T5). Each treatment was conducted with seven replicates. All pots were arranged by following completely randomized design (4). The experiment was conducted at the greenhouse facility, maintaining a temperature of 24 ± 2°C and a 16/8-hour day/night cycle. The plants were watered weekly with sterile water to maintain 50% soil moisture content, and all necessary plant protection measures were followed throughout the experiment.
2.5 Measurement of morpho-anatomical traits of plants
Plants were harvested 90 days after their rhizome plantation. Measurements were taken for leaf growth, as well as the fresh weight of both leaves and roots. The roots were then dried in an oven at 65°C for 48 hours, after which the dry biomass of the root samples was measured. One leaf per plant was collected to determine the nutrient content in the inner leaf gel, with seven replicates for each treatment. Leaf samples were collected in the morning, washed with distilled water, and weighed. Then separated the outer leaf rind and inner leaf gel parts and weighed. The gel content was measured using the formula provided. The remaining samples were homogenized and stored at −20°C until lyophilization.
2.6 Measurement of nutritional properties in the leaf gel
For the analysis of the inner leaf gel, a subsample of 50 mg of lyophilized gel from each sample was heated to ash at 550°C. The resulting extract was digested with 1 mL of 65% nitric acid (HNO3), then diluted to a final volume of 10 mL with deionized water (dH2O) and filtered. Total N content was measured in ashed sample using the Kjeldahl method (26). The total concentration of P was determined through colorimetric analysis. In this process, molybdate and vanadate reacted to form a colored complex, which was measured with a spectrophotometer at a wavelength of 420 nm. Potassium phosphate was used to establish the standard for phosphorus analysis (27). Additionally, the total K concentration was measured using potassium standards (KCl) with a flame photometer (28). All nutrient concentrations were reported as mg/g of dry leaf weight.
The quantification of polyphenols was carried out following the methodology established by Ouahhoud et al. (29). In this protocol, 0.5 mL of a methanolic sample with a concentration of 1 mg/mL. Next, 2 mL of Folin-Ciocalteu reagent was added, followed by 2.5 mL of a 20% sodium carbonate solution. The mixture was incubated for 30 minutes at room temperature, after which the absorbance was measured at 725 nm. A calibration curve was generated using a gallic acid (GA) reference solution, which allows for the quantification of polyphenols. The results were expressed as grams of gallic acid equivalent per 100 g of dry extract.
The phenol-sulfuric acid method was used for quantifying total sugars (30). This assay utilizes the reaction between phenol and concentrated sulfuric acid to detect reducing sugars. This reaction produced a creamy yellow color that corresponds to the concentration of total sugars in the sample. To determine the sugar concentration, optical density measurements were taken at a wavelength of 490 nm (31).
2.7 Analysis of soil health parameters
The pot soil sample was air dried for soil phosphate and nitrate analysis. The soluble phosphate content in the soil was quantified using the method established by Bray & Kurtz (32). In brief, 10 g of soil was mixed with 100 mL of 0.5 N NaHCO3 (pH 8.5). Next, 0.5 g of activated carbon was added to the mixture, which was then incubated at 25°C while shaking at 140 rpm for one hour and filtered at the end. The resulting filtrate was combined with 2.5 mL of sulfo-molybdic reagent. Following this, 0.5 mL of 1% ascorbic acid and 5 mL of distilled water were added to achieve a total volume of 10 mL. The samples were then heated in a water bath at 80°C for 15 minutes. After cooling, the absorbance of the solutions was measured at 650 nm. The soluble phosphate concentration was determined by comparing the absorbance readings against a standard curve prepared from KH2PO4 (10–100 mg).
Nitrate soil content was determined using the protocol established by Chaney (33). In brief, 10 g of soil was combined with 40 mL of 2 M KCl, followed by 30 minutes of shaking at 140 rpm for incubation. The mixture was then filtered and 10 mL of the resulting extract was transferred into glass tubes and combined with 1 mL of NaOH and 1 mL of a 0.5% sodium salicylate solution. The tubes were subjected to evaporation to concentrate on the solution. The residue was reconstituted in 2 mL of sulfuric acid, and then 15 mL each of distilled water and NaOH mixed with sodium potassium tartrate were added. A blank control was prepared using 1 mL of the 0.5% sodium salicylate solution in 10 mL of distilled water. The absorbance of the resultant solution was measured at 415 nm, and the nitrate concentration was quantified against a standard curve derived from sodium nitrate (10–100 mg).
The activities of acid and alkaline phosphatase in soil samples were evaluated using the methodology outlined by Tabatabai and Bremner (34). Specifically, 0.5 g of moist soil was mixed with 2 mL of a modified universal buffer—pH 6.5 for acid phosphatase and pH 11 for alkaline phosphatase—along with 0.5 mL of a 0.05 M p-nitrophenyl phosphate (PNP) substrate solution. The resulting color change, indicating the production of p-nitrophenol (p-NP) from phosphomonoesterase activity, was measured spectrophotometrically at 400 nm. Quantitative analysis of p-NP was carried out using a pre-established calibration curve. A control solution without soil was included for comparison. Phosphomono esterase activity is defined as the amount of enzyme required to release 1 µg of p-NP from 1 g of dried soil at 37°C for one hour (34).
Protease activity was assessed using the protocol established by Ladd and Butler (35). In this assay, 0.5 g of soil was combined with 2.5 mL of 0.2 M phosphate buffer (pH 7.0) and 0.5 mL of a 0.03 M N-benzoyl-l-arginine amide (BAA) substrate solution. The release of ammonium during the enzymatic reaction was measured spectrophotometrically at 690 nm. One unit of protease activity is defined as the amount of enzyme that liberates ammonium equivalents from BAA per hour.
2.8 Data processing and statistical analysis
The assumptions of analysis of variance (ANOVA) were checked first by the Shapiro-Wilk test for normality and Levene’s test for homogeneity of variances using the “car” package in R 4.2.2. (R Core Team 2022). The data were analyzed by one-way ANOVA, followed by post hoc Tukey’s test to separate treatment means if ANOVA results were significant (p< 0.05). Correlation analysis was computed by the GGally library in the R package.
3 Results
3.1 A. vera’s rhizosphere contains various strains of PGPR
All isolates were tested for their PGP abilities and nutrient solubilization traits on agar plates. Among them, four isolates GLAU-BT2, GLAU-BT16, GLAU-BT21, and GLAF3 demonstrated the most promising results across all tested activities. These four isolates were found to produce IAA, as indicated by a color change in the media from yellow to pink following the utilization of L-tryptophan. Highest IAA production was observed with GLAU-BT2 isolate (23.4 ± 3.9 mg/L).
Siderophore production was observed in all four isolates, with GLAU-BT21 showing the highest iron production capacity (solubilization index—1.38 ± 0.03), as evidenced by the orange coloration of the media surrounding the inoculated culture (Supplementary Table S1). None of the bacterial isolates demonstrated nitrogen fixation ability on Jensen agar media.
In terms of nutrient solubilization, all four strains successfully utilized tricalcium phosphate, resulting in positive phosphate solubilization, which formed clear halo zones around their colonies. Among these, GLAU-BT2 had the highest phosphate solubilization index (3.22 ± 0.043). Additionally, all four isolates were able to utilize inorganic potassium, displaying positive results for potassium solubilization and forming clear zones around their colonies, again GLAU-BT2 achieving the maximum potassium solubilization index (3.75 ± 0.023) (Supplementary Table S1).
Furthermore, bacterial isolates GLAU-BT2 (3.33 ± 0.09), GLAU-BT21 (1.57 ± 0.3) and GLAF3 (3.2 ± 0.3) exhibited positive amylase activity. Bacterial isolates GLAU-BT2 (3.04 ± 0.6), GLAU-BT16 (3.16 ± 0.1) and GLAF3 (2.65 ± 0.2) showed positive cellulase activity, while all four bacterial isolates demonstrated protease activity (Supplementary Figure S1). None of the bacterial isolates showed xylanase and pectinase activities.
The phylogenetic analysis based on 16S rRNA gene (~ 1.5 kb) sequencing indicates that the bacterial isolates GLAU-BT2, GLAU-BT16, GLAU-BT21, and GLAF3 are closely related to the genera Paenibacillus, Arthrobacter, Pseudomonas, and Bacillus, respectively (Supplementary Figure S2). The sequences have been submitted to the GenBank NCBI database and have been assigned the following accession numbers: GLAU-BT2 (PV083208), GLAU-BT16 (PV083209), GLAU-BT21 (PV083210), and GLAF3 (OR389156).
3.2 The consortium inoculum promotes growth parameters of plants
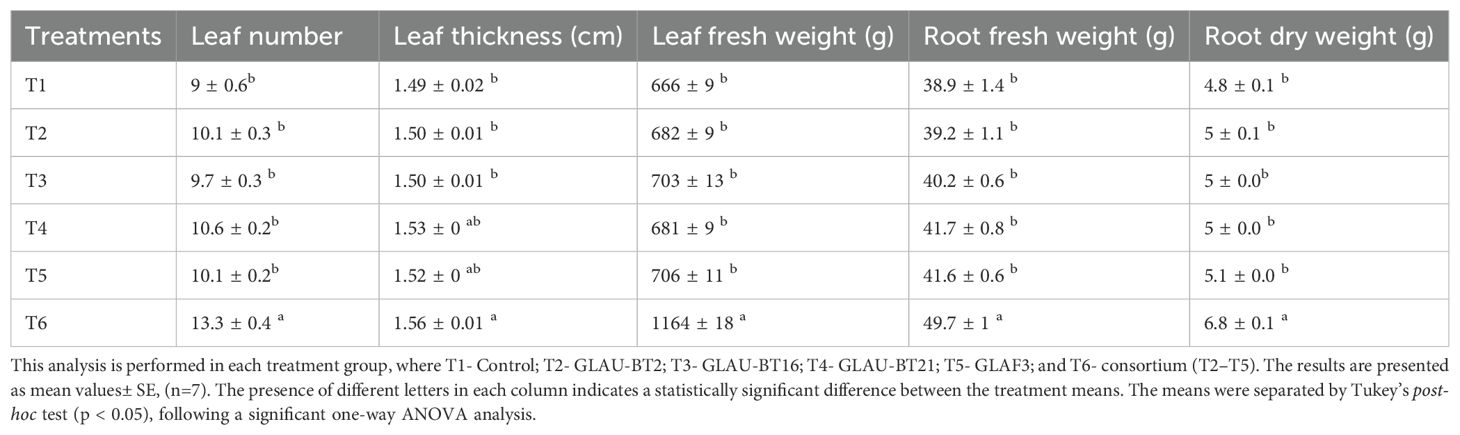
The effect of individual and consortium bacterial inoculums on the growth of A. vera plants was investigated. Our results demonstrated a significant impact of the treatments on several growth parameters, including the number of leaves (F(5,36) = 14.33, p<0.001), fresh weight of leaves (F(5,36) = 269.4, p<0.001), leaf thickness (F(5,36) = 3.561, p=0.01), fresh weight of roots (F(5,36) = 16.76, p<0.001) and dry weight of roots (F(5,36) = 44.35, p<0.001) of the plant. Plants treated with the consortium showed higher growth parameters compared to the other treatments. For instance, bacterial consortium inoculated plants exhibited higher number of leaves (47%) and leaf fresh weight (74%) as compared to control treatment (non-inoculated) (Table 1).
3.3 The consortium inoculum induces leaf gel content and its nutritional properties
The gel content was measured in the leaves of different treatment plants, and it was found to be significantly affected by bacterial treatments (F(5,36) = 34.07, p<0.001) (Figure 1A). The highest gel content was observed in plants treated with a bacterial consortium (33%) as compared to control. Similarly, the nutrient contents, including N (F(5,36) = 100.9, p<0.001), P (F(5,36) = 66.39, p<0.001) and K (F(5,36) = 19.45, p<0.001), were significantly affected by bacterial treatments and found higher in the leaves of consortium-treated plants compared to the others (Figures 1B–D). Additionally, we measured metabolites, specifically polyphenols and total sugar content in the gel. A significantly higher content of polyphenols (F(5,36) = 47.72, p<0.001) and total sugar (F(5,36) = 47.48, p<0.001) were found in bacterial-inoculated plants as compared to control (non-inoculated) plants (Figures 1E, F).
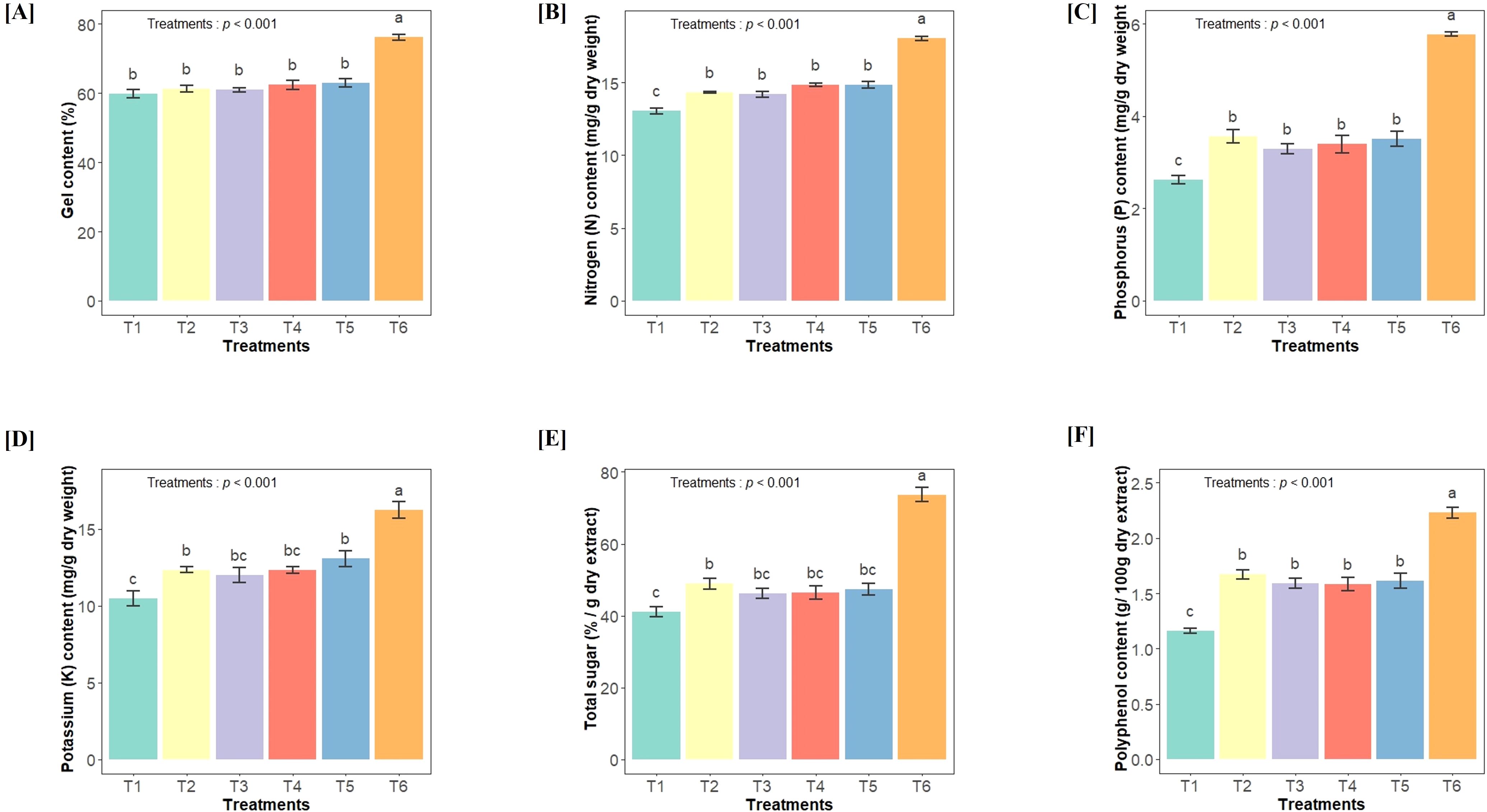
Figure 1. Effect of different bacterial treatments on A vera gel content and its nutritional properties (A) Total gel content in leaf, (B) total N content, (C) total P content, (D) total K content, (E) total sugar, (F) polyphenol content. This analysis is performed in each treatment group, where T1- Control soil (no additional inoculation); T2- GLAU-BT2 inoculation; T3- GLAU-BT16 inoculation; T4- GLAU-BT21 inoculation; T5- GLAF3 inoculation; and T6- consortium inoculation (T2–T5). The results are presented as mean values± SE, (n=7). The presence of different letters on each bar indicates a statistically significant difference between the treatment means. The means were separated by Tukey’s post-hoc test (p < 0.05), following a significant one-way ANOVA analysis.
3.4 Bacterial inoculation improves soil health parameters
To evaluate the impact of different bacterial inoculations on experimental soil, we analyzed the enzymatic activity as well as the soluble phosphate and nitrate contents in the soil. Our results revealed a significant effect of bacterial treatments on the total soluble phosphate (F(5,36) = 37.23, p<0.001) and soluble nitrate (F(5,36) = 32.16, p<0.001) levels, with the soil inoculated with the consortium showing higher concentrations of both nutrients compared to the other treatments (Figures 2A, B). There was no significant effect of the treatments on acid phosphatase activity. However, we did observe a significant effect of bacterial treatments on alkaline phosphatase (F(5,36) = 26.94, p<0.001) and protease (F(5,36) = 30.93, p<0.001) activities in the soil, with the consortium-inoculated soil displaying higher enzymatic activities than the other treatments (Figures 2C–E).
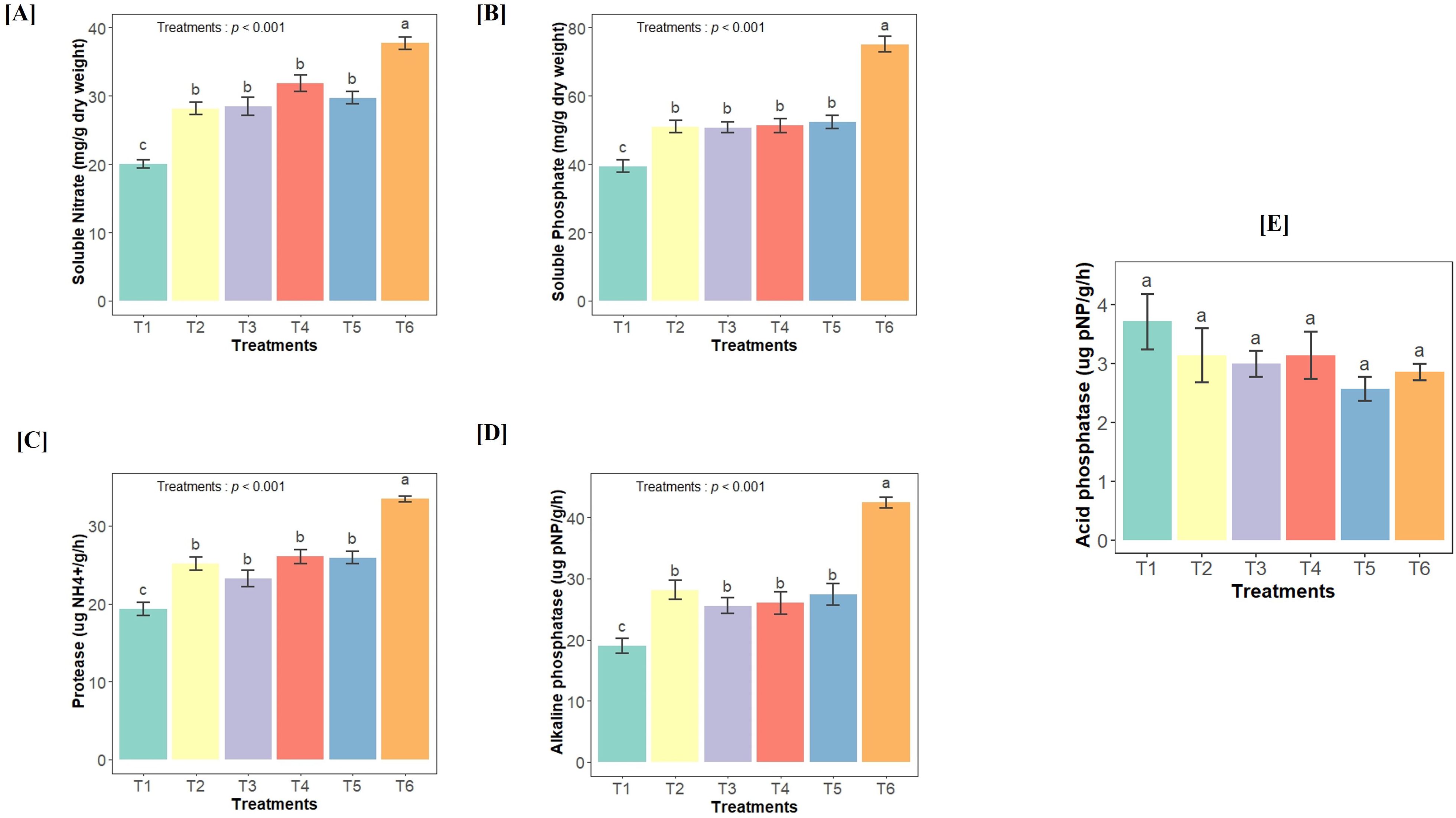
Figure 2. Effect of different bacterial treatments on soil health parameters (A) soluble nitrate content, (B) soluble phosphate content, (C) protease enzyme activity, (D) alkaline phosphatase activity, and (E) acid phosphatase activity. This analysis is performed in each treatment group, where T1- Control soil (no additional inoculation); T2- GLAU-BT2 inoculation; T3- GLAU-BT16 inoculation; T4- GLAU-BT21 inoculation; T5- GLAF3 inoculation; and T6- consortium inoculation (T2–T5). The results are presented as mean values± SE, (n=7). The presence of different letters on each bar indicates a statistically significant difference between the treatment means. The means were separated by Tukey’s post-hoc test (p < 0.05), following a significant one-way ANOVA analysis.
3.5 Soil enzymatic activities correlate with nutritional properties of leaf gel
A correlation analysis was conducted to examine the effect of soil enzymatic activities on the nutrient content of leaf gel. A significant positive correlation was found between alkaline phosphatase and protease activities in the P and N content of leaf gel, respectively. However, no significant correlation was observed between acid phosphatase activity and the nutrient content of the gel. Specifically, alkaline phosphatase exhibited a significant positive correlation with the total P content in the gel for both consortium (0.976) and individual inoculum-treated plants (0.969). Similarly, protease activity showed a significant positive correlation with the total N content in the gel, with values of 0.976 for consortium-treated plants and 0.820 for individual inoculum-treated plants. Both enzymatic activities also displayed positive correlations with the levels of polyphenols and total sugars in plants treated with the consortium (Figure 3).
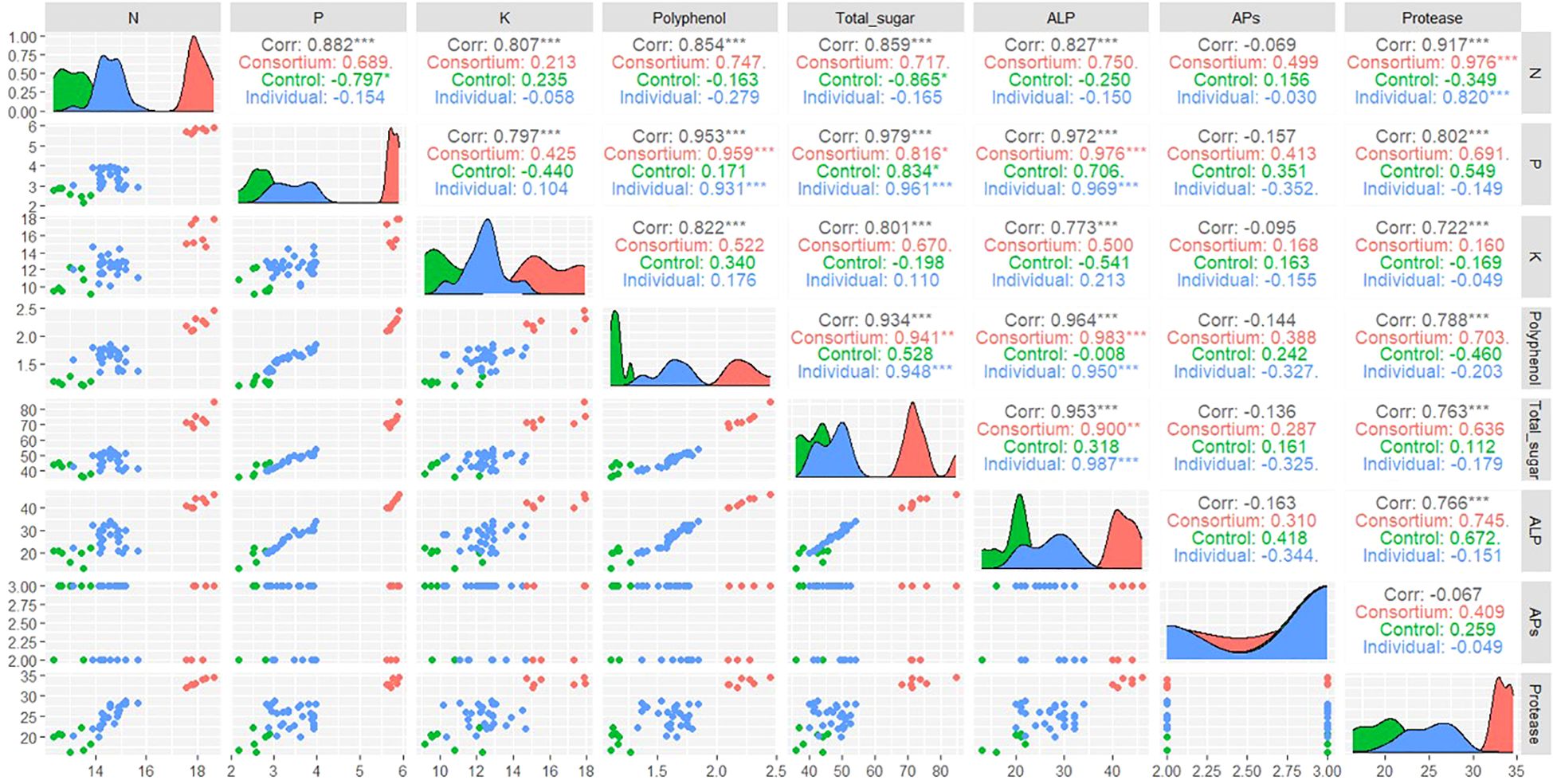
Figure 3. Correlation analysis between soil enzymatic activities and nutritional properties of A. vera’s gel. Analysis was performed in R package with GGally library. Results are shown in overall correlation coefficient (gray color) along with treatment wise (in different color), where Consortium- consortium inoculum of all four bacterial isolates, Control- control soil (no additional inoculation) and Individual- All four bacterial individual inoculation. Asterisk represents significant p value *** (p<0.001), ** (p<0.01), * (p<0.05), (p>0.1). N, Nitrogen; P,Phosphorus; K, Potassium; ALP, alkaline phosphatase; Aps, acid phosphatase.
4 Discussion
The development of sustainable and eco-friendly agricultural technologies has become a vibrant area of research. The use of soil microbes, specially PGPR as a biofertilizers, has attracted significant attention as a sustainable method for enhancing plant production (36). In this study, we investigated the effects of indigenous bacterial isolates within the A. vera rhizosphere, and the results were promising. Our findings indicated that bacterial inoculation treatments positively influenced growth parameters. Specifically, IAA production is responsible for growth promotion as well as development of the plant and their metabolites. Similarly, the nutrient-solubilizing activities of the bacterial isolates were found to significantly enhance the nutritional value of A. vera gel. Several other studies have demonstrated that application of PGPR can improve plant growth parameters such as leaf number, leaf width, plant height, and overall yield of A. vera (11–13). For instance, the inoculation of A. vera with PGPR and mycorrhiza has been associated with increased plant height and nutritional value under normal as well as drought and heavy metal stress conditions (4, 11). Additionally, Rai et al. (37) observed that phosphate-solubilizing bacteria (PSB) improved A. vera growth by enhancing phosphorus dissolution (37). Other studies have also supported the positive effects of PSB on plant growth (38). The consortium inoculation had a significant impact on both fresh and dry weight, while individual treatments did not show such effects. This indicates a synergistic interaction among the four bacterial isolates that promotes plant growth. One possible explanation for this improvement could be enhanced biofilm formation or quorum sensing during consortium inoculation, which may have increased root colonization efficiency (39). As a result, this leads to better overall growth and nutrient uptake. Microbial inoculants, particularly those containing multiple isolates, resulted in the greatest increases in leaf dry weight of A. vera (13). Recent studies have shown that microbial consortium is more effective in enhancing growth parameters, such as dry biomass as compared to individual isolates (38–40). The benefits of PGPR in enhancing plant biomass are often associated with improved nutrient uptake (41–43). This study demonstrates that consortium inoculation enhances leaf and root biomass, highlighting the significance of indigenous microbial populations for nutrient uptake and plant health. While previous research recognizes the benefits of PGPR, their effects can vary based on environmental conditions and host genotype. Our findings emphasize the role of native microflora in promoting host plant growth in saline soil, supporting our hypothesis that these PGPR enhance plant development.
This study examined the effect of PGPR inoculation on the nutritional value and gel yield of A. vera, as the gel is the most commercially valuable part of the plant. The results showed a significant increase in gel content in the leaves of plants treated with a consortium of indigenous bacteria, while the gel content remained constant in the individual treatments and control group. Research has demonstrated that beneficial soil microbes enhance water and mineral absorption in the soil, which can boost plant yield and increase chlorophyll content, leading to greater leaf fresh weight and increased gel amounts in A. vera (12, 44, 45). In addition, inoculation with a consortium having phosphate solubilizing activities led to an increase in P content in gel. Furthermore, N content increased in bacterial-inoculated plant’s gel, suggesting that indigenous bacteria enhance nitrogen uptake in A. vera (13). This effect may be due to the protease activity of the bacteria, which releases bound forms of nitrogen in the soil (46). Other studies have also shown that bacterial inoculation enhances the uptake of macro and micro-nutrients, which may improve stress tolerance in plants (4, 12). In addition, the relationship between nutrient status and the accumulation of secondary metabolites in A. vera gel was also investigated. We measured the polyphenol and total sugar content in dry gel samples. The inoculation of bacterial isolates positively influenced the total sugar and polyphenol contents in both individual and consortium treatments. The increased levels of total soluble sugar and polyphenols highlight their role in salinity resilience and enhance the commercial value of these findings. These findings are consistent with previous studies that reported an increase in total phenol content and specific metabolites (acemannan and aloin) under the inoculation of PGPR (11, 13). Research has also shown that inoculating nutrient-solubilizing bacteria can enhance the production of metabolites in A. vera and other plant species (38, 47, 48).
Nutrient content in soil serves as a critical indicator of soil quality. This study demonstrates the impact of consortium inoculation on soluble phosphate and nitrate levels. The bacterial consortia enhance nutrient availability in the rhizosphere of A. vera, likely due to the nutrient-solubilizing activities of the isolated bacterial strains. Isolated bacterial strains can solubilize nutrients that are unavailable, such as phosphate, potassium, and nitrogen, by producing specific hydrolase enzymes and organic acids that lower soil pH. Concurrent research has shown that certain plant PGPR effectively convert nutrients like phosphate, zinc, and potassium into forms that plants can utilize (38, 49–51). The ability of PGPR to solubilize nutrients significantly increases their availability for plant uptake, thereby improving soil quality (39, 40). Moreover, soil enzyme activities serve as effective bioindicators of soil quality. Our findings indicate that inoculating microbial consortia significantly increased the activities of alkaline phosphatase and protease in the experimental soil. Previous studies have documented comparable outcomes in various plant species (39, 40, 52, 53). Alkaline phosphatase, a key enzyme in the phosphorus cycle, has a strong correlation with P content in leaf gel. This correlation aids in the solubilization of insoluble phosphate, helping to meet plant nutritional requirements (39, 54). In contrast, acid phosphatase content was found to be very low and did not show a correlation with P content. This may be due to the alkaline nature of the experimental soil, which does not support the production of acid phosphatase (53). Protease, another significant enzyme, contributes to the nitrogen cycle by mineralizing nitrates from complex nitrogen compounds such as proteins, DNA, and chitin (55, 56). Overall, the positive correlation between alkaline phosphatase and protease activities with P and N content, respectively, indicates organic matter turnover and improves soil fertility. The inoculation of consortium boosted nutrient availability in the soil, thereby enhancing plant nutrition and growth.
5 Conclusion
Inoculating soil with a consortium of four indigenous bacterial isolates significantly improved soil quality and enhanced key traits of A. vera, such as gel content and nutritional components, including N, P, K, polyphenols, and total sugar. Notably, there was a 33% increase in leaf gel content under consortium inoculation, making this approach both economically and ecologically advisable for sustainable production of A. vera’s gel. Furthermore, consortium inoculation significantly increased soil enzymatic activities, leading to higher levels of soluble nitrate and phosphate in the soil, which positively correlated with the N and P content in leaf gel. Overall, this study lays the groundwork for further research aimed at boosting the production of valuable compounds in A. vera through field trials, while highlighting the role of indigenous nutrient-solubilizing bacteria in promoting host plant growth.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
NC: Formal analysis, Investigation, Methodology, Writing – original draft. HS: Supervision, Validation, Visualization, Writing – review & editing. AV: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their gratitude to Prof. Shoorvir Singh, Head of the Department of Biotechnology at GLA University, Mathura, for his unwavering support during this experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsoil.2025.1576176/full#supplementary-material
References
1. Malmir M, Serrano R, Caniça M, Silva-Lima B, and Silva O. A comprehensive review on the medicinal plants from the genus Asphodelus. Plants. (2018) 7(1):20. doi: 10.3390/plants7010020
2. Adlakha K, Koul B, and Kumar A. Value-added products of Aloe species: Panacea to several maladies. South Afr J Botany. (2022) 147:1124–35. doi: 10.1016/j.sajb.2020.12.025
3. Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. (2008) 13:1599–616. doi: 10.3390/molecules13081599
4. Fatima A, Shabaan M, Ali Q, Malik M, Asghar HN, Aslam M, et al. Integrated application of metal tolerant P. fluorescens and press mud for conferring heavy metal tolerance to Aloe vera (Aloe barbadensis). Plant Stress. (2024) 11:100333. doi: 10.1016/j.stress.2023.100333
5. Moghbeli E, Fathollahi S, Salari H, Ahmadi G, Saliqehdar F, Safari A, et al. Effects of salinity stress on growth and yield of Aloe vera L. J Medicinal Plants Res. (2012) 6:3272–7. doi: 10.5897/JMPR11.1698
6. Jamal-Ali O, Eshaq M, Samaneh F, and Asqar E. Salinity stress effects changed during Aloe vera L. vegetative growth. J Stress Physiol Biochem. (2012) 8:152–8.
7. Yahaya SM, Mahmud AA, Abdullahi M, and Haruna A. Recent advances in the chemistry of nitrogen, phosphorus and potassium as fertilizers in soil: a review. Pedosphere. (2023) 33:385–406. doi: 10.1016/j.pedsph.2022.07.012
8. Vaishnav A, Jain S, Kasotia A, Kumari S, Gaur RK, and Choudhary DK. Molecular mechanism of benign microbe-elicited alleviation of biotic and abiotic stresses for plants. Approaches to Plant Stress their management. (2014), 281–95. doi: 10.1007/978-81-322-1620-9_16
9. Chandel NS, Singh HB, and Vaishnav A. Mechanistic understanding of metabolic cross-talk between Aloe vera and native soil bacteria for growth promotion and secondary metabolites accumulation. Front Plant Sci. (2025) 16:1577521. doi: 10.3389/fpls.2025.1577521
10. Yadav AN, Verma P, Singh B, Chauhan VS, Suman A, and Saxena AK. Plant growth promoting bacteria: biodiversity and multifunctional attributes for sustainable agriculture. Adv Biotechnol Microbiol. (2017) 5:1–6. doi: 10.19080/AIBM.2017.05.555671
11. Khajeeyan R, Salehi A, Dehnavi MM, Farajee H, and Kohanmoo MA. Growth parameters, water productivity and aloin content of Aloe vera affected by mycorrhiza and PGPR application under different irrigation regimes. South Afr J Botany. (2022) 147:1188–98. doi: 10.1016/j.sajb.2021.02.026
12. Khajeeyan R, Salehi A, Movahhedi Dehnavi M, Hamidian M, and Hazrati S. Evaluation of the benefits of plant growth-promoting rhizobacteria and mycorrhizal fungi on biochemical and morphophysiological traits of Aloe barbadensis Mill under water deficit stress. Sci Rep. (2024) 14:14480. doi: 10.1038/s41598-024-64878-9
13. Nikolaou CN, Chatziartemiou A, Tsiknia M, Karyda AG, Ehaliotis C, and Gasparatos D. Calcium-and magnesium-enriched organic fertilizer and plant growth-promoting Rhizobacteria affect soil nutrient availability, plant nutrient uptake, and secondary metabolite production in Aloe vera (Aloe barbadensis Miller) grown under field conditions. Agronomy. (2023) 13:482. doi: 10.3390/agronomy13020482
14. Avliyakulov MA, Kumari M, Rajabov NQ, and Durdiev NK. Characterization of soil salinity and its impact on wheat crop using space-borne hyperspectral data. Geoinformation support Sustain Dev territories. (2020) 26:271–85. doi: 10.35595/2414-9179
15. Kumar A, Sharma AK, and Rani A. Transport of solutes under transient flow conditions–A case study–Yamuna river sub basin (Kosi Kalan to Agra). Int Soil Water Conserv Res. (2015) 3:209–23. doi: 10.1016/j.iswcr.2015.06.004
16. Gordon SA and Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. (1951) 26:192. doi: 10.1104/pp.26.1.192
17. Reang L, Bhatt S, Tomar RS, Joshi K, Padhiyar S, Vyas UM, et al. Plant growth promoting characteristics of halophilic and halotolerant bacteria isolated from coastal regions of Saurashtra Gujarat. Sci Rep. (2022) 12:4699. doi: 10.1038/s41598-022-08151-x
18. Schwyn B and Neilands J. Universal chemical assay for the detection and determination of siderophores. Analytical Biochem. (1987) 160:47–56. doi: 10.1016/0003-2697(87)90612-9
19. Pikovskaya R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. (1948) 17:362–70.
20. Thakur R, Srivastava S, and Yadav S. Multitrait Pseudomonas sp. isolated from the rhizosphere of Bergenia ciliata acts as a growth-promoting bioinoculant for plants. Front Sustain Food Systems. (2023) 7:1097587. doi: 10.3389/fsufs.2023.1097587
21. Saheewala H, Sanadhya S, Upadhyay SK, Mohanty SR, and Jain D. Polyphasic characterization of indigenous potassium-solubilizing bacteria and its efficacy studies on maize. Agronomy. (2023) 13:1919. doi: 10.3390/agronomy13071919
22. Shrestha S, Chio C, Khatiwada JR, Kognou AL, and Qin W. Optimization of multiple enzymes production by fermentation using lipid-producing Bacillus sp. Front Microbiol. (2022) 13:1049692. doi: 10.3389/fmicb.2022.1049692
23. Kotb E, Alabdalall AH, Alghamdi AI, Ababutain IM, Aldakeel SA, Al-Zuwaid SK, et al. Screening for chitin degrading bacteria in the environment of Saudi Arabia and characterization of the most potent chitinase from Streptomyces variabilis Am1. Sci Rep. (2023) 13:11723. doi: 10.1038/s41598-023-38876-2
24. Sritongon N, Boonlue S, Mongkolthanaruk W, Jogloy S, and Riddech N. The combination of multiple plant growth promotion and hydrolytic enzyme producing rhizobacteria and their effect on Jerusalem artichoke growth improvement. Sci Rep. (2023) 13:5917. doi: 10.1038/s41598-023-33099-x
25. Panchami PS, Geetha Thanuja K, and Karthikeyan S. Isolation and characterization of indigenous plant growth-promoting rhizobacteria (PGPR) from cardamom rhizosphere. Curr Microbiol. (2020) 77:2963–81. doi: 10.1007/s00284-020-02116-x
26. Bremner JM. Total nitrogen. In: Methods of soil analysis. John Wiley & Sons, Ltd, Hoboken, NJ, USA (1965). p. 1149–78.
27. Chapman HD and Pratt PF. Methods of analysis for soils, plants and waters. Soil Science. (1962) 93(1):68.
28. Patterson BD, MacRae EA, and Ferguson IB. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Analytical Biochem. (1984) 139:487–92. doi: 10.1016/0003-2697(84)90039-3
29. Ouahhoud S, Khoulati A, Kadda S, Bencheikh N, Mamri S, Ziani A, et al. Antioxidant activity, metal chelating ability and dna protective effect of the hydroethanolic extracts of crocus sativus stigmas, tepals and leaves. Antioxidants. (2022) 11:932. doi: 10.3390/antiox11050932
30. Nielsen SS. Phenol-sulfuric acid method for total carbohydrates. Food Anal Lab manual. (2010), 47–53. doi: 10.1007/978-1-4419-1463-7
31. ABED AKM. Determine of carbohydrates, protein and phenolic compounds content in pollen grains of three date palm Phoenix dactylifera male cultivars. Basrah J For Date Palm Res. (2005) 4.
32. Bray RH and Kurtz LT. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. (1945) 59:39–46. doi: 10.1097/00010694-194501000-00006
33. Chaney K. Effect of nitrogen fertilizer rate on soil nitrate nitrogen content after harvesting winter wheat. J Agric Sci. (1990) 114:171–6. doi: 10.1017/S0021859600072166
34. Tabatabai MA and Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem. (1969) 1:301–7. doi: 10.1016/0038-0717(69)90012-1
35. Ladd JN and Butler JH. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol Biochem. (1972) 4:19–30. doi: 10.1016/0038-0717(72)90038-7
36. Aloo BN, Tripathi V, Makumba BA, and Mbega ER. Plant growth-promoting rhizobacterial biofertilizers for crop production: The past, present, and future. Front Plant Science. (2022) 13:1002448. doi: 10.3389/fpls.2022.1002448
37. Rai A, Rai PK, and Singh S. Characterization of phosphate solubilizing fluorescent pseudomonads from the rhizosphere of Aloe vera (L.). Arch Agron Soil Sci. (2018) 64:1032–40. doi: 10.1080/03650340.2017.1407869
38. Devi R, Kaur T, Negi R, Kour D, Kumar S, Yadav A, et al. Bioformulation of mineral solubilizing microbes as novel microbial consortium for the growth promotion of wheat (Triticum aestivum) under the controlled and natural conditions. Heliyon. (2024) 10. doi: 10.1016/j.heliyon.2024.e33167
39. Ferioun M, Zouitane I, Bouhraoua S, Belahcen D, Srhiouar N, Louahlia S, et al. PGPR consortia promote soil quality and functioning in barley rhizosphere under different levels of drought stress. Ecol Front. (2024) 45(2):444–54. doi: 10.1016/j.ecofro.2024.12.001
40. Jabborova D, KanNepalli A, Davranov K, Narimanov A, Enakiev Y, Syed A, et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci Rep. (2021) 11:22081. doi: 10.1038/s41598-021-01337-9
41. Hungria M, Rondina AB, Nunes AL, Araujo RS, and Nogueira MA. Seed and leaf-spray inoculation of PGPR in brachiarias (Urochloa spp.) as an economic and environmental opportunity to improve plant growth, forage yield and nutrient status. Plant Soil. (2021) 463:171–86. doi: 10.1007/s11104-021-04908-x
42. Hnini M, Rabeh K, and Oubohssaine M. Interactions between beneficial soil microorganisms (PGPR and AMF) and host plants for environmental restoration: A systematic review. Plant Stress. (2024) 10:100391. doi: 10.1016/j.stress.2024.100391
43. Rana KL, Negi R, Sharma B, Yadav A, Devi R, Kaur T, et al. Potential effect of novel endophytic nitrogen fixing diverse species of Rahnella on growth promotion of wheat (Triticum aestivum L.). J Crop Sci Biotechnol. (2024) 22:1–1. doi: 10.1007/s12892-024-00254-3
44. Sheng M, Tang M, Chen H, Yang B, Zhang F, and Huang Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. (2008) 18:287–96. doi: 10.1007/s00572-008-0180-7
45. Yousefi Rad M. Effects of mycorrhiza on quantitative and qualitative traits of Aloe vera. Iranian J Plant Physiol. (2016) 6:1637–42. doi: 10.30495/ijpp.2016.539829
46. Baraniya D, Puglisi E, Ceccherini MT, Pietramellara G, Giagnoni L, Arenella M, et al. Protease encoding microbial communities and protease activity of the rhizosphere and bulk soils of two maize lines with different N uptake efficiency. Soil Biol Biochem. (2016) 96:176–9. doi: 10.1016/j.soilbio.2016.02.001
47. Kavian S, Safarzadeh S, and Yasrebi J. Zinc improves growth and antioxidant enzyme activity in Aloe vera plant under salt stress. South Afr J Botany. (2022) 147:1221–9. doi: 10.1016/j.sajb.2022.04.011
48. Singha PS, Ghosh R, Firdaus SB, and Ghosh D. Effects of Biofertilizers in Improving the Growth and Development of the Traditional Medicinal Plant A. vera L. (Aloe barbadensis Miller). Curr Traditional Med. (2024) 10:97–108. doi: 10.2174/2215083810666230330151402
49. Yadav J, Verma JP, Jaiswal DK, and Kumar A. Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol engineering. (2014) 62:123–8. doi: 10.1016/j.ecoleng.2013.10.013
50. Etesami H and Adl SM. Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. Phyto-Microbiome Stress Regul. (2020), 147–203. doi: 10.1007/978-981-15-2576-6_9
51. Meena VS, Meena SK, Verma JP, Kumar A, Aeron A, Mishra PK, et al. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: a review. Ecol Engineering. (2017) 107:8–32. doi: 10.1016/j.ecoleng.2017.06.058
52. Ren H, Lv C, Fernández-García V, Huang B, Yao J, and Ding W. Biochar and PGPR amendments influence soil enzyme activities and nutrient concentrations in a eucalyptus seedling plantation. Biomass Conversion Biorefinery. (2021) 11:1865–74. doi: 10.1007/s13399-019-00571-6
53. Tirry N, Kouchou A, Laghmari G, Lemjereb M, Hnadi H, Amrani K, et al. Improved salinity tolerance of Medicago sativa and soil enzyme activities by PGPR. Biocatalysis Agric Biotechnol. (2021) 31:101914. doi: 10.1016/j.bcab.2021.101914
54. Khan F, Siddique AB, Shabala S, Zhou M, and Zhao C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants. (2023) 12:2861. doi: 10.3390/plants12152861
55. Hu R, Wang XP, Xu JS, Zhang YF, Pan YX, and Su X. The mechanism of soil nitrogen transformation under different biocrusts to warming and reduced precipitation: From microbial functional genes to enzyme activity. Sci Total Environment. (2020) 722:137849. doi: 10.1016/j.scitotenv.2020.137849
Keywords: Aloe vera, leaf gel content, metabolites, PGPR, saline soil, soil enzymatic activity, soil health
Citation: Chandel NS, Singh HB and Vaishnav A (2025) Disentangling the functioning of native soil microbes in enhancing nutritional value of Aloe vera and soil health parameters. Front. Soil Sci. 5:1576176. doi: 10.3389/fsoil.2025.1576176
Received: 19 February 2025; Accepted: 29 May 2025;
Published: 24 June 2025.
Edited by:
Sapna Langyan, Indian Council of Agricultural Research (ICAR), IndiaCopyright © 2025 Chandel, Singh and Vaishnav. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anukool Vaishnav, YW51a29vbC52YWlzaG5hdkBnbGEuYWMuaW4=
 Neha Singh Chandel
Neha Singh Chandel H. B. Singh
H. B. Singh Anukool Vaishnav
Anukool Vaishnav