Abstract
Objective: This study aims to comprehensively analyze the influence of spontaneous tumor rupture on the prognosis of hepatocellular carcinoma patients following hepatic resection.
Methods: We systematically searched four online electronic databases, including PubMed, Embase, Web of Science, and Cochrane Library, for eligible studies published from inception to March 2021. The main endpoints were overall survival (OS) and disease-free survival (DFS).
Results: This meta-analysis included 21 observational articles with 57,241 cases. The results revealed that spontaneous tumor rupture was associated with worse OS (hazard ratio (HR), 1.65; 95% confidence interval (CI), 1.33–2.05) and DFS (HR, 1.42; 95% CI, 1.12–1.80) in resectable hepatocellular carcinoma patients. This phenomenon was observed in most subgroups, which were classified by recorded survival time, age, country, alpha-fetoprotein (AFP) concentration, liver cirrhosis, and microvascular invasion. However, in subgroups of macrovascular invasion positive, spontaneous tumor rupture was not a risk factor for OS (HR, 1.55; 95% CI, 0.99–2.42) and DFS (HR, 1.23; 95% CI, 0.91–1.65) in hepatocellular carcinoma patients after hepatectomy. For macrovascular invasion negative, compared with non-ruptured hepatocellular carcinoma patients, ruptured hepatocellular carcinoma patients exhibited worse prognosis for OS (HR, 1.55; 95% CI, 0.99–2.42) and DFS (HR, 1.23; 95% CI, 0.91–1.65) following hepatectomy.
Conclusions: Spontaneous tumor rupture was a prognostic risk factor for hepatocellular carcinoma patients after hepatic resection. However, in macrovascular invasion patients, spontaneous tumor rupture was not a prognostic risk factor.
Introduction
Hepatocellular carcinoma (HCC), the sixth most prevalent primary neoplasm, was responsible for around 81,0000 deaths in 2015 worldwide (1, 2). Spontaneous tumor rupture (STR) of HCC is a potentially fatal complication (3). The mechanisms underlying STR remain unclear. Possible reasons include large tumor size, ischemic necrosis, and vascular compression caused by rapid tumor growth (4–6). Although the overall incidence was relatively low (3–26%), the mortality rates of ruptured HCC patients were extremely high (32–75%) in reported literature (3, 7–11). Nowadays, treating STR of HCC is challenging; the current interventions used clinically include conservative treatment, transcatheter arterial chemoembolization (TACE), and hepatic resection (12, 13). Hepatectomy, including emergent and staged (after TACE achieving hemostasis) hepatectomy, provided a better long-term prognosis than palliative treatment in ruptured HCC patients with relatively well-preserved liver functions (13).
Traditionally, STR has recognized as a terminal event of HCC, as it could lead to various symptoms, such as hemorrhagic shock, intraperitoneal hemorrhage, and metastases, and most ruptured HCC patients had portal vein tumor thrombosis (PVTT), impaired liver function, and liver cirrhosis (14–17). As a result, these advanced patients with STR were frequently unable to receive surgical treatment and were compelled to have non-surgical treatment, resulting in a worse long-term prognosis than advanced patients receiving the same therapy without STR (9, 12).
However, whether STR was a prognostic risk factor for HCC patients after hepatic resection remains unclear (18, 19). Consequently, this meta-analysis aims to evaluate the long-term prognosis of patients with or without STR following hepatectomy and explore whether STR affects the prognosis of HCC patients after surgery.
Materials and Methods
Literature Search Strategy
This meta-analysis followed Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines (20). Four online electronic databases (PubMed, Embase, Web of Science, and Cochrane Library) were searched for published literature in English from inception to March 2021. The search strategies included: (“Hepatocellular Carcinoma” OR “Hepatoma” OR “Liver Cell Carcinomas” OR “HCC”) AND (“Rupture”). Furthermore, potentially eligible studies were identified through a thorough inspection from reference lists of all retrieved papers.
Inclusion Criteria
The inclusion criteria for this meta-analysis entailed: (1) Patients in experiment (ruptured HCC) and control (non-ruptured HCC) groups received hepatic resection, including emergent and staged hepatectomy. (2) The included literature is original and includes observational studies (OBSs). (3) The study evaluated the relationship between tumor rupture and prognosis. (4) The primary endpoints as overall survival (OS) or disease-free survival (DFS) were mentioned, and their hazard ratio (HR) and 95% confidence interval (CI) were obtainable or could be calculated.
Exclusion Criteria
The exclusion criteria for this meta-analysis entailed: (1) The relationship between ruptured and non-ruptured HCC in the prognosis of patients has not been explored at the same time. (2) When the duplicate publications were reviewed, the higher-quality or most updated was included. (3) The intervention for patients was not surgery but like TACE alone and palliative chemotherapy. (4) Multiple hepatic metastases, distant organ metastasis, and lymph node metastases were found in patients. (5) The tumor rupture was not spontaneous, but it was caused by trauma.
Data Extraction and Quality Evaluation
Based on pre-determined inclusion/exclusion criteria, two authors performed an independent review, extracting the following information carefully from each included study, including (1) study characteristics (author, country, and publication year), (2) patients' basic characteristics (age, gender, and number of included patients), (3) hepatic features (serum AFP, virus status, and liver cirrhosis), (4) tumor features (tumor number, size, and invasion), (5) therapeutic effect (OS and DFS, and corresponding HR and 95% CI).
The quality of incorporated OBSs was assessed using Newcastle-Ottawa Scale (NOS) that encompassed three aspects (selection of patients, comparability of groups, and evaluation of outcomes). The cumulative scores of articles less than six were considered of low-quality (21).
Statistical Analysis
The pooled HR and 95% CI for OS and DFS were calculated to estimate the relationship between tumor rupture and prognosis. Heterogeneity among included literature was assessed using I2 statistic. For potential heterogeneity, random-effect models were employed for greater reliability. When the number of included articles in each analysis is ≥ 10, Egger's test based on Stata 12.0 software was conducted to evaluate publication bias (22). A sensitivity analysis was conducted to determine robustness of conclusions. P-value <0.05 was considered statistically significant.
Results
Data Collection and Characteristics
A total of 4,285 records were initially yielded from four electronic databases using a pre-designed search strategy. After removing duplicates, 2,952 records remained. Twenty-one studies (13, 18, 19, 23–40) were ultimately included following a strict screening process. The comprehensive literature review and rigorous selection process are displayed in Figure 1.
Figure 1
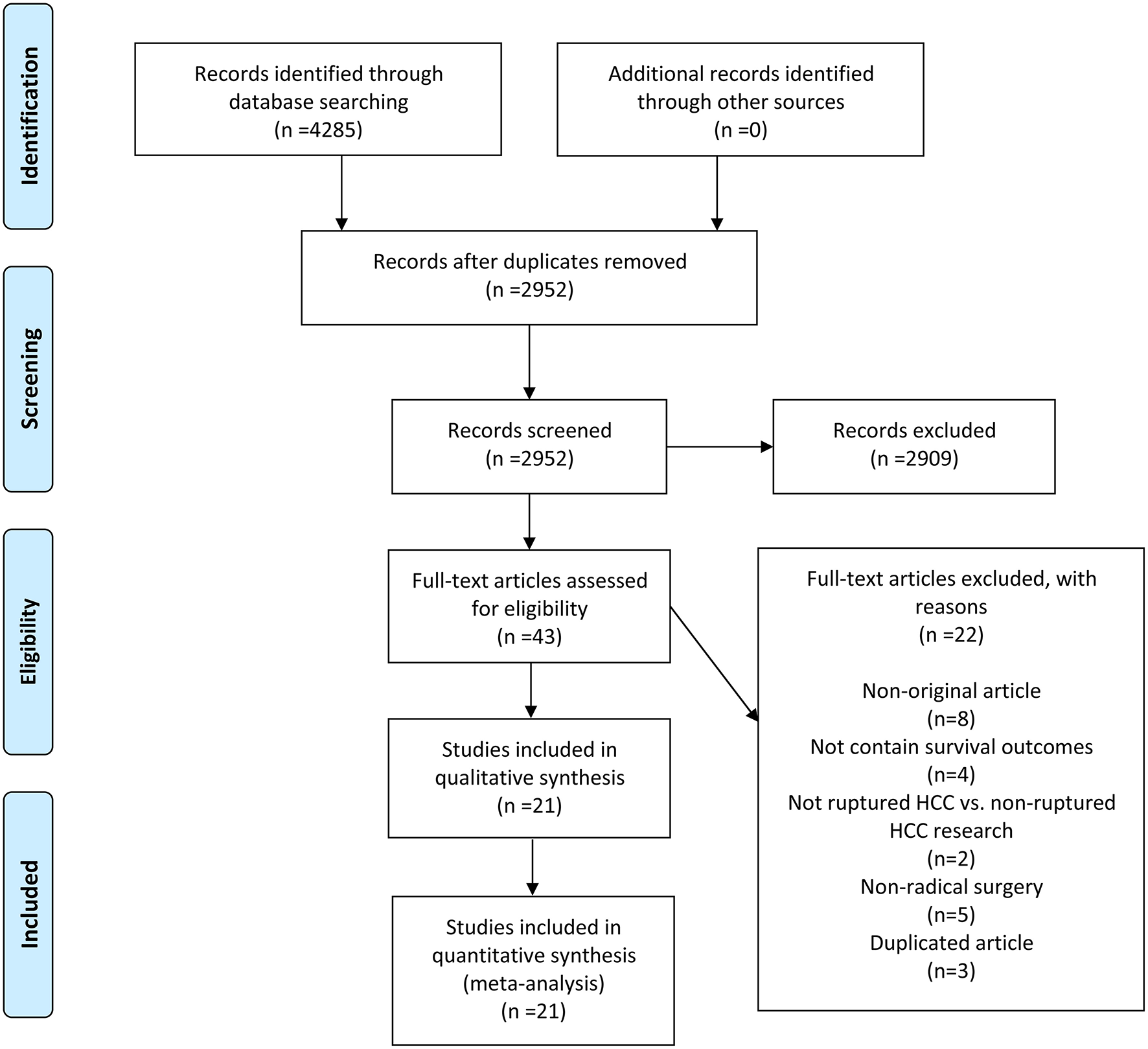
A schematic flow for selecting the articles included in this meta-analysis.
A total of 57,241 patients were enrolled in 21 OBSs mainly originated from Asia (19/21), followed by South America (1/21) and Europe (1/21). Eight studies simultaneously analyzed OS and DFS, ten were with OS alone, and three were only related to DFS. The detailed patients' characteristics of demographic and clinicopathological aspects are shown in Table 1 and Supplementary Table 1. The quality of OBSs was assessed using NOS and assessment outcomes indicated that incorporated articles were of high quality (Supplementary Table 2).
Table 1
| Author | Country | Number of patients | AFP(ng/ml) | Liver cirrhosis (number) | Microvascular invasion (number) | Macrovascular invasion (number) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rupture | Non-rupture | Rupture | Non-rupture | Rupture | Non-rupture | Rupture | Non-rupture | Rupture | Non-rupture | ||
| Aoki et al. (13) | Japan | 1160 | 48548 | 510 cases≥400 504 cases <400 |
9,279 cases≥400 36,431 cases <400 |
579 | 26,473 | NA | NA | 352 | 5,373 |
| Chan et al. (19) | China | 84 | 1,254 | 472 | 91 | 43 | 750 | 52 | 583 | 9 | 102 |
| Cheng et al. (23) | China | 53 | 826 | 66.8 | 507 | 104 | 188 | ||||
| Chua et al. (18) | Singapore | 49 | 98 | NA | NA | 19 | 32 | 27 | 47 | 7 | 11 |
| Fan et al. (24) | China | 211 | NA | NA | 98 | NA | NA | 21 | |||
| Joliat et al. (25) | Switzerland | 14 | 126 | 23 | 18 | 11 | 81 | NA | NA | NA | NA |
| Kwon et al. (26) | Korea | 85 | 186 | 1,2151 | 14,908 | NA | NA | 29 | 58 | 7 | 13 |
| Lee et al. (27) | Korea | 18 | 37 | NA | NA | 11 | 21 | 12 | 26 | 8 | 13 |
| Li et al. (28) | China | 89 | 171 | 138.5 | 73.8 | 73 | 99 | 0 | 0 | NA | NA |
| Miyoshi et al. (29) | Japan | 10 | 295 | 3 cases≥1,000 7 cases <1,000 |
53 cases≥1,000 242 cases <1,000 |
NA | NA | NA | NA | 6 | 81 |
| Mizuno et al. (30) | Japan | 6 | 15 | 3 cases>400 3 cases <400 |
5 cases>400 10 cases <400 |
NA | NA | NA | NA | 5 | 6 |
| Ruan et al. (31) | China | 57 | 57 | 22 | 20 | 28 | NA | 23 | 21 | NA | NA |
| Ruiz et al. (32) | Peru | 253 | 2,651 | 39 | 64 | 29 | |||||
| Tanaka et al. (33) | Japan | 42 | 42 | 78.8 | 49.5 | 13 | 14 | NA | NA | 2 | 3 |
| Uchiyama et al. (34) | Japan | 27 | 1,004 | 168 cases≥400 836 cases <400 |
NA | NA | NA | NA | 40 | ||
| Xiao et al. (35) | China | 53 | 181 | 141 cases≥400 93 cases <400 |
196 | 144 | 234 | ||||
| Yang et al. (36) | China | 143 | 1,090 | 85 cases≥400 58 cases <400 |
420 cases≥400 670 cases <400 |
NA | NA | 97 | 668 | 36 | 172 |
| Yeh et al. (37) | China | 35 | 175 | 100 cases≥400 110 cases <400 |
63 | NA | NA | 116 | |||
| Zhang et al. (38) | China | 41 | 446 | 5788.9 | 3947.1 | 24 | 285 | NA | NA | 1 | 48 |
| Zhao et al. (39) | China | 12 | 70 | 35 cases≥400 47 cases <400 |
65 | 19 | 0 | 0 | |||
| Zhu et al. (40) | China | 89 | 89 | 57 cases≥400 32 cases <400 |
53 cases≥400 36 cases <400 |
76 | 80 | 65 | 60 | 50 | 43 |
Characteristics of all the studies included in the meta-analysis.
AFP, a-fetoprotein; NA, not available.
Effect of STR on OS and DFS
A pooled analysis based on 18 studies including relevant OS data exhibited that STR was potentially related to a worse prognosis of ruptured HCC patients (HR, 1.65; 95% CI, 1.33–2.05) (Figure 2). Consistent with the pooled result of OS, pooled DFS outcomes also illustrated that ruptured HCC patients had a poorer prognosis than non-ruptured HCC patients (HR, 1.42; 95% CI, 1.12–1.80) (Figure 3).
Figure 2
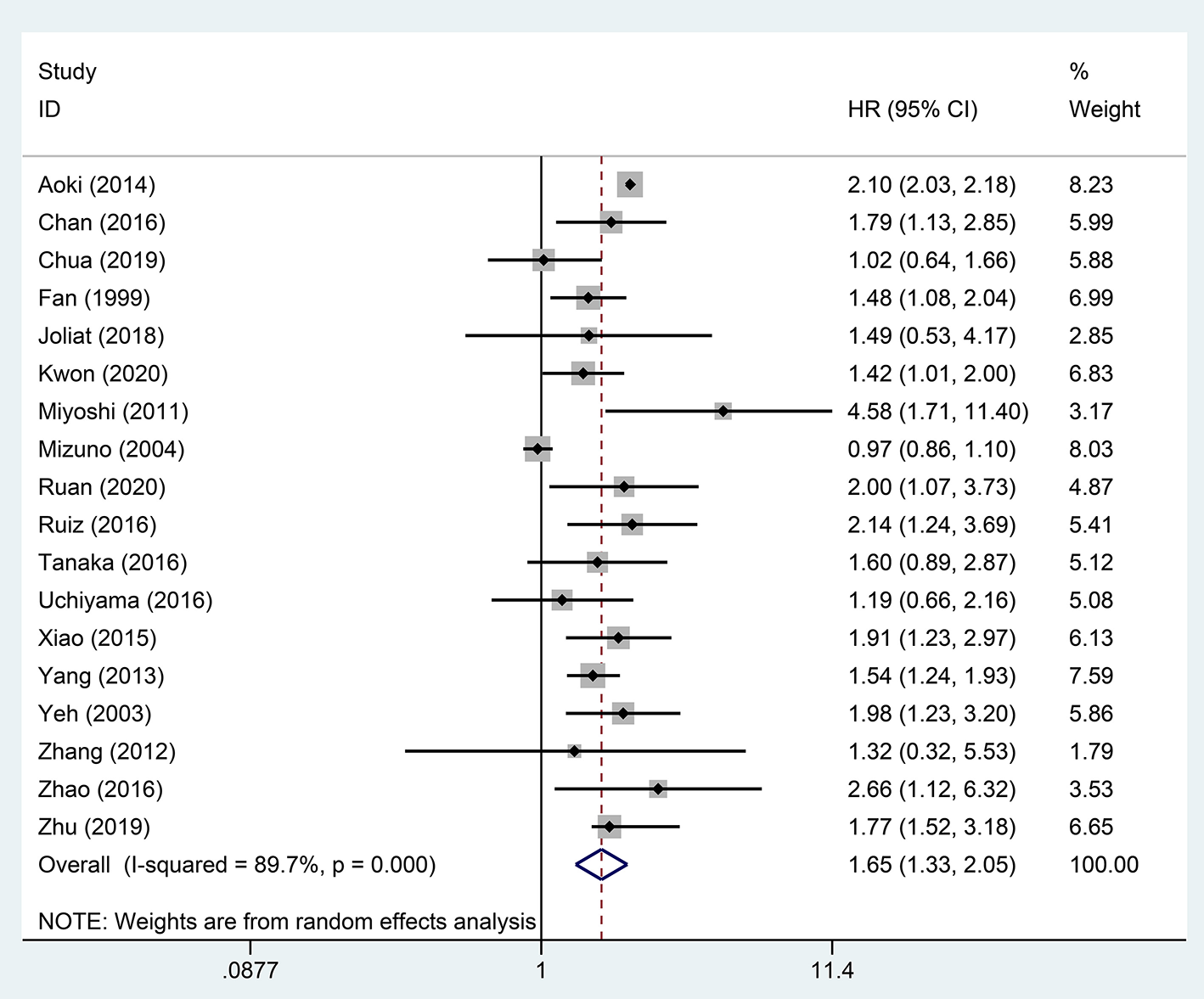
Forest plot of OS of ruptured hepatocellular carcinoma (HCC) patients after hepatectomy (P <0.001).
Figure 3
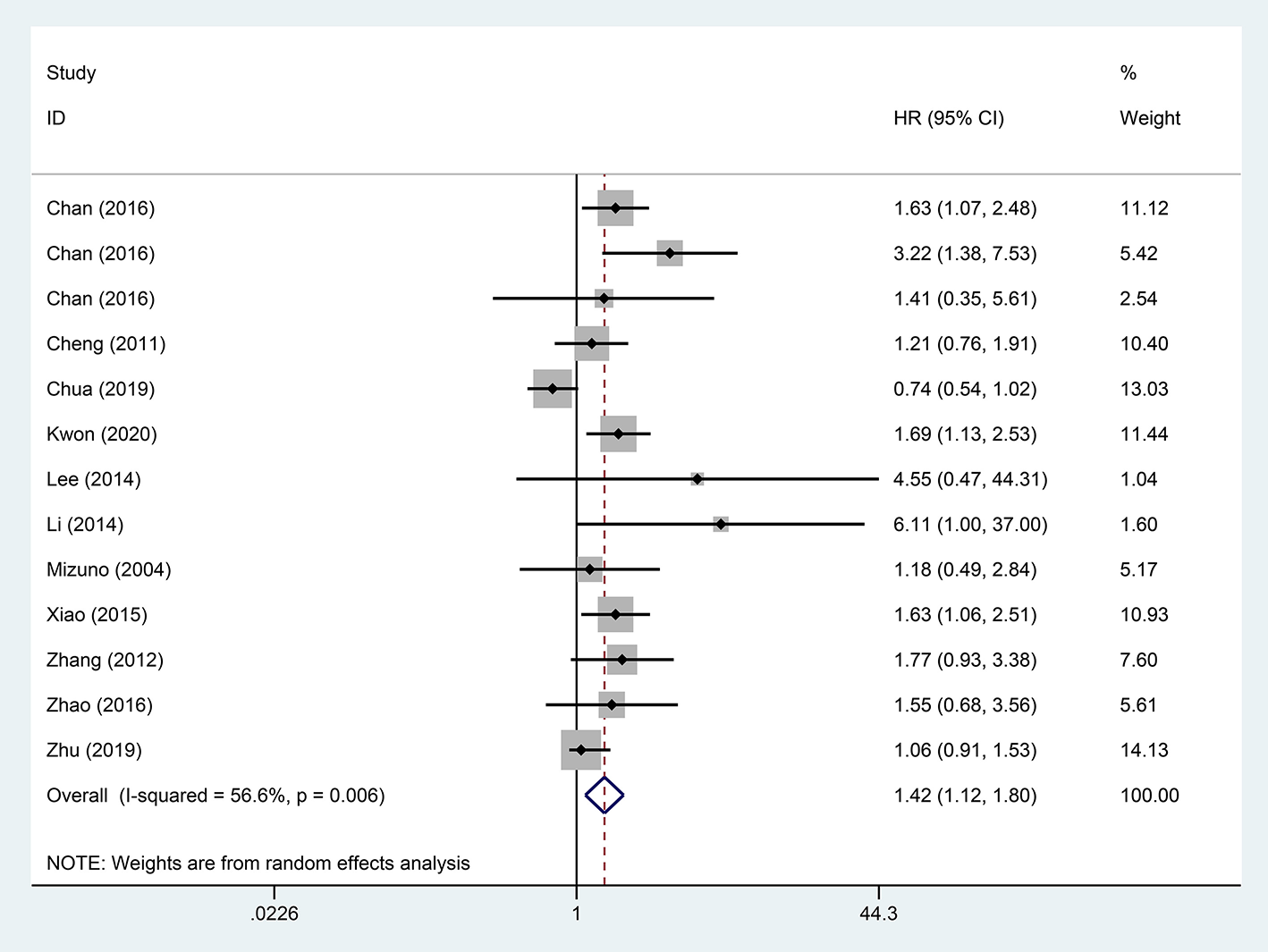
Forest plot of DFS of ruptured hepatocellular carcinoma (HCC) patients following hepatectomy (P = 0.004).
Subgroup Analysis
Subgroups analyses were implemented to explore the effect of various factors on the prognosis of ruptured and non-ruptured HCC patients. We categorized the studies into 3-year OS and 5-year OS groups based on recorded survival time. For subgroups of 5-year OS, non-ruptured HCC patients obtained greater OS than ruptured HCC patients, whereas no statistical difference was found in subgroups of 3-year OS. For subgroups of patients' age ≥ or <60 years old, patients in China or other Asian countries, patients' AFP ≥ or <400 ng/mL, patients with and without liver cirrhosis, and patients' microvascular invasion positive/negative, the analysis results all indicated that STR was associated with worse OS (Table 2). For patients with macrovascular invasion positive patients, STR had no adverse impact on ruptured HCC patients' OS compared to non-ruptured HCC patients (HR, 1.55; 95% CI, 0.99–2.42). However, in macrovascular invasion-negative patients, STR was a prognostic risk factor for HCC patients (HR, 1.67; 95% CI, 1.39–2.01) (Figure 4).
Table 2
| No. of studies | HR | 95%CI | Heterogeneity (I2) (%) | |
|---|---|---|---|---|
| Overall survival (OS) | ||||
| 3-year OS | 3 | 1.87 | 0.80–4.39 | 86.4 |
| 5-year OS | 15 | 1.68 | 1.46–1.94 | 54.8 |
| Age ≥60 years | 5 | 1.78 | 1.19–2.65 | 68.3 |
| Age <60 years | 11 | 1.66 | 1.47–1.88 | 0 |
| China | 9 | 1.68 | 1.47–1.92 | 0 |
| Non-Chinese Asian countries | 7 | 1.50 | 1.00–2.26 | 96.2 |
| AFP≥400 ng/ml | 6 | 1.74 | 1.45–2.09 | 0 |
| AFP <400 ng/ml | 8 | 1.58 | 1.09–2.28 | 95.3 |
| Liver cirrhosis | 7 | 2.09 | 2.02–2.17 | 0 |
| Non-liver cirrhosis | 5 | 1.56 | 1.23–1.98 | 26.2 |
| Microvascular invasion positive | 4 | 1.55 | 1.25–1.91 | 30.4 |
| Microvascular invasion negative | 5 | 1.74 | 1.40–2.17 | 0 |
| Disease-free survival (DFS) | ||||
| Age <60 | 9 | 1.53 | 1.24–1.88 | 31.4 |
| China | 7 | 1.50 | 1.18–1.90 | 36.7 |
| Non-Chinese Asian countries | 4 | 1.22 | 0.67–2.24 | 74.3 |
| AFP≥400 ng/ml | 4 | 1.42 | 1.07–1.90 | 49.9 |
| AFP <400 ng/ml | 4 | 1.35 | 0.93–1.97 | 3.4 |
| Liver cirrhosis | 7 | 1.52 | 1.15–2.01 | 38.5 |
| Microvascular invasion positive | 4 | 1.11 | 0.74–1.65 | 70.6 |
| Microvascular invasion negative | 5 | 1.64 | 1.30–2.06 | 3.6 |
Subgroup analysis of the hepatocellular carcinoma (HCC) rupture on the prognosis of patients with HCC.
AFP, alpha fetoprotein; HR, hazard ratio; CI, confidence interval.
Figure 4
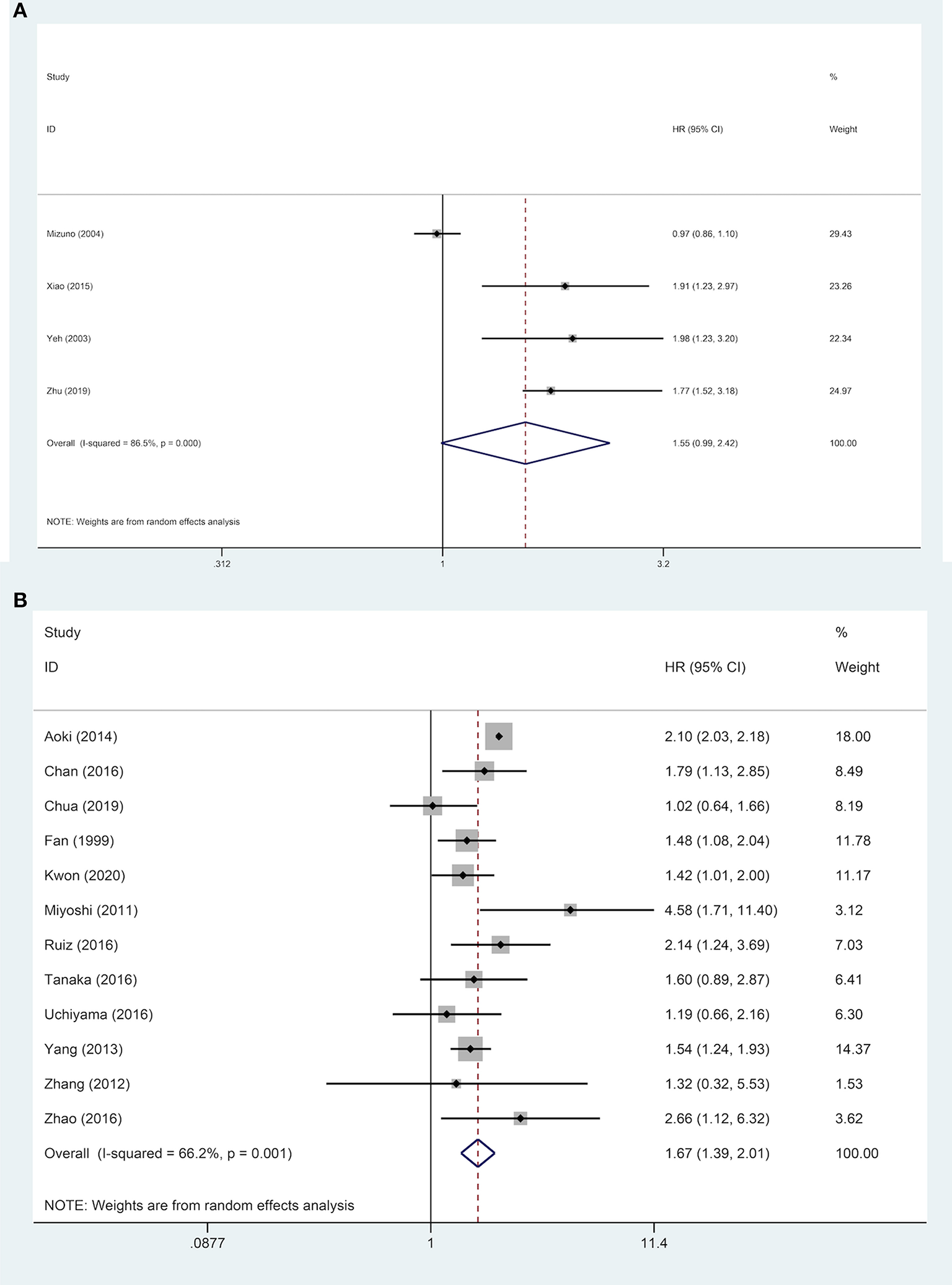
Forest plot of OS of ruptured hepatocellular carcinoma (HCC) patients with macrovascular invasion after hepatectomy (A, macrovascular invasion positive, P = 0.055; B, macrovascular invasion negative, P <0.001).
Nine studies were included to explore the effect of age on DFS of HCC patients. The results demonstrated that in a subgroup of age <60 years old, ruptured HCC patients' DFS was shorter than in the control group. Although no statistical difference was observed between the two groups' DFS regarding other Asian countries, non-ruptured HCC patients achieved better DFS than ruptured HCC patients in China. When patients' AFP concentration ≥ 400 ng/mL, STR is a potential risk factor for patients' DFS. However, in patients with AFP concentration <400 ng/mL, STR was not correlated with HCC patients' DFS. For patients with liver cirrhosis, STR was linked to worse DFS. Similar poor outcomes were also demonstrated in microvascular invasion-negative patients, but in microvascular invasion-positive patients, no significant difference in DFS was identified between the two groups (Table 2). For DFS of patients, STR was not a prognostic risk factor in macrovascular invasion positive patients (HR, 1.23; 95% CI, 0.91–1.65), but it was a risk factor in macrovascular invasion negative patients (HR, 1.48; 95% CI, 1.06–2.05) (Figure 5).
Figure 5
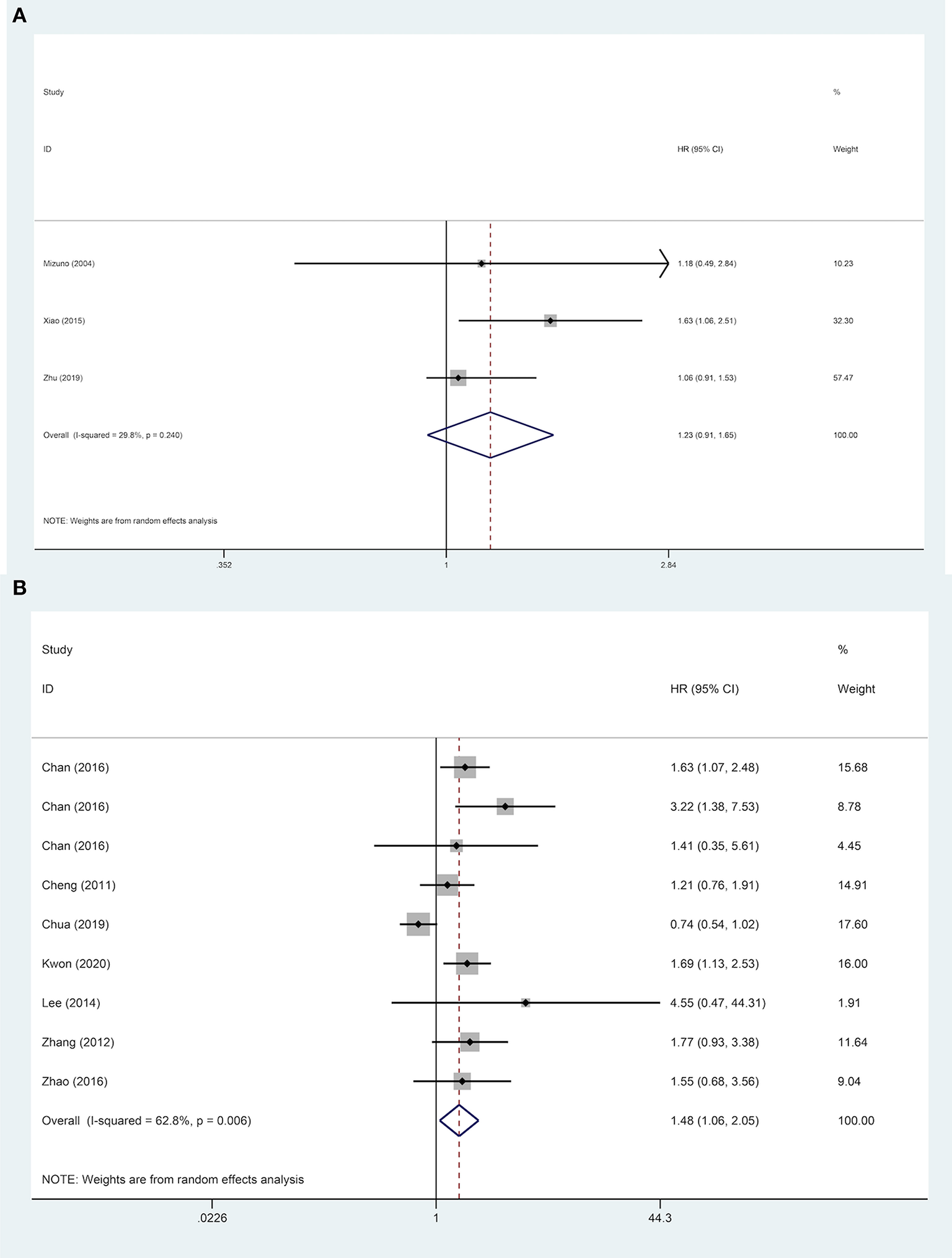
Forest plot of DFS of ruptured hepatocellular carcinoma (HCC) patients with macrovascular invasion after hepatectomy (A, macrovascular invasion positive, P = 0.170; B, macrovascular invasion negative, P = 0.021).
Sensitivity Analysis and Publication Bias
After omitting the included articles in sequence, sensitivity analysis results confirmed the excellent stability of HR for OS. The quantificational Egger's test was employed to evaluate publication bias, and the outcomes revealed no potential publication bias among the included articles on HR for OS (P > 0.05). Additionally, another sensitivity analysis was performed to verify HR robustness for DFS, resulting in reliable results. No potential publication bias was observed for HR for DFS after Egger's test (P > 0.05).
Discussion
Most ruptured HCC patients were in advanced disease stage; among them, many patients exhibited extrahepatic metastasis, PVTT, and impaired liver function (14–17). These tended to cause them to lose the opportunity of surgery and choose conservative treatment options. Therefore, the traditional concept that STR was a prognostic risk factor for HCC patients was mostly based on receiving non-surgical treatment (9, 12, 41, 42). It is worth investigating whether STR remained a prognostic risk factor for those HCC patients undergoing liver resection. The overall findings from this meta-analysis implied that STR was a risk factor in long-term prognosis of HCC patients following hepatic resection, consistent with previous reports (19, 24, 32).
To thoroughly investigate the reasons of STR affecting long-term prognosis of HCC patients after hepatic resection, from previous literature, we inferred that potential reasons were correlated with gender, tumor size, virus status, hepatectomy style, and liver cirrhosis (13, 18, 24, 28, 37, 43–47). STR was more frequently observed in male patients from reported studies (18, 24). The literature revealed that HCC female patients exhibit a better survival rate and low recurrence rate than male patients (43). Then, it was reported that ruptured HCC patients tended to have larger tumor size than non-ruptured HCC patients, and the total tumor volume is a vital prognostic predictor, and larger HCC was associated with a worse OS and DFS (37, 44). From a nationwide survey (1160 ruptured HCC patients), Aoki et al. (13) found that hepatitis B virus (HBV)-infected patients have a higher STR incidence than hepatitis C virus (HCV)-infected patients. According to reports, long-term survival rates of HCC patients with hepatitis B surface antigen (HBsAg) positive was worse than that of HBsAg negative patients following surgery (45). Additionally, staged hepatectomy followed TACE was a prevalent surgical way for ruptured HCC patients. Hanazaki et al. (46) found that preoperative TACE would significantly increase the risk of patients' postoperative recurrence, leading to unsatisfactory long-term prognosis. Besides, numerous studies revealed that ruptured HCC patients were often accompanied by liver cirrhosis, an independent prognostic risk factor affecting prognosis of HCC patients (28, 47). For reduced liver reserve and tolerance, STR was undoubtedly a serious blow to the disease.
Due to high heterogeneity, the situations of ruptured HCC patients were complicated and diverse. We performed subgroup analyses of the prognosis of HCC patients. The analysis result of 5-year OS subgroup revealed that STR was a risk factor, but no statistical difference in survival was observed between the two groups in 3-year OS subgroup, possibly due to limited sample size (three included studies). In addition, long-term follow-up is required after hepatic resection to determine the difference in prognosis.
Our results indicated that STR was correlated with a poorer prognosis for both patients older and younger than 60 years old. There is still controversy regarding whether age affects tumor recurrence and long-term survival of HCC patients following hepatic resection. Numerous studies revealed that advanced age had no adverse effect on the prognosis of patients (48, 49). Meanwhile, a previous study revealed that younger age possibly was a prognostic risk factor for HCC patients as they had more advanced tumor stage and stronger tumor aggressiveness than older HCC patients (50). However, Xu et al. found that younger HCC patients tended to have a better survival outcome regardless of tumor aggressivity (51). Moreover, our meta-analysis indicated that STR was linked to worse prognosis in China and other Asian countries. However, for other Asian countries, DFS result was not statistically different, possibly due to limited sample size. Besides, studies proved that HCV is the major etiology of HCC in Japan, whereas most Chinese HCC patients have an HBV background (52, 53). The main HBV mechanisms contributing to HCC are that HBV-DNA integrates into the host genome and induces genomic instability and insertional mutagenesis of various cancer-related genes (54). However, since HCV is an RNA virus without genes integrating into the host genome, direct cellular programming and indirect inflammatory response are possible mechanisms of inducing HCC (55). Therefore, clinicopathological characteristics and prognoses of HCC caused by different viruses may differ.
We found that STR was a risk factor regardless of subgroups with low/high serum AFP concentrations. AFP, a specific tumor marker for primary HCC, is commonly employed for early screening and diagnosis of HCC; however, its specificity and sensitivity are relatively low (56). Intriguingly, numerous investigations have discovered that several serum markers may assist in diagnosing AFP negative HCC patients (57, 58). High AFP was linked to early recurrence and poor prognosis because it promoted vascular invasion and disease progression (59). Subgroup analyses of liver cirrhosis revealed that STR was a prognostic risk factor in HCC patients with or without liver cirrhosis. Recent years have witnessed a surge in research on the risk factors for HBV-cirrhosis progressing to HCC. According to relevant literature, HBV status, antiviral drugs, and liver cirrhosis severity are potential prognostic factors (60–62).
Subgroup analysis was also used to assess the effect of microvascular invasion on prognosis. The outcomes indicated that for microvascular invasion-negative patients, ruptured HCC patients exhibited a worse prognosis than non-ruptured HCC patients. However, for microvascular invasion-positive patients, whether STR correlates with a worse prognosis remains controversial. Numerous studies have confirmed that microvascular invasion is an independent risk factor for prognosis of HCC patients undergoing hepatic resection and that occult metastases caused by microvascular invasion are a major cause of HCC recurrence following surgery (63, 64). Furthermore, numerous investigations demonstrated a substantial correlation between the existence of microvascular invasion and large tumor size, high AFP concentration, and tumor localization in segment eight (65, 66). Consequently, we speculated that, in addition to the harm caused by microvascular invasion, changes in associated clinicopathological indicators (tumor size, AFP, and tumor localization) might also cause controversies in the above results. Nowadays, it is challenging to detect microvascular invasion in preoperative imaging examination, and its diagnosis still requires validation using postoperative histopathological examination (18).
The most intriguing finding of subgroup analysis was that prognosis of ruptured HCC patients after hepatic resection was opposite depending on different macrovascular invasion status (positive/negative). STR was a significant prognostic risk factor for macrovascular invasion-negative patients; nevertheless, STR was not a prognostic risk factor in macrovascular invasion-positive patients. The possible explanation for this phenomenon is that adverse STR-related prognostic influence was overshadowed by the more harmful macrovascular invasion. In Barcelona Clinic Liver Cancer (BCLC) staging systems, macrovascular invasion HCC patients are classified as an advanced stage (67). When macrovascular invasion is present, the prognosis is extremely poor, with a median survival time of 2.7 months if left untreated (68). In addition, limited included studies in macrovascular positive-subgroup analyses (OS: 4 studies; DFS: 3 studies) might be a reason. PVTT is a prevalent type of HCC macrovascular invasion. There remain numerous controversies regarding the therapeutic options for HCC patients with PVTT. According to BCLC staging system of European and American countries, HCC patients with PVTT were classified as advanced (BCLC-C) stage, and sorafenib as a palliative treatment is recommended for these patients instead of surgery or other active methods (69). However, unlike Western countries, Asia has numerous HCC patients and various treatment methods, and because each kind of HCC is unique, PVTT is not incompatible with hepatic resection (70). Numerous doctors in Asian countries continue to use active methods like surgery to treat patients with well types and liver function, and the result revealed a favorable survival benefit than non-surgical treatment in reported literature (71, 72).
Conservative treatment, TACE, and early/delayed hepatectomy are current treatments for the management of ruptured HCC (12, 13). Conservative treatment alone is suitable for ruptured HCC patients with poor baseline or extensive metastasis (8). The advantage of TACE is its high hemostasis rate, extensive indications, and it can avoid the double blow of general anesthesia and surgery (73–75). Early surgery is suitable for patients with good baseline, and due to insufficient preoperative examinations, the recurrence rate of intrahepatic tumors after surgery is high (75). Delayed hepatectomy could reduce volume of intraoperative bleeding and blood transfusion and improve better short-and long- term prognosis of ruptured HCC patients than early hepatectomy (76). Therefore, delayed surgery (after TACE achieving hemostasis) is a better treatment option for ruptured HCC patients if they are not suitable for emergent surgery.
To the best of our knowledge, this is the first meta-analysis to assess the relationship between STR and prognosis of HCC patients following hepatic resection. Besides, various subgroup analyses were performed to investigate whether the risk effect of STR varied among various subgroups. However, this study has limitations. Firstly, the included studies were retrospective, resulting in potential risks like selection and information biases. Secondly, most populations evaluated in this study were from Asia; therefore, the conclusion does not apply to Western areas with low HCC incidence. Thirdly, since the included studies were highly heterogeneous, relevant data like postoperative recurrence and complications are fully unavailable.
Conclusions
Our study demonstrated that STR was a risk factor for long-term prognosis of HCC patients after hepatectomy. This phenomenon remained consistent in most subgroups stratified by recorded survival time, age, country, AFP concentration, liver cirrhosis, and microvascular invasion. However, STR was not associated with a worse prognosis in macrovascular invasion patients.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
YC designed the research process. JX and JH searched the database for corresponding articles and drafted the meta-analysis. YW and LZ extracted useful information from the articles above. YH and YS used statistical software for analysis. BX polished this article. All authors had read and approved the manuscript and ensured that this was the case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.769233/full#supplementary-material
- HCC
hepatocellular carcinoma
- STR
spontaneous tumor rupture
- TACE
transcatheter arterial chemoembolization
- PVTT
portal vein tumor thrombosis
- OBSs
observational studies
- OS
overall survival
- DFS
disease-free survival
- HR
hazard ratio
- CI
confidence interval
- AFP
alpha-fetoprotein
- NOS
Newcastle-Ottawa scale
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HBsAg
hepatitis B surface antigen
- BCLC
Barcelona clinic liver cancer.
Abbreviations
References
1.
Bray F Ferlay J Soerjomataram I Siegel RL Torre LA Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. 10.3322/caac.21492
2.
Akinyemiju T Abera S Ahmed M Alam N Alemayohu MA Allen C et al . The Burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Dis ease Study (2015). JAMA Oncol. (2017) 3:1683–91. 10.1001/jamaoncol.2017.3055
3.
Lai EC Lau WY . Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. (2006) 141:191–8. 10.1001/archsurg.141.2.191
4.
Hermann RE David TE . Spontaneous rupture of the liver caused by hepatomas. Surgery. (1973) 74:715–9.
5.
Castells L Moreiras M Quiroga S Alvarez-Castells A Segarra A Esteban R et al . Hemoperitoneum as a first manifestation of hepatocellular carcinoma in western patients with liver cirrhosis: effectiveness of emergency treatment with transcatheter arterial embolization. Dig Dis Sci. (2001) 46:555–62. 10.1023/A:1005699132142
6.
Rossetto A Adani GL Risaliti A Baccarani U Bresadola V Lorenzin D et al . Combined approach for spontaneous rupture of hepatocellular carcinoma. World J Hepatol. (2010) 2:49–51. 10.4254/wjh.v2.i1.49
7.
Chen MF Hwang TL Jeng LB Jan YY Wang CS . Clinical experience with hepatic resection for ruptured hepatocellular carcinoma. Hepatogastroenterology. (1995) 42:166–8.
8.
Chen WK Chang YT Chung YT Yang HR . Outcomes of emergency treatment in ruptured hepatocellular carcinoma in the ED. Am J Emerg Med. (2005) 23:730–6. 10.1016/j.ajem.2005.02.052
9.
Liu CL Fan ST Lo CM Tso WK Poon RT Lam CM et al . Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. (2001) 19:3725–32. 10.1200/JCO.2001.19.17.3725
10.
Ong GB Taw JL . Spontaneous rupture of hepatocellular carcinoma. Br Med J. (1972) 4:146–9. 10.1136/bmj.4.5833.146
11.
Tan FL Tan YM Chung AY Cheow PC Chow PK Ooi LL . Factors affecting early mortality in spontaneous rupture of hepatocellular carcinoma. ANZ J Surg. (2006) 76:448–52. 10.1111/j.1445-2197.2006.03750.x
12.
Kirikoshi H Saito S Yoneda M Fujita K Mawatari H Uchiyama T et al . Outcomes and factors influencing survival in cirrhotic cases with spontaneous rupture of hepatocellular carcinoma: a multicenter study. BMC Gastroenterol. (2009) 9:29. 10.1186/1471-230X-9-29
13.
Aoki T Kokudo N Matsuyama Y Izumi N Ichida T Kudo M et al . Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg. (2014) 259:532–42. 10.1097/SLA.0b013e31828846de
14.
Sonoda T Kanematsu T Takenaka K Sugimachi K . Ruptured hepatocellular carcinoma evokes risk of implanted metastases. J Surg Oncol. (1989) 41:183–6. 10.1002/jso.2930410310
15.
Miyamoto M Sudo T Kuyama T . Spontaneous rupture of hepatocellular carcinoma: a review of 172 Japanese cases. Am J Gastroenterol. (1991) 86:67–71.
16.
Xu HS Yan JB . Conservative management of spontaneous ruptured hepatocellular carcinoma. Am Surg. (1994) 60:629–33.
17.
Vergara V Muratore A Bouzari H Polastri R Ferrero A Galatola G et al . Spontaneous rupture of hepatocelluar carcinoma: surgical resection and long-term survival. Eur J Surg Oncol. (2000) 26:770–2. 10.1053/ejso.2000.1001
18.
Chua DW Koh YX Allen JC Chan CY Lee SY Cheow PC et al . Impact of spontaneous rupture on the survival outcomes after liver resection for hepatocellular carcinoma: a propensity matched analysis comparing ruptured versus non-ruptured tumors. Eur J Surg Oncol. (2019) 45:1652–9. 10.1016/j.ejso.2019.03.044
19.
Chan AC Dai JW Chok KS Cheung TT Lo CM . Prognostic influence of spontaneous tumor rupture on hepatocellular carcinoma after interval hepatectomy. Surgery. (2016) 159:409–17. 10.1016/j.surg.2015.07.020
20.
Moher D Liberati A Tetzlaff J Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097
21.
Stang A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z
22.
Egger M Davey Smith G Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629
23.
Cheng CH Lee CF Wu TH Chan KM Chou HS Wu TJ et al . Evaluation of the new AJCC staging system for resectable hepatocellular carcinoma. World J Surg Oncol. (2011) 9:114. 10.1186/1477-7819-9-114
24.
Fan ST Ng IO Poon RT Lo CM Liu CL Wong J . Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. (1999) 134:1124–30. 10.1001/archsurg.134.10.1124
25.
Joliat GR Labgaa I Uldry E Demartines N Halkic N . Recurrence rate and overall survival of operated ruptured hepatocellular carcinomas. Eur J Gastroenterol Hepatol. (2018) 30:792–6. 10.1097/MEG.0000000000001115
26.
Kwon JH Song GW Hwang S Kim KH Ahn CS Moon DB et al . Surgical outcomes of spontaneously ruptured hepatocellular carcinoma. J Gastrointest Surg. (2021) 25:941–53. 10.1007/s11605-020-04555-0
27.
Lee HS Choi GH Kang DR Han KH Ahn SH Kim DY et al . Impact of spontaneous hepatocellular carcinoma rupture on recurrence pattern and long-term surgical outcomes after partial hepatectomy. World J Surg. (2014) 38:2070–8. 10.1007/s00268-014-2502-6
28.
Li J Huang L Liu CF Cao J Yan JJ Xu F et al . Risk factors and surgical outcomes for spontaneous rupture of BCLC stages A and B hepatocellular carcinoma: a case-control study. World J Gastroenterol. (2014) 20:9121–7. 10.3748/wjg.v20.i27.9121
29.
Miyoshi A Kitahara K Kohya N Noshiro H Miyazahi K . Outcomes of patients with spontaneous rupture of hepatocellular carcinoma. Hepatogastroenterology. (2011) 58:99–102.
30.
Mizuno S Yamagiwa K Ogawa T Tabata M Yokoi H Isaji S et al . Are the results of surgical treatment of hepatocellular carcinoma poor if the tumor has spontaneously ruptured?Scand J Gastroenterol. (2004) 39:567–70. 10.1080/00365520410005135
31.
Ruan S Shi N Chen Z Han H Wang H Jin L et al . The role of hyperthermic intraperitoneal chemotherapy in the treatment of spontaneously ruptured hepatocellular carcinoma: a pilot study. Ann Transl Med. (2020) 8:1132. 10.21037/atm-20-5829
32.
Ruiz E Rojas Rojas T Berrospi F Chávez I Luque C Cano L et al . Hepatocellular carcinoma surgery outcomes in the developing world: a 20-year retrospective cohort study at the National Cancer Institute of Peru. Heliyon. (2016) 2:e00052. 10.1016/j.heliyon.2015.e00052
33.
Tanaka S Kaibori M Ueno M Wada H Hirokawa F Nakai T et al . Surgical Outcomes for the Ruptured Hepatocellular Carcinoma: Multicenter Analysis with a Case-Controlled Study. J Gastrointest Surg. (2016) 20:2021–34. 10.1007/s11605-016-3280-2
34.
Uchiyama H Minagawa R Itoh S Kajiyama K Harimoto N Ikegami T et al . Favorable outcomes of hepatectomy for ruptured hepatocellular carcinoma: retrospective analysis of primary R0-hepatectomized patients. Anticancer Res. (2016) 36:379–85.
35.
Xiao CZ Wei W Guo ZX Li SH Zhang YF Wang JH et al . A prognosis model for patients with hepatocellular carcinoma and portal vein tumor thrombus following hepatic resection. Oncol Lett. (2015) 10:2787–94. 10.3892/ol.2015.3677
36.
Yang T Sun YF Zhang J Lau WY Lai EC Lu JH et al . Partial hepatectomy for ruptured hepatocellular carcinoma. Br J Surg. (2013) 100:1071–9. 10.1002/bjs.9167
37.
Yeh CN Lee WC Chen MF . Hepatic resection and prognosis for patients with hepatocellular carcinoma larger than 10 cm: two decades of experience at Chang Gung memorial hospital. Ann Surg Oncol. (2003) 10:1070–6. 10.1245/ASO.2003.03.072
38.
Zhang XF Wei T Liu XM Lv Y . Spontaneous tumor rupture and surgical prognosis of patients with hepatocellular carcinoma. Scand J Gastroenterol. (2012) 47:968–74. 10.3109/00365521.2012.685753
39.
Zhao HC Wu RL Liu FB Zhao YJ Wang GB Zhang ZG et al . A retrospective analysis of long term outcomes in patients undergoing hepatic resection for large (>5 cm) hepatocellular carcinoma. HPB (Oxford). (2016) 18:943–9. 10.1016/j.hpb.2016.08.005
40.
Zhu Q Qiao G Xu C Yu X Zhao J Yu Z et al . Conditional survival in patients with spontaneous tumor rupture of hepatocellular carcinoma after partial hepatectomy: a propensity score matching analysis. HPB. (2019) 21:722–30. 10.1016/j.hpb.2018.10.002
41.
Chearanai O Plengvanit U Asavanich C Damrongsak D Sindhvananda K Boonyapisit S . Spontaneous rupture of primary hepatoma: report of 63 cases with particular reference to the pathogenesis and rationale treatment by hepatic artery ligation. Cancer. (1983) 51:1532–6.
42.
Hirai K Kawazoe Y Yamashita K Kumagai M Nagata K Kawaguchi S et al . Transcatheter arterial embolization for spontaneous rupture of hepatocellular carcinoma. Am J Gastroenterol. (1986) 81:275–9.
43.
Villa E Moles A Ferretti I Buttafoco P Grottola A Del Buono M et al . Natural history of inoperable hepatocellular carcinoma: estrogen receptors' status in the tumor is the strongest prognostic factor for survival. Hepatology. (2000) 32:233–8. 10.1053/jhep.2000.9603
44.
Goh BK Teo JY Chan CY Lee SY Jeyaraj P Cheow PC et al . Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: Implications on the current AJCC staging system. J Surg Oncol. (2016) 113:89–93. 10.1002/jso.24099
45.
Wu CC Ho WL Chen JT Tang JS Yeh DC P'Eng F K . Hepatitis viral status in patients undergoing liver resection for hepatocellular carcinoma. Br J Surg. (1999) 86:1391–6. 10.1046/j.1365-2168.1999.01272.x
46.
Hanazaki K Kajikawa S Shimozawa N Mihara M Shimada K Hiraguri M et al . Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. (2000) 191:381–8. 10.1016/S1072-7515(00)00700-6
47.
Taura K Ikai I Hatano E Yasuchika K Nakajima A Tada M et al . Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery. (2007) 142:685–94. 10.1016/j.surg.2007.05.009
48.
Kim JM Cho BI Kwon CH Joh JW Park JB Lee JH et al . Hepatectomy is a reasonable option for older patients with hepatocellular carcinoma. Am J Surg. (2015) 209:391–7. 10.1016/j.amjsurg.2013.06.010
49.
Tsujita E Utsunomiya T Ohta M Tagawa T Matsuyama A Okazaki J et al . Outcome of repeat hepatectomy in patients with hepatocellular carcinoma aged 75 years and older. Surgery. (2010) 147:696–703. 10.1016/j.surg.2009.10.054
50.
Kim JH Choi MS Lee H Kim DY Lee JH Koh KC et al . Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol. (2006) 21:588–94. 10.1111/j.1440-1746.2005.04127.x
51.
Xu XS Chen W Miao RC Zhou YY Wang ZX Zhang LQ et al . Survival analysis of hepatocellular carcinoma: a comparison between young patients and aged patients. Chin Med J. (2015) 128:1793–800. 10.4103/0366-6999.159356
52.
Ukawa S Okada E Nakamura K Hirata M Nagai A Matsuda K et al . Characteristics of patients with liver cancer in the BioBank Japan project. J Epidemiol. (2017) 27:S43–8. 10.1016/j.je.2016.12.007
53.
Goh GB Chang PE Tan CK . Changing epidemiology of hepatocellular carcinoma in Asia. Best Pract Res Clin Gastroenterol. (2015) 29:919–28. 10.1016/j.bpg.2015.09.007
54.
Levrero M Zucman-Rossi J . Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. (2016) 64(1 Suppl):S84–101. 10.1016/j.jhep.2016.02.021
55.
Dash S Aydin Y Widmer KE Nayak L . Hepatocellular carcinoma mechanisms associated with chronic HCV infection and the impact of direct-acting antiviral treatment. J Hepatocell Carcinoma. (2020) 7:45–76. 10.2147/JHC.S221187
56.
Tangkijvanich P Anukulkarnkusol N Suwangool P Lertmaharit S Hanvivatvong O Kullavanijaya P et al . Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. (2000) 31:302–8. 10.1097/00004836-200012000-00007
57.
Shen Q Fan J Yang XR Tan Y Zhao W Xu Y et al . Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. (2012) 13:817–26. 10.1016/S1470-2045(12)70233-4
58.
Capurro M Wanless IR Sherman M Deboer G Shi W Miyoshi E et al . Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. (2003) 125:89–97. 10.1016/S0016-5085(03)00689-9
59.
Peng SY Chen WJ Lai PL Jeng YM Sheu JC Hsu HC . High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. (2004) 112:44–50. 10.1002/ijc.20279
60.
Chien J Liu J Lee MH Jen CL Batrla-Utermann R Lu SN et al . Risk and predictors of hepatocellular carcinoma for chronic hepatitis B patients with newly developed cirrhosis. J Gastroenterol Hepatol. (2016) 31:1971–7. 10.1111/jgh.13422
61.
Bonino F Oliveri F Colombatto P Brunetto MR . Impact of interferon-alpha therapy on the development of hepatocellular carcinoma in patients with liver cirrhosis: results of an international survey. J Viral Hepat. (1997) 4 (Suppl. 2):79–82. 10.1111/j.1365-2893.1997.tb00183.x
62.
Shim JJ Oh CH Kim JW Lee CK Kim BH . Liver cirrhosis stages and the incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving antiviral therapy. Scand J Gastroenterol. (2017) 52:1029–36. 10.1080/00365521.2017.1335773
63.
Rodríguez-Perálvarez M Luong TV Andreana L Meyer T Dhillon AP Burroughs AK . A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. (2013) 20:325–39. 10.1245/s10434-012-2513-1
64.
Imamura H Matsuyama Y Tanaka E Ohkubo T Hasegawa K Miyagawa S et al . Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. (2003) 38:200–7. 10.1016/S0168-8278(02)00360-4
65.
McHugh PP Gilbert J Vera S Koch A Ranjan D Gedaly R . Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB. (2010) 12:56–61. 10.1111/j.1477-2574.2009.00128.x
66.
Al-Azzawi Y Rouanet E Hendrix RJ Spaho L Malik H Devuni D et al . Segmental Distribution of Hepatocellular Carcinoma Correlates with Microvascular Invasion in Liver Explants Undergoing Transplantation. J Cancer Epidemiol. (2019) 2019:8534372. 10.1155/2019/8534372
67.
Llovet JM Brú C Bruix J . Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. (1999) 19:329–38. 10.1055/s-2007-1007122
68.
Llovet JM Bustamante J Castells A Vilana R Ayuso Mdel C Sala M et al . Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. (1999) 29:62–7. 10.1002/hep.510290145
69.
Forner A Reig ME de Lope CR Bruix J . Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. (2010) 30:61–74. 10.1055/s-0030-1247133
70.
Kudo M Matsui O Izumi N Iijima H Kadoya M Imai Y et al . JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma 2014. Update by the Liver Cancer Study Group of Japan. Liver Cancer. (2014) 3:458–68. 10.1159/000343875
71.
Kokudo T Hasegawa K Matsuyama Y Takayama T Izumi N Kadoya M et al . Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. (2016) 65:938–43. 10.1016/j.jhep.2016.05.044
72.
Zhang ZY Dong KS Zhang EL Zhang LW Chen XP Dong HH . Resection might be a meaningful choice for hepatocellular carcinoma with portal vein thrombosis: a systematic review and meta-analysis. Medicine. (2019) 98:e18362. 10.1097/MD.0000000000018362
73.
Kim JY Lee JS Oh DH Yim YH Lee HK . Transcatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinoma. Eur J Gastroenterol Hepatol. (2012) 24:640–5. 10.1097/MEG.0b013e3283524d32
74.
Han XJ Su HY Shao HB Xu K . Prognostic factors of spontaneously ruptured hepatocellular carcinoma. World J Gastroenterol. (2015) 21:7488–94. 10.3748/wjg.v21.i24.7488
75.
Xu X Chen C Liu Q Huang X . A meta-analysis of TAE/TACE versus emergency surgery in the treatment of ruptured HCC. Cardiovasc Intervent Radiol. (2020) 43:1263–76. 10.1007/s00270-020-02514-5
76.
Zheng YJ Li DL Luo D Chen XP Zhang B Fang C et al . Early versus delayed hepatectomy for spontaneously ruptured hepatocellular carcinoma: a systematic review and meta-analysis. J Invest Surg. (2020) 1:9. 10.1080/08941939.2020.1792009
Summary
Keywords
hepatocellular carcinoma, spontaneous tumor rupture, hepatectomy, prognosis, meta-analysis
Citation
Xu J, Hong J, Wang Y, Zhou L, Xu B, Si Y, He Y and Chen Y (2021) Prognostic Influence of Spontaneous Tumor Rupture in Patients With Hepatocellular Carcinoma After Hepatectomy: A Meta-Analysis of Observational Studies. Front. Surg. 8:769233. doi: 10.3389/fsurg.2021.769233
Received
01 September 2021
Accepted
26 October 2021
Published
16 November 2021
Volume
8 - 2021
Edited by
Alessandro Vitale, University Hospital of Padua, Italy
Reviewed by
Fabricio Coelho, University of São Paulo, Brazil; Matteo Donadon, Humanitas University, Italy
Updates
Copyright
© 2021 Xu, Hong, Wang, Zhou, Xu, Si, He and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yizhou Chen hello666813@126.com
†These authors have contributed equally to this work and share first authorship
This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.