Abstract
Background:
The status of peritoneal surface malignancy (PSM) management in North Africa is undetermined. The aim of this study was to assess and compare current practice and knowledge regarding PSM and examine satisfaction with available treatment options and need for alternative therapies in North Africa.
Methods:
This is a qualitative study involving specialists participating in PSM management in North Africa. The survey analyzed demographic characteristics and current knowledge and opinions regarding PSM management in different institutions. We also looked at goals and priorities, satisfaction with treatment modalities and heated intraperitoneal chemotherapy (HIPEC) usefulness according to specialty, country, years of experience, and activity sector.
Results:
One-hundred and three participants responded to the survey (response rate of 57%), including oncologists and surgeons. 59.2% of respondents had more than 10 years experience and 45.6% treated 20–50 PSM cases annually. Participants satisfaction with PSM treatment modalities was mild for gastric cancer (3/10 [IQR 2–3]) and moderate for colorectal (5/10 [IQR 3–5]), ovarian (5/10 [IQR 3–5]), and pseudomyxoma peritonei (5/10 [IQR 3–5]) type of malignancies. Good quality of life and symptom relief were rated as main priorities for treatment and the need for new treatment modalities was rated 9/10 [IQR 8–9]. The perceived usefulness of systemic chemotherapy in first intention was described as high by 42.7 and 39.8% of respondents for PSM of colorectal and gastric origins, while HIPEC was described as highly useful for ovarian (49.5%) and PMP (73.8) malignancies.
Conclusions:
The management of PSM in the North African region has distinct differences in knowledge, treatments availability and priorities. Disparities are also noted according to specialty, country, years of expertise, and activity sector. The creation of referral structures and PSM networks could be a step forward to standardized PSM management in the region.
Introduction
Peritoneal surface malignancy (PSM) is a rare and challenging pathology resulting from the neoplastic progression of different types of primary malignancies. Due to late diagnosis, rapid progression and limited treatment options, PSM was considered until recently as a terminal illness. Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has emerged as a therapy associated with significant benefits for selected patients with PSM of colorectal (1–3), gastric (4–6), and ovarian origin (7), as well as pseudomyxoma peritonei (8), therefore, changing the historical perception on peritoneal metastases. Other therapies such as perioperative systemic chemotherapy, pressurized intraperitoneal aerosol chemotherapy (PIPAC), and pulsed low dose radiation therapy are also showing promise (9–11), which is resulting in a growing acceptance. However, although a number of centers are venturing in the treatment of peritoneal malignancies and developing specialized programmes, evidence has shown that consideration of this procedure as a treatment option can be low (12). In fact, consideration of these treatment options remains low especially in non-oncologic departments (12), and discrepancies have been noted also between surgical and non-surgical oncologists (13). It appears therefore important to increase awareness and knowledge dissemination regarding these therapies within the cancer care community.
Several studies demonstrated other challenges hindering the acceptance and adoption of PSM treatment strategies, such as the lack of a multidisciplinary approach to patient management (14), the need for specialized training and mentorship but also financial restrictions to establish reference centers for referrals (15–17). These constraints are even worse in low and middle-income countries where cancer care faces additional barriers limiting the access to chemotherapy agents, radiation therapy, and surgical care (18) and where overall healthcare may differ between private and public healthcare systems (19).
North Africa is a diverse region consisting of the five countries forming the Maghreb, namely Morocco, Algeria, Tunisia, Mauritania, and Libya. This region has particular characteristics when it comes to healthcare in general with the public and private sectors playing a complementary role (20) and where cancer incidence has a distincts pattern that completely differs from that of Central and Southern African countries as well as Europe (21). Nonetheless, there is no available data on the knowledge and practices in the management of PSM in this region, except for a few reports (22). The aim of this study was to assess and compare current practice and knowledge regarding PSM and examine satisfaction with available treatment options and need for alternative therapies in North Africa.
Materials and Methods
Study Design and Participants
We conducted a qualitative study among a network of specialized North Africa centers which includes all cancer centers involved in the management of PSM in the private and public sector. Representatives from each one of the five countries were contacted in order to help identify institutions undertaking PSM management and a minimum of two participants per center was required. The study was approved by the institutional review boards of all participating centers and was exempt from ethics committee approval as participants gave their consent to participate in the survey. Two similar studies have been previously conducted in a Swiss oncological network (23) and an Indian peritoneal surface malignancy network (24).
The survey included questions on demographics such as country, subspecialty, year of expertise, and number of patients treated personally with PSM. We also examined PSM management in the different institutions, and specialists current knowledge and opinions concerning carcinomatosis. We requested participants to rate on a 5 point likert scale PSM treatment goals and priorities, namely cure, symptom relief, few side effects, few contraindications, inexpensive, or providing a good quality of life. The usefulness of heated intraperitoneal chemotherapy and systemic chemotherapy (in first and second intention) was also assessed, being either poor, moderate or high for PSM of colorectal, ovarian, gastric, and pseudomyxoma peritonei origins. Participants were also required to rate their satisfaction with treatment options on a scale ranging from frustrated: 0 to perfectly happy: 10, as well as the need for new treatment options with 0: no need and 10: urgent need (23, 24). An additional section was included to analyze current practice in the management of PSM originating from pseudomyxoma peritonei tumors. In total, the survey included 29 questions (Appendix 1).
Statistical Analysis
We reported categorical variables as numbers and percentages, and continuous variables as mean and standard deviations (SD). χ2 tests of association were used to compare respondents characteristics and when more than 25% of the subgroups examined were populated by fewer than five respondents, Fisher's exact test or likelihood ratios were performed. Four subgroup analyses were undertaken comparing satisfaction with current treatment modalities, priorities, and perceived need of new treatment options between participants according to specialty (oncologist vs. surgeons), country, years of experience (> or <10 years of experience) and activity sector (public vs. private). Differences were tested with the non-parametric Mann–Whitney U-test and Kruskal–Wallis test as adequate. All statistical analyses were performed using SPSS version 25. A significant P-value was considered if p < 0.05.
Results
Participants and Institutions Demographics
The representatives of the four North African countries identified 18 cancer care centers including 180 potentially eligible specialists. In total, 103 participants responded to the survey (response rate of 57%), being either oncologists or surgeons directly involved in the management of PSM in private or public healthcare facilities.
Overall, 59.2% of respondents had more than 10 years experience with 45.6% personally treating 20–50 PC cases annually. There was a significantly higher percentage of surgeons with more than 10 years than oncologists (77.27 vs. 47.5, P = 0.045), however, more oncologists reported treating more than 50 PC cases annually (76.9 vs. 43.1 for surgeons, P = 0.009). While 88.3 and 71.8% of participating institutions offered systemic chemotherapy and cytoreductive surgery, respectively, only 26.2% had access to HIPEC, with no difference between the private and public sectors (P = 0.934). Other treatment modalities such as low dose radiotherapy, pressurized intraperitoneal chemotherapy, were possible in 11.7, 2.9% of institutions, respectively, with a significantly higher percentage of availability in the private sector (P = 0.020 and 0.037). Sociodemographic details of the participants in general and according to their subgroups are presented in Table 1.
Table 1
| Global population | Surgeons | oncologists | P | Private | Public | P | |
|---|---|---|---|---|---|---|---|
| Country | |||||||
| Morocco | 75 (72.8) | 32 (72.7) | 43 (72.9) | 0.154 | 33 (94.3) | 42 (61.8) | <0.001 |
| Tunisia | 10 (9.7) | 7 (15.9) | 3 (5.1) | 2 (5.7) | 8 (11.8) | ||
| Algeria | 13 (12.6) | 3 (6.8) | 10 (16.9) | 0 | 13 (19.1) | ||
| Mauritania | 5 (4.9) | 2 (4.5) | 3 (5.1) | 0 | 5 (7.4) | ||
| Activity sector | |||||||
| Private | 35 (25) | 21 (47.7) | 14 (23.7) | 0.011 | – | – | – |
| Public | 68 (66) | 23 (52.3) | 45 (76.3) | ||||
| MDT meeting | |||||||
| Yes | 92 (89.3) | 40 (90.9) | 52 (88.1) | 0.755 | 28 (80) | 64 (94.1) | 0.503 |
| No | 11 (10.7) | 4 (9.1) | 7 (11.9) | 7 (20) | 4 (5.9) | ||
| Years of experience since board qualification | |||||||
| <10 years | 41 (39.8) | 10 (22.7) | 31 (52.5) | 0.045 | 8 (22.9) | 33 (48.5) | 0.248 |
| >10 years | 61 (59.2) | 34 (77.27) | 28 (47.5) | 27 (77.1) | 35 (55.5) | ||
| Specialty | |||||||
| Oncologist | 59 (57.3) | – | – | – | 14 (40) | 45 (66.2) | 0.011 |
| Surgeon | 44 (42.7) | 21 (60) | 23 (33.8) | ||||
| Surgical subspecialties | |||||||
| General surgeon | 29 (72.5) | – | – | – | 8 (80) | 21 (70) | 0.389 |
| Colorectal surgical oncologist | 9 (22.5) | 1 (10) | 8 (26.7) | ||||
| Gynecological surgical oncologist | 2 (5) | 1 (10) | 1 (3.3) | ||||
| Patients with PC personally treated annually | |||||||
| <20 | 33 (26) | 21 (47.7) | 12 (20.3) | 0.009 | 13 (37.1) | 20 (29.4) | 0.723 |
| 20–50 | 47 (45.6) | 4 (9.1) | 1 (1.7) | 15 (42.9) | 32 (47.1) | ||
| >50 | 23 (22.4) | 19 (43.1) | 46 (76.9) | 7 (20) | 16 (23.5) | ||
| Guidelines followed by institution | |||||||
| National | 12 (11.7) | 8 (18.2) | 4 (6.8) | 0.159 | 4 (11.4) | 8 (11.8) | 0.545 |
| French | 81 (78.6) | 31 (70.5) | 50 (84.7) | 26 (74.3) | 55 (80.9) | ||
| PSOGI | 10 (9.7) | 5 (11.4) | 5 (8.5) | 5 (14.3) | 5 (7.4) | ||
| Personal knowledge on PCI calculation method | |||||||
| Yes | 48 (46.6) | 25 (56.8) | 23 (39) | 0.073 | 18 (51.4) | 30 (44.1) | 0.481 |
| No | 55 (53.4) | 19 (43.2) | 36 (61) | 17 (48.6) | 38 (55.9) | ||
| Treatment options offered at institution | |||||||
| Cytoreductive surgery | |||||||
| Yes | 74 (71.8) | 32 (72.7) | 42 (71.2) | 0.863 | 25 (71.4) | 49 (72.1) | 0.946 |
| No | 29 (28.2) | 12 (27.3) | 17 (28.8) | 10 (28.6) | 19 (27.9) | ||
| HIPEC | |||||||
| Yes | 27 (26.2) | 8 (18.2) | 19 (32.2) | 0.109 | 9 (25.7) | 18 (26.5) | 0.934 |
| No | 76 (73.8) | 36 (81.8) | 40 (67.8) | 26 (74.3) | 50 (73.5) | ||
| Systemic chemotherapy | |||||||
| Yes | 91 (88.3) | 32 (72.7) | 59 (100) | <0.001 | 29 (82.9) | 62 (91.2) | 0.330 |
| No | 12 (11.7) | 12 (27.3) | 0 (0) | 6 (17.1) | 6 (8.8) | ||
| Intraperitoneal therapy using a catheter | |||||||
| Yes | 1 (1) | 0 (0) | 1 (1.7) | 1.000 | 1 (2.9) | 0 | 0.340 |
| No | 99 (102) | 44 (100) | 58 (98.3) | 34 (97.1) | 68 (100) | ||
| Pressurized intraperitoneal chemotherapy | |||||||
| Yes | 3 (2.9) | 2 (4.5) | 1 (1.7) | 0.574 | 3 (8.6) | 0 | 0.037 |
| No | 100 (97.1) | 42 (95.5) | 58 (98.3) | 32 (91.4) | 68 (100) | ||
| Low dose radiotherapy | |||||||
| Yes | 12 (11.7) | 5 (11.4) | 7 (11.9) | 0.938 | 8 (22.9) | 4 (5.9) | 0.020 |
| No | 91 (88.4) | 39 (88.6) | 52 (88.1) | 27 (77.1) | 64 (94.1) | ||
| Management of PC of PMP origin in absence of HIPEC | |||||||
| Chemotherapy | |||||||
| Curative | 7 (6.8) | 0 (0) | 7 (11.9) | 1 (2.9) | 6 (8.9) | ||
| Palliative | 4 (3.8) | 1 (2.3) | 3 (5.1) | 0 | 4 (5.9) | ||
| Cytoreductive surgery | 0.003 | 0.331 | |||||
| Curative | 51 (49.5) | 19 (43.2) | 32 (54.2) | 19 (54.3) | 32 (47.1) | ||
| Palliative | 4 (3.8) | 4 (9.1) | 0 (0) | 2 (5.7) | 2 (2.9) | ||
| Transfert to a specialized center | 37 (35.9) | 20 (45.5) | 17 (28.8) | 13 (37.1) | 24 (35.3) | ||
Demographics of participating institutions and specialists.
The bold values mean significant value.
Satisfaction
Details on respondents' satisfaction are described in Figure 1.
Figure 1
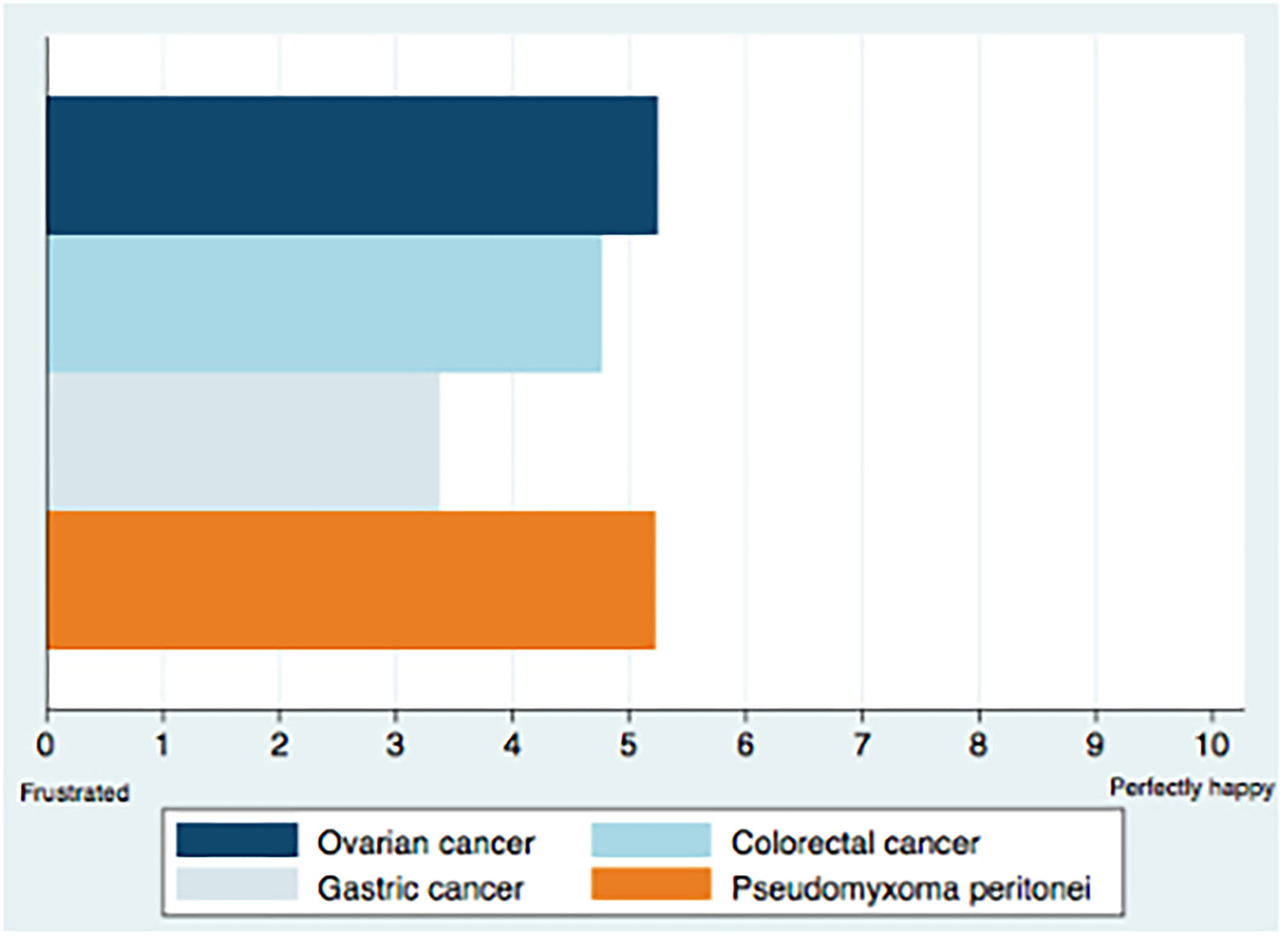
Satisfaction with treatment modalities for PSM.
Participants satisfaction with PSM treatment modalities was examined according to cancer origin and was mild for gastric cancer (3/10 [IQR 2–3]) and moderate for colorectal (5/10 [IQR 3–5]), ovarian (5/10 [IQR 3–5]), and pseudomyxoma peritonei (5/10 [IQR 3–5]) type of malignancies. The need for new treatment modalities was rated 9/10, [IQR 8–9]. The comparison of the degree of satisfaction according to specialty, country, activity sector, and years of experience showed no statistically significant difference between these subgroups. On the other hand, oncologists reported a higher need for new treatment modalities (9.07 ± 0.98) compared to surgeons (8.02 ± 2.26), P = 0.05 (Table 2).
Table 2
| Specialty | Country | Activity sector | Years of experience | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgeon | Oncologist | P- value | Algeria | Morocco | Mauritania | Tunisia | P- value | Public | Private | P- value | <10 years | >10 years | P- value | |
| Priorities (Mean ±SD) | ||||||||||||||
| Cure | 3.66 (±1.36) |
4.22 (±0.96) |
0.035 | 3.77 (±1.23) |
4.05 (±1.17) |
3.20 (±0.83) |
4.10 (±1.28) |
0.205 | 3.99 (±1.16) |
3.97 (±1.22) |
0.982 | 4.20 (±1.05) |
3.87 (±1.23) |
0.161 |
| Symptoms relief | 4.55 (±0.79) |
4.85 (±0.36) |
0.019 | 4.46 (±1.12) |
4.75 (±0.49) |
4.80 (±0.44) |
4.80 (±0.42) |
0.882 | 4.69 (±0.65) |
4.77 (±0.49) |
0.580 | 4.78 (±0.47) |
4.67 (±0.67) |
0.434 |
| Few side effects | 3.80 (±1.06) |
4.27 (±0.84) |
0.023 | 4.08 (±1.03) |
4.09 (±0.98) |
4.20 (±0.83) |
3.80 (±0.91) |
0.775 | 4.10 (±1.02) |
4 (±0.87) |
0.440 | 4.29 (±0.90) |
3.90 (±0.99) |
0.045 |
| Few contraindications | 3.55 (±1.02) |
4.08 (±0.83) |
0.007 | 4.46 (±0.87) |
3.76 (±0.95) |
3.60 (±0.89) |
3.90 (±0.87) |
0.049 | 3.93 (±0.95) |
3.71 (±0.95) |
0.293 | 3.98 (±0.93) |
3.75 (±0.96) |
0.252 |
| Inexpensive | 3.39 (±1.14) |
3.64 (±1.11) |
0.287 | 2.92 (±1.32) |
3.67 (±1.08) |
4.00 (±1) |
3.10 (±0.99) |
0.074 | 3.50 (±1.14) |
3.60 (±1.11) |
0.694 | 3.78 (±1.17) |
3.38 (±1.08) |
0.070 |
| Good quality of life | 4.52 (±0.90) |
4.86 (±0.34) |
0.031 | 4.62 (±1.12) |
4.76 (±0,51) |
4.20 (±1.30) |
4.80 (±0.42) |
0.653 | 4.65 (±0.76) |
4.86 (±0.35) |
0.224 | 4.80 (±0.40) |
4.66 (±0.79) |
0.680 |
|
Satisfaction
(Mean ±SD) |
||||||||||||||
| PC of ovarian origin | 5.61 (±2.83) |
5 (±2.66) |
0.301 | 5.23 (±2.97) |
5.33 (±2.75) |
5 (±2.55) |
4.90 (±2.80) |
0.963 | 5.25 (±2.79) |
5.29 (±2.66) |
0.958 | 5.05 (±2.91) |
5.39 (±2.65) |
0.498 |
| PC of colorectal origin | 5.05 (±2.77) |
4.58 (±2.50) |
0.371 | 5.23 (±3.29) |
4.61(±2.48) | 3.60 (±3.28) |
6 (±2.26) |
0.274 | 4.81 (±2.66) |
4.71 (±2.58) |
0.836 | 4.34 (±2.75) |
5.05 (±2.53) |
0.126 |
| PC of gastric origin | 3.30 (±2.37) |
3.46 (±2.71) |
0.984 | 3.46 (±3.86) |
3.40 (±2.33) |
2.40 (±1.81) |
3.70 (±2.79) |
0.722 | 3.56 (±2.73) |
3.06 (±2.19) |
0.486 | 3.54 (±2.81) |
3.31 (±2.42) |
0.793 |
| PC of PMP origin | 5.68 (±3.35) |
4.90 (± 2.82) |
0.213 | 6 (±2.94) |
5.08 (±3.06) |
3 (±2.64) |
6.50 (±3.06) |
0.138 | 4.93 (±3.09) |
5.83 (±2.98) |
0.157 | 4.93 (±3.00) |
5.49 (±2.65) |
0.396 |
| The need of new treatment modalities | 8.02 (±2.26) |
9.07 (±0.98) |
0.05 | 8.92 (±1.93) |
8.64 (±1.74) |
8.60 (±1.14) |
8.20 (±1.61) |
0.434 | 8.70 (±1.50) |
8.49 (±2.07) |
0.930 | 8.71 (±1.66) |
8.57 (±1.76) |
0.503 |
Subgroup analysis of priorities and degree of satisfaction according to specialty, expertise, country, and activity sector.
The bold values mean significant value.
Goals and Priorities
79.6 and 76.7% of participants rated good quality of life and symptom relief as very important, while few contraindications and inexpensive treatments were rated less important (Figure 2).
Figure 2
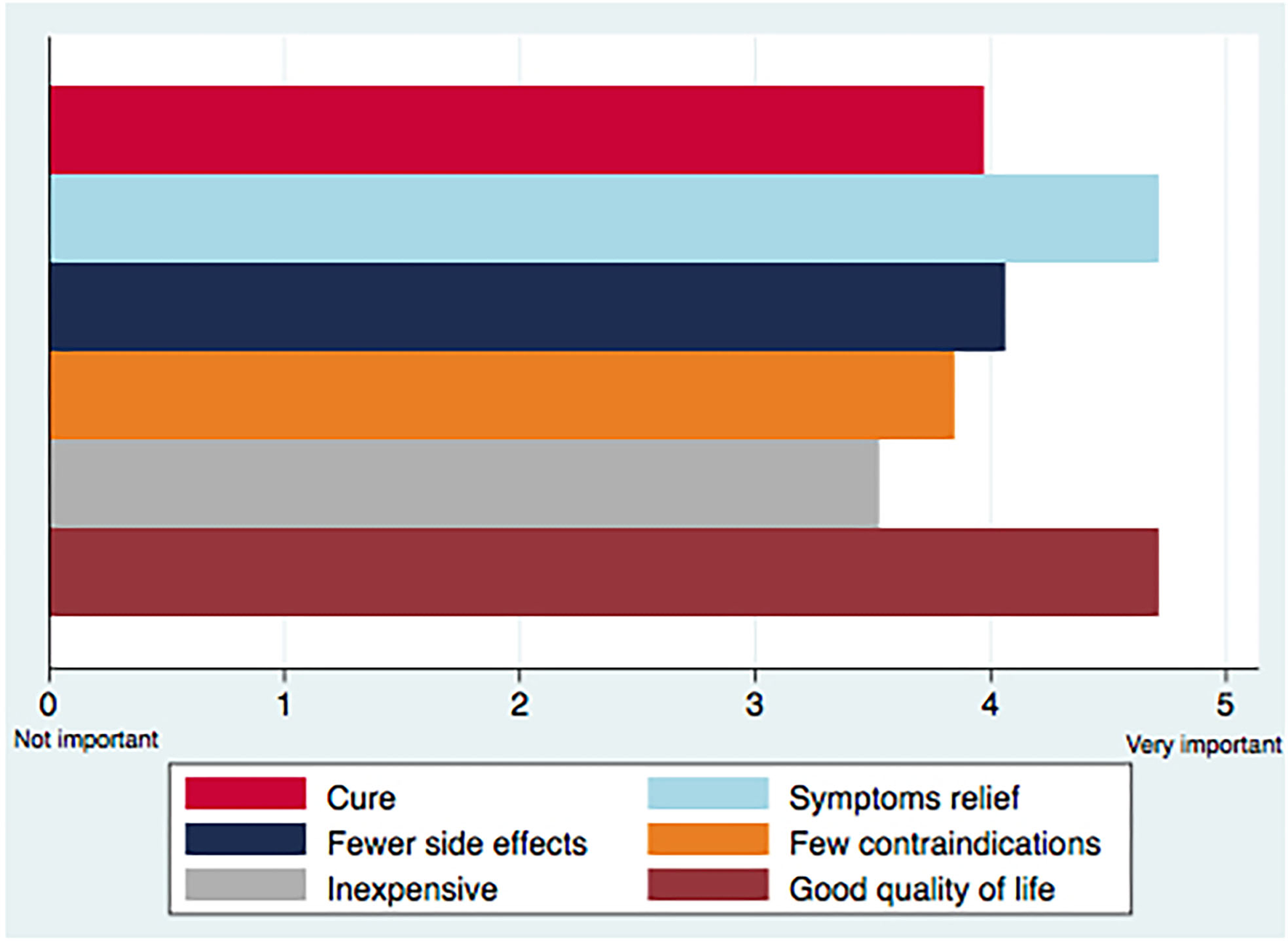
Main priorities in the treatment of peritoneal carcinomatosis.
Looking at treatment goals according to the different subgroups, oncologists rated a statistically significant higher degree of priority for all elements, except for inexpensiveness (P = 0.287), while prioritizing fewer contraindications was different between countries (P = 0.049). No significant variation was established in the priorities according to years of experience and activity sector (Table 2).
Usefulness of Systemic Chemotherapy and HIPEC in Resectable Peritoneal Metastasis
Overall, the perceived usefulness of systemic chemotherapy in first intention was described as high by 42.7 and 39.8% of respondents for PSM of colorectal and gastric origins, while HIPEC was described as highly useful for ovarian (49.5%) and PMP (73.8) malignancies.
Subgroup analysis yielded a significant difference between surgeons and oncologists, with 91.5% oncologists rating systemic chemotherapy as a second line intention for PC of ovarian origin as highly useful, compared to 75% of surgeons (P = 0.044). Differences were also noted in the perceived usefulness for systemic chemotherapy in first line intention for PC of colorectal (P = 0.030) and gastric origin (P = 0.029) between specialists from the private and public sector.
Details on the usefulness of chemotherapy and HIPEC in the management of resectable peritoneal metastasis are demonstrated in Table 3.
Table 3
| Global population | Surgeons | Oncologists | P -value | Public | Private | P- value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poor | Moderate | High | Poor | Moderate | High | Poor | Moderate | High | Poor | Moderate | High | Poor | Moderate | High | |||
| Systemic chemotherapy | |||||||||||||||||
| Colorectal origin | |||||||||||||||||
| First-line intention | 14 (13.6) |
45 (43.7) |
44 (42.7) |
9 (20.5) |
21 (47.7) |
14 (31.8) |
5 (8.5) |
24 (40.7) |
30 (50.8) |
0.079 | 8 (11.8) |
36 (52.9) |
24 (35.3) |
6 (17.1) |
9 (25.7) |
20 (57.1) |
0.030 |
| Second-line intention | 12 (11.7) |
37 (35.9) |
54 (52.4) |
6 (13.6) |
13 (29.5) |
25 (56.8) |
6 (10.2) |
24 (40.7) |
29 (49.2) |
0.494 | 8 (11.8) |
23 (33.8) |
37 (54.4) |
4 (11.4) |
14 (40) |
17 (48.6) |
0.820 |
| Gastric origin | |||||||||||||||||
| First-line intention | 32 (31.1) |
30 (29.1) |
41 (39.8) |
15 (34.1) |
11 (25) |
18 (40.9) |
17 (28.8) |
19 (32.2) |
23 (39) |
0.705 | 24 (35.3) |
14 (20.6) |
30 (44.1) |
8 (22.9) |
16 (45.7) |
11 (31.4) |
0.029 |
| Second-line intention | 41 (40.8) |
31 (30.1) |
30 (29.1) |
17 (38.6) |
12 (27.3) |
15 (34.1) |
25 (42.4) |
19 (32.2) |
15 (25.4) |
0.625 | 28 (41.2) |
19 (27.9) |
21 (30.9) |
14 (40) |
12 (34.3) |
9 (25.7) |
0.768 |
| Ovarian origin | |||||||||||||||||
| Second-line intention | 6 (5.8) |
10 (9.7) |
87 (84.5) |
5 (11.4) |
6 (13.6) |
33 (75) |
1 (1.7) |
4 (6.8) |
54 (91.5) |
0.044 | 3 (4.4) |
8 (11.8) |
57 (83.8) |
3 (8.6) |
2 (5.7) |
30 (85.7) |
0.447 |
| Third-line intention | 40 (39.2) |
32 (31.3) |
30 (29.4) |
16 (36.6) |
12 (27.3) |
16 (36.6) |
24 (41.3) |
20 (34.4) |
14 (24.3) |
0.6 | 28 (41.2) |
19 (27.9) |
21 (30.9) |
12 (35.2) |
13 (38.2) |
9 (26.4) |
0.72 |
| HIPEC | |||||||||||||||||
| Ovarian origin | 11 (10.7) |
41 (39.8) |
51 (49.5) |
5 (11.4) |
13 (29.5) |
26 (59.1) |
6 (10.2) |
28 (47.5) |
25 (42.4) |
0.175 | 7 (10.3) |
26 (38.2) |
35 (51.5) |
4 (11.4) |
15 (42.9) |
16 (45.7) |
0.858 |
| Colorectal origin | 22 (21.4) |
38 (36.9) |
43 (41.7) |
9 (20.5) |
17 (38.6) |
18 (40.9) |
13 (22) |
21 (35.6) |
25 (42.4) |
0.949 | 15 (22.1) |
24 (35.3) |
29 (42.6) |
7 (20) |
14 (40) |
14 (40) |
0.894 |
| Gastric origin | 47 (45.6) |
40 (38.8) |
16 (15.5) |
18 (40.9) |
17 (38.6) |
9 (20.5) |
29 (49.2) |
23 (39) |
7 (11.9) |
0.455 | 30 (44.1) |
28 (41.2) |
10 (14.7) |
17 (48.6) |
12 (34.3) |
6 (17.1) |
0.790 |
| PMP origin | 7 (6.8) |
20 (19.4) |
76 (73.8) |
4 (9.1) |
9 (20.5) |
31 (70.5) |
3 (5.1) |
11 (18.6) |
45 (76.3) |
0.689 | 4 (5.9) |
11 (16.2) |
53 (77.9) |
3 (8.6) |
9 (25.7) |
23 (65.7) |
0.416 |
Perceived clinical usefulness of systemic chemotherapy and HIPEC in resectable peritoneal metastasis.
The bold values mean significant value.
Discussion
PSM can occur as a primary malignancy, or originate from the progression of an array of tumors, such as gastric, colorectal, or ovarian cancers. In the case of metastatic spreading, this can be justified using either one of the two main hypotheses whereby tumor progression can be promoted by anatomical factors such as the local spreading of tumor cells after breaching the initial site or by the “seed-and-soil” hypothesis implying the specific tropism of tumor cells circulating in the lymphatic or vascular systems to certain organs. However, unknown alternative biological routes may also exist (27–29). As such, management options also vary according to tumor origins, and whether the aim of treatment is curative or palliative. This study depicts the heterogeneous knowledge on the management of PSM, differences in priorities, and lack of guideline use and standardization. Furthermore, it underlines potential differences between specialists according to specialty, country, years of expertise, and activity sector. Efforts are needed to disseminate knowledge and awareness through the creation of networks and referral structures in order to offer optimal care to the largest possible number of patients.
This survey targeted the network of cancer care centers involved in the management of PSM in North Africa, which comprises oncologists and surgeons from Morocco, Algeria, Tunisia, and Mauritania. Overall, almost half of participants reported personally treating 20–50 patients annually. However, access to HIPEC or other therapies such as PIPAC or low dose radiotherapy was poor. In case of HIPEC unavailability, 35.9% of respondents choose patient transfer to a specialized center as an alternative, while only 7.6% of respondents sought either palliative chemotherapy or cytoreductive surgery as a substitute, which could convey an overall good understanding of PSM' changing prognosis and the acceptance of HIPEC as a potentially curative treatment option.
The results from our study were comparable to the two previous surveys conducted in the Swiss (23) and Indian networks (24). In fact, similarities were noted between the three networks, with a particular lack of satisfaction for gastric cancer compared to other origins of malignancies, which could be due to the extremely bad prognosis of PSM of this origin (30). That being said, the need for new treatment modalities was also rated as high. On the other hand, although the cost of treatment was the least important treatment goal in all three surveys, other priorities differed with a higher importance given to good quality of life by North African specialists, while cure was ranked as the main goal in the other studies. This could uncover a common misconception about quality of life decline in the treatment of PSM (31). As regards the degree of usefulness of chemotherapy and HIPEC, all three networks recognize a moderate to high usefulness of these therapies in ovarian and colorectal cancer as a first or second line treatment, with less perceived advantages for PC of gastric origin despite regional recommendations that conform to the international guidance (32). We also examined the degree of satisfaction and perceived usefulness of chemotherapy and HIPEC in the case of primary PC originating from pseudomyxoma peritonei, which illustrated a moderate degree of satisfaction and high perceived degree of effectiveness for HIPEC.
Previous studies on the acceptance of PC therapies portrayed a substantial variation in opinions according to factors such as specialty, experience, implementation of multidisciplinary tumor boards and availability of expert centers (13, 26, 33, 34), which could contribute to the underutilization of these treatments. Accordingly, we compared potential differences between specialists and according to years of expertise, activity sector as well as country. When comparing surgeons and oncologists' point of views, some differences in priorities and need for new therapies have been observed. However, no disagreement has been noted regarding chemotherapy and HIPEC use, except for PC of ovarian origin, for which HIPEC indications are still a subject of controversy (25, 35). The ongoing debate between oncologists, surgeons, and other non-oncologic specialists regarding PC has been established in previous studies (12, 13), with suggestions that a multidisciplinary discussion among different specialties is of utmost importance to overcome personal preferences and knowledge gaps, while deciding for treatment or even referral suitability (36, 37). In our study, 89.3% of specialists reported discussing their cases in multidisciplinary team meetings.
In resource restricted contexts such as in the North African region, the prioritization and indications for the curative treatment may also face the constraints of limited critical care and surgical resources management and expertise (22, 38–41). In fact, while only 26.2% of our respondents reported having access to HIPEC, a similar study on practice patterns in PC from the USA identified 65.8% of its participants as having easy access to a HIPEC and CRS specialists, with 42.7% being in the same hospital (33). In such settings, the world health organization (WHO) has identified the private sector as an additional asset allowing the provision of infrastructure, support services, medicines and medical products, as well as a solution to some financial challenges (42). As such, we choose to include the private sector in our analysis, which indeed demonstrated that the significantly higher availability in the private sector of some therapies such as pressurized intraperitoneal chemotherapy and low dose radiotherapy didn't necessarily yield a higher level of satisfaction in the available treatment modalities. Regardless of the activity sector, the level of expertise is considered a better reflection (22), especially as the PSM treatment learning curve can be steep and complex (43).
PSM management has undergone a tremendous change and is still subject to evolving with ongoing randomized clinical trials and emerging new techniques. However, this progress is also perceived as chaotic with many methodological variations according to patients, type and stage of carcinomatosis (44). This is why there is a need for strong collaborations between different specialists and even countries which were more successful in some areas, in order to ensure knowledge dissemination. The creation of organizations could also be a step forward toward consolidating efforts for the effective implementation of these innovative therapies as per the IDEAL framework, as well as developing guidelines regarding patient selection and therapy standardization (44–46). In our context, only 9.7% of respondents reported complying with the peritoneal surface oncology group international guidelines, which could reflect the need for a regional dialogue and a possible adaptation of these recommendations to the North African context, especially as no dissimilarities have been noted between four countries.
This study is exploratory research with some limitations. Firstly, participants' selection was done through designated referees from each country who were in charge of contacting centers, which could result in some selection and reach bias. That being said, all declared cancer care centers in the region were contacted. Also, gynecological surgeons were under-represented in this group, which is due to the fact that gynecological oncological surgery is mostly performed by general oncological surgeons in the North African setting.
Notwithstanding these limitations, this is the first study addressing the current opinions in the North African region, while capturing the differences between specialties and activity sectors, as well as reflecting the real picture of practice in a region where little published data is available on the management of PC.
Conclusion
The assessment of current opinions and knowledge on peritoneal surface malignancy in the North African region indicates a low satisfaction with currently available treatment options and different perspectives between medical oncologists and surgeons. In spite of that, there was a notable homogeneity in treatment practices, which could reflect the possibility of developing a North African collaboration with regional guidelines that allow the adaptation of international recommendations to peritoneal surface malignancy management and that could serve as a template for other low and middle income settings.
Funding
Publication fees supported by the Association Cancer Espoir, Rabat, Morocco.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
AS, SB, and MH have contributed to the conception and the design of the study. AS, SB, HE, AM, MAb, and JB have contributed to the acquisition of the data. AS, SB, and HE contributed to the analysis and the interpretation of data. AS and HE wrote the first draft. MM, AB, and MH critically reviewed the draft for important intellectual content. MAy, FK, ZK, YB, BE, and RM were involved in revising critically the corrected manuscript. All authors read and gave the final approval of the version to be published.
Acknowledgments
The authors would like to thank the board of the AMFROM, the Moroccan Society of Digestive Surgery (SMCD), the Tunisian Society of Surgery (ATS), and Algerian Society of Surgery (ASS), all the participant to the survey and Miss Hanane Benkouya for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.798523/full#supplementary-material
References
1.
Eng OS Turaga KK . Cytoreduction and hyperthermic intraperitoneal chemotherapy in metastatic colorectal cancer. J Surg Oncol. (2019) 119:613–5. 10.1002/jso.25438
2.
Glehen O Kwiatkowski F Sugarbaker PH Elias D Levine EA De Simone M et al . Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J. Clin. Oncol. (2004) 22:3284–92. 10.1200/jco.2004.10.012
3.
Goéré D Souadka A Faron M Cloutier AS Viana B Honoré C et al . Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. (2015) 22:2958–64. 10.1245/s10434-015-4387-5
4.
Manzanedo I Pereira F Rihuete Caro C Pérez-Viejo E Serrano Á Gutiérrez Calvo A et al . Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for gastric cancer with peritoneal carcinomatosis: multicenter study of spanish group of peritoneal oncologic surgery (GECOP). Ann Surg Oncol. (2019) 26:2615–21. 10.1245/s10434-019-07450-4
5.
Honoré C Goéré D Messager M Souadka A Dumont F Piessen G et al . Risk factors of peritoneal recurrence in eso-gastric signet ring cell adenocarcinoma: results of a multicentre retrospective study. Eur J Surg Oncol. (2013) 39:235–41. 10.1016/j.ejso.2012.12.013
6.
Messager M Lefevre JH Pichot-Delahaye V Souadka A Piessen G Mariette C et al . The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg. (2011) 254:684–93; discussion 693. 10.1097/SLA.0b013e3182352647
7.
van Driel WJ Koole SN Sikorska K Schagen van Leeuwen JH Schreuder HWR Hermans RHM et al . Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. (2018) 378:230–40. 10.1056/NEJMoa1708618
8.
Chua TC Moran BJ Sugarbaker PH Levine EA Glehen O Gilly FN et al . Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. (2012) 30:2449–56. 10.1200/JCO.2011.39.7166
9.
Struller F Horvath P Solass W Weinreich F-J Strumberg D Kokkalis MK et al . Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: a phase II study. Ther Adv Med Oncol. (2019) 11:1758835919846402. 10.1177/1758835919846402
10.
Robella M Vaira M De Simone M . Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol. (2016) 14:128. 10.1186/s12957-016-0892-7
11.
Baratti D Kusamura S Azmi N Guaglio M Montenovo M Deraco M . Colorectal peritoneal metastases treated by perioperative systemic chemotherapy and cytoreductive surgery with or without mitomycin C-based HIPEC: a comparative study using the Peritoneal Surface Disease Severity Score (PSDSS). Ann Surg Oncol. (2020) 27:98–06. 10.1245/s10434-019-07935-2
12.
Wang W Tan GHC Skanthakumar T Chia CS Soo KC Teo MCC . Exploring the trend in referrals for consideration of CRS and HIPEC to understand the attitudes of clinicians in the development of a national cancer centre programme in peritoneal disease. Int J Hyperthermia. (2018) 34:551–8. 10.1080/02656736.2017.1387939
13.
Braam HJ Boerma D Wiezer MJ van Ramshorst B . Cytoreductive surgery and HIPEC in treatment of colorectal peritoneal carcinomatosis: experiment or standard care? A survey among oncologic surgeons and medical oncologists. Int J Clin Oncol. (2015) 20:928–34. 10.1007/s10147-015-0816-5
14.
Brandl A Westbrook S Nunn S Arbuthnot-Smith E Mulsow J Youssef H et al . Clinical and surgical outcomes of patients with peritoneal mesothelioma discussed at a monthly national multidisciplinary team video-conference meeting. BJS Open. (2020) 4:260–7. 10.1002/bjs5.50256
15.
Sarpel U Melis M Newman E Pachter HL Berman RS . The development of a peritoneal surface malignancy program: a tale of three hospitals. J Cancer Educ. (2012) 27:670–5. 10.1007/s13187-012-0352-1
16.
González Bayón L Sugarbaker PH Gonzalez Moreno S Vazquez V de L Alves S Moran BJ . Initiation of a program in peritoneal surface malignancy. Surg Oncol Clin N Am. (2003) 12:741–53. 10.1016/S1055-3207(03)00032-2
17.
Barrios P Ramos Bernadó M Crusellas O Sabia D Bijelic L . SO-31 Centralization of care leads to optimal selection and outcomes for patients with peritoneal metastases of colorectal cancer: The Catalonian regional program experience. Ann Oncol. (2020) 31:S228. 10.1016/j.annonc.2020.04.046
18.
Ruff P Al-Sukhun S Blanchard C Shulman LN . Access to cancer therapeutics in low- and middle-income countries. Am Soc Clin Oncol Educ Book. (2016) 35:58–65. 10.1200/EDBK_155975
19.
Basu S Andrews J Kishore S Panjabi R Stuckler D . Comparative performance of private and public healthcare systems in low- and middle-income countries: a systematic review. PLoS Med. (2012) 9:e1001244. 10.1371/journal.pmed.1001244
20.
Semlali H . Positive Practice Environments in Morocco.International Council of Nurses (2010).
21.
Zanetti R Tazi MA Rosso S . New data tells us more about cancer incidence in North Africa. Eur J Cancer. (2010) 46:462–6. 10.1016/j.ejca.2009.11.012
22.
Souadka A Essangri H Majbar MA Benkabbou A Boutayeb S Amrani L et al . Mid-term audit of a national peritoneal surface malignancy program implementation in a low middle income country: the Moroccan experience. Cancers. (2021) 13:1088. 10.3390/cancers13051088
23.
Grass F Martin D Montemurro M Mathevet P Wolfer A Coukos G et al . Current opinion and knowledge on peritoneal carcinomatosis: a survey among a Swiss Oncology Network. Chemotherapy. (2018) 63:143–47. 10.1159/000488774
24.
Martin D Grass F Deo SVS Ashwin KR Maheshwari A Hübner M et al . Current opinion on peritoneal carcinomatosis treatment: a survey of the Indian Society of Peritoneal Surface Malignancies (ISPSM). J Gastrointest Cancer. (2020) 52:1061–6. 10.1007/s12029-020-00538-1
25.
Vermorken JB van Dam P Brand A . HIPEC in advanced epithelial ovarian cancer: why is there controversy?Curr Opin Oncol. (2020) 32:451–8. 10.1097/CCO.0000000000000659
26.
Yoo HJ Hong JJ Ko YB Lee M Kim Y Han HY et al . Current practices of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal surface malignancies: an international survey of oncologic surgeons. World J Surg Oncol. (2018) 16:92. 10.1186/s12957-018-1377-7
27.
Kranenburg O van der Speeten K de Hingh I . Peritoneal metastases from colorectal cancer: defining and addressing the challenges. Front Oncol. (2021) 11:650098. 10.3389/fonc.2021.650098
28.
Sugarbaker PH . Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. (1996) 82:79–100. 10.1007/978-1-4613-1247-5_6
29.
Riihimäki M Hemminki A Sundquist K Sundquist J Hemminki K . Metastatic spread in patients with gastric cancer. Oncotarget. (2016) 7:52307–16. 10.18632/oncotarget.10740
30.
Yonemura Y Elnemr A Endou Y Hirano M . Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol. (2010) 2:85–97. 10.4251/wjgo.v2.i2.85
31.
Chia CS Tan GHC Lim C Soo KC Teo MCC . Prospective quality of life study for colorectal cancer patients with peritoneal carcinomatosis undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. (2016) 23:2905–13. 10.1245/s10434-016-5203-6
32.
Auer RC Sivajohanathan D Biagi J Conner J Kennedy E May T . Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: a clinical practice guideline. Curr Oncol. (2020) 27:146–54. 10.3747/co.27.6033
33.
Bernaiche T Emery E Bijelic L . Practice patterns, attitudes, and knowledge among physicians regarding cytoreductive surgery and HIPEC for patients with peritoneal metastases. Pleura Peritoneum. (2018) 3:20170025. 10.1515/pp-2017-0025
34.
Spiegle G Schmocker S Huang H Victor JC Law C McCart JA et al . Physicians' awareness of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer carcinomatosis. Can J Surg. (2013) 56:237–42. 10.1503/cjs.003912
35.
Friedrich M Friedrich D Kraft C Kuhn W Rogmans C . HIPEC for ovarian cancer: A controversial discussion. In: Gwo-Yax H, Webber K, editors. Ovarian Cancer - Updates in Tumour Biology and Therapeutics.IntechOpen (2021). p. 150–2.
36.
Ramírez-Plaza CP Moreno-Ruiz FJ Pérez-Daga JA . A multidisciplinary approach for peritoneal carcinomatosis and bilobar liver metastases from colorectal cancer: case report and review of the literature. World J Surg Oncol. (2015) 13:233. 10.1186/s12957-015-0654-y
37.
Siddiqui J Brown K Zahid A Young CJ . Current practices and barriers to referral for cytoreductive surgery and HIPEC among colorectal surgeons: a binational survey. Eur J Surg Oncol. (2020) 46:166–72. 10.1016/j.ejso.2019.09.007
38.
Shariff F Bischof D Govindarajan A Prince R Burkes R Haase E et al . Evidence-based strategies for the treatment of peritoneal malignancies during health care resource restriction: the COVID-19 pandemic. Curr Oncol. (2020) 28:40–51. 10.3390/curroncol28010006
39.
Glehen O Kepenekian V Bouché O Gladieff L Honore C RENAPE-BIG-RENAPE . Treatment of primary and metastatic peritoneal tumors in the Covid-19 pandemic. Proposals for prioritization from the RENAPE and BIG-RENAPE groups. J Visc Surg. (2020) 157:S25–S31. 10.1016/j.jviscsurg.2020.04.013
40.
Souadka A Benkabbou A Al Ahmadi B Boutayeb S Majbar MA . Preparing African anticancer centres in the COVID-19 outbreak. Lancet Oncol. (2020) 21:e237. 10.1016/S1470-2045(20)30216-3
41.
Bensbih S Souadka A Diez AG Bouksour O . Patient centered care: focus on low and middle income countries and proposition of new conceptual model. J Med Surg Res. (2020) 1:755–63. 10.46327/msrjg.1.000000000000168
42.
Clarke D Doerr S Hunter M Schmets G Soucat A Paviza A . The private sector and universal health coverage. Bull World Health Organ. (2019) 97:434–5. 10.2471/BLT.18.225540
43.
Souadka A Benkabbou A Majbar MA Essangri H Amrani L Ghannam A et al . CRS and HIPEC: the need for an adaptable learning curve model. J Surg Oncol. (2020) 122:1187–8. 10.1002/jso.26123
44.
Pocard M . Peritoneal carcinomatosis is multifaceted, treatment requires expertise and collaboration with other health care personnel: new avenues of research are open. J Visc Surg. (2019) 156:471–2. 10.1016/j.jviscsurg.2019.09.006
45.
Esquivel J Stojadinovic A Levine EA . The American Society of Peritoneal Surface Malignancies (ASPSM). Ann Surg Oncol. (2011) 18:218–9. 10.1245/s10434-010-1402-8
46.
Tate SJ Torkington J . Pressurized intraperitoneal aerosol chemotherapy: a review of the introduction of a new surgical technology using the IDEAL framework. BJS Open. (2020) 4:206–15. 10.1002/bjs5.50257
Summary
Keywords
peritoneal carcinomatosis, LMIC, surveys and questionnaires, North Africa, HIPEC, PIPAC
Citation
Souadka A, Essangri H, Makni A, Abid M, Ayadi M, Ksantini F, Kordjani Z, Ballah Y, Bouka J, Benkabbou A, Majbar MA, El Khannoussi B, Mohsine R, Boutayeb S and Hubner M (2022) Current Opinion and Practice on Peritoneal Carcinomatosis Management: The North African Perspective. Front. Surg. 9:798523. doi: 10.3389/fsurg.2022.798523
Received
20 October 2021
Accepted
20 January 2022
Published
08 March 2022
Volume
9 - 2022
Edited by
Marco Scarpa, University Hospital of Padua, Italy
Reviewed by
Evgenia Halkia, Tzaneion General Hospital, Greece; Maurizio Cardi, Sapienza Università di Roma, Italy
Updates
Copyright
© 2022 Souadka, Essangri, Makni, Abid, Ayadi, Ksantini, Kordjani, Ballah, Bouka, Benkabbou, Majbar, El Khannoussi, Mohsine, Boutayeb and Hubner.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amine Souadka a.souadka@um5r.ac.ma
This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.