- 1Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Department of Neurosurgery, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 3Department of Neurosurgery, Tianjin Neurological Institute, State Key Laboratory of Experimental Hematology, Laboratory of Post-Neuroinjury Neurorepair and Regeneration in Central Nervous System Tianjin & Ministry of Education, Tianjin Medical University General Hospital, Tianjin, China
- 4Department of Neurosurgery, Nanping First Hospital Affiliated to Fujian Medical University, Fujian, China
Epidural hematomas (EDH), typically requiring surgery, may be managed conservatively in select patients. We investigated atorvastatin (10 mg/day) combined with dexamethasone (2.25 mg/day) as conservative therapy in six EDH patients (GCS ≥ 13, volume < 30 ml) post-trauma. All patients recovered fully without surgery, and literatures support conservative care for stable EDH. Our findings suggest this combination therapy may promote hematoma absorption. In conclusion, atorvastatin/dexamethasone shows promise as a non-surgical EDH option, warranting further investigation.
Introduction
Epidural hematoma (EDH) is a critical neurosurgical condition characterized by the accumulation of blood between the dura mater and the skull's inner surface. It typically results from traumatic injury leading to skull fractures, the laceration of the middle meningeal artery, or dural venous sinuses. EDH accounts for approximately 1%–3% of all head injuries and is most prevalent in young adults due to high-impact trauma such as traffic accidents or falls (1, 2). The classical clinical presentation includes a brief loss of consciousness followed by a lucid interval and subsequent rapid neurological deterioration if left untreated.
The standard management of EDH has traditionally been a prompt surgical evacuation, especially in patients presenting with hematomas greater than 30 cm³ in volume, thickness exceeding 15 mm, or midline shift greater than 5 mm, as these factors are associated with increased morbidity and mortality (1). However, emerging evidence challenges this paradigm, demonstrating that select patients—particularly those with preserved neurologic function (GCS ≥ 14) and stable radiographic parameters—may achieve favorable outcomes through conservative strategies (3, 4). Studies have shown that small to moderate-sized hematomas without significant mass effects or neurological deficits may resolve spontaneously under careful observation (5, 6).
Conservative treatment strategies aim to minimize the risks associated with surgery, such as infection, hemorrhage, and anesthesia-related complications, while relying on the body's physiological mechanisms to reabsorb the hematoma. Recent literature has provided evidence supporting conservative management in carefully selected cases of EDH, emphasizing the importance of patient selection criteria, including hematoma size, location, and the patient's neurological status (4, 7).
Parallel to these developments, pharmacological interventions have emerged as potential adjuncts in managing cranial hematomas. Notably, atorvastatin, a lipid-lowering agent in the statin class, has demonstrated therapeutic benefits beyond its cholesterol-lowering effects. Atorvastatin possesses anti-inflammatory, anti-apoptotic, and anti-angiogenic properties, which have been investigated in chronic subdural hematoma (CSDH) (8). In addition, dexamethasone has been used in the conservative management of CSDH since the 1970s (9), and we found an enhanced anti-inflammatory effect of the combination of atorvastatin and dexamethasone in preclinical trials (10). Therefore, we tried the combination regimen of atorvastatin and dexamethasone in the clinical patients.
In patients with CSDH, atorvastatin has been shown to promote hematoma resolution and improve neurological outcomes. The underlying mechanisms are thought to involve the upregulation of angiogenic factors such as vascular endothelial growth factor (VEGF), enhancement of endothelial progenitor cell (EPC) mobilization, and suppression of inflammatory cytokines, all contributing to improved vascular stability and hematoma absorption (10). Our recent research has demonstrated that atorvastatin can effectively treat CSDH by enhancing lymphatic drainage (11).
Given the pathophysiological similarities between CSDH and EDH—particularly the role of inflammation and lymphatic drainage in hematoma persistence—it is plausible that atorvastatin could benefit patients with EDH. These findings have opened new avenues for non-surgical treatment approaches in select patients with EDH.
In this report, we have explored the role of atorvastatin in the conservative management of EDH. We report a series of six patients who presented with epidural hematomas and were treated conservatively with oral atorvastatin. All patients achieved full recovery without the need for surgical intervention. To our knowledge, this is the first study to evaluate the efficacy of atorvastatin in managing EDH.
Case series
Case presentation
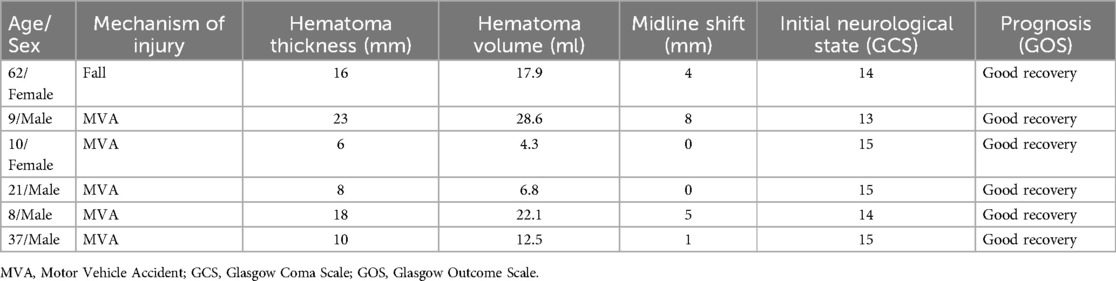
From January 2023 to January 2025, we collected data on six patients with epidural hematomas caused by high falls or motor vehicle accidents (MVA) at Tianjin Medical University General Hospital, Henan Province Kailuan General Hospital, Fujian Province Nanping First Hospital, and Beijing Xuanwu Hospital. The patients ranged from 8-year-old children to 62-year-old adults with a history of acute trauma within 3 days. The average hematoma thickness was 13.5 ± 6.6 mm, the average hematoma volume was 15.4 ± 9.3 ml, and the average midline shift was 3.0 ± 3.2 mm. All patients had a good or slightly impaired consciousness state (GCS ≥ 13) upon admission and immediately underwent CT scans to confirm the location and size of the hematomas. The hematoma volume for all patients was less than 30 ml, but one had a midline shift of more than 5 mm, and three patients had hematoma thickness greater than 15 mm (Figure 1A and Table 1). Due to the patients' stable consciousness, they received conservative treatment with atorvastatin and dexamethasone, along with monitoring of vital signs within 3 days after the injury.
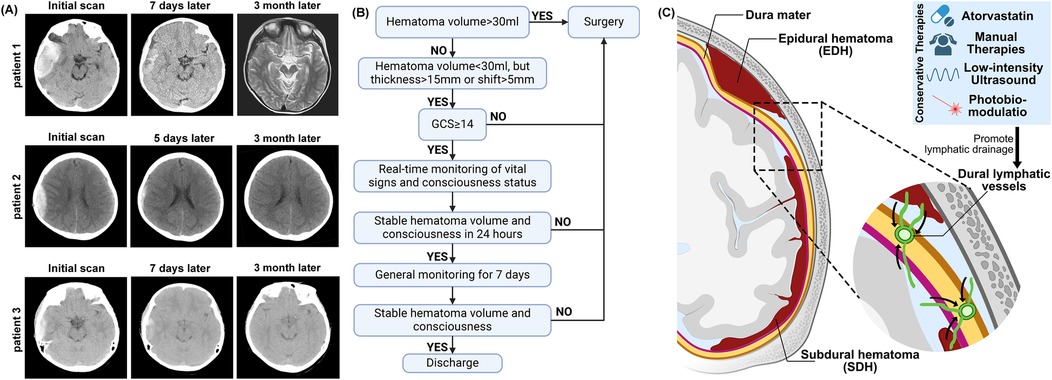
Figure 1. (A) Representative patients head CT shows changes in hematoma at admission and after the treatment. (B) Clinical decision diagram for the treatment of EDH patients. (C) The diagram illustrates the pathological anatomy of EDH and SDH. Targeting the dura mater lymphatic vessels may be a potential approach for treating EDH and SDH.
Treatment protocol
All patients were treated with a combination of atorvastatin and dexamethasone, aiming to promote the resolution of the hematoma and reduce inflammation. Atorvastatin, known for its anti-inflammatory and angiogenic properties, was administered to enhance lymphatic drainage and facilitate hematoma reabsorption. Dexamethasone, a corticosteroid with potent anti-inflammatory effects, was added to reduce cerebral edema and control the inflammatory response. The dosing regimen included Atorvastatin: 10 mg orally once daily.
Dexamethasone: 2.25 mg orally once daily for the first week, 1.5 mg daily for the second week, 0.75 mg daily for the third week, then discontinue. The total dose of the regimen is 31.5 mg. All patients with an initial GCS of 14 or higher and a hematoma volume of less than 30 ml can expect treatment for 24 h under real-time monitoring of vital signs and consciousness assessment. If there is a worsening of consciousness or if a follow-up CT scan after 24 h shows hematoma enlargement, surgery will be performed. If there is no worsening after strict monitoring for 3 days, the monitoring level can be reduced, and the patient can be discharged once a follow-up CT after 7 days shows a reduction in the hematoma (Figure 1B). Patient 2 had an initial GCS score of 13. Due to the guardian's refusal of surgery, the patient entered a conservative treatment process. All patients showed no deterioration in consciousness after admission and underwent CT scan re-examination on the 7th day after injury.
Clinical outcome
All patients recovered well after conservative treatment and did not require delayed surgical intervention. Three months after the injury, the patients were followed up, and all recovered well without any neurological function loss (Glasgow Outcome Scale = 5).
Literature review and discussion
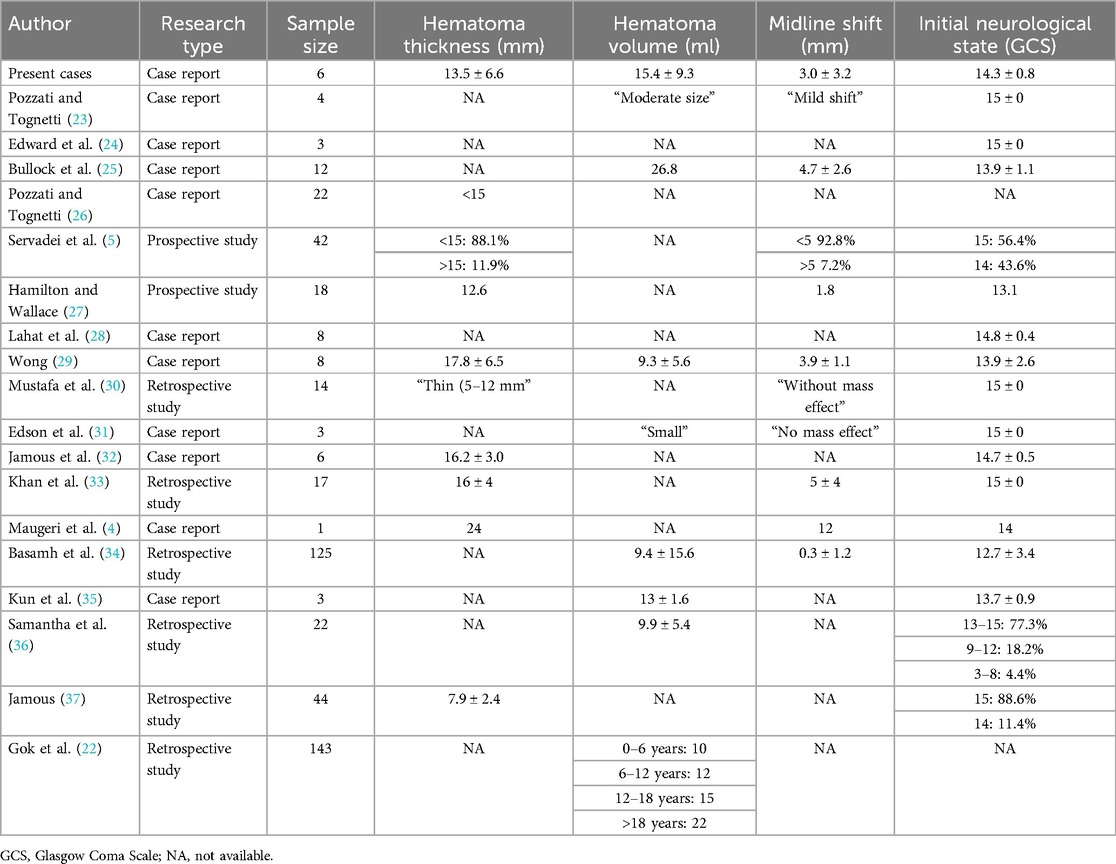
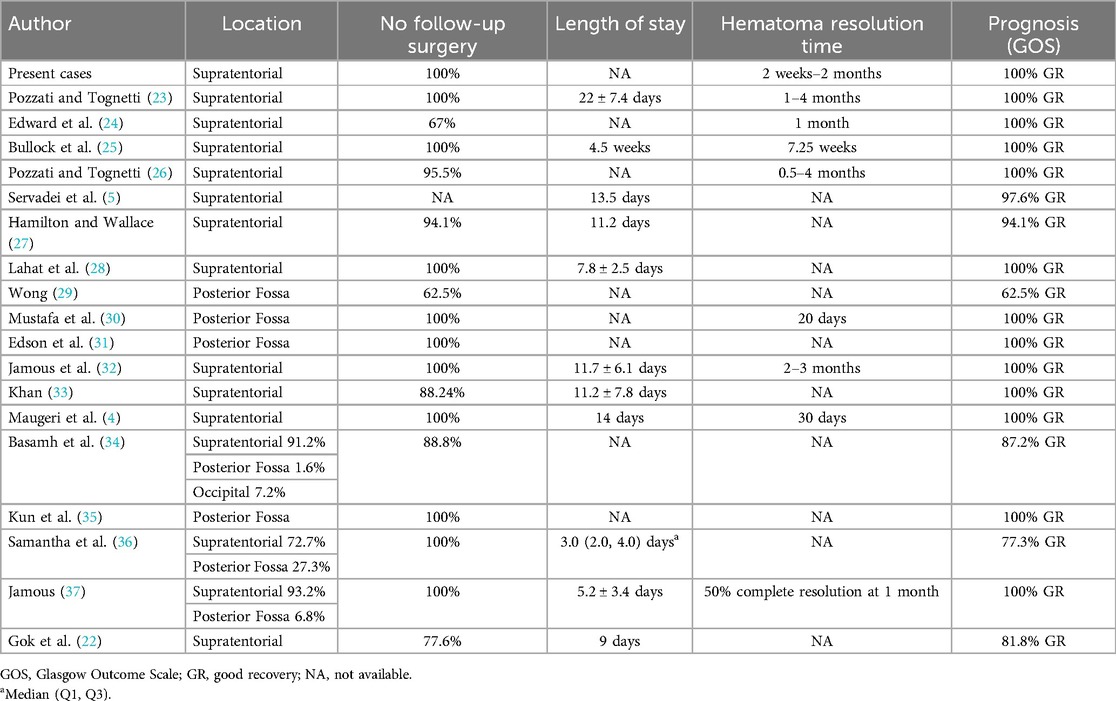
We conducted a literature search on PubMed and Google Scholar using the terms “(epidural hematoma) AND ((Conservative treatment) OR (expectant treatment) OR (medication treatment))” (Tables 2, 3). Compared to previous studies on conservative treatment, our therapy may accelerate hematoma absorption and result in full recovery for the patients (Table 3).
In the present cases, all patients who received a combination of atorvastatin and corticosteroid therapy had faster hematoma absorption and fully recovered their work and social functions within three months. This approach is especially relevant in patients with a Glasgow Coma Scale (GCS) score ≥14 at admission, where spontaneous resolution of the hematoma is possible under careful monitoring (5, 12). Based on the Brain Trauma Foundation guideline (1) and reviewed clinical studies, we conservatively recommend that EDH patients with a GCS ≥ 14 can safely undergo conservative treatment under monitoring (Figure 1B).
In our case series, we observed that patients with EDHs treated conservatively with atorvastatin combined with dexamethasone achieved favorable outcomes without requiring surgical intervention. Notably, all patients demonstrated complete resolution of the hematomas within 2 months, with no neurological deficits at follow-up (GOS = 5). This reinforces previous findings that small EDHs, particularly those without significant neurological deterioration, can be effectively managed conservatively with close observation and serial imaging (4, 5, 12–14).
One of the novel aspects of our study is the use of atorvastatin as an adjunct therapy in the conservative management of EDH. While atorvastatin is widely recognized for its lipid-lowering effects, recent studies have explored its additional benefits, including its anti-inflammatory, anti-apoptotic, and angiogenic properties. In particular, atorvastatin has been shown to promote the resolution of CSDH by enhancing lymphatic drainage and facilitating hematoma absorption. These mechanisms may also extend to EDH, as both conditions share similar pathophysiological features, such as inflammation and impaired lymphatic drainage (8, 10). Dexamethasone is classically used in the treatment of cerebral edema (15), and recent meta-analyses have shown that it reduces mortality from ischemic stroke and cerebral hemorrhage (16). Xuxiang et al. have highlighted the potential benefits of traditional Chinese medicine in enhancing hematoma resolution without surgical intervention (17). In addition to medication, recent studies have found that non-invasive methods such as manual therapy (18), low-intensity ultrasound (19), and phototherapy (20) can promote the brain lymphatic system. These approaches may potentially affect the conservative treatment of EDH (Figure 1C).
However, it is important to note that conservative management remains a highly individualized approach. Patient selection criteria, including age, comorbidities, and the rapidity of hematoma growth, are crucial in determining whether conservative treatment is appropriate. The decision must be made on a case-by-case basis, carefully monitoring neurological status and serial imaging to assess hematoma progression. Gok et al. and Sullivan et al. emphasize the importance of follow-up CT scans in guiding clinical decisions and ensuring that surgical intervention is not delayed in cases of deterioration (21, 22).
While our results are promising, further studies are needed to establish clearer guidelines for using atorvastatin in the conservative management of EDH. Large-scale prospective studies are warranted to validate this treatment regimen's effectiveness and determine the optimal dosage and duration of therapy. Moreover, future research should focus on identifying specific biomarkers or imaging characteristics that could predict which patients are most likely to benefit from conservative treatment, including pharmacological interventions.
Conclusion
Our findings suggest that atorvastatin, when combined with dexamethasone, may offer a promising alternative to surgery for the conservative management of epidural hematomas. This approach, along with careful monitoring and patient selection, may help expand the non-surgical treatment options available for EDH, particularly in patients who do not exhibit significant neurological deterioration at admission. Further clinical investigations are required to optimize this management strategy and validate its long-term efficacy.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CW: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. YT: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. RZ: Data curation, Formal analysis, Writing – review & editing. RC: Data curation, Writing – review & editing. CX: Data curation, Writing – review & editing. JH: Data curation, Writing – review & editing. RJ: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82071390, 82271394, 82071402, 82171359, 82001323, and 82101434) and the Tianjin Key Medical Discipline (Specialty) Construction Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute epidural hematomas. Neurosurgery. (2006) 58(3 Suppl):S7–15; discussion Si–iv. doi: 10.1227/01.NEU.0000210363.91172.A8
2. Irie F, Le Brocque R, Kenardy J, Bellamy N, Tetsworth K, Pollard C. Epidemiology of traumatic epidural hematoma in young age. J Trauma. (2011) 71(4):847–53. doi: 10.1097/TA.0b013e3182032c9a
3. Champeaux C, Lainé G, Gimbert E, Jecko V. Successful conservative management of large vertex epidural hematoma. Neurochirurgie. (2019) 65(6):438–9. doi: 10.1016/j.neuchi.2019.05.002
4. Maugeri R, Anderson DG, Graziano F, Meccio F, Visocchi M, Iacopino DG. Conservative vs. surgical management of post-traumatic epidural hematoma: a case and review of literature. Am J Case Rep. (2015) 16:811–7. doi: 10.12659/AJCR.895231
5. Servadei F, Faccani G, Roccella P, Seracchioli A, Godano U, Ghadirpour R, et al. Asymptomatic extradural haematomas. Results of a multicenter study of 158 cases in minor head injury. Acta Neurochir (Wien). (1989) 96(1–2):39–45. doi: 10.1007/BF01403493
6. Servadei F, Nanni A, Nasi MT, Zappi D, Vergoni G, Giuliani G, et al. Evolving brain lesions in the first 12 h after head injury: analysis of 37 comatose patients. Neurosurgery. (1995) 37(5):899–906; discussion 906–7. doi: 10.1227/00006123-199511000-00008
7. Chan K, Aguilar J, Khu K. Successful conservative management of a large acute epidural hematoma in a patient with arrested hydrocephalus: a case report. Surg Neurol Int. (2022) 13:366. doi: 10.25259/SNI_982_2021
8. Jiang R, Zhao S, Wang R, Feng H, Zhang J, Li X, et al. Safety and efficacy of atorvastatin for chronic subdural hematoma in Chinese patients: a randomized clinicalTrial. JAMA Neurol. (2018) 75(11):1338–46. doi: 10.1001/jamaneurol.2018.2030
9. Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. (1974) 31(2):73–9. doi: 10.1001/archneur.1974.00490380021001
10. Gong Z, Zhan D, Nie M, Li X, Gao C, Liu X, et al. Dexamethasone enhances the efficacy of atorvastatin in inhibiting excessively inflammation-induced abnormal angiogenesis by regulating macrophages. J Neuroinflammation. (2021) 18(1):203. doi: 10.1186/s12974-021-02257-1
11. Yuan J, Liu X, Nie M, Chen Y, Liu M, Huang J, et al. Inactivation of ERK1/2 signaling mediates dysfunction of basal meningeal lymphatic vessels in experimental subdural hematoma. Theranostics. (2024) 14(1):304–23. doi: 10.7150/thno.87633
12. Chen TY, Wong CW, Chang CN, Lui TN, Cheng WC, Tsai MD, et al. The expectant treatment of “asymptomatic” supratentorial epidural hematomas. Neurosurgery. (1993) 32(2):176–9; discussion 179. doi: 10.1227/00006123-199302000-00004
13. Servadei F, Vergoni G. Extradural hematomas: surgical and non-surgical treatment. AJNR Am J Neuroradiol. (1993) 14(2):506–7.8456740
14. Call L, Qiu Q, Morris J, Flaherty B, Vavilala MS, Mills B, et al. Characteristics of pediatric patients with traumatic epidural hematomas who can be safely observed: a clinical validation study. Br J Radiol. (2020) 93(1114):20190968. doi: 10.1259/bjr.20190968
15. Vazquez S, Gold J, Spirollari E, Akmal S, Hanft SJ. The story of dexamethasone and how it became one of the most widely used drugs in neurosurgery. J Neurosurg. (2024) 140(4):1191–7. doi: 10.3171/2023.9.JNS231099
16. Wang Y, Huang L, Li J, Duan J, Pan X, Menon BK, et al. Efficacy and safety of corticosteroids for stroke and traumatic brain injury: a systematic review and meta-analysis. Syst Rev. (2025) 14(1):54. doi: 10.1186/s13643-025-02803-5
17. Qiu XX, Huang JM, Zhou RX. Nonsurgical traditional Chinese medicine in extradural hematomas. Chin Med J (Engl). (1981) 94(4):241–8.6790240
18. Roth C, Stitz H, Roth C, Ferbert A, Deinsberger W, Pahl R, et al. Craniocervical manual lymphatic drainage and its impact on intracranial pressure—a pilot study. Eur J Neurol. (2016) 23(9):1441–6. doi: 10.1111/ene.13055
19. Wu CH, Liao WH, Chu YC, Hsiao MY, Kung Y, Wang JL, et al. Very low-intensity ultrasound facilitates glymphatic influx and clearance via modulation of the TRPV4-AQP4 pathway. Adv Sci (Weinh). (2024) 11(47):e2401039. doi: 10.1002/advs.202401039
20. Salehpour F, Khademi M, Bragin DE, DiDuro JO. Photobiomodulation therapy and the glymphatic system: promising applications for augmenting the brain lymphatic drainage system. Int J Mol Sci. (2022) 23(6):2975. doi: 10.3390/ijms23062975
21. Sullivan TP, Jarvik JG, Cohen WA. Follow-up of conservatively managed epidural hematomas: implications for timing of repeat CT. AJNR Am J Neuroradiol. (1999) 20(1):107–13.9974064
22. Gok H, Celik SE, Yangi K, Yavuz AY, Percinoglu G, Unlu NU, et al. Management of epidural hematomas in pediatric and adult population: a hospital-based retrospective study. World Neurosurg. (2023) 177:e686–92. doi: 10.1016/j.wneu.2023.06.123
23. Pozzati E, Tognetti F. Spontaneous healing of extradural hematomas: report of four cases. Neurosurgery. (1984) 14(6):724–7. doi: 10.1227/00006123-198406000-00012
24. Kaye EM, Cass PR, Dooling E, Rosman NP. Chronic epidural hematomas in childhood: increased recognition and non-surgical management. Pediatr Neurol. (1985) 1(4):255–9. doi: 10.1016/S0887-8994(85)80013-8
25. Bullock R, Smith RM, van Dellen JR. Nonoperative management of extradural hematoma. Neurosurgery. (1985) 16(5):602–6. doi: 10.1227/00006123-198505000-00003
26. Pozzati E, Tognetti F. Spontaneous healing of acute extradural hematomas: study of twenty-two cases. Neurosurgery. (1986) 18(6):696–700. doi: 10.1227/00006123-198606000-00003
27. Hamilton M, Wallace C. Nonoperative management of acute epidural hematoma diagnosed by CT: the neuroradiologist’s role. AJNR Am J Neuroradiol. (1992) 13(3):853–9; discussion 860–2.1590183
28. Lahat E, Livne M, Barr J, Schiffer J, Eshel G. The management of epidural haematomas–surgical versus conservative treatment. Eur J Pediatr. (1994) 153(3):198–201. doi: 10.1007/BF01958986
29. Wong CW. The CT criteria for conservative treatment? But under close clinical observation? Of posterior fossa epidural haematomas. Acta Neurochir (Wien). (1994) 126(2-4):124–7. doi: 10.1007/BF01476421
30. Bozbuğa M, Izgi N, Polat G, Gürel I. Posterior fossa epidural hematomas: observations on a series of 73 cases. Neurosurg Rev. (1999) 22(1):34–40. doi: 10.1007/s101430050006
31. Bor-Seng-Shu E, Aguiar PH, de Almeida Leme RJ, Mandel M, Andrade AF, Marino R Jr. Epidural hematomas of the posterior cranial fossa. Neurosurg Focus. (2004) 16(2):ECP1. doi: 10.3171/foc.2004.16.2.10
32. Jamous MA, Abdel Aziz H, Al Kaisy F, Eloqayli H, Azab M, Al-Jarrah M. Conservative management of acute epidural hematoma in a pediatric age group. Pediatr Neurosurg. (2009) 45(3):181–4. doi: 10.1159/000218200
33. Khan MB, Riaz M, Javed G. Conservative management of significant supratentorial epidural hematomas in pediatric patients. Childs Nerv Syst. (2014) 30(7):1249–53. doi: 10.1007/s00381-014-2391-x
34. Basamh M, Robert A, Lamoureux J, Saluja RS, Marcoux J. Epidural hematoma treated conservatively: when to expect the worst. Can J Neurol Sci. (2016) 43(1):74–81. doi: 10.1017/cjn.2015.232
35. Han K, Li Z, Yin H, Yao W, Lan X, Bo Y. Liquid posterior fossa epidural hematoma in pediatric trauma: a single-center case series. J Neurol Surg. (2018) 79(5):380–5. doi: 10.1055/s-0038-1648225
36. Parker SL, Kabani AA, Conner CR, Choi PA, Withrow JS, Cai C, et al. Management of venous sinus–related epidural hematomas. World Neurosurg. (2020) 138:e241–50. doi: 10.1016/j.wneu.2020.02.089
Keywords: epidural hematoma, conservative treatment, atorvastatin, dexamethasone, meningeal lymphatic system
Citation: Wu C, Tian Y, Zhao R, Chen R, Xu C, Huang J and Jiang R (2025) Conservative therapy of epidural hematoma with atorvastatin combined with glucocorticoids: cases report and literature review. Front. Surg. 12:1587988. doi: 10.3389/fsurg.2025.1587988
Received: 6 March 2025; Accepted: 15 April 2025;
Published: 30 April 2025.
Edited by:
Fulvio Tartara, University Hospital of Parma, ItalyReviewed by:
Yanwei Fang, Second Hospital of Hebei Medical University, ChinaYuanhong Ge, Chengdu Second People's Hospital, China
Copyright: © 2025 Wu, Tian, Zhao, Chen, Xu, Huang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongcai Jiang, amlhbmdyb25nY2FpQHRtdS5lZHUuY24=
†These authors have contributed equally to this work
 Chenrui Wu
Chenrui Wu Yu Tian3,†
Yu Tian3,† Ruichen Zhao
Ruichen Zhao Rongcai Jiang
Rongcai Jiang