- 1Spine and Orthopaedics Department, Olympion General Hospital, Patras, Greece
- 2Embedded Systems Design and Applications (ESDA) Laboratory, Department of Informatics and Telecommunications, University of the Peloponnese, Patras, Greece
- 3University of Patras, Patras, Greece
- 4Biostats, Epirus Science and Technology Park Campus, University of Ioannina, Ioannina, Greece
Study design: A Systematic Review and Meta-Analysis
Purpose: To compare the incidence of New Adjacent Vertebral Fractures (ANVFs) in elderly patients with Osteoporotic Vertebral Compression Fractures (OVCFs) undergoing either percutaneous vertebral augmentation—via Vertebroplasty (PVP) or Kyphoplasty (PKP)—or Conservative Treatment (CT). Additionally, this study aims to identify potential risk factors associated with ANVFs.
Hypothesis: The incidence of ANVFs does not significantly differ between patients managed with CT and those treated with PVP or PKP.
Background: While the optimal treatment for OVCFs remains debated, PVP and PKP offer immediate stabilization, pain relief, and may help correct vertebral body wedging with minimal complications. However, a review of the literature reveals a limited number of meta-analyses comparing CT with PVP/PKP regarding the incidence of ANVFs.
Materials and methods: Following PRISMA guidelines, a systematic search was conducted across PubMed, Cochrane, Web of Science, Scopus and Science Direct to identify studies published between 2005 and 2024 comparing surgical treatment with CT for ANVFs incidence. Nine studies (five RCTs and four retrospective comparative case-control studies) involving 1,930 patients were included in the analysis.
Results: In RCTs, the analysis indicated a significant difference (P < 0.05) in ANVFs incidence favoring the surgical group, with a Relative Risk (RR) of 0.66 (95% CI: 0.44–0.99; P = 0.05); in retrospective studies, no statistically significant difference was found between the surgical and CT groups (OR = 0.87, 95% CI: 0.58–1.31; P = 0.51). Differences in study parameters such as age, total number of participants, surgical approach (unilateral vs. bilateral), etc. were observed but they could not be accurately tested due to the limited number of studies.
Conclusion: This meta-analysis, for the selected RCTs, shows that vertebral augmentation is associated with a lower incidence of ANVFs compared to CT. On the other hand, in the retrospective studies group there was no significant difference in the incidence of ANVFs between the two treatment groups (CT vs. PKP/PVP). Variations in study parameters, such as patient demographics and surgical techniques, may have affected these results. Further high-quality studies are needed to better understand the long-term effects of different treatment strategies on the incidence of ANVFs.
Systematic Review Registration: PROSPERO (CRD420250509815).
Introduction
The ideal treatment approach for Osteoporotic Vertebral Compression Fractures (OVCFs) remains a topic of debate. Conservative treatment (CT) is considered the “gold standard” for OVCFs and typically includes rest, analgesics, braces, etc. While CT can help alleviate pain, they may increase the risk of chronic OVCFs (1, 2). The effectiveness of long-term medication is often restricted due to its adverse side effects (gastrointestinal bleeding, hypostatic pneumonia, deep vein thrombosis, etc. (3–11). These concerns are among the reasons why many authors recommend early surgical intervention for OVCFs in the elderly (6, 12). Percutaneous vertebral body augmentation techniques such as percutaneous vertebroplasty (PVP) and percutaneous balloon kyphoplasty (PKP) for treating symptomatic OVCFs are used to stabilize the fractured vertebra and provide pain relief (6, 13).
However, PVP and PKP may result in surgical complications, primarily related to the injection of PMMA including cement leakage and associated neurological injuries on the nerve roots or spinal cord, etc. (14–16).
With the growing use of vertebral augmentation surgical techniques for OVCFs, spine surgeons have raised increasing concerns about the generation of new OVCFs either adjacent to the augmented vertebra (ANVFs), or re-fractures, e.g., fractures at the vertebral augmentation index level (17) or remote new vertebral fractures. Such occurrences often necessitate a new treatment, increasing patient discomfort and imposing a financial burden on families (16–20) ANVFs have a reported risk of 2%–23% in PKP and up to 52% in PVP (21). Several hypotheses have been suggested to explain the rising incidence of ANVFs following vertebral augmentation such as osteoporosis, biomechanical and balance factors, etc.) (22). Some authors suggest that restoring sagittal balance and physiological loading through vertebral augmentation may help reduce ANVFs, which are primarily attributed to underlying osteoporosis and mechanical alterations caused by spinal deformity (16, 20, 23–30). Clinical prediction models have assessed the likelihood of ANVFs following PVP providing risk factors such as a prior history of OVCFs, bone cement leakage to adjacent intervertebral disc, multi-level vertebral augmentation, distribution of PMMA, BMD, BMI, etc. (31). The long-term impact of vertebral augmentation on ANVFs remains a topic of debate with most of the studies showing no statistically significant difference between CT and PVP (32).
Whether OVCFs should be treated surgically or conservatively is controversial (33, 34). Meta-analyses comparing CT vs. PVP/PKP reported primarily on immediate and intermediate pain reduction and functional outcomes and did not address or analyze the occurrence of ANVFs (35–41). Contradictory results have been generated in studies that compared a CT with a PVP with the passage of time (42–44).
Due to the limited literature comparing CT with PVP/PKP in the development of ANVFs and due to the controversies in the related studies, this meta-analysis aims to determine whether the incidence rate of ANVFs after CT is lower than that following PVP/PKP.
Materials and methods
Literature search
Following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (45), a systematic search was conducted across PubMed, Web of Science, Cochrane, ScienceDirect and Scopus to identify studies published between 2005 and 2024. The process was designed to identify all eligible studies using the following query:
(“vertebroplasty” OR “kyphoplasty” OR “conservative treatment”) AND “new osteoporotic vertebral fractures”
No language filters were used during the search process.
The articles included in this review met the following criteria: Study Design [Randomized Controlled Trials (RCTs) or retrospective cohort studies with a matched control group]; Comparative Analysis [Studies comparing percutaneous vertebroplasty (PVP) or balloon kyphoplasty (PKP) with conservative treatment (CT)]; Patient Population (Studies involving patients with OVCFs); Studies with primary endpoint the incidence of new adjacent OVCFs. In cases, where duplicate or overlapping data were identified in multiple studies, only the study with the most complete and comprehensive data was included in the review.
The criteria for including studies in this review were based on the PICO (46) (Population, Intervention, Comparison, and Outcome) framework. Population (P): Adults or elderly patients with osteoporotic vertebral compression fractures (OVCFs) in the thoracolumbar spine, not caused by neoplasm, trauma, or any other specific condition. Interventional treatment (I): Percutaneous Vertebroplasty (PVP) and Percutaneous Balloon Kyphoplasty (PKP). Comparison-Controls (C): Patients who received Conservative Treatment (CT). Outcome (O): The incidence of adjacent new vertebral fractures (ANVFs) following CT or PVP/PKP.
The inclusion criteria for the Meta-Analysis were as follows: Condition: Symptomatic osteoporotic vertebral compression fractures (OVCFs) in the thoracolumbar spine (T1 to L5 vertebrae); Clinical Presentation: Presence of pain or localized pressure correlating with imaging findings; Diagnostic Confirmation: Preoperative spinal radiographs and MRI confirming new fractures without neurological deficits; Study Size: Studies with more than 30 cases each; Outcome Reporting: Series specifically reporting on new fractures following PVP or PKP and Language: Articles published in English.
The exclusion criteria for the Meta-Analysis were as follows: Study Type: Narrative reviews, systematic reviews, meta-analyses, and case reports; Sample Size: Studies with fewer than 30 participants; Language: Articles published in languages other than English; Intervention: Studies involving additional use of instrumentation; Study Population: Cadaveric studies and studies on pathological fractures, including those caused by hemangiomas, known hematological diseases, infections, or metastatic disease.
From each eligible article, the following information was extracted and documented in an Excel sheet: (1) Authors' name; (2) Year of publication; (3) Type of study; (4) Demographic and baseline characteristics of participants; (5) Type of conservative treatment; (6) Type of surgical treatment; (7) Follow-up details; (8) Outcome data (new OVCF) and (9) Reported complications.
Selection of studies
The search process was blinded, with only article titles and abstracts reviewed initially. Two independent observers assessed the quality and bias of the retrieved studies independently. Articles were selected based on the inclusion criteria to minimize bias in both study selection and data extraction.
Key steps in the review process
Initial Selection: Articles were evaluated based on titles and abstracts. Full texts were retrieved for studies with unclear inclusion/exclusion criteria; Resolution of Doubts: If uncertainties persisted, the decision was made through discussion, with a third reviewer brought in if necessary; Scoring: Each primary study was assigned a score independently by the two reviewers. The final score for each study was the average of the two scores.
Protocol and data extraction
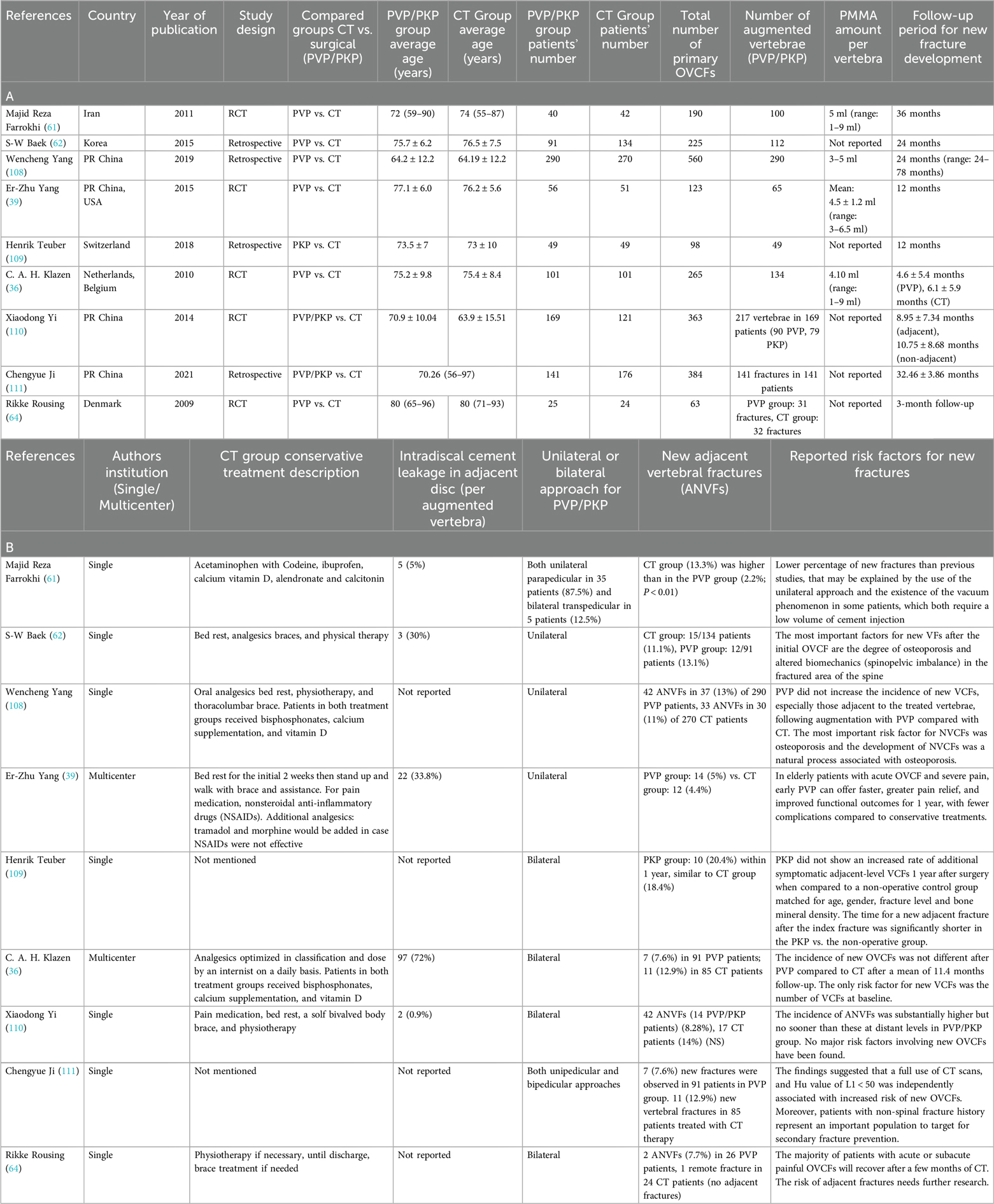
A written study protocol was developed before starting the literature review, including clearly defined eligibility criteria; Two investigators independently extracted relevant data from each trial using a standardized form; The same observers independently extracted data from each article to ensure consistency; This rigorous approach ensured a systematic and unbiased review process. The common characteristics to provide an overview of the 9 studies finally included in the analysis are shown in Tables 1A,B.
The protocol for this meta-analysis has been registered in the PROSPERO database (Registration No: CRD420250509815).
The data supporting this meta-analysis have been deposited in the Mendeley Data repository and can be accessed at DOI: 10.17632/t9c5v4859k.1.
Common clinical characteristics of the selected studies
The primary outcome was adjacent new OVCFs. Perioperative outcomes (PMMA amount injected in each vertebra/per patient); Radiographic outcomes included surgical complications (adjacent intradiscal cement leakage, adjacent new vertebral fractures).
Statistical analysis
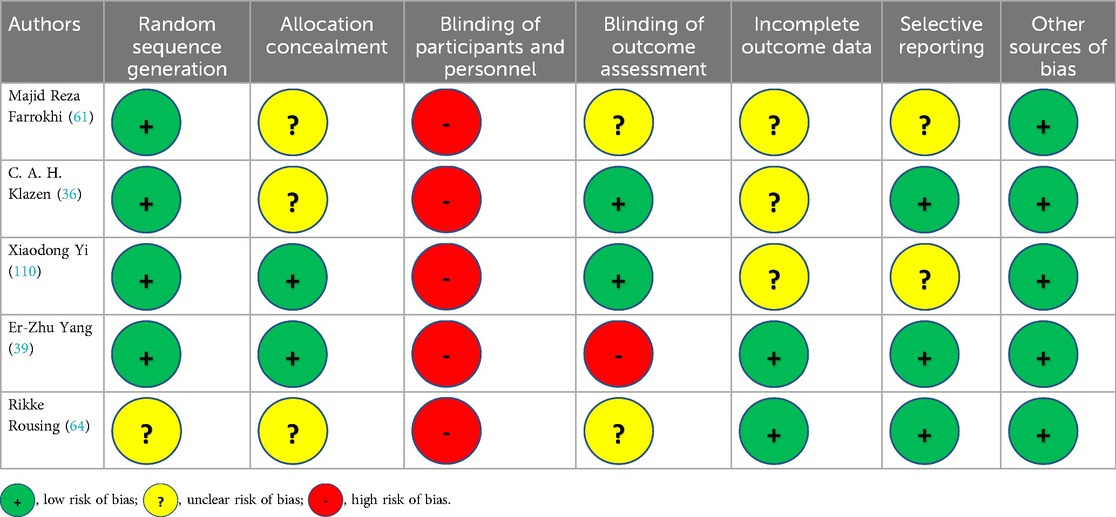
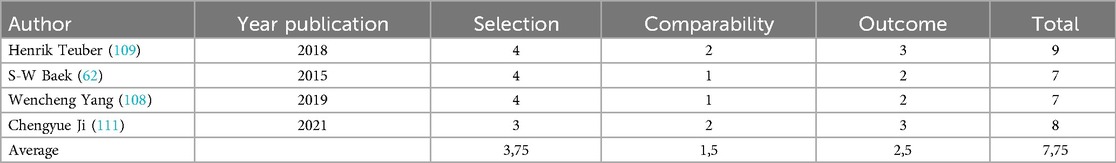
The meta-analysis was conducted in accordance with the recommendations of the Cochrane Collaboration and the guidelines for Quality of Reporting of Meta-analyses (46, 55–57). The analysis of the outcomes was divided to subgroups according to surgical (PVP, PKP) or conservative treatment (CT). RCTs quality was justified using Cochrane Collaboration's “Risk of Bias” tool (58), (Table 2) while for the retrospective studies quality was justified using the Newcastle‒Ottawa scale (NOS) (59) (Table 3). NOS include three areas, patient representation, exposure and outcome determination, and follow-up adequacy, with a maximum total score of 9 for each study. NOS scores of 0–5, 6–7, and 8–9 indicate low, moderate, and high quality, respectively.
Heterogeneity across the studies was assessed using the chi-square test and I2 statistic, with I2 values ranging from 0% to 100% indicating low, moderate, and high heterogeneity at values of 25%, 50%, and 75%, respectively. In this meta-analysis, the pooled estimate was derived under a random-effects model using the inverse variance method (60).
Risk of bias across the studies
The possibility of publication bias was assessed by analyzing a funnel plot using RevMan 5.4. Symmetry in the funnel plot suggests the absence of publication bias, whereas asymmetry may indicate the non-publication of small trials with negative results or a preference for publishing studies with favorable outcomes.
Meta-analysis
A meta-analysis of the mean rate of ANVFs between experimental (surgical interventions, PVP/PKP) and controls (CT) was conducted for the nine studies. The analysis was carried out for retrospective design studies and RCTs and aimed to estimate the pooled effect size. We extracted the odds ratios (ORs) to describe the outcomes of interest data, with its 95% confidence intervals (CIs). The pooled OR was estimated for retrospective studies and the relative risk (RR) for RCTs. The conceptual background of the included studies indicated that all estimates should be based on random-effects models and the inverse variance method. A sensitivity analysis was conducted to assess the impact of individual studies on the overall inference. Additionally, an analysis was performed to evaluate the selection of subgroup approaches for vertebral augmentation, such as comparing unilateral and bilateral approaches in relation to the generation of new fractures. The analysis was conducted with the use of RevMan 5.4 and Meta essentials v.1.5, and significance was set at 0.05 in all cases. Heterogeneity was assessed using the I2 index. Publication bias was assessed using funnel plots and the Egger's test.
Results
Literature search and selection of studies
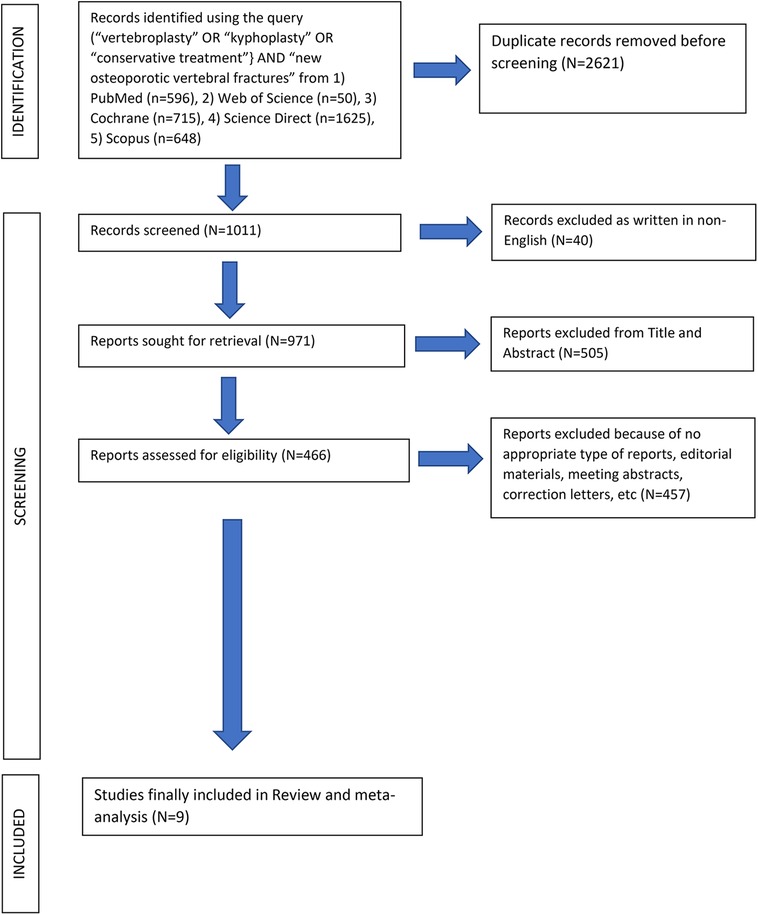
After the computerized search was performed, 3,632 articles were identified from 5 different data bases (PubMed, Web of Science, Cochrane, Science Direct and Scopus). 2,621 papers were identified as duplicates (presented more than once in the search results) and were rejected before screening. In the level 1 screening, 40 records were excluded as these were not written in English language. Of the remaining 971 articles, 505 were excluded reviewing the title and abstract in the screening level 2. From the remaining 466 reports which were assessed for eligibility, 457 articles were excluded in the level III by the unbiased reviewers because of not appropriate type of reports such as editorial materials, meeting abstracts, correction letters, etc. Finally, 9 papers fulfilled all the inclusion criteria and were selected for data extraction and analysis, Figure 1. The 9 studies included in this review were published in the period between 2009 and 2021. Cochrane Collaboration's tool showed low “Risk of Bias” (Table 2) and Newcastle‒Ottawa scale (NOS) showed high quality scores for the retrospective studies (Table 3).
Studies common characteristics
Countries & centers per study: The studies included in our meta-analysis took place in one or in combinations of totally 8 countries (Iran, Korea, PR China, USA, Switzerland, Netherlands, Belgium, Denmark). Seven studies were single-center studies (5 RCTs, 2 Retrospectives); Patients: There were 1,930 patients in the selected studies. 968 patients received conservative and 962 patients received surgical treatment (PVP or PKP). Age of patients: The reported patients' average age ranged from 64 to 80 years in the surgical group and 63.9–80 years in the CT group; Total OVCFs in both groups: The total number of OVCFs in both groups was 2,154, recorded across 1,930 patients. The ratio of index patients to fractures was 1:1.12; Number of primary OVCFs which were augmented (PVP or PKP): 1,107 vertebrae; Unilateral vs. Bilateral approach for vertebral body augmentation: In the studies reviewed, vertebral body augmentation was performed using different approaches: (a) Unilateral Approach used in 4 studies, (b) Bilateral Approach used in 3 studies and (c) Varied Approach (uni- plus bi-lateral) used in 2 studies; Bone cement (PMMA): The amount of injected PMMA per augmented vertebra ranged from 1 to 9 ml and was reported in only three studies (39, 43, 61). PMMA leakage into the adjacent intervertebral disc: Intradiscal PMMA leakage was reported in 3 out of the 9 studies (39, 43, 62). The reported rate of cement leakage ranged from 0.9% to 33.8%. Adjacent new fractures (ANVFs) after surgical and conservative treatment: All 9 studies reported on the incidence of ANVFs; In the CT group, the ANVFs incidence ranged from 7.8% to 35%, whereas in the surgical (PVP/PKP) group, it ranged from 2.6% to 20.4%. Time lapsed from index fracture and ANVFs occurrence in both groups (Surgical, CT): The average time elapsed from the index fracture to the occurrence of new vertebral fractures (ANVFs) in both the surgical and conservative treatment (CT) groups ranged from 3 to 78 months. Conservative treatment: 7/9 studies describe the non-surgical treatment mode (Table 1).
Meta-analysis
Regarding the RCTs, the analysis suggests a statistically significant difference (P = 0.05) between the surgical (PVP & PKP) group and the conservative treatment (CT) group (Figure 2) in the incidence rate of ANVFs. Specifically, it was expected that patients in the surgical group would have a lower rate of adjacent new vertebral fractures compared to those in the CT group, with a relative risk (RR) = 0.66 (95% C.I: 0.44–0.99; P = 0.05). The pooled estimate was calculated using a random effects model with the inverse variance method. The I2 heterogeneity index was 0%, indicating no significant heterogeneity. Sensitivity analysis for this group showed that the inference could be influenced if certain studies were omitted. The funnel plot (Figure 3) is indicative of the symmetry observed indicating absence of publication bias and the Egger's test was non-significant (P = 0.743).
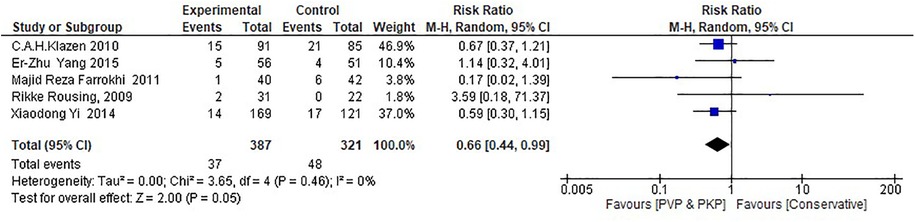
Figure 2. Forest plot for the RCT group. The results indicate a statistically significant difference (P < 0.05) between the experimental (PVP/PKP) surgical group and the control (CT) group.
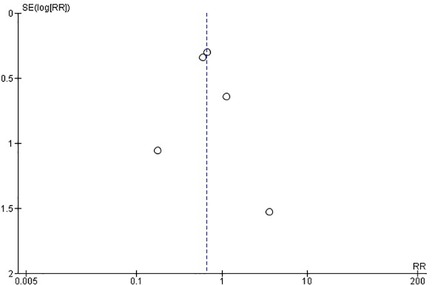
Figure 3. Funnel plot for RCTs, indicating no evidence of publication bias. Egger's test was non-significant (P = 0.743).
Regarding the retrospective studies, the analysis indicates no statistically significant differences between the surgical and the CT group in the incidence rate of ANVFs (Figure 4). It was expected that patients in the surgical group would have a similar rate of ANVFs compared to those in the CT group, with an odds ratio (OR) = 0.87 (95% C.I.: 0.58–1.31; P = 0.51). The pooled estimate was derived using a random effects model with the inverse variance method. The I2 heterogeneity index was 25%, which was not statistically significant. Sensitivity analysis for this group showed that the inference remained unchanged, regardless of which studies could potentially be omitted. In the retrospective studies, the funnel plot is indicative of the symmetry observed indicating almost an absence of publication bias and the Egger's test was non-significant with a P-value equal to 0.749 (Figure 5).
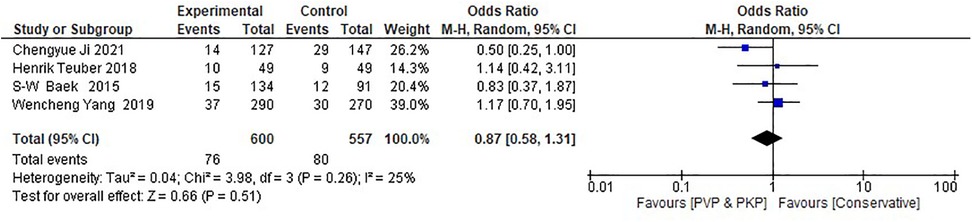
Figure 4. Forest plot for the retrospective studies. The analysis shows no statistically significant difference between the experimental (PVP/PKP) surgical group and the control (CT) group.
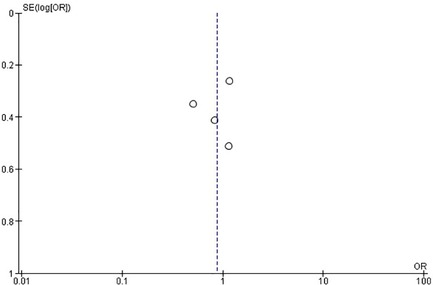
Figure 5. Funnel plot for retrospective studies, suggesting no evidence of publication bias. Egger's test was non-significant (P = 0.749).
Differences between the studies, included in this meta-analysis were observed for other parameters as well (Tables 1A,B). Specifically, regarding age means ranged from 64 to 80 years across studies but in all cases the average estimated between the surgical and CTs were close to a mean difference that did not exceed the 2 years, in any study. The total number of participants was different across studies, but always balanced between the two different treatments, while this, relatively small fluctuation, was accounted for through the weights attributed to each study in the synthesis of the results.
The percutaneous unilateral vs. bilateral approach appears to affect the inference, but still the small number of studies included in this analysis does not allow a clearer view than the one stated in the section regarding RCTs (Figure 6). Similarly, subgroups (Tables 1A,B) that could theoretically differentiate the ANVFs outcomes relating to PMMA (amount, cement leakage, or follow up time), cannot be statistically examined due to the small number of studies included in this meta-analysis. It has to be mentioned though that the evidence provided by the authors in this context includes no indications for major differences.
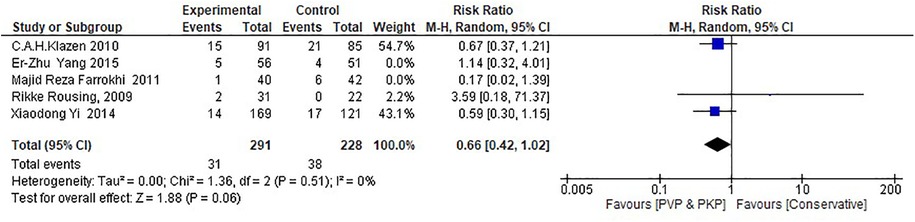
Figure 6. Forest plot for the RCT subgroup analysis examining the correlation between pedicular surgical approaches (unilateral vs. bilateral) in PVP/PKP and the incidence of ANVFs. The unilateral approach has a tendency towards an increased incidence rate of ANVFs; however, this increase is not statistically significant (P = 0.06).
Discussion
Traditionally, the primary treatment for OVCFs is conservative however this treatment is often associated with a poor quality of life, persistent pain, and complications arising from reduced patient mobility (33, 37, 47–53, 63–68).
A frequent complication of OVCFs, either treated surgically or with CT, is the occurrence of new OVCFs in adjacent vertebra (ANVFs). ANVFs, when left untreated, may decrease furthermore the quality of life and increase the morbidity and mortality in elderly patients (63).
Despite the fact that the majority of OVCFs heal without surgery, a relative recent review reported that 15%–35% of patients with an OVCF suffer from persistent intractable back pain, while severely collapsed OVCFs that may cause neurologic deficit, local kyphosis, or chronic pseudarthrosis frequently require surgery (54). Both CT and percutaneous augmentation methods (PKP, PVP) have advantages and disadvantages, and while there are a variety of trials describing the outcomes and complications of each treatment, there is still debate regarding the incidence rates of ANVFs following operative treatment and CT for OVCFs (69).
The reported major predictive risk factors in OVCFs are vertebral collapse, pseudarthrosis, local kyphotic deformity, and neurologic impairment. If prognosis can be predicted at the early fracture stage, some authors recommend more aggressive treatment options, rather than CT (70).
There is still debate and controversy about the effectiveness of PVP and PKP in comparison to CT regarding the incidence of ANVFs. There are numerous reports on indications, results and complications after PVP or PKP. Yet despite the positive findings seen in these reports, there is still conflicting results about surgical indications for PVP and PKP in treating OVCFs, except for cases that had failed CT (37, 71). In contrast to CT, PVP has been reported to afford rapid relief of back and low back pain, permit ambulation, and improve quality of life, however, there have been few reports concerning the long-term clinical efficacy of PVP (61, 65, 72).
While primary PVP may alleviate back pain, this symptom can occasionally reoccur during follow-up, often due to ANVFs. ANVF remain a topic of debate. Some authors suggest that the augmented vertebra has a different modulus of elasticity or stiffness compared to the adjacent fractured vertebra, resulting in increased forces on the surrounding vertebrae (73). In contrast, others argue that cement interdigitation acts as an internal fixation mechanism, strengthening and restoring the anterior column while reducing the flexion moment on the surrounding vertebral bodies (74).
There is an ongoing debate regarding the incidence of ANVFs after CT compared to PVP/PKP, with RCTs reporting varying outcomes—some indicating lower ANVFs rates after PVP/PKP (23), others showing similar rates (34, 36, 37, 75) and some suggesting a lower ANVFs rate with CT (42, 76).
A previous systematic review found that 17 clinical trials on PVP and 12 clinical trials on PKP reported new vertebral fractures. Of the new vertebral fractures following PVP and PKP, 60% and 66%, respectively, occurred adjacent to the augmented vertebra, though the incidence rates for conservative treatment were not mentioned (70). A meta-analysis by Tian et al. (77) investigated the clinical efficacy of PVP for the treatment of OVCF compared to conservative treatment and found no statistically significant difference in the incidence of adjacent vertebral fractures between the two groups. However, inconsistencies in follow-up durations across studies made direct comparisons challenging. In our systematic review, the incidence of adjacent new vertebral fractures in the conservative treatment group ranged from 7.8% to 35% across 9 clinical trials, while in the surgical group (PVP/PKP), it ranged from 2.6% to 20.4%. The Meta-analysis showed that in the RCTs the new fracture incidence was lower in the patients who received PVP/PKP.
Our study aligns with previous research regarding the wide range of follow-up (70). A retrospective study suggested that ANVFs tend to occur earlier than other new fractures in the rest of the spine (78), while long-term studies comparing CT and PVP yielded conflicting results (42–44).
The primary challenge in conducting a meta-analysis comparing studies on PKP and PVP vs. CT in patients with OVCFs is the lack of standardization in CT treatment options (Table 4). To the best of the authors' knowledge, the existing literature includes only six meta-analyses published between 2013 and 2021, along with one narrative review in 2023, that have compared surgical interventions (PKP, PVP) with CT regarding ANVFs incidence (35, 80–85). All of these studies (35, 80–85) concluded that there is no significant difference in the ANVFs incidence rate between PVP/PKP and CT. Our meta-analysis disclosed no significant difference in ANVFs incidence rates between surgical (PVP/PKP) and CT groups in the retrospective studies, this being in accordance to previous meta-analyses. In contrary, in our meta-analysis, when analyzing the RCTs, the vertebral augmentation (PVP/PKP) showed a significantly (P < 0.05) lower ANVFs incidence rate than the CT group. This superiority of interventional treatment (lower incidence rate of ANVFs) in the RCTs group in our meta-analysis, should be evaluated in terms of its significance alongside the benefits highlighted in related research concerning Quality of Life outcomes. This however was not the scope of our meta-analysis and presents a limitation of our study (3, 11, 52, 79, 80, 84–87).
Furthermore, in our meta-analysis, certain parameters varied from study to study. More specifically, the average age ranged from 64 to 80 years across the studies, but in all cases, the age differences between the surgical and CT groups were minimal, with a mean difference of no more than 2 years in any study. The total number of participants varied across the nine studies but remained balanced between the interventional and CT groups. This relatively small variation was accounted for the weights assigned to each study in the result synthesis. In our meta-analysis, the choice between a unilateral or bilateral pedicular approach for vertebral body augmentation appears to marginally influence the incidence of new fractures (P < 0.06), in favor of unilateral approach. This could be because of the two distinct augmentation techniques (PKP, PVP) used in the studies included in our meta-analysis. However, due to the limited number of studies, a more detailed understanding beyond what is discussed in the RCT section is not feasible. Similarly, potential subgroups that could theoretically affect new fractures generation—such as PMMA cement volume per vertebra, cement leakage, or follow-up duration—could not be statistically analyzed because of the limited number of included studies. Nevertheless, the evidence presented does not suggest any major differences in this regard. Conducting a meta-regression analysis for multiple subgroups creation, based on various potential risk factors for ANVFs would require separate analyses for RCTs and retrospective studies. As Thompson and Higgins (88) noted, a meta-regression should generally not be performed when fewer than ten studies are included in a meta-analysis. Since our meta-analysis included only nine studies, RCTs and retrospective studies were analyzed separately due to their distinct data collection methods.
Selected meta-analyses including RCTs exclusively have compared surgical treatments (PVP, PKP) with CT in terms of functional outcomes after treating of OVCFs and reported significantly better functional outcomes and pain relief in the first postoperative year following PVP/PKP (3, 11, 52, 79, 80, 84–87). In contrary to our results in the RCTs group, all these meta-analyses showed no significant differences in the incidence of ANVFs between the surgical and CT groups (Table 4). This difference could be due to the low number of RCTs included in our meta-analysis.
Some authors have emphasized the lack of standardized management strategies for OVCFs and recommended improving the quality of guidelines through multimodal approaches, including CT, surgery, and osteoporosis treatments, such as medications that promote fracture healing (54). However, numerous reports have demonstrated the beneficial effects of PVP without increasing the risk of ANVFs associated with this procedure when compared to CT in treating OVCFs (89). In our meta-analysis, the analysis of the RCTs suggests a statistically significant (P < 0.05) difference in the rate of ANVFs in favor of surgical group, however this was not shown in the retrospective studies group too. According to previous studies, the use of PMMA in PVP effectively stabilizes the fractured vertebral body, leading to pain relief and to an improved ability of performing daily activities (61, 72).
Whether PKP or PVP are associated with lower rates of ANVFs is a widely debated issue. A meta-analysis comparing PKP and PVP concluded that the occurrence of ANVFs in the PKP group did not differ from the PVP group (90).
There are still controversies in the literature regarding factors that may affect the outcomes following PVP and PKP. The time from OVCF to treatment appears to be an important factor that likely influences the outcome. Studies with shorter durations between the onset of pain and randomization tended to show greater effects favoring PVP. Similarly, the diagnostic criteria for enrollment varied, and studies using MRI edema as a criterion showed larger effect sizes in favor of PVP. Despite including studies with these two less favorable inclusion criteria, the pooled results remained significant (84).
Regarding the pros- and cons- of the unilateral compared to the bilateral approach for percutaneous augmentation, both biomechanical data (91–93) and clinical series (94–97) suggest that the unilateral procedure is safe and effective compared to bilateral augmentation. Additionally, comparative studies claim no significant difference in clinical or radiological parameters between uni- and bilateral augmentation (98–100). In our meta-analysis, the RCT subgroup analysis explored the correlation between pedicular approaches (unilateral vs. bilateral) in PVP/PKP and the incidence of new fractures. The unilateral approach appears to decrease the likelihood of new fractures; however, this decrease is only marginally significant (P = 0.06).
The volume of cement to be injected for optimal results remains a point of debate among different authors. Biomechanical studies suggest that smaller cement volumes may be sufficient to restore stiffness to pre-existing damaged levels (101), while others recommend larger volumes to restore vertebral strength and stiffness (102, 103). Some authors have proposed that smaller amounts of cement may be enough to resolve clinical symptoms (104). However, growing evidence suggests that larger cement volumes are associated with better pain resolution (105) and improved restoration of sagittal alignment (106, 107). In the studies included in our meta-analysis, the amount of PMMA injected per vertebra varied significantly among studies, and unfortunately it was reported in only three studies (39, 43, 61) regarding ANVFs.
Conclusion
This meta-analysis for the selected RCTs shows that vertebral augmentation is associated with lower incidence ANVFs compared to CT. On the other hand, in the retrospective studies group there was no significant difference in the incidence of ANVFs between the two treatment groups (CT vs. PKP/PVP). Variations in study parameters, such as patient demographics and surgical techniques, may have affected these results. Further high-quality studies are needed to better understand the long-term effects of different treatment strategies on the incidence of ANVFs.
Future research should adopt standardized diagnostic criteria (e.g., roentgenograms, MRIs, clinical evaluation) to ensure comparable fracture-to-treatment timelines. Additionally, longer follow-up periods are needed to better assess ANVFs rates. Standardizing factors such as PMMA volume, cement injection techniques for PVP/PKP, and conservative treatment protocols will help to reduce variability.
Limitations
This systematic review and meta-analysis included both retrospective studies and RCTs. Both groups consisted of selected comparative studies with similar populations in the experimental and control groups. Cochrane Collaboration's tool showed low “Risk of Bias” for RCTs and Newcastle‒Ottawa scale showed high quality scores for the retrospective studies. The included studies employed various conservative treatment (CT) methods and follow-up protocols. The time from the initial fracture to the development of ANVFs varied across studies in both treatment groups. Two commonly used vertebral body augmentation techniques—PVP and PKP—were applied, sometimes within the same study, despite their distinct surgical effects on fractured vertebrae and associated complications (e.g., kyphosis reduction, cement leakage). The reported surgical approach (unilateral vs. bilateral pedicular access) and the amount of PMMA injected per augmented vertebra also varied between studies. A significant limitation of this review is the absence of functional outcome measures (e.g., pain, quality of life, ODI) in all nine included studies. Since the primary concern for elderly patients is post-treatment quality of life, the lack of such data limits the clinical relevance of the findings. Despite these limitations, our meta-analysis has several strengths. It includes studies from six different countries, demonstrates low publication bias in both retrospective and RCT studies, and involves a comparable number of patients undergoing CT or surgical treatment—enhancing the representativeness of the results. Additionally, the high quality of the included studies supports the validity of our conclusions. These limitations do not compromise the reliability of this meta-analysis.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
PK: Investigation, Formal analysis, Resources, Writing – review & editing, Writing – original draft, Methodology, Validation, Data curation, Supervision, Project administration, Conceptualization. VS: Methodology, Conceptualization, Validation, Data curation, Investigation, Supervision, Writing – review & editing, Resources, Project administration, Writing – original draft. AK: Methodology, Software, Formal analysis, Visualization, Investigation, Data curation, Resources, Writing – review & editing, Writing – original draft, Validation. GD: Resources, Visualization, Project administration, Writing – original draft, Formal analysis, Conceptualization, Methodology, Data curation, Investigation, Validation, Writing – review & editing, Software, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. AI was used very rarely in order to improve the expression of meanings with more clarity in English. In no means AI was used in order to produce original text.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. J Am Med Assoc. (2017) 318:2466–82. doi: 10.1001/jama.2017.19344
2. Dai S, Du Y, Chen L, Xu Y, Hu Q. A mid- and long-term follow-up study on the bilateral pedicle anchoring technique with percutaneous vertebroplasty for the treatment of Kümell’s disease. Front Surg. (2023) 10:1061498. doi: 10.3389/fsurg.2023.1061498
3. Zuo XH, Zhu XXP, Bao HG, Xu CJ, Chen H, Gao XZ, et al. Network meta-analysis of percutaneous vertebroplasty, percutaneous kyphoplasty, nerve block, and conservative treatment for nonsurgical options of acute/subacute and chronic osteoporotic vertebral compression fractures (OVCFs) in short-term and long-term effects. Medicine. (2018) 97:e11544. doi: 10.1097/MD.0000000000011544
4. Fast facts on osteoporosis. National Osteoporosis Foundation. Available at: www.nof.org/professionals/fast_facts_osteo (Accessed September 20, 2024).
5. Mohit A, Orr D. Percutaneous vertebral augmentation in osteoporotic fractures. Curr Opin Orthop. (2007) 16:221–5. doi: 10.1097/BCO.0b013e32813aeafd
6. Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ 3rd. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. (1992) 7:221–7. doi: 10.1002/jbmr.5650070214
7. Abe T, Shibao Y, Takeuchi Y, Mataki Y, Amano K, Hioki S, et al. Initial hospitalization with rigorous bed rest followed by bracing and rehabilitation as an option of conservative treatment for osteoporotic vertebral fractures in elderly patients: a pilot one arm safety and feasibility study. Arch Osteoporos. (2018) 13:134. doi: 10.1007/s11657-018-0547-0
8. Tsujio T, Nakamura H, Terai H, Hoshino M, Namikawa T, Matsumura A, et al. Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk of nonunion. Spine. (2011) 36:1229–35. doi: 10.1097/BRS.0b013e3181f29e8d
9. Hoshino M, Nakamura H, Terai H, Tsujio T, Nabeta M, Namikawa T, et al. Factors affecting neurological deficits and intractable back pain in patients with insufficient bone union following osteoporotic vertebral fracture. Eur Spine J. (2009) 18:1279–86. doi: 10.1007/s00586-009-1041-6
10. Yagi H, Togami K, Suemura M, Tominaga T, Matsushima T, Kido K. Japanese society occupational medicine. Traumatology. (2014) 62:179–83.
11. Mattie R, Laimi K, Yu S, Saltychev M. Comparing percutaneous vertebroplasty and conservative therapy for treating osteoporotic compression fractures in the thoracic and lumbar spine. A systematic review and meta-analysis. J Bone Joint Surg Am. (2016) 98:1041–51. doi: 10.2106/JBJS.15.00425
12. Phillips FM. Minimally invasive treatments of osteoporotic vertebral compression fractures. Spine. (2003) 28(15 Suppl):45–53. doi: 10.1097/01.BRS.0000076898.37566.32
13. Evans AJ, Jensen ME, Kip KE, DeNardo AJ, Lawler GJ, Negin GA, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty. Retrospective report of 245 cases. Radiology. (2003) 226:366–72. doi: 10.1148/radiol.2262010906
14. Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt CJ, et al. Efficacy and safety of vertebroplasty for treatment of painful osteoporotic vertebral fractures: a randomised controlled trial. BMC Musculoskelet Disord. (2008) 9:156. doi: 10.1186/1471-2474-9-156
15. Hadjipavlou AG, Tzermiadianos MN, Katonis PG, Szpalski M. Percutaneous vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures and osteolytic tumours. J Bone Joint Surg Br. (2005) 87:1595–604. doi: 10.1302/0301-620X.87B12.16074
16. Lin EP, Ekholm S, Hiwatashi A, Westesson PL. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. Am J Neuroradiol. (2004) 25:175–80.14970015
17. Lin WC, Cheng TT, Lee YC, Wang T-N, Lui C-C, Yu C-Y. New vertebral osteoporotic compression fractures after percutaneous vertebroplasty: retrospective analysis of risk factors. J Vasc Interv Radiol. (2008) 19(2 Pt 1):225–31. doi: 10.1016/j.jvir.2007.09.008
18. Bouza C, Lopez T, Magro A, Navalpotro L, Amate JM. Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review. Eur Spine J. (2006) 15:1050–67. doi: 10.1007/s00586-005-0048-x
19. Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine. (2006) 31(23):2747–55. doi: 10.1097/01.brs.0000244639.71656.7d
20. Lavelle WF, Cheney R. Recurrent fracture after vertebral kyphoplasty. Spine J. (2006) 6:488–93. doi: 10.1016/j.spinee.2005.10.013
21. Fribourg D, Tang C, Sra P, Delamarter R, Bae H. Incidence of subsequent vertebral fracture after kypholasty. Spine. (2004) 29:2270–7. doi: 10.1097/01.brs.0000142469.41565.2a
22. Li YA, Lin CL, Chang MC, Liu CL, Chen TH, Lai SC. Subsequent vertebral fracture after vertebroplasty: incidence and analysis of risk factors. Spine. (2012) 37:179–83. doi: 10.1097/BRS.0b013e3181f72b05
23. Grafe A, Da Fonseca K, Hillmeier J, Meeder P-J, Libicher M, Nöldge G, et al. Reduction of pain and fracture incidence after kyphoplasty: 1-year outcomes of a prospective controlled trial of patients with primary osteoporosis. Osteoporos Int. (2005) 16(12):2005–12. doi: 10.1007/s00198-005-1982-5
24. Kasperk C, Grafe IA, Schmitt S, Nöldge G, Weiss C, Da Fonseca K, et al. Tree-year outcomes after kyphoplasty in patients with osteoporosis with painful vertebral fractures. J Vasc Interv Radiol. (2010) 21(5):701–9. doi: 10.1016/j.jvir.2010.01.003
25. Kasperk C, Hillmeier J, Nöldge G, Grafe IA, Dafonseca K, Raupp D, et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res. (2005) 20(4):604–12. doi: 10.1359/JBMR.041203
26. Komp M, Ruetten S, Godolias G. Minimally invasive therapy for functionally unstable osteoporotic vertebral fracture by means of kyphoplasty: prospective comparative study of 19 surgically and 17 conservatively treated patients. J Miner Stoffwechs. (2004) 11(supplement 1):13–5.
27. Wardlaw D, Cummings SR, Van Meirhaeghe J, Bastian L, Tillman JB, Ranstam J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. (2009) 373(9668):1016–24. doi: 10.1016/S0140-6736(09)60010-6
28. Movrin I. Adjacent level fracture after osteoporotic vertebral compression fracture: a nonrandomized prospective study comparing balloon kyphoplasty with conservative therapy. Wien Klin Wochenschr. (2012) 124(9-10):304–11. doi: 10.1007/s00508-012-0167-4
29. Zhou J, Ma H, Zou D, Tan R, Wang D, Zheng R. Correlative factors of secondary fracture after percutaneous kyphoplasty for osteoporotic vertebral compression fracture. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2011) 25(10):1180–3.22069969
30. Rho Y-J, Choe WJ, Chun YI. Risk factors predicting the new symptomatic vertebral compression fractures after percutaneous vertebroplasty or kyphoplasty. Eur Spine J. (2012) 21(5):905–11. doi: 10.1007/s00586-011-2099-5
31. Zhong B-Y, He S-C, Zhu H-D, Wu C-G, Fang W, Chen L, et al. Risk prediction of new adjacent vertebral fractures after PVP for patients with vertebral compression fractures: development of a prediction model. Cardiovasc Intervent Radiol. (2017) 40(2):277–84. doi: 10.1007/s00270-016-1492-1
32. Chen Z, Xu L, Shi L, Cao H, Nie M. Long-term outcome of percutaneous vertebroplasty versus conservative treatment for osteoporotic vertebral compression fractures: a retrospective cohort study with three-year follow-up. Front Med (2024) 11:1391243. doi: 10.3389/fmed,2024
33. Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. (2009) 361:569–79. doi: 10.1056/NEJMoa0900563
34. Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. (2009) 361:557–68. doi: 10.1056/NEJMoa0900429
35. Xie L, Zhao ZG, Zhang SJ, Hu YB. Percutaneous vertebroplasty versus conservative treatment for osteoporotic vertebral compression fractures: an updated meta-analysis of prospective randomized controlled trials. Int J Surg. (2017) 47:25–32. doi: 10.1016/j.ijsu.2017.09.021
36. Klazen CA, Lohle PN, de Vries J, Jansen FH, Tielbeek AV, Blonk MC, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (vertos II): an open-label randomised trial. Lancet. (2010) 376:1085–92. doi: 10.1016/S0140-6736(10)60954-3
37. Lee HM, Park SY, Lee SH, Suh SW, Hong JY. Comparative analysis of clinical outcomes in patients with osteoporotic vertebral compression fractures (OVCFs): conservative treatment versus balloon kyphoplasty. Spine J. (2012) 12:998–1005. doi: 10.1016/j.spinee.2012.08.024
38. Blasco J, Martinez-Ferrer A, Macho J, San Roman L, Pomés J, Carrasco J, et al. Effect of vertebroplasty on pain relief, quality of life, and the incidence of new vertebral fractures: a 12-month randomized follow-up, controlled trial. J Bone Miner Res. (2012) 27:1159–66. doi: 10.1002/jbmr.1564
39. Yang EZ, Xu JG, Huang GZ, Xiao WZ, Liu XK, Zeng BF, et al. Percutaneous vertebroplasty versus conservative treatment in aged patients with acute osteoporotic vertebral compression fractures: a prospective randomized controlled clinical study. Spine. (2016) 41:653–60. doi: 10.1097/BRS.0000000000001298
40. Yi HJ, Jeong JH, Im SB, Lee JK. Percutaneous vertebroplasty versus conservative treatment for one level thoracolumbar osteoporotic compression fracture: results of an over 2-year follow-up. Pain Physician. (2016) 19:E743–50.27389117
41. Firanescu CE, de Vries J, Lodder P, Venmans A, Schoemaker MC, Smeets AJ, et al. Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial. Br Med J. (2018) 361:k1551. doi: 10.1136/bmj.k1551
42. Alvarez L, Alcaraz M, Pérez-Higueras A, Granizo JJ, de Miguel I, Rossi RE, et al. Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine. (2006) 31:1113–8. doi: 10.1097/01.brs.0000216487.97965.38
43. Klazen CA, Venmans A, de Vries J, van Rooij WJ, Jansen FH, Blonk MC, et al. Percutaneous vertebroplasty is not a risk factor for new osteoporotic compression fractures: results from VERTOS II. Am J Neuroradiol. (2010) 31:1447–50. doi: 10.3174/ajnr.A2148
44. Ehteshami Rad A, Gray LA, Kallmes DF. Incident vertebral fractures in patients not undergoing vertebroplasty. J Vasc Interv Radiol. (2010) 21:856–60. doi: 10.1016/j.jvir.2010.02.012
45. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
46. Eriksen BM, Frandsen FT. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. (2018) 106(4):420–31. doi: 10.5195/jmla.2018.345
47. Roellinghof M, Siewe J, Zarghooni K, Sobottke R, Alparslan Y, Eysel P, et al. Effectiveness, security and height restoration on fresh compression fractures a comparative prospective study of vertebroplasty and kyphoplasty. Minim Invasive Neurosurg. (2009) 52(5-6):233–7. doi: 10.1055/s-0029-1243631
48. Brunton S, Carmichael B, Gold D, Hull B, Kauffman T, Papaioannou A, et al. Vertebral compression fractures in primary care: recommendations from a consensus panel. J Fam Pract. (2005) 54(9):781–8.16144592
49. Rebolledo JB, Gladnick BP, Unnanuntana A, Nguyen JT, Kepler CK, Lane JM. Comparison of unipedicular and bipedicular balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures: a prospective randomised study. Bone Joint J. (2013) 95(3):401–6. doi: 10.1302/0301-620X.95B3.29819
50. Pfeifer M, Begerow B, Minne HW. Effects of a new spinal orthosis on posture, trunk strength, and quality of life in women with postmenopausal osteoporosis: a randomized trial. Am J Phys Med Rehabil. (2004) 83:177–86. doi: 10.1097/01.PHM.0000113403.16617.93
51. Klazen CA, Lohle PN, de Vries J, Jansen FH, Tielbeek AV, Venmans A, et al. Percutaneous vertebroplasty versus conservative therapy in patients with an acute osteoporotic vertebral compression fracture. Vertos: a randomized controlled trial II. J Vasc Interv Radiol. (2010) 21(suppl 2):S6–7. doi: 10.1016/j.jvir.2009.12.153
52. Liu J, Li X, Tang D, Cui X, Li X, Yao M, et al. Comparing pain reduction following vertebroplasty and conservative treatment for osteoporotic vertebral compression fractures: a meta-analysis of randomized controlled trials. Pain Physician. (2013) 16:455–64.24077192
53. Itshayek E, Miller P, Barzilay Y, Hasharoni A, Kaplan L, Fraifeld S, et al. Vertebral augmentation in the treatment of vertebral compression fractures: review and new insights from recent studies. J Clin Neurosci. (2012) 19:786–91. doi: 10.1016/j.jocn.2011.12.015
54. Jang H-D, Kim E-H, Lee JC, Choi S-W, Kim K, Shin B-J. Current concepts in the management of osteoporotic vertebral fractures: a narrative review. Current concepts in the management of osteoporotic vertebral fractures: a narrative review. Asian Spine J. (2020) 14(6):898–909. doi: 10.31616/asj.2020.0594
55. Clarke M, Horton R. Bringing in all together: lancet-cochrane collaborate on systematic reviews. Lancet. (2001) 357:1728. doi: 10.1016/S0140-6736(00)04934-5
56. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology, a proposal for reporting: meta-analysis of observational studies in epidemiology (MOOSE) group. J Am Med Assoc. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
57. DerSimonian R, Maird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–8. doi: 10.1016/0197-2456(86)90046-2
58. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed. Chichester: John Wiley & Sons (2011).
59. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses (2012). Available at: http://wwwohrica/programs/clinical_epidemiology/Oxfordasp (Accessed September 20, 2024).
60. Dettori JR, Norvell DC, Chapman JR. Fixed-effect vs random-effects models for meta-analysis: 3 points to consider. Global Spine. (2022) 12(7):1624–6. doi: 10.1177/21925682221110527
61. Farrokhi MR, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures clinical article. J Neurosurg Spine. (2011) 14:561–9. doi: 10.3171/2010.12.SPINE10286
62. Baek S-W, Kim C, Chang H. The relationship between the spinopelvic balance and the incidence of adjacent vertebral fractures following percutaneous vertebroplasty. Osteoporos Int. (2015) 26(5):1507–13. doi: 10.1007/s00198-014-3021-x
63. Silverman SL. The clinical consequences of vertebral compression fracture. Bone. (1992) 13(suppl 2):S27–31. doi: 10.1016/8756-3282(92)90193-Z
64. Rousing R, Andersen MO, Jespersen SM, Tomsen K, Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine. (2009) 34(13):1349–54. doi: 10.1097/BRS.0b013e3181a4e628
65. Voormolen MHJ, Mali WPTM, Lohle PNM, Fransen H, Lampmann LE, van der Graaf Y, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. Am J Neuroradiol. (2007) 28(3):555–60.17353335
66. Grohs JG, Matzner M, Trieb K, Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective non-randomized comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech. (2005) 18(3):238–42.15905767
67. Bornemann R, Hanna M, Kabir K, Goost H, Wirtz DC, Pfugmacher R. Continuing conservative care versus crossover to radiofrequency kyphoplasty: a comparative effectiveness study on the treatment of vertebral body fractures. Eur Spine J. (2012) 21(5):930–6. doi: 10.1007/s00586-012-2148-8
68. Estublier C, Chapurlat R, Szulc P. Older men with severe disc degeneration have more incident vertebral fractures-the prospective MINOS cohort study. Rheumatology. (2017) 56(1):37–45. doi: 10.1093/rheumatology/kew327
69. Muratore M, Ferrera A, Masse A, Bistolf A. Osteoporotic vertebral fractures: predictive factors for conservative treatment failure. A systematic review. Eur Spine J. (2017) 27(10):2565–76. doi: 10.1007/s00586-017-5340-z
70. Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. (2006) 31(17):1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b
71. Chow GH, Nelson BJ, Gebhard JS, Brugman JL, Brown CW, Donaldson DH. Functional outcome of thoracolumbar burst fractures managed with hyperextension casting or bracing and easy mobilization. Spine. (1996) 21:2170–5. doi: 10.1097/00007632-199609150-00022
72. Kobayashi K, Shimoyama K, Nakamura K, Murata K. Percutaneous vertebroplasty immediately relieves pain of osteoporotic vertebral compression fractures and prevents prolonged immobilization of patients. Eur Radiol. (2005) 15:360–7. doi: 10.1007/s00330-004-2549-0
73. Berlemann U, Ferguson SJ, Nolte L-P, Heini PF. Adjacent vertebral failure after vertebroplasty. J Bone Joint Surg Br. (2002) 84(5):748–52. doi: 10.1302/0301-620x.84b5.11841
74. Yuan AH, Brown CW, Phillips FM. Osteoporotic spinal deformity: a biomechanical rationale for the clinical consequences and treatment of vertebral body compression fractures. J Spinal Disord Tech. (2004) 17(3):236–42. doi: 10.1097/00024720-200406000-00012
75. Diamond TH, Bryant C, Browne L, Clark AW. Clinical outcomes after acute osteoporotic vertebral fractures: a 2-year non-randomised trial comparing percutaneous vertebroplasty with conservative therapy. Med J Aust. (2006) 184(3):113–7. doi: 10.5694/j.1326-5377.2006.tb00148.x
76. Ma X, Xing D, Ma J, Wang J, Chen Y, Xu W, et al. Risk factors for new vertebral compression fractures after percutaneous vertebroplasty: qualitative evidence synthesized from a systematic review. Spine. (2013) 38(12):E713–22. doi: 10.1097/BRS.0b013e31828cf15b
77. Tian J, Xiang L, Zhou D, Fan Q, Ma B. The clinical efficacy of vertebroplasty on osteoporotic vertebral compression fracture: a meta-analysis. Int J Surg. (2014) 12:1249–53. doi: 10.1016/j.ijsu.2014.10.027
78. Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. Am J Neuroradiol. (2006) 27:217–23.16418388
79. Sanli I, van Kuijk SMJ, de Bie RA, van Rhijn LW, Willems PC. Percutaneous cement augmentation in the treatment of osteoporotic vertebral fractures (OVFs) in the elderly: a systematic review. Eur Spine J. (2020) 29:1553–72. doi: 10.1007/s00586-020-06391-x
80. Zhu R-SS, Kan S-LL, Ning G-ZZ, Chen LX, Cao ZG, Jiang ZH, et al. Which is the best treatment of osteoporotic vertebral compression fractures: balloon kyphoplasty, percutaneous vertebroplasty, or non-surgical treatment? A Bayesian network meta-analysis. Osteoporos Int. (2019) 30:287–98. doi: 10.1007/s00198-018-4804-2
81. Zhang L, Zhai P. A comparison of percutaneous vertebroplasty versus conservative treatment in terms of treatment effect for osteoporotic vertebral compression fractures: a meta-analysis. Surg Innov. (2019) 27(1):19–25. doi: 10.1177/1553350619869535
82. Kawanishi M, Tanaka H, Ito Y, Yamada M, Yokoyama K, Sugie A, et al. Treatment for osteoporotic vertebral fracture - a short review of orthosis and percutaneous vertebroplasty and balloon kyphoplasty. Neurospine. (2023) 20(4):1124–31. doi: 10.14245/ns.2346936.468
83. Halvachizadeh S, Stalder A-L, Bellut D, Hoppe S, Rossbach P, Cianfoni A, et al. A comparison of improvement in pain, adjacent-level fractures, and quality of life between vertebroplasty, kyphoplasty, and nonoperative management.systematic review and meta-analysis of 3 treatment arms for vertebral compression fractures. JBJS Rev. (2021) 9(10):e21.00045. doi: 10.2106/JBJS.RVW.21.00045
84. Anderson AP, Froyshteter BA, Tontz LW Jr. Meta-analysis of vertebral augmentation compared with conservative treatment for osteoporotic spinal fractures. J Bone Miner Res. (2013) 28(2):372–82. doi: 10.1002/jbmr.1762
85. Lou S, Shi X, Zhang X, Lyu H, Li Z, Wang Y. Percutaneous vertebroplasty versus non-operative treatment for osteoporotic vertebral compression fractures: a meta-analysis of randomized controlled trials. Osteoporos Int. (2019) 30:2369–80. doi: 10.1007/s00198-019-05101-8
86. Yuan W-H, Hsu H-C, Lai K-L. Vertebroplasty and balloon kyphoplasty versus conservative treatment for osteoporotic vertebral compression fractures. A meta-analysis. Medicine. (2016) 95(31):e4491. doi: 10.1097/MD.0000000000004491
87. Láinez Ramos-Bossini AJ, López Zúñiga D, Ruiz Santiago F. Percutaneous vertebroplasty versus conservative treatment and placebo in osteoporotic vertebral fractures: meta-analysis and critical review of the literature. Eur Radiol. (2021) 31:8542–53. doi: 10.1007/s00330-021-08018-1
88. Thompson GS, Higgins JT. How should meta-regression analyses be undertaken and interpreted? Stat Med. (2002) 21(11):1559–73. doi: 10.1002/sim.1187
89. Chen D, An ZQ, Song S, Tang JF, Qin H. Percutaneous vertebroplasty compared with conservative treatment in patients with chronic painful osteoporotic spinal fractures. J Clin Neurosci. (2014) 21(3):473–7. doi: 10.1016/j.jocn.2013.05.017
90. Yang H, Liu T, Zhou J, Meng B, Wang G, Zhu X. Kyphoplasty versus vertebroplasty for painful osteoporotic vertebral compression fractures—which one is better? A systematic review and meta-analysis. Int J Spine Surg. (2013) 7:e45–57. doi: 10.1016/j.ijsp.2013.03.001
91. Steinmann J, Tingey CT, Cruz G, Dai Q. Biomechanical comparison of unipedicular versus bipedicular kyphoplasty. Spine. (2005) 30(2):201–5. doi: 10.1097/01.brs.0000150831.46856.87
92. Tohmeh AG, Mathis JM, Fenton DC, Levine AM, Belkof SM. Biomechanical efficacy of unipedicular versus bipedicular vertebroplasty for the management of osteoporotic compression fractures. Spine. (1999) 24(17):1772–6. doi: 10.1097/00007632-199909010-00004
93. Chen B, Li Y, Xie D, Yang X, Zheng Z. Comparison of unipedicular and bipedicular kyphoplasty on the stiffness and biomechanical balance of compression fractured vertebrae. Eur Spine J. (2011) 20(8):1272–80. doi: 10.1007/s00586-011-1744-3
94. Lee S-B, Cho K-S, Huh P-W, Yoo D-S, Kang S-G, Kim D-S, et al. Clinical and radiographic results of unilateral transpedicular balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Acta Neurochir Suppl. (2008) 101:157–60. doi: 10.1007/978-3-211-78205-7_27
95. Kim AK, Jensen ME, Dion JE, Schweickert PA, Kaufmann TJ, Kallmes DF. Unilateral transpedicular percutaneous vertebroplasty: initial experience. Radiology. (2002) 222(3):737–41. doi: 10.1148/radiol.2223010718
96. Chang WS, Lee S-H, Choi WG, Choi G, Jo B-J. Unipedicular vertebroplasty for osteoporotic compression fracture using an individualized needle insertion angle. Clin J Pain. (2007) 23(9):767–73. doi: 10.1097/AJP.0b013e318154b6c3
97. Papadopoulos EC, Edobor-Osula F, Gardner MJ, Shindle MK, Lane JM. Unipedicular balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures: early results. J Spinal Disord Tech. (2008) 21(8):589–96. doi: 10.1097/BSD.0b013e31815d6997
98. Chen C, Wei H, Zhang W, Gu Y, Tang G, Dong R, et al. Comparative study of kyphoplasty for chronic painful osteoporotic vertebral compression fractures via unipedicular versus bipedicular approach. J Spinal Disord Tech. (2011) 24(7):E62–5. doi: 10.1097/BSD.0b013e318228f470
99. Wang Z, Wang G, Yang H. Comparison of unilateral versus bilateral balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. J Clin Neurosci. (2012) 19(5):723–6. doi: 10.1016/j.jocn.2011.08.023
100. Song K-B, Eun J-P, Oh Y-M. Clinical and radiological comparison of unipedicular versus bipedicular balloon kyphoplasty for the treatment of vertebral compression fractures. Osteoporos Int. (2009) 20(10):1717–23. doi: 10.1007/s00198-009-0872-7
101. Liebschner MAK, Rosenberg WS, Keaveny TM. Efects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine. (2001) 26(14):1547–54. doi: 10.1097/00007632-200107150-00009
102. Molloy S, Mathis JM, Belkof SM. The effect of vertebral body percentage fll on mechanical behavior during percutaneous vertebroplasty. Spine. (2003) 28(14):1549–54. doi: 10.1097/01.BRS.0000076831.38265.8D
103. Belkof SM, Mathis JM, Jasper LE, Deramond H. The biomechanics of vertebroplasty: the effect of cement volume on mechanical behavior. Spine. (2001) 26(14):1537–41. doi: 10.1097/00007632-200107150-00007
104. Rollinghof M, Hagel A, Siewe J, Gutteck N, Delank KS, Steinmetz A, et al. Is height restoration possible with a comparatively smaller amount of cement in radiofrequency kyphoplasty using a monopedicle approach? Z Orthop Unfall. (2013) 151(2):156–62. doi: 10.1055/s-0032-1328418
105. Roder C, Boszczyk B, Perler G, Aghayev E, Kulling F, Maestretti G. Cement volume is the most important modifiable predictor for pain relief in BKP: results from SWISS spine, a nationwide registry. Eur Spine J. (2013) 22(10):2241–8. doi: 10.1007/s00586-013-2869-3
106. Xu C, Liu HX, Xu HZ. Analysis of related factors on the deformity correction of balloon kyphoplasty. Am J Neuroradiol. (2014) 35(1):202–6. doi: 10.3174/ajnr.A3617
107. Kruger A, Baroud G, Noriega D, Figiel J, Dorschel C, Ruchholtz S, et al. Height restoration and maintenance after treating unstable osteoporotic vertebral compression fractures by cement augmentation is dependent on the cement volume used. Clin Biomech. (2013) 28(7):725–30. doi: 10.1016/j.clinbiomech.2013.06.007
108. Yang W, Yang J, Liang M. Percutaneous vertebroplasty does not increase the incidence of new fractures in adjacent and nonadjacent vertebral bodies. Clin Spine Surg. (2019) 32(2):E99–E106. doi: 10.1097/BSD.0000000000000734
109. Teuber H, Tiziani S, Halvachizadeh S, Frey D, Sprengel K, Pape HC, et al.Single-level vertebral kyphoplasty is not associated with an increased risk of symptomatic secondary adjacent osteoporotic vertebral compression fractures: a matched case-control analysis. Arch Osteoporos. (2018) 13(1):82. doi: 10.1007/s11657-018-0489-6
110. Yi X, Lu H, Tian F, Wang Y, Li C, Liu H, et al. Recompression in new levels after percutaneous vertebroplasty and kyphoplasty compared with conservative treatment. Arch Orthop Trauma Surg. (2014) 134(1):21–30. doi: 10.1007/s00402-013-1886-3
Keywords: adjacent fracture, kyphoplasty, vertebroplasty, adjacent, vertebra fracture
Citation: Korovessis P, Syrimpeis V, Korovesis A and Dimakopoulos G (2025) Incidence of new osteoporotic adjacent vertebral body fractures. A comparison between conservative treatment and vertebral body augmentation (vertebroplasty, kyphoplasty): a systematic review and meta-analysis. Front. Surg. 12:1594217. doi: 10.3389/fsurg.2025.1594217
Received: 15 March 2025; Accepted: 17 April 2025;
Published: 20 May 2025.
Edited by:
Luca Ambrosio, Campus Bio-Medico University, ItalyReviewed by:
Artur Xhumari, University of Medicine, AlbaniaJuan P. Cabrera, University of Concepcion, Chile
Copyright: © 2025 Korovessis, Syrimpeis, Korovesis and Dimakopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panagiotis Korovessis, a29yb3Zlc3NAb3RlbmV0Lmdy
 Panagiotis Korovessis
Panagiotis Korovessis Vasileios Syrimpeis
Vasileios Syrimpeis Alkis Korovesis3
Alkis Korovesis3