Abstract
Female genital schistosomiasis (FGS) is a chronic manifestation of schistosomiasis, usually caused by Schistosoma haematobium infection, which can be responsible for infertility, ectopic pregnancy, and abortion, and is associated with an increased prevalence of HIV infection. No screening programs are currently recommended for FGS. Colposcopy, the conventionally suggested diagnostic tool for FGS, is also considered a crucial screening tool for cervical cancer (CC). We performed an experimental screening via colposcopy for FGS at primary healthcare centers (PHCCs) in the Boeny region of Madagascar, allowing for the detection of patients with both FGS signs and HPV-related dysplasia (HPV-dy). All suspected FGS cases were treated with praziquantel on the day of colposcopy, and all images of suspected CC or HPV-dy were re-assessed by a gynecologist and, if needed, patients were then provided with additional colposcopy for histologic diagnosis and treatment. We describe three cases of FGS and HPV-related precancerous lesions detected during the project, discussing the state of art of the relationship between CC, FGS and HPV and the real-life challenges encountered in terms of both patient compliance and the diagnostic and treatment cascade. Despite the current diagnostic limitations, a screening for FGS via colposcopy may contribute to the early identification of CC or precancerous lesions. The addition of visual inspection with acetic acid (VIA) during colposcopy for FGS screening could improve its impact on CC screening. In addition, although there is limited evidence of the effectiveness of praziquantel in FGS, treatment should in any case be proposed for suspicious lesions, given its safety and ease of administration. The benefit of combined screening could be maximised by increasing the availability of good quality services and improve awareness of both diseases among women
1 Introduction
Female genital schistosomiasis (FGS) is a chronic manifestation of schistosomiasis, usually caused by Schistosoma haematobium infection, which results from the entrapment of Schistosoma eggs in the genital tract (1). The disease affects up to 56 million women worldwide according to the World Health Organization (WHO) (2) and could be responsible for primary and secondary infertility, ectopic pregnancy, and abortion, and is associated with an increased prevalence of HIV infection (3–5).
Symptoms associated with cervical schistosomiasis are non-specific and consist of dyspareunia, dysmenorrhea, menorrhagia, leukorrhea, lower abdominal pain, and postcoital or intermenstrual bleeding (6). This can pose problems, including mistaken diagnosis with other sexually transmitted infections (STIs), misinterpretation of the symptoms due to the use of contraceptives (i.e., progestin-based contraceptives are associated with bleeding) (7), a risk of stigma, delay in medical evaluation, and improper treatment (8).
The main intervention for schistosomiasis in endemic countries is preventive treatment through mass drug administration (MDA) with praziquantel, which has also been recently suggested for adults in areas with a Schistosoma prevalence ≥10% (9). No screening programs are currently recommended for any form of chronic schistosomiasis, including FGS (8). The diagnostic tool conventionally suggested for FGS is colposcopy (1), which may also be used for cervical cancer (CC) screening to perform a visual inspection with acetic acid or Lugol’s iodine (VIA/VILI) (10).
CC is the fourth most frequent cancer among women, with an estimated 604,000 cases worldwide in 2020, of which 342,000 resulted in death (11). Screening for CC, if applied systematically and with wide coverage, can reduce the incidence of the disease, as observed in northern and western Europe, North America, Australia, and New Zealand (12). Its implementation, however, despite the availability of different algorithms and screening tools, remains very limited in low-resource settings, where about 90% of deaths from CC occur (12).
The most recent WHO guidelines do not support the use of colposcopy as a primary screening method for CC, recommending it for triage after a positive test (via cytology, an HPV test, or naked eye EIA) (10). Nevertheless, in countries with both a high prevalence of schistosomiasis and high incidence of CC (Figure 1), the procedure might have the dual advantage of allowing early diagnosis of HPV-related dysplasia (HPV-dy) or neoplasia along with that of FGS (13, 14).
Figure 1

Worldwide distribution of cervical cancer and S. haematobium. In blue: age-standardized incidence ratio (ASR) for cervical cancer estimated to be higher than 16.7/100,000; in yellow: endemic countries for S. haematobium; in blue/yellow: ASR >16.7/100,000 for cervical cancer and endemic presence of S. haematobium (13, 14).
Clinical management, patients’ attitude to clinical follow-up, and the correct interpretation of screening results may, however, strongly influence the potential benefit of this diagnostic procedure.
Our data were collected in the context of a cross-sectional study, aimed at assessing the prevalence of FGS and HPV infection in the Boeny region of Madagascar, which was selected for its estimated S. haematobium prevalence of more than 50% in the adult population. For this purpose, we established an experimental screening program comprising a colposcopy combined with a symptom-based questionnaire for FGS at primary healthcare centers (PHCCs), allowing for the detection of patients with both FGS and HPV-related dysplasia (HPV-dy). The eligibility criteria were as follows: being aged between 18 and 49 years and not pregnant, being able to provide informed consent, and fluency in either French or Malagasy. The screening consisted of a colposcopy performed at PHCCs by trained midwives and validated by a trained gynecologist through the review of images captured on site. Samples collected using a liquid-based cytology procedure were collected for the detection and typing of HPV. Typing of HPV was performed at the International Agency for Research on Cancer (IARC) in Lyon, France, through a Luminex-based technology (15). The results of the HPV typing were available upon request by the study participants and not reported systematically. All suspected FGS cases were treated with praziquantel the day of colposcopy, while all images of suspected CC or HPV-dy were re-assessed by a gynecologist. If the suspicion was confirmed by the specialist, the patients were called back at the PHCCs and further assessed by a gynecologist via colposcopy for visual inspection with acetic acid and biopsy. Further treatment for patients with histologic confirmation of high-grade HPV-dy or CC was performed at the local hospital.
Among the women who presented with lesions that were suspected to indicate both diseases, three cases were selected for reporting because of the peculiar challenges observed in the diagnostic–therapeutic flow (Supplementary Table 1). These cases were chosen because they reflected (i) the complexity of the clinical management of suspicious lesions in a context with limited possibility of long-term follow-up, (ii) the possible inaccuracy of a histologic diagnosis, and (iii) the potential for patient reluctance to continue the diagnostic–therapeutic pathway.
Qualitative data were additionally collected from two of the three patients described in this case report (patients 1 and 3), specifically the two patients referred for additional medical services, with the aim of exploring drivers for the acceptance or refusal of medical diagnosis and consequent treatment. Data was collected through in-depth interviews driven by a structured topic guide, by a trained local female medical doctor with extensive experience in conducting qualitative data collection. Interviews were performed in Malagasy, recorded, and translated into French and English for analysis.
Ethical approval was obtained from the National Ethics Committee of Madagascar (ref. no. N°052-MSANP/CERBM) and the Ethics Committee Hamburg State Medical Chamber, Germany (ref. no. PV7309). Patients provided written consent for the anonymous use of colposcopy images.
This paper describes three cases of FGS- and HPV-related precancerous lesions detected during the project, discussing the state of the art of the relationship between CC, FGS, and HPV, and the real-life challenges encountered in terms of both patient compliance and the diagnosis and treatment cascade.
2 Case report
2.1 Patient 1
The patient (20 years old) was evaluated on 4 May 2021 in Marovay. She reported one pregnancy brought to term and complained about dyspareunia for more than 6 months prior to consultation, with regular but painful menses and no contraception in place. No chronic or recent therapy nor history of previous treatment with praziquantel was recorded. The colposcopy detected grainy and homogeneous sandy patches, consistent with FGS, together with the finding of cervical contact bleeding. She was consequently referred for gynecological evaluation, performed on 21 May 2021 (Figure 2). On that date she underwent a biopsy with a finding of chronic cervicitis with koilocytosis. A cold knife conization was offered and performed on 12 July 2021 because of the presence of contact bleeding at the examination, the positive VIA screening at the second colposcopy, the young age (20 years), and the high risk of loss to follow-up. Final histology excluded HPV-dy or CC and confirmed FGS only (presence of Schistosoma eggs). HPV detection later confirmed the presence of the cancerogenic HPV strain 45. From the qualitative data collected after her follow-up visit, we report that the patient accepted the additional screening and treatment because she was experiencing clinical consequences from her condition. Specifically, the patient said that she underwent screening because she had “…difficulty conceiving. I also have pain with every period…”. Moreover, when asked why she accepted the proposed treatment, she replied “I just said that I want to be healthy and so I agreed.”
Figure 2
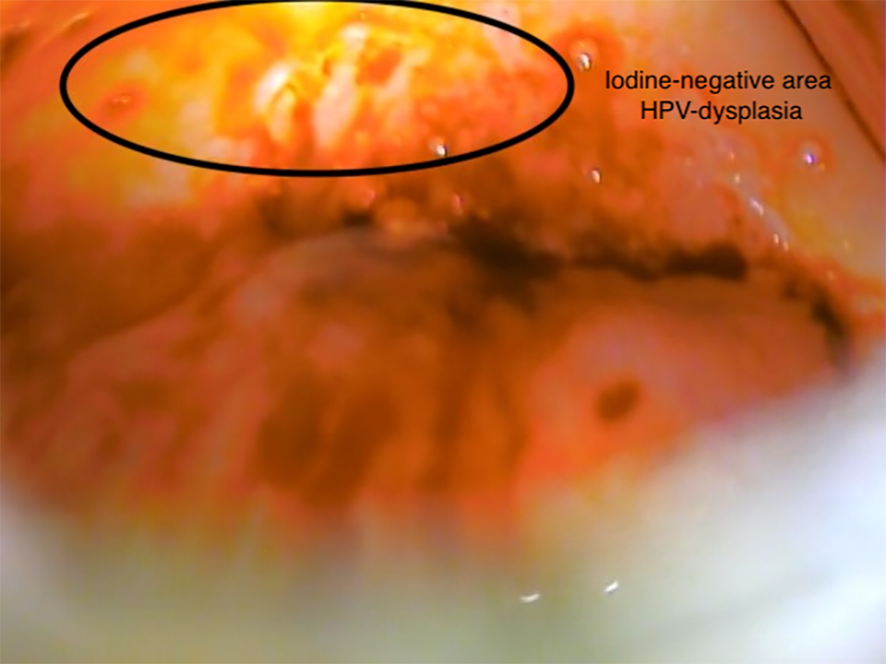
Patient 1 (aged 20 years). Visual inspection with Lugol’s iodine (VILI), iodine-negative area (10 o’clock to 12 o’clock), HPV-dysplasia.
2.2 Patient 2
The patient (38 years old) was evaluated, for the first time, on 6 April 2021 in Marovay (Figure 3). According to her medical history, she reported that she was carrying a subcutaneous contraceptive implant after four pregnancies, one of which ended in a miscarriage. With the exception of dyspareunia, which had arisen in the month prior to consultation, she did not report any other symptoms suggestive of FGS, nor any recent medication, except treatment with praziquantel taken more than a year before the visit. The colposcopy presented grainy sandy patches and areas of doubtful interpretation, for which the patient was referred to the gynecologist, who performed a second colposcopy and biopsy. The patient presented with histologic features consistent with condylomas, koilocytosis, and mild dysplasia, which was not classified, and no presence of Schistosoma eggs. HPV detection later confirmed the presence of cancerogenic HPV strains 31, 35, and 45.
Figure 3
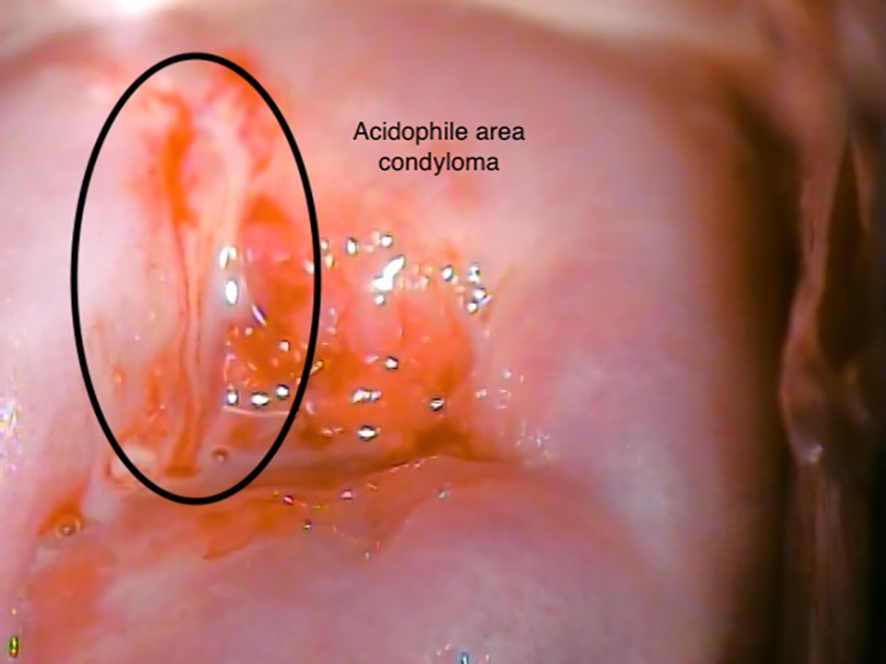
Patient 2 (aged 38 years). Visual inspection with acetic acid (VIA), acidophile area (11 o’clock), condyloma.
2.3 Patient 3
The patient (38 years old) was evaluated on 2 April 2021 in Ankazombornona. She reported no symptoms suggestive of FGS or STIs, had experienced five previous pregnancies, of which one ended with a miscarriage, and had no history of previous treatment with praziquantel. She reported the use of a progestin-based subcutaneous contraceptive implant and a history of irregular menstruation (from four to six times per year). The colposcopy revealed signs compatible with FGS (grainy and homogeneous yellow sandy patches) and lesions that were suspected to be malignancies (Figure 4), which was confirmed by the gynecologist. The second colposcopy for VIA and biopsy was performed on 30 April 2021 (after 49 days) and she was diagnosed with CIN-2 dysplasia without Schistosoma eggs. HPV detection later confirmed the presence of cancerogenic HPV strains 18, 33, and 53. Despite an invitation to come to the hospital to discuss the diagnosis and for subsequent therapeutic indications, the patient never attended the hospital. From the qualitative data collected after her refusal, we report that, despite the positive experience and acceptance of the screening, the treatment was refused because she did not experience major clinical consequences from her condition. Specifically, when asked about the reasons for refusal, she replied “After that, I was pregnant. Besides, me and my family were scared” but also added “if I had not been pregnant, I would have undergone the treatment”.
Figure 4
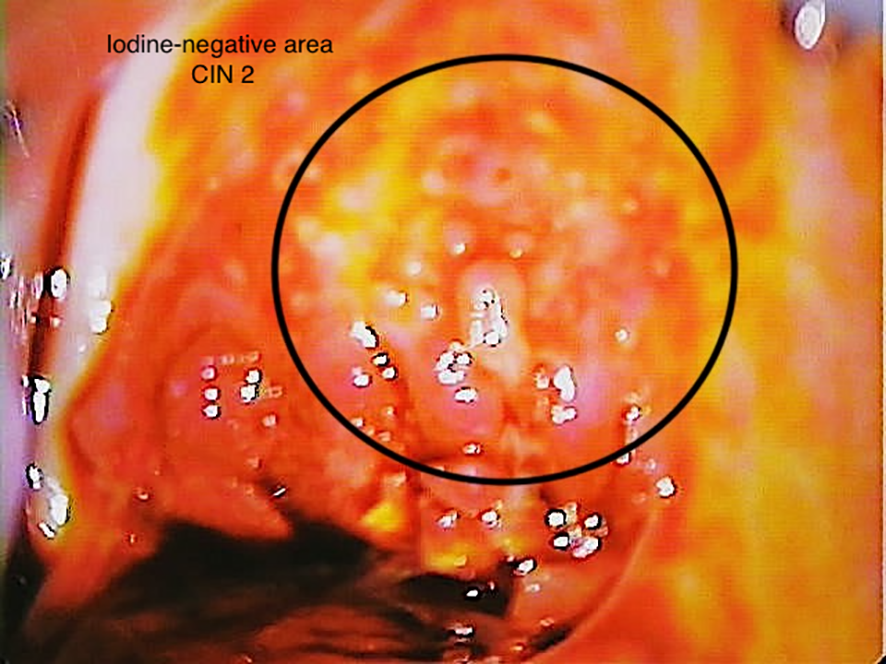
Patient 3 (38 years). Visual inspection with Lugol’s iodine (VILI), iodine-negative area (12 o’clock to 2 o’clock), CIN 2.
3 Discussion
About 90% of the estimated 342,000 deaths from CC in 2020 occurred in low- and middle-income countries (LMICs) (11). In addition, S. haematobium-related schistosomiasis is endemic in many of these countries, and the subsequent burden of FGS remains to be determined (Figure 1).
The association between CC and FGS has been speculated on several times but there is no strong scientific evidence supporting this hypothesis. The relationship between the two conditions remains controversial and needs to be clearly understood (16–21). In particular, based on a few studies of specific populations (i.e., individuals with early-onset CC), it has been hypothesized that FGS may influence HPV acquisition, and indirectly the risk of CC, at two stages: (i) at the acquisition of the infection, as the FGS-associated erosions and ulcerations of the cervical mucosa may facilitate the entrance of the virus (18), and (ii) during viral clearance (22). In this second instance, it is postulated that FGS interferes with the mechanisms of HPV clearance and promotes the rapid propagation of the virus, producing an increase in the viral load and ultimately contributing to the development of cervical precancer (23, 24). Another hypothesis is that chronic inflammation induced by the presence of S. haematobium eggs may contribute to tumor pathogenesis, not only by facilitating HPV acquisition or reducing HPV clearance, but also through epithelial disruption and immune cell recruitment, and by increasing viral oncogenicity (17). Ultimately, the interactions between FGS, STIs, HPV, and HIV are only partially understood. Although HIV is a known risk factor for HPV persistence, precancer and CC due to its ability to reduce the likelihood of HPV clearance and the number of CD4+ cell count (24), the effects of other STIs are still being studied, as is the role of FGS, which would seem to predispose individuals to an increased susceptibility to infection (25).
The uncertainties surrounding and lack of data on the interactions between FGS and CC are also attributable to the difficulties in introducing integrated screening in resource-limited countries (8, 26). Colposcopy is the conventional method used for the diagnosis of FGS and it can also detect CC lesions (1, 10). However, it has some limitations, such as operator dependence and subjectivity, inter- and intra-operator variabilities, and the need for strict quality control and continuous training (27). The lack of specificity is another issue, especially for low-grade lesions (28), as some lesions may be due to infections other than FGS, such as herpes; longstanding bacterial, fungal or protozoal infections; or tuberculosis (29). Finally, the implementation of colposcopy screenings requires adequate infrastructure and important efforts to guarantee large-scale distribution and usage at the point of care (27). Owing to these challenges, colposcopy is scarcely implemented in LMICs, although easier-to-use alternatives, such as hand-held colposcopes, are showing promising results (30, 31). However, colposcopy remains a crucial component of CC screening in both the screen-and-treat and the screen-triage-and-treat approaches, regardless of the algorithm used (10), and it is necessarily performed for sampling when histology is used to make clinical decisions (10). Given the role of colposcopy in screening for CC, FGS is a critical differential diagnosis to account for. The WHO pocket atlas represents so far the most reliable tool worldwide for the identification of FGS signature signs via colposcopy in the cervix and vagina; however, a standardized process for the diagnosis of FGS lesions in the upper genital tract is still missing (32, 33). Microscopy, which is considered the diagnostic standard for schistosomiasis, has limitations for FGS: there is no direct correlation between the presence of S. haematobium eggs in urine and FGS, and its use on wet or pap smears has reported sensitivities of less than 15% (34–37). The identification of eggs in biopsies from cervical–vaginal tissue is currently considered the gold standard for FGS (38), but the invasiveness of the procedure and the need for histologic diagnosis and services limits its large-scale adoption for screening (39, 40). To date, no biomarker has been identified specifically for FGS, so even more complex molecular diagnosis methods are hardly applicable to the disease. Nevertheless, some studies have presented promising results with molecular testing, either self collected or collected in the clinic (38, 41). The current WHO recommendations for CC screening are to implement HPV detection as the initial entry point for screening rather than VIA, owing to quality-assurance limitations (10, 42). The guidelines also recommend follow-up management based on the risk of progression associated with the different HPV serotypes identified. For serotypes with an increased risk of progression (16–18, as found in Patient 3), direct assessment of eligibility for ablation is recommended, while a VIA triage is suggested for the other oncogenic serotypes (10). In this view, the development of biomarkers or molecular tests for the diagnosis of FGS could in the future be used in integrated screening procedures based on sample collection, whether performed by a healthcare professional or self collected.
As seen in Patient 1 (aged 20 years), the typical age of clinical presentation and onset of sequelae may differ between FGS and CC. The natural history of the two diseases and the risk of exposure to the related infectious agents over time are different for FGS and CC. Schistosomiasis is usually contracted in childhood through contact with Schistosoma in fresh water, whereas HPV is usually contracted in adolescence after sexual debut, which can then lead to CC (11, 43). These different patterns can biologically explain the detection of FGS at a younger age than CC. For this reason, FGS screening should ideally start before the age of 30. This threshold, which is the WHO-recommended starting age for CC screening in the general population (25 years for women living with HIV) (10) could have the disadvantage of increasing unnecessary procedures, with consequent higher costs for the health system. Conversely, an advantage might be the diagnosis of FGS at an earlier stage, when it could be more responsive to treatment with praziquantel, the efficacy of which is yet to be proven on lesions (3). In addition, our recent data from Madagascar (44) show that after 30 years of age, the prevalence of the disease is quite high and the disease can be at a more advanced stage and less responsive to praziquantel, meaning that including FGS screening in CC programs at a younger age might have beneficial effects on the burden of FGS and its consequences on reproductive health.
Our cases also show how in limited resources settings, despite the existence of guidelines, there may be the risk of overtreatment. Poor dissemination, imperfect implementation, and the lack of a clear referral system to manage disorders that require more articulated management (as in the case of precancerous cervical lesions) contribute to overtreatment. As an example, in Madagascar, a screening program for CC with VIA for women aged 25–49 years has been established since 2007 (45), although only 8% of women in the target age group for screening underwent VIA assessment at least once (46) and the pap smear has been adopted sporadically in the country (47, 48). This generates uncertainties within medical staff about the concrete possibility of providing adequate care and proper follow-up for patients, which adds to the risk of overtreatment and unnecessary referrals linked to the known low specificity of VIA for CC and its inability to identify HR-HPV serotypes and the related risk of carcinogenesis progression. In the case of Patient 1 we can speculate that treatment was given even in the absence of evident pre-cancerous lesions because of the young age, the high risk of unfavorable outcomes in the event of CC, and the fear of loss to follow-up.
In addition, clinical management is strictly dependent on the quality of the diagnosis, both colposcopy related and histologic, which determines the choice of treatment and follow-up (49, 50). For instance, in our experience with Patient 2, the histologic diagnosis was of poor quality. The histopathology showed the presence of dysplasia, but this condition was not classified according to LAST (lower anogenital squamous terminology) or CIN (cervical intraepithelial neoplasia) terminology (10) and only qualitatively described as “mild”. Nevertheless, a follow-up visit was suggested, ensuring adequate surveillance for dysplasia.
These two cases highlight how strengthening competencies in both the diagnosis and clinical management of precancerous lesions, as well as the presence of accessible and applicable local guidelines for the providers of health services, could contribute to improving the quality of integrated screening and clinical management processes for both FGS and CC (51–53).
The case of Patient 3 highlights another critical element of the weaknesses of health systems in resource-limited settings in the management of CC and HPV-dy. Patient 3, despite the accessibility of free health services, decided not to use them owing to the discovery of a pregnancy and fear of the possible clinical consequences of continuing the follow-up. The typical determinants for healthcare utilization can be divided into supply-sided and demand-sided pillars (54, 55), relating to the attitudes/behaviors toward health services of providers’ and users’, respectively. During our study, the availability of the screening and follow-up service temporarily mitigated the supply barriers but nevertheless did not fully overcome the demand barriers, as in the case of Patient 3. This showed how both the supply and demand pillars must be addressed to improve access to and utilization of healthcare (55). Our qualitative data retrieved from Patients 1 and 3 highlight the importance of health literacy and health education in the decision-making process about the use of medical services. The two patients were confronted with similar clinical manifestations and the outcome that they associated with the condition, namely the ability to conceive, was the driver for the decision-making process in both cases. On one hand, this shows that the health-promotion activities implemented within the project were successful in stressing the consequences of FGS in relation to reproductive health. On the other hand, the campaign failed to reinforce the concept that gynecological disorders can be diverse and lead to several medical consequences. This can be interpreted as the direct effect of a strictly vertical program, which can fail to comprehensively address more general health topics, such as gynecological disorders (56). We believe that these two cases show the potential advantages of and the need for integrated services in which the combined activities of health promotion and health education are broader, addressing both FGS and CC and preventing biased conclusions from the target audience that can lead to individual decisions with critical medical consequences.
Despite the relevance of our cases, some limitations need to be accounted for in our report. First, although the known low HIV prevalence in the country (0.4% in 2021) (57) makes the presence of HIV unlikely, diagnostic tests for both HIV and other STIs were not available. The availability of tests for HIV and other STIs would certainly have improved the description of the cases, especially given the complex interactions between the diseases. Second, qualitative data on service utilization were not available for Patient 2. Patient 3 was lost to follow-up, so it is not possible to provide information on the conclusion of her diagnostic–therapeutic pathway. Finally, histology confirmed the diagnosis of FGS in one out of three cases, in line with the known non-specificity of lesions at colposcopy. Unfortunately, due to the poor quality of the photos, colposcopy images for FGS were not available. Nevertheless, we believe that these three cases are illustrative of what may occur with the introduction of colposcopy in a LMIC. The cases provide insights into how best to optimize the implementation of colposcopy, which, albeit with known limitations for both CC and FGS, remains a good candidate for integrated screening for the two diseases.
In conclusion, our cases show that the screening for FGS by colposcopy, as recommended by the WHO atlas, can contribute to the early identification of CC or precancerous lesions, despite the current diagnostic limitations. The identification of biomarkers for FGS in vaginal samples could increase the potential of integrated HPV and FGS screening programs. Furthermore, although aware of the limitations of the method for both diseases, we believe that the addition of VIA in colposcopy for FGS screenings could improve the impact of colposcopy-based screenings for the detection of CC, and that treatment with praziquantel should in any case be proposed for suspicious lesions. The enhancement of diagnostic services by improving awareness, knowledge, and practices surrounding CC and FGS among users and providers is an urgent measure that should be adopted for the successful implementation of integrated services.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the National Ethics Committee of Madagascar (ref. N°052-MSANP/CERBM) and the Ethics Committee Hamburg State Medical Chamber, Germany (ref. no. PV7309). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
VM: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. ZR: Investigation, Supervision, Validation, Writing – review & editing. J-MK: Data curation, Investigation, Methodology, Writing – review & editing. SR: Investigation, Methodology, Supervision, Writing – review & editing. RiR: Investigation, Project administration, Writing – review & editing. TR: Conceptualization, Methodology, Project administration, Writing – review & editing. RaR: Conceptualization, Investigation, Project administration, Writing – review & editing. PR: Data curation, Formal analysis, Writing – review & editing. TG: Investigation, Methodology, Writing – review & editing. MH: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing. JM: Funding acquisition, Writing – review & editing. RAR: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. DF: Conceptualization, Data curation, Funding acquisition, Investigation, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was primarily funded by the Coalition for Operational Research on NTDs (CORNTD) through the project FIRM-UP (project number NTDSC 210D) and partly funded by the German Centre for Infection research (DZIF) through the project NAMASTE (project number 8008803819).
Acknowledgments
We are grateful to all the participants who agreed to be part of the study, and the midwives and laboratory technicians who contributed to the implementation of the screening campaign.
Conflict of interest
The author MH is employed by St. Elisabeth Krankenhaus, Köln Hohenlind, Germany.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2023.1270852/full#supplementary-material
References
1
World Health Organization . Female genital schistosomiasis: a pocket atlas for clinical health-care professionals. (2015). World Health Organization. Available at: https://apps.who.int/iris/handle/10665/180863 (Accessed July 2, 2023).
2
Hotez PJ Engels D Gyapong M Ducker C Malecela MN . Female genital schistosomiasis. N Engl J Med (2019) 381:2493–5. doi: 10.1056/NEJMp1914709
3
Kjetland EF Leutscher PDC Ndhlovu PD . A review of female genital schistosomiasis. Trends Parasitol (2012) 28:58–65. doi: 10.1016/j.pt.2011.10.008
4
Brodish PH Singh K . Association between schistosoma haematobium exposure and human immunodeficiency virus infection among females in Mozambique. Am J Trop Med Hyg (2016) 94:1040–4. doi: 10.4269/ajtmh.15-0652
5
Kjetland EF Ndhlovu PD Gomo E Mduluza T Midzi N Gwanzura L et al . Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS Lond Engl (2006) 20:593–600. doi: 10.1097/01.aids.0000210614.45212.0a
6
Helling-Giese G Sjaastad A Poggensee G Kjetland EF Richter J Chitsulo L et al . Female genital schistosomiasis (FGS): relationship between gynecological and histopathological findings. Acta Trop (1996) 62:257–67. doi: 10.1016/s0001-706x(96)00027-7
7
Hu Y Zhou K Shan D Yang M . Interventions for vaginal bleeding irregularities with contraceptive implant. Cochrane Database Syst Rev (2021) 2021:CD014649. doi: 10.1002/14651858.CD014649
8
Joint United Nations Programme on HIV/AIDS. No more neglect — Female genital schistosomiasis and HIV — Integrating sexual and reproductive health interventions to improve women’s lives. Available at: https://digitallibrary.un.org/record/3944850 (accessed September 26, 2023)
9
WHO guideline on control and elimination of human schistosomiasis . Available at: https://www.who.int/publications-detail-redirect/9789240041608 (Accessed July 2, 2023).
10
WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. Available at: https://www.who.int/publications-detail-redirect/9789240030824 (Accessed July 2, 2023).
11
Cervical cancer. Available at: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (Accessed July 2, 2023).
12
Arbyn M Weiderpass E Bruni L de Sanjosé S Saraiya M Ferlay J et al . Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health (2020) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
13
Gryseels B Polman K Clerinx J Kestens L . Human schistosomiasis. Lancet Lond Engl (2006) 368:1106–18. doi: 10.1016/S0140-6736(06)69440-3
14
Cancer today. Available at: http://gco.iarc.fr/today/home (Accessed July 2, 2023).
15
Gheit T Tommasino M . Detection of high-risk mucosal human papillomavirus DNA in human specimens by a novel and sensitive multiplex PCR method combined with DNA microarray. Methods Mol Biol Clifton NJ (2011) 665:195–212. doi: 10.1007/978-1-60761-817-1_12
16
Petry KU Scholz U Hollwitz B Von Wasielewski R Meijer CJLM . Human papillomavirus, coinfection with Schistosoma hematobium, and cervical neoplasia in rural Tanzania. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc (2003) 13:505–9. doi: 10.1046/j.1525-1438.2003.13301.x
17
Rafferty H Sturt AS Phiri CR Webb EL Mudenda M Mapani J et al . Association between cervical dysplasia and female genital schistosomiasis diagnosed by genital PCR in Zambian women. BMC Infect Dis (2021) 21:691. doi: 10.1186/s12879-021-06380-5
18
Mosunjac MB Tadros T Beach R Majmudar B . Cervical schistosomiasis, human papilloma virus (HPV), and human immunodeficiency virus (HIV): a dangerous coexistence or coincidence? Gynecol Oncol (2003) 90:211–4. doi: 10.1016/s0090-8258(03)00191-4
19
Szela E Bachicha J Miller D Till M Wilson JB . Schistosomiasis and cervical cancer in Ghana. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet (1993) 42:127–30. doi: 10.1016/0020-7292(93)90625-7
20
North M Dubinchik I Hamid A Elderiny S Sayegh R . Association between cervical schistosomiasis and cervical cancer. A report of 2 cases. J Reprod Med (2003) 48:995–8.
21
Kaizilege GK Kiritta R Chuma C Ndaboine E Ottoman O Elias E et al . Female genital schistosomiasis, a neglected differential of cervical precancerous and cancerous lesion: a wakeup call for on-job training for healthcare workers in endemic areas. Austin J Clin Case Rep (2022) 9:1241.
22
Shattock RJ Griffin GE Gorodeski GI . In vitro models of mucosal HIV transmission. Nat Med (2000) 6:607–8. doi: 10.1038/76138
23
Woodman CBJ Collins SI Young LS . The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer (2007) 7:11–22. doi: 10.1038/nrc2050
24
Campos NG Demarco M Bruni L Desai KT Gage JC Adebamowo SN et al . A proposed new generation of evidence-based microsimulation models to inform global control of cervical cancer. Prev Med (2021) 144:106438. doi: 10.1016/j.ypmed.2021.106438
25
Shukla JD Kleppa E Holmen S Ndhlovu PD Mtshali A Sebitloane M et al . The association between female genital schistosomiasis and other infections of the lower genital tract in adolescent girls and young women: A cross-sectional study in South Africa. J Low Genit Tract Dis (2023) 27:291–6. doi: 10.1097/LGT.0000000000000756
26
Engels D Hotez PJ Ducker C Gyapong M Bustinduy AL Secor WE et al . Integration of prevention and control measures for female genital schistosomiasis, HIV and cervical cancer. Bull World Health Organ (2020) 98:615–24. doi: 10.2471/BLT.20.252270
27
Xue P Ng MTA Qiao Y . The challenges of colposcopy for cervical cancer screening in LMICs and solutions by artificial intelligence. BMC Med (2020) 18:169. doi: 10.1186/s12916-020-01613-x
28
Qin D Bai A Xue P Seery S Wang J Mendez MJG et al . Colposcopic accuracy in diagnosing squamous intraepithelial lesions: a systematic review and meta-analysis of the International Federation of Cervical Pathology and Colposcopy 2011 terminology. BMC Cancer (2023) 23:187. doi: 10.1186/s12885-023-10648-1
29
Colposcopy and treatment of cervical intraepithelial neoplasia: a beginners’ manual . Available at: https://screening.iarc.fr/colpochap.php?chap=9.php&lang=1 (Accessed August 30, 2023).
30
Sturt A Bristowe H Webb E Hansingo I Phiri C Mudenda M et al . Visual diagnosis of female genital schistosomiasis in Zambian women from hand-held colposcopy: agreement of expert image review and association with clinical symptoms. Wellcome Open Res (2023) 8:14. doi: 10.12688/wellcomeopenres.18737.2
31
Søfteland S Sebitloane MH Taylor M Roald BB Holmen S Galappaththi-Arachchige HN et al . A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int J Gynecol Obstet (2021) 153:190–9. doi: 10.1002/ijgo.13538
32
Christinet V Lazdins-Helds JK Stothard JR Reinhard-Rupp J . Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Int J Parasitol (2016) 46:395–404. doi: 10.1016/j.ijpara.2016.02.006
33
Richter J Poggensee G Kjetland EF Helling-Giese G Chitsulo L Kumwenda N et al . Reversibility of lower reproductive tract abnormalities in women with Schistosoma haematobium infection after treatment with praziquantel–an interim report. Acta Trop (1996) 62:289–301. doi: 10.1016/s0001-706x(96)00030-7
34
Orish VN Morhe EKS Azanu W Alhassan RK Gyapong M . The parasitology of female genital schistosomiasis. Curr Res Parasitol Vector-Borne Dis (2022) 2:100093. doi: 10.1016/j.crpvbd.2022.100093
35
Kjetland EF Ndhlovu PD Mduluza T Gomo E Gwanzura L Mason PR et al . Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg (2005) 72:311–9. doi: 10.4269/ajtmh.2005.72.311
36
Holmen SD Kleppa E Lillebø K Pillay P van Lieshout L Taylor M et al . The first step toward diagnosing female genital schistosomiasis by computer image analysis. Am J Trop Med Hyg (2015) 93:80–6. doi: 10.4269/ajtmh.15-0071
37
Pillay P van Lieshout L Taylor M Sebitloane M Zulu SG Kleppa E et al . Cervical cytology as a diagnostic tool for female genital schistosomiasis: Correlation to cervical atypia and Schistosoma polymerase chain reaction. CytoJournal (2016) 13:10. doi: 10.4103/1742-6413.180784
38
Bustinduy AL Randriansolo B Sturt AS Kayuni SA Leustcher PDC Webster BL et al . An update on female and male genital schistosomiasis and a call to integrate efforts to escalate diagnosis, treatment and awareness in endemic and non-endemic settings: The time is now. Adv Parasitol (2022) 115:1–44. doi: 10.1016/bs.apar.2021.12.003
39
Fleming KA Naidoo M Wilson M Flanigan J Horton S Kuti M et al . An essential pathology package for low- and middle-income countries. Am J Clin Pathol (2017) 147:15–32. doi: 10.1093/ajcp/aqw143
40
Alfaro K Maza M Cremer M Masch R Soler M . Removing global barriers to cervical cancer prevention and moving towards elimination. Nat Rev Cancer (2021) 21:607–8. doi: 10.1038/s41568-021-00396-4
41
Sturt AS Webb EL Phiri CR Mweene T Chola N van Dam GJ et al . Genital self-sampling compared with cervicovaginal lavage for the diagnosis of female genital schistosomiasis in Zambian women: The BILHIV study. PloS Negl Trop Dis (2020) 14:e0008337. doi: 10.1371/journal.pntd.0008337
42
Atlas of visual inspection of the cervix with acetic acid for screening, triage, and assessment for treatment. Available at: https://screening.iarc.fr/atlasviadetail.php?Index=3&e=# (Accessed August 30, 2023).
43
McManus DP Dunne DW Sacko M Utzinger J Vennervald BJ Zhou X-N . Schistosomiasis. Nat Rev Dis Primer (2018) 4:13. doi: 10.1038/s41572-018-0013-8
44
Kutz JM Rausche P Rasamoelina T Ratefiarisoa S Razafindrakoto R Klein P et al . Female genital schistosomiasis, human papilloma virus infec?on, and cervical cancer in rural Madagascar: a cross sec? Onal study. Infect Dis Poverty (2023) 12(1):89. doi: 10.1186/s40249-023-01139-3
45
Cancer du col de l’utérus : une meilleure accessibilité améliore le dépistage à Madagascar (2023). OMS Bur Régional Pour Afr. Available at: https://www.afro.who.int/fr/news/cancer-du-col-de-luterus-une-meilleure-accessibilite-ameliore-le-depistage-Madagascar (Accessed July 4, 2023).
46
WHO . Cervical cancer country profiles. Madagascar, World Health Organization - Cervical Cancer Country Profiles. Madagascar. Available at https://cdn.who.int/media/docs/default-source/country-profiles/cervical-cancer/cervical-cancer-mdg-2021-country-profile-en.pdf?sfvrsn=ae28c693_33&download=true (accessed September 26, 2023).
47
Razafimahefa J Ranaliarinosy M Fenomanana J Andriamampionona TF . Prevention strategies of cervical cancer in developing countries: The case of Madagascar. Glob Health Netw Collect (2023). doi: 10.21428/3d48c34a.b847876b
48
Dumont A Bessières N Benbassa A Razafindrafara G Rabearison F Philippe H-J . Dépistage du cancer du col utérin en milieu rural à Madagascar : faisabilité, couverture et incidence. J Gynecol Obstet Hum Reprod (2017) 46:327–32. doi: 10.1016/j.jogoh.2017.03.003
49
Catarino R Petignat P Dongui G Vassilakos P . Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J Clin Oncol (2015) 6:281–90. doi: 10.5306/wjco.v6.i6.281
50
Plan of action for cervical cancer prevention and control 2018-2030 . PAHO/WHO | Pan American Health Organization. Available at: https://www.paho.org/en/documents/plan-action-cervical-cancer-prevention-and-control-2018-2030 (Accessed July 10, 2023).
51
World Health Organization . Cervical cancer screening in developing countries: report of a WHO consultation. Geneva: World Health Organization (2002). p. 75.
52
Kaschula ROC . The practice of pathology in Africa. Arch Pathol Lab Med (2013) 137:752–5. doi: 10.5858/arpa.2011-0587-ED
53
Valls J Baena A Venegas G Celis M González M Sosa C et al . Performance of standardised colposcopy to detect cervical precancer and cancer for triage of women testing positive for human papillomavirus: results from the ESTAMPA multicentric screening study. Lancet Glob Health (2023) 11:e350–60. doi: 10.1016/S2214-109X(22)00545-9
54
Ensor T Cooper S . Overcoming barriers to health service access: influencing the demand side. Health Policy Plan (2004) 19:69–79. doi: 10.1093/heapol/czh009
55
O’Donnell O . Access to health care in developing countries: breaking down demand side barriers. Cad Saude Publica (2007) 23:2820–34. doi: 10.1590/s0102-311x2007001200003
56
World Health Organization . When do vertical (stand-alone) programmes have a place in health systems? (2008). World Health Organization. Regional Office for Europe. Available at: https://apps.who.int/iris/handle/10665/107977 (Accessed July 7, 2023). Regional Office for Europe, Network HE, Policies EO on HS and.
57
World Bank Open Data . Prevalence of HIV, total (% of population ages 15-49) . Madagascar: World Bank Open Data. Available at: https://data.worldbank.org (Accessed August 23, 2023).
Summary
Keywords
female genital schistosomiasis, cervical cancer, HPV, S. haematobium , colposcopy, integrated health services, primary level of care, conization
Citation
Marchese V, Rakotomalala Z, Kutz J-M, Ratefiarisoa S, Rakotomalala R, Rasamoelina T, Rakotozandrindrainy R, Rausche P, Gheit T, Hampl M, May J, Rakotoarivelo RA and Fusco D (2023) Case Report: Three cases of suspected female genital schistosomiasis and precancerous lesions for cervical cancer in a highly endemic country—from clinical management to public health implications. Front. Trop. Dis 4:1270852. doi: 10.3389/fitd.2023.1270852
Received
01 August 2023
Accepted
18 September 2023
Published
11 October 2023
Volume
4 - 2023
Edited by
Amy S. Sturt, University of London, United Kingdom
Reviewed by
Olimpia Lamberti, University of London, United Kingdom; Vanessa Christinet, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Updates
Copyright
© 2023 Marchese, Rakotomalala, Kutz, Ratefiarisoa, Rakotomalala, Rasamoelina, Rakotozandrindrainy, Rausche, Gheit, Hampl, May, Rakotoarivelo and Fusco.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela Fusco, fusco@bnitm.de; Valentina Marchese, valentina.marchese@bnitm.de
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.