Abstract
Malaria is a leading cause of death in school-aged children in sub-Saharan Africa, and non-fatal chronic malaria infections are associated with anaemia, school absence and decreased learning, preventing children from reaching their full potential. Malaria chemoprevention has led to substantial reductions in malaria in younger children in sub-Saharan Africa. In 2022, the WHO updated its recommendations for chemoprevention to older, school-aged children where epidemiologically indicated. To date, there has been limited uptake of these policies which include both extending the age of seasonal malaria chemoprevention in seasonal transmission settings and providing intermittent preventive treatment to school-aged children in perennial transmission settings. In April 2024, a stakeholder meeting was convened in Kigali, Rwanda, to analyse barriers to implementation of malaria chemoprevention targeting school-aged children. Key evidence gaps were identified and needs for coordination and advocacy were highlighted.
Background
Despite widespread implementation of malaria control interventions over the last several decades, progress towards malaria elimination has stalled. The burden of malaria remains high in areas of sub-Saharan Africa, and additional strategies are required to strengthen malaria control (1). While the majority of malaria related mortality occurs in young children, the burden of malaria in older, school-aged children (5–15 years old) is increasingly recognized, negatively impacting children’s health and educational attainment, but also perpetuating malaria transmission within communities and threatening malaria elimination goals (1–3).
Malaria, the disease resulting from infection with Plasmodium parasites, may manifest as severe disease leading to malaria-related mortality, uncomplicated clinical malaria prompting outpatient treatment, or chronic, sub-clinical infections (4, 5). Among school-aged children in Sub-Saharan Africa, malaria is the leading cause of death in (6). In addition to loss of life, frequent episodes of uncomplicated clinical malaria in this age group are associated with anaemia and school absences contributing to negative impacts on education, as well as increased caring duties for family members and additional costs to health systems (7, 8) Chronic, sub-clinical infections are the most common manifestation of malaria in school-aged children, with community surveys in high burden countries revealing that more than 40% of ‘asymptomatic’ school-aged children are infected with malaria parasites (9–12) In fact across the region, prevalence of infection is higher in school-aged children compared to younger children and adults group (9, 10, 13–16). With regard to the health and education of school-aged children themselves, these chronic infections are associated with anaemia, decreased cognitive function, and poor educational outcomes (4, 17–21). This constraint on children’s education ultimately limits them from reaching their full potential and decreases human capital (22). Furthermore, school-aged children have been identified as a primary reservoir for community transmission, contributing to continued risk of infection and mortality in other vulnerable groups (23–26).
Chemoprevention, which is the routine administration of antimalarial drugs at regular intervals to both clear existing infections and prevent new ones, has been a long-standing tool for malaria control and is proposed as a strategy to reduce the burden of malaria in school-aged children 5–15 year olds. First adopted by the World Health Organization (WHO) in 2002 as supplementary antenatal care to decrease adverse effects of malaria in pregnancy, the use of chemoprevention was then expanded to protect younger children through intermittent preventive treatment in infants (IPTi) – now termed perennial malaria chemoprevention (PMC), and seasonal malaria chemoprevention (SMC). Since its adoption as policy in 2012, SMC, which targets children 3–59 months old, now reaches an estimated 53 million children annually and has substantially reduced malaria related morbidity and mortality in younger children (1, 27, 28) In 2022, the WHO updated the guidelines for malaria chemoprevention strategies to broaden their use and support more country-driven, tailored approaches to malaria control (29). These recommendations expanded the age range for provision for chemoprevention through either intermittent preventative treatment of school-aged children (IPTsc) or extending the target age of SMC (ext-age SMC) to older children where epidemiologically indicated.
Since the expansion of the WHO recommendations, however, implementation of chemoprevention in older children has been limited. This slow introduction may be due to many factors. Under the current Global Malaria Technical Strategy, National Malaria Control/Elimination Programs (NMCPs) are supported to develop bespoke approaches to optimize malaria control for their country utilizing national burden stratification and sub-national tailoring of intervention mixes constrained by domestic and donor funding (30, 31). In this context, we sought to bring together key stakeholders from NMCPs, program implementation partners, and researchers in malaria, school health, and education to review the status of malaria chemoprevention targeting school-aged children in sub-Saharan Africa.
Stakeholder meeting proceedings
A group of stakeholders and experts representing eight sub-Saharan African countries and 30 organisations met in Kigali, Rwanda on the sidelines of the Multilateral Initiative on Malaria Conference (MIM) in April 2024 to:
-
Determine the barriers faced by interested National Malaria Control/Elimination Programmes (NMCPs) in implementing IPTsc or ext-age SMC and identify potential solutions.
-
Identify knowledge and research gaps which remain to support program development and prioritization.
-
Enhance knowledge exchange between country programs, implementing partners, and researchers.
Lauren Cohee (LSTM, UK) and Chris Drakeley (LSHTM, UK) began the meeting by providing an overview of malaria in school-aged children, highlighting, as described above, the direct impacts malaria on the health of school-aged children as well as the indirect impacts of malaria in school-aged children on education of school-aged children themselves, community level malaria transmission, and, ultimately human capital.
Suzanne van Hulle (CRS, US/Switzerland) reviewed the 2022 WHO updated guidelines for malaria chemoprevention and summarised key strategy considerations for both IPTsc and ext-age SMC. The similarities and differences between these two approaches to malaria chemoprevention are summarized in Table 1. The key difference in the approaches is that ext-age SMC applies in areas where SMC is already being conducted. If programs are developed to target school-aged children in areas without SMC in younger children, the approach is defined as IPTsc.
Table 1
| Definition | IPTsc | ext-age SMC |
|---|---|---|
| What? | Preventative chemotherapy is the use of medicines, either alone or in combination, to prevent malaria infection and its consequences. It requires giving a full treatment course of an antimalarial medicine to vulnerable populations at designated time points during the period of greatest malarial risk, regardless of whether the recipient is infected. |
|
| Where? | Malaria-endemic settings with moderate to high perennial or seasonal transmission | Areas of seasonal transmission |
| Who? | Age groups* should be identified using local data on the age distribution of malaria admissions and severe disease. | |
| How? | May be delivered via fixed point (e.g. school-based) or community-based (e.g. door-to-door) | |
Definition, setting and target audience of Intermittent Preventive Treatment of malaria in school-aged children (IPTsc) and extended-age Seasonal Malaria Chemoprevention (ext-age SMC), adapted from WHO guidelines and meeting presentation.
*While ext-age SMC has typically be considered up to 10 years of age and IPTsc has generally referred to 5–15 year olds, the current guidelines do not stipulate age restrictions and advise use of local data to determine the target population.
Key considerations for both strategies fall into four core categories: prioritization, timing, delivery point, and drug selection (Figure 1).
Figure 1
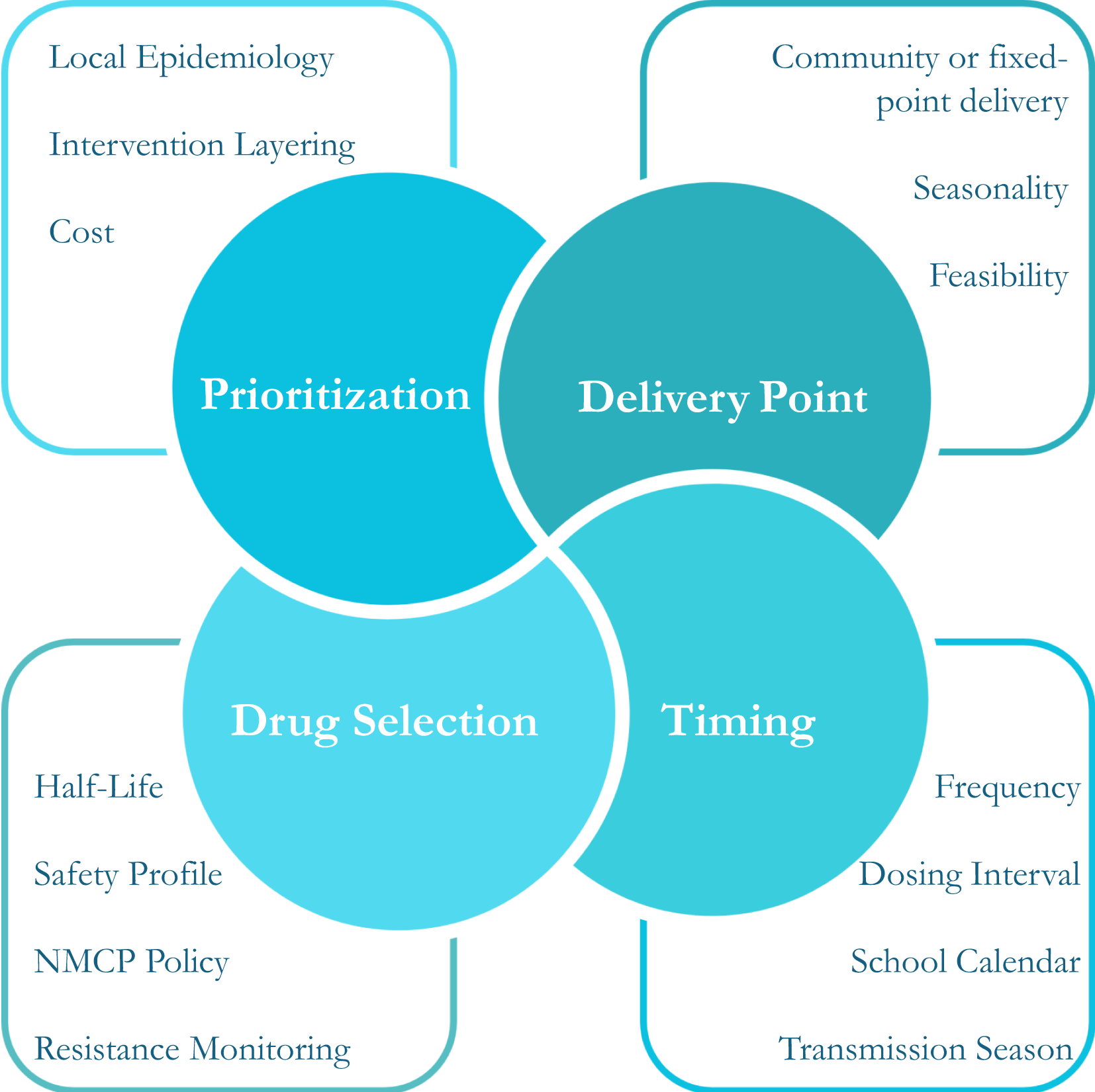
Core decision-making components for chemoprevention in school-aged children.
Local epidemiology, intervention layering, cost and budget constraints inform prioritisation of chemoprevention for school-aged children. In addition, WHO guidelines emphasize that implementation should not compromise interventions targeting younger children who are at highest risk of severe malaria. Optimal delivery strategy, including both delivery point and timing, should be guided by alignment of the school calendar and the seasonality of local malaria transmission, as well as social acceptability and operational feasibility.
For next-age SMC, guidance for drug selection, dosing schedule, and frequency is based on general SMC guidelines. There is limited data on SMC drug regimens other than sulfadoxine-pyrimethamine + amodiaquine (SPAQ), which is widely used across West Africa. Based on the half-life of SPAQ, SMC is recommended every 28 days, with current evidence supporting 3–4 cycles in areas with short transmission seasons, and up to six cycles in settings with longer transmission seasons. In contrast to SMC, no guidance on drug choice for IPTsc is provided. Over 20 years of IPTsc trials, multiple drug regimens have been evaluated, including SPAQ, SP + piperaquine, SP + artesunate, artesunate + amodiaquine and dihydroartemisinin-piperaquine (DP) (32, 33). The IPTsc dosing schedule will depend on the half-life of the drug used and should be informed by local malaria epidemiology with timing to ensure protection at the period of greatest malaria risk (34, 35). For both approaches, it is recommended that first- and second-line malaria treatments are avoided, if safe and effective alternatives are available. Special consideration should be given to treatment of girls post menarche, as data on the safety, efficacy and pharmacokinetics of most antimalarial agents in the first trimester of pregnancy is limited; and potential interactions with other drugs delivered through school-health, or other child-health programmes should be examined (36, 37). All of these considerations present a significant challenge to programs aiming to select the appropriate regimen.
Mahamoudou Touré (University of Sciences, Techniques, and Technologies of Bamako, Mali), Geofrey Makenga (National Institute for Medical Research, Tanzania; University of Antwerp, Belgium), and Lauren Cohee (LSTM, UK) presented progress towards and outcomes of recent, ongoing and planned trials in 5 countries, summarised in Table 2.
Table 2
| Country | Intervention | Study type | Study population | Drug | Frequency | Outcomes | Status and source | ||
|---|---|---|---|---|---|---|---|---|---|
| Malaria | Education | Acceptance | |||||||
| Mali | Ext-age SMC | Pilot non- inferiority randomised clinical trial | Children >3 months and <9 years | SP+AQ vs DP | 4 monthly rounds during transmission season | Yes | No | No | Complete Mali ICEMR |
| Mali | Ext-age SMC | District-wide non-inferiority randomised clinical trial | Children >3 months and <9 years | SP+AQ vs DP | 4 monthly rounds during transmission season | Yes | No | No | Complete - Mali ICEMR (protocol published (38) |
| Burkina Faso | IPTsc | Village-level cluster randomised controlled trial | Children >5 and <15 years | SP+AQ vs DP IVM vs Standard of Care | 4 monthly rounds during transmission season | Yes | No | Yes | Ongoing - MoH, MoE Malaria Consortium (39) |
| Nigeria | IPTsc | Individually randomised controlled trial | Children >5 and <12 years | SP AQ vs DP vs Standard of Care | Seasonal: SP+AQ: every 4 weeks for 4 rounds DP: every 8 weeks for 2 rounds Perennial: SP+AQ: every 4 weeks for 6 rounds DP: every 8 weeks for 4 rounds |
Yes | Yes | No | On-going- Nigeria NMEP, Fosun Pharmaceuticals. Evidence Action |
| Tanzania | IPTsc | Clinical trial | Children >5 and <15 years | DP and ASAQ | 3 Rounds, 4 months apart (once per school term) | Yes | Yes | No | Complete, published (Makenga Baraka, et al., 2023) (32) |
| Tanzania | IPTsc | Implementation study- school- level cluster randomised controlled trial | Children >5 and <15 years | DP | 3 Rounds, 4 months apart | Yes | Yes | Yes | Complete. Methods published (Makenga Seth, et al., 2023) (37) |
| Malawi | IPTsc | Individually randomized, controlled trial | Children in primary grades 1-8 | DP vs SP+CQ (females >10 years old received CQ only) vs Standard of Care | 3 rounds, every 6 weeks, during transmission season | Yes | Yes | No | Complete, unpublished Clinicaltrials.gov NCT05980156 |
| Malawi | IPTsc | Household randomized, controlled trial | Children in primary grades 1-8 | DP vs SP+AQ (females >=13 | 3 rounds, every 6 weeks, during | Yes | Yes | No | On-going Clinicaltrials.gov NCT05980156 |
Summary of trials which are planned, ongoing, or completed after the most recent systematic review of preventive treatment targeting school-aged children. (Cohee et al., 2020).
Defining barriers, knowledge gaps and research priorities
Following introductory presentations and review of recent studies, meeting attendees split into breakout groups to discuss barriers, knowledge gaps and pathways forward to enable effective implementation of chemoprevention in school-aged children. Each group then fed back to the larger group for discussion and consensus building. The main themes that arose are summarized below and in Figure 2.
Figure 2
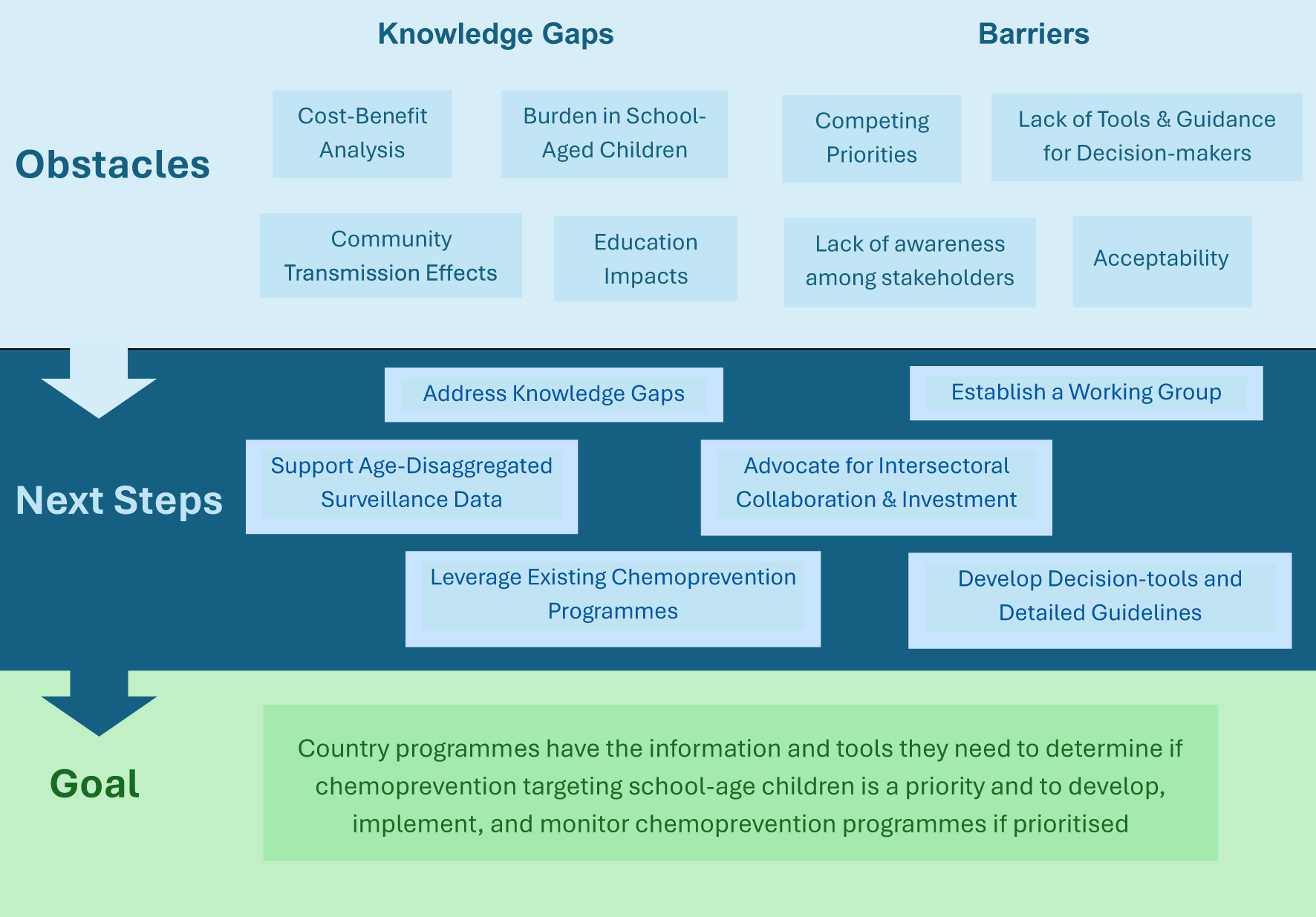
Summary of meeting findings.
Generating additional evidence of the burden of malaria in school-aged children and the direct and indirect impacts of chemoprevention
Access to more granular, age-disaggregated data is required to determine when/where countries should prioritize targeting school-aged children and for monitoring the impact of programs post-implementation
The WHO guidelines emphasise “using local data on the age distribution of malaria admissions and severe disease” to determine when chemoprevention should target older children (29). However, data on the relative burden of malaria in school-aged children is not widely available, as routine surveillance reporting on hospitalisations, severe and uncomplicated malaria is often dichotomized as “under 5 years old” or “over 5 years old”. Consistent access to disaggregated data will also be important for monitoring the impact of chemoprevention programs targeting school-aged children.
Further exploration of the indirect benefits of chemoprevention in school-aged children on education and community-level malaria transmission could reveal substantial dividends
School-aged children have been shown to be the largest infectious reservoir in many settings. The community protective effects of ext-age SMC have already been demonstrated in multiple sites in West Africa (40, 41) and dynamic transmission models suggest IPTsc would reduce clinical malaria in younger children and adults across multiple transmission settings (42). Additional studies in diverse transmission settings would further characterise the extent of transmission reduction potential. Similarly, previously demonstrated positive effects on cognitive function, learning, and educational attainment warrant further evaluation.
Common metrics across diverse study sites could clarify efficacy and strengthen evidence based for policy recommendations
There is some consensus on metrics for malaria-related individual-level outcome measures in studies evaluating impact chemoprevention in school-aged children, e.g. prevalence of infection, anaemia, and incidence of clinical malaria. No consensus exists on metrics for measuring impact on transmission or education. Harmonized metrics for these outcomes is critical in building the evidence base for these indirect, but crucial, outcomes. While these outcomes would be most rigorously evaluated in cluster randomised trials, implementation and pilot studies should not be missed as opportunities to generate evidence. Agreement on optimal study designs for these settings is needed, with input from key decision-makers and funders on outcomes of highest priority. For example, impact on cognition, learning, and education are often measured as secondary outcomes using heterogeneous metrics that limit evidence synthesis. Engaging with colleagues in the education sector to identify meaningful metrics and measuring them may be a key advocacy tool influential in garnering investment from education and broader development sectors.
Improve understanding of the cost-effectiveness of chemoprevention in school-aged children
Limited evidence on the cost-effectiveness and broader economic impacts of chemoprevention targeting school-aged children is a barrier to funding for and prioritization of IPTsc and ext-age SMC
While some costing and cost-effectiveness evidence is available from trials in Kenya and Mali, variations on drug choice, delivery mode, expected coverage and frequency of administration within each country make these data difficult to generalize (43, 44). Additionally, the potential indirect benefits for communities through transmission reduction and improved education are important factors in cost-benefit evaluations and budgetary decision making. For example, by reducing clinical incidence, chemoprevention decreases health system costs in terms of case-management costs and burden on health facilities. Chemoprevention can also better target difficult-to-reach populations, and directly observed therapy improves adherence and may reduce risk of antimalarial resistance. Improved education outcomes and reduced burden on caregivers can also increase individual earning potential, with lifelong benefits (45). It is important that decision-makers and funders consider the myriad knock-on savings benefits of chemoprevention when configuring programme budgets. Further research applying appropriate cost-evaluation methods is needed to support decision-makers to present sufficient justification to prioritise school-aged children within constrained budgets.
Combining malaria chemoprevention with other interventions targeting school-aged children could improve cost efficiency and sustainability
This could include malaria-specific approaches, such as new vector control tools and vaccine strategies in the longer-term. Integration with other established health interventions which target school-aged, such as nutrition, deworming and human papillomavirus (HPV) vaccination programs, may also increase uptake and efficiency.
Barriers and knowledge gaps for effective implementation
Intersectoral collaboration and investment are needed to ensure success in targeting school-aged children
Integration with school systems, Ministries of Education, and national and sub-national school health programs is imperative particularly when schools serve as the primary delivery channel. Similarly, for community-based delivery, integration with community health worker networks, routine adolescent health services, and surveillance systems is important for feasibility, acceptability, and uptake.
Choice of anti-malarial drugs for chemoprevention in school-aged children requires careful consideration
Drug selection for IPTsc or ext-age SMC should be in alignment with national drug policies and resistance mitigation strategies, as well as safe, affordable, and acceptable for the target population. All the identified on-going or planned implementation, pilots, and research studies discussed in the meeting will use either dihydroartemisinin-piperaquine (DP) or sulfadoxine-pyrimethamine plus amodiaquine (SPAQ). These drugs have demonstrated protective efficacy in reducing clinical incidence and parasite carriage in chemoprevention in school-aged children. Addition of single-dose primaquine for gametocytocidal action may be of further interest particular when considering application of chemoprevention targeting school-aged children as a population-level transmission reduction strategy or as an approach to mitigate the potential spread of drug resistant parasites.
In the context of the emergence of artemisinin resistance in Africa and existing wide-spread antifolate resistance, the degree of drug resistance in different contexts as well as the impact of chemoprevention as a potential driver of drug resistance should be considered and monitored. Additional consideration should be taken regarding the safety of drugs administered to adolescents who have begun menstruation and may become pregnant. However, high-risk pregnant adolescents are also a group that may benefit from interventions which clear parasites and decrease anaemia pre-pregnancy. Future drug candidates specifically for use in chemoprevention are also in development (46).
Funding structures and limited resources can hinder introduction of new interventions
Malaria control is a complex and costly endeavour, with many factors influencing the allocation of limited funds. While there is considerable interest among national malaria programs in addressing the burden of malaria in school-aged children, resource allocation remains a critical barrier as the majority of malaria funding comes from external donors, who are currently limited in the ability to meet the funding needs for interventions targeting younger children and malaria case management. Thus, further evidence is needed on the benefits and cost-effectiveness of targeting this age group among competing priorities. In the absence of additional funds, funders and national decision-makers may be limited to budget restructuring, particularly cost-shifting from treatment to prevention. Stronger age-disaggregated surveillance, quantification of community transmission reduction, and evidence of cost-savings or cost-neutrality of chemoprevention are needed to justify allocation of limited funds for school-aged children. Equally, funders must be open to act swiftly in response to growing evidence.
Decision-tools and implementation guidelines are needed to support countries as approaches to target school-aged children with chemoprevention are not ‘one-size fits all’ and require context specific design
Introducing chemoprevention in school-aged children requires consideration of several factors, including timing and frequency of drug administration according to transmission seasons and school calendars, drug choice and alignment with national treatment policies, and combination with other existing interventions (Figure 1). Data gaps in surveillance, delivery cost, projected implementation coverage and performance prevent effective decision-making. Tools and implementation guidance for ext-age SMC are/can be adapted from general guidance for SMC. However, no tools or additional guidance for implementation is currently available for IPTsc. Additional support is needed for implementation and evaluation of school-based delivery in particular.
Attendees also highlighted the vital role of community engagement and social science studies to assess, understand and adapt implementation strategies to different settings. Attention will also need to be paid to reaching out-of-school children when school-based delivery is used.
Looking forward, attendees supported the following next steps:
-
Utilise programmatic implementation, pilot studies, and, if required, clinical trials to generate knowledge in the areas outlined above (indirect benefits on transmission and education, feasibility, acceptability, and cost-benefits). Develop replicable evaluation designs with indicators that support quality impact monitoring and evidence generation to facilitate data sharing across sites.
-
Establish a Working Group to enhance knowledge exchange, amplify existing work, develop consensus on indicators and metrics, and streamline information sharing channels between researchers, funders, and key decision-makers.
-
Advocate at all levels to: communicate the burden of malaria in school-aged children to make the case for investment; enhance collaboration with the education sector, including empowering “champions” in health and education sectors with messaging on positive school outcomes; work with decision-makers to ensure malaria in school-aged children is on the agenda; and highlight the role of interventions targeting school-aged children to accelerate elimination goals.
Conclusions and outcomes
Malaria infection in school-aged children is an important but perhaps under-recognized challenge to malaria control. Program managers, policymakers and researchers in attendance agreed that when combined with accurate age-stratified data on the local burden of disease, the current evidence on the direct impacts of chemoprevention on malaria prevalence and death in school-aged children may be sufficiently compelling for implementation or programmatic consideration. Attendees generally agreed that quantifying and communicating the indirect benefits of chemoprevention on education and community transmission reduction could also heavily influence prioritization. Amidst competing priorities for malaria control, the education sector and school health specialists must play a leading role in advocating for interventions in school-aged children. Vitally, the evidence to support uptake must be compelling to funders and budget-holders for malaria control programmes. Additional tools and more detailed guidance for developing programs, supporting implementation, and evaluating programs are critical for progress. The meeting’s momentum and the report’s outlined next steps are expected to help overcome obstacles and promote broader adoption of chemoprevention in school-aged children. Although delivery of chemoprevention to school-aged children presents challenges, the potential benefits to individual children and communities are likely to be substantial.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
CMo: Writing – original draft, Writing – review & editing, Visualization. IB: Writing – original draft, Writing – review & editing, Visualization. JA: Writing – review & editing. VB: Writing – review & editing. AB: Writing – review & editing. TB: Writing – review & editing. AC: Writing – review & editing. FC: Writing – review & editing. RC: Writing – review & editing. SC: Writing – review & editing. KC: Writing – review & editing. SD: Writing – review & editing. OD: Writing – review & editing. SD: Writing – review & editing. JG: Writing – review & editing. AL: Writing – review & editing. CMa: Writing – review & editing. GM: Writing – review & editing. OM: Writing – review & editing. IM: Writing – review & editing. JN: Writing – review & editing. NO: Writing – review & editing. MP: Writing – review & editing. AS: Writing – review & editing. SS: Writing – review & editing. AT: Writing – review & editing. AT: Writing – review & editing. MT: Writing – review & editing. JV: Writing – review & editing. SV: Writing – review & editing. EW: Writing – review & editing. CD: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. LC: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. DH: Conceptualization, Writing – review & editing. JBY: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The meeting was supported by a grant from Open Philanthropy (GV673604850).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
World malaria report(2024). Available online at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (Accessed May 20, 2025).
2
Nankabirwa J Brooker SJ Clarke SE Fernando D Gitonga CW Schellenberg D et al . Malaria in school-age children in Africa: An increasingly important challenge. Trop Med Int Health. (2014) 19:1294–309. doi: 10.1111/tmi.12374
3
Cohee LM Nankabirwa JI Greenwood B Djimde A Mathanga DP . Time for malaria control in school-age children. Lancet Child Adolesc Health. (2021) 5:537–8. http://www.thelancet.com/article/S2352464221001589/fulltext (Accessed July 17, 2024).
4
Chen I Clarke SE Gosling R Hamainza B Killeen G Magill A et al . Asymptomatic” Malaria: A chronic and debilitating infection that should be treated. PLoS Med. (2016) 13:6–7. doi: 10.1371/journal.pmed.1001942
5
Daily JP Parikh S . Malaria. New Engl J Med. (2025) 392:1320–33. doi: 10.1056/NEJMra2405313
6
Institute for Health Metrics and Evaluation (IHME) . Global Burden of Disease 2021: Findings from the GBD 2021 Study. Seattle, WA: IHME (2024). Available online at: https://www.healthdata.org/research-analysis/library/global-burden-disease-2021-findings-gbd-2021-study (Accessed June 23, 2025).
7
Brooker S Guyatt H Omumbo J Shretta R Drake L Ouma J . Situation analysis of malaria in school-aged children in Kenya - what can be done? Parasitol Today. (2000) 16:183–6. doi: 10.1016/s0169-4758(00)01663-x
8
King N Dewey C Borish D . Determinants of primary school non-enrollment and absenteeism: results from a retrospective, convergent mixed methods, cohort study in rural western Kenya. PLoS One. (2015) 10:7–9. doi: 10.1371/journal.pone.0138362
9
Walldorf JA Cohee LM Coalson JE Bauleni A Nkanaunena K Kapito-Tembo A et al . School-age children are a reservoir of malaria infection in Malawi. PLoS One. (2015) 10:e0134061. doi: 10.1371/journal.pone.0134061
10
Mwandagalirwa MK Levitz L Thwai KL Parr JB Goel V Janko M et al . Individual and household characteristics of persons with Plasmodium falciparum malaria in sites with varying endemicities in Kinshasa Province, Democratic Republic of the Congo. Malar J. (2017) 16:5–6. doi: 10.1186/s12936-017-2110-7
11
Were V Buff AM Desai M Kariuki S Samuels A Ter Kuile FO et al . Socioeconomic health inequality in malaria indicators in rural western Kenya: Evidence from a household malaria survey on burden and care-seeking behaviour. Malar J. (2018) 17:1–10. doi: 10.1186/s12936-018-2319-0
12
Rek J Blanken SL Okoth J Ayo D Onyige I Musasizi E et al . Asymptomatic school-aged children are important drivers of malaria transmission in a high endemicity setting in Uganda. J Infect Dis. (2022) 226:708–13. doi: 10.1093/infdis/jiac169
13
Pinchoff J Chaponda M Shields TM Sichivula J Muleba M Mulenga M et al . Individual and household level risk factors associated with malaria in Nchelenge district, a region with perennial transmission: A serial cross-sectional study from 2012 to 2015. PLoS One. (2016) 11:7. doi: 10.1371/journal.pone.0156717
14
Pullan RL Bukirwa H Staedke SG Snow RW Brooker S . Plasmodium infection and its risk factors in eastern Uganda. Malar J. (2010) 9:1–11. doi: 10.1186/1475-2875-9-2
15
Yeka A Nankabirwa J Mpimbaza A Kigozi R Arinaitwe E Drakeley C et al . Factors associated with malaria parasitemia, anemia and serological responses in a spectrum of epidemiological settings in Uganda. PLoS One. (2015) 10:e0118901. doi: 10.1371/journal.pone.0118901
16
Touré M Sanogo D Dembele S Diawara SI Oppfeldt K Schiøler KL et al . Seasonality and shift in age-specific malaria prevalence and incidence in Binko and Carrière villages close to the lake in Selingué, Mali. Malar J. (2016) 15:1–11. doi: 10.1186/s12936-016-1251-4
17
Fernando D de Silva D Wickremasinghe R . Short-term impact of an acute attack of malaria on the cognitive performance of schoolchildren living in a malaria-endemic area of Sri Lanka. Trans R Soc Trop Med Hyg. (2003) 97:633–9. doi: 10.1016/S0035-9203(03)80093-7
18
Fernando D De Silva D Carter R Mendis KN Wickremasinghe R . A randomized, double-blind, placebo-controlled, clinical trial of the impact of malaria prevention on the educational attainment of school children. Am J Trop Med Hyg. (2006) 74:386–93. https://www.ajtmh.org/view/journals/tpmd/74/3/article-p386.xml (Accessed July 17, 2024).
19
Thuilliez J Sissoko MS Toure OB Kamate P Berthélemy JC Doumbo OK . Malaria and primary education in Mali: a longitudinal study in the village of Donéguébougou. Soc Sci Med. (2010) 71:324–34. doi: 10.1016/j.socscimed.2010.02.027
20
Nankabirwa J Wandera B Kiwanuka N Staedke SG Kamya MR Brooker SJ . Asymptomatic Plasmodium Infection and Cognition among Primary Schoolchildren in a High Malaria Transmission Setting in Uganda. Am J Trop Med Hyg. (2013) 88:1102. https://pmc.ncbi.nlm.nih.gov/articles/PMC3752809/.
21
Haldar K Mohandas N . Malaria, erythrocytic infection, and anemia. In: Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program (Washington (DC): The American Society of Hematology), vol. 87. (2009). Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC2933134/.
22
Bundy DAP de Silva N Horton S Patton GC Schultz L Jamison DT . Child and adolescent health and development: realizing neglected potential. Disease control priorities. In: Child and Adolescent Health and Development, 3rd ed. (Washington (DC): The International Bank for Reconstruction and Development / The World Bank), vol. 8. (2017). p. 1–24. Available at: https://www.ncbi.nlm.nih.gov/books/NBK525272/.
23
Ouédraogo A Tiono AB Diarra A Sanon S Yaro JB Ouedraogo E et al . Malaria morbidity in high and seasonal malaria transmission area of Burkina Faso. PLoS One. (2013) 8:e50036. doi: 10.1371/journal.pone.0050036
24
Stone W Gonçalves BP Bousema T Drakeley C . Assessing the infectious reservoir of falciparum malaria: Past and future. Trends Parasitol. (2015) 31:287–96. http://www.cell.com/article/S147149221500080X/fulltext (Accessed July 17, 2024).
25
Gonçalves BP Kapulu MC Sawa P Guelbéogo WM Tiono AB Grignard L et al . Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun. (2017) 8:1–11. https://www.nature.com/articles/s41467-017-01270-4 (Accessed July 17, 2024).
26
Coalson JE Cohee LM Buchwald AG Nyambalo A Kubale J Seydel KB et al . Simulation models predict that school-age children are responsible for most human-to-mosquito Plasmodium falciparum transmission in southern Malawi. Malar J. (2018) 17:1–12. doi: 10.1186/s12936-018-2295-4
27
Baba E Hamade P Kivumbi H Marasciulo M Maxwell K Moroso D et al . Effectiveness of seasonal malaria chemoprevention at scale in west and central Africa: an observational study. Lancet. (2020) 396:1829–40. https://www.thelancet.com/action/showFullText?pii=S0140673620322273 (Accessed May 20, 2025).
28
Cairns M Ceesay SJ Sagara I Zongo I Kessely H Gamougam K et al . Effectiveness of seasonal malaria chemoprevention (SMC) treatments when SMC is implemented at scale: Case–control studies in 5 countries. PloS Med. (2021) 18:e1003727. doi: 10.1371/journal.pmed.1003727
29
World Health Organisation . Updated WHO recommendations for malaria chemoprevention among children and pregnant women(2022). Available online at: https://www.who.int/news/item/03-06-2022-Updated-WHO-recommendations-for-malaria-chemoprevention-among-children-and-pregnant-women (Accessed May 20, 2025).
30
World Health Organization . Global technical strategy for malaria 2016–2030–2021 update. Geneva: World Health, Organization (2021). Licence: CC BY-NC-SA 3.0 IGO. Global Technical Strategy For Malaria 2016-2030, 2021 update. 2016.
31
WHO Malaria Policy Advisory Group: meeting report. (2024) 1–3 (n.d.). Available online at: https://www.who.int/publications/i/item/9789240105300 (Accessed June 24, 2025).
32
Makenga G Baraka V Francis F Nakato S Gesase S Mtove G et al . Effectiveness and safety of intermittent preventive treatment with dihydroartemisinin–piperaquine or artesunate–amodiaquine for reducing malaria and related morbidities in schoolchildren in Tanzania: a randomised controlled trial. Lancet Glob Health. (2023) 11:e1277–89. http://www.thelancet.com/article/S2214109X23002048/fulltext (Accessed July 17, 2024).
33
Cohee LM Opondo C Clarke SE Halliday KE Cano J Shipper AG et al . Preventive malaria treatment among school-aged children in sub-Saharan Africa: a systematic review and meta-analyses. Lancet Glob Health. (2020) 8:e1499–511. doi: 10.1016/S2214-109X(20)30325-9
34
Braunack-Mayer L Malinga J Masserey T Nekkab N Sen S Schellenberg D et al . Design and selection of drug properties to increase the public health impact of next-generation seasonal malaria chemoprevention: a modelling study. Lancet Glob Health. (2024) 12:e478–90. https://www.thelancet.com/action/showFullText?pii=S2214109X23005508 (Accessed May 21, 2025).
35
Zongo I Compaoré YD . Seasonal malaria chemoprevention: drug design and selection. Lancet Glob Health. (2024) 12:e358–9. https://www.thelancet.com/action/showFullText?pii=S2214109X24000111 (Accessed May 20, 2025).
36
Gatiba P Laury J Steinhardt L Hwang J Thwing JI Zulliger R et al . Contextual factors to improve implementation of malaria chemoprevention in children: A systematic review. Am J Trop Med Hyg. (2023) 110:69. https://pmc.ncbi.nlm.nih.gov/articles/PMC10793032/.
37
Makenga G Seth MD Baraka V Mmbando BP Challe DP Francis F et al . Implementation research of a cluster randomized trial evaluating the implementation and effectiveness of intermittent preventive treatment for malaria using dihydroartemisinin-piperaquine on reducing malaria burden in school-aged children in Tanzania: methodology, challenges, and mitigation. Malar J. (2023) 22. doi: 10.1186/s12936-022-04428-8
38
Toure M Shaffer JG Sanogo D Keita S Keita M Kane F et al . Seasonal Malaria Chemoprevention Therapy in Children Up To 9 Years of Age: Protocol for a Cluster-Randomized Trial Study. JMIR Res Protoc. (2024) 13:e51660. doi: 10.2196/51660
39
Malaria Consortium . (2023). Evaluating intermittent preventive treatment of malaria in school-aged children in Burkina Faso. Available online at: https://www.malariaconsortium.org/media-download-file/202311101150/iptscburkinafaso.pdf.
40
Cissé B Ba EH Sokhna C NDiaye JL Gomis JF Dial Y et al . Effectiveness of seasonal malaria chemoprevention in children under ten years of age in Senegal: A stepped-wedge cluster-randomised trial. PLoS Med. (2016) 13:15. doi: 10.1371/journal.pmed.1002175
41
Soremekun S Conteh B Nyassi A Soumare HM Etoketim B Ndiath MO et al . Household-level effects of seasonal malaria chemoprevention in the Gambia. . Commun Med. (2024) 4:1–11. https://www.nature.com/articles/s43856-024-00503-0 (Accessed May 20, 2025).
42
Suresh JG Zimmermann M Maiteki C Watkins AE Pratt A Mathanga DP et al . Thriving students, thriving communities: modeling the impact and cost-effectiveness of malaria chemoprevention for school-age children across transmission archetypes. Available online at: https://papers.ssrn.com/abstract=4526538 (Accessed May 21, 2025).
43
Temperley M Mueller DH Njagi JK Akhwale W Clarke SE Jukes MCH et al . Costs and cost-effectiveness of delivering intermittent preventive treatment through schools in western Kenya. Malar J. (2008) 7:1–9. doi: 10.1186/1475-2875-7-196
44
Maccario R Rouhani S Drake T Nagy A Bamadio M Diarra S et al . Cost analysis of a school-based comprehensive malaria program in primary schools in Sikasso region, Mali. BMC Public Health. (2017) 17:5–6. doi: 10.1186/s12889-017-4490-6
45
Hamory J Miguel E Walker M Kremer M Baird S . Twenty-year economic impacts of deworming. Proc Natl Acad Sci U S A. (2021) 118. doi: 10.1073/pnas.2023185118
46
Medicines for Malaria Ventures: Rethinking chemoprevention. Available online at: https://www.mmv.org/research-development/rethinking-chemoprevention (Accessed May 20, 2025).
Summary
Keywords
malaria, school - aged children, transmission, education, chemoprevention, intermittent preventive treatment
Citation
Morlino C, Byrne I, Achan J, Baraka V, Barry A, Bousema T, Camara A, Chacky F, Chico RM, Clarke SE, Collins KA, Dagnon SJ-F, Diallo O, Doumbia S, Gerardin J, Hein D, Lusasi AS, Maiteki-Sebuguzi C, Makenga G, Mokuolu OA, Mwenyango I, Nabakooza J, Ogbulafor N, Penny MA, Sadou A, Staedke SG, Tchouatieu AM, Tiono AB, Toure M, Van geertruyden J-P, Van Hulle S, Worrall E, Yaro J-BB, Drakeley C and Cohee LM (2025) Barriers to uptake and implementation of malaria chemoprevention in school-aged children: a stakeholder engagement meeting report. Front. Trop. Dis. 6:1480907. doi: 10.3389/fitd.2025.1480907
Received
19 February 2025
Accepted
09 June 2025
Published
08 September 2025
Volume
6 - 2025
Edited by
Lesley Drake, Imperial College London, United Kingdom
Reviewed by
Ingrid Chen, University of California, San Francisco, United States
Issaka Zongo, Research Institute for Health Sciences (IRSS), Burkina Faso
Updates
Copyright
© 2025 Morlino, Byrne, Achan, Baraka, Barry, Bousema, Camara, Chacky, Chico, Clarke, Collins, Dagnon, Diallo, Doumbia, Gerardin, Hein, Lusasi, Maiteki-Sebuguzi, Makenga, Mokuolu, Mwenyango, Nabakooza, Ogbulafor, Penny, Sadou, Staedke, Tchouatieu, Tiono, Toure, Van geertruyden, Van Hulle, Worrall, Yaro, Drakeley and Cohee.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lauren M. Cohee, lauren.cohee@lstmed.ac.uk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.