- 1Department of Pharmacology, Clinical Pharmacy, and Pharmacy Practice, University of Nairobi, Nairobi, Kenya
- 2Department of Medical Services, Public Health and Sanitation, County Government of Kisumu, Kisumu, Kenya
- 3Department of Pharmacy, Kenyatta National Hospital, Nairobi, Kenya
- 4Department of Research and Training, Jaramogi Oginga Odinga Teaching and Referral Hospital, Kisumu, Kenya
- 5Department of Pharmacology, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
Background: Little is known about the prescription and antibiotic resistance patterns at Kenyatta National Hospital (KNH)’ s critical care units (CCUs). The present study aimed to evaluate these patterns at selected CCUs at KNH.
Methods: This was a descriptive, retrospective cross-sectional study of selected CCUs at KNH between January and December 2017. Data on prescription and antibiotic resistance patterns were abstracted from the medical records of patients ≥13 years old admitted at selected CCUs at KNH during the study period.
Results: 309 patients with a mean age of 40.6 ±17.5 years were recruited; trauma was the leading cause of admission (72/309, 23.53%), and most were male (n=158, 51.1%). Antibiotic therapy was initiated before CCU admission in 304/309 (98.4%) of the patients, documentation of antibiotic indications was low for both empirical (25%) and targeted therapy (41.6%), and ceftriaxone (36.8%), metronidazole (16.9%), and meropenem (12.4%) were predominantly prescribed. Pre-therapy cultures were obtained in 51.1% of cases, with 42.7% yielding positive results and Klebsiella pneumonia (23.9%), Acinetobacter baumanii (16.4%), and Escherichia coli (10.5%) predominating. Furthermore, 67% (n=11) of K. pneumonia isolates were sensitive to meropenem, 82% (n=9) of Acinetobacter baumanii isolates were sensitive to amikacin, 55% (n=6) to meropenem and 27% (n=3) were sensitive to ceftazidime and cefepime. All the A. baumanii isolates were resistant to tigecycline, linezolid, and teicoplanin. Most (86%, n=6) of the E. coli isolates were sensitive to meropenem, 71% (n=5) were sensitive to amikacin, and 43% (n=3) were sensitive to gentamicin.
Conclusions: The high rates of pre-CCU antibiotic initiation, low documentation of therapeutic indications, and widespread resistance to commonly used antibiotics at the Kenyatta National Hospital highlight the urgent need for improved antimicrobial stewardship programs. Moreover, the predominance of multi-drug resistant organisms, particularly K. pneumonia and A. baumanii, and their variable sensitivity patterns to reserve antibiotics like meropenem suggests there is need for regular surveillance and update of antibiotic guidelines.
Background
Antimicrobial resistance (AMR) is an existential danger to patients in all intensive care units (ICUs) worldwide (1). This is according to a position statement by the European Society of Intensive Care Medicine (ESICM), the European Society of Microbiology and Infectious Diseases (ESMID), and the World Alliance Against Antimicrobial Resistance (WAAAR) (1). Patients in these units are at high risk of acquiring AMR bugs because of the intensity of antibiotic treatment and the scope of the invasive procedures (1).
The impact of healthcare-associated infections (HCAIs) in resource-limited settings is severe due to an acute human capital shortage and a high burden of community-acquired infections (2). Bunduki and colleagues reported that the prevalence of HCAIs in Africa varied between 1.6 and 90.2%, with a median of 15% across 92 studies (3). Moreover, they observed that contaminated wounds, extended hospital stays, urinary and venous catheters, and intubation and ventilation were some risk factors associated with HCAIs in Africa (3). Furthermore, of the 6,463 isolates covered by the study, 18.3% were Escherichia coli, 17.3% were Staphylococcus aureus, 17.2% were Klebsiella spp, 10.3% were Pseudomonas spp, and 6.8% were Acinetobacter spp (3). The antibiotic resistance pattern was such that 70.3% of Enterobacterales were resistant to 3rd generation cephalosporins, 70.5% of S. aureus were methicillin-resistant and 55% of the Pseudomonas spp were resistant to all the agents tested (3). A study on the surveillance of respiratory HCAIs among inpatients at three Kenyan hospitals revealed that of the 379 respiratory HCAIs, 60.7% were men, 57.3% were children <18 years old, and the overall incidence was 9.2 per 10,000 patient days (4). In addition, the highest incidence was in the ICUs (4).
A study by Maina et al. in 2023 observed that the prevalence of gram-negative infection at the Nairobi West Hospital was 55.6%, urinary tract infections predominated, and that E. coli and K. pneumonia were the most common isolates recovered at the hospital (5). Additionally, the authors observed that the use of a nasogastric tube and a history of cardiovascular/respiratory disease was significantly associated with gram-negative infections and that 92% of all isolates were multi-drug resistant (5).
The irrational use of antibiotics and increasing AMR trends are significant challenges to patient safety and the general management of critically ill patients in ICUs (6, 7). A retrospective review of 220 admissions at the CCUs of KNH over 2 years reported an 18.5% prevalence rate of rational antibiotic use, 51% inappropriate antibiotic use, and 32.3% incorrect duration of treatment (7). Another study by Momanyi and colleagues at the same hospital reported that antibiotic use was 54.7%, with the highest use at the intensive care units and the isolation wards (8). Furthermore, the study reported that penicillins (46.7%) and cephalosporins (44.7%) were the most prescribed antibiotic classes and were primarily used for treatment and surgical prophylaxis (8).
Kenyatta National Hospital (KNH) is a 2000-bed National Teaching and Referral Hospital in Nairobi, Kenya (9). It is the largest referral hospital in the country and attends to about 60,000 inpatients and 450,000 outpatients annually (9). It serves as a teaching hospital for the University of Nairobi’s Faculty of Health Sciences and the Kenya Medical Training College (KMTC) (9). Healthcare services are provided across its 50 wards, 22 outpatient clinics, accident & emergency departments, and 24 theatres, 16 of which conduct specialized surgeries. Additionally, KNH has 56 critical care unit (CCU) beds, the highest number in Kenya (9). Given the significant challenges of AMR in critical care settings, there is an urgent need for local data to understand the prescription and antibiotic resistance patterns at the CCUs in KNH. The present study aimed to evaluate the prescription and antibiotic resistance patterns at three CCUs at KNH.
Methods
Study setting, sample size, and design
This was a descriptive, retrospective cross-sectional study at the satellite CCU of the Department of Medicine, the main CCU, and the Accident and Emergency Department CCU at KNH. Fisher’s formula (5% error) was used to determine the sample size for medical records which met the inclusion Criteria i.e. >13 years of age, admitted to the selected CCUs as from 1st January to 31st December 2017 and received an antibiotic prescription during their hospital stay. The formula below was used:
Where: N=Estimated sample size, P=Estimated proportion of outcome of interest (Assumed level of compliance of 77% based on a global point prevalence survey conducted in 53 countries by Versporten and colleagues (10), S=Standard error (desired level of precision, permissible error, set at 5%), Z=Z-score value corresponding to 95% confidence interval, which is 1.96. N= 1.962 {0.77(1-0.78)}/0.052. Using this formula, the calculated sample size was found to be 264. This number was inflated by 10% to account for any files with missing information, and the calculated sample size was 290. Sociodemographic and clinical data was then extracted from these files using a pre-designed tool.
Data analysis
Data was analyzed using descriptive statistics on Statistical Package for the Social Sciences (Version 20).
Ethical considerations
This work was reviewed and approved by the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (KNH-UoN ERC) P692/11/2017.
Results
309 patients were recruited during the study period. Most patients were male (158/309, 51.1%), 31 to 40 years old (78/309, 25.24%), had a mean age of 40.6 ± 17.5 years, were mainly from the inpatient wards (129/309, 41.75%), and had primarily suffered from trauma (72/309, 23.53%) (Table 1).

Table 1. Sociodemographic information of patients who presented to selected critical care units of the Kenyatta National Hospital during the study period.
Figure 1 is a summary of the number of antimicrobial agents that were prescribed for the patients at the selected CCU’s during the study period.
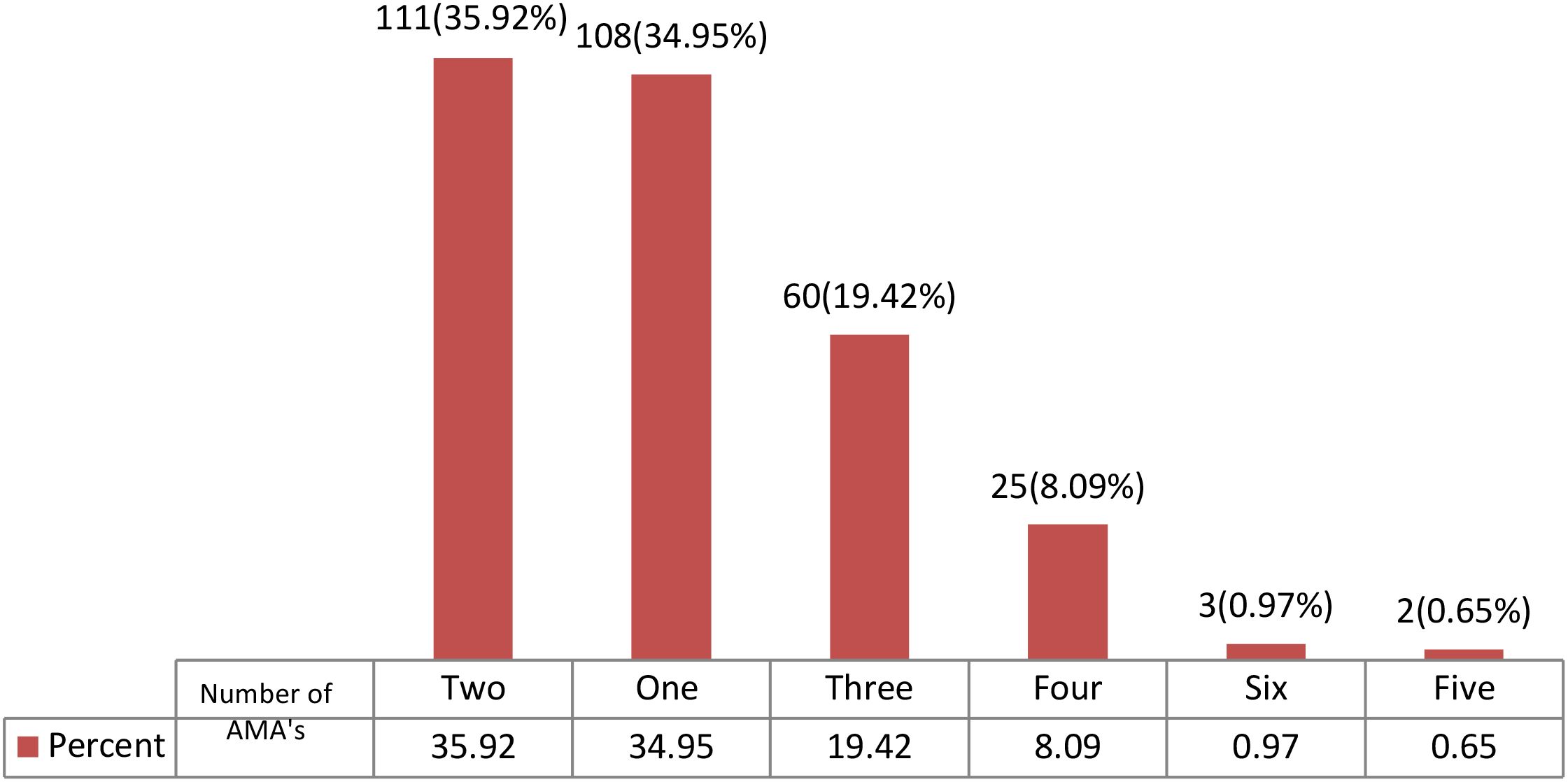
Figure 1. Summary of the number of antimicrobial agents that were prescribed at the critical care unit of the Kenyatta National Hospital during the study period. AMA, Antimicrobial agents.
Most patients were on either two antimicrobial agents (111/309, 35.92%) or one antimicrobial agent (108/3019, 34.95%). There were 2 patients who received a prescription with five antibiotics (Figure 1).
Figure 2 shows the classes of antimicrobial agents which were prescribed for patients at the critical care unit of Kenyatta National Hospital during the study period.
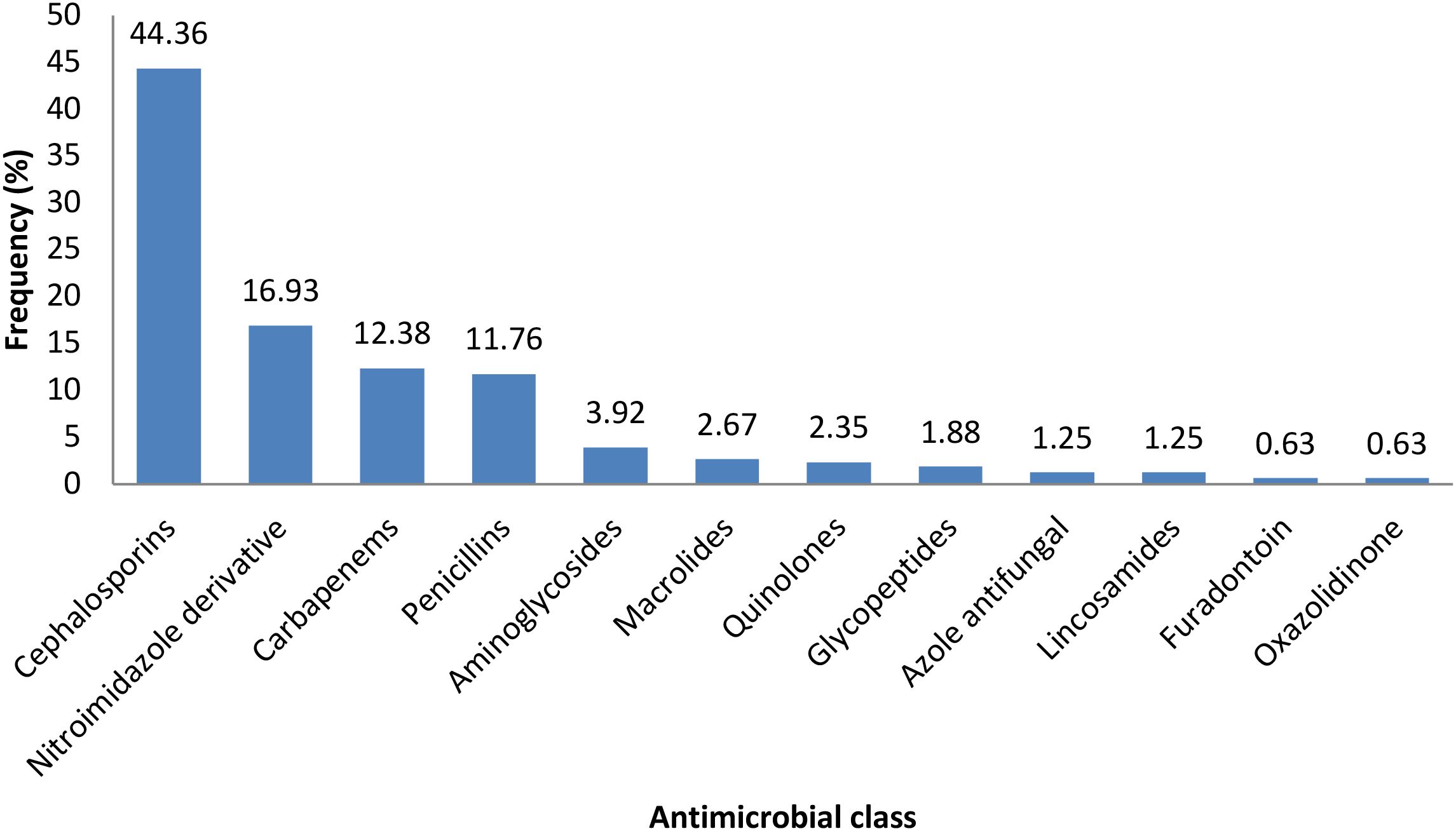
Figure 2. The classes of antimicrobial agents which were prescribed for patients at the critical care unit of Kenyatta National Hospital during the study period.
The most common antibiotic classes were Cephalosporins (137/309, 44.36%), Nitroimidazole derivatives (52/309, 16.93%), and Carbapenems (38/309, 12.38%). Lincosamides. Furadantoins, and oxazolidinediones were the least prescribed antibiotics (Figure 2).
Figure 3 shows the pathogens isolated from samples collected from patients at the critical care unit of the Kenyatta National Hospital during the study period.
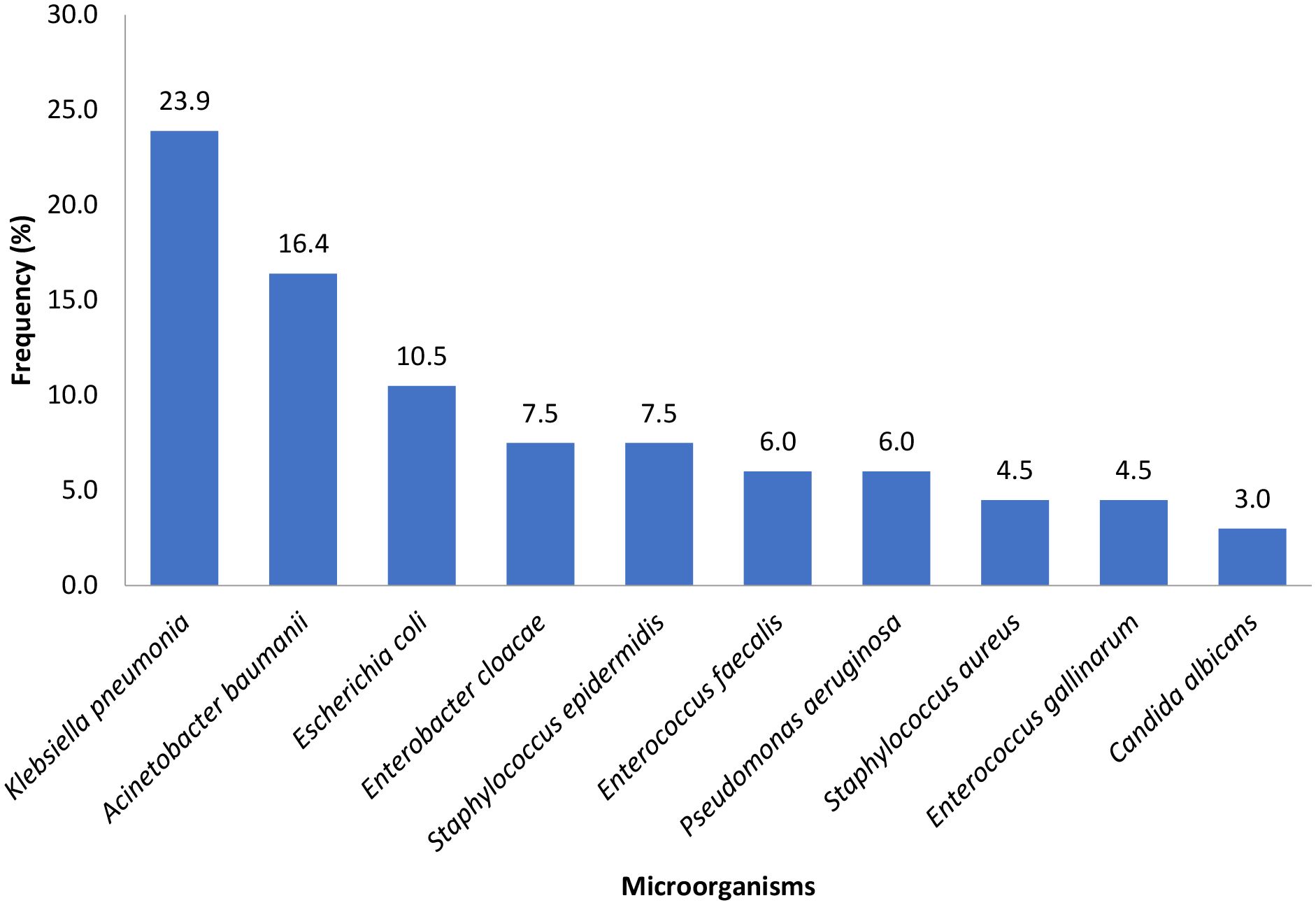
Figure 3. Pathogens isolated from samples collected from patients at the critical care unit of the Kenyatta National Hospital during the study period.
Microorganisms were isolated in 67/158 (42.7%) of the first cultures requested during the CCU stay. The most commonly isolated microorganism were Klebsiella pneumonia (23.9%), followed by Acinetobacter baumanii (16.4%) and Escherichia coli (10.5%) (Figure 3).
Table 2 describes the pattern of antimicrobial resistance of microorganisms recovered from CCU patients at KNH.
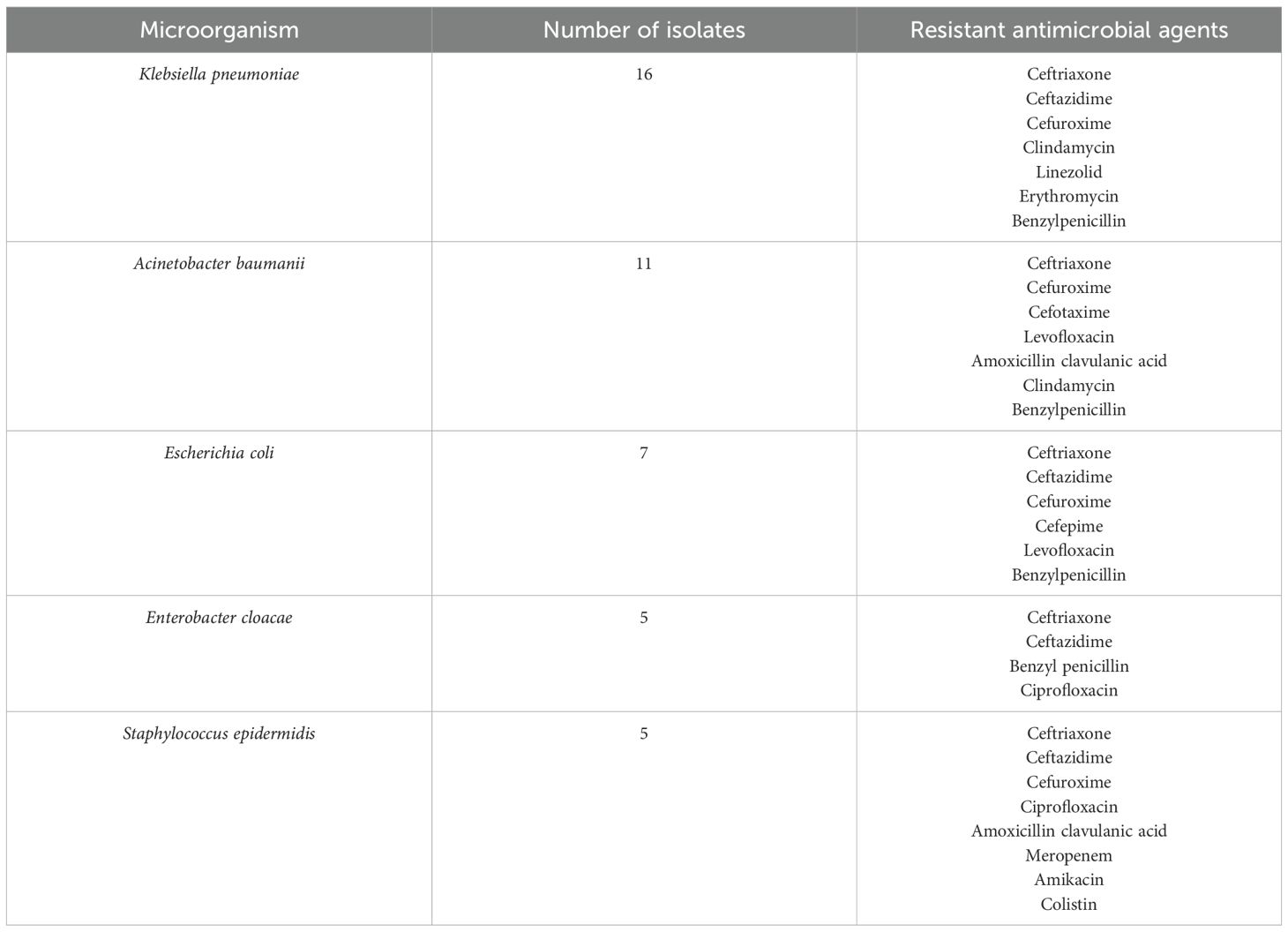
Most of the Klebsiella pneumonia isolates 11 (67%) were sensitive to meropenem, 9(56%) isolates were sensitive to amikacin and 7 (44%) isolates were sensitive to ciprofloxacin. Of the Acinetobacter baumanii isolated, 9 (82%) were sensitive to amikacin, while 6 (55%) were sensitive to meropenem. Only 3 (27%) isolates were sensitive to ceftazidime and cefepime, respectively (Table 2). Moreover, most of the Escherichia coli isolates were sensitive to meropenem 6 (86%), amikacin 5 (71%), and 3 (43%) to gentamycin. None of the E. coli isolates were sensitive to ceftazidime, cefepime, ceftriaxone, cefuroxime, and levofloxacin, among other antimicrobial agents tested against (Table 2).
Table 3 shows the indications for antimicrobial use and adherence to CCU guidelines at the critical care unit at Kenyatta National Hospital during the study period.

Table 3. Indications for the use of antimicrobial agents among the patients admitted at the critical care unit of the Kenyatta National Hospital during the study period.
The indication for antimicrobial prescribing was documented in only 66 (21.4%) of the patients sampled. Most of the prescribing encounters were by generic name 434 (66.7%) with metronidazole 96 (85%) and amoxicillin-clavulanic acid 58 (98%) being the antimicrobial agents that were most prescribed using the brand name.
Discussion
The present study evaluated the prescription and antibiotic resistance patterns at selected critical care units at the Kenyatta National Hospital in Kenya. The most concerning finding was the high prevalence of resistant microorganisms, such as Klebsiella pneumonia and Acinetobacter baumanii, coupled with widespread cephalosporin resistance. These observations mirror other studies in Africa (11–15). A 5-year retrospective review of bloodstream infections and AMR patterns in a CCU of a hospital in South Africa reported that S. aureus (28%), Klebsiella spp (22%), and Acinetobacter spp (18%) were the most common isolates, 59% of K. pneumoniae were extended spectrum β-lactamase (ESBL) producers, and Acinetobacter baumanni isolates showed low susceptibility to aminoglycosides, carbapenems, and cephalosporins (12). Another study in the CCUs of three hospitals in Ethiopia reported that Acinetobacter baumanni was not only the most dominant isolate but that the isolates were resistant to ceftazidime (100%), ceftriaxone (100%) and trimethoprim-sulfamethoxazole (14). Furthermore, Odiase and colleagues reported that Enterobacter sakazakii, K. pneumoniae, and E. coli were the most common isolates at the CCU of a Nigerian hospital. Additionally, most isolates in the study were multi-drug resistant, with the highest resistance observed against cotimoxazole, cefuroxime, and ceftazidime (13).
The high rate of prior antimicrobial exposure and pre-CCU antimicrobial initiation suggest intense selection pressure for antibiotics, a phenomenon well covered in previous studies in critical care settings (16, 17). Equally concerning are the gaps observed in practices around antimicrobial stewardship at the Kenyatta National Hospital. The finding that only 21.4% of prescriptions had documented indications and a paltry 11.7% had documented antimicrobial review represents a significant breakdown in antimicrobial oversight. Similar challenges have been reported across sub-Saharan Africa by Porter, Otieno, Godman, Elton, Wangai, Fuller, Moyo, and their colleagues (18–24), though the rates in this study are much lower than those reported in comparable settings in Nigeria and Ghana (25).
The poor compliance with CCU guidelines for both empirical (25%) and targeted therapy (41.6%) aligns with findings from other parts of Africa and other resource-limited settings (26, 27), highlighting how difficult it is to translate policy to practice. The relatively young patient population (mean age of 40.6 years) and predominance of trauma cases (23.53%) mirror patterns seen in other African CCUs e.g., at the Muhimbili National Hospital, Kilimanjaro Christian Medical Center, Mbeya Referral Hospital, and Bugando Medical Center in Tanzania where the median age of ICU admitted patients was 34 years and 22% were patients who had suffered trauma (28). This observation is important as far as the tailoring of stewardship programs to local needs is concerned.
The culture collection rate of 51.1% was similar to the 51.4% that was reported among patients with catheter-associated urinary tract infections at selected tertiary hospitals in Addis Ababa, Ethiopia (14) but was lower than the rate in Romania, a high-income country (29). A study on the opportunities to enhance diagnostic testing and antimicrobial stewardship in six countries observed that the greatest challenges in diagnostic test utilization were economic in nature (30). Additionally, Gebretekle and colleagues investigated the opportunities and barriers to implementing antibiotic stewardship in a tertiary care hospital in Ethiopia and reported that poor communication between the laboratory, pharmacists, and clinicians and the existing hierarchical culture and academic setting were key barriers in culture testing or lab diagnostics (31). Groumoutis reported on some of the policy gaps in AMS mitigation in CCU’s which included lack of guidelines on de-escalation, poor assessment of ICU nurses, poor staffing norms on infectious disease and antibiotic use experts, and lack of AMS and ICU integration (32). The authors suggested that checklists, algorithms, education of clinicians on infectious diseases and antimicrobial use, antibiotic review, and prompt re-assessment of patients may be strategies that are useful in addressing policy gaps (32).
How can AMS be implemented in CCUs to mitigate AMR? Kaki et al. (33) conducted a systematic review to evaluate the current state of evidence for AMS interventions in the critical care unit (33). They reported that antibiotic restriction/pre-approval, formal consultation with infectious disease specialists, implementation of guidelines and protocols for antibiotic de-escalation, guidelines for antibiotic prophylaxis or treatment in intensive care, formal re-assessment of antibiotics on a pre-specified day of therapy, and implementation of computer-assisted decision support were associated with reducing antimicrobial utilization, shortening average duration of antibiotic therapy, minimizing inappropriate use, and limiting adverse events associated with antibiotics (33). Moreover, stewardship interventions beyond 6 months were associated with reduction in antimicrobial resistance rates (33). Another study by Ture and colleagues reported that establishing an AMS team, limiting the use of broad-spectrum antimicrobials, terminating antimicrobial treatments early, using early warning systems, pursuing infection control, and providing education and feedback are good AMS interventions to mitigate AMR in CCUs (34).
Kpokiri, Michie, and colleagues described a validated behavior change wheel framework which incorporates policies, interventions, and recommendations aimed at guiding the development of antimicrobial stewardship programmes in low-and middle-income countries (35, 36). According to these authors, antimicrobial stewardship policies encompassed those related to communication, marketing, guidelines, regulation, legislation, environmental/social planning, service provision, and fiscal management (35, 36). Some of the interventions proposed included education, persuasion, training, modeling enablement, restriction, coercion and environmental restrictions (35, 36). Moreover, the recommendations provided by the authors as a means of improving antimicrobial stewardship in low-and-middle income countries included increasing awareness of the issues and rational prescribing, use of fliers and posters, online and face-to-face methods, education and training via short courses, workshops, use of local data to educate the prescribers, highlight best practice and address problems (35, 36). Others include the provision of up-to-date guidelines and treatment protocols, enabling models of best practice, and strict protocols to restrict access to reserved products, including restricted pharmacy dispensing (35, 36). Additionally, improvements to lab facilities, more exhaustive coverage of health insurance and regular auditing of practice to identify and address problems were also recommended (35, 36).
What are some of the limitations of this study? First, this research examined only three CCUs over a one-year period, with a sample size of only 309 patients. This approach may not fully capture the variability across different units or healthcare settings in Kenya. Second, the focus on patients aged ≥13 years excludes pediatric populations, where resistance patterns may differ. In addition, the study was not able to correlate the resistance patterns with clinical outcomes including mortality, length of stay, or treatment failure. We are cognizant of the fact that understanding the impact of resistance on patient prognosis would strengthen the argument for implementing stewardship programs.
Conclusions
The high rates of pre-CCU antibiotic initiation, low documentation of therapeutic indications, and widespread resistance to commonly used antibiotics at the Kenyatta National Hospital highlight the urgent need for improved antimicrobial stewardship programs. The predominance of multi-drug-resistant organisms, particularly K. pneumonia and A. baumanii, and their variable sensitivity patterns to reserve antibiotics like meropenem, suggest the need for regular surveillance and guideline updates. Future work may be needed to assess the knowledge, attitudes, and practices of prescribers at the critical care units at KNH and the wards in order to provide information on the gaps that need to be addressed as part of antimicrobial stewardship.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (KNH-UoN ERC) P692/11/2017. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
EO: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. MOl: Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. SO: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DA: Data curation, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. FO: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MOk: Data curation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the administration of the Kenyatta National Hospital for granting them access to patient files to collect the information. They also express special appreciation to the Kenyatta National Hospital Research and programs for funding this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. De Waele JJ, Akova M, Antonelli M, Canton R, Carlet J, De Backer D, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round tavle on multi-drug resistance. Intensive Care Med. (2018) 44:189–96. doi: 10.1007/S00134-017-5036-1/FIGURXES/3
2. Rothe C, Schlaich C, and Thompson S. Healthcare-associated infections in sub-Saharan Africa. J Hosp Infection. (2013) 85:257–67. doi: 10.1016/J.JHIN.2013.09.008
3. Bunduki GK, Masoamphambe E, Fox T, Musaya J, Musicha P, and Feasey N. Prevalence, risk factors, and antimicrobial resistance of endemic healthcare-associated infections in Africa: a systematic review and meta-analysis. BMC Infect Dis. (2024) 24:1–18. doi: 10.1186/S12879-024-09038-0
4. Ndegwa LK, Katz MA, McCormick K, Nganga Z, Mungai A, Emukule G, et al. Surveillance for respiratory health care–associated infections among inpatients in 3 Kenyan hospitals 2010-2012. Am J Infect Control. (2014) 42:985–90. doi: 10.1016/J.AJIC.2014.05.022
5. Maina JW, Onyambu FG, Kibet PS, and Musyoki AM. Multidrug-resistant Gram-negative bacterial infections and associated factors in a Kenyan intensive care unit: a cross-sectional study. Ann Clin Microbiol Antimicrob. (2023) 22:1–11. doi: 10.1186/S12941-023-00636-5/TAVLES/4
6. Zhang Y-Z and Singh S. Antibiotic stewardship programmes in intensive care units: Why, how, and where are they leading us. World J Crit Care Med. (2015) 4:13. doi: 10.5492/WJCCM.V4.I1.13
7. Murila BL, Nyamu DG, Kinuthia RN, and Njogu PM. Rational use of antibiotics and covariates of clinical outcomes in patients admitted to intensive care units of a tertiary hospital in Kenya. Hosp Pract. (2022) 50:151–8. doi: 10.1080/21548331.2022.2054632
8. Momanyi L, Opanga S, Nyamu D, Oluka M, Kurdi A, and Godman B. Antibiotic prescribing patterns at a leading referral hospital in Kenya: a point prevalence survey. J Res Pharm Pract. (2019) 8:149. doi: 10.4103/jrpp.JRPP_18_68
9. Our History – Kenyatta National Hospital. Available online at: https://knh.or.ke/index.php/history/ (Accessed July 22, 2025).
10. Versporten A, Zarb P, Caniaux I, Gros MF, Drapier N, Miller M, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. (2018) 6:e619–29. doi: 10.1016/S2214-109X(18)30186-4
11. Alabi AS, Frielinghaus L, Kaba H, Kösters K, Huson MAM, Kahl BC, et al. Retrospective analysis of antimicrobial resistance and bacterial spectrum of infection in Gabon, Central Africa. BMC Infect Dis. (2013) 13:1–6. doi: 10.1186/1471-2334-13-455
12. Morkel G, Bekker A, Marais BJ, Kirsten G, Van Wyk J, and Dramowski A. Bloodstream infections and antimicrobial resistance patterns in a South African neonatal intensive care unit. Paediatr Int Child Health. (2014) 34:108–14. doi: 10.1179/2046905513Y.0000000082
13. Odiase F and Lofor P. Pathogens and antimicrobial resistance amongst stroke patients in the intensive care unit: A five years review from Benin City, Nigeria. Ann Clin Biomed Res. (2021) 2:53–8. doi: 10.4081/acbr.2021.162
14. Bizuayehu H, Bitew A, Abdeta A, and Ebrahim S. Catheter-associated urinary tract infections in adult intensive care units at a selected tertiary hospital, Addis Ababa, Ethiopia. PloS One. (2022) 17:e0265102. doi: 10.1371/JOURNAL.PONE.0265102
15. Thomas R, Ondongo-Ezhet C, Motsoaledi N, Sharland M, Clements M, and Velaphi S. Incidence, pathogens and antimicrobial resistance of blood and cerebrospinal fluid isolates from a tertiary neonatal unit in South Africa: A 10 year retrospective review. PloS One. (2024) 19:e0297371. doi: 10.1371/journal.pone.0297371
16. Hui C, Lin MC, Jao MS, Liu TC, and Wu RG. Previous antibiotic exposure and evolution of antibiotic resistance in mechanically ventilated patients with nosocomial infections. J Crit Care. (2013) 28:728–34. doi: 10.1016/J.JCRC.2013.04.008
17. Vincent JL, Sakr Y, Singer M, Martin-Loeches I, MacHado FR, Marshall JC, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. (2020) 323:1478–87. doi: 10.1001/JAMA.2020.2717
18. Wangai FK, Masika MM, Lule GN, Karari EM, Maritim MC, Jaoko WG, et al. Bridging antimicrobial resistance knowledge gaps: The East African perspective on a global problem. PloS One. (2019) 14:e0212131. doi: 10.1371/JOURNAL.PONE.0212131
19. Elton L, Thomason MJ, Tembo J, Velavan TP, Pallerla SR, Arruda LB, et al. Antimicrobial resistance preparedness in sub-Saharan African countries. Antimicrob Resist Infect Control. (2020) 9:1–11. doi: 10.1186/S13756-020-00800-Y/TAVLES/6
20. Porter GJ, Owens S, and Breckons M. A systematic review of qualitative literature on antimicrobial stewardship in Sub-Saharan Africa. Glob Health Res Policy. (2021) 6:1–11. doi: 10.1186/S41256-021-00216-0/TAVLES/2
21. Fuller WL, Aboderin AO, Yahaya A, Adeyemo AT, Gahimbare L, Kapona O, et al. Gaps in the implementation of national core elements for sustainable antimicrobial use in the WHO-African region. Front Antibiotics. (2022) 1:1047565/BIBTEX. doi: 10.3389/FRABI.2022.1047565/BIBTEX
22. Godman B, Egwuenu A, Wesangula E, Schellack N, Kalungia AC, Tiroyakgosi C, et al. Tackling antimicrobial resistance across sub-Saharan Africa: current challenges and implications for the future. Expert Opin Drug Saf. (2022) 21:1089–111. doi: 10.1080/14740338.2022.2106368
23. Otieno PA, Campbell S, Maley S, Obinju Arunga T, and Otieno Okumu M. A systematic review of pharmacist-led antimicrobial stewardship programs in sub-saharan africa. Int J Clin Pract. (2022) 2022:3639943. doi: 10.1155/2022/3639943
24. Moyo P, Moyo E, Mangoya D, Mhango M, Mashe T, Imran M, et al. Prevention of antimicrobial resistance in sub-Saharan Africa: What has worked? What still needs to be done? J Infect Public Health. (2023) 16:632–9. doi: 10.1016/J.JIPH.2023.02.020
25. Abubakar U and Salman M. Antibiotic use among hospitalized patients in africa: A systematic review of point prevalence studies. J Racial Ethn Health Disparities. (2024) 11:1308–29. doi: 10.1007/S40615-023-01610-9/TAVLES/3
26. Charani E, Smith I, Skodvin B, Perozziello A, Lucet JC, Lescure FX, et al. Investigating the cultural and contextual determinants of antimicrobial stewardship programmes across low-, middle- and high-income countries—A qualitative study. PloS One. (2019) 14:e0209847. doi: 10.1371/JOURNAL.PONE.0209847
27. Akpan MR, Isemin NU, Udoh AE, and Ashiru-Oredope D. Implementation of antimicrobial stewardship programmes in African countries: a systematic literature review. J Glob Antimicrob Resist. (2020) 22:317–24. doi: 10.1016/J.JGAR.2020.03.009
28. Sawe HR, Mfinanga JA, Lidenge SJ, Mpondo BCT, Msangi S, Lugazia E, et al. Disease patterns and clinical outcomes of patients admitted in intensive care units of tertiary referral hospitals of Tanzania. BMC Int Health Hum Rights. (2014) 14:1–8. doi: 10.1186/1472-698X-14-26/TAVLES/4
29. Novacescu AN, Buzzi B, Bedreag O, Papurica M, Rogobete AF, Sandesc D, et al. Bacterial and fungal superinfections in COVID-19 patients hospitalized in an intensive care unit from timșoara, Romania. Infect Drug Resist. (2022) 15:7001–14. doi: 10.2147/IDR.S390681
30. Jinks T, Subramaniam S, Bassetti M, Gales AC, Kullar R, Metersky ML, et al. Opportunities to enhance diagnostic testing and antimicrobial stewardship: A qualitative multinational survey of healthcare professionals. Infect Dis Ther. (2024) 13:1621–37. doi: 10.1007/s40121-024-00996-1
31. Gebretekle GB, Haile Mariam D, Abebe W, Amogne W, Tenna A, Fenta TG, et al. Opportunities and barriers to implementing antibiotic stewardship in low and middle-income countries: lessons from a mixed-methods study in a tertiary care hospital in Ethiopia. PloS One. (2018) 13:e0208447. doi: 10.1371/journal.pone.0208447
32. Groumoutis JY, Gorman SK, and Beach JE. Identifying opportunities for antimicrobial stewardship in a tertiary intensive care unit: A qualitative study. Can J Crit Care Nurs. (2023) 34:8–17. doi: 10.5737/23688653-3428
33. Kaki R, Elligsen M, Walker S, Simor A, Palmay L, and Daneman N. Impact of antimicrobial stewardship in critical care: a systematic review. J antimicrobial chemotherapy. (2011) 66:1223–30. doi: 10.1093/jac/dkr137
34. Ture Z, Güner R, and Alp E. Antimicrobial stewardship in the intensive care unit. J Intensive Med. (2023) 3:244–53. doi: 10.1016/j.jointm.2022.10.001
35. Michie S, Atkins L, and West R. The behaviour change wheel: a guide to designing interventions. United Kingdom: Silverback Publishing (2014).
Keywords: bacterial profile, prescription patterns, antibiotic resistance patterns, critical care units, Kenyatta National Hospital
Citation: Obegi E, Oluka M, Opanga S, Aywak D, Okalebo F and Okumu M (2025) Prescription and antibiotic resistance patterns at selected critical care units of the largest teaching and referral hospital in Kenya. Front. Trop. Dis. 6:1555008. doi: 10.3389/fitd.2025.1555008
Received: 03 January 2025; Accepted: 28 July 2025;
Published: 28 August 2025.
Edited by:
Alex Owusu-Ofori, Kwame Nkrumah University of Science and Technology, GhanaReviewed by:
Gayathri Govindaraju, Rutgers, The State University of New Jersey, United StatesSantenna Chenchula, All India Institute of Medical Sciences, Bhopal, India
Copyright © 2025 Obegi, Oluka, Opanga, Aywak, Okalebo and Okumu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emmah Obegi, ZW1haG9iZWdpQGdtYWlsLmNvbQ==
 Emmah Obegi
Emmah Obegi Margaret Oluka
Margaret Oluka Sylvia Opanga
Sylvia Opanga Dorothy Aywak
Dorothy Aywak Faith Okalebo
Faith Okalebo Mitchel Okumu
Mitchel Okumu